Abstract
This study was conducted to estimate the effects of exogenous protease on performance, economic evaluation, nutrient digestibility, fecal score, intestinal morphology, blood profile, carcass traits, and meat quality in broilers fed normal diets and diets considered with matrix value. A total of 90, one-day-old Arbor Acres broiler chickens were randomly allocated to 3 dietary treatments with 6 replicates and each replicate of 5 broiler chickens. Treatments were as follows: 1) Basal diet (positive control, PC), 2) Basal diet formulated with full ProAct 360 matrix at 50 g/MT without addition of ProAct 360 (negative control, NC), 3) NC + 50 g/MT ProAct 360 (PA). Supplementation of exogenous protease to nutrient deficient NC diet by matrix values (PA) tended to increase growth performance and significantly improved intestinal morphology compared with the NC group. The PA group had significantly lower fecal score, and higher ATTD of crude protein and amino acids than those of the NC group. Furthermore, supplementation of exogenous protease to NC diet decreased feed cost, resulting in improved profit margin. However, there was no significant difference on carcass yield and relative organ weight. In conclusion, supplementation of exogenous protease using matrix value could be used as economic additive to improve growth, profit margin, digestibility, and gut health in broiler chickens.
Key words: broiler chicken, amino acid digestibility, protein digestibility, feed cost saving, growth performance
INTRODUCTION
Globally, feed consumed by poultry contributes nearly half (463 million metric tons; about 47%) of total feed production annually (Alqaisi et al., 2017; Mottet and Tempio, 2017). Especially, as the poultry industry continues to grow rapidly, availability of feedstuffs starts to be gradually limited, thereby increasing the prices of feed ingredients in recent years (Qi et al., 2022). In addition to higher demand from developing countries and competition with biofuel energy production, global feed prices are sustainably increased (Leroy et al., 2016).
It was estimated that feed costs constitute up to 69%, and the total cost of protein ingredients is about 65 to 70% of the total feed costs in intensive poultry production systems (Mallick et al., 2020). Also, protein ingredients are related to environmental pollution due to nitrogen excretion and ammonia volatilization (Attia et al., 2020). Thus, many researchers have been studying to increase amino acid utilization of protein sources and alleviate negative effects on the environment. The exogenous enzyme is one of the best ways to improve the bioavailability of nutrients in ingredients and eliminate antinutritional factors (Velázquez-De Lucio et al., 2021). Actually, the supplementation of enzymes increased growth performance and crude protein digestion in broiler chickens (Gracia et al., 2003; Moftakharzadeh et al., 2017; Saleh et al., 2019).
In particular, the supplementation of exogenous proteases is one of the effective ways to overcome the above-mentioned problems. Previous research reported that the addition of protease improved protein digestibility and reduced nitrogen excretion, thereby increasing growth performance and reducing environmental impact (Canogullarİ et al., 2009; Vieira et al., 2013; Ndazigaruye et al., 2019; Siegert et al., 2019).
Matrix value which is associated with commercial enzyme represents the amount of nutrients that could be released when the enzyme is supplemented to diets. According to the results of Bedford and Cowieson (2020), a diet containing nonstarch polysaccharide degrading enzyme (NSPase) might boost the energy level of the diet by 100 kcal/kg. If it is only contained at 100 mg/kg, dietary energy density is increased by 100,000 kcal/kg, which is 10 times more than that of fat. For phytase, the amount of calcium (Ca) and available phosphorus (Ave P) increased about 0.09 to 0.10% when phytase was added to diets (Denbow et al., 1995; Mitchell and Edwards, 1996). Consequently, the use of exogenous enzyme in diets could be more economical ways to consider the matrix value of protease by reducing the usage of feed. However, previous studies have been conducted to investigate the effects of enzyme supplementation, including protease on growth performance and nutrient digestibility in broiler chickens by adding enzymes over on top of diets without any consideration of matrix values for the enzymes (Ndazigaruye et al., 2019).
Therefore, we conducted to investigate the effects of exogenous protease on performance, economic evaluation, nutrient digestibility, fecal score, intestinal morphology, blood profile, and meat quality in broilers fed normal diets and diets with consideration of matrix values.
MATERIALS AND MEHODS
Ethics
The experimental protocols describing the management and care of animals were reviewed and approved by the Animal Care and Use Committee of Chungbuk National University (CBNUA-174-22-02).
Preparation of Tested Protease
The protease used in this study was a novel subtilisin protease produced by Bacillus licheniformis (Cupi et al., 2022). This is a sfericase protease, an endopeptidase from the serine protease family, subtilisin subfamily A, MEROPS ID S08.113 (Rawlings et al., 2014). Activity for this protease is defined in New Feed Protease (NFP) units, which measures the enzyme amount required to hydrolyze 1 µmol of para-nitroaniline (pNA) from 1 M 128 substrate Suc-Ala-Ala-Pro-Phe-pNA (Cupi et al., 2022). Enzyme activity in the present study was conducted in an Infinite M200 Pro microtiter plate reader (Tecan Lifesciences, Männedorf, Switzerland) with the amount of released yellow pNA being proportional to the protease activity of the enzyme measured photometrically at a wavelength of 405 nm. The utilized test product was granulated and had 600,000 NFP per g (ProAct 360, DSM Nutritional Products AG, Kaiseraugst, Switzerland).
Nutrient matrix values, including the amount of metabolizable energy (ME) and the percentage of crude protein (CP), lysine, threonine, tryptophan, arginine, valine, iso-leucine, methionine, and cysteine that are anticipated to be released from diets by the action of protease, were used for this study. Matrix values of protease used in this study are presented in Table 1.
Table 1.
Nutrient matrix values of exogenous protease (ProAct 360).
| Items | ProAct 360 Matrix at 50 g/MT inclusion | Amount provided in the diets |
|---|---|---|
| Metabolic energy, kcal/kg | 500000 | 25.000 |
| Crude protein, % | 14423 | 0.721 |
| Available methionine, % | 330 | 0.017 |
| Available methionine + cysteine, % | 955 | 0.048 |
| Available lysine, % | 946 | 0.047 |
| Available threonine, % | 986 | 0.049 |
| Available tryptophan, % | 97 | 0.005 |
| Available arginine, % | 867 | 0.043 |
| Available valine, % | 752 | 0.038 |
| Available iso-leucine, % | 568 | 0.028 |
Experimental Animal and Design
A total of 90, one-day-old Arbor Acres broiler chickens (Cherrybro Co., Eumseong, South Korea) with initial body weight 46.67 ± 7.04 g were placed in 18 cages (100 cm width, 40 cm depth and 45-cm height). The 18 cages were randomly assigned to 3 dietary treatments with 6 replicates of 5 broiler chickens. Treatments were as follows: 1) Basal diet (positive control, PC), 2) Basal diet formulated with full ProAct 360 matrix at 50 g/MT but without addition of ProAct 360 (negative control, NC), 3) NC + 50 g/MT ProAct 360 (PA). PC and PA diets were formulated to meet or exceed National Research Council, and NC diets were formulated to be lower than requirement because matrix value of exogenous protease was considered (NRC, 1994; Table 2, Table 3, Table 4). All broiler chickens were allowed to consume feed and water ad libitum. Each cage was equipped with 2 nipple drinkers connected to a common water supply line. The experiment period was divided into 3 phases: starter phase (0–7 d of age), grower phase (8–21 d of age), and finishing phase (22–33 d of age). The lighting schedule was 23L:1D at 100 lux on d 1, 12L:12D at 30 lux on d 4 until wk 2, and 8L:16D at 30 lux thereafter. The experiment initiation temperature was 33 ± 1°C, and the temperature was lowered by 2°C every week to maintain 24°C at the end of the experiment.
Table 2.
