INTRODUCTION
The WHO Western Pacific Region (WPR) is home to 37 countries/areas and 1.9 billion people. It is economically, culturally, and linguistically diverse, containing some of the world's most populous countries such as China, to some of the least, such as Niue and Tuvalu. Its health systems must serve populations facing the impacts of climate change, from vast geographic areas to tiny island territories, as well as those with a history of colonization. These factors all pose challenges to the equitable provision of health services, including cancer screening.
CONTEXT
Key Objective
This article describes the challenges of implementing lung cancer screening (LCS) in the Western Pacific Region (WPR), outlining lessons learned from international trials and existing real-world programs, as well as successful strategies used in other cancer screening programs.
Knowledge Generated
The WPR has nearly half the global burden for lung cancer mortality, along with great diversity of health services and economic conditions. Future implementation of LCS must consider primary and secondary prevention programs, as existing tobacco strategies may be most effective in low- and middle-income countries where there is insufficient infrastructure to support LCS programs.
Relevance
To enable equitable implementation of LCS, strategies used in other clinical settings need to be tested across the WPR to address a lack of evidence. This includes adapting the strategies used in the six countries and two special administrative regions in the WPR that have experience of implementing LCS programs.
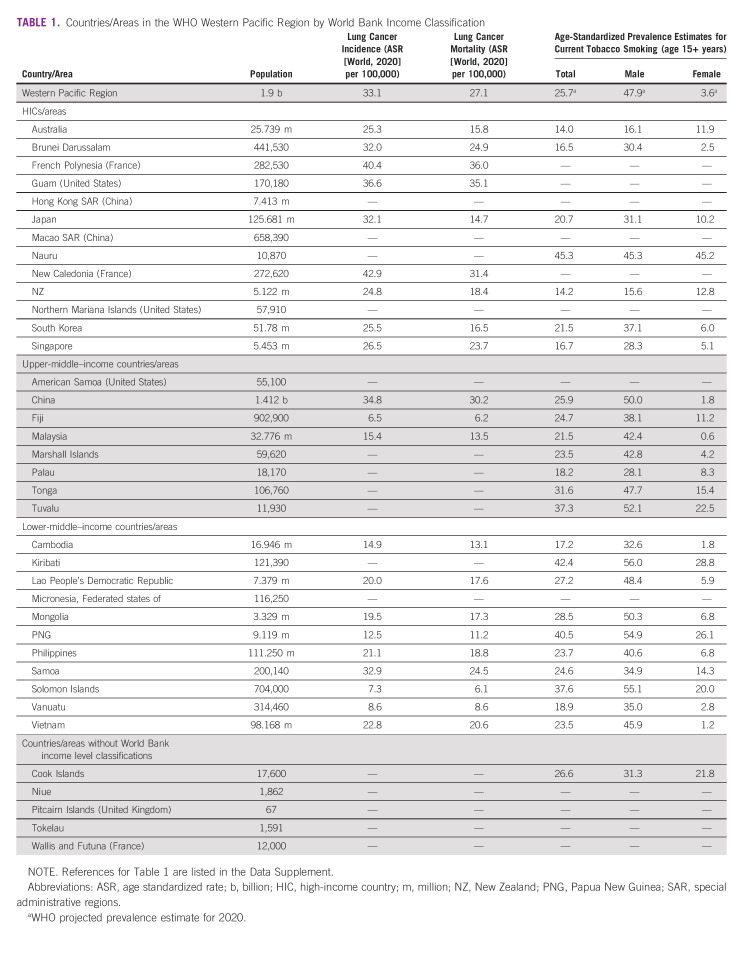
The WPR cancer burden is significant. In 2020, it bore 34.4% of all new cancer cases and 39.4% of all cancer-related deaths globally.1 Lung cancer was the most diagnosed cancer, accounting for 16.1% of all cases and almost a quarter of deaths (22.7%), the highest of any WHO-defined region.1 The WPR has 49.7% of the global burden for lung cancer mortality.1 There is substantial regional variation in lung cancer incidence because of varying rates of tobacco smoking and air quality, with age-standardized rates ranging from 6.5 per 100,000 people in Fiji to 42.2 per 100,000 people in New Caledonia.1-3 There is less variation in mortality as most cases are diagnosed as metastatic disease.4 Survival in high-income countries (HICs) may be better because of improved detection and access to treatment.2 Inequity is evident, with First Nations people in Australia and New Zealand experiencing higher incidence and mortality rates than non-Indigenous people.5,6
This review provides a background to lung cancer in the WPR, including the risk factors and primary and secondary prevention measures. The key considerations for lung cancer screening (LCS) are summarized, highlighting the implementation challenges encountered to date. The review considers broader issues of health equity, other public health priorities, and lessons learned from other cancer screening programs. We highlight the need for greater global advocacy to ensure that, if implemented, LCS is beneficial and not a driver of health inequities.
METHODS
We ran online searches to find peer-reviewed and gray literature about LCS relevant to the WPR. This information was collated, reviewed and summarized to provide examples of relevant research and practice. We compiled lung cancer incidence and mortality data from the International Agency for Research on Cancer and current tobacco smoking, computed tomography (CT) scanner availability, workforce capacity data from WHO fact sheets, and reports for the WPR countries/areas reported. We sourced additional peer-reviewed and gray literature from PubMed and government websites/reports to summarize the status of other cancer screening programs (Data Supplement).
