Summary
Background
Evidence is needed to inform thresholds for glycemic management in neonatal encephalopathy (NE). We investigated how severity and duration of dysglycemia relate to brain injury after NE.
Methods
A prospective cohort of 108 neonates ≥36 weeks gestational age with NE were enrolled between August 2014 and November 2019 at the Hospital for Sick Children, in Toronto, Canada. Participants underwent continuous glucose monitoring for 72 h, MRI at day 4 of life, and follow-up at 18 months. Receiver operating characteristic curves were used to assess the predictive value of glucose measures (minimum and maximum glucose, sequential 1 mmol/L glucose thresholds) during the first 72 h of life (HOL) for each brain injury pattern (basal ganglia, watershed, focal infarct, posterior-predominant). Linear and logistic regression analyses were used to assess the relationship between abnormal glycemia and 18-month outcomes (Bayley-III composite scores, Child Behavior Checklist [CBCL] T-scores, neuromotor score, cerebral palsy [CP], death), adjusting for brain injury severity.
Findings
Of 108 neonates enrolled, 102 (94%) had an MRI. Maximum glucose during the first 48 HOL best predicted basal ganglia (AUC = 0.811) and watershed (AUC = 0.858) injury. Minimum glucose was not predictive of brain injury (AUC <0.509). Ninety-one (89%) infants underwent follow-up assessments at 19.0 ± 1.7 months. A glucose threshold of >10.1 mmol/L during the first 48 HOL was associated with 5.8-point higher CBCL Internalizing Composite T-score (P = 0.029), 0.3-point worse neuromotor score (P = 0.035), 8.6-fold higher odds for CP diagnosis (P = 0.014). While the glucose threshold of >10.1 mmol/L during the first 48 HOL was associated with higher odds of the composite outcome of severe disability or death (OR 3.0, 95% CI 1.0–8.4, P = 0.042), it was not associated with the composite outcome of moderate-to-severe disability or death (OR 0.9, 95% CI 0.4–2.2, P = 0.801). All associations with outcome lost significance after adjusting for brain injury severity.
Interpretation
Maximum glucose concentration in the first 48 HOL is predictive of brain injury after NE. Further trials are needed to assess if protocols to control maximum glucose concentrations improve outcomes after NE.
Funding
Canadian Institutes for Health Research, National Institutes of Health, and SickKids Foundation.
Keywords: Hypoglycemia, Hyperglycaemia, Neonatal neurology, Neonatal encephalopathy, Hypoxic ischemic encephalopathy, MRI, Neurodevelopmental outcomes
Research in context.
Evidence before this study
Hypoglycemia and hyperglycemia are common in patients with neonatal encephalopathy, and have been associated with unfavourable neurophysiological changes, neonatal brain injury, and adverse outcomes. However, evidence informing optimal glycemic thresholds for intervention and neuroprotective glucose management strategies for patients with neonatal encephalopathy is lacking.
Added value of this study
The present study is the first to quantify the relationship between severity and duration of abnormal glycemia in the context of neonatal encephalopathy and outcomes. A glucose threshold of 10.1 mmol/L during the first 48 h of life is a good cut-off for predicting brain injury on magnetic resonance imaging (MRI). Hyperglycemia above 10.1 mmol/L was associated with neuromotor score, internalizing problems, cerebral palsy diagnosis, and a composite outcome of severe disability or death by 18 months, but all outcomes were fully explained by brain injury severity on MRI. Further, we found that during the first 48 h of life, severity of hyperglycemia was inversely related to duration of exposure for predicting the presence of basal ganglia and watershed injury patterns, suggesting that both severity and duration of hyperglycemia should be considered when developing glucose management strategies.
Implications of all the available evidence
Our study uniquely provides evidence for a relationship between severity and duration of hyperglycemia associated with brain injury in the context of neonatal encephalopathy. Randomized controlled trials are needed to assess if protocols to control maximum glucose concentrations can improve outcomes after neonatal encephalopathy.
Introduction
Hypoxic-ischemic encephalopathy (HIE) is the most common cause of neonatal encephalopathy (NE) and is the greatest contributor to disability worldwide.1 Although therapeutic hypothermia has significantly reduced death and disability for newborns with HIE, cooling is only partially neuroprotective,2 justifying the pursuit for modifiable risk factors.
The neonatal brain relies primarily on glucose as the substrate for metabolism.3,4 As newborns transition to extrauterine life, the source of glucose imperative for glucose homeostasis shifts from the continuous placental supply of glucose to the newborn's hepatic glycogen stores.5,6 A healthy newborn can adapt during the transient hypoxic environment of typical labour via anaerobic glycolysis.7 A neonate with HIE, however, undergoes a prolonged period of anaerobic glycolysis, leading to depletion of hepatic glycogen reserves, and hepatic glucose production that cannot meet cerebral metabolic demands.3,6,7 Moreover, in the context of HIE, hyperglycemia might result from a reduced net metabolism of severely damaged tissues or a prolonged rise in stress hormones, and may be further prolonged by therapeutic hypothermia.8,9 Both hypoglycemia and hyperglycemia are potential candidate modifiable risk factors since they are prevalent in NE, and in the era of therapeutic hypothermia, have been associated with unfavourable neurophysiological changes in the neonatal period,10,11 brain injury on neonatal MRI,12,13 and adverse developmental outcomes at preschool age.4,6,14
Evidence informing optimal glycemic thresholds for intervention and neuroprotective glucose management strategies in at risk neonates is limited and conflicting.14,15 Furthermore, normal glucose reference ranges for otherwise healthy term neonates may not be the same for newborns at risk for impaired metabolic adaptation because vulnerability to brain injury and adverse outcomes can differ based on comorbidities, and an individual's ability to make and utilize alternative fuels.15,16 Accordingly, the primary study aim is to utilize continuous glucose monitoring to investigate how severity and duration of hypoglycemia and hyperglycemia relate to brain injury after NE, with the goal of identifying clinically relevant parameters to guide patient management. We hypothesize that there is a threshold severity and duration of hypoglycemia and hyperglycemia that is associated with brain injury on conventional post-rewarming neonatal MRI. In addition, secondary analyses will assess how this identify threshold relates to 18-month neurodevelopmental outcomes in this patient population.
Methods
Study population
A prospective cohort of 108 neonates (≥36 weeks gestational age), born between August 2014 and November 2019, who had an abnormal level of consciousness in addition to seizures and/or abnormalities in tone and/or reflexes, and were admitted to the Neonatal Intensive Care Unit (NICU) at a single centre, The Hospital for Sick Children, were studied. Newborns were excluded for suspected/confirmed congenital malformation, inborn error of metabolism, or congenital infection, or if they weighed <1500 g or could not have the continuous glucose monitor (CGM) inserted within 6 h of life (HOL). A weight cut-off of 1500 g was used based on prior experience with the CGM for patient safety. Neonates eligible/ineligible for therapeutic hypothermia were enrolled. Study data were collected from patient medical records and transport records, and were managed using research electronic data capture (REDCap, Vanderbilt University, Tennessee) hosted at The Hospital for Sick Children.17 Of note, severity of encephalopathy prior to hypothermia was scored using a modified Sarnat scale retrospectively by a single neurologist (EWYT) based on these medical records.
Our institutional protocols for HIE patients undergoing therapeutic hypothermia during this study period include no enteral feeding and total fluid intake (TFI) starting at 60 ml/kg/day followed by titration depending on urine output, renal function, and management of glucose infusion rates (GIR).
