PURPOSE
Atezolizumab plus bevacizumab treatment is a first-line therapy for unresectable hepatocellular carcinoma (HCC) worldwide. The efficacy, safety, and patient-reported outcomes (PROs) of HCC in Thailand have not yet been reported. This study aimed to evaluate the efficacy, safety, and PROs of atezolizumab plus bevacizumab.
MATERIALS AND METHODS
From September 2020 to August 2021, 30 patients with unresectable HCC who met the inclusion criteria of atezolizumab plus bevacizumab as first-line treatment were enrolled. Analysis was assessed for progression-free survival, overall survival, adverse events (AEs), and quality of life (QoL).
RESULTS
The median progression-free survival and overall survival periods were 6.7 and 10.2 months, respectively. The disease control rate was 63.3%. The frequent AEs were proteinuria, hypertension, and hepatitis. Serious AEs included gastrointestinal bleeding, but none of the patients died from serious AEs. The discontinuation rate was 23.3%, and the median number of treatment cycles was 10.5 cycles. In total, 23.3% of the patients continued treatment after 1 year of therapy. The global health status/QoL and physical function scores showed less deterioration at baseline than at 3 and 6 months (median scores = 76.7, 71.6, and 64.1 in QoL and 84.7, 79.6, and 79.0 in physical function, respectively). The HCC18 symptom score index data showed a slow progression of symptom scores from baseline to 3 and 6 months (12.7, 19.6, and 22.3, respectively).
CONCLUSION
This study demonstrates that atezolizumab plus bevacizumab is effective and has a safety profile comparable with that of previous studies as first-line therapy for unresectable HCC in a real-world setting and in Thai populations. Data on PROs also demonstrate benefits in terms of patients' QoL and symptoms.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common cancer of primary liver malignancy and the leading cause of mortality in patients with cancer worldwide, including the Asia-Pacific region, with approximately 800,000 deaths annually.1 HCC arises predominantly from liver cirrhosis but can also be diagnosed in patients with chronic hepatitis B or nonalcoholic steatohepatitis (NASH) who carry metabolic and genomic factors.2,3 Screening protocols (), advanced diagnostic tools, and curative therapies for HCC have been developed over the past decade. Overall survival (OS) remains poor in advanced-stage or unresectable HCC. Numerous randomized controlled clinical studies have been conducted to assess the effectiveness of treatments for advanced HCC.4 Historical studies have been conducted to demonstrate the efficacy of cancer chemotherapy as a single agent or in combination. However, this class of cancer therapy has had no proven benefits on OS in advanced-stage HCC.5,6 Sorafenib, a multityrosine kinase inhibitor with antiangiogenic effects, has shown a survival benefit and has been established as a first-line systemic therapy for patients with advanced HCC or progression from locoregional therapy since 2007.7
CONTEXT
Key Objective
Atezolizumab plus bevacizumab is an emerging first-line standard therapy in unresectable hepatocellular carcinoma (HCC) worldwide. Real-world evidence of efficacy, safety, and patient-reported outcomes needs to be explored, especially in Southeast Asia that has high prevalence of HCC.
Knowledge Generated
This combination therapy in Thai population demonstrated a progression-free survival of 6.7 months and overall survival of 10.2 months that is comparable with pivotal study. Safety profile with patient-reported outcomes also demonstrated manageable and quality-of-life outcomes.
Relevance
This combination therapy is effective for treatment unresectable HCC in Thailand. Although, a country with limited resources and/or budget might lead to poor outcomes of treatment compared with worldwide nation, effective systemic therapy can improve survival and quality of life, which this information may help to set policy of guideline treatment.
New systemic treatments for advanced HCC as first-and second-line systemic treatments have increased in recent years.8 Moreover, these new agents have been approved for advanced-stage HCC and have indicated a cumulative median OS with good liver function and quality of life (QoL). The combination therapy of atezolizumab, a monoclonal antibody targeting programmed death ligand-1, and bevacizumab, a monoclonal antibody against vascular endothelial growth factor, as a first-line treatment for advanced HCC compared with sorafenib revealed a significantly prolonged progression-free survival (PFS), OS, and slow deterioration of QoL in a phase III study.9 To date, combination therapies with atezolizumab plus bevacizumab have changed the paradigm of the standard of care in advanced HCC.
This study aimed to evaluate the overall therapeutic outcomes, safety, and patient-reported outcomes (PROs) of the initial experience of atezolizumab plus bevacizumab for advanced or unresectable HCC as first-line therapy by assessing a prospective cohort of Thai patients with HCC in a real-world clinical practice setting.
