PURPOSE
Tyrosine kinase inhibitors such as sunitinib and pazopanib are the mainstay of treatment of metastatic renal cell carcinoma (mRCC) in India. However, pembrolizumab and nivolumab have shown significant improvement in the median progression-free survival and overall survival among patients with mRCC. In this study, we aimed to determine the cost-effectiveness of the first-line treatment options for the patients with mRCC in India.
METHODS
A Markov state-transition model was used to measure the lifetime costs and health outcomes associated with sunitinib, pazopanib, pembrolizumab/lenvatinib, and nivolumab/ipilimumab among patients with first-line mRCC. Incremental cost per quality-adjusted life-year (QALY) gained with a given treatment option was compared against the next best alternative and assessed for cost-effectiveness using a willingness to pay threshold of one-time per capita gross-domestic product of India. The parameter uncertainty was analyzed using the probabilistic sensitivity analysis.
RESULTS
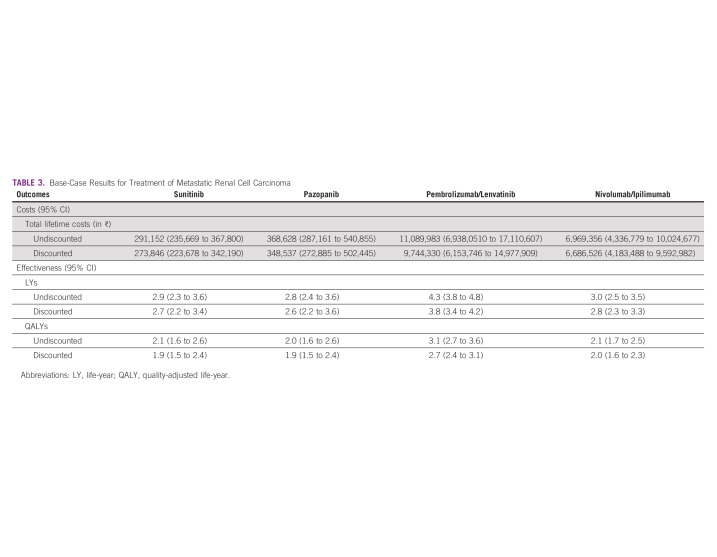
We estimated the total lifetime cost per patient of ₹ 0.27 million ($3,706 US dollars [USD]), ₹ 0.35 million ($4,716 USD), ₹ 9.7 million ($131,858 USD), and ₹ 6.7 million ($90,481 USD) for the sunitinib, pazopanib, pembrolizumab/lenvatinib, and nivolumab/ipilimumab arms, respectively. Similarly, the mean QALYs lived per patient were 1.91, 1.86, 2.75, and 1.97, respectively. Sunitinib incurs an average cost of ₹ 143,269 ($1,939 USD) per QALY lived. Therefore, sunitinib at current reimbursement rates (₹ 10,000 per cycle) has a 94.6% probability of being cost-effective at a willingness to pay threshold of 1-time per capita gross-domestic product (₹ 168,300) in the Indian context.
CONCLUSION
Our findings support the current inclusion of sunitinib under India's publicly financed health insurance scheme.
INTRODUCTION
Approximately 3% of all the adult cancers are renal cell carcinomas (RCCs), and 85% of all kidney tumors are RCCs.1 In India, the incidence of RCC is reported to be about two per 100,000 for males and one per 100,000 for females.2 It is more common in the geriatric population, with the median age of presentation ranging from 50 to 60 years, with clear cell carcinoma being the commonest histologic type accounting for nearly 70%-80% of RCC cases.3-5
CONTEXT
Key Objective
To determine the most cost-effective treatment option for patients with newly diagnosed metastatic renal cell carcinoma in India.
Knowledge Generated
In the Indian context, sunitinib is the treatment of choice for patients with metastatic renal cell carcinoma in India. Pazopanib is a dominated treatment strategy because of its higher overall cost than sunitinib in the Indian context. Moreover, immunotherapeutic combinations such as pembrolizumab/lenvatinib and nivolumab/ipilimumab are not cost-effective at the current prices. However, a 33% reduction in the current price of pembrolizumab (from ₹ 480,000 per cycle to ₹ 321,600 per cycle) is required to make it a cost-effective treatment option compared with nivolumab/ipilimumab.
Relevance
This evidence supports the inclusion of sunitinib in the India's publicly financed health insurance scheme.
In India, patients present with an advanced disease because of lack of screening and reporting.5,6 Until the past decade, the pharmacologic treatment options for metastatic RCC (mRCC) were limited to immunomodulatory cytokines interleukin-2 and interferon-α.7 Targeted therapies such as the tyrosine kinase inhibitors (TKIs, namely, sunitinib, pazopanib, lenvatinib etc), mammalian target of rapamycin inhibitors (everolimus) and antiangiogenesis therapy (bevacizumab) are the mainstay for the treatment of mRCC globally.8
The Indian National Cancer Grid,9 National Comprehensive Cancer Network,8 and Evidence-based Management guidelines10 recommend using TKIs such as sunitinib and pazopanib as the first-line therapy for patients with favorable-risk mRCC. Their high price makes them unaffordable for the majority of Indian patients. However, the introduction of low-cost generics has provided some relief to the patients. Moreover, India's government-funded health insurance program—the Ayushman Bharat Pradhan Mantri Jan Arogya Yojana—has recently included TKIs (such as sunitinib, cabozantinib, and sorafenib) for the treatment of mRCC in its health benefit package.11 This has helped in reducing the financial hardship faced by many Indian patients with mRCC.
