PURPOSE:
Continued smoking after the diagnosis of cancer can markedly worsen oncology treatment side effects, cancer outcomes, cancer mortality, and all-cause mortality. Conversely, mounting evidence demonstrates that smoking cessation by patients with cancer improves outcomes. A cancer diagnosis often serves as a teachable moment, characterized by high motivation to quit. However, too few patients with cancer who smoke are offered evidence-based smoking cessation treatment, and too few engage in such treatment.
METHODS AND MATERIALS:
The National Cancer Institute commissioned Tobacco Control Monograph 23, Treating Smoking in Cancer Patients: An Essential Component of Cancer Care, to review and synthesize the evidence that clarifies the need to intervene with smoking in cancer care.
RESULTS:
Although many patients with newly diagnosed cancer who smoke make quit attempts, many of these are unsuccessful, and among those who successfully quit, relapse is common. Indeed, an estimated 12.2% of adults ever diagnosed with cancer reported they currently smoked (National Health Interview Survey, 2020). Patients with cancer who smoke are likely to benefit from smoking cessation treatments, including counseling and US Food and Drug Administration–approved medications, and there are many effective strategies to increase delivery of smoking cessation treatment in cancer care settings.
CONCLUSION:
Smoking cessation is among the most effective treatment options for improving the likelihood of survival, quality of life, and overall health of patients with cancer who smoke. It is important for cancer care clinicians and patients to realize that it is never too late to quit smoking and that there are clear benefits to doing so, regardless of cancer type.
INTRODUCTION
For more than half a century, tobacco use has been known to cause a broad range of cancers and other adverse health outcomes,1,2 with cigarette smoking being the predominant causal agent. Despite substantial declines in adult tobacco use over many decades, cigarette smoking remains the leading cause of preventable disease and premature death,2 accounting for about 30% of all cancer deaths in the United States.3,4 While smoking is unequaled as a preventable cause of cancer, there has been a paradoxical lack of focus on reducing smoking among patients diagnosed with cancer, perhaps because the adverse effects of smoking in this population may not be widely recognized.
About half of patients with cancer report a history of smoking and 10%-18% report current smoking.5-7 Many patients with cancer try to quit smoking without formal smoking cessation treatment, and data show that, in general, about half or more of patients with cancer are unsuccessful in their attempts to do so.8,9 Unfortunately, a failure to quit is highly consequential. The 2014 and 2020 Surgeon General's reports documented that smoking by patients with cancer poses substantial risks for their recovery from cancer and their well-being.2,10 Continued smoking following a cancer diagnosis increases the risk of cancer-related mortality and the risk of mortality due to other causes, such as heart disease, noncancer pulmonary disease, and stroke.2,11 Furthermore, continued smoking increases the risk of cancer recurrence, second primary cancers, and adverse treatment-related outcomes, including postoperative pulmonary complications, poor surgical healing, and decreased response to systemic drugs and radiation, all of which lead to poorer outcomes and higher health care costs.12-14 These findings have led to calls to designate smoking cessation treatment as the Fourth Pillar of Cancer Care, along with surgery, chemotherapy/immunotherapy, and radiation therapy.15
METHODS
To focus attention on and address continued smoking by patients with cancer, the National Cancer Institute (NCI) commissioned Tobacco Control Monograph 23, Treating Smoking in Cancer Patients: An Essential Component of Cancer Care.16 This Monograph leverages and builds upon the Surgeon General's reports and other evidence by (1) providing a brief overview of the relationship of smoking to cancer outcomes and biological aspects of cancer, (2) reviewing and evaluating the evidence that smoking cessation treatment enhances cessation rates for patients who smoke in general and for patients with cancer in particular, (3) describing and evaluating strategies that have the potential to enhance the delivery of smoking cessation treatment in the cancer care context, (4) identifying medically underserved and vulnerable populations that have high cancer burdens and evaluating their success in quitting and the special challenges they face in doing so, and (5) identifying important research gaps. The Monograph is aimed at researchers, clinicians, health care systems, policymakers, funding agencies, patients with cancer and survivors, and other stakeholders. It is intended to present these audiences with research findings, implementation models, and clear research needs to help support researchers and practitioners in addressing smoking in cancer care.
