PURPOSE
The combination of whole-genome and transcriptome sequencing (WGTS) is expected to transform diagnosis and treatment for patients with cancer. WGTS is a comprehensive precision diagnostic test that is starting to replace the standard of care for oncology molecular testing in health care systems around the world; however, the implementation and widescale adoption of this best-in-class testing is lacking.
METHODS
Here, we address the barriers in integrating WGTS for cancer diagnostics and treatment selection and answer questions regarding utility in different cancer types, cost-effectiveness and affordability, and other practical considerations for WGTS implementation.
RESULTS
We review the current studies implementing WGTS in health care systems and provide a synopsis of the clinical evidence and insights into practical considerations for WGTS implementation. We reflect on regulatory, costs, reimbursement, and incidental findings aspects of this test.
CONCLUSION
WGTS is an appropriate comprehensive clinical test for many tumor types and can replace multiple, cascade testing approaches currently performed. Decreasing sequencing cost, increasing number of clinically relevant aberrations and discovery of more complex biomarkers of treatment response, should pave the way for health care systems and laboratories in implementing WGTS into clinical practice, to transform diagnosis and treatment for patients with cancer.
INTRODUCTION
Cancer is a disease of the genome, where acquired (epi)genetic changes and/or inherited germline aberrations can lead to survival and proliferation of cancerous cells. The advancements of molecular diagnostic assays and development of effective therapies enable cancer eradication and improved survival. The study of the molecular mechanisms responsible for oncogenesis has revealed the importance of genomic aberrations such as single-nucleotide variants, small insertions and deletions, structural variants (SVs) such as copy number aberrations and rearrangements, as well as more complex biomarkers such as tumor mutational burden (TMB), microsatellite instability (MSI), and homologous recombination deficiency (HRD). As the number of clinically actionable and pancancer biomarkers continues to grow and the sequencing technology becomes more accessible, comprehensive molecular approaches such as whole-genome sequencing (WGS) and whole-transcriptome sequencing (WTS), combined also known as whole-genome transcriptome sequencing (WGTS), are becoming increasingly used within cancer diagnostics and are on the horizon for everyday oncology care. This is evidenced by several recent studies validating WGS and WGTS for cancer diagnostics.1-3 How the broad adoption of WGTS for routine cancer care will happen is not yet fully defined, reflecting the work that health care systems across the world must do to prepare for WGTS implementation and bring the benefits of these new developments to patients with cancer.
CONTEXT
Key Objective
How can whole-genome transcriptome sequencing (WGTS) be integrated into health care systems to replace the standard of care for oncology? A panel of 25 global experts in oncology, pathology, genetics, translational research, and health technology assessment discussed key topics for the implementation of WGTS in routine cancer diagnostics and care: technology, health economics and reimbursement, clinical evidence needs, and data analysis and interpretation.
Knowledge Generated
A consensus statement describing the work that health care systems across the world must do to prepare for the WGTS transformation and bring the benefits of these new developments to patients with cancer.
Relevance
The implementation of WGTS has potential to transform diagnosis and treatment for patients with cancer and identify new biomarkers for improved risk stratification and reduction of overtreatment.
Despite differences in national regulations, all countries share common challenges in integrating WGTS into their systems. Here, we address the real-world barriers that are likely to be issues in any system looking to adopt WGTS in routine cancer diagnostics.
METHODS
A panel of 25 global experts in oncology, pathology, genetics, translational research, and health technology assessment were chosen based on their experience using WGTS in oncology in the translational setting or as a routine molecular profiling assay. The experts participated in a 14-day virtual advisory board composed of three working sessions for a total of 4.5 hours and discussions on a virtual engagement platform (Within3) to address key topics related to the implementation of WGTS in routine cancer care and diagnostics: general and technical aspects, health economics and reimbursement, clinical evidence needs, and data analysis and interpretation. To prepare the manuscript, the experts met between November 2020 and September 2021 via conference calls every second month and used Within3 platform to continue the discussion. During this period, the group addressed each topic and discussed opinions until agreement was reached. In addition, the experts identified gaps and required actions to implement WGTS in routine cancer diagnostics and care. This consensus statement summarizes the conclusions of the panel on the basis of existing published evidence and professional experience. The final document was approved by all experts.
RESULTS
Oncology Testing Ready for WGTS Implementation
There is a growing amount of data showing that WGTS profiling of tumor tissue has reached a tipping point in diagnostic and economic value. In a number of cancer types, a precise diagnosis facilitating targeted treatment depends on accurate detection of a fast growing number of clinically relevant genomic alterations4 (Fig 1). Currently used technologies have different limitations. For instance, targeted next-generation sequencing approaches are better at detecting mutations with lower allele frequencies than WGS due to higher sequencing depth but are less comprehensive than WES and WGS and less effective for identification of large genomic aberrations such as SVs.5 Recent feasibility studies of clinical WGTS across pediatric cancers5 and adult solid tumors6 demonstrated that despite 5- to 10-fold lower average sequencing depth typically used for panels, WGTS of matched tissue samples was able to call out all clinically relevant variants (Data Supplement). Karyotyping has limited ability to detect smaller (under 5 Mb), clinically relevant copy number aberrations. Similarly, fluorescence in situ hybridization (FISH) can detect known gene fusions but is not able to detect novel ones or identify cryptic translocations that are clinically relevant.7 WGTS thus offers the advantage of identifying a wide range of actionable aberrations, complex biomarkers, providing insights into the characteristics of the tumor relevant for correct patient diagnosis. In particular, for hematological malignancies, sarcomas, and some other cancer, increasing evidence shows that WGTS would streamline testing and improve diagnosis, risk stratification, and patient management.8-11
FIG 1.
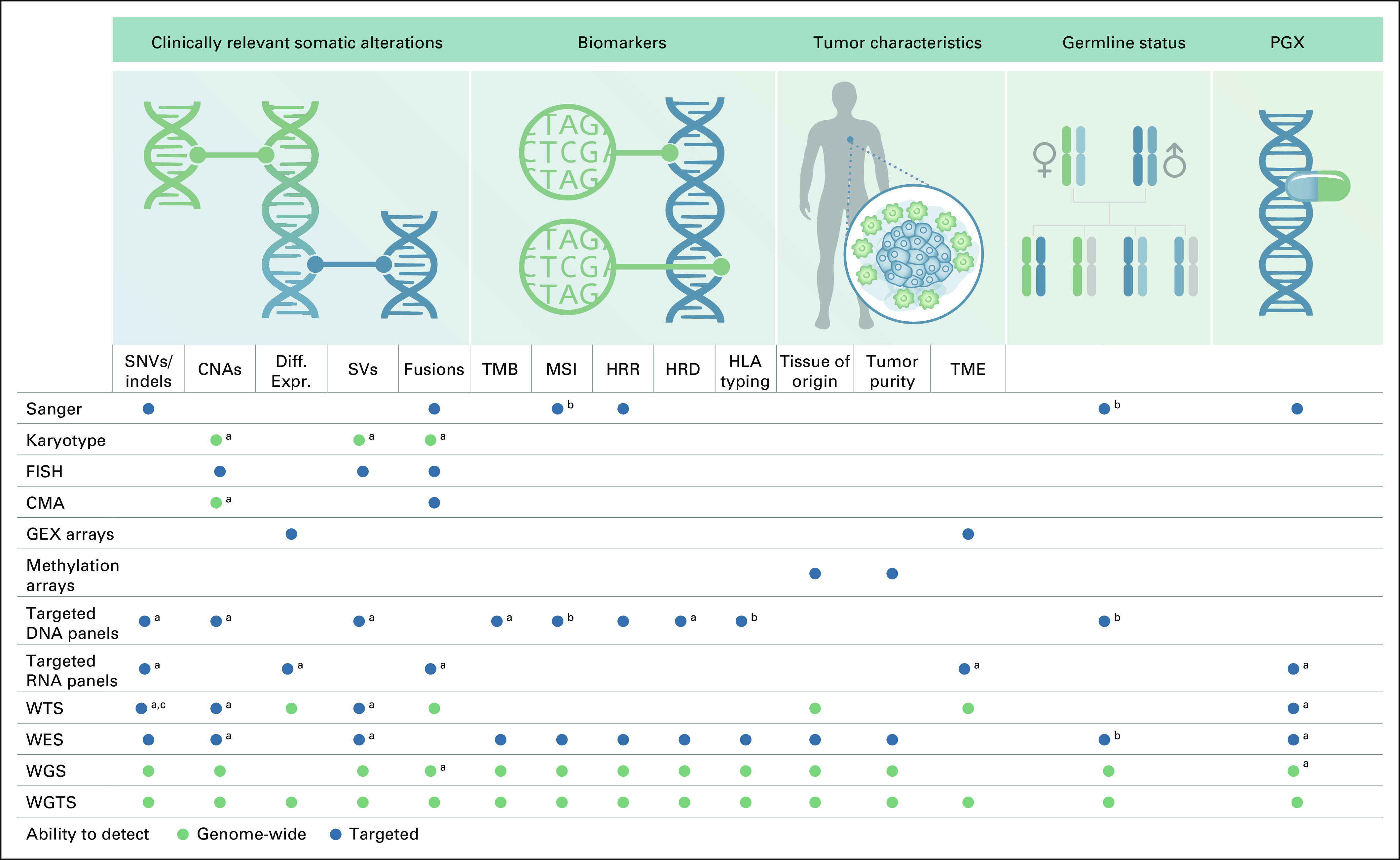
Methods currently used for a precise diagnosis of patients with cancer and tumor characterization. aMethod/design-specific limitations may exist in sensitivity and/or event type or size. bOnly when a normal control is included in the test, which is not routine practice. cAlthough the approach is genome-wide, the analysis is usually done in targeted way because of the complexity of analysis. CMA, chromosomal microarray; CNAs, copy number aberrations; Diff. Expr., differential expressions; FISH, fluorescence in situ hybridization; GEX, gene expression; HLA, human leukocyte antigen; HRD, homologous recombination deficiency; HRR, homologous recombination repair; MSI, microsatellite instability; PGX, pharmacogenomics; SNVs, single-nucleotide variants; TMB, tumor mutational burden; TME, tumor mutational environment; WES, whole-exome sequencing; WGS, whole-genome sequencing; WGTS, whole-genome and transcriptome sequencing; WTS, whole-transcriptome sequencing.
