PURPOSE:
Delivering cancer care by high-functioning multidisciplinary teams promises to address care fragmentation, which threatens care quality, affects patient outcomes, and strains the oncology workforce. We assessed whether the 4R Oncology model for team-based interdependent care delivery and patient self-management affected team functioning in a large community-based health system.
METHODS:
4R was deployed at four locations in breast and lung cancers and assessed along four characteristics of high-functioning teams: recognition as a team internally and externally; commitment to an explicit shared goal; enablement of interdependent work to achieve the goal; and engagement in regular reflection to adapt objectives and processes.
RESULTS:
We formed an internally and externally recognized team of 24 specialties committed to a shared goal of delivering multidisciplinary care at the optimal time and sequence from a patient-centric viewpoint. The team conducted 40 optimizations of interdependent care (22 for breast, seven for lung, and 11 for both cancers) at four points in the care continuum and established an ongoing teamwork adaptation process. Half of the optimizations entailed low effort, while 30% required high level of effort; 78% resulted in improved process efficiency.
CONCLUSION:
4R facilitated development of a large high-functioning team and enabled 40 optimizations of interdependent care along the cancer care continuum in a feasible way. 4R may be an effective approach for fostering high-functioning teams, which could contribute to improving viability of the oncology workforce. Our intervention and taxonomy of results serve as a blueprint for other institutions motivated to strengthen teamwork to improve patient-centered care.
BACKGROUND
Cancer care is complex, interdependent, and difficult to coordinate across multiple specialties, modalities, and settings along a patient's care continuum.1-3 Efforts to organize appropriate timing and sequence of care are often ad hoc, inefficient, and onerous for clinicians and patients.4-6 These challenges cause care breakdowns and delays, worsen patient outcomes, and exacerbate clinician burden and dissatisfaction.1,6-9 The continuing advent of new diagnostics and treatments benefits patient survival but also expands specialties involved, adds appointments per patient, and places growing demands for care coordination on an already stretched oncology workforce.6,10 Left unaddressed, these challenges jeopardize care quality, patient outcomes, and sustainability of the cancer care system.3,6,11,12
Fostering high-functioning teams and intentional teamwork is a promising strategy to enable well-coordinated and efficient delivery of high-quality cancer care, enhance patient experience, and improve viability of the oncology workforce.7,13-15 High-functioning teams are characterized by (1) recognition as a team internally and externally; (2) commitment to an explicit shared goal; (3) enablement of interdependent work to achieve the goal; and (4) engagement in regular reflection to adapt objectives and processes.6,16
The necessity of high-functioning teams has been broadly recognized by oncology organizations and societies.1,7,17,18 In response, over the past two decades, teamwork models emerged in cancer care, with the most common among them being multidisciplinary conferences and clinics (MDCs). MDCs typically occur postdiagnosis as multispecialty meetings and/or patient consults to review cases and recommend treatment plans.19-22 MDCs have been shown to improve treatment planning, quality of care, patient and clinician satisfaction, and patient survival.23-32 However, MDCs, and teamwork in general, encounter considerable adoption barriers, including siloed, specialty-based practice patterns6,8,22,33; concerns about feasibility and requirements for clinicians' time22,33; the challenge of managing teamwork over time along the care continuum, beyond the initial care planning14,19,20; and lacking practical knowledge of how to forge high-functioning teams.20,21
One approach intended to address these barriers is the 4R Oncology model. 4R is Right Information and Right Care for the Right Patient at the Right Time. Featured within the 2016 NCI-ASCO Teams in Cancer Care Delivery initiative, 4R aims to facilitate systematic delivery of interdependent multidisciplinary care by improving teamwork and enabling patient self-management.9 4R promotes high-functioning teams by helping manage care interdependence, a key teamwork principle and a crucial contributor to team effectiveness.34-40 4R has been demonstrated to enhance interdependent care delivery and improve self-management in patients with early breast cancer.41 Herein, we describe the impact of 4R on fostering high-functioning teams in breast and lung cancers to enable delivery of interdependent care along the cancer care continuum. We assess the impact along the four characteristics of high-functioning teams,6,16 report the feasibility of the 4R intervention, and discuss implications of our results for the oncology workforce. The assessment was conducted at Kaiser Permanente Northern California (KPNC)—a large community-based integrated health system. We discuss why our results are generalizable across settings and how our approach may serve as a blueprint to institutions motivated to strengthen teamwork.
METHODS
4R Oncology Model
4R applies project management, a discipline for managing interdependent tasks by multidisciplinary teams, to coordinating timing and sequence of interdependent care. Under 4R, a care team and a patient use a Care Sequence, a personalized, structured care project plan as a roadmap throughout a patient's care trajectory. Care Sequences reflect individual care recommendations emerging from workup and/or treatment planning (MDC or individual) and include oncologic, supportive, primary, comorbidity, and social care. They are developed after diagnosis and updated as needed thereafter. A Care Sequence outlines a course of care indicated for a patient, depicts care timing, sequence, and dependencies, and specifies responsibilities for different types of care.
Importantly, Care Sequences reflect a patient-centric, versus specialty-focused view of care timing and sequencing. Clinical specialties often deliver their care on the basis of internal workflows or ad hoc referrals, and may not have the full view of how their care fits into the overall journey of an individual patient. Care Sequences weave together care events delivered by various specialties in an end-to-end chronology optimal for an individual patient, and indicate when in that chronology different specialties should deliver care to that patient.
4R Optimization Intervention
As previously described,9,42,43 institutional adoption of the 4R Oncology model involves two steps: 4R Optimization, the intervention assessed herein, and 4R Clinical Use, evaluated previously.41 4R Optimization aims to create and sustain the conditions enabling delivery of independent care according to Care Sequences. It starts before 4R launch in the clinic and continues after launch. 4R Optimization includes forming a multidisciplinary team of specialties relevant to care within Care Sequences; conducting collaborative optimizations of timing and sequencing of care to facilitate delivery on the basis of Care Sequences; and establishing an ongoing process for team-based optimizations. Care Sequences serve as a basis for the team formation and structure, as well as tools for facilitating team activities and functioning.
