Abstract
Gamma interferon (IFN-γ)-inducible protein 10 (IP-10) and monokine induced by IFN-γ (Mig) are related CXC chemokines which bind to the CXCR3 receptor and specifically target activated T lymphocytes and natural killer (NK) cells. The production of IP-10 and Mig by various cell types in vitro is strongly dependent on IFN-γ. To determine whether IP-10 and Mig are released during bacterial infection in humans, we measured plasma levels of IP-10 and Mig in patients with melioidosis, a severe gram-negative infection caused by Burkholderia pseudomallei. IP-10 and Mig were markedly elevated in patients with melioidosis on admission, particularly in blood culture-positive patients, and remained elevated during the 72-h study period. Levels of IP-10 and Mig showed a positive correlation with IFN-γ concentrations and also correlated with clinical outcome. In whole blood stimulated with heat-killed B. pseudomallei, neutralization of IFN-γ and tumor necrosis factor alpha (TNF-α) partly attenuated IP-10 and Mig release, while anti-interleukin-12 (IL-12) and anti-IL-18 had a synergistic effect. Stimulation with other bacteria or endotoxin also induced strong secretion of IP-10 and Mig. These data suggest that IP-10 and Mig are part of the innate immune response to bacterial infection. IP-10 and Mig may contribute to host defense in Th1-mediated host defense during infections by attracting CXCR3+ Th1 cells to the site of inflammation.
Chemokines are a family of small chemotactic proteins that play an important role in cell activation and migration of cells from the circulation to the site of inflammation (20, 25). On the basis of the position of their cysteine residues, chemokines are divided into several families (4). The two major families are the CC and CXC chemokine families; the latter can be further divided into two classes based on the presence of the glutamate-leucine-arginine (ELR) motif preceding the CXC sequence. The ELR-containing CXC chemokines, like interleukin-8 (IL-8), have stimulatory and chemotactic activities on neutrophils, while the non-ELR chemokines act mainly on lymphocytes.
Gamma interferon (IFN-γ)-inducible protein 10 (IP-10) and monokine induced by IFN-γ (Mig) are members of the non-ELR CXC chemokine family, which were discovered as products of genes inducible in response to IFN-γ (10, 18, 21). IP-10 and Mig have potent chemotactic activities and predominantly target activated T lymphocytes and natural killer (NK) cells (18, 32). IP-10 and Mig are structurally closely related and also have a common receptor, CXCR3, which is expressed on activated T cells and NK cells but not on monocytes or neutrophils (19). Besides their chemotactic activities, IP-10 and Mig have been shown in vitro to inhibit colony formation by hematopoietic cells (10). In mice, IP-10 and Mig inhibit neovascularization and possess antitumor activity (31).
IP-10 and Mig expression has been found in several animal models of infection, especially infections in which IFN-γ is known to play an important role in host defense (3, 34). In mice infected with Toxoplasma gondii or vaccinia virus, expression of Mig was strongly dependent on IFN-γ, since it was completely prevented after injection of an anti-IFN-γ monoclonal antibody (MAb) and in IFN-γ-deficient mice (3). In contrast, IP-10 expression was not completely dependent on IFN-γ. In humans, expression of IP-10 has been demonstrated in patients with psoriasis, sarcoidosis, tuberculoid leprosy, and viral meningitis (2, 13, 15, 16). Mig expression has also been demonstrated in psoriatic lesions (12). All of these diseases are associated with increased IFN-γ production and a Th1-type immune response. Little is known of the role of IP-10 and Mig in bacterial infections.
Melioidosis is a severe infection caused by the gram-negative bacillus Burkholderia pseudomallei (8). In a murine model of melioidosis, it was shown that IFN-γ plays an essential role in host defense (27). Previously, markedly elevated plasma levels of IFN-γ have been shown in patients with melioidosis, and levels correlated with severity of disease (6, 17). After injection of endotoxin in healthy human volunteers, a well-accepted model of systemic infection, plasma levels of IP-10 and Mig increase, suggesting that IP-10 and Mig are released in response to bacterial infection (F. N. Lauw, D. Pajkrt, C. E. Hack, M. Kurimoto, S. J. H. van Deventer, and T. van der Poll, 39th Intersci. Conf. Antimicrob. Agents Chemother. 1999, abstr. 270). Therefore, in the present study we measured plasma levels of IP-10 and Mig in patients with melioidosis on admission to the hospital and during a 72-h follow-up after starting antibiotic treatment. In addition, we studied in vitro which cytokines contribute to the production of IP-10 and Mig during whole-blood stimulation with heat-killed B. pseudomallei and endotoxin (lipopolysaccharide [LPS]).
MATERIALS AND METHODS
Patients and study design.
