Background:
The Drug Hypersensitivity Quality of Life Questionnaire (DrHy-Q) is not currently available in Chinese. Besides, penicillin allergy (PA) is a worldwide public health challenge, and delabeling inauthentic PA can improve clinical and economic outcomes. However, its effect on health-related quality of life (HRQoL) remains poorly known.
Objective:
The study objective is to translate and validate a Chinese version of DrHy-Q and investigate the effect of PA delabeling on HRQoL using DrHy-Q.
Methods:
A Chinese DrHy-Q was translated then completed by patients with drug allergy labels for psychometric validation. Afterwards, another cohort of patients finished the Chinese DrHy-Q before and after their PA workup for pre–post comparison.
Results:
A total of 130 patients were studied. Sixty-three patients (79.4% female; median age = 59 ± 15 years) completed the Chinese DrHy-Q for validation (mean score = 38.9 ± 23.5). It demonstrated excellent internal consistency (Cronbach α = 0.956; 95% confidence interval [CI], 0.939–0.971) and test–retest reliability (intraclass correlation coefficient = 0.993 [95% CI, 0.969–0.998]). Construct validity was confirmed by its one-dimensional structure in factor analysis. Divergent validity was established because only 2 (out of 9) SF-36 scales showed weak negative correlations to DrHy-Q. Patients with multiple implicated drugs presented significantly higher DrHy-Q scores than those with only a single drug (42.0 ± 22.5 vs 28.7 ± 24.4; P = 0.038), showing discriminant validity. Subsequently, another 67 patients (73.1% female; median age = 56 ± 15 years) underwent PA investigations and completed their pre–post DrHy-Q. A significant drop in DrHy-Q score was shown (40.8 ± 21.7 vs 26.6 ± 22.5; Cohen’s d = 0.964; P < 0.001), reflecting improvement in HRQoL.
Conclusion:
The Chinese DrHy-Q is reliable and valid for HRQoL assessment. PA delabeling significantly benefits patients’ HRQoL. Future larger-scale studies are warranted to corroborate our findings.
Keywords: Delabeling, Drug allergy, Penicillin, Quality of life, Questionnaire, Validation
1. Introduction
Drug hypersensitivity reactions (DHR) are unpredictable, dose-independent adverse drug reactions that clinically resemble allergic reactions [1]. Suspected DHR are commonly encountered in clinical practice, and restrictions due to drug allergy labels can greatly impede future prescription choices. For example, a population-wide study in Hong Kong demonstrated that >7% of the population carried at least 1 physician-diagnosed drug allergy label in their medical records [2]. Among them, β-lactam or penicillin antibiotics were the most implicated class of drugs, contributing to >28% of all drug allergy labels and 2% of the entire Hong Kong population. Alarmingly, the prevalence of penicillin allergy (PA) is even higher in reports from European cohorts with an estimated prevalence of up to 8% to 10% [3]. This global burden of drug allergy is a huge concern, as PA labels are known to be associated with inferior clinical outcomes, prolonged hospitalization, higher medical costs as well as development of multidrug resistant organisms [4]. Delabeling of incorrect PA is safe and can lead to reduction in nonpenicillin antibiotic use, improved clinical outcomes, as well as reduction in future healthcare costs [5–10]. Despite a myriad of detrimental effects associated with PA, there have been surprisingly few studies focusing on patient-reported outcomes (PROs) such as health-related quality of life (HRQoL) and disease perception [11].
HRQoL is an important outcome measure and integral part of allergy research, with extensive literature published for food allergy and asthma. However, research pertaining to measuring HRQoL for DHR remains relatively scarce [12]. One reason is that validated and disease-specific instruments to measure HRQoL for DHR have been lacking until recent years. The Drug Hypersensitivity Quality of Life Questionnaire (DrHy-Q) was first developed and validated in Italy to assess the HRQoL of patients with DHR [13]. DrHy-Q is a self-administered questionnaire that consists of 15 questions on a 5-point Likert scale. The aggregate score is converted to a scale of 0 to 100, where a higher score indicates poorer HRQoL. This tool was subsequently validated and used in a number of European countries such as Turkey and Spain [14–17]. Nevertheless, experience remains limited in Asia, of which Thailand is the only country with its own localized, validated version [18].
Some previous European studies have evaluated the impact of allergy interventions such as desensitization on HRQoL [19, 20]. Yet, no studies have ever been conducted outside of Europe. Provided that there have been reported differences in DrHy-Q scores even among different European countries, there is likely even greater diversity in different populations around the world [17]. Population-specific tools to aid further research on HRQoL for DHR in populations beyond Europe are, therefore, urgently needed. Moreover, DrHy-Q has never been applied to investigate the outcomes and effects of drug allergy delabeling.