Ingredient composition of experimental diets (Starter).1
| Ingredients, % | Starter (d 0–7) |
||
|---|---|---|---|
| PC | NC | PA | |
| Corn | 40.646 | 41.453 | 41.453 |
| Soybean meal (CP 45%) | 33.263 | 33.150 | 33.150 |
| Wheat | 10.000 | 10.000 | 10.000 |
| DDGS 28% | 4.000 | 4.000 | 4.000 |
| Tankage ML-60 | 3.000 | 3.000 | 3.000 |
| MBM 50% | 1.800 | 1.800 | 1.800 |
| Wheat flour | 2.000 | 2.000 | 2.000 |
| Poultry oil | 2.633 | 2.118 | 2.118 |
| L-lysine-SO4 | 0.501 | 0.417 | 0.417 |
| DL-methionine | 0.418 | 0.368 | 0.368 |
| L-threonine | 0.141 | 0.090 | 0.090 |
| L-tryptophan | 0.010 | 0.010 | 0.010 |
| Salt | 0.224 | 0.224 | 0.224 |
| Limestone | 0.445 | 0.445 | 0.445 |
| Mono-dicalcium phosphate | 0.435 | 0.435 | 0.435 |
| Mineral premix2 | 0.220 | 0.220 | 0.220 |
| Vitamin premix3 | 0.150 | 0.150 | 0.150 |
| Choline | 0.100 | 0.100 | 0.100 |
| Phytase1000 (HiPhos 10000 GT) |
0.010 | 0.010 | 0.010 |
| Xylanase (WX 2000) | 0.005 | 0.005 | 0.005 |
| Sand | - | 0.005 | - |
| ProAct 360 | - | - | 0.005 |
| Total | 100.00 | 100.00 | 100.00 |
| Calculated value (%) | |||
| Metabolic energy (ME), kcal/kg | 3000 | 2975 | 3000 |
| Crude protein (CP) | 22.000 | 21.279 | 22.000 |
| Available lysine | 1.310 | 1.263 | 1.310 |
| Available methionine | 0.570 | 0.553 | 0.570 |
| Available threonine | 0.850 | 0.801 | 0.850 |
| Available tryptophan | 0.210 | 0.205 | 0.210 |
| Available SAA | 0.990 | 0.942 | 0.990 |
| Available arginine | 1.510 | 1.467 | 1.510 |
| Available isoleucine | 0.970 | 0.942 | 0.970 |
| Available valine | 1.120 | 1.082 | 1.120 |
| Calcium (Ca) | 0.850 | 0.850 | 0.850 |
| Available P | 0.480 | 0.480 | 0.480 |
| Na | 0.150 | 0.150 | 0.150 |
PC, basal diet; NC, basal diet formulated with full ProAct 360 matrix value at 50 g/MT but without addition of ProAct 360; PA, NC + 50 g/MT ProAct 360.
Provided per kg of diet: 37.5 mg Zn (as ZnSO4), 37.5 mg of Mn (MnO2), 37.5 mg of Fe (as FeSO4·7H2O), 3.75 mg of Cu (as CuSO4·5H2O), 0.83 mg of I (as KI), and 0.23 mg of Se (as Na2SeO3·5H2O).
Provided per kg of diet: 15,000 IU of vitamin A, 3,750 IU of vitamin D3, 37.5 mg of vitamin E, 2.55 mg of vitamin K3, 3 mg of thiamin, 7.5 mg of riboflavin, 4.5 mg of vitamin B6, 24 μg of vitamin B12, 51 mg of niacin, 1.5 mg of folic acid, 0.2 mg of biotin, and 13.5 mg of pantothenic acid.
Table 3.
Ingredient composition of experimental diets (Grower).1
| Ingredients, % | Grower (d 8–21) |
||
|---|---|---|---|
| PC | NC | PA | |
| Corn | 47.357 | 47.552 | 47.552 |
| Soybean meal (CP 45%) | 26.750 | 26.737 | 26.737 |
| Wheat | 10.000 | 10.000 | 10.000 |
| DDGS 28% | 5.000 | 5.000 | 5.000 |
| Tankage ML-60 | 3.000 | 3.000 | 3.000 |
| MBM 50% | 1.700 | 1.700 | 1.700 |
| Wheat flour | 2.000 | 2.000 | 2.000 |
| Poultry oil | 1.700 | 1.700 | 1.700 |
| L-lysine-SO4 | 0.510 | 0.424 | 0.424 |
| DL-methionine | 0.367 | 0.317 | 0.317 |
| L-threonine | 0.141 | 0.090 | 0.090 |
| L-tryptophan | 0.010 | 0.010 | 0.010 |
| Salt | 0.250 | 0.250 | 0.250 |
| Limestone | 0.450 | 0.450 | 0.450 |
| Mono-dicalcium phosphate | 0.300 | 0.300 | 0.300 |
| Mineral premix2 | 0.220 | 0.220 | 0.220 |
| Vitamin premix3 | 0.130 | 0.130 | 0.130 |
| Choline | 0.100 | 0.100 | 0.100 |
| Phytase1000 (HiPhos 10000 GT) |
0.010 | 0.010 | 0.010 |
| Xylanase (WX 2000) | 0.005 | 0.005 | 0.005 |
| Sand | - | 0.005 | - |
| ProAct 360 | - | - | 0.005 |
| Total | 100.00 | 100.00 | 100.00 |
| Calculated value (%) | |||
| Metabolic energy (ME), kcal/kg | 3020 | 2995 | 3020 |
| Crude protein (CP) | 20.500 | 19.779 | 20.500 |
| Available lysine | 1.170 | 1.123 | 1.170 |
| Available methionine | 0.540 | 0.523 | 0.540 |
| Available threonine | 0.770 | 0.721 | 0.770 |
| Available tryptophan | 0.190 | 0.185 | 0.190 |
| Available SAA | 0.900 | 0.852 | 0.900 |
| Arginine | 1.350 | 1.307 | 1.350 |
| Isoleucine | 0.880 | 0.852 | 0.880 |
| Valine | 1.010 | 0.972 | 1.010 |
| Calcium (Ca) | 0.800 | 0.800 | 0.800 |
| Available P | 0.450 | 0.450 | 0.450 |
| Na | 0.150 | 0.150 | 0.150 |
PC, basal diet; NC, basal diet formulated with full ProAct 360 matrix value at 50 g/MT but without addition of ProAct 360; PA, NC + 50 g/MT ProAct 360.
Provided per kg of diet: 37.5 mg Zn (as ZnSO4), 37.5 mg of Mn (MnO2), 37.5 mg of Fe (as FeSO4·7H2O), 3.75 mg of Cu (as CuSO4·5H2O), 0.83 mg of I (as KI), and 0.23 mg of Se (as Na2SeO3·5H2O).
Provided per kg of diet: 15,000 IU of vitamin A, 3,750 IU of vitamin D3, 37.5 mg of vitamin E, 2.55 mg of vitamin K3, 3 mg of thiamin, 7.5 mg of riboflavin, 4.5 mg of vitamin B6, 24 μg of vitamin B12, 51 mg of niacin, 1.5 mg of folic acid, 0.2 mg of biotin, and 13.5 mg of pantothenic acid.
Table 4.
Ingredient composition of experimental diets (Finisher).1
| Ingredients, % | Finisher (d 22–33) |
||
|---|---|---|---|
| PC | NC | PA | |
| Corn | 53.293 | 53.670 | 53.670 |
| Soybean meal (CP 45%) | 23.675 | 23.400 | 23.400 |
| Wheat | 10.000 | 10.000 | 10.000 |
| DDGS 28% | 3.000 | 3.000 | 3.000 |
| Tankage ML-60 | 1.900 | 1.900 | 1.900 |
| MBM 50% | 1.700 | 1.700 | 1.700 |
| Wheat flour | 2.000 | 2.000 | 2.000 |
| Poultry oil | 2.108 | 2.100 | 2.100 |
| L-lysine-SO4 | 0.392 | 0.316 | 0.316 |
| DL-methionine | 0.411 | 0.364 | 0.364 |
| L-threonine | 0.105 | 0.100 | 0.100 |
| L-tryptophan | 0.100 | 0.100 | 0.100 |
| Salt | 0.234 | 0.250 | 0.250 |
| Limestone | 0.438 | 0.450 | 0.450 |
| Mono-dicalcium phosphate | 0.200 | 0.200 | 0.200 |
| Mineral premix2 | 0.220 | 0.220 | 0.220 |
| Vitamin premix3 | 0.130 | 0.130 | 0.130 |
| Choline | 0.080 | 0.080 | 0.080 |
| Phytase1000 (HiPhos 10000 GT) |
0.010 | 0.010 | 0.010 |
| Xylanase (WX 2000) | 0.005 | 0.005 | 0.005 |
| Sand | - | 0.005 | - |
| ProAct 360 | - | - | 0.005 |
| Total | 100 | 100 | 100 |
| Calculated value (%) | |||
| Metabolic energy (ME), kcal/kg | 3100 | 3075 | 3100 |
| Crude protein (CP) | 19.000 | 18.279 | 19.000 |
| Available lysine | 1.010 | 0.963 | 1.010 |
| Available methionine | 0.560 | 0.543 | 0.560 |
| Available threonine | 0.680 | 0.631 | 0.680 |
| Available tryptophan | 0.160 | 0.155 | 0.160 |
| Available SAA | 0.900 | 0.852 | 0.900 |
| Arginine | 1.160 | 1.117 | 1.160 |
| Isoleucine | 0.780 | 0.752 | 0.780 |
| Valine | 0.890 | 0.852 | 0.890 |
| Calcium (Ca) | 0.700 | 0.700 | 0.700 |
| Available P | 0.400 | 0.400 | 0.400 |
| Na | 0.150 | 0.150 | 0.150 |
PC, basal diet; NC, basal diet formulated with full ProAct 360 matrix value at 50 g/MT but without addition of ProAct 360; PA, NC + 50 g/MT ProAct 360.
Provided per kg of diet: 37.5 mg Zn (as ZnSO4), 37.5 mg of Mn (MnO2), 37.5 mg of Fe (as FeSO4·7H2O), 3.75 mg of Cu (as CuSO4·5H2O), 0.83 mg of I (as KI), and 0.23 mg of Se (as Na2SeO3·5H2O).
