Abstract
The objective of this narrative review is to summarize the efficacy and safety of available therapies for rearranged during transfection (RET) fusion-positive non-small cell lung cancer (NSCLC), including in patients with central nervous system (CNS) metastases. Background information is provided on RET rearrangements in NSCLC and the molecular testing options available as well as an overview of clinical guidelines for molecular testing, which recommend broad molecular testing, including for RET rearrangements. The efficacy and safety of potential treatments for RET fusion-positive NSCLC, including multikinase inhibitors, RET-selective inhibitors, pemetrexed-based therapy, and immunotherapies are reviewed from Phase I/II and `real-world’ studies, alongside an overview of primary and secondary resistance mechanisms. The RET-selective inhibitors, selpercatinib and pralsetinib, are preferred first-line therapy options for patients with RET fusion-positive metastatic NSCLC and are recommended as subsequent therapy if RET inhibitors have not been used in the first-line setting.
Keywords: RET fusion-positive NSCLC, treatment, precision medicine, RET rearrangement, oncogene-addiction
This review summarizes the efficacy and safety of available therapies for RET fusion-positive non–small cell lung cancer, including in patients with central nervous system metastases.
Implications for Practice.
This comprehensive review covers a range of topics in RET fusion-positive NSCLC that have direct relevance to cancer care. Clinically relevant background information on RET rearrangements is provided, along with an overview of molecular testing options available and clinical guidelines for molecular testing, noting that application of guideline-based molecular testing in practice is suboptimal. A key focus of the article is on the efficacy and safety of potential treatments for patients with RET fusion-positive NSCLC, including those with CNS metastases.
Introduction
Lung cancer remains the leading cause of death from cancer worldwide despite advances in the understanding of risk, biology and immunologic control, and the advent of newer treatment options.1 Non-small cell lung cancer (NSCLC) is the most common type of lung cancer, accounting for 84% of all lung cancer diagnoses.2
The identification of oncogenic drivers and the subsequent development of targeted therapies (TTs) has established biomarker-based treatment for metastatic NSCLC as standard of care (SOC). International guidelines3-5 recommend that patients whose tumor harbors an actionable molecular aberration should receive the appropriate TT.
Rearranged during transfection (RET) rearrangements were first identified as oncogenic drivers in NSCLC in 2012.6 The proportion of patients with NSCLC who have RET rearrangements (ie, fusion-positive disease) is approximately 1%-2%.7-10 However, in clinical practice, not all patients with NSCLC are tested for RET rearrangements; therefore, the proportion of patients with RET fusion-positive NSCLC who are eligible for TT will be less than 1%-2%. Improvements can be made in precision medicine with increased biomarker testing and use of TTs, although equitable access varies among countries.11
In this review, we summarize the efficacy and safety of all available therapies for RET fusion-positive NSCLC, including in patients with central nervous system (CNS) metastases. To identify relevant published data, a pragmatic, structured literature search was carried out in Embase and MEDLINE from January 1, 2015, to March 12, 2021. Full details of the search strategy, study selection, and data extraction are available in the Supplementary Material.
RET Rearrangements and Molecular Testing
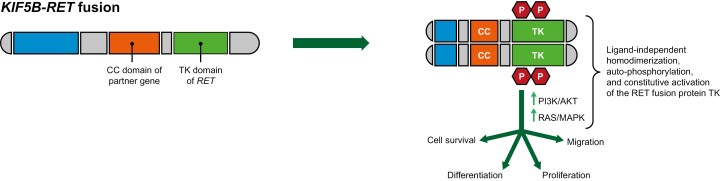
Although RET mutations and fusions have both been identified in several cancers,12 only fusions are known to be involved in NSCLC development.6,9 RET activation typically involves ligand binding, interactions with a co-receptor, and homodimerization, resulting in the formation of a multiprotein complex.13 Most RET fusion proteins lack a transmembrane domain and are chimeric cytosolic proteins that exert their oncogenic influence via constitutive activation of RET kinase domain.14 This enhances activity of various downstream signaling pathways including phosphatidylinositol-3-kinase/protein kinase B, RAS/RAF, and mitogen activated protein kinase,13,14 which, in turn, increase cell proliferation, survival, migration, and differentiation (Figure 1).13
Figure 1.
Schematic model of the mechanism of rearranged during transfection (RET) rearrangements (adapted from13). Intrachromosomal rearrangement (eg, KIF5B-RET fusion) results in ligand-independent homodimerization, auto-phosphorylation, and constitutive activation of the RET fusion protein tyrosine kinase, which leads to increased cell survival, proliferation, migration, and differentiation by activation of downstream pathways including PI3K/AKT and MAPK. Abbreviations: AKT, protein kinase B; MAPK, mitogen activated protein kinase; PI3K, phosphoinositide 3 kinases; RET, rearranged during transfection.
As noted, RET fusions occur in 1%-2% of patients with NSCLC,7-10,15 and 46% of patients develop brain metastases over their lifetime.16RET rearrangements correlate with adenocarcinoma histologic subtype in patients with never-smoking status,17 patients of a younger age (≤60 years),10,18 and more advanced disease stage,9 and potentially may confer higher chemosensitivity (particularly to pemetrexed-based regimens).18,19
At least 45 RET gene fusion partners have been identified in lung cancers, the most common being KIF5B-RET (70%-90%), followed by CCDC6-RET and NCOA4-RET.9,14,20 Although the clinical implications of specific gene fusion partners are currently not well defined, there are data suggesting that the efficacy of RET-selective inhibitory drugs21,22 and some multikinase inhibitors (MKIs; eg, vandetanib) may vary depending on the RET fusion partner (Section 3). A retrospective analysis in patients with RET fusion-positive NSCLC found that selective RET inhibitors were associated with improved survival outcomes versus untreated patients, and irrespective of treatment received, patients with CCDC6-RET fusions had better survival outcomes than those with KIF5B fusions.21 Recent data with pralsetinib from the ARROW trial also indicated that patients with CCDC6-RET-driven disease may have a better prognosis than those with KIF5B-RET driven disease.22
Molecular testing techniques available to detect RET rearrangement include whole genome sequencing, next-generation sequencing (NGS), reverse transcription polymerase chain reaction (RT-PCR), fluorescence in situ hybridization (FISH) and immunohistochemistry (Table 1).23,24 Of these, immunohistochemistry testing is the most convenient, but it has poor sensitivity (false positive up to 40%) and specificity (false negative up to 40%) in the detection of RET rearrangements24 and is generally not used for biomarker testing in the workup for NSCLC.
Table 1.
Differential features of molecular testing techniques (adapted fromBelli et al.24).
| Technique | Specificity | Sensitivity | Detection of fusion partner? | Other advantages or disadvantages |
|---|---|---|---|---|
| IHC | ++ | ++ | No | Convenient but low sensitivity and specificity |
| Break apart FISH | +++ | +++ | Noa | Rapid technique that requires little tissue |
| RT-PCR | +++ | ++/+++ | Yes (known only) | Does not detect unknown fusion partners or those not preselected |
| RNA-sequencing NGS | +++ | +++ | Yes | Expensive, requires high-quality RNA, may detect alterations of uncertain clinical significance and gene expression |
| DNA-sequencing NGS | ++/+++ | ++ | Yes | Use of circulating tumor DNA requires shorter turnaround time and is less invasive than tissue/tumor testing |
aYes if a specific fusion partner probe is used.
FISH, fluorescence in situ hybridization; IHC, immunohistochemistry; NGS, next-generation sequencing; RT-PCR, reverse transcription polymerase chain reaction; ++ indicates moderate; +++ indicates high.
RT-PCR has also been used in RET screening studies using predefined primers to detect RET fusions. The strength of RT-PCR lies in its rapid turn-around time and the ability to identify the specific RET fusion; however, it does not detect unknown fusion partners or variants or those not preselected for the test. Furthermore, poor preservation of RNA in the tumor sample can reduce test sensitivity.
