PURPOSE
To better understand the barriers to accessing standard-of-care radiation therapy (RT) for breast and cervical cancer in sub-Saharan Africa and their impact on outcomes.
METHODS
A comprehensive literature search was completed with a medical librarian. Articles were screened by title, abstract, and full text. Included publications were analyzed for data describing barriers to RT access, available technology, and disease-related outcomes, and further grouped into subcategories and graded according to predefined criteria.
RESULTS
A total of 96 articles were included: 37 discussed breast cancer, 51 discussed cervical cancer, and eight discussed both. Financial access was affected by health care system payment models and combined burdens of treatment-related costs and lost wages. Staffing and technology shortages limit the ability to expand service locations and/or increase capacity within existing centers. Patient factors including use of traditional healers, fear of stigma, and low health literacy decrease the likelihood of early presentation and completion of therapies. Survival outcomes are worse than most high- and middle-income countries and are affected by many factors. Side effects are similar to other regions, but these findings are limited by poor documentation capabilities. Access to palliative RT is more expeditious than definitive management. RT was noted to lead to feelings of burden, lower self-esteem, and worsened quality of life.
CONCLUSION
Sub-Saharan Africa represents a diverse region with barriers to RT that differ on the basis of funding, available technology and staff, and community populations. Although long-term solutions must focus on building capacity by increasing the number of treatment machines and providers, short-term improvements should be implemented, such as interim housing for traveling patients, increased community education to reduce late-stage diagnoses, and use of virtual visits to avoid travel.
INTRODUCTION
Breast cancer (BC) and cervical cancer (CC) represent the first and fourth most common cancers in women worldwide and first and second in sub-Saharan Africa (SSA), respectively.1-4 Nineteen of 20 countries with the highest incidence of CC are in Africa, and 90% of CC deaths in 2020 occurred in less-developed nations.5,6 BC 5-year overall survival (OS) in SSA is below 50%, far lower than high-income (90%) or middle-income countries (66%).4,7 Poor survival outcomes are related to tremendous disparities in access to preventative services and screening, leading to late-stage diagnoses.8-11
CONTEXT
Key Objective
What is the current status of radiation therapy access and outcomes for breast and cervical cancers in sub-Saharan Africa?
Knowledge Generated
Sub-Saharan Africa is a diverse region with varying radiation offerings. Barriers to care include financial toxicities of treatment, prolonged travel to regional clinics and lack of local care opportunities, health literacy, staffing shortages, and unreliable technology, among others. Overall survival for both breast and cervical cancer are poor compared with high-income countries, and understandings of impacts on quality of life and mental health are understudied.
Relevance
The cancer burden in sub-Saharan African continues to grow. As such, it is imperative that an accurate understanding of radiation therapy capacity and treatment outcomes is available to allow for focused discussion of resource allocation.
Additionally, because of advances in prevention and treatment of infectious agents, the average lifespan in SSA has increased, leading to higher cancer burdens.12 Increased incidence necessitates a heightened focus on expanding access to quality cancer care.13 Treatments for locally advanced BC and CC involve chemotherapy and radiation therapy (RT), both with limited access in SSA.14-17
Compounding access, factors such as location, treatment length, and alignment of cultural beliefs represent challenges to providing curative treatments to all indicated patients.18,19 These factors, along with clinical outcomes, are often described as isolated barriers or single-institutional outcomes and are rarely RT-specific. In this review, we aim to better understand the barriers to accessing standard-of-care RT for BC and CC across SSA and their impact on cancer outcomes.
METHODS
The PRISMA Extension for Scoping Reviews checklist was used as a reporting guide.20 The protocol was registered with PROSPERO (ID: CRD42021246847) and reported using PRISMA 2020 guidelines.
Literature Search
A comprehensive literature search was developed with a medical librarian and peer reviewed using Peer Review of Electronic Search Strategies guidelines.21 Searches were conducted on December 30, 2021, in MEDLINE (Ovid), Scopus, Web of Science, and African Index Medicus, and limited to English language articles. Searches were limited to 2016-present to ensure presentation of the current state of RT.
Search strategies were created using medical subject headings and keywords combined with database-specific advanced techniques. Medical subject headings and keywords were identified to represent RT, BC, CC, and SSA. The full search strategy from Ovid Medline is detailed in the Data Supplement. Citations were downloaded into EndNote, deduplicated, and uploaded into Rayyan for screening.
Study Selection/Screening
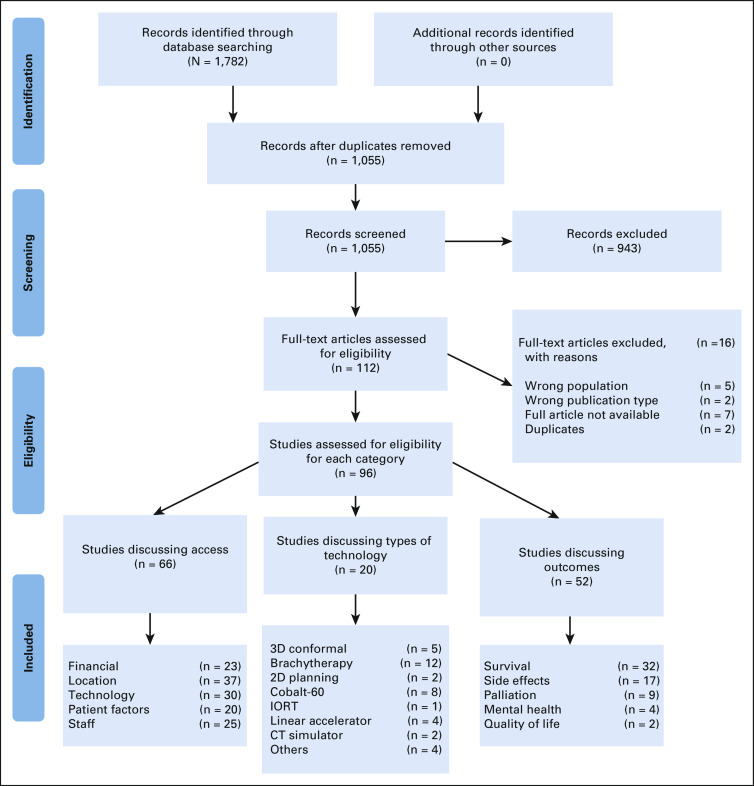
Articles were screened by two independent reviewers (S.E.B.P. and S.A.A. or J.C.B.) to determine eligibility including title, abstract, and full-text review. Blinded screening was conducted in Rayyan with conflicts resolved by group consensus. Full selection is presented in Figure 1.
FIG 1.
PRISMA flowchart. CT, computed tomography; IORT, intraoperative radiation therapy. From Moher D, Liberati A, Tetzlaff J, et al: Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA statement. PLoS Med 6:e1000097, 2009. For more information, visit www.prisma-statement.org.
Eligibility Criteria
Included studies were original studies or summaries of national/regional interventions that assessed the use of RT, including external-beam radiation therapy (EBRT) and brachytherapy, or RT-related outcomes, including survival, toxicities, mental health, palliation, and quality of life (QoL), for BC and CC. There were no exclusions on the basis of patient or tumor characteristics. Additional criteria included human studies, full-text availability, publication after 2015, and from/describing SSA.
Data Extraction/Synthesis
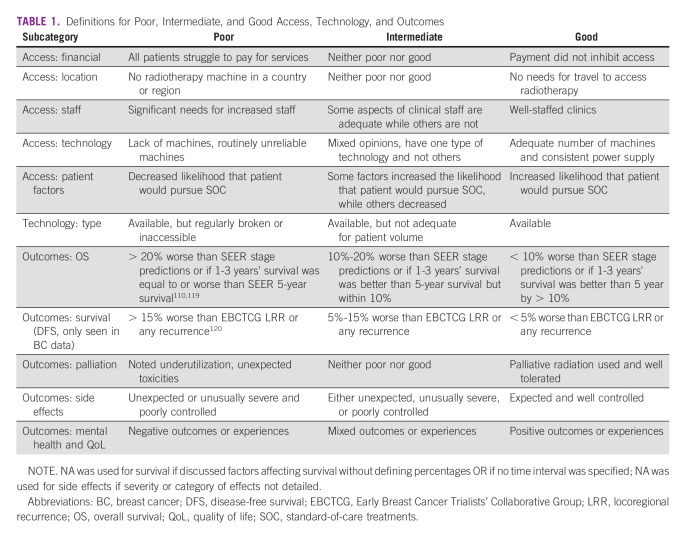
Included studies were analyzed for discussion of RT access and RT-related outcomes. Access was divided into subcategories of financial, location, staff, technology, and patient factors. Technology was specified by type including 2D, 3D conformal (3DCRT), intensity-modulated RT (IMRT), brachytherapy, and other. Outcomes included survival, side effects, QoL, palliation, and mental health. Subcategory data were systematically extracted from the text and categorized as poor, intermediate, or good according to Table 1.
