PURPOSE
The COVID-19 pandemic has affected public health worldwide. The efficacy and safety of COVID-19 vaccines have been evaluated in the general population; however, data on patients with malignancies are limited.
METHODS
This prospective longitudinal observational cohort study was conducted between June and July 2021. Enrolled adult patients with cancer were divided into chemotherapy and nonchemotherapy groups. All participants were immunized with two doses of the ChAdOx1 nCoV-19 or CoronaVac COVID-19 vaccines. The primary outcome was a comparison of the immunogenicity (as assessed by spike protein [anti-S] immunoglobulin G [IgG] antibody titers) of two doses of COVID-19 vaccine in the chemotherapy and nonchemotherapy groups. The secondary outcomes included the anti-S IgG seroconversion rate and vaccine safety in both groups.
RESULTS
Among the 173 enrolled patients with solid cancer, after COVID-19 vaccination, the chemotherapy group had a significantly lower median anti-S IgG titer than the nonchemotherapy group (26 v 237 U/mL, P < .001). A statistically significant difference in anti-S IgG titer was found between groups vaccinated with CoronaVac (7 v 90 U/mL, P < .001), but no difference was found in those vaccinated with ChAdOx1 nCoV-19 (818 v 1061 U/mL, P = .075). The anti-S IgG seroconversion rate was significantly lower in the chemotherapy group than that in the nonchemotherapy group (78.9% v 96.5%, P = .001). No new or serious vaccine-related adverse events were reported.
CONCLUSION
Patients with solid cancer receiving a COVID-19 vaccine while undergoing chemotherapy had lower immunogenicity responses to vaccination than those who were vaccinated while undergoing nonchemotherapy treatment. No statistically significant difference was observed in the COVID-19 vaccine safety profiles between groups.
BACKGROUND
Since December 2019, the SARS-CoV-2 infection has spread globally, resulting in more than 249 million confirmed cases of COVID-19 disease and more than 5 million associated deaths as of December 2021.1
CONTEXT
Key Objective
Our study evaluated whether the patient receiving chemotherapy should be immunized with COVID-19 vaccine.
Knowledge Generated
Patients with cancer receiving the COVID-19 vaccine demonstrated a significantly lower COVID-19 immune response in the chemotherapy-treated group than in the nonchemotherapy-treated group. However, subgroup analysis found that a diminished immune response was appealed only in patients receiving inactivated vaccines. In the chemotherapy-treated group, the doxorubicin/cyclophosphamide regimen might have caused the lowest immune response.
Relevance
Oncologists should be aware of the lower immune response to the COVID-19 vaccine in chemotherapy patients, especially those receiving the inactivated vaccine and doxorubicin/cyclophosphamide regimen. Therefore, proper timing, active cancer treatment, and vaccine type are factors that should be considered when generating the greatest immune response in this patient setting.
Cancer is known to be associated with immunosuppression. Patients with cancer have a higher mortality rate from COVID-19 disease relative to the general population.2,3 A systematic review and analysis of 52 studies reported a COVID-19 mortality rate of up to 25% in solid malignancy patients.4 A recent meta-analysis of 16 studies showed that chemotherapy within the 30 days before a COVID-19 diagnosis increased the risk of death in patients with cancer.5
In the general population, COVID-19 vaccines have a good safety profile and their efficacy against symptomatic disease ranges from 60% to 94%; however, patients with cancer, especially those receiving active treatment, were mostly excluded from the pivotal studies that reached these conclusions.6-10 A recent recommendation from the National Comprehensive Cancer Network COVID-19 Vaccination Advisory Committee is that patients with solid cancer should be immunized against COVID-19 disease when the vaccine is available, whether they are currently receiving active treatment.11
A previous prospective cohort study conducted on 140 patients with either solid (81%) or hematologic malignancies who received either the BNT162b2 (mRNA-based) or mRNA-1273 COVID-19 vaccine showed an excellent anti–SARS-CoV-2 antibody seroconversion rate of 94%. The levels of anti–SARS-CoV-2 antibody were also assessed via the blood test in these populations; the highest antibody levels were observed in patients undergoing clinical surveillance or endocrine therapy, whereas the lowest levels were found in patients treated with chemotherapy or monoclonal antibody.12 Another retrospective study was conducted on 326 patients with solid tumor who were undergoing treatment with anticancer agents (approximately two thirds of them were receiving chemotherapy). The median anti–SARS-CoV-2 immunoglobulin G (IgG) antibody titer after two doses of the BNT162b2 vaccine was lower among patients with cancer than among healthy adults (931 v 2,817 AU/mL, P = .003), and these titers were also significantly different among subgroups of patients undergoing different treatment types, specifically chemotherapy, immune checkpoint inhibitors, or targeted therapy (P = .002); however, only a small number of patients received each treatment type.13
The compromised efficacy by chemotherapy has been reported in the mRNA-based vaccine (BNT162b2 and mRNA-1273).12,13 However, data on the efficacy and safety of viral-vectored and inactivated viral COVID-19 vaccines in immunocompromised patients, especially patients with cancer, are lacking. The purpose of this study was to compare the immunogenicity of the COVID-19 vaccination (with the ChAdOx1 nCoV-19 or Corona Vac vaccines) in patients receiving chemotherapy and those undergoing nonchemotherapy treatment.
METHODS
Study Design
A prospective longitudinal observational cohort study was conducted at Chulabhorn Hospital, Bangkok, Thailand, from June to July 2021. The chemotherapy group was defined as those patients who received their first dose of COVID-19 vaccine while undergoing chemotherapy treatment or within 1 month from their last dose of chemotherapy, and the nonchemotherapy group was defined as those patients who received their first dose of COVID-19 vaccine while undergoing targeted therapy, endocrine therapy, immunotherapy treatment, or clinical surveillance and had either completed adjuvant or palliative chemotherapy for >1 month but not more than 5 years before. Written informed consent was obtained from all the patients. This study was conducted in accordance with the Human Research Ethics Committee of the Chulabhorn Research Institute (reference No. 063/2564). This trial was registered with Thaiclinicaltrials.org (TCTR20221001004).
Patient Population
All patients with solid cancer who visited the Outpatient Department of Medical Oncology at Chulabhorn Hospital from June 7, 2021, to July 12, 2021, were considered for study participation. Patients were eligible if they were age 18 years or older with histologically or cytologically confirmed solid malignancy, in either early or advanced stages, with an Eastern Cooperative Oncology Group performance status score of 0-3, and currently receiving systemic anticancer therapy or on active surveillance/follow-up within 5 years after completing cancer treatment. The main exclusion criteria were hematologic malignancy, concurrent chemoradiotherapy, an absolute neutrophil count < 1,500 cells/μL and/or an platelet count < 100,000/μL, expected life expectancy < 3 months, HIV infection, pregnancy, and breastfeeding.