Cigarette smoking by patients with cancer deserves focused attention for multiple reasons. One is the danger posed by both smoking and cancer in this population, as smoking may adversely affect the biology of the cancer, may interfere with cancer treatment or reduce its success, and may lead to harmful cancer treatment side effects. Moreover, while considerable progress has been made in increasing the delivery of smoking cessation treatment in primary care and other health care settings, less has been made in oncology settings. Finally, the unique characteristics of patients with cancer require a targeted analysis of smoking and cessation treatment in this group. People with cancer who smoke are often highly nicotine-dependent, and the presence of depression, pain, anxiety, fatalism, or treatment side effects may influence their motivation and ability to quit smoking and maintain long-term smoking abstinence.
The Monograph is part of a larger effort by the NCI to address smoking by patients with cancer. Central to that effort is the Cancer MoonshotSM–funded NCI program, the Cancer Center Cessation Initiative (C3I).17 Since 2017, NCI has invested almost $30 million US dollars in C3I to increase the delivery of smoking cessation treatment to patients with cancer, including underserved and vulnerable cancer populations. Since C3I's inception, 52 NCI-Designated Cancer Centers have been funded, and over 75,000 patients with cancer at these centers have received evidence-based cessation treatment services. C3I is also increasing the scientific evidence-base regarding smoking cessation treatment in the context of oncology care,18-20 and the findings from this important initiative are incorporated into the Monograph.
Cigarettes are the most common form of tobacco used by adults, and they are also the type of tobacco product for which the most cessation treatment data exist. However, other forms of tobacco, such as cigars and smokeless tobacco, also play a role in the development of certain cancers, and their continued use is likely to be detrimental to patients. Polytobacco use (the use of more than one tobacco product) and co-use of tobacco with alcohol and other drugs are also common, and some patients with cancer report using e-cigarettes. Where possible, other tobacco products are discussed, and the Monograph identifies new tobacco products and other drug use in patients with cancer as important areas of focus for both clinicians and researchers.
RESULTS
The Effects of Continued Smoking Compared With Cessation
The 2020 Surgeon General's report concluded that there was suggestive evidence of a causal relationship between smoking cessation and improved all-cause mortality among patients currently smoking at the time of their cancer diagnosis on the basis of 10 studies published between 2000 and 2016.10 This Monograph identifies an additional eight studies published between 2017 and 2021 that have examined the association between quitting smoking and all-cause mortality in patient groups diagnosed with different types of cancers.21-28 These studies provide additional evidence that quitting smoking after a cancer diagnosis is associated with reduced all-cause mortality relative to continued smoking.
Identifying Effective Smoking Cessation Treatments
Multiple smoking cessation treatments, including both psychosocial and pharmacotherapy interventions, have been found to be consistently effective in promoting smoking cessation in the general population. This evidence strongly suggests that smoking cessation treatment will be effective and yield important benefits in patients with cancer as well. In addition, the Monograph reviews evidence on methods to enhance the effectiveness of smoking cessation treatment, respond to smoking relapse, and adapt treatment to patients receiving oncology care. However, important research gaps were also identified. For example, while it is clear that quitting smoking can greatly benefit patients with cancer, too little is known about which cessation treatments are most effective and cost-effective in patients with cancer and how they affect specific cancer outcomes, such as cancer treatment effectiveness, toxicity, and survival.
