PURPOSE
The proportion of head and neck cancers (HNCs) with human papillomavirus (HPV) positivity in sub-Saharan Africa (SSA) is poorly characterized. Characterizing this has implications in staging, prognosis, resource allocation, and vaccination policies. This study aims to determine the proportion of HPV-associated HNC in SSA.
MATERIALS AND METHODS
This systematic review included searches from PubMed, EMBASE, Web of Science, African Index Medicus, Google Scholar, and African Journals Online. All English publications reporting the proportion of HNC specimens from SSA patients who tested positive for HPV and/or p16 were included. Study quality was assessed using the National Institutes of Health Quality Assessment Tool for Case Series Studies.
RESULTS
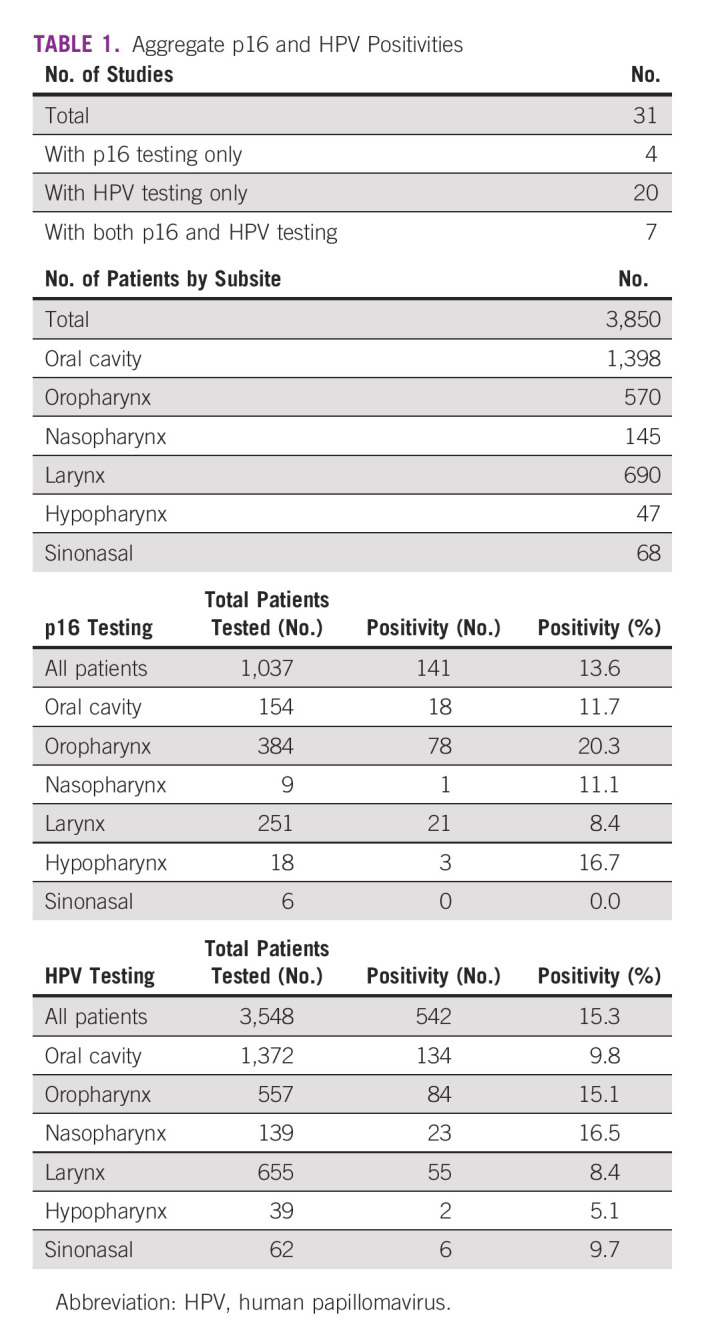
In this systematic review of 31 studies and 3,850 patients, the overall p16 positivity was 13.6% (41 of 1,037 patients tested) with the highest proportion among oropharyngeal cancers (20.3%, 78 of 384 patients) and the overall HPV polymerase chain reaction positivity was 15.3% (542 of 3,548 samples tested) with the highest proportion among nasopharyngeal cancers (16.5%, 23 of 139 patients). Among the 369 HPV strains detected, the most common genotypes were HPV 16 (226 patients, 59.2%) and HPV 18 (78, 20.4%).
CONCLUSION
HPV was found to be associated with a significant proportion of HNC in SSA. The genotypes reported suggest that the nine-valent vaccine and gender-neutral vaccination policies should be considered. Given that these studies may not accurately capture prevalence nor causation of HPV in HNC subsites, additional research is needed to provide a more thorough epidemiologic understanding of HPV-associated HNC in SSA, including risk factors and clinical outcomes.
INTRODUCTION
Head and neck cancers (HNCs) are the seventh most commonly diagnosed malignancy globally.1 Tobacco use and alcohol use are the major risk factors1; however, research over the past several decades has demonstrated strong links between human papillomavirus (HPV) and the development of HNCs, specifically in the oropharynx subsite.2 Although overall rates of HNC are declining, the incidence of HPV-associated squamous cell carcinoma of the oropharyngeal subsite (OPSCC) has increased. In countries such as the United States, the incidence of HPV-associated HNC exceeds that of HPV-associated cervical cancer.3-5 The survival advantage imparted by HPV association in HNC, specifically the oropharyngeal subsite, has resulted in biomarker-driven diagnosis and changes in the latest American Joint Committee on Cancer 8th edition staging system.6 In addition, ongoing research is actively evaluating treatment de-escalation strategies among patients with HPV-associated HNC, with the goal of minimizing toxicity while preserving outcomes.4,6,7
CONTEXT
Key Objective
In sub-Saharan Africa, what is the proportion of head and neck cancers with human papillomavirus (HPV) positivity?
Knowledge Generated
Among 31 studies including 3,850 patients, HPV positivity in the head and neck was assessed most commonly using p16 immunohistochemistry and HPV polymerase chain reaction. The overall p16 positivity was 13.6% with the highest proportion among oropharyngeal cancers (20.3%), and the overall HPV polymerase chain reaction positivity was 15.3% with the highest proportion among nasopharyngeal cancers (16.5%). The areas in which studies lacked in quality were clearly characterized patient populations, reporting of patients who presented consecutively, and detailed results.
Relevance
A portion of head and neck cancers in sub-Saharan African countries test positive for HPV, but additional research is needed to confirm these estimates, assess for risk factors, and characterize clinical outcomes.
Although the proportion of HPV-associated OPSCC is highest in North America at around 60%, regional variations exist; for instance, rates range from 10% in southern Europe to 50% in northern Europe.8 To our knowledge, the proportion of HNCs in sub-Saharan Africa (SSA) associated with HPV is minimally characterized. This is a region of the world that holds the potential for higher proportions given that SSA has the greatest burden of HPV-driven malignancies, including cervical cancer, and the highest global prevalence of HIV,8-20 which predisposes patients to increased rates of oral HPV infection and risk of developing head and neck squamous cell carcinomas.21-26
Characterizing HPV in HNCs is critical in SSA. Accurate data from the SSA region are needed to formulate health policies addressing the prevention, diagnosis, staging, and management of HNCs. Knowledge of the burden of HPV-associated HNC in SSA may influence whether regional ministries of health invest in developing in-country diagnostic capacity and incorporate HPV assessment into national treatment guidelines. The improved prognosis of HPV-associated OPSCC may also change prioritization of limited oncologic resources including radiotherapy.27-30 Finally, identification of a high burden of HPV-associated HNCs could lend support for expanding population-based HPV vaccination strategies.
