Abstract
It is now longer than half a century, humans, animals, and nature of the world are under the influence of exposure to many newly introduced noxious substances. These exposures are nowadays pushing the borders to be considered as the causative or exacerbating factors for many chronic disorders including allergic, autoimmune/inflammatory, and metabolic diseases. The epithelial linings serve as the outermost body’s primary physical, chemical, and immunological barriers against external stimuli. The “epithelial barrier theory” hypothesizes that these diseases are aggravated by an ongoing periepithelial inflammation triggered by exposure to a wide range of epithelial barrier–damaging insults that lead to “epithelitis” and the release of alarmins. A leaky epithelial barrier enables the microbiome’s translocation from the periphery to interepithelial and even deeper subepithelial areas together with allergens, toxins, and pollutants. Thereafter, microbial dysbiosis, characterized by colonization of opportunistic pathogen bacteria and loss of the number and biodiversity of commensal bacteria take place. Local inflammation, impaired tissue regeneration, and remodeling characterize the disease. The infiltration of inflammatory cells to affected tissues shows an effort to expulse the tissue invading bacteria, allergens, toxins, and pollutants away from the deep tissues to the surface, representing the “expulsion response.” Cells that migrate to other organs from the inflammatory foci may play roles in the exacerbation of various inflammatory diseases in distant organs. The purpose of this review is to highlight and appraise recent opinions and findings on epithelial physiology and its role in the pathogenesis of chronic diseases in view of the epithelial barrier theory.
Keywords: Allergy, asthma, autoimmune diseases, barrier dysfunction, epithelial barrier theory, epithelitis, microbiota, tight junctions
1. Introduction
The prevalence of allergic diseases has been rising since 1960s [1, 2]. Around the same time, a set of autoimmune/inflammatory disorders was reported to be breaking out. Together with genetic background and environmental influences, epithelial barrier defect was highlighted to underlie the etiology of these disorders [3–5]. The discovery of the activated T-lymphocyte-mediated keratinocyte apoptosis in atopic dermatitis (AD) was the initial evidence to focus the research on epithelial barrier [4, 6], which was consequently followed by demonstration of the epithelial barrier disruption in asthma, chronic rhinosinusitis (CRS), and inflammatory bowel disease (IBD) [7–9]. The mucosal barrier’s “keep away, wash away and suppress” functions are delicately facilitated by the immune system and encompass tissue and cell-related mechanisms. By forming a physical barrier against external stimuli with a dense lamina reticularis and secreted antimicrobial peptides and IgA antibodies, the “keep away” function prevents the entrance of external milieu including microbes and allergens. The “wash away” function of the epithelium uses mediators, cells, and cytokines present in the inflammation site. Excessive mucus production, ciliary movement, and also the opening of epithelial barriers eradicate mediators and the inflammatory cells from the inflammation site [10]. The allergic inflammation is strictly regulated by means of immune cells with regulatory capacities such as T regulatory cells and B regulatory cells, suppressive cell surface molecules such as CTLA-4 and PD-1, and regulatory cytokines such as interleukin 10 (IL-10), TGF-β, and IL-35 [11].
The epithelial barrier theory defines the impact of urbanization, industrialization, and Westernized lifestyle on skin, airways, and gut mucosa [1, 12], integrating the previous notions; the “Hygiene,” “Biodiversity,” and “Old Friends” hypotheses [13]. After barrier damage, opportunistic pathogenic bacteria colonize the affected organs and skew the microbial burden toward a more proinflammatory state [14]. A series of mutual events lead to persistent barrier leakiness and periepithelial inflammation. Translocation of pathogens, pollutants, and allergens to inter- and subepithelial spaces elicits an inflammatory response termed as “epithelitis” followed by an “expulsion response” that both underlie the pathogenesis of many immune-related diseases. This review aims to summarize novel findings on skin and mucosal barrier dysfunction and their possible contribution in chronic inflammatory diseases.
2. Risk factors of epithelial barrier dysfunction
2.1. Genetic predisposition
The epithelial barrier is the first line of defense to external milieu, whose dysfunction is closely linked with inflammatory disorders. Mutations in the genes of vital epithelial barrier proteins are associated with allergic disorders [15, 16]. The polymorphisms in the genes of type 2 cytokines (IL-4 and IL-13), alarmins (IL-33 and thymic stromal lymphopoietin [TSLP]), inflammation-related proteins (ADAM33 and HLA), and also vitamin D receptor could either reduce or increase the risk and severity of asthma [16, 17]. In addition, CCL20, IL6, IRF4, MUC5AC, TBX21, FADS2, and RUNX1 were reported as asthma-associated genes [18]. Food allergies and AD have T helper type 2 (Th2) inflammation and itch-scratch further impairs skin barrier function, which could further facilitate the entry of toxins and microbes, leading to skin barrier damage [15].
The possible role of epigenetic alternations in epithelial integrity was covered by several studies, revealing the contribution of silent information regulator genes, histone deacetylases, and CpG methylation on tight junction (TJ) barrier integrity in the asthmatic epithelium [19]. The inhibition of histone deacetylases improved epithelial barrier integrity by increased synthesis of TJ molecules. Next-generation sequencing of asthma candidate genes revealed 2 single-nucleotide polymorphisms in the filaggrin (FLG) gene that were suggested to contribute to asthma pathogenesis [20].
2.2. Microbiota changes underlying epithelial functions
The host’s metabolism, epithelial barrier integrity and functions, and immune homeostasis show vital links with microbiota [21]. Close relation between microbial dysbiosis and epithelial barrier dysfunction is also connected with several noncommunicable inflammatory disorders such as allergic, cardiovascular, metabolic, and autoimmune diseases [1, 13, 22, 23]. Local inflammation and progressive damage to epithelial barriers are persuaded by the translocation of the microbes to subepithelial tissues together with environmental agents, and induction of a type 2 expulsion response [1, 24–27]. Besides, microbiota prevents the colonization of the pathogenic microorganisms, regulates, and improves epithelial barrier functions [28]. In barrier defective tissues, type 2 immune responses are initiated against commensal microbes as well as facultative pathogens [29]. Staphylococcus aureus (S. aureus) is the most abundant bacterium colonizing damaged tissues of the respiratory system and the skin. Asthma exacerbations and severity are closely linked with antibody levels against S. aureus [30]. Staphylococcus epidermidis supports the skin barrier by increasing ceramide production in the stratum corneum by sphingomyelinase activity [31]. Beneficial microbiota provides the preservation and healing of the epithelial barrier [32]. On the contrary, resident microbiota could contribute to the strength of the epithelial barrier by mechanisms including the production of metabolites, such as short-chain fatty acids (SCFAs) [33–35]. Restoration of the microbial diversity could be a potential therapeutic approach to sustain the barrier integrity and avoid the onset of inflammatory-driven diseases. Fecal microbiota transplantation represents a promising tool for microbial diversity restoration and prevention of many inflammatory diseases [36]. Additionally, fungal communities known as mycobiota could contribute to the protection of the healthy state [37, 38].
The development of atopic diseases has links to gut dysbiosis. The abundance or low expression of certain bacteria may project about the risk for disease development or protection. Acinetobacter presence on the skin was attributed as “protective” against allergen sensitization [39]. Relatively low abundance of Bacteriodetes have been reported in food-allergic children [40]. The metabolomics research identified microbial metabolites and provided insight into host–microbiota interactions and disease onset [34, 41].
2.3. Environmental influence and pathogenic drivers
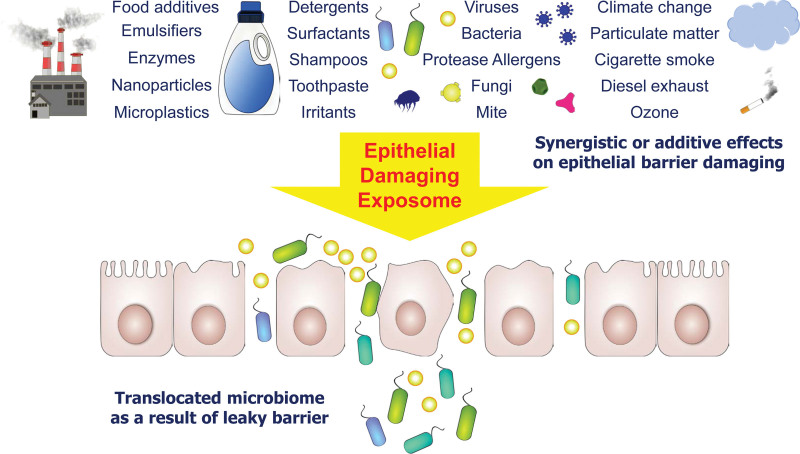
More than 350,000 new substances have been introduced to human lives with almost no control on their health effects after 1960s. Many of them ended up as pollutants [42]. Changes in environmental exposure particularly after 1960s have been proposed to be directly linked with development of autoimmune, allergic, and metabolic diseases [1, 26, 27]. TJs and epithelial barrier integrity could be damaged following encounter upon climate alternations, detergents, surfactants, household cleaners, food additives, particulate matter (PM), diesel exhaust particles, tobacco smoke, microplastics, ozone, and toothpaste, all of which account for the environmental insults (Fig. 1) [1, 12, 13, 26, 27, 43–47].
Figure 1.
Epithelial barrier–damaging agents. A defective epithelial barrier is underlying a number of inflammatory disorders including allergy and autoimmune diseases. Epithelial barriers of the skin, respiratory, or gastrointestinal systems are disrupted upon synergistic or additive effects of a number of agents or factors. These include detergents, shampoos, toothpaste, diesel particles, cigarette smoke, food additives, allergens with protease activity, viruses, ozone, and also alterations in climate. Translocation of the microbiota occurs as the result of leaky epithelial barrier formation.
Climate change is a global problem. As a result of deforestation and the greenhouse effect, the Earth’s CO2 elimination capacity is crippled, which leads to climate change with harmful effects on human health along with animals, plants, agriculture, wildlife, forests, and oceans [12, 48, 49]. Pollutants including PM, CO2, CO, NO, ozone, and volatile organic compounds are among the main driving forces of allergic diseases [50]. The hazardous levels of environmental ozone, PM, and nanomaterials could impair epithelial barrier functions [51]. Wildfires and toxic fumes are hazardous to human health. Wildfire smoke induces oxidative damage and lung inflammation and asthma exacerbations [52]. Animal studies revealed that air pollution has detrimental effects on epithelia, immune cells, and immune responses [53]. Cigarette smoke upregulates inflammatory dendritic cells in the lungs and disrupts the epithelial barrier function by suppressing proinflammatory cytokine and chemokine responses (Fig. 1) [54].