In recent years, the Hong Kong Drug Allergy Delabeling Initiative was established in Hong Kong to conduct territory-wide PA delabeling [10]. It offers us a good opportunity to study, in a vastly different geographical and cultural context, the effects of PA delabeling on different aspects of outcomes including HRQoL. However, all these are not possible without a validated DrHy-Q in Chinese. This study aims to translate and validate the Chinese version of DrHy-Q, as well as to demonstrate its use in assessing HRQoL following PA delabeling.
2. Materials and methods
2.1. Stage 1: Translation and pilot testing
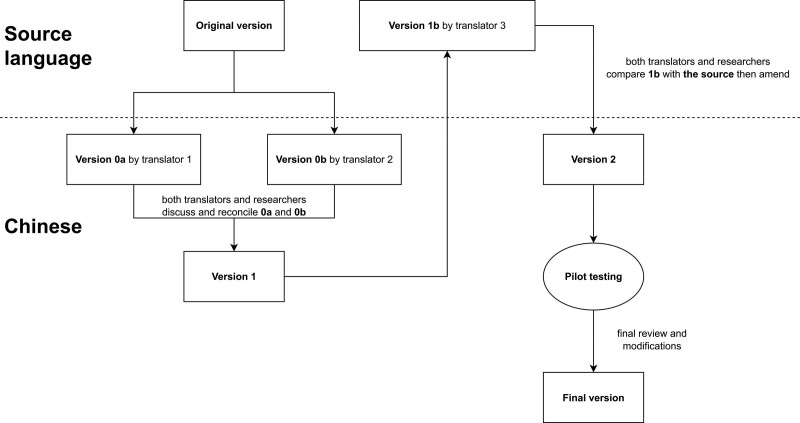
The translation process referenced international guidelines as shown in Fig. 1 [21]. First, the original DrHy-Q was translated into Chinese by 2 translators who discussed and reconciled a single translated version with other researchers of this study. The Chinese version was then back-translated into the source language by another blinded translator. Next, the back-translated questionnaire was reviewed by all the researchers, and the Chinese translation was revised accordingly. The revised version was distributed to a focus group for pilot testing, which resulted in a finalized version.
Figure 1.
Flow of forward- and back-translation of the questionnaire.
2.2. Stage 2: Validation
After cross-cultural adaptation, the Chinese DrHy-Q underwent a validation procedure to confirm its psychometric properties to be consistent with the original version. For this purpose, patients ≥18 years old and labeled allergic to medications were sequentially recruited from Queen Mary Hospital (QMH) and Grantham Hospital (GH) in Hong Kong. They were asked to fill in the Chinese DrHy-Q and the validated Chinese (Hong Kong) version of the 36-Item Short Form Survey (SF-36) simultaneously given. Their demographic and clinical characteristics were also collected. Those who did not complete both questionnaires were excluded.
2.3. Reliability
The reliability of the questionnaire was examined in terms of internal consistency and test–retest reliability. Internal consistency was quantified using Cronbach α, which indicates adequate internal consistency for group comparison if ≥0.70. For clinical application, Cronbach α ≥ 0.90 is often required [22]. Test–retest reliability was assessed by administrating the translated DrHy-Q twice to patients with no major changes in health or allergy status (ie, no allergy assessment, delabeling, or confirmation during the period) with an interval of at least 1 week. Correlation of responses was measured in intraclass correlation coefficient (ICC), which is considered satisfactory if 0.4 to 0.7 using the 2-way mixed consistency model [23].
2.4. Validity
The construct validity, divergent validity, and discriminant validity were checked for the validity of the translated instrument. The construct validity of the translated survey was examined by the exploratory factor analysis with maximum likelihood. Factors were extracted based on the scree plot and parallel analysis. A one-dimensional structure as the original DrHy-Q was expected. Items with factor loadings ≤ 0.40 were excluded.
For divergent validity, the validated Chinese (Hong Kong) SF-36 was used as a comparison tool [24]. Developed in 1992, SF-36 is a generic HRQoL questionnaire that comprises 9 multi-item scales (except health change), each for a health concept such as emotional well-being and physical functioning. Because DrHy-Q is a disease-specific HRQoL questionnaire, we do not expect strong correlations with other generic surveys. As higher scores indicate better health status in SF-36, we hypothesize that no or only a weak negative correlation exists between DrHy-Q and SF-36. This was tested by Spearman correlation between the DrHy-Q score and each of the SF-36 scale scores. Numerically, 0 < ρ ≤ 0.39 implies no or a weak correlation, while 0.40 ≤ ρ ≤ 0.59 and ρ ≥ 0.60, respectively, imply a moderate one and a strong one.