Implementation of Smoking Cessation Treatment in Oncology Care
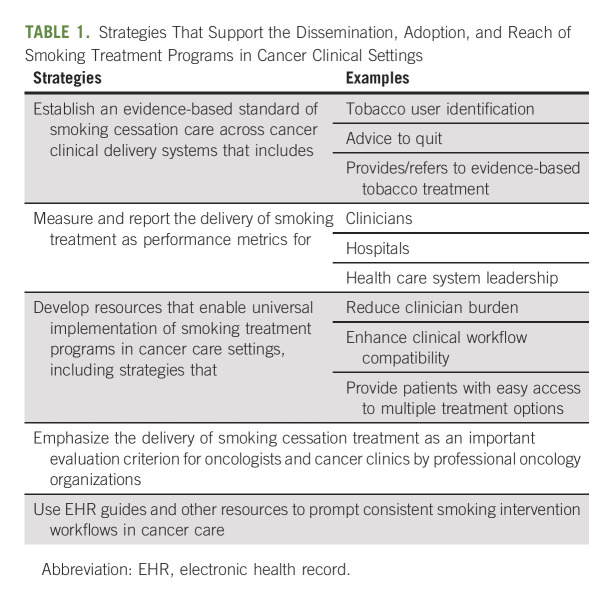
Although continued smoking by patients with cancer increases their risk for adverse cancer outcomes, evidence reviewed for the Monograph shows that treatment for smoking is too rarely offered and delivered in cancer care settings.15,29-33 For example, a 2019 review34 found that few oncology clinicians recommend or arrange smoking cessation treatment or provide follow‐up after a quit attempt. Low rates of addressing smoking cessation in oncology settings help explain why many cancer survivors continue to smoke. This Monograph reviews research that identifies efficient methods to meaningfully increase the delivery of smoking cessation treatment across a wide variety of medical settings (Table 1).
TABLE 1.
Strategies That Support the Dissemination, Adoption, and Reach of Smoking Treatment Programs in Cancer Clinical Settings
Vulnerable Populations
Cancers, including tobacco-related cancers, impose a high burden on individuals, families, and society. This burden is especially high in certain patient populations such as socioeconomically disadvantaged populations, people with behavioral health conditions, and some racial and ethnic minority populations.4 This Monograph addresses how best to help these and other vulnerable and medically underserved populations that experience disparities in cancer outcomes related to smoking. It examines evidence related to their cancer burden, unique challenges that may affect their likelihood of successful smoking cessation, and their response to smoking cessation treatment.
Major Conclusions of the Monograph
On the basis of the evidence reviewed, this Monograph makes the following eight overall conclusions regarding smoking cessation treatment across the cancer care continuum:
Smoking cessation after the diagnosis of cancer is highly likely to reduce all-cause mortality and cancer-specific mortality. Evidence continues to mount that quitting smoking after a cancer diagnosis is causally associated with reduced all-cause mortality and cancer-specific mortality, in comparison with continued smoking. The studies reviewed in this Monograph confirm and expand upon findings of the 2014 and 2020 Surgeon Generals' reports regarding this topic. Laboratory studies provide insight into the mechanisms by which smoking may increase tumor aggressiveness and decrease cancer treatment effectiveness.
Research from the general population indicates that patients with cancer who smoke will benefit from smoking cessation treatments, including both counseling and US Food and Drug Administration–approved medications. Smoking cessation counseling and medication have been shown to be effective in diverse populations of people who smoke. This substantial evidence, including some studies with patients with cancer, clearly supports the delivery of evidence-based smoking cessation treatment as an essential component of cancer care.
Effective strategies exist to increase the delivery of smoking cessation treatment in cancer care settings. Barriers identified by cancer care clinicians include lack of time, lack of specialized training to deliver smoking cessation treatment options, misconceptions about patients' intentions to quit, and difficulties with health insurance reimbursement. Multiple strategies, including use of electronic health record–based clinical workflow tools, can be adopted to address tobacco use for every patient across the cancer care continuum, including those who are screened for or diagnosed with cancer. These strategies can improve the identification of patients who smoke, the offer of smoking cessation treatment, and the delivery of or referral for smoking cessation treatment, and can do so in a low-burden, efficient manner.
Evidence-based smoking cessation treatment should be systematically provided to all patients with cancer, regardless of the type of cancer. However, patients with cancer are not consistently offered and provided such treatment. Many national and international cancer organizations recommend addressing smoking among patients with cancer and provide guidance to cancer care clinicians for effectively delivering smoking cessation treatment. However, the implementation of these evidence-based recommendations has been inconsistent and incomplete, highlighting the need to identify and address barriers to providing smoking cessation intervention that exist for both cancer care clinicians and health care systems.