Detergents and their surfactants sodium lauryl sulphate and sodium benzene sulphanate, which were introduced to our lifes in 1960 with a similar formulation as of today, are regarded as important epithelial barrier disruptors. Public daily exposure to detergents was enhanced as detergent usage was increased, together with additive surfactants and proteolytic enzymes [55]. A direct link between asthma and AD development and detergent exposure, household cleaners and disinfectants has been repeatedly reported [56–58]. Laundry detergent exposure disrupts the epithelial barrier function of skin and bronchial epithelial cells, even at very high dilutions [59, 60]. Professional dishwashing became the state of the art worldwide for common food consuming areas, such as hospitals, armies, and schools after 2000 and their rinse aid containing alcohol ethoxylates show epithelial barrier opening, proinflammatory and cell toxic effects on gut epithelia in very low concentrations [61].
Production and usage of plastics have increased markedly to the levels of 8 billion tons per year. Approximately 1 billion tons of micro- and nanoplastics have been produced each year that contaminate food and water and may trigger inflammation on epithelial cells, leading to microbiota dysbiosis and causing barrier dysfunction in the digestive tract [62, 63]. Again, during the last 20 years, the consumption of processed food has substantially increased and jointly ingestion of emulsifiers may apparently contribute to the development of diseases. Even low concentrations of food emulsifiers may increase intestinal permeability and lead to mucosal damage [64].
2.4. Allergens
The synergistic and additive effect of epithelial barrier–damaging substances is still under investigation; however, it is clear that all of the pollutants are coexposed together with perennial allergens and depending on the time with seasonal allergens. For example, early in the year, birch pollen allergen exposure is always overlapping with air pollutants in March in the Northern Hemisphere [65]. Proteases released by several aeroallergens target transmembrane adhesion proteins such as E-cadherin and transmembrane receptors, damage barrier permeability, facilitate allergen absorption and sensitization, and initiate inflammatory responses (Table 1) [78, 79]. Protease inhibitors could maintain lung homeostasis to stabilize the action of allergens and control apoptosis. Allergen exposure could lead to differential expression of protease inhibitors, which could occur both in the presence or absence of Th2 cytokines, and could damage the lung epithelium [80]. Environmental factors such as climate change and thunderstorms could influence the severity and duration of allergic respiratory diseases directly and indirectly [80, 81]. Increased atmospheric CO2 levels enhance photosynthesis, which leads to prolonged pollination periods. As a result, allergic individuals are exposed to higher pollen concentrations for longer durations. Elevated CO2 concentrations also increase the allergenicity of ragweed pollen and could induce stronger inflammatory responses [82].
Table 1.
Allergens with protease activity
| Allergen type | Allergen source | Allergen name | References |
|---|---|---|---|
| Cockroaches | Periplaneta americana | Per a 10 | [66] |
| Fruits | Actinidia deliciosa | Act d 1 | [67] |
| Ananas comonus | Ana c 2 | ||
| Carica papaya | Papain | ||
| Fungi | Aspergillus fumigatus | Asp f 13 | [68–71] |
| Aspergillus flavus | Asp fl 13 | ||
| Flavus oryzae | Asp o 13 | ||
| Penicillium chrysogenum | Pen ch 13 | ||
| Penicillium citrinum | Pen c 13 | ||
| House dust mites | Dermatophagoides pteronyssinus | Der p 1 | [72–77] |
| Der p 3 | |||
| Der p 9 | |||
| Dermatophagoides farineae | Der f 1 | ||
| Der f 3 |
2.5. Cellular and molecular mechanisms of epithelial barrier impairment
Understanding the molecular mechanisms supporting barrier integrity under physiological circumstances is of great importance. The complex interplay between immunity and TJs is vital for immune homeostasis (Fig. 2). Most epithelial cell lineages express toll-like receptors, and they contribute to epithelial barrier integrity and regulation of the immune responses. Mucus and antimicrobial peptides could avoid damage to epithelial cells and may strengthen the barrier [83]. The barrier integrity is mainly maintained by the regulation of epithelial TJs and their expression levels could be used as a biomarker to evaluate barrier permeability [84]. Air pollutants are important environmental contributors to the epithelial barrier impairment [1]. Wildfire exposure upregulated CRP and IL-1β as inflammation markers [85]. Additionally, the inhalation of chorine-containing disinfectants revealed a stronger immune response and increased frequencies of Th2 cells and eosinophils, in an ovalbumin-induced mouse model [86].
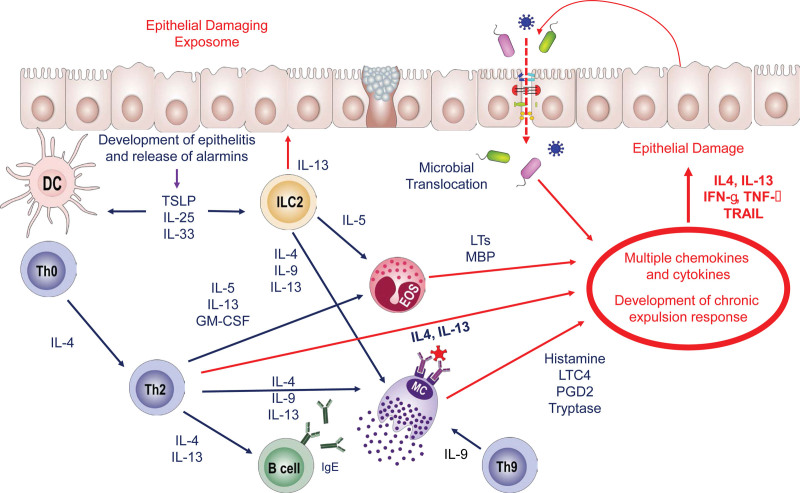
Figure 2.
Immune mechanisms underlying epithelial barrier disruption. The epithelial barrier–damaging exposome disrupts epithelial barrier integrity, which is followed by development of epithelitis and release of alarmins. TSLP, IL-25, and IL-33 are secreted from the epithelial cells, and immune responses are triggered. At the same time, because of disruption of the epithelial barrier, microbial translocation is initiated. Following the differentiation of Th2 cells, type 2 cytokines including IL-4, IL-5, IL-13, and GM-CSF are produced, and B cells isotype-switch to IgE. Eosinophils and mast cells bind IgE, become potentiated thanks to the rich cytokine milieu and start producing their mediators such as LTs, MBP, and histamine, LTC4, PGD2, and tryptase, respectively. Th9 cells produce IL-9, which further potentiates the activity of mast cells. ILC2s produce type 2 cytokines and contribute to the cytokine milieu. The cytokines and mediators collectively initiate the development of a chronic expulsion response including IL-4, IL-13, IFN-γ, TNF-α, and TRAIL, and leads to further epithelial damage. DC, dendritic cell; EOS, eosinophil; GM-CSF, granulocyte colony-stimulating factor; IL, interleukin; ILC, innate lymphoid cell; LTs, leukotrienes; MBP, major basic protein; MC, mast cell; PGD, prostaglandin; TRAIL, TNF-related apoptosis inducing ligand; TSLP, thymic stromal lymphopoietin.
The first step after the injury of epithelial cells is the release of alarmins that were secreted in response to pollutants. IL-33, IL-25, and TSLP secretion have been reported in exposure to epithelial barrier–damaging agents such as PM, ozone, detergents, and dishwasher rinse aid. This step is called epithelitis (Fig. 2). The second step is the dysbiosis in the microbiome with colonizing opportunistic pathogens and decreased numbers and biodiversity of commensal bacteria. An immune response develops to opportunistic pathogens as observed for S. aureus [87, 88].
The third step is the development of an expulsion response (Fig. 3). IL-13, eosinophils, Th2 cells, and group 2 innate lymphoid cells are the main players of epithelial barrier leakiness. This is very similar to eosinophilic expulsion response of parasites such as the lung stage of the larvae of ascaris, schistosoma, and hookworms. Expulsion of every single one of the larvae prevents that an adult does not develop in the lungs and occludes the bronchial tree. In addition, skin parasites such as Sarcoptes scabiei (the itch mite) is expulsed by a type 2 immune response-related eosinophilic dermatitis that initiates an itch-scratch axis. Opening of the epithelial barriers is an essential part of this type 2 immune response.
Figure 3.
The expulsion response against microbiome and opportunistic pathogens. Following epithelial damage, microbiome migrates inside and beneath the epithelium, which consequently triggers cell migration and stimulation of the immune system. Activated immune cells including macrophages, DCs, mast cells, T and B cells, and ILCs migrate to the area and initiate a type 2 expulsion response with Th2 cells, IgE-producing B cells, ILC2, IL-4, IL-5, and IL-13 against opportunistic pathogens, commensals, allergens, and pollutants. The opportunistic pathogens include Staphylococcus aureus, Pneumococcus, Haemophilus, and Moraxella. The inflammatory response together with translocated microbiome and microbial dysbiosis leads to defects in epithelium repair, and misclosure of the barrier, which instigate a vicious cycle of leaky barriers and chronic inflammatory responses as well as microbial dysbiosis. DC, dendritic cell; EOS, eosinophil, IL, interleukin; ILC, innate lymphoid cell; M∅, macrophage; MC, mast cell.
All of these mechanisms are followed up with a continuum of epigenetic regulation. The epithelial stem cells that are isolated from barrier leaky areas cannot make strong TJ barriers, which can be corrected by histone deacetylase inhibition [89]. In addition, histone acetylase activation has been reported in asthmatic bronchial epithelial cells [19]. Epigenetic regulation of epithelial barriers is the reason for chronicity and continuous epithelial barrier defects (Fig. 4).
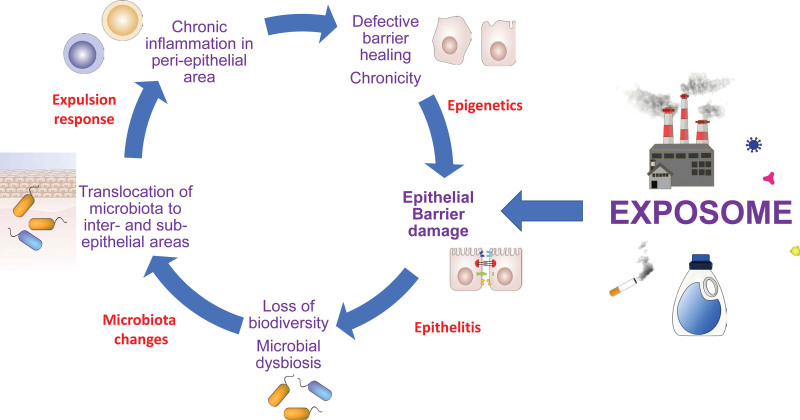
Figure 4.
