Abstract
At least 65 million people around the world suffer from long COVID-19, with the majority of cases occurring in the productive age (36–50 years old). Individuals with long COVID-19 are confounded with multiple organ system dysfunctions, long-term organ injury sequelae, and a decreased quality of life. There is an overlapping of risk factors between long COVID-19 and other postviral infection syndromes, so advances in research could also benefit other groups of patients. Long COVID-19 is the consequence of multiple immune system dysregulation, such as T-cell depletion, innate immune cell hyperactivity, lack of naive T and B cells, and elevated signature of pro-inflammatory cytokines, together with persistent severe acute respiratory syndrome-coronavirus 2 reservoir and other consequences of acute infection. There is an activated condition of mast cells in long COVID-19, with abnormal granulation and excessive inflammatory cytokine release. A study by Weinstock et al. indicates that patients with long COVID-19 suffer the same clinical syndrome as patients with mast cell activation syndrome (MCAS). Diagnosis and treatment of MCAS in patients with long COVID-19 will provide further symptomatic relief, and manage mast cell-mediated hyperinflammation states, which could be useful in the long-term control and recovery of such patients.
Keywords: COVID-19, Long COVID, Mast cells, MCAS
1. Impacts of long COVID
We as a society have entered the 4th year of the COVID-19 pandemic, and many breakthroughs have been achieved, especially in the immunology field. But, up until recently, COVID-19 has infected more than 674 million people worldwide, with 6.86 million documented deaths, likely higher due to undocumented cases [1]. Alarmingly, as with other systemic viral infections such as with Ebstein-Barr virus (EBV), cytomegalo virus, hepatitis C virus, human herpes virus 6, human immunodeficiency virus, and many more, COVID-19 is associated with prolonged multisystemic symptoms that follows the acute infection, termed long COVID-19 [2–4]. At least 65 million people worldwide suffer from long COVID-19, with an incidence estimated at 30% for mild cases, 70% for moderate-to-severe cases, and around 12% for the vaccinated. Most long COVID-19 cases occur between the ages of 36 and 50 years, and the majority experience a mild and nonhospitalized acute infection [5–9].
Individuals with long COVID-19 could develop new onset or worsening of previous underlying multisystemic disorders, ranging from myocardial inflammation, postural orthostatic tachycardia syndrome, abnormal gas exchange, diabetes, pancreatic injury, gut microbiome disturbance, dysautonomia, myalgic encephalitis/chronic fatigue syndrome (ME/CFS), kidney injury, coagulopathy, endothelial dysfunction, stroke, and even infertility, to name a few (Table 1) [5, 6, 8]. In addition, there is also an increased risk of pulmonary embolism, cardiac arrest, heart failure, death, stroke, and new-onset diabetes [5, 6, 8, 10].
Table 1.
| Organ/organ system involvement | Signs and symptoms | Possible pathogenic mechanism |
|---|---|---|
| Central nervous system | Cognitive impairment, fatigue, sleep disorders, memory loss, tinnitus | Dysautonomia, ME/CFS, neuroinflammation, reduced cerebral blood flow, small fiber neuropathy |
| Cardiovascular system | Chest pain, palpitations, fatigue | Cardiac impairment, myocardial inflammation, POTS, coagulopathy, deep vein thrombosis, microangiopathy, endothelial dysfunction, micro clots, pulmonary embolism, stroke |
| Respiratory system | Cough, dyspnea | Abnormal gas exchange |
| Endocrine system | Fatigue, new-onset diabetes | Thyroid injury, pancreas injury, adrenal injury |
| Gastrointestinal tract | Abdominal pain, bloating, nausea, bowel movement disorder, new-onset IBD, increased liver enzymes | Gut microbiome disturbances, viral persistence and reservoir, gut inflammation, liver injury |
| Urogenital tract | Decreased renal function, recurrent urinary tract infection, erectile dysfunction, infertility, irregular menstruation, premenstrual syndrome severity | Renal and urinary tract injury, gonadal injury, reduced sperm count |
| Immune system | Recurrent infection, new-onset autoimmunity, urticaria, allergic rhinitis, and asthma | Immune system dysregulation, persistent viral inflammation, MCAS |
IBD, inflammatory bowel disease; MCAS, mast cell activation syndrome; ME/CFS, myalgic encephalitis/chronic fatigue syndrome; POTS, postural orthostatic tachycardia syndrome.
