Abstract
The tendency to ruminate, magnify, and experience helplessness in the face of pain – known as pain catastrophizing – is a strong predictor of pain outcomes and is associated with adversity. The ability to maintain functioning despite adversity – referred to as resilience – also influences pain outcomes. Understanding the extent to which pain catastrophizing and resilience influence relations between adversity and daily pain in healthy African-American adults could improve pain risk assessment and mitigate racial disparities in the transition from acute to chronic pain. This study included 160 African-American adults (98 women). Outcome measures included daily pain intensity (sensory, affective) and pain impact on daily function (pain interference). Adversity measures included childhood trauma exposure, family adversity, chronic burden from recent stressors, and ongoing perceived stress. A measure of lifetime racial discrimination was also included. Composite scores were created to capture early-life adversity (childhood trauma, family adversity) versus recent-life adversity (perceived stress, chronic burden). Increased pain catastrophizing was correlated with increased adversity (early and recent), racial discrimination, pain intensity, and pain interference. Decreased pain resilience was correlated with increased recent-life adversity (not early-life adversity or racial discrimination) and correlated with increased pain intensity (not pain-related interference). Bootstrapped multiple mediation models revealed that relationships between all adversity/discrimination and pain outcomes were mediated by pain catastrophizing. Pain resilience, however, was not a significant mediator in these models. These findings highlight opportunities for early interventions to reduce cognitive-affective-behavioral risk factors for persisting daily pain among African-American adults with greater adversity exposure by targeting pain catastrophizing.
Keywords: adversity, pain intensity, pain interference, catastrophizing, resilience, mediation
INTRODUCTION
Heightened daily pain in healthy individuals is considered a risk factor for onset of chronic pain (pain persisting ≥ 3 months) [1]. Chronic pain is a major public health challenge, affecting more adults in the United States than heart disease, cancer and diabetes combined, and resulting in over $600 billion annually in medical treatment costs and lost productivity [2]. The burden that chronic pain places on individuals and society warrants the investigation of risk factors that may provide opportunities for early intervention. Biopsychosocial models of pain highlight that the experience of pain involves sensory and affective components that are determined by interactions among not just biological, but also psychological, social, and cultural factors [3]. Racial differences in clinical pain severity and experimental pain responses are well-established: studies show that African Americans (AA) report greater pain severity and pain-related functional disability compared to non-marginalized racial groups with similar pain conditions [2, 4–6]. AAs are also more likely to experience adversity and racial discrimination [7] than non-marginalized racial groups [8]. Racial discrimination represents a unique form of adversity that is disproportionately experienced by AAs [9, 10]. Exposure to adversity and discrimination, in turn, is associated with elevated daily bodily pain, enhanced experimental evoked pain responsiveness, and increased risk for chronic pain [11, 12]. However, the mechanisms through which adversity and/or discrimination lead to negative pain outcomes are still unclear [13–17].
Racial disparities in exposure to adversities and discrimination are well-documented historically and have been relatively resistant to changes in laws and policies designed to mitigate them [18, 19]. AAs are exposed to higher rates of adverse individual experiences (e.g., traumatic events, family adversity, interpersonal violence), including race-based discrimination [20–23], as well as adverse environmental conditions (e.g., housing inequities, neighborhood crime). These “downstream” individual experiences and environmental conditions are driven, in part, by “upstream” structural factors (e.g., systemic racism [24–26]). Adversity is associated with increased risk for developing stress-related mental and physical health conditions, including chronic pain [27]. Whereas some evidence suggests specific types and timing of adversity are associated with pain experiences [11, 28], other studies have failed to detect unique associations between adversity domains and pain outcomes [29, 30]). For instance, both recent and lifetime experiences of discrimination are associated with lower heat tolerance in experimental evoked pain tasks [31] as well as greater clinical pain [17, 32, 33]. Recent theoretical models of adversity posit that event features such as developmental timing are likely to prove more fruitful for understanding biobehavioral responses than approaches that either treat all adverse events the same or assume each subtype has unique neurobiological effects [34]. Examining the effects of early life adversity on risk for chronic pain separately from the effects of adversity in adulthood is supported by clinical and preclinical research [15].
The present study evaluated two cognitive-affective-behavioral processes that may help to explain, in part, the relation between adversity and daily pain. Whereas the first – pain catastrophizing – has received considerable attention in the pain literature as a predictor of clinical and experimental evoked pain outcomes [35], the second – pain resilience – has emerged more recently, and its role as a potential mediator of relations between adversity and pain has yet to be investigated. Pain catastrophizing refers to the tendency to magnify the threat value of pain stimuli, to feel helpless in the context of pain and in one’s ability to cope with it, and by a perceived inability to divert pain-related thoughts in anticipation of, during, or following a painful encounter [36]. Although the 3-factor structure of pain catastrophizing derived from the Pain Catastrophizing Scale (PCS [37]) has been supported in healthy and chronic pain samples [36], the present study focused on the PCS total score, which has been found to be more readily comparable across cultures [38]. Pain catastrophizing has been linked to increased pain sensitivity, greater impairment in those with chronic pain, and is a risk factor for the development of chronic pain [39–41]. Research in clinical and non-clinical settings suggests that pain catastrophizing accounts for up to 31% of the variance in pain ratings [39, 42]. An individual’s tendency to engage in pain catastrophizing may be shaped by environmental factors and early life experiences [42, 43]. For example, pain catastrophizing is associated with specific types of childhood maltreatment [44–46], prior trauma exposure [47], and experiences of racial discrimination in AA adults [48, 49]. Together, evidence for associations between different types of adversity and pain catastrophizing, as well as higher rates of pain catastrophizing observed among AA compared to non-marginalized racial groups [50], suggest that pain catastrophizing could represent one pathway linking adversity and daily pain among AA adults.
Pain resilience has been posed as a construct antithetical to vulnerabilities such as pain catastrophizing, with prior studies suggesting that the two may be separate but interrelated [51]. Resilience is broadly defined as “sustained positive functioning in the face of significant physical or psychological challenges” [52]. Resilience comprises adaptive coping mechanisms (i.e., acceptance) as well as dispositional (i.e., optimism) and environmental factors (i.e., social support) factors that promote well-being [52]. Different indicators of pain resilience have been associated with lower levels of pain catastrophizing [53, 54], greater habituation to experimental heat and cold pain stimuli [55], improved functioning in the presence of pain [56], and higher levels of pain acceptance [57]. Some studies suggest that psychological resilience could serve as a protective factor against the development of chronic pain [55, 58]. While evidence indicates that adversity exposure is negatively associated with resilience and is a risk factor for adverse health outcomes, findings suggest that a U-shaped relationship may exist between adversity exposure and pain outcomes, possibly explained, in part, by the influence of protective factors such as resilience [59]. For example, in a sample of adults with chronic back pain, those exposed to moderate lifetime adversity experienced less impairment than those who reported no adversity or high levels of adversity [60]. Important questions remain unanswered regarding the extent to which developmental timing of adversity contributes to pain resilience, and whether pain resilience protects against the emergence of pain complaints among individuals who experience higher levels of adversity.
Understanding the pathways that link adversity to daily pain in healthy AA adults could highlight opportunities to prevent the development of chronic pain in AAs - particularly those exposed to higher levels of adversity. While pain catastrophizing has been primarily studied in populations with chronic pain, evidence suggests that pain catastrophizing in healthy populations can predict onset of chronic pain [39]. Further investigation is needed to determine the role pain catastrophizing plays in the transition from healthy status to chronic pain. The present study examined how early-life adversity, recent adversity, and racial discrimination were associated with daily pain and pain-related interference with life activities in healthy AA adults. Pain-related life interference captures the extent to which pain disrupts physical, emotional, recreational, and other domains of an individual’s life [61]. Identifying factors that contribute to greater pain-related interference in AA adults could inform the development and refinement of interventions that seek to improve functioning and engagement in important life domains. In addition, this study examined pain catastrophizing and pain resilience as potential mechanisms linking adversity and pain experiences. Pain catastrophizing has been shown to mediate racial differences in experimental pain tolerance [62–64], as well as the relationship between experiences of racial discrimination and chronic pain in AA females [48]. To address lingering questions in the pain literature regarding whether and how different features of adversity are associated with pain intensity and interference, the current study incorporated multiple measures covering a wide range of adversity types across the lifespan. It was hypothesized that adversity would be associated with greater sensory and affective pain intensity and pain interference, and that higher pain catastrophizing and lower pain resilience would account, in part, for this relationship.