Ethics statement
Written consent was obtained from parents/guardians for all participants following a protocol approved by the research ethics board (REB approval number 1000039068) of The Hospital for Sick Children (Toronto, Canada).
Glucose data
All clinically-ordered laboratory and point-of-care testing blood glucose values were collected from the referral and admission hospitals, and were used as study data when CGM data were unavailable (e.g., before CGM application). Glucose values obtained using i-STAT (Abbott Laboratories, Abbott Park, Illinois) were treated as laboratory values due to the gold standard use of glucose oxidase reaction.
All neonates had a CGM comprised of an Enlite™ Sensor (Medtronic Canada, Brampton, Ontario) connected to a blinded Medtronic iPro™2 professional CGM (Medtronic Canada, Brampton, Ontario) inserted into the lateral aspect of the thigh within the first few HOL for approximately 72 h of monitoring. The CGM stores interstitial glucose concentrations and reports averaged glucose data every 5 min once data are uploaded to the Medtronic CareLink™ iPro™ software (Medtronic MiniMed, Northridge, California). Laboratory blood glucose values collected on admission and every 12 HOL as part of standard of care were used to calibrate the CGM. Due to software limitations for determining interstitial glucose concentrations <2.2 and >22.2 mmol/L (<40 and >400 mg/dL), interstitial glucose readings initially reported as 2.2 or 22.2 mmol/L underwent point-to-point verification or manual correction using an equation provided by Medtronic to better estimate their interstitial glucose concentrations.
Since CGM data were unavailable real-time, clinicians detected and treated hypoglycemia and hyperglycemia using intermittent glucose testing collected as part of standard of care. Our institutional protocols for HIE include laboratory glucose testing on admission followed by subsequent testing every 12 h. Glucose levels ≤2.6 mmol/L (≤47 mg/dL) are treated with dextrose boluses and increased GIRs, escalating to glucagon if needed. There is no protocol and no agreed upon threshold for the management of hyperglycemia, with treatment left up to the discretion of the clinical team, including observation, decreased GIRs, or insulin administration.
Minimum and maximum glucose concentrations for the first 12, 24, 48, and 72 HOL for each subject were determined using CGM data when available, supplemented by clinically-ordered laboratory or point-of-care or blood tests when not available (e.g., before CGM initiation, technical gaps in CGM data).
MRI studies
Post-rewarming MRI scans were performed at around day 4 of life18, 19, 20, 21 on a 3T or 1.5T MRI scanner (Siemens Magnetom Prisma or Skyra or Philips Achieva). MRI sequences included: axial volumetric 3-dimensional Fast Low Angle Shot T1-weighted images, axial spin-echo T2-weighted images, and axial diffusion-weighted images (DWI). Blinded to patient history, one pediatric neuroradiologist (EW) reviewed and visually scored basal ganglia and watershed brain injury using the Barkovich scoring system.22 Brain MRIs were then coded as yes/no for exhibiting basal ganglia, watershed, focal infarct, and posterior-predominant injury pattern (posterior predominant white and/or grey matter and/or in the pulvinar and/or anterior medial nuclei of the thalamus previously reported to be associated with neonatal hypoglycemia23).
Neurodevelopmental assessments
As part of standard of care, from March 2016 through May 2021, infants were assessed once by a trained assessor supervised by a registered psychologist at 18–25 months old with the Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III), generating cognitive, language, and motor composite scores according to the scale mean [ ± standard deviation (SD)] of 100 ± 15, with lower scores indicating greater impairment. For infants with cerebral palsy (CP) who could not be tested with the Bayley-III, a cognitive, language, and motor composite score of 70 (−2 SD on Bayley-III) was assigned for infants with a severity of CP classified as Gross Motor Function Classification System (GMFCS) III (n = 1), and composite scores of 45 (−3 SD) were assigned for GMFCS IV-V (n = 1), in accordance with previous literature.24 Infants unable to engage in testing were assigned a motor composite score of 100 if they walked independently by 14 months old (n = 3), and a language composite score of 70 if they were non-verbal at the time of the evaluation (i.e., had not developed any intelligible expressive language; n = 2). Additionally, infants were scored (yes/no) for having moderate-severe disability or death (received ANY Bayley-III composite score ≤85 or had died prior to follow-up) and for having severe disability or death (received ANY Bayley-III composite score ≤70 or had died prior to follow-up were included). Infants also underwent a standardized neurological examination administered by a pediatric neurologist blinded to patient history, yielding a neuromotor score25 and determined the presence/absence of CP. Parents/guardians competed the Child Behavior Checklist, 1.5–5 years (CBCL),26 a standardized parental questionnaire that reports on emotional, social and behavioural problems, where raw scores are summed and converted to age- and sex-specific normalized T-scores. Composite T-scores for Internalizing (Anxiety/depression, Withdrawal, and Somatic complaints) and Externalizing (Attention problems, Aggressive behavior, and Rule-breaking) problems are considered clinically significant when >69 with 65–69 in the borderline clinical range.
Statistical analysis
Statistical analyses were performed using R version 3.5.2 (The R Foundation for Statistical Computing 2018, Vienna, Austria). Regression analyses were used to identify significant confounders for brain injury patterns. Receiver operating characteristic (ROC) curves were used to assess the predictive value of minimum and maximum glucose measures, as well as the duration of hyperglycemia above sequential 1 mmol/L glucose thresholds, with candidate values of 7–10 mmol/L [126–180 mg/dL]) during the first 12, 24, 48, and 72 HOL for each brain injury pattern. Linear and logistic regression analyses were used to assess the relationship between a glucose threshold based on the ROC curve findings and 18-month neurodevelopmental outcomes (Bayley-III cognitive, language, and motor composite scores, moderate-severe disability or death, severe disability or death, CBCL Internalizing and Externalizing Composite T-scores, neuromotor score, CP diagnosis, and death prior to follow-up), adjusting for brain injury severity (basal ganglia and watershed) as per the Barkovich scoring system.22 Briefly, the brain injury severity score is a composite score of basal ganglia and watershed injury based on injury observed on T1, T2, and DWI MRI sequences, with higher scores representing more severe injury. Associations were considered significant at P < 0.050.
Role of the funding source
None of the funders had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Results
Study cohort
Of 182 neonates approached, 112 (62%) were consented. Of the 112, 108 (96%) were enrolled (excluded: 2 had congenital malformations, 1 was discharged prior to any study tests, 1 had contraindication with CGM). Of 108 enrolled, 102 (94%) had MRIs (6 had no MRI prior to death (n = 4) or discharge (n = 2)) at a median age of 5 (interquartile range [IQR], 4-5) days. Of 102 neonates, 97 (95%) underwent 72 h of therapeutic hypothermia, 4 (4%) were rewarmed early between 3 and 34 HOL, and 1 (1%) was not cooled as per the clinical team. Of the neonates rewarmed early, 2 were deemed clinically too well to continue undergoing therapeutic hypothermia, 1 was discontinued due to excessive hemorrhages from disseminated intravascular coagulation, and 1 was transferred to a palliative care home but since survived. The lowest birthweight in this cohort was 2020 g. The study cohort is representative of patients with neonatal encephalopathy at outborn NICUs. Participant demographics are summarized in Table 1.
Table 1.