Immune checkpoint inhibitors such as pembrolizumab and nivolumab in combination with TKIs have shown significant improvement in both progression-free survival (PFS) and overall survival (OS), with fewer toxicities compared with the conventional sunitinib monotherapy.12,13 However, immune checkpoint inhibitors are expensive in the Indian and global markets. Cost-effectiveness analysis can help physicians and payers, particularly in low- and middle-income countries like India, to choose appropriate therapies that offer the maximum value for money.
Published economic evaluation studies comparing TKIs (sunitinib and pazopanib) have reported that pazopanib is a cost-effective treatment option compared with sunitinib.14-16 However, these studies have similar efficacy evidence but different country-specific cost estimates.14-17 Moreover, the clinical trials and systematic reviews show insignificant difference in the PFS and OS between sunitinib and pazopanib.18,19 There is also a dearth of studies providing a comparative analysis of newly approved drugs, making it difficult to make an informed decision. Two studies compared all the first-line treatment options for mRCC and concluded that pembrolizumab/axitinib is a cost-effective treatment option in the context of the United States.20,21 However, these studies used a higher willingness to pay (WTP) threshold, which is not useful in developing countries like India. Thus, the differences in the context of these studies make it difficult to generalize the evidence. We, therefore, aim to bridge this evidence gap, by evaluating the cost-effectiveness of various treatment options for the treatment of patients with newly diagnosed mRCC in India.
METHODS
Overview of the Analysis
We undertook the present cost-effectiveness analysis using a societal perspective as per the methodological guidelines for conducting economic evaluation provided by India's health technology assessment (HTAIn) Agency.22 We compared four options for the treatment of patients with newly diagnosed previously untreated mRCC in India—sunitinib, pazopanib, combination of pembrolizumab/lenvatinib, and nivolumab/ipilimumab. A lifetime horizon was used to measure the health care costs and consequences in the different treatment arms. All future costs and outcomes were discounted at the rate of 3%.22 We followed the methodological guidelines as provided by the Indian reference case for conducting economic evaluations.22 The study findings are reported as per the Consolidated Health Economic Evaluation Reporting Standards.23
Model Structure
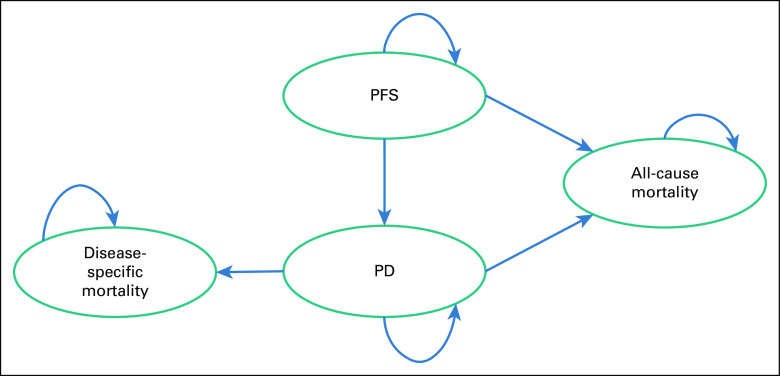
A Markov state-transition model was developed in Microsoft Excel to estimate the lifetime costs and consequences for patients with mRCC (Fig 1). The overall target population for the economic evaluation included adults with previously untreated, advanced, RCC or mRCC. The model consisted of three mutually exclusive health states: PFS, progressive disease (PD), and death. A 6 week cycle length was considered on the basis of the treatment schedule for sunitinib, which is given once daily for 4 weeks, followed by 2 weeks off as per the dosage schedule in the COMPARZ trial.24
FIG 1.
Schematic diagram for the Markov state transition model. PD, progressive disease; PFS, progression-free state.
Once in PD health state, the patients are put on the same second-line targeted therapy or palliative care management irrespective of the type of first-line therapy. No disease-specific mortality was assumed in the PFS state, whereas in the PD state, deaths both from mRCC and all-cause were assumed. The patient enters the model at age 55 years, which is the median age of presentation of mRCC in India.4,5
Treatment Arms
Four treatment arms were modeled: (1) sunitinib (50 mg orally once daily for 4 weeks followed by 2 weeks without treatment); (2) pazopanib (800 mg orally once daily); (3) pembrolizumab (200 mg intravenously once every 3 weeks) plus lenvatinib (20 mg orally once daily); and (4) nivolumab (240 mg intravenously once every 2 weeks) plus ipilimumab 50 mg (four doses intravenously once every 6 weeks).
