PURPOSE:
Community-academic partnerships have the potential to improve access to clinical trials for under-represented minority patients who more often receive cancer treatment in community settings. In 2017, the Memorial Sloan Kettering (MSK) Cancer Center began opening investigator-initiated clinical trials in radiation oncology in targeted community-based partner sites with a high potential to improve diverse population accrual. This study evaluates the effectiveness of a set of implementation strategies for increasing overall community-based enrollment and the resulting proportional enrollment of Hispanic patients on trials on the basis of availability in community-based partner sites.
METHODS:
An interrupted time series analysis evaluating implementation strategies was conducted from April 2018 to September 2021. Descriptive analysis ofHispanic enrollment on investigator-initiated randomized therapeutic radiation trials open at community-based sites was compared with those open only at themain academic center.
RESULTS:
Overall, 84 patients were enrolled in clinical trials in the MSK Alliance, of which 48 (56%) identified as Hispanic. The quarterly patient enrollment pre- vs postimplementation increased from 1.39 (95% CI, –3.67 to 6.46) to 9.42 (95% CI, 2.05 to 16.78; P5 .017). In the investigator-initiated randomized therapeutic radiation trials open in the MSK Alliance, Hispanic representation was 11.5% and 35.9% in twometastatic trials and 14.2% in a proton versus photon trial. Inmatched trials open only at the main academic center, Hispanic representation was 5.6%, 6.0%, and 4.0%, respectively.
CONCLUSION:
A combination of practice-level and physician-level strategies implemented at community-based partner sites was associated with increased clinical trial enrollment, which translated to improved Hispanic representation. This supports the role Q:2 of strategic community-academic partnerships in addressing disparities in clinical trial enrollment.
INTRODUCTION
The National Institutes of Health Revitalization Act of 1993 established guidelines for inclusion of women and minorities in National Institutes of Health–funded clinical trials.1 A subsequent study of nonsurgical National Cancer Institute Clinical Trial Cooperative Group trials in breast, colorectal, lung, and prostate cancers showed that Hispanic patients were 28% less likely to enroll on clinical trials than White patients.2 Between 1996-1998 and 2000-2002, Hispanic enrollment remained unchanged, and then more recent data through 2016 showed that it may even be decreasing.3 Within radiation oncology, a study of prospective trials conducted from 1996 to 2019 found that only 10% of all participants identified as Hispanic, which the authors noted to be lower than their proportional in the US population according to the 2018 census.4 Hispanic representation is even lower among trials testing more advanced technologies, such as proton therapy.4
Community-academic partnerships could potentially improve patient access to clinical trials by bringing trials to the community, where under-represented patients are more often treated for cancer.5 Facilitators in the community include cultural and linguistic competence among staff who are embedded in the community and a greater level of trust.6 However, barriers to enrollment in this setting include inadequate research budgets and support staff (including review boards), inadequate administrative support (including legal), and lack of trial availability (which is related to strict eligibility criteria).7
In 2017, our tertiary cancer center (Memorial Sloan Kettering [MSK]) began opening investigator-initiated clinical trials in radiation oncology at MSK Cancer Alliance community-based partner hospitals.
In the current study, we evaluate and report (1) the effectiveness of select implementation strategies in facilitating general community-based clinical trial accrual in radiation oncology and (2) the impact of enhanced multicenter trial enrollment on Hispanic representation on investigator-initiated randomized trials compared with enrollment only at a main academic center.
METHODS
Data Collection
We included all patients enrolled in radiation oncology investigator-initiated therapeutic clinical trials at the MSK Alliance partner sites from April 2018 to September 2021. Patient-level data included age, self-reported race and ethnicity, trial protocol number, consenting physician, and institution (MSK v MSK Alliance partner). Clinical trial–level data collected included date opened at MSK, date opened in the MSK Alliance site (if applicable), and disease site (ie, genitourinary, breast, thoracic/lung, head and neck, and metastatic). Physician years of experience was estimated from the medical school graduation year, which is publicly available.
In addition, patients enrolled at MSK on select matched investigator-initiated randomized therapeutic radiation trials open during the study period were included. This study was approved by the IRB of MSK.
