Summary
Acute radiation dermatitis (ARD) commonly develops in cancer patients undergoing radiotherapy and is often characterized by erythema, desquamation, and pain. A systematic review was conducted to summarize the current evidence on interventions for the prevention and management of ARD. Databases were searched from 1946 to September 2020 to identify all original studies that evaluated an intervention for the prevention or management of ARD, with an updated search conducted in January 2023. A total of 235 original studies were included in this review, including 149 randomized controlled trials (RCTs). Most interventions could not be recommended due to a low quality of evidence, lack of supporting evidence, or conflicting findings across multiple trials. Photobiomodulation therapy, Mepitel® film, mometasone furoate, betamethasone, olive oil, and oral enzyme mixtures showed promising results across multiple RCTs. Recommendations could not be made solely based on the published evidence due to limited high-quality evidence. As such, Delphi consensus recommendations will be reported in a separate publication.
Keywords: Radiation dermatitis, Clinical practice guidelines, Systematic review, Radiotherapy, Skin toxicity, Prevention, Management
Introduction
Acute radiation dermatitis (ARD) has long been recognized as a common adverse effect of external beam radiotherapy (RT), developing in up to 95% of cancer patients.1 The pathophysiology of ARD is complex and involves radiation-induced damage to both the epidermis and dermis, altered proliferation and differentiation of basal and epidermal keratinocytes, barrier disruption, and a trigger of proinflammatory markers that contribute to ARD-associated symptoms.1,2 ARD, arising within 90 days from the initiation of treatment, is often characterized by changes in skin pigmentation, pain, pruritus, edema, and desquamation (dry and/or moist), with ulceration in severe cases.1, 2, 3 The severity of ARD varies depending on treatment-related factors (e.g., radiation dose, irradiated volume, bolus, concurrent chemotherapy, treatment positioning, etc.) and intrinsic factors (e.g., body mass index, irradiation site, smoking status, and skin pigmentation).1, 2, 3, 4 Approximately 36% of patients develop moderate to severe acute reactions characterized by moist desquamation, which are postulated to be associated with an increased risk of irreversible late side effects developing in the months to years following RT, such as telangiectasia and fibrosis.5
See Article The Lancet Oncology 2023; published online March 27th: DOI: 10.1016/S1470-2045(23)00067-0 This is the first in a Series of two papers on MASCC Clinical Practice Guidelines. (papers 2 appears in The Lancet Oncology)
Despite technological advancements to improve skin dose homogeneity and reduce reaction severity, such as intensity modulated RT (IMRT) and skin-sparing techniques, ARD remains a prominent adverse reaction that can negatively impact patient quality of life (QoL), diminish cosmesis, cause infection or sepsis, and treatment interruptions.1, 2, 3,6 Common QoL issues reported in patients with ARD include decreased self-esteem, embarrassment, increased financial burden associated with treatment, pain, and an impaired ability to complete daily activities.6, 7, 8
Currently, clinical care for ARD is highly variable due to a lack of standardization in approaches to prevent and manage skin reactions, which hampers the homogeneity of clinical practice recommendations worldwide.9 In 2013, the Skin Toxicity Group of the Multinational Association of Supportive Care in Cancer (MASCC) released a clinical practice guideline in attempt to standardize the care of ARD.10 While this guideline was valuable to care-providers at the time, it lacked definitive recommendations on ARD care due to a lack of high-quality evidence and therefore received limited uptake in clinical settings. Other guidelines have also been published by institutions, but suffer from similar issues.9 Therapeutic interventions recommended by these guidelines for both prevention and management include aqueous creams, corticosteroids, and dressings, but a recent narrative review comparing ARD guidelines across cancer institutions revealed significant discrepancies across recommendations, revealing the need for up-to-date, evidence-based guidelines on ARD care.9 An update to the MASCC ARD guidelines is therefore warranted due to publication of numerous high-quality studies since 2013.
MASCC is an international, non-profit, multidisciplinary organization that is dedicated to research into and education of supportive care for cancer patients. The Oncodermatology Study Group comprises experts in dermatology, medical, radiation, dental/oral surgery, and supportive oncology, nursing, health-related QoL, and pharmacovigilance, with a focus on the research and the development of evidence-based guideline recommendations for the care of cancer-related dermatologic (skin, hair, nail) toxicities. Within this MASCC study group, a working group was formed to compile the current literature on interventions for the care of ARD. We present Part One of a two-part publication series on the MASCC Clinical Practice Guidelines for the Prevention and Management of ARD, which aims to perform an extensive systematic review to highlight the available evidence on the prevention and management of ARD. Part Two, involving Delphi-based expert consensus recommendations, will be reported in a separate publication.
Methods
Search strategy and selection criteria
A systematic review of the literature was conducted in consultation with a medical librarian through Ovid MEDLINE®, Embase, and Cochrane Central Register of Controlled Trials databases. This systematic review aimed to update the findings of the 2013 MASCC skin care guidelines on ARD, with a new expanded search to include all study types from 1946 to September 2020. All original studies from the 2013 guidelines were included in this analysis, in addition to any non-randomized studies and relevant publications identified during the search time frame. The search strategy is summarized in Appendix A. In January 2023, an updated search was conducted from September 22nd, 2020 to January 21st, 2023 to identify any new articles.
All works published in the English-language (either full-text articles or abstracts) were included in the analysis if they answered the research question defined according to the Population, Intervention, Comparison, Outcome (PICO) method11: P) patients undergoing external beam RT for cancer; I) any intervention; C) standard of care, placebo, any other intervention, or no intervention; and O) prevention or management of ARD and ARD-associated symptoms. The research question was kept broad to capture all existing literature that included a study population who received RT and were treated with a therapeutic intervention to either prevent or manage ARD. By definition, preventative modalities were considered those administered prior to the start of RT or the onset of any grade ARD and continued throughout the course of RT, while management modalities were those administered during treatment or upon onset of any grade ARD. All randomized and non-randomized studies were included. Works published in a non-English language and/or conducted in animal or in vitro models were excluded.
In accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement,12 two authors (T.B. & S.F.) screened all titles and abstracts independently for eligibility. A third party was consulted for the inclusion of articles in the event of discrepancies (D.G.). Following screening by titles and abstracts, all studies were screened by full-texts; information was extracted from those that met the inclusion criteria, and the extraction was cross-referenced by three reviewers (T.B., S.F., & D.G.) to ensure accuracy.
Data collection and analysis
Among studies included, the following relevant study characteristics were collected: sample size, publication year, patient population and treatment characteristics, outcomes assessed, types of interventions investigated, and key findings. A formal quality of evidence (QoE) assessment was conducted in accordance with the Hadorn criteria for clinical trials (Appendix B)13; any studies identified as having none or minor flaws according to the Hadorn criteria were designated an “adequate” quality of evidence, while those with major flaws were designated a “doubtful” quality of evidence.
Role of funding
No funding was received to complete this study.
Results
Search results
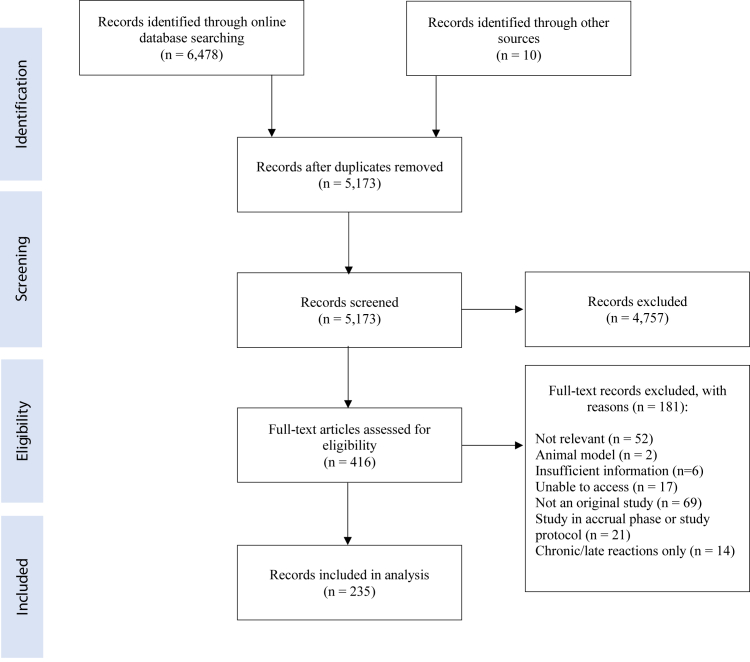
Through the initial database searches, a total of 6478 articles were identified (Fig. 1). After duplicates were removed, 5173 articles remained. After screening by abstracts/titles and full-texts, a total of 235 articles were identified for inclusion in the analysis, with 149 randomized controlled trials (RCTs) and 87 non-randomized studies. The majority of studies evaluated an intervention for the prevention of ARD but had methodological challenges resulting in a doubtful quality of evidence. Interventions were categorized according to the following treatment types: topical non-steroidal agents, topical corticosteroids, barrier films and dressings, laser therapy, natural and miscellaneous agents, growth factors and oral agents, and alternative and multi-component therapies. A summary of key findings according to treatment category has been included in Tables 1 (for prevention modalities) and 2 (for management modalities). Individual characteristics and primary findings of all RCTs have been described in Appendix C (Tables 1–12).
