Abstract
Measuring immune correlates of disease acquisition and protection in the context of a clinical trial is a prerequisite for improved vaccine design. We analyzed binding and neutralizing antibody measurements four weeks post-vaccination as correlates of risk of moderate to severe-critical COVID-19 through 83 days post-vaccination in the phase III, double-blind placebo-controlled phase of ENSEMBLE, an international, randomized efficacy trial of a single dose of Ad26.COV2.S. We also evaluated correlates of protection in the trial cohort. Of the three antibody immune markers we measured, we found most support for 50% inhibitory dilution (ID50) neutralizing antibody titer as a correlate of risk and of protection. The outcome hazard ratio was 0.49 (95% confidence interval 0.29, 0.81; p=0.006) per 10-fold increase in ID50; vaccine efficacy was 60% (43, 72%) at nonquantifiable ID50 (< 2.7 IU50/ml) and increased to 89% (78, 96%) at ID50 = 96.3 IU50/ml. Comparison of the vaccine efficacy by ID50 titer curves for ENSEMBLE-US, the COVE trial of the mRNA-1273 vaccine, and the COV002-UK trial of the AZD1222 vaccine supported that ID50 titer is a correlate of protection across trials and vaccine types.
Introduction
The ENSEMBLE trial (NCT04505722, https://clinicaltrials.gov/ct2/show/NCT04505722) was carried out in Argentina, Brazil, Chile, Colombia, Mexico, Peru, South Africa and the United States to test the efficacy of a single dose of the replication-incompetent human adenovirus type 26 (Ad26)-vectored Ad26.COV2.S vaccine vs. placebo to prevent moderate to severe-critical COVID-19.1,2 Estimated vaccine efficacy against COVID-19 with onset at least 28 days post-injection was 66.1% (95% confidence interval (CI): 55.0 to 74.8) in the primary analysis (median follow-up two months).1 The US Food and Drug Administration (FDA) granted an Emergency Use Authorization to the Ad26.COV2.S vaccine as a single primary vaccination dose for individuals aged ≥18 years and, more recently, as a single homologous or heterologous booster dose for individuals aged ≥18 years.3 The Ad26.COV2.S vaccine has also been issued an Emergency Use Listing by the World Health Organization,4 authorized by the European Commission,5 and approved or authorized in more than 100 countries.6
A validated immune biomarker that correlates with protection7–9 (a “correlate of protection,” or CoP) has several applications including providing evidence for approval of demonstrated-effective vaccines for populations underrepresented in the phase 3 trials (e.g. young children10,11), aiding approval of refined versions of demonstrated-effective vaccines (e.g., strain or schedule changes), aiding approval of candidate vaccines to test efficacy in phase 3 trials, and providing a study endpoint in early-phase trials for comparison and down-selection of candidate next-generation vaccines. A CoP also has population-level applications, including estimating the level of immunity of a population using sero-survey data.12
For most licensed vaccines against viral diseases where a CoP has been established, the CoP is either binding antibodies (bAbs) or neutralizing antibodies (nAbs).8 A growing body of evidence supports these immune markers as CoPs for COVID-19 vaccines. First, both bAbs13 and nAbs14 acquired through infection have been shown to correlate with protection from reinfection, and adoptive transfer of purified convalescent immunoglobulin G (IgG) protected rhesus macaques from SARS-CoV-2 challenge.15 Second, nAb titers elicited by DNA,16 mRNA,17 and adenovirus vectored18 COVID-19 vaccines all correlated with protection of rhesus macaques from SARS-CoV-2 challenge. Third, passive immunization with nAbs had protective efficacy in a phase 3 trial of high risk individuals.19 Fourth, bAbs and nAbs correlated with vaccine efficacy in meta-analyses of phase 3 randomized, placebo-controlled clinical trials.20,21 The evidence provided by correlates analyses of randomized phase 3 trials carries extra weight in the evaluation of CoPs, and is the gold standard for obtaining reliable, unbiased evidence.22
The US Government (USG) COVID-19 Response Team in public-private partnerships with the vaccine developers designed and implemented five harmonized phase 3 COVID-19 vaccine efficacy trials with a major objective being to develop a CoP based on an IgG bAb or nAb assay.23 The first correlates analysis in this program evaluated the mRNA-1273 COVID-19 vaccine in the COVE trial,24 which showed that both IgG bAb and nAb markers measured four weeks post second dose were strongly correlated with the level of mRNA-1273 vaccine efficacy against symptomatic COVID-19, with nAb titer mediating about two-thirds of the vaccine efficacy.25 These findings were consistent with those of the phase 3 COV002-UK trial of the AZD12222 (ChAdOx1 nCoV-19) vaccine, where vaccine efficacy against symptomatic COVID-19 increased with post-injection bAb and nAb markers.26
The ENSEMBLE trial was included in this USG-coordinated effort to identify CoPs. Using the same approach as was used for COVE,25 for one dose of the Ad26.COV2.S vaccine in ENSEMBLE we assessed IgG bAb and nAb markers measured four weeks post one dose of the Ad26.COV2.S vaccine in ENSEMBLE as correlates of risk of COVID-19 and as correlates of protection against COVID-19. (We use “correlate of risk” to indicate a post-vaccination immune marker associated with the rate of COVID-19, and “correlate of protection” to indicate that a correlate of risk is also predictive of vaccine efficacy against COVID-19, which is quantified by estimating a causal parameter that links the marker in some fashion to vaccine efficacy (ref.9 and the Statistical Analysis Plan in ref.27) Three markers were studied: IgG bAbs against SARS-CoV-2 spike protein (spike IgG), IgG bAbs against the spike protein receptor binding domain (RBD IgG), and nAbs measured by a pseudovirus neutralization assay (50% inhibitory dilution, ID50). We report spike IgG and RBD IgG readouts in WHO international units (IU) and calibrated ID50 titers to a WHO international standard, which enables comparing the results to those of the COVE and the COV002-UK trials.
RESULTS
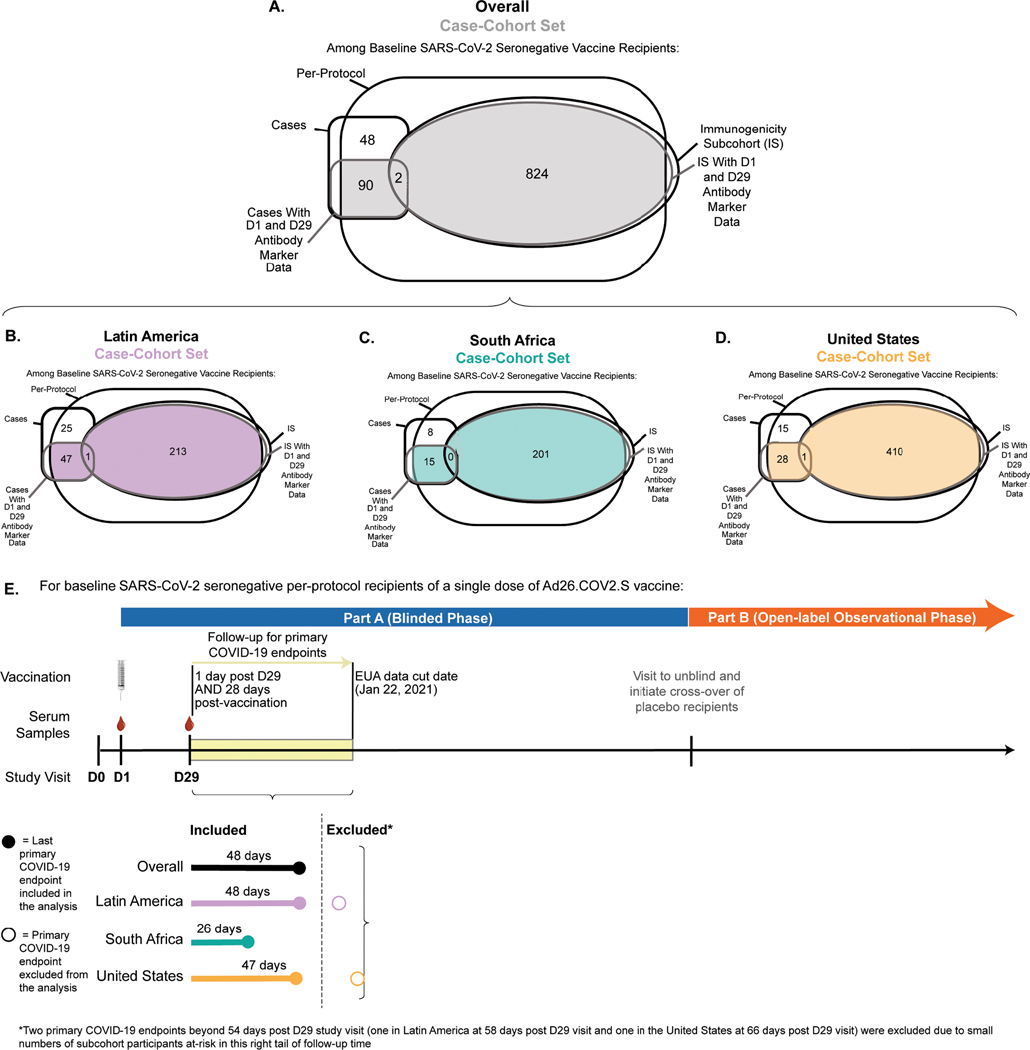
Immunogenicity sub-cohort and case-cohort set
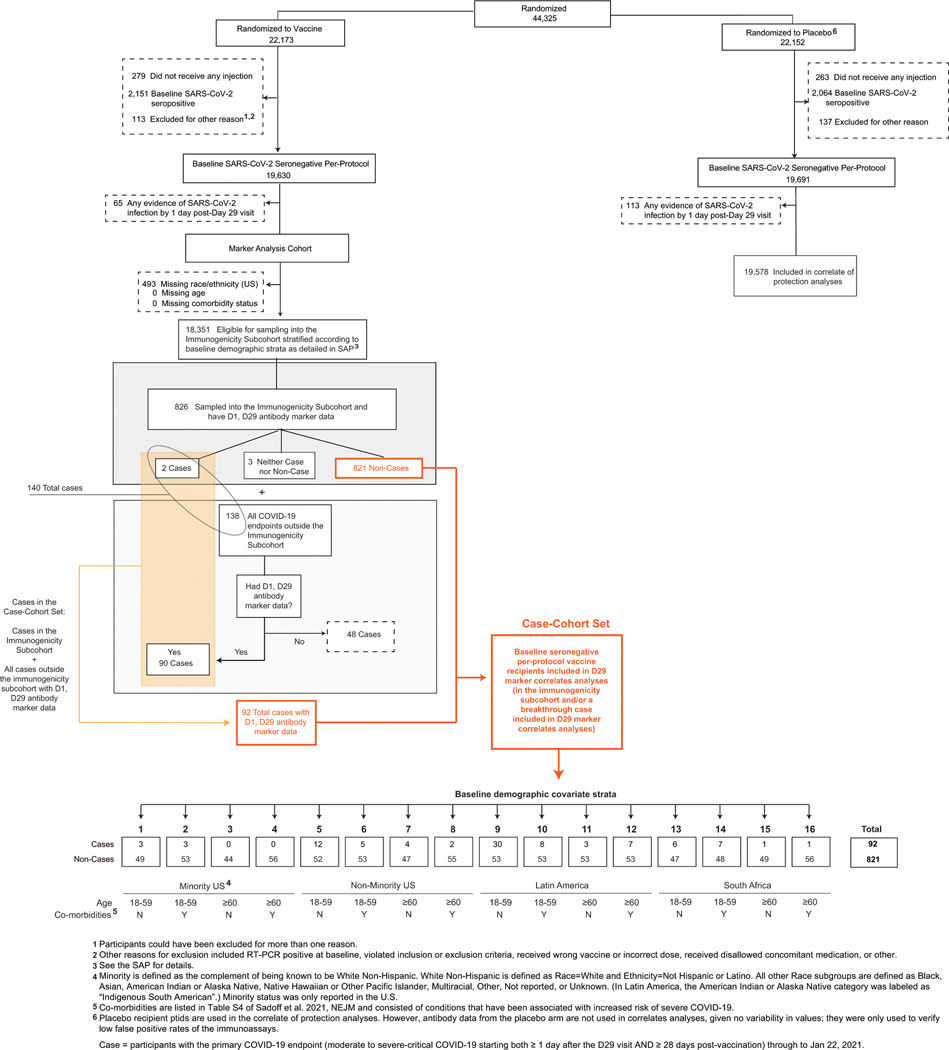
The assessment of immune correlates was based on measurement of the antibody markers at D29 (hereafter, “D29” denotes the Day 29 study visit, with an allowable visit window of +/− three days around 28 days post-injection) in the case-cohort set, comprised of a stratified random sample of the study cohort (the “immunogenicity subcohort”) plus all vaccine recipients with the COVID-19 primary endpoint after D29 (“breakthrough cases”) (Extended Data Fig. 1A). (The sampling design is further detailed in the Statistical Analysis Plan.) Extended Data Fig. 1B–1D describe the case-cohort set overall and by the three geographic regions Latin America (Argentina, Brazil, Chile, Colombia, Mexico, and Peru), South Africa, and United States, with antibody data available from 48, 15, and 29 breakthrough cases, respectively, and from 212, 200, and 409 non-cases, respectively. All analyses of D29 antibody markers restricted to per-protocol, baseline SARS-CoV-2 seronegative participants in the case-cohort set (Supplementary Table 1, Extended Data Fig. 2).
Participant demographics
The demographics and clinical characteristics of the immunogenicity subcohort (N=826 in the vaccine group, N=90 in the placebo group) are reported in Supplementary Table 2. Of all participants in the immunogenicity subcohort, 50.4% were ≥ 60 years old, 51.7% were considered at-risk for severe COVID-19 (defined as having one or more comorbidities associated with elevated risk of severe COVID-191), and 44.8% had been assigned female sex at birth. At U.S. sites 49.3% had minority status (defined as other than White Non-Hispanic). The immunogenicity subcohort was 26.0% Latin America, 23.9% South Africa, and 50.0% United States. Supplementary Tables 3-5 provide demographics and clinical characteristics of the immunogenicity subcohort by geographic region.
COVID-19 endpoint
Correlates analyses were performed based on adjudicated moderate to severe-critical COVID-19. Onset was required to be ≥ 28 days post-vaccination (the day of vaccination defines the D1 study visit) as well as ≥ 1 day post-D29 (the D29 study visit was not always 28 days post-vaccination due to allowable study visit windows, as discussed above), through to January 22, 2021 (the data cut date of the primary analysis).1 This COVID-19 endpoint was selected to be as close as possible to the COVID-19 endpoint used in the primary analysis1 (efficacies against the primary1 vs. correlates analysis “moderate to severe-critical COVID-19” endpoints were very similar), while also seeking inclusiveness of endpoints to aid statistical precision. See Online Methods for details on the analysis databases and exact differences between the two endpoints. The last COVID-19 endpoint included in the correlates analysis occurred 48 days post-D29 (Extended Data Fig. 1E). Of the 92 breakthrough cases with antibody data, 7 were severe-critical (using the same definition as in ref.1), precluding correlates analyses restricted to severe-critical endpoints. Non-cases were defined as baseline seronegative per-protocol participants sampled into the immunogenicity subcohort with no evidence of SARS-CoV-2 infection up to the end of the correlates study period, which is up to 54 days post-D29, the last day such that at least 15 such vaccine recipients were still at risk in the immunogenicity subcohort, but no later than the data cut of January 22, 2021.
