PURPOSE:
Tumor genomic testing (TGT) has become increasingly adopted as part of standard cancer care for many cancers. Despite national guidelines around patient education before TGT, available evidence suggests that most patients' understanding of genomics remains limited, particularly lower-income and minority patients, and most patients are not informed regarding potential incidental germline findings.
METHODS:
To investigate and address limitations in patient understanding of TGT results, a Plan-Do-Study-Act (PDSA) approach is being used to assess needs, identify opportunities for improvement, and implement approaches to optimize patient education. We reviewed published guidelines related to pre-TGT provider-patient education and to identify key points (Plan). A provider quality improvement survey was completed (Do), which highlighted inconsistency in pre-TGT discussion practice across providers and minimal discussion with patients regarding the possibility of incidental germline findings.
RESULTS:
Patient focus groups and interviews (N = 12 patients) were completed with coding of each transcript (Study), which revealed themes including trouble differentiating TGT from other forms of testing, yet understanding that results could tailor therapy. The integration of data across this initial PDSA cycle identified consistent themes and opportunities, which were incorporated into a patient-directed, concise animated video for pre-TGT education (Act), which will form the foundation of a subsequent PDSA cycle. The video addresses how TGT may/may not inform treatment, the process for TGT using existing tissue or liquid biopsy, insurance coverage, and the potential need for germline genetics follow-up because of incidental findings.
CONCLUSION:
This PDSA cycle reveals key gaps and opportunities for improvement in patient education before TGT.
INTRODUCTION
Somatic next-generation sequencing, also known as tumor genomic testing (TGT), has become increasingly adopted as part of standard cancer care for many cancers,1 raising important ethical challenges including uncertainty of results, incidental germline findings, and disparities around TGT options and access.2,3 It is expected that oncology providers will discuss the potential therapeutic implications of TGT (ie, benefits and limitations) with patients. National guidelines are unified in support of reporting incidental germline findings (eg, from tumor-normal or research TGT).4-8 The ASCO Policy Statement4 notes, (1) “Oncology providers should communicate the potential for incidental/secondary germline information…before conducting somatic mutation profiling and should review potential benefits, limitations, and risks before testing; (2) Providers should carefully ascertain patient preferences regarding the receipt of germline information…This may require referral for additional counseling to help the patient clarify preferences; (3) ASCO supports research to determine how to best deliver pretest education, support patient preferences, and understand outcomes of providing incidental/secondary germline information with somatic testing.” Evidence suggests that provider-patient discussions around TGT are inconsistent,9,10 which is complicated further by limited genetics/genomics literacy among patients,11 particularly those who have lower income and those who are medically underserved.12 Additionally, studies have shown that TGT rarely informed therapy selection and that this lack of benefit diminished trust in the provider.13 Taken together, this evidence supports the need for consistency and improved communication between providers and patients in relationship to TGT. To address this need, we engaged in a quality improvement (QI) initiative focused on patient education before TGT using a Plan-Do-Study-Act (PDSA) approach.14
METHODS
Plan
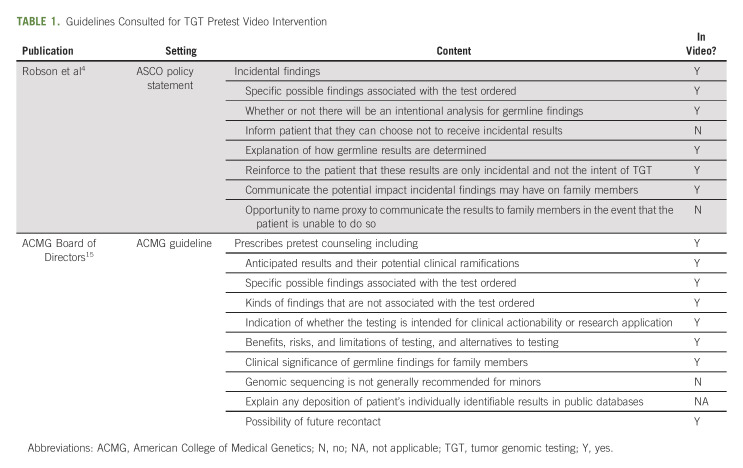
In the planning phase of our QI initiative, we reviewed published guidelines related to pre-TGT provider-patient education and made a list of recommended discussion points and noted whether these discussion points were isolated to one published guideline or found in duplicate (Table 1). Next, to confirm that the experience of providers and patients within our own medical center are in alignment, we sought to assess current Ohio State University Comprehensive Cancer Center oncology provider perspectives on current pre-TGT education practices and barriers to adhering to published pre-TGT guidelines.