MATERIALS AND METHODS
Study Design and Patients
This prospective clinical study evaluated 30 patients with advanced-stage or unresectable HCC treated with atezolizumab plus bevacizumab between September 2020 and August 2021. This was a multicenter study from 10 institutes in Thailand. Patients who met the inclusion criteria for atezolizumab plus bevacizumab as the first-line treatment were enrolled in this study. The inclusion criteria were as follows: HCC diagnosed by radiological evaluation using dynamic imaging, such as computed tomography or magnetic resonance imaging, combined with serum tests for tumor markers or pathologic confirmation, age older than 18 years, Barcelona Clinic Liver Cancer stage C or failure to locoregional therapy, Child–Turcotte–Pugh (CTP) score 5-6 (only class A), and Eastern Cooperative Oncology Group Performance Status (PS) score 0-2. All patients were evaluated for esophageal and gastric varices in an upper gastrointestinal study. When detected or if high-risk bleeding was present, the patient was treated according to the local clinical practice before starting treatment. Among the key exclusion criteria were patients with a known history of autoimmune disease, uncontrolled, or untreated hepatitis B or C, and varices (esophageal and gastric varices). Full eligibility criteria are provided in the Protocol.
The baseline characteristics, therapeutic responses, radiological findings, adverse events (AEs), and PROs were analyzed. Clinical characteristics, including sex, age, HCC etiology, laboratory test results, CTP score, alpha-fetoprotein (AFP) level, tumor size, and previous treatment history, were collected.
Treatment Protocol
Patients received 1,200 mg of atezolizumab plus 15 mg/kg of bevacizumab once daily intravenously every 3 weeks. All patients received the treatment until disease progression or unacceptable AEs. Treatment could continue even beyond tumor progression if a clinical benefit was observed. Patients who developed AEs were allowed to reduce the dose or continue receiving monotherapy with either atezolizumab or bevacizumab according to the toxicity profile.
Evaluation of Therapeutic Response, Safety Profile, Quality of Life, and Follow-Up Schedule
Tumor response was assessed in terms of PFS and OS. PFS, defined as time from screening to the first occurrence of disease progression or death from any cause (whichever occurs first), and OS, defined as time for screening to death from any cause. Response was assessed using computed tomography or magnetic resonance imaging every 8-12 weeks after the initiation of treatment. The response was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) v1.110 through an independent investigation review. Objective response rate (ORR) was assessed as complete response and partial response. Disease control rate (DCR) was assessed by ORR and stable disease (SD).
AEs were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. AEs were recorded during every cycle of therapy. Treatment was interrupted when any AEs were grade 3 or higher or unacceptable AEs were grade 2. The interruption of treatment was observed until AEs recovered to an acceptable grade and then continuous treatment was allowed.
QoL was assessed using a specific questionnaire for cancer and HCC using the EuroQol 5 Dimension 5 Level (EQ-5D-5L), European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire (EORTC-QLC C30), and HCC18 at the initial treatment and every 12 weeks until disease progression occurred or follow-up until patient death. This study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee. Written informed consent was obtained from all the patients.
Statistical Analyses
All statistical analyses were performed using Stata version 15.0 (StataCorp, College Station, TX). The results are expressed as mean ± standard deviation or median (range) and frequencies (%) to describe the baseline characteristics and AEs. The Kaplan-Meier test was used to estimate the probability of OS and PFS as the median time to OS and PFS after receiving treatment. A mixed-effect linear regression model was applied to EQ-5D-5L, EORTC-QLC C30, and HCC18. Statistical significance was set at P < .05.
RESULTS
Thirty patients diagnosed with advanced HCC were administered atezolizumab plus bevacizumab during the study period. All patients were analyzed for efficacy, AEs, and PROs. The median age of the patients was 58 years, and 90% of them were men. Eastern Cooperative Oncology Group PS was predominant in one (90%) patient. All patients had CTP A and Barcelona Clinic Liver Cancer stage C. The median largest tumor size was 6.3 cm. The etiologies of liver cirrhosis were chronic hepatitis B (63.3%), alcoholic hepatitis (16.7%), chronic hepatitis C (10%), and NASH (10%). Sixty percent of patients had a history of fully treated varices before treatment (intervention and medication therapy). Fifty-six percent of patients had distant metastases. The most common sites of metastasis were lung metastasis and intra-abdominal lymphadenopathy in 64.7% and 17.6% of the patients, respectively. Fifty-six percent of the patients had portal vein thrombosis. Sixty-six percent of the patients had previous locoregional therapy, and 50% had previous therapy with transarterial chemoembolization (Table 1).
TABLE 1.