Fig. 1.
Literature search results from 1946 to September 2020.
Table 1.
Characteristics of studies assessing prevention methods for radiation dermatitis.
| Intervention category | Total studies (RCTs) | Total number of patients (Median sample size; range) | Interventions identified (n) | Cancer site included (n) | Key findings | Reference |
|---|---|---|---|---|---|---|
| Topical non-steroidal agents | 42 (29) | 4364 (64; 1–547) | Trolamine emulsions (8); Hyaluronic acid/hyaluronan (5); Urea (2); Heparinoid (Hirudoid ®) (3); Other (27) |
Breast (25); Head and neck (10); Anus (1); Multiple (6) | There was inconsistent evidence supporting the use of trolamine emulsions, hyaluronic acid, and heparinoid for the prevention of ARD. Insufficient evidence was reported to support the use of urea, Xonrid, hydroactive colloid gel, and other interventions. | 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55 |
| Topical corticosteroids | 18 (15) | 1715 (81; 12–219) | Mometasone furoate (8); Betamethasone (6); Other (4) |
Breast (14); Head and neck (3); Multiple (1) | Mometasone furoate and betamethasone were both generally found to be effective in ARD prevention. There is insufficient evidence supporting the use of other corticosteroids, such as hydrocortisone and beclomethasone. | 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73 |
| Barrier films and dressings | 25 (18) | 1856 (39; 2–333) | Mepitel® film (9); 3M™ Cavilon™ No-Sting Barrier Film (4); StrataXRT® (topical film-forming gel) (3); Silver nylon dressing (3); Other (6) |
Breast (15); Head and neck (6); Prostate (2); Other (2) | Mepitel film and Hydrofilm were both generally found to be effective in ARD prevention. There was inconsistent evidence supporting the use of StrataXRT, silver nylon dressings, Cavilon No-Sting barrier film, and other interventions in ARD prevention. | 35,62,74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96 |
| Laser therapy | 10 (5) | 576 (43.5; 25–120) | Photobiomodulation (low-level laser) therapy (9); Photomagnetic therapy (1) | Breast (7); Head and neck (3) | Photobiomodulation (low-level laser) therapy was generally found to be effective in ARD prevention. | 97, 98, 99, 100, 101, 102, 103, 104, 105, 106 |
| Natural and miscellaneous agents | 51 (39) | 5290 (62; 1–686) |
Aloe vera (6); Curcumin (turmeric) (6); Calendula (marigold) (4); Chamomilla recutita (3); Other (38) |
Breast (32); Head and neck (12); Multiple (5); NS (2) |
Topical olive oil was generally found to be effective in ARD prevention. Honey-based products were generally ineffective in ARD prevention. There was inconsistent evidence supporting the use of aloe vera, oral and topical curcumin, topical calendula (marigold)-based products, topical NS-21 skin repair treatment, topical chamomilla recutita, Holoil, topical silymarin, and other interventions for the prevention of ARD. Insufficient evidence was reported to support the use of urea, Xonrid, hydroactive colloid gel, and other interventions. | 21,107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120,121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131,132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155, 156 |
| Growth factors and oral agents | 9 (7) | 1721 (78; 40-1142) | Enzyme mixture (3); Other (6) |
Head and neck (5); Breast (2); Multiple (1); Cervical (1) |
Mixed enzyme tablets were generally found to be effective in ARD prevention. Insufficient evidence was reported to support the use of other interventions. | 157, 158, 159, 160, 161, 162, 163, 164, 165 |
| Alternative and multi-component therapies | 31 (17) | 4963 (90; 1–1358) | Lactokine-based two-step care system (R1 & R2) (3); Lotion (3% urea, polidocanol and hyaluronic acid) (3); Therapeutic touch (1); Other (25) |
Breast (20); Head and neck (5); Multiple (4); Other (2) | Antiperspirant/deodorant use and general skin washing were found to have minimal effects on ARD severity. Insufficient evidence was reported to support the use of any interventions for ARD prevention. | 166, 167, 168, 169, 170, 171, 172, 173, 174, 175, 176, 177, 178, 179, 180, 181, 182, 183, 184, 185, 186, 187, 188, 189, 190, 191, 192, 193, 194, 195, 196 |
“n” indicates the number of studies.
NS, not specified; RCT, randomized controlled trial; ARD, radiation dermatitis.
Table 2.
Characteristics of studies assessing management methods for radiation dermatitis.
| Intervention category | Total studies (RCTs) | Total number of patients (Median sample size; range) | Interventions identified (n) | Cancer site included (n) | Key findings | Reference |
|---|---|---|---|---|---|---|
| Topical non-steroidal agents | 18 (9) | 2800 (61; 1–573) | Heparinoid (Hirudoid ®) (4); Hydroactive colloid gel (Flamigel ®) (3); Other (11) |
Breast (10); Head and neck (2); Multiple (5); Other (1) | There was inconsistent evidence supporting the use of heparinoid (Hirudoid) for the management of ARD. Insufficient evidence was reported to support the use of other interventions. | 15,29,30,39,197, 198, 199, 200, 201, 202, 203, 204, 205, 206, 207, 208, 209, 210 |
| Topical corticosteroids | 2 (2) | 230 (115; 19–211) | Hydrocortisone (1); NS (1) | Head and neck (1); Multiple (1) |
Insufficient evidence was reported to support the use of any interventions for ARD management. | 211,212 |
| Barrier films and dressings | 13 (7) | 1216 (39; 12–357) | Mepilex ® Lite dressings (3); Other (10) | Head and neck (4); Breast (3); Multiple (5); Skin (1) | Mepilex Lite dressings were generally found to be effective in ARD management. Insufficient evidence was reported to support the use of all other interventions for ARD management. | 84,213, 214, 215, 216, 217, 218, 219, 220, 221, 222, 223, 224 |
| Laser therapy | 5 (0) | 96 (4; 1–79) | Photobiomodulation (low-level laser) therapy (3); High level laser therapy (1); NS (1) |
Breast (2); Head and neck (1); Skeletal (1); Anal (1) |
Insufficient evidence was reported to support the use of any interventions for ARD management. | 225, 226, 227, 228, 229 |
| Natural and miscellaneous agents | 9 (4) | 528 (60; 24–126) | Honey (2); Holoil® (containing Hypericum Perforatum/St. John's-wort and Neem Oil) (2); Other (6) |
Breast (4); Head and neck (2); Multiple (3) |
Insufficient evidence was reported to support the use of any interventions for ARD management. | 145,230, 231, 232, 233, 234, 235, 236, 237 |
| Alternative and multi-component therapies | 11 (4) | 422 (30; 1–90) | Hydrotherapy (1); Acupuncture (1); Mixed emulsion (1); Other (11) |
Breast (4); Head and neck (1); Multiple (6) | Insufficient evidence was reported to support the use of any interventions for ARD management. | 179,186,189,200,238, 239, 240, 241, 242, 243, 244 |
“n” indicates the number of studies.
RCT, randomized controlled trial; ARD, radiation dermatitis.
ARD prevention methods
Topical non-steroidal agents
A total of 42 studies assessed topical non-steroidal agents for the prevention of ARD, including 12 and 28 non-randomized studies and RCTs, respectively14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55 (Appendix C, Table 1). The majority of RCTs assessed trolamine-based products (including Biafine ®),14, 15, 16, 17, 18,21,33 hyaluronic acid/hyaluronan-based products,22, 23, 24, 25, 26 and heparinoid (Hirudoid ®).30,31,197 A few RCTs demonstrated promising results in the use of trolamine emulsion,14,21 hyaluronic acid/hyaluronan,22,24,25 3M™ Cavilon™ Durable Barrier Cream,34,35 heparinoid (Hirudoid),30,31 boron-based gel,37 and other emulsions38,47,50,52; however, evidence supporting the use of these interventions was either conflicting or insufficient to produce recommendations for clinical practice.
Topical corticosteroids
A total of 18 studies assessed topical corticosteroids for the prevention of ARD, including three non-randomized studies and 15 RCTs56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73 (Appendix C, Table 2). Topical mometasone furoate (most commonly 0.1% potency) was evaluated in eight RCTs56, 57, 58, 59, 60, 61, 62,72 and demonstrated efficacy across trials of both doubtful and adequate QoE in preventing overall ARD and erythema. Betamethasone further demonstrated prophylactic efficacy in ARD prevention,65, 66, 67, 68, 69,73 suggesting that the current evidence supports the use of both mometasone furoate and betamethasone in preventing ARD and associated symptoms.