Implementation Strategies
Strategies to promote general clinical trial enrollment within the MSK Alliance were guided by Expert Recommendation for Implementing Change8 and were implemented in March 2020. They included the following:
altered financial incentives at the practice level (70% per-patient cost paid to the partner site);
building a coalition of physicians treating metastatic disease (defined as recruiting and cultivating relationships with partners, in this case, through an externally funded grant for practice improvement in radiation therapy for bone metastases);
practice facilitation including sharing contact information to promote physician-physician communication regarding clinical trial issues (ie, patient eligibility, clinical decision making, radiation treatment planning, etc).
Statistical Analysis
A Wilcoxon rank-sum test was used to compare the absolute number of trial enrollments per quarter. In addition, an interrupted time series analysis was conducted to evaluate changes pre- and postimplementation on the basis of both the change in intercept and slope. Descriptive statistics were used to report the pooled proportion of patients reporting Hispanic ethnicity enrolled in each randomized clinical trial.
RESULTS
Participant Characteristics
Overall, 84 patients were enrolled in the Alliance trials during the study period. Participants had a median age of 67 (range 44-89) years. Forty-eight (56%) participants self-identified as Hispanic.
Evaluation of Implementation Strategies' Impact
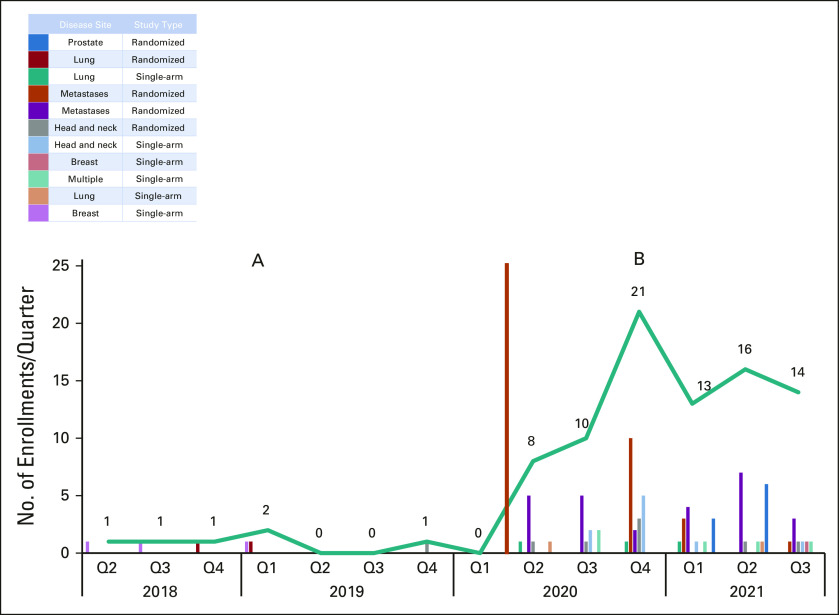
Patient enrollment in the MSK Alliance increased from a mean of 1 per quarter (preimplementation) to 11 per quarter (postimplementation; P = .002; Fig 1). In the interrupted time series analysis, the intercept increased from 1.39 (95% CI, –3.67 to 6.46) before to 9.42 (95% CI, 2.05 to 16.78; P = .017) after implementation. The slope (rate of change in patient enrollments) was not significantly different from zero before the intervention (coefficient –0.14; 95% CI, –1.15 to 0.86; P = .76) or afterward (coefficient 1.29; 95% CI, –0.56 to 3.14; P = .15). The total number of therapeutic clinical trials open in the MSK Alliance before vs after implementation was similar (5 v 8), and patients were enrolled on a diversity of trials (Fig 1).
FIG 1.
Community-based clinical trial enrollment per quarter (A) before and (B) after implementation (orange line) of altered financial incentives, building a coalition (for metastatic disease) and practice facilitation from April 2018 (Q2) to September 2021 (Q3). Q, quarter.
Among 41 radiation oncologists eligible to enroll patients in the Alliance, 9 (22%) accrued at least one patient at any time during the study period. Among enrolling physicians, the total number of patients enrolled per physician ranged from 1 to 36. Physician enrollers had a median of 14 years of experience (interquartile range 9.25-24.25) compared with 28.5 years of experience (interquartile range 13-37.5) for nonenrollers.