TABLE 1.
Guidelines Consulted for TGT Pretest Video Intervention
Do
Provider quality improvement.
A 15-item QI survey was designed in REDCap to assess providers' perspectives on TGT discussions with patients and the specific topics of these discussions. An e-mail invitation was sent to 40 practicing medical oncology providers at Ohio State University Comprehensive Cancer Center to complete a survey that contained both closed-ended and open-ended questions, with completion by 31/40 (77.5% response rate). The survey was exempt from internal review board (IRB) approval, as it was categorized as a QI initiative. Among respondents, all were MD (23/31; 74.2% of respondents) or MD/PhD (8/31; 25.8%), with diverse number of years in practice (range, 1 to 20+). The respondents encompass the majority of solid tumor types. Most providers frequently send TGT and 13/31 (41.9%) of providers send TGT on more than half of the patients. The majority of providers frequently discuss benefits (29/31; 93.6% of providers) and limitations (23/31; 74.2% of providers) of tumor TGT. Fewer discuss risks, including 12/31 (38.7%) that discuss risks with only some or none of the patients. A majority of providers do not discuss potential of incidental germline findings (16/31; 51.6% of providers). Importantly, most providers noted that they do not have adequate time in the context of routine clinical care to provide adequate pre-TGT patient education (19/31; 61.3%). The results of this internal quality assessment showed inconsistencies in pre-TGT discussion practice across providers, and minimal discussion with patients regarding the possibility of incidental germline findings, which aligned with published data on this topic.9,10
Study
Patient focus groups and interviews.
To understand patient perspectives, focus groups and individual interviews were conducted with patients with adult metastatic breast cancer or metastatic lung cancer (total N = 12). Some had prior experience with TGT or other genetic testing, while others did not. This approach allowed us to assess prior experience with TGT and also initial reactions to the possibility of TGT without the bias of knowing the ultimate outcome of testing. Study participation was self-initiated in response to posted fliers or provider-introduced with patient consent for telephone follow-up by the study team. Electronic informed consent was administered through REDCap under Ohio State University IRB. Ohio State University's Recruitment, Intervention, and Survey Shared Resource personnel conducted virtual semistructured focus groups of 3-4 patients at a time as well individual telephone interviews with each patient (n = 5 lung cancer and n = 7 breast cancer). Open-ended questions were used to ascertain patients' knowledge of, experience with, and general perceptions of TGT and genetic testing. Focus groups and individual interviews were recorded and transcribed verbatim. A member of the study team developed an initial codebook, which was further refined by the study team. At least two research assistants coded each transcript, and then met to discuss and compare codes, working through discrepancies until reaching consensus. After coding was complete, the codes were applied to the data in NVIVO 12 so that they could be analyzed to determine major themes.
RESULTS
Participants ranged in age from 28 to 79 years and included one African American man, one African American woman, three White men, and seven White women. Participants' health insurance coverages were as follows: five participants were enrolled in Medicare, one had coverage through a health maintenance organization, and six had conventional private health insurance.
The nature of these discussions with providers varied; some patients reported more extensive conversation before testing (ie, “She broke it down and made it sound really simple and she explained it really well so I knew what was going down before it was going to happen.”), while others were reported simply being informed about testing (“I remember he told me he was going to send my cells off”). Participants were largely aware that their results could help inform and tailor their cancer treatment; one participant noted that the testing was necessary to find out which way to go. Another participant noted, “I just felt like wow this is [TGT], this is a way to find out, you know, what the treatments are going to be available for me now or down the road. So I had, it was a very positive experience.”