Participant demographics.
| Participants enrolled (N = 108) | Participants with MRI (N = 102) | |
|---|---|---|
| Male sex, frequency (%) | 67 (62%) | 64 (63%) |
| Birth weight, mean (SD), g | 3356.5 ± 518.2 | 3360.5 ± 528.5 |
| Head circumference, mean (SD), cm | 34.1 ± 1.4 | 34.1 ± 1.4 |
| Umbilical arterial cord pH, mean (SD) | 7.01 ± 0.16 | 7.01 ± 0.15 |
| Umbilical arterial cord BE, mean (SD) | −15.33 ± 6.69 | −15.17 ± 6.66 |
| Apgar score at 5 min, mean (SD) | 4.2 ± 2.3 | 4.3 ± 2.3 |
| Sarnat score (mild, moderate, severe), frequency (%) | 3 (3%), 90 (83%), 15 (14%) | 3 (3%), 88 (86%), 11 (11%) |
| Gestational age at birth, mean (SD), wk | 39.6 ± 1.4 | 39.6 ± 1.3 |
| Age at first glucose data, median (IQR), h | 1.0 (0.5–2.6) | 1.0 (0.5–2.7) |
| Age at first CGM data, median (IQR), h | 7.7 (6.7–9.0) | 7.8 (6.8–9.0) |
| Day of life at MRI, median (IQR), days | 5 (4-5) | 5 (4-5) |
| Glucose | ||
| First 12 h of life | ||
| Minimum glucose, median (IQR), mmol/L | 3.8 (2.7–4.6) | 3.7 (2.5–4.6) |
| Maximum glucose, median (IQR), mmol/L | 7.0 (5.8–9.4) | 6.9 (5.7–9.3) |
| SD, mean (SD), mmol/L | 1.0 ± 0.7 | 1.0 ± 0.7 |
| Coefficient of variation (SD/mean), mean (SD) | 0.2 ± 0.1 | 0.2 ± 0.1 |
| Hypoglycemia on intermittent testing (n in N (%)) | 71 in 26 (24%) | 71 in 26 (25%) |
| Hypoglycemia on CGM (n in N (%)) | 38 in 1 (1%) | 38 in 1 (1%) |
| Hyperglycemia on intermittent testing (n in N (%)) | 63 in 32 (30%) | 57 in 27 (26%) |
| Hyperglycemia on CGM (n in N (%)) | 358 in 19 (18%) | 319 in 15 (15%) |
| First 24 h of life | ||
| Minimum glucose, median (IQR), mmol/L | 3.5 (2.5–3.9) | 3.4 (2.4–3.8) |
| Maximum glucose, median (IQR), mmol/L | 7.7 (5.9–9.9) | 7.4 (5.8–9.9) |
| SD, mean (SD), mmol/L | 1.0 ± 0.9 | 0.9 ± 0.9 |
| Coefficient of variation (SD/mean), mean (SD) | 0.2 ± 0.1 | 0.2 ± 0.1 |
| Hypoglycemia on intermittent testing (n in N (%)) | 81 in 27 (25%) | 79 in 26 (25%) |
| Hypoglycemia on CGM (n in N (%)) | 177 in 5 (5%) | 177 in 5 (5%) |
| Hyperglycemia on intermittent testing (n in N (%)) | 65 in 33 (31%) | 59 in 28 (27%) |
| Hyperglycemia on CGM (n in N (%)) | 1512 in 26 (24%) | 1334 in 22 (22%) |
| First 48 h of life | ||
| Minimum glucose, median (IQR), mmol/L | 3.2 (2.3–3.8) | 3.4 (2.4–3.8) |
| Maximum glucose, median (IQR), mmol/L | 7.8 (6.3–11.5) | 7.4 (5.8–9.9) |
| SD, mean (SD), mmol/L | 1.2 ± 1.2 | 1.2 ± 1.1 |
| Coefficient of variation (SD/mean), mean (SD) | 0.2 ± 0.1 | 0.2 ± 0.1 |
| Hypoglycemia on intermittent testing (n in N (%)) | 85 in 27 (25%) | 83 in 26 (25%) |
| Hypoglycemia on CGM (n in N (%)) | 359 in 12 (11%) | 343 in 11 (11%) |
| Hyperglycemia on intermittent testing (n in N (%)) | 68 in 34 (31%) | 62 in 29 (28%) |
| Hyperglycemia on CGM (n in N (%)) | 3838 in 33 (31%) | 3449 in 28 (27%) |
| First 72 h of life | ||
| Minimum glucose, median (IQR), mmol/L | 2.9 (2.2–3.4) | 3.1 (2.3–3.7) |
| Maximum glucose, median (IQR), mmol/L | 8.2 (6.3–11.5) | 7.7 (6.3–11.0) |
| SD, mean (SD), mmol/L | 1.2 ± 1.2 | 1.2 ± 1.1 |
| Coefficient of variation (SD/mean), mean (SD) | 0.2 ± 0.1 | 0.2 ± 0.1 |
| Hypoglycemia on intermittent testing (n in N (%)) | 86 in 28 (26%) | 84 in 27 (26%) |
| Hypoglycemia on CGM (n in N (%)) | 460 in 14 (13%) | 444 in 13 (13%) |
| Hyperglycemia on intermittent testing (n in N (%)) | 74 in 36 (33%) | 68 in 31 (30%) |
| Hyperglycemia on CGM (n in N (%)) | 4100 in 35 (32%) | 3711 in 30 (29%) |
| Brain injury patterna | ||
| Basal ganglia, frequency (%) | 24 (22%) | 24 (24%) |
| Watershed, frequency (%) | 17 (16%) | 17 (17%) |
| Focal infarct, frequency (%) | 15 (14%) | 15 (15%) |
| Posterior-predominant, frequency (%) | 31 (29%) | 31 (30%) |
BE, base excess; CGM, continuous glucose monitor; IQR, interquartile range; MRI, magnetic resonance imaging; SD, standard deviation.
Hypoglycemia ≤2.6 mmol/L and hyperglycemia >8.0 mmol/L.
n in N (%) means number of episodes in number (percent) of subjects.
Brain injury pattern categories are not mutually exclusive.
Glucose and brain injury on MRI
Of the 102 neonates who had MRIs, 46 (45%) had an abnormal MRI. Twenty-four (24%) neonates had basal ganglia and 17 (17%) neonates had watershed injury pattern, including 16 (16%) neonates who demonstrated both injury patterns (Supplemental Table S1). Linear regression analyses of the relationship between glucose measures during the first 12, 24, 48, and 72 HOL and each brain injury pattern demonstrated that sex, head circumference, birth weight, and markers of perinatal hypoxic-ischemia (umbilical artery pH, umbilical artery base excess, 5-min Apgar scores, and severity of encephalopathy prior to therapeutic hypothermia) were not significant confounders and were thus were excluded from further analyses.
