PURPOSE:
Timely surgical cavity stereotactic radiosurgery (SRS) is an important adjuvant to brain metastasis resection, with earlier treatment associated with less frequent recurrence. The logistical complexity of treatment organization, however, has resulted in suboptimal start times postsurgically. We implemented a process improvement approach to reduce the time from surgery to adjuvant irradiation of resected brain metastases.
METHODS:
A multidisciplinary working group used process mapping to identify opportunities to reduce visits and shorten treatment times. The care delivery process was modified to streamline perioperative SRS preparation with (1) early patient identification, (2) preoperative intrateam communication, and (3) consolidation of required steps. Plan-Do-Study-Act cycles were used for process improvement. The surgery-to-SRS initiation time interval was the primary outcome. Secondary outcomes included the number of associated patient encounters.
RESULTS:
After implementation, the median (interquartile range) interval from surgery to SRS was reduced 48% from 27 (21-34) to 14 days (13-17; P < .001). The rate of surgical cavity SRS within 30 days increased from 64% (n = 63 of 98) to 97% (n = 60 of 62; P < .001). The median (interquartile range) number of CNS-associated encounters between resection and SRS decreased from 5 (4-6) to 4 (3-5; P < .001). The proportion of patients who had > 1 magnetic resonance imaging/computed tomography between surgery and SRS decreased from 45% (44 of 98) to 13% (8 of 62; P < .001). The time from surgery to systemic therapy resumption/initiation among patients treated within 90 days postoperatively decreased from 35 (24-48) to 32 days (23-40; P = .074). There were no wound complications in either group.
CONCLUSION:
Adjuvant SRS latency and treatment-associated encounters were significantly reduced after care-coordination implementation. This approach reduces patient and health care system burden and can be applied to other scenarios where early postoperative SRS administration is critical.
INTRODUCTION
Surgical resection plus adjuvant irradiation is standard of care for the local control and palliation of large and/or symptomatic brain metastases in patients with good performance status and limited cancer burden.1,2 Such resection cavity irradiation markedly reduces recurrence risk from approximately 50%-20% but is most effective when delivered within 30 days postoperatively.3-6 In addition, systemic cancer-directed treatments are generally held until adjuvant irradiation completion, making rapid delivery important, given that shorter treatment breaks are associated with improved outcomes.7 Stereotactic radiosurgery (SRS), preferred over whole-brain radiation owing to higher conformality and reduced cognitive toxicity, requires several treatment planning steps that introduce logistical complexity which is magnified among recovering surgical patients.8 As many as seven steps including consenting, radiologic examinations, treatment simulation, and physics planning, plus wound healing and staple/suture removal, are required within this time frame.5 Such planning involves clinician, patient, and staff interdependencies, each of whom functions within myriad technical, logistical, and personal constraints. Consequently, even experienced centers report median adjuvant SRS starting approximately 30 days postoperatively, with 50% of patients thus treated beyond this important threshold.1,9 The objective of this project was to significantly reduce the proportion of brain metastasectomy patients receiving adjuvant SRS beyond 30 days postoperatively in a high-volume program using Plan-Do-Study-Act (PDSA) cycles. We hypothesized that a team-based approach to identify and coordinate care would expedite adjuvant SRS treatment.
METHODS
This quality improvement study received an informed consent waiver from the institutional review board and is reported following the Revised Standards for Quality Improvement Reporting Excellence guideline (SQUIRE 2.0).10
Local Context
This study was performed in a National Cancer Institute–designated Comprehensive Cancer Center comprising a main campus at which visits, procedures, neuroimaging, radiation planning, and delivery steps are performed and outpatient facilities with the above capabilities minus neurosurgery. A telemedicine platform, initially operationalized in the acute phase of the COVID-19 pandemic (Q2, 2020), allowed home-based patient encounters.
