Abstract
Although levothyroxine is one of the most prescribed medications in the world, its bioavailability has been reported to be impaired by many factors, including interfering drugs or foods and concomitant diseases, and persistent hypothyroidism with a high dose of levothyroxine is thus elicited. Persistent hypothyroidism can also be induced by noninterchangeability between formulations and poor compliance. To address these issues some strategies have been developed. Novel formulations (liquid solutions and soft gel capsules) have been designed to eliminate malabsorption. Some other delivery routes (injections, suppositories, sprays, and sublingual and transdermal administrations) are aimed at circumventing different difficulties in dosing, such as thyroid emergencies and dysphagia. Moreover, nanomaterials have been used to develop delivery systems for the sustained release of levothyroxine to improve patient compliance and reduce costs. Some delivery systems encapsulating nanoparticles show promising release profiles. In this review, we first summarize the medical conditions that interfere with the bioavailability of oral levothyroxine and discuss the underlying mechanisms and treatments. The efficacy of liquid solutions and soft gel capsules are systematically evaluated. We further summarize the novel delivery routes for levothyroxine and their possible applications. Nanomaterials in the levothyroxine field are then discussed and compared based on their load and release profile. We hope the article provides novel insights into the drug delivery of levothyroxine.
Keywords: LT4, malabsorption, liquid solution, soft gel capsule, injection, nanoparticle
Graphical Abstract
Essential Points.
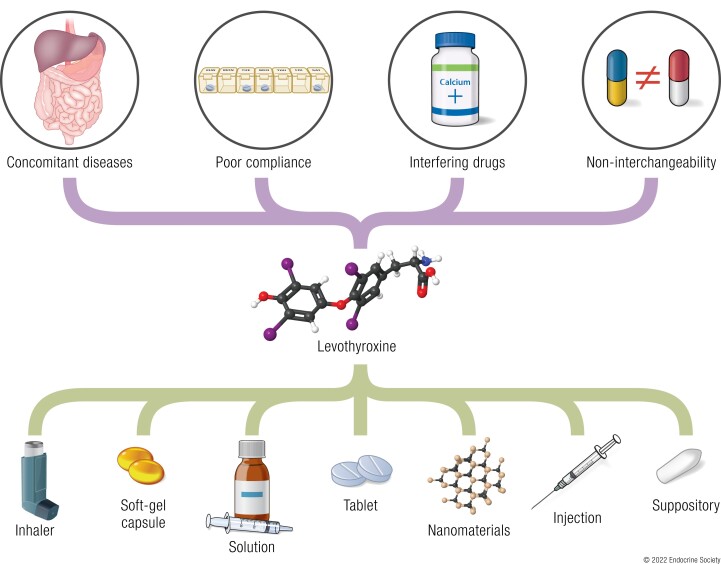
Levothyroxine (LT4) is the mainstay therapy for hypothyroidism, but its bioavailability can be hampered by many conditions, such as concomitant diseases, interfering medications and foods, switch of brands, and noncompliance
A comprehensive medical history taking with necessary examinations is required to find the cause of persistent hypothyroidism
Discontinuation of interferants, administration separation, addressing the concomitant diseases, and close monitoring are generally recommended to eliminate persistent hypothyroidism
Liquid solutions and soft gel capsules are recommended to be used in those with hampered LT4 absorption and who do not allow sufficient time before or after meals for LT4 replacement; liquid solutions can also be used in those with difficulty in swallowing
Intramuscular and subcutaneous injection can serve as sustained-release systems for levothyroxine, especially for noncompliant patients
Subcutaneous implants with nanomaterials can serve as sustained-release platforms for LT4 and thus improve patient compliance
Novel drug delivery systems with larger loads, stable release profile and safety are expected to be developed in the future
The prevalence of overt and subclinical hypothyroidism is estimated to be 3% to 7% in the general population. Hypothyroidism is mainly induced by autoimmune thyroid diseases and thyroidectomy (1, 2). In clinical practice, the diagnosis of hypothyroidism is based on laboratory tests of serum thyrotropin (TSH), thyroxine (T4), and triiodothyronine (T3) levels. Since the 1980s, along with the discovery of T4 deiodination to T3 (3), synthesized levothyroxine (LT4) has gradually replaced desiccated thyroid extracts (DTEs) and has become the mainstay therapy for hypothyroidism (3). LT4 is among the most frequently prescribed medications in the United States (4) and its indications include hypothyroidism and myxedema. It is also used off-label to treat other diseases, such as female infertility with elevated thyroid antibodies, obesity, and depression (5, 6) (Table 1).
Table 1.
The approved indications and off-label uses of levothyroxine
| Overt or subclinical hypothyroidism |
| ȃCongenital |
| ȃIodine deficiency |
| ȃThyroiditis: Hashimoto, subacute granulomatous, postpartum. |
| ȃSecondary: pituitary tumor and treatments used for this pathology (surgery, radiotherapy), Sheehan syndrome, empty sella syndrome, etc. |
| ȃIatrogenic: thyroidectomy, radioiodine therapy, antithyroid drugs, etc. |
| ȃNonantithyroid drug: iodinated contrast mediaa, lithium, tyrosine kinase inhibitors, interferons |
| Myxedema coma |
| Off-label uses |
| ȃSimple goiter |
| ȃFemale infertility with elevated thyroid antibodies |
| ȃCardiovascular diseases: chronic congestive heart failure, myocardial ischemia, coronary artery bypass |
| ȃNeurological disorders: Alzheimer's disease, multiple sclerosis, chorea |
| ȃOrgan transplantation |
| ȃProlonged critical illness |
| ȃHypercholesterolemia |
| ȃObesity |
| ȃUnexplained fatigue |
| ȃDepression |
| ȃCosmetic use (creams)b |
Examples include iohexol, iopamidol, iopromide, ioversol, iobitridol, iomeprol, iodixanol, etc. Oil-soluble media increase the risk of contrast media-induced hypothyroidism compared with water-soluble ones.
Levothyroxine is added into some creams to accelerate the metabolism of epithelium and to reduce subcutaneous adipose tissues.
The daily dose of LT4 is determined by the lean body mass, while age, the etiology of hypothyroidism, comorbidities, and other factors also exert effects (7). In general, patients are initially prescribed a dose of 1.6 to 1.8 µg/kg body weight/day and are tested for thyroid function every 6 to 8 weeks. Once thyroid hormones (THs) remain within the reference ranges, an annual examination is recommended to monitor thyroid function. A persistent TSH elevation despite the administration of a daily dose of >1.9 µg/kg/day is deemed refractory hypothyroidism, which entails further examinations for potential causes. The leading cause of refractory hypothyroidism is interfering medications, since more than 50% of LT4-treated patients are concurrently taking >1 drug for other medical conditions (8). Some concomitant diseases, such as atrophic gastritis, lactose intolerance, and celiac disease might also impair the bioavailability of oral LT4. A switch of brand or generic LT4 and improper storage have also been reported to cause TSH fluctuation in patients, which may be attributed to different pharmacokinetic profiles due to excipients. Poor patient adherence to medication is another common cause of refractory hypothyroidism. Patients may sometimes miss a dose or ingest LT4 with breakfast for convenience, which is observed in 30% to 70% of patients (9, 10).
Tablet is the predominant LT4 formulation. The majority of patients can achieve biological euthyroidism with oral LT4 supplements. In the past 15 years, novel formulations (liquid solutions and soft gel capsules) have been introduced to the market and have shown superior efficacy. These preparations potentially circumvent malabsorption induced by interfering medications and concomitant diseases and improve patient adherence. In addition, alternative routes of LT4 administration have become a new research hotspot. Weekly intramuscular (IM) and subcutaneous (SC) injection represent new methods for sustained drug delivery. LT4 can be rectally administered to patients who have difficulty swallowing. The skin, respiratory tract, and oral cavity can also be alternative routes for drug delivery. Moreover, nanomaterials have been used to develop sustained-release drug delivery systems for LT4. Nanomaterials are supposed to eliminate the inconvenience of daily administration and to improve patient compliance. In summary, the 2 main aims of novel drug delivery systems are to circumvent the impaired bioavailability due to interfering medications and diseases and to develop sustained-release formulations.
This article aims to provide a comprehensive review of conventional and novel drug delivery routes and systems. In addition, LT4 absorption and metabolism and the medical conditions that impair the bioavailability of LT4 are also reviewed.
Levothyroxine Absorption and Metabolism In Vivo
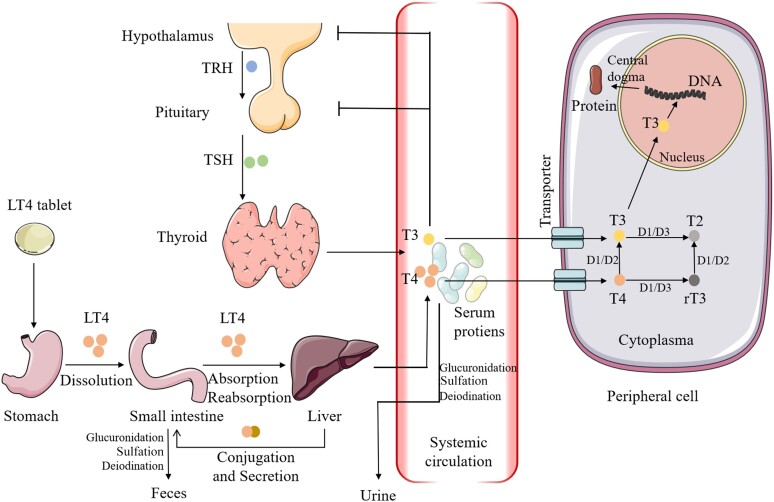
The intraluminal digestion of LT4 tablets comprises disintegration and dissolution in the stomach and absorption mainly in the small intestine (Fig. 1). A physiological gastric pH (1.0-3.0) is essential for tablet dissolution, which removes the sodium ion and converts LT4 into a lipophilic molecule. Several studies have demonstrated that an elevated pH due to Heliobacter pylori infection, autoimmune gastritis, and proton pump inhibitors (PPIs) antagonize the subsequent absorption (11–13). This phenomenon is explained by the ionization status at different environmental pH values (14). As revealed in in vitro studies by Kocic et al and Pabla et al, the dissolution–pH profile is V-shaped (15, 16). The solubility of LT4 tablets at 30 minutes is approximately 100% at pH 1.2 and 2.4. Its solubility decreases substantially to less than 60% at pH 4.0, regardless of the brand. The nadir is reached at pH 5.0, where the percentage of dissolution at 60 minutes decreases to less than 40%. As the environmental pH becomes more alkaline, the dissolution rate gradually increases to 80% to 85% at pH 8.0. More recently, direct in vivo proof for the role of gastric juice pH on LT4 absorption was provided by Virili et al in a retrospective study (17). The LT4 requirement increased with the increasing gastric pH (P = .0007). Notably, generic and brand name tablets exhibit different dissolution–pH profiles, suggesting that excipients and manufacturing techniques influence tablet dissolution (14). Pulverized LT4 tablets were better absorbed than the entire tablet (18). Novel formulations, such as liquid solutions and soft gel capsules, do not require dissolution, and are thus more efficient for hypothyroidism therapy (16).
Figure 1.
The absorption, transportation, metabolism and action of levothyroxine in vivo. Abbreviations: D, deiodinase; LT4, levothyroxine; rT3, reverse triiodothyronine; T1, monoiodotyrosine; T2, diiodotyrosine; T3, triiodothyronine; T4, thyroxine; TRH, thyrotropin-releasing hormone; TSH, thyrotropin. The figure was partly generated using illustrative elements from Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
As no uptake of LT4 was detected in a patient with no jejunum or ileum (19), the small intestine is proposed to be the predominant site for LT4 absorption. Since 1968, the double-radioisotope–labeled T4 method developed by Hays has enabled more precise estimation of exogenous T4 absorption (20). An experiment in 4 healthy volunteers showed overall absorption ranging from 60% to 80%. Specifically, 15 ± 5% was absorbed in the duodenum, 29 ± 14% in the jejunum, and 24 ± 11% in the ileum. After ingestion in the fasting state, absorption rapidly increases and reaches a maximum at 1.5 to 2 hours (19). Coingestion with breakfast or coffee can reduce and delay absorption (21, 22). An intact and normal intestinal mucosal epithelium is required for the absorption of drugs. In patients with celiac disease, intestinal parasitosis, or other intestinal dysfunctions, TSH concentrations remained elevated, even after treatment with increased doses (19, 23–26). In the small intestine, a large portion of LT4 is bound to intraluminal proteins. The mechanism of LT4 transport across the intestinal mucosa is still debated. Since the LT4 molecule is lipophilic, researchers first postulated that passive diffusion was responsible for LT4 transport. However, recent studies have demonstrated that LT4 molecules are also transported by transmembrane proteins, including the organic anion transporting polypeptide family, Na-taurocholate cotransporting polypeptide, L-type amino acid transporter 1 and 2, and monocarboxylate transporter 8 and 10 (27). The expression of these transporters is not limited to enterocytes, as their expression is also detected in peripheral organs. These transporters are responsible for both the influx and the efflux of T4. In addition, the paracellular route may contribute to LT4 absorption (28).
The enterohepatic circulation plays a role in the absorption and metabolism of LT4. After absorption, most LT4 molecules are transported via the mesenteric and portal veins to the liver, where 17% of absorbed LT4 is estimated to be taken up (29). In the liver, some circulating LT4 molecules undergo conjugation, which enhances their water solubility. The main forms of conjugation of T4 produced in the liver are glucuronidation and sulfation. The conjugated T4 is then secreted with bile into the gut. Researchers have also postulated that conjugated T4 could be secreted directly across the bowel wall from the mesenteric circulation (30). The secreted conjugated T4 is then broken down by the intestinal flora and partially reabsorbed (20, 31). Conditions that interfere with bile secretion, such as obstructive disease and cirrhosis, may impair the reabsorption of T4 (32).
Upon entry into the systemic circulation, ∼99.8% of T4 and its biologically active metabolite, T3, are bound to serum carriers, such as thyroxine-binding globulin (TBG), albumin, and transthyretin (33). TBG binds more than 80% of THs. Plasma carriers facilitate the circulation of drugs, stabilize the molecules, and prevent their renal clearance. Conditions increasing the serum TBG concentration (eg, elevated estrogen levels) or enhancing the renal clearance of serum proteins (eg, nephrotic syndrome) can reduce the bioavailability of LT4 (34–36).
Endogenous and exogenous T4 cannot be distinguished by the human body and are handled equally. Thyroid homeostasis is regulated by classic hypothalamic–pituitary–thyroid negative feedback. TSH released by the pituitary gland is a highly sensitive indicator for monitoring thyroid function. A 20% decrease in T4 can increase TSH levels by as much as 2-fold, particularly in patients with no thyroid remnant, as partially functioning thyroids can serve as a buffer (37). The 2 major metabolic routes of LT4 are deiodination and conjugation. The deiodination of T4 produces both T3 and reverse T3 (rT3). The difference between the 2 products is the site of deiodination (the removal of iodine from carbon 5 of the outer ring of T4 by deiodinase type 1 or 2, or the inner ring by deiodinase type 3) (38). T3 is the biologically active form and is approximately 5 times more potent than T4. rT3 has little effect on the human body compared with T3. The common downstream catabolites are diiodotyrosine and monoiodotyrosine. Of note, in the physiological state, the normal ratio of T4:T3 released by the thyroid is approximately 14:1 (39). Four-fifths of circulating T3 are derived from the mono-deiodination of T4, while the remaining 20% is released directly from the thyroid. The mean half-life (T1/2) is 7.5 days for T4 and 1.4 days for T3 (40, 41). THs play important roles in skeletal growth, neurological development, and general health (42). Long-term hypothyroidism increases the risk of neurological deficits and myxedema, whereas long-term hyperthyroidism may lead to cardiovascular diseases and osteoporosis.
Impaired Bioavailability and Malabsorption of Oral LT4 Tablets
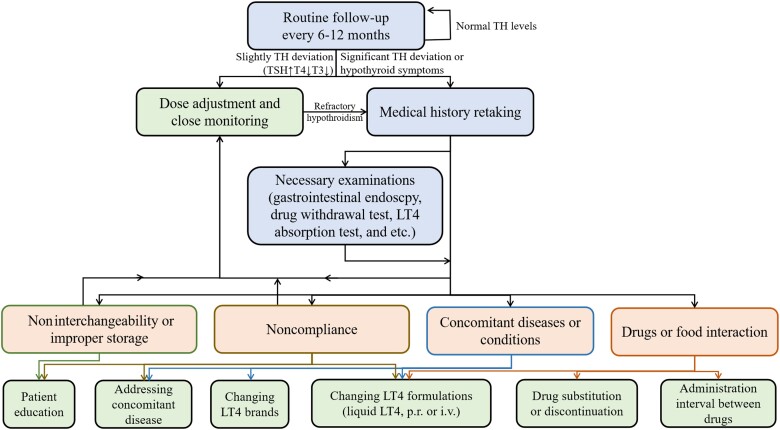
The tablet form is the predominant preparation of LT4, accounting for more than 95% of LT4 prescriptions (43). Generally, a dose of LT4 of 1.6 to 1.8 µg/kg body weight is sufficient for the normalization of thyroid function (44). Unfortunately, more than 30% to 50% of LT4-treated patients are estimated to be either undertreated or overtreated (45–47). Impaired bioavailability of LT4 is a common cause of off-target serum TH levels and has been reported in patients with many medical conditions, including concurrent diseases, coingestion with drugs or food, a switch in the LT4 preparation, improper storage, or poor compliance. Patients will present with elevated TSH levels, and reduced T3 and T4 levels and thus require a dose adjustment (>1.9 µg/kg). Identification of the underlying cause is required to eliminate hypothyroid symptoms (Fig. 2).
Figure 2.
Proposed algorithm for patients with elevated TSH and impaired bioavailability for LT4. Abbreviations: LT4, levothyroxine; T3, triiodothyronine; T4, thyroxine; TH, thyroid hormone; TSH, thyrotropin; p.r., per rectum; i.v., intravenous.
Concomitant Diseases and Conditions
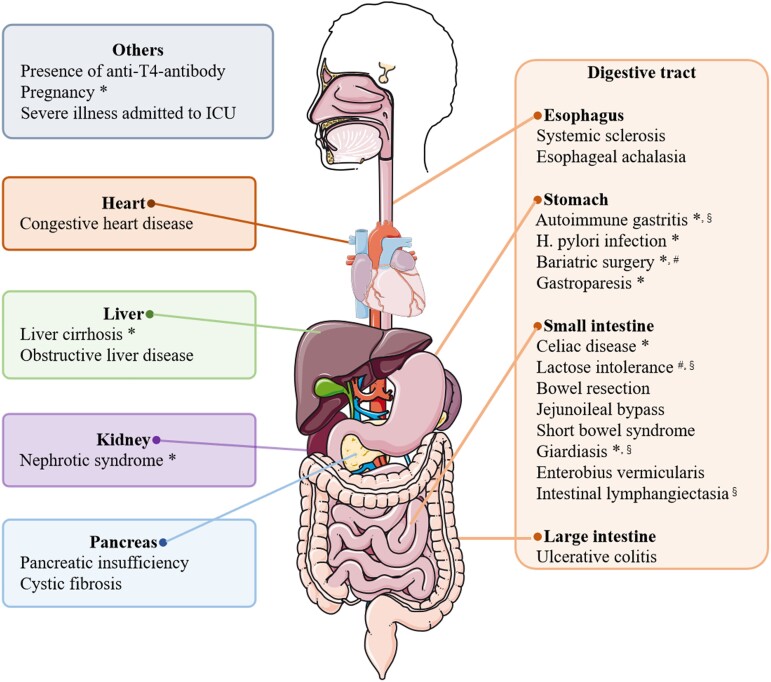
Many concomitant diseases or conditions have been reported to interfere with the efficacy of oral or nonoral LT4 (Fig. 3). The underlying mechanisms of impaired efficacy due to these diseases or medical conditions vary. Some gastric disorders, including H. pylori infection and autoimmune gastritis, reduce drug efficacy via hypochlorhydria. Gastroparesis may delay stomach emptying and enable residual food to adsorb more oral LT4 (48). Similarly, gastric sleeve surgery and other bariatric surgery can cause malabsorption in some LT4-treated patients (49). Recently, systemic sclerosis and esophageal achalasia were found to cause severe hypothyroidism (50, 51). Researchers hypothesized that tablets did not completely reach the stomach and that an inadequate dissolution phase was the main reason for malabsorption.