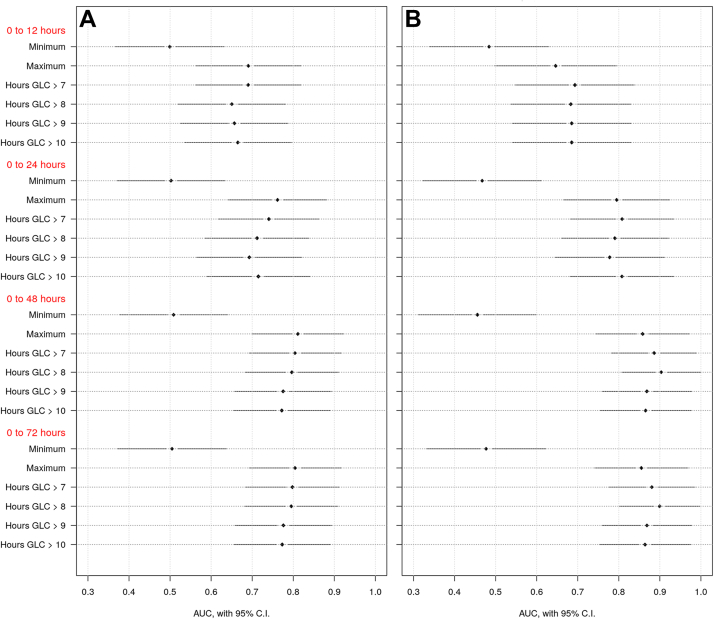
ROC curves were used to assess the predictive value of glucose measures during the first 12, 24, 48, and 72 HOL for each brain injury pattern. Glucose measures in the first 48 HOL were the best predictors for brain injury, with no further improvements when extending to 72 h (Fig. 1). During the first 48 HOL, maximum glucose was the best predictor of basal ganglia injury pattern (Area under receiver operating characteristic curve [AUC] = 0.811), while duration of exposure to glucose >8 mmol/L (>144 mg/dL) best predicted watershed injury pattern (AUC = 0.903). Minimum glucose was not predictive of any brain injury pattern at any timepoint (AUC<0.509). Further, no glucose measures were predictive of focal infarct (AUC<0.455) nor posterior-predominant injury pattern (AUC<0.655).
Fig. 1.
Predictive value of glucose measures during the first 12, 24, 48 and 72 h of life for brain injury patterns on MRI. Area under receiver operating characteristic curve (AUC) and corresponding 95% confidence intervals (CI) are plotted for each glucose measure listed on the y-axis and grouped by the first 12, 24, 48 and 72 h of life. Minimum and maximum glucose levels as well as duration of hyperglycemia above sequential thresholds are presented. Brain injury patterns included A) Basal ganglia and B) Watershed.
Further analyses were performed to determine if a composite predictor of maximum glucose at 48 HOL and duration of exposure to glucose >8 mmol/L would demonstrate stronger predictive ability. The AUC of the ROC curve for this composite predictor for basal ganglia injury was 0.823, which is not much better than the single predictor of maximum glucose (AUC = 0.811). The AUC for the composite predictor for watershed injury is 0.902, which is not better than for the single predictor of duration of glucose >8 mmol/L (AUC = 0.903).
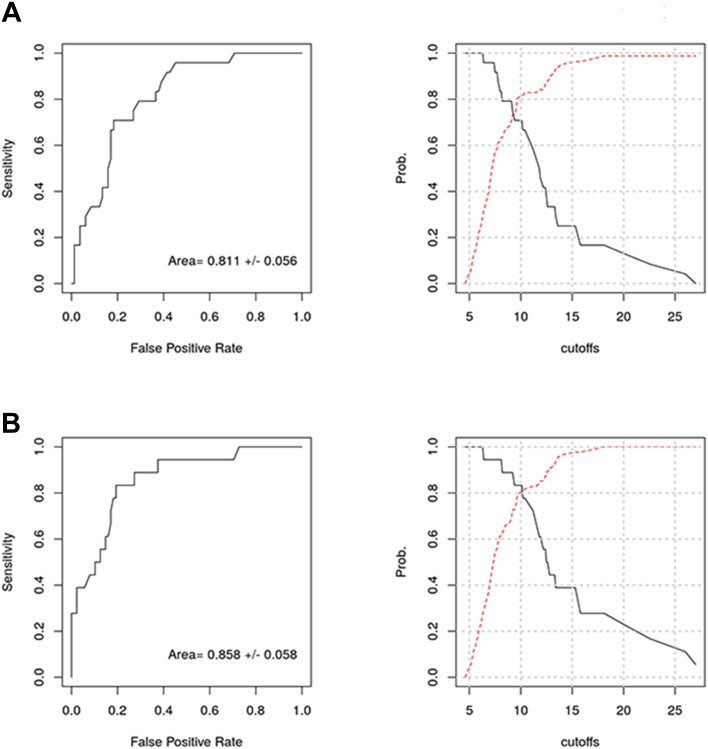
Establishing a most predictive threshold
To establish one glucose measure that best predicted both basal ganglia and watershed injury patterns, maximum glucose at 48 HOL was further investigated since this was the best measure for predicting basal ganglia injury pattern (AUC = 0.811), and only slightly inferior to the best predictor of watershed injury pattern (AUC = 0.858), and simpler to translate into clinical practice than a composite predictor. There are different statistical methods to identify plausible predictive thresholds. One statistical method is to identify the maximum value of the Youden's index for the ROC curve. During the first 48 HOL, a glucose threshold of 10.1 mmol/L (182 mg/dL) results in the maximum Youden's index of 0.53 for basal ganglia injury pattern and 0.64 for watershed injury pattern (Fig. 2). Using this threshold of 10.1 mmol/L results in a 71% sensitivity, 82% specificity, 53% positive predictive value, and 91% negative predictive value for basal ganglia pattern injury. It also results in 83% sensitivity, 81% specificity, 47% positive predictive value, and 96% negative predictive value for watershed pattern injury.
Fig. 2.
Predictive value of maximum glucose during the first 48 h of life for brain injury patterns on MRI. Receiver operating characteristic curves and their associated sensitivity (solid line) and specificity (dotted line) curves for maximum glucose during the first 48 h of life were graphed for each brain injury pattern A) Basal ganglia and B) Watershed.
Relationship between hyperglycemia and glucose infusion rates
The relationship between hyperglycemia and GIR was investigated. Mean GIR was 3.97 ± 1.42 mg/kg/min for neonates with a maximum glucose ≤10.1 mmol/L, and 3.69 ± 1.23 mg/kg/min for neonates with a maximum glucose >10.1 mmol/L, with no significant difference in mean GIR between groups (P = 0.41).
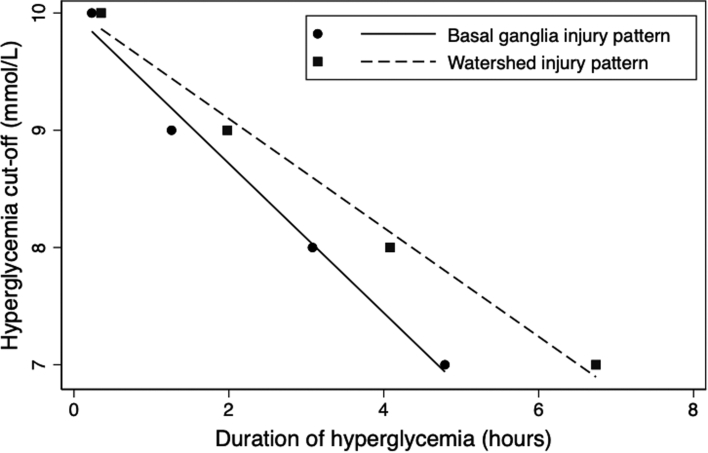
Assessing interaction of severity and duration
ROC curves and their associated sensitivity curves were also used to assess the relationship between severity and duration of exposure to hyperglycemia during the first 48 HOL for basal ganglia and watershed injury patterns. As illustrated in Fig. 3, there is an interaction between severity of hyperglycemia and duration of exposure for predicting the presence of basal ganglia and watershed injury patterns. The higher the hyperglycemic threshold, the shorter the duration of exposure would need to be before there was an increased risk for basal ganglia and watershed injury patterns, and vice versa.