Provided per kg of diet: 15,000 IU of vitamin A, 3,750 IU of vitamin D3, 37.5 mg of vitamin E, 2.55 mg of vitamin K3, 3 mg of thiamin, 7.5 mg of riboflavin, 4.5 mg of vitamin B6, 24 μg of vitamin B12, 51 mg of niacin, 1.5 mg of folic acid, 0.2 mg of biotin, and 13.5 mg of pantothenic acid.
Growth Performance
On 7, 21, and 33 d, all birds and leftover feed in the cages were weighed at each time point to determine the body weight (BW), body weight gain (BWG), feed intake (FI), and feed conversion ratio (FCR). The BWG was calculated as the BW of the previous time point was subtracted from the BW of the current time point. FI was calculated by subtracting the remaining feed amount from the initial feed amount, and FCR was calculated by dividing FI by BWG.
Economic Evaluation
Based on the data obtained from each treatment group, economic evaluation was determined. The indicators of economic evaluation were categorized as total income costs and total deductible costs. The total income cost which means meat cost was obtained in KAPE (Korea Institute for Animal Products Quality Evaluation, Sejong, South Korea) and prices were expressed in dollars (1.56 US$/kg). Briefly, the total income cost is represented by: total income = final body weight (kg) × 1.56 US$/kg. The deductible cost means feed cost which was expended for broilers and this cost was obtained in feed company (Cherrybro Co., Eumseong, South Korea). The feed cost per treatments were as follows: Starter, PC: 0.56 US$/kg; NC: 0.54 US$/kg; PA: 0.52 US$/kg; Grower, PC: 0.55 US$/kg; NC: 0.53 US$/kg; PA: 0.52 US$/kg; Finisher, PC: 0.56 US$/kg; NC: 0.54 US$/kg; PA: 0.52 US$/kg. Briefly, the deductible cost is represented by: total income = overall feed intake (kg) × above mentioned feed cost per treatments. Profitability which means net cost is represented by: profitability = total income cost – total deductible cost. Other deductible costs included initial chick cost, fuel cost, etc., but they were excluded from this evaluation because they were commonly charged regardless of treatments.
Fecal Score
The fecal score was determined following the fecal quality scores of Garcia et al. (2019). Criteria of fecal score were as follow: Score 1: dry and firmed feces with characteristic white uric acid cover; Score 2: mostly dry feces with white uric acid cover; Score 3: moist feces with white uric acid cover; Score 4: wet feces with less white uric acid cover and droppings lose their shape and Score 5: extremely wet feces with little to no white uric acid cover. Fecal assessment in each replicate was conducted through visual fecal scoring by 2 independent evaluators. To avoid erroneous evaluation, evaluators removed previous feces on the fecal plate and then placed white paper on the plates. They assessed first feces on the white paper according to above mentioned criteria in the morning and calculated as the average fecal score for each period (0–7 d; 8–21 d; 22–33 d; overall period, 0–33 d) per treatment by summing the average daily fecal scores of each cage.
Nutrient Digestibility
Broiler chickens were euthanized by cervical dislocation. Diets and feces were analyzed for dry matter (DM), crude protein (CP), and energy using AOAC methods (AOAC, 2000). Determination of the apparent digestibility of nutrients was performed by collecting both total digesta. All experimental diets were mixed with 0.2% Cr2O3 before collecting digesta. The rectum digesta were collected from rectum 3cm before the cloaca to collect fecal without urine. The digesta were gently squeezed, rinsed with saline, and collected in plastic pillboxes. All feed and rectum samples were finely ground after drying for 72 h at 50°C oven and analyzed for DM, CP, energy, and amino acid (AA). The energy was determined using a calorimeter (model 1261, Parr Instrument Company, Moline, IL). Analyses of DM and CP were made according to the methodology described in AOAC methods (AOAC, 2000) and analysis of AA made in high-performance liquid chromatography (HPLC) (SHIMADZU, Model LC-10AT, Shimadzu Corp., Kyoto, Japan) methodology. The apparent total tract digestibility (ATTD) percentage was calculated using the following equation. ATTD% = 100 – [100 × (Cr2O3 in diet/Cr2O3 in rectum digesta) × (nutrient in rectum digesta/nutrient in diet)].
Blood Profiles
On d 33, blood samples (2 mL each) were collected from wing vein of broilers (1 broiler per each cage) using vacuum tubes containing K3EDTA (Becton, Dickinson and Co., Franklin Lakes, NJ), and serum was harvested and stored in a deep freezer at −20°C for further analyses. Concentrations of total protein, blood urea nitrogen (BUN), and creatinine were determined using an UV-visual spectrophotometer (Microlab 200: Merck Laboratory Analyzer, New Delhi, India) with commercial kits (Prism Diagnostic Pvt. Ltd., Mumbai, India).
Ileal Morphology
On d 33, 1 bird per each cage was randomly selected and sacrificed at the end of the experiment to collect ileal tissue samples. A 15 cm ileal segment adjacent to the pyloric valve was freed of mesenteric attachments and rinsed clean with 10% neutral buffered formalin. The intestinal segment was submerged in approximately 20 mL of 10% neutral buffered formalin for 24 h. Slides of intestinal cross-sections (5-µm thick) were processed in low-melt paraffin and stained with hematoxylin and eosin. The stained slides were scanned by a fluorescence microscope (TE2000, Nikon, Tokyo, Japan) with a charge-coupled device camera (DS-Fi1; Nikon, Tokyo, Japan) to measure intestinal morphology. The villus height (VH) was measured from the tip of the villus to the crypt orifice. Crypt depth (CD) was measured from the junction of the villus to the crypt base. And then, the VH-to-CD ratio (VH:CD) was calculated.
Carcass Trait and Relative Organ Weight
One bird per each cage were euthanized on 33 d of age by an intravenous injection of pentobarbital, with cervical dislocation to confirm death. After broilers were euthanized, the abdomen area was opened to excise and weigh the carcass, gastrointestinal tract (gizzard, stomach, duodenum, jejunum, and ileum), liver, spleen, and heart. Carcass yields were calculated relative to the live BW. The weights of the gastrointestinal tract and other organs were recorded and then calculated using the following formula: Relative organ weight (g/kg) = organ weight (g)/live BW (kg).
Meat Quality Traits
At the end of the experiment, 1 bird per each cage was selected to collect breast meat that was cut out and analyzed after freeze-drying. Moisture, protein, fat, and ash were analyzed according to the AOAC methods (AOAC, 1995). Ten grams of sample added with 100 mL of distilled water were homogenized for 30 s using a blender (400 Lab Blender, Seward, UK). The pH levels of the samples were measured using a pH-meter (pH 720, WTW Laboratory Instrument, Germany). The centrifugation method described by Laakkonen et al. (1970) was used to measure water holding capacity (WHC). The breast meat samples (0.5 ± 0.05 g) from each treatment were placed in a centrifugation tube with filter units, heated for 20 min at 80°C, and then cooled for 10 min. The samples were centrifuged at 2,000 rpm for 10 min 4°C, and WHC values were calculated as the change in sample weight. The drip loss (DL) and cooking loss (CL) were measured using the modified method of Sirri et al. (2017). After the product was weighed, it was stored in a refrigerator (JEIO THCH. Co. Ltd., Daejeon, South Korea) for 24 h at 4°C. The sample was then removed from the refrigerator, removed from its package, and blotted with Kimwipes (Yuhan-Kimberly, Seoul, South Korea) to eliminate any liquid from the surface of the sample. The weight of the sample was then recorded one more, and the drip loss was calculated as a weight percentage. A breast meat was placed into a polypropylene bag, cooked for 40 min at 70°C in a water-bath, and cooled down to room temperature for 30 min. CL was calculated by the weight difference of samples before and after cooking.
Statistical Analysis
All data of growth performance, economic evaluation, average fecal score, nutrient digestibility, blood profiles, carcass trait, ileal morphology, and meat quality were statistically analyzed by executing one-way analysis of variance (ANOVA) using JMP 16.0 (SAS Institute Inc., Cary, NC) with post hoc analysis Tukey's honestly significant difference (HSD) test. The incidence of fecal score was statistically analyzed using Pearson's chi-squared test. Variability in the data was expressed as the pooled standard error, P < 0.05 was considered statistically significant, and 0.05 < P < 0.10 was considered statistically tendency.
RESULTS
Growth Performance
The effects of dietary treatment on body weight, body weight gain, feed intake and feed conversion ratio are presented in Table 5. There was no significant difference (P > 0.05) on BW, BWG, FI, and FCR in each period among the treatments. NC group had numerically lower BW at 7, 21, and 33 d than PC and PA group. Moreover, NC group numerically decreased BWG, FI, and increased FCR compared to PC and PA group at 0 to 7, 8 to 21, 22 to 33, and 0 to 33 d. On the other hand, the supplementation of exogenous protease to NC diets (PA) numerically improved BW, BWG, and FCR in each period compared to NC group and reached to similar levels as those of PC groups.
Table 5.