Hematological malignancies.
The hematological malignancies most ready for WGTS adoption into the clinical workflow are acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), and myelodysplastic syndromes.12,13 In routine practice, patients with AML are risk stratified by conventional cytogenetic analysis, FISH, and targeted sequencing. A recent study of 235 patients with AML and patients with myelodysplastic syndromes revealed that WGS detected all SVs detected by conventional cytogenetic analysis as well as new clinically informative genomic events in 17% of patients. This study also highlighted the ability of WGS to refine risk stratification of patients with AML, including those with inconclusive results from cytogenetics.8 Extrapolation of the Human Leukocyte Antigen status from WGS data may be another driving factor for the adoption of this assay before hematopoietic stem-cell transplantation. Thus, for patients with AML, the diagnostic workup could be consolidated into a single comprehensive WGS test.
While WGS provides great number of insights in genomic aberrations occurring at DNA level, WTS analysis enables detection of differentially expressed genes and expressed gene fusions, which may be important for risk group classification as seen in ALL.14,15 Historically, this initial identification has relied on time-consuming approaches using multiple methods including karyotyping, FISH, genomic arrays, measurement of DNA index, and targeted molecular analysis for fusion genes. Gene-expression identification of distinct ALL entities by WTS enables identification of phenocopies of other subtypes16-18 (eg, Ph-like and ETV6-RUNX1–like ALL) and provides verification of rearrangements that may not be conclusive from WGS alone. This is also reflected by the recent WHO classification of lymphoid neoplasms where transcriptome analysis is suggested as a powerful tool for classification of distinct ALL subtypes.19-21 WGS has additional capabilities over WTS, for both AML and ALL, including the precise characterization of SVs and insertions and deletions, the ability to identify rearrangements with intergenic breakpoints that may not encode a fusion chimera but deregulate oncogenes,22 and unambiguous calling of somatic and germline variants. Thus, the combined WGTS approach may offer the most comprehensive molecular analysis than any single genomic sequencing modality and has been implemented for ALL clinical management in some academic and private laboratories in the United States23 and Europe.24,25
Rare cancers, cancers of unknown primary, and pediatric.
For cancers with a frequently long diagnostic odyssey like rare cancers (eg, sarcomas, pediatric cancers, brain cancers) and cancers of unknown primary (CUP), WGTS has the potential to improve and/or refine diagnosis.26 A recent clinical WGS study of 83 patients with sarcoma demonstrated refinement of diagnosis in 14% of cases, nomination of actionable biomarkers in 36% of tumors, and detection of germline cancer predisposition variants in 8% of patients.27 WGS may be particularly impactful in sarcoma given its position as one of the most heterogeneous of all cancers, with the newest WHO classification denoting 175 different soft tissue and bone tumors, many distinguishable by genomic features.28,29 WGTS may also prevent patients from being misassigned to therapies that are unlikely to provide any efficacy while at the same time bringing considerable clinical toxicity to patients and financial burden to the health care system.30
Although considered uncommon as individual cases, rare cancers in aggregate comprise around 25% of all cancer cases.31 Rare cancers are not well served by targeted panel sequencing, whose content and coverage are biased toward mutational events and genes found in common cancers. Recent studies indicate that patients with rare cancers may benefit from molecular stratification using WGTS. For example, a large prospective observational study demonstrated the value of WGTS to guide the clinical management of 1,310 adult patients with advanced rare cancers.32 On the basis of several hundred individual biomarkers and, importantly, several composite biomarkers that WGTS is particularly well suited to capture fully, a multi-institutional molecular tumor board (MTB) made evidence-based recommendations in more than 85% of cases.33 Recommended therapies could be implemented in approximately one third of patients, and resulted in significantly improved response rates compared with prior therapies, leading to a progression-free survival (PFS) ratio of > 1.3 in 36% of these patients. The clinical value of WGTS, however, varied between different entities. Patients with CUP particularly benefited from comprehensive molecular profiling, in terms of more precise diagnostic classification and individually tailored therapy, which was associated with a particularly large prolongation of intrapatient PFS.32,34 In contrast, WGTS did not provide additional benefit in other entities such as bone sarcomas,32 suggesting that more comprehensive analyses of WGTS data are needed in addition to other levels of characterization of these patients. In the Drug Rediscovery Protocol (DRUP), 500 patients started one of the available drugs and were evaluated for treatment outcomes. The overall clinical benefit rate was 33% in both rare and nonrare cancer subgroups.35 Similar studies currently being conducted in various countries will further corroborate the utility of molecular stratification by WGTS in patients with rare cancers and provide a framework for future clinical trials that facilitate drug approval for this underserved patient population.36
Although rare, childhood cancers are the second leading cause of death in children under age 14 years.37 Pediatric cancers are heterogeneous and carry a unique combination of somatic, inherited, and de novo germline mutations. A prospective study on tumor and germline genomes from 309 children showed that inclusion of WGS enabled detection of activating gene fusions and enhancer hijacking in 36% and 8% of tumors, respectively, as well as small intragenic deletions in 15% of tumors and mutational signals associated with pathogenic variants that otherwise would have remained undetected.38 Added values of WTS is the detection of actionable gene fusions, as observed in pediatric patients with neuroblastoma,39 which can help identify patients who would benefit from targeted therapies using NTRK inhibitors.40 Data from 114 pediatric and young adult solid tumors supported the utility of an integrated approach, where WGTS identified additional oncogenic mutations not captured by targeted sequencing in 49% of patients.41
In the multinational precision oncology study INFORM, relapsed pediatric patients with cancer underwent comprehensive molecular profiling including WES, low-coverage WGS, RNA sequencing, and methylation arrays.42 Among 519 patients with complete follow-up, 86% had at least one actionable target and 33% received a matching treatment. In the subgroup of patients with highest target priority level (8% of patients), according to a seven-scale target prioritization algorithm, half of them received targeted treatment with a PFS of 204 days versus 117 days in all other patients. Cancer predisposition syndromes were identified in 7.5% of patients.
Adult solid cancers.
For many solid tumor cancer types, analysis of tumor DNA can help personalize treatment.43-45 In a recent proof-of-principle study,46 WGS characterized genetic lesions and yielded better patient outcomes illustrating the power of a one-size-fits-all test for molecular cancer diagnostics.43,44,47 WGTS also improved the diagnostic yield in advanced patients with non–small-cell lung cancer, pancreatic, CRC, and breast cancer (especially for tumors that come up negative on targeted panels)48 by revealing novel fusion partners of actionable genes such as NTRK2 and NTRK3 and by identifying novel fusions in clinically relevant genes.