Barrier films and dressings
Among studies investigating barrier films and dressings, seven non-randomized studies and 18 RCTs were identified, for a total of 25 studies35,62,74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96 (Appendix C, Table 3). Promising results were found in studies on polyurethane film (Hydrofilm ®),74,96 topical film-forming gel (StrataXRT ®),75, 76, 77 silicone-based polyurethane (Mepitel ® film),78,79,84, 85, 86 3M Cavilon No Sting barrier film,87 and others.90,93 Despite positive findings in some studies, particularly on Hydrofilm and Mepitel film, there were often conflicting findings or a small sample size supporting film and dressing use, suggesting that future research may be needed to support these interventions.
Laser therapy
Photobiomodulation (PBM) (low-level laser) therapy was investigated in ten studies, including four non-randomized and five RCTs97, 98, 99, 100, 101, 102, 103, 104, 105, 106 (Appendix C, Table 4). The majority of studies found PBM to be beneficial in preventing ARD when compared to standard of care and/or placebo in breast cancer patients. Only one RCT was conducted in head and neck patients, which reported similar benefit in the prevention of ARD and associated pain.104
Natural and miscellaneous agents
Natural and miscellaneous agents were assessed in 51 studies, of which 39 were RCTs21,107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120,121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131,132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155, 156 (Appendix C, Table 5). The majority of interventions showed minimal or no benefit protecting against ARD in a randomized setting, including honey-based products,125,126 vitamins,129,130 chamomile-based cream,141 calendula (marigold)-based products,113,116 and products containing glutamine.123,124 Olive oil and a combined olive oil and calcium hydroxide product demonstrated efficacy in two RCTs against placebo143 and standard of care,142 respectively. Across multiple RCTs, support for aloe vera,107,109, 110, 111, 112 oral and topical curcumin,117, 118, 119, 120, 121, 122 topical NS-21 cortisone-free skin repair treatment,131,132 and other interventions was conflicting. While benefits were seen in silymarin,128 adlay bran extract,133 zinc,135 cucumis sativus,139 Boswellia cream (Bosexil®),140 lianbai,145 and other products, a single RCT was considered insufficient to confirm efficacy. The current evidence on natural and miscellaneous agents is insufficient to provide recommendations for ARD prevention.
Growth factors and oral agents
Two studies evaluated topical epidermal growth factors,211,212 while seven evaluated oral agents, such as celecoxib,157 sucralfate,158 pentoxifylline,159 histamines,160 and enzyme mixtures161, 162, 163 (Appendix C, Table 6). Three RCTs consistently supported the use of an oral enzyme mixture containing 100 mg of papain, 40 mg of trypsin, and 40 mg chymotrypsin in ARD prevention, suggesting potential use for oral enzyme compounds in clinical practice. Despite promising findings in epidermal growth factor use in one RCT,164 further research is needed to discern prophylactic benefit. No benefit was found in the use of all other oral agents.
Alternative and multi-component therapies
Alternative and multi-component therapies were assessed in 17 RCTs and 16 non-randomized studies166, 167, 168, 169, 170, 171, 172, 173, 174, 175, 176, 177, 178, 179, 180, 181, 182, 183, 184, 185, 186, 187, 188, 189, 190, 191, 192, 193, 194, 195, 196 (Appendix C, Table 7). Minimal difference was found between patients who used deodorant or antiperspirant and those who washed regularly,167,168 did not use any intervention,169 or used standard of care,170 suggesting that antiperspirant/deodorant use should not be encouraged with the intent of preventing ARD. Similarly, most other interventions had no preventative benefit across RCTs, such as alkaline water,172 or minimal benefit confined to one RCT, such as repalysyal180 or other emulsions.179,182 Based on evidence from multiple RCTs, aluminum/deodorant use and washing with water and soap do not worsen ARD, but there is insufficient evidence to support their use in the prevention of ARD. Overall, evidence supporting the majority of alternative and multi-component therapies is lacking.
ARD management methods
Topical non-steroidal agents and corticosteroids
Non-steroidal and steroidal agents were evaluated in 18 and two studies, respectively, with a total of 11 RCTs (Appendix C, Tables 8 and 9). Conflicting evidence was reported supporting heparinoid (Hirudoid) use in four RCTs.29, 30, 31,197 All other non-steroidal agents, such as trolamine emulsion,15 doxepin,198 and sucralfate199 had insufficient evidence and/or demonstrated minimal benefit. Topical corticosteroids (hydrocortisone211 and an unknown steroid245) were further shown to have no benefit in managing ARD.
Barrier films and dressings
Across seven randomized and six non-randomized studies, Mepilex® Lite dressings,213, 214, 215 Hydrosorb® (hydrogel polyurethane film),216 hydrocolloid dressings,217 and other barrier films and dressings were evaluated for ARD management (Appendix C, Table 10). Mepilex Lite dressings was favoured over aqueous creams213,215 and standard wound care214 in three RCTs. Dry dressings (Tricotex218 and Mepitel film84) showed positive results through single RCTs to manage ARD.
Natural, miscellaneous, alternative, and multi-component therapies
A total of 25 studies evaluated interventions in the natural and miscellaneous145,230, 231, 232, 233, 234, 235, 236, 237 and alternative and multi-component categories179,186,189,200,238, 239, 240, 241, 242, 243, 244 (Appendix C, Tables 11 and 12). Among all interventions, insufficient evidence was available to confirm efficacy. Henna-containing ointment,232 lianbai liquid,145 hydrotherapy,246 acupuncture,244 and an emulsion179 were effective in managing symptoms once ARD developed, but evidence was only shown through a single RCT, highlighting the need for further research confirming efficacy.
Discussion
To our knowledge, this is the most comprehensive and up-to-date systematic review to compile all published clinical trials investigating methods for ARD care. A systematic review was conducted in 2013 by Wong et al.10 in the development of previous MASCC clinical practice guidelines for ARD, identifying 56 relevant RCTs. To develop clinically relevant recommendations on the use of various interventions for ARD, we aimed to include all study types through a systematic approach and identified dozens of different interventions evaluated for ARD prevention and management. A total of 235 original studies were included in this review, including 149 randomized controlled trials (RCTs). Most interventions could not be recommended due to a low quality of evidence, lack of supporting evidence, or conflicting findings across multiple trials. Overall, photobiomodulation therapy, Mepitel® film, mometasone furoate, betamethasone, olive oil, and oral enzyme mixtures showed promising results across multiple RCTs mainly as ARD prevention methods.
Several reviews have been conducted to summarize the current evidence on ARD prevention and management methods. A review by Chan et al. (2014) identified 47 RCTs on interventions for ARD care and recognized six intervention types: oral systemic medications, skin care practices, steroidal topical therapies, non-steroidal topical therapies, dressings, and other.247 An oral enzyme mixture was found to be effective in preventing ARD and minimizing ARD severity, which was similarly found in our review through studies by Dale et al. (2001) and Gujral et al. (2001).161,162 Additionally, similar to our findings, the use of deodorant did not contribute to increased ARD severity among breast cancer patients. Other reviews have also been conducted on this area and produced similar results, but were limited due to their lack of a systematic approach,248,249 restricted inclusion criteria,250,251 or sole focus on one type of intervention.252, 253, 254 Among most reviews, hygienic skin care practices, such as washing with water and/or soap and the use of antiperspirant or deodorant, were consistently found to have minimal impact on ARD severity.
Like other authors who have reviewed ARD care regimens in the past, several key limitations were identified which make it challenging to develop clinical practice recommendations using the current evidence base alone. First, there are limited publications discussing the management of ARD in low-limited income environments. In less developed areas of the world, patients may struggle with poor sanitation and nutrition, as well as access to recommended effective modalities, which all impact skin healing. ARD management may be limited to washing with mild soap and topical steroids, resulting in discontinuation of RT in cases with moist desquamation. It is important for the field of ARD to overcome the barriers in low-resource countries for adequate management of ARD. Another recurring challenge in the field of ARD research has been the high heterogeneity in study design, symptom assessment methods, outcomes reported, and representation of all skin types. Discrepancies in assessment timing thus made comparisons between studies less reliable. Diverse patient demographics and treatment characteristics further added to this challenge, such as dose fractionation (e.g., hypofractionation vs conventional fractionation), use of a boost and/or bolus, use of concurrent systemic agents, RT modality received, use of concurrent chemotherapy, and anatomical location treated. RT-specific demographic information for included studies has been summarized in Appendix D; future research should be undertaken to determine the impact of treatment-specific factors on the efficacy of each intervention. Furthermore, the majority of studies included breast cancer and head and neck cancer patients as these cancer sites are most at-risk of developing ARD given the dose and RT techniques used, the nature of skin folds in these regions; as such, other cancer sites, such as anorectal, skin, or bone cancers, were not adequately reflected in this review due to the limited literature available.