Several risk factors have been identified for developing long COVID-19, such as female sex, type 2 diabetes, previous EBV infection, the occurrence of autoantibodies, previous autoimmune and connective tissue disorders, hyperactivity, and allergic disorders [7, 8]. Because of the overlapping risk factors with other postinfectious syndromes, such as post-EBV ME/CFS, it was postulated that long COVID-19 could share the same pathophysiology, diagnostic strategy, and even therapeutic approaches with these conditions [2, 4, 10, 11].
2. Immunological aspects of long COVID-19
It has been suggested that the occurrence of long COVID-19 is the consequence of multiple pathophysiological mechanisms after the initial acute phase infection. First, systemic inflammatory response syndrome causes tissue injury and organ dysfunction. The resulting immune system hyper-response due to the systemic nature of COVID-19-released numerous pro-inflammatory cytokines; in some individuals, it will result in cytokine storm and severe COVID-19, but in most anti-inflammatory cytokine response will result in immune homeostasis [12–14].
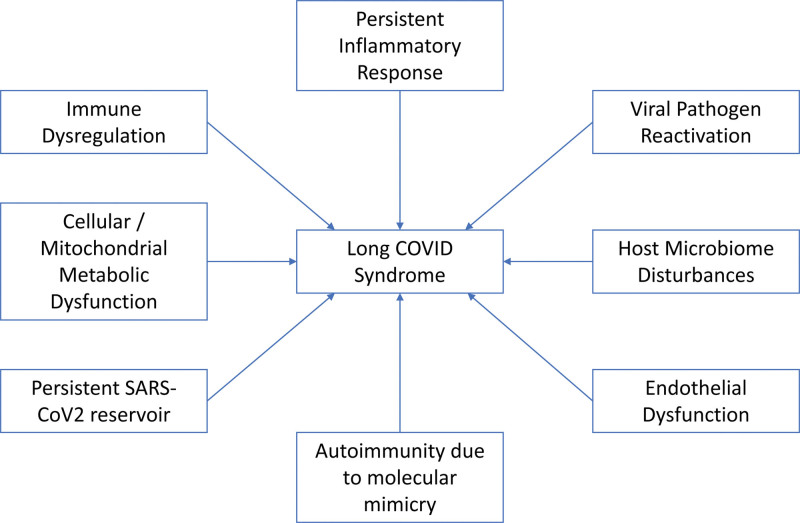
Prolonged immunosuppression in individuals with long COVID-19 will result in a constellation of immune system dysfunction, such as depleted T cells, highly activated innate immune cells, lack of naive T and B cells, and elevated inflammatory cytokines such as type I and type III interferons. The resulting immune dysfunction, in tandem with persistent severe acute respiratory syndrome-coronavirus 2 (SARS-COV2) reservoir, caused an ongoing inflammatory reaction, pathogen reactivation, endothelial damage, host-microbiome dysfunction, autoimmunity due to molecular mimicry, and cellular/mitochondrial metabolism dysfunction (Fig. 1) [3, 4, 15, 16].
Figure 1.
3. Mast cell activation syndrome in long COVID-19
Mast cell, an immune cell derived from the hematopoietic stem cell, is involved in the immediate allergic response. The activation of these cells could be induced by several mechanisms, such as mast cell clonal disorders, allergic disease, neoplastic conditions, physical and autoimmune urticaria, and idiopathically such as in mast cell activation syndrome (MCAS). MCAS is characterized by mast cell hyperactivation and excessive chemokine release (histamine, tryptase, carboxypeptidase A, and chymase). The clinical manifestation of MCAS could involve the cardiopulmonary, gastrointestinal, dermatological, and neurological systems (Table 2) [17, 19].