TABLE 1.
Definitions for Poor, Intermediate, and Good Access, Technology, and Outcomes
Quality Assessment
No randomized controlled trials were identified. Most included studies were retrospective studies or qualitative interviews. By the nature of these designs, risk of bias is elevated. Each article was systematically assessed for bias types, funding sources, and conflicts of interest. A minority of studies were case control or cohort studies, which were analyzed using their respective Newcastle-Ottawa Quality Assessment Scales. Assessments are provided in the Data Supplement.
RESULTS
The number of articles in each category can be found in Figure 1, with trends for themes and grading in Figure 2. A total of 96 articles were included: 37 BC, 51 CC, and eight discussed both. An article breakdown of categories and grades is provided in the Data Supplement.
FIG 2.
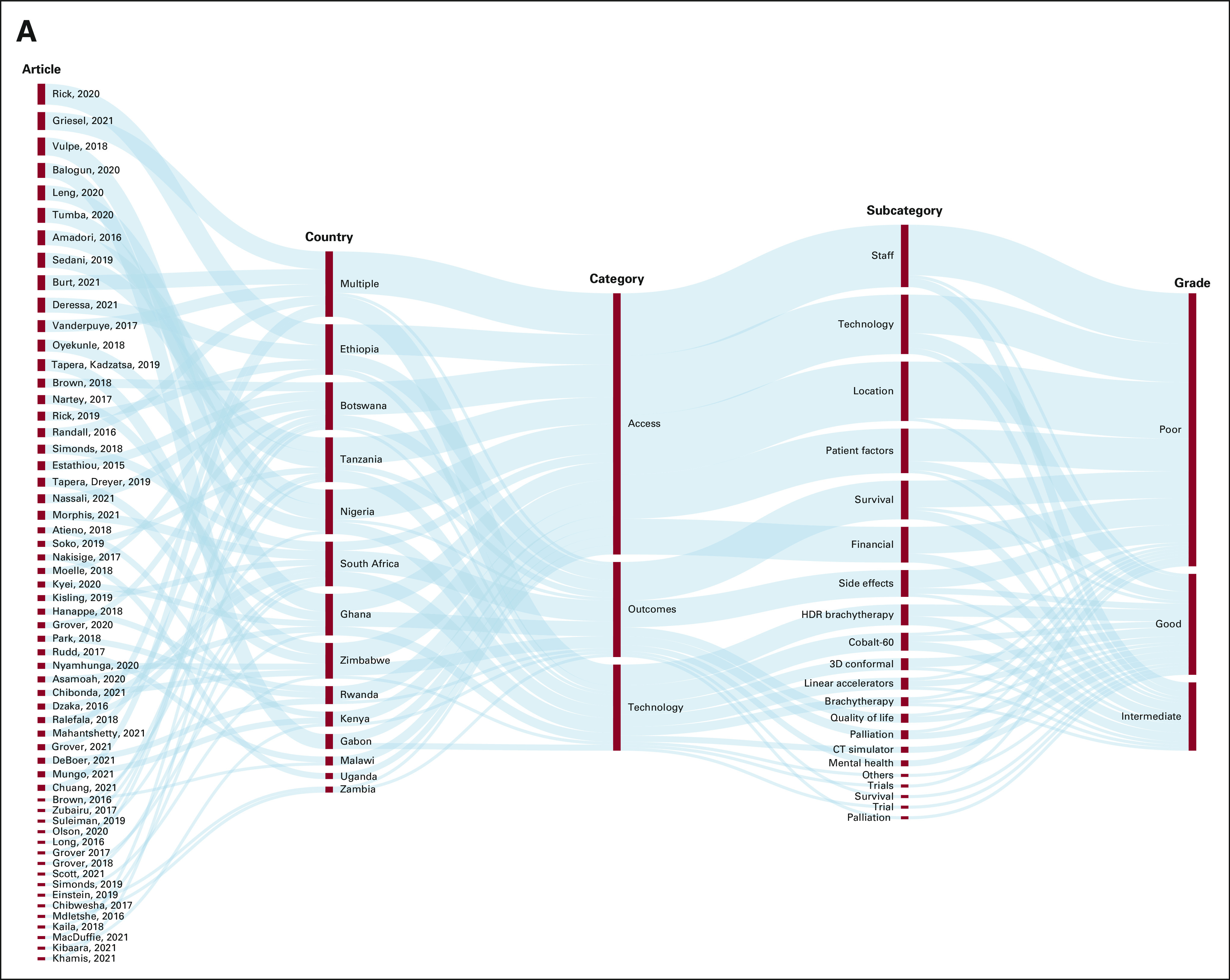
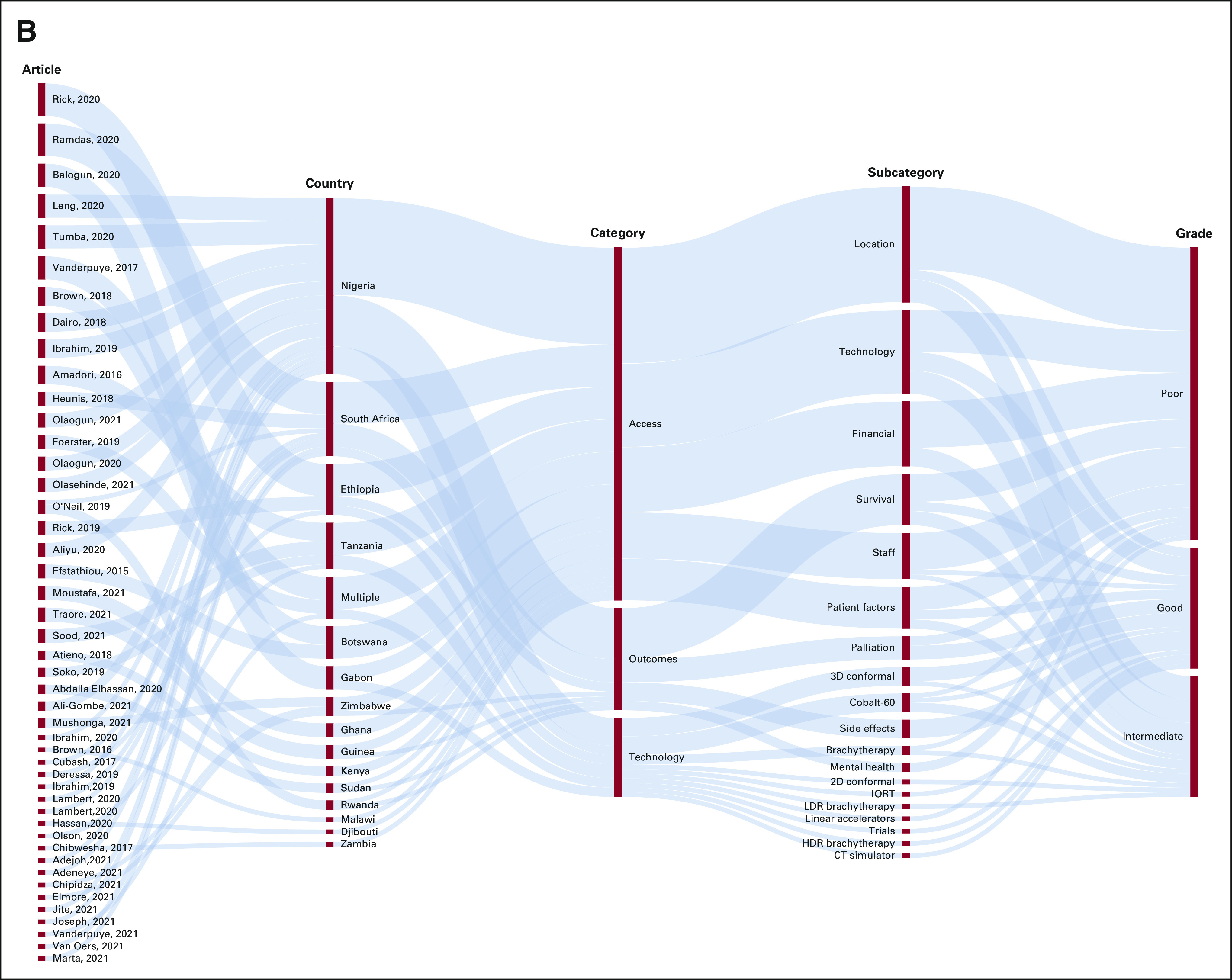
Depictions of trends in the literature regarding radiation access and outcomes throughout SSA. Beginning with specific articles, these outline the most common countries, types of access/outcomes and technology discussed, and the grade given in each article (on the basis of Table 1): (A) cervical cancer and (B) breast cancer. CT, computed tomography; HDR, high dose rate; IORT, intraoperative radiation therapy; LDR, low dose rate; SSA, sub-Saharan Africa.