Setting
The intervention was conducted at four KPNC medical centers. The intervention in breast cancer occurred at the San Francisco center, with collocated breast cancer services. The lung cancer intervention occurred at Oakland (location of thoracic surgery), San Francisco (location of medical oncology), and two centers in Central Valley serviced by a shared clinical group. Multidisciplinary conferences existed for patients with breast and lung cancer at all locations; multidisciplinary clinic existed only for patients with breast cancer in the San Francisco center. 4R Optimization started in July 2020 and was assessed through December 2021. 4R was launched in the clinics with patients in October 2020.
Intervention Scope and Approach
Scope-related inclusion/exclusion was based on perceived need and feasibility of optimization. The intervention focused on care related to surgery and systemic therapy. We further defined scope not by specialty, but by care domain—a set of related multidisciplinary care events and processes. Five care domains were included: surgical care; systemic therapy care; genetics and biomarkers; imaging and other assessment; and supportive care. Preliminary Care Sequences were developed for systemic therapy and surgery, with desired timing and sequence of care. Within Care Sequences, 40 opportunities were identified for optimization of care timing and sequencing. Only opportunities for interspecialty optimizations (related to care involving two or more specialties) were included. We invited 24 specialties relevant to identified optimizations to participate. Although this setting represented a multiteam system,44,45 we considered each specialty group as one team member, including physicians, nurses, nurse navigators, and others, as deemed relevant by each group. The goal of the initiative was to enable delivery of care to patients at optimal time and sequence according to Care Sequences. Impact of optimization on care efficiency was monitored throughout the intervention.
Assessment
The intervention was assessed along the four characteristics of high-functioning teams6,16:
Internal and external recognition as a team. The internal recognition was reported as a proportion of invited specialties who agreed to participate, and as a structure of the resulting team, including team composition and organization. The external recognition was assessed as attainment of formal support for the team from KPNC medical group leadership.
Team's commitment to an explicit shared goal was reported as members' agreement with the goal, provision of input into Care Sequence content, agreement to responsibilities for relevant care, and participation in related optimizations.
Enabling interdependent work to achieve the goal. We report optimizations of interdependent care performed by the team, stratified by cancer type; care process (care domain and point in care continuum); methods used for optimizations; feasibility (level of effort involved in individual optimizations and whether they were completed in the assessment period); and efficiency (whether one or more steps were removed from the care process). The level of effort was calculated as a weighted scale of six metrics: number of specialties involved; number of optimization iterations; time required from a lead; whether capacity barriers had to be addressed (eg, capacity of imaging); whether optimization included establishment of an institutional practice standard; and whether Electronic Medical Report Information Technology or an entity external to KPNC was involved.
Engagement in regular reflection to adapt objectives and processes. We report the establishment of an ongoing iterative learning process to monitor timing and sequencing of care according to Care Sequences and conduct necessary care optimizations.
RESULTS
Internal and External Recognition as a Team
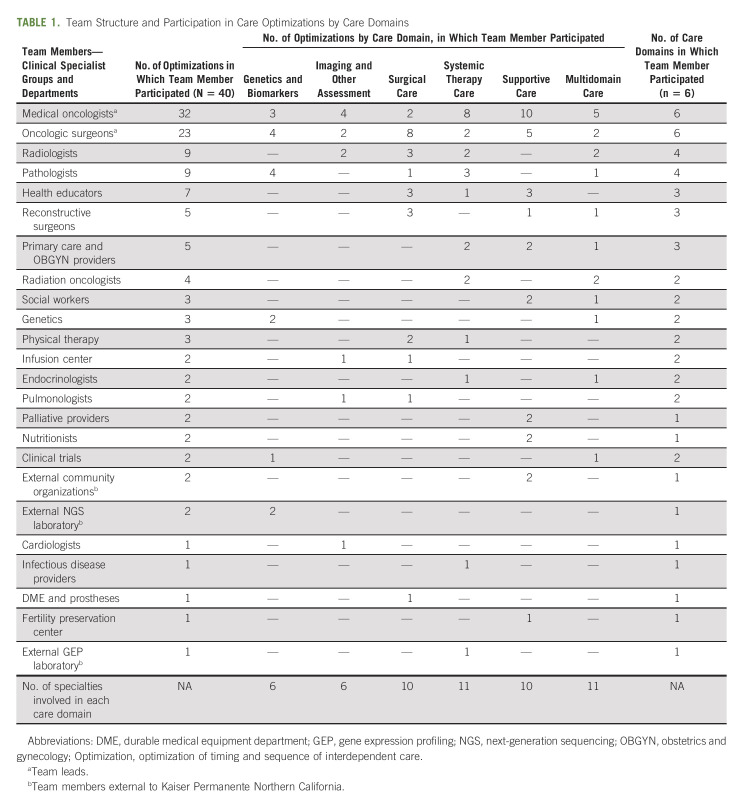
All 24 invited specialties joined the initiative as team members (Table 1) with agreement to assume member responsibilities, including input to relevant Care Sequence content and participation in optimizing interdependent care. Team composition changed over time—starting from 10 members and increasing to 24 during the initiative as optimization opportunities were identified. Members agreed to the team structure and organization. The team was led by medical oncology and surgery. Three members were entities outside KPNC. Members were organized into subteams, each focused on a relevant optimization, with some members joining multiple subteams. KPNC medical group leadership at regional and local levels approved the intervention and supported the team efforts.
TABLE 1.
Team Structure and Participation in Care Optimizations by Care Domains
Team's Commitment to an Explicit Shared Goal
All members agreed to the shared goal. Demonstrating commitment to the goal, members provided input to the content of preliminary Care Sequences related to timing and sequence of care. Team leads integrated input and finalized Care Sequences, resolving inconsistencies by discussion and consensus with relevant members. The following Care Sequences were finalized in breast and lung cancers each: Care Initiation, Surgery, Neoadjuvant Therapy, and Adjuvant Therapy (see example in Fig 1). Two additional ones were finalized in lung cancer—Treatment for Advanced Disease and Chemoradiation. Further commitment to the goal was demonstrated by members' participation in subteams focused on relevant optimizations (Table 1). Subteams worked virtually or asynchronously, convening meetings only when broad practice standards were discussed. More than half (54%; 13/24) of the members engaged in one to two optimizations, and 37% (9/24) in three to nine optimizations. Medical oncologists and surgeons (9%; 2/24) participated in 32 and 23 optimizations, respectively. Most members (71%) participated in optimizations within one to two domains, and 29% engaged in optimizations within three to six domains.
