Abstract
Borrelia hermsii, an agent of tick-borne relapsing fever, was found to contain multiple circular plasmids approximately 30 kb in size. Sequencing of a DNA library constructed from circular plasmid fragments enabled assembly of a composite DNA sequence that is homologous to the cp32 plasmid family of the Lyme disease spirochete, B. burgdorferi. Analysis of another relapsing fever bacterium, B. parkeri, indicated that it contains linear homologs of the B. hermsii and B. burgdorferi cp32 plasmids. The B. hermsii cp32 plasmids encode homologs of the B. burgdorferi Mlp and Bdr antigenic proteins and BlyA/BlyB putative hemolysins, but homologs of B. burgdorferi erp genes were absent. Immunoblot analyses demonstrated that relapsing fever patients produced antibodies to Mlp proteins, indicating that those proteins are synthesized by the spirochetes during human infection. Conservation of cp32-encoded genes in different Borrelia species suggests that their protein products serve functions essential to both relapsing fever and Lyme disease spirochetes. Relapsing fever borreliae replicate to high levels in the blood of infected animals, permitting direct detection and possible functional studies of Mlp, Bdr, BlyA/BlyB, and other cp32-encoded proteins in vivo.
The spirochete genus Borrelia contains many important pathogens of humans and domestic animals, including the causative agents of Lyme disease (the Borrelia burgdorferi sensu lato group) and tick-borne relapsing fever (B. hermsii, B. duttonii, B. parkeri, B. turicatae, and others) (43). Also within this genus are B. anserina (the agent of avian borreliosis), B. recurrentis (the agent of human louse-borne relapsing fever), B. coriaceae (the putative, yet unproven, agent of epizootic bovine abortion), and several other species of unknown pathogenic significance (43). All Borrelia species persist in nature through cycles requiring humans or wild animals and blood-feeding arthropods as hosts (9, 41, 43).
Both tick-borne relapsing fever and Lyme disease borreliae infect their vectors during ingestion of blood and then migrate to the salivary glands to be transmitted via the saliva as the tick feeds on a new vertebrate host. The tick-borne relapsing fever spirochete B. hermsii is transmitted by the argasid tick Ornithodoros hermsi, a relatively fast feeder that usually takes in a complete blood meal in less than 1 h (49). B. hermsii replicates to high levels in the blood of infected mammals, presumably as an adaptation to ensure acquisition by the rapidly feeding tick vectors. Relapsing fever borreliae persistently infect tick salivary glands prior to vector feeding, another adaptation that enhances transmission during the brief period the tick remains attached to its vertebrate host (43). In contrast, Lyme disease spirochetes are transmitted by the bites of ixodid ticks, which attach to their hosts and feed for several days (49). Probably as adaptations to these slow-feeding vectors, B. burgdorferi does not produce high levels of spirochetemia and is generally difficult to observe in host blood samples. The bacteria are generally restricted to the midgut of unfed infected ticks and are found in the tick salivary glands only transiently during vector feeding (43).
Despite these differences, relapsing fever and Lyme disease spirochetes appear to use homologous proteins to facilitate interactions with their arthropod and mammalian hosts. During tick feeding, B. burgdorferi increases production of the surface-exposed lipoprotein OspC (16, 17, 23, 25, 30, 45, 46). After ingestion by its tick vector, B. hermsii increases synthesis of a membrane-bound lipoprotein, Vsp33 (also called VmpC) (44), that is homologous to OspC of B. burgdorferi (31, 32, 59), and this protein continues to be made during persistent infection of the tick salivary glands (44). These observations suggest that OspC and Vsp33 are required by their respective bacteria for either infecting the salivary glands or infecting mammalian hosts immediately following their transmission by tick bite. During mammalian infection, relapsing fever spirochetes produce additional surface proteins (Vsps or Vlps), the genes for which vary through intergenic recombination mechanisms (5, 55), and B. burgdorferi has recently been found to contain a homologous locus (vlsE) that also undergoes genetic recombination during mammalian infection (62). These reports suggest that other genetic similarities might exist between the relapsing fever and Lyme disease spirochetes.
Both B. hermsii and B. burgdorferi carry numerous extrachromosomal elements (7, 14, 27, 42, 60). B. burgdorferi and all other Lyme disease spirochetes examined contain members of a circular plasmid family designated cp32 (1, 3, 13, 15, 37, 52, 54). Individual B. burgdorferi that contain as many as nine different cp32s, which are homologous through most of their DNA sequences, have been identified. The cp32s generally have circumferences of 30 to 32 kb (14, 15, 54), although variants that are approximately 18 kb (10, 37, 52) or as small as 8 to 10 kb in size (19, 24) have been found; a linear 56-kb plasmid containing an entire cp32 has also been characterized (14, 15, 63). The larger B. burgdorferi cp32s encode several antigenic proteins, including members of the Erp, Mlp, and Bdr protein families (S. Porcella, unpublished results; 14, 15, 28, 34, 37, 50, 52, 54, 61, 63, 64), as well as two putative hemolysins, BlyA and BlyB (14, 26, 37). In our studies of B. hermsii, we found that these relapsing fever spirochetes contain multiple circular plasmids that are homologous to the B. burgdorferi cp32s and encode similar proteins synthesized during mammalian infection, suggesting conserved infection mechanisms among different species of the genus Borrelia. These similarities provide the foundation for future studies comparing cp32-encoded proteins of both the relapsing fever and Lyme disease spirochetes during their alternating infections of mammals and ticks.
MATERIALS AND METHODS
Bacteria.
B. hermsii isolate HS1, the type strain of the species, was isolated from an O. hermsii tick collected near Spokane, Wash. (57), and has been cloned by limiting dilution (55). B. hermsii isolates DAH, FRO, HAN, MAN, CON, and YOR were all isolated in the western United States from human blood (27). The HS1 and DAH isolates are genetically indistinguishable at every locus examined previously (27). B. burgdorferi isolate B31-4a was derived from a single colony grown in solid medium (15). Isolates of B. parkeri and B. anserina have been described previously (27). All Borrelia species were grown at 35°C in either Barbour-Stoener-Kelly (BSK)-II (6) or BSK-H (Sigma, St. Louis, Mo.), supplemented with 6% rabbit serum (Sigma).
B. hermsii circular plasmid library construction and analysis.
Supercoiled circular plasmids were purified from B. hermsii isolate HS1 by cesium chloride gradient centrifugation (48). Purified circular DNA was digested with EcoRI and cloned into pUC13 (Bethesda Research Laboratories, Gaithersburg, Md.) to produce a B. hermsii circular plasmid library. Recombinant plasmids pSPR61, pSPR63, pSPR66, pSPR67, and pSPR71 were purified from Escherichia coli using Qiagen Midi kits (Qiagen, Santa Clarita, Calif.).
Additionally, oligonucleotide primers complementary to sequences in the inserts of two of the clones, pSPR67 and pSPR63, were used to PCR amplify a DNA fragment of approximately 10 kb from B. hermsii HS1 to generate PCR#1. This DNA was digested with either EcoRI or BamHI, ends were filled with Taq polymerase, and fragments were cloned into pCR2.1 (Invitrogen, Carlsbad, Calif.).
Total genomic DNA was purified from each of the B. hermsii, B. parkeri, and B. anserina isolates as previously described (48). Total plasmids (both linear and circular) were purified from B. burgdorferi by using a Qiagen Midi kit.
DNA sequencing was performed using either Sequenase (U.S. Biochemical, Cleveland, Ohio) or a model 370A Stretch automated DNA sequencer (Applied Biosystems, Foster City, Calif.).
