Abstract
We compared interleukin-12 (IL-12) and other cytokine activities during and after an acute clinical episode in a matched-pair case-control study of young African children who presented with either mild or severe Plasmodium falciparum malaria. The acute-phase, pretreatment plasma IL-12 and alpha interferon (IFN-α) levels, as well as the acute-phase mitogen-stimulated whole-blood production capacity of IL-12, were significantly lower in children with severe rather than mild malaria. IL-12 levels, in addition, showed strong inverse correlations both with parasitemia and with the numbers of circulating malaria pigment-containing neutrophils. Acute-phase plasma tumor necrosis factor (TNF) and IL-10 levels were significantly higher in those with severe malaria, and the concentrations of both of these cytokines were positively correlated both with parasitemia and with the numbers of pigment-containing phagocytes in the blood. Children with severe anemia had the highest levels of TNF in plasma. In all the children, the levels in plasma and production capacities of all cytokines normalized when they were healthy and parasite free. The results indicate that severe but not mild P. falciparum malaria in young, nonimmune African children is characterized by down-regulated IL-12 activity, contrasting markedly with the up-regulation of both TNF and IL-10 in the same children. A combination of disturbed phagocyte functions resulting from hemozoin consumption, along with reduced IFN-γ responses, may contribute to these differential effects.
Malaria continues to be a global health concern, as evidenced by the 300 million to 500 million clinical cases annually, which result in 1.5 million to 2.7 million deaths (46). Approximately 1 million of those malaria-associated deaths occur in children younger than 5 years (46). Infection with Plasmodium falciparum, the causative agent of severe clinical malaria, in areas of hyperendemicity such as the Province of Moyen Ogooué, Gabon, primarily affects children younger than 5 years due to their nonimmune status (44). Severe falciparum malaria in Gabon is typically characterized by hyperparasitemia and severe anemia, with cerebral malaria being less common.
With the growing problem of antimalarial drug resistance, it is becoming increasingly important to understand the protective immune response to malaria so that novel treatment strategies and interventions can be developed. The host immune response to an invading pathogen depends largely on the development of adaptive immunity mediated by the release of cytokines from T helper (Th) cells, Th1 and Th2. Whereas Th1 cells initiate cell-mediated immunity required for the elimination of intracellular pathogens, Th2 cells mediate humoral immune responses (25, 26). For example, the preferential activation of a Th1 response in C57BL/6 mice is associated with resistance to blood-stage P. chabaudi AS infection, whereas a Th2 response predominates in the susceptible A/J mice (40).
Early events in the cell-mediated immune response required for protection against malaria are initiated by the release of interleukin-12 (IL-12) from monocytes/macrophages, B cells, and perhaps other cell types (4, 25, 43). IL-12-induced protection against the blood stage of P. chabaudi AS and P. berghei XAT is mediated by up-regulation of gamma interferon (IFN-γ) and tumor necrosis factor (TNF), which promote antiparasitic properties, at least in part by generating high levels of nitric oxide (NO) (41, 47). Resistance in the P. chabaudi AS model is associated with early and sustained up-regulation of IL-12 and its receptors (35). As well as inducing protective immunity in this model system, treatment with recombinant IL-12 (rIL-12) corrects severe anemia in P. chabaudi AS-infected susceptible A/J mice by enhancing erythropoiesis (21, 22). Additional studies have shown that administration of rIL-12 before inoculation of mice with P. yoelii or of rhesus monkeys with P. cynomolgi provides 100% protection in both models of malaria through an IFN-γ-dependent (and perhaps NO-dependent) antiplasmodial mechanism (10, 36). Taken together, these studies suggest that protective immunity in malaria is mediated by a cascade of events that involves IL-12-induced production of IFN-γ, TNF, and NO.
Although the role of Th2 cytokines in regulating the immune response to malaria remains unclear, the relative balance between Th1 and Th2 cytokines appears important. Elevated concentrations of TNF and IL-10 in plasma are characteristic of children with malarial anemia and high-density parasitemia (29, 37), but a low IL-10/TNF ratio is specifically associated with malarial anemia (16, 23, 29). These results are consistent with the fact that IL-10 inhibits P. falciparum-induced TNF production (8, 9) and suggest that the lack of an IL-10 response may allow high levels of TNF, which could promote anemia. We have in addition recently demonstrated that IFN-γ-mediated responses are associated with protection against infection with P. falciparum in young African children (19).
To further understand the immune cascade in malaria and the potential relationships with the pathogenesis of severe malarial anemia in particular, we determined the levels of IFN-γ, IFN-α, TNF, IL-10, and IL-12 in plasma before and after treatment of matched groups of children with either mild or severe falciparum malaria. We examined the associations of these cytokines with severe malarial anemia by using either a strict (hemoglobin [Hb] < 50 g/liter) or a broad (hematocrit [Hct] < 25%) definition of anemia. We also compared the cytokine-producing capacities, following mitogen stimulation of whole blood, of the two groups of children, as well as the association between cytokine activity during the acute phase of disease and the numbers of pigment-containing phagocytes.
MATERIALS AND METHODS
Study design.
The study was carried out at the Albert Schweitzer Hospital in Lambaréné, Gabon. Detailed descriptions of the participants, the inclusion criteria used, treatment given, clinical and follow-up surveillance undertaken, and hematological and biochemical methods used have been given elsewhere (15, 18). Briefly, 100 patients with severe malaria (cases) were matched for age, gender, and provenance with 100 patients with mild malaria (controls), where severe malaria was defined as either severe anemia (Hb, <50 g/liter, or Hct, <15%) and/or P. falciparum hyperparasitemia (>250,000 parasites/μl), while mild malaria was defined as P. falciparum parasitemia (1,000 to 50,000 parasites/μl) with Hb of >80 g/liter, glycemia of >50 mg/dl, and no signs of severe malaria. Reinfections with P. falciparum were detected through active follow-up of individuals every 2 weeks. Informed consent for participation in this study was obtained, prior to inclusion, from the parent or guardian of each individual. Ethical clearance for the study was given by the Ethics Committee of the International Foundation of the Albert Schweitzer Hospital in Lambaréné.