Valuation of Consequences
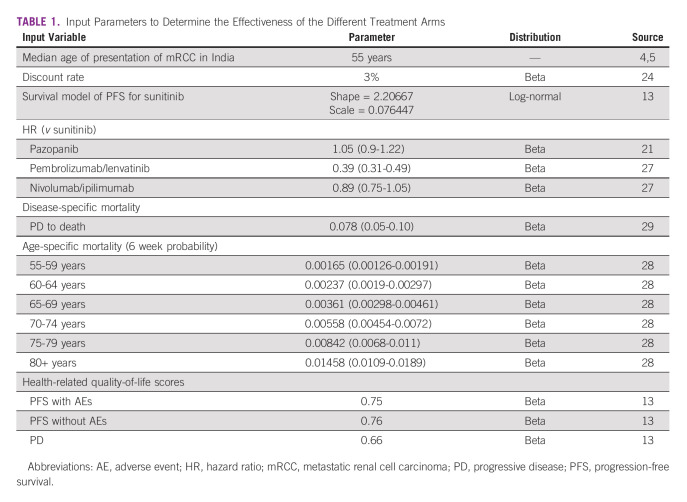
The outcomes were assessed in terms of life-years (LYs) and quality-adjusted life-years (QALYs). The probabilities to stay in the PFS health state were estimated for each cycle for all the arms. The PFS survival data for the sunitinib was extracted using a web-based digitizer software program from the Kaplan-Meier curve using the CLEAR trial.12 We used the CLEAR trial as it is the most recently published randomized clinical trial comparing two different treatment regimens with sunitinib. The probabilities were estimated using the published standard extrapolation technique.25 The PFS data from the Kaplan-Meier curve were extracted to generate pseudoindividual patient-level data. The reconstructed individual patient-level data was then fitted to five standard parametric models (exponential, Weibull, Gompertz, log-normal, and log-logistic). A suitable distribution was selected on the basis of visual inspection and the goodness of fit (Akaike and Bayesian information criteria). The log-normal distribution was the best fit for the sunitinib arm (Data Supplement). The PFS data for the other arms, that is, pazopanib, pembrolizumab/lenvatinib, and nivolumab/ipilimumab, were estimated by applying hazard ratios from the published systematic reviews and network meta-analysis.19,26 Age-specific all-cause mortality rates were obtained from the Sample Registration System lifetables.27 The disease-specific mortality in the PD state was assumed to be the same for all treatment arms as all the patients underwent the same second-line therapy. The probability of death was obtained from the published Indian literature, which aimed to determine the efficacy of second-line treatment among patients with mRCC.28 The detailed input parameters are shown in Table 1.
TABLE 1.
Input Parameters to Determine the Effectiveness of the Different Treatment Arms
Baseline utility values for PFS and PD health state were obtained from the published literature.29 The study also provided disutility values for each of the adverse events (AEs) related to the treatment. These disutility values were applied to the base value and the utility scores were computed for different AEs associated with the treatment of mRCC (Table 2).29 The data on incidence of AEs related to treatment were obtained from published literature (Data Supplement).12,13,24
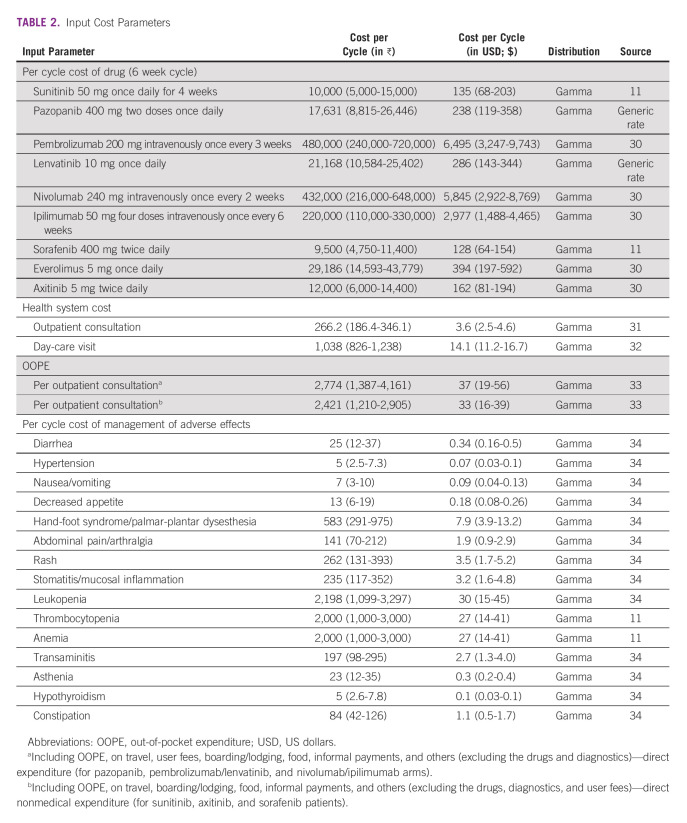
TABLE 2.
Input Cost Parameters
Cost of the Treatment
The costs were estimated from a societal perspective for all the treatment arms. We did not consider the productivity losses incurred by the patient and their caregivers due to the treatment, as per existing Indian reference case for health technology assessment in India.22
The cost of treatment in the PFS state for the sunitinib arm included the reimbursement rate as per the health benefit package under the Pradhan Mantri Jan Arogya Yojana (PMJAY) scheme. The reimbursement rate includes chemotherapeutic agents, recurring investigations, day-care charges, supportive care, and doctor and nursing charges. In addition, the direct nonmedical out-of-pocket expenditure (OOPE; including travel, boarding/lodging, food, informal payment etc) was added to estimate the societal cost. For the other three treatment arms that are not included in the PMJAY scheme, drug acquisition costs, direct nonmedical OOPE (including the user fees), cost of management of grade 3-4 AEs, and the follow-up were incorporated. Routine follow-up costs include cost per outpatient consultation, cost of day-care visit, laboratory investigations, and diagnostic tests (Table 2). Separate incidence rates for each grade 3-4 AEs were applied using the published literature.12,13,24 We assumed that the cost of routine laboratory and diagnostic tests was applied after every 3 months as per the standard treatment guidelines.35
For PD, we included the cost of outpatient consultation, laboratory, and diagnostic tests, as well as second-line therapy. We assumed that the patients would be given second-line therapy for 9 months, after which the patient would be on palliative management. The second-line therapy included oral administration of sorafenib, axitinib, and everolimus as per the standard treatment guidelines35 (Data Supplement). We used the reimbursement rates for sorafenib and market prices for the rest of the drugs.