Effect of supplementing exogenous protease (ProAct 360) on growth performance in broiler chickens.
| Items | PC | NC | PA | SE | P value |
|---|---|---|---|---|---|
| BW, g | |||||
| Initial BW | 46.67 | 46.67 | 46.67 | 0.406 | 1.000 |
| 7 d | 178.83 | 173.33 | 178.17 | 2.771 | 0.336 |
| 21 d | 1055.03 | 1040.37 | 1052.60 | 14.436 | 0.748 |
| 33 d | 1989.50 | 1910.67 | 1999.33 | 34.257 | 0.168 |
| BWG, g | |||||
| 0–7 d | 132.17 | 126.66 | 131.50 | 2.852 | 0.355 |
| 8–21 d | 876.20 | 867.03 | 874.43 | 13.804 | 0.884 |
| 22–33 d | 934.47 | 870.30 | 946.73 | 25.124 | 0.102 |
| 1–33 d | 1942.84 | 1863.99 | 1952.66 | 34.465 | 0.171 |
| FI, g | |||||
| 0–7 d | 158.17 | 157.57 | 154.93 | 2.199 | 0.555 |
| 8–21 d | 1292.00 | 1280.70 | 1262.63 | 19.065 | 0.560 |
| 22–33 d | 1763.50 | 1748.35 | 1759.00 | 29.311 | 0.932 |
| 1–33 d | 3213.67 | 3186.61 | 3176.57 | 30.203 | 0.675 |
| FCR, g/g | |||||
| 0–7 d | 1.21 | 1.26 | 1.19 | 0.027 | 0.262 |
| 8–21 d | 1.48 | 1.49 | 1.45 | 0.033 | 0.722 |
| 22–33 d | 1.90 | 2.07 | 1.87 | 0.077 | 0.164 |
| 0–33 d | 1.66 | 1.73 | 1.63 | 0.034 | 0.169 |
Abbreviations: BW, body weight; BWG, body weight gain; FCR, feed conversion ratio; FI, feed intake; NC, basal diet formulated with full ProAct 360 matrix value at 50 g/MT but without addition of ProAct 360; PA, NC + 50 g/MT ProAct 360; PC, basal diet; SE, standard error. Each value is the mean value of 6 replicates (5 broiler/cage).
Economic Evaluation
The effects of dietary treatment on economic evaluation are presented in Table 6. There was no significant difference (P > 0.05) on total income cost (meat cost) among the treatments. However, NC and PA groups had lower (P < 0.001) total deductible cost (feed cost) than that of the PC group. Moreover, the supplementation of exogenous protease to NC diets (PA) tended (P = 0.079) to improve the profitability compared to PC and NC groups.
Table 6.
Effect of supplementing exogenous protease (ProAct 360) on economic evaluation in broiler chickens.
| Items, $ per bird | PC | NC | PA | SE | P value |
|---|---|---|---|---|---|
| Total income cost1 | |||||
| Meat cost | 3.10 | 2.98 | 3.12 | 0.053 | 0.168 |
| Total deductible cost2 | |||||
| Feed cost | 1.78a | 1.66b | 1.70b | 0.016 | <0.001 |
| Profit margins | |||||
| (Total income cost − Total deductible cost) | 1.33 | 1.32 | 1.40 | 0.054 | 0.079 |
Abbreviations: NC, Basal diet formulated with full ProAct 360 matrix value at 50 g/MT but without addition of ProAct 360; PA, NC + 50 g/MT ProAct 360; PC, basal diet; SE, standard error.
The total income cost consisted of the meat costs obtained from multiplying the average weight (kg) and the unit cost (1.56 $/kg).
Starter, PC: 0.56 US$/kg; NC: 0.54 US$/kg; PA: 0.52 US$/kg; Grower, PC: 0.55 US$/kg; NC: 0.53 US$/kg; PA: 0.52 US$/kg; Finisher, PC: 0.56 US$/kg; NC: 0.54 US$/kg; PA: 0.52 US$/kg. Each value is the mean value of 6 replicates (5 broiler/cage).
Means in the same row with different superscripts differ (P < 0.05).
Fecal Score
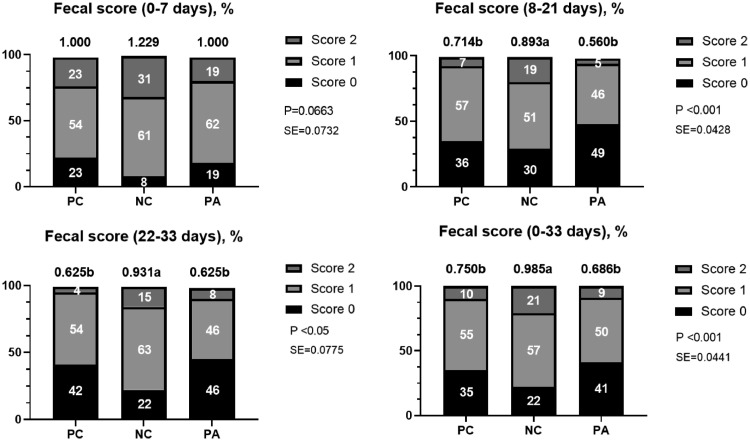
The effects of dietary treatment on fecal score are presented in Figure 1. NC group had significantly higher (P < 0.05) incidence of fecal scores than other treatments and tended (P = 0.0663) to have increased average fecal scores in 0–7 d. Also, the NC group had significantly higher (P < 0.05) incidence of fecal score and increased average fecal score (P < 0.001; P < 0.05; P < 0.001) compared with the other groups in 8 to 21 d, 22 to 33 d, and 0 to 33 d, respectively. Supplementation of exogenous protease to NC diets (PA) alleviated (P < 0.05) high incidence fecal scores and average fecal score by NC diets, and decreased (P < 0.05) high scores and average fecal score to similar levels as those of PC groups in 8 to 21 d, 22 to 33 d, and 0 to 33 d, respectively.
Figure 1.
Effect of supplementing exogenous protease (ProAct 360) on fecal score in broiler chickens. Abbreviations: NC, basal diet formulated with full ProAct 360 matrix value at 50 g/MT but without addition of ProAct 360; PA, NC + 50 g/MT ProAct 360; PC, basal diet; SE, standard error. Fecal score was determined as follow: Score 0: normal feces; Score 1: moist feces; Score 2: watery feces. Each value is the mean value of 6 replicates (5 broiler/cage). abMeans in the same row with different superscripts differ (P < 0.05).
Nutrient Digestibility
The effects of dietary treatment on nutrient digestibility are presented in Table 7. There was no significant difference (P > 0.05) on ATTD of dry matter and energy among the treatments. However, the supplementation of exogenous protease to NC diet (PA) improved (P < 0.05) ATTD of crude protein compared to NC group and there was no significant difference from PC groups.
Table 7.
Effect of supplementing exogenous protease (ProAct 360) on nutrient digestibility in broiler chickens.
| Items | PC | NC | PA | SE | P value |
|---|---|---|---|---|---|
| Dry matter | 80.74 | 80.31 | 81.43 | 0.558 | 0.384 |
| Energy | 79.42 | 78.84 | 80.12 | 0.507 | 0.231 |
| Crude protein | 74.26ab | 72.55b | 75.69a | 0.749 | 0.031 |
| Essential amino acids | |||||
| Arginine | 90.44a | 89.46b | 90.49a | 0.261 | 0.023 |
| Histidine | 82.02 | 81.97 | 83.40 | 0.512 | 0.115 |
| Iso-leucine | 80.78b | 81.04b | 85.76a | 0.474 | <0.001 |
| Leucine | 89.43 | 88.86 | 89.40 | 0.269 | 0.271 |
| Lysine | 87.38 | 86.70 | 87.72 | 0.290 | 0.068 |
| Methionine | 84.69b | 82.95b | 91.01a | 0.727 | <0.001 |
| Phenylalanine | 86.50b | 85.53c | 88.18a | 0.261 | <0.001 |
| Threonine | 81.92a | 80.26b | 83.09a | 0.433 | 0.001 |
| Valine | 78.13b | 79.43b | 83.99a | 0.553 | <0.001 |
| Tryptophan | 86.85 | 86.55 | 86.34 | 0.307 | 0.518 |
| Glycine | 75.82ab | 73.94b | 77.85a | 0.791 | 0.012 |
| Total | 84.61b | 83.85b | 86.35a | 0.324 | <0.001 |
| Nonessential amino acids | |||||
| Alanine | 79.99ab | 78.48b | 81.85a | 0.686 | 0.012 |
| Aspartic acid | 82.34ab | 81.10b | 82.91a | 0.408 | 0.020 |
| Cysteine | 75.03ab | 73.83b | 77.72a | 1.020 | 0.046 |
| Glutamic acid | 87.48b | 86.57b | 89.77a | 0.331 | <0.001 |
| Proline | 83.70 | 83.24 | 84.66 | 0.518 | 0.175 |
| Serine | 85.45 | 84.12 | 85.48 | 0.459 | 0.089 |
| Tyrosine | 87.71 | 86.69 | 86.45 | 0.416 | 0.110 |
| Total | 84.40b | 83.37b | 85.80a | 0.375 | 0.001 |
| Total amino acids | 84.51b | 83.61b | 86.08a | 0.342 | <0.001 |
Abbreviations: NC, basal diet formulated with full ProAct 360 matrix value at 50 g/MT but without addition of ProAct 360; PA, NC + 50 g/MT ProAct 360; PC, basal diet; SE, standard error.
