PURPOSE
A nationwide lockdown was enforced in Brazil starting in March 2020 because of the COVID-19 pandemic when cancer screening activities were reduced. In this study, we evaluated the impact of the COVID-19 pandemic on breast cancer (BC) diagnosis.
METHODS
We extracted data from the medical records of patients age older than 18 years who were diagnosed with BC and started treatment or follow-up in private oncology institutions in Brazil between 2018 and 2021. The primary objective was to compare the stage distribution during the COVID-19 pandemic (2020-2021) with a historical prepandemic control cohort (2018-2019). Early BC was defined as stage I-II and advanced disease as stage IV.
RESULTS
We collected data for 11,753 patients with an initial diagnosis of BC, with 6,493 patients in the pandemic (2020-2021) and 5,260 patients in the prepandemic period (2018-2019). We observed a lower prevalence of early-stage BC (63.6% v 68.4%) and a higher prevalence of advanced-stage BC (16.9 v 12.7%), after the onset of the pandemic (both P < .01). This pattern was similar for both estrogen receptor–positive/human epidermal growth factor receptor 2–negative and human epidermal growth factor receptor 2–positive tumors: significantly decreased in the early stage from 69% to 67% and 68% to 58%, respectively, and a considerable increase in advanced-stage disease from 13% to 15% and 13% to 20%, respectively. For triple-negative BC, there was a significantly higher percentage of patients with advanced-stage disease during the pandemic (17% v 11%). Overall, age 50 years or older and postmenopausal status were associated with a greater risk of advanced stage at diagnosis during the pandemic period.
CONCLUSION
We observed a substantial increase in the number of cases of advanced-stage BC in Brazil during the COVID-19 pandemic.
INTRODUCTION
The first case of the novel coronavirus, also called SARCoV-2, was identified in Wuhan, China, on December 31, 2019. Since then, cases of coronavirus disease 2019, known as COVID-19, began to spread globally. In March 2020, the WHO classified the outbreak of the disease as a pandemic, the month in which the first case was identified in São Paulo, Brazil.1 According to data updated in March 2022 by the WHO, nearly 482 million confirmed cases of COVID-19 have been reported worldwide, with approximately 6.2 million deaths since the beginning of the pandemic.2 According to official estimates provided by the federal government, as of March 2022, almost 30 million cases and 659,000 accumulated deaths have been recorded in Brazil.3
CONTEXT
Key Objective
Our study evaluated the impact of the COVID-19 pandemic on breast cancer (BC) diagnosis.
Knowledge Generated
The results of this study suggest that the proportion of advanced-stage BC has increased significantly since the beginning of the pandemic, especially in tumors with a more aggressive phenotype (human epidermal growth factor receptor 2–positive and triple-negative). As we expected, this increase was accompanied by an equally significant decrease in early-stage BC diagnoses.
Relevance
To our knowledge, our study is the largest reported to date addressing the impact of the COVID-19 pandemic on BC diagnosis. It was the first conducted in a low-middle income country and specifically addressed BC, a condition whose outcomes may be more sensitive to decreased screening and early diagnosis than other cancers because of its highly curable nature when diagnosed at an early stage.
It is widely known that for patients with cancer, timely diagnosis and prompt initiation of treatment are vital to ensure the best results. However, to flatten the curve of the COVID-19 pandemic and consequently reduce the risk of the collapse of the health system, a series of measures were instituted, including lockdowns. These measures, however, led to a reduction or cessation of most elective health services, which, in addition to the widespread reluctance of the population to maintain their routine medical evaluations, resulted in a significant drop in cancer screening and even in the investigation of new clinical abnormalities. This grim reality immediately raised concerns about the potential reversal of decades of progress in breast cancer (BC) early diagnosis, which largely accounts for the significant drop in mortality observed in many countries.4
In this study, we aimed to evaluate the impact of the COVID-19 pandemic on the stage of BC diagnosis in patients from the Oncoclínicas Group, the largest community oncology practice in Brazil. We assessed the stage at BC diagnosis in patients who had their first consultation or started follow-up in the years 2020-2021 when compared with a historical control from 2018 to 2019.
METHODS
Study Design and Population
Oncoclínicas consists of a network of (at the time of this writing) more than 90 oncology outpatient clinics and hospitals present in 13 provinces across Brazil, with a centralized management, standardized patient care procedures, and interconnected patient charts.