Health system costs of outpatient consultation and day-care visit were elicited using data from published studies32,35 and the nationally representative National Health System Cost Database.33 The OOPE estimates were derived from primary data collected as a part of the larger multicentric National Cancer Database for Cost and Quality of Life.32 We used the reimbursement rates,11 generic and market prices,31 and procurement rates of the Rajasthan Medical Service Corporation30 for estimating expenditures on drugs. For the diagnostic services, we used the provider payment rates from the social health insurance scheme for central government employees in India, ie, Central Government Health Scheme.34 All costs are reported in Indian rupee (₹) and converted to US dollar (USD; $) using an exchange rate of $1 USD = ₹ 73.9 for the year 2021.36
The comparative cost-effectiveness was assessed in terms of incremental cost per QALY gained. A WTP threshold equal to per capita gross domestic product (GDP) of India was used to assess the cost-effectiveness as per the guidelines for HTAIn.22,37 The per capita GDP of India for the year 2021 was ₹168,300 ($2,277.4 USD).38
Sensitivity and Threshold Analysis
A probabilistic sensitivity analysis (PSA) was undertaken to test the parameter uncertainty for each scenario. Under PSA, we used gamma distribution for cost parameters and beta distribution for parameters related to effectiveness, risk of complications, OS, and utility scores. For the rest of the parameters in the model, we used uniform distribution. Uncertainty ranges for input parameters were computed from the standard error estimates from the primary data, or data available in the literature. Wherever the measures of dispersion were unavailable, a variation of 20% for clinical parameters; 30% variation for mortality risks, utility scores, and treatment patterns; and 50% variation for cost parameters were assumed on either side of base parameter values. Model results were simulated 1,000 times, and the median value incremental cost-effectiveness ratio along with 95% CI was generated for base estimates using the percentile method.
Extended dominance analysis was undertaken in which each treatment arm was compared against the next best alternative to assess the comparative cost-effectiveness between various treatment arms.
Ethical Approval
The study protocol was approved by the Institute Ethics Committee of the Post Graduate Institute of Medical Education and Research, Chandigarh, India (IEC-03/2020-1565).
RESULTS
Costs and Outcomes
We estimated that a patient with mRCC incurs a lifetime cost of ₹ 273,846 ($3,706 USD), ₹ 348,537 ($4,716 USD), ₹ 9.7 million ($131,858 USD), and ₹ 6.7 million ($90,481 USD) for sunitinib, pazopanib, pembrolizumab/lenvatinib, and nivolumab/ipilimumab treatments, respectively. The overall mean LYs lived with sunitinib, pazopanib, pembrolizumab/lenvatinib, and nivolumab/ipilimumab were 2.70, 2.63, 3.79, and 2.78, respectively. In terms of utility measures, this translates into 1.91, 1.86, 2.75, and 1.97 QALYs, respectively (Table 3).
TABLE 3.
Base-Case Results for Treatment of Metastatic Renal Cell Carcinoma
Cost-Effectiveness
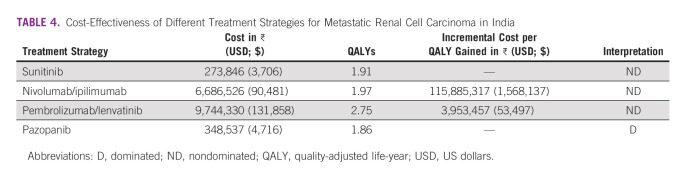
According to Table 4, pazopanib incurs higher cost and statistically insignificant health benefits compared with sunitinib, and is hence dominated. Among the three nondominated options, pembrolizumab/lenvatinib and nivolumab/ipilimumab incur an incremental cost of ₹ 3.9 million ($ 53,497 USD) and ₹ 115.8 million ($1,568,137 USD) per QALY gained, respectively, which are not cost-effective when compared with India's current WTP of 1-time per capita GDP (₹ 168,300). Sunitinib incurs an average cost of ₹ 143,269 ($1,939 USD) per QALY lived, which is a cost-effective treatment strategy in the Indian context when compared with the cost-effectiveness threshold of 1-time per capita GDP.
TABLE 4.
Cost-Effectiveness of Different Treatment Strategies for Metastatic Renal Cell Carcinoma in India
Sensitivity Analysis
Sunitinib, at the current reimbursement rate (₹ 10,000 per cycle), has nearly 95% probability to be cost-effective at the current WTP threshold of one-time per capita GDP (168,300) of India. Similarly, the probability of pembrolizumab/lenvatinib to be cost-effective compared with nivolumab/ipilimumab was 19.9%. A 33% reduction in the current price of pembrolizumab (from ₹ 480,000 per cycle to ₹ 321,600 per cycle) is required to make it a cost-effective treatment option compared with nivolumab/ipilimumab (Fig 2). However, even with a 95% reduction in the current price, nivolumab/ipilimumab is not a cost-effective treatment option compared with sunitinib.