Fig. 3.
Interaction between severity and duration of exposure to hyperglycemia during the first 48 h of life for brain injury patterns on MRI. Duration of hyperglycemia (hours) at the point where sensitivity intersects with specificity on the sensitivity and specificity curves for sequential 1 mmol/L hyperglycemia cut-offs (candidate values of 7–10 mmol/L) during the first 48 h of life were graphed for basal ganglia injury pattern and watershed injury pattern.
Hyperglycemia and neurodevelopmental outcomes
Of the 102 neonates who had MRIs, 91 (89%) underwent neurodevelopmental assessments (6 died prior to follow-up, 3 were lost to follow-up, 2 could not attend due to active COVID-19 pandemic protocols) at a mean age of 19.0 ± 1.7 months. Means for Bayley-III composite scores were 97.9 ± 14.6 for cognitive, 90.9 ± 16.2 for language, and 97.4 ± 16.6 for motor. Forty-one (42%) infants were classified as having moderate-severe disability or death, and 19 (20%) infants were classified as having severe disability or death. Means for CBCL Composite T-scores were 46.7 ± 11.2 for Internalizing and 47.9 ± 10.4 for Externalizing problems. Mean neuromotor score was 0.5 ± 1.2, and 7 (8%) infants who had an 18-month neurodevelopmental assessment were diagnosed with CP.
Since a glucose threshold of 10.1 mmol/L during the first 48 HOL was found to be the optimal cut-off for predicting brain injury, it was investigated whether this threshold could independently predict 18-month outcomes. Analyses were adjusted for basal ganglia and watershed injury severity to determine if associated outcomes were fully explained by brain injury severity on MRI. Linear regression analyses revealed that a glucose threshold of 10.1 mmol/L during the first 48 HOL is associated with worse neuromotor and CBCL Internalizing Composite T-scores. Logistic regression analyses revealed that a glucose threshold of 10.1 mmol/L during the first 48 HOL is associated with higher odds for a CP diagnosis and a higher odds of severe disability or death, but not associated with moderate-severe disability or death. All associations lost significance after adjusting for brain injury severity. No significant associations were found on univariable analysis in the other domains assessed (Table 2). These analyses were then repeated including only the subjects with moderate-severe encephalopathy prior to hypothermia, to focus on the subjects eligible for therapeutic hypothermia, with very similar results and the same conclusions (Supplemental Table S2).
Table 2.
Association of glucose threshold of 10.1 mmol/L during the first 48 h of life and neurodevelopmental outcomes at 18 months.
| Unadjusted regression | Adjusted regressiona | |||
|---|---|---|---|---|
| Linear regression | Coefficient (95% CI) | P value | Coefficient (95% CI) | P value |
| Neuromotor score | 0.3 (0.3–0.6) | 0.035 | 0.2 (−0.3 to 0.8) | 0.386 |
| Bayley-III cognitive composite score | −2.8 (−9.6 to 4.0) | 0.418 | ||
| Bayley-III language composite score | 0.7 (−6.9 to 8.3) | 0.858 | ||
| Bayley-III motor composite score | −6.9 (−14.5 to 0.7) | 0.081 | ||
| CBCL internalizing composite T-score | 5.8 (0.7–10.9) | 0.029 | 1.6 (−4.0 to 7.2) | 0.568 |
| CBCL externalizing composite T-score | 1.5 (−3.4 to 6.3) | 0.552 | ||
| Logistic regression | Coefficient (95% CI) | P value | Coefficient (95% CI) | P value |
| Cerebral palsy diagnosis | 8.6 (1.5–47.6) | 0.014 | 3.1 (0.4–25.5) | 0.299 |
| Death prior to follow-up | 2.7 (0.5–14.2) | 0.243 | ||
| Moderate-severe disability or deathb | 0.9 (0.4–2.2) | 0.807 | ||
| Severe disability or deathc | 3.0 (1.0–8.4) | 0.042 | 1.3 (0.3–4.9) | 0.758 |
Bayley-III, Bayley Scales of Infant and Toddler Development, Third Edition; CBCL, Child Behavior Checklist; CI, confidence interval.
Adjusted for basal ganglia and watershed injury severity.
Patients who received ANY Bayley-III composite score ≤85 or had died prior to follow-up were included.
Patients who received ANY Bayley-III composite score ≤70 or had died prior to follow-up were included.
To determine if a better predictive model could be identified between dysglycemia and 18-month outcomes, a sensitivity analysis was performed. The clinical variables of duration (area under the curve) of dysglycemia, and the mean, median, and standard deviation of glucose values over each day of life were interrogated using Aikake information criterion to determine the best predictive model for 18-month outcomes. The resultant adjusted R-squared were ≤0.33 for the prediction of Bayley and CBCL scores at 18 months. Thus, while glucose >10.1 was strongly associated with brain injury on MRI, this more aggressive analysis approach was not able to generate a stronger predictor for outcomes at 18 months.
Discussion
Utilizing continuous glucose monitoring, neuroimaging, and developmental assessments in NE, we demonstrate that maximum, but not minimum, glucose in the first 48 HOL is most strongly associated with brain injury. Further, we show that there is an interaction between severity and duration of hyperglycemia for predicting brain injury; the higher the hyperglycemic threshold, the shorter the duration of exposure needed to be associated with increased risk for basal ganglia and watershed injury patterns. Moreover, we are the first to posit a data-driven threshold for neonatal hyperglycemia in NE, specifically 10.1 mmol/L during the first 48 HOL, that has good predictive ability for basal ganglia and watershed injury patterns and is associated with select 18-month neurodevelopmental outcomes.
Hypoglycemia and hyperglycemia are common in neonates with NE.6,14 This study finds that 68% of newborns with NE had hypoglycemia (≤2.6 mmol/L; ≤47 mg/dL; 36%) and/or hyperglycemia (>8.0 mmol/L; >144 mg/dL; 49%), including 18% with both, within the first 72 HOL. Our glucose profiles align closely with findings from previous studies.6,12,14 Noteworthy, this study finds that detection of hypoglycemic and hyperglycemic episodes with intermittent glucose testing, when compared to continuous glucose monitoring, is reduced by over 50% after the first 12 HOL, and reduced by another 50% after the first 24 HOL, illustrating that the majority of hypo- and hyperglycemic episodes experienced by patients are missed by current intermittent glucose testing protocols. It is important for neuroprotective glucose management strategies to balance the risks of under- and overtreatment, highlighting the need for evidence-based treatment thresholds for hypo- and hyperglycemia in the context of NE.
Considering previous studies conducted during both the pre- and therapeutic hypothermia era found that hypoglycemia is associated with brain injury on MRI in NE,12,23 it is surprising that this study did not find an association between hypoglycemia and brain injury. Differences in studied glucose measures could account for discrepancies as previous studies likely underrepresented the true degree and frequency of hypoglycemia by utilizing intermittent glucose sampling during the first 24 HOL (resulting in only a few data points per baby), whereas the current study utilized a CGM for the first 72 HOL (resulting in hundreds of data points per day) and used minimum glucose as a glucose measure. Alternatively, it is possible that current strict clinical protocols to monitor and maintain glucose levels above 2.6 mmol/L in our institution have decreased the frequency and duration of hypoglycemia in this study cohort compared to previous studies. Indeed McKinlay et al.27 found that neonatal hypoglycemia was not associated with unfavorable neurological outcome at 2 years when blood glucose concentrations were maintained at ≥2.6 mmol/L with treatment.