Given the increasing number of potentially actionable driver alterations in NSCLC, there is a need for techniques enabling multiplex testing that is not limited to predefined primers. NGS allows for concurrent screening for gene fusions across thousands of genes or the whole genome without knowledge of the possible fusion partners. Multigene sequencing spares valuable tissue biopsies and can be used on both tumor and liquid samples. Typically, NGS diagnostic tests utilize standard NGS panels that can be customized, and these customized tests can be used to identify gene alterations that can be treated with TTs.25 According to the European Society for Medical Oncology (ESMO) recommendations for detection of RET fusions in daily practice and clinical research,24 RNA-based NGS is considered the first choice in RET detection based on better sensitivity, specificity, and ability to detect fusion partner and expression level of gene fusion (Table 1). This is why RNA-based NGS assays should be considered as first choice in the RET screening process. RNA NGS assays require high-quality RNA (ie, the fragile nature of RNA may impact the quality of the assay), whereas DNA NGS assays have more limited sensitivity for detection of fusion genes because they require proper optimization of the panel to include intronic regions.24 Another testing approach is optimizing mutation and fusion detection in NSCLC by sequential DNA and RNA sequencing, which may be more efficient than parallel DNA and RNA NGS for certain subgroups (eg, smoking-associated NSCLC).26 Interestingly, a recent retrospective study (RETING) in which patients with NSCLC were identified as RET fusion positive or negative as part of routine clinical care showed almost complete concordance between NGS and FISH results upon retesting.27RET rearrangements can also be detected using circulating tumor DNA (ctDNA).28 The primary advantages of ctDNA testing are the ability to test for a broad panel of molecular alterations simultaneously, as well as the possibility of sparing the patient invasive procedures and re-collecting the sample easily if needed. The primary concern is the lower sensitivity, especially in patients with fewer extra-thoracic metastatic lesions or lower disease burden.29
Clinical Guidelines for Molecular Testing
After a patient has received a morphological diagnosis of NSCLC, the next consideration is therapy-predictive biomarker testing. National Comprehensive Cancer Network (NCCN) NSCLC Panel and ESMO guidelines recommend testing for a number of key predictive biomarkers after patients have been diagnosed with metastatic NSCLC and ideally before initial treatment.5,30 NCCN guidelines recommend broad molecular testing using a validated test(s) that assesses a minimum of the following potential genetic variants: EGFR mutations (category 1), ALK rearrangements (category 1), BRAF mutations (category 2A), METex14 skipping mutations (category 2A), neurotrophic tyrosine receptor kinase 1/2/3 gene fusions (category 2A), RET rearrangements (category 2A), Kirsten rat sarcoma (KRAS) mutations (category 2A), and ROS1 rearrangements (category 2A).
The ESMO Translational Research and Precision Medicine Working Group launched a project to review the available methods for the detection of RET gene alterations, their potential applications, and strategies for their implementation. The recommended approach is that, for patients with NSCLC with available formalin-fixed paraffin-embedded specimens, NGS should be used to detect RET fusions; if these specimens are unavailable, FISH or RT-PCR is indicated depending on local availability, cost, and/or the amount of tumor cells available for analysis.24
Unfortunately, not all patients will have equal access to therapy-predictive biomarker testing as its availability varies widely between different geopolitical health systems.5 For example, NGS testing is costly and requires specialized dedicated personnel and is therefore not available at all clinical sites for routine clinical care.24,31 Consequently, application of guideline-based molecular testing appears to be inconsistent and suboptimal. Observational data from across Europe indicate considerable variability in uptake of biomarker testing for NSCLC, ranging from 65% to 85% (2011–2016) across Germany, Italy, and Spain for patients with advanced non-squamous NSCLC, although the rate of molecular testing generally increases over time.32 For example, testing for EGFR mutations increased from 71% to 81% during 2014-2017 across five European countries (France, Germany, Italy, Spain, and the UK). Real-world data from Germany found the testing rate for RET alterations in NSCLC in routine care was 26.9% for non-squamous (n = 2921) and 4.5% for squamous (n = 796) histology.33
Potential Treatments for RET Fusion-Positive NSCLC
Until recently, platinum-based doublets (with or without immunotherapy) were the recommended systemic therapy for all patients newly diagnosed with stage IV NSCLC because of the lack of routine molecular testing at the time. With increased testing for RET alterations in clinical practice, the efficacy and safety of a number of potential treatments for RET fusion-positive NSCLC have been evaluated in phase I and II trials (Table 2) and in “real-world” (eg, retrospective or observational) studies. These include MKIs, RET-selective inhibitory drugs, pemetrexed-based chemotherapy, and immunotherapies.
Table 2.
Efficacy and safety summary of phase I and II trials in patients with RET fusion-positive NSCLC.
| Study name | Study design | Patient population | Efficacy summary | Common grade 3 or worse TRAEs | Other safety data |
|---|---|---|---|---|---|
| Broad-spectrum tyrosine kinase inhibitors (MKIs) | |||||
| Vandetanib | |||||
| NCT0182306843 | Phase II, multicenter, open-label trial | Metastatic or recurrent NSCLC with a RET rearrangement (n = 18) | ORR = 18%, Median PFS = 4.5 months, Median OS = 11.6 months |
Hypertension (n = 3), QT prolongation (n = 2), and elevation of aminotransferases (n = 1) |
|
| LURET42 | Phase II, multicenter, open-label trial | Previously treated RET-altered advanced NSCLC (n = 19) | ORR = 47.4% (95% CI 24.4-71.1), Median PFS = 6.5 months (95% CI 3.9-9.3), Median OS = 13.5 months (95% CI 9.8-28.1) |
Hypertension (68.4%), rash acneiform (15.8%), diarrhea (10.5%), and QT corrected interval prolonged (10.5%) | 4 of 19 pts (21%) discontinued treatment due to AEs |
| NCT0158219144 | Phase I trial (in combination with everolimus) | Stage IV NSCLC with an RET rearrangement (n = 13) | ORR = 54%, Median PFS = 4.4 months (95% CI 3.4, NR) |
Diarrhea (21%), thrombocytopenia (16%), QTc prolongation (5%), and rash (5%) | |
| Cabozantinib | |||||
| NCT0163950846 | Phase II single-arm trial | Advanced RET-altered NSCLC (n = 26) [n = 25 response-evaluable] | ORR = 28% (95% CI 12-49), Median PFS = 5.5 months (95% CI 3.8-8.4), Median OS = 9.9 months (95% CI 8.1-NR) |
Asymptomatic lipase elevation (15%), increased ALT (8%), increased AST (8%), thrombocytopenia (8%), and hypophosphatemia (8%) |
19 of 26 patients (73%) required a dose reduction due to TRAEs |
| Lenvatinib | |||||
| NCT0187708354 | Phase II, multicenter, open-label trial | RET fusion-positive lung adenocarcinoma (n = 25) | ORR = 16% (95% CI 4.5-36.1), Median PFS = 7.3 months (95% CI 3.6-10.2), Median OS = NR |
Hypertension (68%), nausea (60%), decreased appetite (52%), diarrhea (52%), and proteinuria (48%) | 6 of 25 pts (24%) discontinued treatment due to TEAEs; 13 of 25 pts (52%) had serious AEs; fatal AEs occurred in 3 pts, 1 of which was considered a TRAE |
| Alectinib | |||||
| ALL-RET20 | Phase I/II, single-arm, open-label, multi-institutional trial | Previously treated RET-altered NSCLC (n = 34) | ORR (RET inhibitor naïve, n = 25) = 4%, Median PFS = 3.4 months (95% CI 2.0-5.4), Median OS = 19.0 months (95% CI 5.4-NR) |
In phase II (n = 28), the following grade ≥3 TRAEs were each reported in 1 pt (4%): pneumonitis, creatine phosphokinase increase, bilirubin increase, hyponatremia, and neutropenia | |
| RXDX-105 a | |||||
| NCT0187781155 | Phase I/Ib, multicenter, open-label, dose-escalation, and dose-expansion trial | RET fusion-positive NSCLC, TKI naïve (n = 31); RET fusion-positive lung cancer, prior TKI (n = 9) | ORR (TKI naïve) = 19% (95% CI 8-38), ORR (prior TKI) = 0% (95% CI 0-34) |
In pts treated with any dose/any tumor type: fatigue (25%), diarrhea (24%), hypophosphatemia (18%), maculopapular rash (18%), non-maculopapular rash (17%), nausea (15%), elevated ALT (14%) or AST (13%), muscle spasms (13%), decreased appetite (11%), and vomiting (10%) | 4 dose-limiting toxicities were reported: rash, fatigue, diarrhea, and hyperbilirubinemia |
| RET-selective inhibitory molecules | |||||
| BOS172738 | |||||
| NCT0378051766 | Phase I dose-escalation and dose-expansion phase trial | RET-altered advanced solid tumors (NSCLC and medullary thyroid cancers) [n = 67] | NSCLC ORR = 33% | Across both cancer types: creatine phosphokinase increase (54%), dyspnea (34%), facial edema, AST elevation, anemia (25% each), neutropenia, diarrhea (22% each), fatigue (21%), and constipation (20%) | |
| Selpercatinib | |||||
| LIBRETTO-00158,59 | Phase I/II, open-label trial | Pts with RET fusion-positive advanced NSCLC had previously received at least platinum-based chemotherapy (n = 247) or were previously untreated (n = 69) | Pts who previously received at least platinum-based chemotherapy: ORR = 61% (95% CI 55-67), PFS = 24.9 months (95% CI 19.3-NR), 2-year OS = 69% (95% CI 62-75), Treatment-naïve pts: ORR = 84% (95% CI 73-92), PFS = 22.0 months (95% CI 13.8-NR), 2-year OS = 69% (95% CI 55-80) |
Safety population of 796 pts with any RET-altered cancer: hypertension (13%), increased ALT (9%), increased AST (6%) | One grade 5 TRAE reported; discontinuation due to TRAEs: 3% (25/796) |
| Pralsetinib | |||||
| ARROW63,65 | Phase I/II, multi-cohort, open-label trial | Pts with RET fusion-positive NSCLC who had previously received at least platinum-based chemotherapy (n = 130) or were previously untreated (n = 107) | Pts with previous platinum-based chemotherapy: ORR = 63.1% (95% CI 54.2-71.4); PFS = 16.4 months (95% CI 11.4-22.3); OS = 44.3 months (95% CI 26.9-44.3), Treatment-naïve pts: ORR = 77.6% (95% CI 68.5-85.1), PFS = 12.6 months (95% CI 9.2-16.6), OS = NR (95% CI 31.9, NR) |
Neutropenia (20%), anemia (12%), and hypertension (12%) |
Discontinuation due to TRAEs: 10% (safety population of 281 patients) |
aThe development of RXDX-105 has been discontinued.