Many patients did perceive clear benefits to TGT, although a few mentioned their results were not informative in treatment decision making. Some patients also demonstrated confusion around the difference between TGT and germline genetic testing. Although the financial aspects of TGT were not a frequent topic of discussion, it was discussed by a few participants, including the need to discuss it with a provider. One participant noted that while they did not have to pay out of pocket for TGT, that is one thing that was not talked about beforehand. Interviews also asked about the potential for incidental findings, including information regarding genetic risks. Participants also realized the potential negative implications; one participant stated, “I mean it would be scary, but at the same time, it's definitely something that needs to be known.”
Act
Based upon the data collected, a concise method of delivering pre-TGT patient education within the clinic flow was needed. We had previously developed a concise, 2-minute animated video focusing on cascade testing for germline inherited risk mutations16 and hypothesized that the video approach would facilitate consistent messaging that addressed patient and provider needs while adhering to national guidelines. We developed a series of three animated educational videos (for lung cancer, breast cancer, and nonspecific metastatic cancer) for patients to view before TGT. Contents in the three videos are nearly identical, but data presented in the video regarding the likelihood of TGT affecting treatment is specific to each cancer type (eg, breast cancer or lung cancer). The video addresses elements included in national guidelines (Table 1) and provider QI survey and patient focus groups (Fig 1), such as how TGT may/may not inform treatment, the process for TGT using existing tissue or liquid biopsy, insurance coverage, and the potential need for germline genetics follow-up because of incidental findings. The videos are approximately 2.5 minutes in length and portray characters of various ethnic and racial groups. These standardized educational videos are designed to be presented to patients at the time of TGT recommendation. This approach will not add substantial burden to providers already facing clinical restraints.
FIG 1.
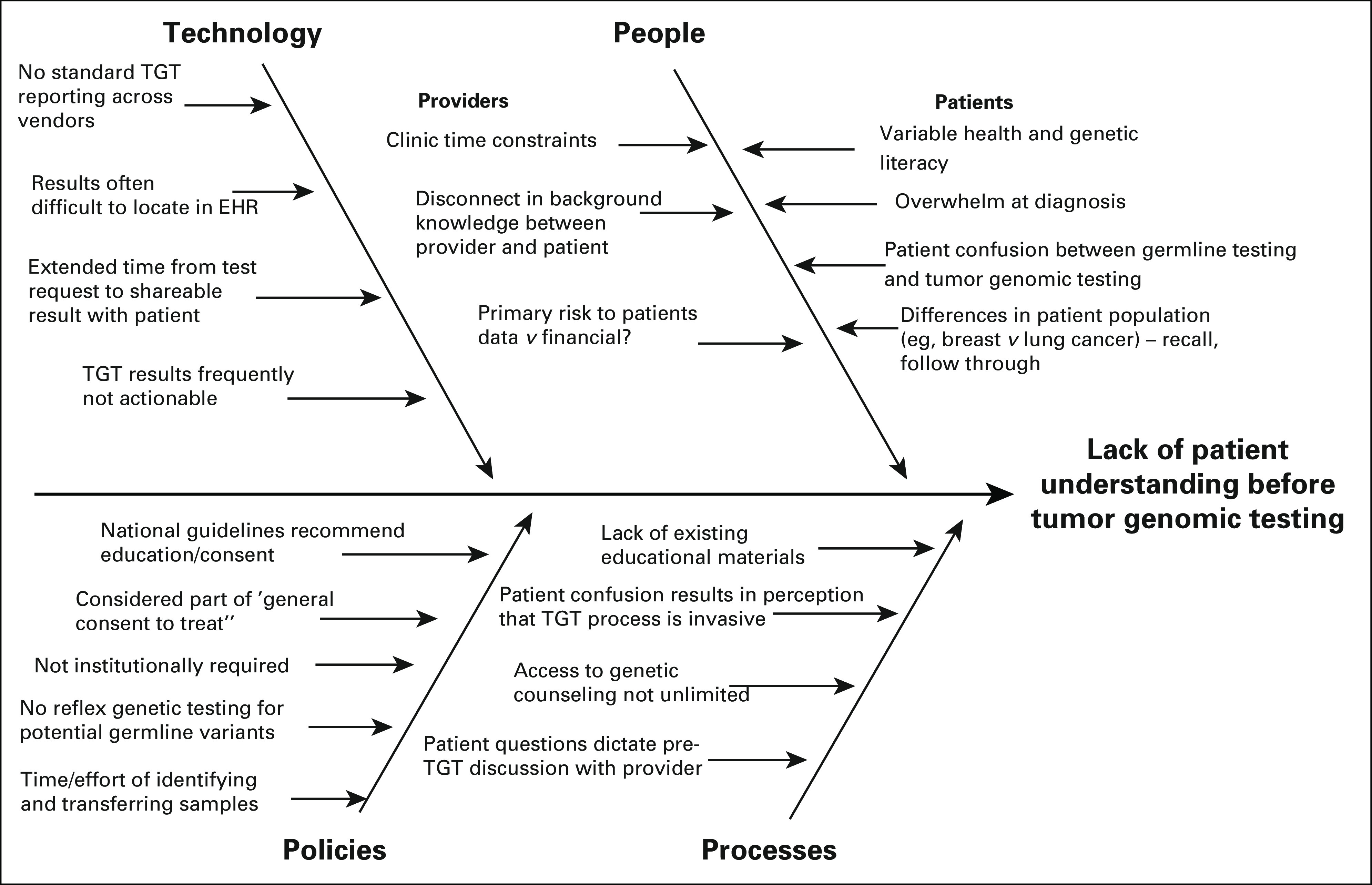
Fishbone diagram incorporating feedback from provider survey and patient focus groups. Themes from 15-question provider quality improvement survey and patient focus groups (n = 12 participants) were aggregated for display and review as part of Plan-Do-Study-Act. EHR, electronic health record; TGT, tumor genomic testing.
DISCUSSION
The available literature suggests that patient education before TGT for oncology patients may not meet recommendations (eg, ASCO Policy Statement4). To help understand why, we reviewed published guidelines related to pre-TGT provider-patient education and identified critical elements for inclusion (Plan), completed a provider QI survey to understand what communication looked like within our own institution (Do), conducted patient focus groups and interviews to understand the patient prospective (Study), and developed TGT educational videos for patients (Act).