COVID-19 Vaccination
In June 2021, The Ministry of Public Health of Thailand launched a national vaccination program for individuals at high risk of SARS-CoV-2 infection and COVID-19 mortality, including patients with cancer. Both ChAdOx1 nCoV-19 (a replication-deficient chimpanzee adenoviral vector containing the SARS-CoV-2 structural surface glycoprotein antigen [spike protein; S] gene [also known as AZD1222 by Oxford/AstraZeneca]) and CoronaVac (a chemically inactivated vaccine by Sinovac) vaccines were approved and available in Thailand.9,10 Both vaccines were administered intramuscularly; their vaccination schedules were two doses administered 21 days apart for CoronaVac and two doses administered 12 weeks apart for ChAdOx1.
Blood samples (6 mL each) were collected before receiving the first dose of ChAdOx1 nCoV-19 or CoronaVac vaccine (for assessing baseline levels of anti–SARS-CoV-2 antibody) and at weeks 8 and 12 after the first vaccine dose. At week 12, blood samples were collected on the same day that the second vaccine dose was administered to patients who received the ChAdOx1 nCoV-19 vaccine. Two additional blood samples were collected at 4 and 24 weeks after the second vaccine dose.
Elecsys Anti–SARS-CoV-2 S Assays
To assess COVID-19 vaccine immunogenicity, the collected blood samples were tested using Elecsys Anti–SARS-CoV-2 S, an immunoassay for the in vitro quantitative determination of antibodies (including IgG) against the SARS-CoV-2 spike (S) protein receptor–binding domain in human serum and plasma. The assay uses a recombinant protein presenting the receptor-binding domain of the S antigen in a double-antigen sandwich assay format, which favors the detection of high-affinity antibodies against SARS-CoV-2 infection. It has been designed for use as an aid to assess the adaptive humoral immune response to SARS-CoV-2 S protein. A cutoff value of ≥ 0.8 U/mL was used for assessing seroconversion, resulting in a clinical sensitivity of 98.8% (95% CI, 98.1 to 99.3) and an analytical specificity of 100% (95% CI, 99.7 to 100).14
Outcome Assessments
The primary outcome of this study was to compare the immunogenicity of two doses of the ChAdOx1 nCoV-19 or CoronaVac vaccine in patients with cancer undergoing chemotherapy and in patients with cancer receiving nonchemotherapy treatment.
The secondary outcomes included the anti-SARS-CoV-2 S antibody seroconversion rate after immunization with COVID-19 vaccine and the safety of these vaccines. Exploratory outcomes were the effects of the COVID-19 vaccine type on the anti-SARS-CoV-2 S antibody titer and seroconversion rate.
Statistical Analysis
All data were collected and managed using REDCap electronic data capture tools.15 To compare characteristics between the chemotherapy and nonchemotherapy groups, Student's t test, Mann-Whitney U test, chi-square test, or Fisher's exact test was used as appropriate.
The median ± interquartile range (IQR) was used to represent the anti–SARS-CoV-2 S antibody titers because of their non-normal distribution. We used a random-intercept linear mixed model, with the natural log-transformed antibody level as the dependent variable. This method incorporates a correlation between repeated blood test results in individuals.
The anti–SARS-CoV-2 S antibody seroconversion rate is presented in percentages.
In addition, we performed a post hoc subgroup analysis to determine the subpopulations with higher anti–SARS-CoV-2 S antibody levels and seroconversion rates. Lastly, vaccine-related adverse events were compared. A P value of <.05 was considered statistically significant. All statistical tests were performed using STATA version 16.0.
RESULTS
Patient Characteristics
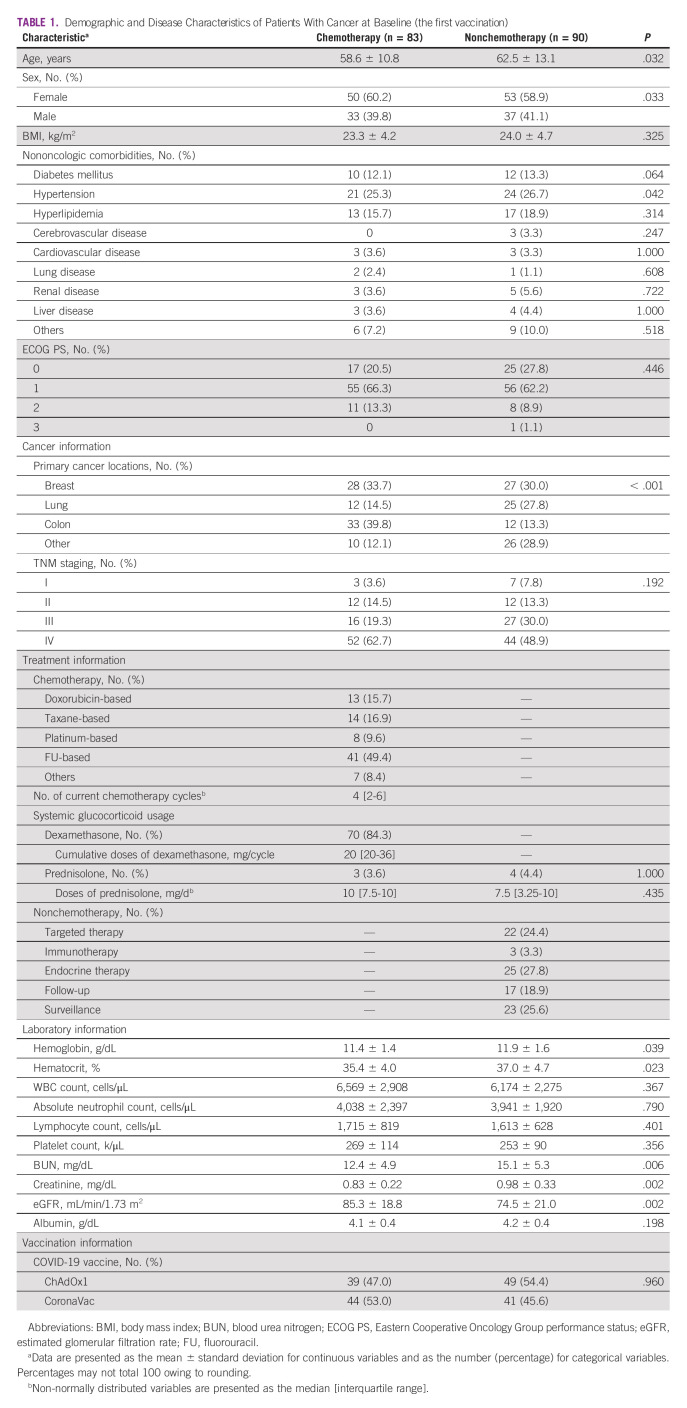
We initially enrolled 185 eligible patients with solid tumors between June 7, 2021, and July 12, 2021. Of these, 12 patients were excluded from the final analysis for various reasons (Fig 1). Among these 173 patients with solid cancer, 83 underwent scheduled chemotherapy (chemotherapy group) and 90 received other treatments or surveillance (nonchemotherapy group). We further stratified patients into four subgroups according to the cancer type: breast, lung, colon, and others. Colorectal cancer was the most common malignancy in the chemotherapy group (39.8%), whereas breast cancer was the most common malignancy in the nonchemotherapy group (30.0%; P < .001). Endocrine therapy (27.8%) and targeted therapy (24.4%) were the two most common treatments administered in the nonchemotherapy group (Table 1).