Assessment of the proportion of HNCs associated with HPV in SSA is an important next step in building capacity for prevention and treatment of HPV-associated HNC in this setting. To address this knowledge gap, we performed a systematic review to report on the proportion of HPV-associated HNCs in SSA. Secondary outcomes of this study were HPV detection methods, the distribution of HPV positivity across head and neck anatomic subsites, and proportion of HPV genotypes. Although current guidelines recommend limiting p16 and HPV testing primarily to oropharyngeal squamous cell carcinomas,4,31 this study includes all HNC subsites given early studies reporting HPV association in other subsites and tumor types32-35 and to broadly capture studies within this systematic review.
MATERIALS AND METHODS
Study Design
This study was registered with the international prospective register of systematic reviews (PROSPERO, registration No. CRD42021252389) and was conducted according to Preferred Reporting Items for Systematic Review and Meta-Analyses guidelines.36
Eligibility Criteria
This systematic review included all cohort, randomized controlled trials, case series, and case-control studies pertaining to HNC, HPV, and SSA. Abstracts from conferences were included if they contained adequate data for inclusion in the analysis. Specifically, studies were required to (1) involve patients with HNC, (2) pertain to patients from SSA, (3) describe HPV in the included study population, (4) report primary data, and (5) not only focus on one or only low-risk HPV subtypes. Head and neck pathologies in addition to squamous cell carcinoma were included given the hypothesized heterogeneity of studies. All publications in English until the date of the search were included.
The primary outcome was the proportion of HPV-associated HNCs reported in SSA. Secondary outcomes included methods used to detect HPV association in HNC, proportion of HNCs with HPV by subsite, and HPV genotypes present in HNCs in SSA.
Of note, the recommended clinical approach to testing for HPV involves p16 immunohistochemistry (IHC) given its high sensitivity and cost-effectiveness with additional testing for high-risk HPV genotypes performed at the discretion of the pathologist.31,37 Additional testing may include HPV polymerase chain reaction (PCR) or HPV in situ hybridization (ISH). The association between p16 status and HPV is strongest for oropharyngeal cancers in comparison with other subsites.31
Data Sources and Search Strategy
The literature search was performed using MeSH terms related to HNC, HPV, and SSA with support from a librarian trained in literature searches. SSA countries were defined by the World Bank.38 Reference lists of pertinent review articles and original research papers identified were also manually screened to identify additional relevant citations (see Appendix Fig A1 and Table A1-A3 for the full search strategy). The electronic databases searched as primary sources included PubMed, EMBASE, Web of Science, and African Index Medicus. Google Scholar and African Journals Online were also searched as secondary sources for any additional, relevant citations. The searches were initially performed on May 6, 2021, and an interval search was performed on May 11, 2022.
Study Selection and Data Collection
Citations identified from the search were uploaded into EndNote. Duplicates were removed. The resultant set of citations was uploaded into Rayyan, a systematic review reference classification platform. The title and abstracts of citations were screened for eligibility by three authors (SO, LM, and MJX). For citations that reported on the same study population, the most recent citation was included. Data were abstracted by two authors (SO and MJX) in a standardized data collection form. Any discrepancies were adjudicated and reconciled between the two authors. Abstracted data included study characteristics, study population, p16 or HPV testing strategy, proportion of p16 or HPV positivity among HNC subsites, and HPV genotypes detected if relevant.
Quality Assessment
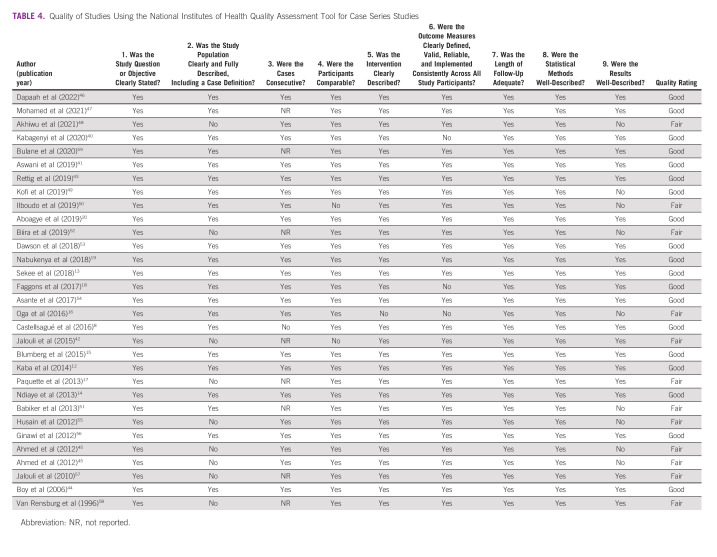
Study quality was assessed using the National Institutes of Health (NIH) Quality Assessment Tool for Case Series Studies by two authors (SO and MJX).39 Discrepancies were reconciled. We defined scores of 1-5 as poor quality, 6-7 as fair, and 8-9 as good.