Figure 3.
Summary of concomitant diseases and conditions which interfere with the bioavailability of LT4. *Impaired bioavailability induced by these conditions has been reported to be relieved by novel formulations (liquid solution and/or soft gel capsule). #Malabsorption induced by these conditions has been reported to be relieved by crushed tablet powder. §Malabsorption induced by these conditions has been reported to be relieved by intravenous or intramuscular injection. The figure was partly generated using illustrative elements from Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
LT4 malabsorption can also be induced by intestinal surgery and diseases. Since ∼70% of oral LT4 is absorbed in the duodenum, jejunum, and ileum, jejunostomy and bowel resection lead to a substantial decrease in drug absorption (52). Some patients with short bowel syndrome were reported to be refractory to oral LT4, and alternative routes, such as rectal administration or SC injection, were recommended (24, 53). Jejunoileal bypass, in which more than 50% of the distal intestine is bypassed, was reported to impair LT4 absorption in 1 patient (54). In addition to surgery, lactose intolerance and celiac disease are among the most frequent intestinal diseases responsible for malabsorption. They may both impair intestinal absorption of drugs and nutrients via hydrolysis and complexation (25, 26, 55). Another cause of LT4 malabsorption is intestinal parasitosis. Giardiasis and Enterobius vermicularis can damage intestinal mucosal cells and subsequently decrease LT4 absorption (23, 56). Notably, critically ill patients may exhibit reduced intestinal absorption of LT4 and increased conversion of T4 to rT3, regardless of the causes of the critical illness (57). Some other intestinal dysfunctions, such as intestinal lymphangiectasia and ulcerative colitis, have been revealed to cause impaired LT4 absorption, although the underlying mechanisms remain unclear (58, 59).
Aside from gastrointestinal disorders, LT4 malabsorption is associated with other diseases involving solid organs or other conditions. Cystic fibrosis was reported to impair LT4 absorption in 1 patient (60). This phenomenon might be explained by cystic fibrosis–associated pancreatic insufficiency and steatorrhea. In addition, liver cirrhosis and obstructive liver disease, which can inhibit enterohepatic circulation, were reported to increase the daily doses of LT4 required (32). An estrogen-induced increase in TBG levels may also play a role in malabsorption due to liver dysfunction. Similarly, an increased need for thyroxine has also been observed in pregnant patients (61). In patients with nephrotic syndrome, T4-bound albumin and free T4 (FT4) are excreted into the urine, and TSH levels are elevated despite a dose increase (35, 36). The presence of LT4 antibodies may also be associated with unsatisfactory TSH levels (62). In addition, a rare case of TH malabsorption was reported to be induced by congestive heart failure, which may cause intestinal dysfunction and chronic passive congestion of the liver (63).
The ideal normalization of TH levels in these patients relies on the elimination of or an improvement in concurrent diseases. In hypothyroid patients with parasitosis or H. pylori infection, TSH levels usually return to the target range with a normal dose after antiparasitic or antibiotic therapy (12, 23). A lactose-free formulation or gluten-free diet can rid patients with lactose intolerance or celiac disease of impaired absorption, respectively (14, 64). In addition, a switch from LT4 tablets to liquid preparations can restore thyroid function in patients with gastrointestinal dysfunction (Fig. 3) (32, 49, 50, 60, 65, 66). Surprisingly, liquid solutions also alleviate the malabsorption induced by some nongastrointestinal disorders, such as nephrotic syndrome (35, 36) and pregnancy (61). Similarly, crushed tablet powder might also resolve hypothyroidism due to bariatric surgery and lactose intolerance (49, 67). Unusual administration routes, including intravenous (IV) injection and IM injection, can also decrease unsuppressed TSH levels in patients with digestive tract disorders (55, 58, 68, 69). Furthermore, clinicians should be aware that severe refractory hypothyroidism is not common in patients with concomitant disease or conditions. Many patients with malabsorption can have a serum TSH level in the target range with an increased dose of LT4 (64, 70).
Drug and Food Interference
A number of drugs and foods have been reported to interfere with the absorption and metabolism of LT4 (Table 2). The most frequently prescribed concomitant drugs were statins, PPIs, and calcium and iron supplements (8, 71). The majority of patients with coingestion are asymptomatic and receive constant doses of LT4.
Table 2.
Summary of interfering drugs, proposed mechanisms, and recommendations
| Mechanism | Drugs | Recommendations for clinicians (in case of drug interactions) |
|---|---|---|
| Direct complexing | Calcium carbonatea | (a) Switch to other drugs of the same class |
| Calcium acetate | (b) Address concomitant diseases | |
| Calcium citrate | (c) Separate the times of ingestion (2-8 hours) | |
| Ferrous sulfatea | (d) Switch to liquid LT4 or capsule | |
| Aluminum hydroxidea | (e) Adjust LT4 doses | |
| Magnesium oxidea | (f) Discontinue interfering medications | |
| Sucralfate | (g) Close monitoring | |
| Cholestyramine | ||
| Colesevelam hydrochloride | ||
| Orlistat | ||
| Simethicone | ||
| Sodium polystyrene sulphonate | ||
| Sevelamer hydrochloridea | ||
| Sevelamer carbonate | ||
| Lanthanum carbonate | ||
| Raloxifene | ||
| Colestipol | ||
| Drug-induced alterations in mucosal transport processes | Chromium picolinate | (a) Adjust LT4 doses |
| Ciprofloxacin | (b) Close monitoring | |
| Rifampin | (c) Address concomitant diseases | |
| Grapefruit juice | (d) Discontinue interfering medications | |
| (e) Switch to other drugs of the same class | ||
| Alkalization | Esomeprazole | (a) Switch to other antacids |
| Pantoprazolea | (b) Address concomitant diseases | |
| Omeprazolea | (c) Switch to liquid LT4 or capsule | |
| Lansoprazole | (d) Adjust LT4 doses | |
| Other proton pump inhibitors | (e) Discontinue interfering medications | |
| Cimetidine | (f) Close monitoring | |
| Papaya | ||
| Acidification | Vitamin C | Of no clinical importance in most cases |
| (a) Discontinue vitamin C | ||
| (b) Adjust LT4 doses Vitamin C can be used in those with LT4 malabsorption |
||
| Acceleration of catabolism of LT4 in the liver (not specific) | Lovastatin | (a) Switch to other drug of the same class |
| Simvastatin | (b) Address concomitant diseases | |
| Lopinavir | (c) Adjust LT4 doses | |
| Ritonavir | (d) Discontinue interfering medications | |
| Nelfinavir | (e) Close monitoring | |
| Phenobarbital | ||
| Nicardipine | ||
| Chloroquine | ||
| Proguanil | ||
| Inhibited hepatic T4 uptake and catabolism | Rifampin | Same as the recommendations for lovastatin and simvastatin |
| Indinavir | ||
| Increased inactivation of T4 via deiodinases | Mifepristone | Same as the recommendations for lovastatin and simvastatin |
| Rifampin | ||
| Carbamazepine | ||
| Sertraline | ||
| Fluoxetine | ||
| Capecitabine | ||
| Sorafenib | ||
| Motesanib | ||
| Selpercatinib | ||
| Increased inactivation of T4 via nondeiodinases | Rifampin | Same as the recommendations for lovastatin and simvastatin |
| Phenytoin | ||
| Imatinib | ||
| Sunitinib | ||
| Inhibition of the mono-deiodination of T4 to T3 | Amiodarone | Same as the recommendations for lovastatin and simvastatin |
| Propranolol | ||
| Propylthiouracil | ||
| Dexamethasone | ||
| Flavonoids | ||
| Iodinated contrast | ||
| Competing for hormone binding sites | Nonsteroidal anti-inflammatory drugs | Same as the recommendations for lovastatin and simvastatin |
| Furosemide | ||
| Dicoumarin | ||
| Clofibrate | ||
| Increasing the serum T4-binding globulin concentration | Estrogen | Same as the recommendations for lovastatin and simvastatin |
| Rifampin | ||
| Capecitabine | ||
| Raloxifene | ||
| Tamoxifen | ||
| Mitotane | ||
| Fluorouracil | ||
| Heroin | ||
| Methadone | ||
| Decreasing the serum T4-binding globulin concentration | Androgen | Similar to the recommendations for vitamin C |
| Enhancing the inhibitory modulation of thyroid hormones on central TSH secretion | Metformin | Similar to the recommendations for vitamin C |
Drugs in italics have not been reported to interact with LT4 in human study. These drugs may affect serum thyroid hormone levels in some observational studies without LT4 dosing. Some other studies revealed the drug interaction by in vitro or animal experiments, or just hypothesis. Notably, most food and beverages impair the absorption of LT4 by direct complexing.
Abbreviations: LT4, levothyroxine; TSH, thyrotropin.
Malabsorption induced by drugs with asterisks have been reported to relieved by novel formulations (liquid solution and/or soft gel capsule).
The mechanisms of interactions with drugs or food vary. It is widely acknowledged that direct complexation and chelation play important roles in the malabsorption of LT4 induced by the intake of many drugs, including calcium and iron supplements (72, 73), aluminum-containing antacids (74), bile acid sequestrants (75, 76), and some foods (eg, fiber-rich foods, coffee, and milk) (22, 77, 78). A simple in vitro binding experiment can quantify the binding ability of interfering agents (72, 78). Drugs may cause the malabsorption of LT4 via reduced gastric dissolution due to alkalization. Gastric pH is impaired by PPIs, H2 antagonists, or other antacids (11, 79). A randomized crossover study demonstrated that IV esomeprazole could decrease the maximum serum concentration (Cmax) and the area under the curve (AUC) from 0 to 12 hours by 12.7% and 14.8%, respectively, in healthy volunteers taking LT4 tablets (11). The results for H2 antagonists are conflicting (79), partially due to their more moderate and slower effects on H+ secretion than those of PPIs. In contrast, vitamin C, which promotes the secretion of gastric acid, presents a potential option to ameliorate malabsorption (80). In addition, some drugs (eg, ciprofloxacin, rifampicin, and chromium picolinate) and grapefruit juice alter intestinal mucosal transporters, including the monocarboxylate transporter family and organic anion transporting polypeptides (81–83).
In addition to drugs that alter the absorption of LT4, some drugs affect the pharmacodynamics and pharmacokinetics of LT4 after absorption. The induction of cytochrome P450 enzyme activity in the liver by statins or protease inhibitors can accelerate the catabolism of LT4, and hence decrease serum T4 levels (84, 85). Moreover, increased inactivation of LT4 via the monodeiodinase induced by mifepristone, rifampicin, and carbamazepine can reduce the T1/2 of LT4 (86–88). Similarly, phenytoin and imatinib inactivate LT4 via nondeiodinative hepatic metabolism and decrease the ratio of T3 to T4 (89, 90). TBG and albumin are other targets for drug interactions. Estrogen can increase TBG and albumin concentrations and decrease FT4 levels, whereas androgen has the opposite effects (34, 91). Interestingly, metformin suppresses TSH levels (92, 93). The potential mechanism may be enhancing the inhibitory modulatory effect of THs on central TSH secretion and changing the affinity and/or the number of TH receptors.
In hypothyroid patients who are suspected of having drug interactions, the diagnosis of malabsorption entails a comprehensive medical history taking. Any diet change or drug used before the increase in TSH levels should be thoroughly investigated. Clinicians should also be alert for frequently prescribed agents interfering with LT4, such as calcium and iron supplements, PPIs, statins, and hypoglycemics. Explorative drug withdrawal tests (tests for thyroid function before and after the withdrawal of a suspected interfering drug) are both diagnostic and therapeutic. Biological hypothyroidism is easily corrected a few days after withdrawal of drugs reducing intestinal absorption, while a longer time (weeks to months) is needed for drugs affecting in vivo metabolism (87, 94). After the identification of the interfering drug, a dosing separation of 2 to 8 hours is generally recommended to avoid direct complexation (95). Besides, patients can switch to another drug of the same class. Ranitidine, for instance, had no effect on T3, T4, and TSH levels, whereas cimetidine reduced the AUC of LT4 by more than 20% compared with the placebo (79). In addition, many cases of drug or food interference can be concealed by an increased daily dose of LT4 (96). Furthermore, novel LT4 preparations are designed to reduce the high TSH levels induced by malabsorption due to calcium and iron supplements, PPIs, coffee, and breakfast (97–101). Liquid formulations are spared from gastric dissolution and are mixed more evenly than solid formulations. As a last resort, the discontinuation of the interfering medication is recommended for patients with severe malabsorption or those who would tolerate drug withdrawal (89, 102).
In addition to drug interactions, food might also impair LT4 absorption via direct complexation. Although the rule of “30 to 60 minutes before breakfast on an empty stomach” is widely recommended, it is inconvenient to follow and may cause poor compliance. The administration time remains debatable and clinical studies are emerging. Dosing before dinner slightly reduces the therapeutic efficacy of LT4 (103), since 2 to 4 hours are usually required for the stomach to empty. Dosing at bedtime is a promising option. In 2010, a randomized, double-blind, crossover trial recruiting 90 patients examined the efficacy of taking LT4 at bedtime (104). Reduced TSH levels and elevated total T3 and total T4 (TT4) levels were observed in patients taking LT4 at bedtime, with no significant difference in quality of life or preference. In addition, it is estimated that fasting for at least 3 hours is required for bedtime administration. More recently, a meta-analysis including 10 prospective and randomized controlled studies revealed no significant difference in TSH and FT3 between patients who took LT4 before breakfast and at bedtime (105). Surprisingly, the FT4 level favored bedtime administration (P = .03). Eating habits and time intervals between dinner and bedtime, which may vary in different countries and cultures, affect the absorption of LT4 ingested at bedtime. Clinicians should take these factors into consideration before informing patients about the possibility of taking LT4 at bedtime.
Interchangeability and Stability of LT4 Preparations
A number of studies have evaluated the interchangeability of LT4 among manufacturers, brands, or batches (106–108), and the results are controversial. This review does not intend to cover all papers in this field and compare the details. Since it is almost impossible to conduct a direct comparative analysis of the bioavailability of all products, we cannot abandon the hypothesis that changes in brands or formulations cause TSH elevations, as supported by some reports (109–111). This phenomenon might be explained by different pharmacokinetic properties due to excipients and manufacturing techniques.
The common excipients in LT4 tablets include lactose monohydrate, cornstarch, carboxymethyl starch, gelatin, and citric acid as well as tens of other excipients that serve as binding materials, dyes, preservatives, or flavoring agents. Acid excipients, such as lactose, mannitol, and sorbitol, can induce drug instability in tablets (112, 113), while basic excipients can be added to improve the stability of LT4 tablets (114). Manufacturers usually change formulations to avoid patent expiration. The changed formulations are sometimes related to altered bioavailability, although bioequivalence between old and new formulations has been confirmed with Food and Drug Administration–approved protocol (115). In March 2017, the removal of lactose and the addition of mannitol and citric acid to the new formulation of LT4 from Merck resulted in complaints from 67% of the 1745 patients (116). The plausible explanation may be altered disintegration and dissolution due to the use of different excipients (117).
As reported in US studies, 20% of patients switch to another LT4 generic preparation at least once within the first year of initiating therapy, and a change in the LT4 preparation is the leading cause (56.0%) of the TH level fluctuation observed in LT4-treated patients (118, 119). From a conventional clinical perspective, doctors should instruct patients not to change preparations if the change will not result in obvious benefits. Interestingly, in 2022, a comparative effectiveness study using data from a national database provided conflicting results (120). By comparing 2780 individuals who switched among generic LT4 preparations with 2780 propensity-matched controls, no significant difference was detected in TSH levels. However, the study did not evaluate the effect of generic to brand and brand to generic switching. The dose adjustment before and after the switch was not evaluated. In addition, only 3 preparations containing similar excipients were included in the study and the conclusion cannot be extended beyond those 3 products. Conservatively, if a switch is necessary, close follow-up of serum TH levels and self-reported symptoms should be conducted.
Improper storage leads to reduced potency of LT4. Normally, the bioequivalence remains unchanged over a shelf life of more than 36 months (107). Exposure to sunlight, high temperatures, humidity, and oxygen significantly accelerates the degradation of LT4 tablets (121, 122). Exposure to direct sunlight for 80 minutes results in more than 60% decomposition (121). Blister packaging may eliminate refractory hypothyroidism due to improper storage (123).
Pseudomalabsorption
Poor compliance is another frequent cause of elevated TSH concentrations in LT4-treated patients. Compared with other common agents taken daily to treat chronic medical conditions, such as insulin and aspirin, LT4 tablets are among the least expensive and easy to take drugs with few side effects. However, as Crilly commented, “no matter how sound the science, our patients do not take their medication as prescribed, particularly when that medication is for a chronic disorder” (9). A cross-sectional study in Pakistan assessed patient adherence to LT4 therapy with the Modified Morisky Adherence Scale (10). Approximately 67.8% of the 289 individuals reported not taking medication at least once, intentionally or unintentionally. Even worse, 27.3% of patients had low adherence to LT4, denoting “frequent” drug omissions. Similarly, a database study conducted in the United States and a questionnaire study conducted in Italy showed that the low and medium adherence rates were 54.1% and 64.5%, respectively (124, 125). Adherence was not satisfying even in women with hypothyroidism who were pregnant, 46% of whom took <80% of the prescribed LT4 (126).
The main social factors associated with poor compliance are older age, irregular medical visits, poor knowledge about medications, lack of assistance when taking medication, affordability, a busy schedule, and the discontinuation of medications when symptomatic relief occurs (10). The coadministration of multiple drugs, administration >1/day, and fasting condition administration were revealed to be drug-related factors contributing to poor compliance (127). In addition, ∼50% of noncompliant patients had concomitant diseases, including diabetes mellitus, Addison disease, obesity, heart failure, migraine and mental disorders (bipolar disorder and depression) (125). It is worth mentioning that some patients with mental disorders may lose their insight and refuse to take drugs without informing their medical practitioners (128, 129).