In addition, there was considerable variability of outcome measures within intra- and inter-agent studies. While many studies used validated scoring scales to measure ARD, many other studies used internally-developed scoring systems, which in turn minimized comparability between studies. With over 50 available assessment methods for ARD severity and associated symptoms, including clinician-reported outcome (CRO) measures, patient-reported outcome measures, and biophysical parameters, it has become difficult to discern the true benefit of interventions in minimizing ARD severity. The high variability in ARD assessment reveals a need for the establishment of an international consensus on a “gold standard” method for ARD measurement. Furthermore, while the most commonly reported outcomes across the majority of studies included moist desquamation incidence, overall ARD grade (based on standardized CRO scales), and subjective measures, such as pain and pruritus, this review revealed other clinically important outcomes that were less often reported and therefore could not be compared. Such outcomes included treatment delays, patient satisfaction with intervention, treatment costs, skin hydration levels, time to ARD resolution, and tolerability of intervention. Despite the vast amount of evidence conducted on ARD, minimal conclusions can be drawn from the evidence alone on the clinical feasibility of each intervention due to the lack of comparability among trials.
Other general trends were found with the proposed agents. The majority of studies had methodological challenges (such as a small sample size and/or lack of blinding), resulting in a low QoE. A limited ability to blind is an inherent flaw in many dermatological trials that is understandably difficult to control; nevertheless, it presents a challenge in the objective assessment of interventions that are visible to observers, such as barrier films and dressings or certain procedural interventions (e.g., acupuncture, massage therapy, laughter therapy). Additionally, the majority of interventions also had few studies evaluating their efficacy. Recommendation of an intervention is only typically justified upon clear demonstration of efficacy across several high-quality randomized trials, yet only few interventions on ARD have been investigated across multiple RCTs. Combined, these challenges made it difficult to assess many of the proposed therapeutic agents.
In addition to common challenges across the field of ARD research, several other limitations were found in our methodology. First, while the 1996 Hadorn criteria for evaluating the quality of research studies has been beneficial in other settings, such as the development of the MASCC Mucositis Clinical Practice Guidelines,255 it may have not have been suitable for the purpose of this review due to the overly generalized assessment criteria. For example, a study was automatically deemed to be of “poor” QoE for using an open-label approach, despite restrictions against blinding with certain interventions. As such, some studies may have been designated a “doubtful” QoE for the sole reason that the intervention could not be blinded. The use of the Hadorn criteria likely overemphasized certain flaws because it was not designed to assess QoE in dermatological trials, thus highlighting the need for a QoE assessment tool tailored specifically to dermatology trials.
Next, since the initial literature search was conducted in September 2020, several RCTs have since been published supporting interventions for the prevention and management of ARD, such as Calendula officinalis,256 photobiomodulation therapy,257 and topical steroid.212 In light of this limitation, an updated search has been conducted from September 22nd, 2020 to January 21st, 2023 and revealed 65 new studies, of which 38 are RCTs; a list of the latest RCTs has been provided in Appendix E, along with a summary of RCT characteristics. RCTs were recently published on interventions already identified through the present review, such as Mepitel film,258 topical corticosteroids,259,260 heparinoid,261 photobiomodulation,257,262,263 aloe vera gel,264 calendula,256 and others. Additional RCTs were identified on new interventions not previously explored before September 2020, such as the use of compound Kushen injection,265 epigallocatechin-3-gallate,201,266 bacterial decolonization,267 and others. For example, in a population of 81 breast cancer patients, Siddiquee et al. (2020)256 reported no difference in grade 2+ ARD severity between patients receiving Calendula and standard of care (p = 0.92); this finding supported previous findings by Sharp et al. (2013)116 and helps to confirm the potential inefficacy of Calendula in preventing ARD. Robijns et al. (2021) added to the existing evidence base on photobiomodulation therapy research in head and neck cancer patients (n = 46), whereby patients who received photobiomodulation therapy were found to have a lower percentage of grade 2–3 ARD when compared to the control group (77.8% control group vs. 28.6% photobiom8odulation group, p = 0.002).257 A 2022 trial on Mepitel film similarly demonstrated benefits of Mepitel film in preventing ARD in a population of 376 patients (15.5% vs. 45.6% p < 0.0001).258 The findings of these recent trials demonstrate a positive trend in the evidence supporting photobiomodulation therapy, topical steroids, and Mepitel film for the prevention of ARD. Overall, among the RCTs identified, 27 demonstrated some significant improvement in RD severity; these latest findings may influence the recommendations made in future guidelines, thus further highlighting a need for regular updates of the literature to reflect ongoing changes in the evidence base.
With regards to the inclusion of studies, we only included studies published in the English language and did not use a translation tool for non-English studies to avoid any risk of mistranslation; this may have resulted in the exclusion of relevant studies. Additionally, there may be non-reporting bias, due to the exclusion of studies that (1) were conducted but never published, and (2) studies that calculated a measure of interest but did not mention this in their report(s), for various reasons. To minimize the risk of non-reporting bias, the databases were searched comprehensively according to the Cochrane Handbook for Systematic Reviews of Interventions268 and under the supervision of a medical librarian, though some bias may still be present.
Lastly, in the past decade, advanced RT modalities have shown promising results in minimizing ARD severity. Our review was limited in that we only included agents directly administered with the intent of reducing skin toxicities and therefore excluded advanced RT techniques. IMRT,269 helical tomotherapy,270 prone positioning (in breast patients),271 proton RT,272 and extreme hypofractionation273 have demonstrated efficacy in reducing ARD severity to some extent in past trials. Additionally, there were limitations in the diversity of populations in many of the clinicals trials reviewed, including underrepresentation of skin of color and English-only speaking populations. Further research should broaden inclusion across languages and skin types to encourage development of evidence-based recommendations on appropriate RT modalities to reduce the burden of ARD in all patients.
Conclusions
In spite of the significant amount of available literature, the evidence supporting interventions for ARD prevention and management is highly variable, most likely due to the differences in study design, outcomes assessed, intervention type, and patient population between studies. Further trials should be conducted on interventions that have shown promising results thus far, such as photobiomodulation therapy, Mepitel film, Hydrofilm, olive oil, oral enzyme mixtures, mometasone furoate, and betamethasone, to confirm efficacy in larger, diverse cohorts of patients. Additional work should also be undertaken to investigate the use of advanced RT modalities in minimizing severe skin toxicity. It is essential that clinical practice guidelines on ARD care not only reflect the current evidence, but the clinical, real-world experience of healthcare providers as well. Expansion of research to include real-world evidence will assist in overcoming the current issues in ARD management with resource-limited environments unable to use any of the potential interventions discussed. As such, to inform evidence-based skin care recommendations on behalf of MASCC, a Delphi consensus process will be reported in a separate publication to reflect the opinions of a panel of experts in treating ARD.
Contributors
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by T.B., D.G., S.F., P.P., L.K., S.F.L., A.W.C., H.C.Y.W., S.M., and S.K., H.L., S.F.L., A.W.C., and H.C.Y.W. performed the literature search. S.C. advised on the analysis methodology. The first draft of the manuscript was written by T.B. and D.G., and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data sharing statement
Not applicable.
Declaration of interests
Julie Ryan Wolf declares BARDA funding for clinical trial for safety of Silverlon dressing for radiation dermatitis and UpToDate coauthor royalties. Tara Behroozian received a travel grant from the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Group (Fall 2022). Edward Chow conducted a clinical trial and received materials free of cost from Mölnlycke Health Care. All other authors declare that they have no conflict of interest.
Acknowledgements
T.B. and D.G. contributed equally to this work. P.B. and E.C. are joint senior authors. J.R.W. and C.v.d.H. are chair and vice chair of the MASCC Oncodermatology Study Group, respectively. All authors are members of the MASCC Oncodermatology Study Group. Thenugaa Rajeswaran BSc (Candidate) and Milena Gojsevic BSc (Candidate) assisted in the data verification. No funding was received.
Footnotes
MASCC does not endorse any brand names of therapeutics identified in this document or in any other documents.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eclinm.2023.101886.
Contributor Information
Tara Behroozian, Email: tara.behroozian@medportal.ca.