This is a national population-based retrospective study, with data collected from curated electronic health records (EHRs) and cancer staging evaluated for all patients age older than 18 years, diagnosed with invasive BC (International Classification of Diseases 10th edition C50), in their first evaluation for treatment, follow-up or second opinion consultation at one of the Oncoclínicas units, between January 1, 2018, and December 31, 2021. The eighth edition of American Joint Committee on Cancer (AJCC) staging module was used to define the initial staging.5
The primary objective was to compare the stage distribution at the first visit during the COVID-19 pandemic (pandemic cohort refers to patients first visited between January 1, 2020, and December 31, 2021) with a historical control cohort of the prepandemic period (prepandemic cohort refers to patients referred between January 1, 2018, and December 31, 2019). Secondary end points were stratified according to BC molecular subtypes, patient age, and menopausal status.
Data Collection
Deidentified data were extracted from the EHRs fed by the Oncoclínicas’ medical team. We combined longitudinal EHR data from all sites in a cloud-based platform that includes structured data with elements from unstructured sources using technology-based abstraction techniques. Trained data curators qualify the data using mCODE standards and predefined ontology and actively search for critical variables in the patient's disease trajectory, including clinicopathological features, patient demographics, treatment exposure, and outcomes. In the present study, we selected patients diagnosed with BC who first visited Oncoclínicas between January 2018 and December 2021.
Statistical Methods
Descriptive statistics were used for demographic data and information about the participants included in our analyses. Categorical data and percentages for each variable were calculated after dichotomization on the basis of BC staging. For age, the median and range were calculated, followed by dichotomization of age younger than 50 or 50 years or older (considered premenopause and postmenopause in case menopausal status was missing).
Univariate and multivariate tests were also performed on the basis of the types of variables to be analyzed, namely staging, age, and tumor subtype (on the basis of immunohistochemical profile: estrogen receptor–positive (ER+), human epidermal growth factor receptor 2–positive [HER2+], and triple negative). We defined early stage as AJCC eighth I and II and advanced stage as AJCC IV. The following tests were initially used: Fisher's exact test, test of proportions, and a generalized linear model on the basis of logistic regression. The variables were only considered significant if they had a minimum significance of P value < .1 (all P values were two-sided). Missing data were disregarded for calculation purposes in the analyses.
The population studied was identified for convenience and represents the total number of cases of International Classification of Diseases-10 C50 diagnosed in the Oncoclínicas. According to historical data, with an average of 2,500 new patients per year, our sample size made it possible to identify differences in the prevalence of stages in the order of 3% when comparing prepandemic and pandemic periods, with a statistical power > 80%.
RESULTS
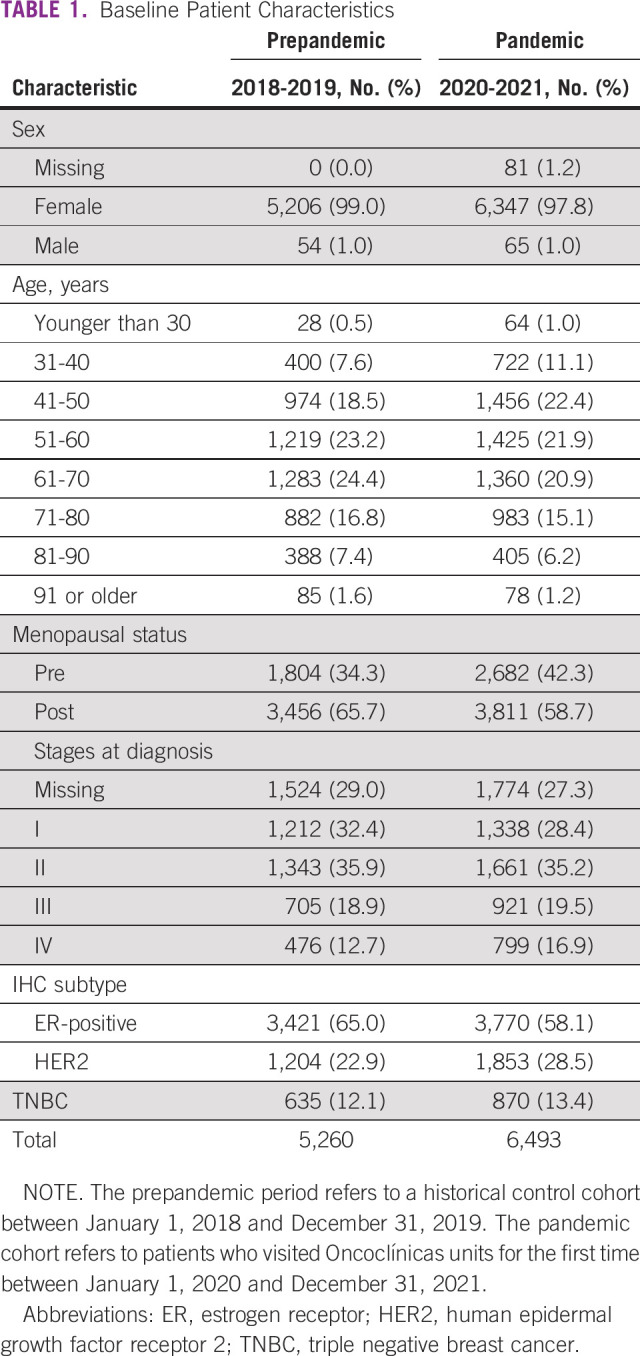
We analyzed data from 11,753 patients diagnosed of invasive BC, comprising 5,260 and 6,493 patients from the prepandemic and pandemic cohorts, respectively. Most patients were female, age older than 50 years, and postmenopausal (Table 1).