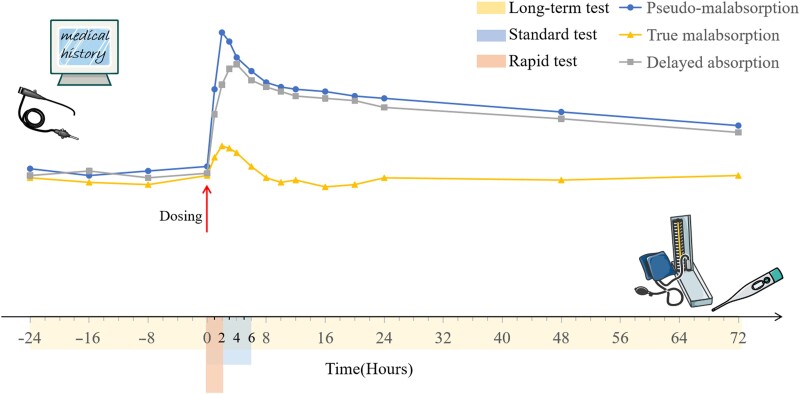
The diagnosis of pseudomalabsorption should exclude other conditions affecting LT4 bioavailability. From a clinical standpoint, most patients with thyroxine pseudomalabsorption deny noncompliance in medical history taking, and they are more likely to admit poor adherence in conversations when clinicians use a casual tone. A completely normal result in the LT4 absorption test in individuals suspected of having malabsorption is strongly suggestive of pseudomalabsorption (130). The LT4 absorption test is defined as the ingestion of a high dose of LT4 in a fasted state followed by serial blood tests for thyroid function. In 2019, the Mayo Clinic published a standardized protocol for the LT4 absorption test, which is described here (Fig. 4) (131). Simsir and colleagues used the protocol to evaluate the absorption ability in 5 patients (132). Two patients had impaired absorption (<60%) and the underlying cause was revealed in subsequent examinations. The remaining 3 individuals were diagnosed with pseudomalabsorption. The increase in FT4 levels compared with baseline also indicates the absorption ability, although the threshold value ranges from 50% to 250% in different studies (132, 133). Since the peak value of T4 is generally observed at 2 hours after dosing, a rapid LT4 absorption test over 2 hours is advocated by Balla et al and Rdzak et al (134, 135).
Figure 4.
The protocol of the LT4 absorption test and the simulated alteration of serum total T4 levels. Patient medical history should be taken and any possible conditions responsible for impaired LT4 bioavailability should be ruled out prior to the test. After fasting overnight, a high dose of LT4 (in tablet form for most patients) is ingested with water by the patient. The standardized dose is determined by the patient's age and body mass index (BMI), namely, 1000 µg for patients aged between 18 and 65 with a BMI <40 kg/m2, 1500 µg for patients aged between 18 and 65 and a BMI ≥40 kg/m2, and 600 µg for patients aged >65. Blood specimens are collected for 6 hours (at 0, 1, 2, 3, 4, and 6 hours, respectively) and are subsequently tested for total T4 and TSH. At the end of the test, vital signs and symptoms are reassessed. In the process, no food or medication is permitted to be ingested. The term of LT4 absorption test can be extended to 72 hours postdosing (without fasting) or be shortened to 2 hours. The percentage of LT4 absorption is calculated with the formula below. A percentage of 60% is defined as normal absorption which indicates a diagnosis of pseudomalabsorption. % Absorbed = [Increment TT4 (µg/dL) × 10/total administered LT4 (µg)] × Vd (L) × 100 Increment TT4 = peak [TT4] – baseline [TT4] Vd (volume of distribution) = 0.442 × BMI. Abbreviations: Vd, volume of distribution. The figure was partly generated using illustrative elements from Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
The therapy for noncompliance is troublesome. Except for patients with specific conditions, such as bedridden individuals needing assistance and people with psychiatric disorders, poor compliance in most patients originates from so-called “human nature,” in other words, forgetfulness. Supervised oral ingestion and a 7-day pillbox are proposed to help (135, 136), but educational booklets have almost no influence on compliance (137). Cappelli et al revealed that liquid LT4 might improve patient compliance compared with tablets (124). The discrepancy in patient compliance between the tablet group and the solution group may be explained by the coingestion of LT4 solution with breakfast. In addition, IM or IV injection of LT4 once or twice weekly may also eliminate hypothyroidism due to pseudomalabsorption (128, 135, 138).
Novel Drug Delivery Formulations
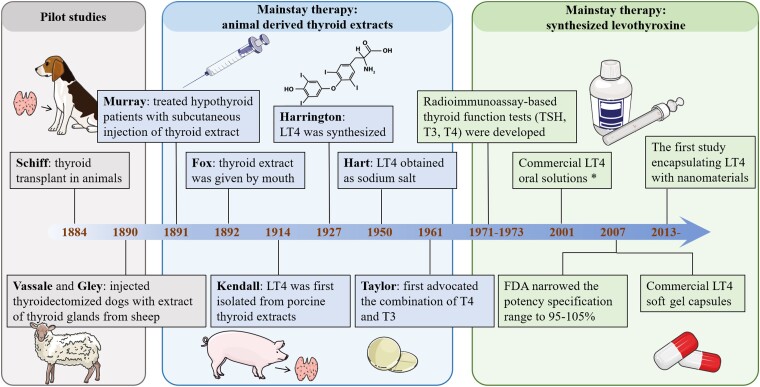
Over a century has elapsed since Murray treated cases of myxedema with a SC injection of sheep thyroid extract for the first time (Fig. 5) (139). Although LT4 was successfully isolated from porcine thyroid extracts in 1914, animal-derived DTE was still the only choice for the treatment of hypothyroidism before the 1940s (140). In 1950, LT4 salt was synthesized successfully and was introduced to the market. LT4 salt rapidly replaced DTE and became the standard therapy for hypothyroidism as there were fewer adverse effects and more accurate dosing (141). LT4 tablets are currently one of the most frequently prescribed drugs worldwide (4). However, as mentioned above, many conditions can reduce the bioavailability of oral LT4 tablets. To overcome this issue, novel formulations (eg, liquid LT4 and soft gel capsules) and new systems (nanomaterial-based systems) with different administration routes (eg, par rectum, IM injection, etc.) have been developed.
Figure 5.
The timeline of seminal discoveries of the mainstay therapy for hypothyroidism and levothyroxine. *The earliest available report about commercial liquid LT4 can be traced back to 2001 (manufacturer: Sanofi-Synthelabo/Henning Berlin). However, there is no doubt that the emergence of extemporaneous oral solution was much earlier since there were lots of articles on the treatment of congenital hypothyroidism and myxedema coma. The Figure was partly generated using illustrative elements from Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
Combined Therapy of T4 and T3
Although the predominant treatment of thyroxine supplementation is LT4 monotherapy, 10% to 15% of patients have persistent or recurrent hypothyroid symptoms (weight gain, fatigue, memory loss, etc.) despite normal TH levels and medical adhesion (142, 143). This phenomenon is assumed to be attributed to a low level of circulating T3, which plays a superior role to T4 in the feedback of the hypothalamus–pituitary–thyroid axis (144). A previous study showed a 10% to 20% reduction in serum T3 levels in ∼1.8 thousand LT4-treated hypothyroid patients compared with the healthy population (145). Similar phenomena were also observed in thyroidectomized rats, which reached euthyroidism only after normalization of serum T3 levels (146).
Based on the theory above, clinicians have returned to combined therapy of T4 plus T3 to eliminate hypothyroid symptoms, with strict indications. Clinicians supported that only hypothyroid patients treated with a stable dose of LT4 for at least 6 months, who had experienced persistent symptoms and had a normal serum TSH level, should receive T4 plus T3 combination therapy (143, 147). However, those with associated comorbidities (eg, autoimmune diseases and psychological dysfunctions) or unrealistic expectations should not receive combination therapy (148, 149). The dose ratio of T3 to T4 in combination therapy is a controversial area. Ratios ranging from 1:3 to 1:17 have been reported in several articles (150–153). Considering that the physiological ratio of T3:T4 secreted by the thyroid gland is approximately 1:14 and the residual thyroid has secretory function, a ratio between 1:8 and 1:13 may be more appropriate (154). Animal-derived DTE has also been introduced to treat refractory hypothyroidism due to combinations of T3 and T4 in a 1:4.22 ratio (155). Animal-derived DTE contains a supraphysiological amount of T3, and cardiac complications should be monitored with caution.
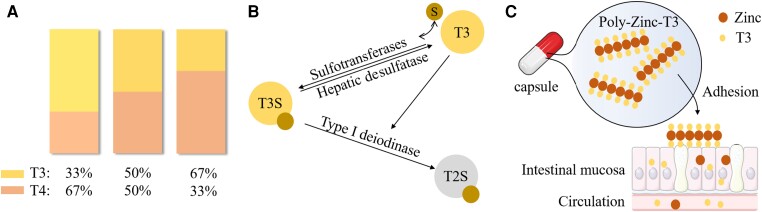
Ratio modification is a challenging task. The previous combination of T3 and T4 added a certain dose of T3 tablets while reducing the T4 administration proportionally. Patients had to take more than 1 pill and/or split pills, which inevitably led to confusion and dose variation (156). Alomari et al developed a novel approach for mixing T3 and T4 using thermal inkjet 2D printing (156). The 2 compounds were printed simultaneously and independently onto the same substrate (Fig. 6A). The ratio modification was easily achieved, enabling the formulation of oral tablets for personalized medicine. Notably, the types of substrate potentially influence the degradation rate of drugs (157).
Figure 6.
(A) The rendering of 2D inkjet printing. The ratio of T3 to T4 can be modified by printer. (B) The counter regulatory mechanism of T3S. T3S is a biologically inactive derivate of T3 and serves as its reserve pool. T3S is mainly converted to inactive T2S by D1. D1 activity is directly regulated by T3, namely, high T3 levels accelerate T3S deiodination, leaving less T3S available for desulfation. Consequently, less T3 in produced. (C) The release and adherence of poly-zinc-T3 in vivo. PZLs leak out from capsules on entry into intestine and adhere to epithelium mucosa through ionic interaction. PZL gradually degrades and T3 is thus released and absorbed. Abbreviations: T2S, 3,3′-diiodothyronine sulfate; T3S, triiodothyronine sulfate; S, sulfate. The figure was partly generated using illustrative elements from Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
Although combined treatment sounds promising, the results from clinical trials comparing monotherapy and combined therapy are conflicting (44, 158). In a previous study involving 19 patients, the TSH levels were normalized only when patients’ T3 levels were not significantly different from those of controls (159). The results are inconsistent with those of more recent double-blind randomized controlled trials (RCTs). Four systematic reviews and meta-analyses based on RCTs failed to show any advantages in quality of life, mood states, or psychometric performance for patients receiving combination therapy (160–163). Although previous meta-analyses reported a preference for combined therapy by patients, a more recent meta-analysis based on 7 blinded RCTs did not support this conclusion (164), suggesting that T3 may serve as a “placebo” and raise patients’ expectations in some nonblinded studies. Taken together, as recommended by the American Thyroid Association, “the use of combined replacement therapy, with the administration of both LT4 and liothyronine (LT3), is generally not recommended due to the low quality of the available evidence. A trial may be considered in those patients with normal values of serum TSH who continue to complain of symptoms of hypothyroidism. Also, in these cases, the presence of coexistent nonthyroid problems should be first ruled out” (44). We further recommend discussing the financial burdens with these patients while addressing concomitant diseases and mitigating their unrealistic expectations.
Novel Sustained-Release T3 System
Liothyronine monotherapy is not commonly recommended for hypothyroid patients (0.12% in The Netherlands in 2011) (165). The major issue limiting the clinical use of T3 is the rapid absorption and clearance rates. An in vivo study confirmed a serum peak that was 42% higher than the baseline only 3 to 4 hours after administration (166). Considering the fact that T3 remains stable with a <10% variance throughout the day in healthy individuals (167), it is no wonder that patients receiving T3 monotherapy or combined therapy present with increasing hyperthyroid symptoms (eg, palpitations, irritability, tremor, and shortness of breath) (168). A clinical trial showed a 3-fold elevation in total T3 and FT3 levels at 2 hours after T3 administration (168). T3 monotherapy failed to suppress TSH levels and patients presented with even more hyperthyroid symptoms, entailing closer monitoring. If TH levels are elevated in the long term, patients will have a higher risk of atrial fibrillation, osteoporosis, and fracture (169, 170). In 2022, a propensity-matched study involving 5342 patients in Korea showed astonishing results (171). The incidences of heart failure and stroke were increased in patients with a longer duration of LT3 use (heart failure incidence rate ratio = 1.664, P = .049, stroke incidence rate ratio = 1.757, P = .025).
Several attempts have been made in the field of sustained-release T3. Hennemann et al designed an in-house slow-release T3 plus T4 preparation and evaluated its pharmacokinetic properties in people with hypothyroidism. With a T3:T4 ratio of 6:125, the preparation showed a delayed and reduced T3 peak time compared with that of normal combined therapy (172). The serum TSH level, however, was somewhat higher and broader than that obtained with LT4 monotherapy. Similar T3 slow-releasing capsules formulated by Bakhteyar et al showed a gradual release profile in a 12-hour period in vitro (173). The release rate may be modulated based on the capsule content and grade, namely, Methocel and SimpleCap/lactose. In addition, osmotic pumps or pellets that are implanted subcutaneously are recommended as parenteral preparations for animals (174). Loaded with doses from 0.001 to 200 mg, these devices could gradually release fixed amounts of T3 daily in a period of up to 90 days. Along a similar line, a novel platform consisting of solid rods prepared with a mixture of T3 and ethylene-vinyl acetate has been developed to be placed in the upper arm (175). In vivo experiments with rats and dogs showed that this platform could release T3 in a relatively stable manner for over 6 months. Human studies are not available at this time.
Another method to create long-acting T3 is the utilization of a T3 derivative. The sulfation of T3 (T3S) can enhance its solubility and stability (176). There are 2 metabolites from T3S: bioactive T3 via desulfation or bioinactive 3,3′-diiodothyronine sulfate generated by deiodinase type 1. The activity of deiodinase type 1 is conversely positively correlated with serum T3 levels. Thus, a counter-regulatory system was constructed based on T3S, T3, and deiodinase type 1 (Fig. 6B). Santini et al conducted a study among 28 thyroidectomized patients administered T3S orally (177). Although serum T3S levels peaked 2 to 4 hours after administration and declined sharply in the next 10 hours, the level of its desulfated (by liver) product, T3, remained steady but increased in serum for more than 48 hours. Even more surprisingly, no adverse events were observed. In 2019, a phase II, open-label, uncontrolled, parallel trial investigating the efficacy and safety of T3S plus T4 was conducted in 36 thyroidectomized individuals (178). A decreased T4/T3 ratio with no reduction in T3 levels was observed compared with LT4 monotherapy. Interestingly, an increase in TSH levels was also observed, potentially due to a decrease in serum T4 levels.
More recently, Da Conceicao and colleagues synthesized poly-zinc-liothyronine (PZL) and loaded it into gelatin capsules (179). After oral administration, PZL can leak out from capsules and adhere to the mucosa of the gastrointestinal tract like a “drug depot” (Fig. 6C). T3 is then released into the intestinal lumen and ultimately absorbed into the bloodstream. An animal study demonstrated an ∼30% lower Cmax, a ∼6 hour later time to peak serum concentration (Tmax), and a plateau time that was ∼3 hours longer than in conventional T3 capsules. The researchers subsequently conducted a phase I, double-blind RCT of 12 healthy volunteers (180). Laboratory tests showed a release profile similar to the previous animal study: a 1 hour later Tmax, an ∼30% lower Cmax, and an ∼6-hour T1/2. The hemodynamic and sleep parameters were not significantly different between the PZL and conventional T3 groups. Capsules prepared with hydrophilic swellable matrix may improve the release pattern. Further trials are expected to test the pharmacokinetics and safety in hypothyroid individuals.
Liquid Solutions
As mentioned above, the absorption of LT4 tablets entails previous gastric disintegration and dissolution, which rely on a normal environmental pH. Liquid solutions intrinsically avoid dissolution. In 2007, liquid LT4 stored in monodose vials (Tirosint SOL) was developed by IBSA, an Italian–Swiss company, and is available on the Italian market. The liquid preparation is primarily composed of 96% ethanol, 85% glycerol, citric acid monohydrate, sodium methyl parahydroxybenzoate, and other excipients (140). A new formula replacing all ethanol with glycerol 85% has been developed for pregnant patients, patients with liver disease or epilepsy, or those allergic to ethanol (181). The liquid solution includes 2 forms, predosed ampoules and oral drops, both of which entail mixing with a glass of water before swallowing. A cross-sectional study in 2017 involving more than 56 000 patients in Italy showed that more than 5% of LT4-treated patients have taken liquid formulations (182). Considering that it was first introduced to the Italian market, the proportion of patients taking liquid solutions is presumed to be lower in other European countries and North America.
The bioequivalence of liquid solutions to other preparations in patients without malabsorption is controversial. Bioequivalence between solid and liquid preparations has been confirmed in crossover clinical trials (183–185). However, these studies were generally conducted before 2011. LT4 solutions were extemporaneously prepared and their formulas were not available, which implies a high risk of nonidenticalness between these solutions and current commercial products. In addition, the pharmacokinetic equivalence of different formulations in healthy volunteers may not translate to equivalence in patients, especially in those with malabsorption (11). In 2012, Yue et al conducted a randomized, 2-way, crossover clinical trial recruiting 84 hypothyroid patients using commercial LT4 products (186). The bioavailability of tablet, solution, and soft gel capsule formulations was evaluated using the standard pharmacokinetic protocol for bioequivalence. T4 was tested from −1 hour predose to 72 hours postdose. The Cmax, Tmax, AUC0-2h, and AUC0-48h were then calculated. The carryover effect was eliminated after a washout period of at least 35 days. No significant differences in the Cmax, AUC0-2h, or AUC0-48h of the liquid solution were observed, while the absorption of the solution was 30 minutes faster than that of the tablet and capsule. An age- and etiology-matched case–control study involving 200 patients revealed a reduced daily dose per kilogram in the group receiving a LT4 solution (187). Studies of patients with congenital hypothyroidism revealed a more suppressed TSH level in infants receiving the LT4 solution (188, 189). The real-world study also showed significantly reduced TSH levels after the switch from a tablet to a liquid preparation (182). A meta-analysis including 6 prospective studies confirmed that patients administered liquid LT4 had reduced TSH levels (4.23 mIU/L, 95% CI 3.69-4.77) compared with the tablet group taking the same dose (190). Taken together, these results show that liquid solutions have higher absorption, faster Tmax, and lower and more stable TSH levels than solid formulations, partially due to better intestinal absorption. In 2021, however, a randomized, crossover study by Markantes et al including 50 patients found that the FT4 level was somewhat lower in the solution group than in the tablet group (1.448 ± 0.232 vs 1.363 ± 0.216 ng/dL, P = .008) (191). The TSH level was also slightly higher in the solution group than in the tablet group. The explanation for these findings may be that changes in the excipient (glycerol substitutes for ethanol) cause altered bioavailability. Further experiments are needed to test this hypothesis.
Liquid formulations have many advantages over solid formulations. First, liquid solutions can be used to treat patients who have difficulty swallowing, such as pediatric patients. Liquid solutions can be administered with milk for infants with congenital hypothyroidism without obvious food interference. Notably, the dose may be reduced, since liquid LT4 at an equivalent dose can lead to increased TSH suppression (192). In addition, a liquid LT4 formulation can be administered directly through feeding tubes in patients receiving enteral feeding, and thus are preferred by nurses due to their convenience (193). The oral administration of a solution via a feeding tube is also a preferred option for patients with myxedema coma when IV LT4 is unavailable (194).