RESULTS
Risk Factors: Tobacco Smoking
Tobacco smoking accounts for approximately 85%-90% of lung cancer cases and two thirds of deaths globally.4,7 Incidence rates largely reflect the maturity of the tobacco epidemic, tending to be greater in HICs.8 However, this is shifting as most people who smoke tobacco now live in low-middle– and low-income countries (LMICs).8 Smoking prevalence across the WPR is high, especially among males, ranging from 15% to 16% in Australia and New Zealand to more than 50% in Papua New Guinea and the Solomon Islands.9 Smoking rates among females are lower, like in Vietnam (1.2%) and China (1.8%), with exceptions being Nauru (45.2%; Table 1).8 Although prevalence rates are decreasing across the WPR for males and females, the region is not on track to achieve the WHO 30% relative reduction in smoking rates.10 Although some countries have achieved marked reductions, such as Australia, where daily tobacco smoking rates have halved in the past three decades, there have been inconsistencies within communities. For instance, smoking rates among Aboriginal and Torres Strait Islander peoples (37.4%), and people living in remote Australia (19.6%) are substantially higher than in the broader population (11%).11
TABLE 1.
Countries/Areas in the WHO Western Pacific Region by World Bank Income Classification
Risk Factors: Beyond Tobacco
Approximately 10%-15% of lung cancer cases in the WPR are detected in people who have never smoked.12 Tuberculosis is an important risk factor as some of the world's highest incidence rates occur in the Philippines and Papua New Guinea.13 Environmental and occupational exposures, family history, and genetic risks are significant contributors.14,15 Exposure to outdoor air pollution, including from particulate matter 2.5 (particles < 2.5 μm), accounts for 14.1% of lung cancer deaths globally.16 New evidence suggests that increasing levels of particulate matter 2.5 increases lung cancer risk in people with certain oncogenic mutations.17 WHO reports that 92% of the world's population lives in areas exceeding air-quality guidelines,18 including countries with increasing industrialization, population density, and vehicle emissions, such as China and Vietnam.19 Indoor air pollution from secondhand smoke, the use of solid fuels for cooking and heating, and radon in soil and water particularly affects women.9 For instance, one in five lung cancer cases in Chinese women is linked to secondhand smoke.20
Exposure to carcinogenic agents such as asbestos is linked to 62.7% of all occupation-attributable cancer deaths globally, the majority being lung cancer.21 Although asbestos use is largely eliminated in HICs and LMICs, there can be a 10- to 30-year lag between exposure and diagnosis.22 In 2016, Australasia and Asian HICs had the highest per-capita deaths from asbestos.21 Asbestos exposure in developing countries is less well quantified but may still be high in countries with large industry and manufacturing activity.22,23 Other agents commonly linked to cancer-related deaths in LMICs include diesel engine exhaust and silica.21
Primary Prevention of Lung Cancer
Tobacco control is the most important approach to the primary prevention of lung cancer.24 The WHO Framework Convention on Tobacco Control is the most influential global plan for reducing the demand of tobacco products.25 The WPR is the only region to have all Member States party to the Convention, demonstrating a commitment to implementing effective interventions, including the WHO MPOWER (Monitoring, smokefree Policies, Offer help to cessation, health Warnings, Enforcing advertising bans, and Raising taxes) measures.26 Implementation of MPOWER measures varies across the WPR, with Australia, Brunei Darussalam, and New Zealand having some of the strongest policies.8 Twenty-four countries in the region have implemented at least one MPOWER measure at best-practice level.27 Implementation continues to improve, with the Cook Islands and Philippines joining the best-practice group for tobacco use cessation services and China for monitoring in 2020.8 However, WPR tobacco smoking rates are decreasing slowly in comparison with other regions.10 This likely reflects challenges in implementing cancer control policies and increasing use of electronic nicotine delivery devices.27,28
Secondary Prevention: LCS
Large-scale international randomized controlled trials of LCS have demonstrated the clinical effectiveness of low-dose computed tomography (LDCT). Two landmark trials, the United States National Lung cancer Screening Trial (NLST), and the Netherlands/Belgium NELSON trial, reported a 20%-24% reduction in lung cancer mortality.29,30 The significant detection of early-stage disease (stage I and II) in both trials (57% and 67.9%, respectively)29,30 addresses the decades-old challenge of lung cancer being diagnosed at a late stage. This stage shift has been noted across the WPR in trials and pilot programs conducted in Japan, China, and Taiwan,31 and the K-LUCAS pilot in South Korea reported that 67% of diagnoses were early-stage.32 Similar findings are reported in real-world programs in the US Veterans Health Affairs Program (71% early-stage lung cancers)33 and five UK programs (81%).90
All screening programs have benefits and harms. Potential LDCT harms include radiation exposure, false positives, unnecessary procedures, overdiagnosis, and potential psychologic distress for participants.34 Innovations across international LDCT trials have shown harm reductions through improved detection and classification of lung nodules, including false positives (from 24% in NLST29 to 1.2% in NELSON trial)29,30 and unnecessary procedures for benign conditions (from 9% in NLST to 0% in NELSON trial).35 However, management protocols for diagnostic investigations and complications must be developed for the WPR, such as those described in the K-LUCAS pilot.32 Thus, although consensus in HICs that LCS benefits outweigh harms, further trials and pilot programs in the WPR are required, as is generating evidence about LCS cost-effectiveness.