Abbreviations: AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; MKIs, multikinase inhibitors; NR, not reached; NSCLC, non-small cell lung cancer; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; pt(s), patient(s); RET, rearranged during transfection; TEAE, treatment-emergent adverse event; TKI, tyrosine kinase inhibitor; TRAE, treatment-related adverse event
Multikinase Inhibitors
Several multitarget agents with anti-RET activity have shown inhibition of RET signaling and proliferation of cells expressing RET rearrangements in preclinical models6,34-36 and demonstrated efficacy in clinical trials in unselected patients with NSCLC.37-40 In general, results of clinical trials in molecularly selected patients with RET fusion-positive NSCLC have shown modest efficacy or equivocal results with MKIs (Table 2).
Vandetanib, Cabozantinib, and Lenvatinib
Vandetanib selectively inhibits vascular endothelial growth factor receptor-2 (VEGFR-2), RET, and EGFR signaling.34,41 Among 19 patients with RET fusion-positive NSCLC who were treated with vandetanib in the phase II LURET trial, 47% experienced an objective response, median progression-free survival (PFS) was 6.5 months, median overall survival (OS) was 13.5 months and OS at 12 months was 52.6%.42 A post hoc analysis demonstrated that patients treated with vandetanib who harbored the CCDC6-RET fusion had a numerically longer PFS and OS than those with the KIF5B-RET fusion.42 Broadly similar findings to those of the LURET study were reported in other phase I or II trials with vandetanib (with or without everolimus)43,44 (Table 2). The most common grade ≥3 treatment-related adverse events (TRAEs) with vandetanib in the LURET trial were hypertension (68.4%), rash acneiform (15.8%), diarrhea (10.5%), and QT corrected interval prolonged (10.5%); 4 of 19 patients (21%) discontinued therapy because of adverse events (AEs)42 (Table 2).
Cabozantinib has low nanomolar (ie, strong) activity against RET, in addition to its activity against MET, VEGFR-2, AXL, TIE2, and KIT.45 In a phase II trial in patients with RET fusion-positive NSCLC,46,47 objective response rate (ORR) was 28%, median PFS was 5.5 months, and median OS was 9.9 months, although 19 of 26 patients (73%) required a dose reduction due to TRAEs (Table 2).
A retrospective global registry study (GLORY) of 165 patients with RET fusion-positive lung cancer across 29 centers showed that the majority of patients were non-smokers (63%), 98% of tumors were classified as adenocarcinoma, and KIF5B was the most common RET fusion partner (72%). ORRs with cabozantinib (n = 21), vandetanib (n = 11), and sunitinib (n = 10) were 37%, 18%, and 22%, respectively. Median PFS was 3.6, 2.9, and 2.2 months, and the median OS was 4.9, 10.2, and 6.8 months, respectively. In the same registry, among 108 patients treated with chemotherapy, the ORR was 52%, and PFS and OS were 6.6 and 23.6 months, respectively.48 However, a retrospective study of 39 Chinese patients with NSCLC (9 evaluable) reported no objective response.49
Lenvatinib has activity against VEGFR1-3, fibroblast growth factor receptors 1-4, RET, and other targets.50-53 A total of 25 patients with lung adenocarcinomas and RET alterations were treated with lenvatinib in a phase II open-label multicenter study in Japan.54 ORR was 16%, and median PFS was 7.3 months. The 12-month OS rate was 40% and 67% for patients with KIF5B-RET or CCDC6-RET fusions, respectively, and median PFS was 3.6 and 9.1 months, respectively. Three fatal AEs were reported, including one considered to be treatment related. Discontinuation of lenvatinib was reported in 6 of 25 patients (24%) due to treatment-emergent AEs (Table 2).
Alectinib
The MKI alectinib showed limited activity in a phase I/II Japanese study in RET fusion-positive advanced NSCLC, which was terminated early because of low recruitment.20 Among 25 RET inhibitor-naïve patients, ORR was 4%, with a median PFS and OS of 3.4 and 19.0 months, respectively (Table 2).
RXDX-105
An exploratory phase I/Ib trial examined the efficacy of the oral VEGFR-sparing MKI RXDX-105.55 RXDX-105 is known to have inhibitory activity against wild-type RET, as well as select mutant proteins (eg, RETM918T), and chimeric oncoproteins generated by RET fusion (KIF5B-RET, CCDC6-RET, NCOA4-RET, and PRKAR1A-RET). Although objective response was reported in 6 of 31 patients (ORR 19%) with RET fusion-positive NSCLC, the exact ORR varied significantly according to gene fusion partner (0% with the KIF5B partner vs. 67% with non-KIF5B partners). The most frequently reported grade ≥3 TRAEs were fatigue, diarrhea, and hypophosphatemia (Table 2). The development of RXDX-105 has been discontinued.
RET-Selective Inhibitory Drugs
Novel highly selective molecules that inhibit RET are recent therapeutic discoveries that target fusion proteins and downstream signaling. Although, in general, these agents appear to have greater efficacy and fewer AEs than MKIs in patients with RET-altered NSCLC (Table 2), head-to-head comparative trials in the RET-altered NSCLC population are lacking. Nevertheless, the RET-selective inhibitors selpercatinib and pralsetinib are preferred first-line therapy options for patients with RET fusion-positive metastatic NSCLC and are recommended as subsequent therapy if RET inhibitors have not been used in the first-line setting.30
Selpercatinib
Selpercatinib is a highly selective and potent RET inhibitor with CNS activity specifically designed to target activated RET signaling. Selpercatinib is approved in the US for metastatic RET fusion-positive NSCLC as well as RET-altered thyroid cancers.56 In the EU, selpercatinib is approved for adults with advanced RET fusion-positive NSCLC not previously treated with a RET inhibitor, as well as RET-altered thyroid cancers.57
In the phase I/II clinical trial LIBRETTO-001,58,59 selpercatinib was evaluated in adolescent and adult patients with any type of solid tumor harboring an activating RET alteration (ie, fusions or mutations). Among 247 patients with NSCLC who had previously received platinum-based chemotherapy (median 2 prior systemic lines), the ORR was 61% and median duration of response (DoR) was 28.6 months. After a median follow-up period of 24.7 months, this population achieved a median PFS of 24.9 months, a 2-year PFS rate of 51% and a 2-year OS rate of 69%.59
For the 69 treatment-naïve patients with NSCLC in LIBRETTO-001, the ORR was 84% and the 2-year OS rate was 69%.59 At a median follow-up of 21.9 months, the median PFS was 22.0 months. Among 796 patients with any RET-altered cancer who received at least one dose of study medication, 3% discontinued treatment because of TRAEs, and one grade 5 TRAE (acute respiratory failure) was observed.59 The most frequently reported grade ≥3 TRAEs were hypertension (13%), increased alanine aminotransferase (ALT) (9%), and increased aspartate aminotransferase (AST) (6%)59 (Table 2). The most frequently reported AEs (≥25% of patients) were edema, diarrhea, fatigue, dry mouth, hypertension, increased ALT/AST, abdominal pain, constipation, rash, nausea, increased blood creatinine, and headache. The safety profile observed in patients with NSCLC was consistent with that of the full safety patient population.59
An off-target effect of selpercatinib is that it inhibits the VEGF signaling pathway, and therefore has the potential to adversely affect wound healing and increase the risk of hypertension and bleeding.56
Pralsetinib
Pralsetinib is a highly potent, oral, selective RET inhibitor that targets RET alterations, regardless of the tissue of origin. It is approved in the US for the treatment of RET-altered lung or thyroid cancers60 and in the EU for RET fusion-positive NSCLC not previously treated with an RET inhibitor.61
The ARROW study62-65 was a multicohort, open-label phase I/II study designed to determine the safety and efficacy of pralsetinib in RET-altered solid tumors. In the NSCLC cohort, for the 130 patients previously treated with a platinum-based regimen, the ORR was 63%.65 Median DoR was 38.8 months, median PFS was 16.4 months, and OS was 44.3 months.
