Abstract
Mannose-binding lectin (MBL) is an important component of the innate immune system. It binds to the arrays of sugars commonly presented by microorganisms and activates the complement system independently of antibody. Despite detailed knowledge of the stereochemical basis of MBL binding, relatively little is known about how bacterial surface structures influence binding of the lectin. Using flow cytometry, we have measured the binding of MBL to a range of mutants of Salmonella enterica serovar Typhimurium and Neisseria gonorrhoeae which differ in the structure of expressed lipopolysaccharide (LPS). For both organisms, the possession of core LPS structures led to avid binding of MBL, which was abrogated by the addition of O antigen (Salmonella serovar Typhimurium) or sialic acid (N. gonorrhoeae). Truncation of the LPS within the core led to lower levels of MBL binding. It was not possible to predict the magnitude of MBL binding from the identity of the LPS terminal sugar alone, indicating that the three-dimensional disposition of LPS molecules is probably also of importance in determining MBL attachment. These results further support the hypothesis that LPS structure is a major determinant of MBL binding.
Mannose-binding lectin (MBL) is a collagenous lectin (collectin) found in the serum of mammals and birds (32). In humans, it is believed to play an important role in innate host immunity by binding to microorganisms and then activating the complement system in an antibody-independent fashion via two MBL-associated serine proteases (MASP-1 and MASP-2) (20, 31). MBL may also interact directly with phagocytic cells to promote the opsonization of bacteria and viruses (2, 14, 22).
The importance of MBL in host defense has been highlighted by the predisposition to certain infections of individuals who have a genetically determined deficiency of the protein (15, 17, 28). MBL deficiency was initially linked to a common opsonic defect, identified in children as a syndrome of recurrent infections such as otitis media and diarrhea (30). This has been supported by the more recent report of a generalized risk of infection in admissions to hospital (29) and reports of specific susceptibilities to infection by human immunodeficiency virus (4), malaria (16), and Neisseria meningitidis (5).
MBL is synthesized in the liver and comprises three identical polypeptide chains associated to form a single subunit with a collagenous triple helix attached to three globular carbohydrate recognition domains (CRD) at the carboxy terminus. Two to six of these subunits then associate to form the functional protein (32). Each of the CRDs is able to bind to members of a family of monosaccharides which includes mannose, N-acetylmannosamine, N-acetylglucosamine, glucose, and fucose (34). The affinity of single interactions is low, with the large number of CRDs giving rise to the higher functional affinity of the intact protein. The regular spacing between the sugar binding sites (49 Å) is thought to be particularly well adapted to the repeating sugars exposed by microorganisms but not the mammalian host (27).
Despite the relatively detailed knowledge of the stereochemical basis of the interaction of MBL with monosaccharides, the binding of MBL to the complex carbohydrate structures of microorganisms is poorly understood. Although the presence of a bacterial capsule was reported to reduce MBL binding (33), other studies suggest that the structure of the lipopolysaccharide (LPS) may be equally or more important. The expression of high-mannose LPS by Salmonella enterica serovar Montevideo is associated with increased MBL binding (14), and overall LPS structure has been shown to be important in determining MBL binding to S. enterica serovar Typhimurium (7, 8, 11) and N. meningitidis (9).
In this investigation, we have studied the influence of LPS structure on the binding of MBL to Salmonella serovar Typhimurium and N. gonorrhoeae. The expression of a full-length (Salmonella serovar Typhimurium) or fully sialylated (N. gonorrhoeae) LPS was required to prevent MBL binding. The expression of some truncated LPS structures allowed the binding of MBL, but the identity of the terminal LPS sugar was not always a reliable predictor of MBL binding. This suggests that the three-dimensional structure of the LPS has an important role in determining MBL attachment to the organism. The expression of certain LPS structures may be an important bacterial virulence mechanism which acts to reduce MBL binding.
(M.D.-J. has submitted this work in partial fulfillment of the M.Sc. degree requirements of the University of East London.)
MATERIALS AND METHODS
Strains of Salmonella serovar Typhimurium.
A series of mutants of Salmonella serovar Typhimurium LT2 was obtained from the Salmonella Genetic Stock Centre (K. E. Sanderson, Department of Biological Sciences, University of Calgary, Calgary, Alberta, Canada). The parent LT2 organism and different mutants derived from it have been described previously (25) and are described in Table 1.
TABLE 1.