Significant barriers to LCS uptake have been identified, including practical barriers (travel time and associated costs, work and/or career responsibilities), the impact of comorbidities, and emotional barriers (fear, shame, fatalism, avoidance, and low risk perception) inclusive of stigma.36-39 To enable equitable participation, LCS programs need to address age, sex differences, current and past smoking status, socioeconomic status, and geographical factors.38
Components of LCS
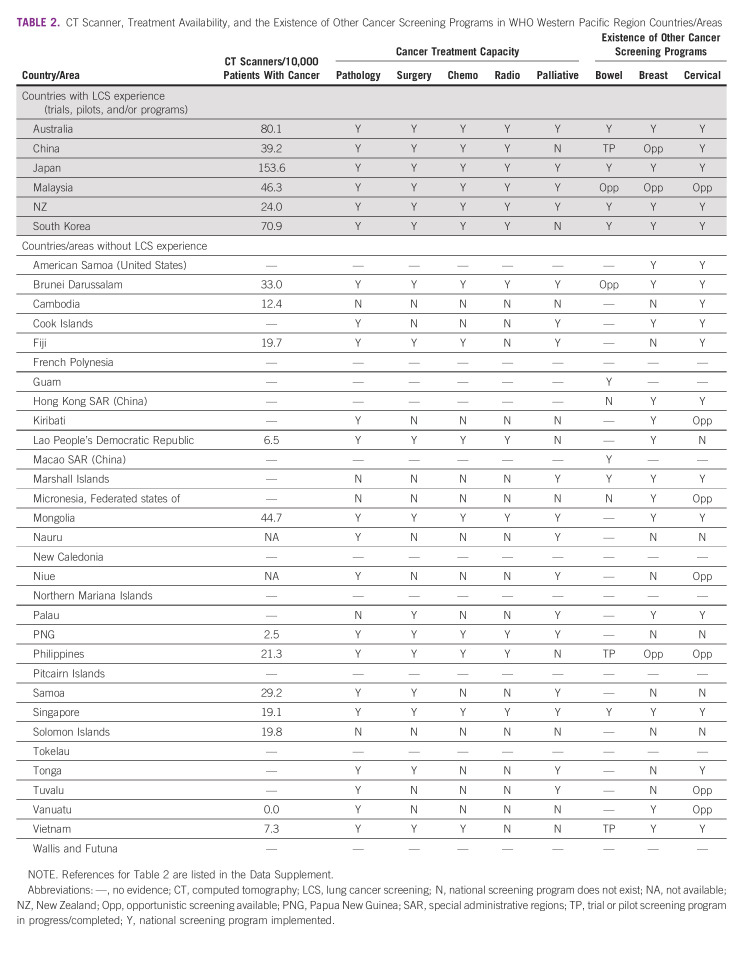
The components of high-quality screening programs for optimal implementation have been recommended in HICs.34,40 Six countries and two special administrative regions in the WPR have experience of implementing LCS (Table 2). In this section, we outline these components and implementation strategies, drawing on LCS exemplars from the WPR. Successful strategies used in the WPR to implement other cancer screening programs are discussed later in this article.
TABLE 2.
CT Scanner, Treatment Availability, and the Existence of Other Cancer Screening Programs in WHO Western Pacific Region Countries/Areas
Selecting the high-risk population.
LCS targets people at high risk in the population. Promotion and awareness raising is essential for increasing LCS knowledge. Common strategies include public awareness campaigns and behavioral interventions using online advertising and printed materials.41,42 There is little published evidence about the most appropriate strategies for the WPR. Recruitment strategies include mailing invitations, community outreach, and mass media.43 Strategies used within the WPR include offering LCS as part of an annual occupational health check44 and invitations from general practitioners.45 Most international trials report large nonresponse rates to invitations.43 For example, in Malaysia, a pilot LCS program was stopped early because of suboptimal recruitment likely due to participant refusal and a fear of diagnosis.46,47 In a Chinese LCS program that achieved a participation rate > 50%, people who had never smoked had higher education levels, a family history of lung cancer, and occupational exposure, and had higher rates of participation.48 Male participants have also been over-represented in international trials,49 and more evidence is needed about the optimal strategies to recruit female participants. A lack of centralized patient registration systems in most WPR countries precludes the systematic mailing of invitations, appointment reminders, and recalls after screening.
Eligibility criteria are typically determined on the basis of criteria of age and smoking history. Risk assessment tools are a more sophisticated way of selecting those at high risk. Inclusion for occupational or secondhand smoke exposure is not currently incorporated into pilot (eg, K-LUCAS) and proposed programs (eg, Australia).50 Asian trials investigating LCS for never-smokers are ongoing.44,51 Across the WPR, risk models are in development stages, and mapping the common causes of lung cancer is critical to developing appropriate risk assessment tools. A recent review highlighted an urgent need for external validation and model optimization for the Chinese population.46
Health care professional education.
Improving health care professional's awareness and knowledge of LCS including benefits and harms is another essential component.52 Primary care practitioners are the key workforce in many jurisdictions who will champion screening. Evidence from HICs shows that primary care practitioners are key in encouraging the participation of high-risk individuals.53,54 In most WPR settings, health care is delivered at multiple levels, with primary care, often nurse-led, typically provided at village/community-based level with referral to provincial and national tertiary settings when necessary. Future LCS implementation will require adaptation to health care settings in the WPR. Shared decision making is a mandatory component of LCS in other jurisdictions, but not yet a common practice in many WPR countries.55-57 The development and use of decision aids appropriate for the WPR requires further study. Finally, patient navigators may be appropriate in health systems across the WPR. To support LCS programs, health care professionals need education in optimal recruitment methods, the provision of smoking cessation support, communicating scan results, reading and reporting LDCT scans, and managing and investigating suspicious nodules and incidental findings. Health care professionals play an important role in reducing stigma in the health system and encouraging inclusivity of all people at high risk. It is likely that additional training to address stigma is needed.