FIG 1.
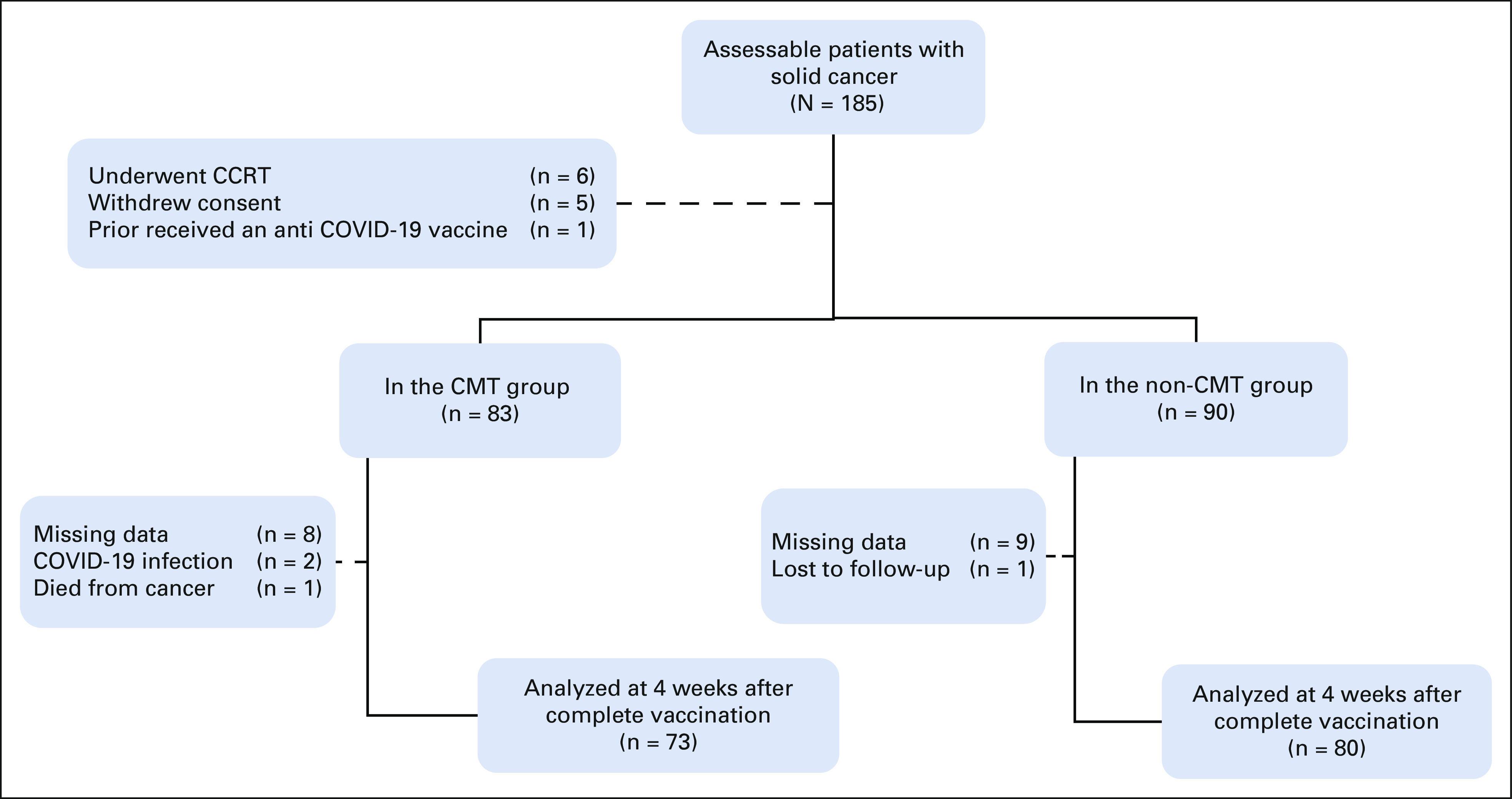
CONSORT diagram. CCRT, concurrent chemoradiotherapy; CMT, chemotherapy; complete vaccination, two doses of the ChAdOx1 nCoV-19 or CoronaVac vaccine; non-CMT, nonchemotherapy.
TABLE 1.
Demographic and Disease Characteristics of Patients With Cancer at Baseline (the first vaccination)
The ChAdOx1 nCoV-19 vaccine was administered to 39 of the 83 patients (47%) in the chemotherapy group and to 49 of the 90 patients (54%) in the nonchemotherapy group (P = .96); the remainder of the study participants received the CoronaVac vaccine.
Postvaccination Anti–SARS-CoV-2 Antibody Levels
In this study, 11.6% (20 of 173) of the enrolled patients were not included in the primary analysis for many reasons (Fig 1). The incidence of documented SARS-CoV-2 infection during the study period was 1.2% (two of 173 patients).