FIG 1.
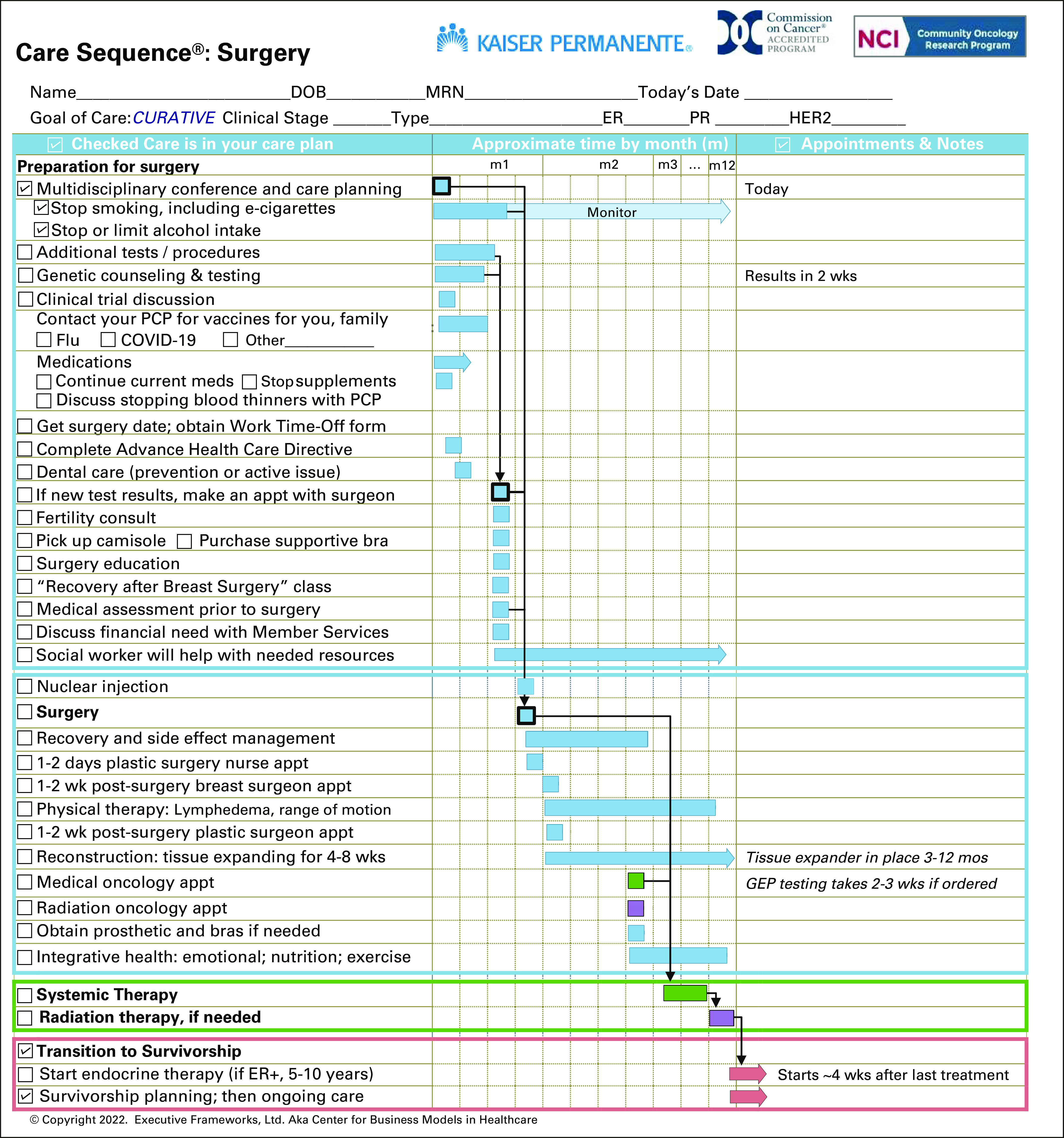
Care Sequence: Surgery. Care Sequences contain a preprinted list of care, which is personalized for each patient by checking boxes and making notes. This Care Sequence is intended for patients indicated for breast surgery, adjuvant systemic therapy, and radiation therapy. Systemic and radiation therapy are described in summary. Patients receive a subsequent Care Sequence specific to adjuvant systemic therapy at an appropriate point in care. Squares are shorter events, such as tests or appointments; bars are longer care processes, for example, genetic counseling and testing or postsurgical recovery. Arrows indicate care dependencies: what care events should be completed before other care starts–responsibilities/resources component is not shown. All content and configuration of the graph may be adapted by an institution to reflect local services and processes. This is an abbreviated schematic for illustration purposes, not an actual Care Sequence template. Actual Care Sequences may contain additional care. ER, estrogen receptor; GEP, gene expression profiling; HER2, human epidermal growth factor receptor 2; PCP, primary care physician; PR, progesterone receptor.