Hispanic Representation on Randomized Trials
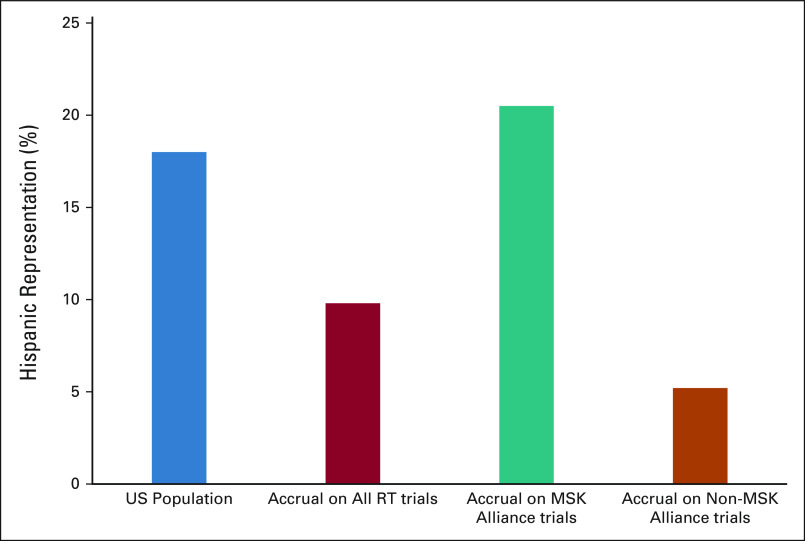
Three investigator-initiated randomized therapeutic radiation clinical trials were open in the Alliance during the study period. The proportion of patients self-identifying as Hispanic was 11.5% (9 of 78) and 35.9% (28 of 78) in two metastatic trials and 14.2% (8 of 56) in a proton vs photon randomized trial for a pooled proportion of 21.2% (45 of 212).
Among three matched randomized trials open only at MSK (non-Alliance), the proportion of patients self-identifying as Hispanic was 5.6% (6 of 107) and 6.0% (4 of 65) in two metastatic trials and 4.0% (4 of 101) in a proton versus photon randomized trial for a pooled proportion of 5.1% (14 of 273; Fig 2).
FIG 2.
Hispanic patient representation in randomized investigator-initiated radiation oncology clinical trials opened in the MSK Cancer Alliance (n = 3) compared with those open only at MSK (non-Alliance, n = 3). Proportions of Hispanic representation in the US population (on the basis of the 2018 US Census) and on all radiation trials (n = 71)4 are included for reference. MSK, Memorial Sloan Kettering; RT, radiation therapy.
DISCUSSION
This study demonstrates that a combination of implementation strategies at the practice and physician level is associated with clinical trial enrollment within a targeted community-academic partnership. A positive consequence of increased community-based clinical trial enrollment was to reduce disparities in representation of minority populations, including Hispanic patients in this study. Such efforts improve access to trials for patients while increasing generalizability of trial results for real-life practice environments.9
Practice-level incentives alone have been shown in a previous randomized study to be ineffective at promoting clinical trial accrual.10 This, combined with large variations in individual physician accrual in our study, leads us to hypothesize that physician-level engagement is critical in enhancing enrollment. For this study, a coalition of physicians treating metastatic disease was convened as part of a National Comprehensive Cancer Network grant.11 Although trial accrual is not an aim of the grant, such structured efforts likely contribute to enhancing trial enrollment. The relatively high accrual on metastatic trials, observed after the creation of the metastases physician coalition, further supports this hypothesis.