FIG 2.
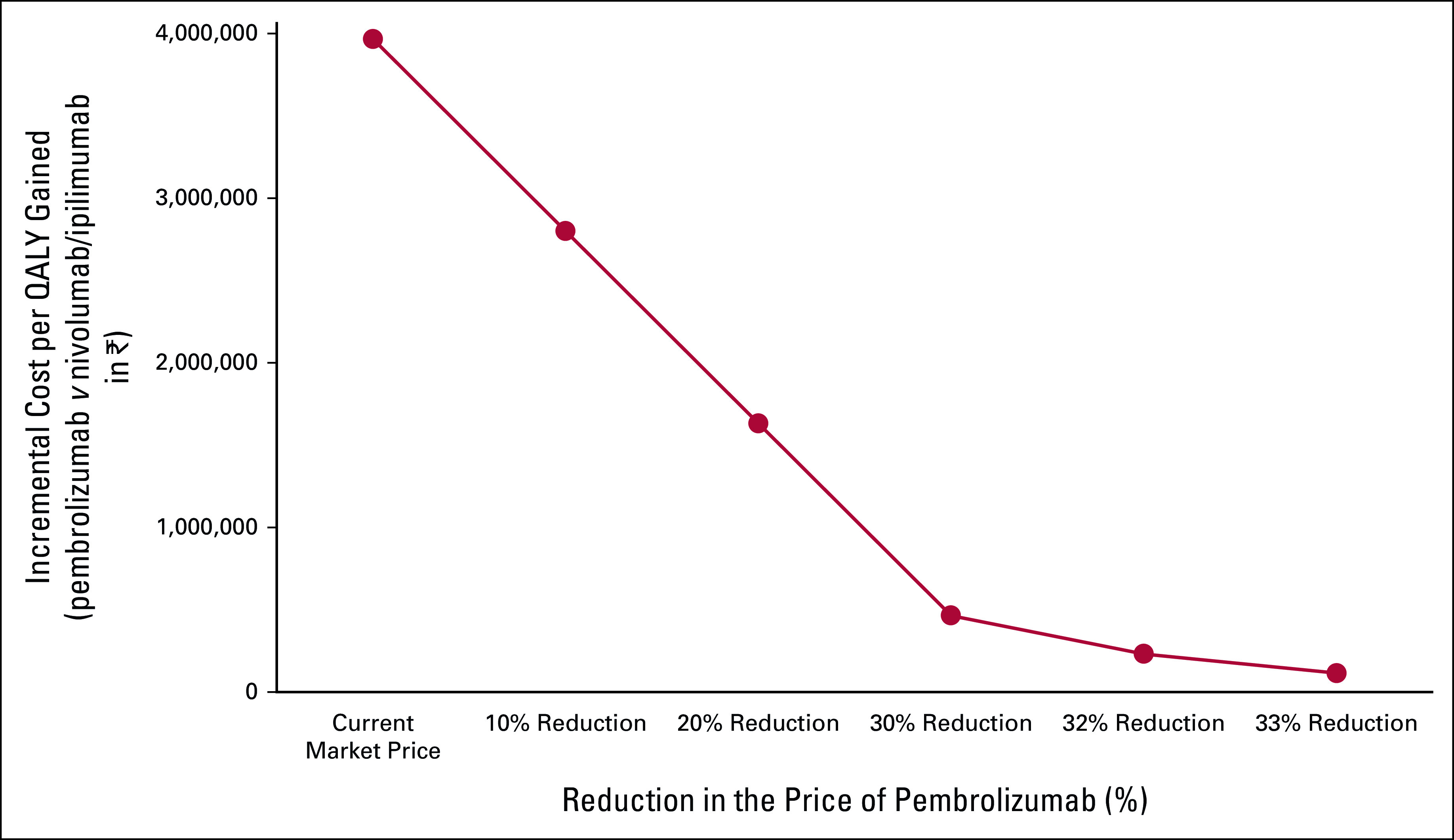
Price threshold analysis: pembrolizumab. QALY, quality-adjusted life-year.
DISCUSSION
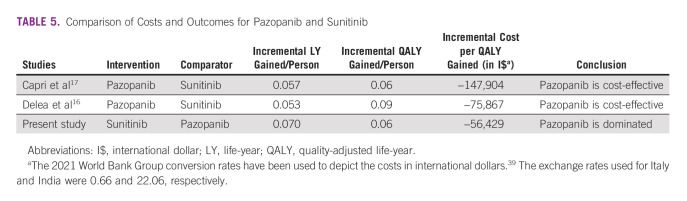
Our study compared four different options for the first-line treatment of mRCC in the Indian context. We concluded that sunitinib is the most cost-effective treatment option. Although the combination of pembrolizumab/lenvatinib provides the maximum health benefits, it is not a cost-effective treatment strategy at current prices. In contrast to other countries, the cost of pazopanib is higher in India compared with sunitinib. As a result, pazopanib is a dominated treatment strategy for patients with first-line mRCC in India as it offers similar health outcomes at a higher overall cost. Therefore, this significantly higher cost of pazopanib bends the results in the favor of sunitinib. Table 5 presents the comparative assessment of our findings in context of other model-based cost-effectiveness studies.
TABLE 5.