Our clinical QI data are in line with the published data and provide further justification for the need for a standardized educational approach. An investigation comparing written versus video education of whole-genome sequencing indicated that study participants retained an equivalent amount of information from either source but had an overall preference for video content.17 More recent work specifically in men considering prostate cancer germline testing, with 71% of men opting for pretest video-based genetic education (relative to genetic counseling), with comparable patient-reported outcomes and uptake of germline testing.18 Pretest video education is being implemented in some settings, for example, Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets tumor and normal testing program, in which patients who desire germline DNA annotation receive pretest genetic counseling via an IRB-approved video.19 Bradbury et al20 completed the Eastern Cooperative Oncology Group-American College of Radiology Imaging Network National Cancer Institute Community Oncology Research Program EAQ152 study, a randomized trial of web-based genetic education versus usual care in patients with advanced cancer undergoing tumor genetic testing, and found that a web-based video intervention increased patient understanding but did not significantly reduce anxiety, depression, or cancer-specific distress.
Although this PDSA effort seeks to address gaps in patient education, there remain other important systematic challenges around widespread implementation of TGT in clinical practice. As TGT increases, there will be increasing burden on medical oncology providers to provide counseling, particularly challenging in community settings where access to genomic experts and genetic counseling may be more limited.21 Furthermore, there may be unintended consequences of TGT, for example, targeted treatment options that require documented mutation may further exacerbate disparities in care if TGT is not equitably available.2,3
These published results and our own qualitative data show that although many patients find value in TGT, key gaps and opportunities for improvement in patient education before TGT exist. No published study to date has systematically evaluated tumor type–specific video and we hypothesize that refined interventions may help to address these gaps. This study does have limitations, including PDSA cycle at a single academic center and ongoing follow-up of downstream effects of PDSA cycle. Patient input from the 12 participants in semistructured interviews provided important initial data; however, additional focus group participants would strengthen the generalizability of the messaging in future iterations of the video. Our future efforts will focus on rigorous assessment of these videos in diverse patient populations to address the need for consistency and improved pretest education before TGT.