Resection Plus SRS Care Coordination Intervention
A working group of neurosurgeons, radiation oncologists, nurses, and medical physicists performed process mapping to identify opportunities to reduce visits and adjuvant radiation latency and the clinical disciplines involved in each step (Fig 1). We focused on three components to consolidate care: (1) performing a single, dual-use, high-resolution magnetic resonance imaging (MRI) (or computed tomography [CT] for patients with magnetic resonance/gadolinium contraindication) on postoperative day 1-2, instead of one on day 1-2 for immediate surgeon feedback plus a second radiation-planning scan after discharge, (2) creating SRS immobilization devices (masks) and simulating hospitalized patients recovering from surgery, allowing treatment planning overlap with wound healing, and (3) offering same-day staple/suture removal plus radiation delivery at outpatient facilities to optionally obviate one in-person visit with the surgical team.
FIG 1.
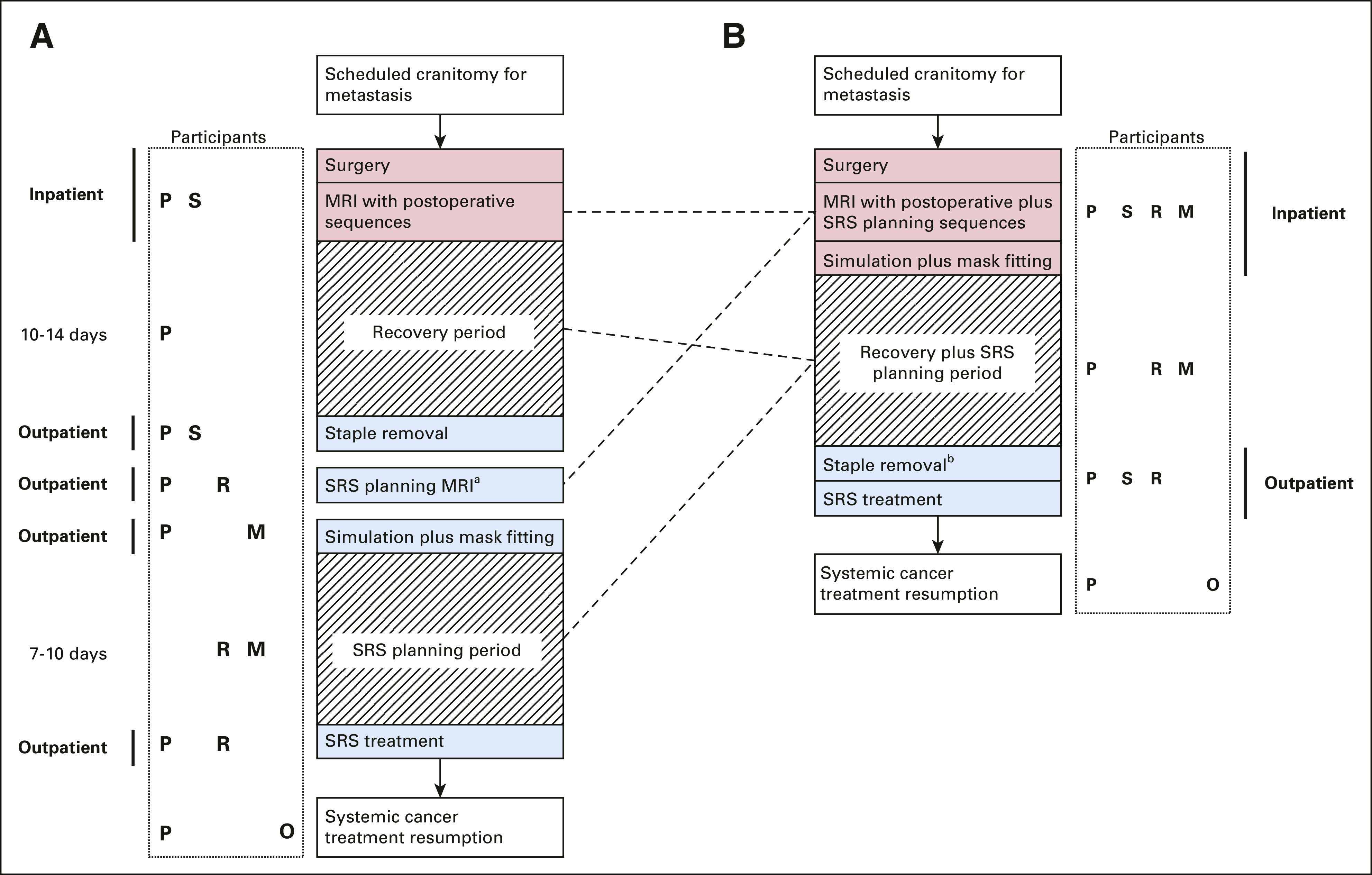
Key steps in brain metastasectomy patient journey (A) preimplementation and (B) postimplementation, with participants at each stage denoted by P, S, R, M, and O. aIf not done as inpatient; bOptionally coordinated to occur at satellite facility the same day as SRS treatment plus virtual postoperative surgeon visit. For patients where magnetic resonance or gadolinium is contraindicated, computed tomography is typically substituted for MRI. M, medical physics; MRI, magnetic resonance imaging; O, medical oncology; P, patient; R, radiation oncology; S, surgical team; SRS, stereotactic radiosurgery.