Embedding smoking cessation interventions.
The integration of smoking cessation interventions into LCS programs maximizes participant benefits and cost-effectiveness. The most effective strategies for incorporating smoking cessation interventions at multiple points in the screening and assessment pathway are not yet identified.58 In the WPR, the effectiveness of mandatory cessation counseling with a physician was tested in the K-LUCAS pilot, which detected a 12.7% (P = .007) change in participant's willingness to quit smoking after screening.59 However, as 14% of LCS participants were recruited from smoking cessation services, they may have had a greater motivation to quit than the participants recruited via the National Screening Program (84%). In the now-implemented Korean National Lung Cancer Screening Program, there was broad support for the mandatory inclusion of in-person smoking cessation counseling, particularly from those eligible for screening (57.6%).60 Evidence in this area is rapidly changing. A microsimulation model of a nurse-led cessation intervention made LCS more cost-effective in the Chinese setting.61 Harnessing successful tobacco cessation initiatives outside LCS, such as the empowerment of community health workers to deliver interventions in LMICs, should be explored in the LCS setting.62 Efforts to support smoking cessation are supported at a policy level as countries in the WPR make progress toward achieving the best practice measures outlined in the MPOWER framework, notably, the increase in the number of countries that offer cost-covered nicotine replacement therapy, and have increased taxation and education campaigns.24
Physical infrastructure and human resources.
Before LCS implementation, WPR countries will need thorough screening and assessment pathways, agreed standards for LDCT scan delivery and results interpretation, and nodule management guidelines to reduce false positives and incidental findings.34 Access to an adequate number of CT machines with the technical capacity for monitored low-radiation doses appropriate for screening and sufficiently qualified and accredited radiologists and radiographers are essential. Australia and Japan have the greatest number of CT machines per capita.48 However, many WPR countries have few CT machines and limited numbers of appropriately skilled thoracic radiologists and radiographers (Table 2). Thoracic surgeons, clinical oncologists, and specialist oncology nurses are essential to the delivery of a LCS program. Robust quality assurance programs must be established alongside national LCS registers to monitor outcomes and enable research and continuous improvement.
Referral pathways, incidental findings, and coordination to treatment.
LCS programs require appropriate and accessible referral pathways and specialist capacity to treat people with identified cancers and manage incidental findings. The most common incidental findings include pulmonary, cardiovascular, and gastrointestinal comorbidities, many of which are clinically insignificant.63 Incidental findings that are clinically significant, such as coronary artery calcifications and osteoporosis, will require further management by primary care physicians. In cases where other cancers are detected, such as breast, adrenal, thyroid, and upper abdominal, referral for specialist clinical evaluation will be required.64 The detection of actionable findings is an advantage of the LDCT scan and presents a positive opportunity to implement standardized reporting recommendations for incidental findings and to minimize unnecessary workup of low-risk nodules. Cancer care and treatment in WPR is challenged by limited infrastructure and workforce capacity (Table 2). A recent WHO survey reported that many WPR countries do not have established referral systems for existing cancer screening programs.65 Challenges include poor access to essential medicines, palliative care and a reliance on out-of-country referrals for cancer treatment.65
Multidisciplinary teams (MDT) make treatment recommendations for people with screen-detected abnormalities and are important in reducing harms associated with overdiagnosis and unnecessary treatments.66 MDTs routinely guide decision making in Australia and New Zealand and are increasingly used in China, Japan, and South Korea.67-69 Most WPR programs offer LCS in metropolitan settings.45 It is critical to consider how people living in rural and remote areas will access screening and appropriate follow-up. This is particularly important, given workforce shortages of medical practitioners in primary and tertiary care outside metropolitan locations. The establishment of efficient and appropriate referral pathways, with the involvement of MDT, the use of standardized nodule management guidelines and leading to appropriately skilled thoracic surgeons, and oncology treatment, remains a challenge for every LCS program that has been implemented.
DISCUSSION
Lessons learnt from LCS implementation should be coupled with learnings from decades of implementation experience of cancer screening and successful public health programs across the WPR. Encouraging innovation, continued workforce expansion, and aligning with and capitalizing on existing health strengthening initiatives will be important for the WPR in the future design and implementation of LCS. Global and regional WHO plans of high relevance to LCS mortality include two overarching regional initiatives70,71 that highlight whole of system health strengthening approaches.71 The WHO global strategy to accelerate the elimination of cervical cancer as a public health problem outlines a series of prevention and treatment targets. As the strategy actions are implemented, improvements in cancer registration and treatment infrastructure and capacity should harnessed to benefit all people diagnosed with any cancer.72
Across the WPR, 89% of countries have cervical screening programs, although only half (48%) have organized population-based programs and only 4% of countries having achieved ever in lifetime screening coverage of > 70%.73 Two thirds of countries have an implemented breast screening program, although about 25% adhere to best practice guidelines on screening age and interval.73 Few WPR countries have national bowel screening available (Table 2).