Each value is the mean value of 6 replicates (5 broiler/cage).
Means in the same row with different superscripts differ (P < 0.05).
For ATTD of essential amino acids, there was no significant difference (P > 0.05) on ATTD of histidine, leucine, and tryptophan among the treatments. However, supplementation of exogenous protease to NC diets (PA) significantly increased (P < 0.05) ATTD of arginine, threonine and glycine compared to NC group. Moreover, PC group had higher (P < 0.05) ATTD of iso-leucine, methionine, phenylalanine, valine, and total essential amino acids than other treatments. A tendency (P = 0.068) was observed for ATTD of lysine as supplementing exogenous protease to NC diets increased ATTD of lysine compared to that of the NC group.
For ATTD of nonessential amino acids, there was no significant difference (P > 0.05) on ATTD of proline and tyrosine among the treatments. Supplementation of exogenous protease to NC diet (PA) improved (P < 0.05) ATTD of alanine, aspartic acid and cysteine compared to NC group. PC group had higher (P < 0.05) ATTD of glutamic acid and total nonessential amino acids than other treatments. In addition, the PA group had the highest (P < 0.05) ATTD of total amino acids compared with the other treatments.
Blood Profiles
The effects of dietary treatment on blood profile are presented in Table 8. There was no significant difference (P > 0.05) on total protein, BUN and creatine among treatments. However, NC group had numerically lower total protein and higher creatine in blood than other treatments, while supplementation of exogenous protease to NC diets (PA) numerically increased total protein and decreased creatinine and had similar values as those of PC groups.
Table 8.
Effect of Effect of supplementing exogenous protease (ProAct 360) on blood profiles in broiler chickens.
| Items | PC | NC | PA | SE | P value |
|---|---|---|---|---|---|
| Total protein, g/dL | 3.08 | 2.92 | 3.13 | 0.089 | 0.229 |
| BUN, mg/dL | 1.20 | 1.17 | 1.20 | 0.164 | 0.987 |
| Creatinine, mg/dL | 0.13 | 0.16 | 0.13 | 0.013 | 0.102 |
Abbreviations: BUN, blood urea nitrogen; NC, basal diet formulated with full ProAct 360 matrix value at 50 g/MT but without addition of ProAct 360; PA, NC + 50 g/MT ProAct 360; PC, basal diet; SE, standard error.
Each value is the mean value of 6 replicates (5 broiler/cage).
Ileal Morphology
The effects of dietary treatment on ileal morphology are presented in Table 9. Supplementation of exogenous protease to NC diets (PA) tended (P = 0.066) to increase the VH compared with that of the PC group. A significant difference (P < 0.05) was observed for crypt depth and VH:CD ratio as supplementing exogenous protease to NC diets (PA) decreased crypt depth and increased VH:CD ratio compared to that of the NC group. Moreover, supplementation exogenous protease to NC diets had similar values as those of PC groups.
Table 9.
Effect of supplementing exogenous protease (ProAct 360) on ileal morphology in broiler chickens.
| Items | PC | NC | PA | SE | P value |
|---|---|---|---|---|---|
| Villus height, μm | 879.60 | 929.82 | 994.40 | 31.825 | 0.066 |
| Crypt depth, μm | 93.31ab | 104.84a | 81.47b | 5.728 | 0.037 |
| VH:CD ratio | 10.05ab | 9.71b | 12.81a | 0.773 | 0.024 |
Abbreviations: NC, Basal diet formulated with full ProAct 360 matrix value at 50 g/MT but without addition of ProAct 360; PA, NC + 50 g/MT ProAct 360; PC, basal diet; SE, standard error; VH:CD, villus height: crypt depth ratio.
Each value is the mean value of 6 replicates (5 broiler/cage).
Means in the same row with different superscripts differ (P < 0.05).
Carcass Yield and Relative Organ Weights
The effects of dietary treatment on carcass yield and relative organ weight are presented in Table 10. There was no significant difference (P > 0.05) on carcass yield and relative organ weight among the treatments.
Table 10.
Effect of supplementing exogenous protease (ProAct 360) on carcass yield and relative organ weights in broiler chickens.
| Items | PC | NC | PA | SE | P value |
|---|---|---|---|---|---|
| Body weight, g | 1970.83 | 1965.00 | 1973.33 | 14.26 | 0.976 |
| Carcass yield, % | 72.60 | 72.73 | 72.92 | 0.324 | 0.789 |
| Relative organ weight, % | |||||
| Liver | 2.90 | 2.74 | 2.79 | 0.245 | 0.885 |
| Heart | 0.53 | 0.58 | 0.52 | 0.042 | 0.574 |
| Spleen | 0.10 | 0.08 | 0.09 | 0.007 | 0.417 |
| Gastrointestinal tract | 6.17 | 6.47 | 6.20 | 0.291 | 0.732 |
Abbreviations: NC, basal diet formulated with full ProAct 360 matrix value at 50 g/MT but without addition of ProAct 360; PA, NC + 50 g/MT ProAct 360; PC, basal diet; SE, standard error.
Each value is the mean value of 6 replicates (5 broiler/cage).
Meat Quality
The effects of dietary treatment on meat quality are presented in Table 11. For proximate composition, there was no significant difference (P > 0.05) on content of moisture, fat, and ash, but supplementation of exogenous protease to NC diet (PA) increased (P < 0.05) content of protein compared with that of the NC group. Moreover, supplementation of exogenous protease to NC diet (PA) alleviated (P < 0.05) reduced water holding capacity and pH produced by NC diet. PA diet did not affect (P > 0.05) water holding capacity but increased (P < 0.05) pH compared to PC group, respectively. The supplementation of exogenous protease to NC diet (PA) tended (P = 0.087) to alleviate drip loss compared with that of the NC group. There was no significant difference (P > 0.05) on meat color among treatments.
Table 11.
Effect of supplementing exogenous protease (ProAct 360) on meat quality in broiler chickens.
| Items | PC | NC | PA | SE | P value |
|---|---|---|---|---|---|
| Proximate composition, % | |||||
| Moisture | 74.27 | 74.72 | 74.42 | 0.324 | 0.623 |
| Protein | 21.78ab | 21.12b | 22.37a | 0.277 | 0.020 |
| Fat | 3.28 | 2.71 | 2.28 | 0.629 | 0.535 |
| Ash | 1.32 | 1.43 | 1.11 | 0.105 | 0.123 |
| Meat quality | |||||
| Water holding capacity, % | 70.59a | 67.78b | 70.02a | 0.567 | 0.008 |
| pH | 5.92b | 5.93b | 6.05a | 0.030 | 0.014 |
| Drip loss, % | 2.23 | 3.99 | 3.35 | 0.527 | 0.087 |
| Cooking loss, % | 12.20 | 12.47 | 11.58 | 0.356 | 0.228 |
| Meat color | |||||
| Lightness | 55.98 | 56.60 | 56.60 | 1.150 | 0.908 |
| Redness | 5.73 | 4.77 | 3.98 | 0.573 | 0.129 |
| Yellowness | 9.98 | 11.40 | 12.09 | 0.738 | 0.155 |
Abbreviations: NC, basal diet formulated with full ProAct 360 matrix value at 50 g/MT but without addition of ProAct 360; PA, NC + 50 g/MT ProAct 360; PC, Basal diet; SE, standard error. Each value is the mean value of 6 replicates (5 broiler/cage).
Means in the same row with different superscripts differ (P < 0.05).
DISCUSSION
In the current study, NC diets (low dietary CP, ME, and imbalance of amino acids) did not affect growth performance in each period and overall period. These results are in agreement with previous studies reporting that the growth performance of broilers fed 1 to 3% low CP diets was unaffected (Salah, 2016; Van Harn et al., 2019). On the other hands, 0.02 to 0.2% low essential amino acid such as lysine, methionine, tryptophan, threonine and arginine and/or 1.6% low ME in diets caused poor BWG and FCR compared to control diets (Lin Law et al., 2019; Maqsood et al., 2022). Moreover, supplementation of exogenous protease to NC diets did not significantly affect growth performance, but numerically improved growth performance including BWG and FCR compared with those of NC groups in the current study. Results of the current study are partially agreement with the finding of numerous studies that protease improved growth performance in broiler chickens (Odetallah et al., 2005; Xu et al., 2017; McCafferty et al., 2022). This disagreement could be due to alternation of feed intake. Previous studies have shown that low protein and/or protease supplementation influenced BWG and FCR, and at the same time positively affected FI. In this study, this nonsignificant results in FI may be attributed to nonsignificant impact on BWG and FCR. Also, this discrepancy might be due to the difference of basal diets, used protease, and dosage of protease between this study and other studies. Other researchers asserted that increase of BWG and FCR could be attributed to better nutrient digestibility rather than FI (Rada et al., 2013; Lin Law et al., 2019; Maqsood et al., 2022).