Implementation occurred in Q3, 2020. Clinical and program coordinator staff were educated on workflow changes and implementation timelines via meetings organized by the working group to create a shared mental model of the new clinical pathways. All patients scheduled for brain metastasectomy (both elective outpatients and inpatients requiring same admission resection) were screened by a dedicated coordinator for eligibility; patients without prior intended field irradiation and not planned for adjuvant irradiation elsewhere (eg, out-of-state) were included. The clinical team then selected one of the three pathways at tumor board meetings or via virtual email huddles between patients' multidisciplinary teams, considering patient-specific factors: (1) planned inpatient radiation consenting and simulation (default), (2) week one outpatient consultation with same-day simulation, or, rarely, (3) inpatient consultation, simulation, and treatment (for anticipated prolonged hospitalization or rapidly progressive tumors).11 The coordinator then communicated with the CNS radiation oncologist to preoperatively schedule these steps. Patients were educated on the workflow at the surgical counseling visit and reinforced during a preoperative coordinator call and by nurses postoperatively in the hospital. Process and outcome measures, and reasons for any delay > 30 days, were collected from treatment records. The comparator preimplementation period for retrospective review was Q1, 2019 until implementation.
Quality Improvement Framework
Two PDSA cycles were completed, with study and refinement after the first 10 and next 40 cases.
Measures
Process measure.
Simulation and mask fitting completion within 14 days postoperatively
Outcome measures.
Time from surgery to simulation, SRS start, and systemic cancer-directed therapy resumption/initiation
Number of CNS-related encounters before adjuvant SRS delivery (radiation oncology and neurosurgery visits, hospitalization, postoperative MRI/CT scans, and simulation).
Statistical Analysis
Differences in continuous variables were compared using the Wilcoxon rank sum test. Differences in proportions for categorical variables were compared using the Pearson chi-squared test.
Program Implementation and Refinement
After each PDSA cycle, physician, nurse, and medical physicist feedback were sought by working group members. Several unanticipated issues were identified after cycle 1: mask fitting was precluded in two patients owing to postoperative discomfort. Consequently, we introduced more routine long acting regional scalp blocks with liposomal bupivacaine for posterior incisions. In response to the feedback that bulky postsurgical head wrap dressings common in our practice sometimes precluded accurate mask fitting, new double dressings with a smaller subjacent dressing to protect the site during simulation, overlaid by a larger head wrap for removal presimulation, were introduced. Finally, to avoid lengthening hospitalizations for patients with Friday surgeries and otherwise ready for weekend discharge, we allowed outpatient simulations the week after surgery at the patient's nearest facility. After cycle 2, the project was expanded beyond an initial pilot to include additional radiation oncologists.