The vicious circle of chronic epithelial barrier dysfunction. Disruption of epithelial barriers are induced by exposome and damaging agents, which is facilitated by genetic defects in barrier-related molecules. Chronic inflammation in the periepithelial area leads to chronic, defective epithelial barrier healing and aggravates the damage. Epigenetics play role in defective barrier healing capacity, which in turn leads to epithelial barrier damage, and is termed as epithelitis. Then, loss of biodiversity and microbial dysbiosis end up with translocation of microbiota to inter- and subepithelial areas. An expulsion response is initiated, leading to chronic inflammation in the periepithelial area.
3. Diseases related to barrier dysfunction
3.1. Allergic diseases
Allergic diseases have established links with epithelial barrier dysfunction [1, 12, 13, 26, 89–92]. Epithelial barrier integrity is important in sensitization to nonprotease allergens. The complex interaction between the skin epithelial barrier, the exposome, and immune cells is vital for understanding the AD pathogenesis [26, 27, 93, 94]. AD pathogenesis is related to TSLP activation of epithelium, dendritic cells, and macrophages that mainly induces Th2 cell differentiation. TSLP impairs epidermal barrier integrity by induced formation of nuclear IL-33/phosphorylated STAT3 complex in human keratinocytes [95]. RNA sequencing of tape-stripped skin samples from patients with AD demonstrated type 2 skewing and the downregulation of proteins related to barrier function, lipid biosynthesis, and metabolism [96]. Blocking IL-4 and IL-13 could be effective therapeutics for AD [97]. Following IL-4Rα blockade, barrier molecules were upregulated [98].
In asthma, defective epithelial barrier eases the entry of environmental toxins and aggravates exacerbations. Several studies demonstrate the role of exposure to environmental agents and the involvement of the immune system, and, therefore, the exposome paradigm can deliver a more ample risk profile in comparison with single predictors [1, 26, 27, 56, 82, 99]. Epithelial damage-initiated tissue environmental exposure leads to the production of IL-33; an alarmin of asthma [100]. IL-5 is an indispensable cytokine of allergic inflammation, whose receptor (IL-5R) expression was recently demonstrated on human airway epithelial cells [101]. IL-5 pathway-interfering biologicals could support barrier integrity by downregulation of eosinophils and their related epithelial barrier–damaging effects [102]. IL-13, a potent biomarker and key cytokine in the pathogenesis of asthma and CRS, alters claudin (CLDN) expression and induces TJ protein aggregation, leading to barrier leakiness [19, 92, 103–105].
Specific gut microbial taxa were shown to be correlated with asthma development [106, 107]. A lower abundance of anti-inflammatory metabolite-producing bacteria together with increased abundance of specific fungi could induce a type 2 immune response [106]. Certain pathogen-associated molecular patterns like lipopolysaccharide could protect against allergic diseases [108]. SCFA byproducts of bacterial fiber fermentation could weaken inflammatory responses. As an example, a mouse model revealed that vancomycin administration during pregnancy was related to the severity of asthma in offspring, which underlines the importance of SCFA in immune homeostasis [109]. On the contrary, respiratory syncytial virus infection can weaken the epithelial barrier integrity by alleviation of epithelial cell proliferation and wound-healing capacity [110, 111].
Functional disruption of the epithelial barrier was also found responsible in the pathogenesis of both allergic rhinitis (AR) and CRS [112]. Occludin, ZO-1, and several CLDNs were downregulated in patients with AR. Environmental and endogenous factors disrupt the integrity of TJs [113]. In addition, corticosteroid therapy upregulated expression of TJ proteins and improved barrier function in AR patients [114]. Inhibition of inflammatory cytokines reestablishes TJ protein expression, which has the capacity as therapeutic approaches. Mucin-1 deficiency leads to a decrease in CLDN-1 via RBFOX3 shortage, which acts to regulate the CLDN1 ubiquitin degradation. Nasal treatment with the inhibitor of ubiquitin-proteasome in mice limited the AR symptoms and restored nasal epithelial barrier function [115]. On the contrary, Pseudomonas aeruginosa exoproteins exerted damage to mucosal barriers in CRS and comorbid asthma patients [116]. The role of the local microbiome–host interactions in the pathogenesis of CRS still requires more investigation to be covered.
Food allergy results from interaction of environmental factors, epithelium, and host-immune responses [117]. Abnormal immune system maturation is associated with intensive hygiene, increased antibiotic use, c-section births, and reduced outdoor encounters. As a consequence, dysbiosis in skin and gut is promoted, all of which contribute to the development of atopy [118–120]. Food allergy could be initiated through the skin [118]. Viral infections, diet, vitamin supplementation, environment, and microbiome together with a damaged epithelial barrier all take part in both the development and prevention of the food allergies [121, 122]. A strong gut mucosal immunity requires diversification of the gut microbiome in early childhood and could protect against food allergy [123]. Butyrate, a SCFA in breast milk, acts as an anti-inflammatory metabolite. It is shown to prevent asthma development in mouse models and its increase can mitigate the childhood food allergy development risk [124–126]. In contrast, diminished levels of butyric acid-producing bacteria were reported in children with egg allergies [127]. Eosinophilic esophagitis (EoE) has a complicated pathology driven by genetic and intrinsic factors, environment, and antigen stimulation. Genes encoding the desmosome-associated proteins and periplakin control cell motility and barrier integrity and could lead to epithelial cell degradation in EoE [128]. IL-13 induces TJ dysfunction in EoE, leading to loss of barrier function [129].
3.2. Autoimmune and metabolic diseases
Compromised barrier integrity was also observed in other inflammatory disorders (Table 2). Autoimmune and metabolic diseases have epithelial barrier damage, which is followed by microbial dysbiosis and consequently affects many organs. In recent years, the gastrointestinal track is facing many harmful agents such as nanoparticles, emulsifiers, enzymes, nanoplastics, and many more, all of which account for intestinal barrier defects and an increase in intestinal permeability. Following intestinal barrier disruption, commensal bacteria infiltrate into tissues and stimulate the immunity. The leaky gut-induced dysbiosis triggers inflammation affecting the entire body and damages the intestinal mucosa [1, 26, 59–64].
Table 2.
Diseases in which epithelial barrier disruption has been linked to pathogenesis
| Disease related to epithelial barrier disruption | References |
|---|---|
| Obesity | [130] |
| Nonalcoholic steatohepatitis | [131] |
| Liver cirrhosis | [87] |
| Multiple sclerosis | [132] |
| Systemic lupus erythematosus | [133] |
| Ankylosing spondylitis | [134] |
| Type 1 diabetes | [135] |
| Autism spectrum disorders | [136] |
| Parkinson disease | [137] |
| Alzheimer disease | [138] |
| Stress-related psychiatric disorders | [139] |
| Chronic depression | [140] |
TJ protein expressions such as occludin and CLDN in intestinal epithelium were downregulated in rheumatoid arthritis patients, while zonulin family peptide levels were significantly increased [141]. These features are complemented by a leaky intestinal barrier, inflammation, and dysbiosis. Gut barrier leakiness associated with rheumatoid arthritis could facilitate the inflammatory cell migration from the gut to the joints [141].
In celiac disease, diminished levels of sealing CLDNs, ZO-1, and occludin displacement in the cell membrane, together with small intestine structural defects, were observed [142]. Patients with celiac disease have a compromised oral epithelial barrier [143]. TJ integrity could be modulated by several microbial products. Butyrate enhances the TJ barrier by the hypoxia response, can diminish TNF-α, and increase TJ-related proteins [144].
Many studies aimed to illuminate the underlying mechanism of IBD with a focus on epithelial barrier dysfunction. Decreased ZO-1 expression has been reported in biopsy samples of patients with IBD [145]. In colitis, metformin as a therapeutic agent improved intestinal mucosal epithelial damage by decreasing the apoptosis of intestinal epithelial cells and increasing TJ proteins [146]. Investigation of quaking (QKI), an RNA-binding protein, in IBD and also in a mouse model of induced colitis revealed the binding of QKI to keap1 mRNA under physiological conditions, and deficiency of QKI was reported to result in diminished antioxidative capacity, and together with increased reactive oxygen species production, this may end up with damage in intestinal epithelial barrier [147]. In relation to cytokine-induced changes in barrier permeability, the JAK-STAT signaling pathway has a vital role in IBD since JAK-STAT signaling pathways regulate the expression and localization of TJ proteins [148].
An important pathogenetic event in autoimmune diseases is the migration of inflammatory cells from barrier defective gut to distant organs [131–135]. Multiple sclerosis has been linked to air pollution in many studies [149]. In a recent study, it was reported in Stockholm that air pollution activates the immune cells in the lungs and that exacerbates multiple sclerosis, namely the brain migrating CCR6 expressing dendritic cells. In rheumatoid arthritis, a link to gut barrier defect has been shown very clearly [141].
There is also a growing body of evidence proposing that epithelial barrier dysfunction and permeability contribute to the pathogenesis of several chronic neurological and psychiatric disorders including Parkinson disease, Alzheimer disease, chronic depression, stress-related psychiatric disorders, and autism spectrum disorders [1]. As an example, a transgenic mouse model of Parkinson disease evaluated the influence of α-synuclein accumulation on the intestinal epithelial barrier in bowel inflammation. Increased caspase-1 activity and increased inflammatory markers were noted. These findings all together revealed increased intestinal barrier permeability and dysbiosis [150].
3.3. COVID-19 and the epithelial barrier theory
Epithelial barriers are vital in defense against severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2) infection, and solidification of this protection could be an important immunomodulatory strategy to prevent lung injury, systemic spread of the virus, and severe COVID-19 [151]. Epithelial cells express various SARS-CoV-2 receptors and related molecules, such as ACE2 [152]. SARS-CoV-2 infection compromises the barrier function of the nasal epithelium [153]. The barrier disruption in COVID-19 was attributed to the interaction between SARS-CoV-2 protein E and human ZO-1 protein [154]. Allergic diseases do not pose a predisposition to COVID-19 and could enhance the antiviral response, as lower number of severe cases were witnessed in atopic patients, with T lymphocytes less affected by SARS-CoV-2 [155, 156].