Detection of parasitemia and pigment-containing cells in blood smears.
A calibrated thick-smear technique was used, with standard Giemsa staining, for the estimation of parasitemias (13). Using a shortened Giemsa staining procedure, the same technique was used for the enumeration of pigment-containing monocytes and neutrophils, expressed as the number of pigment-containing cells per microliter of blood.
Plasma samples.
For immunological assessments, sterile collection tubes containing EDTA were used for collection of venous blood samples, a part of which was separated for use in the whole-blood stimulations described below. After centrifugation of the remaining undiluted whole blood for mononuclear cell separation, plasma was drawn off and stored as aliquots at −80°C until required for cytokine assays. Samples were collected in this way on separate occasions, corresponding to acute (admission, pretreatment) and healthy (infection-free) phases. The latter sample was collected at a time, at least 6 months postadmission, when the child was free of any clinically obvious intercurrent infection and had had at least three consecutive Plasmodium-negative thick blood smears in the active follow-up surveillance period immediately preceding the sample collection. There were three fatalities in the group with severe malaria, all within 48 h of hospitalization and all resulting from multiple complications. These, coupled with subsequent losses to follow-up, meant that we were able to collect healthy-phase samples from 61 and 65 children in the mild and severe cohorts, respectively.
Whole-blood stimulation assays.
Immediately after venous blood collection, 10 μl of mitogen (phytohemagglutinin-L; Sigma, Deisenhofen, Germany), diluted appropriately in RPMI 1640 medium (Seromed, Berlin, Germany), was added to 700 μl of whole blood such that the final concentration of mitogen was 10 μg/ml. This stimulated sample and an unstimulated control sample comprising 700 μl of whole blood to which 10 μl of RPMI 1640 without mitogen was added were incubated for 20 h at 37°C in an atmosphere containing 5% CO2. At the end of this incubation period, the samples were centrifuged and the supernatants were aspirated and stored frozen at −80°C until required for cytokine assays.
Cytokine assays.
The concentrations of TNF, IL-10 (specific only for humans, not virus-derived IL-10), IL-12 (p70 heterodimer and p40 chain), and IFN-γ in supernatants from whole-blood stimulations were determined using enzyme-linked immunosorbent assays obtained commercially (FLEXIA; BioSource, Ratingen, Germany). The assays were performed as specified by the manufacturer. The detection limit in all cases was 1 pg/ml, and values below this level were assigned a concentration of zero. The cytokine activity measured in this way in the supernatants of unstimulated whole-blood samples therefore represented concentrations in plasma, and production capacities were derived by subtraction of these values from the cytokine levels measured in the corresponding stimulated whole-blood samples. Separate measurements of the levels of IL-12 (p70 heterodimer) and IFN-α in fresh-frozen plasma samples were made using commercial ELISA kits from R&D Systems (Minneapolis, Minn.) and from Endogen Inc. (Woburn, Mass.), respectively, with detection limits of 5 and 2.5 pg/ml, respectively.
Statistical analyses.
Correlations between continuous variables were assessed by the Spearman rank test, corrected for ties, where a value of ρ > 0.25 (combined with P < 0.05) was considered significant. Contingency tables, using Fisher's exact test, were used to compare proportions within and between groups. For paired and unpaired analyses, the nonparametric Wilcoxon signed rank and Mann-Whitney U tests, respectively, were used to determine the significance of differences in continuous variables. The level of significance in all cases was set at a two-tailed P of <0.05.
RESULTS
Cytokine levels in plasma.
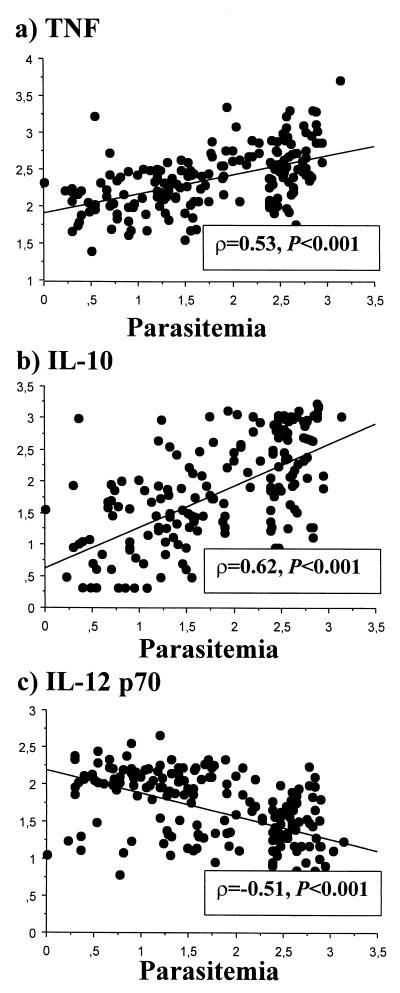
The acute-phase levels of IL-12 p70 in plasma were inversely correlated with those of both IL-10 (P < 0.001, ρ = −0.32) and TNF (P < 0.001, ρ = −0.28) but positively correlated with those of IFN-α (P < 0.001, ρ = 0.28). The concentration of IL-10 in plasma was positively correlated with both that of TNF (P < 0.001, ρ = 0.71) and that of IFN-γ (P < 0.001, ρ = 0.31). Figure 1 shows that the acute-phase levels of both TNF and IL-10 correlated positively with parasitemia (P < 0.001, ρ = 0.53 and ρ = 0.62, respectively), while IL-12 p70 levels were inversely correlated with this parameter (P < 0.001, ρ = −0.51). The levels of IFN-γ and IFN-α in plasma showed no association with parasitemia.