Among 107 treatment-naïve patients, ORR was 78%, median DoR was 13.4 months, median PFS was 12.6 months, and median OS was not reached.65
Among patients with RET fusion-positive NSCLC in the safety population (n = 281), the most frequently reported TRAEs were neutropenia (46%), increased AST (41%), anemia (38%), leukopenia (34%), increased ALT (30%), constipation (26%), hypertension and fatigue (both 25%), and there was one TRAE (pneumonia) leading to death.63 The most frequently reported grade ≥3 TRAEs were neutropenia (20%), anemia (12%), and hypertension (12%)63 (Table 2). At the latest data cutoff, discontinuation due to TRAEs was reported in 10% of patients, and the most common (≥10%) grade ≥3 AEs overall were anemia (23%), hypertension (18%), decreased neutrophil count (14%), pneumonia (13%), and neutropenia (11%).65
Similar to selpercatinib, an off-target effect of pralsetinib is inhibition of the VEGF signaling pathway, with the potential to adversely affect wound healing and increasing the risk of hypertension and bleeding.60
BOS172738
NCT03780517 is a phase I study of a potent and selective oral RET kinase inhibitor, BOS172738, consisting of a dose-escalation and dose-expansion phase for patients with RET-altered advanced solid tumors. Common grade ≥3 TRAEs included elevated creatine phosphokinase, dyspnea, and facial edema (Table 2). ORR was 33%, with a report of response in the CNS, indicating potential activity also in patients with CNS metastasis.66
Pemetrexed-Based Chemotherapy
Findings from several real-world studies indicate that RET rearranged lung cancers are sensitive to pemetrexed-based systemic therapy and that pemetrexed-based regimens appear to be the optimal choice of cytotoxic chemotherapy in patients with RET fusion-positive NSCLC (Table 3).19,67-69 In a retrospective review of 104 patients, median PFS with pemetrexed-based treatment was significantly improved for patients with RET fusion-positive disease compared with KRAS-mutant lung cancers.67 Furthermore, an ORR of 45% was reported,67 which is greater than the previously reported ORR of 28% with cabozantinib.70 PFS was also significantly improved with pemetrexed-based versus non-pemetrexed-based regimens as first- and second-line therapy,19 and PFS results were also favorable for pemetrexed-based regimens compared with the MKI vandetanib or immune checkpoint inhibitors (ICIs).68
Table 3.
Overview of real-world studies (retrospective analyses) with pemetrexed-based regimens in patients with RET fusion-positive NSCLC.
| Study | Patient population | Results |
|---|---|---|
| Drilon et al67 | 104 patients with NSCLC with RET (n = 18) or other (n = 86) rearrangements | Median PFS with pemetrexed-based regimens in RET fusion-positive NSCLC (19 months) similar to ALK- (19 months) and ROS1-rearranged (23 months) disease, and significantly improved vs. KRAS-mutant disease (19 vs. 6 months; P < .001) |
| Lee et al68 | 59 Korean patients | Median PFS results were favorable for pemetrexed-based regimens (9.0 months [95% CI 6.9-11.2] vs. vandetanib (2.9 months [95% CI 2.0-3.8] and ICIs (2.1 months [95% CI 1.6-2.6]). Median OS results were also favorable for pemetrexed-based regimens: 24.1 months (95% CI 15.2-33.0) vs. 9.3 months (95% CI 0.3-18.3) and 12.4 months (95% CI 2.9-21.8) |
| Shen et al19 | 62 Chinese patients | Median PFS significantly improved with pemetrexed-based vs non-pemetrexed-based regimens as first-line (9.2 vs. 5.2 months; P < .01) and second-line (4.9 vs. 2.8 months; P < .05) treatment. Median OS (n = 38) was 35.2 vs. 22.6 months (P = .052) |
| Song et al69 | 11 Chinese patients | Median PFS of first-line pemetrexed-based regimens in patients whose disease recurred or became metastatic after surgery (n = 4) was 7.5 months |
Abbreviations: CI, confidence interval; ICIs, immune checkpoint inhibitors; KRAS, Kirsten rat sarcoma; NSCLC, non-small cell lung cancer; OS, overall survival; PFS, progression-free survival; RET, rearranged during transfection; ROS, reactive oxygen species.
Immune Checkpoint Inhibitors
Although anti-programmed death-1/programmed death-ligand 1 (PD1/PD-L1)-directed ICIs are widely used to treat patients with cancer (including NSCLC), early trials with oncogene-driven advanced NSCLC demonstrated limited efficacy and a possible increased risk of toxicity.71 Results of several real-world studies with ICIs in patients with RET fusion-positive NSCLC have also been disappointing.
RET-altered tumors, along with other oncogenic-driven NSCLC, tend to have both low PD-L1 expression and low tumor mutational burden. This suggests that these cancers could be defined as “biologically cold” and could partially explain the poor outcomes observed in patients with RET fusion-positive NSCLC treated with ICIs.72-75 Moreover, RET has been validated as an inhibitor of major histocompatibility complex class I expression.74 Retrospective studies found that patients with any RET-altered malignancy who received non-ICI therapy were at a decreased risk of disease progression compared with those who received ICIs.76 In patients with RET fusion-positive NSCLC, in which those with PD-L1 expression >50% received first-line pembrolizumab and those with PD-L1 <50% received first-line chemotherapy, PFS was significantly shorter with ICI treatment (2.9 vs. 18.5 months; P < .001).77 In the real-world IMMUNOTARGET study, median PFS was similar for patients with NSCLC with RET fusions treated with anti-PD1/PD-L1 ICI versus the overall cohort (2.1 months; 95% confidence interval [CI] 1.3-4.7 vs. 2.8 months; 95% CI 2.5-3.1), and the difference in OS was also not statistically significant.72 Other retrospective analyses of patients with RET-altered lung cancers also showed poor median PFS with ICI therapy, even in patients with tumors that expressed a higher level of PD-L1,73,78 and similar OS versus untreated patients.21
Brain Metastases in RET-Altered NSCLC
Data from registries and retrospective studies have demonstrated up to 25% of patients with RET-altered NSCLC have brain metastases at diagnosis, and approximately 50% of patients will develop brain metastases during their lifetime.16,79
Preclinical evidence indicated the potential for enhanced intracranial efficacy of selpercatinib and pralsetinib.80 Subsequently, both have demonstrated promising intracranial efficacy in the LIBRETTO-001 and ARROW clinical studies, respectively.
Among 15 patients with measurable intracranial metastases at baseline receiving pralsetinib in the ARROW phase I/II study, 8 (53%) had an intracranial response, including 3 complete responses.65 Median duration of intracranial response was 11.5 months at a median follow-up of 29.7 months.
In the LIBRETTO-001 trial, 106 patients had brain metastasis at baseline, and 26 had measurable disease.59 Among patients with measurable disease, the intracranial ORR was 85% (27% with CR, 58% with partial response [PR]), with a CNS median DoR of 9.4 months at a median follow-up period of 25.8 months. No disease progression was identified. In all 106 patients, median CNS PFS was 19.4 months at a median duration of follow-up of 22.1 months. In 178 patients with no baseline CNS metastasis, the estimated probability of observing intracranial progression at 2 years was 0.7%.
The ongoing LIBRETTO-431 study81 has been designed to determine whether selpercatinib can prevent or delay intracranial progression in patients with NSCLC who begin treatment without intracranial involvement. The ongoing AcceleRET Lung study with pralsetinib in patients with RET fusion-positive NSCLC will also evaluate time to intracranial progression.82 In the retrospective SIREN study evaluating selpercatinib in RET fusion-positive NSCLC, ORR was 100% among eight patients with measurable brain metastases.83 In a real-world study evaluating pralsetinib in RET fusion-positive NSCLC in Italy, patients with measurable brain metastases (n = 6) had an intracranial ORR of 83%, and intracranial disease control rate was 100%.84
Mechanisms of Resistance to RET Inhibition
Acquired resistance can develop with tyrosine kinase inhibitors (TKIs) through activation of alternative mechanisms bypassing the targeted kinase and via secondary on-target mutations that interfere with drug binding (Figure 2).85,86 Multiple distinct mechanisms of resistance are often seen in the same patient, and sequential RET-directed treatment may require combination therapy with inhibitors targeting alternative MAPK effectors.87
Figure 2.
Mechanism of acquired resistance to RET TKIs in RET fusion-positive NSCLC (A) and simplified co-crystal structure of RET-selpercatinib complex (B) (adapted from Lu and Zhou, and Thein et al.85,86). In part B, V804 is a gatekeeper residue and K758 is a gatewall residue; magenta denotes residues where selpercatinib-resistant mutations have been identified. Abbreviations: CC, coiled coil; EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer; RET, rearranged during transfection; RETi, RET inhibitor; TK, tyrosine kinase; TKI, tyrosine kinase inhibitor.
Primary Resistance
MET activation was found to be a targetable mediator of resistance to RET-directed therapy in a retrospective study with selpercatinib. Four single-patient protocols were used to combine selpercatinib with the MET/ALK/ROS1 inhibitor crizotinib in patients with an unusually short benefit from selpercatinib, whereas the combination therapy resulted in extended patient responses. Notably, in three of the four cases, MET amplification was present prior to selpercatinib exposure, indicating an intrinsic tumor resistance.88
MET amplification was observed in 15% of 23 tumor and liquid biopsies from patients with advanced RET fusion-positive NSCLC treated with pralsetinib and selpercatinib.89 Median PFS and duration of therapy in this cohort were 6.3 and 7.2 months, respectively, shorter than has been reported from the phase I/II trials of selpercatinib and pralsetinib, suggesting a bias in the study toward early progressors.