Discriminant validity was also tested by categorizing patients into different subgroups according to their clinical characteristics (type and number of suspected drug allergies, and type and severity of index reactions) and comparing their DrHy-Q scores. Statistical tests applied here include the Mann-Whitney U test and the Kruskal-Wallis H test.
2.5. Stage 3: Application
After linguistic and psychometric validation, the Chinese version was introduced to clinical settings. A cohort of patients ≥18 years old and referred for PA investigation at QMH or GH was enrolled. Only patients with fully completed allergy evaluation with a final allergist-made diagnosis (either with PA confirmed or delabeled) were included. All patients were provided and completed a DrHy-Q prior to their delabeling workup (as pre-responses). Then regardless of their final diagnosis, another DrHy-Q was distributed to collect their post responses 1 week after completion of allergy evaluation. Their demographic and clinical characteristics were gathered as well.
Paired t test was used to compare DrHy-Q scores before and after delabeling. The standardized effect size of the intervention was denoted by Cohen’s d, which represents small, medium or large effect size if ≥ 0.2, ≥ 0.5, or ≥ 0.8, respectively. Their percentage changes in DrHy-Q scores were also calculated and reported.
2.6. Allergy investigation
For delabeling, history taking, skin test ([ST]; skin prick test [SPT] and then intradermal test [IDT] if negative for immediate-type reactions, or delayed IDT and patch test [PT] for nonimmediate reactions), and drug provocation test ([DPT]; nonimmediate type reactions were assessed via follow-up nurse clinic 1 week after DPT) were done in order by the attending allergist. In vitro tests, such as basophil activation test and lymphocyte transformation test, might be ordered at the allergist’s discretion if clinically indicated. Tolerance was confirmed after a negative DPT. On the contrary, patients with a clinically compatible positive result in any of the conducted tests were deemed penicillin allergic.
All statistical analyses were conducted on IBM SPSS Statistics version 28.0 (IBM Co., Armonk, NY, USA). Two-sided P-value <0.05 was considered statistically significant.
This study was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (Chairperson Professor Sydney Tang) on 8 September 2020 (Reference Number: UW20-631). All patients provided informed consent.
3. Results
3.1. Validation
At the validation stage, 63 patients constituted the cohort and completed both DrHy-Q and SF-36. The median age was 59 ± 15 years and the female:male ratio was 3.9:1. The median duration of drug allergy label was 10.0 ± 12.4 years. The leading suspected causes were β-lactam antibiotics (52/63, 82.5%), non-β-lactam antibiotics (24/63, 38.1%), nonsteroidal anti-inflammatory drugs (21/63, 33.3%) and radiocontrast media (9/63, 14.3%). Around three-quarters (48/63, 76.2%) of patients had >1 drug allergy label. Regarding the index event, 6.3% (4/63) of them complained of severe reactions (anaphylaxis or severe cutaneous adverse reactions [SCAR]). For SCAR, all 3 patients were diagnosed with drug reaction with eosinophilia and systemic symptoms. Table 1 summarizes their demographics and other clinical characteristics. The overall mean DrHy-Q score is 38.9 ± 23.5.
Table 1.