METHODS
Study population
This cross-sectional study included 160 healthy adults who self-identified as AA (98 women; 62 men; none identified as transgender), were between the ages of 18 to 45 (inclusive) and were seen for a baseline assessment as part of a larger ongoing longitudinal study. Participants were recruited primarily through online research participant registries, community and university webpages advertising research studies, flyers distributed at local Historically-Black Colleges and Universities, and flyers placed in waiting rooms of local clinics serving the AA community. Participants were excluded if they had a current chronic pain condition (defined as persistent daily or near daily pain of ≥3 months in duration as determined by a modified version of the Persistent Pain Questionnaire [65]), sickle cell disease, medical conditions that might affect the hypothalamic-pituitary-adrenal (HPA) axis or were taking daily medications that could affect pain or stress (i.e., HPA) responses, or met criteria for a substance use disorder in the previous three months. All self-report measures described below were completed by participants via a research electronic data capture (REDCap) system during their initial laboratory visit.
Pain Intensity
The McGill Pain Questionnaire-Short Form (MPQ-SF [66]) was used to assess the intensity of participants’ daily/ongoing pain. Participants indicated on a four-point scale (0 = “None” to 3 = “Severe”) the extent to which they had experienced a variety of pain characteristics. The MPQ-SF provides two subscales that served as primary outcome measures: the MPQ-Sensory (MPQ-S) subscale that contains eleven items and measures the sensory dimension of pain (e.g., throbbing, burning, or aching), with total scores ranging from 0 to 33 (higher scores reflect greater sensory pain intensity); and the MPQ-Affective (MPQ-A) subscale that contains four items and assesses the affective dimension of pain (e.g., tiring, sickening, or punishing), with total scores ranging from 0 to 12 (higher scores reflect greater affective pain intensity). Current reliability was excellent for the MPQ-S (α=0.93) and good for the MPQ-A (α=0.84).
Pain-Related Life Interference
The PROMIS Pain Interference – Short Form (PROMIS-INT [67]) is an 8-item measure that was used to assess the degree to which pain interfered with daily life activities in the past seven days (e.g., “How much did pain interfere with the things you usually do for fun?”). Item scores ranged from 1 (not at all) to 5 (very much), with total scores ranging from 8 to 40 (higher scores reflect greater pain-related interference). Current reliability was excellent for the PROMIS-INT (α=0.95).
Early Life Adversity
The Childhood Trauma Questionnaire (CTQ) [68] is a 28-item, self-report measure used to assess the frequency of different types of abuse experienced as a child and teenager (e.g., “I got hit or beaten so badly that it was noticed by someone like a teacher, neighbor, or doctor”). CTQ item scores ranged from 1 (never true) to 5 (very often true), with total scores ranging from 28 to 140 (higher scores reflect more childhood trauma exposure). Current reliability was good for the CTQ (α=0.84). A brief 10-item version of the Family Adversity Questionnaire (FAQ) was used to assess the number of non-sexual, adverse early-life experiences, including parental incarceration, illness, disability, death and severe poverty (e.g., “Your family often faced serious financial problems because your parents were often out of work”) [69]. Items were dichotomous (0 = No; 1 = Yes), with total scores ranging from 0 to 10 (higher scores reflect more adverse early life experiences). Current reliability was good for the FAQ (α=0.80).
Recent Life Adversity
The Chronic Burden Scale (CBS) is a 21-item scale used to measure the degree to which a variety of stressors (e.g., economic, employment, crime, and legal problems) have been a problem for participants in the past 6 months (e.g., “Housing problems [uncertainty about housing, problems with the landlord, needing to find a new place to live]”) [70]. CBS item scores ranged from 1 (not a problem for me) to 4 (a major problem for me), with total scores ranging from 15 to 84 (higher scores reflect greater burden from chronic stressors). Current reliability was good for the CBS (α=0.86). The Perceived Stress Scale-10 (PSS-10 [71]) is a 10-item measure used to assess the degree to which individuals perceive their lives as stressful in the past month (e.g., “In the last month, how often have you felt difficulties were piling up so high that you could not overcome them?”). PSS-10 item scores ranged from 0 (never) to 4 (very often), with total scores ranging from 0 to 40 (higher scores reflect higher levels of perceived stress). Current reliability was good for the PSS-10 (α=0.86).
Racial/Ethnic Discrimination
The 17-item Brief Perceived Ethnic Discrimination Questionnaire – Community Version (BPEDQ-CV) [72] was used to assess lifetime experiences of discrimination because of one’s race or ethnicity. Participants indicated the frequency of experiences with racial discrimination on a scale of 1 (never) to 5 (very often), with total scores ranging from 17 to 75 (higher scores reflect more experiences with racial discrimination) (e.g., “Because of your race, have policemen or security officers been unfair to you?”). Current reliability was good for the BPDEQ-CV (α=0.88).
Pain Catastrophizing
The Pain Catastrophizing Scale (PCS [37]), a 13-item self-report measure, was used to measure dispositional tendencies to engage in catastrophic thought while experiencing pain (e.g., “I become afraid that the pain will get worse”). Participants indicated the degree to which they experience catastrophic thoughts during a painful experience on a scale of 1 (not at all) to 4 (all the time). Scores range from 0 to 52, with higher scores indicating a greater tendency to engage in catastrophic thoughts. Current reliability was excellent for the PCS (α=0.92).
Pain Resilience
The Pain Resilience Scale (PRS) is a 14-item self-report measure that was used to assess cognitive/affective positivity and behavioral perseverance during a painful experience [73]. Participants were asked to report how frequently they experienced cognitive, emotional, or behavioral responses to pain on a scale of 0 (not at all) to 4 (all the time) (e.g., “When faced with intense or prolonged pain, I don’t let it get me down”). Scores range from 0 to 56, with higher scores indicating higher levels of pain-specific resilience. Current reliability was excellent for the PRS (α=0.95).
Covariates
A brief demographics form was used to determine participant age, gender, and education level (years completed).
Data analysis
Composite scores were computed for early-life adversity (sum of standardized scores for the CTQ and FAQ) and recent-life adversity (sum of standardized scores for the CBS and PSS-10). Theoretical models of stress exposure measurement [74] emphasize conceptual and methodological advantages of composite indices over individual measures: more accurate estimation of stress exposures across multiple contexts; increased predictive power; circumventing problems of highly-correlated individual predictors (i.e., collinearity or suppression effects); and overcoming ‘threshold effects’ [75]. These advantages apply to early-life and recent adversity measurement – individual adversity domains are interconnected and unlikely to operate in isolation. Importantly, prior work evaluating the impact of adversity on mental and physical health outcomes supports distinguishing the unique effects of early-life adversity from recent adversity [76–78].
Primary analyses included multiple mediation models conducted in SPSS v.28 (IBM, Corp.) using the PROCESS macro [79], which employs ordinary least squares regression for path analysis and constructs bias corrected 95% bootstrap confidence intervals for indirect (mediated) effects with a resample rate of 5,000. Models simultaneously evaluated pain catastrophizing and pain resilience as mediators of the relationship between adversity (i.e., early-life adversity, recent-life adversity) or lifetime racial discrimination and pain outcomes (i.e., sensory pain intensity, affective pain intensity, and pain interference). Due to known effects on pain outcomes, all PROCESS models included age, gender, and education (years completed) as covariates [80]. Corrections were made for multiple testing using the false discovery rate approach [81].