We postulate that an efficient strategy for reducing disparities in trial representation is to prioritize targeted partnerships with hospitals that serve under-represented populations, are embedded in underserved communities, and have developed strong relationships on the basis of mutual respect and trust. A majority of accruals of Hispanic patients in the current study originated from our partner site in Miami, FL, which serves a higher proportion of Hispanic patients. Such sites are more likely to have clinicians and research staff who can best deliver culturally appropriate health education and recommendations, perhaps even in their preferred language, particularly benefiting Hispanic patients with limited English proficiency.12
Major academic institutions, which often treat a less diverse patient population, including higher educational level, White, non-Hispanic, and higher socioeconomic status, have a responsibility to advance efforts to increase participant diversity in clinical trials. Such efforts should be supported with tailored and targeted external support grant mechanisms. Funding agencies should prioritize promotion of minority representation in research13 by developing mechanisms that hold grant recipients accountable and encourage minimal goals for diverse study participation. Although reporting requirements already exist, only 10%-30% of cancer trials report ethnicity, specifically Hispanic.3,14,15 In the study by Bero et al4 evaluating representation in radiation oncology, Hispanic ethnicity was reported in 58% trials, which is encouraging.
This study has several limitations. First, the implementation of multiple strategies simultaneously and the lack of a control group limit our ability to identify the key factor driver of practice change and to control for secular trends, respectively. Second, sensitivity analysis of our interrupted time series suggests that the nonzero slope postimplementation was sensitive to the value in quarter 4 2020, which was just before the placement of enrollment caps (January 2021) because of COVID-19–related staffing shortages. In addition, the evaluated randomized trials were open for variable lengths of time, which we did not adjust for. However, such adjustments would increase the effect of accruing patients from community-based sites. We instead opted for a realistic snapshot given the reality of delays associated with opening trials at multiple institutions.
In conclusion, our findings highlight the value in working with and supporting targeted community sites that serve a greater proportion of Hispanic patients to increase representation and reduce disparities in oncology clinical trials. The combination of practice-level financial support, physician-level engagement, and practice facilitation encouraged enrollment generally in clinical trials at partner sites, which positively affected equity in clinical trial accrual. This study adds to a growing body of literature, suggesting that the road to a sustainable solution requires multifaceted approaches, thoughtfully designed academic-community partnerships, and dedicated and funded programs that leverage the capabilities of appropriately targeted competent community partner sites.
ACKNOWLEDGMENT
The authors would like to acknowledge Dr Simon Powell, Chair of the Department of Radiation Oncology at Memorial Sloan Kettering for his leadership in supporting clinical trial enrollment in the MSK Cancer Alliance.
Daniel R. Gomez
Honoraria: Varian Medical Systems, Merck, Bristol Myers Squibb, AstraZeneca, RefleXion Medical, Vindico Medical Education, US Oncology, GRAIL
Consulting or Advisory Role: Olympus Medical Systems, Medtronic, Johnson & Johnson/Janssen
Research Funding: Merck, Varian Medical Systems, AstraZeneca, Bristol Myers Squibb
Travel, Accommodations, Expenses: Varian Medical Systems, AstraZeneca, Merck, Vindico Medical Education, US Oncology, Driver Inc
Jonathan T. Yang
Stock and Other Ownership Interests: Nanocan Therapeutics