RESULTS
Study Selection
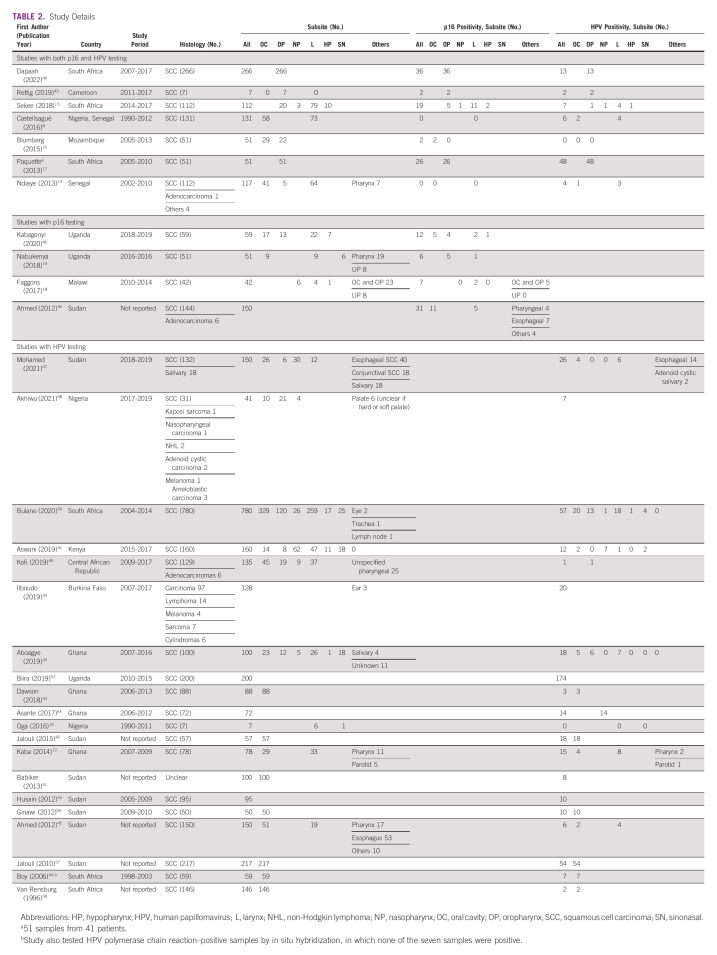
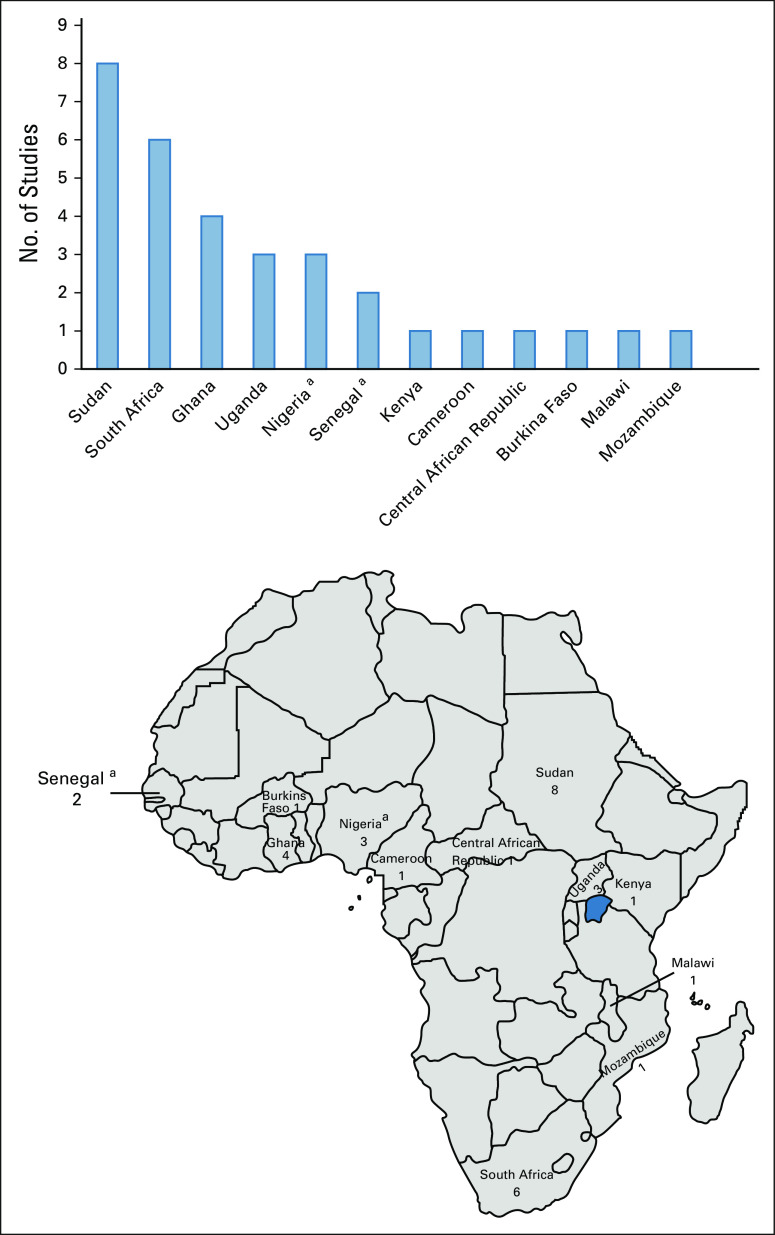
A total of 386 records were identified, of which 31 studies from 12 countries were included (Fig 1). Eight studies were from Sudan, six were from South Africa, four were from Ghana, three were from Uganda, three were from Nigeria, two were from Senegal, and one each was from Kenya, Cameroon, Central African Republic, Burkina Faso, Malawi, and Mozambique (Data Supplement). One study included study populations from both Nigeria and Senegal. Five papers were of prospective studies,13,14,19,40,41 one paper did not report whether samples were collected prospectively or retrospectively,42 and the remaining studies were retrospective.
FIG 1.
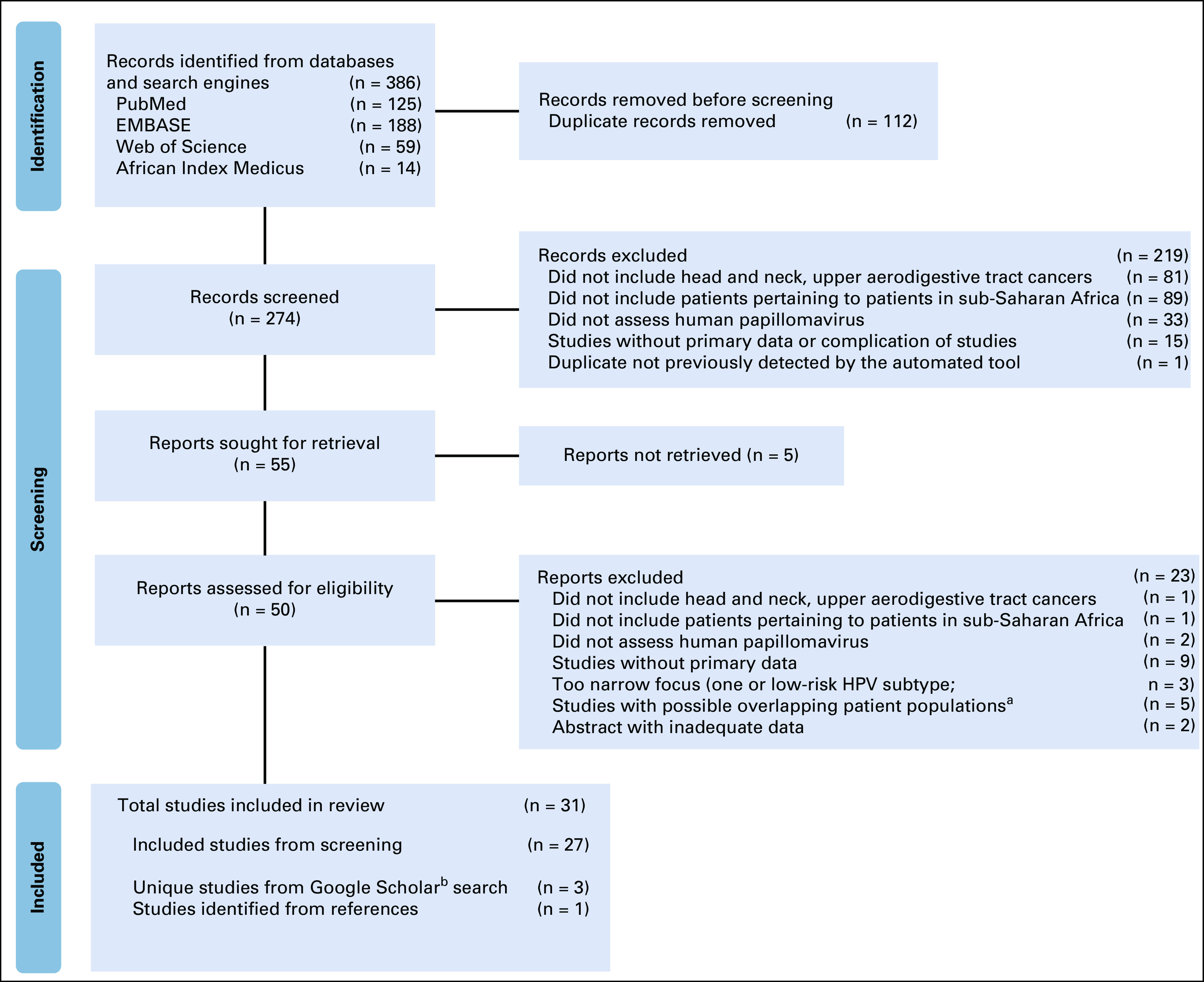
Preferred reporting items for systematic review and meta-analysis flow diagram for study selection and screening process (identification of studies via databases and registers). aStudies with possible overlapping patient populations were the ones in which there were studies from the same country, from the same lead author, and from the similar study period and where there was the possibility of overlapping patient cohorts. In these cases, the more recently published paper was included in the analysis. bGiven differences in the Google Scholar search engine, a search was performed at the completion of the database.