RESULTS
Preliminary Analyses
Participants included 98 female and 62 male African-American adults. Their mean age was 25.9 (SD=6.4) and they completed a mean of 15.7 years of education (SD=3.6). Participants’ mean scores on the MPQ-S and MPQ-A were 5.7 (SD=7.4; range: 0–28) and 1.3 (SD=2.3; range: 0–11), respectively. Their mean raw pain interference score of 10 (SD=4.7; range: 8–33) corresponds to a T-Score of 49.9, which is average for the United States general population. Participants’ mean scores on pain catastrophizing and pain resilience were 9.2 (SD=9.3; range: 0–41) and 33.5 (SD=12.4; range: 0–56), respectively. Bivariate correlations for demographic, adversity, discrimination, pain catastrophizing, pain resilience, and pain outcomes are presented in Table 1. Zero order Pearson correlation analyses showed higher pain catastrophizing was associated with greater adversity (recent-life adversity, early-life adversity), greater racial discrimination, and worse pain outcomes (sensory pain, affective pain, and pain interference). Higher pain catastrophizing was also associated with lower pain resilience. Higher pain resilience was associated with greater recent-life adversity but was not significantly associated with early-life adversity or discrimination. In addition, higher pain resilience was associated with lower sensory and affective pain but was not significantly associated with pain interference. Higher levels of early- and recent-life adversity – but not discrimination - were associated with greater pain interference. Moreover, higher levels of recent-life adversity and discrimination – but not early-life adversity - were associated with higher sensory and affective pain.
Table 1.
Zero-order Pearson Correlations Among Study Variables for African-American Adults.
| Variables | Age | Education | EA | RA | DISC | CAT | RES | MPQ-S | MPQ-A |
|---|---|---|---|---|---|---|---|---|---|
| Age | - | ||||||||
| Education | .21** | - | |||||||
| Early Adversity (EA) | .20* | −.04 | - | ||||||
| Recent Adversity (RA) | .07 | −.07 | .45*** | - | |||||
| Discrimination (DISC) | .15 | −.02 | .36*** | .53*** | - | ||||
| Pain catastrophizing (CAT) | −.13 | −.10 | .27** | .36*** | .23** | - | |||
| Pain resilience (RES) | .07 | .04 | −.06 | −.24** | −.09 | −.41*** | - | ||
| Sensory pain (MPQ-S) | −.11 | −.07 | .04 | .22** | .21* | .38*** | −.27** | - | |
| Affective pain (MPQ-A) | −.16 | −.11 | .003 | .19* | .20* | .32*** | −.23** | .78*** | - |
| Pain interference (INT) | .10 | −.04 | .19* | .22* | .17 | .31*** | −.09 | .19* | .09 |
<p.001;
p<.01;
p<.05.
Multiple Mediation: Early-Life Adversity
The conceptual multiple mediation model via pain catastrophizing and pain resilience is presented in Figure 1. Results of multiple mediation models including early-life adversity are summarized in Table 2. None of the direct effects (c’) of early-life adversity (X) on sensory pain (Y), affective pain (Y), or pain interference (Y) were statistically significant, indicating that early-life adversity was unrelated to these three pain outcomes. However, the indirect (mediated) effects of early-life adversity on all three pain outcomes (i.e., sensory pain intensity, affective pain intensity, pain interference) via pain catastrophizing (a1b1) were significant. That is, higher levels of early-life adversity were associated with more pain catastrophizing, which, in turn, was associated with higher sensory pain, higher affective pain, and greater pain-related interference. None of the indirect effects of early-life adversity on pain outcomes through pain resilience (a2b2), however, were significant.
Figure 1.
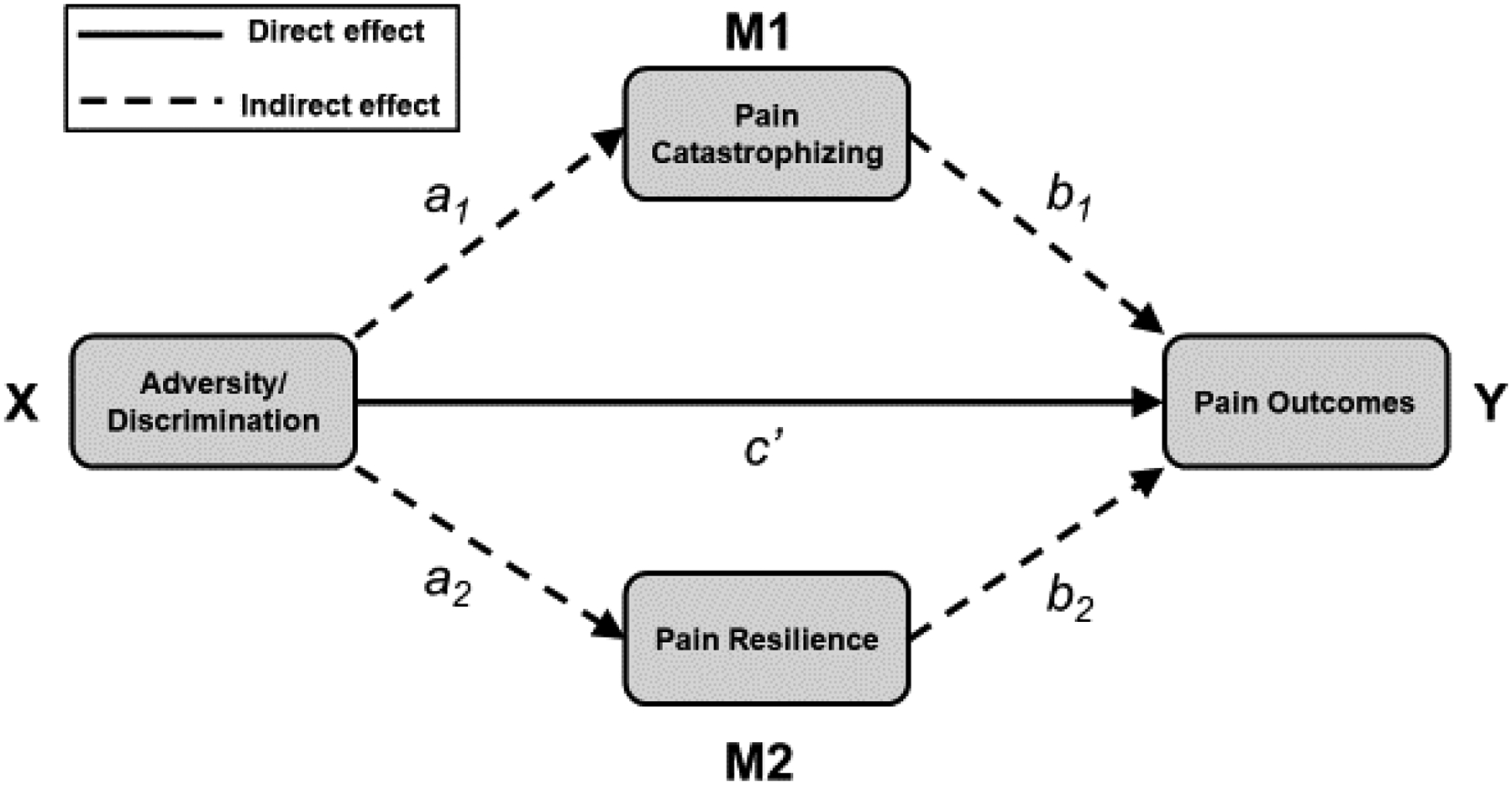
Conceptual multiple mediation model via pain catastrophizing and pain resilience.
Table 2.
Multiple mediation models evaluating the effect of early adversity on pain-related outcomes via pain catastrophizing and pain resilience.
| Direct Effect on Sensory Pain (MPQ-S) | Coefficient | SE | T | p | 95% LLCI | 95% ULCI |
|---|---|---|---|---|---|---|
| Early adversity → MPQ-S | −0.12 | 0.39 | −0.30 | .764 | −0.89 | 0.65 |
| Indirect Effects on MPQ-S (via mediators) | Effect | Boot SE | BootLLCI | BootULCI | Effect size | |
| Pain catastrophizing | 0.44 | 0.23 | 0.09 | 0.98 | 0.95 | |
| Pain resilience | 0.02 | 0.08 | −0.13 | 0.20 | 0.05 | |
| Direct Effect on Affective Pain (MPQ-A) | Coefficient | SE | T | p | 95% LLCI | 95% ULCI |
| Early adversity → MPQ-A | −0.06 | 0.13 | −0.50 | .621 | −0.31 | 0.19 |
| Indirect Effects on MPQ-A (via mediators) | Effect | Boot SE | BootLLCI | BootULCI | Effect size | |
| Pain catastrophizing | 0.11 | 0.06 | 0.02 | 0.24 | 0.93 | |
| Pain resilience | .008 | 0.03 | −0.04 | −0.07 | 0.07 | |
| Direct Effect on Pain Interference (INT) | Coefficient | SE | T | p | 95% LLCI | 95% ULCI |
| Early adversity → INT | 0.24 | 0.25 | 0.96 | .338 | −0.25 | 0.73 |
| Indirect Effects on INT (via mediators) | Effect | Boot SE | BootLLCI | BootULCI | Effect size | |
| Pain catastrophizing | 0.25 | 0.14 | 0.03 | 0.59 | 1.01 | |
| Pain resilience | −.002 | 0.03 | −0.07 | 0.05 | 0.01 | |
Note. LLCI = lower level of 95% confidence interval; ULCI = upper level of 95% confidence interval; effect size = ratio of indirect to total effect.