Multinational Association of Supportive Care in Cancer (MASCC) Oncodermatology Study Group Radiation Dermatitis Guidelines Working Group:
Tara Behroozian, Daniel Goldshtein, Julie Ryan Wolf, Corina van den Hurk, Samuel Finkelstein, Henry Lam, Partha Patel, Lauren Kanee, Shing Fung Lee, Adrian Wai Chan, Henry Chun Yip Wong, Saverio Caini, Simran Mahal, Samantha Kennedy, Edward Chow, and Pierluigi Bonomo
Appendix A. Supplementary data
References
- 1.Singh M., Alavi A., Wong R., Akita S. Radiodermatitis: a review of our current understanding. Am J Clin Dermatol. 2016;17(3):277–292. doi: 10.1007/s40257-016-0186-4. [DOI] [PubMed] [Google Scholar]
- 2.Wei J., Meng L., Hou X., et al. Radiation-induced skin reactions: mechanism and treatment. Cancer Manag Res. 2019;11:167–177. doi: 10.2147/CMAR.S188655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leventhal J., Young M.R. Radiation dermatitis: recognition, prevention, and management. Oncology. 2017;31(12):885–887. 894–899. [PubMed] [Google Scholar]
- 4.Xie Y., Wang Q., Hu T., et al. Risk factors related to acute radiation dermatitis in breast cancer patients after radiotherapy: a systematic review and meta-analysis. Front Oncol. 2021;11:738851. doi: 10.3389/fonc.2021.738851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pignol J.P., Truong P., Rakovitch E., Sattler M.G., Whelan T.J., Olivotto I.A. Ten years results of the Canadian breast intensity modulated radiation therapy (IMRT) randomized controlled trial. Radiother Oncol. 2016;121(3):414–419. doi: 10.1016/j.radonc.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 6.Rzepecki A., Birnbaum M., Ohri N., et al. Characterizing the effects of radiation dermatitis on quality of life: a prospective survey-based study. J Am Acad Dermatol. 2019;86:161–163. doi: 10.1016/j.jaad.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Beamer L.C., Grant M. Using the dermatology life quality index to assess how breast radiodermatitis affects patients' quality of life. Breast Cancer Basic Clin Res. 2019;13 doi: 10.1177/1178223419835547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schnur J.B., Zivin J.G., Mattson D.M.K., et al. Acute skin toxicity-related, out-of pocket expenses in patients with breast cancer treated with external beam radiotherapy: a descriptive, exploratory study. Support Care Cancer. 2012;20(12):3105–3113. doi: 10.1007/s00520-012-1435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finkelstein S., Kanee L., Behroozian T., et al. Comparison of clinical practice guidelines on radiation dermatitis: a narrative review. Support Care Cancer. 2022;30(6):4663–4674. doi: 10.1007/s00520-022-06829-6. [DOI] [PubMed] [Google Scholar]
- 10.Wong R.K.S., Bensadoun R.J., Boers-Doets C.B., et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer. 2013;21(10):2933–2948. doi: 10.1007/s00520-013-1896-2. [DOI] [PubMed] [Google Scholar]
- 11.Aslam S., Emmanuel P. Formulating a researchable question: a critical step for facilitating good clinical research. Indian J Sex Transm Dis. 2010;31(1):47–50. doi: 10.4103/0253-7184.69003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liberati A., Altman D.G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hadorn D.C., Baker D., Hodges J.S., Hicks N. Rating the quality of evidence for clinical practice guidelines. J Clin Epidemiol. 1996;49(7):749–754. doi: 10.1016/0895-4356(96)00019-4. [DOI] [PubMed] [Google Scholar]
- 14.Abbas H., Bensadoun R.J. Trolamine emulsion for the prevention of radiation dermatitis in patients with squamous cell carcinoma of the head and neck. Support Care Cancer. 2012;20(1):185–190. doi: 10.1007/s00520-011-1110-3. [DOI] [PubMed] [Google Scholar]
- 15.Elliott E.A., Wright J.R., Swann R.S., et al. Phase III Trial of an emulsion containing trolamine for the prevention of radiation dermatitis in patients with advanced squamous cell carcinoma of the head and neck: results of Radiation Therapy Oncology Group Trial 99-13. J Clin Oncol. 2006;24(13):2092–2097. doi: 10.1200/JCO.2005.04.9148. [DOI] [PubMed] [Google Scholar]
- 16.Fenig E., Brenner B., Katz A., et al. Topical Biafine and Lipiderm for the prevention of radiation dermatitis: a randomized prospective trial. Oncol Rep. 2001;8(2):305–309. [PubMed] [Google Scholar]
- 17.Fisher J., Scott C., Stevens R., et al. Randomized phase III study comparing Best Supportive Care to Biafine as a prophylactic agent for radiation-induced skin toxicity for women undergoing breast irradiation: radiation Therapy Oncology Group (RTOG) 97-13. Int J Radiat Oncol Biol Phys. 2000;48(5):1307–1310. doi: 10.1016/s0360-3016(00)00782-3. [DOI] [PubMed] [Google Scholar]
- 18.Gosselin T.K., Schneider S.M., Plambeck M.A., Rowe K. A prospective randomized, placebo-controlled skin care study in women diagnosed with breast cancer undergoing radiation therapy. Oncol Nurs Forum. 2010;37(5):619–626. doi: 10.1188/10.ONF.619-626. [DOI] [PubMed] [Google Scholar]
- 19.Simard P.F., Bolton R.M., Tarbell N.J. Anti-inflammatory cream reduces skin damage induced by ionizing radiation. Oncologist. 2009;14(2):197–198. doi: 10.1634/theoncologist.2008-0256. [DOI] [PubMed] [Google Scholar]
- 20.Szumacher E., Wighton A., Franssen E., et al. Phase II study assessing the effectiveness of Biafine cream as a prophylactic agent for radiation-induced acute skin toxicity to the breast in women undergoing radiotherapy with concomitant CMF chemotherapy. Int J Radiat Oncol Biol Phys. 2001;51(1):81–86. doi: 10.1016/s0360-3016(01)01576-0. [DOI] [PubMed] [Google Scholar]
- 21.Rizza L., D'Agostino A., Girlando A., Puglia C. Evaluation of the effect of topical agents on radiation-induced skin disease by reflectance spectrophotometry. J Pharm Pharmacol. 2010;62(6):779–785. doi: 10.1211/jpp.62.06.0015. [DOI] [PubMed] [Google Scholar]
- 22.Liguori V., Guillemin C., Pesce G.F., Mirimanoff R.O., Bernier J. Double-blind, randomized clinical study comparing hyaluronic acid cream to placebo in patients treated with radiotherapy. Radiother Oncol J. 1997;42(2):155–161. doi: 10.1016/s0167-8140(96)01882-8. [DOI] [PubMed] [Google Scholar]
- 23.Maynard A.S., Mitchell C., Calcaterra A. Results of radiaplex in reduction of post-radiation skin reactions in patients with breast cancer. Int J Radiat Oncol. 2010;78(3):S240–S241. [Google Scholar]
- 24.Pinnix C., Perkins G.H., Strom E.A., et al. Topical hyaluronic acid vs. standard of care for the prevention of radiation dermatitis after adjuvant radiotherapy for breast cancer: single-blind randomized phase III clinical trial. Int J Radiat Oncol Biol Phys. 2012;83(4):1089–1094. doi: 10.1016/j.ijrobp.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Primavera G., Carrera M., Berardesca E., Pinnaró P., Messina M., Arcangeli G. A double-blind, vehicle-controlled clinical study to evaluate the efficacy of MAS065D (XClairTM), a hyaluronic acid-based formulation, in the management of radiation-induced dermatitis. Cutan Ocul Toxicol. 2006;25(3):165–171. doi: 10.1080/15569520600860009. [DOI] [PubMed] [Google Scholar]
- 26.Rahimi A., Mohamad O., Albuquerque K., et al. Novel hyaluronan formulation for preventing acute skin reactions in breast during radiotherapy: a randomized clinical trial. Support Care Cancer. 2020;28(3):1481–1489. doi: 10.1007/s00520-019-04957-0. [DOI] [PubMed] [Google Scholar]
- 27.Ferreira E., Reis P. Assessing the effectiveness of urea cream as a prophylactic agent for radiation dermatitis. Cancer Nurs. 2017;40(6):E14. [Google Scholar]
- 28.Momm F., Weissenberger C., Bartelt S., Henke M. Moist skin care can diminish acute radiation-induced skin toxicity. Strahlenther Onkol. 2003;179(10):708–712. doi: 10.1007/s00066-003-1142-9. [DOI] [PubMed] [Google Scholar]
- 29.Ogita M., Sekiguchi K., Akahane K., et al. Moisturizer efficacy for breast radiation-induced dermatitis: a prospective open-label, randomized trial. Radiother Oncol. 2014;111:S61–S62. [Google Scholar]