Second and most importantly, liquid LT4 ameliorates the impaired intestinal absorption of LT4 induced by drug or food interference and gastrointestinal diseases (195). As mentioned above, calcium and iron supplements are common concomitant drugs and can reduce the absorption of LT4 tablets through direct complexation. In 2017, Benvenga et al conducted a prospective, pre–post study of 19 hypothyroid patients with malabsorption due to the coingestion of calcium and iron supplements (196). A follow-up of at least 16 weeks showed a significantly reduced TSH level in the solution group compared with the tablet group (7.48 ± 5.8 vs 1.95 ± 1.3 mU/L, P < .001). Two years later, a retrospective study conducted by the same researchers in 50 patients revealed a similar result (99). PPIs could impair the absorption of LT4 tablets by elevating gastric pH. Vita et al conducted a prospective, pre–post study of 24 hypothyroid patients who took PPIs (197). The mean duration of follow-up was 23.7 weeks. TSH levels were significantly lower in the solution group than in the tablet group (1.7 ± 1.0 vs 5.4 ± 4.3 mU/L, P < .001). Furthermore, liquid LT4 can eliminate malabsorption in patients treated with multiple interfering drugs (100, 198).
Similar results have also been obtained in concomitant disease–associated malabsorption. Fallahi et al studied the TSH levels in patients who underwent bariatric surgery after the switch from tablets to solutions (199). Seventeen patients were followed up for 3 to 8 months after surgery and 2 to 3 months after the switch without dose adjustment. TSH decreased in all 17 patients after the switch (13 Roux-en-Y gastric bypasses: 7.58 ± 3.07 vs 3.80 ± 1.83 mU/L, P < .001; 4 biliary pancreatic diversions: 8.82 ± 2.76 vs 3.12 ± 1.33 mU/L, P < .01). Pregnancy is assumed to have little effect on the malabsorption of LT4 but impairs bioavailability via estrogen-induced TBG elevations. A retrospective cohort study by Cappelli et al in 31 pregnant patients with hypothyroidism showed a decreased daily LT4 dose in 14 patients taking liquid solutions and 17 taking tablets, with no significant difference in age, weight, or TH levels (200). Better absorption of liquid LT4 is a possible explanation. In addition, case reports have shown that liquid LT4 has superior efficacy to tablets in patients with autoimmune gastritis (201), gastroparesis (202), celiac disease (203, 204), giardiasis (66), liver cirrhosis (32), and nephrotic syndrome (35, 36).
However, the weaknesses of these studies are obvious. Among the 31 included articles investigating LT4 formulations, 14 were case reports or case series, 5 were retrospective cohort studies, 2 were prospective cohort studies, and 8 were prospective pre–post self-control studies. Only 2 studies were well designed (randomized and crossover) (11, 98). The levels of evidence were generally low and, obviously, there was a high risk of reporting bias. In addition, studies were conducted in a very limited number of countries, which inevitably restricted the generalization of conclusions. In addition, the settings of the studies were not rigorous. Common weak points in settings included a lack of washout periods, nonstandardized interventions, recruitment of patients with different hypothyroidism etiologies and target TH ranges, selective outcome reporting (TSH, FT3, FT4, or LT4 dose), and unavailability of long-term effects, etc. In conclusion, robust studies are still needed to provide more solid evidence in this field.
The third advantage of liquid LT4 is improvements in patient compliance. As discussed under “Pseudomalabsorption,” patients taking solution adhered much better to LT4 treatment than patients taking tablet (P < .001) (124). The major factor contributing to the discrepancy is the coingestion of breakfast and liquid LT4. In 2014, Cappelli et al revealed for the first time that FT3, FT4, and TSH did not differ between patients taking liquid LT4 with or 30 minutes prior to breakfast (205). Their study was a prospective, pre–post study in 54 patients who were followed up for >6 months after switching the dosing time from breakfast to 30 minutes before breakfast. Since this study did not employ a crossover design and the information on dosing time relied on patients’ self-report and compliance, a randomized, double-blind, placebo-controlled, crossover trial by the same research group in 2016 aimed to provide more solid evidence (98). A real liquid LT4 vial and a placebo were provided to participants to ingest either with their usual breakfast or 30 minutes before breakfast. Patients switched the sequence of the 2 vials after the first 6-week regimen and were followed up for another 6 weeks. Seventy-seven patients completed the study at 12 weeks. No significant differences were observed in FT3, FT4, or TSH, indicating that liquid LT4 can eliminate malabsorption due to coingestion with breakfast. Several prospective or retrospective studies also showed similar results (206–209). More recently, Cappelli et al reported a case of malabsorption due to the coingestion of LT4 with lunch that was corrected by liquid LT4 (97). Surprisingly, a randomized crossover trial conducted by Ducharme et al has demonstrated that food interference could be eliminated by liquid LT4 when taken 15 minutes before high-fat, high-calorie meals (210). In addition, an in vitro study also supported the efficacy of liquid LT4. Bernareggi et al diluted liquid LT4 into several beverages (coffee, milk, tea, and orange juice) at 50 °C and evaluated the stability for up to 20 minutes (211). Liquid chromatography–tandem mass spectrometry revealed that T4 was stable in all beverages after 20 minutes of incubation. In addition, a switch from 30 to 60 minutes before breakfast to the same dose of LT4 in the liquid form taken at breakfast could improve the quality of life in the majority of patients (212). Taken together, these data show that the convenience of the liquid preparation improves patient compliance.
Some other studies have obtained superior results for liquid preparations. For patients with incorrect administration, TSH levels were more stable in those who took liquid solutions or soft gels, as suggested by a real-life study (213). Interestingly, the liquid preparation could ameliorate altered mood states, self-perception of mental well-being, and the TH profile in thyroidectomized patients (214), although the improvement in mood states may not have been attributable to improved thyroid functions but something else. Patient preference for the “novel preparation” is a probable explanation.
There are some disadvantages of liquid preparations. Liquid solutions and soft gel capsules are 2- to 15-fold more expensive than solid formulations, depending on brands, regions, and insurance (215). Patients with low income may not be able to afford the monthly cost of $150 to $240 (although the actual situations are complex and patients may save money through various methods). In addition, the interchangeability of liquid preparations remains debatable. A retrospective study on congenital hypothyroidism raised concerns about the bioequivalence of 2 liquid products (Tirosint and Tifactor) (216). Approximately 10% of patients declared that the solution was distasteful and then returned to the solid LT4 formulation (212). Excipients such as sugar may be added to a formulation to improve its taste. In addition, some patients have incorrect dosing habits. Normally, liquid solution (predosed ampoule and oral drop) should be poured into a glass of water and swallowed. Some patients may simply squeeze the liquid LT4 ampoule directly into their mouth for convenience, which may cause buccal absorption and poor efficacy (217). A figure depicting the correct dosing method should be added to the package leaflet to provide patients with dosing information. Due to presumed better efficacy, liquid LT4 should be avoided or adjusted with carefulness in elderly and central hypothyroid patients, who are more prone to excessive LT4 treatments (218).
The prescription of liquid LT4 is promising and limited. As revealed by a meta-analysis by Laurent et al (219) based on 8 prospective or randomized controlled studies, reduced TSH levels were observed in patients with malabsorption who were taking liquid formulations. However, TSH levels did not differ significantly between tablets and liquid preparations in the nonmalabsorption group. Clinicians should follow the guidelines of the Italian association of clinical endocrinologists, namely, “liquid or gel formulations may be considered in subjects (1) with hampered LT4 absorption or (2) who do not allow sufficient time before or after meals for LT4 replacement” (220). A web-based survey of Italian endocrinologists also supported the prescription of liquid LT4 for patients (3) with unexplained poor biochemical control of hypothyroidism (147). From a clinical standpoint, we also recommend the prescription of liquid LT4 for patients (4) with difficulty swallowing.
Soft Gel Capsules
Commercial soft gel capsules were introduced to the market around the same time as LT4 solutions, but somewhat later (in August 2007) (184, 221, 222). The LT4 powder is dissolved in glycerin solvent and then encapsulated with a soft gelatin shell. After drying, a translucent flat oval capsule is produced (223). Colucci et al conducted a clinical trial in 24 healthy volunteers according to the Food and Drug Administration–approved pharmacokinetic protocol for bioequivalence. The study confirmed the pharmacokinetic equivalence between LT4 soft gel capsules and tablets (221). However, controversy still exists. Data from 104 hypothyroid patients showed that TSH was significantly reduced in the capsule group (1.3 ± 0.9 vs 1.8 ± 1.2 mU/L, P = .02) (224). This difference may be related to a more favorable pharmacokinetic profile of soft gel capsules in patients. The bioequivalence between the solution and capsule has been confirmed in healthy volunteers and patients with interfering medications (198, 225).
Soft gel capsules can also eliminate the impaired bioavailability induced by interfering medications or concomitant disease (195). Malabsorption due to calcium supplements (99), PPIs (226), breakfast (206), H. pylori infection (227), and gastroparesis (202) was eradicated after switching from tablets to capsules. Yue et al conducted a randomized, 2-arm, crossover trial in euthyroid volunteers with a washout period of 45 days (11). Thirty-two individuals were recruited in the LT4 absorption test. In line with previous studies, IV esomeprazole reduced the AUC of tablets. The AUC of the capsules was not altered by esomeprazole. An in vitro study revealed that the dissolution time of soft gel capsules was ∼20 minutes regardless of pH (228), which explains the discrepancy in AUCs in the trial. In 2013, Vita et al found that coffee had little effect on LT4 capsule pharmacokinetics (101). Patients taking soft gel capsules experienced fewer dose adjustments and improved symptoms than patients taking tablets (229). In conclusion, it can be postulated that soft gel capsules have superior efficacy over tablets.
There are some advantages of capsules over liquid solutions. As discussed above, the dosing of liquid solution is inconvenient, requiring breaking a vial, pouring it into a glass of water, and swallowing it. Capsules are obviously easier to take. In addition, the package of capsules is smaller and easy to carry. Although soft gel capsules are expensive, considering the fact that some “hard to treat with pill” patients need more blood tests and dose adjustments, soft gel LT4 is more cost-saving for patients with ≥ 1 dose adjustments annually (230). Of note, certain excipients of soft gel capsules can elicit oral mucositis (231). In conclusion, liquid LT4 and soft gel capsules are ideal second-line therapies for hypothyroidism.
Injectable Preparations
Theoretically, LT4 injections in humans could be administered intravenously, intramuscularly, intra-amniotically, and subcutaneously. LT4 sodium salt is stored in a lucifugal bottle and mixed with saline before injection. The extemporaneous injection can remain stable for more than 24 hours (232).
Intravenous injection
IV LT4 injection is the first-line option for myxedema (233), a rare but lethal crisis commonly due to long-standing undiagnosed hypothyroidism or treatment noncompliance. An IV preparation can also be administered to patients who refuse to receive oral administration (234), those with presurgical preparation of nil by mouth, subtotal ileum resection (235), and intractable hypothyroidism treated with high oral doses of LT4 (236). To conclude, thyroid emergencies and inability of oral LT4 are indications for IV administration. Of note, the appropriate IV equivalent dose is estimated to be 48% to 74% of a previously adequate oral LT4 dose (237). Hence, the American Thyroid Association recommends an IV LT4 dose of 75% of the oral dose (44).
IV injection is strongly not recommended for long-term treatment due to its rapid and great effect on TH homeostasis. LT4 IV injection can augment the calorigenic effect of norepinephrine within minutes and thus exerts direct, rapid effects on the myocardium, which may lead to cardiac arrest (238). In addition, previous research has shown a positive correlation between the dose of IV LT4 and mortality in patients with myxedema (239). A 3- to 5-day course of IV LT4 with subsequent oral tablets is recommended to prevent potential adverse events and to save costs (240, 241).
In the absence of IV injection, the nasogastric route of oral preparation is an alternative for hypothyroid crisis (242).
Intramuscular injection
IM injection is an alternative route for patients with myxedema or difficulty in swallowing. Different from IV injection, IM injection can serve as a sustained-release drug delivery system and be administered for quite a long period. In 2008, a novel feedback control system simulator was developed by Eisenberg et al (153). The model was quantified from pharmacokinetic and physiological human data and was validated against several independent clinical data sets. As simulated by the model, a dose of 800 µg weekly or 400 µg twice weekly can maintain serum T3, T4, and TSH in the target range in patients with athyreosis. The simulation has been confirmed by case reports. Peynirci et al reported a 32-year-old female patient with elevated TSH (98.22 mIU/L) (243). Poor adherence and malabsorption were excluded by examinations and LT4 absorption tests. She was started with 200 µg/day IM LT4 and then switched to weekly IM injection at 500 µg. After the treatment for >1 year, her hypothyroid symptoms were relieved, and her thyroid function returned to a normal range. Three patients receiving IM LT4 injections for 1 to 2 years were reported to have remained biochemically euthyroid without any adverse events (244). Similar cases have also been reported by other researchers (245–247). The weekly dose of IM injection ranges from 400 to 1000 µg. The dosage can be administered once a week or split into 2 doses per week. Thyroid function should be monitored with care in patients with transient hyperthyroid symptoms due to burst release.
Subcutaneous injection
Similar to IM injection, SC injection can serve as a convenient sustained-release system. SC injection produces smaller fluctuations in TSH levels and slower release into the circulation than IM injection (153). An individual with hypothyroidism refractory to oral LT4 due to severe malabsorption was reported to achieve euthyroidism after receiving a SC LT4 injection at a dose of 500 µg/week (248). The advantages and disadvantages of this route are obvious. Patients can easily perform the injections themselves, similar to insulin injection. However, some patients may experience considerable discomfort at injection sites (236). Topf et al successfully restored the TH levels of a female patient treated with SC LT4 injections. A split injection of 500 µg/week in 2 sites of the abdomen minimized local pain (24).
Intra-amniotic injection
IA LT4 injection is administered in rare cases of dyshormonogenesis with fetal goiters. Fetal goiters in the uterus are induced by maternal thyroid dysfunction of various causes (either secondary to antithyroid treatment for maternal hyperthyroidism, or compensatory to maternal hypothyroidism). Genetic mutations of thyroid-specific proteins also play a role in the development of fetal goiters (249–251). The conditions are usually detected by ultrasound examinations or amniocentesis in the second or third trimesters (252). Addressing maternal diseases and LT4 replacement is necessary for preventing potential fetal tracheal compression or other developmental malformations (253). A 10 to 150 µg/kg/day dose via IA injection at an interval of 1 to 4 weeks is recommended, depending on the goiter sizes and fetal serum TH levels (250, 254, 255). Of note, fetal goiters induced by mutations, which affect the transport and metabolism of T4, may respond more poorly to LT4 supplements (256).
Suppositories
Rectal administration is an alternative route to treat hypothyroidism in patients who are unable to take oral formulations. The indications for rectal administration include short bowel syndrome and gastrointestinal tract obstruction. Since LT4 suppositories are not available in most medical institutions, IV LT4 preparations or extemporary tablet-dissolved solutions can be alternatives. Interestingly, although rectal administration can bypass the hepatic first-pass effect, plasma TH levels are normalized only with an additional 100% of the dose or more (257, 258).
Two mechanisms could explain the increased demand for LT4 in the rectum. On the one hand, rectal pH impairs the absorption of LT4. Hamada et al demonstrated that although LT4 suppository content was uniform and stable over 90 days, its release rate correlated directly with rectal pH (259). A 240-minute in vitro experiment showed an ∼20% release at pH 8.2 and undetectable release at pH <7.2. Considering that rectal mucosal pH generally ranges from 7.2 to 7.9 (260), a low pH may be the cause of a LT4 suppository dose increase. On the other hand, the expression levels of TH transporters differ between the small intestine and the rectum, which, in part, may explain the differences in the bioavailability of T4 after oral and rectal administration (261).
Oral Cavity
The sublingual and buccal mucosa are highly vascularized, hence drug degradation by intestinal enzymes can be avoided. Liquid solutions were administered via the sublingual route to correct refractory hypothyroidism (262). The presence of ethanol in the solution favors the permeation of LT4 through mucosal epithelium.
Respiratory Tract
The upper respiratory mucosa may be an alternative target for noninvasive drug delivery. Agu et al dissolved LT4 sodium salt hydrate in buffered saline and tested its permeability and toxicity in respiratory mucosa cells (Calu-3 cells) (263). A cumulative permeation of 0.90 ± 0.53 µM was achieved in the apical to basolateral direction at 37 °C over 3 hours. Real-time polymerase chain reaction showed expression of sodium-dependent TH transporters on the apical membrane of Calu-3 cells, which were significantly affected by pH and temperature. Their study denoted the respiratory epithelium as a promising route for LT4 administration without significant cytotoxicity. The spray remains untested in animal models.
External Application on the Skin
LT4 can be added to creams to reduce deposits of adipose tissue on the skin. Ideally, LT4 should enter the skin layer but not cross it (264). According to a previous study by Santini et al, only 10% of LT4 applied to bare skin was transferred through the epidermis, whereas a greater portion of LT4 was degraded by type III deiodinase in the skin (265). Azarbayjani et al further decreased the permeability to almost an undetectable level by encapsulating LT4 into sustained-release nanoparticles (266, 267). However, 50% to 70% of LT4 was gradually released in vitro during the first 15 to 30 minutes, with the remainder being released in the next 2 to 7 hours, denoting an unsatisfying sustained-release profile. Padula et al suggested that a microemulsion system could include LT4 in reverse micelles and reduce LT4 skin permeation to ∼1% of that in dimethyl-β-cyclodextrin solution, which is widely used in creams (268). An additional transdermal film could further increase the dermal retention of LT4.
LT4 and Nanomaterials
Nanomaterials are materials that have at least 1 dimension at the nanoscale size (1-100 nm), or are composed of nanomaterials as basic units. In recent years, various nanomaterials have been used to develop sustained-release LT4 systems (195).
Some researchers have tried to deliver LT4 into the systemic circulation via a SC route. Kashanian et al loaded LT4 into 3-(aminopropyl) triethoxysilane modified porous silicon (Fig. 7A) (269). The height of the porous silicon membrane was ∼2 µm and it bound LT4 through hydrogen bonding. 3-(Aminopropyl) triethoxysilane was added to dramatically increase the affinity to this hydrophobic drug molecule. An in vitro experiment demonstrated a first-order release profile of 87% of the total contents of 21 µg in an ∼14-day period. This study provided a promising method for cutaneous LT4 delivery, but the load remains to be increased and an animal experiment is needed to evaluate the potential effects of the administration method on the skin.
Figure 7.
(A) The structure of 3-(aminopropyl)triethoxysilane-functionalized, oxidized porous silicon film. The LT4 molecules bind to porous silicon with hydrogen bonding. (B) The rendering of nanofluidic drug delivery implants and the cross section of microchannels on nanofluidic membranes. The implant is ∼2.5 cm in length. The silicon nanochannel membranes are 6 × 6 mm wide and 730 µm in height. The length and width of the 3 channels (inlet, connecting and outlet) are 3 µm. (C) The rendering of poly(caprolactone)-based subcutaneous implant. (D) The rendering of LT4-loaded poly ethylene glycol 100 stearate–coated solid lipid nanoparticles. (E) The process of sol to gel transformation. Thermosensitive triblock undergoes polymerization. (F) The rendering of microneedle patch. Abbreviation: PEEK, polyether ether ketone. The figure was partly generated using illustrative elements from Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
A SC implantable device was designed by Geninatti et al for the sustained release of several hormones, including LT4 (270). Approximately 18 mg of LT4 were loaded into 2.5-cm-long cylinders comprising 5 components (Fig. 7B). LT4 was stored in the drug reservoir and gradually permeated through the silicon nanochannel membranes. The membrane was composed of >160 macrochannels and each macrochannel contained >1000 inlet microchannels. Every inlet channel ended with 2 connecting nanochannels, which were 3 to 40 nm in height. LT4 flowed through the inlet and connecting channels and finally arrived at the outlet channels. The drug was then released into the SC space and systemic circulation. In vivo experiments revealed a 0-order release profile lasting for more than 15 days. Although this is an invasive drug delivery route, long-acting effectiveness may be achieved if the device volume is reduced. In doing so, LT4 should be dissolved in a more soluble, nontoxic medium, and the capsule should be minimized to an acceptable size (271). Animal experiments are needed to confirm the safety and pharmacokinetics of the device in vivo.