TABLE 1.
Baseline Patient Characteristics
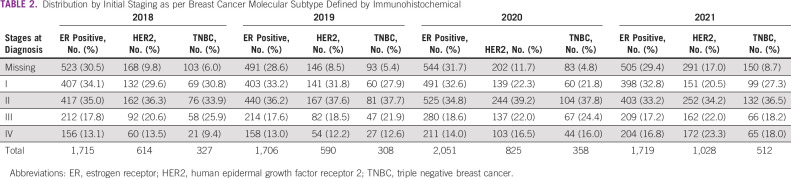
In the prepandemic cohort, the proportion of patients with stage I-IV BC was 32.5%, 35.9%, 18.9%, and 12.7%, respectively. Information was not available for approximately 29% (n = 1,524) of the cases. In the pandemic cohort, the same distribution by stage I-IV was 28.4%, 35.2%, 19.5%, and 17%, respectively, with missing information in 27% (n = 1,774) of the patients.
Comparing the pandemic with the prepandemic cohort, we observed a lower prevalence of patients with early-stage BC (63.6% v 68.4%; two-sided P < .001) and (mainly through the year 2021), a higher prevalence of patients with advanced-stage BC (16.9 v 12.7%; P < .001; Fig 1A).
FIG 1.
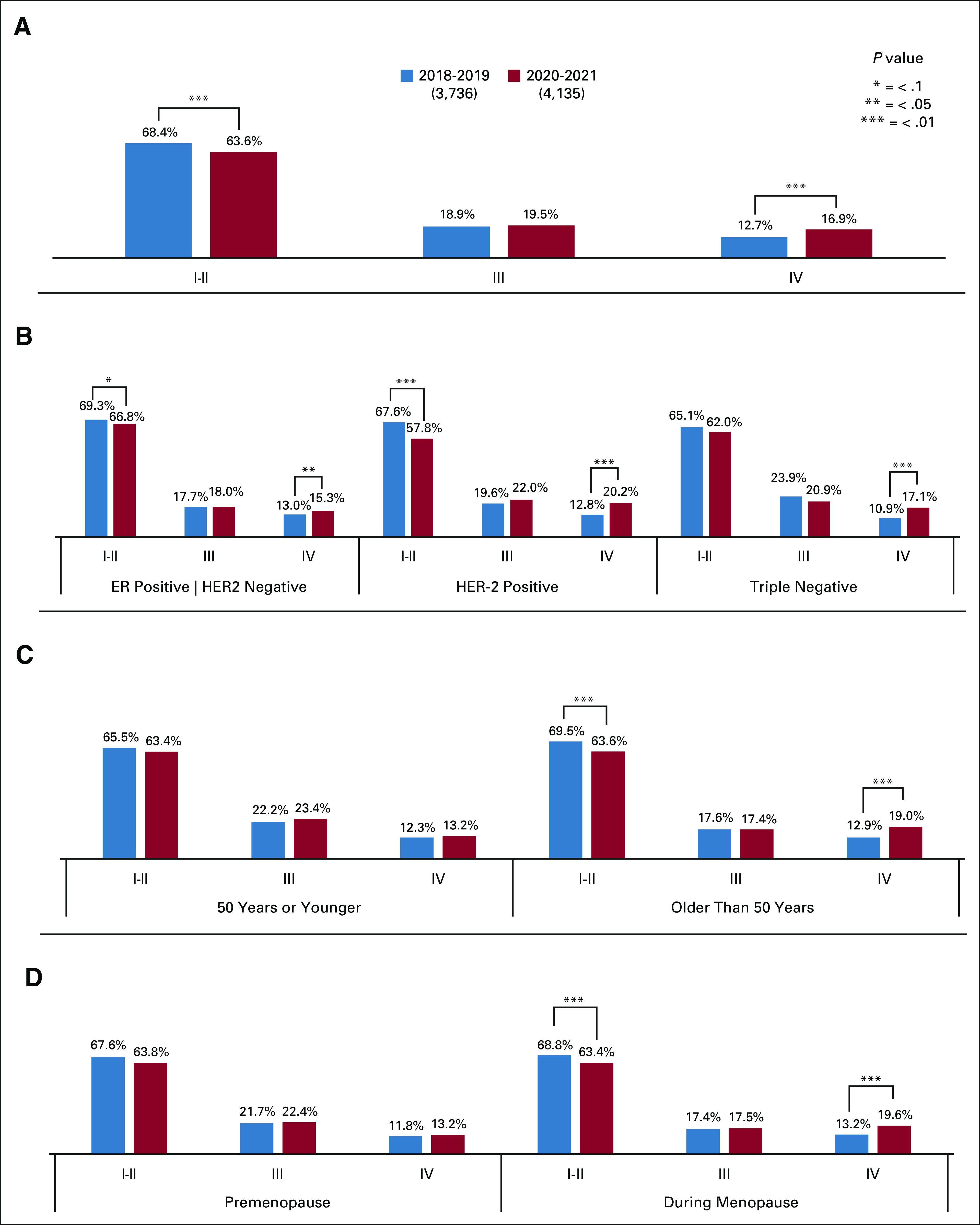
(A) Distribution of cases of early and advanced BC prepandemic and during the pandemic. (B) Distribution of BC cases by immunohistochemical profile in the prepandemic period and during the pandemic. (C) Distribution of BC cases by age ( 50 or younger v older than 50 years) in the prepandemic period and during the pandemic. (D) Distribution of BC cases by menopausal status in the prepandemic period and during the pandemic. BC, breast cancer.
Among the ER+/HER2- tumors (n = 7,191), there was a numerically lower prevalence of patients in the early-stage (67% v 69%; P = .061) and a significantly higher proportion of patients with advanced-stage BC (15% v 13%; P = .026) in the pandemic cohort than in the prepandemic years (Table 2 and Fig 1B).
TABLE 2.
Distribution by Initial Staging as per Breast Cancer Molecular Subtype Defined by Immunohistochemical