Enabling Interdependent Work to Achieve the Goal
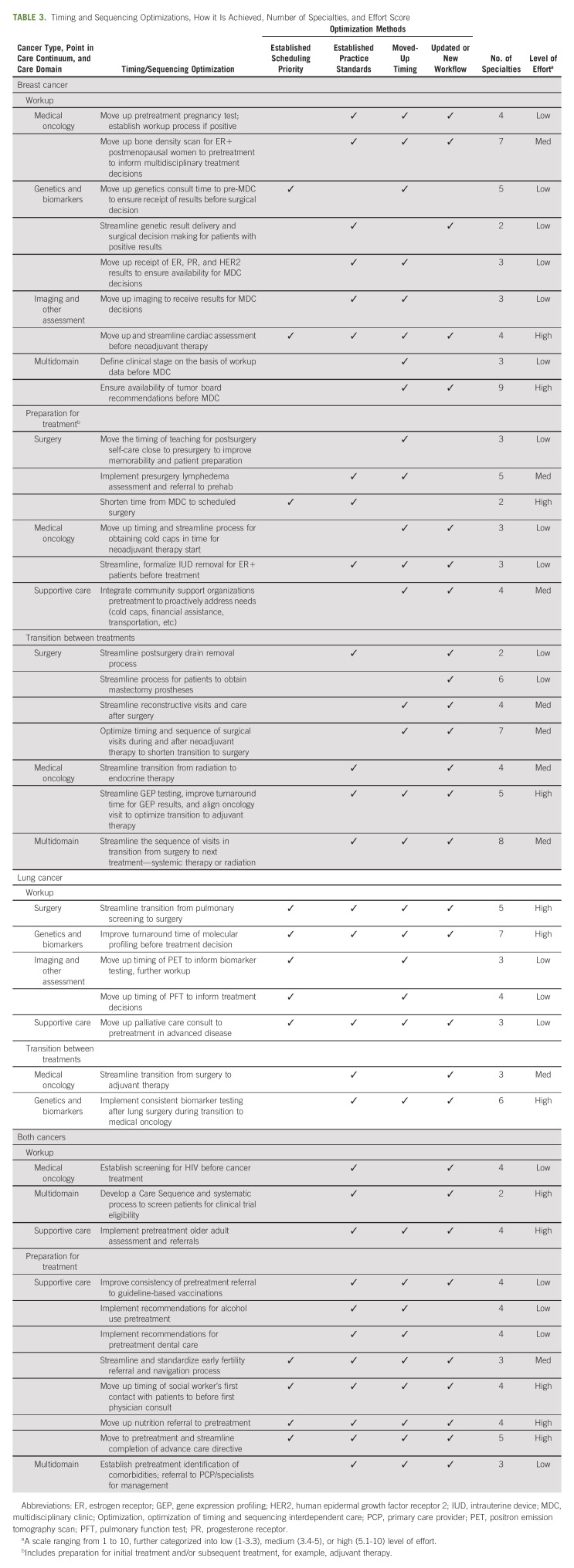
The team performed 40 optimizations, summarized in Table 2 and detailed in Table 3. Initially, 23 optimizations were identified in breast cancer and 17 in lung cancer. Eleven optimizations were further determined by subteams as benefiting both cancers and were applied accordingly, resulting in 22 breast cancer optimizations, seven lung cancer optimizations, and 11 optimizations in both.
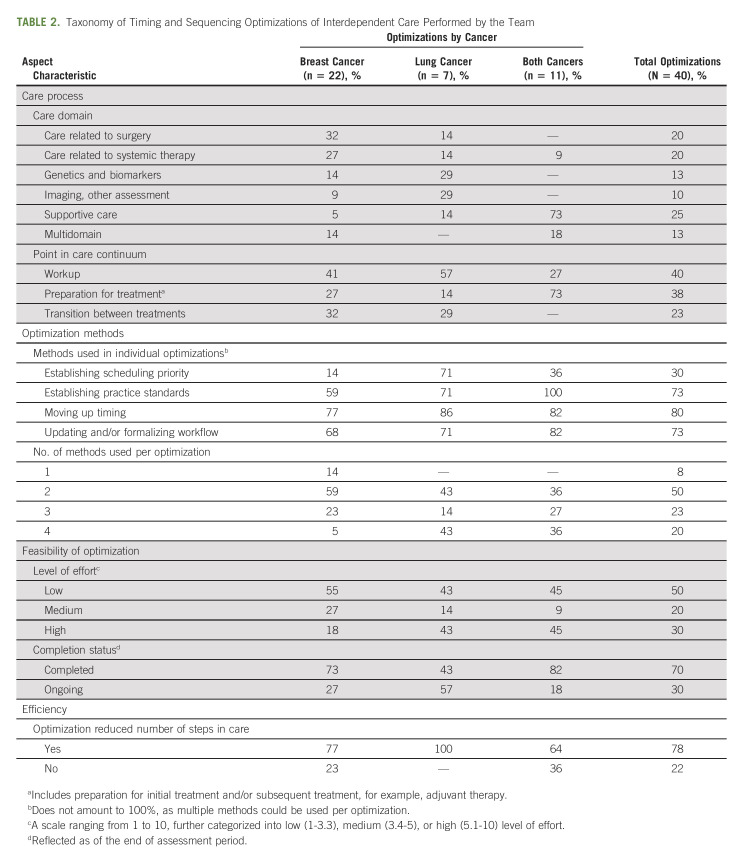
TABLE 2.
Taxonomy of Timing and Sequencing Optimizations of Interdependent Care Performed by the Team
TABLE 3.
Timing and Sequencing Optimizations, How it Is Achieved, Number of Specialties, and Effort Score
The distribution of optimizations across care domains was relatively even overall and varied by cancer type. Most breast cancer optimizations addressed surgery and systemic therapy, most lung cancer optimizations focused on genetics and biomarkers and imaging and other assessment, and optimizations in both cancers centered primarily on supportive care.
Optimizations fell into several points along the cancer care continuum: workup, preparation for treatment (initial and/or subsequent, such as adjuvant therapy), and transition between treatments.
Four methods were used to conduct optimizations (Table 2). Most breast cancer optimizations required one or two methods, while most optimizations in lung and both cancers required three or four methods. Moving up timing of care in a patient's care trajectory was the most common method in lung and breast cancers, while establishing standards was the most common method for optimizations applied to both cancers. Establishing scheduling priority was used in 71% of lung cancer optimizations, as they addressed genetics and biomarkers and/or imaging, where capacity is typically limited and requires prioritization of patients for timely care.