Local innovation and adaptation of screening programs to suit the resources and settings across the WPR has been successful. In both HIC and LMIC settings, cervical and breast cancer education, screening, and prevention programs have been delivered through community and occupational outreach models and dedicated screening days in countries including Hong Kong, Papua New Guinea, the Federated States of Micronesia, Cambodia, China, and Vietnam.74-78 Community health workers have delivered smoking cessation advice and clinical interventions but cite a need for more education to increase confidence in delivering cessation support and talking about cancer.62,78-80
Endorsement of primary care practitioners is an important facilitator to participation in screening programs, including for culturally and linguistically diverse people in HICs.81 Community education,82 multimedia, and SMS messaging approaches have demonstrated effectiveness in increasing knowledge and participation in cancer screening programs.83,84
Increasing global inequity is evident in cancer mortality. In HICs, primary prevention, including tobacco control and vaccination for hepatitis B and human papillomavirus, prevent cancers developing; greater resources to implement screening programs lead to more cases being diagnosed early with better treatment outcomes; and a greater availability and accessibility of treatment result in a higher proportion of cases being treated with curative intent, compared with LMICs. Using cervical cancer as an example, many HICs are on track to eliminate cervical cancer as a public health problem within our lifetime. However, nearly 90% of all cervical cancer deaths occur in LMICs; these deaths are preventable.
Participation barriers in cancer screening and poor access to treatment have a greater impact on marginalized and underserved populations.80 A lack of culturally safe services, language barriers, cultural beliefs about cancer, fatalistic attitudes, and the ongoing impacts of trauma and colonization contribute to First Nations People in countries such as Australia and New Zealand participating in national programs at lower rates compared with non-Indigenous people.85 Ethnic minorities and people living in lower socioeconomic areas participate at lower rates and experience worse cancer outcomes for screen preventable cancers.80 In the Pacific, identified barriers include a lack of knowledge and awareness among the general population, high-risk participants, and healthcare workers, limited access to health facilities, cultural beliefs, and cost.47 Financial barriers have been associated with delayed diagnosis or lower adherence to treatment in Vietnam,80 Australia, China,86 and New Zealand.87 Distance to services and spending a long time away from home are significant barriers. Financial toxicity after cancer treatment disproportionately affects people from rural areas, on low incomes, and from ethnic minorities.86,87 The critical importance of working directly with underserved populations to codesign recruitment materials and tailor models of service delivery to the population and setting has been an essential learning from programs to date and should be incorporated into any future LCS program design.88,89
In conclusion, lung cancer is a leading cause of global mortality. Evidence shows that LCS can reduce lung cancer mortality and detect disease at an early stage. However, substantial implementation challenges remain, which will continue to influence and shape the implementation of primary and secondary prevention programs across the WPR. To our knowledge, this is the first review to consider challenges across the entire WPR. Increasing health challenges associated with the impacts of climate change, aging populations, and an increased burden of noncommunicable diseases across the 37 WPR states create a substantial task in the prioritization of health spending. Systematic assessments of lung cancer burden, human and technical resource availability, and local cost-effectiveness will be needed across the WPR to inform health planning.
As evidence emerges, more LCS programs will be implemented and require a combination of primary and secondary prevention measures. It is critical that they are designed and delivered with equity considerations at their core. However, resource limitations and infrastructure challenges will mean that many LMICs will rely on tobacco control measures as the primary prevention strategy to reduce the impact of lung cancer. Thus, continued support for strengthening the implementation of the MPOWER measures in each country is critical. There is a need for greater global advocacy to ensure the innovation of LCS does not further drive cancer inequities.
Emily Stone
Honoraria: AstraZeneca, MSD
Consulting or Advisory Role: BMSi
Travel, Accommodations, Expenses: AstraZeneca
No other potential conflicts of interest were reported.
SUPPORT
C.N. is supported by a Mid-Career Research Fellowship from the Victorian Government acting through the Victorian Cancer Agency.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: Claire Nightingale, Claire Bavor, Nicole M. Rankin
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Emily Stone
Honoraria: AstraZeneca, MSD
Consulting or Advisory Role: BMSi
Travel, Accommodations, Expenses: AstraZeneca
No other potential conflicts of interest were reported.
REFERENCES
- 1.International Agency for Research on Cancer : Cancer Today: World Health Organisation. Lyon, France, International Agency for Research on Cancer, 2020 [Google Scholar]
- 2.Torre LA, Siegel RL, Jemal A: Lung Cancer Statistics. London, United Kingdom, Springer International Publishing, 2016, pp 1-19 [Google Scholar]
- 3.Sharma R: Mapping of global, regional and national incidence, mortality and mortality-to-incidence ratio of lung cancer in 2020 and 2050. Int J Clin Oncol 27:665-675, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Groot PM, Wu CC, Carter BW, et al. : The epidemiology of lung cancer. Transl Lung Cancer Res 7:220-233, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Australian Institute of Health and Welfare : Cancer in Aboriginal and Torres Strait Islander People of Australia. Canberra, Australia, Australian Institute of Health and Welfare, 2018 [Google Scholar]