A more promising SC implant was manufactured by Stewart and colleagues (272). The rod-shaped implants with dimensions of 2.5 × 40 mm were prepared with biodegradable poly(caprolactone) (PCL) of 2 mean weights (light PCL, L-PCL, mass average molar mass = 550 Da; heavy PCL, H-PCL, mass average molar mass = 50 000 Da) (Fig. 7C). An implant made of 100% H-PCL could load ∼40 mg LT4. An increased proportion of L-PCL in the ingredients was observed to increase the drug loadings by 20% to 25%. Polyethylene glycol (PEG) was added to the implant. Frustratingly, it was incompatible with LT4 and caused significant drug degradation. An in vitro study in bovine serum albumin solution revealed a linear release after a burst release in the first 24 hours. The addition of L-PCL could prevent unwanted fast release over this period. The average release rate was 30 to 100 µg/day and was close to the oral dose of LT4 (44). A cumulative percentage of 8% to 20% was revealed after 98 days in vitro. Presumptuously, the SC implant could work for 1.3 to 3.4 years if the linear release profile continued. A subsequent in vivo experiment was conducted in healthy Wistar rats by inserting half size of the implants into the skin under sedation. Blood samples were collected and tested for 4 weeks with high-performance liquid chromatography (273). No burst release was observed in the first 24 hours and the plasma drug concentration reached a Cmax of 10 to 20 ng/mL at 14 to 21 days, depending on the ratio of H-PCL to L-PCL. Considering that the obtained plasma concentration was >2000 times that required for healthy humans (274) and the weight of the average human body is >300 times the weight of rats, the size of implants providing a therapeutic LT4 dose can be reduced to 5 to 14 times that of the current implants. A phase I trial is supposed to reveal the performance of these implants in healthy individuals, and to evaluate their operation convenience and potential adverse events, including pain and skin infection.
Other researchers have coated LT4 nanoparticles to develop slow-releasing oral solutions. Kashanian et al encapsulated LT4 with PEG stearate coated solid lipid nanoparticles to increase the intestinal absorption rate and to construct a sustained-release solution (Fig. 7D) (275). Although the particles remained stable in the gastric environment and showed a gradual release of drug up to 60 hours in vitro, there were some unneglectable flaws to address. A 40% to 50% burst release of total contents was observed in the first 12 hours. Moreover, only 70% of total LT4 was able to be released from nanoparticles. Thicker nanoparticles with a lower proportion of lipids may solve this problem. Of note, these solid lipid nanoparticles were stable over 6 months (276).
Chitosan has been a hotspot in recent years due to its unique properties, such as biocompatibility, biodegradability, safety, and mucoadhesivity. Rostami et al encapsulated LT4 with chitosan nanoparticles at an average diameter of 220 nm (277). In vitro release in phosphate saline buffer revealed a 0-order profile in 15 to 20 hours. Interestingly, energy applied from the outside, such as ultrasound, could accelerate the liberation of LT4 from polymers.
Moreover, Kamali et al designed an injectable SC long-acting LT4 preparation with thermosensitive polymers (278). Poly D, L-lactic-co-glycolic acid (PLGA) and PEG are linked and form PLGA–PEG–PLGA triblock polymers, which are water insoluble and biocompatible. The triblock was dissolved in biocompatible water-soluble N-methyl pyrrolidone (NMP) and existed in soluble form at room temperature. LT4 was mixed with triblock NMP solution before SC injection. Upon reaching the interstitial space at body temperature, NMP leaks out from the triblock and the triblock undergoes a thermosensitive reaction in seconds. Consequently, an in situ implant loaded with 3 mg of LT4 forms in the SC space (Fig. 7E). In vitro experiments revealed a 500-hour gradual release of LT4 after a burst release of ∼20% of the total contents in the first 24 hours. Taking cellular toxicity into consideration, a 50% triblock NMP solution showed the best biocompatibility of all concentrations. However, a 25 °C to 30 °C transition temperature from sol to gel limits the application of thermosensitive polymers in clinical practice.
Microneedle patch (MNP) is another promising approach for parenteral LT4 delivery. Two Iraq researchers, Ghazi and Al-Mayahy, manufactured MNPs using a micro-molding technique (279). LT4 sodium was dissolved in deionized water with Tween 80 and hyaluronic acid. The percentages of Tween 80 and hyaluronic acid could be changed for different mechanical strength and shape-forming ability. After stirring and removing bubbles, the polymeric mixture was poured into molds and was kept for drying. In this study, microneedle formulation composed of 50% w/v of hyaluronic acid, 1% v/v of Tween 80, and 0.05% w/v of LT4 (50 µg) was found to achieve the best balance between mechanical strength and shape-forming ability. Ultimately, the patch consisted of 10 × 10 microneedles on a 0.64 cm2 area (Fig. 7F). The pyramid-shaped microneedle was 500 µm in height and 300 µm at the base. An in vitro study showed that 96% to 98% of loaded LT4 was gradually released in 60 minutes. A subsequent ex vivo study on human skin revealed a similar release profile in 420 minutes. The study provided a novel insight into the transdermal delivery of LT4. However, the current loaded dose of MNP (50 µg) showed no superiority over oral preparations. In addition, the application of MNPs cause slight erythema. The in vivo release profile and skin irritation should be evaluated in further animal and human studies.
The combination of nanomaterials with LT4 in most of these studies aimed to construct sustained-release drug delivery systems or to increase bioavailability. However, a large proportion of articles only extended the studies to in vitro experiments and few of them proceeded to animal or human studies (179, 180, 272). The pharmacokinetic properties and biocompatibility remained untested. Some nanomaterials have intrinsic unsolved problems, including a low LT4 release rate from polymers, a low drug load, burst release, short acting time, inconvenient administration, or chemical instability. The road for LT4-combined nanomaterials to be used in clinical therapy is long.
Conclusions
Liquid solutions and soft gel capsules are the most exciting advances in LT4 in the past 20 years. These formulations can circumvent malabsorption due to other medications and diseases, and improve patient compliance. Liquid solutions can also be used in patients with difficulty in swallowing. Many studies have evaluated the efficacy of LT4 solutions and capsules in different populations or under different conditions. However, the debate is still ongoing since most studies have intrinsic flaws in study designs. Randomized, crossover, multicenter studies are needed to provide more solid evidence.
Aside from oral formulations, some researchers have evaluated less common drug delivery routes, including injections, suppositories, sublingual administration, sprays, and transdermal administration. These studies are meaningful explorations.
Moreover, the combination of nanomaterials and LT4 delivery is a novel and interesting field. Sustained-release systems have been developed with various nanomaterials. The load, burst release and the linear release rate are major issues to be considered in the design and development process. Some of these formulations (PCL and PZL) have exhibited a promising release profile and are proposed for in vivo studies or human studies. More nanomaterials are expected to be used to improve the release profile.
Abbreviations
- AUC
area under the curve
- BMI
body mass index
- Cmax
maximum serum concentration
- DTE
desiccated thyroid extract
- FT4
free T4
- H-PCL
heavy PCL
- IA
intra-amniotic
- IM
intramuscular
- IV
intravenous
- L-PCL
light PCL
- LT3
liothyronine
- LT4
levothyroxine
- MNP
microneedle patch
- NMP
N-methyl pyrrolidone
- PCL
poly(caprolactone)
- PEG
polyethylene glycol
- PLGA
poly D, L-lactic-co-glycolic acid
- PPI
proton pump inhibitor
- PZL
poly-zinc-liothyronine
- RCT
randomized controlled trial
- rT3
reverse triiodothyronine
- SC
subcutaneous
- T1/2
half-life
- T3
triiodothyronine
- T3S
sulfation of T3
- T4
thyroxine
- TBG
thyroxine-binding globulin
- TH
thyroid hormone
- Tmax
amount of time at the peak serum concentration
- TSH
thyrotropin
- TT4
total thyroxine
Contributor Information
Hanqing Liu, Department of Breast and Thyroid Surgery, Renmin Hospital of Wuhan University, Wuhan 430060, Hubei, People’s Republic of China.
Wei Li, Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (Ministry of Education), School of Pharmaceutical Sciences, Wuhan University, Wuhan 430060, Hubei, People's Republic of China.
Wen Zhang, Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (Ministry of Education), School of Pharmaceutical Sciences, Wuhan University, Wuhan 430060, Hubei, People's Republic of China.
Shengrong Sun, Department of Breast and Thyroid Surgery, Renmin Hospital of Wuhan University, Wuhan 430060, Hubei, People’s Republic of China.
Chuang Chen, Department of Breast and Thyroid Surgery, Renmin Hospital of Wuhan University, Wuhan 430060, Hubei, People’s Republic of China.
Funding
This research was supported by the grants from Beijing Xisike Clinical Oncology Research Foundation (Y-SY201901-0189), Fundamental Research Funds for the Central Universities (2042019kf0229), Major Technology Innovation of Hubei Province (2019AEA170), and Fundamental Research Funds for the Central Universities of China (no. 2042021kf0073).
References
- 1. Chaker L, Bianco AC, Jonklaas J, Peeters RP. Hypothyroidism. Lancet. 2017;390(10101):1550‐1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160(4):526‐534. [DOI] [PubMed] [Google Scholar]
- 3. McAninch EA, Bianco AC. The history and future of treatment of hypothyroidism. Ann Intern Med. 2016;164(1):50‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kane S. The Top 200 of 2021. Accessed February 19, 2022. https://clincalc.com/DrugStats/Top300Drugs.aspx
- 5. Burlacu M-C, Attanasio R, Hegedus L, et al. Use of thyroid hormones in hypothyroid and euthyroid patients: a THESIS* survey of Belgian specialists *THESIS: Treatment of Hypothyroidism in Europe by Specialists: an international survey. Thyroid Res. 2022;15(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vardarli I, Brandenburg T, Hegedus L, et al. A questionnaire survey of German thyroidologists on the use of thyroid hormones in hypothyroid and euthyroid patients: the THESIS (treatment of hypothyroidism in Europe by specialists: an international survey) collaborative. Exp Clin Endocrinol Diabetes. 2022;130(9):577‐586. [DOI] [PubMed] [Google Scholar]
- 7. Jonklaas J. Optimal thyroid hormone replacement. Endocr Rev. 2022;43(2):366‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Irving SA, Vadiveloo T, Leese GP. Drugs that interact with levothyroxine: an observational study from the Thyroid Epidemiology, Audit and Research Study (TEARS). Clin Endocrinol (Oxf). 2015;82(1):136‐141. [DOI] [PubMed] [Google Scholar]
- 9. Crilly M. Thyroxine adherence in primary hypothyroidism. Lancet. 2004;363(9420):1558. [DOI] [PubMed] [Google Scholar]
- 10. Kumar R, Shaukat F. Adherence to levothyroxine tablet in patients with hypothyroidism. Cureus. 2019;11(5):e4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yue CS, Benvenga S, Scarsi C, Loprete L, Ducharme MP. When bioequivalence in healthy volunteers may not translate to bioequivalence in patients: differential effects of increased gastric pH on the pharmacokinetics of levothyroxine capsules and tablets. J Pharm Pharm Sci. 2015;18(5):844‐855. [DOI] [PubMed] [Google Scholar]
- 12. Gupta P, Johnson JT, Soumya SL, Cherian KE, Kapoor N, Paul TV. A case of H. pylori infection presenting as refractory hypothyroidism. J Family Med Prim Care. 2020;9(7):3770‐3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Virili C, Brusca N, Capriello S, Centanni M. Levothyroxine therapy in gastric malabsorptive disorders. Front Endocrinol (Lausanne). 2021;11:621616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Virili C, Antonelli A, Santaguida MG, Benvenga S, Centanni M. Gastrointestinal malabsorption of thyroxine. Endocr Rev. 2019;40(1):118‐136. [DOI] [PubMed] [Google Scholar]
- 15. Kocic I, Homsek I, Dacevic M, Parojcic J, Miljkovic B. An investigation into the influence of experimental conditions on in vitro drug release from immediate-release tablets of levothyroxine sodium and its relation to oral bioavailability. AAPS PharmSciTech. 2011;12(3):938‐948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pabla D, Akhlaghi F, Zia H. A comparative pH-dissolution profile study of selected commercial levothyroxine products using inductively coupled plasma mass spectrometry. Eur J Pharm Biopharm. 2009;72(1):105‐110. [DOI] [PubMed] [Google Scholar]
- 17. Virili C, Bruno G, Santaguida MG, et al. Levothyroxine treatment and gastric juice pH in humans: the proof of concept. Endocrine. 2022;77(1):102‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yamamoto T. Tablet formulation of levothyroxine is absorbed less well than powdered levothyroxine. Thyroid. 2003;13(12):1177‐1181. [DOI] [PubMed] [Google Scholar]
- 19. Hays MT. Localization of human thyroxine absorption. Thyroid. 1991;1(3):241‐248. [DOI] [PubMed] [Google Scholar]
- 20. Hays MT. Absorption of oral thyroxine in man. J Clin Endocrinol Metab. 1968;28(6):749‐756. [DOI] [PubMed] [Google Scholar]
- 21. Bach-Huynh T-G, Nayak B, Loh J, Soldin S, Jonklaas J. Timing of levothyroxine administration affects serum thyrotropin concentration. J Clin Endocrinol Metab. 2009;94(10):3905‐3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Benvenga S, Bartolone L, Pappalardo MA, et al. Altered intestinal absorption of L-thyroxine caused by coffee. Thyroid. 2008;18(3):293‐301. [DOI] [PubMed] [Google Scholar]
- 23. Figueiredo Radaeli R, Diehl LA. Increased levothyroxine requirement in a woman with previously well-controlled hypothyroidism and intestinal giardiasis. Arq Bras Endocrinol Metabol. 2011;55(1):81‐84. [DOI] [PubMed] [Google Scholar]
- 24. Topf A, Pleininger T, Motloch LJ, et al. Subcutaneous administration of levothyroxine: a novel approach to refractory hypothyroidism—a review and a case report. Arch Endocrinol Metab. 2021;65(5):664‐668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Asik M, Gunes F, Binnetoglu E, et al. Decrease in TSH levels after lactose restriction in Hashimoto's thyroiditis patients with lactose intolerance. Endocrine. 2014;46(2):279‐284. [DOI] [PubMed] [Google Scholar]
- 26. Cellini M, Santaguida MG, Gatto I, et al. Systematic appraisal of lactose intolerance as cause of increased need for oral thyroxine. J Clin Endocrinol Metab. 2014;99(8):E1454‐E1458. [DOI] [PubMed] [Google Scholar]
- 27. Chung CW, Mo EY, Jung GS, et al. Decreased expression of ileal thyroid hormone transporters in a hypothyroid patient: a case report. Front Endocrinol (Lausanne). 2021;12:664839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lennernas H. Intestinal permeability and its relevance for absorption and elimination. Xenobiotica. 2007;37(10-11):1015‐1051. [DOI] [PubMed] [Google Scholar]
- 29. Choe W, Hays MT. Absorption of oral thyroxine. Endocrinologist. 1995;5(3):222‐228. [Google Scholar]
- 30. DiStefano JJ, 3rd, Sternlicht M, Harris DR. Rat enterohepatic circulation and intestinal distribution of enterally infused thyroid hormones. Endocrinology. 1988;123(5):2526‐2539. [DOI] [PubMed] [Google Scholar]
- 31. Virili C, Centanni M. “With a little help from my friends”—the role of microbiota in thyroid hormone metabolism and enterohepatic recycling. Mol Cell Endocrinol. 2017;458(C):39‐43. [DOI] [PubMed] [Google Scholar]
- 32. Benvenga S, Capodicasa G, Perelli S, Ferrari SM, Fallahi P, Antonelli A. Increased requirement of replacement doses of levothyroxine caused by liver cirrhosis. Front Endocrinol (Lausanne). 2018;9(150):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ianiro G, Mangiola F, Di Rienzo TA, et al. Levothyroxine absorption in health and disease, and new therapeutic perspectives. Eur Rev Med Pharmacol Sci. 2014;18(4):451‐456. [PubMed] [Google Scholar]
- 34. Arafah BM. Increased need for thyroxine in women with hypothyroidism during estrogen therapy. N Engl J Med. 2001;344(23):1743‐1749. [DOI] [PubMed] [Google Scholar]
- 35. Benvenga S, Vita R, Di Bari F, Fallahi P, Antonelli A. Do not forget nephrotic syndrome as a cause of increased requirement of levothyroxine replacement therapy. Eur Thyroid J. 2015;4(2):138‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Iqbal S, Wan WY, Mitchell NE. It's not lupus this time! A case of worsening hypothyroidism in a patient with nephrotic syndrome. Cureus J Med Sci. 2022;14(5):e25355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hoermann R, Midgley JEM, Larisch R, Dietrich JW. Recent advances in thyroid hormone regulation: toward a new paradigm for optimal diagnosis and treatment. Front Endocrinol (Lausanne). 2017;8:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mol JA, Visser TJ. Rapid and selective inner ring deiodination of thyroxine sulfate by rat-liver deiodinase. Endocrinology. 1985;117(1):8‐12. [DOI] [PubMed] [Google Scholar]
- 39. Wartofsky L. Combination L-T3 and L-T4 therapy for hypothyroidism. Curr Opin Endocrinol Diabetes Obes. 2013;20(5):460‐466. [DOI] [PubMed] [Google Scholar]
- 40. Gietka-Czernel M, Hubalewska-Dydejczyk A, Kos-Kudla B, et al. Expert opinion on liquid L-thyroxine usage in hypothyroid patients and new liquid thyroxine formulation—Tirosint SOL. Endokrynol Pol. 2020;71(5):441‐465. [DOI] [PubMed] [Google Scholar]