The patients included in the present study were also part of a previous investigation in which the release of IFN-γ and IFN-γ-inducing cytokines was studied (17), and all were part of a clinical trial comparing the efficacy of intravenous imipenem and ceftazidime in suspected severe melioidosis (29). Clinical outcomes were similar for the two treatment groups, and therefore data were combined for the present investigation. Informed consent was obtained from all patients or attending relatives. The patients (more than 14 years old) were all admitted to Sappasitprasong Hospital, Ubon Ratchathani, Thailand, with suspected severe melioidosis. On admission, blood, urine, and throat swab specimens plus, where available, specimens of sputum and pus were collected for culture. Clinical data and baseline APACHE II score were recorded at study entry. A total of 86 consecutive patients (43 males and 43 females) with a median age of 50 years (range, 16 to 85 years) were studied. For 64 patients, the diagnosis of melioidosis was confirmed by positive cultures for B. pseudomallei. Positive blood cultures were found for 34 patients (of whom 16 patients died), while for the other 30 patients, B. pseudomallei was isolated only from sites other than blood (2 patients died). The remaining 22 patients were not culture positive for B. pseudomallei and are referred to subsequently as patients with diseases other than melioidosis. Of these patients, 15 were diagnosed with other infections: clinical sepsis in 9 patients (of whom 4 died) with positive blood cultures in 4 patients (Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Staphylococcus aureus), pneumonia in 2 patients (positive cultures for S. aureus in 1 patient, who died), urinary tract infection in 1 patient, and tuberculosis in 3 patients. In three patients, liver and/or splenic abscesses without positive cultures were found, one patient was diagnosed with hepatocellular carcinoma, and for three patients no final diagnosis was made (one died). The median APACHE II score for patients with bacteremic melioidosis was 16 (range, 4 to 30), for nonbacteremic melioidosis patients was 9.5 (range, 1 to 24), and for the group with diseases other than melioidosis was 13.5 (range, 5 to 24).
Blood samples (EDTA anticoagulated) were collected directly before the start of antibiotic treatment (time zero) and at 12, 24, 48, and 72 h afterwards. In addition, blood was collected from 12 healthy adult volunteers (patients' relatives or hospital staff, all resident in Ubon Ratchathani or the surrounding provinces). Plasma was separated immediately and stored at −70°C until assays were performed.
Whole-blood stimulation.
Heat-killed B. pseudomallei, P. aeruginosa, E. coli, Streptococcus pneumoniae, and S. aureus were prepared from clinical isolates. Each isolate was suspended in bacteriological culture medium (Todd-Hewitt broth for B. pseudomallei and Trypticase soy broth for all other bacteria) and incubated overnight in 5% CO2 at 37°C. This suspension was diluted in fresh medium the next morning and incubated until log-phase growth was obtained. Thereafter, 10-fold dilutions of this suspension were made and plated on blood agar plates for CFU counts. Bacteria were harvested by centrifugation, washed twice in pyrogen-free 0.9% NaCl, resuspended in 20 ml of 0.9% NaCl, and heat inactivated for 60 min at 80°C. A 500-μl sample on a blood agar plate did not show growth of bacteria. LPS derived from E. coli (serotype O111:B4) was obtained from Sigma (St. Louis, Mo.).
Whole blood was collected from six healthy individuals aseptically using a sterile collecting system consisting of a butterfly needle connected to a syringe (Becton Dickinson & Co, Rutherford, N.J.). Anticoagulation was obtained using endotoxin-free heparin (Leo Pharmaceutical Products B.V., Weesp, The Netherlands; final concentration, 10 U/ml of blood). Whole blood, diluted 1:1 in pyrogen-free RPMI 1640 (Bio Whittaker, Verviers, Belgium), was stimulated for 24 h at 37°C with 107 CFU of heat-killed bacteria or 10 ng of LPS per ml in the presence or absence of mouse anti-human tumor necrosis factor (TNF) (MAK 195; final concentration, 10 μg/ml), anti-IFN-γ (clone 25718.11), anti-IL-12 (24910.1), or anti-IL-18 (52713.11) (all anti-mouse immunoglobulin G [IgG]; R&D Systems, Abingdon, United Kingdom; final concentration, 10 μg/ml for all). MAK 195F was generously provided by Knoll AG, Ludwigshafen, Germany. During in vitro cell stimulation, these concentrations of the MAbs completely neutralize the activity of recombinant human TNF (rhTNF), rhIFN-γ, rhIL-12, and rhIL-18 when added at 10- to 100-fold-higher concentrations compared to levels detected after whole-blood stimulation with heat-killed B. pseudomallei (17), and no cross-reactivity with rhIP-10 or rhMig was observed (information on the neutralizing capacities of the MAbs used was provided by the manufacturer). Control mouse IgG (R&D Systems) was used at the appropriate concentrations. After the incubation, supernatant was obtained after centrifugation and stored at −20°C until assays were performed.
Assays.