- 6.Gurney J, Stanley J, McLeod M, et al. : Disparities in cancer-specific survival between Māori and non-Māori New Zealanders, 2007-2016. JCO Glob Oncol 6:766-774, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sung H, Ferlay J, Siegel RL, et al. : Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209-249, 2021 [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization : WHO Report on the Global Tobacco Epidemic, 2021: Addressing New and Emerging Products. Geneva, Switzerland, World Health Organization, 2021 [Google Scholar]
- 9.Corrales L, Rosell R, Cardona AF, et al. : Lung cancer in never smokers: The role of different risk factors other than tobacco smoking. Crit Rev Oncol Hematol 148:102895, 2020 [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization : WHO Global Report on Trends in Prevalence of Tobacco Smoking 2000-2025. Geneva, Switzerland, World Health Organization, 2018 [Google Scholar]
- 11.Greenhalgh EM, Maddox R, van der Sterren A, et al. : 8.3 Prevalence of Tobacco Use Among Aboriginal and Torres Strait Islander Peoples. Melbourne, Australia, Cancer Council Australia, 2021 [Google Scholar]
- 12.Schwartz AG, Cote ML: Epidemiology of Lung Cancer. London, United Kingdom, Springer International Publishing, 2016, pp 21-41 [Google Scholar]
- 13.The World Bank : Incidence of Tuberculosis: World Health Organisation. Washington, DC, The World Bank, 2020 [Google Scholar]
- 14.Wang F, Tan F, Wu Z, et al. : Lung cancer risk in non-smoking females with a familial history of cancer: A multi-center prospective cohort study in China. J Natl Cancer Cent 1:108-114, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu C-W, Ku Y-T, Huang C-Y, et al. : The BUILT study: A single-center 5-year experience of lung cancer screening in Taiwan. Int J Med Sci 18:3861-3869, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.GBD 2017 Risk Factor Collaborators, Afshin A, Gakidou E, et al. : Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study. Lancet (London, England) 392:1923-1994, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swanton C, Hill W, Lim E, et al. : LBA1—Mechanism of action and an actionable inflammatory axis for air pollution induced non-small cell lung cancer: Towards molecular cancer prevention. Ann Oncol 33:S1413-S1469, 2022 [Google Scholar]
- 18.Turner MC, Andersen ZJ, Baccarelli A, et al. : Outdoor air pollution and cancer: An overview of the current evidence and public health recommendations. CA Cancer J Clin 70:460-479, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jamrozik E, Musk AW: Respiratory health issues in the Asia-Pacific region: An overview. Respirology 16:3-12, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Islami F, Chen W, Yu X, et al. : Cancer deaths and cases attributable to lifestyle factors and infections in China, 2013. Ann Oncol 28:2567-2574, 2017 [DOI] [PubMed] [Google Scholar]
- 21.GBD 2016 Occupational Carcinogens Collaborators : Global and regional burden of cancer in 2016 arising from occupational exposure to selected carcinogens: A systematic analysis for the Global Burden of Disease Study 2016. Occup Environ Med 77:151-159, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spyratos D, Zarogoulidis P, Porpodis K, et al. : Occupational exposure and lung cancer. J Thorac Dis 5:S440-S445, 2013. (suppl 4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stayner L, Welch LS, Lemen R: The worldwide pandemic of asbestos-related diseases. Annu Rev Public Health 34:205-216, 2013 [DOI] [PubMed] [Google Scholar]
- 24.De Pinho Campos K, Kashiwabara M, Teakle A, et al. : Investing in tobacco control: Twelve years of MPOWER measures and progress in the Western Pacific Region. Asian Pac J Cancer Prev 21:9-16, 2020 [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization : WHO Framework Convention on Tobacco Control. Geneva, Switzerland, World Health Organisation, 2003 [Google Scholar]
- 26.World Health Organization Regional Office for the Western Pacific : Regional Action Plan for Tobacco Control in the Western Pacific (2020-2030). Manilla, Philippines, World Health Organization Regional Office for the Western Pacific, 2020 [Google Scholar]
- 27.The Lancet Regional Health-Western Pacific : Tobacco control in the Western Pacific region. Lancet Reg Health West Pac 10:100179, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varghese C, Clem Carlos M, Shin H-R: Cancer burden and control in the Western Pacific Region: Challenges and opportunities. Ann Glob Health 80:358-369, 2014 [DOI] [PubMed] [Google Scholar]
- 29.The National Lung Screening Trial Research Team, Adams AM, Berg CD, et al. : Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 365:395-409, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Koning HJ, van der Aalst CM, de Jong PA, et al. : Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med 382:503-513, 2020 [DOI] [PubMed] [Google Scholar]
- 31.Triphuridet N, Henschke C: >Landscape on CT screening for lung cancer in Asia<>. Lung Cancer (Auckl) 10:107-124, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee J, Kim Y, Kim HY, et al. : Feasibility of implementing a national lung cancer screening program: Interim results from the Korean Lung Cancer Screening Project (K-LUCAS). Transl Lung Cancer Res 10:723-736, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kinsinger LS, Anderson C, Kim J, et al. : Implementation of lung cancer screening in the Veterans Health Administration. JAMA Intern Med 177:399-406, 2017 [DOI] [PubMed] [Google Scholar]
- 34.Bade BC, Brasher PB, Luna BW, et al. : Reviewing lung cancer screening: The who, where, when, why, and how. Clin Chest Med 39:31-43, 2018 [DOI] [PubMed] [Google Scholar]
- 35.Crosbie PA, Balata H, Evison M, et al. : Implementing lung cancer screening: Baseline results from a community-based “Lung Health Check” pilot in deprived areas of Manchester. Thorax 74:405-409, 2019 [DOI] [PubMed] [Google Scholar]