- 41. Gottwald-Hostalek U, Kahaly GJ. Triiodothyronine alongside levothyroxine in the management of hypothyroidism? Curr Med Res Opin. 2021;37(12):2099‐2106. [DOI] [PubMed] [Google Scholar]
- 42. Leung AM. Thyroid function in pregnancy. J Trace Elem Med Biol. 2012;26(2-3):137‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vaisman M, Spina LDC, Eksterman LF, et al. Comparative bioavailability of two oral L-thyroxine formulations after multiple dose administration in patients with hypothyroidism and its relation with therapeutic endpoints and dissolution profiles. Arzneimitt Forsch Drug Res. 2001;51(3):246‐252. [DOI] [PubMed] [Google Scholar]
- 44. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American thyroid association task force on thyroid hormone replacement. Thyroid. 2014;24(12):1670‐1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Okosieme OE, Belludi G, Spittle K, Kadiyala R, Richards J. Adequacy of thyroid hormone replacement in a general population. QJM. 2011;104(5):395‐401. [DOI] [PubMed] [Google Scholar]
- 46. Taylor PN, Iqbal A, Minassian C, et al. Falling threshold for treatment of borderline elevated thyrotropin levels-balancing benefits and risks evidence from a large community-based study. JAMA Intern Med. 2014;174(1):32‐39. [DOI] [PubMed] [Google Scholar]
- 47. Yaylaci S, Tosun O, Sahin O, et al. Misuse of levothyroxine and the rate of achieving target thyroid-stimulating hormone in levothyroxine treatment. Biomed Res (India). 2017;28(6):2661‐2665. [Google Scholar]
- 48. Khraisha OS, Al-Madani MM, Peiris AN, Paul TK. Gastroparesis—a novel cause of persistent thyroid stimulating hormone elevation in hypothyroidism. J Louisiana State Med Soc. 2015;167(2):47‐49. [PubMed] [Google Scholar]
- 49. Fain K, Rojas AP, Peiris AN. Hypothyroidism following gastric sleeve surgery resolved by ingesting crushed thyroxine tablets. Proc (Bayl Univ Med Cent). 2020;33(1):38‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lobasso A, Nappi L, Barbieri L, et al. Severe hypothyroidism due to the loss of therapeutic efficacy of L-thyroxine in a patient with esophageal complication associated with systemic sclerosis. Front Endocrinol (Lausanne). 2017;8(241):241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Van Tellingen V, Finken MJJ, Israels J, Hendriks YMC, Kamp GA, van Santen HM. Poorly controlled congenital hypothyroidism due to an underlying Allgrove syndrome. Horm Res Paediatr. 2016;86(6):420‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Smyrniotis V, Vaos N, Arkadopoulos N, Kostopanagiotou G, Theodoraki K, Lambrou A. Severe hypothyroidism in patients dependent on prolonged thyroxine infusion through a jejunostomy. Clin Nutr. 2000;19(1):65‐67. [DOI] [PubMed] [Google Scholar]
- 53. Tuncel D, Ince Z, Aygun E, Coban A. An alternative route of treatment in transient hypothyroxinemia of prematurity: rectal administration of levothyroxine. J Clin Res Pediatr Endocrinol. Published online October 1, 2021. Doi: 10.4274/jcrpe.galenos.2021.2021.0153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bevan JS, Munro JF. Thyroxine malabsorption following intestinal-bypass surgery. Int J Obes. 1986;10(3):245‐246. [PubMed] [Google Scholar]
- 55. Munoz-Torres M, Varsavsky M, Alonso G. Lactose intolerance revealed by severe resistance to treatment with levothyroxine. Thyroid. 2006;16(11):1171‐1173. [DOI] [PubMed] [Google Scholar]
- 56. Fritzen R. Malabsorption of levothyroxine due to Enterobius vermicularis. Exp Clin Endocrinol Diabetes. 2007;115:S80. [Google Scholar]
- 57. Imberti R, Ferrari M, Albertini R, Rizzo V, Tinelli C. Increased levothyroxine requirements in critically ill patients with hypothyroidism. Minerva Anestesiol. 2010;76(7):500‐503. [PubMed] [Google Scholar]
- 58. Menendez A, Sura S. Case report: use of biweekly intramuscular levothyroxine therapy in a pediatric patient with acquired hypothyroidism and malabsorption from intestinal lymphangiectasia. Horm Res Paediatr. 2021;94(Suppl 2):126. [Google Scholar]
- 59. Virili C, Stramazzo I, Santaguida MG, et al. Ulcerative colitis as a novel cause of increased need for levothyroxine. Front Endocrinol (Lausanne). 2019;10:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Giuffrida G, Magazzu G, Campenni A, et al. Cystic fibrosis as a cause of malabsorption and increased requirement of levothyroxine. Thyroid. 2020;30(7):1095‐1096. [DOI] [PubMed] [Google Scholar]
- 61. Mandel SJ, Larsen PR, Seely EW, Brent GA. Increased need for thyroxine during pregnancy in women with primary hypothyroidism. N Engl J Med. 1990;323(2):91‐96. [DOI] [PubMed] [Google Scholar]
- 62. Lozanov B, Gorcheva D, Lozanov LB, Koleva V, Refetoff S. Insufficiency of levothyroxine therapy in autoimmune hypothyroidism: effect of glucocorticoid administration. Acta Endocrinologica-Bucharest. 2017;13(4):515‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Surks MI, Schadlow AR, Stock JM, Oppenheimer JH. Determination of iodothyronine absorption and conversion of L-thyroxine (T4) to L-triiodothyronine (T3) using turnover rate techniques. J Clin Invest. 1973;52(4):805‐811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Collins D, Wilcox R, Nathan M, Zubarik R. Celiac disease and hypothyroidism. Am J Med. 2012;125(3):278‐282. [DOI] [PubMed] [Google Scholar]
- 65. Fallahi P, Ferrari SM, Marchi S, De Bortoli N, Ruffilli I, Antonelli A. Patients with lactose intolerance absorb liquid levothyroxine better than tablet levothyroxine. Endocrine. 2017;57(1):175‐178. [DOI] [PubMed] [Google Scholar]
- 66. Tortora A, La Sala D, Vitale M. Switch from tablet levothyroxine to oral solution resolved reduced absorption by intestinal parasitosis. Endocrinol Diabetes Metab Case Rep. Published online March 21, 2019. Doi: 10.1530/EDM-19-0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jojima T, Shinzawa T, Ohira E, et al. Switching from the tablet to the powder formulation of levothyroxine corrects severe hypothyroidism in a patient with lactose intolerance. Endocr J. 2022;69(8):941‐945. [DOI] [PubMed] [Google Scholar]
- 68. Jauk B, Mikosch P, Gallowitsch HJ, et al. Unusual malabsorption of levothyroxine. Thyroid. 2000;10(1):93‐95. [DOI] [PubMed] [Google Scholar]
- 69. Seppel T, Rose F, Schlaghecke R. Chronic intestinal giardiasis with isolated levothyroxine malabsorption as reason for severe hypothyroidism—implications for localization of thyroid hormone absorption in the gut. Exp Clin Endocrinol Diabetes. 1996;104(2):180‐182. [DOI] [PubMed] [Google Scholar]
- 70. Checchi S, Montanaro A, Pasqui L, et al. L-thyroxine requirement in patients with autoimmune hypothyroidism and parietal cell antibodies. J Clin Endocrinol Metab. 2008;93(2):465‐469. [DOI] [PubMed] [Google Scholar]
- 71. Machado-Alba J, Valencia-Marulanda J, Jimenez-Canizales C, Salazar V, Romero D. Thyroid hormone prescription patterns in a Colombian population. Pan Am J Public Health. 2014;36(2):80‐86. [PubMed] [Google Scholar]
- 72. Campbell NRC, Hasinoff BB, Stalts H, Rao B, Wong NCW. Ferrous sulfate reduces thyroxine efficacy in patients with hypothyroidism. Ann Intern Med. 1992;117(12):1010‐1013. [DOI] [PubMed] [Google Scholar]
- 73. Singh N, Singh PN, Hershman JM. Effect of calcium carbonate on the absorption of levothyroxine. Jama. 2000;283(21):2822‐2825. [DOI] [PubMed] [Google Scholar]
- 74. Liel Y, Sperber AD, Shany S. Nonspecific intestinal adsorption of levothyroxine by aluminum hydroxide. Am J Med. 1994;97(4):363‐365. [DOI] [PubMed] [Google Scholar]
- 75. Northcutt RC, Stiel JN, Hollifield JW, StantEG, Jr. The influence of cholestyramine on thyroxine absorption. JAMA. 1969;208(10):1857‐1861. [PubMed] [Google Scholar]
- 76. Weitzman SP, Ginsburg KC, Carlson HE. Colesevelam hydrochloride and lanthanum carbonate interfere with the absorption of levothyroxine. Thyroid. 2009;19(1):77‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chon DA, Reisman T, Weinreb JE, Hershman JM, Leung AM. Concurrent milk ingestion decreases absorption of levothyroxine. Thyroid. 2018;28(4):454‐457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Liel Y, HarmanBoehm I, Shany S. Evidence for a clinically important adverse effect of fiber-enriched diet on the bioavailability of levothyroxine in adult hypothyroid patients. J Clin Endocrinol Metab. 1996;81(2):857‐859. [DOI] [PubMed] [Google Scholar]
- 79. Jonderko G, Jonderko K, Marcisz C, Kotulska A. Effect of cimetidine and ranitidine on absorption of [125I]levothyroxine administered orally. Acta Pharmacol Sin. 1992;13(5):391‐394. [PubMed] [Google Scholar]
- 80. Jubiz W, Ramirez M. Effect of vitamin C on the absorption of levothyroxine in patients with hypothyroidism and gastritis. J Clin Endocrinol Metab. 2014;99(6):E1031‐E1034. [DOI] [PubMed] [Google Scholar]
- 81. Goldberg AS, Tirona RG, Asher LJ, Kim RB, Van Uum SHM. Ciprofloxacin and Rifampin have opposite effects on levothyroxine absorption. Thyroid. 2013;23(11):1374‐1378. [DOI] [PubMed] [Google Scholar]
- 82. Lilja JJ, Laitinen K, Neuvonen PJ. Effects of grapefruit juice on the absorption of levothyroxine. Br J Clin Pharmacol. 2005;60(3):337‐341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. John-Kalarickal J, Pearlman G, Carlson HE. New medications which decrease levothyroxine absorption. Thyroid. 2007;17(8):763‐765. [DOI] [PubMed] [Google Scholar]
- 84. Kisch E, Segall HS. Interaction between simvastatin and L-thyroxine. Ann Intern Med. 2005;143(7):547. [DOI] [PubMed] [Google Scholar]
- 85. Sahajpal R, Ahmed RA, Hughes CA, Foisy MM. Probable interaction between levothyroxine and ritonavir: case report and literature review. Am J Health Syst Pharm. 2017;74(8):587‐592. [DOI] [PubMed] [Google Scholar]
- 86. Deluca F, Arrigo T, Pandullo E, Siracusano MF, Benvenga S, Trimarchi F. Changes in thyroid function tests induced by 2 month carbamazepine treatment in L-thyroxine-substituted hypothyroid children. Eur J Pediatr. 1986;145(1-2):77‐79. [DOI] [PubMed] [Google Scholar]
- 87. Guarda FJ, Findling J, Yuen KCJ, Fleseriu M, Nachtigall LB. Mifepristone increases thyroid hormone requirements in patients with central hypothyroidism: a multicenter study. J Endocr Soc. 2019;3(9):1707‐1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Nolan SR, Self TH, Norwood JM. Interaction between rifampin and levothyroxine. South Med J. 1999;92(5):529‐531. [DOI] [PubMed] [Google Scholar]
- 89. de Groot JWB, Zonnenberg BA, Plukker JTM, van Der Graaf WTA, Links TP. Imatinib induces hypothyroidism in patients receiving levothyroxinc. Clin Pharmacol Ther. 2005;78(4):433‐438. [DOI] [PubMed] [Google Scholar]
- 90. Faber J, Lumholtz IB, Kirkegaard C, et al. The effects of phenytoin (diphenylhydantoin) on the extrathyroidal turnover of thyroxine, 3,5,3′-triiodothyronine, 3,3′,5′-triiodothyronine, and 3′,5′-diiodothyronine in man. J Clin Endocrinol Metab. 1985;61(6):1093‐1099. [DOI] [PubMed] [Google Scholar]
- 91. Arafah BM. Decreased levothyroxine requirement in women with hypothyroidism during androgen therapy for breast cancer. Ann Intern Med. 1994;121(4):247‐251. [DOI] [PubMed] [Google Scholar]
- 92. Al-Alusi MA, Du L, Li N, et al. Metformin does not suppress serum thyrotropin by increasing levothyroxine absorption. Thyroid. 2015;25(10):1080‐1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Isidro ML, Penin MA, Nemina R, Cordido F. Metformin reduces thyrotropin levels in obese, diabetic women with primary hypothyroidism on thyroxine replacement therapy. Endocrine. 2007;32(1):79‐82. [DOI] [PubMed] [Google Scholar]
- 94. Csako G, McGriff NJ, Rotman-Pikielny P, Sarlis NJ, Pucino F. Exaggerated levothyroxine malabsorption due to calcium carbonate supplementation in gastrointestinal disorders. Ann Pharmacother. 2001;35(12):1578‐1583. [DOI] [PubMed] [Google Scholar]
- 95. Butner LE, Fulco PP, Feldman G. Calcium carbonate-induced hypothyroidism. Ann Intern Med. 2000;132(7):595. [DOI] [PubMed] [Google Scholar]
- 96. Wegrzyn NM. Malabsorption of L-T4 due to drip coffee: a case report using predictors of causation. J Acad Nutr Diet. 2016;116(7):1073‐1076. [DOI] [PubMed] [Google Scholar]
- 97. Cappelli C, Pirola I, Castellano M. Liquid levothyroxine formulation taken during lunch in Italy: a case report and review of the literature. Case Rep Endocrinol. 2020;2020:8858887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Cappelli C, Pirola I, Daffini L, et al. A double-blind placebo-controlled trial of liquid thyroxine ingested at breakfast: results of the TICO study. Thyroid. 2016;26(2):197‐202. [DOI] [PubMed] [Google Scholar]
- 99. Morini E, Catalano A, Lasco A, Morabito N, Benvenga S. In thyroxine-replaced hypothyroid postmenopausal women under simultaneous calcium supplementation, switch to oral liquid or softgel capsule l-thyroxine ensures lower serum TSH levels and favorable effects on blood pressure, total cholesterolemia and glycemia. Endocrine. 2019;65(3):569‐579. [DOI] [PubMed] [Google Scholar]
- 100. Vita R, Di Bari F, Benvenga S. Oral liquid levothyroxine solves the problem of tablet levothyroxine malabsorption due to concomitant intake of multiple drugs. Expert Opin Drug Deliv. 2017;14(4):467‐472. [DOI] [PubMed] [Google Scholar]
- 101. Vita R, Saraceno G, Trimarchi F, Benvenga S. A novel formulation of L-thyroxine (L-T4) reduces the problem of L-T4 malabsorption by coffee observed with traditional tablet formulations. Endocrine. 2013;43(1):154‐160. [DOI] [PubMed] [Google Scholar]
- 102. Deiana L, Marini S, Mariotti S. Ingestion of large amounts of papaya fruit and impaired effectiveness of levothyroxine therapy. Endocr Pract. 2012;18(1):98‐100. [DOI] [PubMed] [Google Scholar]
- 103. Ala S, Akha O, Kashi Z, Asgari H, Bahar A, Sasanpour N. Dose administration time from before breakfast to before dinner affect thyroid hormone levels? Caspian J Intern Med. 2015;6(3):134‐140. [PMC free article] [PubMed] [Google Scholar]
- 104. Bolk N, Visser TJ, Nijman J, Jongste IJ, Tijssen JGP, Berghout A. Effects of evening vs morning levothyroxine intake a randomized double-blind crossover trial. Arch Intern Med. 2010;170(22):1996‐2003. [DOI] [PubMed] [Google Scholar]
- 105. Pang X, Pu T, Xu L, Sun R. Effect of l-thyroxine administration before breakfast vs at bedtime on hypothyroidism: a meta-analysis. Clin Endocrinol (Oxf). 2020;92(5):475‐481. [DOI] [PubMed] [Google Scholar]
- 106. Gottwald-Hostalek U, Uhl W, Wolna P, Kahaly GJ. New levothyroxine formulation meeting 95-105% specification over the whole shelf-life: results from two pharmacokinetic trials. Curr Med Res Opin. 2017;33(2):169‐174. [DOI] [PubMed] [Google Scholar]
- 107. Lipp H-P, Hostalek U. A new formulation of levothyroxine engineered to meet new specification standards. Curr Med Res Opin. 2019;35(1):147‐150. [DOI] [PubMed] [Google Scholar]
- 108. Eisenberg M, DiStefanoJJ, III. TSH-based protocol, tablet instability, and absorption effects on L-T-4 bioequivalence. Thyroid. 2009;19(2):103‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Berg JA, Mayor GH. A study in normal human volunteers to compare the rate and extent of levothyroxine absorption from Synthroid(R) and Levoxine(R). J Clin Pharmacol. 1992;32(12):1135‐1140. [PubMed] [Google Scholar]
- 110. Copeland PM. 2 Cases of therapeutic failure associated with levothyroxine brand interchange. Ann Pharmacother. 1995;29(5):482‐485. [DOI] [PubMed] [Google Scholar]
- 111. Dong BJ, Brown CH. Hypothyroidism resulting from generic levothyroxine failure. J Am Board Fam Pract. 1991;4(3):167‐170. [PubMed] [Google Scholar]
- 112. Kaur N, Suryanarayanan R. Investigating the influence of excipients on the stability of levothyroxine sodium pentahydrate. Mol Pharm. 2021;18(7):2683‐2693. [DOI] [PubMed] [Google Scholar]
- 113. Ledeti I, Romanescu M, Circioban D, et al. Stability and compatibility studies of levothyroxine sodium in solid binary systems-instrumental screening. Pharmaceutics. 2020;12(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Patel H, Stalcup A, Dansereau R, Sakr A. The effect of excipients on the stability of levothyroxine sodium pentahydrate tablets. Int J Pharm. 2003;264(1-2):35‐43. [DOI] [PubMed] [Google Scholar]
- 115. Olveira G, Almaraz MC, Soriguer F, et al. Altered bioavailability due to changes in the formulation of a commercial preparation of levothyroxine in patients with differentiated thyroid carcinoma. Clin Endocrinol (Oxf). 1997;46(6):707‐711. [DOI] [PubMed] [Google Scholar]
- 116. Casassus B. Pharmacovigilance risks of reformulation: French patients complain after Merck modifies levothyroxine pills. Br Med J. 2018;360:k714. [DOI] [PubMed] [Google Scholar]
- 117. Abou-Taleb BA, Bondok M, Nounou MI, Khalafallah N, Khalil S. Are multisource levothyroxine sodium tablets marketed in Egypt interchangeable? Ann D Endocrinol. 2018;79(1):23‐29. [DOI] [PubMed] [Google Scholar]
- 118. Hennessey JV, Malabanan AO, Haugen BR, Levy EG, Pharmacovigilance Task Force A . Adverse event reporting in patients treated with levothyroxine: results of the pharmacovigilance task force survey of the American Thyroid Association, American Association of Clinical Endocrinologists, and the Endocrine Society. Endocr Pract. 2010;16(3):357‐370. [DOI] [PubMed] [Google Scholar]
- 119. Brito JP, Deng Y, Ross JS, et al. Rates of, and factors associated with, switching among generic levothyroxine preparations in commercially insured American adults. Endocrine. 2022;76(2):349‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Brito JP, Deng Y, Ross JS, et al. Association between generic-to-generic levothyroxine switching and thyrotropin levels among US adults. JAMA Intern Med. 2022;182(4):418‐425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Abdallah S, Mohamed I. Factor affecting photo and thermal stability of levothyroxine sodium. Br J Pharm Res. 2016;10(2):1‐11. [Google Scholar]