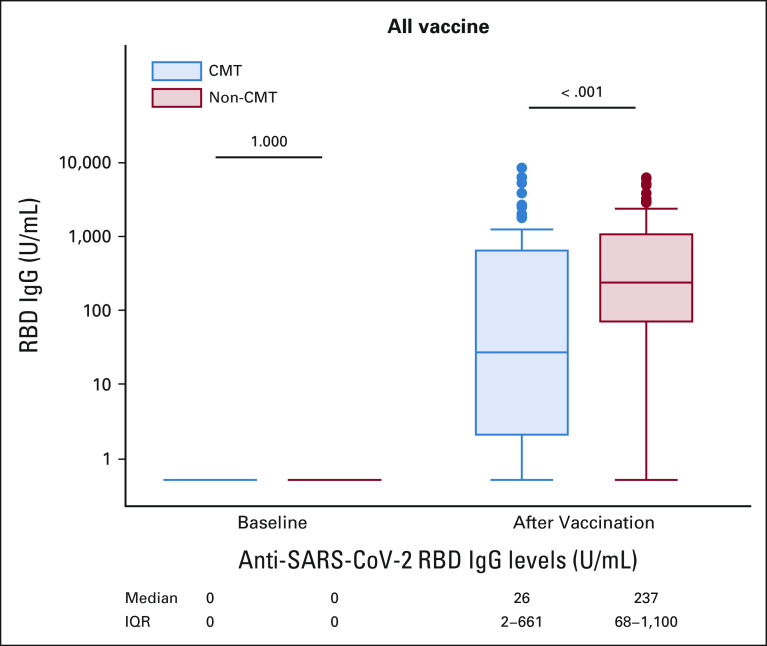
Regarding the primary study outcome, of the patients who completed vaccination with ChAdOx1 nCoV-19 or CoronaVac vaccine, those in the chemotherapy group had significantly lower median anti–SARS-CoV-2 S IgG titers than those in the nonchemotherapy group (26 v 237 U/mL, P < .001; Fig 2).
FIG 2.
Anti–SARS-CoV-2 RBD IgG levels before and after COVID-19 vaccination (all vaccine). The anti–SARS-CoV-2 RBD IgG levels in patients in CMT and non-CMT treatment groups were zero at baseline. At 4 weeks after two-dose vaccination, the anti–SARS-CoV-2 RBD IgG levels in patients receiving CMT were 26 U/mL and 267 U/mL in non-CMT treatment (P < .001). CMT, chemotherapy; IgG, immunoglobulin G; IQR, interquartile range; non-CMT, nonchemotherapy; RBD, receptor-binding domain.
When this comparison was repeated for vaccine-type subgroups, no significant difference was observed for those who received the ChAdOx1 nCoV-19 vaccine (818 v 1,061 U/mL, P = .075; Fig 3).
FIG 3.
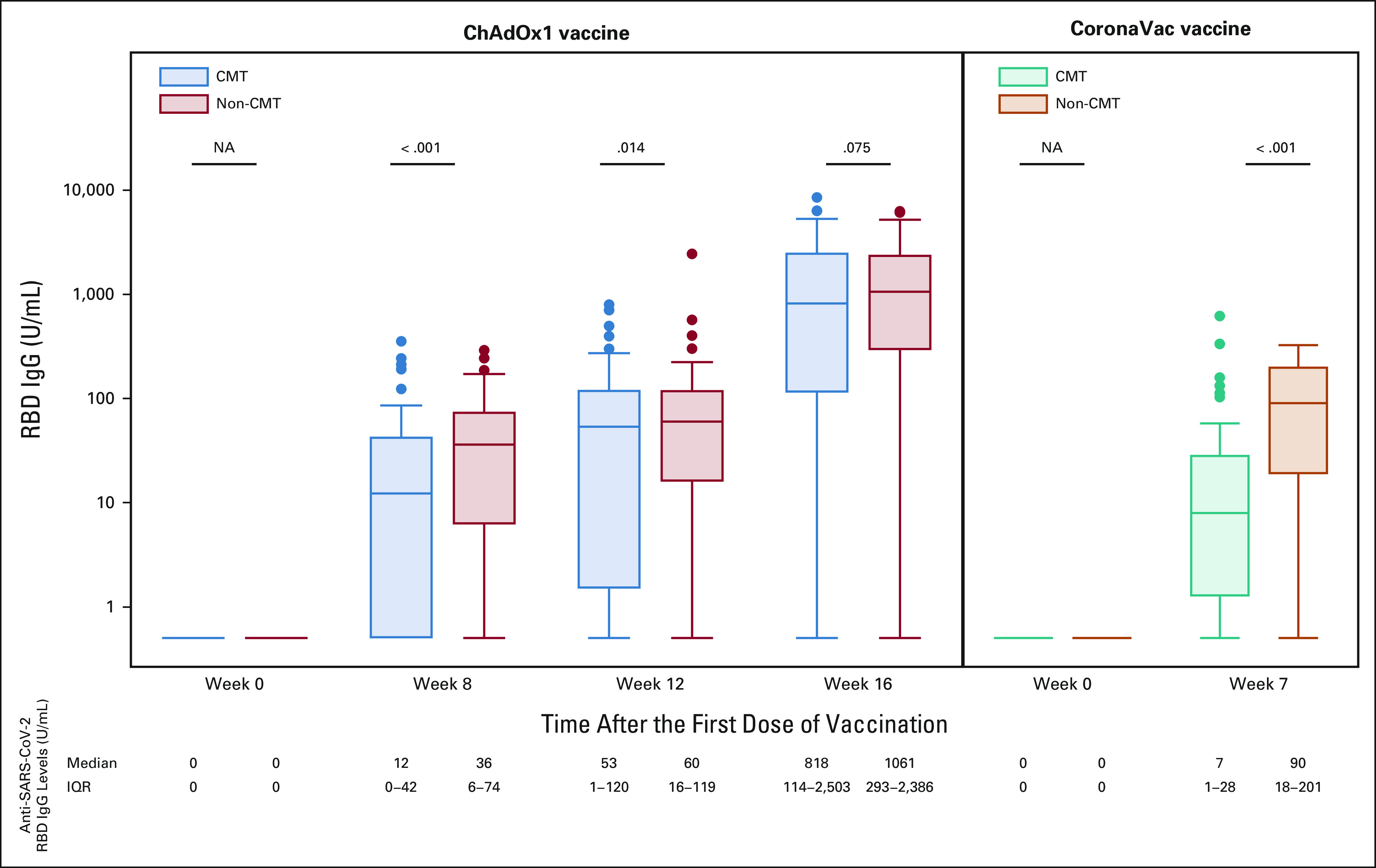
Anti–SARS-CoV-2 RBD IgG levels before and after COVID-19 vaccination (ChAdOx1 and CoronaVac vaccine). In the ChAdOx1 vaccination group (left), there was no difference in the anti–SARS-CoV-2 S RBD IgG levels at 4 weeks after completing vaccination between chemotherapy and nonchemotherapy groups, 818 and 1,061 U/mL, respectively (P = .075). Patients receiving the CoronaVac vaccination (right) showed a statistically significant difference in anti–SARS-CoV-2 RBD IgG levels between the two groups, 7 U/mL in the CMT group and 90 U/mL in the non-CMT group (P < .001). CMT, chemotherapy; IgG, immunoglobulin G; IQR, interquartile range; NA, not available; non-CMT, nonchemotherapy; RBD, receptor-binding domain.