As a part of the epithelial barrier theory, air pollution, causing respiratory epithelial barrier dysfunction, may contribute to local chronic inflammation, microbiome dysbiosis, and impaired antiviral immune response against SARS-CoV-2, all of which contribute to the high incidence and excess mortality from COVID-19. This was observed at the beginning of the pandemic in Northern Italy. In addition, air pollution and epithelial barrier dysfunction contribute also to the higher prevalence of several comorbidities of COVID-19, such as diabetes, chronic obstructive pulmonary disease, and obesity, which can be identified as risk factors for severe diseases and mortality [157, 158].
4. Conclusions
Diseases related to the epithelial barrier theory have 5 common criteria: (1) they show increased prevalence in the last decades, which is not affected with the improved method of diagnosis; (2) they show epithelial barrier defect and epithelitis, which is characterized by the release of alarmins; (3) microbial dysbiosis with the loss of numbers and diversity of commensal bacteria and colonization of opportunistic pathogens is a common feature; (4) different and practically irrelevant diseases that fulfill these criteria appear in multimorbidities; and (5) they show increased inflammatory biomarkers in the circulation (circulating microinflammation).
Novel strategies for the prevention and treatment of allergic, autoimmune, and metabolic diseases require a thorough understanding of the underlying processes involved in epithelial barrier damage. Multiple immune regulatory mechanisms become dominant in leaky barrier areas to reduce the level of inflammation and avoid extensive tissue injury. Accordingly, the barrier hypothesis brings together several hypotheses that were proposed to explain the origins of allergic diseases. The biodiversity, hygiene, and old friends hypotheses are all associated with immune regulatory mechanisms, loss of biodiversity, and epithelial barrier leakiness.
The barrier hypothesis suggests a need for avoidance of the environmental cues and warrants further studies on safe levels of exposure to potentially harmful substances discussed here, such as inhaled and ingested detergents, ingestion of processed foods containing emulsifiers, exposure to PM, diesel exhaust, microplastics, and certain nanoparticles.
A comprehensive understanding of the barrier hypothesis is essential for the prevention, early intervention, and development of novel therapeutic approaches. Indeed, many treatment plans target protecting or repairing the epithelial barrier, such as avoidance of barrier-disrupting substances, development of safer products, identification of leaky barrier biomarkers, innovative treatments for reestablishing tissue-specific barrier elements, suppressing the colonization of opportunistic pathogens, dietary interventions, and microbiome-based therapies.
Conflicts of interest
CAA has received research grants from the Swiss National Science Foundation, European Union (EU CURE, EU Syn-Air-G), Novartis Research Institutes, (Basel, Switzerland), Stanford University (Redwood City, CA), Seed Health (Boston, USA) and SciBase (Stockholm, Sweden); is the Co-Chair for EAACI Guidelines on Environmental Science in Allergic Diseases and Asthma; Chair of the EAACI Epithelial Cell Biology Working Group; is on the Advisory Boards of Sanofi/Regeneron (Bern, Switzerland; New York, USA), Stanford University Sean Parker Asthma Allergy Center (CA, USA), Novartis (Basel, Switzerland), Glaxo Smith Kline (Zurich, Switzerland), Bristol-Myers Squibb (New York, USA), Seed Health (Boston, USA), and SciBase (Stockholm, Sweden); and is the Editor-in-Chief of Allergy. MA has received research grants from Swiss National Science Foundation, Bern; research grant from the Stanford University; Leading House for the Latin American Region, Seed Money Grant. She is in the Scientific Advisory Board member of Stanford University Sean Parker Asthma Allergy Center (CA, USA); Advisory Board member of LEO Foundation Skin Immunology Research Center (Kopenhagen, Denmark); and Scientific Co-Chair of World Allergy Congress (WAC) Istanbul, 2022, Scientific Programme Committee Chair, EAACI. KN is the Director of the World Allergy Organization Center of Excellence for Stanford, Advisor at Cour Pharma, Consultant for Excellergy, Red tree ventures, Eli Lilly, and Phylaxis, Co-founder of Before Brands, Alladapt, Latitude, and IgGenix; and National Scientific Committee member at Immune Tolerance Network (ITN), and National Institutes of Health (NIH) clinical research centers, outside the submitted work; patents include “Mixed allergen composition and methods for using the same,” “Granulocyte-based methods for detecting and monitoring immune system disorders,” and “Methods and Assays for Detecting and Quantifying Pure Subpopulations of White Blood Cells in Immune System Disorders.” The remaining authors declare no conflicts of interest.
Author contributions
Conceptualization: Umut Can Kucuksezer, Cevdet Ozdemir, Cezmi A. Akdis.
Formal analysis: Umut Can Kucuksezer, Cevdet Ozdemir, Cezmi A. Akdis.
Investigation: Duygu Yazici, Yagiz Pat, Yasutaka Mitamura, Manru Li, Na Sun, Paolo D’Avino, Xiangting Bu, Xueyi Zhu, Ismail Ogulur.
Project administration: Umut Can Kucuksezer, Cevdet Ozdemir, Cezmi A. Akdis.
Writing - original draft: Umut Can Kucuksezer, Cevdet Ozdemir, Cezmi A. Akdis, Ismail Ogulur.
Writing - review & editing: Umut Can Kucuksezer, Cevdet Ozdemir, Duygu Yazici, Yagiz Pat, Yasutaka Mitamura, Manru Li, Na Sun, Paolo D’Avino, Xiangting Bu, Xueyi Zhu, Mubeccel Akdis, Kari Nadeau, İsmail Ogulur, Cezmi A. Akdis.
Footnotes
Umut Can Kucuksezer and Cevdet Ozdemir contributed equally to this article as co-first authors.
Published online 30 March 2023
References
- 1.Akdis CA. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions?. Nat Rev Immunol 2021;21:739–751. [DOI] [PubMed] [Google Scholar]
- 2.Platts-Mills TA. The allergy epidemics: 1870-2010. J Allergy Clin Immunol 2015;136:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trautmann A, Schmid-Grendelmeier P, Kruger K, Crameri R, Akdis M, Akkaya A, Bröcker E-B, Blaser K, Akdis CA. T cells and eosinophils cooperate in the induction of bronchial epithelial cell apoptosis in asthma. J Allergy Clin Immunol 2002;109:329–37. [DOI] [PubMed] [Google Scholar]
- 4.Trautmann A, Akdis M, Kleemann D, Altznauer F, Simon HU, Graeve T, Noll M, Bröcker EB, Blaser K, Akdis CA. T cell-mediated Fas-induced keratinocyte apoptosis plays a key pathogenetic role in eczematous dermatitis. J Clin Invest 2000;106:25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kraehenbuhl JP, Pringault E, Neutra MR. Review article: intestinal epithelia and barrier functions. Aliment Pharmacol Ther. 1997;11 Suppl 3:3–8; discussion 8; discussion -9. [DOI] [PubMed] [Google Scholar]
- 6.Zimmermann M, Koreck A, Meyer N, Basinski T, Meiler F, Simone B, Woehrl S, Moritz K, Eiwegger T, Schmid-Grendelmeier P, Kemeny L, Akdis CA. TNF-like weak inducer of apoptosis (TWEAK) and TNF-alpha cooperate in the induction of keratinocyte apoptosis. J Allergy Clin Immunol 2011;127:200–7, 207.e1. [DOI] [PubMed] [Google Scholar]
- 7.Dong X, Ding M, Zhang J, Ogulur I, Pat Y, Akdis M, Gao Y, Akdis CA. Involvement and therapeutic implications of airway epithelial barrier dysfunction in type 2 inflammation of asthma. Chin Med J (Engl) 2022;135:519–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basinski TM, Holzmann D, Eiwegger T, Zimmermann M, Klunker S, Meyer N, Schmid-Grendelmeier P, Jutel M, Akdis CA. Dual nature of T cell-epithelium interaction in chronic rhinosinusitis. J Allergy Clin Immunol 2009;124:74–80.e1. [DOI] [PubMed] [Google Scholar]
- 9.Pott J, Maloy KJ. Epithelial autophagy controls chronic colitis by reducing TNF-induced apoptosis. Autophagy 2018;14:1460–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erjefalt JS, Uller L, Malm-Erjefalt M, Persson CG. Rapid and efficient clearance of airway tissue granulocytes through transepithelial migration. Thorax 2004;59:136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kucuksezer UC, Ozdemir C, Cevhertas L, Ogulur I, Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy and allergen tolerance. Allergol Int 2020;69:549–560. [DOI] [PubMed] [Google Scholar]
- 12.Celebi Sozener Z, Cevhertas L, Nadeau K, Akdis M, Akdis CA. Environmental factors in epithelial barrier dysfunction. J Allergy Clin Immunol 2020;145:1517–1528. [DOI] [PubMed] [Google Scholar]
- 13.Pat Y, Ogulur I. The epithelial barrier hypothesis: a 20-year journey. Allergy 2021;76:3560–3562. [DOI] [PubMed] [Google Scholar]
- 14.Moffatt MF, Cookson WO. The lung microbiome in health and disease. Clin Med (Lond) 2017;17:525–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev 2011;242:233–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ranjbar M, Whetstone CE, Omer H, Power L, Cusack RP, Gauvreau GM. The genetic factors of the airway epithelium associated with the pathology of asthma. Genes (Basel) 2022;13:1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gauvreau GM, Bergeron C, Boulet LP, Cockcroft DW, Cote A, Davis BE, Leigh R, Myers I, O'Byrne PM, Sehmi R. Sounding the alarmins-the role of alarmin cytokines in asthma. Allergy 2023;78:402–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vercelli D, Bleecker ER. Strength in numbers: the quest for asthma genes. J Allergy Clin Immunol 2019;144:413–415. [DOI] [PubMed] [Google Scholar]