HPV Testing
Of the 31 studies included (Table 1), seven tested by both p16 IHC and HPV PCR,8,13-15,17,43 one study used HPV PCR and HPV ISH,44 four studies used p16 IHC only,18,19,40,45 and the remaining 19 studies used HPV PCR only. Among the seven studies that tested by both p16 IHC and PCR, four studies tested the whole patient population,13,15,17,46 one study tested p16 only on the samples that were reported as HPV-positive by PCR,8 and two studies tested by HPV only on the samples that tested positive by p16 IHC.14,43 For the study that used both HPV PCR and HPV ISH, the ISH was only tested on the samples that were positive by HPV PCR.44
TABLE 1.
Aggregate p16 and HPV Positivities
HPV Association
A total of 3,850 cases of HNCs were collectively reported in the literature. Of these, the following subsites were represented (in decreasing frequency): oral cavity (n = 1,398, 36.3%), larynx (n = 690, 17.9%), oropharynx (n = 570, 14.8%), nasopharynx (n = 145, 3.8%), sinonasal (n = 68, 1.8%), and hypopharynx (n = 47, 1.2%). Six studies included pathologic diagnoses other than squamous cell carcinoma,14,45,47-50 and one study reported the pathologic diagnoses of samples that tested positive for HPV but not the overall cohort.51
p16 IHC
Among patients, 1,037 underwent IHC staining for p16 (Table 1) with an overall p16 positivity rate of 13.6% (n = 105). The highest proportion was noted in oropharyngeal (20.3%, 78 of 384 patients), hypopharyngeal (16.7%, 3 of 18 patients), and oral cavity cancers (11.7%, 18 of 154 patients). When we assessed the 20 studies that only include head and neck squamous cell carcinomas of the upper aerodigestive tract,8,13,15-19,40-44,46,52-58 the proportion of specimens that were positive for p16 IHC (Data Supplement) was similar to that when assessing all the studies (Table 1).
HPV ISH
To our knowledge, the study by Boy et al (2006)44 was the only study to test for HPV with HPV ISH. In their retrospective study of patients from South Africa, they tested ISH on samples that tested positive by HPV PCR. Among those seven samples, none were positive on HPV ISH (Table 2). When the 20 studies that only include head and neck squamous cell carcinomas of the upper aerodigestive tract were assessed,8,13,15-19,40-44,46,52-58 the proportion of specimens that were positive for HPV (Data Supplement) was similar to that when assessing all the studies (Table 1).
TABLE 2.
Study Details
HPV Genotypes
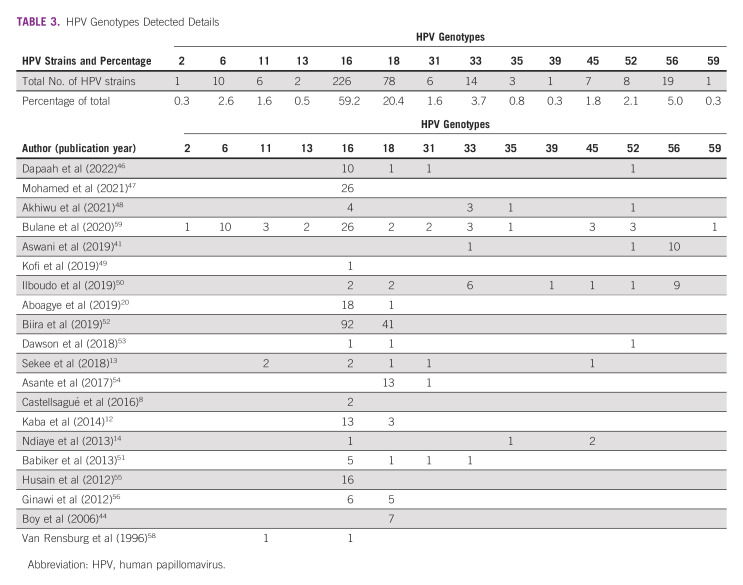
For HPV testing, 3,548 patients were tested by HPV PCR (Table 1) with an overall positivity of 15.3% (n = 542). The nasopharyngeal subsite had the highest proportion of HPV positivity (16.5%, 23 of 139 patients), followed by oropharynx (15.1%, 84 of 557 patients) and oral cavity (9.8%, 134 of 1,372 patients). Specific HPV genotypes were reported for 382 strains detected across all subsites (Table 3). The most commonly reported was HPV 16 (226, 59.2%), HPV 18 (78, 20.4%), and HPV 56 (19, 5.0%). Among the three papers that reported on patients with oropharyngeal cancer and HPV genotypes,13,46,59 the most common were HPV 16 (17 of 27, 63.0%) and HPV 6, 13, and 52 (2 of 27, 7.4% each).
TABLE 3.
HPV Genotypes Detected Details
Risk Factors
Seven studies reported tobacco use in patients, which ranged from 20.0% to 66.8%.18,19,40,41,46,48,57 Alcohol use was reported by five studies, ranging from 8.0% to 85.0%.19,40,41,46,48 Sexual history was reported in two studies.40,41
The proportion of HIV-positive patients ranged from 4.9% to 27.1% among four studies.19,40,41,48 There were two papers that reported HIV and HPV coinfection among patients with head and neck squamous cell carcinoma.18,40 In the study by Kabagenyi et al,40 6.8% of patients (4 of 59 patients) were HIV-positive and had tumors that were p16-positive. Similarly, in the study by Faggons et al (2017),18 the proportion of coinfection was 5.9% (1 of 17 patients). Anatomic subsites were combined in these results, and no studies reported clinical outcomes of HIV- and HPV-coinfected patients.
Quality Assessment and Risk of Bias Within Studies
On the basis of the NIH Quality Assessment Tool for Case Series Studies, 19 studies were rated as good quality and 12 studies were deemed fair (Table 4). The areas in which the most studies lacked in quality were clearly characterized patient populations, reporting of patients who presented consecutively, and well-described results.
TABLE 4.