- 122. Hamad ML, Engen W, Morris KR. Impact of hydration state and molecular oxygen on the chemical stability of levothyroxine sodium. Pharm Dev Technol. 2015;20(3):314‐319. [DOI] [PubMed] [Google Scholar]
- 123. Benvenga S, Papi G, Antonelli A. Refractory hypothyroidism due to improper storage of levothyroxine tablets. Front Endocrinol (Lausanne). 2017;8:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Cappelli C, Castello R, Marini F, et al. Adherence to levothyroxine treatment among patients with hypothyroidism: a northeastern Italian survey. Front Endocrinol. 2018;9:699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Hepp Z, Lage MJ, Espaillat R, Gossain VV. The association between adherence to levothyroxine and economic and clinical outcomes in patients with hypothyroidism in the US. J Med Econ. 2018;21(9):912‐919. [DOI] [PubMed] [Google Scholar]
- 126. Siscart J, Oros M, Serna MC, Perejon D, Galvan L, Ortega M. Adherence to treatment for hypothyroidism in pregnancy and relationship with thyrotropin control: a retrospective observational cohort study. BMC Pregnancy Childbirth. 2022;22(1):168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Bezerra YM, Pereira I, Sarubbi RD, Viana Júnior AB, Quidute ARP. [Polypharmacy simulation and pharmacotherapy perceptions among students from a university in Ceará: a pilot study.] Simulação de polimedicação e percepções sobre farmacoterapia em estudantes de universidade no Ceará: estudo-piloto. Rev Bras Educ Méd. 2021;45(3):e158. [Google Scholar]
- 128. Kubota S, Fukata S, Matsuzuka F, Kuma K, Miyauchi A. Successful management of a patient with pseudomal absorption of levothyroxine. Int J Psychiatry Med. 2003;33(2):183‐188. [DOI] [PubMed] [Google Scholar]
- 129. Van Wilder N, Bravenboer B, Herremans S, Vanderbruggen N, Velkeniers B. Pseudomalabsorption of levothyroxine: a challenge for the endocrinologist in the treatment of hypothyroidism. Eur Thyroid J. 2017;6(1):52‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Molines L, Fromont I, Morlet-Barla N, Nogueira J-P, Valero R, Vialettes B. L-thyroxine pseudomalabsorption: a factitious disease. Presse Medicale. 2007;36(10):1390‐1394. [DOI] [PubMed] [Google Scholar]
- 131. Gonzales KM, Stan MN, MorrisJC, III, Bernet V, Castro MR. The levothyroxine absorption test: a four-year experience (2015-2018) at the Mayo Clinic. Thyroid. 2019;29(12):1734‐1742. [DOI] [PubMed] [Google Scholar]
- 132. Simsir IY, Soyaltin UE, Ozgen AG. Levothyroxine absorption test results in patients with TSH elevation resistant to treatment. Endocrine. 2019;64(1):118‐121. [DOI] [PubMed] [Google Scholar]
- 133. Elbasan O, Yavuz DG. Refractory hypothyroidism to levothyroxine treatment: five cases of pseudomalabsorption. Acta Endocrinol (Buchar). 2020;16(3):339‐345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Balla M, Jhingan RM, Rubin DJ. Rapid levothyroxine absorption testing: a case series of nonadherent patients. Int J Endocrinol Metab. 2015;13(4):e31051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Rdzak GM, Whitman LM, Inzucchi SE. Levothyroxine pseudo-malabsorption: testing and treatment in the outpatient setting. Ther Adv Endocrinol Metab. 2018;9(7):217‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Livadariu E, Valdes-Socin H, Burlaeu MC, Vulpoi C, Daly AF, Beckers A. Pseudomalabsorption of thyroid hormones: case report and review of the literature. Ann Endocrinol. 2007;68(6):460‐463. [DOI] [PubMed] [Google Scholar]
- 137. Crilly M, Esmail A. Randomised controlled trial of a hypothyroid educational booklet to improve thyroxine adherence. Br J Gen Pract. 2005;55(514):362‐368. [PMC free article] [PubMed] [Google Scholar]
- 138. Toro-Diez AD, Sola-Sanchez E, Mangual-Garcia M. Effect of once weekly oral levothyroxine therapy. Endocrinol Diabetes Metab Case Rep. Published online July 1, 2021. Doi: 10.1530/EDM-21-0045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Wiersinga WM. Thyroid hormone replacement therapy. Horm Res. 2001;56(Suppl. 1):74‐81. [DOI] [PubMed] [Google Scholar]
- 140. Vita R, Fallahi P, Antonelli A, Benvenga S. The administration of L-thyroxine as soft gel capsule or liquid solution. Expert Opin Drug Deliv. 2014;11(7):1103‐1111. [DOI] [PubMed] [Google Scholar]
- 141. Virili C, Trimboli P, Centanni M. Novel thyroxine formulations: a further step toward precision medicine. Endocrine. 2019;66(1):87‐94. [DOI] [PubMed] [Google Scholar]
- 142. Appelhof BC, Fliers E, Wekking EM, et al. Combined therapy with levothyroxine and liothyronine in two ratios, compared with levothyroxine monotherapy in primary hypothyroidism: a double-blind, randomized, controlled clinical trial. J Clin Endocrinol Metab. 2005;90(5):2666‐2674. [DOI] [PubMed] [Google Scholar]
- 143. Wiersinga WM. T4 + T3 combination therapy: an unsolved problem of increasing magnitude and complexity. Endocrinol Metab. 2021;36(5):938‐951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Abdalla SM, Bianco AC. Defending plasma T3 is a biological priority. Clin Endocrinol (Oxf). 2014;81(5):633‐641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Gullo D, Latina A, Frasca F, Le Moli R, Pellegriti G, Vigneri R. Levothyroxine monotherapy cannot guarantee euthyroidism in all athyreotic patients. PLoS One. 2011;6(8):e22552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Escobar-Morreale HF, Obregon MJ, Escobar del Rey F, Morreale de Escobar G. Replacement therapy for hypothyroidism with thyroxine alone does not ensure euthyroidism in all tissues, as studied in thyroidectomized rats. J Clin Invest. 1995;96(6):2828‐2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Negro R, Attanasio R, Nagy EV, Papini E, Perros P, Hegedus L. Use of thyroid hormones in hypothyroid and euthyroid patients; the 2019 Italian survey. Eur Thyroid J. 2020;9(1):25‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Jonklaas J, Bianco AC, Cappola AR, et al. Evidence-based use of levothyroxine/liothyronine combinations in treating hypothyroidism: a consensus document. Thyroid. 2021;31(2):156‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Wiersinga WM, Duntas L, Fadeyev V, Nygaard B, Vanderpump MP. 2012 ETA guidelines: the use of L-T4 + L-T3 in the treatment of hypothyroidism. Eur Thyroid J. 2012;1(2):55‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Celi FS, Zemskova M, Linderman JD, et al. The pharmacodynamic equivalence of levothyroxine and liothyronine: a randomized, double blind, cross-over study in thyroidectomized patients. Clin Endocrinol (Oxf). 2010;72(5):709‐715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Pilo A, Iervasi G, Vitek F, Ferdeghini M, Cazzuola F, Bianchi R. Thyroidal and peripheral production of 3,5,3′-triiodothyronine in humans by multicompartmental analysis. Am J Physiol. 1990;258(4):E715‐E726. [DOI] [PubMed] [Google Scholar]
- 152. Yavuz S, Linderman JD, Smith S, Zhao X, Pucino F, Celi FS. The dynamic pituitary response to escalating-dose TRH stimulation test in hypothyroid patients treated with liothyronine or levothyroxine replacement therapy. J Clin Endocrinol Metab. 2013;98(5):E862‐E866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Eisenberg M, Samuels M, DiStefanoJJ, III. Extensions, validation, and clinical applications of a feedback control system simulator of the hypothalamo-pituitary-thyroid axis. Thyroid. 2008;18(10):1071‐1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. DiStefano J III, Jonklaas J. Predicting optimal combination LT4 + LT3 therapy for hypothyroidism based on residual thyroid function. Front Endocrinol (Lausanne). 2019;10:746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Heald AH, Premawardhana L, Taylor P, et al. Is there a role for natural desiccated thyroid in the treatment of levothyroxine unresponsive hypothyroidism? Results from a consecutive case series. Int J Clin Pract. 2021;75(12):e14967. [DOI] [PubMed] [Google Scholar]
- 156. Alomari M, Vuddanda PR, Trenfield SJ, et al. Printing T-3 and T-4 oral drug combinations as a novel strategy for hypothyroidism. Int J Pharm. 2018;549(1-2):363‐369. [DOI] [PubMed] [Google Scholar]
- 157. Vakili H, Wickstrom H, Desai D, Preis M, Sandler N. Application of a handheld NIR spectrometer in prediction of drug content in inkjet printed orodispersible formulations containing prednisolone and levothyroxine. Int J Pharm. 2017;524(1-2):414‐423. [DOI] [PubMed] [Google Scholar]
- 158. Gadiraju S, Lee CJ, Cooper DS. Levothyroxine dosing following bariatric surgery. Obes Surg. 2016;26(10):2538‐2542. [DOI] [PubMed] [Google Scholar]
- 159. Fish LH, Schwartz HL, Cavanaugh J, Steffes MW, Bantle JP, Oppenheimer JH. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism—role of triiodothyronine in pituitary feedback in humans. N Engl J Med. 1987;316(13):764‐770. [DOI] [PubMed] [Google Scholar]
- 160. Escobar-Morreale HF, Botella-Carretero JI, de Escobar GM. Treatment of hypothyroidism with levothyroxine or a combination of levothyroxine plus L-triiodothyronine. Best Pract Res Clin Endocrinol Metab. 2015;29(1):57‐75. [DOI] [PubMed] [Google Scholar]
- 161. Grozinsky-Glasberg S, Fraser A, Nahshoni E, Weizman A, Leibovici L. Thyroxine-triiodothyronine combination therapy versus thyroxine monotherapy for clinical hypothyroidism: meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2006;91(7):2592‐2599. [DOI] [PubMed] [Google Scholar]
- 162. Joffe RT, Brimacombe M, Levitt AJ, Stagnaro-Green A. Treatment of clinical hypothyroidism with thyroxine and triiodothyronine: a literature review and metaanalysis. Psychosomatics. 2007;48(5):379‐384. [DOI] [PubMed] [Google Scholar]
- 163. Ma C, Xie J, Huang X, et al. Thyroxine alone or thyroxine plus triiodothyronine replacement therapy for hypothyroidism. Nucl Med Commun. 2009;30(8):586‐593. [DOI] [PubMed] [Google Scholar]
- 164. Akirov A, Fazelzad R, Ezzat S, Thabanes L, Sawka AM. A systematic review and meta-analysis of patient preferences for combination thyroid hormone treatment for hypothyroidism. Front Endocrinol (Lausanne). 2019;10(477):477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. de Jong NW, Baljet GM. Use of T4, T4 + T3, and T3 in the Dutch population in the period 2005-2011. Eur Thyroid J. 2012;1(2):135‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Saravanan P, Siddlque H, Simmons DJ, Greenwood R, Dayan CM. Twenty-four hour hormone profiles of TSH, free T3 and free T4 in hypothyroid patients on combined T3/T4 therapy. Exp Clin Endocrinol Diabetes. 2007;115(4):261‐267. [DOI] [PubMed] [Google Scholar]
- 167. Russell W, Harrison RF, Smith N, et al. Free triiodothyronine has a distinct circadian rhythm that is delayed but parallels thyrotropin levels. J Clin Endocrinol Metab. 2008;93(6):2300‐2306. [DOI] [PubMed] [Google Scholar]
- 168. Jonklaas J, Burman KD. Daily Administration of short-acting liothyronine is associated with significant triiodothyronine excursions and fails to alter thyroid-responsive parameters. Thyroid. 2016;26(6):770‐778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Bauer DC, Ettinger B, Nevitt MC, Stone KL, Study Osteoporotic Fractures Res G . Risk for fracture in women with low serum levels of thyroid-stimulating hormone. Ann Intern Med. 2001; 134(7):561‐568. [DOI] [PubMed] [Google Scholar]
- 170. Biondi B, Fazio S, Palmieri EA, et al. Effects of chronic subclinical hyperthyroidism from levothyroxine on cardiac morphology and function. Effetti dell'ipertiroidismo subclinico cronico da terapia con levotiroxina su morfologia e funzione cardiaca. Cardiologia. 1999;44(5):443‐449. [PubMed] [Google Scholar]
- 171. Yi W, Kim BH, Kim M, et al. Heart failure and stroke risks in users of liothyronine with or without levothyroxine compared with levothyroxine alone: a propensity score-matched analysis. Thyroid. 2022;32(7):764‐771. [DOI] [PubMed] [Google Scholar]
- 172. Hennemann G, Docter R, Visser TJ, Postema PT, Krenning EP. Thyroxine plus low-dose, slow-release triiodothyronine replacement in hypothyroidism: proof of principle. Thyroid. 2004;14(4):271‐275. [DOI] [PubMed] [Google Scholar]
- 173. Bakhteyar H, Cassone C, Kohan HG, Sani SN. Kinetic analysis of drug release from compounded slow-release capsules of liothyronine sodium (T3). Int J Pharm Compd. 2017;21(5):418‐425. [PubMed] [Google Scholar]
- 174. Bianco AC, Anderson G, Forrest D, et al. American Thyroid association guide to investigating thyroid hormone economy and action in rodent and cell models. Thyroid. 2014;24(1):88‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Idrees T, Price JD, Piccariello T, Bianco AC. Sustained release T3 therapy: animal models and translational applications. Front Endocrinol (Lausanne). 2019;10:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Santini F, Chiovato L, Bartalena L, et al. Study of serum 3,5,3′-triiodothyronine sulfate concentration in patients with systemic non-thyroidal illness. Eur J Endocrinol. 1996;134(1):45‐49. [DOI] [PubMed] [Google Scholar]
- 177. Santini F, Giannetti M, Ricco I, et al. Steady-state serum T-3 concentrations for 48 hours following the oral administration of a single dose of 3,5,3′-triiodothyronine sulfate (T3S). Endocr Pract. 2014;20(7):680‐689. [DOI] [PubMed] [Google Scholar]
- 178. Santini F, Ceccarini G, Pelosini C, et al. Treatment of hypothyroid patients with L-thyroxine (L-T4) plus triiodothyronine sulfate (T3S). A phase II, open-label, single center, parallel groups study on therapeutic efficacy and tolerability. Front Endocrinol (Lausanne). 2019;10(826):826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Da Conceicao RR, Fernandes GW, Fonseca TL, Bocco BMLC, Bianco AC. Metal coordinated poly-zinc-liothyronine provides stable circulating triiodothyronine levels in hypothyroid rats. Thyroid. 2018;28(11):1425‐1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Dumitrescu AM, Hanlon EC, Arosemena M, et al. Extended absorption of liothyronine from poly-zinc-liothyronine: results from a phase 1, double-blind, randomized, and controlled study in humans. Thyroid. 2022;32(2):196‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Bornikowska K, Gietka-Czernel M, Raczkiewicz D, Glinicki P, Zgliczynski W. Improvements in quality of life and thyroid parameters in hypothyroid patients on ethanol-free formula of liquid levothyroxine therapy in comparison to tablet LT4 form: an observational study. J Clin Med. 2021;10(22):5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Ferrara R, Ientile V, Arcoraci V, et al. Treatment pattern and frequency of serum TSH measurement in users of different levothyroxine formulations: a population-based study during the years 2009-2015. Endocrine. 2017;58(1):143‐152. [DOI] [PubMed] [Google Scholar]
- 183. Al-Numani D, Scarsi C, Ducharme MP. Levothyroxine soft capsules demonstrate bioequivalent pharmacokinetic exposure with the European reference tablets in healthy volunteers under fasting conditions. Int J Clin Pharmacol Ther. 2016;54(2):135‐143. [DOI] [PubMed] [Google Scholar]
- 184. Grussendorf M, Vaupel R, Wegscheider K. Bioequivalence of L-thyroxine tablets and a liquid L-thyroxine solution in the treatment of hypothyroid patients. Med Klin. 2004;99(11):639‐644. [DOI] [PubMed] [Google Scholar]
- 185. Koytchev R, Lauschner R. Bioequivalence study of levothyroxine tablets compared to reference tablets and an oral solution. Arzneimittel-Forschung-Drug Res. 2004;54(10):680‐684. [DOI] [PubMed] [Google Scholar]
- 186. Yue CS, Scarsi C, Ducharme MP. Pharmacokinetics and potential advantages of a new oral solution of levothyroxine vs. other available dosage forms. Arzneimittel-Forschung-Drug Res. 2012;62(12):631‐636. [DOI] [PubMed] [Google Scholar]
- 187. Negro R, Valcavi R, Agrimi D, Toulis KA. Levothyroxine liquid solution versus tablet for replacement treatment in hypothyroid patients. Endocr Pract. 2014;20(9):901‐906. [DOI] [PubMed] [Google Scholar]
- 188. Cassio A, Monti S, Rizzello A, et al. Comparison between liquid and tablet formulations of levothyroxine in the initial treatment of congenital hypothyroidism. J Pediatr. 2013;162(6):1264‐1269.e1-2. [DOI] [PubMed] [Google Scholar]
- 189. Peroni E, Vigone MC, Mora S, et al. Congenital hypothyroidism treatment in infants: a comparative study between liquid and tablet formulations of levothyroxine. Horm Res Paediatr. 2014;81(1):50‐54. [DOI] [PubMed] [Google Scholar]
- 190. Virili C, Giovanella L, Fallahi P, et al. Levothyroxine therapy: changes of TSH levels by switching patients from tablet to liquid formulation. a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2018;9(10):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Markantes GK, Dimitropoulos K, Mamali I, et al. Therapeutic equivalence of a new preparation of liquid levothyroxine with tablets in patients with overt primary hypothyroidism. Eur Thyroid J. 2021;10(1):59‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Cherella CE, Wassner AJ. Update on congenital hypothyroidism. Curr Opin Endocrinol Diabetes Obesity. 2020;27(1):63‐69. [DOI] [PubMed] [Google Scholar]
- 193. Pirola I, Daffini L, Gandossi E, et al. Comparison between liquid and tablet levothyroxine formulations in patients treated through enteral feeding tube. J Endocrinol Invest. 2014;37(6):583‐587. [DOI] [PubMed] [Google Scholar]
- 194. Rajendran A, Bhavani N, Nair V, Pavithran PV, Menon VU, Kumar H. Oral levothyroxine is an effective option for myxedema coma: a single-centre experience. Eur Thyroid J. 2021;10(1):52‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Liu H. Data from: Supplemental materials for the review “Levothyroxine: impaired bioavailability and novel drug delivery formulations”. Mendeley Data 2022. Deposited March 25, 2022.