Strains of Salmonella serovar Typhimuriuma
| Strain | Relevant genotype | Chemotypeb |
|---|---|---|
| LT2 | WT | Smooth |
| SA1355 | LT2 WT | Smooth |
| SL3770 | LT2 WT | Smooth |
| SH7770 | rfaP | Smooth, reduced phosphates linked to Hep I |
| SA1627 | rfb | Ra (no O antigen) |
| SL4489 | rfaB rfb | Ra (no O antigen), lacks Gal II attached to Glc I |
| SL733 | rfaK | Rb1, deficient in outer core N-acetylglucosamine and partly deficient in side chains |
| SL3748 | rfaI | Rb3, lacks Gal I attached to Glc I |
| SL3750 | rfaJ | Lacks Glc II attached to the Gal II |
| SL1306 | galE | Rc, terminating at Glc I in the outer core |
| SL1248, SL1250 | rfaB galE | Lack some LPS galactosyl II transferase activity with the LPS terminated at Glc I in the outer core and lacking Gal II attached to Glc I |
| SL3769 | rfaG | Rd1, lacking the Glc I attached to Hep II |
| SL3789 | rfaF | Rd2, lacks the heptosyltransferase that adds the Hep II to the inner core |
| SA1377 | rfaC | Deep rough, terminates at KDOI |
| SL1102 | rfaE | Deep rough, terminates at KDOI |
| SL3600 | rfaD | Deep rough, terminates at KDOI |
Obtained from the Salmonella Stock Centre.
Phenotypes are described by Schnaitman and Klena (25).
Strains of N. gonorrhoeae.
A series of isogenic mutants of N. gonorrhoeae MS11-E1 was constructed by the insertion of a chloramphenicol acetyltransferase (CAT) cartridge, driven by a gonococcal opa gene promoter, into the gene of interest, as previously described (24, 26). The galE mutant possesses a truncated LPS lacking the sialic acid acceptor in the outer core (24); rfaK carries a null mutation in the GlcNAc transferase resulting from the insertion of the CAT cartridge at the ClaI site in the gene. It lacks the β-GlcNAc linked to the Glc II and thus has no γ-oligosaccharide. The rfaF mutant (26) and the rfaD mutant (this study) carry null mutations and lack all three α-, β-, and γ-oligosaccharides; they also cannot be sialylated. The rfaF gene encodes heptosyltransferase which adds the second α1,3-linked backbone heptose (Hep II) to the inner core. The rfaD mutant has the central XbaI-to-EcoRI region deleted and replaced with the promoter CAT cartridge and is defective in the epimerization of ADP-d-glycero-d-manno-heptose to ADP-l-glycero-d-manno-heptose. Such mutants accumulate ADP-d-glycero-d-manno-heptose and synthesize defective LPS which lacks ld-heptose but contains a small amount of the incorrect dd form (1).
Growth and preparation of bacteria.
Salmonella serovar Typhimurium strains were removed from frozen storage at −70°C and cultured for 16 to 18 h at 37°C on blood agar. Immediately prior to each experiment the bacteria were suspended in Veronal-buffered saline supplemented with 5 mM CaCl2 and 5 mM MgCl2 (VBS++; A540 = 1). In certain experiments, Salmonella serovar Typhimurium was cultured for 16 to 18 h on minimal medium plates (Columbia agar; Difco, West Molesey, United Kingdom).
N. gonorrhoeae strains were prepared similarly on GC agar (Difco) supplemented with Vitox (Oxoid, Basingstoke, United Kingdom) at 37°C-6%- CO2 and suspended in VBS++. To compare sialylated and nonsialylated gonococci, GC agar plates were supplemented with cytidine 5′-monophospho-N-acetylneuraminic acid (CMP-NANA) (50 μg/ml).
Purification of MBL.
MBL was purified from human, ethanol-fractionated plasma paste (kindly donated by C. Dash, Blood Products Laboratory, Elstree, United Kingdom) by the method of Kilpatrick (13), modified to include positive affinity removal of human immunoglobulin (M. P. Kelly, D. L. Jack, B. Mandanda, R. C. G. Pollok, N. Klein, M. W. Turner, M. J. G. Farthing, submitted for publication).
Conjugation of FITC to anti-MBL.
The storage buffer of a 1-mg/ml solution of monoclonal anti-MBL antibody (clone 131-1; State Serum Institute, Copenhagen, Denmark) was exchanged for conjugation buffer (18 mM Na2CO3, 30 mM NaHCO3, 0.135 M NaCl [pH 9.5]), using a centrifuge ultrafiltration device (Ultrafree MC; Millipore, Watford, United Kingdom) according to the manufacturer's instructions. To this a solution of fluorescein isothiocyanate (FITC; 1 mg/ml in conjugation buffer) was added (0.3 ml/ml of antibody solution), and the resulting mixture was incubated at room temperature for 3.5 h. The buffer was exchanged for phosphate-buffered saline (PBS) using a ultrafiltration membrane, which also removed unconjugated FITC from the antibody preparation. The conjugated antibody was aliquoted and stored at −70°C until use.
Assay for MBL binding to microorganisms.