Quality of Studies Using the National Institutes of Health Quality Assessment Tool for Case Series Studies
DISCUSSION
Although recognition of HPV-associated OPSCC has changed treatment paradigms in high-resource health systems,6,7 less is known about the proportions of HNCs with HPV association in SSA. Meanwhile, the greatest burden of HPV-driven malignancies intersects with the highest prevalence of HIV infection in this setting.23,60 To our knowledge, this is the first systematic review assessing associations between HPV and malignancies in head and neck subsites in SSA. Two international studies, one meta-analysis and one internationally coordinated analysis of head and neck carcinomas, previously reported global proportions of HPV-associated HNSCC to be 31.5% and 12.2%.8,10 These studies, however, included no data on oropharyngeal cancers from SSA and limited data from other head and neck subsites of patients from SSA. Our systematic review noted an overall HPV association in 13.6% of patients on the basis of p16 testing and 15.3% on the basis of HPV testing. The slightly higher rate noted with HPV testing may be due to incidental infection of HPV.
In terms of HPV association in the oropharynx, studies have highlighted regional variations although data from SSA countries are sparse. In a systematic review by Carlander et al61 (2021), 31 studies involving 49,564 patients with OPSCC were assessed and the worldwide positivity ranged from 0% to 85%, with wide geographic variation. The highest rates were found in Northern European countries, the United States, Lebanon, China, and South Korea. Additional international studies have reported HPV-associated OPSCC to be 45.8% and 22.4%.8,10 Our study found HPV association for OPSCC in 20.3% of patients on the basis of p16 testing and 15.1% of patients on the basis of HPV testing. Although these rates are among the lower ranges reported globally, Chaturvedi et al62 (2013) noted that between 1988 and 2002, HPV-positive OPSCC tumors increased among younger males in developed countries. As more of the SSA country economies continue to develop, there is a potential for a surge in HPV-positive OPSCC to rates comparable with developed countries. In addition, these lower rates found in studies centered in SSA are in the context of limited testing for HPV association in many SSA nations. Indeed, Fagan et al63 (2020) reported that 16 of 17 head and neck surgeons in SSA surveyed did not have routine access to p16 testing.
Interestingly, the proportion of nasopharyngeal cancers (NPC) with HPV positivity as tested by HPV PCR was the greatest among subsites at 16.5%. Globally, NPC is most prevalent in Asia and SSA and associated with the Epstein-Barr virus (EBV).64 Stenmark et al33 (2014) noted that patients with HPV-positive/EBV-negative NPCs have worse prognosis than patients who are HPV-negative/EBV-positive. Given the small sample size and heterogeneous nature of studies of this systematic review, more testing is needed to confirm the association and prognostication of HPV in NPC.
Currently, p16 IHC and/or HPV testing is not routinely recommended as part of guidelines for HNCs in SSA.65,66 This is due to challenges related to reagent cost or supply and/or technical aspects of p16 and HPV testing.67 However, our data highlight a need for HPV testing to be included as part of standard care practices. Certainly, knowledge of HPV positivity conveys important prognostic implications and could help inform treatment decisions. Given the heterogeneity of the existing studies, routine p16 and HPV testing of HNC would provide a more comprehensive understanding of the epidemiology of HPV-associated HNC and OPSCC in SSA. Without this information, which is incorporated into the latest tumor staging system, patients in SSA without access to p16 testing could be at a disadvantage because of not receiving prognostic information and limiting their treatment options with the risk of unnecessary toxcitiy.63 Cost is a critical barrier to implementation of p16 testing; assessing cost and considering newer point-of-care testing could aid in increased adoption of HPV testing in SSA.68 Furthermore, with active research into treatment de-escalation for patients with HPV-associated OPSCC, an understanding of the burden of HPV-associated HNC in SSA could lead to reprioritization and reallocation of limited resources for patients with HNC.
Finally, knowledge of the prevalence of subtypes is highly relevant to vaccination programming. Currently, the quadrivalent HPV vaccine covering genotypes 6, 11, 16, and 18 is available in SSA through Gavi, the Vaccine Alliance, for prevention of cervical cancer.69 In the case of cervical cancer, studies have reported that high-risk subtypes including HPV 45 are more prevalent in SSA,23 which may influence future adoption of the nine-valent vaccine that covers for HPV 6, 11, 16, 18, 31, 33, 45, 52, and 58.70 In this study, we found that HPV 16 is the most prevalent genotype detected in HNCs and oropharyngeal cancers. Among all subsites, HPV 18 and HPV 56 were the next most common, the latter of which is currently not covered by the nine-valent vaccine. These data highlight a need to re-evaluate population-based vaccine strategies, particularly given that the potential benefit may extend beyond cervical cancer and include meaningful reduction of the burden of HNC.
A major limitation of this systematic review is the heterogeneity of methodologies among the included studies, minimal data on risk factors, and limited staging and outcomes data. Only 7 of 31 studies tested for both p16 and HPV status. Currently, the standard for testing is to use p16 IHC first as a surrogate for HPV association in tumors involving the oropharynx with follow-up high-risk HPV genotyping used in select scenarios.31 Given that HPV positivity in the absence of p16 positivity may indicate incidental HPV infection, the 20 studies that only tested using HPV PCR would require additional testing such as HPV ISH to confirm the HPV integration. These tests also require training and standardized interpretation, which may influence the results. Furthermore, only six studies reported patient risk factor data,18,19,40,41,48,57 only two papers provided staging information,19,41 and only one paper reported long-term clinical outcomes.19 These variables are critically needed to understand the epidemiology of and context of HPV-associated HNC in SSA.
In conclusion, HPV-associated HNCs comprise a significant proportion of HNCs in SSA. The genotypes reported suggest that the nine-valent vaccine and gender-neutral vaccination policies should be re-evaluated. Given the growing burden of malignancy in SSA, increased research is needed to understand the epidemiology of HPV-associated HNCs, long-term clinical outcomes, and how these data influence treatment guidelines and vaccination policies.
ACKNOWLEDGMENT
We are grateful for Evan Whitaker, MD, from the University of California San Francisco library who helped formulate and advise our search strategy.
APPENDIX
FIG A1.
Number of studies per country. aOne study included patients from both Nigeria and Senegal.
TABLE A1.