IP-10 and Mig levels were measured by enzyme-linked immunosorbent assay according to the instructions of the manufacturer. In short, mouse anti-human IP-10 (4 μg/ml) and mouse anti-human Mig (1 μg/ml) were used as coating antibodies, biotinylated goat anti-human IP-10 (50 ng/ml) and goat anti-human Mig (4 μg/ml) were used as detection antibodies, and rhIP-10 and rhMig were used as standards. All IP-10 reagents were from R&D Systems, and all Mig reagents were from PharMingen (San Diego, Calif.). The detection limits of the assays were 20 pg/ml (IP-10) and 8 pg/ml (Mig).
Statistical analysis.
Values are given as medians and ranges. Differences between controls and/or patient groups were analyzed by the Mann-Whitney U test. Changes in time during antibiotic treatment were analyzed by one-way analysis of variance. These two tests were performed after log transformation of the data. Spearman's ρ was used to determine correlation coefficients. Data from the in vitro stimulations are expressed as mean ± standard error (SE) for six donors. Statistical analysis was performed by the Wilcoxon test. A P of <0.05 was considered to represent a significant difference.
RESULTS
IP-10 and Mig concentrations on admission.
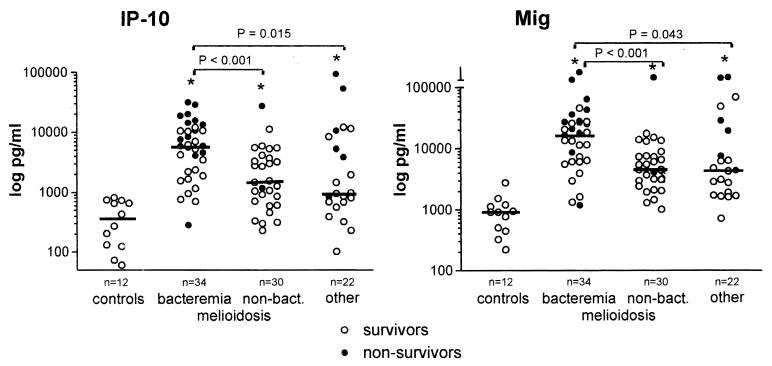
The median plasma concentration of IP-10 in healthy controls was 352 (range, 61 to 821) pg/ml (Fig. 1). In patients with melioidosis, markedly elevated levels of IP-10 were found compared to controls (P < 0.001), with significantly higher concentrations in patients with positive blood cultures (5,923 [282 to 31,713] pg/ml) than in patients with nonbacteremic melioidosis (1,511 [230 to 27,355] pg/ml; P < 0.001). In patients with bacteremic melioidosis, levels of IP-10 were higher in patients who died then in patients who survived (9,545 [282 to 31,713] pg/ml versus 2,924 [703 to 11,996] pg/ml; P = 0.004). IP-10 plasma concentrations correlated positively with APACHE II scores (ρ = 0.64; P < 0.001). In patients with diseases other than melioidosis, IP-10 levels were increased compared to controls (965 [103 to 93,732] pg/ml; P = 0.001) but significantly lower than in patients with bacteremic melioidosis (P = 0.015). In the group with diseases other than melioidosis, IP-10 concentrations were higher in nonsurviving patients than in surviving patients (7,986 [839 to 93,732] pg/ml and 832 [103 to 12,174] pg/ml, respectively; P = 0.018).
FIG. 1.
Plasma concentrations of IP-10 and Mig on admission in patients with culture-proven melioidosis (bacteremic and nonbacteremic), patients with diseases other than melioidosis, and healthy controls. Horizontal lines represent medians. ∗, P < 0.05 versus controls. P values reflect differences between groups by the Mann-Whitney U test.
Mig was detectable in the plasma of healthy controls at a median concentration of 914 (220 to 2,772) pg/ml (Fig. 1). Mig concentrations were markedly increased in melioidosis patients (P < 0.001), with higher levels in patients with positive blood cultures then in patients with nonbacteremic melioidosis (18,265 [1,185 to 407,000] pg/ml and 5,036 [1,017 to 167,000] pg/ml, respectively; P < 0.001). Patients with positive blood cultures who died had higher Mig plasma concentrations than patients who survived (27,761 [1,185 to 407,000] pg/ml and 9,409 [1,330 to 46,024] pg/ml, respectively; P = 0.001). Mig levels showed a positive correlation with APACHE II scores (ρ = 0.72; P < 0.001). In patients with diseases other than melioidosis, Mig concentrations were elevated compared to controls (4,425 [717 to 172,000] pg/ml; P < 0.001), although significantly lower than in bacteremic melioidosis patients (P = 0.043), with higher levels in patients who died than in patients who survived (24,310 [4,425 to 172,000] pg/ml and 2,880 [717 to 69,791] pg/ml, respectively; P = 0.008).
In patients with culture-proven melioidosis and also in the total patient population, IP-10 and Mig plasma levels showed a strong positive correlation (Table 1). Since the production of IP-10 and Mig is strongly IFN-γ dependent in both in vitro and mouse studies, we examined correlations between both IP-10 and Mig concentrations and IFN-γ levels in these patients (the IFN-γ levels have been reported previously [17]). Both IP-10 and Mig showed a positive, although weak, correlation with IFN-γ (Table 1).