- 36.Yoon S, Wang K, Luo Y, et al. : Cancer screening literacy among Vietnamese population: Does annual checkup improve cancer screening literacy? Asian Pac J Cancer Prev 22:927-933, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naidu C, Wiseman N, Harris N: Factors associated with low screening participation and late presentation of cancer amongst women in the Pacific island countries and territories: A systematic review. Asian Pac J Cancer Prev 22:1451-1458, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ali N, Lifford KJ, Carter B, et al. : Barriers to uptake among high-risk individuals declining participation in lung cancer screening: A mixed methods analysis of the UK Lung Cancer Screening (UKLS) trial. BMJ Open 5:e008254, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olson RE, Goldsmith L, Winter S, et al. : Emotions and lung cancer screening: Prioritising a humanistic approach to care. Health Soc Care Commun 30:e5259-e5269, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organization : A Short Guide to Cancer Screening. Increase Effectiveness, Maximise Benefits and Minimize Harm. Geneva, Switzerland, World Health Organization, 2022 [Google Scholar]
- 41.Cardarelli R, Roper KL, Cardarelli K, et al. : Identifying community perspectives for a lung cancer screening awareness campaign in Appalachia Kentucky: The Terminate Lung Cancer (TLC) Study. J Cancer Educ 32:125-134, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Springer SM, McFall A, Hager P, et al. : Lung cancer screening: An emerging cancer control issue presents opportunities for an awareness campaign in rural Michigan. Cancer Causes Control 29:1257-1263, 2018 [DOI] [PubMed] [Google Scholar]
- 43.Rankin NM, McWilliams A, Marshall HM: Lung cancer screening implementation: Complexities and priorities. Respirology 25:5-23, 2020. (suppl 2) [DOI] [PubMed] [Google Scholar]
- 44.Kakinuma R, Muramatsu Y, Asamura H, et al. : Low-dose CT lung cancer screening in never-smokers and smokers: Results of an eight-year observational study. Transl Lung Cancer Res 9:10-22, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng YI, Davies MPA, Liu D, et al. : Implementation planning for lung cancer screening in China. Prec Clin Med 2:13-44, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rajadurai P, How SH, Liam CK, et al. : Lung cancer in Malaysia. J Thorac Oncol 15:317-323, 2020 [DOI] [PubMed] [Google Scholar]
- 47.Elia CR, Devine S: Barriers and enablers for cervical cancer screening in the Pacific: A systematic review of the literature. Pac J Reprod Health 1:372-382, 2018 [Google Scholar]
- 48.Liang D, Shi J, Li D, et al. : Participation and yield of a lung cancer screening program in Hebei, China. Front Oncol 11:795528, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schütte S, Dietrich D, Montet X, et al: Participation in lung cancer screening programs: Are there gender and social differences? A systematic review. Public Health Rev 39:23, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cancer Australia : Report on the Lung Cancer Screening Enquiry. Surry Hills, NSW, Australia, Cancer Australia, 2020 [Google Scholar]
- 51.Kang H-R, Cho JY, Lee SH, et al. : Role of low-dose computerized tomography in lung cancer screening among never-smokers. J Thorac Oncol 14:436-444, 2019 [DOI] [PubMed] [Google Scholar]
- 52.Ortmeyer K, Ma GX, Kaiser LR, Erkmen C: Effective educational approaches to training physicians about lung cancer screening. J Cancer Educ 37:52-57, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li J, Chung S, Wei EK, Luft HS: New recommendation and coverage of low-dose computed tomography for lung cancer screening: Uptake has increased but is still low. BMC Health Serv Res 18:525, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Draucker CB, Rawl SM, Vode E, et al. : Understanding the decision to screen for lung cancer or not: A qualitative analysis. Health Expect 22:1314-1321, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goto Y, Miura H: Challenges in promoting shared decision making: Towards a breakthrough in Japan. Z Evid Fortbild Qual Gesundhwes 171:84-88, 2022 [DOI] [PubMed] [Google Scholar]
- 56.Schaede U, Mahlich J, Nakayama M, et al. : Shared decision-making in patients with prostate cancer in Japan: Patient preferences versus physician perceptions. J Glob Oncol 4:1-9, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang R, Gionfriddo MR, Ting HH, et al. : Shared decision-making in the People's Republic of China: Current status and future directions. Patient Prefer Adher 9:1129-1141, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Joseph AM, Rothman AJ, Almirall D, et al. : Lung cancer screening and smoking cessation clinical trials. SCALE (Smoking Cessation Within the Context of Lung Cancer Screening) Collaboration. Am J Respir Crit Care Med 197:172-182, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee J, Lim J, Kim Y, et al. : Development of protocol for Korean Lung Cancer Screening Project (K-LUCAS) to evaluate effectiveness and feasibility to implement National Cancer Screening Program. Cancer Res Treat 51:1285-1294, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park J, Lee J, Kim Y: Public opinion on implementing the National Lung Cancer Screening Program in Korea. Transl Lung Cancer Res 10:1355-1367, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yuan J, Sun Y, Xu F, et al. : Cost-effectiveness of lung cancer screening combined with nurse-led smoking cessation intervention: A population-based microsimulation study. Int J Nurs Stud 134:104319, 2022 [DOI] [PubMed] [Google Scholar]
- 62.Nguyen N, Nguyen T, Chapman J, et al. : Tobacco cessation in Vietnam: Exploring the role of village health workers. Glob Public Health 13:1265-1275, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morgan L, Choi H, Reid M, et al. : Frequency of incidental findings and subsequent evaluation in low-dose computed tomographic scans for lung cancer screening. Ann Am Thorac Soc 14:1450-1456, 2017 [DOI] [PubMed] [Google Scholar]
- 64.Penha D, Pinto E, Monaghan C, et al. : Incidental findings on lung cancer screening: pictorial essay and systematic checklist. J Bras Pneumol 48:e20210371, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.World Health Organization Regional Office for the Western Pacific : Progress on the Prevention and Control of Noncommunicable Diseases in the Western Pacific Region: Country Capacity Survey 2019. Manilla, Philippines, World Health Organization Regional Office for the Western Pacific, 2021 [Google Scholar]