Table 2.
| Organ system | Clinical symptoms |
|---|---|
| Systemic | Anaphylaxis, syncope, fatigue |
| Dermatologic | Flushing, skin rash, pruritus, urticaria |
| Cardiovascular | Hypotension, shock, chest pain, tachycardia |
| Respiratory | Wheezing |
| Musculoskeletal | Arthralgia, myalgia, degenerative disc disease, osteoporosis/osteopenia |
| Gastrointestinal | Nausea, vomitus, abdominal pain, gastroesophageal reflux, diarrhea, esophagitis, abdominal cramp, bloating, malabsorption |
| Neurological | Cognitive impairment, brain fog, dizziness, vertigo, migraine, paresthesia, peripheral neuropathy |
In the long COVID-19, a persistent inflammatory state will activate specific mast cell genes that will cause an abnormal mast cell activation control. Activation of toll-like receptors in SARS-CoV2 infection causes the formation of autoantibodies that could interact with mast cell immunoglobulin receptor and then activates them. Mast cells are one of the main producers of inflammatory cytokines of COVID-19 due to the abundance of angiotensin converting enzyme-2 receptors. The location of mast cells in the pulmonary perivascular space, where their maturation happens, also puts these cells in the front line of immune hyper-responsive condition caused by COVID-19. In addition, mast cell stimulation will release many pro-inflammatory cytokines, such as platelet-activating factor, histamine, heparin, tryptase, prostaglandins, leukotriene, and chemokines (IL-1β and IL-6). Furthermore, SARS-CoV2 infection also increased substance P secretion by immune cells and the activation of the G-protein X2 receptor by protein cross-linking with PSD-95/Dlg/ZO-1. All of these things will increase mast cell activation and the occurrence of MCAS in patients with long COVID-19 [2, 4, 16, 20].
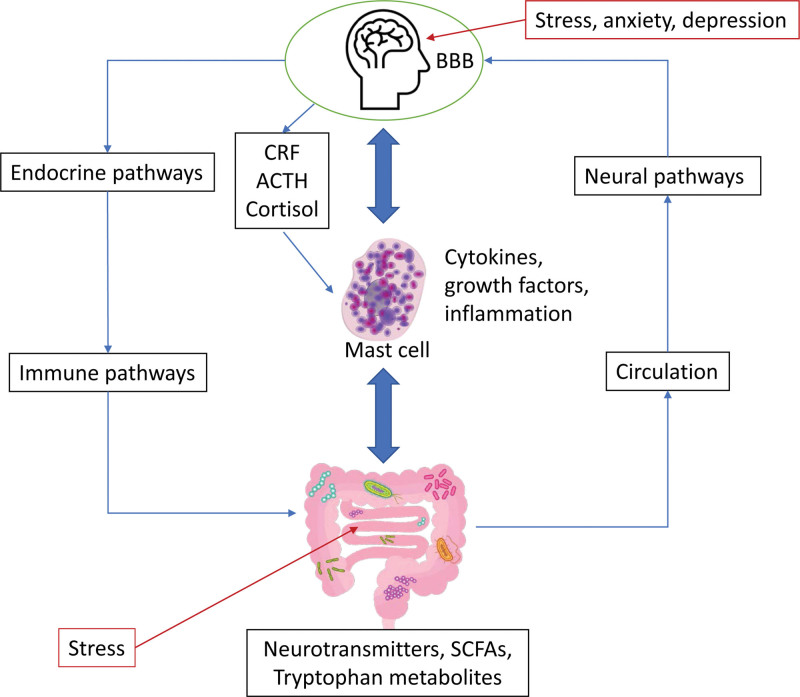
An observational study conducted by Weinstock et al. [21] shows that patients with long COVID-19 have virtually identical mast cell activation symptoms and severity as previously diagnosed MCAS patients without long COVID-19. Notable similar symptoms in long COVID-19 compared with MCAS patients are physical weakness, brain fog, tachycardia, insomnia, shortness of breath, migraines, paresthesia, arthralgia, dizziness, eye complaints, nasal complaints, tinnitus, dry mouth, constipation, easy bruising, flushing, vertigo, wheezing, bone pain, weight loss, rashes, abdominal pain, and skin lesions [21]. There are several explanations for these overlapping symptoms aside from what has been mentioned above, such as blood-brain barrier disturbances and neuroinflammation through microglia activation. These observations showed that MCAS could cause deleterious central and peripheral effects in patients with acute COVID-19 and may contribute to the progression to long COVID-19 (Fig. 2) [3, 6, 22].