Consulting or Advisory Role: Debiopharm Group, Galera Therapeutics, AstraZeneca, Nanocan Therapeutics
Research Funding: AstraZeneca, Kazia Therapeutics, X-RAD Therapeutics
Nancy Y. Lee
Consulting or Advisory Role: Merck, Pfizer, Merck Serono, Sanofi, Mirati Therapeutics, Roche/Genentech
Research Funding: AstraZeneca, Pfizer (Inst)
Andreas Rimner
Honoraria: More Health
Consulting or Advisory Role: AstraZeneca, Merck, Boehringer Ingelheim
Research Funding: Varian Medical Systems (Inst), Boehringer Ingelheim (Inst), Pfizer (Inst), AstraZeneca (Inst), Merck (Inst)
Yoshiya Yamada
Employment: Memorial Sloan-Kettering Cancer Center
Leadership: Chordoma Foundation
Honoraria: Varian Medical Systems, BrainLAB, Vision RT
Speakers' Bureau: BrainLAB, Vision RT, Varian Medical Systems
Research Funding: Varian Medical Systems
Travel, Accommodations, Expenses: Philips Healthcare
Other Relationship: University of Wollongong
Michael J. Zelefsky
Honoraria: Accuray
Consulting or Advisory Role: Alpha Tau, Boston Scientific
Patents, Royalties, Other Intellectual Property: Ferring provided partial funding for a clinical trial (Inst)
Other Relationship: Editor in Chief for Brachytherapy
Noah S. Kalman
Consulting or Advisory Role: Naveris
Rupesh R. Kotecha
Honoraria: Accuray, Novocure, Elekta, BrainLAB, Elsevier, ViewRay, PeerView
Consulting or Advisory Role: ViewRay, Novocure
Speakers' Bureau: Novocure
Research Funding: Medtronic (Inst), Blue Earth Diagnostics (Inst), Novocure (Inst), GT Medical Technologies (Inst), AstraZeneca (Inst), Exelixis (Inst), ViewRay (Inst), BrainLAB (Inst)
Travel, Accommodations, Expenses: PeerView
Minesh P. Mehta
Leadership: Oncoceutics
Stock and Other Ownership Interests: Chimerix
Consulting or Advisory Role: Karyopharm Therapeutics, Mevion Medical Systems, ZappRx, Sapience Therapeutics, Xoft
Patents, Royalties, Other Intellectual Property: WARF patent 14/934,27, Topical vasoconstrictor preparations and methods for protecting cells during cancer chemotherapy and radiotherapy
Uncompensated Relationships: Xcision Medical Systems, ViewRay
Michael D. Chuong
Employment: Fertility and IVF Center of Miami
Honoraria: ViewRay, Sirtex Medical
Consulting or Advisory Role: ViewRay, Advanced Accelerator Applications
Speakers' Bureau: ViewRay, Sirtex Medical
Research Funding: AstraZeneca/MedImmune, ViewRay
Travel, Accommodations, Expenses: ViewRay
Andrew L. Salner
Consulting or Advisory Role: Best Doctors Inc
Jamie S. Ostroff
Patents, Royalties, Other Intellectual Property: UptoDate
David G. Pfister
Consulting or Advisory Role: Boehringer Ingelheim, Incyte
Research Funding: Boehringer Ingelheim (Inst), AstraZeneca (Inst), Exelixis (Inst), Novartis (Inst), MedImmune (Inst), Merck (Inst), Genentech/Roche (Inst), Lilly (Inst), Bayer (Inst), Eisai (Inst), Regeneron (Inst), Atara Biotherapeutics (Inst), MeiraGTx (Inst), Hookipa Pharma (Inst)
Fumiko Chino
This author is a Consultant Editor for JCO Oncology Practice. Journal policy recused the author from having any role in the peer review of this manuscript.
Jillian Tsai
Honoraria: Varian Medical Inc
Consulting or Advisory Role: Varian Medical Systems
Erin F. Gillespie
Other Relationship: eContour
No other potential conflicts of interest were reported.
SUPPORT
Supported in part by a National Comprehensive Cancer Network (NCCN) Pfizer EMBRACE Community-Partnership grant and a National Institutes of Health (NIH) core grant to Memorial Sloan Kettering (P30 CA008748).
AUTHOR CONTRIBUTIONS
Conception and design: Diana Lin, Minesh P. Mehta, Joseph E. Panoff, Andrew L. Salner, Jamie S. Ostroff, Oren Cahlon, David G. Pfister, Fumiko Chino, Jillian Tsai
Administrative support: Minesh P. Mehta
Provision of study materials or patients: Nancy Y. Lee, Minesh P. Mehta, Andrew L. Salner
Collection and assembly of data: Nahomy Ledesma Vicioso, Diana Lin, Jonathan T. Yang, Erin F. Gillespie
Data analysis and interpretation: Nahomy Ledesma Vicioso, Diana Lin, Daniel R. Gomez, Jonathan T. Yang, Nancy Y. Lee, Andreas Rimner, Yoshiya Yamada, Michael J. Zelefsky, Noah S. Kalman, Charles E. Rutter, Rupesh R. Kotecha, Minesh P. Mehta, Michael D. Chuong, Lisa C. Diamond, Noah J. Mathis, Oren Cahlon, David G. Pfister, Zhigang Zhang, Fumiko Chino, Jillian Tsai, Erin F. Gillespie