Access: Financial
Financial payment models differ throughout SSA. Most frequently, out-of-pocket (OOP) payment methods are used.22,23 In this model, treatment poses too great a financial burden, even with insurance, to allow for adequate care. Additionally, costs varied substantially. For example, while living on approximately 1 US dollar (USD) per day, the cost of RT was 1,200 USD in Kenya compared with 1.5-25 USD in Ethiopia, a burdensome but less debilitating cost.22,23 Furthermore, Ethiopia developed a mixed model with government, private, and NGO contributions; 70% of patients may still require OOP payments.23,24 In Gabon, patient costs decreased from 51% to 22% of health spending from 2008 to 2020 because of increased government healthcare allocations, improving access for low-income patients.25 Similarly, in Botswana, government subsidies comprise a significant portion of low-income patient coverage.26 National insurance covers just 5% of Nigerians; because of financial constraints, only 22 of 66 patients for whom breast RT was recommended were referred, and only five completed treatment.27 Without additional subsidies, RT remains inaccessible.28 In Tanzania, care is free if patients pay for biopsies; although less expensive, this often remains unaffordable.29 Treatment length affects costs as some hospitals charge for entire RT courses, while others charge per fraction, potentially leading to incomplete treatments on the basis of inability to continue paying.30
Payment models affect treatment completion rates. In comparing one Ugandan, one Namibian, and two Nigerian hospitals, rates of treatment initiation within the first year after diagnosis ranged from 38% to 82% in facilities with largely OOP payments compared with 98.7%-100% in a free center.31 Financial burdens lead to delays, unplanned breaks, or nonstandard treatments.32-37 They affect treatment options, particularly with new technologies such as intraoperative RT (IORT), which are ineligible for aid and, therefore, only available for wealthy South Africans.38 Insurance may help offset impacts, increasing RT utilization.39
Compounding costs of workup and treatment, patients are often paying for transportation and temporary housing because of distance from treatment facilities.35,36,40,41 Travel needs limit employment, increasing financial toxicity.42 Bearing these consequences in mind, the choice of treatment options in BC, mastectomy or lumpectomy and RT, are affected by the ramifications of travel logistics and lost wages.43 After completion of therapy, early recurrences may be missed because of unaffordability of surveillance imaging.44 Financial barriers affect all aspects of care from initial presentation through long-term follow-up and contribute to lack of survivorship care.45
Access: Location
Approximately 90% of African RT capacity is concentrated in Northern and Southern Africa, with nearly 60% within Egypt and South Africa alone.44,46 In one study, only 2% of patients with CC and no patient with BC completed RT despite this being standard of care.47 As of the writing of included publications (2016-2021), 26 of 54 African countries had no RT, including Malawi, Chad, Cameroon, Guinea, Burundi, Cape Verde, the Democratic Republic of Congo, Seychelles, Eritrea, and Niger; some may have increased capacity since publication.48-55 In countries with RT, access is often limited to poorly maintained tertiary centers or cost-prohibitive private hospitals.50 In Botswana, RT is available at one private hospital serving 1.3 million residents.26,56,57 It is not uncommon to find a single RT center within a country; this was noted in Zimbabwe, Zambia, Uganda, and Ethiopia.23,58-60 Having single national centers leads to delays; while awaiting RT for CC, an Ethiopian cohort noted 16 deaths and 44.4% progression to a higher stage if waiting for more than 60 days.24 Ethiopia, however, is actively working to expand RT access to six regional centers.23 Ghana and Sudan have two centers each, and, within Ghana, 78% of patients live within 45 km of a RT center.44,61,62 Access is improved in South Africa with 86.4% of referred postmastectomy patients and 78.6% of breast-conserving surgery patients completing adjuvant RT because of proximity.63
Centralized RT limits access because of encumbrances of travel. This places a larger burden on community clinics to diagnose oncologic processes, posing a risk for misdiagnoses by nurses who manage rural facilities without physician oversight, loss of documents as they shuffle between centers, and limited communication in referrals.23,26 Despite referring to the closest center, in a Nigerian cohort, 41.9% and 12.4% of patients traveled more than 3 and 8 hours, respectively, for care, with distances up to 800 miles.29,32 The closest center may be out of the country, leading to sharp decreases in the percentage of successful referrals and a need for caregivers to accompany patients, increasing the burden of lost wages.45,51 In Botswana, interim housing is available but always at capacity, leading to hospital admissions in overburdened wards.60 Living near RT centers increases the likelihood of guideline compliance, improves follow-up, and expands the number of treatment options.33,36-38,64,65 In patients with BC, 66% completed RT in a Namibian center, 15% in Uganda, and < 5% in a Nigerian hospital without on-site RT.31
Access: Staff
Staff volume varies greatly between countries and centers. In Kenyatta National Hospital, lack of staff is postulated to negatively affect outcomes with only four practicing radiation oncologists within the country managing care for approximately 82,000 new cancer cases annually.22 Because of lack of trained staff, a Botswana center rejected a cobalt-60 machine with the potential to significantly increase treatment volumes.60 Some centers are forced to turn away patients because of long lines, high patient volumes, or limited physician presence.26,35 Low staffing, accompanied by physician/staff strikes and clinic closures, lengthens wait times. In a center with five oncologists and a machine operating 8 hours a day, median wait time for consultations was 40 days and time from consultation to treatment was 130.5 days.29,32,42,49,58,66 Additionally, there is a shortage of pathologists in SSA. Although some patients underwent surgery without a pathologic cancer diagnosis, they were less likely to accept recommended adjuvant therapies.37,42,62,66,67 Specific to CC, a shortage of gynecologic oncologists places more pressure on RT services for early-stage patients who may be surgical candidates.33,42,44,50,68,69 The use of multidisciplinary clinics may help improve staffing, referral systems, and delays as patients only need one visit to arrange care.70
Limited training capacity poses an additional barrier. A Gabonese clinic recently converted to 3DCRT with help from Moroccan staff who spent 18 months facilitating the transition, leading to an appropriately staffed clinic.25 Ethiopia has started a dual radiation/medical oncology training program for 7-10 trainees each year.23 Partly because of government-sponsored education requiring trainees to practice locally, this has tripled the number of oncologists in the country between 2017 and 2020.23 Dual training, however, is challenging as more advanced radiation technologies and complex systemic options become available as mastering the progressively expanding toolbox of treatment options and techniques is difficult for any one individual.71 Staffing concerns extend beyond providers and lead to struggles with managing a vast referral network and recordkeeping, limiting provision adequate survivorship care with incomplete documentation.24,42,67,72
Access: Technology and Technology Type
SSA has just 0.115 radiation machines per one million people, far less than the recommended 4, and many oncology facilities are without RT.25,33,45,54,62,71,72 Even in areas with a growing RT capacity such as Botswana, the growth in cancer incidences outpaces that of RT services.60 In other areas, issues surround the lack of brachytherapy, affecting CC outcomes despite adequate access to EBRT.35 In a survey of health care providers from 23 countries, 44.8% were without EBRT and 52.5% lacked brachytherapy.69 In a second survey, 70% of facilities had RT access with 78% of these having linear accelerators (linacs), 42% having cobalt-60 machines, 60% using 3DCRT, and 22% using IMRT.55 Reviews of available technologies can be found in Table 2.
TABLE 2.