The definition and management of neonatal hyperglycemia are even more highly variable.28 In accordance with the other study that investigated the relationship between hyperglycemia and brain injury after therapeutic hypothermia for neonatal HIE,12 this study finds that maximum glucose is associated with brain injury. Consequently, this study determined that a glucose threshold of 10.1 mmol/L during the first 48 HOL has good predictive ability for brain injury, suggesting that maintaining glucose levels below 10.1 mmol/L could potentially lead to a reduction in brain injury after NE. Of note, we recently reported widespread changes in brain microstructure associated with higher maximum glucose during the first day of life in this same cohort,13 further highlighting the importance of managing hyperglycemia and monitoring the sequelae of brain injury in these regions. Additionally, the current study found that during the first 48 HOL, severity of hyperglycemia was inversely related to duration of exposure for predicting the presence of basal ganglia and watershed injury patterns, suggesting that both severity and duration of hyperglycemia should be considered when developing glucose management strategies.
Treatment thresholds for hyperglycemia have been better studied in other patient populations. For instance, proposed treatment thresholds for neurocritical care patients are based on available evidence to prevent neuroglycopenia with the recommendation to be more cautious when treating patients with more severe brain injury: severe traumatic brain injury target range of 6–10 mmol/L (110–180 mg/dL); acute ischemic stroke <10 mmol/L (<180 mg/dL); subarachnoid/intracerebral hemorrhage <7.8–8.3 mmol/L (<140–150 mg/dL) and >6.1–6.7 mmol/L (>110–120 mg/dL).29 Additionally, standard practice for treating hyperglycemic preterm neonates with insulin utilizes a target range of 8–10 mmol/L (144–180 mg/dL).30 These target treatment ranges are comparable to the thresholds identified in this study. Adding to this, our study has demonstrated that the use of the maximum glucose concentration at any time within the first 48 HOL has promise in guiding glucose management after NE. Moreover, similar to studies investigating hyperglycemia in preterm neonates,31,32 our study has illustrated that duration should also be considered. Further, although previous studies have demonstrated that intensive insulin therapy (IIT) may not be an optimal treatment option for specific types of neurocritical care patients29 or preterm neonates,30 it is worth investigating whether patients with NE can benefit from IIT since other treatments (e.g., therapeutic hypothermia) have proven to be beneficial after NE whilst not beneficial for other age groups.33
Prior to therapeutic hypothermia, one study reported hyperglycemia was not associated with adverse outcomes (e.g., death, Griffith's Quotient <87, or significant motor disability) at 2 years of age after NE.34 Since therapeutic hypothermia,14,35,36 findings align with results from the current study in that hyperglycemia is associated with select 18-month neurodevelopmental outcomes in neonates with NE. Here, a glucose threshold of 10.1 mmol/L during the first 48 HOL is associated with higher CBCL Internalizing Composite T-scores, worse neuromotor scores, higher odds for a CP diagnosis, and higher odds for severe disability or death. However, these associations lose significance after adjusting for basal ganglia and watershed injury severity, suggesting the mechanism for the relationship between hyperglycemia and 18-month outcomes can be fully explained by severity of basal ganglia and watershed brain injury on early MRI. Thus, there does not seem to be a relationship between hyperglycemia and outcomes independent of brain injury severity.
Increased behavioural problems have been associated with watershed injury,37,38 and CP diagnosis has been shown to be dependent on basal ganglia and watershed injury,38 supporting our current findings. Although no clear associations were found between hyperglycemia and Bayley-III composite scores at 18 months, hyperglycemia may impact skills that do not emerge until later in development. This is plausible since the relationship between hyperglycemia and outcomes seems to mediated by the degree of basal ganglia and watershed injury, and previous studies have shown that basal ganglia injury is associated with impaired motor and cognitive outcomes, while watershed injury is associated with less severe cognitive deficits at 30 months of age.25 Severity of watershed injury is associated with impaired language-related abilities at 4 years.39 Additionally, watershed injury is associated with lower overall cognitive ability, perceptual reasoning skills, and auditory working memory during adolescence.40 Together, this highlights the importance of follow up of this cohort to assess later outcomes, including assessments at 3 and 5 years which is ongoing.
A limitation of studying a cohort of infants with NE is the difficulty of adequately separating the contributions of hypoxia-ischemia and dysglycemia. It is interesting to note that available metrics for HIE, including laboratory markers and clinical encephalopathy severity, did not demonstrate significant association with brain injury severity in this cohort. In particular, Sarnat staging is known to be a strong predictor for outcomes. One possible reason for not finding such an association in this cohort is that the majority of this cohort (83%) presented with moderate encephalopathy, and only a small proportion (14%) had severe encephalopathy. This likely limited the power of this study for detecting this association. As well, since Sarnat staging prior to hypothermia was only performed by bedside clinicians, scoring was re-assessed retrospectively using strict criteria by a single neonatal neurologist to try to improve accuracy. However, this retrospective review may still have limited the accuracy of scoring in this cohort.
This cohort reported a high rate (42%) of moderate-severe disability or death (44% if only consider subjects eligible for therapeutic hypothermia), which is in keeping with the findings of the cooling trials but is high compared to more contemporary NE cohorts.41 The main difference between this cohort and others is that all subjects enrolled in this study were out-born and transported to our NICU from a large provincial catchment area. This suggests that, while our cohort may be representative of out-born centers, it may represent a more severely impacted cohort of children than the contemporary trials in NE. However, the higher rate of adverse outcomes may be seen as a strength of this study, as it could increase the power to detect significant associations.
The main limitation of this prospective observational study is that causality cannot be determined. Randomized controlled trials are needed to determine causality and validate appropriate glycemic treatment thresholds. It is possible that more severe hypoxic-ischemic insult could result in higher infant stress and need for medications that could increase glucose levels, highlighting the need for further investigation to determine if hyperglycemia is on the causal pathway of brain injury or simply a marker of illness severity. Our current findings of no association between hypoglycemia and brain injury disagree with prior studies that demonstrate an association between hypoglycemia and outcomes in the context of NE.14,23 One reason for this may be that only 19 patients in this cohort had only hypoglycemic episodes, limiting the power of this study to detect this association. Moreover, while it is possible that some hyperglycemic episodes are related to overcorrection of hypoglycemia, most episodes of hyperglycemia are not related to previous hypoglycemia because only 18% of the study cohort had both hypo- and hyperglycemia within the first 72 HOL. Another study limitation is the use of MRI data in our predictive modelling. Although MRI measurements are only a biomarker of future outcomes, we chose to investigate a potential predictive hyperglycemia treatment threshold for brain injury pattern on MRI for an association with 18-month outcomes, and then adjust for brain injury severity because previous studies have found that deviations from normoglycemia may impact neurodevelopmental outcomes that are undetectable until later in development.27,42 Consequently, when the data becomes available, future investigations will examine how severity and duration of glycemia relate to 3- and 5-year outcome data for this cohort. This study was also limited by the blood samples used for glucose value determination and CGM calibration. Glucose values are affected by the source of the blood sample and whether whole blood or plasma glucose is obtained.6,15 However, blood samples were quite homogeneous in this study and plasma glucose was always measured.