Figure 2.
Stress induced by SARS-CoV2 infection will increase the release of corticotropin-releasing factor, adrenocorticotropic hormone, and cortisol. Stress conditions in the gut will also induce alteration in the gut microbiota, neurotransmitter and short-chain fatty acid release, and tryptophan metabolism. These pathophysiological mechanisms will induce the activation and degranulation of mast cells, thus releasing inflammatory cytokines. Adapted from Batiha et al. [15].
4. Diagnosis and treatment options for MCAS in long COVID-19
There are two diagnostic criteria to diagnose mast cell disorders; the previous one developed by WHO is useful to diagnose clonal disorders, but not MCAS, due to the necessity for demonstrating increased mast cell numbers, whether in tissue or bone marrow biopsy. However, in MCAS, there is no increase in mast cell numbers or infiltration, only activation and the release of inflammatory cytokines, so newer diagnostic criteria proposed by Akin et al. [17]. will be more appropriate. In the new diagnostic criteria, MCAS could be diagnosed if the following criteria are met:
-
(1)
Episodic symptoms consistent with mast cell mediator release affecting two or more organ systems are evidenced as follows:
-
(a)
Skin: urticaria, angioedema, flushing
-
(b)
Gastrointestinal: nausea, vomiting, diarrhea, abdominal cramping
-
(c)
Cardiovascular: hypotensive syncope or near syncope, tachycardia
-
(d)
Respiratory: wheezing
-
(e)
Naso-ocular: conjunctival injection, pruritus, nasal stuffiness.
-
(a)
-
(2)
A decrease in the frequency or severity; or the resolution of symptoms with antimediator therapy: H1 and H2 histamine receptor antagonists, anti-leukotriene medications (cysLT receptor blockers or 5-LO inhibitor), or mast cell stabilizers (cromolyn sodium).
-
(3)
Evidence of an elevation in a validated urinary or serum marker of mast cell activation: documentation of elevation of the marker above the patient’s baseline during a symptomatic period on at least 2 occasions; or if baseline tryptase levels are persistent >15 ng, documentation of elevation of the tryptase above baseline on one occasion. Total serum tryptase is recommended as the marker of choice; less specific (also from basophils) 24-hour urine histamine metabolites or 11-beta-prostaglandin F2.
-
(4)
Primary (clonal) and secondary mast cell activation disorders were ruled out.
After ruling out conditions that could mimic MCAS, such as endocrine, cardiovascular, psychologic, pharmacological, and neurologic causes, the first thing to do is to avoid known and specific triggers. Commonly inducing triggers include heat, changes in temperature, pressure, cold, rubbing, exercise, emotional swing, stress, sleep deprivation, and some medications such as opiates, Non Steroidal Anti Inflammatory Drugs, muscle relaxants, antibiotics such as quinolones, and histamine-releasing foods [18, 19].
Usually, MCAS is treated by antihistamines (H1 and H2 blockers), inhibition of synthesis of mediators (zileuton and aspirin), inhibition of mediator release (sodium-cromoglycate), and inhibition of degranulation of mast cells by anti-IgE. Antihistamine, especially H1 receptor blockers, are the cornerstone of the treatment, with a combination of nonsedating (cetirizine, fexofenadine, loratadine, desloratadine, and levocetirizine) and sedating (diphenhydramine, hydroxyzine, cyproheptadine, doxepin, and ketotifen) at doses that could be titrated as indicated up to four times the recommended dose. In addition, a combination with H2 antihistamines (ranitidine, cimetidine, and famotidine) could provide a synergistic histamine receptor blockade and relieve gastrointestinal symptoms [18, 19, 23].