Breakdown of Technology by Country
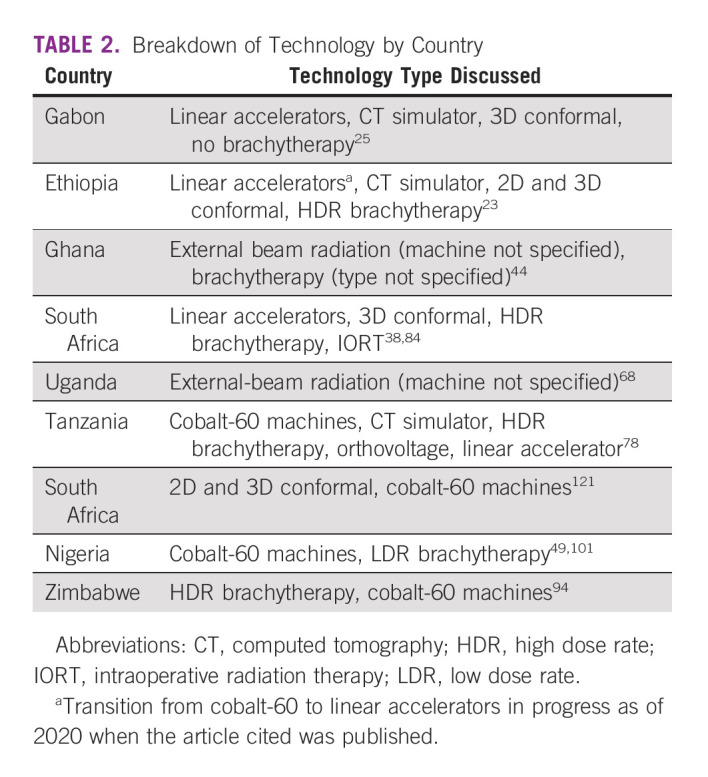
In addition to equipment, access to technology is affected by unreliable power grids, the cost of exchanging cobalt sources, unstable supplies of concurrent chemotherapy, and broken-down equipment.14,23,27,32,58,60,73 In one survey, four of seven RT facilities noted downtime at least weekly.71 These challenges lead to delays and decrease the number of patients treated in a given period. Capacity could increase with additional and/or more reliable machines.29,32,71 The ability to obtain generators or purchase linacs with built-in generators helps decrease delays by increasing machine reliability.23 Unreliable equipment leads to non–standard-of-care treatments and/or the need to travel long distances.36,74 Some institutions have increased capacity and are participating in research.75,76 Only 4% of surveyed centers noted participation in clinical trials; reasons included limited funding, high workload, and inabilities to meet trial requirements at their center.14
Innovative technologies may increase capacity. An RT planning assistant with automated plan generation from computed tomography scans led to all plans being subsequently approved by treating physicians in one study.77 Certain technologies, such as IORT, may increase access by allowing for single treatments; patients presenting with advanced disease do not qualify for this option, limiting utility.38 International partnerships can also help train physicians, physicists, and others in using new technologies as they become available.78 In planning any expansions, it is essential to first understand the demographics, cancer incidences, and availability of therapies to ensure resources are used efficiently.60
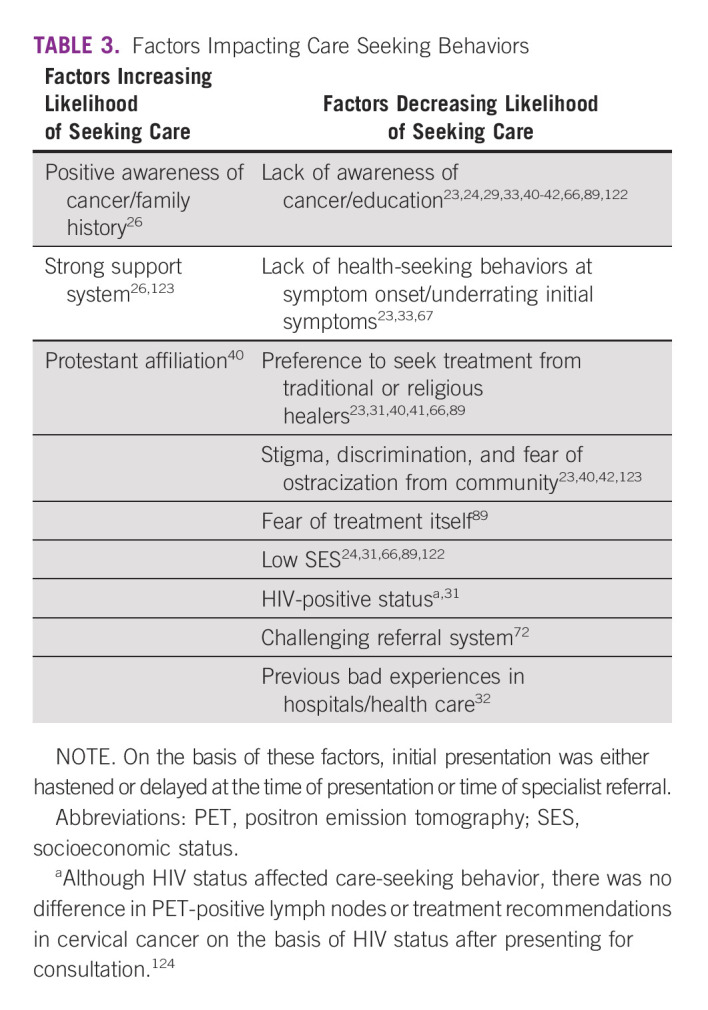
Access: Patient Factors
Social and cultural beliefs, health literacy, and other patient-specific factors affect care utilization positively and negatively. These factors are outlined in Table 3. Expanding on the theme of misinformation, a survey on RT-specific beliefs noted that 83% of respondents felt RT would decrease their lifespan and over half believed RT was poison, would cause their cancer to spread, or would make them radioactive.46 Although this decreased care-seeking, one analysis noted that education, use of traditional healers, and low socioeconomic status did not affect initiation of treatment after consultations were completed.32 The COVID-19 pandemic altered treatment decisions secondary to increased delays, fear of exposures, and generalized unease38; once treated, patients were more likely to be lost to follow-up if they were older or had advanced presentations.45
TABLE 3.
Factors Impacting Care Seeking Behaviors
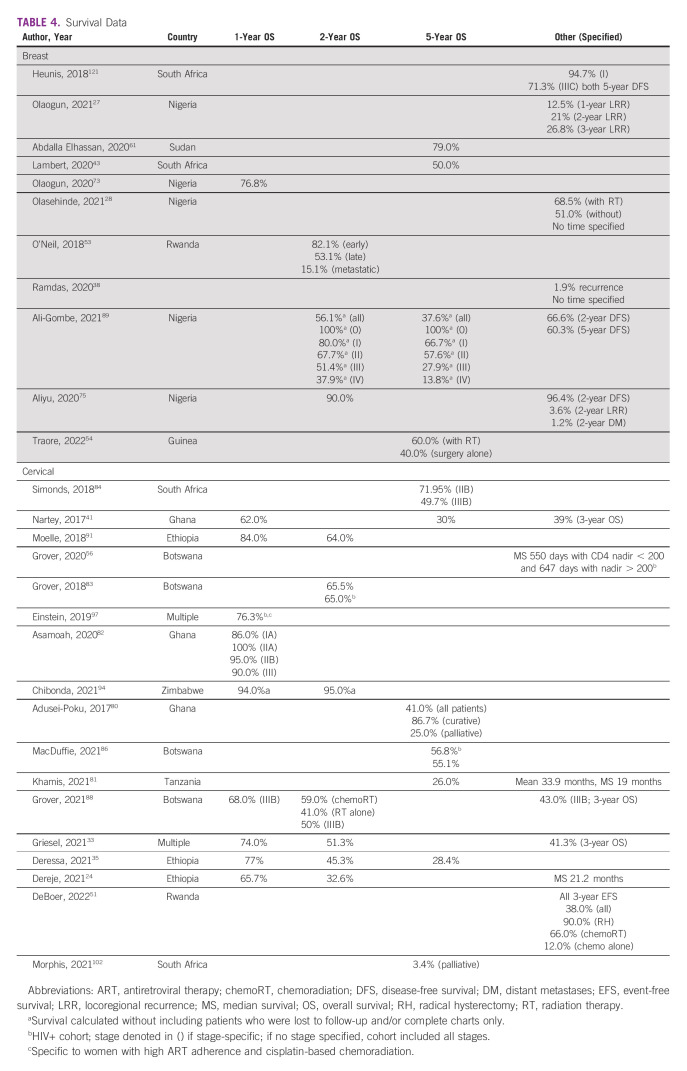
Outcomes: Survival
Numerical survival data, including overall and progression-free survival, can be seen in Table 4 along with data on locoregional and distant recurrences. Factors that improve survival include curative-intent treatments, higher parity, no previous medical history, and guideline adherence.33,35,41,79-81 Despite the importance of guideline compliance, only 5.2% of patients received guideline-compliant treatment with curative intent, while 2.4% received curative-intent treatment with minor deviations and 8.2% received curative-intent treatment with major deviations.33 Factors that decrease survival include late-stage presentations, treatment breaks, loss to follow-up, poor performance status, undifferentiated tumors, older age, poor nutrition, and long wait times.24,34,36,41,43,61,79-83 In one series, compared with wait times of < 60 days, all-cause mortality was three times higher for waits between 120 and 179 days and 5.8 times higher if > 180 days.24 Some patients with CC were given chemotherapy while awaiting RT availability; within this cohort, survival was similar to those given upfront chemoradiation.51 HIV status was shown in separate studies to negatively affect survival or have no impact, a finding that may be mediated by CD4 count.34,56,79,84-86
TABLE 4.