SARS-CoV-2 lineages causing COVID-19 endpoints
Fig. 1 in ref.2 (which reports the results of the final efficacy analysis) shows the distribution of SARS-CoV-2 lineages among COVID-19 endpoint cases for each country in the trial over time during the double-blind period of the trial (September 21, 2020 through July 9, 2021). Data in this figure through January 22, 2021 are relevant for the current work. With “reference” referring to the Wuhan-Hu-1 strain harboring the D614G point mutation and “other” referring to sequences with substitutions departing from reference not resulting in another SARS-CoV-2 lineage or variant, the results show two lineages in the US, at approximately equal prevalence (reference, other); almost all lineages beta in South Africa; and lineages reference, zeta, and other in Latin America in similar proportions. For the US most “other” lineages were close genetically to reference. These data are consistent with the preliminary sequencing data provided in ref.1
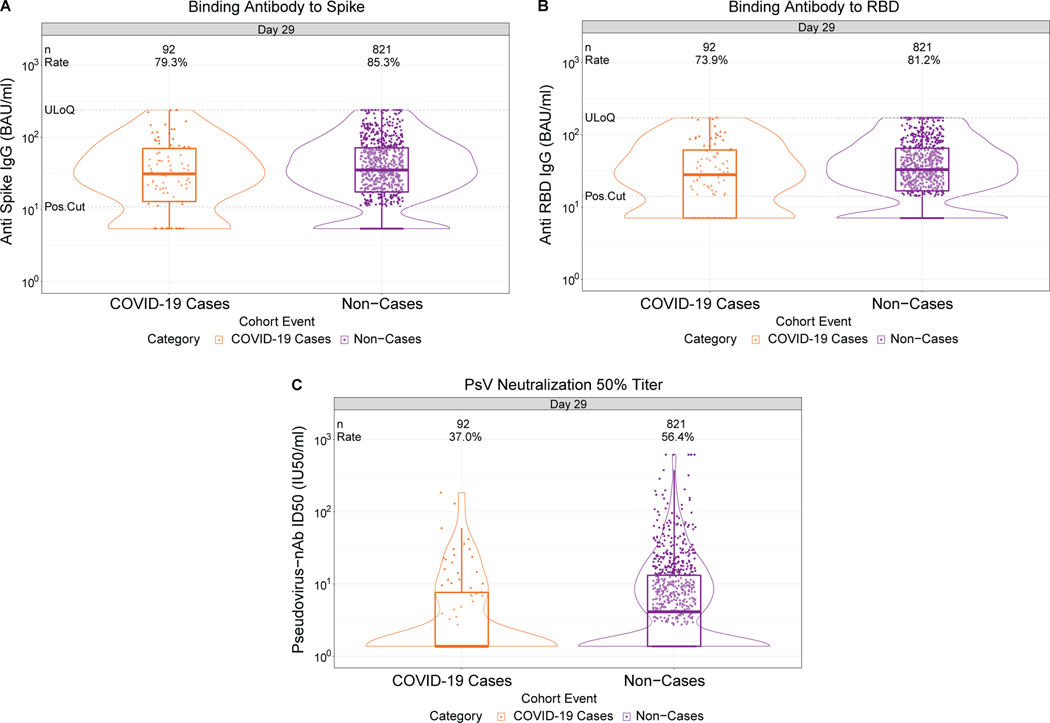
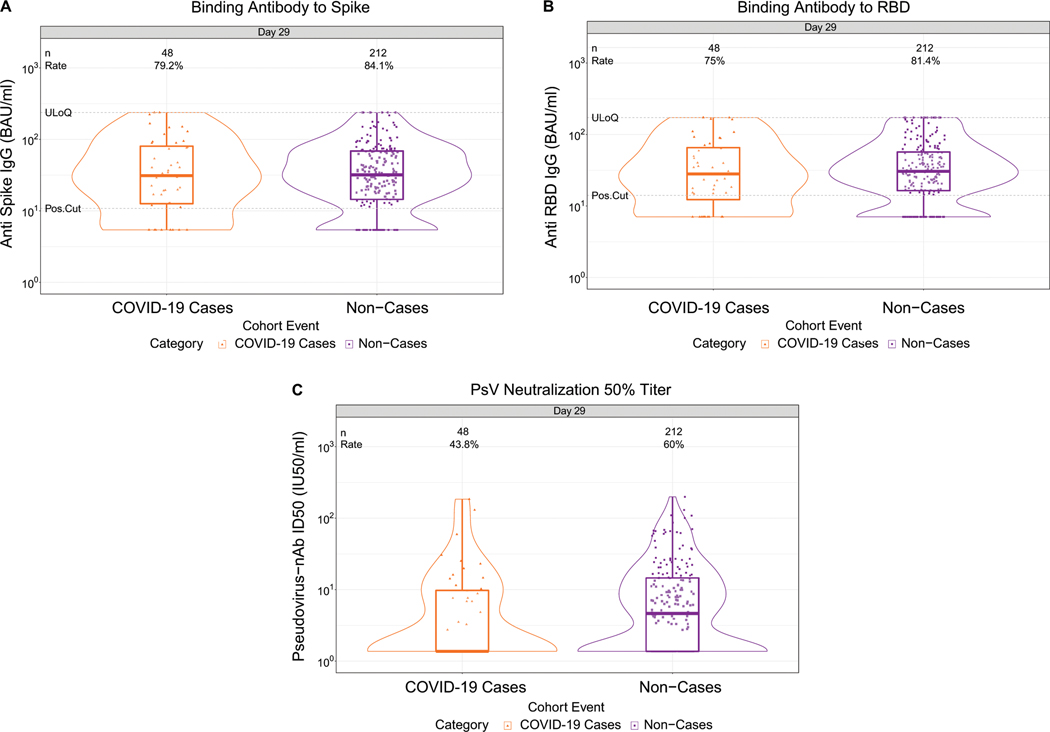
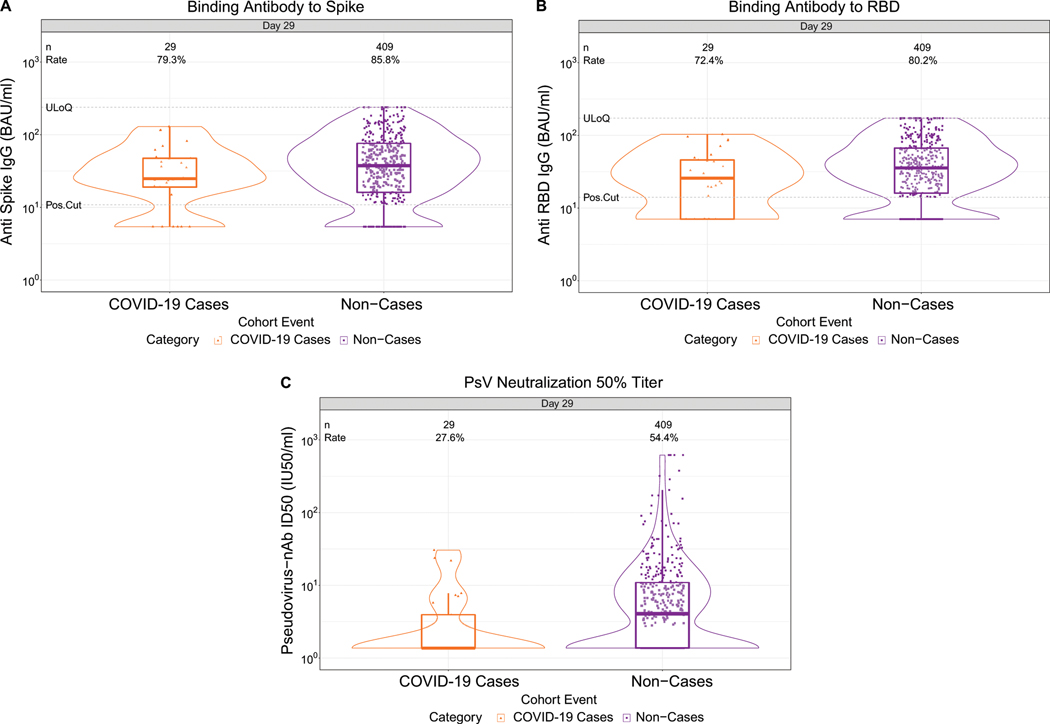
Fig. 1. D29 antibody marker level by COVID-19 outcome status.
(A) Anti-spike IgG concentration, (B) anti-receptor binding domain (RBD) IgG concentration, and (C) pseudovirus (PsV) neutralization ID50 titer. Data points are from baseline SARS-CoV-2 seronegative per-protocol vaccine recipients in the set [(A-C): N=92 cases, 821 non-cases]. The violin plots contain interior box plots with upper and lower horizontal edges the 25th and 75th percentiles of antibody level and middle line the 50th percentile, and vertical bars the distance from the 25th (or 75th) percentile of antibody level and the minimum (or maximum) antibody level within the 25th (or 75th) percentile of antibody level minus (or plus) 1.5 times the interquartile range. At both sides of the box, a rotated probability density curve estimated by a kernel density estimator with a default Gaussian kernel is plotted. Frequencies of participants with detectable responses were computed with inverse probability of sampling weighting. Pos.Cut, Dectectability/Positivity cut-off. Detectable response for spike IgG was defined by IgG > 10.8424 BAU/ml and for RBD IgG was defined by IgG > 14.0858 BAU/ml. ULoQ, upper limit of quantitation. ULoQ = 238.1165 BAU/ml for spike IgG and 172.5755 BAU/ml for RBD IgG. LLoQ, lower limit of quantitation. Seroresponse for ID50 was defined by a quantifiable value > LLoQ (2.7426 IU50/ml). ULoQ = 619.3052 IU50/ml for ID50. Cases are baseline SARS-CoV-2 seronegative per-protocol vaccine recipients with the primary COVID-19 endpoint (moderate to severe-critical COVID-19 with onset both ≥ 1 day post D29 and ≥ 28 days post-vaccination) up to 54 days post D29 but no later than January 22, 2021.
Lower D29 antibody marker levels in cases vs. non-cases
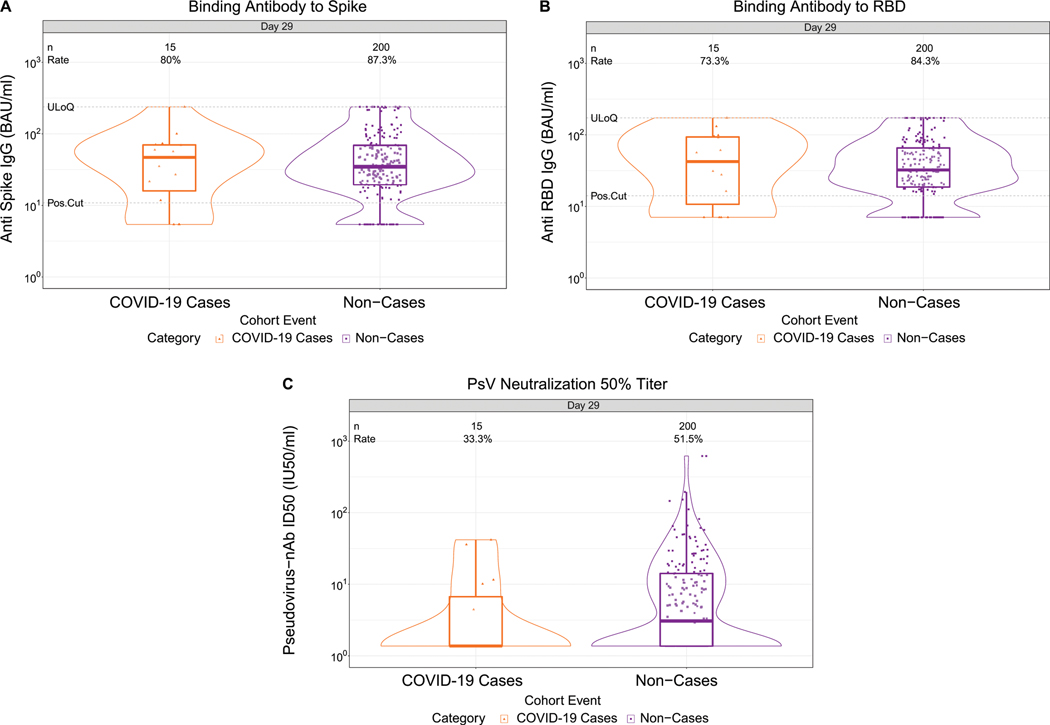
At D29, 85.3% (95% CI: 82.0%, 88.0%) and 81.2% (77.7%, 84.3%) of vaccine recipient non-cases had a detectable spike IgG response (defined by IgG > 10.8424 BAU/ml) or detectable RBD IgG response (defined by IgG > 14.0858 BAU/ml), respectively, whereas 56.4% (52.1%, 60.6%) had quantifiable ID50 nAb titer (Fig. 1, Table 1 ). For each D29 marker, the response rate was lower in cases than in non-cases; this difference was largest for ID50 [response rate difference: −19.5% (95% CI: −29.7%, −8.2%)] (Table 1). For each D29 marker, the geometric mean value was also lower in cases than in non-cases, with ID50 again having the greatest difference [3.22 IU50/ml (95% CI: 2.50, 4.15) in cases vs. 4.95 (4.42, 5.55) in non-cases, ratio = 0.65 (0.52, 0.81)]. The bAb markers had slightly higher case/non-case geometric ratios, with 95% CI upper bounds close to 1. Similar results were seen in each ENSEMBLE geographic region (Supplementary Table 6, Extended Data Figs 3-5), with D29 ID50 nAb titer in United States participants having the greatest response rate difference [cases minus non-cases; −26.8% (−41.6%, −6.3%)] and the lowest geometric mean ratio [cases/non-cases; 0.55 (0.41, 0.72)] across all markers and geographic regions.
Table 1. D29 antibody marker response rates and geometric means by COVID-19 outcome status.
Analysis based on baseline SARS-CoV-2 seronegative per-protocol vaccine recipients in the case-cohort set. Median (interquartile range) days from vaccination to D29 was 29 (2).
| COVID-19 Cases1 | Non-Cases in Immunogenicity Subcohort2 | Comparison | ||||||
|---|---|---|---|---|---|---|---|---|
| D29 Marker | N | Proportion with Antibody Response3 (95% CI) | Geometric Mean (GM) (95% CI) | N | Proportion with Antibody Response3`(95% CI) | Geometric Mean (GM) (95 % CI) | Response Rate Difference (Cases – Non-Cases) | Ratio of GM (Cases/Non-Cases) |
| Anti Spike IgG (BAU/ml) | 92 | 79.3% (69.7%, 86.5%) | 28.98 (23.09, 36.39) | 821 | 85.3% (82.0%, 88.0%) | 33.96 (31.04, 37.16) | −5.9% (−16%, 1.9%) | 0.85 (0.71, 1.02) |
| Anti RBD IgG (BAU/ml) | 92 | 73.9% (63.9%, 82.0%) | 27.54 (22.32, 33.97) | 821 | 81.2% (77.7%, 84.3%) | 32.49 (29.95, 35.26) | −7.3% (−17.8%, 1.5%) | 0.85 (0.71, 1.01) |
| Pseudovirus-nAb ID50 (IU50/ml) | 92 | 37.0% (27.6%, 47.4%) | 3.22 (2.50, 4.15) | 821 | 56.4% (52.1%, 60.6%) | 4.95 (4.42, 5.55) | −19.5% (−29.7%, −8.2%) | 0.65 (0.52, 0.81) |
Cases are baseline SARS-CoV-2 seronegative per-protocol vaccine recipients with the primary COVID-19 endpoint (moderate to severe-critical COVID-19 with onset that was both ≥ 28 days post-vaccination and ≥ 1 day post-D29) up to 54 days post D29, but no later than the data cut (January 22, 2021).
Non-cases/Controls are baseline seronegative per-protocol vaccine recipients sampled into the immunogenicity subcohort with no evidence of SARS-CoV-2 infection up to the end of the correlates study period, which is up to 54 days post D29 but no later than the data cut (January 22, 2021). See Extended Data Fig. 2. CI, confidence interval; GM, geometric mean.
Antibody response defined by detectable IgG concentration above the antigen-specific positivity cut-off (10.8424 BAU/ml for spike, 14.0858 BAU/ml for RBD) or by quantifiable ID50 > LLOQ = 2.7426 IU50/ml.
RBD, receptor-binding domain.