Aggregate p16 and HPV Positivities for Studies Only Including Patients With Squamous Cell Carcinoma
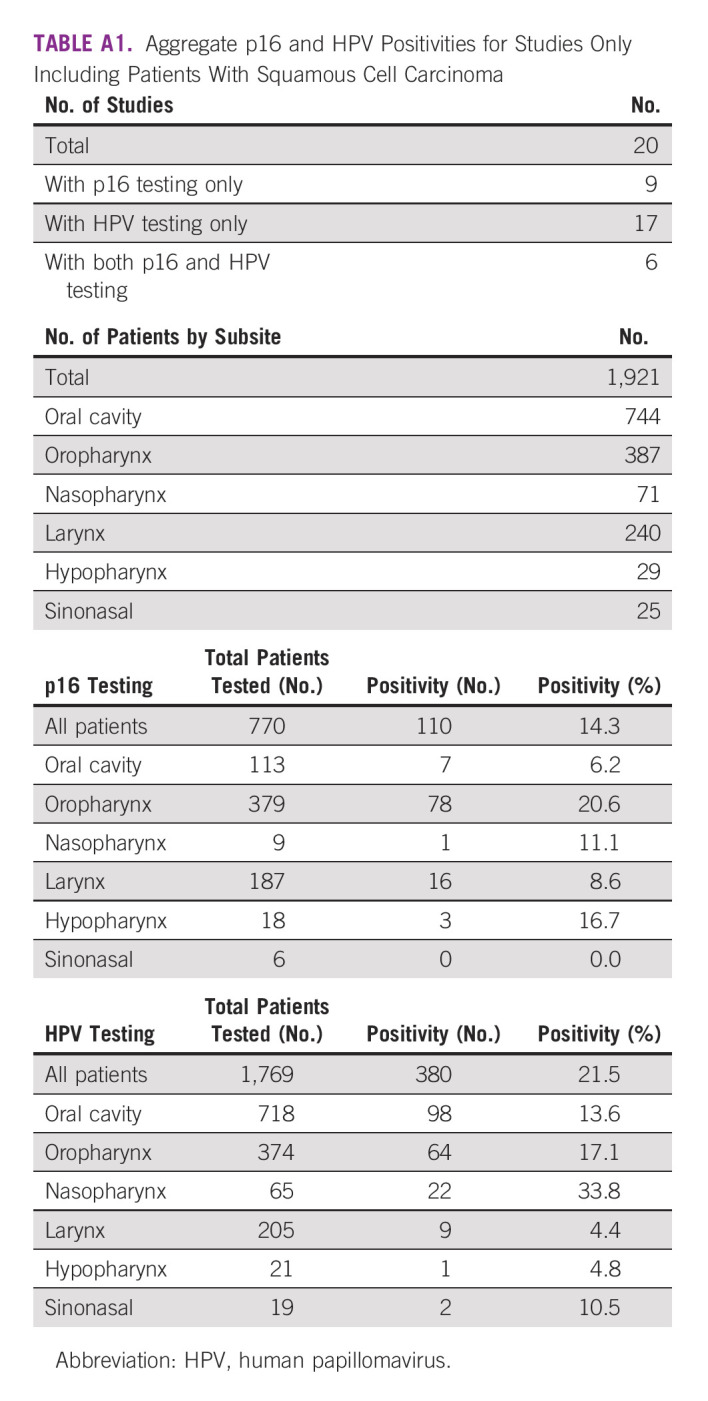
TABLE A2.
Aggregate p16 and HPV Positivities for Studies With the Study Period From 2015 Onward
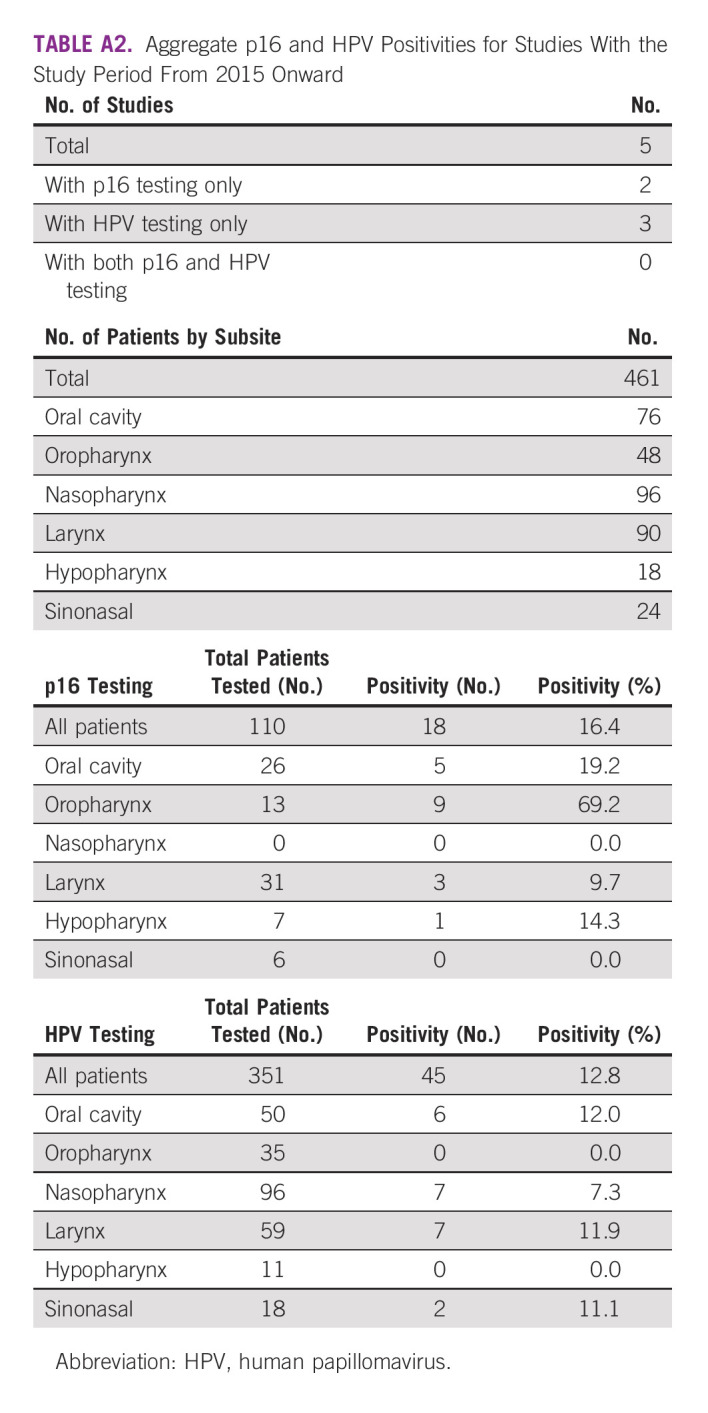
TABLE A3.
Aggregate p16 and HPV Positivities for Studies With Study Periods Before 2015
Katherine Van Loon
Research Funding: Celgene (Inst)
Geoffrey Buckle
Research Funding: Celgene
Mary Jue Xu
Employment: Merck
Stock and Other Ownership Interests: Merck
Travel, Accommodations, Expenses: AXOGEN, AbbVie, PolarityTE, OptiNose
Samuel Okerosi
Travel, Accommodations, Expenses: Krishna Chemists
Patrick Ha
Consulting or Advisory Role: Rakuten Medical, Checkpoint Surgical, Privo Technologies
Research Funding: Stryker (Inst), Medtronic (Inst), Ethicon (Inst)
No other potential conflicts of interest were reported.
SUPPORT
D.N. received funding from the National Cancer Institute (K08CA263299).