Survival Data
Specific to CC, use of concurrent chemotherapy and brachytherapy improved survival, the type of brachytherapy (low v high dose rate) had no impact, and baseline anemia worsened survival.36,56,79,81,83,85-88 One multinational study notes nearly identical survival compared with 20 years before.33 Specific to BC, a lack of human epidermal growth factor receptor 2–targeted agents, large subset of triple-negative and/or undifferentiated tumors, and expensive chemotherapy worsened survival.53,89 Survival data, however, are challenging to obtain because of low socioeconomic status and long distances traveled for treatments leading to decreased ability to access and afford survivorship care; one study noted 40.3% of patients lost to follow-up by 5 years, with another noting 91.2% of patients lost by 3 years.33,61,73,84
Outcomes: Side Effects
Ninety-four percent of patients, including all cancers, were noted to complete treatment uninterrupted in an Ethiopian series, alluding to limited severe acute toxicities.90 Within CC, studies reported differing rates of toxicities; one noted 27% of patients with adverse effects, while another noted 18% grade 3+ toxicities.34,74 In two series with EBRT alone, acute side effects included radiation dermatitis (9%), diarrhea (12%), ulcerated sores (52.8%), dysuria (7.5%), thrombocytopenia (5.6%), and anorexia (5.6%).91,92 One series documented late toxicities with vesicovaginal fistula (18%), radiation proctitis (31%), subcutaneous fibrosis (41%), vaginal strictures (14%), and dysuria (50%).91 Secondary cancers were discussed, although this risk is low with modern RT.93 Larger fields had higher rates of severe toxicities.76
With brachytherapy, both vesicovaginal and rectovaginal fistulas were noted, but the most common toxicity was vaginal stenosis.94 One study noted higher bladder and rectum doses than recommended by the American Brachytherapy Society, with maximum doses of 82.5%-95.7% of the prescribed dose compared with a recommended < 80% for bladder and < 65% for rectum.95 Acutely pain control was poor in brachytherapy procedures, with some women noting they “would've rather died from CC.”96 Aside from increased leukopenia, no differences were noted in toxicities on the basis of HIV status.83,97,98
Within BC, toxicities were only discussed in studies assessing a new technology or fractionation.38,75,99 Within hypofractionated postmastectomy RT, there were no grade 3+ toxicities, 50.6% grade 2 skin toxicities, and 19.2% grade 2 nausea.75 Postmastectomy RT with IMRT spared higher dose volumes to organs at risk but increased low-dose spillage compared with 3DCRT.99 Major complications with IORT were rare (2.8%) and included fat necrosis and skin erythema.38 Notably, side effects are challenging to report because of poor documentation.81
Outcomes: Palliation
Palliative RT is available in many centers with treatments ranging from 1 to 11 fractions and documented responses rates as high as 87%.58,65,100-102 This was mostly used for bone and brain metastases, but was available for pain, bleeding, obstructive symptoms, vaginal discharge, and neurologic symptoms.65,90,100,101 In a Nigerian series, palliative RT was offered to 23.2% of patients, with BC and CC comprising 43.5% and 16.1% of this cohort, respectively.103 In a Zimbabwean series, only 19.7% of patients with metastases were offered palliative RT.65 Despite performance status improvements and symptom relief, utilization is low overall. Notably, wait times for palliative RT are significantly less than definitive treatments, decreased from a median of 150 days to 0-15.90,100-102,104,105
Outcomes: Mental Health and Quality of Life
QoL and mental health are affected by complex treatments, toxicities, and changing family relationship dynamics. Qualitative studies noted negative impacts when patients had unmet informational needs, particularly regarding sexual side effects.96,106 The impact of delays is often underestimated, and those with longer wait times had an increased likelihood of psychiatric hospitalization in the first year of treatment.67 Survey data demonstrated that early-stage diagnoses, higher education, religiosity, supportive providers, and being married were protective.96,107 Education was protective in measures of caregiver burnout; however, 72% of respondents noted some burnout.108
Specific to brachytherapy, patients noted emotional distress because of fear, pain, and humiliation in regard to multiple providers and staff being present in treatments.96 In a survey of body image, patients with BC, compared with other cancers, noted higher scores of body dysmorphia, specifically in physical and sexual attractiveness, leading to lower self-esteem, increased tension, and decreased interest in life.109
DISCUSSION
Access to RT affects outcomes in SSA in numerous ways. Clinics are sparse, and obtaining treatment often requires cumbersome travel arrangements that affect financial stability, job security, and availability of socioemotional support. Sociocultural beliefs, such as use of traditional healers and stigmas surrounding cancer diagnoses, cause delayed care-seeking. Compounding limited access, many patients are unable to complete treatments because of financial and social burdens of fractionated RT. These barriers lead SSA to have worse OS in BC and CC compared with high- and middle-income nations.
Five-year OS rates for BC ranged from 38% to 79%, far lower than the United States, where survival is 99% in localized disease and 86% with regional involvement.43,54,61,89,110 Increased de novo metastatic disease likely drives these rates lower.111 High-income countries manage BC like a chronic disease with long-term survivors using indefinite systemic therapies; however, long-term drugs are cost-prohibitive to many in low-income countries. For CC, US all-stage 5-year OS is 66%; SSA cohorts ranged from 3.4% for palliative cases, 26% for all-comers, and 86.7% for localized disease, highlighting that SSA nations vary greatly in outcomes, with some touting cure rates that rival high-income nations, while others lag behind.35,41,80,81,84,86,102
Although the presence of barriers is ubiquitous, there is tremendous diversity between countries. Some nations, including Gabon and South Africa, have reasonable technological availability to deliver RT to their populations, while other nations lack RT altogether. Palliative RT had decreased wait times; however, this is likely because of urgency; these patients often present with symptomatic lesions leading to uncontrolled pain or bleeding that require rapid control. Although this speed is necessary, the competition for machine availability poses a risk of further prolonging wait times for definitive cases. Long-term improvements in accessibility for all require increased technology and staffing to allow for more rural centers, increasing regional capacity and reducing current travel burdens. Although large-scale developments take time, strategies should be implemented to increase interim capacity such as temporary lodging, education initiatives, and utilization of hypofractionated regimens and virtual visits.
Systematic initiatives are imperative. Partnerships between governments and outside organizations, such as in Gabon, may help expedite increased RT access by securing funding and providing training for local providers to deliver services, promoting sustainability.25 Programs to increase the number of trained oncologists and retention strategies are needed, like in Ethiopia; however, careful consideration of dual radiation/medical oncology program development is essential.23 Additional support staff in existing centers can improve efficiency, allowing for increased patient volume by permitting providers to focus exclusively on treatment planning–related care.
Equitable access requires more facilities within remote regions of SSA. While awaiting large-scale increases in the amount and location of available technologies, several strategies may help improve short-term access. Local lodging and resources can be provided for patients with long-distance traveling to reduce financial burdens, potentially building upon an existing Botswana model.60 These programs could be government-sponsored in nations that lack RT services to increase the feasibility of their citizens successfully traveling abroad for treatment. Additionally, physicians should be encouraged to adopt hypofractionated schedules, which decrease costs and improve completion rates, particularly as long-term data regarding five-fraction BC regimens become available. In CC, single-insertion brachytherapy procedures could be considered to decrease travel demands of multifraction courses; however, this increases needs for anesthesia support and inpatient admission that may limit utility.
The COVID-19 pandemic has increased utilization of virtual visits; allowing initial consultations to take place remotely can decrease travel burdens while still permitting necessary treatment-related discussions.112 Virtual multidisciplinary clinics may be possible as single visits significantly decreased wait times by allowing multiple providers to speak with patients in one encounter, preventing delays caused by referrals.70 This allows access to subspecialty providers that are otherwise inaccessible in rural clinics. As patients complete oncologic care, regional centers can manage follow-up, offloading the demands of oncologic providers and limiting needs for continued travel while still monitoring long-term toxicities and recurrences.
Additionally, negative impacts of stigma, treatment misconceptions, and fear can be combated with a focus on health literacy to dispel myths, normalize screening and treatment-seeking behaviors, and encourage women to be cognizant of symptoms that warrant medical attention.9,113-115 Particularly for women from lower educational backgrounds who were more likely to agree with statements about stigmatizing cancer beliefs, these programs can significantly improve screening uptake.116 A lack of care-seeking behavior leads to delays in presentation, late-stage diagnoses, and worse outcomes. Earlier diagnoses can allow for use of ultrahypofractionation, decreasing treatment lengths. Educational efforts in Ethiopia succeeded in increasing screening rates, which in turn improved survival by allowing timely access to curative treatments.117 Similarly, increased vaccination programming can, in the long term, lead to a decrease in CC incidence, a shift seen in developed nations, and thus, a decrease in the need for additional RT infrastructure.118
Although this review contains a comprehensive summary of the available literature, there are notable limitations. Included studies represent only 15 of 48 SSA countries, which may limit generalizability. Additionally, a significant portion of data was derived from retrospective studies which, though prone to biases, brings to light the significant need for additional research to elucidate barriers, and identity strategies to improve the treatment capacity in SSA.
As SSA's cancer burden increases, infrastructure designed to improve availability of oncologic treatments is essential as poor access directly relates to worse survival. Although long-term improvements require monetary commitments and large-scale national buy-in, small systematic changes such as use of telehealth, locoregional clinic follow-up, and increased availability of low-cost temporary housing can bridge gaps. Additional efforts are needed to determine country-specific solutions, assess technological needs, and expand the pool of qualified physicians and professionals in SSA.
ACKNOWLEDGMENT
Alluvial charts were created using https://rawgraphs.io/.
Christina Small
Other Relationship: Society for Women in Radiation Oncology
No other potential conflicts of interest were reported.