In conclusion, this study uniquely provides evidence for a relationship between severity and duration of hyperglycemia that is associated with brain injury in the context of NE. Since minimum glucose was not associated with brain injury in our cohort, we were unable to determine a potential treatment threshold for neonatal hypoglycemia in patients with NE. Randomized controlled trials are needed to verify if controlling maximum blood glucose concentrations could improve outcomes after NE.
Contributors
EWYT conceived and designed the study. DK collected the data. DK and EWYT verified the underlying data. DK, EWYT, and RB analyzed the data. DK drafted the manuscript. All authors had full access to all the data, interpreted the data, critically revised the manuscript for important intellectual content, approved the final submitted version of the manuscript, and had final responsibility for the decision to submit for publication.
Data sharing statement
All data sharing requests should be submitted to the corresponding author (EWYT) for consideration. After publication, access to anonymized data might be granted for non-commercial research at the discretion of the corresponding author.
Declaration of interests
VC reports research funding from Xeon Pharmaceuticals Inc. outside the submitted work. EW reports research funding from Ontario Brain Institute and Pediatric Epilepsy Research Foundation outside of the submitted work, and leadership in the American Society of Neuroradiology and the Canadian League Against Epilepsy. TW reports leadership in the American Academy of Clinical Neuropsychology. EWYT reports research funding from SickKids Foundation and UCB Biopharma outside the submitted work, speaker fees from Columbia University in New York and the Children's Hospital of Fudan University in Shanghai, expert testimony fees from Bennett Jones LLP and Gowling WLG, and leadership roles in the Pediatric Academic Societies Meeting and the Newborn Brain Society. All other authors declare no competing interests.
Acknowledgements
This work was supported by the Canadian Institutes for Health Research [MOP-133710, PJT-166076], the National Institutes of Health [R01 HD101419], and the SickKids Foundation awarded to EWYT. Continuous glucose monitors were provided by Medtronic Canada. The authors thank the families and children who have participated in this study. The authors also thank the NOGIN research team, including the SickKids Acute Care Transport Services team for recruiting study subjects, and Ashley Coppin for transporting the neonates to/from the MRI scanner and clinical data collection.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.101914.
Contributor Information
Daphne Kamino, Email: daphne.kamino@sickkids.ca.
Elysa Widjaja, Email: elysa.widjaja@sickkids.ca.
Rollin Brant, Email: rollin@stat.ubc.ca.
Linh G. Ly, Email: linh.ly@sickkids.ca.
Eva Mamak, Email: eva.mamak@sickkids.ca.
Vann Chau, Email: vann.chau@sickkids.ca.
Aideen M. Moore, Email: aideen.moore@sickkids.ca.
Tricia Williams, Email: tricia.williams@sickkids.ca.
Emily W.Y. Tam, Email: emily.tam@utoronto.ca.
Appendix A. Supplementary data
References
- 1.Gunn A.J., Thoresen M. Neonatal encephalopathy and hypoxic-ischemic encephalopathy. Handb Clin Neurol. 2019;162:217–237. doi: 10.1016/B978-0-444-64029-1.00010-2. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs S.E., Berg M., Hunt R., Tarnow-Mordi W.O., Inder T.E., Davis P.G. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013;2013(1) doi: 10.1002/14651858.CD003311.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Douglas-Escobar M., Weiss M.D. Hypoxic-ischemic encephalopathy: a review for the clinician. JAMA Pediatr. 2015;169(4):397–403. doi: 10.1001/jamapediatrics.2014.3269. [DOI] [PubMed] [Google Scholar]
- 4.Basu S.K., Salemi J.L., Gunn A.J., Kaiser J.R., CoolCap Study Group Hyperglycemia in infants with hypoxic-ischemic encephalopathy is associated with improved outcomes after therapeutic hypothermia: a post hoc analysis of the CoolCap Study. Arch Dis Child Fetal Neonatal Ed. 2017;102:F299–F306. doi: 10.1136/archdischild-2016-311385. [DOI] [PubMed] [Google Scholar]
- 5.Aynsley-Green A. Glucose: a fuel for thought! J Paediatr Child Health. 1991;27(1):21–30. doi: 10.1111/j.1440-1754.1991.tb00340.x. [DOI] [PubMed] [Google Scholar]
- 6.Basu S.K., Kaiser J.R., Guffey D., et al. Hypoglycemia and hyperglycemia are associated with unfavourable outcome in infants with hypoxic ischemic encephalopathy: a post hoc analysis of the CoolCap Study. Arch Dis Child Fetal Neonatal Ed. 2016;101:F149–F155. doi: 10.1136/archdischild-2015-308733. [DOI] [PubMed] [Google Scholar]
- 7.Boardman J.P., Hawdon J.M. Hypoglycemia and hypoxic-ischemic encephalopathy. Dev Med Child Neurol. 2015;57(Suppl 3):29–33. doi: 10.1111/dmcn.12729. [DOI] [PubMed] [Google Scholar]
- 8.Jensen E.C., Bennet L., Hunter C.J., Power G.C., Gunn A.J. Post-hypoxic hypoperfusion is associated with suppression of cerebral metabolism and increased tissue oxygenation in near-term fetal sheep. J Physiol. 2006;572(Pt 1):131–139. doi: 10.1113/jphysiol.2005.100768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidson J.O., Fraser M., Naylor A.S., Roelfsema V., Gunn A.J., Bennet L. Effect of cerebral hypothermia on cortisol and adrenocorticotropic hormone responses after umbilical cord occlusion in preterm fetal sheep. Pediatr Res. 2008;63(1):51–55. doi: 10.1203/PDR.0b013e31815b8eb4. [DOI] [PubMed] [Google Scholar]
- 10.Pinchefsky E.F., Hahn C.D., Kamino D., et al. Hyperglycemia and glucose variability are associated with worse brain function and seizures in neonatal encephalopathy: a prospective cohort study. J Pediatr. 2019;209:23–32. doi: 10.1016/j.jpeds.2019.02.027. [DOI] [PubMed] [Google Scholar]
- 11.Kamino D., Almazrooei A., Pang E.W., et al. Abnormalities in evoked potentials associated with abnormal glycemia and brain injury in neonatal hypoxic-ischemic encephalopathy. Clin Neurophysiol. 2021;132:307–313. doi: 10.1016/j.clinph.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basu S.K., Ottolini K., Govindan V., et al. Early glycemic profile is associated with brain injury patterns on magnetic resonance imaging in hypoxic ischemic encephalopathy. J Pediatr. 2018;203:137–143. doi: 10.1016/j.jpeds.2018.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tam E.W.Y., Kamino D., Shatil A.S., et al. Hyperglycemia associated with acute brain injury in neonatal encephalopathy. Neuroimage Clin. 2021;32 doi: 10.1016/j.nicl.2021.102835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montaldo P., Caredda E., Pugliese U., et al. Continuous glucose profile during therapeutic hypothermia in encephalopathic infants with unfavorable outcome. Pediatr Res. 2020;88:218–224. doi: 10.1038/s41390-020-0827-4. [DOI] [PubMed] [Google Scholar]