The majority of all optimizations required low or medium effort. The numbers of high-effort optimizations were similar across cancers, but proportionally more optimizations in lung (45%; 3/7) and both cancers (45%; 5/11) required high effort than those in breast cancer (18%; 4/22). Most optimizations (70%; 28/40) were completed within the assessment period, while 27% (6/22) breast cancer optimizations, 18% (2/11) of optimizations in both cancers, and 57% (4/7) of lung cancer optimizations required further optimization cycles. These were identified later in the assessment period, for example, implementing consistent biomarker testing after lung surgery; were related to a capacity constraint (eg, operating room capacity); or required a broad institutional consensus, for example, implementing pretreatment older adult assessment and referrals (data not shown).
Most optimizations in breast and both cancers (77% and 64%, respectively), and all lung cancer optimizations resulted in improved process efficiency.
Regular Reflection by the Team to Adapt Objectives and Processes
Following the dynamic sustainability framework,46 the team established a learning, problem-solving, and adaptation process, making the effort of fostering a high-functioning team dynamic and ongoing. This entailed monitoring of new opportunities to optimize care timing and sequencing, involving new specialties and forming new subteams. After the 4R launch in the clinics, a feedback loop from team members was established, which enabled continuous identification of new optimization needs. Care Sequences were adjusted to reflect the results of optimizations. This adaptive approach allowed the team to reframe 11 optimizations to apply to both breast and lung cancers, thus expanding their impact, as described above. Going forward, monitoring for new opportunities will be supported by a data-driven dashboard.
DISCUSSION
We assessed how a 4R Optimization intervention, a component of the 4R Oncology model, affected team functioning in breast and lung cancers at four locations of a community-based integrated health system. 4R facilitated development of a high-functioning team along the four characteristics of such teams. We formed an internally and externally recognized team of 24 specialties committed to a shared goal of enabling interdependent care delivery at the optimal time and sequence from a patient-centric viewpoint. The team enabled interdependent work with 40 optimizations of care timing and sequencing and established a learning process for ongoing teamwork adaptation. Optimizations addressed six care domains at several points along the care continuum. Half of the optimizations entailed low effort, while 30% required a high level of effort. Most optimizations resulted in improved process efficiency.
Our results suggest that 4R represents a promising and practical approach to forging high-functioning teams, which may help address challenges of multidisciplinary teamwork, dovetail with other teamwork models, and contribute to viability of the oncology workforce. Below, we discuss these implications, highlight opportunities for further model enhancement, and suggest how our results can serve as a blueprint for other institutions.
Specialty-based, siloed approach to care is a recognized barrier to multidisciplinary teamwork.1,6,8,33 Using patient-centered Care Sequences to orient teamwork, 4R allowed us to assemble a team of 24 diverse specialties who changed many of their silo-focused practice patterns (such as scheduling and consult workflows) to align 40 types of interdependent care from a patient-centric view of care timing and sequence. This suggests that 4R may help develop important team competencies, such as providing patient-centered care.47
Our results indicate that 4R helps address another challenge—organizing teamwork longitudinally, along the patient care continuum.14,19,20 4R helped us improve teamwork at several points in the care continuum—workup, preparation for initial and subsequent treatments, and transitions in care. Other team-based care models, such as MDC or Oncology Medical Home, do not address the challenge of longitudinal teamwork: MDCs focus on point-in-time treatment decision making, and Oncology Medical Home provides the overall structure and metrics for team-based practice but does not provide specific tools for team functioning.19-22,48 These models address needs outside of the 4R scope, making them synergistic. Integrating them with 4R may improve a broad scope of teamwork and should be evaluated.
Perhaps the greatest obstacle to teamwork is concern about the feasibility of establishing and sustaining high-functioning teams.22,33,49 We showed that 4R can facilitate an ambitious scope of teamwork in an attainable way. The team performed 40 optimizations of interdependent care in two cancers in a relatively short time. Half of the optimizations required low effort, indicating that not all teamwork is arduous. However, we were able to also carry out optimizations requiring a high level of effort (30%; 12/40). Strategies enabling feasibility included straightforward optimization methods; structuring teamwork in subteams; using virtual and asynchronous communication; and identifying synergies between two cancers to expand the impact. Participation burden was low for most specialties, but medical oncology and surgery had higher involvement as team leads to make the initiative successful. Institutions aspiring to conduct similar optimizations should plan accordingly.
Our assessment showed that 4R enabled efficiency of care delivery in 78% of conducted optimizations, suggesting that it may support feasibility of both establishing and sustaining teamwork. However, we did not evaluate direct impact of 4R on clinician time required to deliver the optimized care, which must be done in the future to address the inefficiency concern. Broadly, on the basis of this and previous 4R evaluations, we believe that 4R can contribute to sustainability of the oncology workforce by improving team functioning, reducing the burden of ad hoc coordination of interdependent care, and streamlining care delivery. Previous studies demonstrated that using 4R in the clinic increased clinicians' satisfaction and ability to manage multidisciplinary care,50 as well as improved patient self-management.41 These factors also support workforce viability and reduce burden. Future studies should thoroughly examine how the ongoing use of the overall 4R Oncology model affects oncology workforce.
This intervention was conducted at an integrated health system. However, our results are generalizable to other settings, such as nonintegrated systems, academic institutions, and accountable care organizations. The optimizations performed at KPNC addressed obstacles to teamwork common to other settings, such as siloed practices and challenges with interdependent care.4,5,8,9,42 4R Optimization was shown to be feasible, practical, and thus repeatable. Our intervention may serve as a blueprint for other institutions motivated to create high-functioning teams and optimize care delivery. The taxonomy of our results may help institutions frame intervention scope, including cancer types and care domains, identify needed optimizations, prioritize them on the basis of required effort, form a multidisciplinary team, and use relevant optimization methods to collaboratively conduct optimizations.
Our assessment had limitations. The intervention did not include important care domains, such as radiation therapy and survivorship, which will be addressed in future efforts. Our assessment did not evaluate impact on actual care at the patient level. Such evaluation is underway, and data from three optimizations indicate positive impact, including shortening turnaround time for molecular profiling before treatment decision in lung cancer,51 improving completion of advance care directives in breast cancer,52 and implementing pretreatment older adult assessment and referrals.53,54 We have not assessed intervention impact on clinical outcomes and hope to do so in the future.