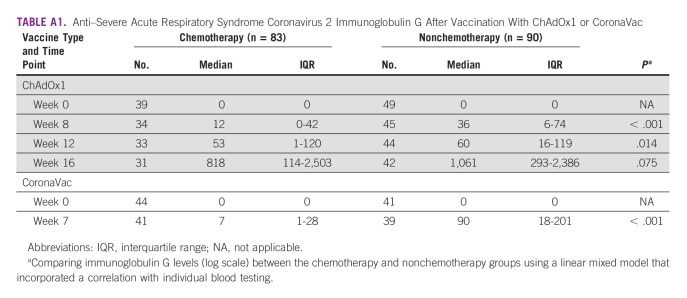
However, at weeks 8 and 12 after the first dose of ChAdOx1 nCoV-19 vaccination, a statistically significant difference in median anti–SARS-CoV-2 S IgG titers was observed between the CMT and non-CMT groups (Appendix Table A1). Concerning the CoronaVac vaccine, the median anti–SARS-CoV-2 S IgG titer was 7 U/mL in the chemotherapy group compared with 90 U/mL in the nonchemotherapy group, with a statistically significant difference (P < .001; Fig 3).
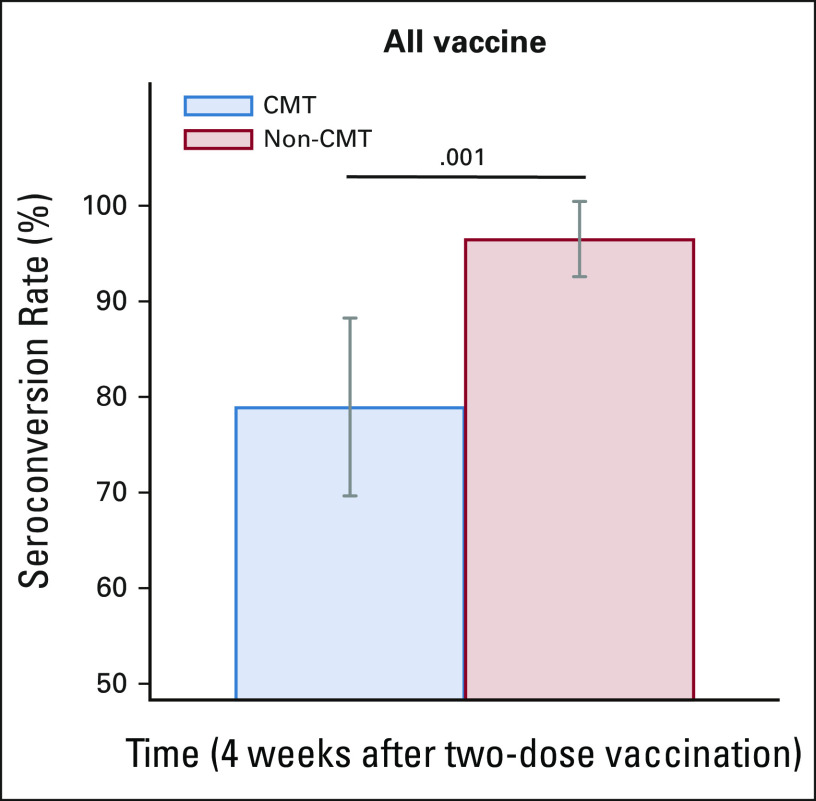
Regarding secondary study outcomes, the anti–SARS-CoV-2 S antibody seroconversion rate after vaccination in the chemotherapy group was significantly lower than that in the nonchemotherapy group (78.9% [95% CI, 68.1 to 87.5] v 96.5% [95% CI, 80.1 to 99.3], P = .001; Fig 4).
FIG 4.
Anti–SARS-CoV-2 IgG seroconversion rates after COVID-19 vaccination (all vaccine). The anti–SARS-CoV-2 IgG seroconversion rates between the CMT and non-CMT groups at 4 weeks after two-dose vaccination were 78.9% (95% CI, 68.1 to 87.5) and 96.5% (95% CI, 80.1 to 99.3), respectively, P = .001. The grey bars indicate 95% CIs. CMT, chemotherapy; IgG, immunoglobulin G; non-CMT, nonchemotherapy.
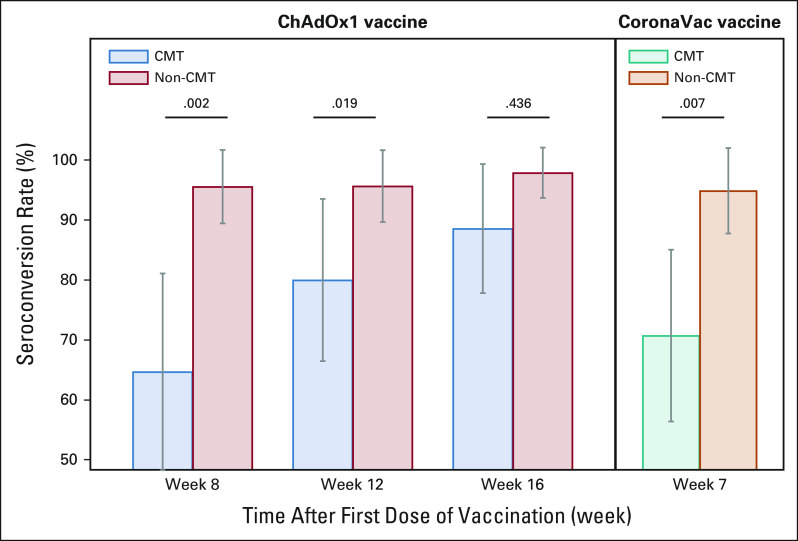
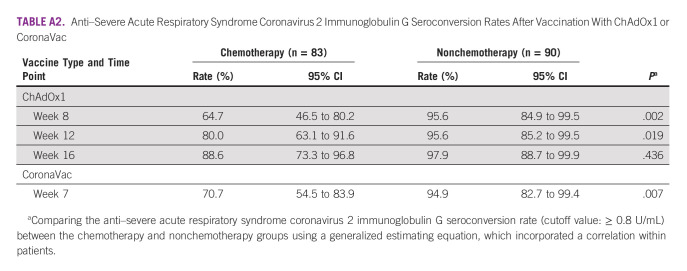
The pattern of differences in the anti–SARS-CoV-2 S IgG seroconversion rate was consistent with that of the primary end point, as described above. There was no significant difference in the seroconversion rates between the chemotherapy and nonchemotherapy groups among the subgroups that received the ChAdOx1 nCoV-19 vaccine at 1 month after completing vaccination (88.6% v 97.9%, P = .436), whereas a statistically significant difference was observed between these two groups at weeks 8 and 12 after the first dose of ChAdOx1 vaccination (Appendix Table A2). Among the subgroup of patients who received the CoronaVac vaccine, the chemotherapy group had a significantly lower rate of anti–SARS-CoV-2 S IgG seroconversion than the nonchemotherapy group (70.7% v 94.9%, P = .007; Fig 5).