FIG. 1.
Association between acute-phase cytokine levels in plasma and parasitemia at admission. Data are logarithms of cytokine concentrations in plasma (picograms per milliliter) (y axis) plotted against logarithms of parasitemia (number of parasites per microliter of blood) (x axis), with corresponding correlation coefficients (ρ) and statistical significance of associations (P) calculated by the Spearman rank correlation test, for TNF (a), IL-10 (b), and IL-12 p70 (c).
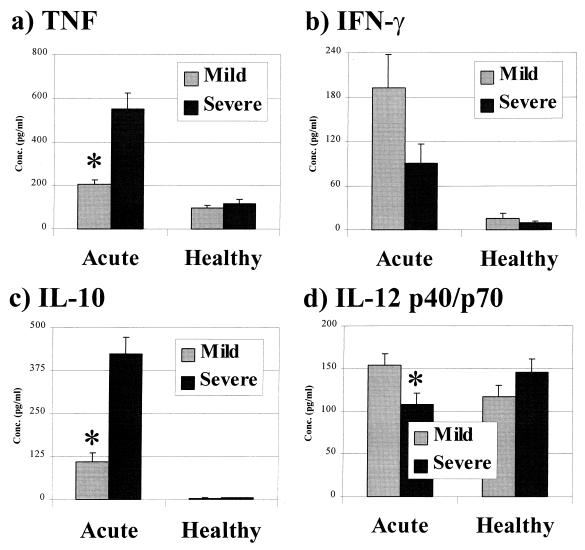
As illustrated in Fig. 2d, the concentration of IL-12 p40/p70 was significantly higher in the plasma of those with mild malaria than in the plasma of those with severe malaria at admission (P < 0.001), and measurements of IL-12 p70 levels alone showed the same significant difference between the groups (mean ± standard error of the mean [SEM], mild versus severe: 113 ± 8 versus 30 ± 3 pg/ml; P < 0.001). Both TNF and IL-10 were present at significantly higher concentrations in the plasma of those with severe malaria (Fig. 2a and c). The concentration of IFN-γ did not differ significantly in the two groups at this time (Fig. 2b), but the level of IFN-α was significantly higher in those with mild malaria (mean ± SEM, mild versus severe: 22 ± 2 versus 16 ± 1 pg/ml; P = 0.002). Comparisons were also made, within the group presenting with severe malaria, between those with and without anemia. Those classified as having severe malarial anemia, using an Hb of <50 g/liter as a cutoff, had significantly higher plasma TNF levels (579 ± 82 versus 409 ± 60 pg/ml; P = 0.030) and a significantly lower IL-10/TNF ratio (0.59 ± 0.10 versus 1.04 ± 0.14; P = 0.028) than those without anemia, while the levels of other cytokines were equivalent between the groups (data not shown). Segregation based on a broader definition of anemia, using an Hct of <25% as the cutoff, revealed the same significant difference in TNF levels (649 ± 93 versus 328 ± 69 pg/ml; P < 0.001) between those with and those without anemia but showed no difference in other parameters (data not shown). In healthy-phase plasma samples, all cytokines measured were present at similar concentrations in the two groups (Fig. 2). Pairwise analyses showed that the levels of TNF, IL-10, and IFN-γ in healthy-phase plasma samples of both groups were significantly lower compared to their corresponding acute-phase values (P < 0.001 in all cases), as can be seen in Fig. 2. However, compared to their respective acute-phase levels, healthy-phase levels of IL-12 p40/p70 in plasma were significantly lower (P = 0.027) in the group with mild malaria but significantly higher (P = 0.019) in those admitted with severe malaria (Fig. 2d). In subgroups segregated according to the presence or absence of anemia at admission, healthy-phase cytokine levels were similar (data not shown).
FIG. 2.
Cytokine levels in plasma in the presence and absence of acute P. falciparum infection. Bars represent the mean concentrations (with SEM indicated) of different cytokines in the plasma of groups of children, segregated according to their clinical presentation (mild or severe) at admission, measured in the presence (acute) or absence (healthy) of parasitemia. Comparisons of cytokine concentrations between groups at the different times were made using the nonparametric Wilcoxon signed rank test. ∗, P < 0.001. Note that the cytokine concentrations given here comprise activity measured in supernatants of unstimulated whole blood and represent background values for the production capacity estimates in Fig. 3.
Cytokine production capacity.
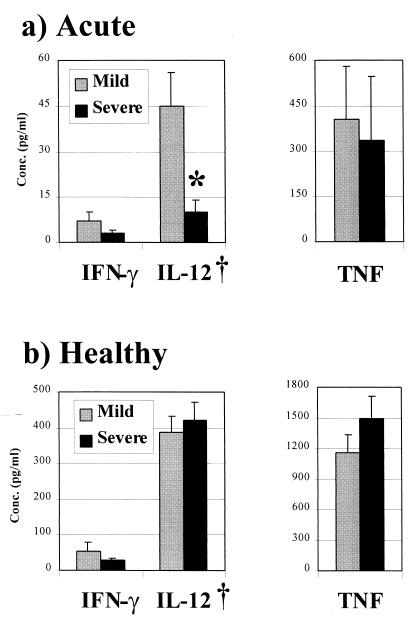
Under the conditions used, mitogen stimulation of whole blood did not result in a detectable elevation of IL-10 production. In acute-phase samples, the IL-12 p40/p70 production capacity was significantly lower in whole-blood samples of those with severe malaria than of those with mild malaria (P < 0.001) (Fig. 3a). TNF production capacities were similar in those with mild malaria and those with severe malaria (Fig. 3a), while IFN-γ production was stimulated in only 30% of whole-blood samples, and then at only negligible levels, with no significant difference between the groups (Fig. 3a). Pairwise analyses showed that the mitogen-stimulated production of all three of these cytokines was significantly higher in healthy-phase samples from both groups (Fig. 3b; P < 0.001 in all cases) than in the respective acute-phase samples. Thus, healthy-phase TNF and IFN-γ production was, respectively, between 3- and 4-fold and between 8- and 9-fold higher in the two groups, while IL-12 production was 9-fold higher in those who presented with mild malaria but over 40-fold higher in those who presented with severe malaria. In the latter group, at this time, there were also nonsignificant trends for lower IFN-γ production but higher TNF production than in the mild-malaria control group (Fig. 3b). No differences were found at any time in the cytokine production capacities in groups segregated according to the presence or absence of anemia at admission (data not shown).