In a retrospective analysis of NGS data from 95 patients with RET fusion-positive NSCLC who were treated with a RET inhibitor, primary resistance (disease progression with 6 months) developed in 23% of patients, and KRAS and SMARCA4 mutations were identified only in poor responders, suggesting a role for these co-mutations in primary resistance.90
Secondary Resistance
For the overall drug class, TKIs either occupy both the front and the back clefts of the drug-binding pockets by passing through the gate that separates the front and back clefts or bind only the front cleft. A significant number of cases of mutations leading to TKI drug resistance can be traced back to those occurring at the “gatekeeper” residue in the hinge region of the kinase, directly preventing or weakening the interaction with the inhibitor molecule.91 In contrast, for RET-selective inhibitory drugs, crystal structure studies of RET-kinase-selpercatinib and RET-kinase-pralsetinib complexes have shown that they both dock one end in the front cleft of the drug-binding pocket without inserting through the gate; this novel binding mode is responsible for their high-affinity binding and their ability to avoid disruption of the gatekeeper mutations. However, it also leaves them vulnerable to non-gatekeeper mutations, such as solvent front mutations (those that reside in the solvent front of the adenosine triphosphate-binding pocket in the catalytic region of the kinase domain).91
ctDNA and tissue from patients with RET fusion-positive NSCLC and RET mutation-positive medullary thyroid cancer who had subsequently developed disease progression after an initial response to selpercatinib was examined.92 Solvent front mutations were identified at residue glycine (G)810 that limited the inhibitory activity of selpercatinib, pralsetinib, cabozantinib, and vandetanib because of the prevention of drug binding as evaluated in enzyme and cell-based assays. Although tissue was examined from only a small cohort of patients in this study, the approach utilizing a combination of preclinical and clinical studies is a novel one that could result in the acceleration of development of a next-generation selective RET TKI capable of inhibiting both solvent front and gatekeeper mutations.92 Acquired RET mutations affecting the RET G810 residue in the kinase solvent front were also identified in a small proportion of tissue and/or plasma samples from patients with RET fusion-positive NSCLC following treatment with selpercatinib or pralsetinib.89,93 Other RET mutations of potential relevance to the development of resistance to RET-selective inhibitory drugs include RET L730 and V804.91,93
MET amplification can also be an acquired/secondary mechanism of resistance to RET inhibitors.88,89MET amplification was identified in post-treatment tissue/plasma biopsies in patients with RET fusion-positive NSCLC resistant to treatment with selpercatinib or pralsetinib. Thus, resistance to selective RET inhibition may be driven by RET-independent mechanisms such as acquired MET amplification.88,89 Secondary RET mutations, novel RET rearrangements and MET/MYC amplifications were also identified after RET inhibitor therapy in patients with RET fusion-positive NSCLC.90
Upfront combination treatments may be explored to prevent potentially resistant clones from emerging. To maximize the potential patient benefit, identifying the appropriate patients for each specific combination, determining when combination strategies should be implemented, and testing the potential of alternating different treatment regimens will all be critical to prevent or delay resistance.86,87
RET Fusions as a Mechanism of Resistance to Other Primary Oncogene Drivers
RET-selective inhibitory drugs may have a role in patients whose tumors become resistant to other primary oncogene drivers, such as EGFR mutations.94 Twelve patients having advanced EGFR-mutated NSCLC with an acquired RET fusion detected from tissue or plasma following osimertinib therapy received selpercatinib in combination with osimertinib, across three selpercatinib compassionate access programs.95 Among the evaluable patients, five had a response (50%), which included four confirmed PRs and one unconfirmed PR. For patients who experienced a response, the median DoR was 11 months (range 7.4 to >16.7). Thus, for patients with EGFR-mutant NSCLC with an acquired RET fusion as a mechanism of EGFR inhibitor resistance, the addition of selpercatinib to osimertinib is feasible and potentially effective but remains investigational. This combination will be evaluated prospectively in the phase II ORCHARD study (NCT03944772).
Future Directions and Conclusions
The recent FDA approvals of selpercatinib and pralsetinib have led to expanded treatment options for patients with RET fusion-positive NSCLC. Current NCCN guidelines30 indicate that RET rearrangements are considered “established biomarkers,” reflecting the recently published clinical trial data for their corresponding TTs. Selpercatinib and pralsetinib are first-line therapy options for patients with RET fusion-positive metastatic NSCLC and are recommended as subsequent therapy if RET inhibitors have not been used as first-line therapy.
Next-generation RET inhibitors such as TPX-0046, which has demonstrated activity in drug-resistant and drug-naïve RET-driven preclinical cancer models, may also provide treatment options in the future, although further research is needed. Results of an ongoing phase I/II trial with this agent (NCT04161391) in patients with advanced solid tumors harboring RET fusions or mutations will be of interest. Other next-generation RET inhibitors, such as LOXO-260 and TAS0953/HM06,96 have also demonstrated robust activity in preclinical models of RET alterations, suggesting the potential to extend durable disease control for patients with RET-altered cancers following the development of acquired resistance to RET-selective drugs. For example, TAS0953/HM06 has shown inhibitory effects against a range of mutations, including RET solvent front mutations.96 An ongoing trial with LOXO-260 (NCT05225259) will evaluate the drug in patients with RET-altered tumors that did not or are no longer responsive to treatment with currently available RET inhibitors.
Novel combinations for overcoming drug resistance such as the addition of the MET inhibitor crizotinib to selpercatinib in patients with increased MET expression may be a useful strategy in the treatment of RET fusion-positive NSCLC, although patient numbers are limited.88 In four selpercatinib-treated patients with RET fusion-positive NSCLC with MET amplification (identified in post-treatment biopsies), the addition of crizotinib to selpercatinib provided clinical benefits such as relief of bone pain and/or partial responses.88 Cabozantinib or tivantinib (an MET inhibitor that has been evaluated in advanced solid tumors) may also be beneficial in this setting, although data are currently not available for these combinations.
Of interest will be findings from the ongoing phase III LIBRETTO-431 trial, which will compare the efficacy of selpercatinib versus SOC chemotherapy with or without pembrolizumab in untreated patients with locally advanced/metastatic RET fusion-positive non-squamous NSCLC.81 In addition, AcceleRET Lung, an international, open-label, randomized, phase III study, will evaluate the efficacy and safety of pralsetinib versus SOC for first-line treatment of advanced/metastatic RET fusion-positive NSCLC (NCT04222972).82,97
TTs are also under evaluation in settings such as early disease and adjuvant treatment of NSCLC. The LIBRETTO-432 phase III trial in patients with RET fusion-positive NSCLC with early-stage disease (IB-IIIA) will evaluate the efficacy and safety of adjuvant selpercatinib versus placebo following definitive radiotherapy or surgery with a curative intent (NCT04819100).98 The phase II NAUTIKA1 trial will evaluate multiple therapies in biomarker-selected patients with resectable stages IB-III NSCLC (NCT04302025), including pralsetinib neoadjuvant treatment in the RET fusion-positive cohort.
Despite the benefits of TT, lack of awareness and inconsistent application of guideline-based molecular testing is widespread across all regions of the world.99 As more actionable targets are identified, there is a greater need for the use of multigene testing techniques to optimize sample analysis and reduce time before treatment commencement.
RET-selective inhibitory drugs, such as selpercatinib and pralsetinib, have demonstrated favorable efficacy and tolerability in patients with RET-altered cancers and have additionally shown promising efficacy in patients with brain metastases, which eventually develop in approximately half of patients with RET fusion-positive NSCLC. Selpercatinib and pralsetinib are preferred first-line therapy options for patients with RET fusion-positive metastatic NSCLC and are recommended as subsequent therapy if RET inhibitors have not been used in the first-line setting. Next-generation RET inhibitors are currently under development to overcome the solvent front RET mutations that emerge in some patients treated with selective RET inhibitors.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Philana Fernandes (Lilly), Greg Plosker (Rx Communications, Mold, UK), and Karen Goa (Rx Communications, Mold, UK) for medical writing assistance during the preparation of this manuscript.
President of WALCE Onlus (www.womenagainstlungcancer.eu).
Contributor Information
Silvia Novello, University of Turin, Department of Oncology, AOU San Luigi, Orbassano (Turin), Italy.
Raffaele Califano, The Christie NHS Foundation Trust and Division of Cancer Sciences, The University of Manchester, Manchester, UK.
Niels Reinmuth, Thoracic Oncology Department, Asklepios Lung Clinic, Munich-Gauting, Germany.
Antonella Tamma, Medical Oncology, Eli Lilly and Company, Indianapolis, IN, USA.
Tarun Puri, Medical Oncology, Eli Lilly and Company, Indianapolis, IN, USA.