RESULTS
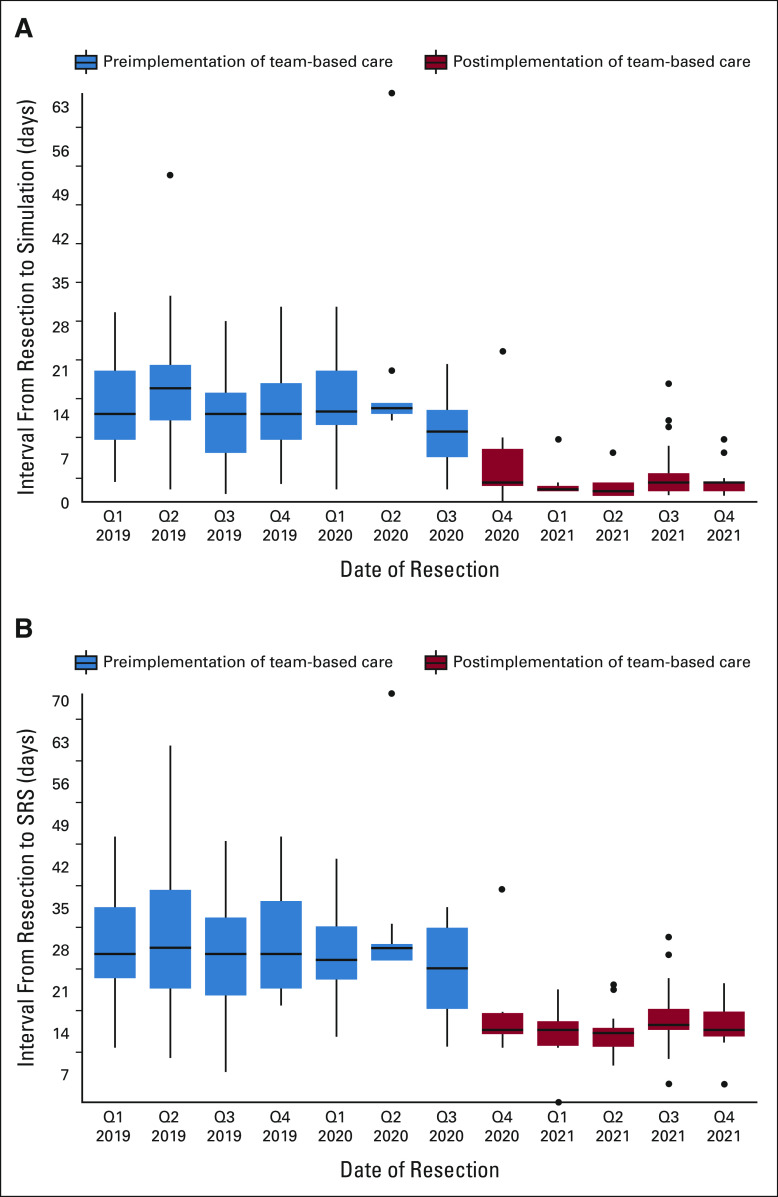
Ninety-eight consecutive preimplementation and 62 consecutive postimplementation patients were assessed; all survived at least to SRS. A similar proportion presented as inpatients (60% v 59%, respectively). The results are shown in Figure 2. The median (interquartile range [IQR]) time to SRS was reduced 48% from 27 (21-34) to 14 days (13-17; P < .001), and the proportion receiving SRS by 30 days increased from 64% (n = 63 of 98) to 97% (n = 60 of 62; P < .001). The simulation completion rate within 14 days increased from 52% (n = 51 of 98) to 97% (n = 60 of 62; P < .001). The median CNS-related encounters (IQR) were reduced from 5 (4-6) to 4 (3-5; P < .001), and the proportion of patients with > 1 MRI/CT between surgery and SRS decreased from 45% (44 of 98) to 13% (8 of 62; P < .001). The median postoperative hospitalization duration (IQR) was equivalent at 4 days (2-7 preimplementation; 3-7 postimplementation). Among patients who initiated or restarted systemic cancer therapies within 90 days postoperatively (n = 60 of 98 preimplementation, 32 of 62 postimplementation; P = .2), the median interval (IQR) from resection to systemic therapy was 35 days (24-48) preimplementation versus 32 (23-40) postimplementation (P = .074). The two postimplementation patients with > 30-day SRS latency had unrelated medical problems that took precedence over brain metastasis care. The median (IQR) follow-up among survivors in the postimplementation group was 9.1 (7.2-11.1) months and 28.0 (24.1-35.3) months in the preimplementation group. There were no issues with postoperative scalp edema altering mask fitting by SRS initiation and no wound healing complications by 3 months postoperatively in patients in either arm with follow-up to this point.