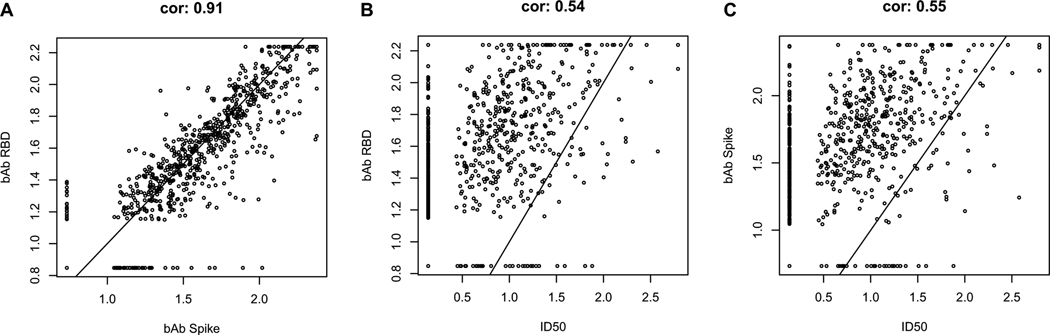
The D29 bAb markers were highly correlated with each other (Spearman rank r = 0.91), whereas they were only moderately correlated with ID50 (r = 0.55 for spike IgG and ID50; r = 0.54 for RBD IgG and ID50) (Extended Data Fig. 6). For each D29 marker, the reverse cumulative distribution function curve in the context of the overall vaccine efficacy estimate is shown in Supplementary Fig. 1.
As expected because the population is baseline seronegative, frequencies of placebo recipients with detectable or quantifiable responses at D29 were near zero (e.g., for ID50, 0.6% and 0% for cases and non-cases, respectively) (Supplementary Fig. 2).
D29 antibody marker levels correlate with risk
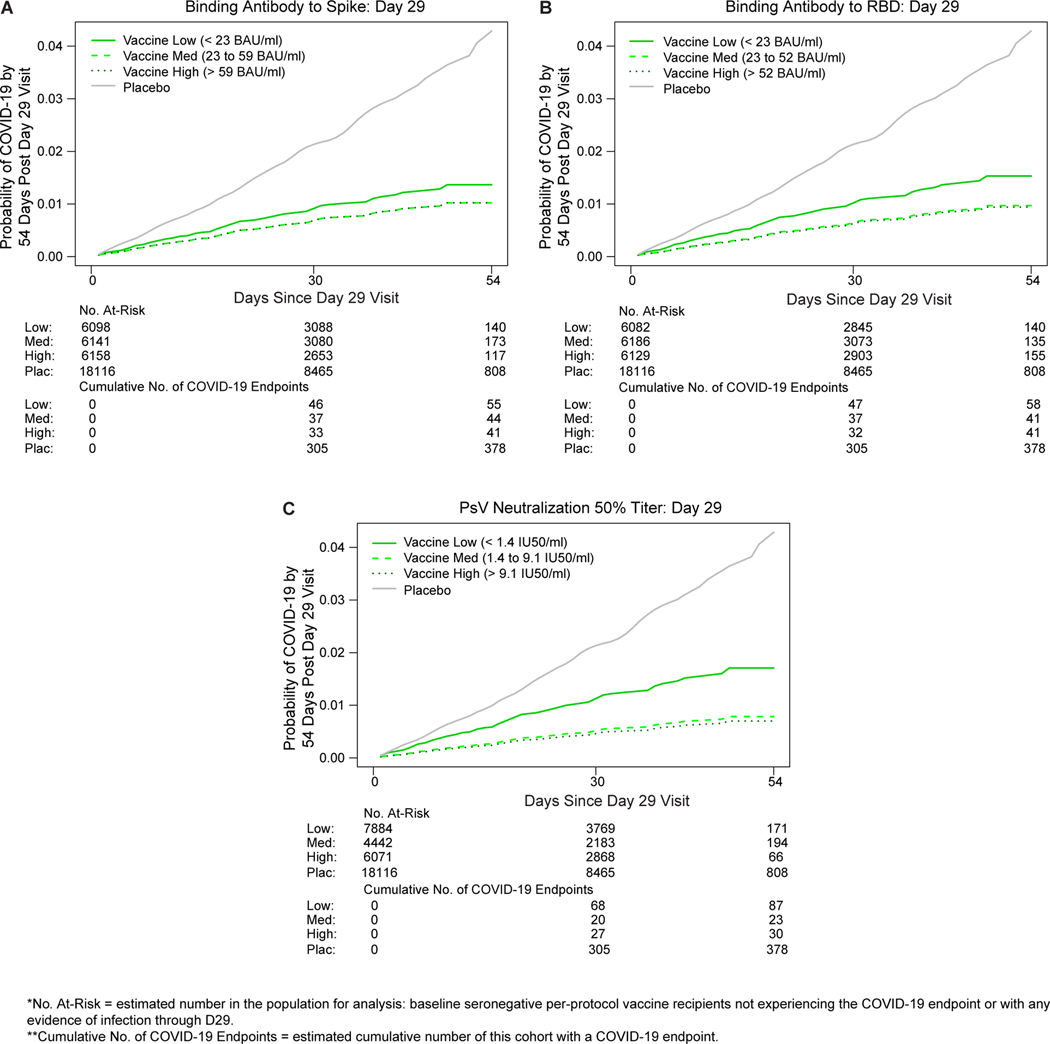
The cumulative incidence of COVID-19 for vaccine recipient subgroups defined by D29 antibody marker tertile (Fig. 2A-C) show that COVID-19 risk decreased with increasing tertile. The hazard ratio (High vs. Low tertile) was significantly less than one for ID50, estimate 0.41 (95% CI: 0.22, 0.75), and there were weak trends toward inverse correlates for the two IgG markers, estimate 0.75 (0.42, 1.32) for spike IgG and 0.61 (0.34, 1.09) for RBD IgG. Only ID50 passed the pre-specified family-wise error rate (FWER) multiplicity-adjusted p-value threshold for testing whether the hazard rate of COVID-19 differed across the Low, Medium, and High tertiles (Table 2A; p=0.003, FWER-adjusted p=0.011) (multiplicity adjustment was performed over the six categorical and quantitative markers). Evidence for the spike and RBD bAb markers as inverse correlates of risk across tertiles was weaker, with unadjusted p-values 0.50 and 0.16, respectively (Table 2A).
Fig. 2. COVID-19 risk by D29 antibody marker level.
The plots show covariate-adjusted cumulative incidence of COVID-19 by Low, Medium, High tertile of D29 antibody marker level in baseline SARS-CoV-2 seronegative per-protocol participants. (A) Anti-spike IgG concentration; (B) anti-receptor binding domain (RBD) IgG concentration; (C) pseudovirus (PsV) neutralization ID50 titer. Baseline covariates adjusted for were baseline risk score and geographic region.
Table 2. Covariate-adjusted hazard ratio of COVID-19 (A) across D29 antibody marker tertiles or (B) per 10-fold increase in D29 quantitative marker.
Analysis based on baseline SARS-CoV-2 seronegative per-protocol vaccine recipients in the case-cohort set. Baseline covariates adjusted for: baseline risk score, geographic region.
| A. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| D29 Immunologic Marker | Tertile* | No. Cases/No. At-Risk** | Attack Rate | Hazard Ratio (Across Tertiles) | P-Value (2-sided) | Overall P-Value¶ | FDR-Adjusted P-Value† | FWER-Adjusted P-Value† | |
| Point Est. | 95% CI | ||||||||
| Anti Spike IgG (BAU/ml) | Low | 55/6,098 | 0.0090 | 1 | N/A | N/A | 0.498 | 0.499 | 0.493 |
| Medium | 44/6,141 | 0.0072 | 0.75 | (0.42, 1.32) | 0.316 | ||||
| High | 41/6,158 | 0.0067 | 0.75 | (0.42, 1.32) | 0.316 | ||||
| Anti RBD IgG (BAU/ml) | Low | 58/6,082 | 0.0095 | 1 | N/A | N/A | 0.162 | 0.189 | 0.255 |
| Medium | 41/6,186 | 0.0066 | 0.63 | (0.35, 1.12) | 0.118 | ||||
| High | 41/6,129 | 0.0067 | 0.61 | (0.34, 1.09) | 0.095 | ||||
| Pseudovirus-nAb ID50 (IU50/ml) | Low | 87/7,884 | 0.0110 | 1 | N/A | N/A | 0.003 | 0.015 | 0.011 |
| Medium | 23/4,442 | 0.0052 | 0.46 | (0.24, 0.86) | 0.016 | ||||
| High | 30/6,071 | 0.0049 | 0.41 | (0.22, 0.75) | 0.004 | ||||
| Placebo | 378/18,116 | 0.0209 | |||||||
|
| |||||||||
| B. | |||||||||
|
| |||||||||
| D29 Immunologic Marker | No. Cases/No. At-Risk** | Hazard Ratio (Per 10-fold Increase) | P-Value (2-sided) | FDR-Adjusted P-Value† | FWER-Adjusted P-Value† | ||||
| Point Est. | 95% CI | ||||||||
| Anti Spike IgG (BAU/ml) | 140/18,395 | 0.69 | (0.41, 1.16) | 0.162 | 0.189 | 0.255 | |||
| Anti RBD IgG (BAU/ml) | 140/18,395 | 0.59 | (0.33, 1.06) | 0.079 | 0.150 | 0.144 | |||
| Pseudovirus-nAb ID50 (IU50/ml) | 140/18,395 | 0.49 | (0.29, 0.81) | 0.006 | 0.015 | 0.016 | |||
BAU, binding antibody units/ml; CI, confidence interval; FDR, false discovery rate; FWER, family-wise error rate; RBD, receptor binding domain; ID50, 50% inhibitory dilution titer.
Tertiles: Spike IgG: Low is < 23 BAU/ml, Medium is 23 to 59 BAU/ml, High is > 59 BAU/ml; RBD IgG: Low is < 23 BAU/ml, Medium is 23 to 52 BAU/ml, High is > 52 BAU/ml; ID50: Low is < 1.4 IU50/ml, Medium is 1.4 to 9.1 IU50/ml, High is > 9.1 IU50/ml.
No. at-risk = estimated number in the population for analysis, i.e. baseline SARS-CoV-2 seronegative per-protocol vaccine recipients not experiencing the COVID-19 endpoint or with evidence of SARS-CoV-2 infection through D29; no. cases = numbers of this cohort with an observed COVID-19 endpoint (with onset ≥ 1 day post D29 and ≥ 28 days post vaccination). The total count across all tertiles for each marker (140) differs from the case numbers in Fig. 1 (92) because the former number is the estimated number of all vaccine breakthrough cases within each tertile including ones without D1, D29 antibody marker data.
The overall p-value is from a generalized Wald test of whether the hazard rate of COVID-19 differed across the Low, Medium, and High subgroups.
q-value and FWER are computed over the set of p-values both for quantitative markers and categorical markers using the Westfall and Young permutation method (10000 replicates).
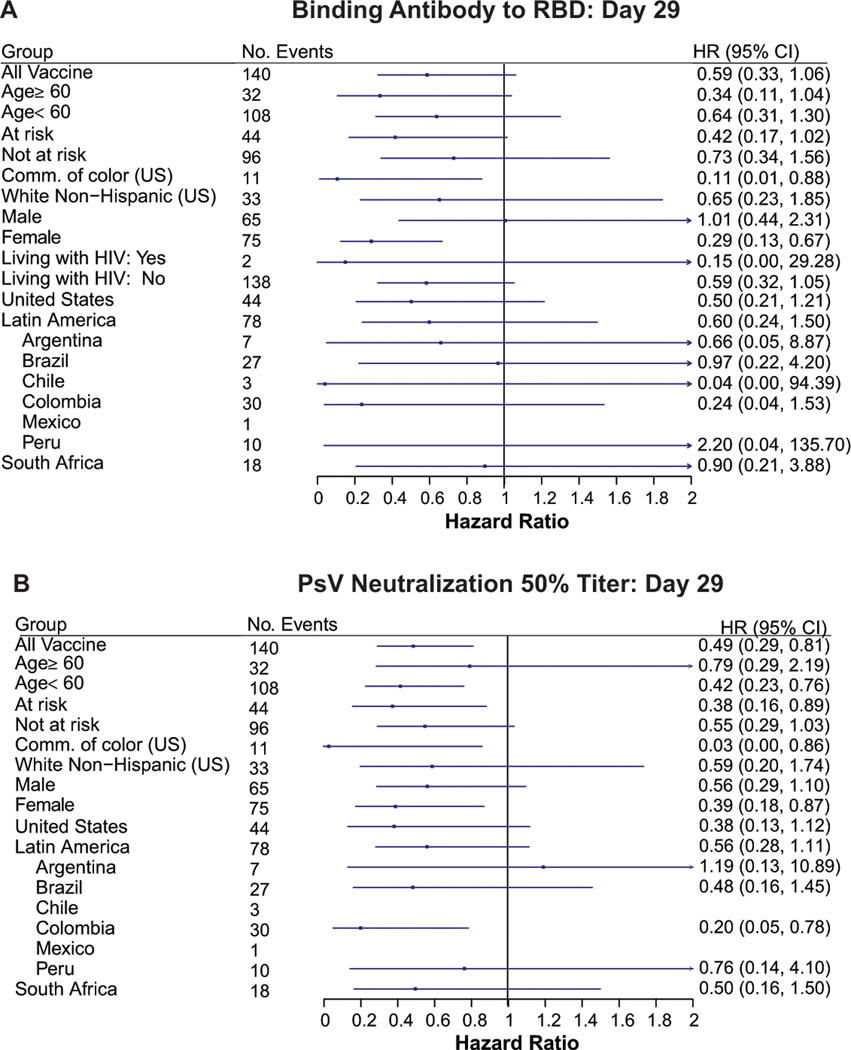
Similar results were observed for the D29 quantitative markers, with estimated hazard ratio per 10-fold increase in antibody marker level clearly indicating an inverse correlate of risk for ID50, estimate 0.49 (0.29, 0.81), with estimates less than one for each IgG marker yet with 95% CIs including 1.0: estimate 0.69 (95% CI: 0.41, 1.16) for spike IgG and 0.59 (0.33, 1.06) for RBD IgG (Table 2B). Again, only ID50 passed the multiple testing correction (FWER-adjusted p=0.016). (Supplementary Table 7 shows the hazard ratios per standard deviation-increase in each D29 marker.) An additional post-hoc analysis was done reporting Cox model fits for each antibody marker with a set of demographic factors also in the model (Supplementary Table 8). The results are similar, e.g., the estimated hazard ratio per 10-fold increase in ID50 is 0.49 (0.30, 0.80). Extended Data Fig. 7 shows analogous results across pre-specified subgroups of vaccine recipients for RBD IgG and ID50, respectively. The point estimates indicate stronger correlates of risk for participants assigned female vs. male sex at birth and for communities of color vs. White Non-Hispanics in the U.S., generating potential hypotheses about the role of sex and race/ethnicity on vaccine-induced immunity. However, because the 95% confidence intervals overlap, these apparent differences could be false positives.