- 19.Wawrzyniak P, Krawczyk K, Acharya S, Tan G, Wawrzyniak M, Karouzakis E, Dreher A, Jakiela B, Altunbulakli C, Sanak M, O'Mahony L, Nadeau K, Akdis CA. Inhibition of CpG methylation improves the barrier integrity of bronchial epithelial cells in asthma. Allergy 2021;76:1864–1868. [DOI] [PubMed] [Google Scholar]
- 20.Gao W, Gong J, Mu M, Zhu Y, Wang W, Chen W, Han G, Hu H, Bao P. The pathogenesis of eosinophilic asthma: a positive feedback mechanism that promotes Th2 immune response via filaggrin deficiency. Front Immunol 2021;12:672312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou K, Wu ZX, Chen XY, Wang JQ, Zhang D, Xiao C, Zhu D, Koya JB, Wei L, Li J, Chen Z-S. Microbiota in health and diseases. Signal Transduct Target Ther 2022;7:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol 2021;19:55–71. [DOI] [PubMed] [Google Scholar]
- 23.Witkowski M, Weeks TL, Hazen SL. Gut microbiota and cardiovascular disease. Circ Res 2020;127:553–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alemao CA, Budden KF, Gomez HM, Rehman SF, Marshall JE, Shukla SD, Donovan C, Forster SC, Yang IA, Keely S, Mann ER, El Omar EM, Belz GT, Hansbro PM. Impact of diet and the bacterial microbiome on the mucous barrier and immune disorders. Allergy 2021;76:714–734. [DOI] [PubMed] [Google Scholar]
- 25.Bjerre RD, Holm JB, Palleja A, Solberg J, Skov L, Johansen JD. Skin dysbiosis in the microbiome in atopic dermatitis is site-specific and involves bacteria, fungus and virus. BMC Microbiol 2021;21:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Celebi Sozener Z, Ozdel Ozturk B, Cerci P, Turk M, Gorgulu Akin B, Akdis M, Altiner S, Ozbey U, Ogulur I, Mitamura Y, Yilmaz I, Nadeau K, Ozdemir C, Mungan D, Akdis CA. Epithelial barrier hypothesis: Effect of the external exposome on the microbiome and epithelial barriers in allergic disease. Allergy 2022;77:1418–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitamura Y, Ogulur I, Pat Y, Rinaldi AO, Ardicli O, Cevhertas L, Brüggen M-C, Traidl-Hoffmann C, Akdis M, Akdis CA. Dysregulation of the epithelial barrier by environmental and other exogenous factors. Contact Dermatitis 2021;85:615–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eyerich S, Eyerich K, Traidl-Hoffmann C, Biedermann T. Cutaneous barriers and skin immunity: differentiating a connected network. Trends Immunol 2018;39:315–327. [DOI] [PubMed] [Google Scholar]
- 29.Kim J, Kim BE, Berdyshev E, Bronova I, Bin L, Bae J, Kim S, Kim H-Y, Lee UH, Kim MS, Kim H, Lee J, Hall CF, Hui-Beckman J, Chang Y, Bronoff AS, Hwang D, Lee H-Y, Goleva E, Ahn K, Leung DYM. Staphylococcus aureus causes aberrant epidermal lipid composition and skin barrier dysfunction. Allergy 2023. [DOI] [PubMed] [Google Scholar]
- 30.Sorensen M, Klingenberg C, Wickman M, Sollid JUE, Furberg AS, Bachert C, Bousquet J. Staphylococcus aureus enterotoxin sensitization is associated with allergic poly-sensitization and allergic multimorbidity in adolescents. Allergy 2017;72:1548–1555. [DOI] [PubMed] [Google Scholar]
- 31.Zheng Y, Hunt RL, Villaruz AE, Fisher EL, Liu R, Liu Q, Cheung GYC, Li M, Otto M. Commensal Staphylococcus epidermidis contributes to skin barrier homeostasis by generating protective ceramides. Cell Host Microbe 2022;30:301–313.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altunbulakli C, Reiger M, Neumann AU, Garzorz-Stark N, Fleming M, Huelpuesch C, Castro-Giner F, Eyerich K, Akdis CA, Traidl-Hoffmann C. Relations between epidermal barrier dysregulation and Staphylococcus species-dominated microbiome dysbiosis in patients with atopic dermatitis. J Allergy Clin Immunol 2018;142:1643–1647.e12. [DOI] [PubMed] [Google Scholar]
- 33.Zheng L, Kelly CJ, Battista KD, Schaefer R, Lanis JM, Alexeev EE, Wang RX, Onyiah JC, Kominsky DJ, Colgan SP. Microbial-derived butyrate promotes epithelial barrier function through IL-10 receptor-dependent repression of claudin-2. J Immunol 2017;199:2976–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang L, Liu L, Zhou W, Yang C, Mai G, Li H, Chen Y. Gut microbiota-derived butyrate regulates gut mucus barrier repair by activating the macrophage/WNT/ERK signaling pathway. Clin Sci (Lond) 2022;136:291–307. [DOI] [PubMed] [Google Scholar]
- 35.Kleuskens MTA, Haasnoot ML, Herpers BM, Ampting M, Bredenoord AJ, Garssen J, Redegeld FA, van Esch BCAM. Butyrate and propionate restore interleukin 13-compromised esophageal epithelial barrier function. Allergy 2022;77:1510–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marrs T, Walter J. Pros and cons: is faecal microbiota transplantation a safe and efficient treatment option for gut dysbiosis? Allergy 2021;76:2312–2317. [DOI] [PubMed] [Google Scholar]
- 37.Vitte J, Michel M, Malinovschi A, Caminati M, Odebode A, Annesi-Maesano I, Caimmi DP, Cassagne C, Demoly P, Heffler E, Menu E, Nwaru BI, Sereme Y, Ranque S, Raulf M, Feleszko W, Janson C, Galán C; EAACI Task Force on Allergic Bronchopulmonary Aspergillosis. Fungal exposome, human health, and unmet needs: a 2022 update with special focus on allergy. Allergy 2022;77:3199–3216. [DOI] [PubMed] [Google Scholar]
- 38.Leonardi I, Gao IH, Lin WY, Allen M, Li XV, Fiers WD, De Celie MB, Putzel GG, Yantiss RK, Johncilla M, Colak D, Iliev ID. Mucosal fungi promote gut barrier function and social behavior via Type 17 immunity. Cell 2022;185:831–846.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruokolainen L, Parkkola A, Karkman A, Sinkko H, Peet A, Hamalainen AM, von Hertzen L, Tillmann V, Koski K, Virtanen SM, Niemelä O, Haahtela T, Knip M. Contrasting microbiotas between finnish and estonian infants: exposure to acinetobacter may contribute to the allergy gap. Allergy 2020;75:2342–2351. [DOI] [PubMed] [Google Scholar]
- 40.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A 2010;107:12204–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barker-Tejeda TC, Zubeldia-Varela E, Obeso D. A step closer to understanding the relationship between host and gut microbiota metabolism. Allergy 2022;77:1638–1640. [DOI] [PubMed] [Google Scholar]
- 42.Wang Z, Walker GW, Muir DCG, Nagatani-Yoshida K. Toward a global understanding of chemical pollution: a first comprehensive analysis of national and regional chemical inventories. Environ Sci Technol 2020;54:2575–2584. [DOI] [PubMed] [Google Scholar]
- 43.Smyth T, Veazey J, Eliseeva S, Chalupa D, Elder A, Georas SN. Diesel exhaust particle exposure reduces expression of the epithelial tight junction protein Tricellulin. Part Fibre Toxicol 2020;17:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doyle AD, Masuda MY, Pyon GC, Luo H, Putikova A, LeSuer WE, Flashner S, Rank MA, Nakagawa H, Kita H, Wright BL. Detergent exposure induces epithelial barrier dysfunction and eosinophilic inflammation in the esophagus. Allergy 2023;78:192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yazici D, Pat Y, Mitamura Y, Akdis CA, Ogulur I. Detergent-induced eosinophilic inflammation in the esophagus: a key evidence for the epithelial barrier theory. Allergy 2023. [DOI] [PubMed] [Google Scholar]
- 46.D’Amato G, Akdis CA. Desert dust and respiratory diseases: further insights into the epithelial barrier hypothesis. Allergy 2022;77:3490–3492. [DOI] [PubMed] [Google Scholar]
- 47.Yang F-M, Hu M-C, Weng C-M, Chuang H-C, Lan Y-T, Su B-H, Heo K-S, Kuo H-P. Loss of PP4 contributes to diesel exhaust particles-induced epithelial barrier integrity disruption and alarmins release. Allergy 2022. [DOI] [PubMed] [Google Scholar]
- 48.Manisalidis I, Stavropoulou E, Stavropoulos A, Bezirtzoglou E. Environmental and health impacts of air pollution: a review. Front Public Health 2020;8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nadeau KC, Agache I, Jutel M, Annesi Maesano I, Akdis M, Sampath V, D'Amato G, Cecchi L, Traidl-Hoffmann C, Akdis CA. Climate change: a call to action for the United Nations. Allergy 2022;77:1087–1090. [DOI] [PubMed] [Google Scholar]
- 50.Gonzalez-Martin J, Kraakman NJR, Perez C, Lebrero R, Munoz R. A state-of-the-art review on indoor air pollution and strategies for indoor air pollution control. Chemosphere 2021;262:128376. [DOI] [PubMed] [Google Scholar]
- 51.Olesiejuk K, Chalubinski M. How does particulate air pollution affect barrier functions and inflammatory activity of lung vascular endothelium?. Allergy 2023;78:629–638. [DOI] [PubMed] [Google Scholar]
- 52.Xu R, Yu P, Abramson MJ, Johnston FH, Samet JM, Bell ML, Haines A, Ebi KL, Li S, Guo Y. Wildfires, global climate change, and human health. N Engl J Med 2020;383:2173–2181. [DOI] [PubMed] [Google Scholar]
- 53.Fitch MN, Phillippi D, Zhang Y, Lucero J, Pandey RS, Liu J, Brower J, Allen MS, Campen MJ, McDonald JD, Lund AK. Effects of inhaled air pollution on markers of integrity, inflammation, and microbiota profiles of the intestines in Apolipoprotein E knockout mice. Environ Res 2020;181:108913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Danov O, Wolff M, Bartel S, Bohlen S, Obernolte H, Wronski S, Jonigk D, Hammer B, Kovacevic D, Reuter S, Krauss-Etschmann S, Sewald K. Cigarette Smoke affects dendritic cell populations, epithelial barrier function, and the immune response to viral infection With H1N1. Front Med (Lausanne) 2020;7:571003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bajpai D, Tyagi VK. Laundry detergents: an overview. J Oleo Sci 2007;56:327–40. [DOI] [PubMed] [Google Scholar]