AUTHOR CONTRIBUTIONS
Conception and design: Sara E. Beltrán Ponce, Jean C. Bikomeye, Kirsten M.M. Beyer
Administrative support: Jean C. Bikomeye
Provision of study materials or patients: Rita Sieracki
Collection and assembly of data: Sara E. Beltrán Ponce, Sarah Adamma Abunike, Rita Sieracki
Data analysis and interpretation: Sara E. Beltrán Ponce, Sarah Adamma Abunike, Nixon Niyonzima, Pius Mulamira, Solomon Kibudde, Saryleine Ortiz de Choudens, Malika Siker, Christina Small, Kirsten M.M. Beyer
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Christina Small
Other Relationship: Society for Women in Radiation Oncology
No other potential conflicts of interest were reported.
REFERENCES
- 1.WHO : Cervical Cancer Overview. Geneva, WHO , 2022 [Google Scholar]
- 2.Jedy-Agba E, Joko WY, Liu B, et al. : Trends in cervical cancer incidence in sub-Saharan Africa. Br J Cancer 123:148-154, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sung H, Ferlay J, Siegel RL, et al. : Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209-249, 2021 [DOI] [PubMed] [Google Scholar]
- 4.WHO: WHO—International Agency for Research on Cancer : Breast Cancer Outcomes in Sub-Saharan Africa. 2021 [Google Scholar]
- 5.Panorama Global: TogetHER for Health : The Global Burden of Cervical Cancer. 2022 [Google Scholar]
- 6.WHO: WHO—Regional Office for Africa : Cervical Cancer. 2022 [Google Scholar]
- 7.WHO : Breast Cancer. Geneva, WHO , 2021 [Google Scholar]
- 8.Akinyemiju TF, McDonald JA, Lantz PM: Health care access dimensions and cervical cancer screening in South Africa: Analysis of the world health survey. BMC Public Health 15:382, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yimer NB, Mohammed MA, Solomon K, et al. : Cervical cancer screening uptake in sub-Saharan Africa: A systematic review and meta-analysis. Public Health 195:105-111, 1952 [DOI] [PubMed] [Google Scholar]
- 10.Friebel-Klingner TM, Luckett R, Bazzett-Matabele L, et al. : Clinical and sociodemographic factors associated with late stage cervical cancer diagnosis in Botswana. BMC Womens Health 21:267, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jedy-Agba E, McCormack V, Adebamowo C, et al. : Stage at diagnosis of breast cancer in sub-Saharan Africa: A systematic review and meta-analysis. Lancet Glob Health 4:e923-e935, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimani KN, Namukwaya E, Grant L, et al. : Cancer and palliative care in Africa. Eur J Cancer Care (Engl) 26:e12655, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Jemal A, Bray F, Forman D, et al. : Cancer burden in Africa and opportunities for prevention. Cancer 118:4372-4384, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Burt LM, McCormak M, Lecuru F, et al. : Cervix cancer in sub-Saharan Africa: An assessment of cervical cancer management. JCO Glob Oncol 7:173-182, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher BJ, Daugherty LC, Einck JP, et al. : Radiation oncology in Africa: Improving access to cancer care on the African continent. Int J Radiat Oncol Biol Phys 89:458-461, 2014 [DOI] [PubMed] [Google Scholar]
- 16.National Comprehensive Cancer Network : Breast Cancer (Version 4.2022). https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
- 17.National Comprehensive Cancer Network : Cervical Cancer (Version 1.2002). https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf
- 18.Akuoko CP, Armah E, Sarpong T, et al. : Barriers to early presentation and diagnosis of breast cancer among African women living in sub-Saharan Africa. PLoS One 12:e0171024, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morhason-Bello IO, Odedina F, Rebbeck TR, et al. : Challenges and opportunities in cancer control in Africa: A perspective from the African Organisation for Research and Training in Cancer. Lancet Oncol 14:e142-e151, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Tricco AC, Lillie E, Zarin W, et al. : PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med 169:467-473, 2018 [DOI] [PubMed] [Google Scholar]
- 21.McGowan J, Sampson M, Salzwedel DM, et al. : PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol 75:40-46, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Atieno OM, Opanga S, Martin A, et al. : Pilot study assessing the direct medical cost of treating patients with cancer in Kenya; findings and implications for the future. J Med Econ 21:878-887, 2018 [DOI] [PubMed] [Google Scholar]
- 23.Rick TJ, Habtamu B, Abreha A, et al. : Ethiopia: How the care of 100 million people pivots on a single cobalt teletherapy machine. Int J Radiat Oncol Biol Phys 106:230-235, 2020 [DOI] [PubMed] [Google Scholar]
- 24.Dereje N, Addissie A, Worku A, et al. : Association between waiting time for radiotherapy initiation and disease progression among women with cervical cancer in Addis Ababa, Ethiopia. Int J Cancer 149:1284-1289, 2021 [DOI] [PubMed] [Google Scholar]
- 25.Balogun O, James L, Chepkemoi L, et al. : Radiation therapy in Gabon: Multi-institutional collaboration as a paradigm for growth in the African Radiation Oncology Sector. Int J Radiat Oncol Biol Phys 106:663-668, 2020 [DOI] [PubMed] [Google Scholar]
- 26.Brown CA, Kohler RE, John O, et al. : Multilevel factors affecting time to cancer diagnosis and care quality in Botswana. Oncologist 23:1453-1460, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olaogun JG, Agodirin OS, Etonyeaku AC, et al. : Management of locally advanced breast cancer: Challenges and treatment outcomes in an emerging tertiary hospital in South-Western Nigeria. J Clin Diagn Res 15:PC01-PC05, 2021 [Google Scholar]
- 28.Olasehinde O, Alatise O, Omisore A, et al. : Contemporary management of breast cancer in Nigeria: Insights from an institutional database. Int J Cancer 148:2906-2914, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sedani A, Soliman AS, Msami K, et al. : Impact of initiating screening programs on referral and management of cervical cancer in Tanzania. J Glob Oncol 5:JGO1800052, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marta GN, Ramiah D, Kaidar-Person O, et al. : The financial impact on reimbursement of moderately hypofractionated postoperative radiation therapy for breast cancer: An international consortium report. Clin Oncol (R Coll Radiol) 33:322-330, 2021 [DOI] [PubMed] [Google Scholar]
- 31.Foerster M, Anderson BO, McKenzie F, et al. : Inequities in breast cancer treatment in sub-Saharan Africa: Findings from a prospective multi-country observational study. Breast Cancer Res 21:93, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leng J, Ntekim AI, Ibraheem A, et al. : Infrastructural challenges lead to delay of curative radiotherapy in Nigeria. JCO Glob Oncol 6:269-276, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Griesel M, Seraphin TP, Mezger NCS, et al. : Cervical cancer in sub-Saharan Africa: A multinational population-based cohort study of care and guideline adherence. Oncologist 26:e807-e816, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nyamhunga A, Ndlovu N, Kadzatsa W, et al. : Chemoradiation in stage IIIB cancer of the uterine cervix: A review of the Zimbabwean experience. JCO Glob Oncol 6:1554-1564, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deressa BT, Assefa M, Tafesse E, et al. : Contemporary treatment patterns and survival of cervical cancer patients in Ethiopia. BMC Cancer 21:1102, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park PH, Davey S, Fehr AE, et al. : Patient characteristics, early outcomes, and implementation lessons of cervical cancer treatment services in rural Rwanda. J Glob Oncol 4:1-11, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sood R, Masalu N, Connolly RM, et al. : Invasive breast cancer treatment in Tanzania: Landscape assessment to prepare for implementation of standardized treatment guidelines. BMC Cancer 21:527, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramdas Y, Benn CA, van Heerden M: First intraoperative radiation therapy center in Africa: First 2 years in operation, including COVID-19 experiences. JCO Glob Oncol 6:1696-1703, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chipidza FE, Mushonga M, Kanda C, et al. : Utilization and predictors of postmastectomy radiation receipt in an Oncology Center in Zimbabwe. Breast Cancer Res Treat 189:701-709, 2021 [DOI] [PubMed] [Google Scholar]
- 40.Tapera O, Dreyer G, Kadzatsa W, et al. : Determinants of access and utilization of cervical cancer treatment and palliative care services in Harare, Zimbabwe. BMC Public Health 19:1018, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nartey Y, Hill PC, Amo-Antwi K, et al. : Factors contributing to the low survival among women with a diagnosis of invasive cervical cancer in Ghana. Int J Gynecol Cancer 27:1926-1934, 2017 [DOI] [PubMed] [Google Scholar]
- 42.Randall TC, Ghebre R: Challenges in prevention and care delivery for women with cervical cancer in sub-Saharan Africa. Front Oncol 6:160, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lambert M, Mendenhall E, Kim AW, et al. : Health system experiences of breast cancer survivors in urban South Africa. Womens Health (Lond) 16:174550652094941, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vulpe H, Asamoah FA, Maganti M, et al. : External beam radiation therapy and brachytherapy for cervical cancer: The experience of the National Centre for Radiotherapy in Accra, Ghana. Int J Radiat Oncol Biol Phys 100:1246-1253, 2018 [DOI] [PubMed] [Google Scholar]