Multiple Mediation: Recent-Life Adversity
Results of multiple mediation models targeting recent-life adversity are summarized in Table 3. None of the direct effects (c’) of recent life adversity (X) on sensory pain (Y), affective pain (Y), or pain interference (Y) were statistically significant, indicating that recent-life adversity was unrelated to these three pain outcomes. However, the indirect (mediated) effects of recent-life adversity on all three pain outcomes (i.e., sensory pain intensity, affective pain intensity, pain interference) via pain catastrophizing (a1b1) were significant. That is, higher levels of recent-life adversity were associated with more pain catastrophizing, which, in turn, was associated with higher sensory pain, higher affective pain, and greater pain-related interference. None of the indirect effects of recent-life adversity on pain outcomes through pain resilience (a2b2), however, were significant.
Table 3.
Multiple mediation models evaluating the effect of recent adversity on pain-related outcomes via pain catastrophizing and pain resilience.
| Direct Effect on Sensory Pain (MPQ-S) | Coefficient | SE | t | p | 95% LLCI | 95% ULCI |
|---|---|---|---|---|---|---|
| Recent adversity → MPQ-S | 0.52 | 0.40 | 1.28 | .201 | −0.28 | 1.31 |
| Indirect Effects on MPQ-S (via mediators) | Effect | Boot SE | BootLLCI | BootULCI | Effect size | |
| Pain catastrophizing | 0.47 | 0.20 | 0.11 | 0.92 | 0.81 | |
| Pain resilience | 0.11 | 0.11 | −0.06 | 0.36 | 0.19 | |
| Direct Effect on Affective Pain (MPQ-A) | Coefficient | SE | t | p | 95% LLCI | 95% ULCI |
| Recent adversity → MPQ-A | 0.17 | 0.13 | 1.29 | .201 | −0.09 | 0.43 |
| Indirect Effects on MPQ-A (via mediators) | Effect | Boot SE | BootLLCI | BootULCI | Effect size | |
| Pain catastrophizing | 0.10 | 0.05 | 0.01 | 0.22 | 0.75 | |
| Pain resilience | 0.03 | 0.03 | −0.02 | 0.12 | 0.25 | |
| Direct Effect on Pain Interference (INT) | Coefficient | SE | t | p | 95% LLCI | 95% ULCI |
| Recent adversity → INT | 0.28 | 0.26 | 1.08 | .284 | −0.23 | 0.79 |
| Indirect Effects on INT (via mediators) | Effect | Boot SE | BootLLCI | BootULCI | Effect size | |
| Pain catastrophizing | 0.30 | 0.15 | 0.05 | 0.64 | 1.07 | |
| Pain resilience | −0.02 | 0.05 | −0.14 | 0.09 | 0.07 | |
Note. LLCI = lower level of 95% confidence interval; ULCI = upper level of 95% confidence interval; effect size = ratio of indirect to total effect.
Multiple Mediation: Racial Discrimination
Results of multiple mediation models including lifetime racial discrimination are summarized in Table 4. The direct effect (c’) of discrimination (X) on affective pain (Y) was significant, indicating that discrimination predicted affective pain. However, the direct effects (c’) of discrimination (X) on sensory pain (Y) and pain interference (Y) were not statistically significant. The indirect (mediated) effects of discrimination on all three pain outcomes (i.e., sensory pain intensity, affective pain intensity, pain interference) via pain catastrophizing (a1b1) were significant. That is, higher levels of discrimination were associated with more pain catastrophizing, which, in turn, was associated with higher sensory pain, higher affective pain, and greater pain-related interference. None of the indirect effects of discrimination on pain outcomes through pain resilience (a2b2), however, were significant.
Table 4.
Multiple mediation models evaluating the effect of lifetime racial discrimination on pain-related outcomes via pain catastrophizing and pain resilience.
| Direct Effect on Sensory Pain (MPQ-S) | Coefficient | SE | t | p | 95% LLCI | 95% ULCI |
|---|---|---|---|---|---|---|
| Discrimination → MPQ-S | 1.14 | 0.65 | 1.76 | .080 | −0.14 | 2.41 |
| Indirect Effects on MPQ-S (via mediators) | Effect | Boot SE | BootLLCI | BootULCI | Effect size | |
| Pain catastrophizing | 0.60 | 0.29 | 0.13 | 1.25 | 0.88 | |
| Pain resilience | 0.08 | 0.13 | −0.11 | 0.39 | 0.12 | |
| Direct Effect on Affective Pain (MPQ-A) | Coefficient | SE | t | p | 95% LLCI | 95% ULCI |
| Discrimination → MPQ-A | 0.45 | 0.21 | 2.15 | .034 | .035 | 0.86 |
| Indirect Effects on MPQ-A (via mediators) | Effect | Boot SE | BootLLCI | BootULCI | Effect size | |
| Pain catastrophizing | 0.13 | 0.07 | 0.12 | 0.29 | 0.82 | |
| Pain resilience | 0.03 | 0.04 | −0.03 | 0.12 | 0.18 | |
| Direct Effect on Pain Interference (INT) | Coefficient | SE | t | p | 95% LLCI | 95% ULCI |
| Discrimination → INT | 0.28 | 0.42 | 0.66 | .509 | −0.55 | 1.10 |
| Indirect Effects on INT (via mediators) | Effect | Boot SE | BootLLCI | BootULCI | Effect size | |
| Pain catastrophizing | 0.40 | 0.21 | 0.08 | 0.89 | 1.02 | |
| Pain resilience | −.009 | 0.05 | −0.12 | 0.09 | 0.02 | |
Note. LLCI = lower level of 95% confidence interval; ULCI = upper level of 95% confidence interval; effect size = ratio of indirect to total effect.
DISCUSSION
The burden of pain in the United States falls disproportionately on AA adults [5]. Although heightened exposure to adversity and racial/ethnic discrimination is likely to increase risk for acute and chronic pain among AA adults [11, 12], the pathways through which these exposures influence pain experiences have yet to be elucidated [13–17]. The present study evaluated the extent to which two cognitive-affective-behavioral processes - pain catastrophizing and pain resilience - could explain relations between different features of adversity and pain outcomes. Pain catastrophizing is one of the most robust and well-characterized predictors of clinical pain [35]. Pain resilience has received comparatively less attention in the literature but is implicated in dual-factor models of chronic pain, which propose that risk and resilience are not simply two sides of the same coin but capture distinct mechanistic pathways [82]. Consistent with hypotheses, pain catastrophizing statistically mediated (in part) relations between adversity and daily pain-related outcomes (i.e., sensory and affective pain intensity, pain-related life interference). Importantly, greater pain catastrophizing was independently associated with higher levels of lifetime racial discrimination and greater exposure to both early- and recent-life adversity. Future studies should evaluate potential mechanisms linking discrimination and adversity to pain catastrophizing, including alterations in social threat hypervigilance and stress response systems [83]. Contrary to hypotheses, pain resilience did not mediate any of the relations between early/recent adversity or racial discrimination and pain outcomes. Together, these findings suggest that early interventions should target pain catastrophizing among healthy AA adults to mitigate risk for persistent daily pain, which, in turn, confers increased risk for transition to chronic pain [84, 85].