WGTS can identify a wider range of markers, including complex markers, than other techniques used in cancer genomic workup (Fig 1). WTGS detects all relevant markers covered by panels in tumors with purity above 20% (assuming a tumor sequencing depth of approximately 90×) and those caused by SVs including copy neutral intragenic SVs disrupting tumor suppressor genes. Furthermore, detecting deletions in low purity samples is challenging using panel-based sequencing, while a recent analysis shows that in 9% of solid tumors, a cancer driver is affected by a homozygous disruption, which includes clinically relevant genes like PTEN and TP53.49 The same study also revealed that 13% of clinically relevant gene fusions arise through complex rearrangements involving three or more genomic segments, which would likely remain undetected with targeted next-generation sequencing but are effectively detected using WTS. A WGTS approach is likely the most sophisticated and sensitive way to characterize cancers with HRD, for which poly (ADP-ribose) polymerase inhibitors is currently or soon to be approved. The genome-wide view shows the signature HRD genomic lesions, namely the loss of heterozygosity, telomeric allelic imbalance, and large-scale state transitions, yielding a personalized HRD score that is likely the best response predictor of poly (ADP-ribose) polymerase inhibitors sensitivity.50 Like TMB, the establishment of universal HRD signatures and thresholds would help maximize the value of HRD for patient stratification.51 WGS recently detected clear signs of HRD deficiency in a broad range of tumor types including the most common ones (ovarian, breast, pancreatic, prostate, and lung) and others such as biliary and urinary tract cancer (5%-6% HRD frequency), suggesting new therapeutic opportunities for difficult-to-treat cancer types.52
Taken together, mounting evidence shows that WGTS enables improved diagnosis over existing clinical tests used in oncology. As new clinically relevant biomarkers are identified, WGTS will have the capability to quickly report them without designing a new assay. Furthermore, WGTS provides better understanding of cancer etiology, including the currently still enigmatic role of noncoding mutations53 and biology behind characteristic mutational signatures that can be discerned in tumors,54,55 the expansion of germline analyses with polygenic risk scores56 and pharmacogenomics,57 and also valuable input for emerging applications, including cfDNA analyses (eg, for panel design or shallow sequencing-based residual MRD detection),58 personalized vaccines,59 and microenvironment characterization, including infiltrating immune cell decomposition.60 For all these reasons, WGTS is poised to replace a wide range of other types of molecular testing (Fig 1); however, the health care systems in most countries must address key practical barriers to implementation.
DISCUSSION
Practical Factors for WGTS Implementation
Sample collection and biobanking.
Currently, formalin-fixed, paraffin-embedded (FFPE) samples are those routinely collected in most clinical laboratories and fresh-frozen (FF) samples are available in only a limited number of centers. Importantly, samples for high-quality WGTS analyses must contain undegraded and chemically intact nucleic acid. FF samples are far less damaged and provide less noise in genome-wide WGTS results. However, routine collection of FF samples and standardized biobanking creates logistic and infrastructure issues, eg, infrastructures to store samples and train personnel, where some of these would be solved by centralization (Fig 2). The Hartwig Medical Foundation2 and 100.000 Genomes Project in the United Kingdom61 showed that WGS can be performed on FFPE and gives similar diagnostic accuracy of known driver mutations as panels. However, genome-wide analyses, including the evaluation of potentially novel cancer drivers, accurate detection of copy number, and SVs as well as complex biomarker (MSI, HRD) assessment, turned out to be very challenging or impossible to perform on FFPE input material. Although The New York Genome Center has validated FFPE specimens for WGS and WTS3 and shown the performance with matched frozen samples, FFPE does have a downstream effect on the variant calling and requires additional data sets for complex biomarker discovery.
FIG 2.
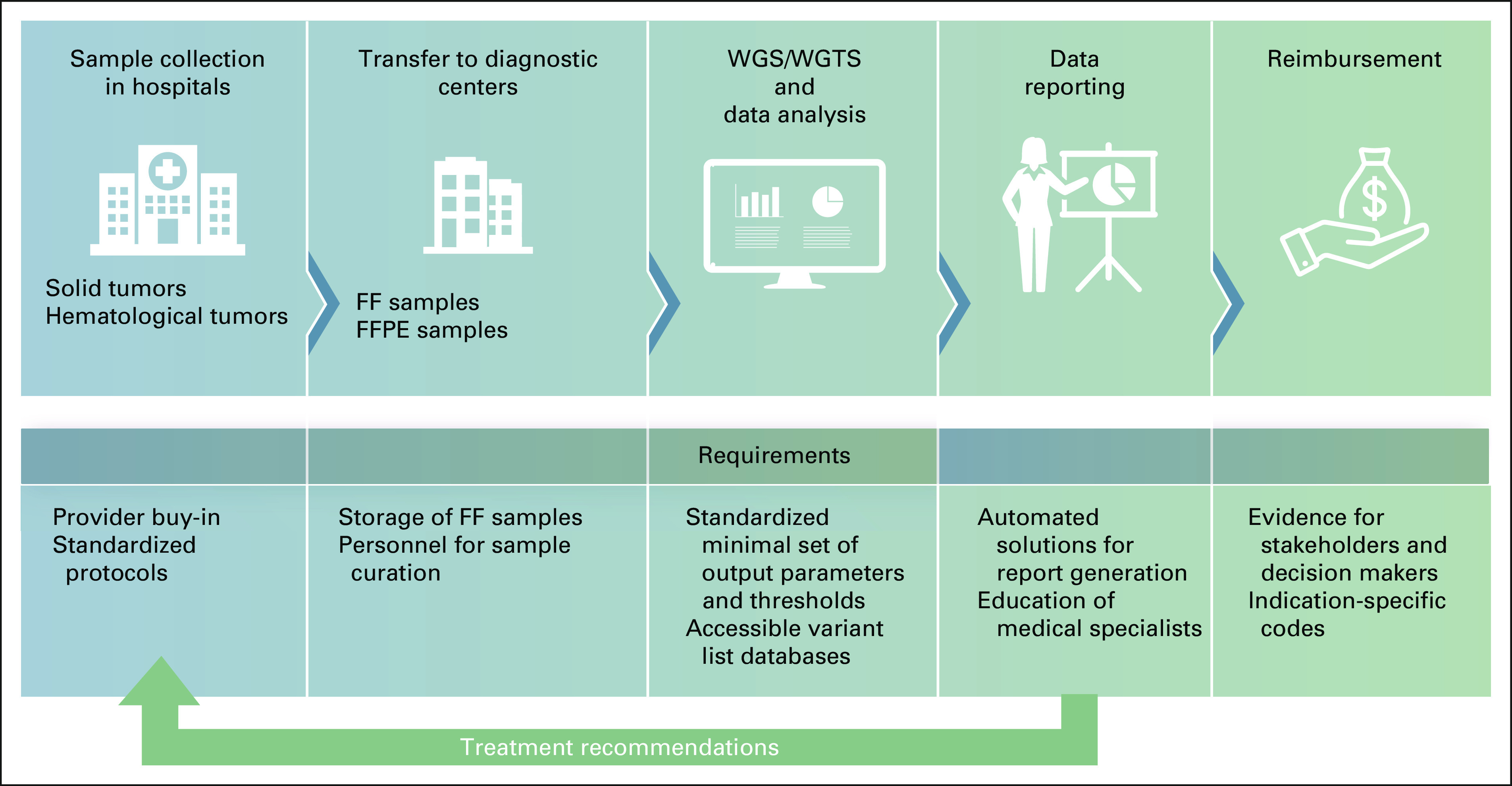
Centralized approach enabling effective WGTS-based precision medicine. FF, fresh frozen; FFPE, formalin-fixed paraffin-embedded; WGS, whole-genome sequencing; WGTS, whole-genome transcriptome sequencing.
Since FFPE tumor samples do still bring key value to initial diagnoses on the basis of histopathologic evaluation and tumor type classification and staging, a likely future scenario is that FFPE specimen will be collected for histology as well as FF biopsies for molecular diagnostics (Data Supplement).
Data analysis, interpretation, and reporting.