In the current study, meat cost as total income cost was not affected by any treatments, whereas feed cost as the total deductible cost was lower in birds fed low dietary ME, CP, and amino acids (NC) and supplementation of exogenous protease to NC diet (PA). Moreover, the supplementation of exogenous protease to NC diets tended to improve profit margin. These results were consistent with the results of Rehman et al. (2017), reporting that the supplementation of protease to low protein and low amino acid diet led to higher profit margins in broilers. The variation in profit margin mainly occurred to the body weight, feed intake, feed cost, as well as livability of birds (Rehman et al., 2017). We did not observe any significant difference in total income cost because there was no significant difference on final body weight. However, we were able to reduce raw ingredient usages such as soybean meal and crystalline lysine by considering the matrix value of exogenous protease, thereby decreasing significantly total deductible cost. According to Banson et al. (2015), feed cost of total cost in poultry production accounts for about 70%. Especially, high feed cost is attributed to the using protein feed ingredient such as soybean meal. This improvement of profit margin in this study could be attributed to use of low protein ingredient such as soybean meal. Similar results were obtained in the studies of Onu et al. (2011) and Anuradha and Barun (2015), who reported a decrease in feed cost and an improvement in profit margin when supplementing dietary enzymes.
We did not observe the ATTD of dry matter and energy in the current study. However, NC diet lacking nutrients decreased the ATTD of CP and most amino acids, including essential and nonessential amino acids, whereas the supplementation of exogenous protease to NC diet (PA) improved the ATTD of CP and most amino acids. In consistent with the current study, supplementation of exogenous protease to diets increased the apparent AA digestibility of arginine, threonine, isoleucine, aspartate, lysine, serine, and cysteine (Angel et al., 2011; Shad et al., 2022). Soybean meal have been known to have substantial antinutritional factors, and thus it caused poor digestibility and growth performance (Erdaw et al., 2017). Conversely, exogenous protease supplementation could diminish negative effects of its antinutritional factors thereby resulting in beneficial effects such as improvement of AA utilization and growth (Tajudeen et al., 2022). Furthermore, other researchers have reported that surplus amino acids by an imbalance of dietary amino acids increased the excretion of amino acids (Selle et al., 2020a,b). Thus, reduction of CP and AAs digestibility in NC diet might be attributed to these antinutritional factors of soybean meal and imbalance of ingested amino acids. On the other hand, by supplementing exogenous protease to NC diets, we were able to meet the nutrient requirements and hydrolyze antinutritional factors such as lectins or trypsin inhibitors and thus improvement of digestibility may be attributed to above-mentioned action.
Broilers are not able to digest and absorb protein and amino acids present in their diets, and thus resulting in increased undigested protein into the ceca of broilers (Widyaratne and Drew, 2011; Moughan et al., 2014). Moreover, previous studies have shown that undigested protein accelerated the proliferation of pathogenic bacteria (e.g., E. coli and clostridia), leading to reduce fecal consistency and dry matter content of feces (Nollet et al., 1999; Pieper et al., 2012). In the present study, a nutrient-deficient NC diet (low protein and imbalance of amino acids) caused an increased incidence of the high fecal score and average fecal score, while the supplementation of exogenous protease alleviated fecal score. As mentioned above, the increased fecal score might be due to undigested nitrogen derived from an imbalance of dietary amino acids. The fecal score was also decreased when the broilers fed NC diet supplemented with exogenous protease. Although many factors such as CP level, raw ingredients, animal health, drinking system, and stocking density could affect the development of fecal score (Dunlop et al., 2016), improvement of these parameters in the current study might be contributed to the fact that addition of exogenous protease to the nutrient deficient diet resulted in minimal protein waste and improved nitrogen retention (Yu et al., 2007).
Total protein in blood is influenced by protein intake and dietary protein quality (Liu et al., 2019). For example, it means that the total protein level in the blood is up-regulated as protein utilization is increased by improving the digestibility of protein by high-quality protein sources. BUN can be used as a parameter of protein status as well as nitrogen utilization (Kohn et al., 2005; Nguyen et al., 2018). Creatinine is related to kidney function (Saleh et al., 2020). In the current study, despite the increased digestibility of CP, we did not observe any change of total protein in blood among the treatment. This result is in agreement with the results of Corzo et al. (2005) and Hernández et al. (2012), who reported that total protein in serum was not affected regardless of dietary protein levels. However, Corzo et al. (2009) and Ahmadi et al. (2015) reported that total protein in blood will only be affected when diets ingested by the animals are deficient in AA. Semeniuk and Grela (2011) observed a high negative correlation between the biological value of the feed and BUN. Other researchers reported that BUN was decreased due to improved nitrogen digestibility (Whang and Easter, 2000; Pavan et al., 2012). Also, creatine levels were decreased by supplementation of exogenous protease (Dongare et al., 2013). However, there was no significant difference on BUN and creatine in the present study. This discrepancy might be due to the difference in basal diets, used protease, and protease dosage between this study and other studies. In addition, this might be because the nutrient imbalance in the diet was not severe enough to change the blood parameters.
VH, CD, and VH:CD ratio are related to nutrient absorption and indicators of gut health (Choct, 2006). In the current study, a nutrient-deficient NC diet caused increased CD and decreased VH:CD ratio, whereas the supplementation of exogenous protease to NC diet improved these parameters by reducing CD and increasing VH:CD ratio. This result is in agreement with the results of previous studies reporting that protease supplementation improved VH and VH:CD ratio in the intestine (Yuan et al., 2008; Nabizadeh et al., 2017). Also, improved ATTD of CP and amino acids were observed in the current study, which might have been affected by improved intestinal development. Because arginine plays an important role in epithelial proliferation and recovery (Tan et al., 2010), increased ATTD of arginine by protease supplementation might affect improved intestinal morphology.
Carcass yield and relative organ weight were not affected by the supplementation of exogenous protease. No difference of carcass yield might be related to the similar final body weight of these birds. Also, there was no significant difference on relative organ weight. The intestinal organ weight is affected by the passage time of digesta (Shakouri et al., 2009); prolonged accumulation of un-digesta in the gastrointestinal tract could induce an increase in the size of the gastrointestinal tract and organs as a response to intestinal motility and digestive excretions. The results of meat quality in the present study were consistent with previous studies (Xu et al., 2017; Saleh et al., 2020). In these studies, pH in breast muscle increased, but drip loss in breast muscle tended to decrease when birds were fed diets supplemented with protease. These results were in agreement with previous results that supplementation of protease decreased drip loss and increased pH in breast meat of broiler (Xu et al., 2017). Also, these results were supported by the results of Calvo et al. (2017) that high pH positively correlated with low drip loss. Moreover, supplementation of protease increased amino acids content in breast muscle. Other researchers reported that protease influenced meat colors such as lightness, redness, and yellowness (Ndazigaruye et al., 2019). However, we did not observe any changes in meat color. There were many factors affecting the meat quality of broilers (Mir et al., 2017). Therefore, further research was needed to estimate the effects of exogenous protease on meat quality.
CONCLUSIONS
The reduction of dietary nutrients produced by considering matrix value caused adverse effects on broiler growth, digestibility, ileal morphology and meat quality including protein content, water holding capacity, pH. However, the supplementation of 50 g/MT exogenous protease to diets considered matrix value numerically improve growth performance, and significantly improved AA utilization, gut health and meat quality. In addition, it was found to have the positive effects of improving profit margin by reducing feed cost. In conclusion, supplementation of exogenous protease (ProAct 360) to diets with consideration of matrix value could efficiently maintain growth performance and health and improve profit margin of producers.
ACKNOWLEDGMENTS
This research was funded by DSM Nutritional Products, Ltd. and Chungbuk National University BK21 Program (2022).