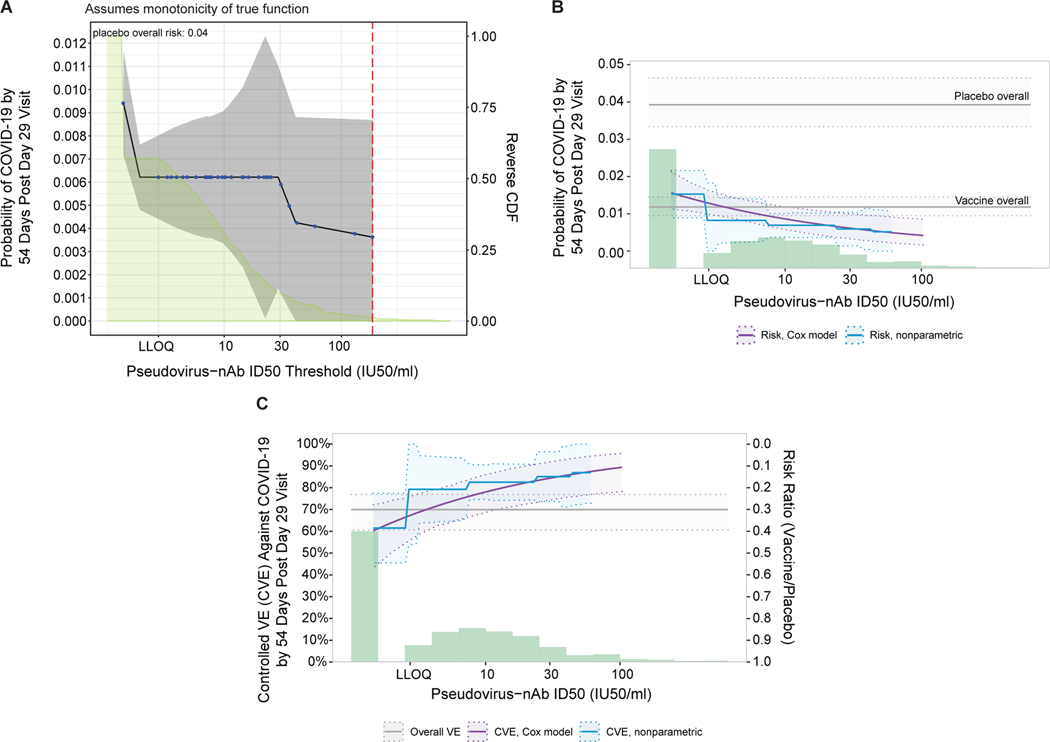
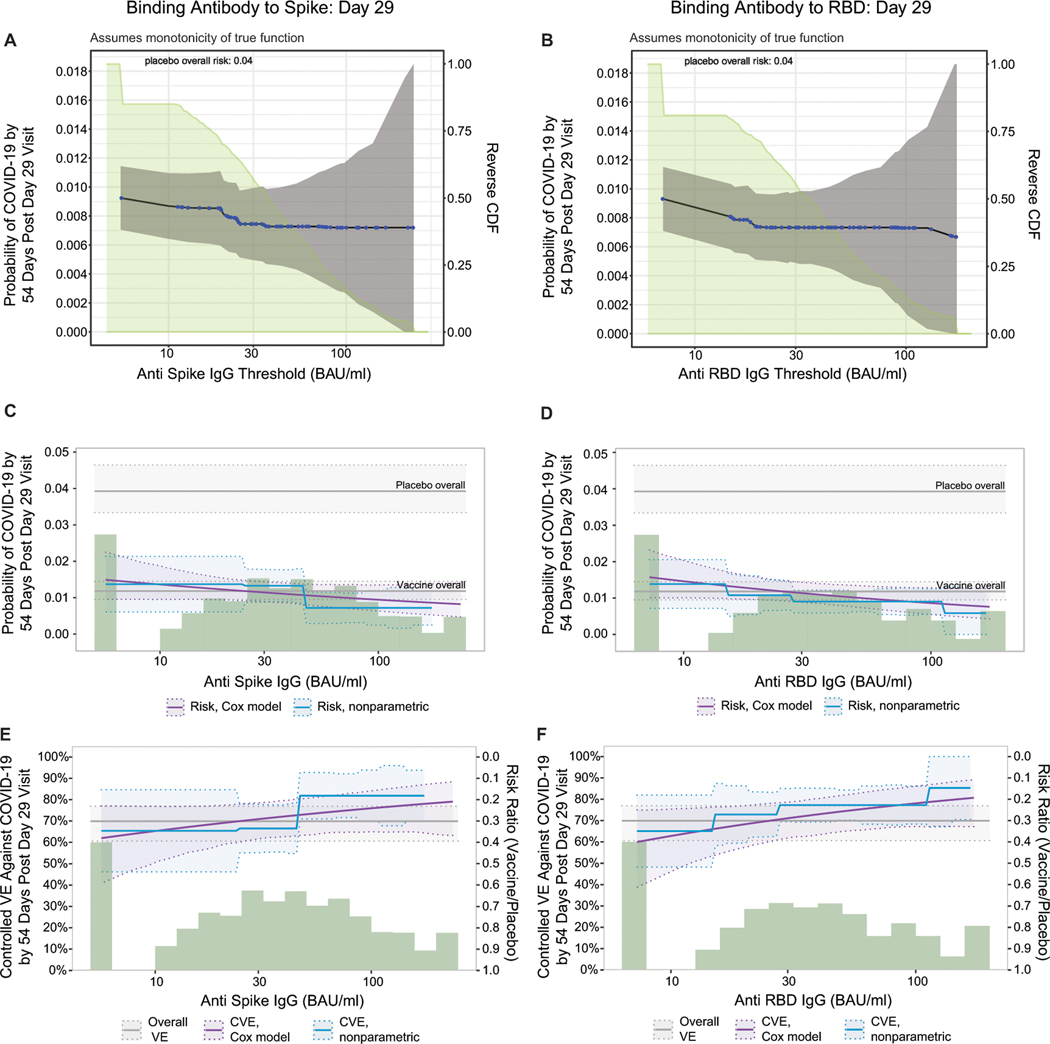
When vaccine recipients were divided into subgroups defined by having an antibody marker level above a specific threshold and varying the threshold over the range of values, nonparametric regression showed that cumulative incidence of COVID-19 (from 1 to 54 days post-D29) decreased as the ID50 threshold increased (Fig. 3A). This decrease in risk was steepest across increasing thresholds closer to the assay lower limit of quantitation (LLOQ = 2.74 IU50/ml) and was more gradual across higher increasing thresholds. The risk estimate for COVID-19 was 0.009 (95% CI: 0.007, 0.012) for all vaccine recipients and decreased to 0.006 (0.004, 0.009) for vaccine recipients with any quantifiable ID50 titer, whereas at the highest threshold examined (>185 IU50/ml) the risk estimate was 0.004 (95% CI: 0.000, 0.009). The bAb markers also showed decreases in risk (although less pronounced) with increasing threshold value (Extended Data Fig. 8A, 8B).
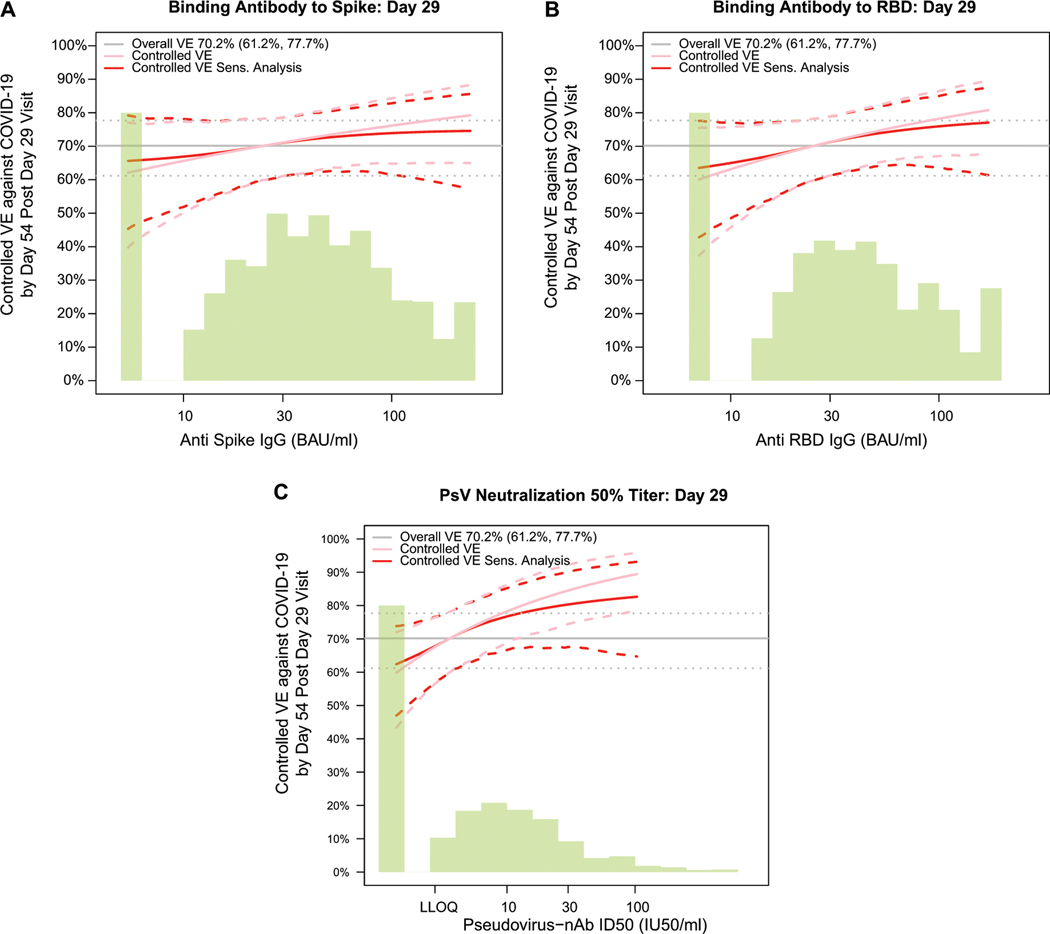
Fig. 3. Analyses of D29 ID50 titer as a correlate of risk and as a correlate of protection.
Analyses were performed in baseline SARS-CoV-2 seronegative per-protocol vaccine recipients. (A) Covariate-adjusted cumulative incidence of COVID-19 by 54 days post D29 by D29 ID50 titer above a threshold. The blue dots are point estimates at each COVID-19 primary endpoint linearly interpolated by solid black lines; the gray shaded area is pointwise 95% confidence intervals (CIs). The estimates and CIs were adjusted using the assumption that the true threshold-response is nonincreasing. The upper boundary of the green shaded area is the estimate of the reverse cumulative distribution function (CDF) of D29 ID50 titer. The vertical red dashed line is the D29 ID50 threshold above which no COVID-19 endpoints occurred (in the time frame of 1 to 54 days post D29). (B) Covariate-adjusted cumulative incidence of COVID-19 by 54 days post D29 by D29 ID50 titer, estimated using (solid purple line) a Cox model or (solid blue line) a nonparametric method. Each point on the curve represents the covariate-adjusted cumulative COVID-19 incidence at the given D29 ID50 titer value. The dotted black lines indicate bootstrap point-wise 95% CIs. The upper and lower horizontal gray lines are the overall cumulative incidence of COVID-19 from 1 to 54 days post D29 in placebo and vaccine recipients, respectively. (C) Vaccine efficacy (solid purple line) by D29 ID50 titer, estimated using a Cox proportional hazards implementation of Gilbert et al.44 Each point on the curve represents the vaccine efficacy at the given D29 ID50 titer value. The dashed black lines indicate bootstrap point-wise 95% CIs. Vaccine efficacy (solid blue line) by Day 29 ID50 titer, estimated using a nonparametric implementation of Gilbert et al.44 (described in the SAP). The blue shaded area represents the 95% CIs. In (B) and (C), the green histogram is an estimate of the density of Day 29 ID50 titer and the horizontal gray line is the overall vaccine efficacy from 1 to 54 days post D29, with the dotted gray lines indicating the 95% CIs. Baseline covariates adjusted for were baseline risk score and geographic region. LLOQ, limit of quantitation. In (B, C), curves are plotted over the range from LLOQ/2 to the 97.5th percentile = 96.3 IU50/ml.
Fig. 3B and Extended Data Fig. 8C, 8D show the Cox modeling results in terms of estimated cumulative incidence of COVID-19 (from 1 to 54 days post-D29) across D29 marker levels. For each antibody marker, COVID-19 risk decreased as antibody marker level increased. Across the full range of D29 ID50 values examined (nonquantifiable ID50 < 2.74 IU50/ml to 96.3 IU50/ml, the 97.5th percentile value), estimated risk decreased from 0.016 (0.011, 0.021) to 0.004 (0.002, 0.008), a 4-fold reduction in risk (Fig. 3B). For D29 RBD IgG, estimated risk also decreased across the range of values examined, from 0.016 (0.010, 0.025) at negative response (7 BAU/ml) to 0.008 (0.004, 0.013) at 173 BAU/ml (the 97.5th percentile), a 2-fold reduction in risk (Extended Data Fig. 8D). Results for D29 spike IgG were similar (Extended Data Fig. 8C).
Vaccine efficacy increases with D29 antibody marker level
Fig. 3C and Extended Data Fig. 8E, 8F show estimated vaccine efficacy against COVID-19 (from 1 to 54 days post-D29) across a range of levels of a given D29 antibody marker. For each marker, estimated vaccine efficacy rose with increasing marker level. This increase was greatest for ID50 titer: At nonquantifiable D29 ID50, estimated vaccine efficacy was 60% (95% CI 43, 72%); this increased to 78% (69, 86%) at 9.9 IU50/ml and to 89% (78, 96%) at 96.3 IU50/ml (purple curve, Fig. 4C). Nonparametric estimation of the vaccine efficacy-by-D29 ID50 curve suggests that vaccine recipients with nonquantifiable ID50 titer had an estimated vaccine efficacy of 60% with a jump in vaccine efficacy just above the LLOQ to 79% (blue curve, Fig. 3C).
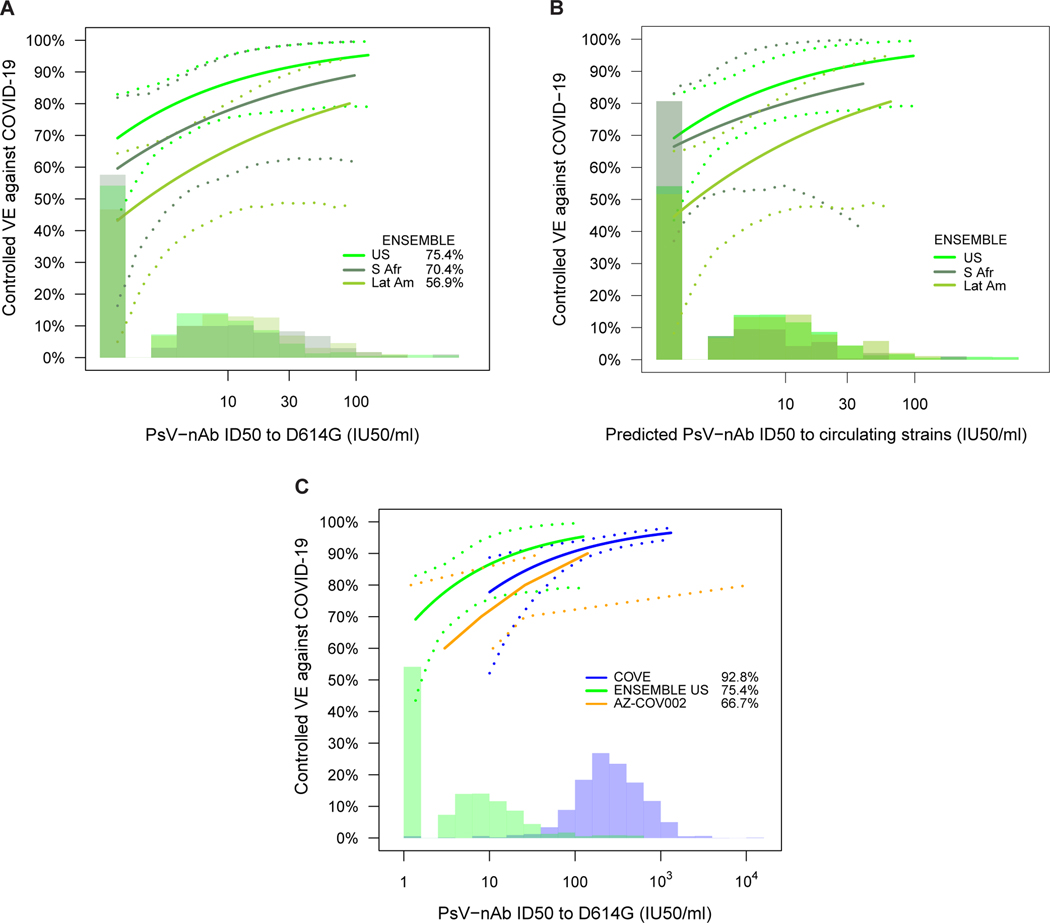
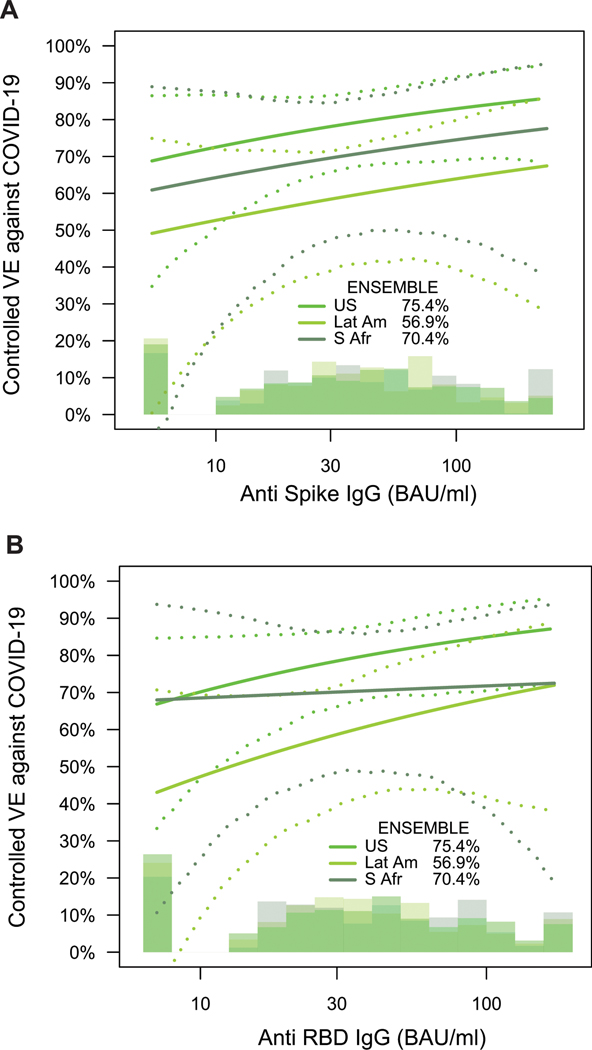
Fig. 4. Vaccine efficacy (solid lines) in baseline SARS-CoV-2 seronegative per-protocol vaccine recipients by A) D29 pseudovirus (PsV)-nAb ID50 titer to D614G in ENSEMBLE by geographic region (US, United States; Lat Am, Latin America; S Afr, South Africa); B) D29 predicted geometric mean PsV-nAb ID50 titer to strains that circulated during follow-up in each designated geographic region (see Supplementary Note 2); and C), D57 ID50 titer to D614G in COVE, D29 ID50 titer to D614G in ENSEMBLE (US), D56 ID50 titer to D614G in COV002, all estimated using the Cox proportional hazards implementation of Gilbert et al.44.