The benefits of specialized centers for WGTS diagnostics extend to comprehensive data analysis, including flexible and more automated solutions for clinical interpretation, reporting, and data storage (Fig 2), which will not only improve diagnostic efficiency and precision but also reduce costs and analysis/test heterogeneity. Actionable variant lists62 should be stored in databases to ensure analysis of genomic regions known to be useful for diagnosis and treatment. General-purpose, genome-wide variant callers could be sensitized to perform better in diagnostically relevant parts of the genome. This approach would increase detection sensitivity significantly for low tumor purity samples and in cases that lack matched normal tissue. For molecular reporting, tools for summarizing results require focus on clinical relevance rather than returning all molecular findings.
The turnaround time for WGTS data should be considered, and several groups have reported it to be < 10-14 working days, similar as current standard-of-care (SoC) molecular diagnostics.2,5 Independent of the time required to analyze data, rapid delivery of WGTS depends on several factors: blood and tissue processing, sample extraction, pathology review, MTB meeting schedule, and integration of a joint panel WGTS/MTB report sign-off. These challenges are similar to those faced by large diagnostic panels.
Automated solutions will solve many of the problems surrounding cancer genome interpretation and report drafting. Automated systems could create reports that include detailed information on relevant molecular events including complex tumor characteristics like MSI, HRD, and TMB and reference to actionability options. Importantly, these systems should be user-friendly and broadly available and should use well-curated and up-to-date knowledge bases to annotate and enrich molecular reports63 (Data Supplement). Data sharing at national/international level will also be essential to fuel research to further improve patient-adapted treatment and follow-up. Data sharing is expected to improve clinical interpretation of WGTS by providing practical guidelines and supporting the development of standards for the use of clinical WGTS. Furthermore, connecting data sources offers opportunities for developing data-driven treatment stratification using a patient-like-me approach. However, collection of standardized data and obtaining proper patient consent and/or legislative approval that allows for learning and improving cycles is not a standard mechanism in most health care systems. Consenting procedures are also complicated by the fact that WGS increases the chance of incidental genomic findings. Internationally, there is currently no consensus on how to address secondary findings,64 but perhaps, a larger challenge is that different medical specialists (eg, oncologist versus geneticist) have different perspectives on this topic.65 It is essential for effective WGTS implementation that medical specialists, ethicists, legal experts, and patients develop national guidelines and procedures for informing and consenting patients regarding incidental findings and data reuse.
Regulatory Landscape
Regulatory approval of WGTS solutions is likely to pose challenges as validation of all individual genes genome-wide is not feasible. In practice, genome-wide variant calling accuracy is assessed using gold standard reference controls,66,67 in addition to orthogonal validation for specific biomarkers that are already tested in routine diagnostics.2,3,5 Emerging WGTS-based biomarkers, like mutational signatures, expression profiles, and immune signatures should be regarded research-grade until formal validation is demonstrated in properly controlled studies.
While it is beyond the scope of this manuscript to describe the regulatory landscape of each country (Data Supplement), in general, clinical laboratories are bound to follow the requirements for the development of procedures and establish the performance (validation) of their assay as per the jurisdiction of the regulatory agencies under which they fall. Importantly, as WGTS involves the possibility of incidental germline findings, specific attention is required for implementing appropriate counseling, consenting, and opt-out procedures, following regional regulations and laws. Overall, assay validation of WGTS follows the same basic principles for validating other molecular tests. As the field matures and the laboratories gain experience, it is expected that these guidelines will evolve.
Reimbursement Landscape
In Europe, WGTS is not widely reimbursed for patients with cancer; however, access has been made available in a few countries through innovative programs. For example, following the 100,000 Genomes Project, National Health Service (NHS) England has created a genomic test directory which includes WGS testing of pediatric neurologic and solid tumors, sarcomas, and hematological malignancies.68 In the Netherlands, molecular cancer diagnostics are typically not reimbursed separately but is part of a disease-specific diagnosis-treatment code for which the reimbursement is calculated based on the average costs of the SoC procedures for that specific patient group (excluding expensive targeted treatments). In 2020, after a motion in the Dutch House of Representative, health care authorities decided that WGS would be reimbursed and included in the basic health care insurance for CUP patients. In France, patients with advanced cancer and a selected number of rare diseases have access to WGS through one of the two platforms publicly funded. The list of indications is regularly updated to include evidence on WGS benefit to patients after critical review by the national health authority (H.A.S.).69
In the United Kingdom, cancer molecular testing is commissioned by the NHS and offered as recommended by The National Institute for Health and Care Excellence (NICE) or as available through the NHS genomic testing centers that have been created.
In Australia, WGS is covered through a Medicare Benefits Schedule item to test the germline for monogenic disorders in pediatric diseases, but no WGTS reimbursement is available for oncology indications.
In the United States, while coverage and reimbursement of germline WGS has accelerated in recent years for pediatric patients with suspected genetic diseases, there is currently little-to-no coverage for oncology WGS applications. Of note, when testing is performed for patients in the inpatient setting (eg, during initial ALL workup), hospitals are generally reimbursed through a bundled diagnosis-related group rate. Therefore, specific coverage of a test is not required, and tumor types where WGTS offsets the aggregated cost of performing traditional SoC tests are likely to be adopted faster. In the outpatient setting, insurance coverage will be dictated through coverage policies, which are influenced by published evidence of clinical utility and recommendations from professional society guidelines.
The Real-World Cost of Cancer Health Care and Improved Outcomes
In the end, no matter how accurate or effective, WGTS must be cost-effective and affordable to convince clinical stakeholders to adopt the technology. So, what does a cancer diagnostic workup cost today? This can vary between tumor types and be based on the number of biomarkers recommended. As the number of actionable biomarkers with on-label therapies increases over time, it has become more cost-efficient to use gene panels compared with iterative single gene tests. For example, for non–small-cell lung cancer, the tumor type with the most established actionable biomarkers, the most frequently conducted tests are targeted gene panels, followed by IHC analysis for programmed cell death protein ligand 1, at a per-patient cost of $2,260 USD ± $1,217 US dollars (USD).70 Larger, more comprehensive multigene panels are in general more costly than targeted panels and cost between $1,750 USD and $4,000 USD.71 As more complex biomarkers like TMB and HRD enter SoC, approaches like Comprehensive Genomic Profiling and WGTS will increasingly be required.
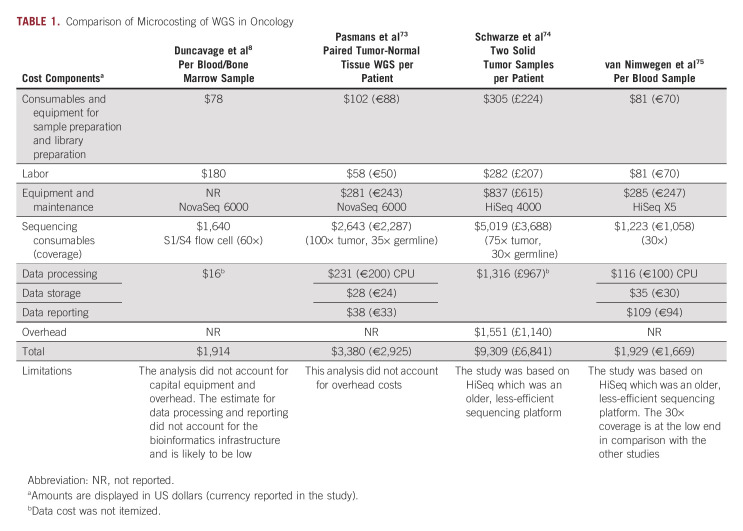
Several groups have started to report data on the cost of WGTS testing. A number of factors can affect the cost of clinical testing: depth of sequencing72; sequencing of tumor only or tumor + normal; breadth of reporting; sequencing platform and chemistry; throughput; data storage costs; bioinformatic pipeline development, optimization, and maintenance; and personnel costs in a clinical vs translational setting. Microcosting estimates from four studies range between $9,309 USD and $1,914 USD (Table 1) and are higher than SoC testing but decrease over time and approach the costs of large panels. The main challenge to come to an overall favorable health economic evaluation resides in monetizing the range of benefits of WGTS, including the value of experimental treatment options, sustainability of testing infrastructure, and contributions to a learning care system that allow for reduction of overtreatment of future patients (Data Supplement).
TABLE 1.