Predicted protein sequences from B. hermsii cp32 open reading frames (ORFs) were analyzed during October 1999 by BLAST-P searches from the Institute for Genomic Research B. burgdorferi strain B31 genome web site (http://www.tigr.org/tdb/CMR/gbb/htmls/SeqSearch.html) and the National Center for Biotechnology Information web site (http://www.ncbi.nlm.nih.gov/blast/blast.cgi).
PCR analysis of B. hermsii circular plasmids.
Cloned fragments of B. hermsii cp32s were linked by PCR of genomic HS1 DNA. Reaction conditions for short amplifications (less than 2 kb) consisted of 25 cycles of 94°C for 30 s, 50°C for 30 s, and extension at 65°C for 2 min. PCR amplifications for fragments greater than 5 kb were performed using the Expand Long Template PCR system (Boehringer Mannheim, Indianapolis, Ind.) with 10 cycles of 94°C for 10 s, 50°C for 30 s, and 68°C for 10 min, followed by 20 cycles with the time of each successive extension step increasing by 20 s.
Southern blot analysis.
DNA molecules of different sizes were separated by pulsed-field agarose gel electrophoresis or two-dimensional chloroquine agarose gel electrophoresis and transferred to nylon membranes as previously described (54). Membranes were incubated overnight with radiolabeled probes at either 55°C (high stringency) or 45°C (low stringency) (22). Blots were washed in either 0.2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% sodium dodecyl sulfate (SDS) at 55°C (high-stringency wash conditions) or 2× SSC–0.1% SDS at room temperature (low-stringency wash conditions). Hybridized probes were visualized by autoradiography.
Probes for Southern blotting were produced by PCR (54) from appropriate recombinant plasmid clones using oligonucleotides listed in Table 1. Three different B. hermsii cp32 probes were produced using pSPR67 as template. Two probes, one derived from the B. burgdorferi B31 erpK promoter region (22) and one from an internal region of B31 erpM that is almost entirely composed of GAA and AAA codons, were produced from plasmids pBLS491 and pBLS492, respectively (15, 50). Probes were purified by dilution in 2 ml of water and concentrated to a final volume of approximately 60 μl in Centricon-100 spin columns (Amicon, Beverly, Mass.) (54). Purified probes were labeled with [α-32P]dATP (DuPont, Boston, Mass.) by random priming (Life Technologies, Gaithersburg, Md.).
TABLE 1.
Oligonucleotides used to produce DNA probes
| Probe | Our designation | Sequence (5′ to 3′) |
|---|---|---|
| B. hermsii PF-144 | BH-2 | CTTATCTCAAATTCATGACTTGCG |
| BH-21 | TTTAGAAATACGTCAGTGGGGATG | |
| B. hermsii PF-145 | BH-35 | CTGTTATTGATTTTAGAGGGTTTG |
| BH-36 | GATATAAATAGCAAGCCAAGAATG | |
| B. hermsii mlpL | BH-40 | CCCTTGATCACATACAAAGTG |
| BH-41 | CTACATTACAAGCGCTTGATG | |
| B. burgdorferi erpK | E-506 | AACTTTTTTTTACATCTTCACCAC |
| promoter | E-513 | CTGTTTGTTAATATGTAATAGCTG |
| B. burgdorferi erpM | E-718 | TTCTTTTTCTGCTTTAACCCTAGC |
| poly(GAA-AAA) | E-721 | AATCTAAAGATAAAGTTGAGGAAG |
Recombinant Mlp proteins and immunoblot analyses.
Polyhistidine-tagged recombinant MlpL and MlpK proteins were synthesized as follows. The mlpL and partial mlpK genes were PCR amplified from pSPR67 and pSPR66, respectively, ligated into plasmid pET-15b (Novagen, Madison, Wis.), and transformed into E. coli BL21(DE3) (Novagen). Recombinant MlpL and MlpK proteins were expressed and purified as specified by the manufacturer (Novagen). Purified proteins were quantified using the Bio-Rad (Hercules, Calif.) protein assay, and approximately 10 μg of each protein was subjected to polyacrylamide gel electrophoresis and transferred to Hybond-P polyvinylidene difluoride membranes (Amersham, Little Chalfont, England). Membranes were blocked for 2 h in phosphate-buffered saline–Tween 20 (PBS-T; 100 mM sodium phosphate, 100 mM NaCl, 0.1% [vol/vol] Tween 20) containing 5% nonfat dry milk. Membranes were then incubated for 1 h with a 1:200 diluted serum sample from either a human patient diagnosed with relapsing fever or an uninfected individual, washed thoroughly with PBS-T, and incubated for 1 h with anti-human immunoglobulin-horseradish peroxidase conjugate (diluted 1:20,000; Amersham). Bound secondary antibody was detected by enhanced chemiluminescence (Amersham). Serum samples from 10 clinically diagnosed relapsing fever patients and 19 uninfected humans were generously provided by Donald Anderson (Sacred Heart Medical Center, Spokane, Wash.) and Martin Schriefer and David Dennis (Centers for Disease Control and Prevention, Fort Collins, Colo.).
Nucleotide sequence accession numbers.
The sequences of the inserts of pSPR61-L, pSPR61-R, pSPR63, pSPR66, pSPR67, and pSPR71 have been submitted to GenBank and given accession numbers AF209439, AF209440, AF209441, AF209442, AF123078, and AF209443, respectively. The sequences of the EcoRI- and BamHI-digested fragments of PCR#1 have accession numbers AF209444 through AF209449. The sequence of PCR#2 has accession number AF209450. Annotated sequence of the composite B. hermsii cp32 illustrated in Fig. 3 is available from http://jenner.mi.uky.edu/bstevens/Bhcp32.htm.
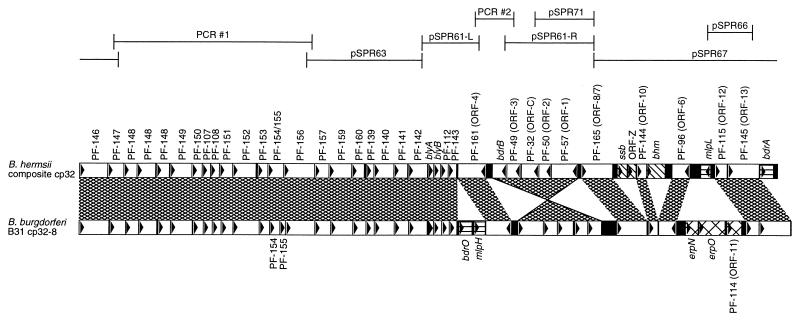
FIG. 3.
Alignment of the composite B. hermsii cp32 and a typical B. burgdorferi cp32, cp32-8 of isolate B31 (this plasmid contains all genes carried by the majority of studied B. burgdorferi cp32s) (14). Base pair 1 of both plasmids is defined as the first base pair of the PF-146 ORF (14). The names or PF numbers of each ORF are indicated, as are previously used ORF designations (15, 19, 52, 54, 63). Two different B. hermsii mlp alleles (mlpL and mlpK) were discovered, both of which occupy the same location of two different cp32 plasmids. Homologous sequences are indicated by the cross-hatched areas between the two plasmids. Note that the five genes spanning PF-57 to bdr on the two plasmids are inverted with respect to each other. Loci found in different locations on the B. hermsii and B. burgdorferi cp32s are indicated by horizontal hatching. ORFs located only on the B. hermsii cp32 are indicated by wide cross-hatching, while those found only on B. burgdorferi cp32s are indicated by diagonal hatching. B. hermsii cp32 regions encompassed by circular DNA library clones or PCR products are indicated above the plasmid maps.
RESULTS
Characterization of B. hermsii circular plasmids.