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Administrative support: Katherine Van Loon, Joyce Aswani
Collection and assembly of data: Samuel Okerosi, Lillian Wairimu Mokoh, Brandon Asuman Niyibizi, Aslam Nkya, Mary Jue Xu
Data analysis and interpretation: Samuel Okerosi, Fidel Rubagumya, Brandon Asuman Niyibizi, Aslam Nkya, Katherine Van Loon, Geoffrey Buckle, Stephen Bent, Johannes J. Fagan, Dianna Ng, Mary Jue Xu
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Katherine Van Loon
Research Funding: Celgene (Inst)
Geoffrey Buckle
Research Funding: Celgene
Mary Jue Xu
Employment: Merck
Stock and Other Ownership Interests: Merck
Travel, Accommodations, Expenses: AXOGEN, AbbVie, PolarityTE, OptiNose
Samuel Okerosi
Travel, Accommodations, Expenses: Krishna Chemists
Patrick Ha
Consulting or Advisory Role: Rakuten Medical, Checkpoint Surgical, Privo Technologies
Research Funding: Stryker (Inst), Medtronic (Inst), Ethicon (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, et al. : Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394-424, 2018 [DOI] [PubMed] [Google Scholar]
- 2.D'Souza G, Dempsey A: The role of HPV in head and neck cancer and review of the HPV vaccine. Prev Med 53:S5-S11, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC-how many cancers are linked with HPV each year? https://www.cdc.gov/cancer/hpv/statistics/cases.htm
- 4.Ang KK, Harris J, Wheeler R, et al. : Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363:24-35, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. : Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 29:4294-4301, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lydiatt WM, Patel SG, O'Sullivan B, et al. : Head and neck cancers-major changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual. CA Cancer J Clin 67:122-137, 2017 [DOI] [PubMed] [Google Scholar]
- 7.Wirth LJ, Burtness B, Nathan CAO, et al. : Point/counterpoint: Do we de-escalate treatment of HPV-associated oropharynx cancer now? And how?. Am Soc Clin Oncol Educ book 39:364-372, 2019 [DOI] [PubMed] [Google Scholar]
- 8.Castellsagué X, Alemany L, Quer M, et al. : HPV involvement in head and neck cancers: Comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst 108:djv403, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Faggons CE, Mabedi C, Shores CG, Gopal S: Review: Head and neck squamous cell carcinoma in sub-Saharan Africa. Malawi Med J 27:79-87, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ndiaye C, Mena M, Alemany L, et al. : HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: A systematic review and meta-analysis. Lancet Oncol 15:1319-1331, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Jedy-Agba EE, Dareng EO, Adebamowo SN, et al. : The burden of HPV associated cancers in two regions in Nigeria 2012-2014. Cancer Epidemiol 45:91-97, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaba G, Dzudzor B, Gyasi RK, et al. : Human papillomavirus genotypes in a subset of head and neck squamous cell carcinoma. West Afr J Med 33:121-124, 2014 [PubMed] [Google Scholar]
- 13.Sekee TR, Burt FJ, Goedhals D, et al. : Human papillomavirus in head and neck squamous cell carcinomas in a South African cohort. Papillomavirus Res 6:58-62, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ndiaye C, Alemany L, Diop Y, et al. : The role of human papillomavirus in head and neck cancer in Senegal. Infect Agents Cancer 8:14, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blumberg J, Monjane L, Prasad M, et al. : Investigation of the presence of HPV related oropharyngeal and oral tongue squamous cell carcinoma in Mozambique. Cancer Epidemiol 39:1000-1005, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oga EA, Schumaker LM, Alabi BS, et al. : Paucity of HPV-related head and neck cancers (HNC) in Nigeria. PLoS One 11:e0152828, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paquette C, Evans MF, Meer SS, et al. : Evidence that alpha-9 human papillomavirus infections are a major etiologic factor for oropharyngeal carcinoma in black South Africans. Head Neck Pathol 7:361-372, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faggons CE, Mabedi CE, Liomba NG, et al. : Human papillomavirus in head and neck squamous cell carcinoma: A descriptive study of histologically confirmed cases at Kamuzu Central Hospital in Lilongwe, Malawi. Malawi Med J 29:142-145, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nabukenya J, Hadlock TA, Arubaku W: Head and neck squamous cell carcinoma in Western Uganda: Disease of uncertainty and poor prognosis. OTO Open 2:2473974X18761868, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aboagye E, Agyemang-Yeboah F, Duduyemi BM, Obirikorang C: Human papillomavirus detection in head and neck squamous cell carcinomas at a Tertiary Hospital in Sub-Saharan Africa. Scientific World J 2019:2561530, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beachler DC, D'Souza G: Oral human papillomavirus infection and head and neck cancers in HIV-infected individuals. Curr Opin Oncol 25:503-510, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ceccarelli M, Rullo EV, Facciola A, et al. : Head and neck squamous cell carcinoma and its correlation with human papillomavirus in people living with HIV: A systematic review. Oncotarget 9:17171-17180, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Vuyst H, Alemany L, Lacey C, et al. : The burden of human papillomavirus infections and related diseases in sub-Saharan Africa. Vaccine 31:F32-F46, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu G, Sharma M, Tan N, Barnabas RV: HIV-positive women have higher risk of human papilloma virus infection, precancerous lesions, and cervical cancer. AIDS 32:795-808, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beachler DC, Abraham AG, Silverberg MJ, et al. : Incidence and risk factors of HPV-related and HPV-unrelated head and neck squamous cell carcinoma in HIV-infected individuals. Oral Oncol 50:1169-1176, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beachler DC, Weber KM, Margolick JB, et al. : Risk factors for oral HPV infection among a high prevalence population of HIV-positive and at-risk HIV-negative adults. Cancer Epidemiol Biomarkers Prev 21:122-133, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atun R, Jaffray DA, Barton MB, et al. : Expanding global access to radiotherapy. Lancet Oncol 16:1153-1186, 2015 [DOI] [PubMed] [Google Scholar]
- 28.Barton MB, Frommer M, Shafiq J: Role of radiotherapy in cancer control in low-income and middle-income countries. Lancet Oncol 7:584-595, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Abdel-Wahab M, Bourque JM, Pynda Y, et al. : Status of radiotherapy resources in Africa: An International Atomic Energy Agency analysis. Lancet Oncol 14:e168-e175, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Elmore SNC, Polo A, Bourque JM, et al. : Radiotherapy resources in Africa: An International Atomic Energy Agency update and analysis of projected needs. Lancet Oncol 22:e391-e399, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis JS Jr., Beadle B, Bishop JA, et al. : Human papillomavirus testing in head and neck carcinomas: Guideline from the College of American Pathologists. Arch Pathol Lab Med 142:559-597, 2018 [DOI] [PubMed] [Google Scholar]
- 32.Oliver JR, Lieberman SM, Tam MM, et al. : Human papillomavirus and survival of patients with sinonasal squamous cell carcinoma. Cancer 126:1413-1423, 2020 [DOI] [PubMed] [Google Scholar]