DrHy-Q scores of patients with different demographic and clinical characteristics
| N (%) | DrHy-Q score | P | |
|---|---|---|---|
| Total | 63 (100.0) | 38.9 ± 23.5 | NA |
| Age | |||
| ≥65 y | 21 (33.3) | 42.5 ± 29.0 | 0.704 |
| <65 y | 42 (66.7) | 37.1 ± 20.3 | |
| Sex | |||
| Female | 50 (79.4) | 41.4 ± 23.0 | 0.074 |
| Male | 13 (20.6) | 29.0 ± 23.7 | |
| Duration of allergy label | |||
| <1 y | 9 (14.3) | 47.8 ± 19.3 | 0.138 |
| ≥1 y | 54 (85.7) | 37.4 ± 23.9 | |
| Implicated drug | |||
| β-Lactam antibiotics | 52 (82.5) | 39.6 ± 22.8 | NA† |
| Non-β-lactam antibiotics | 24 (38.1) | 41.3 ± 23.9 | |
| NSAID | 21 (33.3) | 42.9 ± 22.7 | |
| Radiocontrast media | 9 (14.3) | 39.8 ± 21.3 | |
| Others | 28 (44.4) | 39.9 ± 24.1 | |
| Number of implicated drug(s) | |||
| Single | 15 (23.8) | 28.7 ± 24.4 | *0.038 |
| Multiple | 48 (76.2) | 42.0 ± 22.5 | |
| Type of index reaction | |||
| Immediate (<1 h) | 22 (34.9) | 40.6 ± 23.7 | 0.735 |
| Nonimmediate (≥1 h) | 27 (42.9) | 39.4 ± 24.0 | |
| Unknown | 14 (22.2) | 35.1 ± 23.4 | |
| Severity of index reaction | |||
| Anaphylaxis or SCAR | 4 (6.3) | 57.5 ± 19.6 | 0.073 |
| Others | 59 (93.7) | 37.6 ± 23.3 | |
| Clinical manifestations | |||
| Urticaria | 23 (36.5) | 40.1 ± 22.8 | 0.226 |
| Anaphylaxis | 1 (1.6) | 73.3 ± NA | |
| Non-SCAR cutaneous reactions | 23 (36.5) | 39.4 ± 23.4 | |
| SCAR | 3 (4.8) | 52.2 ± 20.2 | |
| Others | 13 (20.6) | 30.0 ± 24.1 |
DrHy-Q, Drug Hypersensitivity Quality of Life Questionnaire; NSAID, nonsteroidal anti-inflammatory drugs; SCAR, severe cutaneous adverse reactions.
Bold denotes variable reaching statistical significance.
The groups are not mutually exclusive because patients could be subjected to multiple suspected drug allergies.
3.2. Reliability
Excellent global internal consistency was established with a Cronbach α of 0.956 (95% confidence interval [CI], 0.939–0.971), meeting the criterion for clinical application. Nine patients completed DrHy-Q the second time and confirmed the questionnaire’s test–retest reliability (ICC = 0.993 [95% CI, 0.969–0.998]).
3.3. Validity
In terms of construct validity, a 1-dimensional structure, which explained up to 62.9% of the total variance, was revealed via exploratory factor analysis. No survey item was excluded because all factor loadings exceeded 0.40 (Table 2).
Table 2.
Factor loading of items in the Chinese version of DrHy-Q
| Item | Factor loading |
|---|---|
| Since I am unable to take drugs every illness limits me more than other people. | 0.720 |
| I am afraid of being administered a drug during an emergency to which I am allergic. | 0.808 |
| I feel frightened due to my problem of allergy reaction. | 0.838 |
| The problem of adverse reaction to drugs affects my life. | 0.863 |
| I would like the allergist’s opinion before taking drugs prescribed by other specialists. | 0.590 |
| Even a little discomfort for me is a problem. | 0.861 |
| The fact that I cannot use medication safely made me feel different from others. | 0.844 |
| I feel anxious due to my problem of allergy reaction. | 0.871 |
| For each disease I would be confident that there is a drug that I can safely take. | 0.613 |
| I am afraid I could not deal with the pain. | 0.723 |
| I feel anguished due to my problem of allergy reaction. | 0.863 |
| I worry every time I take a drug different from ones that cause my allergic reactions. | 0.826 |
| I give up leisure activities (sport, vacations, and trips) because of my problem. | 0.666 |
| I’m in a bad mood due to my problem of allergy reaction. | 0.697 |
| The idea of taking a medicine makes me feel anxious. | 0.799 |
Divergent validity was demonstrated in view of the differentiation between SF-36 and DrHy-Q. Only 2 SF-36 scales were weakly and negatively correlated to DrHy-Q (Table 3). Both (emotional well-being [Spearman ρ = −0.390; P = 0.002] and social functioning [Spearman ρ = −0.349; P = 0.005]) concern mental health impairment. The other 7 scales did not display statistically significant associations with DrHy-Q.
Table 3.
Spearman correlation between DrHy-Q score and SF-36 scales
| SF-36 scale | Spearman ρ | P value |
|---|---|---|
| Physical functioning | −0.205 | 0.107 |
| Role limitations due to physical health | −0.180 | 0.157 |
| Role limitations due to emotional problems | −0.216 | 0.089 |
| Energy/fatigue | −0.245 | 0.053 |
| Emotional well-being | −0.390 | *0.002 |
| Social functioning | −0.349 | *0.005 |
| Pain | −0.192 | 0.133 |
| General health | −0.164 | 0.198 |
| Health change | −0.208 | 0.102 |
DrHy-Q, Drug Hypersensitivity Quality of Life Questionnaire; SF-36, 36-Item Short Form Survey.
Bold denotes correlations reaching statistical significance.