- 66.Stone E, Rankin N, Currow D, et al. : Optimizing lung cancer MDT data for maximum clinical impact—A scoping literature review. Transl Lung Cancer Res 9:1629-1638, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maeng CH, Ahn HK, Oh SY, et al. : Practice patterns of multidisciplinary team meetings in Korean cancer care and patient satisfaction with this approach. Korean J Intern Med 35:205-214, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu C, Zhao L, Wu F, et al. : The multidisciplinary team plays an important role in the prediction of small solitary pulmonary nodules: A propensity-score-matching study. Ann Transl Med 7:740, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ichikawa M, Uematsu K, Yano N, et al. : Implementation rate and effects of multidisciplinary team meetings on decision making about radiotherapy: An observational study at a single Japanese institution. BMC Med Inform Decis Making 22:111, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.World Health Organization Western Pacific Region : Western Pacific Regional Strategy for Health Systems Based on the Values of Primary Health Care. Geneva, Switzerland, World Health Organization, 2010 [Google Scholar]
- 71.World Health Organization Regional Office for the Western Pacific : Western Pacific Regional Action Plan for the Prevention and Control of Noncommunicable Diseases (2014-2020). Manilla, Philippines, World Health Organization Regional Office for the Western Pacific, 2014 [Google Scholar]
- 72.World Health Organization : Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem. Geneva, Switzerland, World Health Organization, 2020 [Google Scholar]
- 73.World Health Organization : Cervical Cancer Country Profiles 2021. https://www.who.int/teams/noncommunicable-diseases/surveillance/data/cervical-cancer-profiles [Google Scholar]
- 74.Vallely AJB, Saville M, Badman SG, et al. : Point-of-care HPV DNA testing of self-collected specimens and same-day thermal ablation for the early detection and treatment of cervical pre-cancer in women in Papua New Guinea: A prospective, single-arm intervention trial (HPV-STAT). Lancet Glob Health 10:e1336-e1346, 2022 [DOI] [PubMed] [Google Scholar]
- 75.Sy AU, Hernandez BY, Tareg A, et al. : Acceptability and feasibility of a community based participatory research project comparing cytology and urine HPV DNA testing for cervical cancer screening in Yap, Federated States of Micronesia. Cancer Epidemiol 50:283-288, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ueda Y, Kawana K, Yanaihara N, et al. : Development and evaluation of a cervical cancer screening system in Cambodia: A collaborative project of the Cambodian society of Gynecology and Obstetrics and Japan Society of Obstetrics and Gynecology. J Obstet Gynaecol Res 45:1260-1267, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gong Y, Peng P, Bao P, et al. : The implementation and first-round results of a community-based colorectal cancer screening program in Shanghai, China. Oncologist 23:928-935, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wong CL, Choi KC, Chen J, et al. : A community health worker–led multicomponent program to promote cervical cancer screening in South Asian women: A cluster RCT. Am J Prev Med 61:136-145, 2021 [DOI] [PubMed] [Google Scholar]
- 79.Kumar K, Mohammadnezhad M: Primary health care workers perspective towards cancer in Fiji: A qualitative study. Prim Health Care Res Dev 23:e1, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.So WKW, Chan DNS, Law BMH, et al. : Achieving equitable access to cancer screening services to reduce the cancer burden in the Asia-Pacific region: Experience from Hong Kong. Lancet Reg Health West Pac 29:100587, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Javanparast S, Baum F, Labonte R, et al. : The experience of community health workers training in Iran: A qualitative study. BMC Health Serv Res 12:291, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fang CY, Lee M, Feng Z, et al. : Community-based cervical cancer education: Changes in knowledge and beliefs among Vietnamese American women. J Commun Health 44:525-533, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schliemann D, Tan MM, Hoe WMK, et al. : mHealth interventions to improve cancer screening and early detection: Scoping review of reviews. J Med Internet Res 24:e36316, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schliemann D, Su TT, Paramasivam D, et al. : Effectiveness of mass and small media campaigns to improve cancer awareness and screening rates in Asia: A systematic review. JCO Glob Oncol 5:1-20, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Davies A, Gurney J, Garvey G, et al. : Cancer care disparities among Australian and Aotearoa New Zealand Indigenous peoples. Curr Opin Support Palliat Care 15:162-168, 2021 [DOI] [PubMed] [Google Scholar]
- 86.Jing J, Feng R, Zhang X, et al. : Financial toxicity and its associated patient and cancer factors among women with breast cancer: A single-center analysis of low-middle income region in China. Breast Cancer Res Treat 181:435-443, 2020 [DOI] [PubMed] [Google Scholar]
- 87.Lao C, Kuper-Hommel M, Laking G, et al. : Evidence of inequitable use of chemotherapy in New Zealand colorectal cancer patients. NZ Med J 133:15-26, 2020 [PubMed] [Google Scholar]
- 88.MacDonald EJ, Geller S, Sibanda N, et al. : Reaching under-screened/never-screened indigenous peoples with human papilloma virus self-testing: A community-based cluster randomised controlled trial. Aust N Z J Obstet Gynaecol 61:135-141, 2021 [DOI] [PubMed] [Google Scholar]
- 89.Christou A, Katzenellenbogen JM, Thompson SC: Australia's National Bowel Cancer Screening Program: Does it work for Indigenous Australians? BMC Public Health 10:373, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Balata H, Ruparel M, O’Dowd E, et al. : Analysis of the baseline performance of five UK lung cancer screening programmes. Lung Cancer 161:136-140, 2021 [DOI] [PubMed] [Google Scholar]