- 196. Benvenga S, Di Bari F, Vita R. Undertreated hypothyroidism due to calcium or iron supplementation corrected by oral liquid levothyroxine. Endocrine. 2017;56(1):138‐145. [DOI] [PubMed] [Google Scholar]
- 197. Vita R, Saraceno G, Trimarchi F, Benvenga S. Switching levothyroxine from the tablet to the oral solution formulation corrects the impaired absorption of levothyroxine induced by proton-pump inhibitors. J Clin Endocrinol Metab. 2014;99(12):4481‐4486. [DOI] [PubMed] [Google Scholar]
- 198. Benvenga S. Liquid and softgel capsules of 1-thyroxine results lower serum thyrotropin levels more than tablet formulations in hypothyroid patients. J Clin Transl Endocrinol. 2019;18:100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Fallahi P, Ferrari SM, Camastra S, et al. TSH Normalization in bariatric surgery patients after the switch from l-thyroxine in tablet to an oral liquid formulation. Obes Surg. 2017;27(1):78‐82. [DOI] [PubMed] [Google Scholar]
- 200. Cappelli C, Negro R, Pirola I, Gandossi E, Agosti B, Castellano M. Levothyroxine liquid solution versus tablet form for replacement treatment in pregnant women. Gynecol Endocrinol. 2016;32(4):290‐292. [DOI] [PubMed] [Google Scholar]
- 201. Fallahi P, Ferrari SM, Ruffilli I, Antonelli A. Reversible normalisation of serum TSH levels in patients with autoimmune atrophic gastritis who received L-T4 in tablet form after switching to an oral liquid formulation: a case series. BMC Gastroenterol. 2016;16(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Reardon DP, Yoo PS. Levothyroxine tablet malabsorption associated with gastroparesis corrected with gelatin capsule formulation. Case Rep Endocrinol. 2016;2016:1316724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Asamoah E. Levothyroxine sodium oral solution to control thyroid function in a patient with hypothyroidism and celiac disease. Clin Case Rep. 2021;9(5):e04170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Baldini IM, Cocino C, Seghezzi S, Cappellini MD. TSH-suppressive therapy: a thorny issue. Eur J Case Rep Intern Med. 2017;4(2):000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Cappelli C, Pirola I, Gandossi E, Formenti A, Castellano M. Oral liquid levothyroxine treatment at breakfast: a mistake? Eur J Endocrinol. 2014;170(1):95‐99. [DOI] [PubMed] [Google Scholar]
- 206. Cappelli C, Pirola I, Gandossi E, et al. Thyroid hormone profile in patients ingesting soft gel capsule or liquid levothyroxine formulations with breakfast. Int J Endocrinol. 2016;2016:9043450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Marina M, Ceda GP, Aloe R, Gnocchi C, Ceresini G. Circulating concentrations of free thyroxine after an oral intake of liquid LT4 taken either during fasting conditions or at breakfast. Acta Bio-Med. 2016;87(3):247‐252. [PMC free article] [PubMed] [Google Scholar]
- 208. Morelli S, Reboldi G, Moretti S, Menicali E, Avenia N, Puxeddu E. Timing of breakfast does not influence therapeutic efficacy of liquid levothyroxine formulation. Endocrine. 2016;52(3):571‐578. [DOI] [PubMed] [Google Scholar]
- 209. Pirola I, Gandossi E, Brancato D, et al. TSH Evaluation in hypothyroid patients assuming liquid levothyroxine at breakfast or 30 minutes before breakfast. J Endocrinol Invest. 2018;41(11):1301‐1306. [DOI] [PubMed] [Google Scholar]
- 210. Ducharme M, Scarsi C, Bettazzi E, Mautone G, Lewis Y, Celi FS. A novel levothyroxine solution results in similar bioavailability whether taken 30 or just 15 minutes before a high-fat high-calorie meal. Thyroid. 2022;32(8):897‐904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Bernareggi A, Grata E, Pinorini MT, Conti A. Oral liquid formulation of levothyroxine is stable in breakfast beverages and may improve thyroid patient compliance. Pharmaceutics. 2013;5(4):621‐633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Guglielmi R, Grimaldi F, Negro R, et al. Shift from levothyroxine tablets to liquid formulation at breakfast improves quality of life of hypothyroid patients. Endocr Metab Immune Disord-Drug Targets. 2018;18(3):235‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Trimboli P, Scappaticcio L, De Bellis A, et al. Different formulations of levothyroxine for treating hypothyroidism: a real-life study. Int J Endocrinol. 2020;2020:4524759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Lombardi CP, Bocale R, Barini A, et al. Comparative study between the effects of replacement therapy with liquid and tablet formulations of levothyroxine on mood states, self-perceived psychological well-being and thyroid hormone profile in recently thyroidectomized patients. Endocrine. 2017;55(1):60‐68. [DOI] [PubMed] [Google Scholar]
- 215. Price guide (Drug.com) . Levothyroxine Prices, Coupons and Patient Assistance Programs. Accessed February 19, 2022. https://www.drugs.com/price-guide/levothyroxine
- 216. Tuli G, Munarin J, de Sanctis L. Comparison among two liquid formulations of L-thyroxine in the treatment of congenital hypothyroidism in the first month of life: a pilot study. Front Endocrinol (Lausanne). 2022;13:860775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Benvenga S, Di Bari F. Intestinal absorption and buccal absorption of liquid levothyroxine. Endocrine. 2017;58(3):591‐594. [DOI] [PubMed] [Google Scholar]
- 218. Formenti AM, Mazziotti G, Giubbini R, Giustina A. Treatment of hypothyroidism: all that glitters is gold? Endocrine. 2016;52(3):411‐413. [DOI] [PubMed] [Google Scholar]
- 219. Laurent I, Tang S, Astère M, et al. Liquid L-thyroxine versus tablet L-thyroxine in patients on L- thyroxine replacement or suppressive therapy: a meta-analysis. Endocrine 2018;61(1):28‐35. Doi: 10.1007/s12020-018-1574-8 [DOI] [PubMed] [Google Scholar]
- 220. Guglielmi R, Frasoldati A, Zini M, et al. Italian Association of Clinical Endocrinologists statement-replacement therapy for primary hypothyroidism: a brief guide for clinical practice. Endocr Pract. 2016;22(11):1319‐1326. [DOI] [PubMed] [Google Scholar]
- 221. Colucci P, D'Angelo P, Mautone G, Scarsi C, Ducharme MP. Pharmacokinetic equivalence of a levothyroxine sodium soft capsule manufactured using the New Food and Drug Administration potency guidelines in healthy volunteers under fasting conditions. Ther Drug Monit. 2011;33(3):355‐361. [DOI] [PubMed] [Google Scholar]
- 222. U.S. Food and Drug Administration . Drug Approval Package of Tirosint (levothyroxine sodium capsules) Company: Institut Biochimique SA (IBSA) NDA: 22121. Accessed March 12, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/022121_tirosint_toc.cfm
- 223. Benvenga S. (Soft) capsules of wisdom: preventing myo-inositol malabsorption caused by coffee. Expert Opin Drug Deliv. 2012;9(10):1177‐1179. [DOI] [PubMed] [Google Scholar]
- 224. Di Donna V, Paragliola RM, de Waure C, Papi G, Pontecorvi A, Corsello SM. Is levothyroxine requirement the same for tablet and soft gel formulations? Endocrine. 2018;59(2):458‐460. [DOI] [PubMed] [Google Scholar]
- 225. Tanguay M, Girard J, Scarsi C, Mautone G, Larouche R. Pharmacokinetics and comparative bioavailability of a levothyroxine sodium oral solution and soft capsule. Clin Pharmacol Drug Dev. 2019;8(4):521‐528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Vita R, Benvenga S. Tablet levothyroxine (L-T4) malabsorption induced by proton pump inhibitor: a problem that was solved by switching to L-T4 in soft gel capsule. Endocr Pract. 2014;20(3):E38‐E41. [DOI] [PubMed] [Google Scholar]
- 227. Santaguida MG, Virili C, Del Duca SC, et al. Thyroxine softgel capsule in patients with gastric-related T-4 malabsorption. Endocrine. 2015;49(1):51‐57. [DOI] [PubMed] [Google Scholar]
- 228. Fiorini G, Ribichini D, Pasquali R, Vaira D. In vivo dissolution of levothyroxine soft gel capsules. Intern Emerg Med. 2016;11(8):1151‐1152. [DOI] [PubMed] [Google Scholar]
- 229. Ernst FR, Sandulli W, Elmor R, Welstead J, Sterman AB, Lavan M. Retrospective study of patients switched from tablet formulations to a gel cap formulation of levothyroxine: results of the control switch study. Drugs R D. 2017;17(1):103‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Carter C, Elmor R, Sandulli W. The economic impact of optimizing therapy in ‘hard-to-treat’ and ‘harder-to-treat’ hypothyroid patients by changing the levothyroxine formulation from tablets to gel caps. Value Health. 2017;20(9):A920. [Google Scholar]
- 231. Messina E, Ferrau F, Cannavo S. Oral mucositis induced by treatment with soft gel formulation of levothyroxine. Endocrine. 2018;59(1):226‐227. [DOI] [PubMed] [Google Scholar]
- 232. Stadalman KA, Kelner MJ, Box K, Dominguez A, Rigby JF. Stability of levothyroxine sodium 0.4 mu g/mL in 0.9% sodium chloride injection. Prog Transpl. 2009;19(4):354‐357. [DOI] [PubMed] [Google Scholar]
- 233. Papi G, Corsello SM, Pontecorvi A. Clinical concepts on thyroid emergencies. Front Endocrinol (Lausanne). 2014;5:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Avichal D, Kravets I. Intravenous levothyroxine during hemodialysis in a patient with hypothyroidism and non-adherence to oral medications. AACE Clin Case Rep. 2020;6(5):e230‐e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Schoemig CS, Robinson M-E, von Oettingen JE. Treatment of congenital hypothyroidism in a newborn with malabsorption after subtotal ileum resection. Endocrinol Diabetes Metabol Case Rep. 2018;2018:170156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Hays MT. Parenteral thyroxine administration. Thyroid. 2007;17(2):127‐129. [DOI] [PubMed] [Google Scholar]
- 237. Ritter MJ, Gupta S, Hennessey JV. Alternative routes of levothyroxine administration for hypothyroidism. Curr Opin Endocrinol Diabetes Obes. 2020;27(5):318‐322. [DOI] [PubMed] [Google Scholar]
- 238. Bacci V, Schussler GC, Bhogal RS, Carter AC. Cardiac-arrest after intravenous administration of levothyroxine. JAMA. 1981;245(9):920. [PubMed] [Google Scholar]
- 239. Yamamoto T, Fukuyama J, Fujiyoshi A. Factors associated with mortality of myxedema coma: report of eight cases and literature survey. Thyroid. 1999;9(12):1167‐1174. [DOI] [PubMed] [Google Scholar]
- 240. Barlow BT, Roberts RJ, Newman K, Harrison SK, Sin JH. Economic evaluation of a pharmacist-led 5-day therapeutic hold of IV levothyroxine at an academic medical center. Hosp Pharm. 2020;57(1):20‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Marino KK, Crowley KE, Tran LK, et al. Intravenous levothyroxine stewardship program at a tertiary academic medical center. Am J Health Syst Pharm. 2021;78(13):1200‐1206. [DOI] [PubMed] [Google Scholar]
- 242. Hariharan R, Maulik K, Karunakar P, et al. Is oral levothyroxine as effective as its intravenous form in myxoedema crisis with catecholamine-refractory shock? J Paediatr Child Health. 2021;58(4):717‐718. [DOI] [PubMed] [Google Scholar]
- 243. Peynirci H, Taskiran B, Erturk E, Sisman P, Ersoy C. Is parenteral levothyroxine therapy safe in intractable hypothyroidism? J Natl Med Assoc. 2018;110(3):245‐249. [DOI] [PubMed] [Google Scholar]
- 244. Aponte PA, Mitre N, Feldt MM. More than one way to skin a thyroid. Managing pediatric primary hypothyroidism with weekly intramuscular levothyroxine: a case series report. Endocr Rev. 2014; 35(3). [DOI] [PubMed] [Google Scholar]
- 245. Kempke J, Hussain H, Bhan B, Graves L. Treatment of thyroxine malabsorption. J Endocrinol Metab. 2015;5(1-2):192‐195. [Google Scholar]
- 246. Laycock K, Beale C, Peters C, Anderson J. Intramuscular levothyroxine: a solution to a mental health related thyroid crisis. BMJ Case Rep. 2019;12(8):e228147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Taylor PN, Tabasum A, Sanki G, et al. Weekly intramuscular injection of levothyroxine following myxoedema: a practical solution to an old crisis. Case Rep Endocrinol. 2015;2015:169194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Groener JB, Lehnhoff D, Piel D, Nawroth PP, Schanz J, Rudofsky G. Subcutaneous application of levothyroxine as successful treatment option in a patient with malabsorption. Am J Case Rep. 2013;14:48‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Caron P, Moya CM, Malet D, et al. Compound heterozygous mutations in the thyroglobulin gene (1143delC and 6725G3 -> A R2223H) resulting in fetal goitrous hypothyroidism. J Clin Endocrinol Metab. 2003;88(8):3546‐3553. [DOI] [PubMed] [Google Scholar]
- 250. Ghazi AAM, Ordookhani A, Pourafkari M, et al. Intrauterine diagnosis and management of fetal goitrous hypothyroidism: a report of an Iranian family with three consecutive pregnancies complicated by fetal Goiter. Thyroid. 2005;15(12):1341‐1347. [DOI] [PubMed] [Google Scholar]
- 251. Stoupa A, Chehade GAH, Kariyawasam D, et al. First case of fetal goitrous hypothyroidism due to SLC5A5/NIS mutations. Eur J Endocrinol. 2020;183(5):K1‐K5. [DOI] [PubMed] [Google Scholar]
- 252. Machado CM, Castro JM, Campos RA, Oliveira MJ. Graves' disease complicated by fetal goitrous hypothyroidism treated with intra-amniotic administration of levothyroxine. BMJ Case Rep. 2019;12(8):e230457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Blidda S, Rasmussen AK, Sundberg K, Brocks V, Skovbo P, Feldt-Rasmussen U. Graves' disease in two pregnancies complicated by fetal goitrous hypothyroidism: successful in utero treatment with levothyroxine. Thyroid. 2011;21(1):75‐81. [DOI] [PubMed] [Google Scholar]
- 254. Gruner C, Kollert A, Wildt L, Dorr HG, Beinder E, Lang N. Intrauterine treatment of fetal goitrous hypothyroidism controlled by determination of thyroid-stimulating hormone in fetal serum—a case report and review of the literature. Fetal Diagn Ther. 2001;16(1):47‐51. [DOI] [PubMed] [Google Scholar]
- 255. Nemescu D, Tanasa IA, Stoian DL, Navolan DB, Vinturache AE. Conservative in utero treatment of fetal dyshormonogenetic goiter with levothyroxine, a systematic literature review. Exp Ther Med. 2020;20(3):2434‐2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Vasudevan P, Powell C, Nicholas AK, et al. Intrauterine death following intraamniotic triiodothyronine and thyroxine therapy for fetal goitrous hypothyroidism associated with polyhydramnios and caused by a thyroglobulin mutation. Endocrinol Diabetes Metab Case Rep. 2017:17-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Ybarra M, dos Santos TJ, Cabido Pinheiro CT, Dichtchekenian V, Damiani D. Rectal levothyroxine for the treatment of hypothyroidism: a case study. Pediatrics. 2018; 142(2):e20173317. [DOI] [PubMed] [Google Scholar]
- 258. Obeidat KA, Saadeh NA, As'ad A, Bakkar S. Successful management of hypothyroidism in gastric outlet obstruction using levothyroxine rectal enemas: a case report. Am J Case Rep. 2018;19:903‐905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Hamada Y, Masuda K, Okubo M, Nakasa H, Sekine Y, Ishii I. Pharmaceutical studies of levothyroxine sodium hydrate suppository provided as a hospital preparation. Biol Pharm Bull. 2015;38(4):625‐628. [DOI] [PubMed] [Google Scholar]
- 260. Bitterman W, Spencer RJ, Huizenga KA, Shorter RG. Contact pH of rectal mucosa in humans and dogs. Dis Colon Rectum. 1969;12(2):96‐98. [DOI] [PubMed] [Google Scholar]
- 261. Kashiwagura Y, Uchida S, Tanaka S, et al. Clinical efficacy and pharmacokinetics of levothyroxine suppository in patients with hypothyroidism. Biol Pharm Bull. 2014;37(4):666‐670. [DOI] [PubMed] [Google Scholar]
- 262. Peirce C, Ippolito S, Lanas A, et al. Treatment of refractory and severe hypothyroidism with sublingual levothyroxine in liquid formulation. Endocrine. 2018;60(1):193‐196. [DOI] [PubMed] [Google Scholar]
- 263. Agu RU, Mactavish J, Yeung PK, Imran SA. Thyroid hormone (levothyroxine) replacement via the respiratory route by inhalation: in vitro exploratory studies. Expert Opin Drug Deliv. 2016;13(2):195‐205. [DOI] [PubMed] [Google Scholar]
- 264. Padula C, Pappani A, Santi P. In vitro permeation of levothyroxine across the skin. Int J Pharm. 2008;349(1-2):161‐165. [DOI] [PubMed] [Google Scholar]
- 265. Santini F, Vitti P, Chiovato L, et al. Role for inner ring deiodination preventing transcutaneous passage of thyroxine. J Clin Endocrinol Metab. 2003;88(6):2825‐2830. [DOI] [PubMed] [Google Scholar]
- 266. Azarbayjani AF, Khu JV, Chan YW, Chan SY. Development and characterization of skin permeation retardants and enhancers: a comparative study of levothyroxine-loaded PNIPAM, PLA, PLGA and EC microparticles. Biopharm Drug Dispos. 2011;32(7):380‐388. [DOI] [PubMed] [Google Scholar]
- 267. Azarbayjani AF, Venugopal JR, Ramakrishna S, Lim PFC, Chan YW, Chan SY. Smart polymeric nanofibers for topical delivery of levothyroxine. J Pharm Pharm Sci. 2010;13(3):400‐410. [DOI] [PubMed] [Google Scholar]
- 268. Padula C, Nicoli S, Santi P. Innovative formulations for the delivery of levothyroxine to the skin. Int J Pharm. 2009;372(1-2):12‐16. [DOI] [PubMed] [Google Scholar]
- 269. Kashanian S, Rostami E, Harding FJ, McInnes SJP, Al-Bataineh S, Voelcker NH. Controlled delivery of levothyroxine using porous silicon as a drug nanocontainer. Aust J Chem. 2016;69(2):204‐211. [Google Scholar]
- 270. Geninatti T, Hood RL, Bruno G, et al. Sustained administration of hormones exploiting nanoconfined diffusion through nanochannel membranes. Materials (Basel). 2015;8(8):5276‐5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Ferrati S, Fine D, You J, et al. Leveraging nanochannels for universal, zero-order drug delivery in vivo. J Control Release. 2013;172(3):1011‐1019. [DOI] [PubMed] [Google Scholar]
- 272. Stewart SA, Dominguez-Robles J, Utomo E, et al. Poly(caprolactone)-based subcutaneous implant for sustained delivery of levothyroxine. Int J Pharm. 2021;607:121011. [DOI] [PubMed] [Google Scholar]
- 273. Stewart SA, Waite D, Dominguez-Robles J, et al. HPLC Method for levothyroxine quantification in long-acting drug delivery systems. Validation and evaluation of bovine serum albumin as levothyroxine stabilizer. J Pharm Biomed Anal. 2021;203:114182. [DOI] [PubMed] [Google Scholar]
- 274. Kang M-J, Chung H-R, Oh Y-J, Shim Y-S, Yang S, Hwang IT. Three-year follow-up of children with abnormal newborn screening results for congenital hypothyroidism. Pediatr Neonatol. 2017;58(5):442‐448. [DOI] [PubMed] [Google Scholar]
- 275. Kashanian S, Rostami E. PEG-stearate coated solid lipid nanoparticles as levothyroxine carriers for oral administration. J Nanopart Res. 2014;16(3):2293. [Google Scholar]
- 276. Rostami E, Kashanian S, Azandaryani AH. Preparation of solid lipid nanoparticles as drug carriers for levothyroxine sodium with in vitro drug delivery kinetic characterization. Mol Biol Rep. 2014;41(5):3521‐3527. [DOI] [PubMed] [Google Scholar]
- 277. Rostami E, Kashanian S, Askari M. The effect of ultrasound wave on levothyroxine release from chitosan nanoparticles. Paper presented at: 4th International Conference on Ultrafine Grained and Nano-Structured Materials (UFGNSM 2013); November 2013, Tehran, Iran.
- 278. Kamali H, Khodaverdi E, Kaffash E, et al. Optimization and in vitro evaluation of injectable sustained-release of levothyroxine using PLGA-PEG-PLGA. J Pharm Innov. 2020;16(4):688‐698. [Google Scholar]
- 279. Ghazi RF, Al-Mayahy MH. Levothyroxine sodium loaded dissolving microneedle arrays for transdermal delivery. ADMET DMPK. 2022;10(3):213‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]