Comparison of Microcosting of WGS in Oncology
In conclusion, WGTS is ultimately expected to transform diagnosis and treatment for patients with cancer. It is able to streamline diagnosis by making more efficient use of scarce/limited tumor material (especially in case of biopsies of metastases) by replacing a multiplicity of patient-testing platforms or cascade testing with one powerful technology that is also future proof as it can rapidly be extended to report new biomarkers. This is important because newly developed, targeted therapies can be life-saving game-changers in some patients. Going forward, WGTS can also be the foundation for a learning system for oncology diagnostics, wherein cumulative WGTS data plus clinical and outcome data could, supported by machine learning and artificial intelligence (AI) approaches, guide clinicians in a patients like mine assessment. The assessment would identify pathogenic markers to guide treatment, allow patient-group stratifications to enable targeted rather than cookbook treatment, expand access to treatment options (drug repurposing and early experimental drug access), accelerate development of new drugs, and identify new biomarkers for improved stratification and reduction of overtreatment. A refined diagnosis of future patients could increase life expectancy and save patient exposure to ineffective therapy. Finally, (inter)national standardization amongst clinical laboratories will be necessary to allow wider adoption and access to WGTS as a routine method for patients with cancer.
ACKNOWLEDGMENT
The authors thank Lucia Speroni and Diana Colgan for their assistance with manuscript preparation and review of the manuscript.
Edwin Cuppen
Consulting or Advisory Role: Illumina (Inst), InteRNA
Travel, Accommodations, Expenses: Illumina
Olivier Elemento
Stock and Other Ownership Interests: Volastra Therapeutics, Owkin, OneThree Biotech
Richard Rosenquist
Honoraria: AbbVie, Illumina, Roche, Janssen, AstraZeneca
Consulting or Advisory Role: Illumina, AbbVie
Travel, Accommodations, Expenses: Illumina
Svetlana Nikic
Employment: Illumina
Stock and Other Ownership Interests: Illumina
Maarten IJzerman
Honoraria: RTI Health Solutions, Illumina
Consulting or Advisory Role: Creativ-Ceutical, Illumina (Inst)
Research Funding: Illumina (Inst)
Travel, Accommodations, Expenses: Illumina (Inst)
Isabelle Durand Zaleski
Honoraria: Abbott Laboratories, BMS, MSD Oncology, Pfizer, Illumina
Consulting or Advisory Role: BMS, MSD Oncology, Pfizer
Geert Frederix
Honoraria: Illumina (Inst)
Consulting or Advisory Role: Illumina (Inst)
Lars-Åke Levin
Employment: Janssen
Stock and Other Ownership Interests: AstraZeneca
Research Funding: Boehringer Ingelheim (Inst), Janssen (Inst)
Charles G. Mullighan
Stock and Other Ownership Interests: Amgen
Honoraria: Amgen, Illumina
Consulting or Advisory Role: Illumina, Faze, Beam Therapeutics
Speakers' Bureau: Amgen, Pfizer
Research Funding: Loxo, Pfizer, AbbVie
Patents, Royalties, Other Intellectual Property: Inventor on a pending patent application related to gene-expression signatures for detection of underlying Philadelphia chromosome “like events and therapeutic targeting in leukemia (PCT/US2012/069228), WO 2021/022076 A1. This Patent Highlight shows representative PROTAC compounds bound to JAK2, where ruxolitinib and baricitinib bind to the human JAK2 JH1. Furthermore, representative data illustrate protein degradation, cytotoxicity, and effect of the JAKSTAT signaling pathway of the PROTAC compounds in MHHCALL-4 cells, Marcus Fisher, Fatemeh Keramatnia, Kevin Mcgowan, Jaeki Min, Gisele A. Nishiguchi, Jeanine Price, Zoran Rankovic, Das Sourav, Charles G. Mullighan, Yunchao Chang 2021 Substituted N-(2-(2,6-Dioxopiperidin-3-YL)-1,3-Dioxoisoindolin-5-YL)Arylsulfonamide Analogs as Modulators of Cereblon Protein, Application No: PCT/US2021/051648 Filed: September 23, 2021. Patent pending (Inst)
Travel, Accommodations, Expenses: Amgen, Illumina
Reinhard Buettner
Stock and Other Ownership Interests: Gnothis Inc
Honoraria: AstraZeneca, AbbVie, Bayer, Bristol Myers Squibb, Boehringer Ingelheim, Merck Serono, MSD, Novartis, Qiagen, Pfizer, Roche, Illumina
Research Funding: Roche (Inst)
Trevor J. Pugh
Honoraria: Merck, AstraZeneca, Illumina, PACT Pharma
Consulting or Advisory Role: Chrysalis Biomedical Advisors, Axiom Healthcare Strategies, Canadian Pension Plan Investment Board
Research Funding: Roche
Patents, Royalties, Other Intellectual Property: Hybrid-capture sequencing for dete rmining immune cell clonality
Carlos Caldas
Consulting or Advisory Role: AstraZeneca/MedImmune, Illumina
Research Funding: AstraZeneca (Inst), Genentech/Roche (Inst), Servier (Inst)
Fabrice Andre
Stock and Other Ownership Interests: PEGASCY
Consulting or Advisory Role: Guardant Health (Inst), MedImmune (Inst), Gilead Sciences (Inst), Relay therapeutics (Inst)
Research Funding: AstraZeneca (Inst), Novartis (Inst), Pfizer (Inst), Lilly (Inst), Roche (Inst), Daiichi (Inst)
Travel, Accommodations, Expenses: Novartis, Roche, GlaxoSmithKline, AstraZeneca
Ilse Custers
Honoraria: Illumina
Travel, Accommodations, Expenses: Illumina
Elias Campo
Honoraria: EUSA Pharma, AstraZeneca, Takeda
Consulting or Advisory Role: Illumina, AbbVie
Research Funding: AstraZeneca
Patents, Royalties, Other Intellectual Property: Author on a patent licensed to NanoStrig Technologies, Author in the protectedt bioinformatic IgCaller pipeline
Hans van Snellenberg
Research Funding: Illumina (Inst)
Anna Schuh
Stock and Other Ownership Interests: Illumina, SERENOx
Consulting or Advisory Role: AbbVie, Roche, Janssen, AstraZeneca
Research Funding: Johnson & Johnson, Illumina (Inst), Oxford Nanopore Technologies (Inst)
Travel, Accommodations, Expenses: AbbVie, Janssen Oncology
Christof von Kalle
Stock and Other Ownership Interests: GeneWerk
Consulting or Advisory Role: Roche/Genentech, Novartis, Pfizer
Patents, Royalties, Other Intellectual Property: Patent applications in molecular diagnostics
Torsten Haferlach
Employment: MLL Munich Leukemia Laboratory
Leadership: MLL Munich Leukemia Laboratory
Consulting or Advisory Role: Illumina
Stefan Fröhling
Honoraria: PharmaMar, Roche, Lilly, Amgen
Consulting or Advisory Role: Bayer, Illumina, Roche
Research Funding: AstraZeneca (Inst), PharmaMar (Inst), Pfizer (Inst), Roche (Inst)
Travel, Accommodations, Expenses: PharmaMar, Roche, Lilly, Amgen
Vaidehi Jobanputra
Honoraria: Illumina
No other potential conflicts of interest were reported.
SUPPORT
Hartwig Medical Foundation (Hans van Snellenberg, Edwin Cuppen) receives funding from Hartwig Foundation, the Dutch Cancer Foundation (KWF), ZonMW and Illumina Inc. R.R. received funding from the Swedish Cancer Society, the Swedish Research Council, the Knut and Alice Wallenberg Foundation, Karolinska Institutet, Karolinska University Hospital, and Radiumhemmets Forskningsfonder, Stockholm. University of Twente, Enschede, The Netherlands (M.I.) receives funding from Illumina Inc. C.G.M. is supported by NCI CA 197695. S.F. received support from the National Center for Tumor Diseases and the German Cancer Consortium. C.C. is supported by funding from CRUK (grant numbers A17197, A27657 and A29580) and a European Research Council Advanced Award (grant number 694620). T.J.P. holds the Canada Research Chair in Translational Genomics and is supported by a Senior Investigator Award from the Ontario Institute for Cancer Research and the Gattuso-Slaight Personalized Cancer Medicine Fund.