FIG 5.
Anti–SARS-CoV-2 IgG seroconversion rates after COVID-19 vaccination (ChAdOx1 and CoronaVac vaccine). In the ChAdOx1 vaccination group (left), the anti–SARS-CoV-2 IgG seroconversion rate in the non-CMT group was significantly higher than that in the CMT group at weeks 8 and 12 (P = .002 and P = .019), whereas the seroconversion rate at week 16 showed no difference. There were 88.6% (95% CI, 73.3 to 96.8) in the CMT group and 97.9% (95% CI, 88.7 to 99.9) in the non-CMT group, P = .436. In the CoronaVac vaccination group (right), the anti–SARS-CoV-2 IgG seroconversion rate in the non-CMT group was significantly higher than that in the CMT group (94.9% [95% CI, 82.7 to 99.4] and 70.7% [95% CI, 54.5 to 83.9], respectively; P = .007). The grey bars indicate 95% CIs. CMT, chemotherapy; IgG, immunoglobulin G; non-CMT, nonchemotherapy.
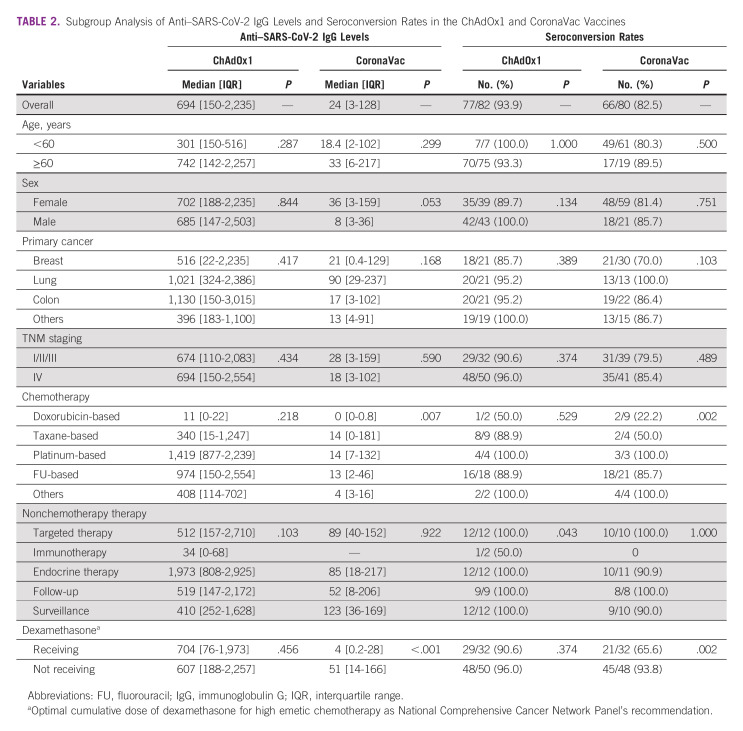
In subgroup analysis of COVID-19 vaccine types, patients with breast cancer who received doxorubicin/cyclophosphamide (AC) regimens and vaccination with CoronaVac had the lowest anti–SARS-CoV-2 S IgG level and seroconversion rate, compared with those who received other chemotherapy regimens (0 [IQR, 0-0.8] U/mL [P = .007] and 22.2% [P = .002], respectively). Furthermore, patients who used dexamethasone as a preventative treatment for chemotherapy-induced nausea and/or vomiting had a significantly lower median anti–SARS-CoV-2 S IgG level and seroconversion rate than patients who did not use dexamethasone (4 [IQR, 0.2-28] U/mL [P < .001] and 65.6% [P = .002], respectively). However, other patient characteristics such as age, primary cancer type, cancer stage, and baseline lymphocyte count did not significantly affect the median anti–SARS-CoV-2 S IgG level and seroconversion rate, regardless of the COVID-19 vaccine type (Table 2).
TABLE 2.
Subgroup Analysis of Anti–SARS-CoV-2 IgG Levels and Seroconversion Rates in the ChAdOx1 and CoronaVac Vaccines
Adverse Events
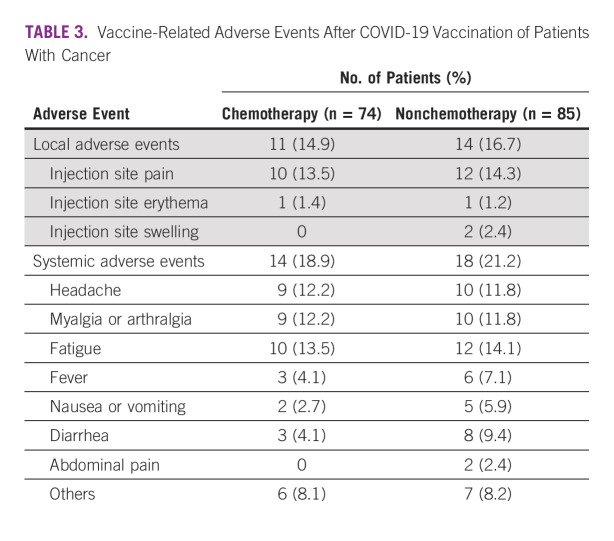
There were no differences in the reported local or systemic adverse events between the chemotherapy and nonchemotherapy groups (Table 3). All vaccine-related adverse events were mild to moderate in severity, but were easily manageable. Local adverse events, most of which were injection site pain, occurred in 15.7% (n = 25) of study participants. Systemic adverse events (n = 32, 20.1%), fatigue (n = 22, 13.8%), headache (n = 19, 12.0%), and myalgia (n = 19, 12.0%) were the most frequent adverse events. No new or serious adverse events (either life-threatening conditions or those requiring hospitalization) were reported in our study.