TABLE 1.
Correlations between IP-10, Mig, and IFN-γ on admission in patients with clinically suspected melioidosis
| Correlation | Patients with melioidosis (n = 64)
|
Total patient population (n = 86)
|
||
|---|---|---|---|---|
| ρ | P | ρ | P | |
| IP-10—Mig | 0.88 | <0.001 | 0.87 | <0.001 |
| IP-10—IFN-γ | 0.49 | <0.001 | 0.51 | <0.001 |
| Mig—IFN-γ | 0.44 | 0.034 | 0.52 | 0.009 |
IP-10 and Mig during follow-up.
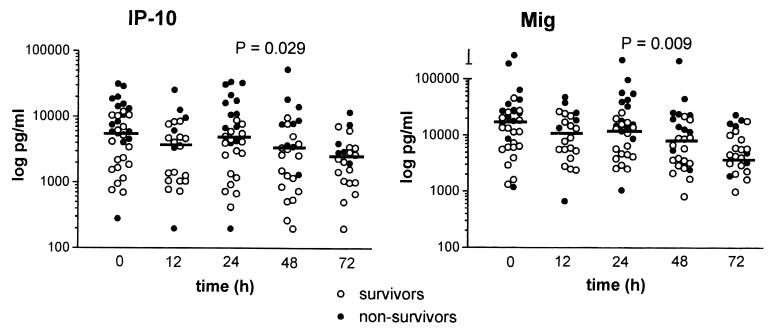
Patients with culture-proven melioidosis were monitored for 72 h following the start of antibiotic therapy with either ceftazidime or imipenem. Since the type of antibiotic treatment had no effect on IP-10 and Mig levels, data for the two treatment groups were combined (data not shown). In patients with positive blood cultures for B. pseudomallei, both IP-10 and Mig concentrations decreased significantly over time during antibiotic treatment (72-h IP-10: 2,328 [200 to 11,885] pg/ml; 72-h Mig, 4,893 [993 to 23,606] pg/ml; P = 0.029 and 0.009, respectively) (Fig. 2). However, IP-10 and Mig levels at 72 h after the start of antibiotic therapy were still elevated compared to levels in healthy controls (both, P < 0.001). In patients with nonbacteremic melioidosis, IP-10 and Mig concentrations did not decrease significantly during antibiotic treatment and remained elevated until the end of the 72-h study period (data not shown).
FIG. 2.
Plasma levels of IP-10 and Mig in patients with bacteremic melioidosis during antibiotic treatment. Horizontal lines represent medians. P values indicate changes in time analyzed by one-way analysis of variance.
Regulation of IP-10 and Mig production in whole blood.
The production of IP-10 and Mig by various cell types in vitro is strongly dependent on IFN-γ (7, 11). Also, costimulation with TNF was needed for optimal IFN-γ-induced IP-10 and Mig release by human neutrophils (11). Melioidosis is associated with elevated plasma concentrations of several proinflammatory cytokines (6, 17, 30). To obtain insight into the role of endogenous cytokines in IP-10 and Mig release during melioidosis, we incubated whole blood with heat-killed B. pseudomallei (amount equivalent to 107 CFU/ml) in the presence of neutralizing antibodies against cytokines which are important for IP-10 and Mig release and which are known to be important in the pathogenesis of melioidosis. Incubation of whole blood for 24 h at 37°C without heat-killed B. pseudomallei resulted in detectable levels of IP-10 (299 ± 94 pg/ml) and Mig (352 ± 109 pg/ml). Incubation with heat-killed B. pseudomallei increased IP-10 levels to 4,448 ± 955 pg/ml and Mig levels to 3,851 ± 650 pg/ml (both, P < 0.05 versus control). Addition of control IgG did not influence IP-10 and Mig concentrations. Addition of either anti-IFN-γ or anti-TNF decreased the release of IP-10 significantly and, more potently, that of Mig (Table 2). In contrast, anti-IL-12 or anti-IL-18 alone did not significantly inhibit IP-10 or Mig production, but the combination of anti-IL-12 and anti-IL-18 resulted in synergistic inhibition of both IP-10 and Mig release. Addition of both anti-IFN-γ and anti-IL-12 induced a further decrease in IP-10 and Mig production relative to the addition of anti-IFN-γ only, while the combination of anti-IFN-γ and anti-IL-18 had no additional inhibitory effect.
TABLE 2.