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- Ahmadi M., Yaghobfar A., Tabatabaei S.H. Study of effects difference levels of crude protein and amino acid of diet on intestinal morphological and blood biological parameters of poultry. Biol. Forum. 2015;7:666–670. Research Trend. [Google Scholar]
- Alqaisi O., Ndambi O.A., Williams R.B. Time series livestock diet optimization: cost-effective broiler feed substitution using the commodity price spread approach. Agric. Food Econ. 2017;5:1–19. [Google Scholar]
- Angel C.R., Saylor W., Vieira S.L., Ward N. Effects of a monocomponent protease on performance and protein utilization in 7-to 22-day-old broiler chickens. Poult. Sci. 2011;90:2281–2286. doi: 10.3382/ps.2011-01482. [DOI] [PubMed] [Google Scholar]
- Anuradha P., Barun R. Effect of supplementation of fiber degrading enzymes on performance of broiler chickens fed diets containing de-oiled rice bran. Asian J. Anim. Vet. Adv. 2015;10:179–184. [Google Scholar]
- AOAC . 16th ed. AOAC International; Gaithersburg, MD: 1995. Official Methods of Analysis of AOAC International. [Google Scholar]
- AOAC . 17th ed. AOAC International; Gaithersburg, MD: 2000. Official Methods of Analysis of AOAC International. [Google Scholar]
- Attia Y.A., Bovera F., Wang J., Al-Harthi M.A., Kim W.K. Multiple amino acid supplementations to low-protein diets: effect on performance, carcass yield, meat quality and nitrogen excretion of finishing broilers under hot climate conditions. Animals. 2020;10:973. doi: 10.3390/ani10060973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banson E.K., Muthusamy G., Kondo E. The import substituted poultry industry; evidence from Ghana. Int. J. Agric. For. 2015;5:166–175. [Google Scholar]
- Bedford M.R., Cowieson A.J. Matrix values for exogenous enzymes and their application in the real world. J. Appl. Poult. Res. 2020;29:15–22. [Google Scholar]
- Calvo L., Segura J., Toldrá F., Flores M., Rodríguez A.I., López-Bote C.J., Rey A.I. Meat quality, free fatty acid concentration, and oxidative stability of pork from animals fed diets containing different sources of selenium. Food. Sci. Technol. Int. 2017;23:716–728. doi: 10.1177/1082013217718964. [DOI] [PubMed] [Google Scholar]
- Canogullarİ S., Baylan M., Ayasan T. Threonine requirement of laying Japanese quails. J. Anim. Vet. Adv. 2009;8:1539–1541. [Google Scholar]
- Choct M. Enzymes for the feed industry: past, present and future. Worlds Poult. Sci. J. 2006;62:5–16. [Google Scholar]
- Corzo A., Fritts C.A., Kidd M.T., Kerr B.J. Response of broiler chicks to essential and non-essential amino acid supplementation of low crude protein diets. Anim. Feed. Sci. Technol. 2005;118:319–327. [Google Scholar]
- Corzo A., Loar R.E., II, Kidd M.T. Limitations of dietary isoleucine and valine in broiler chick diets. Poult. Sci. 2009;88:1934–1938. doi: 10.3382/ps.2009-00109. [DOI] [PubMed] [Google Scholar]
- Cupi D., Thorsen M., Elvig-Jørgensen S.G., Wulf-Andersen L., Berti-Sorbara J.O., Cowieson A.J., Faruk M.U. Efficacy and safety profile of a subtilisin protease produced by fermentation in bacillus licheniformis to be used as a feed additive. Heliyon. 2022;8:e10030. doi: 10.1016/j.heliyon.2022.e10030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denbow D.M., Ravindran V., Kornegay E.T., Yi Z., Hulet R.M. Improving phosphorus availability in soybean meal for broilers by supplemental phytase. Poult. Sci. 1995;74:1831–1842. doi: 10.3382/ps.0741831. [DOI] [PubMed] [Google Scholar]
- Dongare P.P., Dhande S.R., Kadam V.J. Standardization of carbon tetrachloride-induced hepatotoxicity in the rat. Am. J. Pharm. Tech. Res. 2013;3 [Google Scholar]
- Dunlop M.W., Moss A.F., Groves P.J., Wilkinson S.J., Stuetz R.M., Selle P.H. The multidimensional causal factors of ‘wet litter’ in chicken-meat production. Sci. Total Environ. 2016;562:766–776. doi: 10.1016/j.scitotenv.2016.03.147. [DOI] [PubMed] [Google Scholar]
- Erdaw M.M., Wu S., Iji P.A. Growth and physiological responses of broiler chickens to diets containing raw, full-fat soybean and supplemented with a high-impact microbial protease. Asian-Australas. J. Anim. Sci. 2017;30:1303. doi: 10.5713/ajas.16.0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia B.A., Aguirre-Reyes A.T., Decena K., Sulabo R. Effect of a performance enhancer mixture as replacement for antibiotic growth promoters on production performance, excreta quality and carcass characteristics of broilers. Philipp. J. Vet. Anim. Sci. 2019;45:34–47. [Google Scholar]
- Gracia M.I., Aranibar M., Lazaro R., Medel P., Mateos G.G. Alpha-amylase supplementation of broiler diets based on corn. Poult. Sci. 2003;82:436–442. doi: 10.1093/ps/82.3.436. [DOI] [PubMed] [Google Scholar]
- Hernández F., López M., Martínez S., Megías M.D., Catalá P., Madrid J. Effect of low-protein diets and single sex on production performance, plasma metabolites, digestibility, and nitrogen excretion in 1-to 48-day-old broilers. Poult. Sci. 2012;91:683–692. doi: 10.3382/ps.2011-01735. [DOI] [PubMed] [Google Scholar]
- Kohn R.A., Dinneen M.M., Russek-Cohen E. Using blood urea nitrogen to predict nitrogen excretion and efficiency of nitrogen utilization in cattle, sheep, goats, horses, pigs, and rats. J. Anim. Sci. 2005;83:879–889. doi: 10.2527/2005.834879x. [DOI] [PubMed] [Google Scholar]
- Laakkonen E., Wellington G.H., Sherbon J.N. Low-temperature, long-time heating of bovine muscle 1. Changes in tenderness, water-binding capacity, pH and amount of water-soluble components. J. Food. Sci. 1970;35:175–177. [Google Scholar]
- Leroy G., Baumung R., Boettcher P., Scherf B., Hoffmann I. Sustainability of crossbreeding in developing countries; definitely not like crossing a meadow…. Animal. 2016;10:262–273. doi: 10.1017/S175173111500213X. [DOI] [PubMed] [Google Scholar]
- Lin Law F., Idrus Z., Soleimani Farjam A., Juan Boo L., Awad E.A. Effects of protease supplementation of low protein and/or energy diets on growth performance and blood parameters in broiler chickens under heat stress condition. Ital. J. Anim. Sci. 2019;18:679–689. [Google Scholar]
- Liu X., Yin J., Kim I.H. Effect of protease derived from Pseudoalteromonas arctica supplementation on growth performance, nutrient digestibility, meat quality, noxious gas emission and blood profiles in finishing pigs. J. Anim. Physiol. Anim. 2019;103:1926–1933. doi: 10.1111/jpn.13202. [DOI] [PubMed] [Google Scholar]
- Mallick P., Muduli K., Biswal J.N., Pumwa J. Broiler poultry feed cost optimization using linear programming technique. J. Oper. Strateg. Plan. 2020;3:31–57. [Google Scholar]
- Maqsood M.A., Khan E.U., Qaisrani S.N., Rashid M.A., Shaheen M.S., Nazir A., Talib H., Ahmad S. Interactive effect of amino acids balanced at ideal lysine ratio and exogenous protease supplemented to low CP diet on growth performance, carcass traits, gut morphology, and serum metabolites in broiler chicken. Trop. Anim. Health. Prod. 2022;54:1–10. doi: 10.1007/s11250-022-03184-w. [DOI] [PubMed] [Google Scholar]
- McCafferty K.W., Morgan N.K., Cowieson A.J., Choct M., Moss A.F. Varying apparent metabolizable energy concentrations and protease supplementation affected broiler performance and jejunal and ileal nutrient digestibility from 1 to 35 d of age. Poult. Sci. 2022;101:101911. doi: 10.1016/j.psj.2022.101911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir N.A., Rafiq A., Kumar F., Singh V., Shukla V. Determinants of broiler chicken meat quality and factors affecting them: a review. J. Food. Sci. Technol. 2017;54:2997–3009. doi: 10.1007/s13197-017-2789-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell R.D., Edwards H.M., Jr Effects of phytase and 1, 25-dihydroxycholecalciferol on phytate utilization and the quantitative requirement for calcium and phosphorus in young broiler chickens. Poult. Sci. 1996;75:95–110. doi: 10.3382/ps.0750095. [DOI] [PubMed] [Google Scholar]
- Moftakharzadeh S.A., Moravej H., Shivazad M. Effect of using the matrix values for NSP-degrading enzymes on performance, water intake, litter moisture and jejunal digesta viscosity of broilers fed barley-based diet. Acta Sci. Anim. 2017;39:65–72. [Google Scholar]
- Mottet A., Tempio G. Global poultry production: current state and future outlook and challenges. Worlds Poult. Sci. 2017;73:245–256. [Google Scholar]
- Moughan P.J., Ravindran V., Sorbara J.O.B. Dietary protein and amino acids—consideration of the undigestible fraction. Poult. Sci. 2014;93:2400–2410. doi: 10.3382/ps.2013-03861. [DOI] [PubMed] [Google Scholar]