- 33.Stenmark MH, McHugh JB, Schipper M, et al. : Nonendemic HPV-positive nasopharyngeal carcinoma: Association with poor prognosis. Int J Radiat Oncol Biol Phys 88:580-588, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li H, Torabi SJ, Yarbrough WG, et al. : Association of Human Papillomavirus Status at Head and Neck Carcinoma Subsites with Overall Survival. JAMA Otolaryngol Head Neck Surg 144:519-525, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian S, Switchenko JM, Jhaveri J, et al. : Survival outcomes by high-risk human papillomavirus status in nonoropharyngeal head and neck squamous cell carcinomas: A propensity-scored analysis of the National Cancer Data Base. Cancer 125:2782-2793, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Page MJ, McKenzie JE, Bossuyt PM, et al. : The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372:n71, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Augustin JG, Lepine C, Morini A, et al. : HPV detection in head and neck squamous cell carcinomas: What is the issue?. Front Oncol 10:1751, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sub-Saharan Africa. The World Bank . https://data.worldbank.org/country/ZG
- 39.Study Quality Assessment Tools. National Heart, Lung, and Blood Institute . https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. 2021
- 40.Kabagenyi F, Otiti J, Namwagala J, et al. : A descriptive study of human papilloma virus in upper aero-digestive squamous cell carcinoma at Uganda Cancer Institute assessed by p16 immunohistochemistry. Cancers Head Neck 5:10, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aswani J, Anzala O, Mwang’ombe N: High risk human papillomavirus in head and neck squamous cell carcinoma patients at Kenyatta national Hospital, Kenya. Afr J Health Sci 32:1-14, 2019 [Google Scholar]
- 42.Jalouli M, Jalouli J, Ibrahim SO, et al. : Comparison between single PCR and nested PCR in detection of human papilloma viruses in paraffin-embedded OSCC and fresh oral mucosa. In Vivo 29:65-70, 2015 [PubMed] [Google Scholar]
- 43.Rettig EM, Gooi Z, Bardin R, et al. : Oral human papillomavirus infection and head and neck squamous cell carcinoma in Rural Northwest Cameroon. OTO Open 3:2473974X18818415, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boy S, Rensburg EJV, Engelbrecht S, et al. : HPV detection in primary intra-oral squamous cell carcinomas--commensal, aetiological agent or contamination?. J Oral Pathol Med 35:86-90, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Ahmed HG, Mustafa SA, Warille E: Human papilloma virus attributable head and neck cancer in the Sudan assessed by p16INK4A immunostaining. Asian Pac J Cancer Prev 13:6083-6086, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Dapaah G, Hille J, Faquin WC, et al. : The prevalence of human papillomavirus-positive oropharyngeal squamous cell carcinoma at one of the Largest Tertiary Care Centers in Sub-Saharan Africa. Arch Pathol Lab Med 146:1018-1023, 2022 [DOI] [PubMed] [Google Scholar]
- 47.Mohamed FE, Aldayem LN, Hemaida MA, et al. : Molecular detection of human papillomavirus-16 among Sudanese patients diagnosed with squamous cell carcinoma and salivary gland carcinoma. BMC Res Notes 14:56, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akhiwu BI, Akhiwu HO, Afolaranmi TO, et al. : Characterization of high risk human papilloma virus genotypes associated with oropharyngeal cancers in a Nigerian population. Pan Afr Med J 38:40, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kofi B, Mossoro-Kpinde CD, Mboumba Bouassa RS, et al. : Infrequent detection of human papillomavirus infection in head and neck cancers in the Central African Republic: A retrospective study. Infect Agents Cancer 14:9, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ilboudo M, Zohoncon TM, Traore EMA, et al. : Characterization of high-risk oncogenic human papillomavirus genotypes in histologically confirmed ear, nose and throat (ENT) cancers in Burkina Faso. Asian Pac J Cancer Prev 20:3429-3435, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Babiker AY, Eltom FM, Abdalaziz MS, et al. : Screening for high risk human papilloma virus (HR-HPV) subtypes, among Sudanese patients with oral lesions. Int J Clin Exp Med 6:275-281, 2013 [PMC free article] [PubMed] [Google Scholar]
- 52.Biira AM, Kityamuwesi R, Rwenyonyi CM: HIV-1 infected individuals are at a higher risk of HPV 16 associated oral and oropharyngeal squamous cell carcinoma than HPV 18. Eur J Immunol 49:1068, 2019 [Google Scholar]
- 53.Dawson RNT, Nartey NO, Kwamin F, et al. : Human papillomavirus DNA prevalence and type distribution in oral squamous cell carcinoma in Ghana. Translational Res Oral Oncol 3:2057178X1878712, 2018 [Google Scholar]
- 54.Asante DB, Asmah RH, Adjei AA, et al. : Detection of human papillomavirus genotypes and Epstein-Barr virus in nasopharyngeal carcinomas at the Korle-Bu Teaching Hospital, Ghana. Scientific World J 2017:2721367, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Husain N, Abdul Rahman A, Osman H, Elmubarak A: Human papillomavirus type 16 in Sudanese patients with upper respiratory and digestive tract squamous cell carcinoma. Histopathology 61:160-161, 2012 [Google Scholar]
- 56.Ginawi IAM, Mahgoub EA, Ahmed HG: Immunophenotyping of HPV types 16 and 18 among Sudanese patients with oral lesions. Oman Med J 27:201-206, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jalouli J, Ibrahim SO, Sapkota D, et al. : Presence of human papilloma virus, herpes simplex virus and Epstein-Barr virus DNA in oral biopsies from Sudanese patients with regard to toombak use. J Oral Pathol Med 39:599-604, 2010 [DOI] [PubMed] [Google Scholar]
- 58.Van Rensburg EJ, Engelbrecht S, Van Heerden WF, et al. : Human papillomavirus DNA in oral squamous cell carcinomas from an African population sample. Anticancer Res 16:969-973, 1996 [PubMed] [Google Scholar]
- 59.Bulane A, Goedhals D, Seedat RY, et al. : Human papillomavirus DNA in head and neck squamous cell carcinomas in the Free State, South Africa. J Med Virol 92:227-233, 2020 [DOI] [PubMed] [Google Scholar]
- 60.Ortblad KF, Lozano R, Murray CJ: The burden of HIV: Insights from the global burden of disease study 2010. AIDS 27:2003-2017, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carlander AF, Jakobsen KK, Bendtsen SK, et al. : A contemporary systematic review on repartition of HPV-positivity in oropharyngeal cancer worldwide. Viruses 13:1326, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chaturvedi AK, Anderson WF, Lortet-Tieulent J, et al. : Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol 31:4550-4559, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fagan JJ, Wetter J, Otiti J, et al. : Is AJCC/UICC staging still appropriate for head and neck cancers in developing countries?. OTO Open 4:2473974X20938313, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen YP, Chan ATC, Le QT, et al. : Nasopharyngeal carcinoma. Lancet 394:64-80, 2019 [DOI] [PubMed] [Google Scholar]
- 65.Zafereo M, Yu J, Onakoya PA, et al. : African Head and Neck Society Clinical Practice guidelines for thyroid nodules and cancer in developing countries and limited resource settings. Head Neck 42:1746-1756, 2020 [DOI] [PubMed] [Google Scholar]
- 66.Koh WJ, Anderson BO, Carlson RW: NCCN resource-stratified and harmonized guidelines: A paradigm for optimizing global cancer care. Cancer 126:2416-2423, 2020 [DOI] [PubMed] [Google Scholar]
- 67.NCCN Harmonized Guidelines For Sub-Saharan Africa. 2021. [Google Scholar]
- 68.Jang D, Shah A, Arias M, et al. : Performance of AmpFire HPV assay on neck cervical lymph node aspirate and oropharyngeal samples. J Virol Methods 279:113840, 2020 [DOI] [PubMed] [Google Scholar]
- 69.Tanzania Support for Human Papillomavirus Vaccine (HPV) Demonstration Programme [Press Release]. 2016 [Google Scholar]
- 70.Joura EA, Giuliano AR, Iversen OE, et al. : A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med 372:711-723, 2015 [DOI] [PubMed] [Google Scholar]