- 45.Dairo MD, Adamu DB, Onimode YA, et al. : Characteristics and determinants of patients discontinuation of breast cancer follow-up care at the Radiation Oncology Department, University College Hospital, Ibadan, Nigeria. Int J Breast Cancer 2018:1597964, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soko GF, Burambo AB, Mngoya MM, et al. : Public awareness and perceptions of radiotherapy and their influence on the use of radiotherapy in Dar es Salaam, Tanzania. J Glob Oncol 5:1-10, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olson AC, Afyusisye F, Egger J, et al. : Cancer incidence and treatment utilization patterns at a regional cancer center in Tanzania from 2008-2016: Initial report of 2,772 cases. Cancer Epidemiol 67:101772, 2020 [DOI] [PubMed] [Google Scholar]
- 48.Brown E, Gorman D, Knowles G, et al. : The Edinburgh Malawi Cancer Partnership: Helping to establish multidisciplinary cancer care in Blantyre, Malawi. J R Coll Physicians Edinb 46:14-17, 2016 [DOI] [PubMed] [Google Scholar]
- 49.Tumba N, Adewuyi SA, Eguzo K, et al. : Radiotherapy waiting time in Northern Nigeria: Experience from a resource-limited setting. Ecancermedicalscience 14:10972020, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mahantshetty U, Lavanya G, Grover S, et al. : Incidence, treatment and outcomes of cervical cancer in low- and middle-income countries. Clin Oncol (R Coll Radiol) 33:e363-e371, 2021 [DOI] [PubMed] [Google Scholar]
- 51.DeBoer RJ, Umutoni V, Bazzett-Matabele L, et al. : Cervical cancer treatment in Rwanda: Resource-driven adaptations, quality indicators, and patient outcomes. Gynecol Oncol 164:370-378, 2022 [DOI] [PubMed] [Google Scholar]
- 52.Hassan R, Sheeraye M, Astini C, et al. : Current practice in diagnosis and treatment of breast cancer in Cheicko Balbala Hospital Djibouti. Ann Ital Chir 91:592-597, 2020 [PubMed] [Google Scholar]
- 53.O'Neil DS, Keating NL, Dusengimana JMV, et al. : Quality of breast cancer treatment at a rural cancer center in Rwanda. J Glob Oncol 4:1-11, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Traore B, Keita M, Toure A, et al. : Impact of surgery associated with radiotherapy on the prognosis of breast cancer—Guinea Breast Cancer Cohort Study. Cancer Rep (Hoboken) 5:e1554, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vanderpuye V, Dadzie MA, Huo D, et al. : Assessment of breast cancer management in sub-Saharan Africa. JCO Glob Oncol 7:1593-1601, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grover S, Mehta P, Wang Q, et al. : Association between CD4 count and chemoradiation therapy outcomes among cervical cancer patients with HIV. J Acquir Immune Defic Syndr 85:201-208, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deressa BT, Cihoric N, Badra EV, et al. : Breast cancer care in northern Ethiopia—Cross-sectional analysis. BMC Cancer 19:393, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tapera O, Kadzatsa W, Nyakabau AM, et al. : Sociodemographic inequities in cervical cancer screening, treatment and care amongst women aged at least 25 years: Evidence from surveys in Harare, Zimbabwe. BMC Public Health 19:428, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chibwesha C, Pinder LF, Musonda A, et al. : A comprehensive assessment of breast and cervical cancer control infrastructure in Zambia. J Cancer Policy 13:24-29, 2017 [Google Scholar]
- 60.Efstathiou JA, Heunis M, Karumekayi T, et al. : Establishing and delivering quality radiation therapy in resource-constrained settings: The story of Botswana. J Clin Oncol 34:27-35, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abdalla Elhassan SI: The five-year survival rate of breast cancer at Radiation and Isotopes Centre Khartoum, Sudan. Heliyon 6:e04615, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moustafa M, Mali ME, Lopez-Verdugo F, et al. : Surveying and mapping breast cancer services in Ghana: A cross-sectional pilot study in the Eastern Region. BMJ Open 11:e051122, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cubasch H, Joffe M, Ruff P, et al. : Breast conservation surgery versus total mastectomy among women with localized breast cancer in Soweto, South Africa. PLoS One 12:e0182125, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O'Neil DS, Chen WC, Ayeni O, et al. : Breast cancer care quality in South Africa's public health system: An evaluation using American Society of Clinical Oncology/National Quality Forum measures. J Glob Oncol 5:1-16, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mushonga M, Nyakabau AM, Ndlovu N, et al. : Patterns of palliative radiotherapy utilization for patients with metastatic breast cancer in Harare, Zimbabwe. JCO Glob Oncol 7:1212-1219, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rudd P, Gorman D, Meja S, et al. : Cervical cancer in southern Malawi: A prospective analysis of presentation, management, and outcomes. Malawi Med J 29:124-129, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nassali MN, Melese T, Modimowame J, et al. : Timelines to cervical cancer diagnosis and treatment at a tertiary hospital in Botswana. Int J Womens Health 13:385-393, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakisige C, Schwartz M, Ndira AO: Cervical cancer screening and treatment in Uganda. Gynecol Oncol Rep 20:37-40, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chuang L, Rainville N, Byrne M, et al. : Cervical cancer screening and treatment capacity: A survey of members of the African Organisation for Research and Training in Cancer (AORTIC). Gynecol Oncol Rep 38:100874, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grover S, Chiyapo SP, Puri P, et al. : Multidisciplinary gynecologic oncology clinic in Botswana: A model for multidisciplinary oncology care in low- and middle-income settings. J Glob Oncol 3:666-670, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vanderpuye VD, Olopade OI, Huo D: Pilot survey of breast cancer management in sub-Saharan Africa. J Glob Oncol 3:194-200, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mungo C, Randa M, Shauri A, et al. : Characteristics, stage at presentation, and status of women with cervical cancer at a major referral center in western Kenya. Int J Gynecol Obstet 156:173-174, 2022 [DOI] [PubMed] [Google Scholar]
- 73.Olaogun JG, Omotayo JA, Ige JT, et al. : Socio-demographic, pattern of presentation and management outcome of breast cancer in a semi-urban tertiary health institution. Pan Afr Med J 36:363, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hanappe M, Nicholson LT, Elmore SNC, et al. : International radiotherapy referrals from rural Rwanda: Implementation processes and early clinical outcomes. J Glob Oncol 4:1-12, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aliyu UM, Kehinde A: Tolerance and outcome of hypofractionated post-mastectomy radiotherapy among elderly breast cancer patients in a specialized center in Nigeria. Transl Cancer Res 9:6833-6840, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ralefala TB, van Wijk L, Saidu R: A retrospective study of stage IB node-negative cervical cancer treated with adjuvant radiation with standard pelvic versus central small pelvic fields. S Afr J Gynaecol Oncol 10:11-15, 2018 [Google Scholar]
- 77.Kisling K, Zhang L, Simonds H, et al. : Fully automatic treatment planning for external-beam radiation therapy of locally advanced cervical cancer: A tool for low-resource clinics. J Glob Oncol 5:1-9, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Amadori D, Serra P, Bucchi L, et al. : The Mwanza Cancer Project. Lancet Oncol 17:146-148, 2016 [DOI] [PubMed] [Google Scholar]
- 79.Bakari LL, Akarro RR, Msengwa AS, et al. : Some traits on the outcome of the treatment of cervical cancer in Tanzania: A case study of Ocean Road Cancer Institute (ORCI). Tanzania J Health Res 21:1-9, 2019 [Google Scholar]
- 80.Adusei-Poku P, Opoku S, Antwi W: Survival rate of cervical cancer: A five year review at the National Center for Radiotherapy and Nuclear Medicine, Korle-Bu Teaching Hospital, Accra, Ghana. Eur J Cancer 72:S172, 2017 [Google Scholar]
- 81.Khamis SI, Mrema AS, Katanga J, et al. : Survival in cervical cancer and its predictors at ocean road cancer institute from January to December 2012. JCO Glob Oncol 7:734-739, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Asamoah FA, Yarney J, Scott A, et al. : Comparison of definitive cervical cancer management with chemotherapy and radiation between two centers with variable resources and opportunities for improved treatment. JCO Glob Oncol 6:1510-1518, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grover S, Bvochora-Nsingo M, Yeager A, et al. : Impact of human immunodeficiency virus infection on survival and acute toxicities from chemoradiation therapy for cervical cancer patients in a limited-resource setting. Int J Radiat Oncol Biol Phys 101:201-210, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Simonds HM, Botha MH, Neugut AI, et al. : Five-year overall survival following chemoradiation among HIV-positive and HIV-negative patients with locally advanced cervical carcinoma in a South African cohort. Gynecol Oncol 151:215-220, 2018 [DOI] [PubMed] [Google Scholar]