Funding
Medical writing was funded by Eli Lilly and Company.
Conflict of Interest
Silvia Novello reported consulting/advisory relationships (speakers bureau or advisor) with Eli Lilly, MSD, Roche, BMS, Takeda, Pfizer, AstraZeneca, and Boehringer Ingelheim. Raffaele Califano reported honoraria and consultancy fees from AstraZeneca, Boeringher Ingelheim, Lilly Oncology, Roche, Pfizer, MSD, Bristol Myers Squibb, Takeda, Bayer, Ipsen, Janssen, and Novartis, grants paid to Institution for conduct of clinical trials or contracted research from Roche, AstraZeneca, Pfizer, Clovis, Lilly Oncology, MSD, BMS, Abbvie, Takeda, and Novartis, stock ownership with The Christie Private Care; non-remunerated activities include Principal Investigator for trials with Roche, AstraZeneca, Pfizer, Clovis, Lilly Oncology, MSD, BMS, Abbvie, Takeda, and Novartis; other non-remunerated membership: ESMO and EORTC. Niels Reinmuth reported honoraria for educational lectures and advisory services from AstraZeneca, Boehringer Ingelheim, BMS, MSD, Pfizer, Roche, Lilly, Takeda, Merck, Sanofi, and Janssen. Antonella Tamma and Tarun Puri are employees of Eli Lilly and Company with stock options.
Author Contributions
Conception/design: A.T., T.P. Provision of study material: A.T., T.P. Collection and/or assembly of data: A.T., T.P. Data analysis and interpretation: All authors. Manuscript writing: All authors. Final approval: All authors.
Data Availability
No new data were generated or analyzed in support of this research.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2. Cancer.org. Key statistics for lung cancer 2020. Available at https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html. Accessed September, 2021.
- 3. Hanna N, Johnson D, Temin S, et al. Systemic therapy for stage IV non-small cell lung cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(30):3484-3515. [DOI] [PubMed] [Google Scholar]
- 4. Hanna NH, Robinson AG, Temin S, et al. Therapy for stage IV non-small cell lung cancer with driver alterations: ASCO and OH (CCO) joint guideline update. J Clin Oncol. 2021;39(9):1040-1091. [DOI] [PubMed] [Google Scholar]
- 5. European Society for Medical Oncology. Clinical practice guidelines. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Updated version published 15 September 2020 by the ESMO Guidelines Committee. Available at https://www.esmo.org/content/download/347819/6934778/1/ESMO-CPG-mNSCLC-15SEPT2020.pdf. Accessed November 15, 2021.
- 6. Lipson D, Capelletti M, Yelensky R, et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat Med. 2012;18(3):382-384. 10.1038/nm.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kato S, Subbiah V, Marchlik E, et al. RET aberrations in diverse cancers: next-generation sequencing of 4,871 patients. Clin Cancer Res. 2017;23(8):1988-1997. 10.1158/1078-0432.ccr-16-1679. [DOI] [PubMed] [Google Scholar]
- 8. Mulligan LM. RET revisited: expanding the oncogenic portfolio. Nat Rev Cancer. 2014;14(3):173-186. 10.1038/nrc3680. [DOI] [PubMed] [Google Scholar]
- 9. Wang R, Hu H, Pan Y, et al. RET fusions define a unique molecular and clinicopathologic subtype of non-small cell lung cancer. J Clin Oncol. 2012;30(35):4352-4359. 10.1200/jco.2012.44.1477. [DOI] [PubMed] [Google Scholar]
- 10. Tsuta K, Kohno T, Yoshida A, et al. RET-rearranged non-small cell lung carcinoma: a clinicopathological and molecular analysis. Br J Cancer. 2014;110(6):1571-1578. 10.1038/bjc.2014.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS.. Lung cancer. Lancet. 2021;398(10299):535-554. 10.1016/s0140-6736(21)00312-3. [DOI] [PubMed] [Google Scholar]
- 12. Zhao Z, Fu T, Gao J, et al. Identifying novel oncogenic RET mutations and characterising their sensitivity to RET-specific inhibitors. J Med Genet 2020:jmedgenet-2019-106546. 10.1136/jmedgenet-2019-106546. Epub ahead of print. PMID: 32284345. [DOI] [PubMed] [Google Scholar]
- 13. Stinchcombe TE. Current management of RET rearranged non-small cell lung cancer. Ther Adv Med Oncol 2020;12:1758835920928634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Regua AT, Najjar M, Lo HW.. RET signaling pathway and RET inhibitors in human cancer. Front Oncol. 2022;12:932353. 10.3389/fonc.2022.932353. PMID: 35957881; PMCID: PMC9359433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Takeuchi K. Discovery stories of RET fusions in lung cancer: a mini-review. Front Physiol. 2019;10:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Drilon A, Lin JJ, Filleron T, et al. Frequency of brain metastases and multikinase inhibitor outcomes in patients with RET-rearranged lung cancers. J Thorac Oncol 2018;13(10):1595-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ju YS, Lee WC, Shin JY, et al. A transforming KIF5B and RET gene fusion in lung adenocarcinoma revealed from whole-genome and transcriptome sequencing. Genome Res. 2012;22(3):436-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Drilon A, Hu ZI, Lai GGY, et al. Targeting RET-driven cancers: lessons from evolving preclinical and clinical landscapes. Nat Rev Clin Oncol. 2018;15(3):151-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shen T, Pu X, Wang L, et al. Association between RET fusions and efficacy of pemetrexed-based chemotherapy for patients with advanced NSCLC in China: a multicenter retrospective study. Clin Lung Cancer. 2020;21(5):e349-e354. 10.1016/j.cllc.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 20. Takeuchi S, Yanagitani N, Seto T, et al. Phase 1/2 study of alectinib in RET-rearranged previously-treated non-small cell lung cancer (ALL-RET). Transl Lung Cancer Res 2021;10(1):314-325. 10.21037/tlcr-20-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tan AC, Seet AOL, Lai GGY, et al. Molecular characterization and clinical outcomes in RET-rearranged NSCLC. J Thorac Oncol 2020;15(12):1928-1934. [DOI] [PubMed] [Google Scholar]
- 22. Gadgeel SM, Gainor J, Cappuzzo F, et al. Relationship between RET fusion partner and treatment outcomes in patients (pts) with non-small cell lung cancer (NSCLC) from the phase I/II ARROW study and real-world data (RWD) [abstract no. 984P]. Ann Oncol. 2022;33(suppl 7):S1001S448-S100S1002. 10.1016/j.annonc.2022.07.1111. [DOI] [Google Scholar]
- 23. Ferrara R, Auger N, Auclin E, et al. Clinical and translational implications of RET rearrangements in non-small cell lung cancer. J Thorac Oncol 2018;13(1):27-45. [DOI] [PubMed] [Google Scholar]
- 24. Belli C, Penault-Llorca F, Ladanyi M, et al. ESMO recommendations on the standard methods to detect RET fusions and mutations in daily practice and clinical research. Ann Oncol. 2021;32(3):337-350. 10.1016/j.annonc.2020.11.021. [DOI] [PubMed] [Google Scholar]
- 25. Doostparast Torshizi A, Wang K.. Next-generation sequencing in drug development: Target identification and genetically stratified clinical trials. Drug Discov Today. 2018;23(10):1776-1783. 10.1016/j.drudis.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 26. Cohen D, Hondelink LM, Solleveld-Westerink N, et al. Optimizing mutation and fusion detection in NSCLC by sequential DNA and RNA sequencing. J Thorac Oncol 2020;15(6):1000-1014. [DOI] [PubMed] [Google Scholar]
- 27. Conde E, Hernandez S, Caminoa A, et al. RET fusion testing in advanced non-small cell lung carcinoma patients: the RETING study. Abstract presented at: 2021 World Conference on Lung Cancer; September 8–14, 2021; Worldwide virtual event.
- 28. Supplee JG, Milan MSD, Lim LP, et al. Sensitivity of next-generation sequencing assays detecting oncogenic fusions in plasma cell-free DNA. Lung Cancer. 2019;134(Aug):96-99. [DOI] [PubMed] [Google Scholar]
- 29. Aggarwal C, Thompson JC, Black TA, et al. Clinical implications of plasma-based genotyping with the delivery of personalized therapy in metastatic non-small cell lung cancer. JAMA Oncol 2019;5(2):173-180. 10.1001/jamaoncol.2018.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. National Comprehensive Cancer Network. NCCN guidelines: non-small cell lung cancer, version 3.2022. Available at NCCN.org. Accessed August 29, 2022.