Patient Characteristics
Overall Efficacy Outcomes of Atezolizumab Plus Bevacizumab
The median number of cycles of treatment was 10.5 cycles. The median follow-up period was 10.1 months (95% CI, 6.9 to not applicable). Seven patients received atezolizumab plus bevacizumab therapy 1 year after the initial treatment. The overall median PFS and OS periods were 6.7 and 10.2 months (95% CI, 5.1 to 10.5 and 5.1 to not applicable), respectively (Fig 1). The radiological therapeutic response to combination therapy is shown in Figure 2. In the RECIST evaluation, partial response and SD were observed in seven patients (23.3%) and 12 (40%) patients, respectively. The overall DCR was 63.3%. Progressive disease was observed in 11 (36.7%) patients, and none of the patients had a complete response. Four patients (36% of patients with progressive disease) had continuous therapy after disease progression by imaging evaluation but clinical benefit (clinical, laboratory, and QoL) of treatment.
FIG 1.
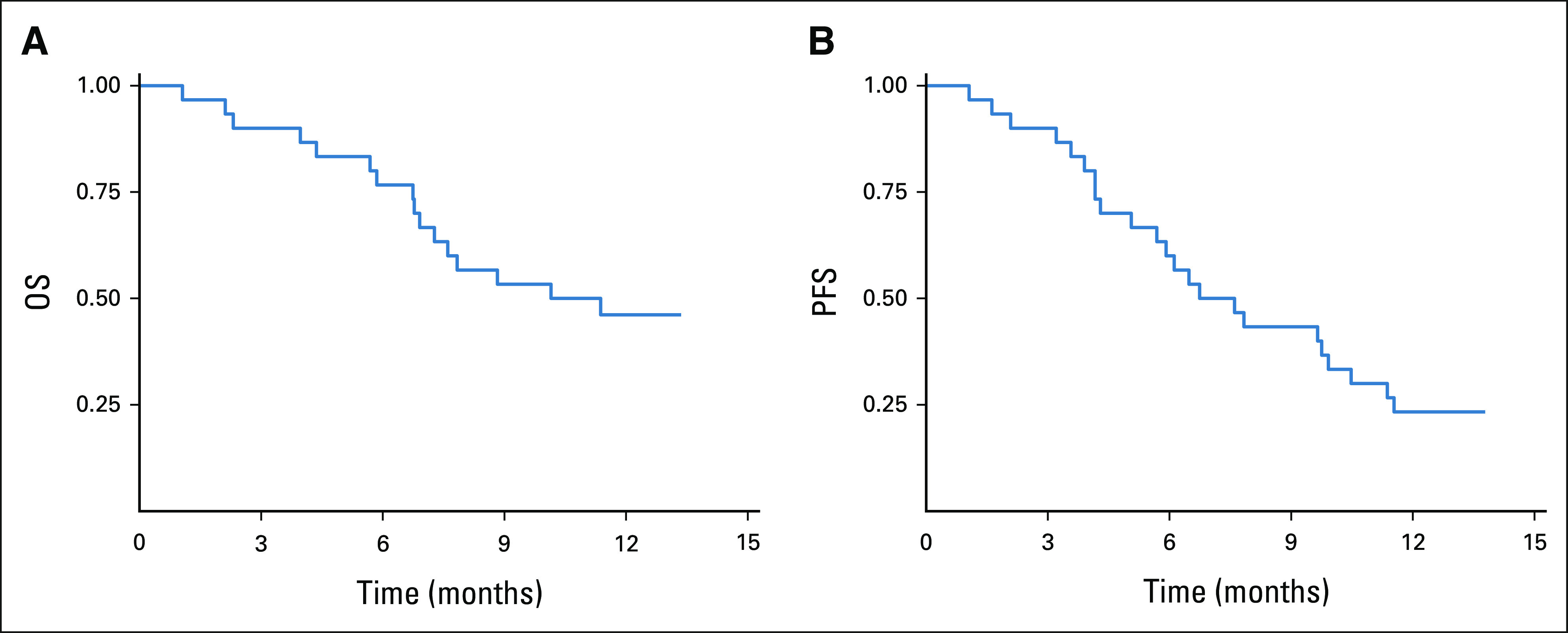
(A) OS and (B) PFS curves of the patients treated with atezolizumab plus bevacizumab. OS, overall survival; PFS, progression-free survival.
FIG 2.
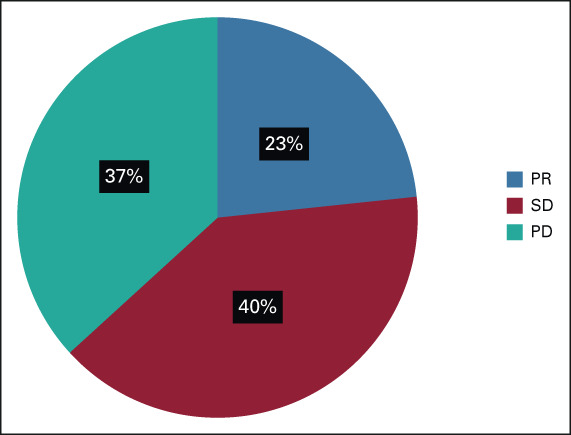
Assessment of the overall response rate using the Response Evaluation Criteria in Solid Tumors. PD, progressive disease; PR, partial response; SD, stable disease.