FIG 2.
Time (A) from surgery to SRS simulation and (B) from surgery to first SRS treatment. Q, quarter; SRS, stereotactic radiosurgery.
DISCUSSION
This project resulted in significantly reduced adjuvant SRS latency and near universal achievement of SRS within 30 days postoperatively, an important threshold for optimizing control of these tumors. In addition, reduced patient encounters and scans highlight this approach's efficiency for patients and health care systems. On a larger basis, this is likely to result in fewer nosocomial complications, and we anticipate that additional cost savings of potentially of reduced recurrence substantially outweighs the costs of care coordination and modified local anesthetic and dressing use patterns.12 Long-term clinical data are required to evaluate survival outcomes: We hypothesize that such an approach may even yield survival benefits in patients dependent on CNS control, particularly with shortened cancer treatment breaks.13 Importantly, we did not observe any wound healing risk with this initiative.
Strengths of this work include its generalizability. Although multidisciplinary teams confer improved outcomes including prolonged survival in breast, colorectal, and other cancers, this is the first description to our knowledge of multidisciplinary process integration for brain metastases directly improving practice efficiency.14,15 Achieving a shared goal required and resulted in team members understanding their roles, communicating clearly and effectively, and coordinating in a synergistic manner. We expect program implementation to be sustainable and increasingly seamless as providers become more familiar with the process and its utility. Furthermore, as brain metastasis treatment often requires surgery plus radiotherapy, many centers treating patients with cancer house both services, making such coordination transferable to other contexts; this importantly contributes to a lack of insurance denials delaying irradiation in our practice, although not necessarily all others. Achieving early-adjuvant SRS without inpatient planning has also been reported using same-day SRS planning-plus-treatment workflows (eg, with Gamma Knife or certain linear accelerator systems).16 Such care delivery modifications may also be warranted in other contexts, such as sarcomas where treatment at around 4 weeks postoperatively is recommended.17
This work benefited from an integrated cancer center context encompassing a compliant population, seven radiation planning and delivery facilities (with on-site nursing) in a three-state geography, and ready mask and digital record transfer. The process was further supported by a telemedicine platform reducing in-person visits, and a physics team able to render treatment plans within days of simulation. Availability of these processes could partially limit the generalizability of this work.
Existing literature supports high local control and low radiation-associated complication rates with accelerated adjuvant treatment. Although some studies suggest postoperative cavity contraction over time (which may reduce SRS treatment field sizes and consequently potentially alter the risk of radiation necrosis), this has not been shown to be clinically meaningful; regardless, this is an important area of further study.5,6,16,18 In addition, further study is needed to understand why systemic therapy resumption was not accelerated as much as adjuvant SRS (eg, patient fatigue or interdisciplinary communication), with potential interventions to improve this (eg, via symptom management or better patient education and coordination with medical oncologists). Additional prospective patient-reported insight is needed for further program improvement, to identify any unintended consequences (eg, out-of-pocket costs), and to ultimately improve outcomes and experiences. Finally, additional powering and follow-up are needed to understand effects of treatment sequencing and timing given widely disparate toxicity profiles among cancer treatments and their compatibility with SRS.
ACKNOWLEDGMENT
The authors wish to thank our patients, their families and other caregivers, and all providers who participated in brain metastasis care.