- 31. Legras A, Barritault M, Tallet A, et al. Validity of targeted next-generation sequencing in routine care for identifying clinically relevant molecular profiles in non-small cell lung cancer: results of a 2-year experience on 1343 samples. J Mol Diagn. 2018;20(4):550-564. 10.1016/j.jmoldx.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 32. Kerr KM, Bibeau F, Thunnissen E, et al. The evolving landscape of biomarker testing for non-small cell lung cancer in Europe. Lung Cancer. 2021;154:161-175. 10.1016/j.lungcan.2021.02.026. Epub 2021 Feb 22. PMID: 33690091. [DOI] [PubMed] [Google Scholar]
- 33. Griesinger F, Eberhardt W, Nusch A, et al. Biomarker testing in non-small cell lung cancer in routine care: analysis of the first 3,717 patients in the German prospective, observational, nation-wide CRISP registry (AIO-TRK-0315). Lung Cancer. 2021;152(Feb):174-184. [DOI] [PubMed] [Google Scholar]
- 34. Carlomagno F, Vitagliano D, Guida T, et al. ZD6474, an orally available inhibitor of KDR tyrosine kinase activity, efficiently blocks oncogenic RET kinases. Cancer Res. 2002;62(24):7284-7290. [PubMed] [Google Scholar]
- 35. Kohno T, Ichikawa H, Totoki Y, et al. KIF5B-RET fusions in lung adenocarcinoma. Nat Med. 2012;18(3):375-377. 10.1038/nm.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Matsubara D, Kanai Y, Ishikawa S, et al. Identification of CCDC6-RET fusion in the human lung adenocarcinoma cell line, LC-2/ad. J Thorac Oncol 2012;7(12):1872-1876. [DOI] [PubMed] [Google Scholar]
- 37. Herbst RS, Sun Y, Eberhardt WEE, et al. Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small cell lung cancer (ZODIAC): a double-blind, randomised, phase 3 trial. Lancet Oncol. 2010;11(7):619-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Natale RB, Thongprasert S, Greco FA, et al. Phase III trial of vandetanib compared with erlotinib in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2011;29(8):1059-1066. 10.1200/jco.2010.28.5981. [DOI] [PubMed] [Google Scholar]
- 39. Lee JS, Hirsh V, Park K, et al. Vandetanib versus placebo in patients with advanced non-small-cell lung cancer after prior therapy with an epidermal growth factor receptor tyrosine kinase inhibitor: a randomized, double-blind phase III trial (ZEPHYR). J Clin Oncol. 2012;30(10):1114-1121. 10.1200/jco.2011.36.1709. [DOI] [PubMed] [Google Scholar]
- 40. De Boer RH, Arrieta O, Yang C-H, et al. Vandetanib plus pemetrexed for the second-line treatment of advanced non-small-cell lung cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2011;29(8):1067-1074. 10.1200/jco.2010.29.5717. [DOI] [PubMed] [Google Scholar]
- 41. Wedge SR, Ogilvie DJ, Dukes M, et al. ZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration. Cancer Res. 2002;62(16):4645-4655. [PubMed] [Google Scholar]
- 42. Yoh K, Seto T, Satouchi M, et al. Final survival results for the phase II study of vandetanib in previously treated patients with RET-rearranged advanced non-small cell lung cancer. Lung Cancer. 2021;155(May):40-45. 10.1016/j.lungcan.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 43. Lee SH, Lee JK, Ahn MJ, et al. Vandetanib in pretreated patients with advanced non-small cell lung cancer-harboring RET rearrangement: a phase II clinical trial. Ann Oncol. 2017;28(2):292-297. 10.1093/annonc/mdw559. [DOI] [PubMed] [Google Scholar]
- 44. Subbiah V, Cascone T, Hess KR, et al. Multi-kinase RET inhibitor vandetanib combined with mTOR inhibitor everolimus in patients with RET rearranged non-small cell lung cancer. J Clin Oncol. 2018;36(15 suppl):9035-9035. 10.1200/jco.2018.36.15_suppl.9035. [DOI] [Google Scholar]
- 45. Yakes FM, Chen J, Tan J, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. 2011;10(12):2298-2308. 10.1158/1535-7163.mct-11-0264. [DOI] [PubMed] [Google Scholar]
- 46. Drilon A, Rekhtman N, Arcila M, et al. Cabozantinib in patients with advanced RET-rearranged non-small cell lung cancer: an open-label, single-centre, phase 2, single-arm trial. Lancet Oncol. 2016;17(12):1653-1660. 10.1016/s1470-2045(16)30562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Drilon A, Somwar R, Smith R, et al. A phase 2 study of cabozantinib for patients with advanced RET-rearranged lung cancers. J Thorac Oncol 2017;12(1 Suppl 1):S286-S287. [Google Scholar]
- 48. Gautschi O, Milia J, Filleron T, et al. Targeting RET in patients with RET-rearranged lung cancers: results from the global, multicenter RET registry. J Clin Oncol. 2017;35(13):1403-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xing P, Yang N, Mu Y, et al. The clinical significance of RET gene fusion among Chinese patients with lung cancer. Transl Cancer Res 2020;9(10):6455-6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Matsui J, Yamamoto Y, Funahashi Y, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer. 2008;122(3):664-671. [DOI] [PubMed] [Google Scholar]
- 51. Okamoto K, Kodama K, Takase K, et al. Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against RET gene fusion-driven tumor models. Cancer Lett. 2013;340(1):97-103. 10.1016/j.canlet.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 52. Tohyama O, Matsui J, Kodama K, et al. Antitumor activity of lenvatinib (E7080): An angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res 2014;2014:638747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Matsui J, Funahashi Y, Uenaka T, et al. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin Cancer Res. 2008;14(17):5459-5465. 10.1158/1078-0432.ccr-07-5270. [DOI] [PubMed] [Google Scholar]
- 54. Hida T, Velcheti V, Reckamp KL, et al. A phase 2 study of lenvatinib in patients with RET fusion-positive lung adenocarcinoma. Lung Cancer. 2019;138(Dec):124-130. 10.1016/j.lungcan.2019.09.011. [DOI] [PubMed] [Google Scholar]
- 55. Drilon A, Fu S, Patel MR, et al. A phase I/Ib trial of the VEGFR-sparing multikinase RET inhibitor RXDX-105. Cancer Discov 2019;9(3):384-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Selpercatinib (Retevmo). US prescribing information. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213246s000lbl.pdf. Accessed February 15, 2022.
- 57. Union Register of medicinal products – Public health – European Commission. Available at https://ec.europa.eu/health/documents/community-register/html/h1527.htm. Accessed September 6, 2022.
- 58. Drilon A, Oxnard GR, Tan DSW, et al. Efficacy of selpercatinib in RET fusion-positive non-small cell lung cancer. N Engl J Med. 2020;383(9):813-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Drilon A, Subbiah V, Gautschi O, et al. Selpercatinib in patients with RET fusion-positive non-small cell lung cancer: updated safety and efficacy from the registrational LIBRETTO-001 phase I/II trial. J Clin Oncol 2023;41(2):385-394. 10.1200/JCO.22.00393 PMID: 36122315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pralsetinib (Gavreto). US prescribing information. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/214701s000lbl.pdf. Accessed February 15, 2022.
- 61. Pralsetinib (Gavreto). Summary of product characteristics. Available at https://www.ema.europa.eu/en/documents/product-information/gavreto-epar-product-information_en.pdf. Accessed February 15, 2022.