Inhibition of mediator release by sodium-cromoglycate and, to a lesser extent, ketotifen has been shown to improve skin, gastrointestinal and neuropsychiatric symptoms. As an inhibitor of mediator synthesis, aspirin, montelukast, and zileuton could also provide a synergistic effect. Furthermore, there is no reason to avoid aspirin or other Non Steroidal Anti Inflammatory Drugs once the diagnosis of MCAS has been made if the patient has tolerated these medications previously [18, 19, 24].
The combination of histamine blockade, stabilization of mast cells, and mediator inhibition could provide additional benefits in MCAS and long COVID-19 patients beyond mast cell-mediated symptoms. In addition, it has been shown that histamine-dependent mechanisms also mediate T-cell disorders in long COVID-19, so these treatment modalities could also be used to manage hyperinflammation states and provide therapeutic benefits [20, 21].
Long-term prognosis of MCAS in long COVID-19 patients is unknown, as there are no longitudinal observation studies finished yet. Prognosis is largely related to the severity of preceding infections and underlying comorbid. MCAS also increases the risk of anaphylaxis, which could prove to be a risk for patient survival.
5. Conclusion
Long COVID-19 may progress in association with the development of MCAS. The emergence of MCAS during the course of SARS-CoV2 infection is linked to the severity of the infection and the occurrence of MCAS. Application of antihistamines, inhibition of synthesis/release of mediators, and suppression of mast cell activation could help control MCAS-associated long COVID-19 symptoms. Early recognition and treatment of MCAS in long COVID-19 may reduce systemic complications and increase patients’ quality of life.
Acknowledgements
Assistance with the study: none.
Financial support and sponsorship: none.
Conflicts of interest
The authors have no financial conflicts of interest.
Author contributions
Stevent Sumantri and Iris Rengganis contributed to the article equally, including the initial concept, drafting, revision, and approval for the final version.
Footnotes
Published online 30 March 2023
References
- 1.COVID-19 Data Explorer - Our World in Data. [Google Scholar]
- 2.Umesh A, Pranay K, Pandey RC, Gupta MK. Evidence mapping and review of long-COVID and its underlying pathophysiological mechanism. Infection 2022;50:1053–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castanares-Zapatero D, Chalon P, Kohn L, Dauvrin M, Detollenaere J, Maertens de Noordhout C, Primus-de Jong C, Cleemput I, Van den Heede K. Pathophysiology and mechanism of long COVID: a comprehensive review. Ann Medicine 2022;54:1473–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol 2023;21:133–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonsaksen T, Leung J, Price D, Ruffolo M, Lamph G, Kabelenga I, Thygesen H, Geirdal AØ. Self-reported long COVID in the general population: sociodemographic and health correlates in a cross-national sample. Life 2022;12:901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mizrahi B, Sudry T, Flaks-Manov N, Yehezkelli Y, Kalkstein N, Akiva P, Ekka-Zohar A, Ben David SS, Lerner U, Bivas-Benita M, Greenfeld S. Long covid outcomes at one year after mild SARS-CoV-2 infection: nationwide cohort study. BMJ 2023;380:e072529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perlis RH, Santillana M, Ognyanova K, Safarpour A, Trujillo KL, Simonson MD, Green J, Quintana A, Druckman J, Baum MA, Lazer D. Prevalence and correlates of long COVID symptoms among US adults. JAMA Netw Open 2022;5:e2238804E2238804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Mahoney LL, Routen A, Gillies C, Ekezie W, Welford A, Zhang A, Karamchandani U, Simms-Williams N, Cassambai S, Ardavani A, Wilkinson T J, Hawthorne G, Curtis F, Kingsnorth AP, Almaqhawi A, Ward T, Ayoubkhani D, Banerjee A, Calvert M, Shafran R, Stephenson