Recent work suggests that exposure to adversity may increase pain catastrophizing via increased psychological distress [86]. The present findings extend this work by showing that pain catastrophizing statistically mediates the effects of a broad array of adversity types on daily pain outcomes among AA adults. We demonstrated that the indirect mediating effects of pain catastrophizing are present regardless of adversity timing, are observed for lifetime experiences of racial discrimination, and extend to composite measures of early-life adversity (including emotional and physical neglect, physical and sexual abuse, and non-traumatic adverse events). That indirect effects were observed for past (childhood) adverse events on current daily pain suggests that these pathways may operate even in the absence of current (adulthood) adverse events. One interpretation of these findings is that pain catastrophizing could exhibit enduring (trait-like) effects on pain in adulthood long after the early adverse events from which it emerged. This is consistent with evidence that pain catastrophizing exhibits both trait and state characteristics [87]. Notably, the present findings further suggest that pain catastrophizing could emerge in AA adults following adverse events that involve social (i.e., rejection, exclusion, or loss) - but not necessarily physical - pain [88]. Social pain has been defined as the unpleasant emotional response elicited by actual or threatened separation from people or social groups [89]. However, in the absence of previous assessment of pain catastrophizing in childhood, these interpretations remain speculative. Social and physical pain have been found to share neural pathways and inflammatory consequences [90], which suggests that psychological distress and catastrophizing responses to social pain could influence an individual’s vulnerability for negative physical pain outcomes. It is critical for future studies to determine for whom psychological distress contributes to the emergence of pain catastrophizing following adversity and to elucidate the mechanisms by which this process occurs. Given the transdiagnostic importance of catastrophic thinking across mental health outcomes that frequently co-occur with chronic pain (e.g., mood disorders) [91], one key question is whether disorder-specific distress (e.g., depressive, anxious, and posttraumatic stress symptoms) explains the emergence of pain catastrophizing following adversity. Answers to these questions could inform the development and refinement of interventions designed to help individuals manage the dimensions of distress most relevant to pain catastrophizing in the aftermath of adversity and, ultimately, to reduce risk for developing chronic pain.
Exposure to adversity and racial/ethnic discrimination was associated with greater pain-related interference via elevations in pain catastrophizing. This finding is consistent with the fear-avoidance model of chronic pain, which proposes that pain catastrophizing increases risk for subsequent pain severity and disability through increases in pain-related fear and avoidance behaviors [92]. One important contribution of this study is to demonstrate that this pathway operates in healthy AA adults without acute injury or chronic pain, adding to prior work in a Dutch cohort showing that pain catastrophizing predicted the development of low back pain with disability among pain-free individuals [93]. Individual differences in pain catastrophizing among AA adults accounted, in part, for relations between adversity and the degree to which subclinical pain interfered with the performance and enjoyment of daily activities. Research in adults without chronic pain suggests that “catastrophizers” preferentially attend to threatening pain-related stimuli, even after controlling for negative affectivity [94]. Hence, pain catastrophizing could negatively impact task completion and social activity engagement by shifting attention away from these activities toward painful sensations or cues [95]. Dual process models of pain further posit, with conflicting empirical support [96, 97], that greater pain catastrophizing is more strongly associated with heightened attentional bias to pain threat among individuals with lower attentional control. Future studies are needed to evaluate potential mechanisms (e.g., cognitive appraisals, pain behaviors) linking pain catastrophizing to pain-related interference in healthy AA adults, as well as potential moderators of this relation (e.g., attentional control, social support).
Consistent with expectations, pain resilience was strongly and negatively correlated with pain catastrophizing. However, unlike pain catastrophizing, none of the hypothesized indirect (mediated) pathways from adversity and discrimination measures to pain outcomes through pain resilience were significant. Resilience in the context of pain includes a behavioral perseverance component (i.e., doing things despite pain) and a cognitive-affective component (i.e., maintaining positive – and managing negative - emotions and cognitions during pain) [98]. Resilience has also been defined more broadly as the ability to bounce back from adversity [99]. Bivariate correlations revealed that greater recent adversity was associated with lower pain resilience, and neither early adversity nor discrimination were associated with pain resilience. One interpretation of these findings is that pain resilience may only emerge as a mediator of relations between adversity types and pain outcomes once pain has become chronic. According to this perspective, resilience among chronic pain patients is captured by a combination of low pain-related life interference and high pain severity [100] that may not be characteristic of healthy individuals. Whereas pain resilience was negatively correlated with both pain intensity and life interference among persons living with HIV and chronic pain [101], pain resilience was negatively correlated with pain intensity but unrelated to pain-related life interference among healthy AA adults in the present study. Individuals with back pain exhibit significant indirect effects of pain resilience on subsequent pain intensity and physical dysfunction via self-efficacy and kinesiophobia, supporting the inclusion of pain resilience in an expanded fear-avoidance model of pain alongside pain catastrophizing [102]. Future studies are needed to evaluate whether this model can account for variation in pain risk among healthy individuals, the role of adversity in determining pain resilience, and potential mediators that can account for relations between pain resilience and pain experiences. One promising potential mediator to target is the experience of positive emotions. Indirect effects of higher resilience on lower pain catastrophizing via elevations in daily positive emotions were found in a study of chronic pain patients [103], raising the possibility that positive emotions could protect against development of pain by disrupting cognitive-affective processes involved in pain catastrophizing [58]. Positive emotions could influence expectations about pain perception, which are known to influence placebo analgesia and neural circuits that subserve nociception and pain relief [104]. Future studies evaluating an expanded fear-avoidance model should also consider affective-motivational factors that may influence relations between pain catastrophizing and pain experiences, including goal attainment [105].
The present study contributes to understanding of how exposure to adversity and racial/ethnic discrimination are associated with daily pain complaints in healthy AA adults. However, findings should be interpreted cautiously in light of several important limitations. First, given the reliance on cross-sectional and correlational data, it is not possible to establish temporal precedence or make causal inferences. Fear-avoidance models of pain posit bidirectional relations between state- or trait-like factors (i.e., catastrophizing, resilience) and pain-related outcomes [92, 102]. Hence, it is possible that higher levels of pain catastrophizing resulted from greater pain intensity and interference among healthy AA adults. For example, pain catastrophizing may strengthen as pain itself becomes more chronic, in a maladaptive attempt to cope with pain-related distress and uncertainty. Second, similar mediation patterns across adversity types could have been due to overlap among these measures, consistent with evidence that adverse events frequently co-occur [106]. However, correlations among early adversity, recent adversity, and racial discrimination were moderate in size (.36 ≤ r’s ≤ .53), which suggests that these measures captured unique aspects of exposure to adverse events. Third, reliance on self-report measures of early adversity could have contributed to retrospective recall bias, though reports of adverse childhood experiences are relatively stable over time [107]. Fourth, pain catastrophizing was not assessed in early childhood, and therefore we are unable to determine whether this cognitive-affective-behavioral process was present before exposure to adverse events in adulthood. Finally, the absence of a comparison group precludes speculation regarding whether the mediation pathways observed in this study would extend to other demographic groups. Future research is needed to determine whether the strength and significance of these indirect effects vary across different racial and ethnic groups, including if experiences of adversity and discrimination are similarly associated pain catastrophizing for non-minoritized racial/ethnic groups.
CONCLUSIONS
Understanding the factors that contribute to daily pain complaints and pain interference among healthy AA adults could help to minimize racial disparities in chronic pain risk by identifying opportunities for prevention. Interrupting the transition from acute to chronic pain among AA adults is critical given lingering racial disparities in pain treatment [5] and evidence that once chronic, pain becomes difficult to treat. Non-pharmacological interventions for chronic pain are associated with only small-to-moderate - and often short-term - benefits on pain [108, 109]. The present findings have at least two important clinical implications. First, adversity across the lifespan, including experiences of racial discrimination, is associated with worse daily pain regardless of the timing of events. Exposure to adverse events could aid in the identification of healthy AA adults at elevated risk for developing persistent daily pain complaints. Importantly, daily pain risk was linked not only to adverse childhood experiences, whose importance for health outcomes is increasingly recognized in medical settings, but also to experiences of racial discrimination across the lifespan. The latter should be included in routine screening for chronic pain risk among marginalized adults. Moreover, efforts to prevent the downstream effects of racial discrimination on daily pain among AA adults must be accompanied by efforts to modify the upstream structural conditions from which these health inequities emerge [110]. Second, among AA adults reporting higher levels of adversity or racial discrimination, interventions specifically targeting pain catastrophizing [111] could help to reduce the risk for transition from acute to chronic pain. Both cognitive behavioral and acceptance and commitment therapies have shown promise as interventions that can mitigate the effects of pain catastrophizing [111]. These interventions may be particularly salient for AA adults, who report higher levels of pain catastrophizing compared to NHW adults [50], and could be added to existing guidelines for primary prevention of chronic pain that focus on pain education, modifiable lifestyle factors, and stress management [112].