The binding of MBL to all bacteria was determined by a flow cytometric procedure described previously (9). Briefly, a 50-μl aliquot of organism suspension was centrifuged, and the pellet was resuspended in VBS++ containing MBL of differing concentrations. Suspensions were incubated at 37°C for 30 min and then centrifuged. The cell pellet was washed and resuspended in VBS++ containing 10 μg of FITC-anti-MBL per ml. This mixture was incubated at 37°C for 30 min before centrifugation and washing of the cell pellet. Samples were resuspended in 100 μl of PBS and fixed by the addition of 100 μl of 2% (wt/vol) formaldehyde–2% (wt/vol) glucose in PBS for flow cytometry. Organisms were identified on the basis of size and granularity.
Evaluation of binding data and statistical analyses.
MBL binding was expressed as median fluorescence intensity (MFI), and binding was arbitrarily categorized into three levels, low (MFI < 5), moderate (5 to 20), and high (>20), as described by Neth et al. (21). Kruskal-Wallis H tests were used to determine the significance of differences in MBL binding to different organisms. In each case, comparisons were made with the background fluorescence in the absence of MBL.
RESULTS
Binding of MBL to Salmonella serovar Typhimurium.
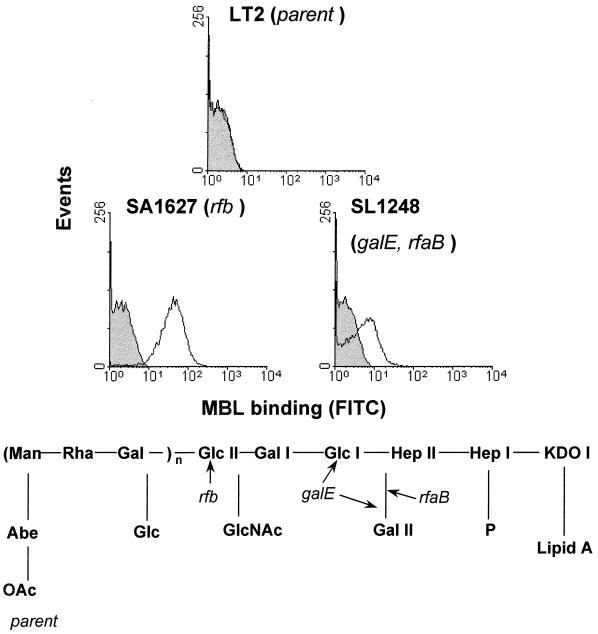
In this study, flow cytometry was used to investigate the interaction of MBL with Salmonella serovar Typhimurium LT2 and 16 mutants. Clear differences in binding were observed; representative MBL binding profiles are illustrated in Fig. 1.
FIG. 1.
Representative flow cytometric profiles of MBL binding to the parent (LT2) and two mutants of Salmonella serovar Typhimurium. MBL was added at a final concentration of 3.4 μg/ml. The control (filled histogram) shows samples incubated with FITC-anti-MBL alone, with the experimental samples (open histogram) incubated with MBL and then FITC-anti-MBL. The results shown are representative of five separate experiments. The scheme below shows the LPS structure for the LT2 organism, with the truncation points of SL1248 and SL1627 marked.
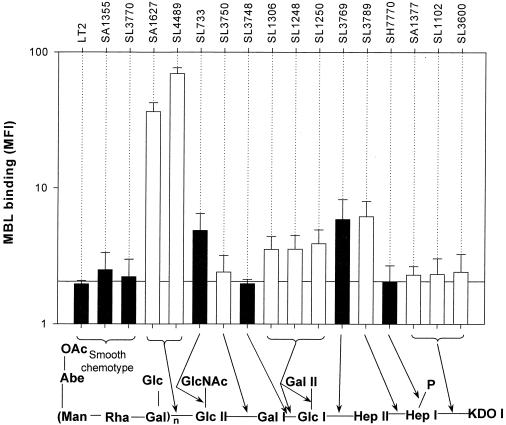
The 17 Salmonella strains studied could be grouped into three categories of MBL binding (see Materials and Methods). Using a physiologically high concentration of MBL (3.4 μg/ml) (15), there was high binding to SA1627 and SL4489, with moderate binding to SL3769 and SL3789 (Fig. 2). There was low but nevertheless statistically significant binding (P < 0.05) to SL733, SL1250, SL1248, SL3600, SL1306, and SA1377 and no detectable binding to the parent LT2, SL3748, SL1102, SH7770, SL3750, SA1355, and SL3770 organisms.
FIG. 2.
The influence of Salmonella serovar Typhimurium LPS structure on MBL binding. MBL was added at a final concentration of 3.4 μg/ml. Each bar represents the mean MBL binding (median fluorescence) + upper 95% confidence interval of at least three separate experiments. Adjacent bars with the same shading represent organisms which have similar LPS phenotypes. The reference line indicates the mean background MFI from experiments performed in the absence of MBL, i.e., no MBL binding. The diagram below shows the LPS structure of Salmonella serovar Typhimurium; the arrows indicate the primary enzymatic defect in the LPS structure.