A similar pattern was observed in HER2+ tumors (n = 3,056), with a significantly lower prevalence of patients with early-stage (58% v 68%; P < .01) and a higher proportion of advanced-stage BC (20% v 13%; P < .01) in the pandemic period than in the prepandemic cohort (Table 2 and Fig 1B).
For triple-negative BC (n = 1,505), there was a trend toward a lower prevalence of early-stage BC (62% v 65%; P > .1) and a higher prevalence of advanced-stage BC (17% v 11%; two-sided P < .01) in the pandemic cohort (Table 2 and Fig 1B).
A higher prevalence of advanced-stage BC was observed in patients age older than 50 years (19% v 13%) and postmenopausal status (20% v 13%) in the pandemic cohort versus prepandemic years (Figs 1C and 1D, respectively).
DISCUSSION
The COVID-19 pandemic has had a negative impact on global access to health care and has not spared oncology. The results of this retrospective database study suggest that the proportion of advanced-stage BC at Oncoclínicas has increased significantly since the beginning of the pandemic, mainly in tumors with a more aggressive phenotype such as HER2+ and triple-negative; unsurprisingly, this increase was accompanied by an equally meaningful decrease in the diagnosis of BC at an early stage. To the best of our knowledge, this study is the largest reported so far addressing this important issue and the first to be conducted in a low-middle income country. Furthermore, it specifically addresses BC, a condition whose outcomes may be more sensitive to decreases in screening and early diagnosis than in other types of cancer because of its highly curable nature when diagnosed at earlier stages.