Funding.
This work was supported, in part, by grants from the National Institutes of Health (U54 MD007593, U54MD007586, R01MH108155, R01MD010757, R01DA040966, R01HL164823). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics Approval
This study was approved by the institutional review boards (IRB) at Meharry Medical College and the University of Mississippi Medical Center.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
REFERENCES
- 1.Works T, et al. , Traumatic Exposure History as a Risk Factor for Chronic Pain in Adult Patients with Sickle Cell Disease. Health & Social Work, 2016. 41(1): p. 42–50. [DOI] [PubMed] [Google Scholar]
- 2.Institute of Medicine, Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. 2011. [PubMed]
- 3.Bevers K, Watts L, Kishino ND, & Gatchel RJ, The Biopsychosocial model of the assessment, prevention, and treatment of chronic pain.. US Neurology, 2016. 12(2): p. 98–104. [Google Scholar]
- 4.Rahim-Williams B, et al. , A Quantitative Review of Ethnic Group Differences in Experimental Pain Response: Do Biology, Psychology, and Culture Matter? Pain Medicine, 2012. 13(4): p. 522–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson KO, Green CR, and Payne R, Racial and ethnic disparities in pain: causes and consequences of unequal care. J Pain, 2009. 10(12): p. 1187–204. [DOI] [PubMed] [Google Scholar]
- 6.Day MA and Thorn BE, The relationship of demographic and psychosocial variables to pain-related outcomes in a rural chronic pain population. Pain, 2010. 151(2): p. 467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiske ST, What we know now about bias and intergroup conflict, the problem of the century. Current Directions in Psychological Science, 2002. 11(4): p. 123–128. [Google Scholar]
- 8.Williams DR, Neighbors HW, and Jackson JS, Racial/ethnic discrimination and health: Findings from community studies. American Journal of Public Health, 2003. 93(2): p. 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pieterse AL and Carter RT, An examination of the relationship between general life stress, racism-related stress, and psychological health among Black men. Journal of Counseling Psychology, 2007. 54(1): p. 101–109. [Google Scholar]
- 10.Pieterse AL, Carter RT, and Ray KV, Racism-Related Stress, General Life Stress, and Psychological Functioning Among Black American Women. Journal of Multicultural Counseling and Development, 2013. 41(1): p. 36–46. [Google Scholar]
- 11.You DS, et al. , Cumulative childhood adversity as a risk factor for common chronic pain conditions in young adults. Pain Medicine, 2019. 20(3): p. 486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown TT, et al. , Discrimination hurts: The effect of discrimination on the development of chronic pain. Soc Sci Med, 2018. 204: p. 1–8. [DOI] [PubMed] [Google Scholar]
- 13.Von Korff M, et al. , Childhood psychosocial stressors and adult onset arthritis: Broad spectrum risk factors and allostatic load. Pain, 2009. 143(1–2): p. 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imbierowicz K and Egle UT, Childhood adversities in patients with fibromyalgia and somatoform pain disorder. European Journal of Pain, 2003. 7(2): p. 113–119. [DOI] [PubMed] [Google Scholar]
- 15.Burke NN, et al. , Psychological stress in early life as a predisposing factor for the development of chronic pain: Clinical and preclinical evidence and neurobiological mechanisms. Journal of Neuroscience Research, 2016. [DOI] [PubMed] [Google Scholar]
- 16.Burgess DJ, et al. , The effect of perceived racial discrimination on bodily pain among older African American men. Pain Med, 2009. 10(8): p. 1341–52. [DOI] [PubMed] [Google Scholar]
- 17.Edwards RR, The association of perceived discrimination with low back pain. Journal of Behavioral Medicine, 2008. 31(5): p. 379–389. [DOI] [PubMed] [Google Scholar]
- 18.Williams DR, Lawrence JA, and Davis BA, Racism and health: evidence and needed research. Annual review of public health, 2019. 40: p. 105–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams DR and Mohammed SA, Discrimination and racial disparities in health: evidence and needed research. Journal of Behavioral Medicine, 2009. 32(1): p. 20–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myers HF, et al. , Cumulative burden of lifetime adversities: Trauma and mental health in low-SES African Americans and Latino/as. Psychol Trauma, 2015. 7(3): p. 243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carter RT, Racism and Psychological and Emotional Injury: Recognizing and Assessing Race-Based Traumatic Stress. The Counseling Psychologist, 2007. 35(1): p. 13–105. [Google Scholar]
- 22.Sorenson SB, Violence against women - Examining ethnic differences and commonalities. Evaluation Review, 1996. 20(2): p. 123–145. [DOI] [PubMed] [Google Scholar]
- 23.West CM, Black women and intimate partner violence - New directions for research. Journal of Interpersonal Violence, 2004. 19(12): p. 1487–1493. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs DE, Environmental Health Disparities in Housing. American Journal of Public Health, 2011. 101(S1): p. S115–S122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.York E and Hall M, Neighborhood problems across the rural-urban continuum: Geographic trends and racial and ethnic disparities. The ANNALS of the American Academy of Political and Social Science, 2017. 672(1): p. 238–256. [Google Scholar]
- 26.Churchwell K, et al. , Call to Action: Structural Racism as a Fundamental Driver of Health Disparities: A Presidential Advisory From the American Heart Association. Circulation, 2020. 142(24). [DOI] [PubMed] [Google Scholar]
- 27.Nelson CA, et al. , Adversity in childhood is linked to mental and physical health throughout life. BMJ, 2020: p. m3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buscemi V, et al. , The Role of Perceived Stress and Life Stressors in the Development of Chronic Musculoskeletal Pain Disorders: A Systematic Review. J Pain, 2019. 20(10): p. 1127–1139. [DOI] [PubMed] [Google Scholar]
- 29.Davis DA, Luecken LJ, and Zautra AJ, Are Reports of Childhood Abuse Related to the Experience of Chronic Pain in Adulthood?: A Meta-analytic Review of the Literature. The Clinical Journal of Pain, 2005. 21(5): p. 398–405. [DOI] [PubMed] [Google Scholar]
- 30.Marin TJ, et al. , A Systematic Review of the Prospective Relationship between Child Maltreatment and Chronic Pain. Children, 2021. 8(9): p. 806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodin BR, et al. , Perceived racial discrimination, but not mistrust of medical researchers, predicts the heat pain tolerance of African Americans with symptomatic knee osteoarthritis. Health Psychol, 2013. 32(11): p. 1117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burgess DJ, et al. , The association between perceived discrimination and underutilization of needed medical and mental health care in a multi-ethnic community sample. Journal of Health Care for the Poor and Underserved, 2008. 19(3): p. 894–911. [DOI] [PubMed] [Google Scholar]