FIG. 3.
Mitogen-stimulated whole-blood cytokine production capacity in the presence or absence of acute P. falciparum infection. Bars represent the mean concentrations (with SEM indicated), after subtraction of background levels in unstimulated cultures, of cytokines measured in the supernatants of phytohemagglutinin-stimulated whole-blood cultures in the presence (a) or absence (b) of parasitemia. Groups are segregated according to their clinical presentation (mild or severe) at admission. Comparisons of cytokine production capacities between groups at the different times were made using the nonparametric Wilcoxon signed rank test. ∗, P < 0.001; †, IL-12 p40/p70.
Production capacities of the three cytokines measured were positively correlated in healthy-phase samples (TNF versus IFN-γ, ρ = 0.61, P < 0.001; TNF versus IL-12 p40/p70, ρ = 0.81, P < 0.001; IL-12 p40/p70 versus IFN-γ, ρ = 0.50, P < 0.001), but these associations were either absent or weaker in the acute-phase samples (TNF versus IFN-γ, ρ = 0.11, P not significant; TNF versus IL-12 p40/p70, ρ = 0.30, P < 0.001; IL-12 p40/p70 versus IFN-γ, ρ = 0.10, P not significant). The IL-12 p40/p70 production capacity in acute-phase samples was inversely correlated with plasma IL-10 levels (ρ = −0.26, P < 0.001), but no other associations between cytokine production capacities and corresponding plasma cytokine levels were found.
Parasitemia was inversely correlated with the IL-12 p40/p70 production capacity in acute-phase samples (ρ = −0.33, P < 0.001) but showed no association with the mitogen-stimulated whole-blood production of either TNF or IFN-γ.
Pigment-containing cells and acute-phase cytokine activity.
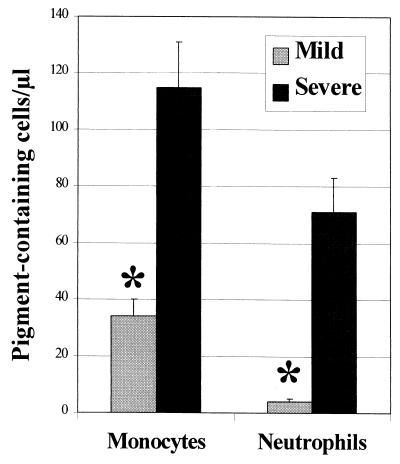
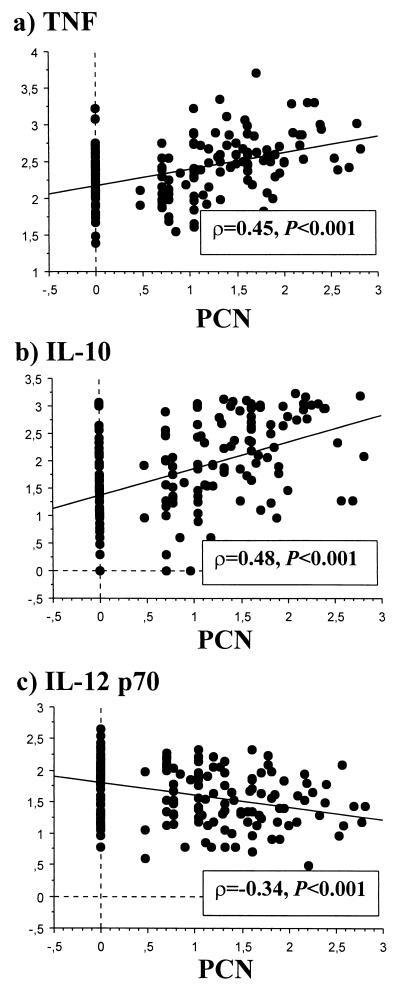
At admission, 4- and 18-fold-larger numbers of pigment-containing monocytes (PCM) and neutrophils (PCN), respectively, were present in the blood of those with severe than of those with mild malaria (Fig. 4). The numbers of both PCM and of PCN were positively correlated with parasitemia (ρ = 0.35 and ρ = 0.72, respectively; P < 0.001 in both cases). The levels of both TNF and IL-10 in plasma were positively correlated with the numbers of PCN (Fig. 5a and b), but the level of IL-12 p70 in plasma (Fig. 5c), as well as the IL-12 p40/p70 production capacity (ρ = −0.35, P < 0.001), showed an inverse correlation with the numbers of PCN. Similar but nonsignificant trends were seen for the same cytokine activity and the numbers of PCM (IL-12 p70: ρ = −0.23, P = 0.002). IFN-γ and IFN-α showed no association with either PCM or PCN numbers (data not shown).
FIG. 4.
Numbers of pigment-containing cells in the peripheral blood of groups of children segregated according to their clinical presentation at admission. Bars represent the mean numbers (with SEM indicated), in cells per microliter of blood, of malaria pigment-containing monocytes and neutrophils detected in the peripheral blood at admission in the two groups of children. Comparisons between the groups were made using the nonparametric Wilcoxon signed rank test. ∗, P < 0.001. These data were originally reported by Kun et al. (15).
FIG. 5.