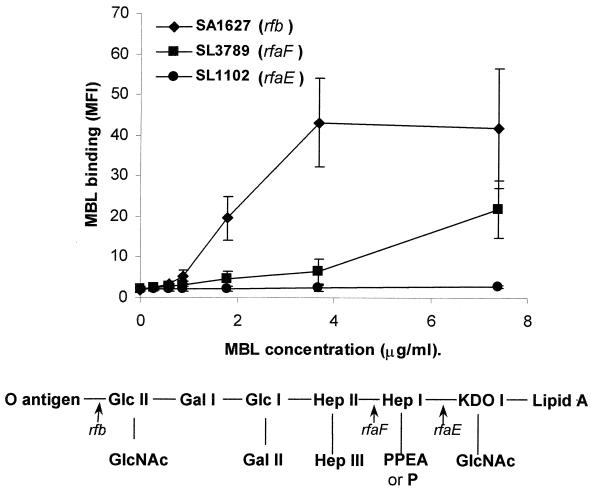
To determine the concentration dependence of binding, organisms were incubated with MBL at concentrations ranging from zero to 7.5 μg/ml. We observed three distinct patterns of concentration-dependent binding; an example of each is shown in Fig. 3. When organisms that bound high levels of MBL (e.g., SA1627) were incubated with increasing concentrations of MBL, lectin binding increased rapidly from low concentrations to a plateau of maximal binding at 3.4 μg/ml. With organisms that bound moderate levels of MBL (e.g., SL3789), binding increased more gradually, with no plateau observed over the range of MBL concentrations studied. For the organisms that bound little or no MBL at 3.4 μg/ml (for example, the deep rough chemotype SL1102), no binding was observed at any concentration.
FIG. 3.
Dose-dependent MBL binding to three different mutants of Salmonella serovar Typhimurium. Binding of MBL is expressed as mean MBL binding (median fluorescence) ± 95% confidence intervals from three experiments. The scheme below represents the LPS structure of the organisms analyzed.
To confirm that the binding of MBL was due to an interaction of the C-type lectin domain with bacterial sugars, competition experiments were performed using known antagonists of MBL. The addition of 25 mM N-acetylglucosamine or 5 mM EDTA to the MBL preparation before incubation with organisms inhibited binding by 75 and 95%, respectively. The addition of 25 mM galactose in the same experiments had no effect on MBL binding. This pattern is compatible with previous analyses of the sugar specificity and calcium dependence of the C-type lectin domain (6).
Construction of isogenic mutants of N. gonorrhoeae.
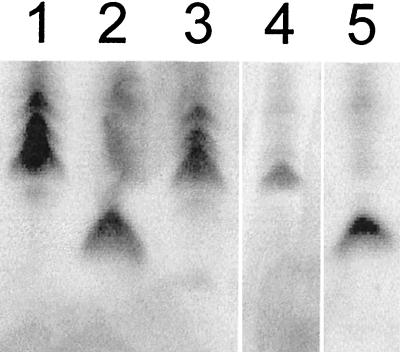
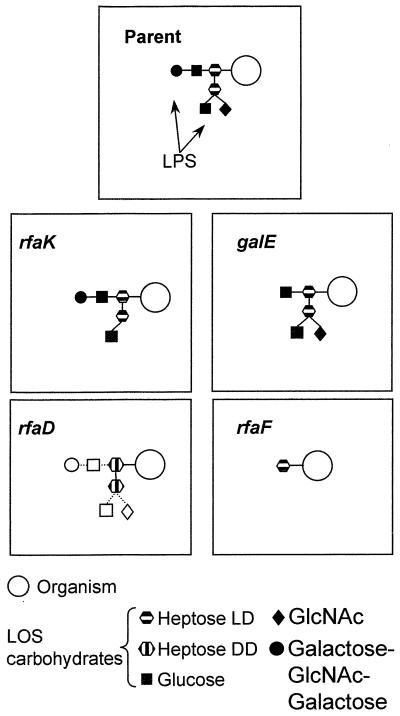
Whole cell lysates of the gonococcal mutants and parental strain were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and silver stained to visualize the LPS (Fig. 4). The galE and rfaF mutants (lanes 2 and 5) exhibited a single truncated band as previously described (24, 26). The rfaD mutant (lane 4) produced a pattern similar to that of the parent but with an increased amount of lower-molecular-weight product. The schematic structures illustrated in Fig. 5 highlight the effect of the mutations on the structure of the LPS molecule.
FIG. 4.
Silver-stained gel after SDS-PAGE showing the LPS profiles of the gonococcal mutants. Lane 1, MS11-E1 parental strain; lane 2, galE mutant; lane 3, rfaK mutant; lane 4, rfaD mutant; lane 5, rfaF mutant.
FIG. 5.