- 56.Siracusa A, De Blay F, Folletti I, Moscato G, Olivieri M, Quirce S, Raulf-Heimsoth M, Sastre J, Tarlo SM, Walusiak-Skorupa J, Zock J-P. Asthma and exposure to cleaning products - a European academy of allergy and clinical immunology task force consensus statement. Allergy 2013;68:1532–45. [DOI] [PubMed] [Google Scholar]
- 57.Folletti I, Zock JP, Moscato G, Siracusa A. Asthma and rhinitis in cleaning workers: a systematic review of epidemiological studies. J Asthma 2014;51:18–28. [DOI] [PubMed] [Google Scholar]
- 58.Brant A, Hole A, Cannon J, Helm J, Swales C, Welch J, Taylor AN, Cullinan P. Occupational asthma caused by cellulase and lipase in the detergent industry. Occup Environ Med 2004;61:793–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xian M, Wawrzyniak P, Ruckert B, Duan S, Meng Y, Sokolowska M, Globinska A, Zhang L, Akdis M, Akdis CA. Anionic surfactants and commercial detergents decrease tight junction barrier integrity in human keratinocytes. J Allergy Clin Immunol 2016;138:890–893.e9. [DOI] [PubMed] [Google Scholar]
- 60.Wang M, Tan G, Eljaszewicz A, Meng Y, Wawrzyniak P, Acharya S, Altunbulakli C, Westermann P, Dreher A, Yan L, Wang C, Akdis M, Zhang L, Nadeau KC, Akdis CA. Laundry detergents and detergent residue after rinsing directly disrupt tight junction barrier integrity in human bronchial epithelial cells. J Allergy Clin Immunol 2019;143:1892–1903. [DOI] [PubMed] [Google Scholar]
- 61.Ogulur I, Pat Y, Aydin T, Yazici D, Ruckert B, Peng Y, Kim J, Radzikowska U, Westermann P, Sokolowska M, Dhir R, Akdis M, Nadeau K, Akdis CA. Gut epithelial barrier damage caused by dishwasher detergents and rinse aids. J Allergy Clin Immunol 2023;151:469–484. [DOI] [PubMed] [Google Scholar]
- 62.Huang Z, Weng Y, Shen Q, Zhao Y, Jin Y. Microplastic: A potential threat to human and animal health by interfering with the intestinal barrier function and changing the intestinal microenvironment. Sci Total Environ 2021;785:147365. [DOI] [PubMed] [Google Scholar]
- 63.Jin Y, Lu L, Tu W, Luo T, Fu Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci Total Environ 2019;649:308–317. [DOI] [PubMed] [Google Scholar]
- 64.Aungst BJ. Intestinal permeation enhancers. J Pharm Sci 2000;89:429–42. [DOI] [PubMed] [Google Scholar]
- 65.Biedermann T, Winther L, Till SJ, Panzner P, Knulst A, Valovirta E. Birch pollen allergy in Europe. Allergy 2019;74:1237–1248. [DOI] [PubMed] [Google Scholar]
- 66.Tamaki FK, Pimentel AC, Dias AB, Cardoso C, Ribeiro AF, Ferreira C, Terra WR. Physiology of digestion and the molecular characterization of the major digestive enzymes from Periplaneta americana. J Insect Physiol 2014;70:22–35. [DOI] [PubMed] [Google Scholar]
- 67.Gonzalez-Rabade N, Badillo-Corona JA, Aranda-Barradas JS, Oliver-Salvador Mdel C. Production of plant proteases in vivo and in vitro–a review. Biotechnol Adv 2011;29:983–96. [DOI] [PubMed] [Google Scholar]
- 68.Behnsen J, Lessing F, Schindler S, Wartenberg D, Jacobsen ID, Thoen M, Zipfel PF, Brakhage AA. Secreted Aspergillus fumigatus protease Alp1 degrades human complement proteins C3, C4, and C5. Infect Immun 2010;78:3585–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Caraballo L, Valenta R, Puerta L, Pomes A, Zakzuk J, Fernandez-Caldas E, Acevedo N, Sanchez-Borges M, Ansotegui I, Zhang L, van Hage M, Abel-Fernández E, Karla Arruda L, Vrtala S, Curin M, Gronlund H, Karsonova A, Kilimajer J, Riabova K, Trifonova D, Karaulov A. The allergenic activity and clinical impact of individual IgE-antibody binding molecules from indoor allergen sources. World Allergy Organ J 2020;13:100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen JC, Chuang JG, Su YY, Chiang BL, Lin YS, Chow LP. The protease allergen Pen c 13 induces allergic airway inflammation and changes in epithelial barrier integrity and function in a murine model. J Biol Chem 2011;286:26667–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tai HY, Tam MF, Chou H, Peng HJ, Su SN, Perng DW, Shen H-D. Pen ch 13 allergen induces secretion of mediators and degradation of occludin protein of human lung epithelial cells. Allergy 2006;61:382–8. [DOI] [PubMed] [Google Scholar]
- 72.Fattouh R, Pouladi MA, Alvarez D, Johnson JR, Walker TD, Goncharova S, Inman MD, Jordana M. House dust mite facilitates ovalbumin-specific allergic sensitization and airway inflammation. Am J Respir Crit Care Med 2005;172:314–21. [DOI] [PubMed] [Google Scholar]
- 73.Li B, Zou Z, Meng F, Raz E, Huang Y, Tao A, Ai Y. Dust mite-derived Der f 3 activates a pro-inflammatory program in airway epithelial cells via PAR-1 and PAR-2. Mol Immunol 2019;109:1–11. [DOI] [PubMed] [Google Scholar]
- 74.Dumez ME, Herman J, Campizi V, Galleni M, Jacquet A, Chevigne A. Orchestration of an uncommon maturation cascade of the house dust mite protease allergen quartet. Front Immunol 2014;5:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vidal-Quist JC, Ortego F, Hernandez-Crespo P. Contribution of cysteine and serine proteases to proteolytic digestion in an allergy-eliciting house dust mite. J Insect Physiol 2021;133:104285. [DOI] [PubMed] [Google Scholar]
- 76.Sun G, Stacey MA, Schmidt M, Mori L, Mattoli S. Interaction of mite allergens Der p3 and Der p9 with protease-activated receptor-2 expressed by lung epithelial cells. J Immunol 2001;167:1014–21. [DOI] [PubMed] [Google Scholar]
- 77.Kukreja N, Sridhara S, Singh BP, Arora N. Effect of proteolytic activity of Epicoccum purpurascens major allergen, Epi p 1 in allergic inflammation. Clin Exp Immunol 2008;154:162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gaspar R, de Matos MR, Cortes L, Nunes-Correia I, Todo-Bom A, Pires E, Veríssimo P. Pollen proteases play multiple roles in allergic disorders. Int J Mol Sci 2020;21:3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Soh WT, Zhang J, Hollenberg MD, Vliagoftis H, Rothenberg ME, Sokol CL, Robinson C, Jacquet A. Protease allergens as initiators-regulators of allergic inflammation. Allergy 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Karaaslan C, Karaguzel D, Sarac BE, Sucularli C, Bilgic HA, Kalayci O. The expression profile of protease inhibitors in the airway epithelial cells after allergen (Der p 1) stimulation. Int Arch Allergy Immunol 2022;183:25–33. [DOI] [PubMed] [Google Scholar]
- 81.Hew M, Lee J, Varese N, Aui PM, McKenzie CI, Wines BD, Aumann H, Rolland JM, Mark Hogarth P, van Zelm MC, O'Hehir RE. Epidemic thunderstorm asthma susceptibility from sensitization to ryegrass (Lolium perenne) pollen and major allergen Lol p 5. Allergy 2020;75:2369–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rauer D, Gilles S, Wimmer M, Frank U, Mueller C, Musiol S, Vafadari B, Aglas L, Ferreira F, Schmitt-Kopplin P, Durner J, Winkler JB, Ernst D, Behrendt H, Schmidt-Weber CB, Traidl-Hoffmann C, Alessandrini F. Ragweed plants grown under elevated CO(2) levels produce pollen which elicit stronger allergic lung inflammation. Allergy 2021;76:1718–1730. [DOI] [PubMed] [Google Scholar]
- 83.Burgueno JF, Abreu MT. Epithelial Toll-like receptors and their role in gut homeostasis and disease. Nat Rev Gastroenterol Hepatol 2020;17:263–278. [DOI] [PubMed] [Google Scholar]
- 84.Serek P, Oleksy-Wawrzyniak M. The effect of bacterial infections, probiotics and zonulin on intestinal barrier integrity. Int J Mol Sci 2021;22:11359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Prunicki MM, Dant CC, Cao S, Maecker H, Haddad F, Kim JB, Snyder M, Wu J, Nadeau K. Immunologic effects of forest fire exposure show increases in IL-1beta and CRP. Allergy 2020;75:2356–2358. [DOI] [PubMed] [Google Scholar]
- 86.Shim JS, Lee HS, Park DE, Won Lee J, Bae B, Chang Y, Kim J, Kim HY, Kang H-R. Aggravation of asthmatic inflammation by chlorine exposure via innate lymphoid cells and CD11c(intermediate) macrophages. Allergy 2020;75:381–391. [DOI] [PubMed] [Google Scholar]
- 87.Ponziani FR, Zocco MA, Cerrito L, Gasbarrini A, Pompili M. Bacterial translocation in patients with liver cirrhosis: physiology, clinical consequences, and practical implications. Expert Rev Gastroenterol Hepatol 2018;12:641–656. [DOI] [PubMed] [Google Scholar]
- 88.Andersen K, Kesper MS, Marschner JA, Konrad L, Ryu M, Kumar Vr S, Kulkarni OP, Mulay SR, Romoli S, Demleitner J, Schiller P, Dietrich A, Müller S, Gross O, Ruscheweyh H-J, Huson DH, Stecher B, Anders H-J. Intestinal dysbiosis, barrier dysfunction, and bacterial translocation account for CKD-related systemic inflammation. J Am Soc Nephrol 2017;28:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wawrzyniak P, Wawrzyniak M, Wanke K, Sokolowska M, Bendelja K, Ruckert B, Globinska A, Jakiela B, Kast JI, Idzko M, Akdis M, Sanak M, Akdis CA. Regulation of bronchial epithelial barrier integrity by type 2 cytokines and histone deacetylases in asthmatic patients. J Allergy Clin Immunol 2017;139:93–103. [DOI] [PubMed] [Google Scholar]
- 90.Singh N, Diebold Y, Sahu SK, Leonardi A. Epithelial barrier dysfunction in ocular allergy. Allergy 2022;77:1360–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Masterson JC, Biette KA, Hammer JA, Nguyen N, Capocelli KE, Saeedi BJ, Harris RF, Fernando SD, Hosford LB, Kelly CJ, Campbell EL, Ehrentraut SF, Ahmed FN, Nakagawa H, Lee JJ, McNamee EN, Glover LE, Colgan SP, Furuta GT. Epithelial HIF-1alpha/claudin-1 axis regulates barrier dysfunction in eosinophilic esophagitis. J Clin Invest 2019;129:3224–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Soyka MB, Wawrzyniak P, Eiwegger T, Holzmann D, Treis A, Wanke K, Kast JI, Akdis CA. Defective epithelial barrier in chronic rhinosinusitis: the regulation of tight junctions by IFN-gamma and IL-4. J Allergy Clin Immunol 2012;130:1087–1096.e10. [DOI] [PubMed] [Google Scholar]