Association between acute-phase cytokine levels in plasma and numbers of pigment-containing neutrophils at admission. Data are logarithms of cytokine concentrations in plasma (picograms per milliliter) (y axis) plotted against logarithm of PCN numbers per microliter of blood (x axis), with corresponding correlation coefficients (ρ) and statistical significance of associations (P) calculated by the Spearman rank correlation test, for TNF (a), IL-10 (b), and IL-12 p70 (c).
Segregation according to the presence or absence of anemia at admission showed a significantly larger number of PCM in those with anemia, regardless of the definition used, and also of PCN when using the broader definition of anemia (Table 1).
TABLE 1.
Numbers of pigment-containing cells in children with severe malaria segregated according to the presence or absence of anemia at admissiona
| Cell type | No. of cells/μl of blood in children withb:
|
Pd | No. of cells/μl of blood in children withc:
|
Pd | ||
|---|---|---|---|---|---|---|
| Hb < 50 g/L (n = 39) | Hb > 50 g/L (n = 61) | Hct < 25% (n = 70) | Hct > 25% (n = 30) | |||
| PCM | 116 (176) | 40 (87) | 0.002 | 82 (179) | 38 (40) | 0.001 |
| PCN | 28 (66) | 30 (58) | NS | 38 (69) | 15 (35) | 0.016 |
Values given are median numbers of pigment-containing cells per microliter of blood; interquartile ranges are given in parentheses.
Strict definition of anemia, using the Hb level as cutoff, above or below 50 g/liter.
Broad definition of anemia, using the Hct level as cutoff, above or below 25%.
Differences between groups assessed by the nonparametric Mann-Whitney U test. NS, nonsignificant.
DISCUSSION
The principal findings of this study concern IL-12 activity in the acute phase of P. falciparum malaria, which is characterized in particular by both the lower levels in plasma and the dramatically reduced leukocyte production capacity of this cytokine in children with severe compared to mild malaria. In addition, IL-12 showed inverse correlations with both parasitemia and the numbers of PCN, paralleled by the acute-phase plasma profile of IFN-α. These profiles are in striking contrast to those we and others have described for either TNF or IL-10, which are consistently higher in children with severe malaria, as discussed below. Mitogen-stimulated leukocyte production of IL-12 and IFN-γ, as we observed with samples taken from these children during infection-free conditions, is normally coordinated and associated. Acute P. falciparum malaria clearly has a profound effect on this equilibrium, which could be a key factor influencing the well-described disturbance of cell-mediated immunity in individuals acutely infected with P. falciparum. In the same context, the known effects of hemozoin on monocyte functions may also have an important role to play.
Impairment of cellular immunological responses with specificity for both parasite and nonparasite antigens is a feature of acute P. falciparum infection. These effects are manifested, for example, through a reduction in demonstrable in vitro parasite antigen-specific lymphoproliferative activity, which is thought to be principally, if not exclusively, the result of temporary relocation or sequestration of activated lymphocytes away from the peripheral blood compartment (2, 7, 34). The posttreatment repopulation of the peripheral circulation with T-cell subsets, whose kinetics are related to the severity of the malarial attack, is consistent with these ideas (11). In a separate study, we have shown that Gabonese children with P. falciparum hyperparasitemia have significantly lower levels of IFN-γ-secreting CD4+ T cells in the peripheral blood than do children with uncomplicated malaria (45). The reduced IL-12 activity we observed in the present study specifically in those with severe malaria, a majority of whom presented with hyperparasitemia, may thus be related to the more marked reduction in the frequency of the CD4+ T-cell subset in these individuals, since it is known that IFN-γ can potentiate the production of IL-12 from human monocytes (6). The trend toward reduced IFN-γ activity in those with severe malaria, as noted here as well as in an earlier study of cell-mediated immune responses in the same children (19), is clearly consistent with these other findings. Reduced T-cell-mediated IFN-γ activity in the peripheral circulation of these children may therefore be directly implicated in the reduced acute-phase plasma IL-12 levels and the corresponding reduction in IL-12 production capacity we observed in the same individuals. The lower concentration of IFN-α in plasma that we observed in children with severe malaria may be of additional significance in the context of mechanisms with putative protective roles, since it is known that this cytokine promotes the production of nitric oxide (NO) by human blood mononuclear cells (38). In a separate study with individuals from this same study cohort, we have shown that mononuclear cells from those who presented with mild malaria have higher IFN-α-induced NO activity (30).
The principal cellular sources of human IL-12 include dendritic cells, monocytes, and neutrophils (3, 42). The constitutive production of IL-12 by monocytes is inhibited following phagocytosis of small amounts of hemozoin (24). Under the same in vitro conditions, monocyte production of both TNF and IL-10 is enhanced after consumption of this metabolic waste product of parasite-mediated hemoglobin digestion (24). Ingestion of hemozoin may conceivably, therefore, have differential effects on cytokine production by phagocytic cells, directly enhancing TNF and IL-10 production but suppressing IL-12 production through either direct or indirect mechanisms or both. IL-10 inhibits the production of IL-12 from monocytes (42), an effect corroborated by our own observations in this study of an inverse correlation between IL-12 production capacity and plasma IL-10 levels. Down-regulation of human monocyte IL-12 production, in the absence of similar effects on other proinflammatory cytokines, is a well-known phenomenon (27). Our data suggest that neutrophil-mediated IL-12 production during acute P. falciparum malaria may be particularly susceptible to these inhibitory effects, but confirmation of this would require, for example, in vitro experiments similar to those already performed with hemozoin-fed monocytes/macrophages. In addition, the principal cellular sources of IL-12 during malaria infection have yet to be defined, making it impossible, at this stage, to attribute a defined role for specific cytokine production to individual cell types.