Effects of neutralizing MAbs against proinflammatory cytokines on IP-10 and Mig production during whole-blood stimulation with heat-killed B. pseudomallei or E. coli LPSa
| MAb | % Inhibition
|
|||
|---|---|---|---|---|
| IP-10
|
Mig
|
|||
| B. pseudomallei | LPS | B. pseudomallei | LPS | |
| Control | 2.8 ± 7.2 | 1.1 ± 10.3 | 2.5 ± 8.9 | 5.4 ± 14.7 |
| Anti-IFN-γ | 19.0 ± 6.2∗ | 50.2 ± 5.5∗ | 35.1 ± 6.3∗ | 67.7 ± 6.0∗ |
| Anti-IL-12 | 4.3 ± 5.9 | 59.1 ± 3.9∗ | 3.8 ± 6.7 | 61.5 ± 7.8∗ |
| Anti-IL-18 | 0.6 ± 7.2 | 26.1 ± 6.2∗ | 8.4 ± 4.7 | 32.9 ± 6.8∗ |
| Anti-TNF | 22.1 ± 1.1∗ | 36.7 ± 3.6∗ | 27.6 ± 8.6∗ | 58.6 ± 3.1∗ |
| Anti-IL-12 + anti-IL-18 | 28.4 ± 5.4∗ | 65.0 ± 2.5∗ | 29.1 ± 10.5∗ | 71.9 ± 6.4∗ |
| Anti-IFN-γ + anti-IL-12 | 33.8 ± 5.9∗# | ND | 46.1 ± 6.2∗# | ND |
| Anti-IFN-γ + anti-IL-18 | 21.3 ± 7.5∗ | ND | 31.8 ± 9.1∗ | ND |
Data are means ± SE for six healthy donors and expressed as percent inhibition relative to incubation with heat-killed B. pseudomallei only. Whole blood, diluted 1:1 in RPMI, was stimulated for 24 h at 37°C with 107 CFU of heat-killed B. pseudomallei per ml or LPS (10 ng/ml) in the presence or absence of neutralizing antibodies against TNF, IFN-γ, IL-12, or IL-18 (final concentration of all, 10 μg/ml). Incubation without stimulus resulted in low levels of IP-10 (653 ± 110 pg/ml) and Mig (253 ± 96 pg/ml). IP-10 levels after stimulation with heat-killed B. pseudomallei were 4,448 ± 955 pg/ml, and Mig levels were 3,851 ± 650 pg/ml. Stimulation with LPS increased IP-10 concentrations to 12,594 ± 1,686 pg/ml and Mig levels to 2,858 ± 807 pg/ml. *, P < 0.05 versus control antibody; #, P < 0.05 versus anti-IFN-γ. ND, not determined.
We have shown previously that plasma levels of IP-10 and Mig increase after an intravenous bolus injection of LPS in humans (Lauw et al., abstr.). In accordance with these results, incubation of whole blood with LPS resulted in elevated concentrations of IP-10 (12,594 ± 1,686 pg/ml) and Mig (2,858 ± 807 pg/ml; both, P < 0.05 versus control). In contrast to the results found with heat-killed B. pseudomallei, addition of anti-IL-12 strongly inhibited IP-10 and Mig release, while anti-IL-18 also significantly attenuated IP-10 and Mig release, although to a lesser extent than anti-IL-12 (Table 2). The combination of anti-IL-12 and anti-IL-18 further decreased the release of IP-10 and Mig slightly, although this difference was not significant compared to the effect of anti-IL-12 only. Addition of anti-IFN-γ strongly inhibited LPS-induced IP-10 and Mig release, while anti-TNF also had a strong inhibitory effect (Table 2).
To determine whether other bacteria can stimulate the release of IP-10 and Mig, we compared the effect of heat-killed B. pseudomallei with that of other gram-negative bacteria (i.e., heat-killed P. aeruginosa and E. coli) and gram-positive bacteria (heat-killed S. pneumoniae and S. aureus) during whole-blood stimulation in vitro. As shown in Table 3, all bacteria were potent inducers of IP-10 and Mig production.
TABLE 3.
IP-10 and Mig release during whole-blood stimulation with different bacteriaa
| Bacteria | Concn (pg/ml)
|
|
|---|---|---|
| IP-10 | Mig | |
| B. pseudomallei | 6,299 ± 836 | 1,794 ± 282 |
| P. aeruginosa | 4,171 ± 701 | 1,903 ± 449 |
| E. coli | 7,005 ± 619 | 2,328 ± 386 |
| S. pneumoniae | 2,899 ± 992 | 3,379 ± 1,659 |
| S. aureus | 10,444 ± 1,800 | 3,247 ± 569 |
Data are means ± SE for six healthy subjects. Whole blood, diluted 1:1 in RPMI, was stimulated for 24 h at 37°C with different heat-killed bacteria (final concentration, 107 CFU/ml). Incubation of whole blood for 24 h at 37°C without bacteria resulted in detectable levels of IP-10 (963 ± 344 pg/ml) and Mig (352 ± 109 pg/ml).