- 30.Ogita M., Sekiguchi K., Akahane K., et al. Damage to sebaceous gland and the efficacy of moisturizer after whole breast radiotherapy: a randomized controlled trial. BMC Cancer. 2019;19(1):125. doi: 10.1186/s12885-019-5334-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sekiguchi K., Akahane K., Ogita M., et al. Efficacy of heparinoid moisturizer as a prophylactic agent for radiation dermatitis following radiotherapy after breast-conserving surgery: a randomized controlled trial. Jpn J Clin Oncol. 2018;48(5):450–457. doi: 10.1093/jjco/hyy045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iacovelli N.A., Naimo S., Bonfantini F., et al. Preemptive treatment with Xonrid®, a medical device to reduce radiation induced dermatitis in head and neck cancer patients receiving curative treatment: a pilot study. Support Care Cancer. 2017;25(6):1787–1795. doi: 10.1007/s00520-017-3569-z. [DOI] [PubMed] [Google Scholar]
- 33.Ingargiola R., De Santis M.C., Iacovelli N.A., et al. A monocentric, open-label randomized standard-of-care controlled study of XONRID®, a medical device for the prevention and treatment of radiation-induced dermatitis in breast and head and neck cancer patients. Radiat Oncol. 2020;15(1):193. doi: 10.1186/s13014-020-01633-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laffin N., Smyth W., Heyer E., Fasugba O., Abernethy G., Gardner A. Effectiveness and acceptability of a moisturizing cream and a barrier cream during radiation therapy for breast cancer in the tropics: a randomized controlled trial. Cancer Nurs. 2015;38(3):205–214. doi: 10.1097/NCC.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 35.Graham P.H., Plant N., Graham J.L., et al. A paired, double-blind, randomized comparison of a moisturizing durable barrier cream to 10% glycerine cream in the prophylactic management of postmastectomy irradiation skin care: trans Tasman Radiation Oncology Group (TROG) 04.01. Int J Radiat Oncol Biol Phys. 2013;86(1):45–50. doi: 10.1016/j.ijrobp.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 36.Løkkevik E., Skovlund E., Reitan J.B., Hannisdal E., Tanum G. Skin treatment with bepanthen cream versus no cream during radiotherapy: a randomized controlled trial. Acta Oncol. 1996;35(8):1021–1026. doi: 10.3109/02841869609100721. [DOI] [PubMed] [Google Scholar]
- 37.Aysan E., Idiz U.O., Elmas L., Saglam E.K., Akgun Z., Yucel S.B. Effects of boron-based gel on radiation-induced dermatitis in breast cancer: a double-blind, placebo-controlled trial. J Invest Surg. 2017;30(3):187–192. doi: 10.1080/08941939.2016.1232449. [DOI] [PubMed] [Google Scholar]
- 38.Pastore F., Rese A., Panelli G., Pepe A., Toledo D., Iorio V. PDRN-based cream in the prevention and treatment of radiodermatitis in Head and nech cancer: our experience. Radiother Oncol. 2019;133:S366–S367. [Google Scholar]
- 39.Censabella S., Claes S., Orlandini M., Braekers R., Bulens P. Efficacy of a hydroactive colloid gel versus historical controls for the prevention of radiotherapy-induced moist desquamation in breast cancer patients. Eur J Oncol Nurs. 2017;29:1–7. doi: 10.1016/j.ejon.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 40.Rudge R. Colloidal oatmeal emollient as an alternative skincare approach in radiotherapy: a feasibility study. J Radiother Pract. 2016;15(4):322–333. [Google Scholar]
- 41.Maramaldi G., Riva A., Togni S., Cesarone M.R., Corti A., Belcaro G. Leniqol cream in radio-dermatitis. Minerva Med. 2019;21(1):1–5. [Google Scholar]
- 42.Tao Y., Auperin A., Sire C., et al. Multicenter Randomized Double-Blind, Placebo-Controlled Trial GORTEC (Groupe Oncologie Radiotherapie Tete et Cou) 2009-01 Evaluating the Effect of the Regenerating Agent on Radiodermatitis of Head and Neck Cancer Patients. Int J Radiat Oncol Biol Phys. 2017;99(3):590–595. doi: 10.1016/j.ijrobp.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 43.Evensen F., Bjordal K., Anne B.J. Effects of Na-sucrose octasulfate on skin and mucosa reactions during radiotherapy of head and neck cancers&A randomized prospective study. Acta Oncol. 2001;40(6):751–755. doi: 10.1080/02841860152619188. [DOI] [PubMed] [Google Scholar]
- 44.Maramaldi G., Corti A., Meneghin M., et al. Evaluation of the soothing effect of Eupilen topical formulation on radiotherapy-induced dermatitis. Minerva Med. 2018;20(2):27–31. [Google Scholar]
- 45.Falkowski S., Trouillas P., Duroux J.L., Bonnetblanc J.M., Clavère P. Radiodermatitis prevention with sucralfate in breast cancer: fundamental and clinical studies. Support Care Cancer. 2011;19(1):57–65. doi: 10.1007/s00520-009-0788-y. [DOI] [PubMed] [Google Scholar]
- 46.Kouloulias V., Asimakopoulos C., Tolia M., et al. Sucralfate gel as a radioprotector against radiation induced dermatitis in a hypo-fractionated schedule: a non-randomized study. Hippokratia. 2013;17(2):126–129. [PMC free article] [PubMed] [Google Scholar]
- 47.Maiche A., Isokangas O.P., Gröhn P. Skin protection by sucralfate cream during electron beam therapy. Acta Oncol. 1994;33(2):201–203. doi: 10.3109/02841869409098406. [DOI] [PubMed] [Google Scholar]
- 48.Wells M., Macmillan M., Raab G., et al. Does aqueous or sucralfate cream affect the severity of erythematous radiation skin reactions? A randomised controlled trial. Radiother Oncol. 2004;73(2):153–162. doi: 10.1016/j.radonc.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 49.Cleary J.F., Anderson B.M., Eickhoff J.C., Khuntia D., Fahl W.E. Significant suppression of radiation dermatitis in breast cancer patients using a topically applied adrenergic vasoconstrictor. Radiat Oncol. 2017;12(1):201. doi: 10.1186/s13014-017-0940-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ben-David M.A., Elkayam R., Gelernter I., Pfeffer R.M. Melatonin for prevention of breast radiation dermatitis: a phase II, prospective, double-blind randomized trial. Isr Med Assoc J. 2016;18(3–4):188–192. [PubMed] [Google Scholar]
- 51.Ghasemi A., Ghashghai Z., Akbari J., Yazdani-Charati J., Salehifar E., Hosseinimehr S.J. Topical atorvastatin 1% for prevention of skin toxicity in patients receiving radiation therapy for breast cancer: a randomized, double-blind, placebo-controlled trial. Eur J Clin Pharmacol. 2019;75(2):171–178. doi: 10.1007/s00228-018-2570-x. [DOI] [PubMed] [Google Scholar]
- 52.Leonardi M.C., Gariboldi S., Ivaldi G.B., et al. A double-blind, randomised, vehicle-controlled clinical study to evaluate the efficacy of MAS065D in limiting the effects of radiation on the skin: interim analysis. Eur J Dermatol. 2008;18(3):317–321. doi: 10.1684/ejd.2008.0396. [DOI] [PubMed] [Google Scholar]
- 53.Berger A., Regueiro C., Hijal T., et al. Interest of supportive and barrier protective skin care products in the daily prevention and treatment of cutaneous toxicity during radiotherapy for breast cancer. Breast Cancer Basic Clin Res. 2018;12 doi: 10.1177/1178223417752772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stefanelli A., Forte L., Medoro S., et al. Topical use of phytotherapic cream (Capilen® cream) to prevent radiodermatitis in breast cancer: a prospective historically controlled clinical study. G Ital Dermatol venereol Organo Uff Soc Ital Dermatol Sifilogr. 2014;149(1):107–113. [PubMed] [Google Scholar]
- 55.Schreck U., Paulsen F., Bamberg M., Budach W. Intraindividual comparison of two different skin care conceptions in patients undergoing radiotherapy of the head-and-neck region creme or powder?: creme or powder? Strahlenther Onkol. 2002;178(6):321–329. doi: 10.1007/s00066-002-0912-0. [DOI] [PubMed] [Google Scholar]
- 56.Boström A., Lindman H., Swartling C., Berne B., Bergh J. Potent corticosteroid cream (mometasone furoate) significantly reduces acute radiation dermatitis: results from a double-blind, randomized study. Radiother Oncol. 2001;59(3):257–265. doi: 10.1016/s0167-8140(01)00327-9. [DOI] [PubMed] [Google Scholar]
- 57.Dunn K., Hindley A., Wood L., et al. Mometasone furoate significantly reduces radiation dermatitis in patients undergoing breast radiation therapy: a double-blind randomized control trial in 120 patients. Int J Radiat Oncol. 2013;87(2):S115. [Google Scholar]