Leigha Senter
Consulting or Advisory Role: AstraZeneca/Merck, GlaxoSmithKline
Speakers' Bureau: AstraZeneca/Merck
Heather Hampel
Stock and Other Ownership Interests: Genome Medical, GI OnDemand
Consulting or Advisory Role: InVitae, Genome Medical, Promega, 23andMe, GI OnDemand, Natera
Carolyn J. Presley
Consulting or Advisory Role: PotentiaMetrics, Onc Live
Daniel G. Stover
Consulting or Advisory Role: Novartis
No other potential conflicts of interest were reported.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
SUPPORT
Supported by National Institutes of Health (NIH) Grant No. 1R21CA259985 (L.S., S.R.H., H.H., A.E.T., C.J.P., D.G.S.), P30CA016058 (T.S., A.E.T.), UH2CA239105 (D.G.S.), 1R01 CA215151 (A.E.T.), The project described was supported by Award No. UL1TR002733 from the National Center for Advancing Translational Sciences.
L.S. and D.V. contributed equally to this work. C.N.L., S.R.H., and D.G.S. co-directed this work equally.
AUTHOR CONTRIBUTIONS
Conception and design: Leigha Senter, Marcy Haynam, Elizabeth J. Adams, Heather Hampel, Amanda E. Toland, Carolyn J. Presley, Tasleem J. Padamsee, Daniel G. Stover
Administrative support: Deloris Veney, Marcy Haynam
Provision of study materials or patients: Deloris Veney, Carolyn J. Presley
Collection and assembly of data: Leigha Senter, Taylor Surplus, Elizabeth J. Adams, Daniel G. Stover
Data analysis and interpretation: Leigha Senter, Deloris Veney, Heather Hampel, Carolyn J. Presley, Clara N. Lee, Shelly R. Hovick, Daniel G. Stover
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Patient Understanding of Tumor Genomic Testing: A Quality Improvement Effort
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Leigha Senter
Consulting or Advisory Role: AstraZeneca/Merck, GlaxoSmithKline
Speakers' Bureau: AstraZeneca/Merck
Heather Hampel
Stock and Other Ownership Interests: Genome Medical, GI OnDemand
Consulting or Advisory Role: InVitae, Genome Medical, Promega, 23andMe, GI OnDemand, Natera
Carolyn J. Presley
Consulting or Advisory Role: PotentiaMetrics, Onc Live
Daniel G. Stover
Consulting or Advisory Role: Novartis
No other potential conflicts of interest were reported.