The dashed lines indicate bootstrap point-wise 95% CIs. The follow-up periods for the VE assessment were: A) ENSEMBLE-US, 1 to 53 days post D29; ENSEMBLE-Lat Am, 1 to 48 days post D29; ENSEMBLE-S Afr, 1 to 40 days post D29; B) COVE (doses D1, D29), 7 to 100 days post D57; ENSEMBLE-US, 1 to 53 days post D29; COV002 (doses D0, D28; VE defined as 1-relative risk of whether or not an event occurred = 28 days post-D28 till the end of the study period). The histograms are an estimate of the density of D29 ID50 titer in ENSEMBLE (including by geographic region in A, B). The blue histograms are an estimate of the density of ID50 titer in baseline SARS-CoV-2 negative per-protocol vaccine recipients in COVE. Curves are plotted over the range from 10 IU50/ml to 97.5th percentile of marker for COVE and from 2.5th percentile to 97.5th percentile for ENSEMBLE. Baseline covariates adjusted for were: ENSEMBLE, baseline risk score and geographic region; COVE: baseline risk score, comorbidity status, and Community of color status; COV002: baseline risk score.
Two sensitivity analyses (see the SAP for details) were performed to evaluate how strong unmeasured confounding would have to be to overturn an inference that D29 antibody marker impacted vaccine efficacy. The first sensitivity analysis, based on E-values,28 assessed the robustness of the inference that vaccine efficacy is greater at High vs. Low ID50 tertile. The results indicated some robustness to confounding of this inference for ID50 but not for the bAb markers (Supplementary Table 9). The second sensitivity analysis “flattened” the estimated vaccine efficacy-by-D29 antibody marker level curve by assuming a certain amount of unmeasured confounding. Estimated vaccine efficacy still increased with D29 ID50 titer (Extended Data Fig. 9).
Vaccine efficacy rises with D29 ID50 titer in each region
Vaccine efficacy increased with D29 ID50 titer in each geographic region (Fig. 4A). The US curve was shifted upwards compared to the South Africa curve, which was in turn shifted upwards compared to the Latin America curve. The curves also indicated higher vaccine efficacy at nonquantifiable ID50 in the US (69%; 95% CI: 43, 83%) compared to in South Africa (60%; 16, 82%) and in Latin America (43%; 5, 64%); however, the confidence intervals overlapped. Extended Data Fig. 10 shows similar results for spike IgG and for RBD IgG, where vaccine efficacy also increased with D29 bAb marker level (with the exception that vaccine efficacy appeared to remain constant in South Africa with increasing D29 RBD IgG concentration) and the lowest bAb levels were needed in the US out of the three regions to mark a given level of vaccine efficacy. (Participant demographic characteristics of geographic region subgroups of the immunogenicity subcohort are shown in Supplementary Tables 3-5; response rates and magnitudes are shown by case/non-case status, for each geographic region, in Supplementary Table 6 and Extended Data Figs 3-5; and Supplementary Fig. 3 shows the distribution of the number of days from D29 until COVID-19 endpoint occurrence or until right-censoring, stratified by case/non-case status and by geographic region).
Vaccine efficacy by circulating-matched D29 ID50 titer
In the U.S., the circulating strains during follow-up were Wuhan-like, being genetically and antigenically similar to the D614G strain against which neutralizing antibodies were measured. In contrast, in South Africa beta predominantly circulated and in Latin America several variants circulated, such that for these regions the correlates analyses had a mismatch where antibodies were measured to D614G and vaccine efficacy was measured against circulating strains different from D614G. One model for a correlate of protection, the ‘variant-invariant CoP model’, states that the level of ID50 against a circulating strain required to achieve a certain vaccine efficacy value against that strain is constant across strains. To evaluate this model, we repeated the analysis of Fig. 4A using a new D29 ID50 marker for each of the three geographic regions, defined as the predicted geometric mean ID50 to the strains that circulated during follow-up in the given geographic region, with the prediction based on measurement of neutralization titers of Ad26.COV2.S vaccine recipients to a panel of variants (see Supplementary Note 2). The vaccine efficacy curves for the U.S. and South Africa become closer together when creating this greater match of the ID50 measurements to circulating strains, providing some support for the model (Fig. 4B). For example, for South Africa VE is 81% (57, 98%) at ID50 = 10 IU50/ml averaged to the South Africa circulating strains (beta variant), compared to the U.S. where VE is 86% (75, 95%) at ID50 = 10 IU50/ml to D614G that circulated in the U.S. In contrast, for Latin America the vaccine efficacy curve based on ID50 to circulating strains did not change noticeably compared to the curve based on ID50 to D614G. This is explained by the fact that more than 90% of the placebo arm COVID-19 endpoints in Latin America through January 22, 2021 were of the ancestral lineage.
Cross-trial cross-platform comparison of ID50 titer as a CoP
We next compared the vaccine efficacy-by-ID50 titer curves for three double-blind, placebo-controlled COVID-19 vaccine efficacy trials: ENSEMBLE (one dose: D1; VE curve by D29 ID50 titer), COVE (two doses: D1, D29; VE curve by D57 ID50 titer), and the COV002 (United Kingdom) trial29 of the AZD1222 (ChAdOx1 nCoV-19) chimpanzee adenoviral-vectored COVID-19 vaccine (two doses: D0, D28; VE curve by D56 ID50 titer). In this comparison for ENSEMBLE we restricted to the US (ENSEMBLE-US) in order to match COVE in its restriction to the US.
In each trial, vaccine efficacy rose with increasing ID50 titer (Fig. 4C). Comparison at high and at low ID50 titers is hindered by the limited overlap of adenovirus-vectored and mRNA vaccine-elicited ID50 titers, with span of values (IU50/ml) from 2.5th to 97.5th percentile 1.4 to 96.3 in ENSEMBLE (the span in ENSEMBLE-US is 1.4 to 98) vs. 32 to 1308 in COVE. In the intersection of these ID50 titer spans (32 to 96.3 IU50/ml) (the only titer spans where vaccine efficacy levels can be directly compared), the point estimates of vaccine efficacy are similar and the confidence bands show large overlap. While the confidence intervals of the curves in ENSEMBLE-US are wide, the lower overall vaccine efficacy in ENSEMBLE-US compared to COVE could be explained by the lower ID50 titers, consistent with results of meta-analyses.21,30
DISCUSSION
We report that each D29 antibody marker evaluated was an inverse correlate of risk of moderate to severe-critical COVID-19 over 83 days post Ad26.COV2.S vaccination, with strongest evidence for ID50 titer, passing the pre-specified multiple testing correction bar. We found that vaccine efficacy increased with higher D29 antibody marker levels, with results supporting the importance of achieving quantifiable antibodies; negative binding antibody response and nonquantifiable neutralization corresponded to moderate vaccine efficacy of about 60%. We found that the risk of COVID-19 decreases incrementally with D29 neutralization titers (Fig. 3) and that non-zero risk remains at highest titers, and estimated vaccine efficacy increases incrementally from 60% at nonquantifiable titers to 90% at highest titers, which supports a relative, not an absolute, correlate of protection. The moderate vaccine efficacy in vaccine recipients with nonquantifiable neutralizing antibodies indicates that this marker did not fully mediate vaccine efficacy: other immune responses or immune markers at other time points or not quantifiable in serum must have contributed to vaccine efficacy. Memory B cells, Fc effector functions, CD4+ and CD8+ T cells (at least for severe disease) all likely contribute to protection.31 Overall, our findings are a step towards establishing an immune marker surrogate endpoint for adenovirus-vectored COVID-19 vaccines, and potentially a surrogate endpoint that might prove useful across vaccine platforms.
Strengths of our study include the fact that analyses were pre-specified; the fact that the data come from the double-blind follow-up period of a randomized, placebo-controlled phase 3 vaccine efficacy trial; and the restriction to SARS-CoV-2 naïve individuals, ensuring that only vaccine-elicited immune responses are studied as correlates. (The latter restriction could also be viewed as a limitation, as a correlate of protection may be altered by prior infection and/or vaccination and the global proportion of SARS-CoV-2 naïve individuals is declining.32) In the continuing follow-up of ENSEMBLE, participants who experienced the COVID-19 endpoint have been receiving vaccinations, and future analyses are planned to assess the same antibody markers as immune correlates in these individuals. The degree to which each evaluated D29 antibody marker predicts vaccine efficacy against SARS-CoV-2 strains other than those circulating during the trial period, as well as over longer follow-up periods will be important for informing the use of any of these biomarkers as a surrogate endpoint in practice.
The estimated relationship of ID50 titer with vaccine efficacy differed between the US, Latin America and South Africa, which might be explained by the greater match of the vaccine strain to the reference strain (which predominated in the US) compared with the different strains that circulated in Latin America and South Africa. In support of this hypothesis, Ad26.COV2.S efficacy against moderate to severe-critical COVID-19 with onset ≥ 28 days post-vaccination was reported to be higher against the reference strain [58.2% (95% CI: 35.0%, 73.7%)] than against non-reference lineages [44.4% (34.6%, 52.8%)], particularly against gamma [36.5% (14.1%, 53.3%)], over a median follow-up of 121 days post-vaccination.2 Another potential explanation for the apparent difference in the estimated relationship of ID50 titer with vaccine efficacy by geographic region is that COVID-19 cases tended to occur earlier in South Africa than in the other two geographic regions, and the longer follow-up in the US. This longer follow-up may have allowed expansion of neutralizing antibody breadth, which is associated with improved coverage of SARS-CoV-2 variants over time.33 An additional potential explanation may be a lower placebo arm attack rate in the US (as greater antibody levels may be needed to protect against greater exposure8). However, a post-hoc interaction test in a marginalized Cox model for whether the association of quantitative D29 ID50 titer with COVID-19 differed across the three geographic regions yielded p = 0.83, such that there is not statistical evidence for a differential correlate by region.
We found that in ENSEMBLE, the pseudovirus neutralization assay readout (D29 ID50 titer) had stronger evidence as a correlate than either of the binding antibody assay readouts. However, given that the hazard ratio estimates per 10-fold increase of each of the D29 binding antibody markers were less than 1.0, the binding and pseudovirus neutralization assay readouts were substantially correlated, and the fact that both assays were strong inverse correlates of risk (of similar strength as ID50 nAb titer) in the COVE25 and COV002 (Ad-vectored)26 trials, we believe it likely that both binding antibody markers are also correlates (albeit weaker ones) for the Ad26.COV2.S vaccine. However, even the two Ad-vectored vaccines (Ad26.COV2.S and AZD1222) differ (one vs. two doses, with one implication potentially increased avidity of post dose two antibodies); pre-fusion stabilized vs. native-like spike; human vs. chimpanzee adenovirus). Moreover, different variants [(B.1.177 and B.1.1.7 (alpha)] were circulating at the sites at which COV002 was conducted.26 Future correlates analyses should help clarify whether the binding antibody markers are also correlates for Ad26.COV2.S.
In the range of overlapping titers, similar vaccine efficacy by nAb ID50 curves were observed in ENSEMBLE-US and COVE. In both trials, the vast majority of circulating strains were similar to the reference strain1,2,34 (used in the nAb assay). Thus, the most transportable correlate across vaccine platforms may involve assessing nAbs against circulating strains, which can be evaluated in the future.
Our study has limitations. First, other Ad26.COV2.S-induced immune responses (e.g. spike-specific T-cell responses,35 Fc effector antibody functions36) were not assessed. Analyses of D29 spike-specific antibody-dependent cellular phagocytosis (ADCP) are underway; future work will address how ADCP and other immune markers may work together with bAb and/or nAb markers as correlates of protection. A second limitation is the relatively short follow-up (slightly over two months post-D29), which prevented assessment of D29 antibody marker correlates over longer term risk. Measurement of the D29 markers in vaccine breakthrough COVID-19 events occurring after the cut-off of the primary analysis will enable a future analysis of correlates for COVID-19 through 6–7 months. The primary analysis of ENSEMBLE showed waning of overall vaccine efficacy from 67% at 2–3 months post-vaccination1 to 53% at 6–7 months post-vaccination, with the waning evidently restricted to variants of concern,2 yet antibody levels did not decrease from 2 to 7 months. The future analyses may help understand these results by directly assessing D29 antibodies as correlates for COVID-19 through 6–7 months. A third limitation is that the study took place before emergence of the delta and omicron variants (with analysis pooled over all SARS-CoV-2 strains which were mainly reference, beta, zeta, and other1,2) and before any boosters were given. Future work is being planned to assess in ENSEMBLE levels of post-vaccination nAbs against spike-pseudotyped viruses of each sufficiently-prevalent variant of concern as correlates of risk and protection against COVID-19 with the matched variant of concern: these include beta in South Africa and gamma, lambda, and mu in Latin America. The region-specific differences in circulating strains comprise a fourth limitation, in that it is not possible to assess whether strain and/or geographic region has an isolated impact on the correlates of risk and protection. A fifth limitation is that the comparison of vaccine efficacy by antibody marker curves across efficacy trials did not use a common reference covariate distribution in the adjustment for prognostic factors, and the estimates of vaccine efficacy by antibody marker can be biased if a confounder of the effect of the marker on COVID-19 risk was not accounted for. Additionally, the primary endpoints differed among studies (COVE, COV002: symptomatic COVID-19 of any severity vs. ENSEMBLE: moderate to severe-critical COVID-19; all 14 days post second dose/vaccination in baseline seronegative participants). However, in the ENSEMBLE primary efficacy analysis only 1 case was mild out of 117 symptomatic COVID-19 events in the vaccine group and only 3 of 351 in the placebo group1, supporting similarity of the endpoints across the three trials.
Our study evaluated antibody levels measured 4 weeks post-vaccination (Day 29) as correlates of COVID-19 occurrence over the subsequent 54 days, whose results can be approximately interpreted as outcome-proximal correlates for vaccine recipients’ average antibody level during follow-up for 54 days after Day 29. Alternative ‘outcome-proximal’ correlates analyses measure antibody levels over time and assess their association with the instantaneous hazard of COVID-19 occurrence, which account for the fact that antibody levels change over time; these two types of analyses address distinct questions. Antibody levels of one-dose Ad26.COV2.S recipients do not decrease from Day 29 to Day 71 and slightly increase,37 suggesting that antibody dynamics do not play a major role in complicating the interpretation of the current results given the short-term follow-up of 54 days.