Subgroup analysis based on index drugs and reactions also suggested good discriminant validity. Multiple implicated drugs predicted significantly higher DrHy-Q scores, that is, poorer HRQoL than those with only a single trigger (42.0 ± 22.5 vs 28.7 ± 24.4; P = 0.038). The DrHy-Q scores based on different clinical features are also listed in Table 1.
3.4. Application
In the application phase, a total of 67 patients met all inclusion criteria (referred for and completed PA workup, finished both pre–post DrHy-Q) and were included for analysis: 73.1% of them were female and the median age was 56 ± 15 years (Table 4). The median duration of drug allergy label was 11.0 ± 14.3 years ago. More than half (41/67, 61.2%) of the cohort had multiple drug allergy labels. The average DrHy-Q score before allergist consultation is 40.8 ± 21.7.
Table 4.
Demographic and clinical characteristics of patients referred for penicillin allergy delabeling
| N (%) or mean ± SD | |
|---|---|
| Demographics | |
| Age (median, y) | 56 ± 15 |
| Female sex | 49 (73.1) |
| Duration of allergy label | |
| Median, y | 11.0 ± 14.3 |
| Implicated drug | |
| β-Lactam antibiotics | 67 (100.0) |
| Non-β-lactam antibiotics | 23 (34.3) |
| NSAID | 11 (16.4) |
| Other | 14 (20.9) |
| Number of implicated drug(s) | |
| Single | 26 (38.8) |
| Multiple | 41 (61.2) |
| Type of index reaction | |
| Immediate (<1 h) | 25 (37.3) |
| Nonimmediate (≥1 h) | 16 (23.9) |
| Unknown | 26 (38.8) |
| DrHy-Q score | |
| Baseline | 40.8 ± 21.7 |
| After allergy investigation | 26.6 ± 22.5 |
| Percentage change (%) | −39.7 ± 39.3 |
DrHy-Q, Drug Hypersensitivity Quality of Life Questionnaire; NSAID, nonsteroidal anti-inflammatory drugs; SD, standard deviation.
3.5. Ninety-seven percent of patients could safely use β-lactam after workup
During workup, all of them showed negative ST and proceeded to DPT. Eventually, 94% of them (63/67) were delabeled after a negative DPT for at least 1 penicillin. On the contrary, 4 had their recorded allergies confirmed after positive DPT, 2 of whom have further undertaken tolerance tests and confirmed their tolerance to another penicillin/β-lactam.
3.6. Delabeling leads to on average 40% improvement in HRQoL
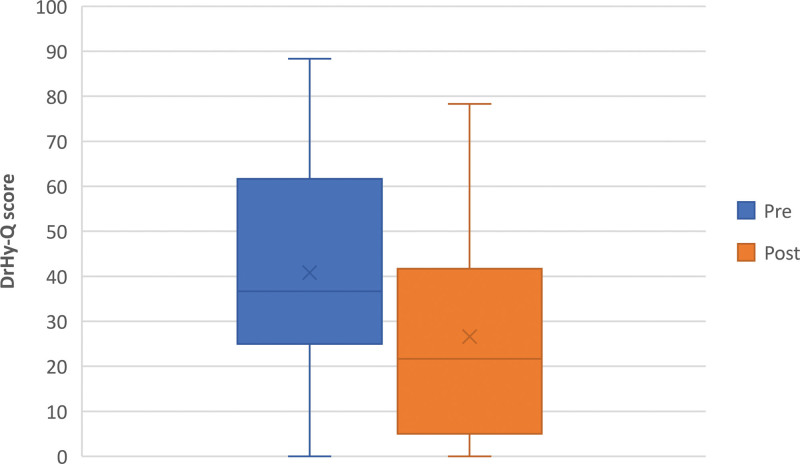
After diagnosis, the majority (58/67, 86.6%) of patients reported better HRQoL (ie, lower DrHy-Q score), whereas 3 sustained their scores (1 of them gave the lowest possible DrHy-Q score in both pre- and post-questionnaires already) and 6 reported worse HRQoL. The overall DrHy-Q score significantly dropped to 26.6 ± 22.5 after delabeling (P < 0.001), implying a major improvement in HRQoL (Fig. 2). A large effect size was reflected by a Cohen d of 0.964 (95% CI, 0.668–1.248), and the average percentage change in the DrHy-Q score was −39.7 ± 39.3. Specifically, among the 4 patients with genuine PA, those with tolerated alternative still showed certain improvement (percentage change in the DrHy-Q score = −12.0 ± 6.6), while the remaining half demonstrated worsened HRQoL (percentage change in the DrHy-Q score = +14.8 ± 14.4).