N. gonorrhoeae MS11-E1 parent strain and four isogenic mutants derived from it. The parent organism has the L3,7,9 immunotype, with the mutants possessing truncated LPS structures due to insertional inactivation of relevant genes. The galE mutant terminates at Glc I within the lacto-N-neotetraose moiety and cannot be sialylated; rfaF lacks α-, β-, and γ-oligosaccharides; rfaK lacks GlcNAc attached to Hep II. rfaD is unable to convert l-glycero-d-manno-heptose to d-glycero-d-manno-heptose, and some LPS molecules will be truncated at these residues; however, there is evidence (Fig. 4) that some LPS molecules are completed despite this defect (represented by the broken lines and unfilled symbols). LOS, lipooligosaccharide.
MBL binding to N. gonorrhoeae.
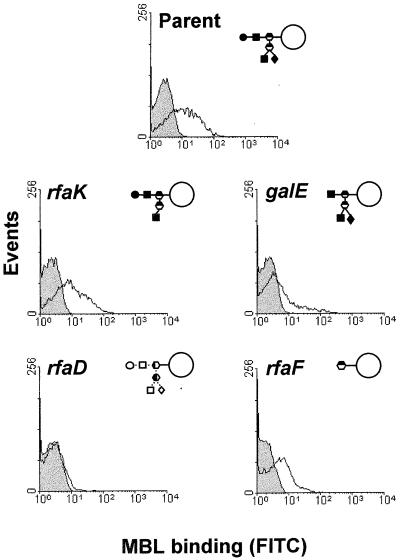
There were marked differences in the binding of MBL to the different N. gonorrhoeae mutants studied (Fig. 6). At a physiologically high concentration of MBL (7.5 μg/ml), there was detectable binding to the parent organism and rfaK mutant grown to stationary phase on GC agar. There was slightly less binding to the rfaF organism and low levels of binding to the galE and rfaD mutants.
FIG. 6.
Representative flow cytometric profiles of MBL binding to the parent and four mutants of N. gonorrhoeae. The control (filled histogram) shows samples incubated with FITC-anti-MBL alone. The experimental samples (unfilled histogram) were incubated with MBL (7.5 μg/ml) and then FITC-anti-MBL. The results shown are representative of five separate experiments.
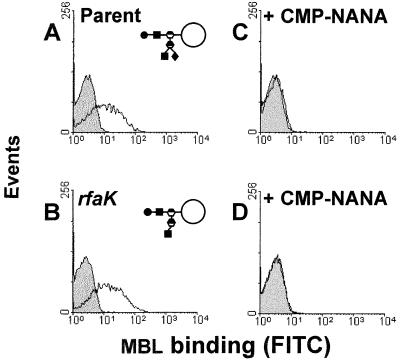
To determine the effect of LPS sialylation on MBL binding, the two organisms which had LPS structures containing the sialic acid acceptor site (parent and rfaK) were grown on solid medium containing 50 μg of CMP-NANA per ml and then incubated with MBL as usual (Fig. 7). Gonococci grown in the presence of CMP-NANA will exogenously sialylate their LPS, and this process completely inhibited MBL binding to these organisms.
FIG. 7.
Effect of sialylation on MBL binding to the parent and rfaK organisms of N. gonorrhoeae. The control (filled histogram) shows samples incubated with FITC-anti-MBL alone; the experimental samples (unfilled histograms) were incubated with MBL (7.5 μg/ml) and then FITC-anti-MBL. (A and B) MBL binding to bacteria grown to stationary phase on GC plates; (C and D) experiments with gonococci grown on GC agar plates supplemented with CMP-NANA (50 μg/ml). The experiment was repeated four times, and representative profiles are shown.
DISCUSSION
The basis of protection against infection mediated by MBL is the primary recognition of the target organism by the carbohydrate recognition domains of the protein followed by activation of the complement system and/or the recruitment of phagocytic cells (32). A recent study has shown that MBL is able to bind to a wide range of the bacteria and yeasts that commonly cause infections in children. MBL bound in this way activated complement on the organisms tested in a concentration-dependent manner (21).
Previous work had indicated that bacterial encapsulation significantly inhibited the binding of MBL to whole organisms, similar to the effect of capsule on other immune processes (33). Our own studies with N. meningitidis have indicated that LPS structure, rather than and independently of encapsulation, may be the major determinant of MBL binding to these bacteria, with the subsequent activation of complement (9). This is supported by other studies which have also suggested that LPS structure and composition are important in determining the binding of MBL to bacteria (7, 8, 12, 14). Using Salmonella serovar Typhimurium and N. gonorrhoeae mutants with specific LPS mutations, we have attempted to identify whether LPS is a common determinant of MBL binding.