In conclusion, we assessed how 4R Optimization, a component of the 4R Oncology model, affected team functioning in a community-based integrated health system. 4R fostered a large high-functioning team and enabled 40 optimizations of interdependent care in two cancers along the cancer care continuum in a feasible and practical way. Our results suggest that 4R may be an effective approach to teamwork and could contribute to viability of the oncology workforce. Our intervention and taxonomy of the results may serve as a blueprint for other institutions motivated to strengthen teamwork.
Lori C. Sakoda
Stock and Other Ownership Interests: Johnson & Johnson
Research Funding: AstraZeneca (Inst)
Julia R. Trosman
Consulting or Advisory Role: Genentech (Inst)
Research Funding: The Merck Foundation (Inst), Pfizer (Inst), National Comprehensive Cancer Network (Inst), Genentech (Inst)
Christine B. Weldon
Consulting or Advisory Role: Genentech (Inst)
Speakers' Bureau: Genentech
Research Funding: National Comprehensive Cancer Network (Inst), Pfizer (Inst), The Merck Foundation (Inst), Genentech (Inst), Lilly (Inst)
Travel, Accommodations, Expenses: Genentech (Inst)
Chun Ng
Employment: The Permanente Medical Group NorCal
Elaine Yu
Employment: Genentech/Roche
Stock and Other Ownership Interests: Roche
Research Funding: Genentech/Roche (Inst)
Patents, Royalties, Other Intellectual Property: Listed as inventor on a pending patent application for Display screen with icon or graphical user interface
Travel, Accommodations, Expenses: Genentech/Roche
Arliene Ravelo
Employment: Genentech/Roche
Stock and Other Ownership Interests: Roche
Raymond Liu
Research Funding: Genentech
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
The data described in the manuscript have not been previously presented or published.
SUPPORT
Kaiser Permanente and Center for Business Models in Health Care received funding for this project from Genentech, Inc. A.R. and E.Y. are employees of Genentech, Inc. C.W. and J.T. acknowledge funding support from NCI grant: UG1 CA189828, ECOG-ACRIN NCORP Research Base.
AUTHOR CONTRIBUTIONS
Conception and design: Christine B. Weldon, Nancy Gordon, Thea Abbe, Jed Katzel, Chun Ng, Megumi Tomita, Stephanie Ossowski, Sharon Likely Sprague, Arliene Ravelo, Elaine Yu, Julia R. Trosman
Financial support: Arliene Ravelo, Elaine Yu
Administrative support: Nancy Gordon
Provision of study materials or patients: Thea Abbe, Marti Hennings, Henie James, Megumi Tomita, Sharon Likely Sprague, Anna Dowling
Collection and assembly of data: Christine B. Weldon, Nancy Gordon, Thea Abbe, Marti Hennings, Henie James, Chun Ng, Megumi Tomita, Stephanie Ossowski, Sharon Likely Sprague, Anna Dowling, Kimberly Beringer, Arliene Ravelo, Elaine Yu, Julia R. Trosman
Data analysis and interpretation: Christine B. Weldon, Nancy Gordon, Lori C. Sakoda, Julia R. Trosman
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Fostering a High-Functioning Team in Cancer Care Using the 4R Oncology Model: Assessment in a Large Health System and a Blueprint for Other Institutions
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Lori C. Sakoda
Stock and Other Ownership Interests: Johnson & Johnson
Research Funding: AstraZeneca (Inst)
Julia R. Trosman
Consulting or Advisory Role: Genentech (Inst)
Research Funding: The Merck Foundation (Inst), Pfizer (Inst), National Comprehensive Cancer Network (Inst), Genentech (Inst)
Christine B. Weldon
Consulting or Advisory Role: Genentech (Inst)
Speakers' Bureau: Genentech
Research Funding: National Comprehensive Cancer Network (Inst), Pfizer (Inst), The Merck Foundation (Inst), Genentech (Inst), Lilly (Inst)
Travel, Accommodations, Expenses: Genentech (Inst)
Chun Ng
Employment: The Permanente Medical Group NorCal
Elaine Yu
Employment: Genentech/Roche
Stock and Other Ownership Interests: Roche
Research Funding: Genentech/Roche (Inst)
Patents, Royalties, Other Intellectual Property: Listed as inventor on a pending patent application for Display screen with icon or graphical user interface
Travel, Accommodations, Expenses: Genentech/Roche
Arliene Ravelo
Employment: Genentech/Roche
Stock and Other Ownership Interests: Roche
Raymond Liu
Research Funding: Genentech
No other potential conflicts of interest were reported.