AUTHOR CONTRIBUTIONS
Conception and design: Edwin Cuppen, Olivier Elemento, Richard Rosenquist, Svetlana Nikic, Maarten IJzerman, Isabelle Durand Zaleski, Lars-Åke Levin, Charles G. Mullighan, Reinhard Buettner, Trevor J. Pugh, Sean Grimmond, Carlos Caldas, Ilse Custers, Hans van Snellenberg, Anna Schuh, Christof von Kalle, Torsten Haferlach, Stefan Fröhling, Vaidehi Jobanputra
Financial support: Isabelle Durand Zaleski
Administrative support: Isabelle Durand Zaleski, Carlos Caldas
Provision of study materials or patients: Torsten Haferlach
Collection and assembly of data: Svetlana Nikic, Lars-Åke Levin, Trevor J. Pugh, Carlos Caldas, Hans van Snellenberg, Hidewaki Nakagawa, Stefan Fröhling
Data analysis and interpretation: Olivier Elemento, Maarten IJzerman, Isabelle Durand Zaleski, Geert Frederix, Lars-Åke Levin, Charles G. Mullighan, Carlos Caldas, Fabrice Andre, Elias Campo, Hidewaki Nakagawa, Stefan Fröhling
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Edwin Cuppen
Consulting or Advisory Role: Illumina (Inst), InteRNA
Travel, Accommodations, Expenses: Illumina
Olivier Elemento
Stock and Other Ownership Interests: Volastra Therapeutics, Owkin, OneThree Biotech
Richard Rosenquist
Honoraria: AbbVie, Illumina, Roche, Janssen, AstraZeneca
Consulting or Advisory Role: Illumina, AbbVie
Travel, Accommodations, Expenses: Illumina
Svetlana Nikic
Employment: Illumina
Stock and Other Ownership Interests: Illumina
Maarten IJzerman
Honoraria: RTI Health Solutions, Illumina
Consulting or Advisory Role: Creativ-Ceutical, Illumina (Inst)
Research Funding: Illumina (Inst)
Travel, Accommodations, Expenses: Illumina (Inst)
Isabelle Durand Zaleski
Honoraria: Abbott Laboratories, BMS, MSD Oncology, Pfizer, Illumina
Consulting or Advisory Role: BMS, MSD Oncology, Pfizer
Geert Frederix
Honoraria: Illumina (Inst)
Consulting or Advisory Role: Illumina (Inst)
Lars-Åke Levin
Employment: Janssen
Stock and Other Ownership Interests: AstraZeneca
Research Funding: Boehringer Ingelheim (Inst), Janssen (Inst)
Charles G. Mullighan
Stock and Other Ownership Interests: Amgen
Honoraria: Amgen, Illumina
Consulting or Advisory Role: Illumina, Faze, Beam Therapeutics
Speakers' Bureau: Amgen, Pfizer
Research Funding: Loxo, Pfizer, AbbVie
Patents, Royalties, Other Intellectual Property: Inventor on a pending patent application related to gene-expression signatures for detection of underlying Philadelphia chromosome “like events and therapeutic targeting in leukemia (PCT/US2012/069228), WO 2021/022076 A1. This Patent Highlight shows representative PROTAC compounds bound to JAK2, where ruxolitinib and baricitinib bind to the human JAK2 JH1. Furthermore, representative data illustrate protein degradation, cytotoxicity, and effect of the JAKSTAT signaling pathway of the PROTAC compounds in MHHCALL-4 cells, Marcus Fisher, Fatemeh Keramatnia, Kevin Mcgowan, Jaeki Min, Gisele A. Nishiguchi, Jeanine Price, Zoran Rankovic, Das Sourav, Charles G. Mullighan, Yunchao Chang 2021 Substituted N-(2-(2,6-Dioxopiperidin-3-YL)-1,3-Dioxoisoindolin-5-YL)Arylsulfonamide Analogs as Modulators of Cereblon Protein, Application No: PCT/US2021/051648 Filed: September 23, 2021. Patent pending (Inst)
Travel, Accommodations, Expenses: Amgen, Illumina
Reinhard Buettner
Stock and Other Ownership Interests: Gnothis Inc
Honoraria: AstraZeneca, AbbVie, Bayer, Bristol Myers Squibb, Boehringer Ingelheim, Merck Serono, MSD, Novartis, Qiagen, Pfizer, Roche, Illumina
Research Funding: Roche (Inst)
Trevor J. Pugh
Honoraria: Merck, AstraZeneca, Illumina, PACT Pharma
Consulting or Advisory Role: Chrysalis Biomedical Advisors, Axiom Healthcare Strategies, Canadian Pension Plan Investment Board
Research Funding: Roche
Patents, Royalties, Other Intellectual Property: Hybrid-capture sequencing for dete rmining immune cell clonality
Carlos Caldas
Consulting or Advisory Role: AstraZeneca/MedImmune, Illumina
Research Funding: AstraZeneca (Inst), Genentech/Roche (Inst), Servier (Inst)
Fabrice Andre
Stock and Other Ownership Interests: PEGASCY
Consulting or Advisory Role: Guardant Health (Inst), MedImmune (Inst), Gilead Sciences (Inst), Relay therapeutics (Inst)
Research Funding: AstraZeneca (Inst), Novartis (Inst), Pfizer (Inst), Lilly (Inst), Roche (Inst), Daiichi (Inst)
Travel, Accommodations, Expenses: Novartis, Roche, GlaxoSmithKline, AstraZeneca
Ilse Custers
Honoraria: Illumina
Travel, Accommodations, Expenses: Illumina
Elias Campo
Honoraria: EUSA Pharma, AstraZeneca, Takeda
Consulting or Advisory Role: Illumina, AbbVie
Research Funding: AstraZeneca
Patents, Royalties, Other Intellectual Property: Author on a patent licensed to NanoStrig Technologies, Author in the protectedt bioinformatic IgCaller pipeline
Hans van Snellenberg
Research Funding: Illumina (Inst)
Anna Schuh
Stock and Other Ownership Interests: Illumina, SERENOx
Consulting or Advisory Role: AbbVie, Roche, Janssen, AstraZeneca
Research Funding: Johnson & Johnson, Illumina (Inst), Oxford Nanopore Technologies (Inst)
Travel, Accommodations, Expenses: AbbVie, Janssen Oncology
Christof von Kalle
Stock and Other Ownership Interests: GeneWerk
Consulting or Advisory Role: Roche/Genentech, Novartis, Pfizer
Patents, Royalties, Other Intellectual Property: Patent applications in molecular diagnostics
Torsten Haferlach
Employment: MLL Munich Leukemia Laboratory
Leadership: MLL Munich Leukemia Laboratory
Consulting or Advisory Role: Illumina
Stefan Fröhling
Honoraria: PharmaMar, Roche, Lilly, Amgen
Consulting or Advisory Role: Bayer, Illumina, Roche
Research Funding: AstraZeneca (Inst), PharmaMar (Inst), Pfizer (Inst), Roche (Inst)
Travel, Accommodations, Expenses: PharmaMar, Roche, Lilly, Amgen
Vaidehi Jobanputra
Honoraria: Illumina
No other potential conflicts of interest were reported.