- 33.Walker Taylor JL, et al. , Pain, Racial Discrimination, and Depressive Symptoms among African American Women. Pain Management Nursing, 2018. 19(1): p. 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith KE and Pollak SD, Rethinking concepts and categories for understanding the neurodevelopmental effects of childhood adversity. Perspectives on psychological science, 2021. 16(1): p. 67–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petrini L and Arendt-Nielsen L, Understanding Pain Catastrophizing: Putting Pieces Together. Frontiers in Psychology, 2020. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quartana PJ, Campbell CM, and Edwards RR, Pain catastrophizing: a critical review. Expert Review of Neurotherapeutics, 2009. 9(5): p. 745–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sullivan MJL, Bishop SR, and Pivik J, The Pain Catastrophizing Scale: Development and validation. Psychological Assessment, 1995. 7(4): p. 524–532. [Google Scholar]
- 38.Ikemoto T, et al. , A systematic review of cross-cultural validation of the pain catastrophizing scale. European Journal of Pain, 2020. 24(7): p. 1228–1241. [DOI] [PubMed] [Google Scholar]
- 39.Katz J, et al. , Pain catastrophizing as a risk factor for chronic pain after total knee arthroplasty: a systematic review. Journal of Pain Research, 2015: p. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinez-Calderon J, et al. , Pain Catastrophizing and Function In Individuals With Chronic Musculoskeletal Pain: A Systematic Review and Meta-Analysis. The Clinical Journal of Pain, 2019. 35(3): p. 279–293. [DOI] [PubMed] [Google Scholar]
- 41.Meints SM, et al. , The relationship between catastrophizing and altered pain sensitivity in patients with chronic low-back pain. Pain, 2019. 160(4): p. 833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sullivan MJL, et al. , Theoretical Perspectives on the Relation Between Catastrophizing and Pain. The Clinical Journal of Pain, 2001. 17(1): p. 52–64. [DOI] [PubMed] [Google Scholar]
- 43.Turk DC and Okifuji A, Psychological factors in chronic pain: Evolution and revolution. Journal of Consulting and Clinical Psychology, 2002. 70(3): p. 678–690. [DOI] [PubMed] [Google Scholar]
- 44.Macdonald TM, et al. , The association between childhood maltreatment and pain catastrophizing in individuals with immune-mediated inflammatory diseases. Journal of Psychosomatic Research, 2021. 145: p. 110479. [DOI] [PubMed] [Google Scholar]
- 45.Sansone RA, Watts DA, and Wiederman MW, Childhood trauma and pain and pain catastrophizing in adulthood: a cross-sectional survey study. Prim Care Companion CNS Disord, 2013. 15(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taghian NR, et al. , Associations between Childhood Abuse and Chronic Pain in Adults with Substance Use Disorders. Substance Use & Misuse, 2021. 56(1): p. 87–92. [DOI] [PubMed] [Google Scholar]
- 47.Horsham S and Chung MC, Investigation of the relationship between trauma and pain catastrophising: The roles of emotional processing and altered self-capacity. Psychiatry Research, 2013. 208(3): p. 274–284. [DOI] [PubMed] [Google Scholar]
- 48.Terry EL, et al. , Everyday Discrimination in Adults with Knee Pain: The Role of Perceived Stress and Pain Catastrophizing. Journal of Pain Research, 2020. Volume 13: p. 883–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richardson EJ, et al. , The Negative Effect of Social Discrimination on Pain Tolerance and the Moderating Role of Pain Catastrophizing. Journal of Clinical Psychology in Medical Settings, 2022. [DOI] [PubMed] [Google Scholar]
- 50.Meints SM, Miller MM, and Hirsh AT, Differences in pain coping between black and white Americans: a meta-analysis. The Journal of pain, 2016. 17(6): p. 642–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith BW and Zautra AJ, Vulnerability and resilience in women with arthritis: Test of a two-factor model. Journal of Consulting and Clinical Psychology, 2008. 76(5): p. 799–810. [DOI] [PubMed] [Google Scholar]
- 52.Sturgeon JA and Zautra AJ, Psychological Resilience, Pain Catastrophizing, and Positive Emotions: Perspectives on Comprehensive Modeling of Individual Pain Adaptation. Current Pain and Headache Reports, 2013. 17(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kratz AL, Davis MC, and Zautra AJ, Pain acceptance moderates the relation between pain and negative affect in female osteoarthritis and fibromyalgia patients. Annals of Behavioral Medicine, 2007. 33(3): p. 291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vowles KE, McCracken LM, and Eccleston C, Processes of change in treatment for chronic pain: The contributions of pain, acceptance, and catastrophizing. European Journal of Pain, 2007. 11(7): p. 779–787. [DOI] [PubMed] [Google Scholar]
- 55.Smith BW, et al. , The Role of Resilience and Purpose in Life in Habituation to Heat and Cold Pain. The Journal of Pain, 2009. 10(5): p. 493–500. [DOI] [PubMed] [Google Scholar]
- 56.Wright LJ, Zautra AJ, and Going S, Adaptation to early knee osteoarthritis: The role of risk, resilience, and disease severity on pain and physical functioning. Annals of Behavioral Medicine, 2008. 36(1): p. 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramírez-Maestre C, Esteve R, and López AE, The Path to Capacity: Resilience and Spinal Chronic Pain. Spine, 2012. 37(4): p. E251–E258. [DOI] [PubMed] [Google Scholar]
- 58.Zautra AJ, Johnson LM, and Davis MC, Positive affect as a source of resilience for women in chronic pain. Journal of Consulting and Clinical Psychology, 2005. 73(2): p. 212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dienstbier RA, Arousal and physiological toughness: Implications for mental and physical health. Psychological Review, 1989. 96(1): p. 84–100. [DOI] [PubMed] [Google Scholar]
- 60.Seery MD, et al. , Lifetime exposure to adversity predicts functional impairment and healthcare utilization among individuals with chronic back pain. Pain, 2010. 150(3): p. 507–515. [DOI] [PubMed] [Google Scholar]
- 61.Amtmann D, et al. , Development of a PROMIS item bank to measure pain interference. Pain, 2010. 150(1): p. 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meints SM and Hirsh AT, In Vivo Praying and Catastrophizing Mediate the Race Differences in Experimental Pain Sensitivity. The Journal of Pain, 2015. 16(5): p. 491–497. [DOI] [PubMed] [Google Scholar]
- 63.Meints SM, et al. , Pain-Related Rumination, But Not Magnification or Helplessness, Mediates Race and Sex Differences in Experimental Pain. The Journal of Pain, 2017. 18(3): p. 332–339. [DOI] [PubMed] [Google Scholar]
- 64.Forsythe LP, et al. , Race and Sex Differences in Primary Appraisals, Catastrophizing, and Experimental Pain Outcomes. Journal of Pain, 2011. 12(5): p. 563–572. [DOI] [PubMed] [Google Scholar]
- 65.Bruehl S, et al. , How accurate are parental chronic pain histories provided by offspring? Pain, 2005. 115(3): p. 390–397. [DOI] [PubMed] [Google Scholar]
- 66.Melzack R, THE SHORT-FORM MCGILL PAIN QUESTIONNAIRE. Pain, 1987. 30(2): p. 191–197. [DOI] [PubMed] [Google Scholar]
- 67.National Institutes of Health, http://www.nihpromis.org/,.