It was possible to detect striking differences in the capacity of the different LPS mutants of both Salmonella serovar Typhimurium and N. gonorrhoeae to bind MBL, which confirms the importance of these structures in the attachment of this collectin to bacteria. There were similarities between the two species in the influence of certain LPS mutations on MBL binding. In both cases the expression of core LPS (Ra chemotypes of Salmonella serovar Typhimurium and N. gonorrhoeae grown in the absence of CMP-NANA) was associated with the binding of the maximum amount of MBL. Truncations at deeper LPS structures generally resulted in reduced levels of binding.
The carbohydrate recognition domain of MBL is thought to form an association with the terminal sugar of microbial saccharide structures through an interaction with both the 3- and 4-OH groups of the sugar (34). The presence of glucose as the terminal LPS structure was associated with MBL binding (Salmonella serovar Typhimurium SL733, SL1306, SL1248, and SL1250; N. gonorrhoeae galE strain), with a higher level of binding to mutants terminating with heptose sugars (Salmonella serovar Typhimurium SL3769 and SL3789; N. gonorrhoeae rfaF strain). However, the terminal sugar did not always determine lectin binding. The nonsialylated forms of N. gonorrhoeae (parent and rfaK) both bound MBL avidly, but both terminate with galactose or possibly with N-acetylgalactosamine, neither of which is a ligand for MBL (6). MBL might therefore have recognized branch sugars deeper within the LPS, although the removal of N-acetylglucosamine from the rfaK mutant did not affect binding compared to the parent organism. These results indicate that it is not always possible to predict the binding of MBL simply on the basis of the linear LPS sequence, and we suggest that the three-dimensional structure of LPS makes an important contribution to the binding of MBL to gram-negative bacteria.
The relevance of MBL binding to Salmonella serovar Typhimurium can be assessed by comparing the reported bactericidal properties of MBL toward Salmonella serovar Typhimurium strains bearing differing LPS structures. In early studies, Kawakami et al. (11) showed that MBL (then known functionally as Ra reactive factor) was bactericidal for Ra but not the Rb and Rd chemotypes. It was therefore interesting that we detected MBL binding to the Rb (SL733) and Rd (SL3769 and SL3789) chemotypes, but at reduced levels compared to the Ra chemotype. This magnitude of binding may be sufficient to increase phagocytic killing through increased opsonization with C3b but does not initiate direct complement-mediated killing. This will require further investigation.
The finding that the addition of sialic acid (N. gonorrhoeae) or O antigen (Salmonella serovar Typhimurium) abrogated MBL binding is likely to be of clinical significance. Gonococci are unable to add sialic acid to their LPS in the absence of the activated sugar, CMP-NANA, which can be found in serum (19). Nonsialylated gonococci are more adherent to epithelial surfaces, but in this form a significant proportion of strains are sensitive to the lytic activity of complement and would probably survive poorly in serum after migration across the epithelial barrier of the urethra (10, 23). Gonococci become serum resistant only by the sialylation of their LPS. MBL has been shown to be present at extravascular sites in association with inflammatory processes (3, 18; Kelly et al., submitted) and may therefore be able to protect the host against gonococcal infection before LPS sialylation occurs. This would be likely to occur through complement activation, similar to that described previously for nonsialylated meningococci (9).
Smooth Salmonella serovar Typhimurium strains express a range of LPS molecules with different numbers of attached O antigens (35). In contrast to N. gonorrhoeae, it appears that Salmonella serovar Typhimurium adds O antigen to the lipid A-LPS core in the periplasmic space (35), and therefore it is unlikely that MBL would have access to incomplete LPS structures on the external surface of the intact microorganism. The effect of the number of O antigens or the effect of damage mediated by other immune mechanisms on MBL binding remains to be assessed.
The results presented here suggest that the three-dimensional structure of the LPS molecule is important in determining MBL binding. For both Salmonella serovar Typhimurium and N. gonorrhoeae, the construction of a complete LPS was necessary to avoid MBL binding, suggesting that such avoidance is an important step in bacterial virulence. This is another instance in which the nature of LPS may be expected to alter the host response to infection by gram-negative bacteria.
ACKNOWLEDGMENT
The financial support of the Wellcome Trust, United Kingdom, grants 052227 and 040400 is gratefully acknowledged.