T, Sterne J, Ward H, Evans RA, Zaccardi F, Wright S, Khunti K. The prevalence and long-term health effects of long Covid among hospitalised and non-hospitalised populations: a systematic review and meta-analysis. EClinicalMedicine 2023;55:101762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fainardi V, Meoli A, Chiopris G, Motta M, Skenderaj K, Grandinetti R, Bergomi A, Antodaro F, Zona S, Esposito S. Long COVID in children and adolescents. Life 2022;12:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehandru S, Merad M. Pathological sequelae of long-haul COVID. Nat Immunol 2022;23:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mumtaz A, Sheikh AAE, Khan AM, Khalid SN, Khan J, Nasrullah A, Sagheer S, Sheikh AB. COVID-19 vaccine and long COVID: a scoping review. Life 2022;12:1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Q, Wang Y, Sun Q, Knopf J, Herrmann M, Lin L, Jiang J, Shao C, Li P, He X, Hua F, Niu Z, Ma C, Zhu Y, Ippolito G, Piacentini M, Estaquier J, Melino S, Weiss FD, Andreano E, Latz E, Schultze JL, Rappuoli R, Mantovani A, Mak TW, Melino G, Shi Y. Immune response in COVID-19: what is next? Cell Death Differ 2022;29:1107–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nile SH, Nile A, Qiu J, Li L, Jia X, Kai G. COVID-19: pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev 2020;53:66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal 2020;10:102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Batiha GES, Al-kuraishy HM, Al-Gareeb AI, Welson NN. Pathophysiology of post-COVID syndromes: a new perspective. Virol J 2022;19:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monje M, Iwasaki A. The neurobiology of long COVID. Neuron 2022;110:3484–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akin C, Valent P, Metcalfe DD. Mast cell activation syndrome: proposed diagnostic criteria. J Allergy Clin Immunol 2010;126:1099–1104.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akin C. Mast cell activation syndromes. J Allergy Clin Immunol 2017;140:349–355. [DOI] [PubMed] [Google Scholar]
- 19.Castells M, Butterfield J. Mast cell activation syndrome and mastocytosis: initial treatment options and long-term management. J Allergy Clin Immunol Pract 2019;7:1097–1106. [DOI] [PubMed] [Google Scholar]
- 20.Szukiewicz D, Wojdasiewicz P, Watroba M, Szewczyk G. mast cell activation syndrome in COVID-19 and female reproductive function: theoretical background vs. accumulating clinical evidence. J Immunol Res 2022;2022:9534163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weinstock LB, Brook JB, Walters AS, Goris A, Afrin LB., Molderings GJ. Mast cell activation symptoms are prevalent in long-COVID. Int J Infect Dis 2021;112:217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ortona E, Malorni W. Long COVID: to investigate immunological mechanisms and sex/gender related aspects as fundamental steps for a tailored therapy. Eur Respir J 2022;59:2102245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamilton MJ. Nonclonal mast cell activation syndrome: a growing body of evidence. Immunol Allergy Clin North Am 2018;38:469–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jennings SV, Finnerty CC, Hobart JS, Martín-Martínez M, Sinclair KA, Slee VM, Agopian J, Akin C, Álvarez-Twose I, Bonadonna P, Bowman AS, Brockow K, Bumbea H, de Haro C, Fok JS, Hartmann K, Hegmann N, Hermine O, Kalisiak M, Katelaris CH, Kurz J, Marcis P, Mayne D, Mendoza D, Moussy A, Mudretzkyj G, Vaia NN, Niedoszytko M, Elberink HO, Orfao A, Radia DH, Rosenmeier S, Ribada E, Schinhofen W, Schwaab J, Siebenhaar F, Triggiani M, Tripodo G, Velazquez R, Wielink Y, Wimazal F, Yigit T, Zubrinich C, Valent P. Mast cell diseases in practice and research: issues and perspectives raised by patients and their recommendations to the scientific community and beyond. J Allergy Clin Immunol Pract 2022;10:2039–2051. [DOI] [PubMed] [Google Scholar]