REFERENCES
- 1.Levit L, Balogh E, Nass S, et al., editors. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Washington, DC, National Academies Press (US): 2013. [PubMed] [Google Scholar]
- 2. Gorin SS, Haggstrom D, Han PKJ, et al. Cancer care coordination: A systematic review and meta-analysis of over 30 years of empirical studies. Ann Behav Med. 2017;51:532–546. doi: 10.1007/s12160-017-9876-2. [DOI] [PubMed] [Google Scholar]
- 3. Weaver SJ, Jacobsen PB. Cancer care coordination: Opportunities for healthcare delivery research. Transl Behav Med. 2018;8:503–508. doi: 10.1093/tbm/ibx079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weldon CB, Trosman JR, Gradishar WJ, et al. Barriers to the use of personalized medicine in breast cancer. J Oncol Pract. 2012;8:e24–e31. doi: 10.1200/JOP.2011.000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chollette V, Beasley DD, Abdiwahab E, et al. Health information systems approach to managing task interdependence in cancer care teams. J Oncol Pract. 2017;13:154–156. doi: 10.1200/JOP.2016.020156. [DOI] [PubMed] [Google Scholar]
- 6.Nass SJ, Patlak M, Zevon E, et al., editors. The National Academies Collection: Reports Funded by National Institutes of Health. Washington, DC: National Academies Press (US); 2019. Developing and sustaining an effective and resilient oncology careforce: Proceedings of a workshop. [PubMed] [Google Scholar]
- 7. Kosty MP, Hanley A, Chollette V, et al. National Cancer Institute-American Society of Clinical Oncology teams in cancer care project. J Oncol Pract. 2016;12:955–958. doi: 10.1200/JOP.2016.018127. [DOI] [PubMed] [Google Scholar]
- 8. Taplin SH, Weaver S, Chollette V, et al. Teams and teamwork during a cancer diagnosis: Interdependency within and between teams. J Oncol Pract. 2015;11:231–238. doi: 10.1200/JOP.2014.003376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Trosman JR, Carlos RC, Simon MA, et al. Care for a patient with cancer as a project: Management of complex task interdependence in cancer care delivery. J Oncol Pract. 2016;12:1101–1113. doi: 10.1200/JOP.2016.013573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shulman LN, Sheldon LK, Benz EJ. The future of cancer care in the United States—Overcoming workforce capacity limitations. JAMA Oncol. 2020;6:327–328. doi: 10.1001/jamaoncol.2019.5358. [DOI] [PubMed] [Google Scholar]
- 11. Hlubocky FJ, Taylor LP, Marron JM, et al. A call to action: Ethics committee roundtable recommendations for addressing burnout and moral distress in oncology. JCO Oncol Pract. 2020;16:191–199. doi: 10.1200/JOP.19.00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takvorian SU, Balogh E, Nass S, et al. Developing and sustaining an effective and resilient oncology careforce: Opportunities for action. J Natl Cancer Inst. 2020;112:663–670. doi: 10.1093/jnci/djz239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kosty MP, Bruinooge SS, Cox JV. Intentional approach to team-based oncology care: Evidence-based teamwork to improve collaboration and patient engagement. J Oncol Pract. 2015;11:247–248. doi: 10.1200/JOP.2015.005058. [DOI] [PubMed] [Google Scholar]
- 14. Rosen MA, DiazGranados D, Dietz AS, et al. Teamwork in healthcare: Key discoveries enabling safer, high-quality care. Am Psychol. 2018;73:433–450. doi: 10.1037/amp0000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Van Houtven CH, Hastings SN, Colon-Emeric C. A path to high-quality team-based care for people with serious illness. Health Aff (Millwood) 2019;38:934–940. doi: 10.1377/hlthaff.2018.05486. [DOI] [PubMed] [Google Scholar]
- 16. Vogel AL, Hall KL. Creating the conditions for implementing team principles in cancer care. J Oncol Pract. 2016;12:964–969. doi: 10.1200/JOP.2016.018218. [DOI] [PubMed] [Google Scholar]
- 17.Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC, National Academies Press (US): 2001. [PubMed] [Google Scholar]
- 18.National Cancer Institute: NCI Healthcare Teams & Teamwork Processes in Cancer Care Delivery. 2022. https://healthcaredelivery.cancer.gov/healthcare/#:∼:text=The%20goal%20of%20the%20Healthcare,%2C%20and%20cancer%2Drelated%20outcomes . [Google Scholar]
- 19. Fennell ML, Das IP, Clauser S, et al. The organization of multidisciplinary care teams: Modeling internal and external influences on cancer care quality. J Natl Cancer Inst Monogr. 2010;2010:72–80. doi: 10.1093/jncimonographs/lgq010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taplin SH, Weaver S, Salas E, et al. Reviewing cancer care team effectiveness. J Oncol Pract. 2015;11:239–246. doi: 10.1200/JOP.2014.003350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prabhu Das I, Baker M, Altice C, et al. Outcomes of multidisciplinary treatment planning in US cancer care settings. Cancer. 2018;124:3656–3667. doi: 10.1002/cncr.31394. [DOI] [PubMed] [Google Scholar]
- 22. Osarogiagbon RU. Making the evidentiary case for universal multidisciplinary thoracic oncologic care. Clin Lung Cancer. 2018;19:294–300. doi: 10.1016/j.cllc.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 23. Oxenberg J, Papenfuss W, Esemuede I, et al. Multidisciplinary cancer conferences for gastrointestinal malignancies result in measureable treatment changes: A prospective study of 149 consecutive patients. Ann Surg Oncol. 2015;22:1533–1539. doi: 10.1245/s10434-014-4163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schmidt HM, Roberts JM, Bodnar AM, et al. Thoracic multidisciplinary tumor board routinely impacts therapeutic plans in patients with lung and esophageal cancer: A prospective cohort study. Ann Thorac Surg. 2015;99:1719–1724. doi: 10.1016/j.athoracsur.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 25. Freeman RK, Ascioti AJ, Dake M, et al. The effects of a multidisciplinary care conference on the quality and cost of care for lung cancer patients. Ann Thorac Surg. 2015;100:1834–1838. doi: 10.1016/j.athoracsur.2015.05.056. discussion 1838. [DOI] [PubMed] [Google Scholar]
- 26. Richardson B, Preskitt J, Lichliter W, et al. The effect of multidisciplinary teams for rectal cancer on delivery of care and patient outcome: Has the use of multidisciplinary teams for rectal cancer affected the utilization of available resources, proportion of patients meeting the standard of care, and does this translate into changes in patient outcome? Am J Surg. 2016;211:46–52. doi: 10.1016/j.amjsurg.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 27. Agarwal PD, Phillips P, Hillman L, et al. Multidisciplinary management of hepatocellular carcinoma improves access to therapy and patient survival. J Clin Gastroenterol. 2017;51:845–849. doi: 10.1097/MCG.0000000000000825. [DOI] [PubMed] [Google Scholar]