- 15.Harding J.E., Harris D.L., Hegarty J.E., Alsweiler J.M., McKinlay C.J. An emerging evidence base for the management of neonatal hypoglycemia. Early Hum Dev. 2017;104:51–56. doi: 10.1016/j.earlhumdev.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Kempen A.A.M.W., Eskes P.F., Nuytemans D.H.G.M., et al. Lower versus traditional treatment threshold for neonatal hypoglycemia. N Engl J Med. 2020;382:534–544. doi: 10.1056/NEJMoa1905593. [DOI] [PubMed] [Google Scholar]
- 17.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap) –a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barkovich A.J., Miller S.P., Bartha A., et al. MR imaging, MR spectroscopy, and diffusion tensor imaging of sequential studies in neonates with encephalopathy. AJNR Am J Neuroradiol. 2006;27:533–547. [PMC free article] [PubMed] [Google Scholar]
- 19.Thayyil S., Chandrasekaran M., Taylor A., et al. Cerebral magnetic resonance biomarkers in neonatal encephalopathy: a meta-analysis. Pediatrics. 2010;125:e382–e395. doi: 10.1542/peds.2009-1046. [DOI] [PubMed] [Google Scholar]
- 20.Bednarek N., Mathur A., Inder T., Wilkinson J., Neil J., Shimony J. Impact of therapeutic hypothermia on MRI diffusion changes in neonatal encephalopathy. Neurology. 2012;78:1420–1427. doi: 10.1212/WNL.0b013e318253d589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chau V., Poskitt K.J., Dunham C.P., Hendson G., Miller S.P. Magnetic resonance imaging in the encephalopathic term newborn. Curr Pediatr Rev. 2014;10:28–36. doi: 10.2174/157339631001140408120336. [DOI] [PubMed] [Google Scholar]
- 22.Barkovich A.J., Hajnal B.L., Vigneron D., et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR Am J Neuroradiol. 1998;19:143–149. [PMC free article] [PubMed] [Google Scholar]
- 23.Wong D.S.T., Poskitt K.J., Chau V., et al. Brain injury patterns in hypoglycemia in neonatal encephalopathy. AJNR Am J Neuroradiol. 2013;34:1456–1461. doi: 10.3174/ajnr.A3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weeke L.C., Groenendaal F., Mudigonda K., et al. A novel magnetic resonance imaging score predicts neurodevelopmental outcome after perinatal asphyxia and therapeutic hypothermia. J Pediatr. 2018;192:33–40.e2. doi: 10.1016/j.jpeds.2017.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller S.P., Ramaswamy V., Michelson D., et al. Patterns of brain injury in term neonatal encephalopathy. J Pediatr. 2005;146:453–460. doi: 10.1016/j.jpeds.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 26.Achenbach T.M., Rescorla L.A. University of Vermont, Research Center for Children, Youth, & Families; Burlington, VT: 2001. Manual for the ASEBA school-age forms & profiles. [Google Scholar]
- 27.McKinlay C.J.D., Alsweiler J.M., Ansell J.M., et al. Neonatal glycemia and neurodevelopmental outcomes at 2 years. N Engl J Med. 2015;373:1507–1518. doi: 10.1056/NEJMoa1504909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinchefsky E.F., Schneider J., Basu S., Tam E.W.Y. Gale C, Newborn Brain Society Guidelines and Publications Committee. Nutrition and management of glycemia in neonates with neonatal encephalopathy treated with hypothermia. Semin Fetal Neonatal Med. 2021;26 doi: 10.1016/j.siny.2021.101268. [DOI] [PubMed] [Google Scholar]
- 29.Godoy D.A., Napoli M.D., Rabinstein A.A. Treating hyperglycemia in neurocritical care patients: benefits and perils. Neurocrit Care. 2010;13(3):425–438. doi: 10.1007/s12028-010-9404-8. [DOI] [PubMed] [Google Scholar]
- 30.Alsweiler J.M., Harding J.E., Bloomfield F.H. Tight glycemic control with insulin in hyperglycemic preterm babies: a randomized controlled trial. Pediatrics. 2012;129(4):639–647. doi: 10.1542/peds.2011-2470. [DOI] [PubMed] [Google Scholar]
- 31.Auerbach A., Eventov-Friedman S., Arad I., et al. Long duration of hyperglycemia in the first 96 hours of life is associated with severe intraventricular hemorrhage in preterm infants. J Pediatr. 2013;163(2):388–393. doi: 10.1016/j.jpeds.2013.01.051. [DOI] [PubMed] [Google Scholar]
- 32.Kermorvant-Duchemin E.K., Le Meur G., Plaisant F., et al. Thresholds of glycemia, insulin therapy, and risk for severe retinopathy in premature infants: a cohort study. PLoS Med. 2020;17(12) doi: 10.1371/journal.pmed.1003477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moler F.W., Silverstein F.S., Holubkov R., et al. Therapeutic Hypothermia after in-hospital cardiac arrest in children. N Engl J Med. 2017;376(4):318–329. doi: 10.1056/NEJMoa1610493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nadeem M., Murray D.M., Boylan G.B., Dempsey E.M., Ryan C.A. Early blood glucose profile and neurodevelopmental outcome at two years in neonatal hypoxic-ischemic encephalopathy. BMC Pediatr. 2011;11:10. doi: 10.1186/1471-2431-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spies E.E., Lababidi S.L., McBride M.C. Early hyperglycemia is associated with poor gross motor outcome in asphyxiated term newborns. Pediatr Neurol. 2014;50:586–590. doi: 10.1016/j.pediatrneurol.2014.01.043. [DOI] [PubMed] [Google Scholar]
- 36.Chouthai N.S., Sobczak H., Khan R., Subramanian D., Raman S., Raghavendra R. Hyperglycemia is associated with poor outcome in newborn infants undergoing therapeutic hypothermia for hypoxic ischemic encephalopathy. J Neonatal Perinatal Med. 2015;8:125–131. doi: 10.3233/NPM-15814075. [DOI] [PubMed] [Google Scholar]
- 37.Martinez-Biarge M., Bregant T., Wusthoff C.J., et al. White matter and cortical injury in hypoxic-ischemic encephalopathy: antecedent factors and 2-year outcome. J Pediatr. 2012;161:799–807. doi: 10.1016/j.jpeds.2012.04.054. [DOI] [PubMed] [Google Scholar]
- 38.Hayes B.C., Ryan S., McGarvey C., et al. Brain magnetic resonance imaging and outcome after hypoxic ischaemic encephalopathy. J Matern Fetal Neonatal Med. 2016;29:777–782. doi: 10.3109/14767058.2015.1018167. [DOI] [PubMed] [Google Scholar]
- 39.Steinman K.J., Gorno-Tempini M.L., Glidden D.V., et al. Neonatal watershed brain injury on magnetic resonance imaging correlates with verbal IQ at 4 years. Pediatrics. 2009;123:1025–1030. doi: 10.1542/peds.2008-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee B.L., Gano D., Rogers E.E., et al. Long-term cognitive outcomes in term newborns with watershed injury caused by neonatal encephalopathy. Pediatr Res. 2022;92(2):505–512. doi: 10.1038/s41390-021-01526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shakaran S., Laptook A.R., Pappas A., et al. Effect of depth and duration of cooling on death or disability at age 18 Months among neonates with hypoxic-ischemic encephalopathy: a randomized clinical trial. JAMA. 2017;318(1):57–67. doi: 10.1001/jama.2017.7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McKinlay C.J.D., Alsweiler J.M., Anstice N.S., et al. Association of neonatal glycemia with neurodevelopmental outcomes at 4.5 years. JAMA Pediatr. 2017;171(10):972–983. doi: 10.1001/jamapediatrics.2017.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.