Overall Safety Outcomes
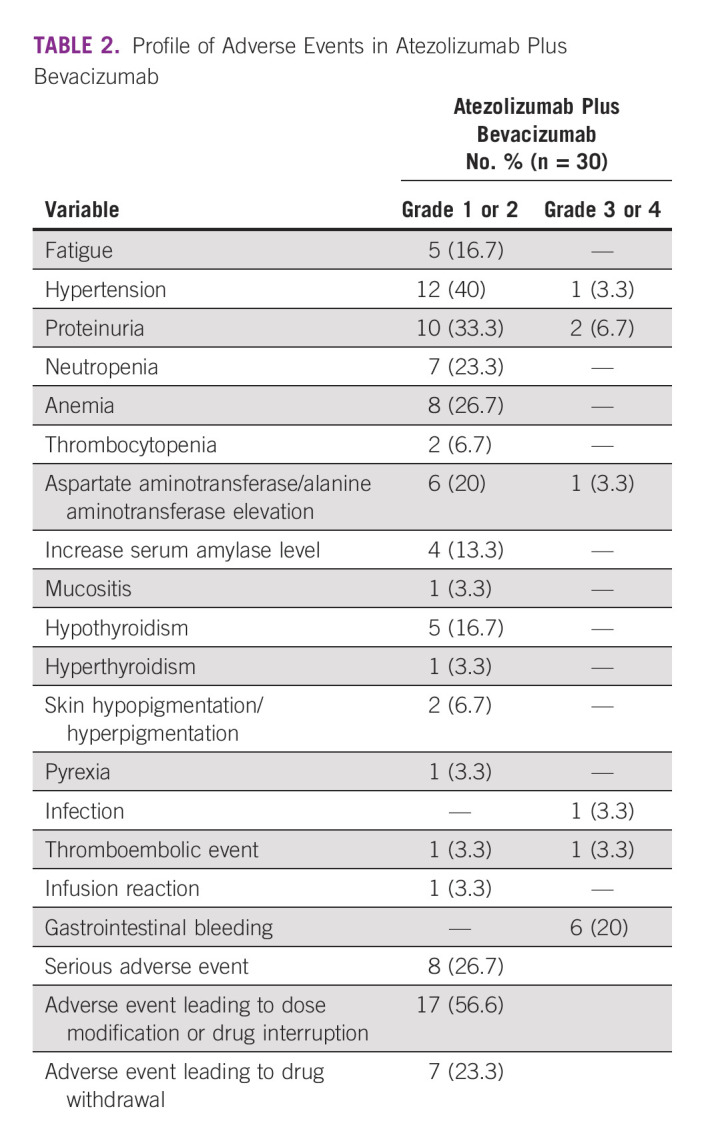
The treatment-related AEs are presented in Table 2. The overall incidence rate of AEs above grade 3 was 20% (n = 6). The most frequent AEs of any grade were hypertension, followed by proteinuria, hematologic toxicities (anemia and neutropenia), aspartate aminotransferase (AST) and alanine aminotransferase elevation, and hypothyroidism. The most frequent grade 3 AE was gastrointestinal bleeding in six (20%) patients. The observed immune-related AEs were commonly found in 16.7% of hypothyroidism without severe toxicities. Serious AEs occurred in eight (26.7%) patients, and most of the AEs were gastrointestinal bleeding, sepsis, and thromboembolic events without any death from these serious AEs. AEs leading to dose modification or interruption and withdrawal of drugs were observed in 56.6% (n = 17) and 23.3% (n = 7) of the patients, respectively. One patient died due to an infection unrelated to the drug, and none of the patients died from an AE (Table 2).
TABLE 2.