Figure 2.
Pre- and postdelabelling DrHy-Q. DrHy-Q, Drug Hypersensitivity Quality of Life Questionnaire.
4. Discussion
This study confirms the validity of our Chinese version of DrHy-Q as well as demonstrates that the positive impact PA delabeling has on HRQoL.
Our high Cronbach α and ICC (>0.95) evidenced the reliability of the Chinese DrHy-Q. Together with the unidimensional structure, which is consistent with the original and most other translated versions, these findings proved that the Chinese DrHy-Q is an excellent instrument for measuring a single construct using multiple aspects [13, 14, 16–18].
Our results confirmed the divergence between DrHy-Q and SF-36 as hypothesized. As widely reported, DHR exerted certain detriments poorly detectable by generic surveys, highlighting the uniqueness and significance of DrHy-Q [13, 16]. Comparatively, we appreciated stronger associations between DrHy-Q and mental health-related SF-36 scales. Likewise, in the Dutch study, DrHy-Q was least correlated to health change and physical functioning but most to energy/fatigue and emotional well-being (in terms of Spearman ρ) [17]. Other studies comparing DrHy-Q with the Psychological General Well-Being Index also reported certain correlations, signifying the momentous influence of DHR history on patients’ mental well-being [14, 15].
In concordance with other validation studies, the Chinese DrHy-Q could differentiate patients with multiple and single implicated drugs [16–18]. This can be justified by the fact that multiple labels imply greater uncertainty and limitations in drug use, as well as worse clinical outcomes secondary to the prescription restrictions associated with such labels. Interestingly, female patients and patients suffering from severe reactions tended to have worse DrHy-Q scores, although not reaching statistical significance possibly due to our relatively small sample size. With this in mind, further study with more enrolled subjects would be useful to determine if these factors are indeed significant.
Moreover, to our best knowledge, it is the first study to investigate the impact of drug allergy delabeling on patients’ HRQoL in Asia. We demonstrated that delabeling incorrect PA can improve HRQoL and the psychological burden of drug allergy patients. It is worth noting that, the DrHy-Q score did not reach zero even after the removal of PA labels, suggesting a possible long-lasting impact on HRQoL and mental health despite debunked PA claims. Given that data was only collected at 2 time points in our study, future research with serial data of longer term should be conducted to study the long-term effects of allergy interventions.
Interestingly, our findings diverged from an earlier Spanish study, which reported improvement in HRQoL after drug allergy evaluation regardless of test outcomes [20]. In our cohort, the HRQoL of patients with confirmed allergy appeared to slightly worsen upon confirmation by allergy investigations. This may be explained by cultural differences between European and Asian populations, or disparities in clinical practice in different territories. Future larger-scale, multicenter studies are needed to study these factors. More validated instruments should be made available to facilitate international collaboration and comparison, especially in non-European countries.
PA delabeling is essentially confirming tolerance to penicillins. In other words, it is a collection of medical procedures/interventions, comprising history taking and risk stratification, ST (SPT, [delayed] IDT, and PT), DPT and even in vitro tests and postdelabeling counseling. In this study, we only assessed and demonstrated that PA delabeling as a whole is effective in enhancing HRQoL. Future dedicated study, which measures patients’ responsiveness to each particular procedure or intervention, is warranted. For example, whether tailored, in-depth postdelabeling counseling, or longer postdelabeling follow-up, could further boost HRQoL and other PROs such as willingness to reuse penicillins. Regardless, despite a very small cohort size, we have shown that tolerance tests might be able to improve the HRQoL of truly penicillin-allergic patients. This finding requires corroboration from future investigations.
Until now, HRQoL research in the area of drug allergy has been largely lacking. Little is known about the impact of DHR and related interventions from the patient’s perspective, especially in the long term. It is even rarer to have PROs designated as the primary outcome in clinical studies [25]. With the ever-rising importance of patient-centered and personalized care, the role of PROs in research and clinical practice, for example, as a point-of-care indicator of treatment response, warrants further attention. Furthermore, more PRO instruments specific to allergic diseases such as anaphylaxis should be developed and validated.
This study had several limitations. The sample size was relatively small so subtle differences may not be detected. In this pilot study, we mainly focused on and collected variables related to the clinical features of DHR per se, such as causative agent, timing, and severity of reactions, and it is possible that certain confounders exist but are out of our scope of investigation. More variables such as comorbidities and other PROs should be incorporated for more comprehensive assessment in future studies. In addition, interregional and interethnic comparisons could not be performed because the characteristics of other patient cohorts and clinical protocols were not available. This may be achieved by future multinational studies with predesigned protocols to ensure the clinical and research practice would not significantly vary across the studied countries.