Given the interest in assessing correlates against severe COVID-19 and the fact that many Ad26.COV2.S-induced antibody responses show increased magnitude and affinity maturation over time post-D29,33,38 the study’s scope of a single clinical endpoint (moderate to severe-critical COVID-19) and a single antibody measurement timepoint (D29) are further limitations. Currently, antibody responses are being assayed in D29 and D71 samples from the remaining ~300 vaccine breakthrough COVID-19 events during the entire double-blinded period. Planning is underway to assess correlates for COVID-19 over longer-term follow-up, for severe COVID-19, for asymptomatic SARS-CoV-2 infection, and for viral load.
Another important question is how vaccine efficacy depends on SARS-CoV-2 spike features (e.g., amino acid motifs, distances to the vaccine insert, neutralization sensitivity scores), and whether/how the immune correlates depend on these spike features. Future work is planned to address these questions, with the overarching objective being to build a general model for predicting vaccine efficacy across SARS-CoV-2 strains/spike features and time since vaccination, based on D29 and possibly also D71 antibody markers. The data from the additional vaccine breakthrough cases discussed above will provide an opportunity to construct and evaluate such a model. In the meantime, the contribution of the current correlates study is to establish that pseudovirus neutralization assay readouts are a correlate of risk for COVID-19 for the Ad26.COV2.S vaccine, and to provide proof of concept that this marker is likely also a correlate of protection for this vaccine. After the additional evidence about this marker as a correlate of protection is gathered as indicated above, it should be possible to define whether and how to use this marker as a surrogate endpoint for predicting vaccine efficacy.
Online Methods
Trial design, study cohort, COVID primary endpoints, and case/non-case definitions
Enrolment for the ENSEMBLE trial began on September 21, 2020. A total of 44,325 participants were randomized (1:1 ratio) to receive a single injection of Ad26.COV2.S or placebo on Day 1. Serum samples were taken on D1 and on D29 for potential antibody measurements. Antibody measurements were evaluated as correlates against the moderate to severe-critical COVID-19 endpoint defined in the main text.
While the correlates analysis only included COVID-19 primary endpoints up to Jan 22nd, 2021 (the cut-off date of the primary analysis1), the correlates analysis was performed using the analysis database of the final analysis.2 Compared to the analysis database of the primary analysis, the analysis database of the final analysis includes changes to the SAP and protocol, as well as information that became available only after the database lock date on cases up to Jan 22nd, 2021. Specifically, for the primary analysis, the definition of the moderate to severe-critical COVID-19 endpoint was algorithmically programmed according to the protocol definition (with only severe-critical being assessed by the Case Severity Adjudication Committee). After the primary analysis, the severity was assessed by the (blinded) adjudication committee for all case definitions. This also includes central confirmation results which were obtained after the primary analysis on COVID-19 primary endpoints with an onset prior to Jan 22nd. Other differences between the moderate to severe-critical COVID-19 endpoint for the correlates analysis vs. that for the primary analysis are: (1) both analyses included endpoints that occurred at least 28 days post-vaccination, where the correlates analysis additionally required that endpoints occurred after the D29 visit (which could have occurred +/− 3 days around 28 days post-vaccination, based on the allowable study visit windows), when the markers were measured; (2) the correlates analysis only required RT-PCR SARS-CoV-2 positivity of a nasal swab at a local laboratory (with or without central confirmation), whereas the primary analysis required that participants with RT-PCR SARS-CoV-2 positivity of a nasal swab at a local laboratory must also have a respiratory tract sample confirmed to be RT-PCR SARS-CoV-2 positive at a central laboratory using the m-2000 SARS-CoV-2 real-time RT-PCR assay (Abbott).1
Correlates analyses were performed in baseline SARS-CoV-2 seronegative participants in the per-protocol cohort, with the same definition of “per-protocol” as in Sadoff et al.1 Within this correlates analysis cohort, cases were COVID-19 primary endpoints in vaccine recipients starting both ≥ 1 day post-D29 and ≥ 28 days post-vaccination up to the end of the correlates study period, which is up to 54 days post D29, but no later than the data cut (January 22, 2021). Participants with any evidence of SARS-CoV-2 infection, such as a positive nucleic acid amplification test or rapid antigen test result, up to D29, were excluded. Correlates analyses were also done counting endpoints starting seven days after D29 or later through the same data cut, under the rationale that the D29 antibody marker measurements in participants who are diagnosed with the COVID-19 endpoint between 1–6 days post-D29 may possibly be influenced by SARS-CoV-2 infection. The point estimates of both analyses were similar; we report only the results that start counting COVID-19 endpoints at both ≥ 1 day post-D29 and ≥ 28 days post-vaccination, given the greater precision (approximately 35% more vaccine breakthrough cases).
Within the correlates analysis cohort, non-cases/controls were vaccine recipients sampled into the immunogenicity subcohort with no evidence of SARS-CoV-2 infection up to the end of the correlates study period, which is up to 54 days post D29 but no later than the data cut (January 22, 2021).
Solid-phase electrochemiluminescence S-binding IgG immunoassay (ECLIA)
Serum IgG binding antibodies against spike and serum IgG binding antibodies against RBD were quantitated using a validated solid-phase electrochemiluminescence S-binding IgG immunoassay and MSD Discovery Workbench software (version 4.0) as previously described.25 Within an assay run, each human serum test sample was added to the precoated wells in duplicates in an 8-point dilution series. Antibodies bound to spike or to RBD were detected using an MSD SULFO-TAG anti-human IgG detection antibody (Meso Scale Diagnostics, #R32AJ-1, goat polyclonal), diluted to 1X from a 200X vendor-provided stock. Conversion of arbitrary units/ml (AU/ml) readouts to bAb units/ml (BAU/ml) based on the World Health Organization 20/136 anti SARS-CoV-2 immunoglobulin International Standard39 was also as previously described.25 Antibody response was defined by detectable IgG concentration above the antigen-specific positivity cut-off (10.8424 BAU/ml for spike, 14.0858 BAU/ml for RBD).
Pseudovirus neutralization assay
Neutralizing antibody activity was measured at Monogram in a formally validated assay (detailed in Huang et al.40) that utilized lentiviral particles pseudotyped with full-length SARS-CoV-2 Spike protein. The lentiviral particles also contained a firefly luciferase (Luc) reporter gene, enabling quantitative measurement (via relative luminescence units, RLU) of infection of HEK 293T cells transiently transfected to express human ACE2 cell surface receptor protein and the TMPRSS2 protease. Supplementary Table 10 provides the assay limits. Readouts from the Monogram assay (also used in the immune correlates analysis of the COV002 trial of the ChAdOx1 nCoV-19 (AZD1222) vaccine 26) have been calibrated to those from the Duke pseudovirus neutralization assay (used in the immune correlates analysis of the COVE trial of the mRNA-1273 vaccine25) based on the World Health Organization 20/136 anti SARS-CoV-2 immunoglobulin International Standard39 and conversion to International Units/ml (IU50/ml), enabling direct comparison of vaccine efficacy at a given ID50 titer in ENSEMBLE to vaccine efficacy at the same ID50 titer in COVE or in COV002. Neutralizing antibody seroresponse was defined by quantifiable ID50 greater than the lower limit of quantitation (LLOQ), 2.7426 IU50/ml.
Ethics
All experiments were performed in accordance with the relevant guidelines and regulations. All participants whose serum samples were assayed in this work provided informed consent.
Statistical methods
All data analyses were performed as pre-specified in the Statistical Analysis Plan (SAP) (available as a supplementary file), with one exception. We had originally prespecified to include COVID-19 primary endpoints through the last COVID-19 primary endpoint with antibody data in the vaccine arm, and to let the time of this COVID-19 primary endpoint set the total duration of follow-up for the correlates analyses. However, after learning that the marginalized Cox modeling method yielded confidence intervals about the vaccine-efficacy-by-D29-marker-level curve that were wider than they should be based on statistical theory (precipitated by only a few vaccine recipients in the immunogenicity subcohort being at-risk for COVID-19 at 66 days, the time of the last COVID-19 primary endpoint with antibody data in the vaccine arm), we revised this rule to set follow-up through to the last time point at which there were still 15 participants from the immunogenicity subcohort still at risk, which corresponded to 54 days post D29. Consequently, two COVID-19 primary endpoints and some non-cases beyond 54 days post D29 were excluded from the analysis. The point estimates of the vaccine-efficacy-by-D29-marker-level curve were very similar for the two choices (follow-up through 54 vs. 66 days post D29).
Case-cohort set included in the correlates analyses
A case-cohort41 sampling design was used to randomly sample participants for D1, D29 antibody marker measurements. This random sample was stratified by the following baseline covariates: randomization arm, baseline SARS-CoV-2 serostatus, and 16 baseline demographic covariate strata defined by all combinations of: underrepresented minority (URM) within the US vs. non-URM within the US vs. Latin America vs. South Africa participant, age 18–59 vs. age ≥ 60, and presence vs. absence of comorbidities (see the SAP for details, as well as Extended Data Fig. 2 and Supplementary Table 1).
Covariate adjustment
All correlates analyses adjusted for the logit of predicted COVID-19 risk score built from machine learning of data from placebo arm participants (see Supplementary Note 1 and Supplementary Table 11) and geographic region (US, South Africa, Latin America).
Correlates of risk in vaccine recipients
All correlates of risk and protection analyses were performed in per-protocol baseline seronegative participants with no evidence of SARS-CoV-2 infection or right-censoring up to D29. For each of the three D29 markers, the covariate-adjusted hazard ratio of COVID-19 (either across marker tertiles or per 10-fold increase in the quantitative marker) was estimated using inverse probability sampling weighted Cox regression models with 95% CIs and Wald-based p-values. These Cox model fits were also used to estimate marker-conditional cumulative incidence of COVID-19 through 54 days post-D29 in per-protocol baseline seronegative vaccine recipients, with 95% CIs computed using the percentile bootstrap. The Cox models were fit using the survey package42 for the R language and environment for statistical computing.43 The same marker-conditional cumulative incidence of COVID-19 parameter was also estimated using nonparametric dose-response regression with influence-function-based Wald-based 95% CIs.44 Point and 95% CI estimates about marker-threshold-conditional cumulative incidence were computed by nonparametric targeted minimum loss-based regression.45
Correlates of protection
Controlled vaccine efficacy
For each marker, vaccine efficacy by marker level was estimated by a causal inference approach using both Cox proportional hazards estimation and nonparametric monotone dose-response estimation.44 The causal parameter being estimated is one minus the probability of COVID-19 by 54 days for the vaccine group supposing the D29 marker is set to a given level for all vaccine recipients, divided by this probability for the placebo arm; see the Statistical Analysis Plan (Section 12.3.2, 15.1) for details. Two sensitivity analyses of the robustness of results to potential unmeasured confounders of the impact of antibody markers on COVID-19 risk were also conducted, which specified a certain amount of confounding that made it harder to infer a correlate of protection (see the SAP for details). One of the sensitivity analyses was based on E-values28 and assessed the robustness of the inference that vaccine efficacy is greater for the upper marker tertile compared to the lower marker tertile. The other sensitivity analysis estimated how much vaccine efficacy increases with quantitative D29 antibody marker despite the specified unmeasured confounder.
Hypothesis testing
For hypothesis tests for D29 marker correlates of risk, Westfall-Young multiplicity adjustment46 was applied to obtain false-discovery rate adjusted p-values and family-wise error rate (FWER) adjusted p-values. Permutation-based multi-testing adjustment was performed over both the quantitative marker and tertilized marker CoR analyses. All p-values were two-sided.
Cross-trial comparisons
Calibration of ID50 nAb titers between the Duke neutralization assay (COVE trial samples) and the Monogram PhenoSense neutralization assay (COV002 and ENSEMBLE trial samples), performed using the WHO Anti-SARS CoV-2 Immunoglobulin International Standard (20/136) and Approach 1 of Huang et al.40 (with arithmetic mean as the calibration factor) is described in the supplementary material of Gilbert, Montefiori, McDermott et al.25
Software and data quality assurance
The analysis was implemented in R version 4.0.343; code was verified using mock data.
Extended Data
Extended Data Fig. 1:
Case-cohort set and trial timeline.
Extended Data Fig. 2:
Flowchart of study participants.
Extended Data Fig. 3:
D29 antibody marker level in participants in Latin America by COVID-19 outcome status.
Extended Data Fig. 4:
D29 antibody marker level in participants in South Africa by COVID-19 outcome status.
Extended Data Fig. 5:
D29 antibody marker level in participants in the United States by COVID-19 outcome status.
Extended Data Fig. 6:
Correlations of D29 antibody markers in baseline SARS-CoV-2 seronegative per-protocol vaccine recipients in the immunogenicity subcohort.
Extended Data Fig. 7:
Covariate-adjusted hazard ratios of COVID-19 per 10-fold increase in each Day 29 antibody marker in baseline SARS-CoV-2 seronegative per-protocol vaccine recipients in subgroups.
Extended Data Fig. 8:
Analyses of spike IgG and receptor binding domain (RBD) IgG as correlates of risk and as correlates of protection.
Extended Data Fig. 9:
Vaccine efficacy with sensitivity analysis by D29 (A) anti-spike IgG concentration, (B) anti-receptor binding domain (RBD) IgG concentration, or (C) pseudovirus (PsV) neutralization ID50 titer.
Extended Data Fig. 10:
Vaccine efficacy (solid lines) in baseline SARS-CoV-2 seronegative per-protocol vaccine recipients by A) D29 spike IgG or B) D29 receptor binding domain (RBD) IgG in ENSEMBLE by geographic region (US, United States; Lat Am, Latin America; S Afr, South Africa), estimated using the Cox proportional hazards implementation of Gilbert et al.44
Supplementary Material
Acknowledgments
This work was partially funded by the Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, under Government Contract Nos. HHSO100201700018C with Janssen and 75A50122C00008 with Labcorp – Monogram Biosciences; by the National Institutes of Health, National Institute of Allergy and Infectious Diseases (NIAID) under Public Health Service Grants UM1 AI068635 (HVTN SDMC) (PBG), UM1 AI068614 (HVTN LOC) (LC), and R37AI054165 (PBG); and by the Intramural Research Program of the NIAID Scientific Computing Infrastructure at Fred Hutch, under ORIP grant S10OD028685. This work was also supported by Janssen Research and Development, an affiliate of Janssen Vaccines and Prevention and part of the Janssen pharmaceutical companies of Johnson & Johnson.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the Department of Health and Human Services or its components.
We also thank Craig Magaret for assistance with SARS-CoV-2 sequencing data.
Footnotes
Code Availability Statement
All analyses were done reproducibly based on publicly available R scripts hosted on the GitHub collaborative programming platform (https://github.com/CoVPN/correlates_reporting2).