- 68.Bernstein DP, et al. , INITIAL RELIABILITY AND VALIDITY OF A NEW RETROSPECTIVE MEASURE OF CHILD-ABUSE AND NEGLECT. American Journal of Psychiatry, 1994. 151(8): p. 1132–1136. [DOI] [PubMed] [Google Scholar]
- 69.Kessler RC and Magee WJ, CHILDHOOD ADVERSITIES AND ADULT DEPRESSION - BASIC PATTERNS OF ASSOCIATION IN A UNITED-STATES NATIONAL SURVEY. Psychological Medicine, 1993. 23(3): p. 679–690. [DOI] [PubMed] [Google Scholar]
- 70.Gurung RAR, et al. , “HIV is not my biggest problem: The impact of HIV and chronic burden on depression in women at risk for AIDS. Journal of Social and Clinical Psychology, 2004. 23(4): p. 490–511. [Google Scholar]
- 71.Cohen S, Kamarck T, and Mermelstein R, A GLOBAL MEASURE OF PERCEIVED STRESS. Journal of Health and Social Behavior, 1983. 24(4): p. 385–396. [PubMed] [Google Scholar]
- 72.Brondolo E, et al. , The perceived ethnic discrimination questionnaire: Development and preliminary validation of a community version. Journal of Applied Social Psychology, 2005. 35(2): p. 335–365. [Google Scholar]
- 73.Slepian PM, et al. , Development and initial validation of the pain resilience scale. The Journal of Pain, 2016. 17(4): p. 462–472. [DOI] [PubMed] [Google Scholar]
- 74.Rutter M, Stress, coping and development: Some issues and some questions. Journal of child psychology and psychiatry, 1981. 22(4): p. 323–356. [DOI] [PubMed] [Google Scholar]
- 75.Ettekal I, et al. , Comparing alternative methods of measuring cumulative risk based on multiple risk indicators: Are there differential effects on children’s externalizing problems? PloS one, 2019. 14(7): p. e0219134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stern KR and Thayer ZM, Adversity in childhood and young adulthood predicts young adult depression. International journal of public health, 2019. 64(7): p. 1069–1074. [DOI] [PubMed] [Google Scholar]
- 77.Klaassens ER, et al. , Adulthood trauma and HPA-axis functioning in healthy subjects and PTSD patients: a meta-analysis. Psychoneuroendocrinology, 2012. 37(3): p. 317–31. [DOI] [PubMed] [Google Scholar]
- 78.Tarullo AR and Gunnar MR, Child maltreatment and the developing HPA axis. Hormones and behavior, 2006. 50(4): p. 632–639. [DOI] [PubMed] [Google Scholar]
- 79.Hayes AF, Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. 2017: Guilford publications. [Google Scholar]
- 80.Fillingim RB, Individual differences in pain: understanding the mosaic that makes pain personal. Pain, 2017. 158(Suppl 1): p. S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Benjamini Y and Hochberg Y, CONTROLLING THE FALSE DISCOVERY RATE - A PRACTICAL AND POWERFUL APPROACH TO MULTIPLE TESTING. Journal of the Royal Statistical Society Series B-Methodological, 1995. 57(1): p. 289–300. [Google Scholar]
- 82.Sturgeon JA and Zautra AJ, Resilience: a new paradigm for adaptation to chronic pain. Current pain and headache reports, 2010. 14(2): p. 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Goosby BJ, Cheadle JE, and Mitchell C, Stress-related biosocial mechanisms of discrimination and African American health inequities. Annual Review of Sociology, 2018. 44: p. 319–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sng BL, et al. , Incidence and risk factors for chronic pain after caesarean section under spinal anaesthesia. Anaesthesia and Intensive Care, 2009. 37(5): p. 748–752. [DOI] [PubMed] [Google Scholar]
- 85.Williams RA, et al. , The contribution of job satisfaction to the transition from acute to chronic low back pain. Archives of Physical Medicine and Rehabilitation, 1998. 79(4): p. 366–374. [DOI] [PubMed] [Google Scholar]
- 86.Huber FA, et al. , The association between adverse life events, psychological stress, and pain-promoting affect and cognitions in native Americans: results from the Oklahoma study of native American pain risk. Journal of Racial and Ethnic Health Disparities, 2022. 9(1): p. 215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dumenci L, et al. , Disentangling trait versus state characteristics of the Pain Catastrophizing Scale and the PHQ‐8 Depression Scale. European Journal of Pain, 2020. 24(8): p. 1624–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eisenberger NI, Social pain and the brain: controversies, questions, and where to go from here. Annual review of psychology, 2015. 66(1): p. 601–629. [DOI] [PubMed] [Google Scholar]
- 89.MacDonald GE and Jensen-Campbell LA, Social pain: Neuropsychological and health implications of loss and exclusion. 2011: American Psychological Association. [Google Scholar]
- 90.Sturgeon JA and Zautra AJ, Social pain and physical pain: shared paths to resilience. Pain management, 2016. 6(1): p. 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gellatly R and Beck AT, Catastrophic thinking: A transdiagnostic process across psychiatric disorders. Cognitive Therapy and Research, 2016. 40(4): p. 441–452. [Google Scholar]
- 92.Vlaeyen JW and Linton SJ, Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain, 2000. 85(3): p. 317–332. [DOI] [PubMed] [Google Scholar]
- 93.Picavet HSJ, Vlaeyen JW, and Schouten JS, Pain catastrophizing and kinesiophobia: predictors of chronic low back pain. American journal of epidemiology, 2002. 156(11): p. 1028–1034. [DOI] [PubMed] [Google Scholar]
- 94.Crombez G, et al. , The effects of catastrophic thinking about pain on attentional interference by pain: no mediation of negative affectivity in healthy volunteers and in patients with low back pain. Pain research and management, 2002. 7(1): p. 31–39. [DOI] [PubMed] [Google Scholar]
- 95.Eccleston C and Crombez G, Pain demands attention: A cognitive–affective model of the interruptive function of pain. Psychological bulletin, 1999. 125(3): p. 356. [DOI] [PubMed] [Google Scholar]
- 96.Heathcote LC, et al. , The relationship between adolescents’ pain catastrophizing and attention bias to pain faces is moderated by attention control. Pain, 2015. 156(7): p. 1334–41. [DOI] [PubMed] [Google Scholar]
- 97.Crombez G, et al. , Attentional bias to pain-related information: a meta-analysis. Pain, 2013. 154(4): p. 497–510. [DOI] [PubMed] [Google Scholar]
- 98.Ankawi B, et al. , Validation of the pain resilience scale in a chronic pain sample. The Journal of Pain, 2017. 18(8): p. 984–993. [DOI] [PubMed] [Google Scholar]
- 99.Masten AS, Ordinary magic: Resilience processes in development. American psychologist, 2001. 56(3): p. 227. [DOI] [PubMed] [Google Scholar]
- 100.Karoly P and Ruehlman LS, Psychological “resilience” and its correlates in chronic pain: findings from a national community sample. Pain, 2006. 123(1–2): p. 90–97. [DOI] [PubMed] [Google Scholar]
- 101.Gonzalez CE, et al. , Pain-specific resilience in people living with HIV and chronic pain: beneficial associations with coping strategies and catastrophizing. Frontiers in psychology, 2019. 10: p. 2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Slepian PM, Ankawi B, and France CR, Longitudinal analysis supports a fear-avoidance model that incorporates pain resilience alongside pain catastrophizing. Annals of Behavioral Medicine, 2020. 54(5): p. 335–345. [DOI] [PubMed] [Google Scholar]
- 103.Ong AD, Zautra AJ, and Reid MC, Psychological resilience predicts decreases in pain catastrophizing through positive emotions. Psychology and aging, 2010. 25(3): p. 516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Colloca L, et al. , Placebo analgesia: psychological and neurobiological mechanisms. Pain, 2013. 154(4): p. 511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vervoort T and Trost Z, Examining affective-motivational dynamics and behavioral implications within the interpersonal context of pain. The Journal of Pain, 2017. 18(10): p. 1174–1183. [DOI] [PubMed] [Google Scholar]
- 106.Felitti VJ, et al. , Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults - The adverse childhood experiences (ACE) study. American Journal of Preventive Medicine, 1998. 14(4): p. 245–258. [DOI] [PubMed] [Google Scholar]
- 107.Dube SR, et al. , Assessing the reliability of retrospective reports of adverse childhood experiences among adult HMO members attending a primary care clinic. Child Abuse & Neglect, 2004. 28(7): p. 729–737. [DOI] [PubMed] [Google Scholar]
- 108.Eccleston C, de C Williams AC, and Morley S, Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane database of systematic reviews, 2009(2). [DOI] [PubMed] [Google Scholar]
- 109.Chou R, et al. , Nonpharmacologic therapies for low back pain: a systematic review for an American College of Physicians clinical practice guideline. Annals of internal medicine, 2017. 166(7): p. 493–505. [DOI] [PubMed] [Google Scholar]
- 110.Gee GC and Ford CL, Structural racism and health inequities: Old issues, New Directions. Du Bois Review: Social Science Research on Race, 2011. 8(1): p. 115–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schütze R, et al. , How can we best reduce pain catastrophizing in adults with chronic noncancer pain? A systematic review and meta-analysis. The journal of Pain, 2018. 19(3): p. 233–256. [DOI] [PubMed] [Google Scholar]
- 112.Gatchel RJ, et al. , Research agenda for the prevention of pain and its impact: report of the work group on the prevention of acute and chronic pain of the Federal Pain Research Strategy. The Journal of Pain, 2018. 19(8): p. 837–851. [DOI] [PubMed] [Google Scholar]