To conclude, our study showed that the Chinese DrHy-Q was a valid and reliable instrument for HRQoL assessment among patients with DHR history. PA delabeling was an effective means to improve the HRQoL of patients with suspected PA. The potential of DrHy-Q as a point-of-care tool warrants further exploration.
Conflicts of interest
The authors have no financial conflicts of interest.
Author contributions
HWFM researched the data and drafted the manuscript. ETSC, JSHY, EL, DLYL and VC researched the data. PHL conceptualized the study and critically reviewed and edited the manuscript. All authors contributed to and approved the final version before submission.
Footnotes
Published online 30 March 2023
References
- 1.Demoly P, Adkinson NF, Brockow K, Castells M, Chiriac AM, Greenberger PA, Khan DA, Lang DM, Park H-S, Pichler W, Sanchez-Borges M, Shiohara T, Thong BY-H. International consensus on drug allergy. Allergy 2014;69:420–437. [DOI] [PubMed] [Google Scholar]
- 2.Li PH, Yeung HHF, Lau C-S, Au EYL. Prevalence, incidence, and sensitization profile of β-lactam antibiotic allergy in Hong Kong. JAMA Netw Open 2020;3:e204199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mak HWF, Yeung MHY, Wong JCY, Chiang V, Li PH. Differences in beta-lactam and penicillin allergy: beyond the west and focusing on Asia-Pacific. Front Allergy 2022;3:1059321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattingly TJ, Fulton A, Lumish RA, Williams AMC, Yoon SJ, Yuen M, Heil EL. The cost of self-reported penicillin allergy: a systematic review. J Allergy Clin Immunol Pract 2018;6:1649–1654.e4. [DOI] [PubMed] [Google Scholar]
- 5.Siew LQC, Li PH, Watts TJ, Thomas I, Ue KL, Caballero MR, Rutkowski K, Till SJ, Pillai P, Haque R. Identifying low-risk beta-lactam allergy patients in a UK tertiary centre. J Allergy Clin Immunol Pract 2019;7:2173–2181.e1. [DOI] [PubMed] [Google Scholar]
- 6.Powell N, Honeyford K, Sandoe J. Impact of penicillin allergy records on antibiotic costs and length of hospital stay: a single-centre observational retrospective cohort. J Hosp Infect 2020;106:35–42. [DOI] [PubMed] [Google Scholar]
- 7.Steenvoorden L, Bjoernestad EO, Kvesetmoen TA, Gulsvik AK. De-labelling penicillin allergy in acutely hospitalized patients: a pilot study. BMC Infect Dis 2021;21:1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sobrino-Garcia M, Moreno EM, Munoz-Bellido FJ, Gracia-Bara MT, Laffond E, Doña I, Martín C, Macías EM, de Arriba S, Campanón V, Gallardo A, Dávila I. Analysis of the costs associated with the elective evaluation of patients labelled as allergic to beta-lactams or nonsteroidal antiinflamatory agents. Front Pharmacol 2020;11:584633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krah NM, Jones TW, Lake J, Hersh AL. The impact of antibiotic allergy labels on antibiotic exposure, clinical outcomes, and healthcare costs: a systematic review. Infect Control Hosp Epidemiol 2021;42:530–548. [DOI] [PubMed] [Google Scholar]
- 10.Kan AKC, Hui HKS, Li TS, Chiang V, Wong JCY, Chan TS, Kwan IYK, Shum WZ, Yeung MSC, Au EYL, Ho CTK, Lau CS, Li PH. Comparative effectiveness, safety, and real-world outcomes of a nurse-led, protocol-driven penicillin allergy evaluation from the Hong Kong drug allergy delabelling initiative (HK-DADI). J Allergy Clin Immunol Pract 2023;11:474–480.e2. [DOI] [PubMed] [Google Scholar]
- 11.Bavbek S, Ozyigit LP, Baiardini I, Braido F, Roizen G, Jerchow E. Placebo, nocebo and patient reported outcome measures in drug allergy. J Allergy Clin Immunol Pract 2023;11:371–379. [DOI] [PubMed] [Google Scholar]
- 12.Baiardini I, Braido F, Brandi S, Canonica GW. Allergic diseases and their impact on quality of life. Ann Allergy Asthma Immunol 2006;97:419–429. [DOI] [PubMed] [Google Scholar]