Competing Interests
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/downloads/coi_disclosure.docx and declare: P.B.G., Y.F., D.B., A.L., O.H., Y.L., C.Y., B.B., L.W.P.v.d.L., A.K., M.C., A.K.R., M.P.A., J.G.K., L.C., and L.N.C. had support (in the form of grant payments to their institutions) from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health for the submitted work; O.A.-A. had support from the NIH Vaccine Immunology Program for the submitted work; C.J.P. had support from BARDA for the contract testing provided by Labcorp-Monogram Biosciences for the submitted work and is a shareholder in and corporate officer of Labcorp; A.L. declares support (in the form of grant payments to his institution) within the past 36 months from Janssen Pharmaceuticals for work on the HVTN 706 HIV vaccine efficacy trial and from the World Health Organization for writing the statistical analysis plan for the WHO’s Solidarity Trial for COVID-19 vaccines, as well as consulting fees within the past 36 months received from Harvard Medical School for statistical consulting on individualized medicine software; O.H. had support (in the form of payments to his institution) from the Bill and Melinda Gates Foundation within the past 36 months; N.S.H. had support (in the form of payments to his institution) from the National Science Foundation for the submitted work; S.R. had partial support from the Department of Health and Human Services Biomedical Advanced Research and Development Authority (BARDA) (in the form of contract payments to his institution) for the submitted work, has stock and/or stock options in Johnson & Johnson, and is an employee of Janssen; D.J.S. had partial support from BARDA (in the form of contract payments to his institution) for the submitted work, has stock and/or stock options in Johnson & Johnson, and is an employee of Janssen; A.V. had partial support from BARDA (in the form of contract payments to her institution) for the submitted work, had all patent rights transferred to Johnson & Johnson, has stock and/or stock options in Johnson & Johnson, and is an employee of Janssen; M.L.G. had partial support from BARDA (in the form of contract payments to his institution) for the submitted work, has shares in Johnson & Johnson, and is an employee of Johnson & Johnson; G.A.V.R. had partial support from BARDA (in the form of contract payments to her institution) for the submitted work, has stock and/or stock options in Johnson & Johnson, and is an employee of Janssen; M.C. declares an honorarium from Pfizer within the past 36 months for serving on a scientific panel to evaluate submitted funding proposals on use of talazoparib in prostate cancer submitted in response to a joint call by the National Comprehensive Cancer Network and Pfizer; J.S. declares support for the submitted work from the Janssen Pharmaceutical Companies of Johnson & Johnson and partial support (in the form of funding to his institution) from BARDA for the submitted work, declares support within the past 36 months from the Janssen Pharmaceutical Companies of Johnson & Johnson and BARDA funding for part of this work, has patents on invention of the Janssen COVID-19 vaccine, and has Johnson & Johnson stock and stock options; G.E.G. declares payment for speaking at a Johnson and Johnson-sponsored session at a conference within the past 36 months (payment donated to charity) and for serving on the Johnson and Johnson COVID-19 Vaccine Advisory Board (fees donated to charity), as well as receipt of 500,000 doses of Ad26.COV2.S vaccine donated by the United States Government/Johnson and Johnson for the Sisonke study; B.G. received consulting fees from Janssen within the past 36 months and declares receiving payments from Merck, Janssen, and GSK within the last 36 months for participation on advisory boards, as well as receipt of Truvada provided to her institution from Gilead within the past 36 months for a PrEP demonstration project; P.A.G. declares support (in the form of grant payments to his institution) from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health within the past 36 months and consulting fees from Johnson and Johnson in the past 36 months; J.H. had partial support from BARDA (in the form of contract payments to her institution) for the submitted work, is a shareholder of Janssen Pharmaceuticals, and is an employee of Janssen Pharmaceuticals, C.T. had partial support from BARDA (in the form of contract payments to her institution) for the submitted work, holds stock in Janssen Pharmaceuticals, and is an employee of Janssen Pharmaceuticals; F.S. had partial support from BARDA (in the form of contract payments to his institution) for the submitted work, has restricted shares in Johnson & Johnson as part of compensation for employment, has shares in GlaxoSmithKline as compensation for past employment, and is employed by Johnson & Johnson; H.S. had partial support from BARDA (in the form of contract payments to her institution) for the submitted work, is a holder of Johnson & Johnson stock, and is an employee of Janssen Vaccines & Prevention BV; M.D. had partial support from BARDA (in the form of contract payments to her institution) for the submitted work, is a shareholder of Johnson & Johnson stock (has Johnson & Johnson Company stocks and stock options), and is an employee of Johnson & Johnson; K.M.N. declares grants paid to her institution within the past 36 months from Pfizer to conduct clinical trials of COVID vaccines (no salary support from this grant received) and support (in the form of grant payments to her institution) within the past 36 months from the National Institutes of Health; P.B.G. is a member of the BMGF COVID-19 Correlates Working Group (unpaid/no compensation); R.A.K. had support (in the form of intramural funding from NIAID) for the submitted work; R.O.D. declares that BARDA provided funding to Janssen and to Monogram Biosciences for the submitted work. All other authors declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years, and no other relationships or activities that could appear to have influenced the submitted work.
Ethics Committees
The COV3001 (ENSEMBLE) study was reviewed and approved by the following local ethics committees and IRBs:
Argentina: ANMAT - Administración Nacional de Medicamentos, Alimentos y Tecnologia Médica (Capital Federal, La Plata, Ramos Mejia – Buenos Aires; Ciudad Autonoma de Buenos Aires), Comite de Etica Dr Carlos Barclay (Capital Federal, Buenos Aires; Ciudad Autonoma de Buenos Aires), Comision Conjunta de Investigacion en Salud – CCIS (La Plata, Ramos Mejia - Buenos Aires), Comite de Bioetica de Fundacion Huesped (Ciudad Autonoma de Buenos Aires), Comité de Docencia e Investigación DIM Clínica Privada (Ramos Mejia, Buenos Aires), Comité de Ética en Investigación Clínica y Maternidad Suizo Argentina (Ciudad Autonoma de Buenos Aires), Comité de Ética en Investigación de CEMIC (Ciudad Autonoma de Buenos Aires), Comite de Etica en Investigacion DIM Clinica Privada (Ramos Mejia, Buenos Aires), Comite de Etica Hospital Italiano de La Plata (La Plata, Buenos Aires), Comite de Etiica en Investigacion Hospital General de Agudos J.M. Ramos Mejia (Ciudad Autonoma de Buenos Aires), Comitéde ética del Instituto Médico Platense (CEDIMP) (La Plata, Buenos Aires), IBC Fundacion Huesped (Ciudad Autonoma de Buenos Aires), IBC Helios Salud (Ciudad Autonoma de Buenos Aires), IBC Hospital General de Agudos J.M. Ramos Mejia (Ciudad Autonoma de Buenos Aires)
Brazil: ANVISA – Agência Nacional de Vigilância Sanitária (Salvador, Bahia; Barretos, Campinas, São Paulo, São Jose Rio Preto, Ribeirão Preto, São Caetano do Sul – São Paulo; Santa Maria, Porto Alegre – Rio Grande do Sul; Natal, Rio Grande do Norte; Para, Pará; Belo Horizonte, Minas Gerais; Rio de Janeiro, Nova Iguaçu – Rio de Janeiro; Curitiba, Paraná; Brasília, Distrito Federal; Campo Grande, Mato Grosso do Sul; Criciúma, Santa Catarina; Cuiabá, Mato Grosso), CONEP - Comissão Nacional de Ética em Pesquisa (Salvador, Bahia; São Paulo, São Paulo; Santa Maria, Rio Grande do Sul; Para, Pará;), CAPPESq – Comissão de Ética de Análise para Projetos de Pesquisa – HCFMUSP (São Paulo, São Paulo), CEP da Faculdade de Medicina de São José do Rio Preto – FAMERP (São Jose Rio Preto, São Paulo), CEP da Faculdade de Medicina do ABC/SP (São Paulo, São Paulo), CEP da Fundação Pio XII - Hospital do Câncer de Barretos/SP (Barretos, São Paulo), CEP da Liga Norteriograndense Contra o Câncer (Natal, Rio Grande do Norte), CEP da Pontificia Universidade Catolica de Campinas / PUC Campinas (Campinas, São Paulo), CEP da Real Benemérita Associaçao Portuguesa de Beneficência - Hospital São Joaquim (São Paulo, São Paulo), CEP da Santa Casa de Misericórdia de Belo Horizonte (Belo Horizonte, Minas Gerais), CEP da Secretaria Municipal De Saúde do Rio de Janeiro – SMS/RJ (Rio de Janeiro, Rio de Janeiro), CEP da Universidade de São Caetano do Sul (CEP da Universidade de São Caetano do Sul, São Paulo), CEP da Universidade Federal de Mato Grosso do Sul – UFMS (Campo Grande, Mato Grosso do Sul), CEP da Universidade Federal de Minas Gerais (Belo Horizonte, Minas Gerais), CEP do Centro de Referência e Treinamento DST/AIDS (São Paulo, São Paulo), CEP do do INI-Ipec/Fiocruz (Rio de Janeiro, Rio de Janeiro), CEP do Grupo Hospitalar Conceição / RS (Porto Alegre, Rio Grande do Sul), CEP do Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto/USP (Ribeirão Preto, São Paulo), CEP do Hospital de Clinicas da Universidade Federal do Parana - HCUFPR / PR (Curitiba, Paraná), CEP do Hospital de Clínicas de Porto Alegre/HCPA (Porto Alegre, Rio Grande do Sul), CEP do Hospital Geral de Nova Iguaçu (Nova Iguaçu, Rio do Janeiro), CEP do Hospital Municipal São José (Criciúma, Santa Catarina), CEP do Hospital Pró-Cardíaco/RJ (Rio de Janeiro, Rio de Janeiro), CEP do Hospital Sírio Libanês (São Paulo, Sao Paulo), CEP do Hospital Universitário Júlio Muller / MT (Cuiabá, Mato Grosso), CEP do Hospital Universitário Professor Edgard Santos – UFBA (Salvador, Bahia), CEP do Instituto de Cardiologia do Distrito Federal (Brasília, Distrito Federal), CEP do Instituto de Infectologia Emílio Ribas/SP (São Paulo, Sao Paulo), CEP do Instituto de Saude e Bem Estar da Mulher - ISBEM / SP (São Paulo, Sao Paulo), CEP em Seres Humanos do HFSE - Hospital Federal dos Servidores do Estado (Rio de Janeiro, Rio de Janeiro), CONEP - Comissão Nacional de Ética em Pesquisa (Brasília, Distrito Federal, Salvador, Bahia; Belo Horizonte, Minas Gerais; Cuiabá, Mato Grosso; Campo Grande, Mato Grosso do Sul; Nova Iguaçu, Rio Janeiro – Rio Janeiro; Barretos, Campinas, Sao Jose Rio Preto, São Caetano do Sul, Sao Paulo, Ribeirão Preto – Sao Paulo; Porto Alegre, Rio Grande do Sul; Natal, Rio Grande do Norte; Curitiba, Paraná; Criciúma, Santa Catarina)
Chile: Comité de Ética de Investigación en Seres Humanos (Santiago, Region Met), Comité Ético Científico Servicio de Salud Metropolitano Central (Santiago, Region Met), Instituto de Salud Pública de Chile (Santiago, Region Met; Talca, Temuco), Comité Ético-Científico Servicio de Salud Metropolitano Sur Oriente (Talca, Santiago), Comité de Evaluación Ética Científica Servicio de Salud Araucanía Sur Temuco (Temuco), Comité Ético Científico Servicio de Salud Metropolitano Central (Viña del Mar)
Colombia: CEI de la Fundación Cardiovascular de Colombia (Floridablanca), Comité de Ética en Investigación Clínica de la Costa (Barranquilla), INVIMA - Instituto Nacional de Vigilancia de Medicamentos y Alimentos (Colombia) (Barranquilla), Comite de Etica en Investigacion de la E.S.E. Hospital Mental de Antioquia (Santa Marta), Comite de Etica en la Investigacion CAIMED (Bogotá), INVIMA - Instituto Nacional de Vigilancia de Medicamentos y Alimentos (Colombia) (Bogotá), Comite Corporativo de Etica en Investigacion de la Fundacion Santa Fe de Bogota (Bogotá), Comité de Ética e Investigación Biomédica de la Fundación Valle del Lili (Cali), Comite de Etica e Investigacion IPS Universitaria (Medellin), Comite de Etica en Investigacion Asustencial Cientifica de Alta Complejidad (Bogotá), Comite de Etica en Investigacion Biomedica de la Corporacion Cientifica Pediatrica de Cali (Cali), Comité de Ética en Investigación Clínica de la Costa (Barranquilla), Comite de Etica en Investigacion de la E.S.E. Hospital Mental de Antioquia (Barrio Barzal Villavicencio), Comite de Etica en Investigacion del area de la Salud de la Universidad del Norte (Barranquilla), Comite de Etica en Investigacion Medplus Centro de Recuperación Integral S.A.S (Bogotá), Comité de Ética en Investigaciones CEI-FOSCAL (Floridablanca), Comite de Etica en la Investigacion CAIMED (Bogotá), Comite de Etica para Investigacion Clinica(CEIC) de la Fundacion Centro de Investigacion Clinica CIC (Medellin), Comite de Investigaciones y Etica en Investigaciones Hospital Pablo Tobon Uribe (Medellin), INVIMA - Instituto Nacional de Vigilancia de Medicamentos y Alimentos (Colombia) (Barranquilla, Bogotá, Cali, Floridablanca, Medellin
Mexico: CEI del Hospital Civil de Guadalajara Fray Antonio Alcalde (Guadalajara, Jalisco), CEI Hospital La Mision (Tijuana, Baja California Norte), CI del Hospital Civil de Guadalajara Fray Antonio Alcalde (Guadalajara, Jalisco), CI Hospital La Mision (Tijuana, Baja California Norte), Comite de Bioseguridad del Instituto Nacional de Salud Publica (Mexico, Distrito Federal; Cuernavaca, Morelos), Comite de Etica en Investigacion del Instituto Nacional de Salud Publica (Mexico, Distrito Federal; Cuernavaca, Morelos), Comité de Bioseguridad del Hospital La Misión S.A. de C.V. (Tijuana, Baja California Norte; Oaxaca, Oaxaca; Merida, Yucatán; Tijuana, Baja California Norte), Comité de Bioseguridad de la Coordinación de Investigación en Salud (IMSS) (Mexico, Estado de Mexico), Comité de Bioseguridad de Médica Rio Mayo (CLINBOR) (Mexico, Distrito Federal), Comité de Bioseguridad del Hospital Universitario “Dr. José Eleuterio González” (Monterrey, Nuevo León), COFEPRIS (Comisión Federal para la Protección contra Riesgos Sanitarios) (Cuernavaca, Morelos; Mexico, Distrito Federal; Monterrey, Nuevo León; Oaxaca, Oaxaca; Merida, Yucatán), Comite de Etica de la Fac de Med de la UANL y Hospital Universitario “Dr. Jose Eleuterio Gonzalez” (Monterrey, Nuevo León), Comite de Etica en Investigacion de la Unidad de Atencion Medica e Investigacion en Salud S.C. (Merida, Yucatán), Comite de Etica en Investigacion de Medica Rio Mayo S.C. (Mexico, Distrito Federal), Comite de Etica en Investigacion de Oaxaca Site Management Organization, S.C. (Oaxaca, Oaxaca), Comite de Etica en Investigacion del Centro Medico Nacional Siglo XXI (IMSS) (Mexico, Estado do Mexico), Comité de Investigación de la Coordinación de Investigación en Salud (IMSS) (Mexico, Estado do Mexico), Comite de Investigacion de la Unidad de Atencion Medica e Investigacion en Salud S.C. (Merida, Yucatán), Comite de Investigacion de Oaxaca Site Management Organization, S.C. (Oaxaca, Oaxaca), Comité de Investigación del Hospital Universitario José Eleuterio González (Monterrey, Nuevo León), Comite de Investigacion Medica Rio Mayo, S.C. (Mexico, Distrito Federal)
Peru: Comite Nacional Transitorio de Etica en Invest. de los Ensayos Clinicos de la enfermedad COVID-19 (Iquitos - Maynas, Loreto; Lima, San Miguel – Lima), INS - Instituto Nacional de Salud (Peru) (Lima, San Miguel – Lima; Callao; Iquitos – Maynas, Loreto)