REFERENCES
- 1. Jobanputra V, Wrzeszczynski KO, Buttner R, et al. Clinical interpretation of whole-genome and whole-transcriptome sequencing for precision oncology. Semin Cancer Biol. 2022;84:23–31. doi: 10.1016/j.semcancer.2021.07.003. [DOI] [PubMed] [Google Scholar]
- 2. Roepman P, de Bruijn E, van Lieshout S, et al. Clinical validation of whole genome sequencing for cancer diagnostics. J Mol Diagn. 2021;23:816–833. doi: 10.1016/j.jmoldx.2021.04.011. [DOI] [PubMed] [Google Scholar]
- 3. Wrzeszczynski KO, Felice V, Abhyankar A, et al. Analytical validation of clinical whole-genome and transcriptome sequencing of patient-derived tumors for reporting targetable variants in cancer. J Mol Diagn. 2018;20:822–835. doi: 10.1016/j.jmoldx.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Van Hoeck A, Tjoonk NH, van Boxtel R, et al. Portrait of a cancer: Mutational signature analyses for cancer diagnostics. BMC Cancer. 2019;19:457. doi: 10.1186/s12885-019-5677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shukla N, Levine MF, Gundem G, et al. Feasibility of whole genome and transcriptome profiling in pediatric and young adult cancers. Nat Commun. 2022;13:2485. doi: 10.1038/s41467-022-30233-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Samsom KG, Schipper LJ, Roepman P, et al. Feasibility of whole-genome sequencing-based tumor diagnostics in routine pathology practice. J Pathol. 2022;258:179–188. doi: 10.1002/path.5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martín-Garcia D, Navarro A, Valdés-Mas R, et al. CCND2 and CCND3 hijack immunoglobulin light-chain enhancers in cyclin D1(-) mantle cell lymphoma. Blood. 2019;133:940–951. doi: 10.1182/blood-2018-07-862151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Duncavage EJ, Schroeder MC, O'Laughlin M, et al. Genome sequencing as an alternative to cytogenetic analysis in myeloid cancers. N Engl J Med. 2021;384:924–935. doi: 10.1056/NEJMoa2024534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hollein A, Twardziok SO, Walter W, et al. The combination of WGS and RNA-Seq is superior to conventional diagnostic tests in multiple myeloma: Ready for prime time? Cancer Genet. 2020;242:15–24. doi: 10.1016/j.cancergen.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 10. Prendergast SC, Strobl AC, Cross W, et al. Sarcoma and the 100,000 Genomes Project: Our experience and changes to practice. J Pathol Clin Res. 2020;6:297–307. doi: 10.1002/cjp2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wong M, Mayoh C, Lau LMS, et al. Whole genome, transcriptome and methylome profiling enhances actionable target discovery in high-risk pediatric cancer. Nat Med. 2020;26:1742–1753. doi: 10.1038/s41591-020-1072-4. [DOI] [PubMed] [Google Scholar]
- 12. Docking TR, Parker JDK, Jadersten M, et al. A clinical transcriptome approach to patient stratification and therapy selection in acute myeloid leukemia. Nat Commun. 2021;12:2474. doi: 10.1038/s41467-021-22625-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen X, Wang F, Zhang Y, et al. Fusion gene map of acute leukemia revealed by transcriptome sequencing of a consecutive cohort of 1000 cases in a single center. Blood Cancer J. 2021;11:112. doi: 10.1038/s41408-021-00504-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jeha S, Choi J, Roberts KG, et al. Clinical significance of novel subtypes of acute lymphoblastic leukemia in the context of minimal residual disease-directed therapy. Blood Cancer Discov. 2021;2:326–337. doi: 10.1158/2643-3230.BCD-20-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Paietta E, Roberts KG, Wang V, et al. Molecular classification improves risk assessment in adult BCR-ABL1-negative B-ALL. Blood. 2021;138:948–958. doi: 10.1182/blood.2020010144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gu Z, Churchman ML, Roberts KG, et al. PAX5-driven subtypes of B-progenitor acute lymphoblastic leukemia. Nat Genet. 2019;51:296–307. doi: 10.1038/s41588-018-0315-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lilljebjorn H, Henningsson R, Hyrenius-Wittsten A, et al. Identification of ETV6-RUNX1-like and DUX4-rearranged subtypes in paediatric B-cell precursor acute lymphoblastic leukaemia. Nat Commun. 2016;7:11790. doi: 10.1038/ncomms11790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li JF, Dai YT, Lilljebjorn H, et al. Transcriptional landscape of B cell precursor acute lymphoblastic leukemia based on an international study of 1,223 cases. Proc Natl Acad Sci USA. 2018;115:E11711–E11720. doi: 10.1073/pnas.1814397115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khoury JD, Solary E, Abla O, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: Myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36:1703–1719. doi: 10.1038/s41375-022-01613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alaggio R, Amador C, Anagnostopoulos I, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: Lymphoid neoplasms. Leukemia. 2022;36:1720–1748. doi: 10.1038/s41375-022-01620-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization (WHO) WHO Classification of Tumours Online. https://tumourclassification.iarc.who.int/welcome/
- 22. Montefiori LE, Bendig S, Gu Z, et al. Enhancer hijacking drives oncogenic BCL11B expression in lineage ambiguous stem cell leukemia. Cancer Discov. 2021;11:2846–2867. doi: 10.1158/2159-8290.CD-21-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Inaba H, Azzato EM, Mullighan CG. Integration of next-generation sequencing to treat acute lymphoblastic leukemia with targetable lesions: The St. Jude Children's Research Hospital approach. Front Pediatr. 2017;5:258. doi: 10.3389/fped.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evaluation of genome sequencing as a diagnostic test in acute leukemia. ISRCTN identifier: ISRCTN6698714.
- 25. Walter W, Shahswar R, Stengel A, et al. Clinical application of whole transcriptome sequencing for the classification of patients with acute lymphoblastic leukemia. BMC Cancer. 2021;21:886. doi: 10.1186/s12885-021-08635-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cobain EF, Wu YM, Vats P, et al. Assessment of clinical benefit of integrative genomic profiling in advanced solid tumors. JAMA Oncol. 2021;7:525–533. doi: 10.1001/jamaoncol.2020.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schipper LJ, Monkhorst K, Samsom KG, et al. Clinical impact of prospective whole genome sequencing in sarcoma patients. Cancers. 2022;14:436. doi: 10.3390/cancers14020436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Choi JH, Ro JY. The 2020 WHO classification of tumors of soft tissue: Selected changes and new entities. Adv Anat Pathol. 2021;28:44–58. doi: 10.1097/PAP.0000000000000284. [DOI] [PubMed] [Google Scholar]
- 29. Sbaraglia M, Bellan E, Dei Tos AP. The 2020 WHO classification of soft tissue tumours: News and perspectives. Pathologica. 2021;113:70–84. doi: 10.32074/1591-951X-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rosenquist R, Cuppen E, Buettner R, et al. Clinical utility of whole-genome sequencing in precision oncology. Semin Cancer Biol. 2021;84:32–39. doi: 10.1016/j.semcancer.2021.06.018. [DOI] [PubMed] [Google Scholar]
- 31.NIH National Cancer Institute . About Rare Cancers. https://www.cancer.gov/pediatric-adult-rare-tumor/rare-tumors/about-rare-cancers [Google Scholar]
- 32. Horak P, Heining C, Kreutzfeldt S, et al. Comprehensive genomic and transcriptomic analysis for guiding therapeutic decisions in patients with rare cancers. Cancer Discov. 2021;11:2780–2795. doi: 10.1158/2159-8290.CD-21-0126. [DOI] [PubMed] [Google Scholar]
- 33. Jahn A, Rump A, Widmann TJ, et al. Comprehensive cancer predisposition testing within the prospective MASTER trial identifies hereditary cancer patients and supports treatment decisions for rare cancers. Ann Oncol. 2022;33:1186–1199. doi: 10.1016/j.annonc.2022.07.008. [DOI] [PubMed] [Google Scholar]
- 34. Möhrmann L, Werner M, Oleś M, et al. Comprehensive genomic and epigenomic analysis in cancer of unknown primary guides molecularly-informed therapies despite heterogeneity. Nat Commun. 2022;13:4485. doi: 10.1038/s41467-022-31866-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hoes LR, van Berge Henegouwen JM, van der Wijngaart H, et al. Patients with rare cancers in the drug rediscovery protocol (DRUP) benefit from genomics—Guided treatment. Clin Cancer Res. 2022;28:1402–1411. doi: 10.1158/1078-0432.CCR-21-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.The Drug Rediscovery Protocol (DRUP Trial) NCT02925234. https://clinicaltrials.gov/ct2/show/NCT02925234 [Google Scholar]
- 37. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 38. Newman S, Nakitandwe J, Kesserwan CA, et al. Genomes for Kids: The scope of pathogenic mutations in pediatric cancer revealed by comprehensive DNA and RNA sequencing. Cancer Discov. 2021;11:3008–3027. doi: 10.1158/2159-8290.CD-20-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kimura S, Sekiguchi M, Watanabe K, et al. Association of high-risk neuroblastoma classification based on expression profiles with differentiation and metabolism. PLoS One. 2021;16:e0245526. doi: 10.1371/journal.pone.0245526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. FDA approves an oncology drug that targets a key genetic driver of cancer, rather than a specific type of tumor. https://www.fda.gov/news-events/press-announcements/fda-approves-oncology-drug-targets-key-genetic-driver-cancer-rather-specific-type-tumor.