- 93.Rapin A, Rehbinder EM, Macowan M, Pattaroni C, Carlsen KCL, Harris NL, Jonassen CM, Landrø L, Lossius AH, Nordlund B, Rudi K, Skjerven HO, Staff AC, Söderhäll C, Ubags N, Vettukattil R, Marsland BJ. The skin microbiome in the first year of life and its association with atopic dermatitis. Allergy 2023. [DOI] [PubMed] [Google Scholar]
- 94.Warnberg Gerdin S, Lie A, Asarnoj A, Borres MP, Lodrup Carlsen KC, Fardig M, Konradsen JR, Monceyron Jonassen C, Olsson Mägi C-A, Rehbinder EM, Rudi K, Skjerven HO, Staff AC, Söderhäll C, Tedner SG, van Hage M, Vettukattil R, Nordlund B. Impaired skin barrier and allergic sensitization in early infancy. Allergy 2022;77:1464–1476. [DOI] [PubMed] [Google Scholar]
- 95.Dai X, Muto J, Shiraishi K, Utsunomiya R, Mori H, Murakami M, Sayama K. TSLP impairs epidermal barrier integrity by stimulating the formation of nuclear IL-33/phosphorylated STAT3 complex in human keratinocytes. J Invest Dermatol 2022;142:2100–2108.e5. [DOI] [PubMed] [Google Scholar]
- 96.Pavel AB, Renert-Yuval Y, Wu J, Del Duca E, Diaz A, Lefferdink R, Fang MM, Canter T, Rangel SM, Zhang N, Krueger JG, Paller AS, Guttman-Yassky E. Tape strips from early-onset pediatric atopic dermatitis highlight disease abnormalities in nonlesional skin. Allergy 2021;76:314–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Berdyshev E, Goleva E, Bissonnette R, Bronova I, Bronoff AS, Richers BN, Garcia S, Ramirez-Gama M, Taylor P, Praestgaard A, Agueusop I, Jurvilliers P, Boguniewicz M, Levit NA, Rossi AB, Zhang A, Leung DYM. Dupilumab significantly improves skin barrier function in patients with moderate-to-severe atopic dermatitis. Allergy 2022;77:3388–3397. [DOI] [PubMed] [Google Scholar]
- 98.Bangert C, Rindler K, Krausgruber T, Alkon N, Thaler FM, Kurz H, Ayub T, Demirtas D, Fortelny N, Vorstandlechner V, Bauer WM, Quint T, Mildner M, Jonak C, Elbe-Bürger A, Griss J, Bock C, Brunner PM. Persistence of mature dendritic cells, T(H)2A, and Tc2 cells characterize clinically resolved atopic dermatitis under IL-4Ralpha blockade. Sci Immunol 2021;6:eabe2749. [DOI] [PubMed] [Google Scholar]
- 99.Pat Y, Ogulur I, Yazici D, Mitamura Y, Cevhertas L, Kucukkase OC, Mesisser SS, Akdis M, Nadeau K, Akdis CA. Effect of altered human exposome on the skin and mucosal epithelial barrier integrity. Tissue Barriers 2022:2133877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chan BCL, Lam CWK, Tam LS, Wong CK. IL33: roles in allergic inflammation and therapeutic perspectives. Front Immunol 2019;10:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barretto KT, Brockman-Schneider RA, Kuipers I, Basnet S, Bochkov YA, Altman MC, Jarjour NN, Gern JE, Esnault S. Human airway epithelial cells express a functional IL-5 receptor. Allergy 2020;75:2127–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Agumadu VC, Ramphul K, Mejias SG, Sonaye R, Sombans S, Lohana P. A review of three new anti-interleukin-5 monoclonal antibody therapies for severe asthma. Cureus 2018;10:e3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mukherjee M, Agache I. IL-13 signature in severe adult asthmatics with airway neutrophilia: A new endotype to treat! Allergy 2021;76:1964–1966. [DOI] [PubMed] [Google Scholar]
- 104.Pat Y, Ruckert B, Ogulur I, Yazici D, Perez-Diego M, Kucukkase OC, Li M, Akdis CA. Differentiation of bronchial epithelial spheroids in the presence of IL-13 recapitulates characteristic features of asthmatic airway epithelia. Allergy 2022;77:2229–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sugita K, Steer CA, Martinez-Gonzalez I, Altunbulakli C, Morita H, Castro-Giner F, Kubo T, Wawrzyniak P, Rückert B, Sudo K, Nakae S, Matsumoto K, O'Mahony L, Akdis M, Takei F, Akdis CA. Type 2 innate lymphoid cells disrupt bronchial epithelial barrier integrity by targeting tight junctions through IL-13 in asthmatic patients. J Allergy Clin Immunol 2018;141:300–310.e11. [DOI] [PubMed] [Google Scholar]
- 106.Fujimura KE, Sitarik AR, Havstad S, Lin DL, Levan S, Fadrosh D, Panzer AR, LaMere B, Rackaityte E, Lukacs NW, Wegienka G, Boushey HA, Ownby DR, Zoratti EM, Levin AM, Johnson CC, Lynch SV. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med 2016;22:1187–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Arrieta MC, Arevalo A, Stiemsma L, Dimitriu P, Chico ME, Loor S, Vaca M, Boutin RCT, Morien E, Jin M, Turvey SE, Walter J, Parfrey LW, Cooper PJ, Finlay B. Associations between infant fungal and bacterial dysbiosis and childhood atopic wheeze in a nonindustrialized setting. J Allergy Clin Immunol 2018;142:424–434.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schuijs MJ, Willart MA, Vergote K, Gras D, Deswarte K, Ege MJ, Madeira FB, Beyaert R, van Loo G, Bracher F, von Mutius E, Chanez P, Lambrecht BN, Hammad H. Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science 2015;349:1106–10. [DOI] [PubMed] [Google Scholar]
- 109.Alhasan MM, Cait AM, Heimesaat MM, Blaut M, Klopfleisch R, Wedel A, Conlon TM, Yildirim AÖ, Sodemann EB, Mohn WW, Bereswill S, Conrad ML. Antibiotic use during pregnancy increases offspring asthma severity in a dose-dependent manner. Allergy 2020;75:1979–1990. [DOI] [PubMed] [Google Scholar]
- 110.Andersson CK, Iwasaki J, Cook J, Robinson P, Nagakumar P, Mogren S, Fleming L, Bush A, Saglani S, Lloyd CM. Impaired airway epithelial cell wound-healing capacity is associated with airway remodelling following RSV infection in severe preschool wheeze. Allergy 2020;75:3195–3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kast JI, McFarlane AJ, Globinska A, Sokolowska M, Wawrzyniak P, Sanak M, Schwarze J, Akdis CA, Wanke K. Respiratory syncytial virus infection influences tight junction integrity. Clin Exp Immunol 2017;190:351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Velasco E, Delicado-Miralles M, Hellings PW, Gallar J, Van Gerven L, Talavera K. Epithelial and sensory mechanisms of nasal hyperreactivity. Allergy 2022;77:1450–1463. [DOI] [PubMed] [Google Scholar]
- 113.Nur Husna SM, Tan HT, Md Shukri N, Mohd Ashari NS, Wong KK. Nasal epithelial barrier integrity and tight junctions disruption in allergic rhinitis: overview and pathogenic insights. Front Immunol 2021;12:663626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Doulaptsi M, Wils T, Hellings PW, Martens K, Farre R, Vicario M, Fokkens W, Prokopakis E, Steelant B. Mometasone furoate and fluticasone furoate are equally effective in restoring nasal epithelial barrier dysfunction in allergic rhinitis. World Allergy Organ J 2021;14:100585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang C, Wang Y, Liao W, Liang T, Liu W, Xie J, Wang X, Yang P, Lu W, Zhang X. MUC1 deficiency induces the nasal epithelial barrier dysfunction via RBFOX3 shortage augment ubiquitin-proteasomal degradation in allergic rhinitis pathogenesis. Allergy 2022;77:1596–1599. [DOI] [PubMed] [Google Scholar]
- 116.Tuli JF, Ramezanpour M, Cooksley C, Psaltis AJ, Wormald PJ, Vreugde S. Association between mucosal barrier disruption by Pseudomonas aeruginosa exoproteins and asthma in patients with chronic rhinosinusitis. Allergy 2021;76:3459–3469. [DOI] [PubMed] [Google Scholar]
- 117.Sindher SB, Long A, Chin AR, Hy A, Sampath V, Nadeau KC, Chinthrajah RS. Food allergy, mechanisms, diagnosis and treatment: innovation through a multi-targeted approach. Allergy 2022;77:2937–2948. [DOI] [PubMed] [Google Scholar]
- 118.Brough HA, Nadeau KC, Sindher SB, Alkotob SS, Chan S, Bahnson HT, Leung DYM, Lack G. Epicutaneous sensitization in the development of food allergy: what is the evidence and how can this be prevented? Allergy 2020;75:2185–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lee-Sarwar KA, Chen YC, Lasky-Su J, Kelly RS, Zeiger RS, O’Connor GT, Bacharier LB, Jia X, Beigelman A, Gold DR, Laranjo N, Bunyavanich S, Weiss ST, Litonjua AA, Brennan PJ. Early-life fecal metabolomics of food allergy. Allergy 2023;78:512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Su KW, Cetinbas M, Martin VM, Virkud YV, Seay H, Ndahayo R, Rosow R, Elkort M, Gupta B, Kramer E, Pronchick T, Reuter S, Sadreyev RI, Huang J-L, Shreffler WG, Yuan Q. Early infancy dysbiosis in food protein-induced enterocolitis syndrome: a prospective cohort study. Allergy 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Peters RL, Mavoa S, Koplin JJ. An overview of environmental risk factors for food allergy. Int J Environ Res Public Health 2022;19:722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Avino G, Riggioni C, Comberiati P. Immune-epithelial barrier interactions mediate intestinal adaptation to diverse diets. Allergy 2022;77:1636–1637. [DOI] [PubMed] [Google Scholar]
- 123.Rachid R, Stephen-Victor E, Chatila TA. The microbial origins of food allergy. J Allergy Clin Immunol 2021;147:808–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Paparo L, Nocerino R, Ciaglia E, Di Scala C, De Caro C, Russo R, Trinchese G, Aitoro R, Amoroso A, Bruno C, Di Costanzo M, Passariello A, Messina F, Agangi A, Napolitano M, Voto L, Gatta GD, Pisapia L, Montella F, Mollica MP, Calignano A, Puca A, Berni Canani R. Butyrate as a bioactive human milk protective component against food allergy. Allergy 2021;76:1398–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Frei R, Heye K, Roduit C. Environmental influences on childhood allergies and asthma - the farm effect. Pediatr Allergy Immunol 2022;33:e13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Roduit C, Frei R, Ferstl R, Loeliger S, Westermann P, Rhyner C, Schiavi E, Barcik W, Rodriguez-Perez N, Wawrzyniak M, Chassard C, Lacroix C, Schmausser-Hechfellner E, Depner M, von Mutius E, Braun-Fahrländer C, Karvonen AM, Kirjavainen PV, Pekkanen J, Dalphin J-C, Riedler J, Akdis C, Lauener R, O'Mahony L; PASTURE/EFRAIM study group. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy 2019;74:799–809. [DOI] [PubMed] [Google Scholar]