Profile of Adverse Events in Atezolizumab Plus Bevacizumab
Patients-Reported Outcomes
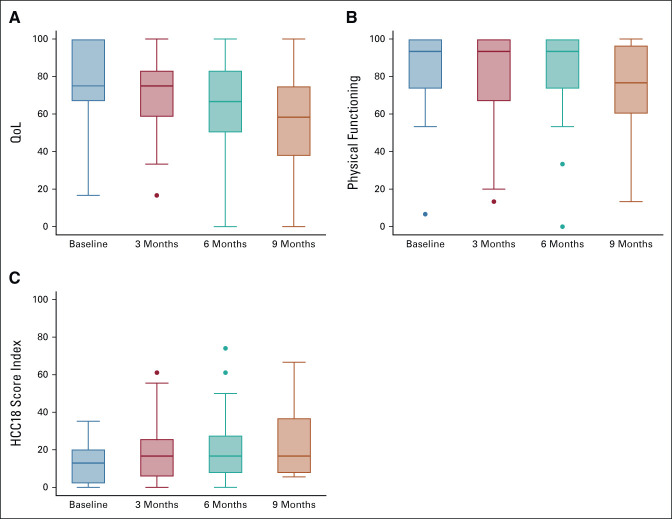
QoL and PROs of atezolizumab plus bevacizumab in this study were assessed using a specific questionnaire for cancer and HCC by EQ-5D-5L, EORTC-QLC C30, and HCC18. The mean global health status/QoL scores were 76.67, 71.57, 64.10, and 55.20 at baseline, 3, 6, and 9 months, respectively. The coefficients at baseline and 3 months were not significantly different (–5.58; 95% CI, –16.44 to 5.27), and when compared with baseline, the coefficients at 6 and 9 months were significantly lower (–17.82; 95% CI, –29.81 to –5.84 and –33.46; 95% CI, –47.83 to –19.06, respectively). Similarly, the physical function scores were 84.67, 79.61, 78.97, and 72.5 at baseline, 3, 6, and 9 months, respectively. The score was statistically maintained from baseline to 3 months (–5.96; 95% CI, –15.27 to 3.34) and significantly declined at 6 and 9 months (–12.58; 95% CI, –22.87 to –2.29 and –23.76; 95% CI, –36.18 to –11.40, respectively). In addition, the HCC symptom score index slowly increased from 12.69, 19.63, 22.31, and 23.06 at baseline, 3, 6, and 9 months, respectively (Fig 3).
FIG 3.
(A) The global health status/QoL and (B) physical function score based on the EORTC-QLC C30 and (C) HCC symptoms score index using the EORTC-QLQ-HCC18 at baseline, 3, 6, 9, and 12 months of atezolizumab and bevacizumab treatment. EORTC-QLC C30, European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire; HCC, hepatocellular carcinoma; QoL, quality of life.
DISCUSSION
Atezolizumab and bevacizumab are first-line standard therapies for advanced-stage/unresectable HCC. We reported the initial real-world evidence of the use of atezolizumab plus bevacizumab for unresectable HCC in Thai patients with unresectable HCC. Thus, combination therapy is feasible and effective. The 6.7-month median PFS period in this study was similar to that reported in a landmark phase III study (6.8 months).8 Although the treatment protocol was consistent with that of IMbrave150, our study evaluated tumor response every 8-12 weeks. While the 6-week interval of the phase III trial may not have been feasible in real life, it's worth considering the timing of assessment response, especially in terms of PFS, with same result of progression. Furthermore, the median OS period in this study was 10.2 months shorter than that in the pivotal trial (19.2 months).11 In addition, the 12-month survival rate in our patients was lower than that in patients in the pivotal trial (23.3% v 67%). This result may be explained by the fact that in our study, that is the real-world data. The patient characteristic in this study shows that most patients (90%) were ECOG PS1, while in the IMbrave150 trial only 38% of patients treated with atezolizumab plus bevacizumab had ECOG PS1. BCLC stage C was reported for 100% of patients, as compared to roughly 80% in IMbrave with higher number of patients had previous locoregional therapy. This result might be affected that real-world patients has more aggressive factors than in clinical trial. Twenty percent of patients received subsequent therapy after disease progression in atezolizumab plus bevacizumab because of problems with reimbursement molecular targeting agent (MTA) in our country, and the Child–Pugh score with PS decreased after progression, which might affect the OS period. However, the overall treatment response in our real-life study was 23.3%, which is nearly the same as that in the phase III study, which revealed a treatment response of 27%.9 In addition, the DCR was similar to that reported in the IMbrave 150 study (63.3% v 74%).
The most common infection experienced by the patients in our study, which represents the Asia-Pacific population, was viral hepatitis. Most of the patients in this study had viral hepatitis B (63.3%), viral hepatitis C (10%), and nonviral hepatitis (26.7%), which were the causes of HCC. The rate of viral hepatitis B in this study is higher than that in the IMbrave study (viral hepatitis B, 49%). This might have resulted in the shortened survival in our data because of different geographic data, socioeconomic status, and standard of care and supportive care of patients with cirrhosis in our population. However, the exploratory subgroup analysis of the IMbrave 150 study favored immunotherapy for patients with viral hepatitis infection over nonviral hepatitis in terms of PFS and OS.12 The emerging result of the poor effectiveness of immunotherapy in patients with NASH/nonalcoholic fatty liver disease with HCC is most probably due to an altered immune environment.13 Furthermore, subgroup analysis needs to be explored.