Comparison of Costs and Outcomes for Pazopanib and Sunitinib
According to the literature, the use of immunotherapy provides better health outcomes in terms of PFS and OS than sunitinib and pazopanib.19,26,39 Our study corroborates the above-mentioned finding that the immunotherapy and TKI combination provides more LYs and QALYs compared with single-agent TKIs. However, at current prices, the pembrolizumab/lenvatinib and nivolumab/ipilimumab combinations are expensive for developing countries like India compared with the incremental health benefits attributable to them. Therefore, these drugs are not cost-effective at current prices.
The findings of our model are in concurrence with existing clinical and epidemiological evidence available in the Indian as well as the global context. The median progression-free survival (mPFS) and median OS (mOS) in our model for sunitinib (mPFS, > 9 months; mOS, 27 months) and pazopanib (mPFS, < 9 months; mOS, 27 months) are consistent with the current Indian literature. Two Indian studies estimated the mPFS for sunitinib patients to be 11.4 months and 9 (3–43) months, respectively, which is consistent with our model estimates.42,43 Similarly, the mOS for the sunitinib arm is reported to be 22.6 months and 28.2 months, respectively, which is in line with our study findings.42,44 There is a significant lack of evidence with respect to the other two arms (ie, pembrolizumab/lenvatinib and nivolumab/ipilimumab) in the Indian context; however, a single-center study from India reported 1-year OS among patients treated with an immunotherapy (pembrolizumab or nivolumab)-TKI (axitinib or lenvatinib) combination to be 92%. This corroborates with our study outcomes that estimate the 1-year OS to be 88.8% and 85.1% among the pembrolizumab/lenvatinib and nivolumab/ipilimumab arms, respectively.45
A recently published study comparing all the frequently used first-line treatment regimens estimated 2.1 (2.99 LYs), 2.6 (3.44 LYs), and 2.4 (3.21 LYs) QALYs in sunitinib, pembrolizumab/lenvatinib, and nivolumab/ipilimumab treatment arms, respectively.20 This is in line with our study results, which estimated 1.9 (2.7 LYs), 2.7 (3.7 LYs), and 2.0 (2.8 LYs) in sunitinib, pembrolizumab/ipilimumab, and nivolumab/ipilimumab arms, respectively. In our study, pazopanib was estimated to incur fewer progression-free LYs (1.26 LYs) than sunitinib, which incurred 1.3 progression-free LYs. Many studies that compare sunitinib and pazopanib have also reported more progression-free LYs (1.17 and 1.15, respectively) with the sunitinib arm.14-16
We would like to highlight a few merits of our study. First,to our knowledge, this study is the first to examine the cost-effectiveness of the treatment options for mRCC in the Indian context. Second, we included all the possible first-line treatment options available, making the analysis comprehensive and close to real-world practices. Third, we have obtained OOPE estimates from the primary data being collected as a part of an ongoing multicentric study for assessing the economic burden among patients with cancer in India.32 Fourth, we incorporated the reimbursement rates set up under India's publicly funded national health insurance scheme wherever available to make our analysis policy-relevant.11,46 Finally, we used the survival data from published Indian studies to make our results representative in the Indian context.
However, there are certain limitations to this analysis. First, we used a 4/2 regimen instead of a 2/1 regimen for the sunitinib treatment as the literature considers the former regimen and there would not be major cost differences between the two. Second, we did not consider the cost of grade 1-2 AEs, which has resulted in a slight underestimation of costs. However, since none of the immunotherapeutic treatments is cost-effective, the exclusion of such costs further strengthens our conclusions. Third, we did not take into account the indirect costs due to loss of productivity incurred by the patients as well as the caregivers, which are in line with Indian health technology assessment guidelines, which do not recommend the inclusion of indirect costs in the base case.22 Finally, we did not perform separate subgroup analysis according to the favorable-, intermediate-, and poor-risk categories in our study because of the lack of robust Indian evidence on the same.
We performed the analysis from the societal perspective and have not presented the costs separately from the health system and patients' perspective. This is in line with the methodological principles outlined by the HTAIn. Inclusion of a treatment in a largely publicly financed insurance program such as PMJAY may lower the overall cost due to economies of scale. However, we did consider a wide variation in prices during the PSA, which did not alter the overall conclusions on cost-effectiveness. Hence, our study results are robust to these variations in contextual factor of health care financing and delivery.
In conclusion, at the current reimbursement rate, sunitinib is the cost-effective option for treatment of mRCC in India. The immunotherapeutic agents (such as nivolumab, pembrolizumab etc) provide significant clinical benefits, but they are very expensive drugs to be considered cost-effective for use in the Indian context. Therefore, further consideration should be made to promote the manufacturing and introduction of low-cost generics and regulate the price of these expensive drugs to make it cost-effective and affordable for Indian patients. Finally, the screening strategies for early-stage detection of mRCC (along the lines of screening for breast, oral, and cervical cancers) should be implemented to reduce the economic and clinical burden of the disease in India.
Nidhi Gupta
Employment: Grecian Multispeciality Hospital, Mohali, Punjab, India
Vipul Aggarwal
Stock and Other Ownership Interests: NH, Apollo
Sudeep Gupta
Research Funding: Roche (Inst), Sanofi (Inst), Johnson & Johnson (Inst), Amgen (Inst), Celltrion (Inst), Oncostem Diagnostics (Inst), Novartis (Inst), AstraZeneca (Inst), Intas (Inst)
No other potential conflicts of interest were reported.