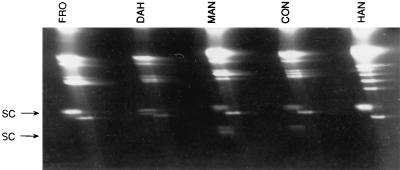
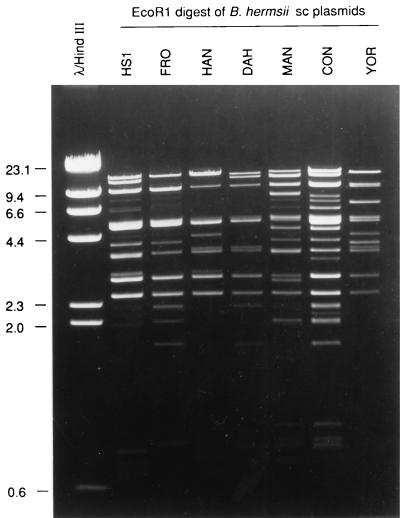
Two-dimensional agarose gel analysis of total DNA preparations from several isolates of B. hermsii demonstrated some DNA molecules that migrated more slowly in the second dimension after treatment (Fig. 1), indicating they were supercoiled in structure (40, 54). Electron microscopy (EM) analysis of spread DNA samples from these same isolates all showed circular molecules estimated to be 28 to 30 kb in size (data not shown). However, EcoRI digestion analysis of purified circular DNA showed that the fragment sizes added up to significantly more than the size based on EM analysis (Fig. 2). Therefore, we suspected that the population of 28- to 30-kb circular plasmids was comprised of multiple similar-sized molecules that varied in sequence.
FIG. 1.
Two-dimensional agarose gel of total DNA from five B. hermsii isolates. Molecules determined to have supercoiled (SC) structure lagged behind the linear molecules after treatment and are shown by the arrow.
FIG. 2.
EcoRI digestion and agarose gel analysis of CsCl2 gradient-purified supercoiled (sc) plasmids from seven B. hermsii isolates.
Circular plasmid DNA from B. hermsii isolate HS1 was digested with EcoRI and cloned to produce a recombinant plasmid library. Preliminary sequence analyses indicated that five recombinant clones, pSPR61, pSPR63, pSPR66, pSPR67, and pSPR71, contained different inserts and were completely sequenced, with insert sizes of 6,500, 5,092, 1,546, 10,099, and 2,252 bp, respectively. All five cloned DNA fragments contained ORFs homologous to those found on the B. burgdorferi cp32 family of plasmids (Fig. 3). The inserts of recombinant plasmids pSPR61 and pSPR71 contained similar but unique sequences, as did pSPR66 and pSPR67, indicating that they were cloned fragments of at least two different but homologous circular plasmids. Several identified ORFs are homologs of B. burgdorferi genes whose proteins are involved in mammalian infection; these are discussed in greater detail below. Many of the B. burgdorferi cp32 ORFs are as yet unnamed, so we refer to such ORFs by the paralog family (PF) number recently assigned by Casjens and coworkers (14).
Recombinant plasmid pSPR67 contained the 3′ end of a homolog of the B. burgdorferi cp32 PF-165 (ORF-8/7) genes (15, 54), while pSPR61 and pSPR71 both contained similar 5′ ends of PF-165 genes. However, the pSPR61 and pSPR71 inserts did not originate from the same B. hermsii plasmid, since their sequences were approximately 90% identical, with significant differences in the noncoding region between their PF-57 and PF-165 paralogs. Either pSPR61 or pSPR71 could create an in-frame fusion with pSPR67 that would encode a complete PF-165 protein homologous to those found on B. burgdorferi cp32s (Fig. 3). To confirm that the recombinant plasmid insert sequences were oriented as illustrated in Fig. 3, total B. hermsii HS1 DNA was subjected to PCR using four sets of oligonucleotide primer pairs, in which one primer was complementary to pSPR61 and the other was complementary to pSPR67. In all cases, PCR yielded amplicons of the anticipated sizes (data not shown), indicating that the cloned inserts of pSPR67 and either pSPR61 or pSPR71 (or a similar, unidentified DNA sequence) represented contiguous fragments of the same circular plasmid.
The other terminus of the pSPR61 insert contained the 3′ end of an ORF homologous to the B. burgdorferi PF-142 genes (14, 37), while the insert of recombinant plasmid pSPR63 contained the 5′ end of a PF-142 homolog. PCR of B. hermsii HS1 DNA with four oligonucleotide primer pairs (one of each pair complementary to either pSPR61 or pSPR63) all yielded amplicons as expected if the inserts of pSPR61 and pSPR63 were physically linked in B. hermsii as diagrammed in Fig. 3 (data not shown). These two DNA fragments probably were not derived from the same B. hermsii plasmid, since the PF-142 gene fragments cloned in pSPR61 and pSPR63 do not overlap in frame at the EcoRI site. Alternatively, it is possible that both cloned fragments were derived from a single B. hermsii plasmid containing a 1-bp insertion that created a frameshift near this EcoRI site. The overlap of the pSPR61 and pSPR63 inserts corresponds with amino acid 152 of the B. burgdorferi PF-142 homologs, with both inserts containing >50% amino acid coding identity with B. burgdorferi homologs in the region flanking the overlap, making it unlikely that any intervening DNA was missed by our studies. Additionally, the above-described PCR studies with pSPR61 and pSPR63 primer pairs yielded amplicons with sizes that indicated B. hermsii does not contain additional DNA in this region.
Sequence analysis of recombinant plasmid pSPR61 revealed that it contained two EcoRI fragments, which we have reported separately as pSPR61-L and pSPR61-R. PCR of B. hermsii HS1 plasmid DNA with oligonucleotides derived from sequences of the two fragments indicated that the pSPR61-L and pSPR61-R inserts were derived from a plasmid(s) having the orientation shown in Fig. 3. Additionally, PCR produced a 728-bp sequence, PCR#2 (Fig. 3), not represented in the recombinant plasmid library clones analyzed.
Since the recombinant plasmid library was constructed from B. hermsii circular plasmids, we next used PCR of purified B. hermsii HS1 DNA to determine the linkage between the inserts of pSPR67 and pSPR63. PCR with three combinations of oligonucleotide primer pairs, having one binding site in each cloned fragment, produced amplicons that indicated the sequences were separated by approximately 10 kb (data not shown). PCR#1, the product of one such amplification, was cloned and sequenced; this analysis revealed two slightly different DNA amplicons, indicating further that there are multiple related plasmids in these bacteria.
The successful PCR linkage of all the ends of the three large B. hermsii plasmid fragments indicated that they were derived from circular DNA. Such a plasmid would have a size equal to that of the inserts of pSPR63, pSPR61L, pSPR61R, and pSPR67 plus the PCR#1 and PCR#2 amplicons, a total of approximately 30.3 kb. This size is consistent with the results determined by EM contour length measurements of B. hermsii circular plasmids described above. In comparison, the cp32 plasmids of B. burgdorferi isolate B31 range between 29.8 and 30.9 kb (14). Alignment of the composite B. hermsii plasmid with a representative B. burgdorferi cp32 indicated that the plasmids of both Borrelia species are largely homologous, containing many of the same ORFs in the same relative positions (Fig. 3). Due to the similarities in size and sequence with the B. burgdorferi cp32 plasmids, we designate the assembled B. hermsii plasmid a cp32, with the caveat that the diagram in Fig. 3 possibly includes fragments from more than one such B. hermsii plasmid.