- Nabizadeh A., Golian A., Hassanabadi A., Zerehdaran S. Effects of nutrient density and exogenous enzymes in starter diet on performance, intestinal microflora, gut morphology and immune response of broiler chickens. Braz. J. Poult. Sci. 2017;19:509–518. [Google Scholar]
- National Research Council . Nutrient Requirements of Poultry. National Academies Press; Washington, DC: 1994. [Google Scholar]
- Ndazigaruye G., Kim D.H., Kang C.W., Kang K.R., Joo Y.J., Lee S.R., Lee K.W. Effects of low-protein diets and exogenous protease on growth performance, carcass traits, intestinal morphology, cecal volatile fatty acids and serum parameters in broilers. Animals. 2019;9:226. doi: 10.3390/ani9050226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen D.H., Lee S.I., Cheong J.Y., Kim I.H. Influence of low-protein diets and protease and bromelain supplementation on growth performance, nutrient digestibility, blood urine nitrogen, creatinine, and faecal noxious gas in growing–finishing pigs. Can. J. Anim. Sci. 2018;98:488–497. [Google Scholar]
- Nollet H., Deprez P., Van Driessche E., Muylle E. Protection of just weaned pigs against infection with F18+ Escherichia coli by non-immune plasma powder. Vet. Microbiol. 1999;65:37–45. doi: 10.1016/s0378-1135(98)00282-x. [DOI] [PubMed] [Google Scholar]
- Odetallah N.H., Wang J.J., Garlich J.D., Shih J.C.H. Versazyme supplementation of broiler diets improves market growth performance. Poult. Sci. 2005;84:858–864. doi: 10.1093/ps/84.6.858. [DOI] [PubMed] [Google Scholar]
- Onu P.N., Madubuike F.N., Onu D.O., Ekenyem B.U. Performance and economic analysis of broiler starter chicks fed enzyme supplemented sheep manure-based diets. ARPN J. Agric. Biol. Sci. 2011;6:14–19. [Google Scholar]
- Pavan R., Jain S., Kumar A. Properties and therapeutic application of bromelain: a review. Biotechnol. Res. Int. 2012;2012:976203. doi: 10.1155/2012/976203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper R., Kröger S., Richter J.F., Wang J., Martin L., Bindelle J., Htoo J.K., Smolinski D.V., Vahjen W., Zentek J., Van Kessel A.G. Fermentable fiber ameliorates fermentable protein-induced changes in microbial ecology, but not the mucosal response, in the colon of piglets. J. Nutr. 2012;142:661–667. doi: 10.3945/jn.111.156190. [DOI] [PubMed] [Google Scholar]
- Qi Y.Y., Zhang K.Y., Tian G., Bai S.P., Ding X.M., Wang J.P., Peng H.W., Lv L., Xuan Y., Zeng Q.F. Effects of dietary corn germ meal levels on growth performance, serum biochemical parameters, meat quality, and standardized ileal digestibility of amino acids in Pekin ducks. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada V., Foltyn M., Lichovníková M., Musilová A. Effects of protease supplementation of low protein broiler diets on growth parameters and carcass characteristic. Mendelnet. 2013;2013:268–272. [Google Scholar]
- Rawlings N.D., Waller M., Barrett A.J., Bateman A. MEROPS: the database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 2014;42:503–509. doi: 10.1093/nar/gkt953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman Z.U., Kamran J., Abd El-Hack M.E., Alagawany M., Bhatti S.A., Ahmad G., Saleem A., Ullah Z., Yameen R.M.K., Ding C. Influence of low-protein and low-amino acid diets with different sources of protease on performance, carcasses and nitrogen retention of broiler chickens. Anim. Prod. Sci. 2017;58:1625–1631. [Google Scholar]
- Salah A.A. Effect of low-protein in iso-energetic diets on performance, carcass characteristics, digestibilities and plasma lipids of broiler chickens. Egypt. Poult. Sci. J. 2016;36:251–262. [Google Scholar]
- Saleh A.A., Dawood M.M., Badawi N.A., Ebeid T.A., Amber K.A., Azzam M.M. Effect of supplemental serine-protease from Bacillus licheniformis on growth performance and physiological change of broiler chickens. J. Appl. Anim. Res. 2020;48:86–92. [Google Scholar]
- Saleh A.A., Kirrella A.A., Abdo S.E., Mousa M.M., Badwi N.A., Ebeid T.A., Nada A.L., Mohamed M.A. Effects of dietary xylanase and arabinofuranosidase combination on the growth performance, lipid peroxidation, blood constituents, and immune response of broilers fed low-energy diets. Animals. 2019;9:467. doi: 10.3390/ani9070467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selle P.H., Chrystal P.V., Liu S.Y. The cost of deamination in reduced-crude protein broiler diets. Proc. Aust. Poult. Sci. Symp. 2020;31:63–66. [Google Scholar]
- Selle P.H., de Paula Dorigam J.C., Lemme A., Chrystal P.V., Liu S.Y. Synthetic and crystalline amino acids: alternatives to soybean meal in chicken-meat production. Animals. 2020;10:729. doi: 10.3390/ani10040729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semeniuk W., Grela E.R. Effect of a reduced protein content in the nutrition of growing-finishing pigs fed a restricted or ad libitum diet on nitrogen parameters in their blood and urine. Med. Weter. 2011;67:339–342. [Google Scholar]
- Shad A.A., Ahmad T., Iqbal M.F., Asad M.J. Effects of a novel protease from bacillus subtilis K-5 in low protein corn distiller dried grains with solubles (cDDGS) based diets on performance and nutrients digestibility in broiler chickens. Braz. J. Poult. Sci. 2022;24:1–12. [Google Scholar]
- Shakouri M.D., Iji P.A., Mikkelsen L.L., Cowieson A.J. Intestinal function and gut microflora of broiler chickens as influenced by cereal grains and microbial enzyme supplementation. J. Anim. Physiol. Anim. Nutr. 2009;93:647–658. doi: 10.1111/j.1439-0396.2008.00852.x. [DOI] [PubMed] [Google Scholar]
- Siegert W., Zuber T., Sommerfeld V., Krieg J., Feuerstein D., Kurrle U., Rodehutscord M. Prececal amino acid digestibility and phytate degradation in broiler chickens when using different oilseed meals, phytase and protease supplements in the feed. Poult. Sci. 2019;98:5700–5713. doi: 10.3382/ps/pez355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirri F., Petracci M., Zampiga M., Meluzzi A. Effect of EU electrical stunning conditions on breast meat quality of broiler chickens. Poult. Sci. 2017;96:3000–3004. doi: 10.3382/ps/pex048. [DOI] [PubMed] [Google Scholar]
- Tajudeen H., Hosseindoust A., Ha S.H., Moturi J., Mun J.Y., Lee C.B., Park J.W., Lokhandel A., Ingale S.L., Kim J.S. Effects of dietary level of crude protein and supplementation of protease on performance and gut morphology of broiler chickens. Eur. Poult. Sci. 2022;86:1–12. [Google Scholar]
- Tan B., Yin Y., Kong X., Li P., Li X., Gao H., Li X., Huang R., Wu G. L-arginine stimulates proliferation and prevents endotoxin-induced death of intestinal cells. Amino Acids. 2010;38:1227–1235. doi: 10.1007/s00726-009-0334-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Harn J., Dijkslag M.A., Van Krimpen M.M. Effect of low protein diets supplemented with free amino acids on growth performance, slaughter yield, litter quality, and footpad lesions of male broilers. Poult. Sci. 2019;98:4868–4877. doi: 10.3382/ps/pez229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velázquez-De Lucio B.S., Hernández-Domínguez E.M., Villa-Garcia M., Diaz-Godinez G., Mandujano-Gonzalez V., Mendoza-Mendoza B., Alvarez-Cervantes J. Exogenous enzymes as zootechnical additives in animal feed: a review. Catalysts. 2021;11:851. [Google Scholar]
- Vieira S.L., Angel C.R., Miranda D.J.A., Favero A., Cruz R.F.A., Sorbara J.O.B. Effects of a monocomponent protease on performance and protein utilization in 1- to 26-day-of-age turkey poults. J. Appl. Poult. Res. 2013;22:680–688. [Google Scholar]
- Whang K.Y., Easter R.A. Blood urea nitrogen as an index of feed efficiency and lean growth potential in growing-finishing swine. Asian-Australas. J. Anim. Sci. 2000;13:811–816. [Google Scholar]
- Widyaratne G.P., Drew M.D. Effects of protein level and digestibility on the growth and carcass characteristics of broiler chickens. Poult. Sci. 2011;90:595–603. doi: 10.3382/ps.2010-01098. [DOI] [PubMed] [Google Scholar]
- Xu X., Wang H.L., Pan L., Ma X.K., Tian Q.Y., Xu Y.T., Long S.F., Zhang Z.H., Piao X.S. Effects of coated proteases on the performance, nutrient retention, gut morphology and carcass traits of broilers fed corn or sorghum based diets supplemented with soybean meal. Anim. Feed. Sci. Technol. 2017;223:119–127. [Google Scholar]
- Yu B., Wu S.T., Liu C.C., Gauthier R., Chiou P.W. Effects of enzyme inclusion in a maize–soybean diet on broiler performance. Anim. Feed. Sci. Technol. 2007;134:283–294. [Google Scholar]
- Yuan J., Yao J., Yang F., Yang X., Wan X., Han J., Wang Y., Chen X., Liu Y., Zhou Z., Zhou N., Feng X. Effects of supplementing different levels of a commercial enzyme complex on performance, nutrient availability, enzyme activity and gut morphology of broilers. Asian-Australas. J. Anim. 2008;21:692–700. [Google Scholar]