Compared with real-world evidence data, data in Asian patients with advanced-stage HCC who receive atezolizumab plus bevacizumab have limitations. Regarding real-world evidence publication data, mostly from Japan and Korea, Ando et al investigated early tumor response and safety in 40 patients with HCC who were treated with atezolizumab plus bevacizumab. They found an ORR of 22.5% on the basis of modified RECIST, but 24 patients in this study had previous treatment with MTA.14 Hiraoka et al evaluated 171 patients with HCC from Japan with an early response at 6 weeks of atezolizumab plus bevacizumab. Ninety-six patients underwent systemic therapy. The ORR and DCR at 6 weeks in this study were 10.6% and 79.6%, respectively.15 Hayakawa et al reported 52 patients undergoing atezolizumab/bevacizumab treatment with ORR and DCR (15.4% and 57.7%, respectively), but only 23 patients received atezolizumab/bevacizumab as the first-line therapy. They suggested AFP response to be a predictive marker.16
In terms of PFS evidence data, Iwamoto et al retrospectively analyzed 61 patients receiving atezolizumab/bevacizumab with a PFS period of 5.4 months. A DCR of 86.3% was observed in this study, and AE rates were higher than grade 3 (29.4%). However, 23 (62.7%) patients had prior experience with at least one line of MTA, hampering direct comparison.17 In Korea, Cheon et al retrospectively reviewed 121 patients with HCC treated with atezolizumab/bevacizumab as first-line therapy. The ORR and DCR were 24% and 76%, respectively, with a median PFS period of 6.5 months, and the median OS was not reached at the time of analysis. AEs with grades 3 and 4 in this study were observed in 28.9% of the patients, and the most frequent was AST elevation (10.7%). The dose interruption rate was only 8.3%. They suggested that AFP elevation, best response to SD or progressive disease, and baseline neutrophil-to-lymphocyte ratio ≥ 5 were associated with poor PFS.18 The Asian real-world evidence and our study are comparable outcomes of treatment with pivotal studies.
Safety profiles of atezolizumab and bevacizumab in the IMbrave 150 trial were assessed. The most common grade AEs were hypertension, fatigue, proteinuria, and AST elevation, and 56.5% of patients had grade 3 and 4 toxicities. The most common grade 3 and 4 AEs were hypertension and AST elevation, which corresponded to our study and other real-world studies. We documented treatment discontinuation because of AE in seven (23.3%) patients, which also fit the trial data. Gastrointestinal bleeding is a major cause of AEs in patients receiving a bevacizumab-containing regimen. Approximately 3% of patients experience gastrointestinal bleeding, which leads to discontinuation. Although we assessed the high risk of bleeding with esophagogastroduodenoscopy before the initiation of atezolizumab/bevacizumab and intervention or medication control, our study showed gastrointestinal bleeding in six (20%) patients, which seemed to be higher than that in the IMbrave study and other studies (approximately 3%-7%), requiring the discontinuation of bevacizumab. This is probably due to the limitation of the number of patients and less strict patient inclusion and exclusion criteria for treatment in real-life settings, according to local clinical practice for evaluating esophageal or gastric varices. However, none of the patients in this study died from these AEs. As recently reported, an important AE seems to be hypertension in atezolizumab/bevacizumab-treated patients (up to 30% of patients with grade 3),19 which was reported at a significantly lower frequency in our study, and this may implicate underdiagnosis in real-life practice.
PROs of the IMbrave 150 study were published separately and showed benefits in terms of QoL, function, and disease symptoms with atezolizumab/bevacizumab compared with sorafenib.20 Our study reported that comparable outcomes with atezolizumab/bevacizumab could maintain the QoL (mean global health status score), physical function, and HCC symptoms during therapy before progression and prolong time to deterioration.
Our study has some limitations. Although this was a multicenter and prospective observational study, this study had a small sample size because of limited reimbursement of atezolizumab/bevacizumab in our country and different standards of care in local clinical practice guidelines. Country-specific reasons are a limitation of reimbursement in systemic therapy. Most patients have more clinical symptoms, prior locoregional treatment (transarterial chemoembolization beyond progression or distant metastasis), higher BCLC stage, and subsequential systemic therapy after progression that might affect the overall survival that shortens time compared to a pivotal study. The strength of this study was that this was a multicenter prospective study and the first study in the Asia-Pacific region, where HCC is common. The present study partly confirmed the effectiveness and AEs associated with PROs (QoL, functions, and symptoms) of atezolizumab/bevacizumab in patients with HCC. Thus, further accumulation of treatment analyses for a larger population is required to confirm its effectiveness.