REFERENCES
- 1.Drazek E S, Stein D C, Deal C D. A mutation in the Neisseria gonorrhoeae rfaD homolog results in altered lipooligosaccharide expression. J Bacteriol. 1995;177:2321–2327. doi: 10.1128/jb.177.9.2321-2327.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ezekowitz R A B, Kuhlman M, Groopman J E, Byrn R A. A human serum mannose-binding protein inhibits in vitro infection by the human immunodeficiency virus. J Exp Med. 1989;169:185–196. doi: 10.1084/jem.169.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garred P, Brygge K, Sorensen C H, Madsen H O, Thiel S, Svejgaard A. Mannan-binding protein—levels in plasma and upper-airways secretions and frequency of genotypes in children with recurrence of otitis media. Clin Exp Immunol. 1993;94:99–104. doi: 10.1111/j.1365-2249.1993.tb05984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garred P, Madsen H O, Balsev U, Hofmann B, Pedersen C, Gerstoft J, Svejgaard A. Susceptibility to HIV infection and progression of AIDS in relation to variant alleles of mannose-binding lectin. Lancet. 1997;349:236–240. doi: 10.1016/S0140-6736(96)08440-1. [DOI] [PubMed] [Google Scholar]
- 5.Hibberd M L, Sumiya M, Summerfield J A, Booy R, Levin M. Association of variants of the gene for mannose-binding lectin with susceptibility to meningococcal disease. Meningococcal Research Group. Lancet. 1999;353:1049–1053. doi: 10.1016/s0140-6736(98)08350-0. [DOI] [PubMed] [Google Scholar]
- 6.Holmskov U, Jensenius J C. Structure and function of collectins: humoral C-type lectins with collagenous regions. Behring Inst Mitt. 1993;93:224–235. [PubMed] [Google Scholar]
- 7.Ihara I, Harada Y, Ihara S, Kawakami M. A new complement-dependent bactericidal factor found in nonimmune mouse sera: specific binding to polysaccharide of Ra chemotype Salmonella. J Immunol. 1982;128:1256–1260. [PubMed] [Google Scholar]
- 8.Ihara S, Takahashi A, Hatsuse H, Sumitomo K, Doi K, Kawakami M. Major component of Ra-reactive factor, a complement-activating bactericidal protein, in mouse serum. J Immunol. 1991;146:1874–1879. [PubMed] [Google Scholar]
- 9.Jack D L, Dodds A W, Anwar N, Ison C A, Law S K A, Frosch M, Turner M W, Klein N J. Activation of complement by mannose-binding lectin on isogenic mutants of Neisseria meningitidis serogroup B. J Immunol. 1998;160:1346–1353. [PubMed] [Google Scholar]
- 10.Jarvis G A. Recognition and control of neisserial infection by antibody and complement. Trends Microbiol. 1995;3:198–201. doi: 10.1016/s0966-842x(00)88921-0. [DOI] [PubMed] [Google Scholar]
- 11.Kawakami M, Ihara I, Ihara S, Suzuki A, Fukui K. A group of bactericidal factors conserved by vertebrates for more than 300 million years. J Immunol. 1984;132:2578–2581. [PubMed] [Google Scholar]
- 12.Kawakami M, Ihara I, Suzuki A, Harada Y. Properties of a new complement-dependent bactericidal factor specific for Ra chemotype Salmonella in sera of conventional and germ-free mice. J Immunol. 1982;129:2198–2201. [PubMed] [Google Scholar]
- 13.Kilpatrick D C. Isolation of human mannan binding lectin, serum amyloid P component and related factors from Cohn fraction III. Transfus Med. 1997;7:289–294. doi: 10.1046/j.1365-3148.1997.d01-40.x. [DOI] [PubMed] [Google Scholar]
- 14.Kuhlman M, Joiner K, Ezekowitz R A. The human mannose-binding protein functions as an opsonin. J Exp Med. 1989;169:1733–1745. doi: 10.1084/jem.169.5.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lipscombe R J, Sumiya M, Hill A V, Lau Y L, Levinsky R J, Summerfield J A, Turner M W. High frequencies in African and non-African populations of independent mutations in the mannose binding protein gene. Hum Mol Genet. 1992;1:709–715. doi: 10.1093/hmg/1.9.709. . (Erratum, 2:342, 1993.) [DOI] [PubMed] [Google Scholar]
- 16.Luty A J F, Kun J F J, Kremsner P G. Mannose-binding lectin plasma levels and gene polymorphisms in Plasmodium falciparum malaria. J Infect Dis. 1998;178:1221–1224. doi: 10.1086/515690. [DOI] [PubMed] [Google Scholar]
- 17.Madsen H O, Garred P, Kurtzhals J A, Lamm L U, Ryder L P, Thiel S, Svejgaard A. A new frequent allele is the missing link in the structural polymorphism of the human mannan-binding protein. Immunogenetics. 1994;40:37–44. doi: 10.1007/BF00163962. [DOI] [PubMed] [Google Scholar]
- 18.Malhotra R, Wormald M R, Rudd P M, Fischer P B, Dwek R A, Sim R B. Glycosylation changes of IgG associated with rheumatoid arthritis can activate complement via the mannose-binding protein. Nat Med. 1995;1:237–243. doi: 10.1038/nm0395-237. [DOI] [PubMed] [Google Scholar]