Continued smoking after a cancer diagnosis is associated with higher health care utilization and greater health care costs in comparison with quitting smoking. Direct non–health care costs, such as transportation and caregiving, may also be increased with continued smoking after a cancer diagnosis. Smoking cessation interventions in patients with cancer are highly likely to be cost-effective.
Medically underserved and vulnerable populations of patients with cancer who smoke are very likely to benefit from using the evidence-based smoking cessation treatments identified as effective in the general population of people who smoke. Medically underserved and vulnerable populations are faced with multiple factors at the individual, community, institutional or health care system, and societal levels that may impede access to smoking cessation treatment and cessation success. Importantly, substantial evidence indicates that medically underserved and vulnerable populations overall (ie, noncancer populations) benefit from evidence-based smoking cessation treatment, providing evidence that these populations with cancer will benefit as well.
The tobacco product marketplace and consumer use patterns are changing for both the general population and patients with cancer, posing challenges for researchers and cancer care clinicians. Research is needed to monitor the use and effects of diverse tobacco products, both conventional and new, by patients with cancer, including their effects on smoking cessation and relapse and their potential deterrence of patients' using evidence-based smoking cessation treatments such as counseling and US Food and Drug Administration–approved medications.
Continued research is needed to identify effective cessation interventions for patients with cancer who smoke and to better understand the effects of smoking cessation on cancer outcomes. Relatively few well-powered randomized controlled trials of smoking cessation treatments in patients with cancer have been conducted. Additional research is needed to identify the effectiveness of smoking cessation interventions in increasing abstinence among patients with cancer, including which intervention strategies are most effective; the effects of smoking cessation treatment and resulting abstinence on cancer-related outcomes (eg, all-cause and cancer-specific mortality); and health care system changes and implementation strategies that are especially effective in engaging persons with cancer in evidence-based smoking cessation treatment.
In addition to these major conclusions, the Monograph contains numerous specific conclusions and identifies other major unmet research needs.
DISCUSSION
In summary, smoking worsens oncologic and other outcomes among patients with cancer, whereas quitting smoking brings numerous benefits. Moreover, effective smoking cessation treatments exist and can be integrated into cancer care clinical settings in multiple ways to promote cessation. Patients, families, and their clinicians should recognize that among the treatment options available to patients with cancer who smoke, smoking cessation is among the most effective in terms of improving the likelihood of survival, quality of life, and overall health. As this Monograph demonstrates, helping patients with cancer quit smoking is a fundamental, but largely unrealized, obligation of and opportunity for oncology care clinicians and programs. The strategies and research needs identified in NCI Tobacco Control Monograph 23 are intended to assist the oncology community to meet this responsibility efficiently and effectively.
ACKNOWLEDGMENT
The authors gratefully acknowledge the many contributors to and reviewers of this NCI Tobacco Control Monograph.
Douglas R. Lowy
Patents, Royalties, Other Intellectual Property: I am an inventor of technology that underlies the L1-based prophylactic virus-like particle (VLP) HPV vaccine and technology that underlies an L2-based candidate prophylactic HPV vaccine (Inst), Merck, GlaxoSmithKline
Michele Bloch
Patents, Royalties, Other Intellectual Property: My spouse is a co-inventor on patents obtained by the National Cancer Institute stemming from his government employment. He is now a retired federal employee (I)
No other potential conflicts of interest were reported.
SUPPORT
Supported by the National Cancer Institute, National Institutes of Health.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Administrative support: Timothy B. Baker
Data analysis and interpretation: Michael C. Fiore
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Treating Smoking in Cancer Patients: An Essential Component of Cancer Care—The New National Cancer Institute Tobacco Control Monograph
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Douglas R. Lowy
Patents, Royalties, Other Intellectual Property: I am an inventor of technology that underlies the L1-based prophylactic virus-like particle (VLP) HPV vaccine and technology that underlies an L2-based candidate prophylactic HPV vaccine (Inst), Merck, GlaxoSmithKline
Michele Bloch
Patents, Royalties, Other Intellectual Property: My spouse is a co-inventor on patents obtained by the National Cancer Institute stemming from his government employment. He is now a retired federal employee (I)
No other potential conflicts of interest were reported.