TABLE 3.
Vaccine-Related Adverse Events After COVID-19 Vaccination of Patients With Cancer
DISCUSSION
Patients with cancer may face severe COVID-19 morbidity and mortality.2-4 Impaired humoral and cellular immunity has been observed in these patients. In addition, bone marrow suppression, a side effect of chemotherapy, may impede immunologic responses to both infection and immunization. Hence, the COVID-19 vaccine–induced antibody response may be poor in patients with cancer, especially those undergoing chemotherapy.
The efficacy of some COVID-19 vaccines has been studied in patients with cancer, although data regarding the mRNA COVID-19 vaccines are available,12,13 and data on viral-vectored and/or inactivated viral COVID-19 vaccines are still limited. To address this gap, the present study investigated the immunogenicity and safety of viral-vectored and inactivated viral COVID-19 vaccines in patients with solid cancers. Specifically, this study compared the anti–SARS-CoV-2 S IgG levels and seroconversion rates in patients with cancer who were receiving ongoing chemotherapy or nonchemotherapy treatments, while also receiving two-dose COVID-19 vaccination with either the ChAdOx1 nCoV-19 or CoronaVac vaccine, both of which were available in Thailand during the study period.
Our study found that the group of patients with cancer vaccinated with two doses of COVID-19 vaccine while receiving ongoing chemotherapy had a significantly lower median anti–SARS-CoV-2 S IgG level and seroconversion rate than the nonchemotherapy group. Similarly, a retrospective cohort study conducted on 326 patients with cancer showed that the mRNA-based COVID-19 vaccine BNT162b2 was safe and effective in patients with cancer undergoing active treatment (62.9% with chemotherapy, 16.9% with immune checkpoint inhibitors, and 11.7% with targeted therapy), similar to that in healthy adults. A multivariate analysis found that the percentage of individuals who were still seronegative for anti–SARS-CoV-2 S IgG after vaccination was higher in the chemotherapy-treated group (18.8%) than in the immunotherapy-treated group (9.1%) or the targeted therapy-treated group (2.6%; P = .02).13 Another prospective cohort study conducted on 366 patients with solid malignancies confirmed the favorable efficacy of two-dose vaccination with BNT162b2 in patients undergoing active cancer treatment and found no difference in the vaccine efficacy assessment data between these patients who were receiving active treatment or not (median anti–SARS-CoV-2 IgG titer:1,530 v 2,256 AU/mL, respectively [P = .29]; and anti–SARS-CoV-2 IgG seroconversion rate [seroconversion defined as ≥50 AU/mL IgG titer] 91.2% v 89%, respectively [P = .56]).16 As in the mRNA-based COVID-19 vaccine studies, the present study observed relatively high rates of immune response in the subset of patients with cancer who were not being treated with immunosuppressive agents, as compared with those in the subset who received immunosuppressants.17,18
Interestingly, we found that the patients with cancer who were vaccinated with the inactivated viral COVID-19 vaccine (CoronaVac) had a lower immune anti–SARS-CoV-2 antibody response and seroconversion rate than those who were vaccinated with the viral-vectored vaccine (ChAdOx1 nCoV-19) in both the chemotherapy (7 v 818 U/mL and 70.7% v 88.6%, respectively) and nonchemotherapy (90 v 1,061 U/mL and 94.9% v 97.9%, respectively) groups. The difference in the mechanisms of action of whole-cell inactivated and viral vector vaccines could result in differences in antibody responses. The results of this analysis are consistent with data from the general population, which reported that adenovirus-vectored vaccines are more effective than inactivated viral vaccines. A recent prospective cohort study conducted in a Thai population reported a more rapid decline in the short-term immune response, as reflected by the anti–SARS-CoV-2 antibody levels, to COVID-19 vaccination in health care workers vaccinated with CoronaVac than in individuals vaccinated with ChAdOx1 nCoV-19.19 Nevertheless, no previous study has compared the performance of these two types of vaccination in an oncologic setting.
The current study observed that the anti–SARS-CoV-2 S IgG seroconversion rate after the first dose of ChAdOx1 nCoV-19 in the nonchemotherapy group was high (95.6%), but the mean IgG titer in this group was low (36-60 U/mL) because the cutoff value used for seroconversion was low, 0.8 U/mL. Poor anti–SARS-CoV-2 antibody response and seroconversion rates were found in the subgroup of CoronaVac-vaccinated patients treated with the AC regimen and dexamethasone. These findings could be the result of a low cellular immunity response in these patients because of bone marrow suppression from doxorubicin and cyclophosphamide and the very high dose of dexamethasone that is routinely prescribed for emesis prevention. The side effects of AC regimens are widely recognized; therefore, it was not surprising that the lowest post–COVID-19 vaccination anti–SARS-CoV-2 antibody titers and seroconversion rates were observed in the AC regimen subgroup compared with the other chemotherapy treatment subgroups in both the virus-vectored and inactivated viral vaccine groups. Although, these differences were significant only in the latter group. By contrast, there was no immunogenicity difference between the subgroups treated with higher and lower dexamethasone doses, nor was there any relation between COVID-19 vaccine immunogenicity and age, sex, Eastern Cooperative Oncology Group performance status, primary cancer type and stage, laboratory data, or vaccine type. Moreover, two patients in the current study were diagnosed with mild symptomatic COVID-19 disease. Both patients underwent chemotherapy. One patient received the CoronaVac vaccine, and the other received the ChAdOx1 nCoV-19 vaccine. None of the participants died from this cause. No new or serious vaccine-related adverse events were reported in any of the enrolled patients with cancer, regardless of treatment type. The incidence of documented COVID-19 disease in the enrolled patients during this study was lower (1.2%) than that reported in a previous study (2.5%), likely owing to the strict maintenance of social distancing by patients with cancer.20
Our study has several limitations. First, this study used the measurement of anti–SARS-CoV-2 S IgG as a surrogate for determining vaccine immunogenicity. However, the results of the anti–SARS-CoV-2 S IgG assay are highly correlated with those of a SARS-CoV-2 neutralizing antibody assay.21 Second, the findings of our subgroup analysis need to be interpreted with appropriate caution because the subgroups were each composed of a small number of patients with cancer; thus, larger studies are required to verify these results.