DISCUSSION
IP-10 and Mig are members of the non-ELR CXC chemokine family, which are potent chemoattractants for activated T lymphocytes and NK cells (10). They were identified as products of genes induced in response to IFN-γ (18, 21). The production of IP-10 and Mig can be induced strongly by IFN-γ in a large variety of cells in vitro, including monocytes-macrophages, neutrophils, epithelial cells, and endothelial cells (7, 10, 11, 28). In murine infection models, expression of IP-10 and Mig has been demonstrated in multiple organs and was largely dependent on IFN-γ (3). Increased expression of IP-10 and Mig has been observed in various clinical conditions in humans (2, 12, 13, 15, 16), but little is known of the expression of IP-10 and Mig and their relation to IFN-γ during bacterial infection.
In this study we have demonstrated that plasma concentrations of IP-10 and Mig are elevated markedly and for a prolonged time in patients with melioidosis. Melioidosis is a severe infection caused by the gram-negative bacillus B. pseudomallei and an important cause of illness and death in parts of Southeast Asia (8). This patient population was selected because IFN-γ was shown to be important for host defense in a mouse model of melioidosis (27). In addition, elevated plasma levels of IFN-γ have been found in a high proportion of patients with melioidosis (6, 17). The clinical presentation of melioidosis varies from mild localized disease to acute fulminant septicemia. IP-10 and Mig concentrations were higher in patients with bacteremic disease, and higher levels were associated with a fatal outcome. Concentrations of IP-10 and Mig showed a positive, although weak, correlation with IFN-γ levels. These data indicate that during severe melioidosis, IP-10 and Mig levels correlate with severity of disease and with IFN-γ levels, although this latter correlation was not as strong as previously found in vitro and in mice (3, 11, 28).
Most chemokines can bind to more than one chemokine receptor, but IP-10 and Mig specifically bind to CXCR3 (4, 19). Another recently identified non-ELR CXC chemokine, IFN-γ-inducible T-cell α chemoattractant, also selectively binds to CXCR3 (9). CXCR3 is only expressed on activated T lymphocytes and NK cells, and not on other leukocytes (19). Recently, it has been demonstrated that CXCR3 (and CCR5) is preferentially expressed on Th1-type lymphocytes (5, 24, 26). A Th1-type immune response is associated with the release of Th1-type cytokines, such as IFN-γ and IL-2, and known to enhance cell-mediated immunity, which is important for host defense against intracellular pathogens (1). In vitro studies have demonstrated that B. pseudomallei can survive intracellularly within phagocytes (14). This suggests that during melioidosis, IP-10 and Mig may be important for the activation and attraction of CXCR3± Th1 cells to the site of inflammation, which can contribute to host defense against B. pseudomallei by the additional production of Th1-type cytokines.
To obtain more insight into the role of IFN-γ in the regulation of IP-10 and Mig production during bacterial infection, we incubated whole blood with heat-killed B. pseudomallei and LPS in the presence and absence of neutralizing antibodies against IFN-γ. Interestingly, neutralization of IFN-γ only reduced the release of IP-10 and Mig slightly. The effect of anti-IFN-γ on Mig release was stronger than the effect on IP-10 production. This is concordant with previous studies, which demonstrated that Mig release is more dependent on IFN-γ than is IP-10 production (3, 10). These data suggest that the effect of B. pseudomallei on IP-10 and Mig production is not completely dependent on IFN-γ.
To study the involvement of other cytokines, we performed whole-blood stimulations with neutralizing antibodies against a number of cytokines that are elevated in patients with melioidosis, i.e., TNF, IL-12, and IL-18 (17, 30). TNF has been shown to play an essential synergistic role with IFN-γ in the production of IP-10 and Mig in vitro (11, 28). In line with these results, addition of anti-TNF significantly inhibited heat-killed B. pseudomallei-stimulated IP-10 and Mig production. IL-12 is a potent stimulator of IFN-γ production, and IL-18 synergistically enhances IL-12-induced IFN-γ release (23, 33). Previously, we found that addition of anti-IL-12 or anti-IL-18 strongly but not completely inhibited IFN-γ production during whole-blood stimulation with heat-killed B. pseudomallei (17), which may explain why neither anti-IL-12 nor anti-IL-18 alone had any effect. The combination of anti-IL-12 and IL-18 had an additional inhibitory effect on IFN-γ production, which may have provided a decrease in IFN-γ production sufficient to inhibit IP-10 and Mig release. The combination of anti-IL-12 and anti-IFN-γ had an additional inhibitory effect, suggesting that IL-12 and IFN-γ influence IP-10 and Mig production in part by independent mechanisms. In contrast to the effects on heat-killed B. pseudomallei-stimulated IP-10 and Mig production, neutralization of IFN-γ or TNF strongly inhibited LPS-induced IP-10 and Mig release, while anti-IL-12 and anti-IL-18 also had a potent inhibitory effect. This suggests that the stimulatory effect of B. pseudomallei on IP-10 and Mig production is only partially mediated through LPS and is consistent with previous studies which suggest that B. pseudomallei endotoxin is considerably less potent than LPS from E. coli (22). Likely, B. pseudomallei is capable of potently stimulating the production of IP-10 and Mig from leukocytes either directly or through mediators other than the cytokines studied.