REFERENCES
- 1.U.S. Department of Health, Education, and Welfare . Smoking and Health: Report of the Advisory Committee to the Surgeon General of the Public Health Service. Washington, DC: U.S. Department of Health, Education, and Welfare, Public Health Service, Centers for Disease Control and Prevention; 1964. [Google Scholar]
- 2.U.S. Department of Health and Human Services . The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 3. Islami F, Sauer AG, Miller KD, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018;68:31–54. doi: 10.3322/caac.21440. [DOI] [PubMed] [Google Scholar]
- 4.American Cancer Society . Cancer Facts & Figures 2021. Atlanta, GA: American Cancer Society; 2021. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf [Google Scholar]
- 5. Warren GW, Kasza KA, Reid ME, et al. Smoking at diagnosis and survival in cancer patients. Int J Cancer. 2013;132:401–410. doi: 10.1002/ijc.27617. [DOI] [PubMed] [Google Scholar]
- 6.National Center for Health Statistics . National Health Interview Survey, 2019. Public-Use Data File and Documentation. 2021. https://www.cdc.gov/nchs/nhis/2019nhis.htm [Google Scholar]
- 7.National Cancer Institute . Cancer Trends Progress Report: Cancer Survivors and Smoking. 2020. https://progressreport.cancer.gov/after/smoking [Google Scholar]
- 8. Gritz ER, Talluri R, Domgue JF, et al. Smoking behaviors in survivors of smoking-related and non-smoking-related cancers. JAMA Netw Open. 2020;3:e209072. doi: 10.1001/jamanetworkopen.2020.9072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jassem J. Tobacco smoking after diagnosis of cancer: Clinical aspects. Transl Lung Cancer Res. 2019;8(suppl 1):S50–S58. doi: 10.21037/tlcr.2019.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.U.S. Department of Health and Human Services . Smoking Cessation. A Report of the Surgeon General. Atlanta, GA: U.S Department of Health and Human Services, Centers for Disease Control and Prevention, National Cancer for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2020. [Google Scholar]
- 11. Wang TW, Walton K, Jamal A, et al. State-specific cessation behaviors among adult cigarette smokers—United States, 2014–2015. Prev Chronic Dis. 2019;16:e26. doi: 10.5888/pcd16.180349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martínez Ú, Brandon KO, Sutton SK, et al. Does smoking abstinence predict cancer patients' quality of life over time? Psychooncology. 2019;28:1702–1711. doi: 10.1002/pon.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shields PG, Herbst RS, Arenberg D, et al. Smoking cessation, version 1.2016: NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14:1430–1468. doi: 10.6004/jnccn.2016.0152. [DOI] [PubMed] [Google Scholar]
- 14. Warren GW, Cartmell KB, Garrett-Mayer E, et al. Attributable failure of first-line cancer treatment and incremental costs associated with smoking by patients with cancer. JAMA Netw Open. 2019;2:e191703. doi: 10.1001/jamanetworkopen.2019.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fiore MC, D'Angelo H, Baker T. Effective cessation treatment for patients with cancer who smoke—The fourth pillar of cancer care. JAMA Netw Open. 2019;2:e1912264. doi: 10.1001/jamanetworkopen.2019.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.U.S. National Cancer Institute . Treating Smoking in Cancer Patients: An Essential Component of Cancer Care. National Cancer Institute Tobacco Control Monograph 23. Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 2022. [Google Scholar]
- 17. Croyle R, Morgan G, Fiore M. Addressing a core gap in cancer care: The NCI Cancer MoonshotSM initiative to help oncology patients stop smoking. N Engl J Med. 2019;380:512–515. doi: 10.1056/NEJMp1813913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. D'Angelo H, Rolland B, Adsit R, et al. Tobacco treatment program implementation at NCI cancer centers: Progress of the NCI Cancer Moonshot-Funded Cancer Center Cessation Initiative. Cancer Prev Res (Phila) 2019;12:735–740. doi: 10.1158/1940-6207.CAPR-19-0182. [DOI] [PubMed] [Google Scholar]