Genes of the borrelial paralog families 144 (ORF-10), 145 (ORF-13), and 113 (mlp) have been found only on members of the cp32 plasmid family, and there are no known similar genes in any non-Borrelia organism. Additionally, members of paralog families 144 and 145 are well conserved among all cp32 family members (3, 14, 15, 24, 52, 54). For these reasons, probes derived from the B. hermsii PF-144, PF-145, and mlpL genes were used in Southern blot analyses of B. hermsii plasmids.
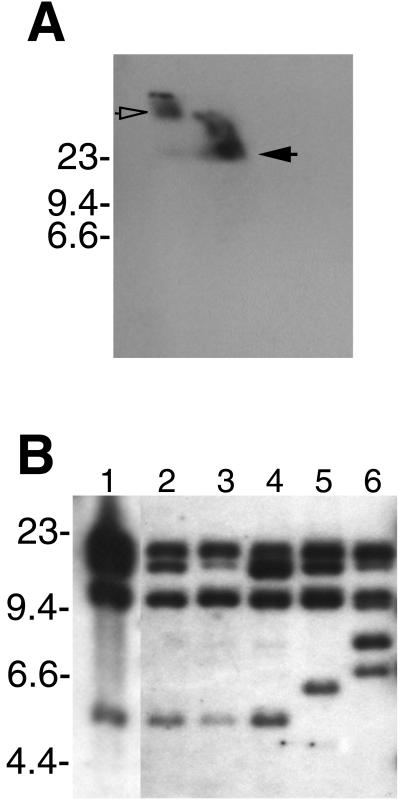
The PF-144 probe was used in Southern blot analysis of B. hermsii HS1 DNA separated by two-dimensional electrophoresis, which confirmed that the bacteria contain circular cp32-related DNA species (Fig. 4A). The linear DNA seen in Fig. 4A is probably sheared cp32, but it may also include linear plasmids with homology to cp32, similar to the linear lp56 of B. burgdorferi (14, 64).
FIG. 4.
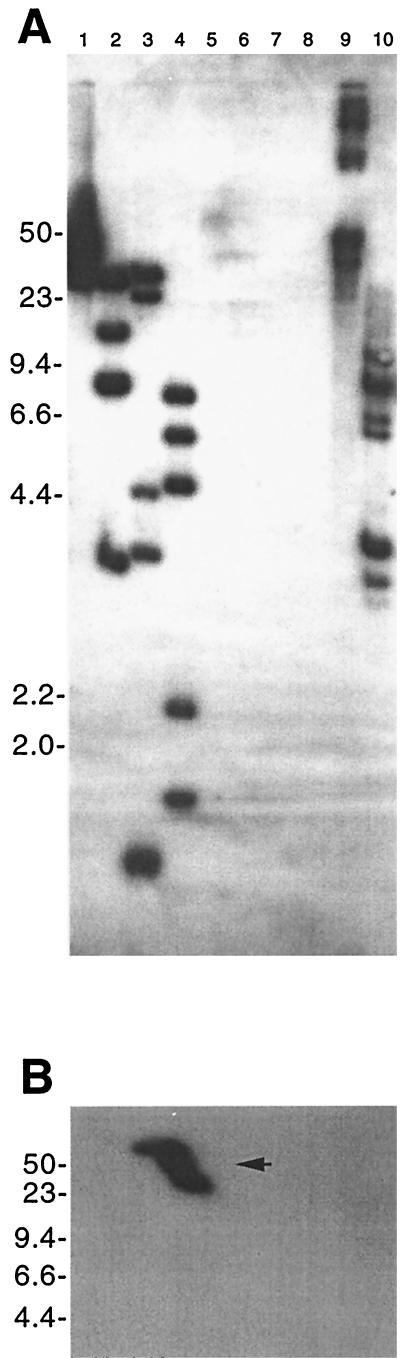
Southern blot analysis of B. hermsii DNA hybridized with a probe derived from the identified B. hermsii cp32 PF-144 locus. (A) Genomic DNA of isolate DAH separated by two-dimensional agarose gel electrophoresis. Circular DNA is indicated by an open arrow, and linear DNA is indicated by a filled arrow. (B) Genomic DNA of isolates DAH, HS1, FRO, MAN, CON, and YOR (lanes 1, 2, 3, 4, 5, and 6, respectively) digested with EcoRI. Molecular size markers (in kilobases) are indicated to the left of each panel.
Individual B. burgdorferi bacteria that contain up to nine different cp32 family members have been identified (1, 14, 15, 37). To further examine the multiplicity of B. hermsii cp32s, genomic DNA from HS1 and five other B. hermsii isolates were digested with restriction endonucleases, blotted, and incubated with B. hermsii cp32-derived probes. The PF-144 probe hybridized with four or more DNA fragments from each of the six B. hermsii isolates (Fig. 4B). The banding patterns of isolates HS1 and DAH were identical, further indicating that these two isolates are very similar (27), while diversity was seen among the other B. hermsii isolates. Multiple bands were also observed when using probes derived from the mlpL and PF-145 loci, even with high-stringency hybridization and wash conditions (data not shown).
B. hermsii plasmids encode antigenic proteins homologous to proteins of B. burgdorferi.
Humans and other mammals infected with B. burgdorferi produce antibodies directed against the Mlp (Porcella, unpublished; 61) and Bdr (64) proteins, indicating that those proteins are synthesized during mammalian infection. Additionally, the B. burgdorferi cp32 blyA and blyB genes encode putative hemolytic proteins (26). Sequencing of the cloned B. hermsii cp32 fragments indicated that isolate HS1 contained at least two mlp genes (mlpK and mlpL, cloned in recombinant plasmids pSPR66 and pSPR67, respectively), two bdr genes (bdrA and bdrB), and a blyAB locus.
The sequences of B. burgdorferi mlp paralogs can vary considerably between different bacteria (14, 37, 61), and our sequencing data indicated that this was also true of the B. hermsii paralogs. The B. hermsii mlpK and mlpL genes were identified on different, overlapping cloned cp32 fragments, and the deduced MlpK and MlpL proteins share approximately 48% identical amino acids. Compared to the B. burgdorferi B31 proteins, MlpK was most similar to B31 MlpF (located on cp32-6, 58% identity) and MlpL was most similar to B31 MlpB (located on cp32-2, 46% identity) (Fig. 5). Despite sequence variability, borrelial Mlp proteins fall into two classes based on size, hydrophilicity profiles, and antigenic cross-reactivity (37, 61), with the HS1 MlpK being a member of class 2 and MlpL being a member of class 1.
FIG. 5.
(A) Alignment of the B. hermsii MlpL and B. burgdorferi B31 MlpF proteins. (B) Alignment of the B. hermsii MlpK and B. burgdorferi B31 MlpB proteins. The complete sequence of the mlpK gene is unknown, since recombinant plasmid pSPR66 contains only part of this gene. Homologous amino acids predicted to be located in proteins of both species are boxed.
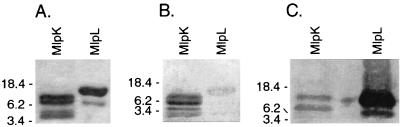
Serum samples from humans and laboratory animals infected with relapsing fever spirochetes produce antibodies that recognize antigens with sizes similar to those of the MlpK and MlpL proteins (4, 20, 39, 47). Recombinant MlpK and MlpL proteins (rMlpK and rMlpL, respectively) were produced for immunoblot analyses with relapsing fever patient sera, which indicated that Mlp proteins are synthesized by B. hermsii during mammalian infection. The serum samples from 2 of 10 patients examined contained detectable levels of antibodies reactive with both recombinant proteins, although with different intensities (Fig. 6). For example, the serum from patient C-1 contained antibodies that reacted strongly with rMlpK but only weakly with rMlpL, while the converse was true for serum from patient H-1 (Fig. 6). Immunoblot signal variations were probably due to different Mlp protein sequences of the B. hermsii that infected the tested patients, since similar variations have been observed in analyses of Lyme disease patient serum reactivities to different recombinant B. burgdorferi Mlp proteins (Porcella, unpublished; 61). Control immunoblots using serum samples from 19 different uninfected humans did not show any detectable antibody binding to the recombinant Mlp proteins (data not shown).