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Implementation Strategies to Increase Clinical Trial Enrollment in a Community-Academic Partnership and Impact on Hispanic Representation: An Interrupted Time Series Analysis
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Daniel R. Gomez
Honoraria: Varian Medical Systems, Merck, Bristol Myers Squibb, AstraZeneca, RefleXion Medical, Vindico Medical Education, US Oncology, GRAIL
Consulting or Advisory Role: Olympus Medical Systems, Medtronic, Johnson & Johnson/Janssen
Research Funding: Merck, Varian Medical Systems, AstraZeneca, Bristol Myers Squibb
Travel, Accommodations, Expenses: Varian Medical Systems, AstraZeneca, Merck, Vindico Medical Education, US Oncology, Driver Inc
Jonathan T. Yang
Stock and Other Ownership Interests: Nanocan Therapeutics
Consulting or Advisory Role: Debiopharm Group, Galera Therapeutics, AstraZeneca, Nanocan Therapeutics
Research Funding: AstraZeneca, Kazia Therapeutics, X-RAD Therapeutics
Nancy Y. Lee
Consulting or Advisory Role: Merck, Pfizer, Merck Serono, Sanofi, Mirati Therapeutics, Roche/Genentech
Research Funding: AstraZeneca, Pfizer (Inst)
Andreas Rimner
Honoraria: More Health
Consulting or Advisory Role: AstraZeneca, Merck, Boehringer Ingelheim
Research Funding: Varian Medical Systems (Inst), Boehringer Ingelheim (Inst), Pfizer (Inst), AstraZeneca (Inst), Merck (Inst)
Yoshiya Yamada
Employment: Memorial Sloan-Kettering Cancer Center
Leadership: Chordoma Foundation
Honoraria: Varian Medical Systems, BrainLAB, Vision RT
Speakers' Bureau: BrainLAB, Vision RT, Varian Medical Systems
Research Funding: Varian Medical Systems
Travel, Accommodations, Expenses: Philips Healthcare
Other Relationship: University of Wollongong
Michael J. Zelefsky
Honoraria: Accuray
Consulting or Advisory Role: Alpha Tau, Boston Scientific
Patents, Royalties, Other Intellectual Property: Ferring provided partial funding for a clinical trial (Inst)
Other Relationship: Editor in Chief for Brachytherapy
Noah S. Kalman
Consulting or Advisory Role: Naveris
Rupesh R. Kotecha
Honoraria: Accuray, Novocure, Elekta, BrainLAB, Elsevier, ViewRay, PeerView
Consulting or Advisory Role: ViewRay, Novocure
Speakers' Bureau: Novocure
Research Funding: Medtronic (Inst), Blue Earth Diagnostics (Inst), Novocure (Inst), GT Medical Technologies (Inst), AstraZeneca (Inst), Exelixis (Inst), ViewRay (Inst), BrainLAB (Inst)
Travel, Accommodations, Expenses: PeerView
Minesh P. Mehta
Leadership: Oncoceutics
Stock and Other Ownership Interests: Chimerix
Consulting or Advisory Role: Karyopharm Therapeutics, Mevion Medical Systems, ZappRx, Sapience Therapeutics, Xoft
Patents, Royalties, Other Intellectual Property: WARF patent 14/934,27, Topical vasoconstrictor preparations and methods for protecting cells during cancer chemotherapy and radiotherapy
Uncompensated Relationships: Xcision Medical Systems, ViewRay
Michael D. Chuong
Employment: Fertility and IVF Center of Miami
Honoraria: ViewRay, Sirtex Medical
Consulting or Advisory Role: ViewRay, Advanced Accelerator Applications
Speakers' Bureau: ViewRay, Sirtex Medical
Research Funding: AstraZeneca/MedImmune, ViewRay
Travel, Accommodations, Expenses: ViewRay
Andrew L. Salner
Consulting or Advisory Role: Best Doctors Inc
Jamie S. Ostroff
Patents, Royalties, Other Intellectual Property: UptoDate
David G. Pfister
Consulting or Advisory Role: Boehringer Ingelheim, Incyte
Research Funding: Boehringer Ingelheim (Inst), AstraZeneca (Inst), Exelixis (Inst), Novartis (Inst), MedImmune (Inst), Merck (Inst), Genentech/Roche (Inst), Lilly (Inst), Bayer (Inst), Eisai (Inst), Regeneron (Inst), Atara Biotherapeutics (Inst), MeiraGTx (Inst), Hookipa Pharma (Inst)
Fumiko Chino
This author is a Consultant Editor for JCO Oncology Practice. Journal policy recused the author from having any role in the peer review of this manuscript.