SUPPORT
Supported by Department of Health Research, Ministry of Health and Family Welfare, Government of India vide Grant No. F.No.T.11011/02/2017-HR/3100291.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Administrative support: Shankar Prinja
Provision of study materials or patients: Ashish Singh, Nidhi Gupta, Nikita Mehra, Manjunath Nookala Krishnamurthy, Partha Sarathi Roy, Pankaj Malhotra, Sudeep Gupta, Lalit Kumar, Amal Kataki, Shankar Prinja
Collection and assembly of data: Ashish Singh, Nidhi Gupta, Nikita Mehra, Vipul Aggarwal, Manjunath Nookala Krishnamurthy, Partha Sarathi Roy, Pankaj Malhotra, Sudeep Gupta, Lalit Kumar, Amal Kataki, Shankar Prinja
Data analysis and interpretation: Dharna Gupta, Ashish Singh, Nidhi Gupta, Pankaj Bahuguna, Shankar Prinja
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Nidhi Gupta
Employment: Grecian Multispeciality Hospital, Mohali, Punjab, India
Vipul Aggarwal
Stock and Other Ownership Interests: NH, Apollo
Sudeep Gupta
Research Funding: Roche (Inst), Sanofi (Inst), Johnson & Johnson (Inst), Amgen (Inst), Celltrion (Inst), Oncostem Diagnostics (Inst), Novartis (Inst), AstraZeneca (Inst), Intas (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Ljungberg B, Campbell SC, Weikert S, et al. : The epidemiology of renal cell carcinoma. Eur Urol 60:615-621, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Abraham GP, Cherian T, Mahadevan P, et al. : Detailed study of survival of patients with renal cell carcinoma in India. Indian J Cancer 53:572-574, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Mandrekar S, Amoncar S, Raiturkar SP, et al. : A histopathological study of renal cell carcinoma at a tertiary care hospital. Indian J Pathol Oncol 8:193-197, 2021 [Google Scholar]
- 4.Prakash G, Joshi A, Anand A, et al. : Kidney cancer demographics and outcome data from 2013 at a tertiary cancer hospital in India. Indian J Cancer 54:601-604, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Pallagani L, Choudhary GR, Pandey H, et al. : Epidemiology and clinicopathological profile of renal cell carcinoma: A review from Tertiary Care Referral Centre. J Kidney Cancer VHL 8:1-6, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ray RP, Mahapatra RS, Khullar S, et al. : Clinical characteristics of renal cell carcinoma: Five years review from a tertiary hospital in Eastern India. Indian J Cancer 53:114-117, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Tongaonkar H, Noronha V, Joshi A, et al. : Current evidence and the evolving role of sunitinib in the management of renal cell carcinoma. Indian J Cancer 53:102-108, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Motzer RJ, Jonasch E, Boyle S, et al. : NCCN guidelines insights: Kidney cancer, version 1.2021: Featured updates to the NCCN guidelines. J Natl Compr Canc Netw 18:1160-1170, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Draft Guidelines 2020—NCG. https://tmc.gov.in/ncg/index.php/guidelines/draft-guidelines-2020 [Google Scholar]
- 10.EBM Guidelines—TATA Memorial Hospital. https://tmc.gov.in/tmh/index.php/en/education-and-research/publications/evidence-based-management-guidelines-ebm [Google Scholar]
- 11.Health Benefit Package—2.0|Official Website Ayushman Bharat Pradhan Mantri Jan Arogya Yojana|National Health Authority. https://pmjay.gov.in/node/1128 [Google Scholar]
- 12.Motzer R, Alekseev B, Rha SY, et al. : Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med 384:1289-1300, 2021 [DOI] [PubMed] [Google Scholar]
- 13.Motzer RJ, Tannir NM, McDermott DF, et al. : Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 378:1277-1290, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amdahl J, Diaz J, Park J, et al. : Cost-effectiveness of pazopanib compared with sunitinib in metastatic renal cell carcinoma in Canada. Curr Oncol 23:e340-e354, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amdahl J, Diaz J, Sharma A, et al. : Cost-effectiveness of pazopanib versus sunitinib for metastatic renal cell carcinoma in the United Kingdom. PLoS One 12:e0175920, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delea TE, Amdahl J, Diaz J, et al. : Cost-effectiveness of pazopanib versus sunitinib for renal cancer in the United States. J Manag Care Spec Pharm 21:46-54, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capri S, Porta C, Condorelli C, et al. : An updated cost-effectiveness analysis of pazopanib versus sunitinib as first-line treatment for locally advanced or metastatic renal cell carcinoma in Italy. J Med Econ 23:1579-1587, 2020 [DOI] [PubMed] [Google Scholar]
- 18.Motzer RJ, Rini BI, Bukowski RM, et al. : Sunitinib in patients with metastatic renal cell carcinoma. JAMA 295:2516-2524, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Riaz IB, He H, Ryu AJ, et al. : A living, interactive systematic review and network meta-analysis of first-line treatment of metastatic renal cell carcinoma. Eur Urol 80:712-723, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Li S, Li J, Peng L, et al. : Cost-effectiveness of frontline treatment for advanced renal cell carcinoma in the era of immunotherapies. Front Pharmacol 12:718014, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bensimon AG, Zhong Y, Swami U, et al. : Cost-effectiveness of pembrolizumab with axitinib as first-line treatment for advanced renal cell carcinoma. Curr Med Res Opin 36:1507-1517, 2020 [DOI] [PubMed] [Google Scholar]