In conclusion, the combination of atezolizumab and bevacizumab in this study demonstrates real-life efficacy and safety profiles in Thai patients with HCC compared with other studies. Gastrointestinal bleeding is a major AE associated with this drug combination therapy. Careful assessment before therapy and follow-up is required. Data on PROs also demonstrate benefits in terms of patients' QoL, physical function, and HCC symptoms.
ACKNOWLEDGMENT
The authors acknowledge Thai Society of Clinical Oncology (TSCO) for funding and education. The authors would like to thank MedResNet (Medical Research Network for Social Co, Ltd), Thailand for data management and support. Finally, the authors were most appreciative for all patients' participation in this research.
Chanchai Charonpongsuntorn
Research Funding: AstraZeneca
Suebpong Tanasanvimon
Honoraria: Eisai, MSD, Roche Canada, Bristol Myers Squibb/Celgene, Amgen, Lilly, Merck, Novartis, Ipsen, AstraZeneca
Consulting or Advisory Role: Eisai, MSD, Bristol Myers Squibb/Celgene, Merck, Amgen
Travel, Accommodations, Expenses: Fresinius Krabi
Ekkamol Phaibulvatanapong
Honoraria: Roche
Consulting or Advisory Role: Roche
Naiyarat Prasongsook
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Roche, Novartis, Bristol Myers Squibb/Celgene
Travel, Accommodations, Expenses: AstraZeneca, Roche
Nuttapong Ngamphaiboon
Consulting or Advisory Role: MSD, Novartis, Amgen, Eisai, Merck
Speakers' Bureau: Roche, Eisai, MSD, Novartis
Research Funding: MSD (Inst), Pfizer (Inst), Roche (Inst), Exelixis (Inst), RAPT Therapeutics (Inst), BeiGene (Inst)
Travel, Accommodations, Expenses: Roche
Ekaphop Sirachainan
Honoraria: MSD, Sanofi/Aventis, Merck, Amgen, Roche, Mundipharma, AstraZeneca, LF Asia, Diethelm Keller Group, Bristol Myers Squibb, Boehringer Ingelheim, Taiho Pharmaceutical, Dr Reddy's Laboratories, Zuellig Pharma, Meda Pharmaceuticals, Pfizer, Novo Nordisk, Novartis
Consulting or Advisory Role: Roche, Taiho Oncology, MSD Oncology, Novartis, Bristol Myers Squibb/Celgene
Travel, Accommodations, Expenses: MSD, AstraZeneca, Pfizer, Amgen, American Taiwan Biopharm, Taiho Pharmaceutical, Eisai
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented as an oral presentation at The Japanese Society of Medical Oncology Annual meeting (JSMO2022), February 17, 2022, Kyoto, Japan.
CLINICAL TRIAL INFORMATION
TCTR20200921003
AUTHOR CONTRIBUTIONS
Conception and design: Chanchai Charonpongsuntorn, Suebpong Tanasanvimon, Songwit Payapwattanawong, Jitlada Juengsamarn, Nuttapong Ngamphaiboon, Ekaphop Sirachainan
Administrative support: Chanchai Charonpongsuntorn, Suebpong Tanasanvimon, Teerada Siripoon
Provision of study materials or patients: Chanchai Charonpongsuntorn, Suebpong Tanasanvimon, Krittiya Korphaisarn, Songwit Payapwattanawong, Teerada Siripoon, Nussara Pakvisal, Naiyarat Prasongsook, Ekaphop Sirachainan
Collection and assembly of data: Chanchai Charonpongsuntorn, Suebpong Tanasanvimon, Krittiya Korphaisarn, Songwit Payapwattanawong, Teerada Siripoon, Nussara Pakvisal, Jitlada Juengsamarn, Ekkamol Phaibulvatanapong, Jarin Chindaprasirt, Naiyarat Prasongsook, Kittipong Udomdamrongkul, Nuttapong Ngamphaiboon, Ekaphop Sirachainan
Data analysis and interpretation: Chanchai Charonpongsuntorn, Suebpong Tanasanvimon, Krittiya Korphaisarn, Jarin Chindaprasirt, Nuttapong Ngamphaiboon, Ekaphop Sirachainan
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Chanchai Charonpongsuntorn
Research Funding: AstraZeneca
Suebpong Tanasanvimon
Honoraria: Eisai, MSD, Roche Canada, Bristol Myers Squibb/Celgene, Amgen, Lilly, Merck, Novartis, Ipsen, AstraZeneca
Consulting or Advisory Role: Eisai, MSD, Bristol Myers Squibb/Celgene, Merck, Amgen
Travel, Accommodations, Expenses: Fresinius Krabi
Ekkamol Phaibulvatanapong
Honoraria: Roche
Consulting or Advisory Role: Roche
Naiyarat Prasongsook
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Roche, Novartis, Bristol Myers Squibb/Celgene
Travel, Accommodations, Expenses: AstraZeneca, Roche
Nuttapong Ngamphaiboon
Consulting or Advisory Role: MSD, Novartis, Amgen, Eisai, Merck
Speakers' Bureau: Roche, Eisai, MSD, Novartis
Research Funding: MSD (Inst), Pfizer (Inst), Roche (Inst), Exelixis (Inst), RAPT Therapeutics (Inst), BeiGene (Inst)
Travel, Accommodations, Expenses: Roche
Ekaphop Sirachainan
Honoraria: MSD, Sanofi/Aventis, Merck, Amgen, Roche, Mundipharma, AstraZeneca, LF Asia, Diethelm Keller Group, Bristol Myers Squibb, Boehringer Ingelheim, Taiho Pharmaceutical, Dr Reddy's Laboratories, Zuellig Pharma, Meda Pharmaceuticals, Pfizer, Novo Nordisk, Novartis
Consulting or Advisory Role: Roche, Taiho Oncology, MSD Oncology, Novartis, Bristol Myers Squibb/Celgene
Travel, Accommodations, Expenses: MSD, AstraZeneca, Pfizer, Amgen, American Taiwan Biopharm, Taiho Pharmaceutical, Eisai
No other potential conflicts of interest were reported.