Nelson S. Moss
Consulting or Advisory Role: AstraZeneca
Research Funding: GT Medical Technologies (Inst)
Samantha Brown
Research Funding: AACR (Inst)
Justin Chen
Stock and Other Ownership Interests: MindMed, Moderna Therapeutics, Tonix Pharmaceuticals, Ocugen, Ocuphire Pharma, Innerscope Hearing Technologies, Heat Biologics
Brandon S. Imber
Honoraria: GT Medical Technologies
Luke Pike
Stock and Other Ownership Interests: Clovis Oncology, Schrodinger, Novavax
Consulting or Advisory Role: Blackstone, Deerfield Management, Third Rock Ventures, Aviko, Monograph Capital, Roivant, Galera Therapeutics, Dynamo Therapeutics, Myst Therapeutics, Turnstone Bio, Best Doctors Inc
Katherine S. Panageas
Stock and Other Ownership Interests: AstraZeneca, Pfizer, Sunesis Pharmaceuticals
Cameron Brennan
Stock and Other Ownership Interests: AVEO
Patents, Royalties, Other Intellectual Property: Co-inventor on patents licensed through Memorial Sloan Kettering to Elucida Oncology
Viviane Tabar
Stock and Other Ownership Interests: BlueRock Therapeutics (I)
Honoraria: BlueRock Therapeutics (I)
Consulting or Advisory Role: BlueRock Therapeutics (I)
Research Funding: BlueRock Therapeutics (I)
Patents, Royalties, Other Intellectual Property: BlueRock Therapeutics (I)
Travel, Accommodations, Expenses: BlueRock Therapeutics (I)
Kathryn Beal
Stock and Other Ownership Interests: MMT (I)
No other potential conflicts of interest were reported.
SUPPORT
Supported in part through the NIH/NCI Cancer Center Support Grant No. P30 CA008748.
AUTHOR CONTRIBUTIONS
Conception and design: Nelson S. Moss, Tarek Y. El Ahmadieh, Kathryn Beal
Administrative support: Nelson S. Moss
Provision of study materials or patients: Nelson S. Moss, Brandon S. Imber, Luke Pike, Cameron Brennan, Viviane Tabar, Kathryn Beal
Collection and assembly of data: Tarek Y. El Ahmadieh, Justin Chen
Data analysis and interpretation: Nelson S. Moss, Tarek Y. El Ahmadieh, Samantha Brown, Anne S. Reiner, Katherine S. Panageas
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Integrated Multidisciplinary Brain Metastasis Care Reduces Patient Visits and Shortens Time to Adjuvant Irradiation
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Nelson S. Moss
Consulting or Advisory Role: AstraZeneca
Research Funding: GT Medical Technologies (Inst)
Samantha Brown
Research Funding: AACR (Inst)
Justin Chen
Stock and Other Ownership Interests: MindMed, Moderna Therapeutics, Tonix Pharmaceuticals, Ocugen, Ocuphire Pharma, Innerscope Hearing Technologies, Heat Biologics
Brandon S. Imber
Honoraria: GT Medical Technologies
Luke Pike
Stock and Other Ownership Interests: Clovis Oncology, Schrodinger, Novavax
Consulting or Advisory Role: Blackstone, Deerfield Management, Third Rock Ventures, Aviko, Monograph Capital, Roivant, Galera Therapeutics, Dynamo Therapeutics, Myst Therapeutics, Turnstone Bio, Best Doctors Inc
Katherine S. Panageas
Stock and Other Ownership Interests: AstraZeneca, Pfizer, Sunesis Pharmaceuticals
Cameron Brennan
Stock and Other Ownership Interests: AVEO
Patents, Royalties, Other Intellectual Property: Co-inventor on patents licensed through Memorial Sloan Kettering to Elucida Oncology
Viviane Tabar
Stock and Other Ownership Interests: BlueRock Therapeutics (I)
Honoraria: BlueRock Therapeutics (I)
Consulting or Advisory Role: BlueRock Therapeutics (I)
Research Funding: BlueRock Therapeutics (I)
Patents, Royalties, Other Intellectual Property: BlueRock Therapeutics (I)
Travel, Accommodations, Expenses: BlueRock Therapeutics (I)
Kathryn Beal
Stock and Other Ownership Interests: MMT (I)
No other potential conflicts of interest were reported.