In conclusion, we found that during severe gram-negative bacterial infection in humans, IP-10 and Mig plasma concentrations were elevated markedly and correlated with the severity of disease and clinical outcome. In whole blood in vitro, not only B. pseudomallei but also other gram-negative and gram-positive bacteria as well as E. coli LPS were able to induce IP-10 and Mig release. These data suggest that the release of IP-10 and Mig is part of the innate immune response to bacterial infection. IP-10 and Mig may contribute to Th1-mediated host defense during infections by attracting CXCR3+ Th1 cells to the site of inflammation.
ACKNOWLEDGMENTS
T. van der Poll is a fellow of the Royal Netherlands Academy of Arts and Science. The clinical component of the study was part of the Wellcome-Mahidol University Oxford Tropical Medicine Research Programme, supported by the Wellcome Trust of Great Britain.
We thank the Director of Sappasitprasong Hospital for his continued support and the medical and nursing staff of the Department of Medicine for their help. Yupin Suputtamongkol, Mike Smith, and Brian Angus helped with collection of specimens.
REFERENCES
- 1.Abbas A K, Murphy K M, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 2.Agostini C, Cassatella M, Zambello R, Trentin L, Gasperini S, Perin A, Piazza F, Siviero M, Facco M, Dziejman M, Chilosi M, Qin S, Luster A D, Semenzato G. Involvement of the IP-10 chemokine in sarcoid granulomatous reactions. J Immunol. 1998;161:6413–6420. [PubMed] [Google Scholar]
- 3.Amichay D, Gazzinelli R T, Karupiah G, Moench T R, Sher A, Farber J M. Genes for chemokines MuMig and Crg-2 are induced in protozoan and viral infections in response to IFN-gamma with patterns of tissue expression that suggest nonredundant roles in vivo. J Immunol. 1996;157:4511–4520. [PubMed] [Google Scholar]
- 4.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 5.Bonecchi R, Bianchi G, Bordignon P P, D'Ambrosio D, Lang R, Borsatti A, Sozzani S, Allavena P, Gray P A, Mantovani A, Sinigaglia F. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown A E, Dance D A B, Suputtamongkol Y, Chaowagul W, Kongchareon S, Webster H K, White N J. Immune cell activation in melioidosis: increased serum levels of interferon-gamma and soluble interleukin-2 receptors without change in soluble CD8 protein. J Infect Dis. 1991;163:1145–1148. doi: 10.1093/infdis/163.5.1145. [DOI] [PubMed] [Google Scholar]
- 7.Cassatella M A, Gasperini S, Calzetti F, Bertagnin A, Luster A D, McDonald P P. Regulated production of the interferon-gamma-inducible protein-10 (IP-10) chemokine by human neutrophils. Eur J Immunol. 1997;27:111–115. doi: 10.1002/eji.1830270117. [DOI] [PubMed] [Google Scholar]
- 8.Chaowagul W, White N J, Dance D A B, Wattanagoon Y, Naigowit P, Davis T M E, Looareesuwan S, Pitakwatchara N. Melioidosis: a major cause of community-acquired septicemia in northeastern Thailand. J Infect Dis. 1989;159:890–899. doi: 10.1093/infdis/159.5.890. [DOI] [PubMed] [Google Scholar]
- 9.Cole K E, Strick C A, Paradis T J, Ogborne K T, Loetscher M, Gladue R P, Lin W, Boyd J G, Moser B, Wood D E, Sahagan B G, Neote K. Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J Exp Med. 1998;187:2009–2021. doi: 10.1084/jem.187.12.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farber J M. Mig and IP-10: CXC chemokines that target lymphocytes. J Leukocyte Biol. 1997;61:246–257. [PubMed] [Google Scholar]
- 11.Gasperini S, Marchi M, Calzetti F, Laudanna C, Vicentini L, Olsen H, Murphy M, Liao F, Farber J, Cassatella M A. Gene expression and production of the monokine induced by IFN-γ (MIG), IFN-inducible T cell α chemoattractant (I-TAC), and IFN-γ-inducible protein-10 (IP-10) chemokines by human neutrophils. J Immunol. 1999;162:4928–4937. [PubMed] [Google Scholar]
- 12.Goebeler M, Toksoy A, Spandau U, Engelhardt E, Brocker E B, Gillitzer R. The C-X-C chemokine Mig is highly expressed in the papillae of psoriatic lesions. J Pathol. 1998;184:89–95. doi: 10.1002/(SICI)1096-9896(199801)184:1<89::AID-PATH975>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 13.Gottlieb A B, Luster A D, Posnett D N, Carter D M. Detection of a gamma interferon-induced protein IP-10 in psoriatic plaques. J Exp Med. 1988;168:941–948. doi: 10.1084/jem.168.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones A L, Beveridge T J, Woods D E. Intracellular survival of Burkholderia pseudomallei. Infect Immun. 1996;64:782–790. doi: 10.1128/iai.64.3.782-790.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan G, Luster A D, Hancock G, Cohn Z A. The expression of a gamma interferon-induced protein (IP-10) in delayed immune responses in human skin. J Exp Med. 1987;166:1098–1108. doi: 10.1084/jem.166.4.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lahrtz F, Piali L, Nadal D, Pfister H W, Spanaus K S, Baggiolini M, Fontana A. Chemotactic activity on mononuclear cells in the cerebrospinal fluid of patients with viral meningitis is mediated by interferon-gamma inducible protein-10 and monocyte chemotactic protein-1. Eur J Immunol. 1997;27:2484–2489. doi: 10.1002/eji.1830271004. [DOI] [PubMed] [Google Scholar]