Our results are consistent with those of other studies. Zhou et al6 reported a cohort of patients diagnosed with some types of cancer at the UC San Diego Health Cancer Services outpatient clinic. In this series, among 442 patients with BC, there was a percentage decrease in the number of patients presenting with clinical stage I from 51.3% in 2020 to 63.9% in 2019, as well as a higher prevalence of metastatic cases (6.2% v 1.9%).
In an interesting presentation at ASCO 2021, Lloyd et al7 reported the results of a retrospective analysis of 1,930 patients diagnosed with invasive BC between 2016 and 2020 using the Beth Israel Deaconess Medical Center Cancer Registry. The proportion of patients diagnosed with stage III and IV BC in 2020 was almost double that observed between 2016 and 2019 (12.6% v 6.6%; P < .001). In addition to the year of diagnosis, lower income and increased Charlson Comorbidity Index were associated with a more advanced stage at diagnosis in a multivariate analysis. Although in our study we did not address medical comorbidities, the identification of age older than 50 years and postmenopausal status as factors associated with a greater odds of metastatic disease at presentation appears in line with the results of Lloyd et al7, since this is the most vulnerable population, generally associated with other unfavorable health conditions, which was more reclusive during the lockdown and its aftermath.
The main reason for this increase in the number of advanced cases might be directly related to the reduction in the execution of screening tests such as mammography, to a greater difficulty in accessing health services as a result of lockdowns and/or to the reluctance of the population to break isolation because of the risk of infection. According to a retrospective analysis that used Optum's deidentified Clinformatics Data Mart Database (which includes Medicare and commercially insured members), the percentage of patients who were screened for BC decreased by 49.5% between March and June of 2020 compared with the same period in 2019.8 Yabroff et al9 also observed a significant decline in the number of cancer pathology reports, including BC, during 2020 in a population-based cancer registry pathology of patients from Georgia and Louisiana (USA), which was 40% lower in some months than in the same period in 2019.
Our study was carried out in a population served exclusively by private health services, but because of the peculiarities of public health services in low- and middle-income countries such as Brazil, we believe that the impact of the COVID-19 pandemic could be even more impactful. Epidemiological cancer data in Brazil are still inconsistent owing to the low capillarity of cancer registry services in all states of the country. The National Cancer Institute (INCA) publishes a document every three years that estimates, through a mathematical model, the incidence and mortality from cancer in different states of Brazil.10 Consequently, the production of data regarding the impact of the COVID-19 pandemic on the diagnosis and treatment of BC in Brazil is greatly impaired.
Two studies published in 2022 evaluated the impact of COVID-19 on the early diagnosis of BC by public health services in our country. Moterani Júnior et al studied three Brazilian public databases regarding the performance of mammography between the beginning of 2017 and end of 2020. There was a drop in the monthly average of mammograms from 14.9/1,000 in 2019 to 9.25/1,000 in 2020,11 raising the possibility of later diagnoses in subsequent years of screening, as demonstrated in this study. A second study carried out at the public oncology service in the state of São Paulo compared BC diagnoses made in 2019 with those made in 2020. The number of diagnoses performed in 2020 (pandemic period) was 59 compared with 115 in the previous year. However, the percentage of symptomatic cases (palpable masses) was 79.7% in 2020 and 50.4% in 2019 (P < .001), confirming the delay in the diagnosis of BC caused by the COVID-19 pandemic.12
Our study has some limitations, two of which are important to highlight. First, this was a retrospective and observational study; therefore, causality could not be assessed. Second, our analysis included patients diagnosed with BC who were evaluated during the first consultation at Oncoclínicas between 2018 and 2021. Thus, this selection criterion included patients who sought a second opinion, potentially because of relapsed/recurrent disease, with a prior diagnosis of early-stage disease in another clinic. However, we believe that this ascertainment bias is equally distributed across the years.
In summary, we observed a substantial increase in cases of advanced-stage BC at Oncoclínicas sites, most likely related to delays in BC diagnosis because of the COVID-19 pandemic. The impact appeared to be greater in tumors with a more aggressive phenotype (HER2+ and triple-negative) and in older adults, potentially because of stricter confinement in this subpopulation. Unfortunately, a potential negative impact on treatment outcomes should be expected, which has already been reported in another study.4
The COVID-19 pandemic was the first sanitary emergency to emerge in a globalized world. The response was swift in most countries, but a price would be paid for the (probably unavoidable) neglect of other health conditions. In BC, a condition that tends to have a long natural course, the full impact of the pandemic, may take years to be understood. However, as shown in our study, the early consequences were evident. Notably, the impact may be even greater in developing nations, who, even before the pandemic, had been slow in reducing late-stage diagnoses and had not yet experienced meaningful reductions in BC mortality.