- 28. Serper M, Taddei TH, Mehta R, et al. Association of provider specialty and multidisciplinary care with hepatocellular carcinoma treatment and mortality. Gastroenterology. 2017;152:1954–1964. doi: 10.1053/j.gastro.2017.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smeltzer MP, Rugless FE, Jackson BM, et al. Pragmatic trial of a multidisciplinary lung cancer care model in a community healthcare setting: Study design, implementation evaluation, and baseline clinical results. Transl Lung Cancer Res. 2018;7:88–102. doi: 10.21037/tlcr.2018.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bilfinger TV, Albano D, Perwaiz M, et al. Survival outcomes among lung cancer patients treated using a multidisciplinary team approach. Clin Lung Cancer. 2018;19:346–351. doi: 10.1016/j.cllc.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 31. Ray MA, Faris NR, Fehnel C, et al. Survival impact of an enhanced multidisciplinary thoracic oncology conference in a regional community health care system. JTO Clin Res Rep. 2021;2:100203. doi: 10.1016/j.jtocrr.2021.100203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Meltzer C, Nguyen NT, Zhang J, et al. Survival associated with consolidated multidisciplinary care in head and neck cancer: A retrospective cohort study. Otolaryngol Head Neck Surg. doi: 10.1177/01945998211057852. [epub ahead of print on November 9, 2021] [DOI] [PubMed] [Google Scholar]
- 33. Osarogiagbon RU. "Like heart valve clinic, it probably saves lives, but… Who has time for that?" The challenge of disseminating multidisciplinary cancer care in the United States. Cancer. 2018;124:3634–3637. doi: 10.1002/cncr.31396. [DOI] [PubMed] [Google Scholar]
- 34. Kiggundu MN. Task interdependence and job design: Test of a theory. Organ Behav Hum Perform. 1983;31:145–172. doi: 10.1016/0030-5073(83)90118-6. [DOI] [PubMed] [Google Scholar]
- 35. Saavedra R, Earley PC, Van Dyne L. Complex interdependence in taskperforming groups. J Appl Psychol. 1993;78:61. [Google Scholar]
- 36. Langfred CW, Moye NA. Effects of task autonomy on performance: An extended model considering motivational, informational, and structural mechanisms. J Appl Psychol. 2004;89:934–945. doi: 10.1037/0021-9010.89.6.934. [DOI] [PubMed] [Google Scholar]
- 37. Wageman R. Interdependence and group effectiveness. Adm Sci Q. 1995;40:145–180. [Google Scholar]
- 38. Wageman R, Gardner H, Mortensen M. The changing ecology of teams: New directions for teams research. J Organ Behav. 2012;33:301–315. [Google Scholar]
- 39.Salas E, Dickinson T, Converse S, et al. Toward an understanding of team performance and training. In: Swezey R, Salas EE, editors. Teams: Their Training and Performance. New York, NY, Ablex Publishing; 1992. pp. 3–29. [Google Scholar]
- 40.Hackman JR. Leading Teams: Setting the Stage for Great Performances. Boston, MA, Harvard Business School Press; 2002. [Google Scholar]
- 41. Trosman JR, Weldon CB, Rapkin BD, et al. Evaluation of the novel 4R oncology care planning model in breast cancer: Impact on patient self-management and care delivery in safety-net and non-safety-net centers. JCO Oncol Pract. 2021;17:e1202–e1214. doi: 10.1200/OP.21.00161. [DOI] [PubMed] [Google Scholar]
- 42. Weldon CB, Friedewald SM, Kulkarni SA, et al. Radiology as the point of cancer patient and care team engagement: Applying the 4R model at a patient's breast cancer care initiation. J Am Coll Radiol. 2016;13:1579–1589. doi: 10.1016/j.jacr.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Trosman J, Weldon C, Kircher S, et al. Innovating cancer care delivery: The example of the 4R Oncology model for colorectal cancer patients. Curr Treat Options Oncol. 2019;20:11 doi: 10.1007/s11864-019-0608-7. [DOI] [PubMed] [Google Scholar]
- 44. Weaver SJ, Che XX, Petersen LA, et al. Unpacking care coordination through a multiteam system lens: A conceptual framework and systematic review. Med Care. 2018;56:247–259. doi: 10.1097/MLR.0000000000000874. [DOI] [PubMed] [Google Scholar]
- 45. Verhoeven DC, Chollette V, Lazzara EH, et al. The anatomy and physiology of teaming in cancer care delivery: A conceptual framework. J Natl Cancer Inst. 2021;113:360–370. doi: 10.1093/jnci/djaa166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chambers DA, Glasgow RE, Stange KC. The dynamic sustainability framework: Addressing the paradox of sustainment amid ongoing change. Implement Sci. 2013;8:117. doi: 10.1186/1748-5908-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chollette V, Weaver SJ, Huang G, et al. Identifying cancer care team competencies to improve care coordination in multiteam systems: A modified Delphi study. JCO Oncol Pract. 2020;16:e1324–e1331. doi: 10.1200/OP.20.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Woofter K, Kennedy EB, Adelson K, et al. Oncology Medical Home: ASCO and COA standards. JCO Oncol Pract. 2021;17:475–492. doi: 10.1200/OP.21.00167. [DOI] [PubMed] [Google Scholar]
- 49. Kedia SK, Ward KD, Collins AC, et al. "All boats will rise": Physicians' perspectives on multidisciplinary lung cancer care in a community-based hospital setting. Support Care Cancer. 2020;28:1765–1773. doi: 10.1007/s00520-019-04950-7. [DOI] [PubMed] [Google Scholar]
- 50.Trosman JR, Kulkarni SA, Baer RP, et al. Does the 4R Oncology model improve clinicians’ effectiveness in patient-facing planning of complex cancer care? J Clin Oncol 40, (suppl; abstr 1542)
- 51. Ossowski S, Neeman E, Borden C, et al. Improving time to molecular testing results in patients with newly diagnosed, metastatic non-small cell lung cancer (NSCLC) JCO Oncol Pract. 2022;18:e1874–e1884. doi: 10.1200/OP.22.00260. [DOI] [PubMed] [Google Scholar]
- 52.Ossowski S, Lyon L, Linehan ES, et al. Increasing advance directive completion within the 4R Oncology model in breast cancer patients prior to surgery in a racially diverse patient population. J Clin Oncol 40, (suppl; abstr e13511)
- 53.Shaia J, Liu R, Sun X, et al. Nurse navigator initiated geriatric assessments in hematology-oncology clinics. J Clin Oncol 40, (suppl; abstr 12051)
- 54.Arora A, Sun X, Shaia J, et al. G8 and CARG toxicity score can predict emergency room (ER) visits, hospitalizations, and mortality in older patients with newly diagnosed cancer. J Clin Oncol 40, (suppl; abstr 12055)