REFERENCES
- 1. Stover DG, Wagle N. Precision medicine in breast cancer: Genes, genomes, and the future of genomically driven treatments. Curr Oncol Rep. 2015;17:15. doi: 10.1007/s11912-015-0438-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yusuf RA, Rogith D, Hovick SR, et al. Attitudes toward molecular testing for personalized cancer therapy. Cancer. 2015;121:243–250. doi: 10.1002/cncr.28966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marron JM, Joffe S. Ethical considerations in genomic testing for hematologic disorders. Blood. 2017;130:460–465. doi: 10.1182/blood-2017-01-734558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Robson ME, Bradbury AR, Arun B, et al. American Society of Clinical Oncology policy statement update: Genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2015;33:3660–3667. doi: 10.1200/JCO.2015.63.0996. [DOI] [PubMed] [Google Scholar]
- 5. DeLeonardis K, Hogan L, Cannistra SA, et al. When should tumor genomic profiling prompt consideration of germline testing? J Oncol Pract. 2019;15:465–473. doi: 10.1200/JOP.19.00201. [DOI] [PubMed] [Google Scholar]
- 6. Daly MB, Pilarski R, Berry M, et al. NCCN guidelines insights: Genetic/familial high-risk assessment: Breast and ovarian, version 2.2017. J Natl Compr Canc Netw. 2017;15:9–20. doi: 10.6004/jnccn.2017.0003. [DOI] [PubMed] [Google Scholar]
- 7. Green RC, Berg JS, Grody WW, et al. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med. 2013;15:565–574. doi: 10.1038/gim.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li MM, Datto M, Duncavage EJ, et al. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: A joint consensus recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn. 2017;19:4–23. doi: 10.1016/j.jmoldx.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yabroff KR, Zhao J, de Moor JS, et al. Factors associated with oncologist discussions of the costs of genomic testing and related treatments. J Natl Cancer Inst. 2019;112:498–506. doi: 10.1093/jnci/djz173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pentz RD, Pocock RH, Pinheiro AM, et al. Physician communication and patient understanding of molecular testing of tumors. J Clin Oncol. 2017;35 doi: 10.1002/onco.13930. abstr e18217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lea DH, Kaphingst KA, Bowen D, et al. Communicating genetic and genomic information: Health literacy and numeracy considerations. Public Health Genomics. 2011;14:279–289. doi: 10.1159/000294191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaphingst KA, Blanchard M, Milam L, et al. Relationships between health literacy and genomics-related knowledge, self-efficacy, perceived importance, and communication in a medically underserved population. J Health Commun. 2016;21:58–68. doi: 10.1080/10810730.2016.1144661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stover DG, Reinbolt RE, Adams EJ, et al. Prospective decision analysis study of clinical genomic testing in metastatic breast cancer: Impact on outcomes and patient perceptions. JCO Precis Oncol. doi: 10.1200/PO.19.00090. 10.1200/PO.19.00090 [epub ahead of print on November 18, 2019] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taylor MJ, McNicholas C, Nicolay C, et al. Systematic review of the application of the plan–do–study–act method to improve quality in healthcare. BMJ Qual Saf. 2014;23:290–298. doi: 10.1136/bmjqs-2013-001862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. ACMG Board of Directors Points to consider for informed consent for genome/exome sequencing Genet Med 15 748 749 2013. 23970068 [Google Scholar]
- 16. Aeilts AM, Carpenter KM, Hovick SR, et al. The impact of a cascade testing video on recipients' knowledge, cognitive message processing, and affective reactions: A formative evaluation. J Genet Couns. 2021;30:656–664. doi: 10.1002/jgc4.1345. [DOI] [PubMed] [Google Scholar]
- 17. Sanderson SC, Suckiel SA, Zweig M, et al. Development and preliminary evaluation of an online educational video about whole-genome sequencing for research participants, patients, and the general public. Genet Med. 2016;18:501–512. doi: 10.1038/gim.2015.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Russo J, McDougall C, Bowler N, et al. Pretest genetic education video versus genetic counseling for men considering prostate cancer germline testing: A patient-choice study to address urgent practice needs. JCO Precis Oncol. doi: 10.1200/PO.21.00238. 10.1200/PO.21.00238 [epub ahead of print on September 1, 2021] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hyman DM, Solit DB, Arcila ME, et al. Precision medicine at Memorial Sloan Kettering Cancer Center: Clinical next-generation sequencing enabling next-generation targeted therapy trials. Drug Discov Today. 2015;20:1422–1428. doi: 10.1016/j.drudis.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bradbury AR, Lee J-W, Gaieski JB, et al. A randomized study of genetic education versus usual care in tumor profiling for advanced cancer in the ECOG-ACRIN Cancer Research Group (EAQ152) Cancer. 2022;128:1381–1391. doi: 10.1002/cncr.34063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Demeshko A, Pennisi DJ, Narayan S, et al. Factors influencing cancer genetic somatic mutation test ordering by cancer physician. J Transl Med. 2020;18:431. doi: 10.1186/s12967-020-02610-7. [DOI] [PMC free article] [PubMed] [Google Scholar]