REFERENCES
- 1.Villanueva A: Hepatocellular carcinoma. N Engl J Med 380:1450-1462, 2019 [DOI] [PubMed] [Google Scholar]
- 2.Arbuthnot P, Kew M: Hepatitis B virus and hepatocellular carcinoma. Int J Exp Pathol 82:77-100, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanwal F, Kramer JR, Mapakshi S, et al. : Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology 155:1828-1837.e2, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Llovet JM, Villanueva A, Lachenmayer A, et al. : Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat Rev Clin Oncol 12:436, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Bruix J: Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol 48:S20-S37, 2008. (suppl 1) [DOI] [PubMed] [Google Scholar]
- 6.Qin S, Bai Y, Lim HY, et al. : Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol 31:3501-3508, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Llovet JM, Ricci S, Mazzaferro V, et al. : Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359:378-390, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Kudo M: Systemic therapy for hepatocellular carcinoma: Latest advances. Cancers 10:412, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finn RS, Qin S, Ikeda M, et al. : Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 382:1894-1905, 2020 [DOI] [PubMed] [Google Scholar]
- 10.Schwartz LH, Seymour L, Litière S, et al. : RECIST 1.1—Standardisation and disease-specific adaptations: Perspectives from the RECIST Working Group. Eur J Cancer 62:138-145, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng AL, Qin S, Ikeda M, et al. : Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol 76:862-873, 2022 [DOI] [PubMed] [Google Scholar]
- 12.Cheng AL, Qin S, Ikeda M, et al. : IMbrave150: Efficacy and safety results from a Ph III study evaluating atezolizumab (Atezo) + bevacizumab (Bev) vs sorafenib (Sor) as first treatment (Tx) for patients (Pts) with unresectable hepatocellular carcinoma (HCC). Ann Oncol 30:ix186-ix187, 2019 [Google Scholar]
- 13.Pfister D, Núñez NG, Pinyol R, et al. : NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature 592:450-456, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ando Y, Kawaoka T, Kosaka M, et al. : Early tumor response and safety of atezolizumab plus bevacizumab for patients with unresectable hepatocellular carcinoma in real-world practice. Cancers (Basel) 13:3958, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiraoka A, Kumada T, Tada T, et al. : Atezolizumab plus bevacizumab treatment for unresectable hepatocellular carcinoma: Early clinical experience. Cancer Rep (Hoboken) 5:e1464, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayakawa Y, Tsuchiya K, Kurosaki M, et al. : Early experience of atezolizumab plus bevacizumab therapy in Japanese patients with unresectable hepatocellular carcinoma in real-world practice. Invest New Drugs 40:392-402, 2022 [DOI] [PubMed] [Google Scholar]
- 17.Iwamoto H, Shimose S, Noda Y, et al. : Initial experience of atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma in real-world clinical practice. Cancers (Basel) 13:2786, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheon J, Yoo C, Hong JY, et al. : Efficacy and safety of atezolizumab plus bevacizumab in Korean patients with advanced hepatocellular carcinoma. Liver Int 42:674-681, 2022 [DOI] [PubMed] [Google Scholar]
- 19.Jiang L, Tan X, Li J, et al. : Incidence and risk of hypertension in cancer patients treated with atezolizumab and bevacizumab: A systematic review and meta-analysis. Front Oncol 11:726008, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galle PR, Finn RS, Qin S, et al. : Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): An open-label, randomised, phase 3 trial. Lancet Oncol 22:991-1001, 2021 [DOI] [PubMed] [Google Scholar]