- 127.Yamagishi M, Akagawa S, Akagawa Y, Nakai Y, Yamanouchi S, Kimata T, Hashiyada M, Akane A, Tsuji S, Kaneko K. Decreased butyric acid-producing bacteria in gut microbiota of children with egg allergy. Allergy 2021;76:2279–2282. [DOI] [PubMed] [Google Scholar]
- 128.Shoda T, Kaufman KM, Wen T, Caldwell JM, Osswald GA, Purnima P, Zimmermann N, Collins MH, Rehn K, Foote H, Eby MD, Zhang W, Ben-Baruch Morgenstern N, Ballaban AY, Habel JE, Kottyan LC, Abonia JP, Mukkada VA, Putnam PE, Martin LJ, Rothenberg ME. Desmoplakin and periplakin genetically and functionally contribute to eosinophilic esophagitis. Nat Commun 2021;12:6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wheeler JC, Vanoni S, Zeng C, Waggoner L, Yang Y, Wu D, Uddin J, Karns R, Kottyan L, Mukkada V, Rothenberg ME, Hogan SP. 17beta-Estradiol protects the esophageal epithelium from IL-13-induced barrier dysfunction and remodeling. J Allergy Clin Immunol 2019;143:2131–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cox AJ, West NP, Cripps AW. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol 2015;3:207–15. [DOI] [PubMed] [Google Scholar]
- 131.Mouries J, Brescia P, Silvestri A, Spadoni I, Sorribas M, Wiest R, Mileti E, Galbiati M, Invernizzi P, Adorini L, Penna G, Rescigno M. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J Hepatol 2019;71:1216–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Camara-Lemarroy CR, Silva C, Greenfield J, Liu WQ, Metz LM, Yong VW. Biomarkers of intestinal barrier function in multiple sclerosis are associated with disease activity. Mult Scler 2020;26:1340–1350. [DOI] [PubMed] [Google Scholar]
- 133.Kim JW, Kwok SK, Choe JY, Park SH. Recent advances in our understanding of the link between the intestinal microbiota and systemic lupus erythematosus. Int J Mol Sci 2019;20:4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ciccia F, Guggino G, Rizzo A, Alessandro R, Luchetti MM, Milling S, Saieva L, Cypers H, Stampone T, Di Benedetto P, Gabrielli A, Fasano A, Elewaut D, Triolo G. Dysbiosis and zonulin upregulation alter gut epithelial and vascular barriers in patients with ankylosing spondylitis. Ann Rheum Dis 2017;76:1123–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sorini C, Cosorich I, Lo Conte M, De Giorgi L, Facciotti F, Luciano R, Rocchi M, Ferrarese R, Sanvito F, Canducci F, Falcone M. Loss of gut barrier integrity triggers activation of islet-reactive T cells and autoimmune diabetes. Proc Natl Acad Sci U S A 2019;116:15140–15149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fiorentino M, Sapone A, Senger S, Camhi SS, Kadzielski SM, Buie TM, Kelly DL, Cascella N, Fasano A. Blood-brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Mol Autism 2016;7:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.van ISCD, Derkinderen P. The intestinal barrier in Parkinson’s Disease: current state of knowledge. J Parkinsons Dis 2019;9:S323–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kohler CA, Maes M, Slyepchenko A, Berk M, Solmi M, Lanctot KL, Carvalho AF. The gut-brain axis, including the microbiome, leaky gut and bacterial translocation: mechanisms and pathophysiological role in Alzheimer’s disease. Curr Pharm Des 2016;22:6152–6166. [DOI] [PubMed] [Google Scholar]
- 139.Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci 2015;9:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Maes M, Kubera M, Leunis JC, Berk M. Increased IgA and IgM responses against gut commensals in chronic depression: further evidence for increased bacterial translocation or leaky gut. J Affect Disord 2012;141:55–62. [DOI] [PubMed] [Google Scholar]
- 141.Tajik N, Frech M, Schulz O, Schalter F, Lucas S, Azizov V, Dürholz K, Steffen F, Omata Y, Rings A, Bertog M, Rizzo A, Iljazovic A, Basic M, Kleyer A, Culemann S, Krönke G, Luo Y, Überla K, Gaipl US, Frey B, Strowig T, Sarter K, Bischoff SC, Wirtz S, Cañete JD, Ciccia F, Schett G, Zaiss MM. Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat Commun 2020;11:1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vanuytsel T, Tack J, Farre R. The role of intestinal permeability in gastrointestinal disorders and current methods of evaluation. Front Nutr 2021;8:717925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sanchez-Solares J, Sanchez L, Pablo-Torres C, Diaz-Fernandez C, Sorensen P, Barber D, Gomez-Casado C. Celiac disease causes epithelial disruption and regulatory T cell recruitment in the oral mucosa. Front Immunol 2021;12:623805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kinashi Y, Hase K. Partners in leaky gut syndrome: intestinal dysbiosis and autoimmunity. Front Immunol 2021;12:673708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kuo WT, Zuo L, Odenwald MA, Madha S, Singh G, Gurniak CB, Abraham C, Turner JR. The tight junction protein ZO-1 is dispensable for barrier function but critical for effective mucosal repair. Gastroenterology 2021;161:1924–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang J, Chen C, Ren Y, Zhou X, Yu S. Metformin alleviates intestinal epithelial barrier damage by inhibiting endoplasmic reticulum stress-induced cell apoptosis in colitis cell model. Zhejiang Da Xue Xue Bao Yi Xue Ban 2021;50:627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang W, Zhai D, Bai Y, Xue K, Deng L, Ma L, Du T, Ye Z, Qu D, Xiang A, Chen G, Zhao Y, Wang L, Lu Z. Loss of QKI in macrophage aggravates inflammatory bowel disease through amplified ROS signaling and microbiota disproportion. Cell Death Discov 2021;7:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lei H, Crawford MS, McCole DF. JAK-STAT pathway regulation of intestinal permeability: pathogenic roles and therapeutic opportunities in inflammatory bowel disease. Pharmaceuticals (Basel) 2021;14:840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cortese A, Lova L, Comoli P, Volpe E, Villa S, Mallucci G, La Salvia S, Romani A, Franciotta D, Bollati V, Basso S, Guido I, Quartuccio G, Battistini L, Cereda C, Bergamaschi R. Air pollution as a contributor to the inflammatory activity of multiple sclerosis. J Neuroinflammation 2020;17:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pellegrini C, D’Antongiovanni V, Miraglia F, Rota L, Benvenuti L, Di Salvo C, Testa G, Capsoni S, Carta G, Antonioli L, Cattaneo A, Blandizzi C, Colla E, Fornai M. Enteric alpha-synuclein impairs intestinal epithelial barrier through caspase-1-inflammasome signaling in Parkinson’s disease before brain pathology. NPJ Parkinsons Dis 2022;8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fiorito S, Soligo M, Gao Y, Ogulur I, Akdis CA, Bonini S. Is the epithelial barrier hypothesis the key to understanding the higher incidence and excess mortality during COVID-19 pandemic? The case of Northern Italy. Allergy 2022;77:1408–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Radzikowska U, Ding M, Tan G, Zhakparov D, Peng Y, Wawrzyniak P, Wang M, Li S, Morita H, Altunbulakli C, Reiger M, Neumann AU, Lunjani N, Traidl-Hoffmann C, Nadeau KC, O'Mahony L, Akdis C, Sokolowska M. Distribution of ACE2, CD147, CD26, and other SARS-CoV-2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID-19 risk factors. Allergy 2020;75:2829–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hatton CF, Botting RA, Duenas ME, Haq IJ, Verdon B, Thompson BJ, Spegarova JS, Gothe F, Stephenson E, Gardner AI, Murphy S, Scott J, Garnett JP, Carrie S, Powell J, Khan CMA, Huang L, Hussain R, Coxhead J, Davey T, Simpson AJ, Haniffa M, Hambleton S, Brodlie M, Ward C, Trost M, Reynolds G, Duncan CJA. Delayed induction of type I and III interferons mediates nasal epithelial cell permissiveness to SARS-CoV-2. Nat Commun 2021;12:7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shepley-McTaggart A, Sagum CA, Oliva I, Rybakovsky E, DiGuilio K, Liang J, Bedford MT, Cassel J, Sudol M, Mullin JM, Harty RN. SARS-CoV-2 Envelope (E) protein interacts with PDZ-domain-2 of host tight junction protein ZO1. PLoS One 2021;16:e0251955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shi W, Gao Z, Ding Y, Zhu T, Zhang W, Xu Y. Clinical characteristics of COVID-19 patients combined with allergy. Allergy 2020;75:2405–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang HP, Sun YL, Wang YF, Yazici D, Azkur D, Ogulur I, Azkur AK, Yang Z-W, Chen X-X, Zhang A-Z, Hu J-Q, Liu G-H, Akdis M, Akdis CA, Gao Y-D. Recent developments in the immunopathology of COVID-19. Allergy 2023;78:369–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Grasselli G, Greco M, Zanella A, Albano G, Antonelli M, Bellani G, Bonanomi E, Cabrini L, Carlesso E, Castelli G, Cattaneo S, Cereda D, Colombo S, Coluccello A, Crescini G, Forastieri Molinari A, Foti G, Fumagalli R, Iotti GA, Langer T, Latronico N, Lorini FL, Mojoli F, Natalini G, Pessina CM, Ranieri VM, Rech R, Scudeller L, Rosano A, Storti E, Thompson BT, Tirani M, Villani PG, Pesenti A, Cecconi M; COVID-19 Lombardy ICU Network. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med 2020;180:1345–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, Akdis CA, Gao Y-D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020;75:1730–1741. [DOI] [PubMed] [Google Scholar]