Jillian Tsai
Honoraria: Varian Medical Inc
Consulting or Advisory Role: Varian Medical Systems
Erin F. Gillespie
Other Relationship: eContour
No other potential conflicts of interest were reported.
REFERENCES
- 1.National Institutes of Health . Policy and Compliance. National Institutes of Health; 2017. NIH policy and guidelines on the inclusion of women and minorities as subjects in clinical research.https://grants.nih.gov/policy/inclusion/women-and-minorities/guidelines.htm#:~:text=It%20is%20the%20policy%20of,that%20inclusion%20is%20inappropriate%20with [Google Scholar]
- 2. Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials. JAMA. 2004;291:2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 3. Duma N, Vera Aguilera J, Paludo J, et al. Representation of minorities and women in oncology clinical trials: Review of the past 14 years. J Oncol Pract. 2018;14:e1–e10. doi: 10.1200/JOP.2017.025288. [DOI] [PubMed] [Google Scholar]
- 4. Bero EH, Rein LE, Banerjee A, et al. Characterization of underrepresented populations in modern era clinical trials involving radiation therapy. Pract Radiat Oncol. 2021;11:453–459. doi: 10.1016/j.prro.2021.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Institute of Medicine Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care . In: Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Smedley BD, Stith AY, Nelson AR, editors. Washington, DC: National Academies Press (US); 2003. [PubMed] [Google Scholar]
- 6. Cunningham-Erves J, Barajas C, Mayo-Gamble TL, et al. Formative research to design a culturally-appropriate cancer clinical trial education program to increase participation of African American and Latino communities. BMC Public Health. 2020;20:840. doi: 10.1186/s12889-020-08939-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Go RS, Frisby KA, Lee JA, et al. Clinical trial accrual among new cancer patients at a community-based cancer center. Cancer. 2006;106:426–433. doi: 10.1002/cncr.21597. [DOI] [PubMed] [Google Scholar]
- 8. Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: Results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10:21. doi: 10.1186/s13012-015-0209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vuong I, Wright J, Nolan MB, et al. Overcoming barriers: Evidence-based strategies to increase enrollment of underrepresented populations in cancer therapeutic clinical trials—A narrative review. J Cancer Educ. 2020;35:841–849. doi: 10.1007/s13187-019-01650-y. [DOI] [PubMed] [Google Scholar]
- 10. Parker C, Snyder R, Jefford M, et al. A randomized controlled trial of an additional funding intervention to improve clinical trial enrollment. J Natl Compr Cancer Netw. 2017;15:1104–1110. doi: 10.6004/jnccn.2017.0150. [DOI] [PubMed] [Google Scholar]
- 11.Dana-Farber Cancer Institute EMBRACE (Ending Metastatic Breast Cancer for Everyone): A Comprehensive Approach to Improve the Care of Patients with Metastatic Breast Cancer Pfizer; 2015.https://www.pfizer.com/node/469076 [Google Scholar]
- 12. Pelto DJ, Sadler GR, Njoku O, et al. Adaptation of a cancer clinical trials education program for African American and Latina/o community members. Health Educ Behav. 2016;43:381–388. doi: 10.1177/1090198115610555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Winkfield KM, Flowers CR, Patel JD, et al. American Society of Clinical Oncology strategic plan for increasing racial and ethnic diversity in the oncology workforce. J Clin Oncol. 2017;35:2576–2579. doi: 10.1200/JCO.2017.73.1372. [DOI] [PubMed] [Google Scholar]
- 14. Geller SE, Koch AR, Roesch P, et al. The more things change, the more they stay the same: A study to evaluate compliance with inclusion and assessment of women and minorities in randomized controlled trials. Acad Med. 2018;93:630–635. doi: 10.1097/ACM.0000000000002027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Loree JM, Anand S, Dasari A, et al. Disparity of race reporting and representation in clinical trials leading to cancer drug approvals from 2008 to 2018. JAMA Oncol. 2019;5:e191870. doi: 10.1001/jamaoncol.2019.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]