Cristiano A.A. Resende
Honoraria: Roche, BMS Brazil, MSD Oncology, Pfizer, Novartis, Daiichi Sankyo/AstraZeneca
Consulting or Advisory Role: Daiichi Sankyo/AstraZeneca
Matheus Costa e Silva
Employment: Grupo Oncoclínicas, Rede D'Or São Luiz
Rafael D. Paes
Speakers' Bureau: MSD Oncology
Rodrigo Dienstmann
Employment: Oncoclínicas
Stock and Other Ownership Interests: Trialing Health
Consulting or Advisory Role: Roche, Boehringer Ingelheim, Foundation Medicine
Speakers' Bureau: Roche, Ipsen, Sanofi, MSD Oncology, Servier, Amgen, Libbs, AstraZeneca, Lilly, GlaxoSmithKline
Research Funding: Merck
Carlos H.E. Barrios
Stock and Other Ownership Interests: MedSIR, Tummi
Honoraria: Novartis, Roche/Genentech, Pfizer, GlaxoSmithKline, Sanofi, Boehringer Ingelheim, Eisai, MSD, Lilly, Bayer, AstraZeneca, Zodiac Pharma
Consulting or Advisory Role: Boehringer Ingelheim, Roche/Genentech, Novartis, GlaxoSmithKline, Eisai, Pfizer, AstraZeneca, Libbs, MSD Oncology, United Medical, Lilly
Research Funding: Pfizer (Inst), Novartis (Inst), Amgen (Inst), AstraZeneca (Inst), Boehringer Ingelheim (Inst), GlaxoSmithKline (Inst), Roche/Genentech (Inst), Lilly (Inst), Sanofi (Inst), Taiho Pharmaceutical (Inst), Mylan (Inst), Merrimack (Inst), Merck (Inst), AbbVie (Inst), Astellas Pharma (Inst), Biomarin (Inst), Bristol Myers Squibb (Inst), Daiichi Sankyo (Inst), Abraxis BioScience (Inst), AB Science (Inst), Asana Biosciences (Inst), Medivation (Inst), Exelixis (Inst), ImClone Systems (Inst), LEO Pharma (Inst), Millennium (Inst), Janssen (Inst), Clinica Atlantis (Inst), INC Research (Inst), Halozyme (Inst), Covance (Inst), Celgene (Inst), inVentiv Health (Inst), Merck KGaA (Inst), Shanghai Henlius Biotech (Inst), Polyphor (Inst), PharmaMar (Inst)
Travel, Accommodations, Expenses: Roche/Genentech, Novartis, Pfizer, BMS Brazil, AstraZeneca, MSD Oncology, Lilly
Aline C. Goncalves
Consulting or Advisory Role: Lilly, Novartis, Novartis, Pfizer, United Medical
Speakers' Bureau: AstraZeneca/Daiichi Sankyo, Pfizer, Novartis, Lilly
Andreza K.B.A. Souto
Honoraria: MSD Oncology, Pfizer, AstraZeneca, Grunenthal, United Medical, GlaxoSmithKline
Consulting or Advisory Role: MSD Oncology, GlaxoSmithKline, AstraZeneca
Leandro C. Oliveira
Honoraria: AstraZeneca
Consulting or Advisory Role: AstraZeneca
Speakers' Bureau: AstraZeneca
Tomás Reinert
Honoraria: Novartis, AstraZeneca, Pfizer, Lilly, Pierre Fabre, Libbs
Consulting or Advisory Role: Lilly, Novartis
Research Funding: AstraZeneca (Inst), Libbs (Inst)
Eduardo C. Millen
Honoraria: Roche
Felipe Zerwes
Speakers' Bureau: LIBBS, MSD Oncology, Novartis, AstraZeneca
Max S. Mano
Employment: Grupo Oncoclinicas
Stock and Other Ownership Interests: Fleury Group, Hypera Pharma
Honoraria: Roche/Genentech, Novartis, Oncologia Brasil, Lilly/ImClone, MSD Oncology, Grupo Fleury, DASA, Libbs, Gilead Sciences, Daiichi Sankyo
Consulting or Advisory Role: Novartis, Roche, Pfizer, AstraZeneca, Lilly/ImClone, MSD, Gilead Sciences
Travel, Accommodations, Expenses: Roche, Daiichi Sankyo
Uncompensated Relationships: Roche
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented as a poster at the 2022 ASCO Annual Meeting (abstr 10559), June 6, 2022, Chicago, IL.