- 62. Gainor JF, Curigliano G, Kim D-W, et al. Pralsetinib for RET fusion-positive non-small cell lung cancer (ARROW): a multi-cohort, open-label, phase 1/2 study. Lancet Oncol. 2021;22(7):959-969. 10.1016/s1470-2045(21)00247-3. [DOI] [PubMed] [Google Scholar]
- 63. Griesinger F, Curigliano G, Thomas M, et al. Safety and efficacy of pralsetinib in RET fusion–positive non-small cell lung cancer including as first-line therapy: update from the ARROW trial. Ann Oncol. 2022;33(11):1168-1178. 10.1016/j.annonc.2022.08.002. [DOI] [PubMed] [Google Scholar]
- 64. Curigliano G, Gainor JF, Griesinger F, et al. Safety and efficacy of pralsetinib in patients with advanced RET fusion-positive non-small cell lung cancer: Update from the ARROW trial. J Clin Oncol 2021;39(15 suppl):9089 [plus poster presented at the 2021 American Society of Clinical Oncology virtual annual meeting; June 4–8, 2021. 10.1200/jco.2021.39.15_suppl.9089. [DOI] [Google Scholar]
- 65. Besse B, Griesinger F, Curigliano G, et al. Updated efficacy and safety data from the phase I/II ARROW study of pralsetinib in patients (pts) with advanced RET fusion+ non-small cell lung cancer (NSCLC). Ann Oncol. 2022;33(suppl 7):S1083S448-S108S1084. 10.1016/j.annonc.2022.07.1293. [DOI] [Google Scholar]
- 66. Schoffski P, Cho BC, Italiano A, et al. BOS172738, a highly potent and selective RET inhibitor, for the treatment of RET-altered tumors including RET-fusion+ NSCLC and RET-mutant MTC: Phase 1 study results. J Clin Oncol. 2021;39(15 suppl):3008-3008. 10.1200/jco.2021.39.15_suppl.3008. [DOI] [Google Scholar]
- 67. Drilon A, Bergagnini I, Delasos L, et al. Clinical outcomes with pemetrexed-based systemic therapies in RET-rearranged lung cancers. Ann Oncol. 2016;27(7):1286-1291. 10.1093/annonc/mdw163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lee J, Ku BM, Shim JH, et al. Characteristics and outcomes of RET-rearranged Korean non-small cell lung cancer patients in real-world practice. Jpn J Clin Oncol. 2020;50(5):594-601. [DOI] [PubMed] [Google Scholar]
- 69. Song Z, Yu X, Zhang Y.. Clinicopathologic characteristics, genetic variability and therapeutic options of RET rearrangements patients in lung adenocarcinoma. Lung Cancer. 2016;101(Nov):16-21. 10.1016/j.lungcan.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 70. Drilon AE, Sima CS, Somwar R, et al. Phase II study of cabozantinib for patients with advanced RET-rearranged lung cancers. J Clin Oncol. 2015;33(15 suppl 1):8007-8007. 10.1200/jco.2015.33.15_suppl.8007. [DOI] [Google Scholar]
- 71. Mhanna L, Guibert N, Milia J, et al. When to consider immune checkpoint inhibitors in oncogene-driven non-small cell lung cancer?. Curr Treat Options Oncol. 2019;20(7):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mazieres J, Drilon A, Lusque A, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol. 2019;30(8):1321-1328. 10.1093/annonc/mdz167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Offin M, Guo R, Wu SL, et al. Immunophenotype and response to immunotherapy of RET-rearranged lung cancers. JCO Precis Oncol 2019;3:PO.18.00386. 10.1200/PO.18.00386. Epub 2019 May 16. PMID: 31192313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Brea EJ, Oh CY, Manchado E, et al. Kinase regulation of human MHC class I molecule expression on cancer cells. Cancer Immunol Res 2016;4(11):936-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gainor JF, Shaw AT, Sequist LV, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin Cancer Res. 2016;22(18):4585-4593. 10.1158/1078-0432.ccr-15-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hegde A, Andreev-Drakhlin AY, Roszik J, et al. Responsiveness to immune checkpoint inhibitors versus other systemic therapies in RET-aberrant malignancies. ESMO Open 2020;5(5):e000799. 10.1136/esmoopen-2020-000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Marra A, Belli C, Passaro A, et al. Clinical implications of RET rearrangements in non-squamous NSCLC patients: an Italian single-institution study. 21st National Congress of Italian Association of Medical Oncology 2019.
- 78. Bhandari NR, Hess LM, Han Y, Zhu YE, Sireci AN.. Efficacy of immune checkpoint inhibitor therapy in patients with RET fusion-positive non-small cell lung cancer. Immunotherapy 2021;13(11):893-904. 10.2217/imt-2021-0035. [DOI] [PubMed] [Google Scholar]
- 79. Lee J, Ku BM, Shim JH, et al. P2.14-54 high incidence of CNS metastases in advanced or recurrent non-small cell lung cancer patients with RET fusion. J Thorac Oncol 2019;14(10):S851-S852. [Google Scholar]
- 80. Subbiah V, Velcheti V, Tuch BB, et al. Selective RET kinase inhibition for patients with RET-altered cancers. Ann Oncol. 2018;29(8):1869-1876. 10.1093/annonc/mdy137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Solomon BJ, Zhou CC, Drilon A, et al. Phase III study of selpercatinib versus chemotherapy ± pembrolizumab in untreated RET positive non-small cell lung cancer. Future Oncol. 2021;17(7):763-773. 10.2217/fon-2020-0935. [DOI] [PubMed] [Google Scholar]
- 82. Besse B, Felip E, Kim ES, et al. AcceleRET lung: a phase 3 study of first-line pralsetinib in patients with RET-fusion+ advanced/metastatic NSCLC [abstract no. PUL01.02]. J Thorac Oncol 2021;16(1 suppl):S44-S45. [Google Scholar]
- 83. Illini O, Hochmair MJ, Fabikan H, et al. Selpercatinib in RET fusion-positive non-small cell lung cancer (SIREN): a retrospective analysis of patients treated through an access program. Ther Adv Med Oncol 2021;13(June 11):17588359211019617588359211019675. 10.1177/17588359211019675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Passaro A, Lo Russo G, Passiglia F, et al. Pralsetinib in RET fusion-positive non-small cell lung cancer: a real-world data (RWD) analysis from the Italian expanded access program (EAP). Ann Oncol. 2022;33(suppl 7):S1065S448-S1065S554. 10.1016/j.annonc.2022.07.1248. [DOI] [PubMed] [Google Scholar]
- 85. Lu C, Zhou Q.. Diagnostics, therapeutics and RET inhibitor resistance for RET fusion-positive non-small cell lung cancers and future perspectives. Cancer Treat Rev. 2021;96:102153. 10.1016/j.ctrv.2021.102153. Epub 2021 Jan 16. PMID: 33773204. [DOI] [PubMed] [Google Scholar]
- 86.. Thein KZ, Velcheti V, Mooers BHM, Wu J, Subbiah V.. Precision therapy for RET-altered cancers with RET inhibitors. Trends Cancer. 2021;7(12):1074-1088. 10.1016/j.trecan.2021.07.003. Epub 2021 Aug 12. PMID: 34391699; PMCID: PMC8599646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Rosen EY, Won HH, Zheng Y, et al. The evolution of RET inhibitor resistance in RET-driven lung and thyroid cancers. Nat Commun. 2022;13(1):1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Rosen EY, Johnson ML, Clifford SE, et al. Overcoming MET-dependent resistance to selective RET inhibition in patients with RET fusion-positive lung cancer by combining selpercatinib with crizotinib. Clin Cancer Res. 2021;27(1):34-42. 10.1158/1078-0432.ccr-20-2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lin JJ, Liu SV, McCoach CE, et al. Mechanisms of resistance to selective RET tyrosine kinase inhibitors in RET fusion-positive non-small cell lung cancer. Ann Oncol. 2020;31(12):1725-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Marinello A, Vasseur D, Conci N, et al. Mechanism of primary and secondary resistance to RET inhibitors in patients with RET-positive advanced NSCLC [abstract no. 1007P]. Ann Oncol. 2022;33(suppl 7):S1013S448-S101S1014. 10.1016/j.annonc.2022.07.1133. [DOI] [Google Scholar]
- 91. Subbiah V, Shen T, Terzyan SS, et al. Structural basis of acquired resistance to selpercatinib and pralsetinib mediated by non-gatekeeper RET mutations. Ann Oncol. 2021;32(2):261-268. 10.1016/j.annonc.2020.10.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Solomon BJ, Tan L, Lin JJ, et al. RET solvent front mutations mediate acquired resistance to selective RET inhibition in RET-driven malignancies. J Thorac Oncol 2020;15(4):541-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Gainor JF, Curigliano G, Doebele RC, et al. Analysis of resistance mechanisms to pralsetinib (BLU-667) in patients with RET fusion-positive non-small cell lung cancer (NSCLC) from the ARROW study [poster]. 2020 North America Conference on Lung Cancer; Oct 16‒17, 2020.
- 94. Lu C, Cheng J-T, Kang J, et al. Clinical outcomes of NSCLC patients with acquired RET rearrangement after resistance to osimertinib. J Clin Oncol. 2019;37(15 Suppl):e20626-e20626. 10.1200/jco.2019.37.15_suppl.e20626. [DOI] [Google Scholar]
- 95. Rotow J, Patel J, Hanley M, et al. Combination osimertinib plus selpercatinib for EGFR-mutant non-small cell lung cancer (NSCLC) with acquired RET fusions. J Thorac Oncol 2021;16(3):S230. 10.1016/j.jtho.2021.01.150. [DOI] [Google Scholar]
- 96. Miyazaki I, Ishida K, Kato M, et al. Discovery of TAS0953/HM06, a novel next generation RET-specific inhibitor capable of inhibiting RET solvent front mutations [abstract]. AACR-NCI-EORTC Virtual International Conference on Molecular Targets and Cancer Therapeutics; Oct 7–10, 2021.
- 97. Besse B, Felip E, Clifford C, et al. AcceleRET lung: a phase III study of first-line pralsetinib in patients (pts) with RET-fusion+ advanced/metastatic non-small cell lung cancer (NSCLC). J Clin Oncol. 2020;38(15 suppl):TPS9633-TPS9633. 10.1200/jco.2020.38.15_suppl.tps9633. [DOI] [Google Scholar]
- 98. Goldman J, Besse B, Wu Y, et al. LIBRETTO-432: a placebo-controlled phase 3 study of adjuvant selpercatinib in stage IB-IIIA RET fusion-positive NSCLC [abstract no. P01.01]. J Thorac Oncol 2021;16(10):S975-S976. [Google Scholar]
- 99. Smeltzer MP, Wynes MW, Lantuejoul S, et al. The International Association for the Study of Lung Cancer Global Survey on Molecular Testing in Lung Cancer. J Thorac Oncol 2020;15(9):1434-1448. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were generated or analyzed in support of this research.