The profiles of pre- and posttreatment levels of TNF and IL-10 in plasma that we describe here are consistent with those reported in earlier studies of young African children and show that during the acute stages of infection with P. falciparum, both TNF and IL-10 levels are raised and are higher in the plasma of children with severe malaria than of those with mild malaria (5, 12, 14, 16, 17, 28, 29, 37). Elevated concentrations of IFN-γ in plasma during acute P. falciparum malaria, as we saw here, have been reported in some but not all studies (23, 28, 29). We also found higher TNF levels in plasma and an altered IL-10/TNF ratio in those with malaria-associated anemia, which confirms earlier findings (29), as do the pre- and posttreatment kinetics of cytokine production in ex vivo mitogen-stimulated whole-blood cultures (14, 23). The strong correlations we observed between the acute-phase levels of both TNF and IL-10 and parasitemia confirm our own earlier findings (14, 23), but this is the first study, to our knowledge, to report the existence of close associations between such cytokine activity and the numbers of circulating hemozoin-containing phagocytes detectable during human infections. As discussed above in the context of IL-12, these findings provide further evidence that at least a part of the production of certain cytokines during an acute malaria episode is directly attributable to the effects of hemozoin on phagocytic cells, an idea strongly supported by the results of in vitro studies (1, 24, 32, 33, 39). Our data demonstrate tight associations between the presence of severe anemia, high TNF levels, and large numbers of circulating hemozoin-containing monocytes. Since such monocytes are thought to be a direct indication of the longevity of a given infection (20, 31), we conclude that hemozoin-induced TNF production probably plays a role in either initiation or exacerbation of anemia as a clinical outcome of chronic, uncontrolled parasitemia.
Among the members of the human cytokine network so far described, IL-12 plays the most important role in enhancing Th-1-associated immunity (43). In malaria, IL-12 mediates host-protective mechanisms in a number of different experimental models, including both primates and mice, which has led to speculation about its potential usefulness as either an antimalarial prophylactic measure or a Th-1-type response-promoting adjuvant for antimalarial vaccines (10, 22, 36). We interpret the results of the study described here, providing evidence of an association between reduced IL-12 activity and susceptibility to severe malaria in humans, as lending strong support to such ideas. We nevertheless recognize that the issue of cause and effect, in the context of disease outcome and reduced or suppressed immune responses during malaria infection, remains unresolved.
ACKNOWLEDGMENTS
We thank the children and their families for their participation in this study. We also thank Anselme Ndzengué and Marcel Nkeyi for their excellent technical assistance. We are grateful to Swissair for the free transport of study material.
This study was supported in part by the Fortune program of the Medical Faculty, University of Tübingen; by the WHO-TDR; and by the European Union (INCO-DC IC18 CT98 0370).
Footnotes
This paper is dedicated to the memory of the late Robert N. Mshana.
REFERENCES
- 1.Arese P, Schwartzer E. Malaria pigment (haemozoin): a very active ‘inert’ substance. Ann Trop Med Parasitol. 1997;91:501–516. doi: 10.1080/00034989760879. [DOI] [PubMed] [Google Scholar]
- 2.Brasseur P, Agrapart M, Ballet J J, Druilhe P, Warrell M J, Tharavanij S. Impaired cell-mediated immunity in Plasmodium falciparum-infected patients with high parasitemia and cerebral malaria. Clin Immunol Immunopathol. 1983;27:38–50. doi: 10.1016/0090-1229(83)90054-5. [DOI] [PubMed] [Google Scholar]
- 3.Cassatella M A, Meda L, Gasperini S, D'Andrea A, Ma X, Trinchieri G. Interleukin-12 production by human polymorphonuclear leukocytes. Eur J Immunol. 1995;25:1–5. doi: 10.1002/eji.1830250102. [DOI] [PubMed] [Google Scholar]
- 4.Crutcher J M, Stevenson M M, Sedegah M, Hoffman S L. Interleukin-12 and malaria. Res Immunol. 1995;146:552–559. doi: 10.1016/0923-2494(96)83031-8. [DOI] [PubMed] [Google Scholar]
- 5.Grau G E, Taylor T E, Molyneux M E, Wirima J J, Vassalli P, Hommel M, Lambert P-H. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med. 1989;320:1586–1591. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- 6.Hayes M P, Wang J, Norcross M A. Regulation of interleukin-12 expression in human monocytes: selective priming by interferon-γ of lipopolysaccharide-inducible p35 and p40 genes. Blood. 1995;86:646–650. [PubMed] [Google Scholar]
- 7.Ho M, Webster H K, Looareesuwan S, Supanaranond W, Phillips R E, Chanthavanich P, Warrell D A. Antigen-specific immunosuppression in human malaria due to Plasmodium falciparum. J Infect Dis. 1986;153:763–771. doi: 10.1093/infdis/153.4.763. [DOI] [PubMed] [Google Scholar]
- 8.Ho M, Sexton M, Tongtawe P, Looareesuwan S, Suntharasamai P, Webster K. Interleukin-10 inhibits tumor necrosis factor production but not antigen-specific proliferation in acute Plasmodium falciparum malaria. J Infect Dis. 1995;172:838–844. doi: 10.1093/infdis/172.3.838. [DOI] [PubMed] [Google Scholar]