- 19.Mandrell R E, Lesse A J, Sugal J V, Shero M, Griffiss J M, Cole J A, Parsons N J, Smith H, Morse S A, Apicella M A. In vitro and in vivo modification of Neisseria gonorrhoeae lipooligosaccharide epitope structure by sialylation. J Exp Med. 1990;171:1649–1664. doi: 10.1084/jem.171.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsushita M, Fujita T. Activation of the classical complement pathway by mannose-binding protein in association with a novel C1s-like serine protease. J Exp Med. 1992;176:1497–1502. doi: 10.1084/jem.176.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neth O, Jack D L, Dodds A W, Holzel H, Klein N J, Turner M W. Mannose-binding lectin binds to a range of clinically relevant microorganisms and promotes complement deposition. Infect Immun. 2000;68:688–693. doi: 10.1128/iai.68.2.688-693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polotsky V Y, Belisle J T, Mikusova K, Ezekowitz R A B, Joiner K A. Interaction of human mannose-binding protein with Mycobacterium avium. J Infect Dis. 1997;175:1159–1168. doi: 10.1086/520354. [DOI] [PubMed] [Google Scholar]
- 23.Ram S, Sharma A K, Simpson S D, Gulati S, McQuillen D P, Pangburn M K, Rice P A. A novel sialic acid binding site on factor H mediates serum resistance of sialylated Neisseria gonorrhoeae. J Exp Med. 1998;187:743–752. doi: 10.1084/jem.187.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robertson B D, Frosch M, van Putten J P. The role of galE in the biosynthesis and function of gonococcal lipopolysaccharide. Mol Microbiol. 1993;8:891–901. doi: 10.1111/j.1365-2958.1993.tb01635.x. [DOI] [PubMed] [Google Scholar]
- 25.Schnaitman C A, Klena J D. Genetics of lipopolysaccharide biosynthesis in enteric bacteria. Microbiol Rev. 1993;57:655–682. doi: 10.1128/mr.57.3.655-682.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwan E T, Robertson B D, Brade H, van Putten J P M. Gonococcal rfaF mutants express Rd2 chemotype LPS and do not enter epithelial host cells. Mol Microbiol. 1995;15:267–275. doi: 10.1111/j.1365-2958.1995.tb02241.x. [DOI] [PubMed] [Google Scholar]
- 27.Sheriff S, Chang C Y, Ezekowitz R A B. Human mannose-binding protein carbohydrate recognition domain trimerizes through a triple α-helical coiled-coil. Nat Struct Biol. 1994;1:789–794. doi: 10.1038/nsb1194-789. [DOI] [PubMed] [Google Scholar]
- 28.Sumiya M, Super M, Tabona P, Levinsky R J, Arai T, Turner M W, Summerfield J A. Molecular basis of opsonic defect in immunodeficient children. Lancet. 1991;337:1569–1570. doi: 10.1016/0140-6736(91)93263-9. [DOI] [PubMed] [Google Scholar]
- 29.Summerfield J A, Sumiya M, Levin M, Turner M W. Mannose-binding protein gene mutations are associated with childhood infections in a consecutive hospital series. Br Med J. 1997;314:1229–1232. doi: 10.1136/bmj.314.7089.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Super M, Thiel S, Lu J, Levinsky R J, Turner M W. Association of low levels of mannan-binding protein with a common defect of opsonisation. Lancet. 1989;ii:1236–1239. doi: 10.1016/s0140-6736(89)91849-7. [DOI] [PubMed] [Google Scholar]
- 31.Thiel S, Vorup-Jensen T, Stover C M, Schwaeble W, Laursen S B, Poulsen K, Willis A C, Eggleton P, Hansen S, Holmskov U, Reid K B M, Jensenius J. A second serine protease associated with mannan-binding lectin that activates complement. Nature. 1997;386:506–510. doi: 10.1038/386506a0. [DOI] [PubMed] [Google Scholar]
- 32.Turner M W. Mannose-binding lectin: the pluripotent molecule of the innate immune system. Immunol Today. 1996;17:532–540. doi: 10.1016/0167-5699(96)10062-1. [DOI] [PubMed] [Google Scholar]
- 33.van Emmerik L C, Kuijper E J, Fijen C A, Dankert J, Thiel S. Binding of mannan-binding protein to various bacterial pathogens of meningitis. Clin Exp Immunol. 1994;97:411–416. doi: 10.1111/j.1365-2249.1994.tb06103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weis W I, Drickamer K, Hendrickson W A. Structure of a C-type mannose-binding protein complexed with an oligosaccharide. Nature. 1992;360:127–134. doi: 10.1038/360127a0. [DOI] [PubMed] [Google Scholar]
- 35.Whitfield C, Amor P A, Köplin R. Modulation of the surface architecture of gram-negative bacteria by the action of surface polymer:lipid A-core ligase and by determinants of polymer chain length. Mol Microbiol. 1997;23:629–638. doi: 10.1046/j.1365-2958.1997.2571614.x. [DOI] [PubMed] [Google Scholar]