- 58.Ho A.Y., Olm-Shipman M., Zhang Z., et al. A randomized trial of mometasone furoate 0.1% to reduce high-grade acute radiation dermatitis in breast cancer patients receiving postmastectomy radiation. Int J Radiat Oncol Biol Phys. 2018;101(2):325–333. doi: 10.1016/j.ijrobp.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 59.Liao Y., Feng G., Dai T., et al. Randomized, self-controlled, prospective assessment of the efficacy of mometasone furoate local application in reducing acute radiation dermatitis in patients with head and neck squamous cell carcinomas. Medicine (Baltimore) 2019;98(52):e18230. doi: 10.1097/MD.0000000000018230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller R.C., Schwartz D.J., Sloan J.A., et al. Mometasone furoate effect on acute skin toxicity in breast cancer patients receiving radiotherapy: a phase III double-blind, randomized trial from the North Central Cancer Treatment Group N06C4. Int J Radiat Oncol Biol Phys. 2011;79(5):1460–1466. doi: 10.1016/j.ijrobp.2010.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Olm-Shipman M., Gelblum D., Lacouture M.E., et al. Efficacy of mometasone furoate in the reduction of moderate/severe radiation dermatitis in breast cancer patients following mastectomy. Int J Radiat Oncol. 2016;96(2):S5. [Google Scholar]
- 62.Shaw S.Z., Nien H.H., Wu C.J., Lui L.T., Su J.F., Lang C.H. 3M Cavilon No-Sting Barrier Film or topical corticosteroid (mometasone furoate) for protection against radiation dermatitis: a clinical trial. J Formos Med Assoc. 2015;114(5):407–414. doi: 10.1016/j.jfma.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 63.Meghrajani C.F., Co H.S., Arcillas J.G., Maaño C.C., Cupino N.A. A randomized, double-blind trial on the use of 1% hydrocortisone cream for the prevention of acute radiation dermatitis. Expet Rev Clin Pharmacol. 2016;9(3):483–491. doi: 10.1586/17512433.2016.1126506. [DOI] [PubMed] [Google Scholar]
- 64.Sperduti A., Cashell A., Rocca C., et al. A feasibility study of an internal control methodology using hydrocortisone cream for the management of skin reactions in patients receiving radical radiation therapy for cancers of the head and neck. J Radiother Pract. 2006;5(4):211–218. [Google Scholar]
- 65.Erridge S.C., McCabe M., Porter M.K., Simpson P., Stillie A.L. Prospective audit showing improved patient-assessed skin toxicity with use of betamethasone cream for those at high risk of radiation dermatitis. Radiother Oncol. 2016;121(1):143–147. doi: 10.1016/j.radonc.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 66.Menon A., Prem S.S., Kumari R. Topical betamethasone valerate as a prophylactic agent to prevent acute radiation dermatitis in head and neck malignancies: a randomized, open-label, phase 3 trial. Int J Radiat Oncol Biol Phys. 2021;109(1):151–160. doi: 10.1016/j.ijrobp.2020.08.040. [DOI] [PubMed] [Google Scholar]
- 67.Omidvari S., Saboori H., Mohammadianpanah M., et al. Topical betamethasone for prevention of radiation dermatitis. Indian J Dermatol Venereol Leprol. 2007;73(3):209. doi: 10.4103/0378-6323.32755. [DOI] [PubMed] [Google Scholar]
- 68.Ulff E., Maroti M., Serup J., Falkmer U. A potent steroid cream is superior to emollients in reducing acute radiation dermatitis in breast cancer patients treated with adjuvant radiotherapy. A randomised study of betamethasone versus two moisturizing creams. Radiother Oncol. 2013;108(2):287–292. doi: 10.1016/j.radonc.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 69.Uysal B., Gamsız H., Dincoglan F., et al. Comparative evaluation of topical corticosteroid and moisturizer in the prevention of radiodermatitis in breast cancer radiotherapy. Indian J Dermatol. 2020;65(4):279–283. doi: 10.4103/ijd.IJD_607_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shukla P.N., Gairola M., Mohanti B.K., Rath G.K. Prophylactic beclomethasone spray to the skin during postoperative radiotherapy of carcinoma breast: a prospective randomized study. Indian J Cancer. 2006;43(4):180–184. doi: 10.4103/0019-509x.29424. [DOI] [PubMed] [Google Scholar]
- 71.Schmuth M., Wimmer M.A., Hofer S., et al. Topical corticosteroid therapy for acute radiation dermatitis: a prospective, randomized, double-blind study. Br J Dermatol. 2002;146(6):983–991. doi: 10.1046/j.1365-2133.2002.04751.x. [DOI] [PubMed] [Google Scholar]
- 72.Hindley A., Zain Z., Wood L., et al. Mometasone furoate cream reduces acute radiation dermatitis in patients receiving breast radiation therapy: results of a randomized trial. Int J Radiat Oncol Biol Phys. 2014;90(4):748–755. doi: 10.1016/j.ijrobp.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 73.Ulff E., Maroti M., Serup J., Nilsson M., Falkmer U. Prophylactic treatment with a potent corticosteroid cream ameliorates radiodermatitis, independent of radiation schedule: a randomized double blinded study. Radiother Oncol. 2017;122(1):50–53. doi: 10.1016/j.radonc.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 74.Schmeel L.C., Koch D., Schmeel F.C., et al. Hydrofilm polyurethane films reduce radiation dermatitis severity in hypofractionated whole-breast irradiation: an objective, intra-patient randomized dual-center assessment. Polymers. 2019;11(12):E2112. doi: 10.3390/polym11122112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ahn S., Sung K., Kim H.J., et al. Reducing radiation dermatitis using a film-forming silicone gel during breast radiotherapy: a pilot randomized-controlled trial. In Vivo. 2020;34(1):413–422. doi: 10.21873/invivo.11790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Benstead K., Spencer S., Foroudi F., Ho H., Kai C. StrataXRT is as effective as Mepitel Film in reducing the incidence of moist desquamation in breast cancer patients undergoing post-mastectomy radiotherapy. J Med Imaging Radiat Oncol. 2019;63:87-. added to CENTRAL: 30 June 2020 | 2020 Issue 06. [Google Scholar]
- 77.Chan R.J., Blades R., Jones L., et al. A single-blind, randomised controlled trial of StrataXRT® - a silicone-based film-forming gel dressing for prophylaxis and management of radiation dermatitis in patients with head and neck cancer. Radiother Oncol. 2019;139:72–78. doi: 10.1016/j.radonc.2019.07.014. [DOI] [PubMed] [Google Scholar]
- 78.Herst P.M., Bennett N.C., Sutherland A.E., Peszynski R.I., Paterson D.B., Jasperse M.L. Prophylactic use of Mepitel Film prevents radiation-induced moist desquamation in an intra-patient randomised controlled clinical trial of 78 breast cancer patients. Radiother Oncol. 2014;110(1):137–143. doi: 10.1016/j.radonc.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 79.Møller P.K., Olling K., Berg M., et al. Breast cancer patients report reduced sensitivity and pain using a barrier film during radiotherapy - a Danish intra-patient randomized multicentre study. Tech Innov Patient Support Radiat Oncol. 2018;7:20–25. doi: 10.1016/j.tipsro.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morgan K. Radiotherapy-induced skin reactions: prevention and cure. Br J Nurs Mark. 2014;23(16):S24. doi: 10.12968/bjon.2014.23.Sup16.S24. S26–S32. [DOI] [PubMed] [Google Scholar]
- 81.Oshin F., McBrayne L., Bratt M., et al. A retrospective chart review on the prophylactic use of mepitel film for breast cancer patients undergoing chest wall irradiation: a single-institution experience. J Med Imaging Radiat Sci. 2020;51(3):S3–S4. [Google Scholar]
- 82.Yee C., Lam E., Gallant F., et al. A feasibility study of mepitel film for the prevention of breast radiation dermatitis in a Canadian center. Pract Radiat Oncol. 2021;11(1):e36–e45. doi: 10.1016/j.prro.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 83.Rades D., Narvaez C.A., Splettstößer L., et al. A randomized trial (RAREST-01) comparing Mepitel® Film and standard care for prevention of radiation dermatitis in patients irradiated for locally advanced squamous cell carcinoma of the head-and-neck (SCCHN) Radiother Oncol. 2019;139:79–82. doi: 10.1016/j.radonc.2019.07.023. [DOI] [PubMed] [Google Scholar]
- 84.Wooding H., Yan J., Yuan L., et al. The effect of Mepitel Film on acute radiation-induced skin reactions in head and neck cancer patients: a feasibility study. Br J Radiol. 2018;91(1081):20170298. doi: 10.1259/bjr.20170298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu G. Intervention time of mepitel film to prevent radiodermatitis in NPC: a multi center RCT study. Cancer Nurs. 2017;40(6S):E14–E15. [Google Scholar]