- 9.Ho M, Schollardt T, Snape S, Looareesuwan S, Suntharasamai P, White N J. Endogenous interleukin-10 modulates proinflammatory response in Plasmodium falciparum malaria. J Infect Dis. 1998;178:520–525. doi: 10.1086/515640. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman S, Crutcher J, Puri S, Ansari A, Villinger F, Franke E, Singh P, Finkelman F, Gately M, Dutta G, Sedegah M. Sterile protection of monkeys against malaria after administration of interleukin-12. Nat Med. 1997;3:80–83. doi: 10.1038/nm0197-80. [DOI] [PubMed] [Google Scholar]
- 11.Hviid L, Kurtzhals J A L, Goka B Q, Oliver-Commey J O, Nkrumah F K, Theander T G. Rapid reemergence of T cells into peripheral circulation following treatment of severe and uncomplicated Plasmodium falciparum malaria. Infect Immun. 1997;65:4090–4093. doi: 10.1128/iai.65.10.4090-4093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kern P, Hemmer C J, Van Damme J, Gruss H J, Dietrich M. Elevated tumor necrosis factor alpha and interleukin-6 serum levels as markers for complicated Plasmodium falciparum malaria. Am J Med. 1989;87:139–143. doi: 10.1016/s0002-9343(89)80688-6. [DOI] [PubMed] [Google Scholar]
- 13.Kremsner P G, Zotter G M, Feldmeier H, Graninger W, Rocha R M, Wiedermann G. A comparative trial of three regimens for treating uncomplicated falciparum malaria in Acre, Brazil. J Infect Dis. 1988;158:1368–1371. doi: 10.1093/infdis/158.6.1368. [DOI] [PubMed] [Google Scholar]
- 14.Kremsner P G, Winkler S, Brandts C, Wildling E, Jenne L, Graninger W, Prada J, Bienzle U, Juillard P, Grau G E. Prediction of accelerated cure in Plasmodium falciparum malaria by the elevated capacity of tumor necrosis factor production. Am J Trop Med Hyg. 1995;53:532–538. doi: 10.4269/ajtmh.1995.53.532. [DOI] [PubMed] [Google Scholar]
- 15.Kun J F J, Schmidt-Ott R J, Lehman L G, Lell B, Luckner D, Greve B, Matousek P, Kremsner P G. Merozoite surface antigen 1 and 2 genotypes and rosetting of Plasmodium falciparum in severe and mild malaria in Lambaréné, Gabon. Trans R Soc Trop Med Hyg. 1998;92:110–114. doi: 10.1016/s0035-9203(98)90979-8. [DOI] [PubMed] [Google Scholar]
- 16.Kurtzhals J A L, Adabayeri V, Goka B Q, Akanmori B D, Oliver-Commey J O, Nkrumah F K, Behr C, Hviid L. Low plasma concentrations of interleukin 10 in severe malarial anemia compared with cerebral and uncomplicated malaria. Lancet. 1998;351:1768–1772. doi: 10.1016/S0140-6736(97)09439-7. [DOI] [PubMed] [Google Scholar]
- 17.Kwiatkowski D, Hill A V S, Sambou I, Twumasi P, Castracane J, Manogue K R, Cerami A, Brewster D R, Greenwood B M. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated malaria. Lancet. 1990;336:1201–1204. doi: 10.1016/0140-6736(90)92827-5. [DOI] [PubMed] [Google Scholar]
- 18.Luckner D, Lell B, Greve B, Lehman L G, Schmidt-Ott R J, Matousek P, Herbich K, Schmid D, Mba R, Kremsner P G. No influence of socioeconomic factors on severe malarial anaemia, hyperparasitaemia or reinfection. Trans R Soc Trop Med Hyg. 1998;92:478–481. doi: 10.1016/s0035-9203(98)90882-3. [DOI] [PubMed] [Google Scholar]
- 19.Luty A J F, Lell B, Schmidt-Ott R, Lehman L G, Luckner D, Greve B, Matousek P, Herbich K, Schmid D, Migot-Nabias F, Deloron P, Nussenzweig R S, Kremsner P G. Interferon-γ responses are associated with resistance to reinfection with Plasmodium falciparum in young African children. J Infect Dis. 1999;179:980–988. doi: 10.1086/314689. [DOI] [PubMed] [Google Scholar]
- 20.Metzger W G, Mordmüller B G, Kremsner P G. Malaria pigment in leucocytes. Trans R Soc Trop Med Hyg. 1995;89:637–638. doi: 10.1016/0035-9203(95)90423-9. [DOI] [PubMed] [Google Scholar]
- 21.Mohan K, Stevenson M. Interleukin-12 corrects severe anemia during blood-stage Plasmodium chabaudi AS in susceptible A/J mice. Exp Hematol. 1998;26:45–52. [PubMed] [Google Scholar]
- 22.Mohan K, Sam H, Stevenson M. Therapy with a combination of low doses of interleukin 12 and chloroquine completely cures blood-stage malaria, prevents severe anemia, and induces immunity to reinfection. Infect Immun. 1999;67:513–519. doi: 10.1128/iai.67.2.513-519.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mordmüller B G, Metzger W G, Juillard P, Brinkman B M N, Verweij C L, Grau G E, Kremsner P G. Tumor necrosis factor in Plasmodium falciparum malaria: high plasma level is associated with fever, but high production capacity is associated with rapid fever clearance. Eur Cytokine Netw. 1997;8:29–35. [PubMed] [Google Scholar]
- 24.Mordmüller B G, Turrini F, Long H, Kremsner P G, Arese P. Neutrophils and monocytes from subjects with the Mediterranean G6PD variant: effect of Plasmodium falciparum hemozoin on G6PD activity, oxidative burst and cytokine production. Eur Cytokine Netw. 1998;9:239–246. [PubMed] [Google Scholar]
- 25.Mosmann T, Coffman R. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 26.Mosmann T R. Regulation of immune responses by T cells with different cytokine secretion phenotypes: role of a new cytokine, cytokine synthesis inhibitory factor (IL10) Int Arch Allergy Appl Immunol. 1991;94:110–115. doi: 10.1159/000235340. [DOI] [PubMed] [Google Scholar]
- 27.Mosser D M, Karp C L. Receptor-mediated subversion of macrophage cytokine production by intracellular pathogens. Curr Opin Immunol. 1999;11:406–411. doi: 10.1016/s0952-7915(99)80068-5. [DOI] [PubMed] [Google Scholar]