South Africa: Department Agriculture, Forestry and Fisheries (DAFF) (Port Elizabeth, Mthatha – Eastern Cape; Cape Town, Worcester – Western Cape; Durban, Ladysmith, Vulindlela – KwaZulu-Natal; Johannesburg, Pretoria, Mamelodi East, Soweto, Tembisa – Gauteng; Rustenburg, Klerksdorp – North West; Bloemfontein, Free State; Middelburg, Mpumalanga; Dennilton, Limpopo), Pharma Ethics (Port Elizabeth, Eastern Cape; Durban, Ladysmith – KwaZulu-Natal; Cape Town, Western Cape; Pretoria, Mamelodi East, Johannesburg, Tembisa – Gauteng; Rustenburg, Klerksdorp – North West; Bloemfontein, Free State; Middelburg, Mpumalanga; Dennilton, Limpopo), SAHPRA - South African Health Products Regulatory Authority (Port Elizabeth, Mthatha – Eastern Cape; Cape Town, Worcester – Western Cape; Durban, Ladysmith, Vulindlela – KwaZulu-Natal; Johannesburg, Pretoria, Mamelodi East, Soweto, Tembisa – Gauteng; Rustenburg, Klerksdorp – North West; Bloemfontein, Free State; Middelburg, Mpumalanga; Dennilton, Limpopo), WIRB (Mamelodi East, Pretoria – Gauteng; Ladysmith, KwaZulu-Natal; Bloemfontein, Free State; Cape Town, Western Cape; Dennilton, Limpopo), Wits Health Consortium (Soweto, Johannesburg – Gauteng; Ladysmith, KwaZulu-Natal; Mthatha, Eastern Cape), Wits Institutional Biosafety Committee (Soweto, Pretoria, Johannesburg, Tembisa – Gauteng; Rustenburg, Klerksdorp – North West; Mthatha, Eastern Cape), University of Cape Town HREC (Cape Town, Worcester – Western Cape); University of Cape Town Institute of Infectious Disease & Molecular Medicine (Cape Town, Worcester – Western Cape), University of Cape Town Institutional Biosafety Committee (Cape Town, Worcester – Western Cape), SAMRC Human Research Ethics Committee Scientific Review (Durban, KwaZulu-Natal), Sefako Makgatho University Research Ethics Committee (SMUREC) (Pretoria, Gauteng), University of KwaZulu Natal Institutional Biosafety Committee (Durban, KwaZulu-Natal), University of KwaZulu-Natal Ethics (Durban, Vulindlela – KwaZulu-Natal), University of Stellenbosch Ethics Committee (Cape Town, Western Cape), University of KwaZulu Natal Institutional Biosafety Committee (Vulindlela, KwaZulu-Natal)
United States: Advarra IBC (Detroit, MI; Chapel Hill, NC; Boston, MA; Seattle, WA; Winston-Salem, NC; Austin, TX; Peoria, IL; Huntsville, AL; Long Beach, CA; Tucson, AZ), Biomedical Institute of New Mexico - IBC (Albuquerque, NM), Birmingham VA Medical Center - Alabama- IBC (Birmingham, AL), Clinical Biosafety Services (Hollywood, FL), Columbia University IBC (New York, NY), Copernicus Group IRB (Austin, Dallas, Houston, San Antonio – TX; Rochester, New York, Bronx, Binghamton – NY; Hillsborough, Hackensack, Newark, New Brunswick – NJ; West Palm Beach, Coral Gables, Hollywood, Miami, Orlando, Gainesville, Tampa, Hallandale Beach, Pinellas Park, The Villages, Jacksonville, Deland – FL; Fort Worth, Dallas, San Antonio – TX; Norfolk, Charlottesville – VA; Matairie, New Orleans – LA; Nashville, Knoxville, Memphis, Bristol – TN; Cincinnati, Cleveland, Columbus, Akron – OH; Detroit, Ann Arbor, Grand Rapids – MI; Philadelphia, Pittsburgh – PA; Stanford, San Diego, San Francisco, Oakland, Long Beach, Anaheim, Sacramento, West Hollywood – CA, Las Vegas, Reno – NV; Chicago, Peoria – IL; Omaha, NE; Mobile, Birmingham, Huntsville – AL; St Louis, Greer, Kansas City – MO; Boston, MA; Harrisburg, SD; Decatur, Atlanta, Savannah – GA; Baltimore, Rockville, Annapolis – MD; New Haven, Hartford – CT; Chapel Hill, Raleigh, Fayetteville, Charlotte, Durham, Winston-Salem – NC; Indianapolis, Valparaiso, Evansville – IN; Seattle, WA; Aurora, CO; Lexington, Louisville – KY; Murray, West Jordan, Salt Lake City – UT; Phoenix, Tucson, Glendale – AZ; Spartanburg, Columbia, North Charleston, Anderson, Charleston, Mount Pleasant – SC; Portland, Medford, Corvallis – OR; Albuquerque, Gallup – NM; Little Rock, AR; Jackson, MS; Newport News, VA, Minneapolis, MN; Lenexa, KS), WIRB (Hackensack, NJ; Dallas, TX; Baltimore, MD; Chicago, IL; Aurora, CO; Winston-Salem, NC; Minneapolis, MN; Orlando, Miami, Gainesville – FL; Philadelphia, Pittsburgh – PA; Boston, MA; St Louis, MO; Bronx, New York, NY; New Brunswick, NJ; Phoenix, AZ; Birmingham, AL; Louisville, KY; Albuquerque, NM; New Orleans, LA; Baltimore, MD; San Francisco, CA; Tampa, FL; Aurora, CO; Columbia, SC; Decatur, GA; Reno, NV; Raleigh, NC; Little Rock, AS), Clinical Biosafety Services (Dallas, San Antonio – TX; San Diego, CA; Lexington, KY; Murray, UT; Greer, Kansas City, St Louis – MO; Rockville, MD; Las Vegas, NV; Cincinnati, Columbus, Akron – OH; Phoenix, Tucson, Glendale – AZ; North Charleston, Anderson – SC; Orlando, Pinellas Park, The Villages, Miami – FL; Birmingham, AL; Valparaiso, Evansville – IN; Lenexa, KS), Columbia University IBC (Bronx, New York), Durham VA Medical Center-IBC (Raleigh, NC), Emory University IRB (Decatur, GA), Environmental Health and Safety Office (Atlanta, GA), Institutional Biosafety Committee (New Orleans, LA), James A. Haley Veterans Hospital_IBC (Tampa, FL), Jesse Brown VA Medical Center- IBC (Chicago, IL), Mass General Brigham IBC (Boston, MA), Mount Sinai- Icahn School of Medicine IBC (New York, NY), New York Blood Center IBC (New York, NY), OHSU IBC (Portland, OR), Partners Institutional Biosafety Committee (Boston, MA), Rocky Mountain Regional VA Medical Center-IBC (Aurora, CO), Rush University Medical Center (Chicago, IL), Rush University Medical Center IBC (Chicago, IL), Rutgers Institutional Biosafety Committee (New Brunswick, NJ), Saint Louis University IBC (St Louis, MO), Saint Michael’s Medical Center IRB (Newark, NJ), Southeast Louisiana Veterans Health Care System IBC (New Orleans, LA), St. Jude Children’s Research Hospital IBC Committee (Memphis, TN), St. Jude Children’s Research Hospital IRB (Memphis, TN), Stanford University Administrative Panel on Human Subjects in Medical Research (Stanford, CA), Temple University – IBC (Philadelphia, PA), The University of Chicago Institutional Biosafety Committee (Chicago, IL), UAMS IBC (Little Rock, AS), UIC IBC (Chicago, IL), University of Alabama at Birmingham Institutional Biosafety Committee (Birmingham, AL), University of Arkansas IRB (Little Rock, AS), University of Kentucky Biological Safety (Lexington, KY), University of Kentucky IRB (Lexington, KY), University of Louisville IRB (Louisville, KY), University of Miami-IBC (Miami, FL), University of Mississippi Medical Center IRB (Jackson, MI), University of Pennsylvania Institutional Biosafety Committee (Philadelphia, PA), University of Pittsburgh IBC (Pittsburgh, Pennsylvania), University of South Florida IRB (Tampa, FL), University of Utah Institutional Biosafety Committee (Salt Lake City, UT), University of Utah IRB (Salt Lake City, UT), UTHealth – IBC (Houston, TX), VA Baltimore Research & Education Foundation (BREF)- IBC (Baltimore, MD), VA Central Arkansas Veterans Healthcare System-IBC (Little Rock, AS), VA James J. Peters Department of VA Medical Center-IBC (Bronx, NY), VA Medical Center - Atlanta-IBC (Decatur, GA), VA Medical Center San Francisco- IBC (San Francisco, CA), VA North Florida/South Georgia IBC (Gainesville, FL), VA North Texas Health Care System IBC (Dallas, TX), VA San Diego Healthcare System IBC (Phoenix, AZ), VA Sierra Nevada Health Care System-IBC (Reno, NV), Vanderbilt University Instituitional Review Board (Nashville, TN), Washington University IBC (St Louis, MO), WCG IBCS (Houston, TX; Orlando, FL), Western Institutional Review Board (San Diego, CA; Detroit, MI; New Orleans, LA; New York, NY), WIRB - IBCS Services (Chicago, IL; New Orleans, LA; Oakland, CA; Minneapolis, MN; Columbus, OH; Lexington, KY), WJB Dorne VA Medical Center IBC (Columbia, SC)
Data Availability Statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. The data supporting the findings of this study may be obtained from the authors upon reasonable request.
References
- 1.Sadoff J, Gray G, Vandebosch A, et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med 2021; 384(23): 2187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sadoff J, Gray G, Vandebosch A, et al. Final Analysis of Efficacy and Safety of Single-Dose Ad26.COV2.S. N Engl J Med 2022; 386(9): 847–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US Food and Drug Administration. Janssen COVID-19 Vaccine. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/janssen-covid-19-vaccine Last updated 19 Jan, 2022. Access date 23 Jan, 2022. [Google Scholar]
- 4.World Health Organization. COVID-19 vaccines WHO EUL issued. https://extranet.who.int/pqweb/vaccines/vaccinescovid-19-vaccine-eul-issued Access date 24 Jan, 2022. [Google Scholar]
- 5.European Commission. European Commission authorises fourth safe and effective vaccine against COVID-19. Press release. 11 March 2021. https://ec.europa.eu/commission/presscorner/detail/en/ip_21_1085 Access date 24 Jan, 2022. [Google Scholar]
- 6.VIPER Group COVID19 Vaccine Tracker Team. COVID19 Vaccine Tracker. https://covid19.trackvaccines.org/vaccines/approved/ Access date 10 Jun, 2022. [Google Scholar]
- 7.Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol 2010; 17(7): 1055–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plotkin SA, Gilbert PB. “Correlates of Protection” in Plotkin’s Vaccines (Seventh Edition). Plotkin SA, Orenstein WA, Offit PA, Edwards KM, Eds. (Elsevier, 2018), chap. 3. [Google Scholar]
- 9.Plotkin SA, Gilbert PB. Nomenclature for immune correlates of protection after vaccination. Clin Infect Dis 2012; 54(11): 1615–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walter EB, Talaat KR, Sabharwal C, et al. Evaluation of the BNT162b2 Covid-19 Vaccine in Children 5 to 11 Years of Age. N Engl J Med 2022; 386(1): 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.US Food and Drug Administration. FDA News Release. FDA Authorizes Pfizer-BioNTech COVID-19 Vaccine for Emergency Use in Children 5 through 11 Years of Age. 29 Oct, 2021. https://www.fda.gov/news-events/press-announcements/fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use-children-5-through-11-years-age Access date 3 Feb, 2022. [Google Scholar]
- 12.Duarte N, Yanes-Lane M, Arora RK, et al. Adapting Serosurveys for the SARS-CoV-2 Vaccine Era. Open Forum Infect Dis 2022; 9(2): ofab632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lumley SF, O’Donnell D, Stoesser NE, et al. Antibody Status and Incidence of SARS-CoV-2 Infection in Health Care Workers. N Engl J Med 2021; 384(6): 533–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Addetia A, Crawford KHD, Dingens A, et al. Neutralizing Antibodies Correlate with Protection from SARS-CoV-2 in Humans during a Fishery Vessel Outbreak with a High Attack Rate. J Clin Microbiol 2020; 58(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMahan K, Yu J, Mercado NB, et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 2021; 590(7847): 630–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu J, Tostanoski LH, Peter L, et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science 2020; 369(6505): 806–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corbett KS, Nason MC, Flach B, et al. Immune correlates of protection by mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. Science 2021; 373(6561): eabj0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He X, Chandrashekar A, Zahn R, et al. Low-dose Ad26.COV2.S protection against SARS-CoV-2 challenge in rhesus macaques. Cell 2021; 184(13): 3467–73.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Brien MP, Forleo-Neto E, Musser BJ, et al. Subcutaneous REGEN-COV Antibody Combination to Prevent Covid-19. N Engl J Med 2021; 385(13): 1184–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021; 27(7): 1205–11. [DOI] [PubMed] [Google Scholar]
- 21.Earle KA, Ambrosino DM, Fiore-Gartland A, et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 2021; 39(32): 4423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO Ad Hoc Expert Group on the Next Steps for Covid-19 Vaccine Evaluation, Krause PR, Fleming TR, et al. Placebo-Controlled Trials of Covid-19 Vaccines - Why We Still Need Them. N Engl J Med 2021; 384(2): e2. [DOI] [PubMed] [Google Scholar]
- 23.Koup RA, Donis RO, Gilbert PB, Li AW, Shah NA, Houchens CR. A government-led effort to identify correlates of protection for COVID-19 vaccines. Nature Medicine 2021; 27: 1493–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med 2021; 384(5): 403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilbert PB, Montefiori DC, McDermott AB, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 2022; 375(6576): 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng S, Phillips DJ, White T, et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med 2021; 27(11): 2032–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilbert PB, Montefiori DC, McDermott AB, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 2021: eab3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann Intern Med 2017; 167(4): 268–74. [DOI] [PubMed] [Google Scholar]
- 29.Voysey M, Costa Clemens SA, Madhi SA, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet 2021; 397(10277): 881–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cromer D, Steain M, Reynaldi A, et al. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet Microbe 2022; 3(1): e52–e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldblatt D, Alter G, Crotty S, Plotkin SA. Correlates of protection against SARS-CoV-2 infection and COVID-19 disease. Immunol Rev 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization. Interim statement on hybrid immunity and increasing population seroprevalence rates. 1 Jun 2022. Available at: https://www.who.int/news/item/01-06-2022-interim-statement-on-hybrid-immunity-and-increasing-population-seroprevalence-rates Access date 8 Sept 2022. [Google Scholar]
- 33.Barouch DH, Stephenson KE, Sadoff J, et al. Durable Humoral and Cellular Immune Responses 8 Months after Ad26.COV2.S Vaccination. N Engl J Med 2021; 385(10): 951–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pajon R, Paila YD, Girard B, et al. Initial analysis of viral dynamics and circulating viral variants during the mRNA-1273 Phase 3 COVE trial. Nat Med 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Z, Mateus J, Coelho CH, et al. Humoral and cellular immune memory to four COVID-19 vaccines. doi: 10.1101/2022.03.18.484953 Posted 21 Mar, 2022. Access date 22 Mar, 2022. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richardson SI, Manamela NP, Motsoeneng BM, et al. SARS-CoV-2 Beta and Delta variants trigger Fc effector function with increased cross-reactivity. Cell Rep Med 2022; 3(2): 100510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sadoff J, Le Gars M, Brandenburg B, et al. Durable antibody responses elicited by 1 dose of Ad26.COV2.S and substantial increase after boosting: 2 randomized clinical trials. Vaccine 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alter G, Yu J, Liu J, et al. Immunogenicity of Ad26.COV2.S vaccine against SARS-CoV-2 variants in humans. Nature 2021; 596(7871): 268–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Institute for Biological Standards and Control (NIBSC). Instructions for use of First WHO International Standard for anti-SARS-CoV-2 Immunoglobulin (Version 3.0, Dated 17/12/2020) NIBSC code: 20/136 https://www.nibsc.org/science_and_research/idd/cfar/covid-19_reagents.aspx Access date Jul 29, 2021. [Google Scholar]
- 40.Huang Y, Borisov O, Kee JJ, et al. Calibration of two validated SARS-CoV-2 pseudovirus neutralization assays for COVID-19 vaccine evaluation. Sci Rep 2021; 11(1): 23921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prentice RL. A Case-Cohort Design for Epidemiologic Cohort Studies and Disease Prevention Trials. Biometrika 1986; 73(1): 1–11. [Google Scholar]
- 42.Lumley T. Complex Surveys: A Guide to Analysis Using R (vol. 565, John Wiley & Sons, 2010). [Google Scholar]
- 43.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. 2022. [Google Scholar]
- 44.Gilbert PB, Fong Y, Kenny A, Carone M. A Controlled Effects Approach to Assessing Immune Correlates of Protection. kxac024, 10.1093/biostatistics/kxac24. Biostatistics 2022. [DOI] [PubMed] [Google Scholar]
- 45.van der Laan L, Zhang W, Gilbert PB. arXiv:2107.11459 [stat.ME] https://arxiv.org/abs/2107.11459. 2021. [Google Scholar]
- 46.Westfall PH, Young SS. Resampling-Based Multiple Testing: Examples and Methods for P-Value Adjustment (vol. 279, Wiley Series in Probability and Statistics, John Wiley & Sons, 1993). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. The data supporting the findings of this study may be obtained from the authors upon reasonable request.