AUTHOR CONTRIBUTIONS
Conception and design: Cristiano A.A. Resende, Matheus Costa e Silva, Rodrigo Dienstmann, Carlos H.E. Barrios, Aline C. Goncalves, Andreza K.B.A. Souto, Diocesio A.P. Andrade, Mauro P. Passos, Paulo L. Moraes, Bruno L. Ferrari, Max S. Mano
Financial support: Bruno L. Ferrari
Administrative support: Bruno L. Ferrari
Provision of study materials or patients: Heloísa M. Fernandes Cruz, Diocesio A.P. Andrade, Eduardo C. Millen, Felipe Zerwes, Bruno L. Ferrari
Collection and assembly of data: Cristiano A.A. Resende, Heloísa M. Fernandes Cruz, Matheus Costa e Silva, Rafael D. Paes, Rodrigo Dienstmann, Aline C. Goncalves, Fanny G. A. Cascelli, Andreza K.B.A. Souto, Tomás Reinert, Diocesio A.P. Andrade, Mauro P. Passos, Eduardo C. Millen, Felipe Zerwes
Data analysis and interpretation: Cristiano A.A. Resende, Heloísa M. Fernandes Cruz, Matheus Costa e Silva, Rafael D. Paes, Rodrigo Dienstmann, Carlos H. E. Barrios, Aline C. Goncalves, Andreza K.B.A. Souto, Leandro C. Oliveira, Diocesio A.P. Andrade, Mauro P. Passos, Bruno L. Ferrari, Max S. Mano
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Cristiano A.A. Resende
Honoraria: Roche, BMS Brazil, MSD Oncology, Pfizer, Novartis, Daiichi Sankyo/AstraZeneca
Consulting or Advisory Role: Daiichi Sankyo/AstraZeneca
Matheus Costa e Silva
Employment: Grupo Oncoclínicas, Rede D'Or São Luiz
Rafael D. Paes
Speakers' Bureau: MSD Oncology
Rodrigo Dienstmann
Employment: Oncoclínicas
Stock and Other Ownership Interests: Trialing Health
Consulting or Advisory Role: Roche, Boehringer Ingelheim, Foundation Medicine
Speakers' Bureau: Roche, Ipsen, Sanofi, MSD Oncology, Servier, Amgen, Libbs, AstraZeneca, Lilly, GlaxoSmithKline
Research Funding: Merck
Carlos H.E. Barrios
Stock and Other Ownership Interests: MedSIR, Tummi
Honoraria: Novartis, Roche/Genentech, Pfizer, GlaxoSmithKline, Sanofi, Boehringer Ingelheim, Eisai, MSD, Lilly, Bayer, AstraZeneca, Zodiac Pharma
Consulting or Advisory Role: Boehringer Ingelheim, Roche/Genentech, Novartis, GlaxoSmithKline, Eisai, Pfizer, AstraZeneca, Libbs, MSD Oncology, United Medical, Lilly
Research Funding: Pfizer (Inst), Novartis (Inst), Amgen (Inst), AstraZeneca (Inst), Boehringer Ingelheim (Inst), GlaxoSmithKline (Inst), Roche/Genentech (Inst), Lilly (Inst), Sanofi (Inst), Taiho Pharmaceutical (Inst), Mylan (Inst), Merrimack (Inst), Merck (Inst), AbbVie (Inst), Astellas Pharma (Inst), Biomarin (Inst), Bristol Myers Squibb (Inst), Daiichi Sankyo (Inst), Abraxis BioScience (Inst), AB Science (Inst), Asana Biosciences (Inst), Medivation (Inst), Exelixis (Inst), ImClone Systems (Inst), LEO Pharma (Inst), Millennium (Inst), Janssen (Inst), Clinica Atlantis (Inst), INC Research (Inst), Halozyme (Inst), Covance (Inst), Celgene (Inst), inVentiv Health (Inst), Merck KGaA (Inst), Shanghai Henlius Biotech (Inst), Polyphor (Inst), PharmaMar (Inst)
Travel, Accommodations, Expenses: Roche/Genentech, Novartis, Pfizer, BMS Brazil, AstraZeneca, MSD Oncology, Lilly
Aline C. Goncalves
Consulting or Advisory Role: Lilly, Novartis, Novartis, Pfizer, United Medical
Speakers' Bureau: AstraZeneca/Daiichi Sankyo, Pfizer, Novartis, Lilly
Andreza K.B.A. Souto
Honoraria: MSD Oncology, Pfizer, AstraZeneca, Grunenthal, United Medical, GlaxoSmithKline