- 28.Mshana R N, Boulandi J, Mshana N M, Mayombo J, Mendome G. Cytokines in the pathogenesis of malaria: levels of IL-1β, IL-4, IL-6, TNF-α, and IFN-γ in plasma of healthy individuals and malaria patients in a holoendemic area. J Clin Lab Immunol. 1991;34:131–139. [PubMed] [Google Scholar]
- 29.Othoro C, Lal A A, Nahlen B, Koech D, Orago A S S, Udhayakumar V. A low interleukin-10 tumor necrosis factor-α ratio is associated with malaria anemia in children residing in a holoendemic malaria region in West Kenya. J Infect Dis. 1999;179:279–282. doi: 10.1086/314548. [DOI] [PubMed] [Google Scholar]
- 30.Perkins D J, Kremsner P G, Schmid D, Misukonis M A, Kelly M A, Weinberg J B. Blood mononuclear cell nitric oxide production and plasma cytokine levels in healthy Gabonese children with prior mild or severe malaria. Infect Immun. 1999;67:4977–4981. doi: 10.1128/iai.67.9.4977-4981.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phu N H, Day N, Diep P T, Ferguson D J P, White N J. Intraleucocytic malaria pigment and prognosis in severe malaria. Trans R Soc Trop Med Hyg. 1995;89:200–204. doi: 10.1016/0035-9203(95)90496-4. [DOI] [PubMed] [Google Scholar]
- 32.Pichyangkul S, Saengkrai P, Webster H K. Plasmodium falciparum pigment induces monocytes to release high levels of tumor necrosis factor-α and interleukin-1β. Am J Trop Med Hyg. 1994;51:430–435. [PubMed] [Google Scholar]
- 33.Prada J, Malinowski J, Muller S, Bienzle U, Kremsner P G. Hemozoin differentially modulates the production of interleukin 6 and tumor necrosis factor in murine malaria. Eur Cytokine Netw. 1995;6:109–112. [PubMed] [Google Scholar]
- 34.Riley E M, Andersson G, Otoo L N, Jepsen S, Greenwood B M. Cellular immune responses to Plasmodium falciparum antigens in Gambian children during and after an acute attack of falciparum malaria. Clin Exp Immunol. 1988;73:17–22. [PMC free article] [PubMed] [Google Scholar]
- 35.Sam H, Stevenson M M. In vivo IL-12 production and IL-12 receptors beta1 and beta2 mRNA expression in the spleen are differentially up-regulated in resistant B6 and susceptible A/J mice during early blood-stage Plasmodium chabaudi AS malaria. J Immunol. 1999;162:1582–1589. [PubMed] [Google Scholar]
- 36.Sedegah M, Finkelman F, Hoffman S L. Interleukin 12 induction of interferon gamma-dependent protection against malaria. Proc Natl Acad Sci USA. 1994;91:10700–10702. doi: 10.1073/pnas.91.22.10700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaffer N, Grau G E, Hedberg K, Davachi F, Lyamba B, Hightower A W, Breman J G, Phuc N D. Tumor necrosis factor and severe malaria. J Infect Dis. 1991;163:96–101. doi: 10.1093/infdis/163.1.96. [DOI] [PubMed] [Google Scholar]
- 38.Sharara A I, Perkins D J, Misukonis M A, Chan S U, Dominitz J A, Weinberg J B. Interferon (IFN)-alpha activation of human blood mononuclear cells in vitro and in vivo for nitric oxide synthase (NOS) type 2 mRNA and protein expression—possible relationship of induced NOS2 to the anti-hepatitis C effects of IFN-alpha in vivo. J Exp Med. 1997;186:1495–1502. doi: 10.1084/jem.186.9.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sherry B A, Alava G, Tracey K J, Martiney J, Cerami A, Slater A F. Malaria-specific metabolite hemozoin mediates the release of several potent endogenous pyrogens (TNF, MIP-1alpha, and MIP-1beta) in vitro, and altered thermoregulation in vivo. J Inflamm. 1995;45:85–96. [PubMed] [Google Scholar]
- 40.Stevenson M, Tam M. Differential induction of helper T cell subsets during blood-stage Plasmodium chabaudi AS infection in resistant and susceptible mice. Clin Exp Immunol. 1993;92:77–83. doi: 10.1111/j.1365-2249.1993.tb05951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stevenson M M, Tam M F, Wolf S F, Sher A. IL-12-induced protection against blood-stage Plasmodium chabaudi AS requires IFN-gamma and TNF-alpha and occurs via a nitric oxide-dependent mechanism. J Immunol. 1995;155:2545–2556. [PubMed] [Google Scholar]
- 42.Storkus W J, Tahara H, Lotze M T. Interleukin-12. In: Thomson A W, editor. The cytokine handbook. 3rd ed. San Diego, Calif: Academic Press Inc.; 1998. pp. 391–425. [Google Scholar]
- 43.Trinchieri G. IL-12 and its role in the generation of Th1 cells. Immunol Today. 1993;14:335. doi: 10.1016/0167-5699(93)90230-I. [DOI] [PubMed] [Google Scholar]
- 44.Wildling E, Winkler S, Kremsner P G, Brandts C, Jenne L, Wernsdorfer W H. Malaria epidemiology in the province of Moyen Ogooue, Gabon. Trop Med Parasitol. 1995;46:77–82. [PubMed] [Google Scholar]
- 45.Winkler S, Willheim M, Baier K, Schmid D, Aichelburg A, Graninger W, Kremsner P G. Frequency of cytokine-producing T cells in patients of different age groups with Plasmodium falciparum malaria. J Infect Dis. 1999;179:209–216. doi: 10.1086/314571. [DOI] [PubMed] [Google Scholar]
- 46.World Health Organization. World malaria situation in 1994. Weekly Epidemiol Rec. 1997;72:269–276. [Google Scholar]
- 47.Yoshimoto T, Yoneto T, Waki S, Nariuchi H. Interleukin-12-dependent mechanisms in the clearance of blood-stage murine malaria parasite Plasmodium berghei XAT, an attenuated variant of P. berghei NK65. J Infect Dis. 1998;177:1674–1681. doi: 10.1086/515301. [DOI] [PubMed] [Google Scholar]