- 17.Lauw F N, Simpson A J H, Prins J M, Smith M D, Kurimoto M, van Deventer S J H, Speelman P, Chaowagul W, White N J, van der Poll T. Elevated plasma concentrations of interferon-γ (IFN-γ) and the IFN-γ-inducing cytokines interleukin-18 (IL-18), IL-12 and IL-15 in severe melioidosis. J Infect Dis. 1999;180:1878–1885. doi: 10.1086/315155. [DOI] [PubMed] [Google Scholar]
- 18.Liao F, Rabin R L, Yannelli J R, Koniaris L G, Vanguri P, Farber J M. Human Mig chemokine: biochemical and functional characterization. J Exp Med. 1995;182:1301–1314. doi: 10.1084/jem.182.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loetscher M, Gerber B, Loetscher P, Jones S A, Piali L, Clark-Lewis I, Baggiolini M, Moser B. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184:963–969. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luster A D. Chemokines—chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 21.Luster A D, Ravetch J V. Biochemical characterization of a gamma interferon-inducible cytokine (IP-10) J Exp Med. 1987;166:1084–1097. doi: 10.1084/jem.166.4.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuura M, Kawahara K, Ezaki T, Nakano M. Biological activities of lipopolysaccharide of Burkholderia (Pseudomonas) pseudomallei. FEMS Microbiol Lett. 1996;137:79–83. doi: 10.1111/j.1574-6968.1996.tb08086.x. [DOI] [PubMed] [Google Scholar]
- 23.Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, Akita K, Namba M, Tanabe F, Konishi K, Fukuda S, Kurimoto M. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 24.Qin S, Rottman J B, Myers P, Kassam N, Weinblatt M, Loetscher M, Koch A E, Moser B, Mackay C R. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Investig. 1998;101:746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rollins B J. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 26.Sallusto F, Lenig D, Mackay C R, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santanirand P, Harley V S, Dance D A B, Drasar B S, Bancroft G J. Obligatory role of gamma interferon for host survival in a murine model of infection with Burkholderia pseudomallei. Infect Immun. 1999;67:3593–3600. doi: 10.1128/iai.67.7.3593-3600.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sauty A, Dziejman M, Taha R A, Iarossi A S, Neote K, Garcia-Zepeda E A, Hamid Q, Luster A D. The T cell-specific CXC chemokines IP-10, Mig, and I-TAC are expressed by activated human bronchial epithelial cells. J Immunol. 1999;162:3549–3558. [PubMed] [Google Scholar]
- 29.Simpson A J H, Suputtamongkol Y, Smith M D, Angus B J, Rajanuwong A, Wuthiekanun V, Howe P A, Walsh A L, Chaowagul W, White N J. Comparison of imipenem and ceftazidime as therapy for severe melioidosis. Clin Infect Dis. 1999;29:381–387. doi: 10.1086/520219. [DOI] [PubMed] [Google Scholar]
- 30.Suputtamongkol Y, Kwiatkowski D, Dance D A B, Chaowagul W, White N J. Tumor necrosis factor in septicemic melioidosis. J Infect Dis. 1992;165:561–564. doi: 10.1093/infdis/165.3.561. [DOI] [PubMed] [Google Scholar]
- 31.Tannenbaum C S, Tubbs R, Armstrong D, Finke J H, Bukowski R M, Hamilton T A. The CXC chemokines IP-10 and Mig are necessary for IL-12-mediated regression of the mouse RENCA tumor. J Immunol. 1998;161:927–932. [PubMed] [Google Scholar]
- 32.Taub D D, Lloyd A R, Conlon K, Wang J M, Ortaldo J R, Harada A, Matsushima K, Kelvin D J, Oppenheim J J. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J Exp Med. 1993;177:1809–1814. doi: 10.1084/jem.177.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trinchieri G. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood. 1994;84:4008–4027. [PubMed] [Google Scholar]
- 34.Vester B, Muller K, Solbach W, Laskay T. Early gene expression of NK cell-activating chemokines in mice resistant to Leishmania major. Infect Immun. 1999;67:3155–3159. doi: 10.1128/iai.67.6.3155-3159.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]