REFERENCES
- 1. Mahajan A, Ahmed S, McAleer MF, et al. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: A single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18:1040–1048. doi: 10.1016/S1470-2045(17)30414-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Soliman H, Ruschin M, Angelov L, et al. Consensus contouring guidelines for postoperative completely resected cavity stereotactic radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2018;100:436–442. doi: 10.1016/j.ijrobp.2017.09.047. [DOI] [PubMed] [Google Scholar]
- 3. Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: A randomized trial. JAMA. 1998;280:1485–1489. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 4. Iorio-Morin C, Masson-Côté L, Ezahr Y, et al. Early Gamma Knife stereotactic radiosurgery to the tumor bed of resected brain metastasis for improved local control. J Neurosurg. 2014;121(suppl):69–74. doi: 10.3171/2014.7.GKS141488. [DOI] [PubMed] [Google Scholar]
- 5. Bander ED, Yuan M, Reiner AS, et al. Durable 5-year local control for resected brain metastases with early adjuvant SRS: The effect of timing on intended-field control. Neurooncol Pract. 2021;8:278–289. doi: 10.1093/nop/npab005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roth O'Brien DA, Poppas P, Kaye SM, et al. Timing of adjuvant fractionated stereotactic radiosurgery affects local control of resected brain metastases. Pract Radiat Oncol. 2021;11:e267–e275. doi: 10.1016/j.prro.2021.01.011. [DOI] [PubMed] [Google Scholar]
- 7. Hanna TP, King WD, Thibodeau S, et al. Mortality due to cancer treatment delay: Systematic review and meta-analysis. BMJ. 2020;371:m4087. doi: 10.1136/bmj.m4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 9. Brennan C, Yang TJ, Hilden P, et al. A phase 2 trial of stereotactic radiosurgery boost after surgical resection for brain metastases. Int J Radiat Oncol Biol Phys. 2014;88:130–136. doi: 10.1016/j.ijrobp.2013.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): Revised publication guidelines from a detailed consensus process: Table 1. BMJ Qual Saf. 2016;25:986–992. doi: 10.1136/bmjqs-2015-004411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moss NS, Beal K, Tabar V. Brain metastasis-a distinct oncologic disease best served by an integrated multidisciplinary team approach. JAMA Oncol. 2022;8:1252–1254. doi: 10.1001/jamaoncol.2022.1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goodman RA, Solomon SL. Transmission of infectious diseases in outpatient health care settings. JAMA. 1991;265:2377–2381. [PubMed] [Google Scholar]
- 13. Sperduto PW, Mesko S, Li J, et al. Survival in patients with brain metastases: Summary report on the updated diagnosis-specific graded prognostic assessment and definition of the eligibility quotient. J Clin Oncol. 2020;38:3773–3784. doi: 10.1200/JCO.20.01255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kesson EM, Allardice GM, George WD, et al. Effects of multidisciplinary team working on breast cancer survival: Retrospective, comparative, interventional cohort study of 13 722 women. BMJ. 2012;344:e2718. doi: 10.1136/bmj.e2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bilfinger TV, Albano D, Perwaiz M, et al. Survival outcomes among lung cancer patients treated using a multidisciplinary team Approach. Clin Lung Cancer. 2018;19:346–351. doi: 10.1016/j.cllc.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 16. Jagannathan J, Yen CP, Ray DK, et al. Gamma Knife radiosurgery to the surgical cavity following resection of brain metastases: Clinical article. J Neurosurg. 2009;111:431–438. doi: 10.3171/2008.11.JNS08818. [DOI] [PubMed] [Google Scholar]
- 17. Dickie CI, Haas R, O'Sullivan B. Adjuvant radiation for soft tissue sarcomas. Am Soc Clin Oncol Ed Book. 2015;35:e634–e642. doi: 10.14694/EdBook_AM.2015.35.e634. [DOI] [PubMed] [Google Scholar]
- 18. Atalar B, Choi CYH, Harsh GR, et al. Cavity volume dynamics after resection of brain metastases and timing of postresection cavity stereotactic radiosurgery. Neurosurgery. 2013;72:180–185. doi: 10.1227/NEU.0b013e31827b99f3. [DOI] [PubMed] [Google Scholar]