FIG. 6.
Analyses of recombinant B. hermsii Mlp proteins. Purified proteins were separated by SDS-polyacrylamide gel electrophoresis and either stained with Coomassie brilliant blue (A) or transferred to polyvinylidene difluoride membranes and immunoblotted with serum samples from relapsing fever patients C-1 (B) and H-1 (C). Some purified proteins appeared as multiple bands, presumably due to multimerization or degradation. Molecular mass standards (in kilodaltons) are shown on the left of each panel.
All B. burgdorferi cp32 plasmids contain a locus encoding one or two erp genes between PF-96 and PF-115 (1, 14, 15, 50–52, 54), a region occupied by the mlp locus in the composite B. hermsii cp32 (Fig. 3). Low-stringency Southern blot analyses were performed to search for erp homologs elsewhere in the B. hermsii genome. Two probes were derived from highly conserved sequences of the B. burgdorferi erp loci: the erp promoter region and a 200-bp region found in many erp genes that is rich in GAA and AAA codons. Both probes hybridized with multiple B. burgdorferi restriction fragments, but neither probe hybridized with DNA from B. hermsii (data not shown). We conclude that either B. hermsii lacks homologs of the erp genes or, if they are present, their sequences are divergent beyond our level of detection.
Other ORFs found on both B. burgdorferi and B. hermsii cp32s.
Alignment of the composite B. hermsii cp32 with a typical B. burgdorferi cp32 indicated extensive regions of homology between the two species (Fig. 3). Many of the ORFs on the B. hermsii cp32 are also in the same relative position and orientation on B. burgdorferi cp32s, although the DNA sequences of the two species are not identical. For example, a pair of oligonucleotide primers directs PCR amplification of a region spanning the PF-115 and PF-145 loci (52) of all Lyme disease spirochetes and has been used in differentiating these bacteria from other Borrelia species (3). As expected, the corresponding regions of the B. hermsii cp32 fragments cloned in pSPR6 and pSPR67 lack both oligonucleotide binding sites.
The B. hermsii and B. burgdorferi cp32s also differ in having opposite orientations for the five-gene cluster spanning PF-57 through the adjacent bdr allele (Fig. 3). Unlike all known members of the B. burgdorferi cp32 family (14, 19, 24, 52, 63), the B. hermsii PF-57-to-bdr regions are not flanked by inverted repeats, nor do the immediately flanking sequences resemble those of B. burgdorferi cp32s.
An earlier study, using high-stringency Southern blotting with probes derived from cp32s of B. burgdorferi isolate Sh-2-82, indicated hybridization with DNA from Lyme disease spirochetes but not from B. hermsii (48). Sequencing of the B. burgdorferi Sh-2-82 DNA fragments revealed that they were derived from the bdr-to-erp regions of two different cp32s (pSPR13 and pSPR14) and from a cp32 PF-147-to-PF-108 region (pSPR9) (data not shown). These regions of B. hermsii and B. burgdorferi cp32s share an average 60% or less identical nucleotides with interspersed unrelated sequences, differences that apparently prevented hybridization between the two species' DNAs under the conditions used in the earlier studies (48).
Novel ORFs on B. hermsii cp32.
The cp32 fragment cloned in recombinant plasmid pSPR67 contained three ORFs unrelated to any sequences on B. burgdorferi cp32 plasmids (Fig. 3). One, designated bhm (B. hermsii methyltransferase), encodes a protein homologous to the BalI methylase of Brevibacterium albidum and the StsI methylase of Streptococcus sanguis (GenBank accession numbers D82028 and D11101, respectively). The predicted Bhm protein bears little similarity to the putative nucleotide methylases on the lp56 and lp25 plasmids of B. burgdorferi (14, 24). A second unique ORF, ssb, is homologous to genes encoding single-stranded DNA binding proteins found on the chromosomes of B. burgdorferi (24) and many other bacteria. None of the B. burgdorferi plasmids contain ssb homologs (14, 24), but similar ssb genes are found on the E. coli plasmid R64 and related conjugative plasmids (38). A third ORF, ORF-Z, is not related to any gene or protein previously reported to GenBank.
Homologs of B. hermsii cp32s in other relapsing fever spirochetes.
Having found multiple cp32 plasmids in both B. hermsii and B. burgdorferi, we examined isolates of other Borrelia species for cp32-related plasmids. DNA from B. parkeri and B. anserina were digested, blotted, and hybridized with the B. hermsii cp32 PF-144 probe. At low stringency, this probe hybridized with multiple DNA fragments from both B. parkeri and B. burgdorferi, although not with any DNA from B. anserina (Fig. 7A). Hybridization of the PF-144 probe with B. parkeri DNA separated by two-dimensional gel electrophoresis indicated the presence of related linear DNA in that species but no circular forms (Fig. 7B). During the course of our work, others reported that a probe derived from a B. turicatae bdr homolog also hybridized with multiple fragments of B. parkeri DNA (11, 12). These data indicate that B. parkeri contains multiple plasmids related to the B. hermsii and B. burgdorferi cp32 plasmids, although all such plasmids appear to be linear. The isolate of B. anserina used in our studies does not appear to contain cp32-related plasmids, although it may contain DNAs with sequences too divergent from those of B. hermsii for our detection.
FIG. 7.
Southern blot analysis of DNA from other Borrelia species hybridized with a probe derived from the identified B. hermsii cp32 PF-144 locus. (A) Genomic DNA from B. parkeri (lanes 1 to 4), B. anserina (lanes 5 to 8), and B. burgdorferi (lanes 9 and 10). DNAs were either uncut (lanes 1, 5, and 9) or cut with EcoRI (lanes 2, 6, and 10), EcoRV (lanes 3 and 7), or HindIII (lanes 4 and 8) and separated by pulsed-field agarose gel electrophoresis. Uncut circular DNAs form a broad smear extending from the gel well under the electrophoresis conditions used in these experiments (54). (B) B. parkeri genomic DNA separated by two-dimensional agarose gel electrophoresis. Linear DNA is marked with an arrow. Molecular size markers (in kilobases) are indicated to the left of each panel.
DISCUSSION
We found that B. hermsii HS1 contains circular plasmids that by EM contour length measurement had sizes of approximately 30 kb. Fragments of these plasmids were linked by overlapping sequences to assemble a composite circular DNA sequence of 30,295 bp in circumference, with extensive homology to the cp32 family of B. burgdorferi plasmids. Sequencing of B. hermsii HS1 cp32 fragments indicated that these clonal bacteria contain at least two different cp32s, while Southern blotting suggested four or more such plasmids in this strain. The five other B. hermsii isolates examined also contain multiple cp32s. Two other species of relapsing fever spirochetes, B. parkeri and B. turicatae, also contain linear DNAs related to the B. hermsii and B. burgdorferi cp32 plasmids (this work and references 11 and 12).