- 13.Baiardini I, Braido F, Fassio O, Calia R, Canonica GW, Romano A. Development and validation of the Drug hypersensitivity quality of life questionnaire. Ann Allergy Asthma Immunol 2011;106:330–335. [DOI] [PubMed] [Google Scholar]
- 14.Bavbek S, Kepil Özdemir S, Doğanay Erdoğan B, Karaboga I, Büyüköztürk S, Gelincik A, Yilmaz I, Göksel Ö, Berna Dursun A, Karakaya G, Fuat Kalyoncu A, Özseker F, Pasaoglu Karakis G, Öner Erkekol F, Köycü G, Keren M, Baiardini I, Romano A. Turkish version of the drug hypersensitivity quality of life questionnaire: assessment of reliability and validity. Qual Life Res 2016;25:101–109. [DOI] [PubMed] [Google Scholar]
- 15.Gastaminza G, Ruiz-Canela M, Baiardini I, Andrés-López B, Corominas M. Psychometric validation of the Spanish version of the DHRQoL questionnaire. J Investig Allergol Clin Immunol 2016;26:322–323. [DOI] [PubMed] [Google Scholar]
- 16.Dias de Castro E, Barbosa J, Mesquita AM, Caires A, Ribeiro L, Cernadas JR, Baiardini I. Drug hypersensitivity quality of life questionnaire: validation procedures and first results of the Portuguese version. Health Qual Life Outcomes 2021;19:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moayeri M, Van Os-Medendorp H, Baiardini I, Röckmann H. Assessment of validity and reliability of drug hypersensitivity quality of life questionnaire: the Dutch experience. Eur Ann Allergy Clin Immunol 2017;49:129–134. [PubMed] [Google Scholar]
- 18.Chongpison Y, Rerknimitr P, Hurst C, Mongkolpathumrat P, Palapinyo S, Chularojanamontri L, Srinoulprasert Y, Rerkpattanapipat T, Chanprapaph K, Disphanurat W, Chakkavittumrong P, Tovanabutra N, Srisuttiyakorn C, Sukasem C, Tuchinda P, Baiardini I, Klaewsongkram J. Reliability and validity of the Thai drug hypersensitivity quality of life questionnaire: a multi-center study. Int J Qual Health Care 2019;31:527–534. [DOI] [PubMed] [Google Scholar]
- 19.Kepil Özdemir S, Görgülü B, Doğanay Erdoğan B, Dursun AB, Göksel Ö, Öztürk AB, Işik SR, Bavbek S. Effect of drug desensitization on drug hypersensitivity-related quality of life. J Allergy Clin Immunol Pract 2021;9:1738–1741.e1. [DOI] [PubMed] [Google Scholar]
- 20.Gastaminza G, Ruiz-Canela M, Andrés-López B, Barasona Villarejo MJ, Cabañas R, García-Núñez I, José Goikoetxea M, Julio Laguna J, Lobera T, López-San Martín M, Martín-Lázaro J, Mielgo-Ballesteros R, Moreno E, Del Carmen Moya-Quesada M, Ortega-Rodríguez N, Rojas-Perez-Ezquerra P, Rosado A, Salas M, Sánchez-Morillas L, Vila-Albelda C, Corominas M. Quality of life in patients with allergic reactions to medications: influence of a drug allergy evaluation. J Allergy Clin Immunol Pract 2019;7:2714–2721. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. WHO Guidelines on Translation and Adaptation of Instruments. Geneva, Switzerland: World Health Organization; 2018; [Google Scholar]
- 22.Bland JM, Altman DG. Statistics notes: Cronbach’s alpha. BMJ 1997;314:572572572. 572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosner B. Fundamentals of Biostatistics. 8th edition. ed. Cengage Learning; 2016. [Google Scholar]
- 24.Lam CLK, Gandek B, Ren XS, Chan MS. Tests of scaling assumptions and construct validity of the Chinese (HK) version of the SF-36 health survey. J Clin Epidemiol 1998;51:1139–1147. [DOI] [PubMed] [Google Scholar]
- 25.Baiardini I, Bousquet PJ, Brzoza Z, Canonica GW, Compalati E, Fiocchi A, Fokkens W, van Wijk RG, La Grutta S, Lombardi C, Maurer M, Pinto AM, Ridolo E, Senna GE, Terreehorst I, Todo Bom A, Bousquet J, Zuberbier T, Braido F; Global Allergy and Asthma European Network. Recommendations for assessing patient-reported outcomes and health-related quality of life in clinical trials on allergy: a GA2LEN taskforce position paper. Allergy 2010;65:290–295. [DOI] [PubMed] [Google Scholar]