- 41. Shukla N, Levine M, Gundem G, et al. Feasibility and clinical utility of cancer whole genome and transcriptome sequencing for pediatric and young adult solid tumors. J Clin Oncol. 2021;39 suppl 15; abstr 3063. [Google Scholar]
- 42. van Tilburg CM, Pfaff E, Pajtler KW, et al. The Pediatric Precision Oncology INFORM Registry: Clinical outcome and benefit for patients with very high-evidence targets. Cancer Discov. 2021;11:2764. doi: 10.1158/2159-8290.CD-21-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Priestley P, Baber J, Lolkema MP, et al. Pan-cancer whole-genome analyses of metastatic solid tumours. Nature. 2019;575:210–216. doi: 10.1038/s41586-019-1689-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sicklick JK, Kato S, Okamura R, et al. Molecular profiling of cancer patients enables personalized combination therapy: The I-PREDICT study. Nat Med. 2019;25:744–750. doi: 10.1038/s41591-019-0407-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Walter W, Pfarr N, Meggendorfer M, et al. Next-generation diagnostics for precision oncology: Preanalytical considerations, technical challenges, and available technologies. Semin Cancer Biol. 2022;84:3–15. doi: 10.1016/j.semcancer.2020.10.015. [DOI] [PubMed] [Google Scholar]
- 46. van der Velden DL, Hoes LR, van der Wijngaart H, et al. The Drug Rediscovery protocol facilitates the expanded use of existing anticancer drugs. Nature. 2019;574:127–131. doi: 10.1038/s41586-019-1600-x. [DOI] [PubMed] [Google Scholar]
- 47.Study of Molecular Profile-Related Evidence to Determine Individualized Therapy for Advanced or Poor Prognosis Cancers. https://ClinicalTrials.gov/show/NCT02534675 [Google Scholar]
- 48. Tsang ES, Grisdale CJ, Pleasance E, et al. Uncovering clinically relevant gene fusions with integrated genomic and transcriptomic profiling of metastatic cancers. Clin Cancer Res. 2021;27:522–531. doi: 10.1158/1078-0432.CCR-20-1900. [DOI] [PubMed] [Google Scholar]
- 49. Shale C, Cameron DL, Baber J, et al. Unscrambling cancer genomes via integrated analysis of structural variation and copy number. Cell Genomics. 2022;2:100112. doi: 10.1016/j.xgen.2022.100112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. de Luca XM, Newell F, Kazakoff SH, et al. Using whole-genome sequencing data to derive the homologous recombination deficiency scores. NPJ Breast Cancer. 2020;6:33. doi: 10.1038/s41523-020-0172-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ngoi NYL, Tan DSP. The role of homologous recombination deficiency testing in ovarian cancer and its clinical implications: Do we need it? ESMO Open. 2021;6:100144. doi: 10.1016/j.esmoop.2021.100144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nguyen L, W M Martens J, Van Hoeck A, et al. Pan-cancer landscape of homologous recombination deficiency. Nat Commun. 2020;11:5584. doi: 10.1038/s41467-020-19406-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Elliott K, Larsson E. Non-coding driver mutations in human cancer. Nat Rev Cancer. 2021;21:500–509. doi: 10.1038/s41568-021-00371-z. [DOI] [PubMed] [Google Scholar]
- 54. Alexandrov LB, Kim J, Haradhvala NJ, et al. The repertoire of mutational signatures in human cancer. Nature. 2020;578:94–101. doi: 10.1038/s41586-020-1943-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Degasperi A, Zou X, Amarante TD, et al. Substitution mutational signatures in whole-genome-sequenced cancers in the UK population. Science. 2022;376:science.abl9283. doi: 10.1126/science.abl9283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang Y, Zhu M, Ma H, et al. Polygenic risk scores: The future of cancer risk prediction, screening, and precision prevention. Inflamm Regen. 2021;1:129–149. doi: 10.1515/mr-2021-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Miteva-Marcheva NN, Ivanov HY, Dimitrov DK, et al. Application of pharmacogenetics in oncology. Biomark Res. 2020;8:32. doi: 10.1186/s40364-020-00213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zviran A, Schulman RC, Shah M, et al. Genome-wide cell-free DNA mutational integration enables ultra-sensitive cancer monitoring. Nat Med. 2020;26:1114–1124. doi: 10.1038/s41591-020-0915-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shemesh CS, Hsu JC, Hosseini I, et al. Personalized cancer vaccines: Clinical landscape, challenges, and opportunities. Mol Ther. 2021;29:555–570. doi: 10.1016/j.ymthe.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chuah S, Chew V. High-dimensional immune-profiling in cancer: Implications for immunotherapy. J Immunother Cancer. 2020;8:e000363. doi: 10.1136/jitc-2019-000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Robbe P, Popitsch N, Knight SJL, et al. Clinical whole-genome sequencing from routine formalin-fixed, paraffin-embedded specimens: Pilot study for the 100,000 Genomes Project. Genet Med. 2018;20:1196–1205. doi: 10.1038/gim.2017.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wagner AH, Walsh B, Mayfield G, et al. A harmonized meta-knowledgebase of clinical interpretations of somatic genomic variants in cancer. Nat Genet. 2020;52:448–457. doi: 10.1038/s41588-020-0603-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tamborero D, Dienstmann R, Rachid MH, et al. Support systems to guide clinical decision-making in precision oncology: The Cancer Core Europe Molecular Tumor Board Portal. Nat Med. 2020;26:992–994. doi: 10.1038/s41591-020-0969-2. [DOI] [PubMed] [Google Scholar]
- 64.ESHG, ACMG Differ Starkly in Recommendations for Reporting Secondary Findings from Genomic Tests. https://www.genomeweb.com/molecular-diagnostics/eshg-acmg-differ-starkly-recommendations-reporting-secondary-findings-genomic#.YxGAjnbMKUk [Google Scholar]
- 65. Gourna EG, Armstrong N, Wallace SE. Compare and contrast: A cross-national study across UK, USA and Greek experts regarding return of incidental findings from clinical sequencing. Eur J Hum Genet. 2016;24:344–349. doi: 10.1038/ejhg.2015.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Espejo Valle-Inclan J, Besselink NJM, de Bruijn E, et al. A multi-platform reference for somatic structural variation detection. Cell Genomics. 2022;2:100139. doi: 10.1016/j.xgen.2022.100139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Arora K, Shah M, Johnson M, et al. Deep whole-genome sequencing of 3 cancer cell lines on 2 sequencing platforms. Sci Rep. 2019;9:19123. doi: 10.1038/s41598-019-55636-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.National Health Service (NHS) National Genomic Test Directory. https://www.england.nhs.uk/publication/national-genomic-test-directories/ [Google Scholar]
- 69.France Médecine Génomique 2025. Préindications d'accès au séquençage génomique; https://pfmg2025.aviesan.fr/le-plan/indications-dacces-au-sequencage-genomique/ [Google Scholar]
- 70. van de Ven M, Koffijberg H, Retel V, et al. Real-world utilization of biomarker testing for patients with advanced non-small cell lung cancer in a tertiary referral center and referring hospitals. J Mol Diagn. 2021;23:484–494. doi: 10.1016/j.jmoldx.2021.01.004. [DOI] [PubMed] [Google Scholar]
- 71. IJzerman MJ, de Boer J, Azad A, et al. Towards routine implementation of liquid biopsies in cancer management: It is always too early, until suddenly it is too late. Diagnostics (Basel) 2021;11:103. doi: 10.3390/diagnostics11010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Genome sequencing in myeloid cancers. N Engl J Med. 2021;384:e106. doi: 10.1056/NEJMc2106014. [DOI] [PubMed] [Google Scholar]
- 73. Pasmans CTB, Tops BBJ, Steeghs EMP, et al. Micro-costing diagnostics in oncology: From single-gene testing to whole- genome sequencing. Expert Rev Pharmacoecon Outcomes Res. 2021;21:413–414. doi: 10.1080/14737167.2021.1917385. [DOI] [PubMed] [Google Scholar]
- 74. Schwarze K, Buchanan J, Fermont JM, et al. The complete costs of genome sequencing: A microcosting study in cancer and rare diseases from a single center in the United Kingdom. Genet Med. 2020;22:85–94. doi: 10.1038/s41436-019-0618-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. van Nimwegen KJ, van Soest RA, Veltman JA, et al. Is the $1000 genome as near as we think? A cost analysis of next-generation sequencing. Clin Chem. 2016;62:1458–1464. doi: 10.1373/clinchem.2016.258632. [DOI] [PubMed] [Google Scholar]