Comparison of the ORFs found on the B. hermsii cp32 DNAs with those on B. burgdorferi cp32s revealed the presence of homologous genes in the two species, including members of the mlp, bdr, and blyAB gene families. These similarities suggest common functions for cp32-encoded proteins in the different Borrelia species. Furthermore, the presence of similar genes in both B. hermsii and B. burgdorferi presents opportunities to compare protein synthesis patterns and functions in the two species of bacteria during mammalian and tick infections. For example, the Mlp proteins of both B. hermsii and B. burgdorferi are synthesized in vivo, as indicated by the reactivity against recombinant Mlps with serum samples from both relapsing fever (this work) and Lyme disease patients (Porcella, unpublished; 61). Since B. hermsii achieves high levels of bacteremia in the mammalian bloodstream, spirochetes can be examined directly in blood smears from infected animals. Future studies can determine the time course of Mlp synthesis in both mammals and ticks, which of the Mlp proteins are produced, and the location of Mlps in the bacterial cell. Similar in vivo studies on the synthesis and function of the Bdr antigens, BlyA/BlyB putative hemolysins, and other cp32-encoded proteins conserved in relapsing fever Borrelia species can also be performed.
All analyzed Lyme disease spirochete cp32s contain erp genes, which encode antigenic proteins synthesized during the initial stages of mammalian infection (2, 28, 50, 53, 56, 58). However, no such gene was found on B. hermsii cp32s, nor was there hybridization evidence of erp genes elsewhere in the B. hermsii genome. These data suggest that Erp proteins perform functions essential for Lyme disease borrelial infection that are not required by relapsing fever borreliae. Additional analyses of B. hermsii and other spirochetes of this genus will undoubtedly reveal additional differences reflecting the distinct life histories of Borrelia species.
The B. hermsii cp32 fragments cloned in recombinant plasmids pSPR66 and pSPR67 contained similar sequences, although differences were found throughout the two plasmid inserts, with regions of near identity separated by regions without recognizable similarity. The B. hermsii cp32 fragment cloned in pSPR66 appears to be badly damaged, since its PF-115 and PF-145 ORFs contain multiple frameshifts and premature termination codons, suggesting that the products of PF-115 and PF-145 are not essential for B. hermsii cp32 maintenance. Further comparative studies will identify whether the DNA near the B. hermsii cp32 mlp locus is a hot spot for mutation and recombination. Additionally, the apparent mutations in the cp32 fragment cloned in pSPR66 may indicate that relapsing fever spirochetes harbor degenerated plasmids, as do the Lyme disease borreliae (14).
Phage-like particles have been observed in B. hermsii cultures (9), and since there is evidence that B. burgdorferi cp32s may be bacteriophage genomes (14, 21), B. hermsii cp32s might also be prophages. That hypothesis is further supported by the presence of the B. hermsii cp32 ssb gene, since bacteriophages often encode single-stranded DNA binding proteins (29). Such bacteriophages may be capable of transferring DNA between B. hermsii and thus prove to be useful genetic tools.
The B. hermsii composite cp32 contains an additional novel gene, bhm, encoding a putative nucleotide methylase. Earlier reports indicated the presence of methylated DNA in B. hermsii and other relapsing fever spirochetes (33, 35), possibly consequences of the bhm gene product. The presence of a nucleotide methylase in these bacteria may indicate a DNA restriction mechanism, which could have important consequences on the development of a B. hermsii recombinant genetic system. DNA methylation can play other roles in prokaryotes, including gene regulation and the packaging of bacteriophage DNA into capsids (36). Nucleotide methylases may also protect bacteria from toxic substances, such as modifying rRNA to mediate resistance to macrolide antibiotics (18).
Based on their location on almost all borrelial plasmids and homologies with plasmid proteins of other bacterial species, the proteins of paralog families 57, 50, 32, and 49 have been proposed to be involved with plasmid replication and partition (8, 14, 19, 51, 63). Sequencing and hybridization studies indicate that plasmids of different B. burgdorferi isolates often contain nearly identical PF-32 and PF-49 gene pairs (R. Iyer, O. Kalu, I. Schwartz, and B. Stevenson, Abstr. 99th Gen. Meet. Am. Soc. Microbiol., abstr. D/B-260, 1999; 10, 51), suggesting that the products of these two genes specifically interact with each other. Additionally, all cp32s in individual B. burgdorferi encode PF-32 and PF-49 proteins with no more than 65% identical amino acids (51), which may account for the compatibility of the different plasmids. The PF-32 and PF-49 proteins encoded by the pSPR61-L B. hermsii cp32 fragment share 76 and 75% identical amino acids with their B. burgdorferi B31 cp32-6 homologs but less than 65% identity with proteins encoded by the other B31 cp32s. The similarity of the B. hermsii cp32 and B. burgdorferi cp32-6 putative segregation genes raises the intriguing possibility that these two plasmids are more closely related to each other than are most of the B. burgdorferi B31 cp32s. This implies that either B. hermsii and B. burgdorferi have exchanged DNA at some time or the divergence of cp32s predated the divergence of the relapsing fever and Lyme disease borreliae from their common ancestor.
Many species within the genus Borrelia contain multiple plasmids of the cp32 family (this work and references 11, 12, 15, 37, 54, and 63). The cp32 plasmids of B. hermsii and B. burgdorferi contain many homologous genes, most of which are located in similar positions and orientations. This conservation of sequences indicates that they serve important functions, either for survival of the bacteria or for maintenance of the plasmids. Production of conserved proteins during mammalian infection also implies essential functions, perhaps facilitating interactions with tissues of the mammalian or arthropod hosts. We propose that comparative studies of cp32-encoded proteins of B. hermsii and B. burgdorferi will help elucidate the mechanisms underlying the pathogenicity of these bacteria.
ACKNOWLEDGMENTS
This study was funded in part by grants RO1-AI44254 from the National Institutes of Health and 949 from the University of Kentucky Chandler Medical Center Research Fund to Brian Stevenson.
Stephen Porcella and Brian Stevenson contributed equally to the work described in this report.
We thank Donald Anderson, Jr., Martin Schriefer, and David Dennis for providing sera, Lou Lieto for technical assistance, Patti Rosa for helpful encouragement, and Sherwood Casjens and Wolf Zückert for valuable discussions and for constructive comments on the manuscript.