- 85.Gizaw M, Addissie A, Getachew S, et al. : Cervical cancer patients presentation and survival in the only oncology referral hospital, Ethiopia: A retrospective cohort study. Infect Agent Cancer 12:61, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.MacDuffie E, Bvochora-Nsingo M, Chiyapo S, et al. : Five-year overall survival following chemoradiation therapy for locally advanced cervical carcinoma in women living with and without HIV infection in Botswana. Infect Agent Cancer 16:55, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Scott AA, Yarney J, Vanderpuye V, et al. : Outcomes of patients with cervical cancer treated with low- or high-dose rate brachytherapy after concurrent chemoradiation. Int J Gynecol Cancer 8:670-678, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grover S, Ning MS, Bale M, et al. : Chemoradiation versus radiation alone in stage IIIB cervical cancer patients with or without human immunodeficiency virus. Int J Gynecol Cancer 31:1220-1227, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ali-Gombe M, Mustapha MI, Folasire A, et al. : Pattern of survival of breast cancer patients in a tertiary hospital in South West Nigeria. Ecancermedicalscience 15:1192, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rick T, Habtamu B, Tigeneh W, et al. : Patterns of care of cancers and radiotherapy in Ethiopia. J Glob Oncol 5:1-8, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moelle U, Mathewos A, Aynalem A, et al. : Cervical cancer in Ethiopia: The effect of adherence to radiotherapy on survival. Oncologist 23:1024-1032, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kibaara M, Degu A: Assessment of adverse events among cervical cancer patients at Kenyatta National Hospital. J Oncol Pharm Pract 29:326-332, 2023 [DOI] [PubMed] [Google Scholar]
- 93.Suleiman SA, Qi Y, Chen Z, et al. : Monte Carlo study of organ doses and related risk for cancer in Tanzania from scattered photons in cervical radiation treatment involving Co-60 source. Physica Med 62:13-19, 2019 [DOI] [PubMed] [Google Scholar]
- 94.Chibonda S, Ndlovu N, Tsikai N, et al. : High dose rate intra-cavitary brachytherapy with cobalt 60 source for locally advanced cervical cancer: The Zimbabwean experience. Infect Agent Cancer 16:1, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oyekunle EO, Ibhade R, Akinlade BI: Assessment of cartesian co-ordinates-based bladder and rectal dose and variability with tandem-ring angles first Nigerian experience in high-dose-rate brachytherapy. Br J Radiol 91:20180258, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dzaka AD, Maree JE: Experiences of women receiving high dose rate brachytherapy for cervical cancer at an academic hospital. S Afr J Gynaecol Oncol 8:42-45, 2016 [Google Scholar]
- 97.Einstein MH, Ndlovu N, Lee J, et al. : Cisplatin and radiation therapy in HIV-positive women with locally advanced cervical cancer in sub-Saharan Africa: A phase II study of the AIDS malignancy consortium. Gynecol Oncol 153:20-25, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mdletshe S, Munkupa H, Lishimpi K: Acute toxicity in cervical cancer HIV-positive vs. HIV-negative patients treated by radical chemo-radiation in Zambia. S Afr J Gynaecol Oncol 8:37-41, 2016 [Google Scholar]
- 99.Adeneye S, Akpochafor M, Adegboyega B, et al. : Evaluation of three-dimensional conformal radiotherapy and intensity modulated radiotherapy techniques for left breast post-mastectomy patients: Our experience in Nigerian Sovereign Investment Authority-Lagos University Teaching Hospital Cancer Center, South-West Nigeria. Eur J Breast Health 17:247-252, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ibrahim H, Abdullahi A: Factors influencing the use of palliative external beam radiotherapy for advanced breast cancer patients in the University College Hospital, Ibadan. West Afr J Radiol 27:12-17, 2020 [Google Scholar]
- 101.Ibrahim H, Yaroko AA: Palliative external beam radiotherapy for advanced breast cancer patients with brain metastasis in the university college hospital Ibadan. Ann Afr Med 18:127-131, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morphis AK, Napo H, Joubert G: Retrospective review of 37.4 Gy in 11 fractions for the palliation of advanced cervical cancer. South Afr J Gynaecol Oncol 13:36-41, 2021 [Google Scholar]
- 103.Joseph AO, Salako O, Alabi A, et al. : Cancer pain control in a Nigerian oncology clinic: Treating the disease and not the patient. Pan Afr Med J 40:104, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ibrahim H, Bello U: Dose fractionation schemes for palliative external beam radiotherapy on painful bone metastasis from breast cancer. Borno Med J 17:1-9, 2020 [Google Scholar]
- 105.Kaila I, Maree JE: An exploration into the level and characteristics of pain experienced by South African women treated for cervical cancer. Int J Africa Nurs Sci 8:141-148, 2018 [Google Scholar]
- 106.Long D, Friedrich-Nel HS, Joubert G: Patients' informational needs while undergoing brachytherapy for cervical cancer. Int J Qual Health Care 28:200-208, 2016 [DOI] [PubMed] [Google Scholar]
- 107.Kyei KA, Yakanu F, Donkor A, et al. : Quality of life among cervical cancer patients undergoing radiotherapy. Pan Afr Med J 35:125, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jite IE, Adetunji AA, Folasire AM, et al. : Caregiver burden and associated factors amongst carers of women with advanced breast cancer attending a radiation oncology clinic in Nigeria. Afr J Prim Health Care Fam Med 13:e1-e8, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Van Oers H, Schlebusch L: Breast cancer patients’ experiences of psychological distress, hopelessness, and suicidal ideation. J Nat Sci Med 4:250-257, 2021 [Google Scholar]
- 110.American Cancer Society : Survival Rates for Breast Cancer. Atlanta, GA, American Cancer Society, 2022 [Google Scholar]
- 111.Thrift-Perry M, Cabanes A, Cardoso F, et al. : Global analysis of metastatic breast cancer policy gaps and advocacy efforts across the patient journey. Breast 41:93-106, 2018 [DOI] [PubMed] [Google Scholar]
- 112.Mann DM, Chen J, Chunara R, et al. : COVID-19 transforms health care through telemedicine: Evidence from the field. J Am Med Inform Assoc 27:1132-1135, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fiander AN: The prevention of cervical cancer in Africa. Womens Health (Lond) 7:121-132, 2011 [DOI] [PubMed] [Google Scholar]
- 114.Brinton LA, Figueroa JD, Awuah B, et al. : Breast cancer in sub-Saharan Africa: Opportunities for prevention. Breast Cancer Res Treat 144:467-478, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chinn J, Tewari KS: Multimodality screening and prevention of cervical cancer in sub-Saharan Africa: A collaborative model. Curr Opin Obstet Gynecol 32:28-35, 2020 [DOI] [PubMed] [Google Scholar]
- 116.Williams MS, Kenu E, Adanu A, et al. : Awareness and beliefs about cervical cancer, the HPV vaccine, and cervical cancer screening among Ghanaian women with diverse education levels. J Cancer Educ 34:897-903, 2019 [DOI] [PubMed] [Google Scholar]
- 117.Abu SH, Woldehanna BT, Nida ET, et al. : The role of health education on cervical cancer screening uptake at selected health centers in Addis Ababa. PLoS One 15:e0239580, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mix JM, Van Dyne EA, Saraiya M, et al. : Assessing impact of HPV vaccination on cervical cancer incidence among women aged 15–29 years in the United States, 1999–2017: An ecologic study. Cancer Epidemiol Biomarkers Prev 30:30-37, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.American Cancer Society : Survival Rates for Cervical Cancer. Atlanta, GA, American Cancer Society, 2022 [Google Scholar]
- 120.McGale P, Taylor C, Correa C, et al. : Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 383:2127-2135, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Heunis M, Lombe DC, McCaul M: Retrospective analysis of radiotherapy outcomes in breast cancer radiotherapy at a single institution. S Afr J Gynaecol Oncol 10:1-4, 2018 [Google Scholar]
- 122.Zubairu IH, Theyra HE, Nuhu T: Demographic pattern of cervical cancer patients seen in a radiotherapy treatment facility in Northern Nigeria. Indian J Gynecol Oncol 15:52, 2017 [Google Scholar]
- 123.Adejoh SO, Olorunlana A, Adejayan A: Patients' experiences of family members' reactions to diagnosis of breast cancer and support in the management of breast cancer in Lagos, Nigeria. Palliat Support Care 19:592-597, 2021 [DOI] [PubMed] [Google Scholar]
- 124.Simonds H, Botha MH, Ellmann A, et al. : HIV status does not have an impact on positron emission tomography-computed tomography (PET-CT) findings or radiotherapy treatment recommendations in patients with locally advanced cervical cancer. Int J Gynecol Cancer 29:1252-1257, 2019 [DOI] [PubMed] [Google Scholar]