- 22.Health Technology Assessment: India (HTAIn)—HTAIn Manual. https://htain.icmr.org.in/index.php/documents/publications/htain-manual [Google Scholar]
- 23.Husereau D, Drummond M, Petrou S, et al. : Consolidated Health Economic Evaluation Reporting Standards (CHEERS)—Explanation and elaboration: A report of the ISPOR Health Economic Evaluation publication guidelines good reporting practices task force. Value Health 16:231-250, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Motzer RJ, Hutson TE, Cella D, et al. : Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 369:722-731, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Guyot P, Ades A, Ouwens MJ, Welton NJ: Enhanced secondary analysis of survival data: Reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol 12:9, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nocera L, Karakiewicz PI, Wenzel M, et al. : Clinical outcomes and adverse events after first-line treatment in metastatic renal cell carcinoma: A systematic review and network meta-analysis. J Urol 207:16-24, 2022 [DOI] [PubMed] [Google Scholar]
- 27.Registrar General and Census Commissioner of India : SRS Bulletin 2014. https://censusindia.gov.in/vital_statistics/SRS_Bulletins/SRS%20Bulletin%20-Sepetember%202014pdf [Google Scholar]
- 28.Ramaswamy A, Joshi A, Noronha V, et al. : Patterns of care and clinical outcomes in patients with metastatic renal cell carcinoma-results from a Tertiary Cancer Center in India. Clin Genitourin Cancer 15:e345-e355, 2017 [DOI] [PubMed] [Google Scholar]
- 29.de Groot S, Redekop WK, Versteegh MM, et al. : Health-related quality of life and its determinants in patients with metastatic renal cell carcinoma. Qual Life Res 27:115-124, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drugs, Surgical and Sutures. http://www.rmsc.health.rajasthan.gov.in/content/raj/medical/rajasthan-medical-services-corporation-ltd-/en/Approved-Rate-Lists/DrugsRC.html# [Google Scholar]
- 31.Online Pharmacy India|Buy Medicines from India's Trusted Medicine Store: 1mg.com. https://www.1mg.com/ [Google Scholar]
- 32.Prinja S, Dixit J, Gupta N, et al. : Development of National Cancer Database for Cost and Quality of Life (CaDCQoL) in India: A protocol. BMJ Open 11:e048513, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Department of Community Medicine and School of Public Health PGIMER Chandigarh. https://www.healtheconomics.pgisph.in/costing_web/index.php?action=Cost_data [Google Scholar]
- 34.CGHS Rate List—CGHS: Central Government Health Scheme. https://cghs.gov.in/index1.php?lang=1&level=3&sublinkid=5948&lid=3881 [Google Scholar]
- 35.Prakash G, Menon S: Guidelines for Management of Urological Cancers. Mumbai, India, Tata Memorial Center, 2020 [Google Scholar]
- 36.US Dollar to Indian Rupee Spot Exchange Rates for 2021. https://www.exchangerates.org.uk/USD-INR-spot-exchange-rates-history-2021.html [Google Scholar]
- 37.Cost-Effectiveness Threshold. YHEC—York Health Economics Consortium. https://yhec.co.uk/glossary/cost-effectiveness-threshold/ [Google Scholar]
- 38.GDP Per Capita (Current US$)—India|Data. https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=IN [Google Scholar]
- 39.Riaz IB, Ryu AJ, Yao Y, et al. : A systematic review and network meta-analysis of first-line treatment options in patients metastatic renal cell carcinoma. J Clin Oncol 38, 2020. (suppl 6; abstr 709) [Google Scholar]
- 40.Gupta N, Prinja S, Patil V, Bahuguna P: Cost-effectiveness of temozolamide for treatment of glioblastoma multiforme in India. JCO Glob Oncol 7:108-117, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Conversion Rates—Purchasing Power Parities (PPP)—OECD Data. theOECD. http://data.oecd.org/conversion/purchasing-power-parities-ppp.htm [Google Scholar]
- 42.Ravi T, Menon H, Prabhash K, et al. : Sunitinib in metastatic renal cell carcimoma: A single-center experience. Indian J Cancer 50:268-273, 2013 [DOI] [PubMed] [Google Scholar]
- 43.Patil S, Thungappa S, Kumar K, et al. : Retrospective multicentric analysis of Indian patients with metastatic renal cell carcinoma on first-line sunitinib 2/1 schedule. Ann Oncol 28:x84, 2017 [Google Scholar]
- 44.Kumar KA, Sadashivudu G, Krishnamani KV, et al. : Managing metastatic renal cell carcinoma-challenges, pitfalls, and outcomes in the real world. Indian J Med Paediatr Oncol 37:260-264, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rauthan A, Patil P, Murthy NY, et al. : Combination of immunotherapy and tyrosine kinase inhibitor in first-line metastatic renal cell carcinoma: A real-world Indian experience. J Clin Oncol 39:e16576, 2021. (15 suppl) [Google Scholar]
- 46.About Pradhan Mantri Jan Arogya Yojana (PM-JAY)|Official Website Ayushman Bharat Pradhan Mantri Jan Arogya Yojana|National Health Authority. https://pmjay.gov.in/about/pmjay [Google Scholar]