REFERENCES
- 1.Akins D R, Caimano M J, Yang X, Cerna F, Norgard M V, Radolf J D. Molecular and evolutionary analysis of Borrelia burgdorferi 297 circular plasmid-encoded lipoproteins with OspE- and OspF-like leader peptides. Infect Immun. 1999;67:1526–1532. doi: 10.1128/iai.67.3.1526-1532.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akins D R, Porcella S F, Popova T G, Shevchenko D, Baker S I, Li M, Norgard M V, Radolf J D. Evidence for in vivo but not in vitro expression of a Borrelia burgdorferi outer surface protein F (OspF) homologue. Mol Microbiol. 1995;18:507–520. doi: 10.1111/j.1365-2958.1995.mmi_18030507.x. [DOI] [PubMed] [Google Scholar]
- 3.Amouriaux P, Assous M, Margarita D, Baranton G, Saint Girons I. Polymerase chain reaction with the 30-kb circular plasmid of Borrelia burgdorferi B31 as a target for detection of the Lyme borreliosis agents in cerebrospinal fluid. Res Microbiol. 1993;144:211–219. doi: 10.1016/0923-2508(93)90046-5. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee S N, Banerjee M, Fernando K, Burgdorfer W, Schwan T G. Tick-borne relapsing fever in British Columbia, Canada: first isolation of Borrelia hermsii. J Clin Microbiol. 1998;36:3505–3508. doi: 10.1128/jcm.36.12.3505-3508.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbour A G. Antigenic variation of a relapsing fever Borrelia species. Annu Rev Microbiol. 1990;44:155–171. doi: 10.1146/annurev.mi.44.100190.001103. [DOI] [PubMed] [Google Scholar]
- 6.Barbour A G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 7.Barbour A G. Plasmid analysis of Borrelia burgdorferi, the Lyme disease agent. J Clin Microbiol. 1988;26:475–478. doi: 10.1128/jcm.26.3.475-478.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbour A G, Carter C J, Bundoc V, Hinnebusch J. The nucleotide sequence of a linear plasmid of Borrelia burgdorferi reveals similarities to those of circular plasmids of other prokaryotes. J Bacteriol. 1996;178:6635–6639. doi: 10.1128/jb.178.22.6635-6639.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbour A G, Hayes S F. Biology of Borrelia species. Microbiol Rev. 1986;50:381–400. doi: 10.1128/mr.50.4.381-400.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caimano M J, Yang X, Popova T G, Clawson M L, Akins D R, Norgard M V, Radolf J D. Molecular and evolutionary characterization of the cp32/18 family of supercoiled plasmids in Borrelia burgdorferi 297. Infect Immun. 2000;68:1574–1586. doi: 10.1128/iai.68.3.1574-1586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlyon J A, Marconi R T. Cloning and molecular characterization of a multicopy, linear plasmid-carried, repeat motif-containing gene from Borrelia turicatae, a causative agent of relapsing fever. J Bacteriol. 1998;180:4974–4981. doi: 10.1128/jb.180.18.4974-4981.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlyon J A, Roberts D M, Marconi R T. Evolutionary and molecular analyses of the Borrelia bdr super gene family: delineation of distinct sub-families and demonstration of the genus wide conservation of putative functional domains, structural properties and repeat motifs. Microb Pathog. 2000;28:89–105. doi: 10.1006/mpat.1999.0326. [DOI] [PubMed] [Google Scholar]
- 13.Casjens S, Huang W M. Linear chromosomal physical and genetic map of Borrelia burgdorferi, the Lyme disease agent. Mol Microbiol. 1993;8:967–980. doi: 10.1111/j.1365-2958.1993.tb01641.x. [DOI] [PubMed] [Google Scholar]
- 14.Casjens S, Palmer N, van Vugt R, Huang W M, Stevenson B, Rosa P, Lathigra R, Sutton G, Peterson J, Dodson R J, Haft D, Hickey E, Gwinn M, White O, Fraser C. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs of an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol. 2000;35:490–516. doi: 10.1046/j.1365-2958.2000.01698.x. [DOI] [PubMed] [Google Scholar]
- 15.Casjens S, van Vugt R, Tilly K, Rosa P A, Stevenson B. Homology throughout the multiple 32-kilobase circular plasmids present in Lyme disease spirochetes. J Bacteriol. 1997;179:217–227. doi: 10.1128/jb.179.1.217-227.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coleman J L, Gebbia J A, Piesman J, Degen J L, Bugge T H, Benach J L. Plasminogen is required for efficient dissemination of B. burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell. 1997;89:1111–1119. doi: 10.1016/s0092-8674(00)80298-6. [DOI] [PubMed] [Google Scholar]
- 17.de Silva A M, Telford S R, Brunet L R, Barthold S W, Fikrig E. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996;183:271–275. doi: 10.1084/jem.183.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dowding J, Davies J. Mechanisms and origins of plasmid-determined antibiotic resistance. In: Schlessinger D, editor. Microbiology—1974. Washington, D.C.: American Society for Microbiology; 1975. pp. 179–186. [Google Scholar]
- 19.Dunn J J, Buchstein S R, Butler L-L, Fisenne S, Polin D S, Lade B N, Luft B J. Complete nucleotide sequence of a circular plasmid from the Lyme disease spirochete, Borrelia burgdorferi. J Bacteriol. 1994;176:2706–2717. doi: 10.1128/jb.176.9.2706-2717.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dworkin M S, Anderson D E, Schwan T G, Shoemaker P C, Banerjee S N, Kassen B O, Burgdorfer W. Tick-borne relapsing fever in the northwestern United States and southwestern Canada. Clin Infect Dis. 1998;26:122–131. doi: 10.1086/516273. [DOI] [PubMed] [Google Scholar]
- 21.Eggers C H, Samuels D S. Molecular evidence for a new bacteriophage of Borrelia burgdorferi. J Bacteriol. 1999;181:7308–7313. doi: 10.1128/jb.181.23.7308-7313.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Hage N, Lieto L D, Stevenson B. Stability of erp loci during Borrelia burgdorferi infection: recombination is not required for chronic infection of immunocompetent mice. Infect Immun. 1999;67:3146–3150. doi: 10.1128/iai.67.6.3146-3150.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fingerle V, Liegl G, Munderloh U, Wilske B. Expression of outer surface proteins A and C of Borrelia burgdorferi in Ixodes ricinus ticks removed from humans. Med Microbiol Immunol. 1998;187:121–126. doi: 10.1007/s004300050083. [DOI] [PubMed] [Google Scholar]
- 24.Fraser C M, Casjens S, Huang W M, Sutton G G, Clayton R, Lathigra R, White O, Ketchum K A, Dodson R, Hickey E K, Gwinn M, Dougherty B, Tomb J-F, Fleischmann R D, Richardson D, Peterson J, Kerlavage A R, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams M D, Gocayne J, Weidmann J, Utterback T, Watthey L, McDonald L, Artiach P, Bowman C, Garland S, Fujii C, Cotton M D, Horst K, Roberts K, Hatch B, Smith H O, Venter J C. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 25.Gilmore R D, Piesman J. Inhibition of Borrelia burgdorferi migration from the midgut to the salivary glands following feeding by ticks on OspC-immunized mice. Infect Immun. 2000;68:411–414. doi: 10.1128/iai.68.1.411-414.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guina T, Oliver D B. Cloning and analysis of a Borrelia burgdorferi membrane-interactive protein exhibiting haemolytic activity. Mol Microbiol. 1997;24:1201–1213. doi: 10.1046/j.1365-2958.1997.4291786.x. [DOI] [PubMed] [Google Scholar]
- 27.Hinnebusch B J, Barbour A G, Restrepo B I, Schwan T G. Population structure of the relapsing fever spirochete Borrelia hermsii as indicated by polymorphism of two multigene families that encode immunogenic outer surface lipoproteins. Infect Immun. 1998;66:432–440. doi: 10.1128/iai.66.2.432-440.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lam T T, Nguyen T-P K, Montgomery R R, Kantor F S, Fikrig E, Flavell R A. Outer surface proteins E and F of Borrelia burgdorferi, the agent of Lyme disease. Infect Immun. 1994;62:290–298. doi: 10.1128/iai.62.1.290-298.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lehnherr H, Bendtsen J D, Preuss F, Ilyina T V. Identification and characterization of the single-stranded DNA-binding protein of bacteriophage P1. J Bacteriol. 1999;181:6463–6468. doi: 10.1128/jb.181.20.6463-6468.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leuba-Garcia S, Martinez R, Gern L. Expression of outer surface proteins A and C of Borrelia afzelii in Ixodes ricinus ticks and in the skin of mice. Zentbl Bakteriol. 1998;287:475–484. doi: 10.1016/s0934-8840(98)80187-4. [DOI] [PubMed] [Google Scholar]