Consulting or Advisory Role: MSD Oncology, GlaxoSmithKline, AstraZeneca
Leandro C. Oliveira
Honoraria: AstraZeneca
Consulting or Advisory Role: AstraZeneca
Speakers' Bureau: AstraZeneca
Tomás Reinert
Honoraria: Novartis, AstraZeneca, Pfizer, Lilly, Pierre Fabre, Libbs
Consulting or Advisory Role: Lilly, Novartis
Research Funding: AstraZeneca (Inst), Libbs (Inst)
Eduardo C. Millen
Honoraria: Roche
Felipe Zerwes
Speakers' Bureau: LIBBS, MSD Oncology, Novartis, AstraZeneca
Max S. Mano
Employment: Grupo Oncoclinicas
Stock and Other Ownership Interests: Fleury Group, Hypera Pharma
Honoraria: Roche/Genentech, Novartis, Oncologia Brasil, Lilly/ImClone, MSD Oncology, Grupo Fleury, DASA, Libbs, Gilead Sciences, Daiichi Sankyo
Consulting or Advisory Role: Novartis, Roche, Pfizer, AstraZeneca, Lilly/ImClone, MSD, Gilead Sciences
Travel, Accommodations, Expenses: Roche, Daiichi Sankyo
Uncompensated Relationships: Roche
No other potential conflicts of interest were reported.
REFERENCES
- 1.Vuagnat P, Frelaut M, Ramtohul T, et al. : COVID-19 in breast cancer patients: A cohort at the Institut Curie hospitals in the Paris area. Breast Cancer Res 22:55, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organisation : Coronavirus Disease (COVID-19) Pandemic, 2022. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 [Google Scholar]
- 3.Brasil Ministério da Saúde: Coronavırus Brasil, 2022. https://covid.saude.gov.br [Google Scholar]
- 4.Maringe C, Spicer J, Morris M, et al. : The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: A national, population-based, modelling study. Lancet Oncol 21:1023-1034, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giuliano A, Edge S, Hortobagyi G, et al. : Eighth edition of the AJCC Cancer Staging manual: Breast cancer. Ann Surg Oncol 25:1783-1785, 2018 [DOI] [PubMed] [Google Scholar]
- 6.Zhou J, Kane S, Ramsey C, et al. : Comparison of early- and late-stage breast and colorectal cancer diagnoses during vs before the COVID-19 Pandemic. JAMA Netw Open 5:e2148581, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lloyd M, Stephens S, Hong J, et al. : The impact of COVID-19 on breast cancer stage at diagnosis. J Clin Oncol 39, 2021. (suppl 15; abstr 528) [Google Scholar]
- 8.Kim A, Gitlin M, Fadli E, et al. : Reductions in cancer screening: The consequene of changes in routine care during the COVID-19 pandemic. J Clin Oncol 39, 2021. (suppl 15; abstr 10550) [Google Scholar]
- 9.Yabroff K, Wu XC, Negoita S, et al. : Association of the COVID-19 pandemic with patterns of statewide cancer services. J Natl Cancer Inst 114:907-909, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Estimativa 2020: Incidência de Câncer no Brasil. Rio de Janeiro: INCA—Instituto Nacional de Câncer José Alencar Gomes da Silva, 2020, https://www.inca.gov.br/estimativa [Google Scholar]
- 11.Moterani Júnior NJW, Monterani VC, Monterani LBBG, et al. : Impact of coronavirus disease 2019 pandemic on breast cancer screening and detection of high-risk mammographic findings. Rev Assoc Med Bras 68:842-846, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Negrao EMS, Cabello C, Conz L, et al. : The COVID-19 pandemic impact on breast cancer diagnosis: A retrospective study. Rev Bras Ginecol Obstet 44:871-877, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]