- 19. D'Angelo H, Ramsey AT, Rolland B, et al. Pragmatic application of the RE-AIM framework to evaluate the implementation of tobacco cessation programs within NCI-Designated Cancer Centers. Front Public Health. 2020;8:221. doi: 10.3389/fpubh.2020.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. D'Angelo H, Webb Hooper M, Burris JL, et al. Achieving equity in the reach of smoking cessation services within the NCI Cancer Moonshot-funded Cancer Center Cessation Initiative. Health Equity. 2021;5:424–430. doi: 10.1089/heq.2020.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barnett TE, Lu Y, Gehr AW, et al. Smoking cessation and survival among people diagnosed with non-metastatic cancer. BMC Cancer. 2020;20:726. doi: 10.1186/s12885-020-07213-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Day AT, Dahlstrom KR, Lee R, et al. Impact of a tobacco treatment program on abstinence and survival rates among current smokers with head and neck squamous cell carcinoma. Head Neck. 2020;42:2440–2452. doi: 10.1002/hed.26268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gemine RE, Ghosal R, Collier G, et al. Longitudinal study to assess impact of smoking at diagnosis and quitting on 1-year survival for people with non-small cell lung cancer. Lung Cancer. 2019;129:1–7. doi: 10.1016/j.lungcan.2018.12.028. [DOI] [PubMed] [Google Scholar]
- 24. Hansen JM, Nagle CM, Ibiebele TI, et al. A healthy lifestyle and survival among women with ovarian cancer. Int J Cancer. 2020;147:3361–3369. doi: 10.1002/ijc.33155. [DOI] [PubMed] [Google Scholar]
- 25. Hawari FI, Obeidat NA, Rimawi D, et al. Smoking cessation care can translate to lower hazard of death in the short-run in cancer patients—A retrospective cohort study to demonstrate the value of smoking cessation services within the treatment phase of cancer. BMC Cancer. 2019;19:580. doi: 10.1186/s12885-019-5778-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Romaszko-Wojtowicz A, Buciński A, Doboszyńska A. Impact of smoking on multiple primary cancers survival: A retrospective analysis. Clin Exp Med. 2018;18:391–397. doi: 10.1007/s10238-018-0498-1. [DOI] [PubMed] [Google Scholar]
- 27. Sheikh M, Mukeriya A, Shangina O, et al. Postdiagnosis smoking cessation and reduced risk for lung cancer progression and mortality: A prospective cohort study. Ann Intern Med. 2021;174:1232–1239. doi: 10.7326/M21-0252. [DOI] [PubMed] [Google Scholar]
- 28. Wang T, Townsend MK, Simmons V, et al. Prediagnosis and postdiagnosis smoking and survival following diagnosis with ovarian cancer. Int J Cancer. 2020;147:736–746. doi: 10.1002/ijc.32773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goldstein AO, Ripley-Moffitt CE, Pathman DE, et al. Tobacco use treatment at the U.S. National Cancer Institute's designated cancer centers. Nicotine Tob Res. 2013;15:52–58. doi: 10.1093/ntr/nts083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Toll BA, Brandon TH, Gritz ER, et al. Assessing tobacco use by cancer patients and facilitating cessation: An American Association for Cancer Research policy statement. Clin Cancer Res. 2013;19:1941–1948. doi: 10.1158/1078-0432.CCR-13-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gritz ER, Toll BA, Warren GW. Tobacco use in the oncology setting: Advancing clinical practice and research. Cancer Epidemiol Biomarkers Prev. 2014;23:3–9. doi: 10.1158/1055-9965.EPI-13-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chang EHE, Braith A, Hitsman B, et al. Treating nicotine dependence and preventing smoking relapse in cancer patients. Expert Rev Qual Life Cancer Care. 2017;2:23–39. doi: 10.1080/23809000.2017.1271981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gallaway MS, Tai E, Rohan EA. Smoking cessation treatment programs offered at hospitals providing oncology services. J Smok Cessat. 2019;14:65–71. doi: 10.1017/jsc.2018.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Price SN, Studts JL, Hamann HA. Tobacco use assessment and treatment in cancer patients: A scoping review of oncology care clinician adherence to clinical practice guidelines in the U.S. Oncologist. 2019;24:229–238. doi: 10.1634/theoncologist.2018-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]