- 31.Marconi R T, Samuels D S, Schwan T G, Garon C T. Identification of a protein in several species of Borrelia related to OspC of the Lyme disease spirochetes. J Clin Microbiol. 1993;31:2577–2583. doi: 10.1128/jcm.31.10.2577-2583.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Margolis N, Hogan D, Cieplak W, Jr, Schwan T G, Rosa P A. Homology between Borrelia burgdorferi OspC and members of the family of Borrelia hermsii variable major proteins. Gene. 1994;143:105–110. doi: 10.1016/0378-1119(94)90613-0. [DOI] [PubMed] [Google Scholar]
- 33.Meier J T, Simon M I, Barbour A G. Antigenic variation is associated with DNA rearrangements in a relapsing fever Borrelia. Cell. 1985;41:403–409. doi: 10.1016/s0092-8674(85)80013-1. [DOI] [PubMed] [Google Scholar]
- 34.Miller J C, El-Hage N, Babb K, Stevenson B. Borrelia burgdorferi B31 Erp proteins that are dominant immunoblot antigens of animals infected with isolate B31 are recognized by only a subset of human Lyme disease patient sera. J Clin Microbiol. 2000;38:1569–1574. doi: 10.1128/jcm.38.4.1569-1574.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Norton Hughes C A, Johnson R C. Methylated DNA in Borrelia species. J Bacteriol. 1990;172:6602–6604. doi: 10.1128/jb.172.11.6602-6604.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noyer-Weidner M, Trautner T A. Methylation of DNA in prokaryotes. In: Jost J P, Saluz H P, editors. DNA methylation: molecular biology and biological significance. Basel, Switzerland: Birkhäuser Verlag; 1993. pp. 39–108. [Google Scholar]
- 37.Porcella S F, Popova T G, Akins D R, Li M, Radolf J D, Norgard M V. Borrelia burgdorferi supercoiled plasmids encode multicopy tandem open reading frames and a lipoprotein gene family. J Bacteriol. 1996;178:3293–3307. doi: 10.1128/jb.178.11.3293-3307.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruvolo P P, Keating K M, Williams K R, Chase J W. Single-stranded binding proteins (SSBs) from prokaryotic transmissible plasmids. Proteins. 1991;9:120–134. doi: 10.1002/prot.340090206. [DOI] [PubMed] [Google Scholar]
- 39.Sambri V, Marangoni A, Massaria F, Farencena A, La Placa M, Cevenini R. Functional activities of antibodies directed against surface lipoproteins of Borrelia hermsii. Microbiol Immunol. 1995;39:623–627. doi: 10.1111/j.1348-0421.1995.tb02252.x. [DOI] [PubMed] [Google Scholar]
- 40.Samuels D S, Garon C F. Coumermycin A1 inhibits growth and induces relaxation of supercoiled plasmids in Borrelia burgdorferi, the Lyme disease agent. Antimicrob Agents Chemother. 1993;37:46–50. doi: 10.1128/aac.37.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwan T G. Ticks and Borrelia: model systems for investigating pathogen-arthropod interactions. Infect Agents Dis. 1996;5:167–181. [PubMed] [Google Scholar]
- 42.Schwan T G, Burgdorfer W, Garon C F. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect Immun. 1988;56:1831–1836. doi: 10.1128/iai.56.8.1831-1836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwan T G, Burgdorfer W, Rosa P A. Borrelia. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 7th ed. Washington, D.C.: American Society for Microbiology; 1999. pp. 746–758. [Google Scholar]
- 44.Schwan T G, Hinnebusch B J. Bloodstream- versus tick-associated variants of a relapsing fever bacterium. Science. 1998;280:1938–1940. doi: 10.1126/science.280.5371.1938. [DOI] [PubMed] [Google Scholar]
- 45.Schwan T G, Piesman J. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J Clin Microbiol. 2000;38:382–388. doi: 10.1128/jcm.38.1.382-388.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwan T G, Piesman J, Golde W T, Dolan M C, Rosa P A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shang E S, Skare J T, Exner M M, Blanco D R, Kagan B L, Miller J N, Lovett M A. Isolation and characterization of the outer membrane of Borrelia hermsii. Infect Immun. 1998;66:1082–1091. doi: 10.1128/iai.66.3.1082-1091.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simpson W J, Garon C F, Schwan T G. Borrelia burgdorferi contains repeated DNA sequences that are species specific and plasmid associated. Infect Immun. 1990;58:847–853. doi: 10.1128/iai.58.4.847-853.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sonenshine D E. Biology of ticks. Vol. 1. New York, N.Y: Oxford University Press; 1991. [Google Scholar]
- 50.Stevenson B, Bono J L, Schwan T G, Rosa P. Borrelia burgdorferi Erp proteins are immunogenic in mammals infected by tick bite, and their synthesis is inducible in cultured bacteria. Infect Immun. 1998;66:2648–2654. doi: 10.1128/iai.66.6.2648-2654.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stevenson B, Casjens S, Rosa P. Evidence of past recombination events among the genes encoding the Erp antigens of Borrelia burgdorferi. Microbiology. 1998;144:1869–1879. doi: 10.1099/00221287-144-7-1869. [DOI] [PubMed] [Google Scholar]
- 52.Stevenson B, Casjens S, van Vugt R, Porcella S F, Tilly K, Bono J L, Rosa P. Characterization of cp18, a naturally truncated member of the cp32 family of Borrelia burgdorferi plasmids. J Bacteriol. 1997;179:4285–4291. doi: 10.1128/jb.179.13.4285-4291.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stevenson B, Schwan T G, Rosa P A. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63:4535–4539. doi: 10.1128/iai.63.11.4535-4539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stevenson B, Tilly K, Rosa P A. A family of genes located on four separate 32-kilobase circular plasmids in Borrelia burgdorferi B31. J Bacteriol. 1996;178:3508–3516. doi: 10.1128/jb.178.12.3508-3516.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stoenner H G, Dodd T, Larsen C. Antigenic variation in B. hermsii. J Exp Med. 1982;156:1297–1311. doi: 10.1084/jem.156.5.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suk K, Das S, Sun W, Jwang B, Barthold S W, Flavell R A, Fikrig E. Borrelia burgdorferi genes selectively expressed in the infected host. Proc Natl Acad Sci USA. 1995;92:4269–4273. doi: 10.1073/pnas.92.10.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thompson R S, Burgdorfer W, Russell R, Francis B J. Outbreak of tick-borne relapsing fever in Spokane County, Washington. JAMA. 1969;210:1045–1050. [PubMed] [Google Scholar]
- 58.Wallich R, Brenner C, Kramer M D, Simon M M. Molecular cloning and immunological characterization of a novel linear-plasmid-encoded gene, pG, of Borrelia burgdorferi expressed only in vivo. Infect Immun. 1995;63:3327–3335. doi: 10.1128/iai.63.9.3327-3335.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilske B, Preac-Mursic V, Jauris S, Hofmann A, Pradel I, Soutschek E, Schwab E, Will G, Wanner G. Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein of Borrelia burgdorferi. Infect Immun. 1993;61:2182–2191. doi: 10.1128/iai.61.5.2182-2191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu Y, Johnson R C. Analysis and comparison of plasmid profiles of Borrelia burgdorferi sensu lato strains. J Clin Microbiol. 1995;33:2679–2685. doi: 10.1128/jcm.33.10.2679-2685.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang X, Popova T G, Hagman K E, Wikel S K, Schoeler G B, Caimano M J, Radolf J D, Norgard M V. Identification, characterization, and expression of three new members of the Borrelia burgdorferi Mlp (2.9) lipoprotein gene family. Infect Immun. 1999;67:6008–6018. doi: 10.1128/iai.67.11.6008-6018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang J-R, Hardham J M, Barbour A G, Norris S J. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell. 1997;89:1–20. doi: 10.1016/s0092-8674(00)80206-8. [DOI] [PubMed] [Google Scholar]
- 63.Zückert W R, Meyer J. Circular and linear plasmids of Lyme disease spirochetes have extensive homology: characterization of a repeated DNA element. J Bacteriol. 1996;178:2287–2298. doi: 10.1128/jb.178.8.2287-2298.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zückert W R, Meyer J, Barbour A G. Comparative analysis and immunological characterization of the Borrelia Bdr protein family. Infect Immun. 1999;67:3257–3266. doi: 10.1128/iai.67.7.3257-3266.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]