In conclusion, COVID-19 immunization induced lower anti–SARS-CoV-2 antibody responses when administered to patients with cancer actively receiving chemotherapy than when administered to patients with cancer receiving nonchemotherapy treatment, especially for patients who received the inactivated viral COVID-19 vaccine. Therefore, oncologists should be concerned about the proper timing of vaccination to generate maximum immunization in patients with cancer. Finally, further studies should be conducted to determine the ideal COVID-19 vaccination timing for patients with cancer to achieve the highest vaccine efficacy.
APPENDIX
TABLE A1.
Anti–Severe Acute Respiratory Syndrome Coronavirus 2 Immunoglobulin G After Vaccination With ChAdOx1 or CoronaVac
TABLE A2.
Anti–Severe Acute Respiratory Syndrome Coronavirus 2 Immunoglobulin G Seroconversion Rates After Vaccination With ChAdOx1 or CoronaVac
Archara Supavavej
Speakers' Bureau: Pfizer, AstraZeneca
Teerapat Ungtrakul
Research Funding: AstraZeneca/Merck, Roche, Taiho Oncology
Travel, Accommodations, Expenses: AstraZeneca/Merck, Roche
No other potential conflicts of interest were reported.
SUPPORT
Supported by Chulabhorn Royal Academy and National Vaccine Institute, Thailand.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Walaipan Tantiyavarong, Prakongboon Sungkasubun, Worawit Chaiwiriyawong, Chumut Phanthunane, Wisut Lamlertthon, Kriangkrai Tawinprai, Pichaya Tantiyavarong, Chayanee Samdaengpan
Administrative support: Kriangkrai Tawinprai
Provision of study materials or patients: Worawit Chaiwiriyawong, Piyarat Limpawittayakul, Wisut Lamlertthon
Collection and assembly of data: Walaipan Tantiyavarong, Archara Supavavej, Piyarat Limpawittayakul, Bowon Weerasubpong, Chumut Phanthunane, Teerapat Ungtrakul, Chayanee Samdaengpan
Data analysis and interpretation: Walaipan Tantiyavarong, Chumut Phanthunane, Pichaya Tantiyavarong, Chayanee Samdaengpan
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Archara Supavavej
Speakers' Bureau: Pfizer, AstraZeneca
Teerapat Ungtrakul
Research Funding: AstraZeneca/Merck, Roche, Taiho Oncology
Travel, Accommodations, Expenses: AstraZeneca/Merck, Roche
No other potential conflicts of interest were reported.
REFERENCES
- 1.World Health Organization : Coronavirus disease (COVID-19) weekly epidemiological update and weekly operational update. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19–-7-december-2021
- 2.Liang W, Guan W, Chen R, et al. : Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol 21:335-337, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyashita H, Mikami T, Chopra N, et al. : Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann Oncol 31:1088-1089, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saini KS, Tagliamento M, Lambertini M, et al. : Mortality in patients with cancer and coronavirus disease 2019: A systematic review and pooled analysis of 52 studies. Eur J Cancer 139:43-50, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yekedüz E, Utkan G, Ürün Y: A systematic review and meta-analysis: The effect of active cancer treatment on severity of COVID-19. Eur J Cancer 141:92-104, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polack FP, Thomas SJ, Kitchin N, et al. : Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med 383:2603-2615, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baden LR, El Sahly HM, Essink B, et al. : Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 384:403-416, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadoff J, Gray G, Vandebosch A, et al. : Safety and efficacy of single-dose Ad26.COV2.S vaccine against COVID-19. N Engl J Med 384:2187-2201, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voysey M, Costa Clemen SA, Madhi SA, et al. : Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 397:99-111, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palacios R, Batista AP, Albuquerque CSN, et al. : Efficacy and safety of a COVID-19 inactivated vaccine in healthcare professionals in Brazil: The PROFISCOV study. SSRN Electron J 10.2139/ssrn.3822780 https://ssrn.com/abstract=3822780 [DOI] [Google Scholar]
- 11.Steven P, Lindsey RB, Brahm S, et al. : Recommendations of the National Comprehensive Cancer Network (NCCN) Advisory Committee on COVID-19 Vaccination and Pre-exposure Prophylaxis, NCCN: Cancer and COVID-19 Vaccination Version 5.0, 2022 [Google Scholar]
- 12.Addeo A, Shah PK, Bordry N, et al. : Immunogenicity of SARS-CoV-2 messenger RNA vaccines in patients with cancer. Cancer Cell 39:1091-1098, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ligumsky H, Safadi E, Etan T, et al. : Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine among actively treated cancer patients. J Natl Cancer Inst 114:203-209, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roche Diagnostics: Elecsys anti-SARS-CoV-2 S package insert 2020–09, V1.0, material numbers 09289267190 and 09289275190. Roche Diagnostics, Indianapolis, IN, 2020.
- 15.Harris A, Taylor R, Thielke R, et al. : Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377-381, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelli F, Fabbri A, Onorato A, et al. : Effects of active cancer treatment on safety and immunogenicity of COVID-19 mRNA-BNT162b2vaccine: Preliminary results from the prospective observational Vax-On study. Ann Oncol 33:107-108, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herishanu Y, Avivi I, Aharon A, et al. : Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood 137:3165-3173, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyarsky BJ, Werbel WA, Avery RK, et al. : Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA 325:1784-1786, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jantarabenjakul W, Chantasrisawad N, Puthanakit T, et al. : Short-term immune response after inactivated SARS-CoV-2 (CoronaVac®, Sinovac) and ChAdOx1 nCoV-19 (Vaxzevria®, Oxford-AstraZeneca) vaccinations in health care workers (published online ahead of print 2021 Oct 31). Asian Pac J Allergy Immunol 40:269-277, 2022 [DOI] [PubMed] [Google Scholar]
- 20.Peeters M, Verbruggen L, Teuwen L, et al. : Reduced humoral immune response after BNT162b2 coronavirus disease 2019 messenger RNA vaccination in cancer patients under antineoplastic treatment. ESMO Open 6:100274, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oosting SF, van der Veldt AAM, GeurtsvanKesse CH, et al. : mRNA-1273 COVID-19 vaccination in patients receiving chemotherapy, immunotherapy, or chemoimmunotherapy for solid tumours: a prospective, multicentre, non-inferiority trial. Lancet Oncol 22:1681-1691, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]