- 86.Yan J., Yuan L., Wang J., et al. Mepitel Film is superior to Biafine cream in managing acute radiation-induced skin reactions in head and neck cancer patients: a randomised intra-patient controlled clinical trial. J Med Radiat Sci. 2020;67(3):208–216. doi: 10.1002/jmrs.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Graham P., Browne L., Capp A., et al. Randomized, paired comparison of No-Sting Barrier Film versus sorbolene cream (10% glycerine) skin care during postmastectomy irradiation. Int J Radiat Oncol Biol Phys. 2004;58(1):241–246. doi: 10.1016/s0360-3016(03)01431-7. [DOI] [PubMed] [Google Scholar]
- 88.Lam A.C., Yu E., Vanwynsberghe D., et al. Phase III randomized pair comparison of a barrier film vs. standard skin care in preventing radiation dermatitis in post-lumpectomy patients with breast cancer receiving adjuvant radiation therapy. Cureus. 2019;11(6):e4807. doi: 10.7759/cureus.4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aquino-Parsons C., Lomas S., Smith K., et al. Phase III study of silver leaf nylon dressing vs standard care for reduction of inframammary moist desquamation in patients undergoing adjuvant whole breast radiation therapy. J Med Imaging Radiat Sci. 2010;41(4):215–221. doi: 10.1016/j.jmir.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 90.Niazi T.M., Vuong T., Azoulay L., et al. Silver clear nylon dressing is effective in preventing radiation-induced dermatitis in patients with lower gastrointestinal cancer: results from a phase III study. Int J Radiat Oncol Biol Phys. 2012;84(3):e305–e310. doi: 10.1016/j.ijrobp.2012.03.062. [DOI] [PubMed] [Google Scholar]
- 91.Vuong T., Franco E., Lehnert S., et al. Silver leaf nylon dressing to prevent radiation dermatitis in patients undergoing chemotherapy and external beam radiotherapy to the perineum. Int J Radiat Oncol Biol Phys. 2004;59(3):809–814. doi: 10.1016/j.ijrobp.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 92.Arimura T., Ogino T., Yoshiura T., et al. Effect of film dressing on acute radiation dermatitis secondary to proton beam therapy. Int J Radiat Oncol Biol Phys. 2016;95(1):472–476. doi: 10.1016/j.ijrobp.2015.10.053. [DOI] [PubMed] [Google Scholar]
- 93.Chang C. A randomized clinical comparison of a non-alcohol barrier film and standard care for radiotherapy skin protection in nasopharyngeal cancer patients. Support Care Cancer. 2013;21(S1):1–301. [Google Scholar]
- 94.Whaley Jonathan T, Kirk M., Cengel K., McDonough J., Bekelman J., Christodouleas J.P. Protective effect of transparent film dressing on proton therapy induced skin reactions. Radiat Oncol. 2013;8(1):19. doi: 10.1186/1748-717X-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu G., Yuan L. Observation on new soft silicone dressing to prevent radioactive skin lesion in breast cancer. Cancer Nurs. 2016;39(6):S39–S40. [Google Scholar]
- 96.Schmeel L.C., Koch D., Stumpf S., et al. Prophylactically applied Hydrofilm polyurethane film dressings reduce radiation dermatitis in adjuvant radiation therapy of breast cancer patients. Acta Oncol. 2018;57(7):908–915. doi: 10.1080/0284186X.2018.1441542. [DOI] [PubMed] [Google Scholar]
- 97.DeLand M.M., Weiss R.A., McDaniel D.H., Geronemus R.G. Treatment of radiation-induced dermatitis with light-emitting diode (LED) photomodulation. Laser Surg Med. 2007;39(2):164–168. doi: 10.1002/lsm.20455. [DOI] [PubMed] [Google Scholar]
- 98.Fife D., Rayhan D.J., Behnam S., et al. A randomized, controlled, double-blind study of light emitting diode photomodulation for the prevention of radiation dermatitis in patients with breast cancer. Dermatol Surg. 2010;36(12):1921–1927. doi: 10.1111/j.1524-4725.2010.01801.x. [DOI] [PubMed] [Google Scholar]
- 99.Gorji E., Djavid G., Nicofar A., et al. Evaluation of low-level laser therapy (630 nm) in prevention of radiation-induced dermatitis in breast cancer patients. Ann Surg Oncol. 2013;20:57. [Google Scholar]
- 100.Robijns J., Censabella S., Claes S., et al. Prevention of acute radiodermatitis by photobiomodulation: a randomized, placebo-controlled trial in breast cancer patients (TRANSDERMIS trial) Laser Surg Med. 2018 doi: 10.1002/lsm.22804. [DOI] [PubMed] [Google Scholar]
- 101.Strouthos I., Chatzikonstantinou G., Tselis N., et al. Photobiomodulation therapy for the management of radiation-induced dermatitis: a single-institution experience of adjuvant radiotherapy in breast cancer patients after breast conserving surgery. Strahlenther Onkol. 2017;193(6):491–498. doi: 10.1007/s00066-017-1117-x. [DOI] [PubMed] [Google Scholar]
- 102.Park J.H., Byun H.J., Lee J.H., et al. Feasibility of photobiomodulation therapy for the prevention of radiodermatitis: a single-institution pilot study. Laser Med Sci. 2020;35(5):1119–1127. doi: 10.1007/s10103-019-02930-1. [DOI] [PubMed] [Google Scholar]
- 103.Roma A. Low level laser therapy in skin reactions post radiotherapy. Laser Surg Med. 2013;45(S25):1–93. [Google Scholar]
- 104.Zhang X., Li H., Li Q., et al. Application of red light phototherapy in the treatment of radioactive dermatitis in patients with head and neck cancer. World J Surg Oncol. 2018;16(1):222. doi: 10.1186/s12957-018-1522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Simonova-Pushkar L.I., Kulinich G.V., Gertman V.Z., Belogurova L.V. Prevention and treatment of post-radiation skin reactions after radiation therapy of breast cancer with photo-magnetic therapy. Photodiagnosis Photodyn Ther. 2012;9:S29–S30. [Google Scholar]
- 106.Robijns J., Censabella S., Claes S., et al. Biophysical skin measurements to evaluate the effectiveness of photobiomodulation therapy in the prevention of acute radiation dermatitis in breast cancer patients. Support Care Cancer. 2019;27(4):1245–1254. doi: 10.1007/s00520-018-4487-4. [DOI] [PubMed] [Google Scholar]
- 107.Ahmadloo N., Kadkhodaei B., Omidvari S., et al. Lack of prophylactic effects of aloe vera gel on radiation induced dermatitis in breast cancer patients. Asian Pac J Cancer Prev. 2017;18(4):1139–1143. doi: 10.22034/APJCP.2017.18.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Haddad P., Amouzgar-Hashemi F., Samsami S., Chinichian S., Oghabian M.A. Aloe vera for prevention of radiation-induced dermatitis: a self-controlled clinical trial. Curr Oncol. 2013;20(4):e345–e348. doi: 10.3747/co.20.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Heggie S., Bryant G.P., Tripcony L., et al. A Phase III study on the efficacy of topical aloe vera gel on irradiated breast tissue. Cancer Nurs. 2002;25(6):442–451. doi: 10.1097/00002820-200212000-00007. [DOI] [PubMed] [Google Scholar]
- 110.Hoopfer D., Holloway C., Gabos Z., et al. Three-Arm randomized phase III trial: quality aloe and placebo cream versus powder as skin treatment during breast cancer radiation therapy. Clin Breast Cancer. 2015;15(3):181–190.e1-4. doi: 10.1016/j.clbc.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 111.Olsen D.L., Raub W., Bradley C., et al. The effect of aloe vera gel/mild soap versus mild soap alone in preventing skin reactions in patients undergoing radiation therapy. Oncol Nurs Forum. 2001;28(3):543–547. [PubMed] [Google Scholar]
- 112.Williams M.S., Burk M., Loprinzi C.L., et al. Phase III double-blind evaluation of an aloe vera gel as a prophylactic agent for radiation-induced skin toxicity. Int J Radiat Oncol Biol Phys. 1996;36(2):345–349. doi: 10.1016/s0360-3016(96)00320-3. [DOI] [PubMed] [Google Scholar]
- 113.Fenton-Kerimian M., Cartwright F., Peat E., et al. Optimal topical agent for radiation dermatitis during breast radiotherapy: a pilot study. Clin J Oncol Nurs. 2015;19(4):451–455. doi: 10.1188/15.CJON.451-455. [DOI] [PubMed] [Google Scholar]
- 114.Maurizi F., Bavasso A., Maronta D., Moroni G., Ceccolini M., Bunkheila F. Two different calendula creams for the prophylactic management of acute skin toxicity: a pilot prospective study. Radiother Oncol. 2012;103:S483–S484. [Google Scholar]
- 115.Pommier P., Gomez F., Sunyach M.P., D'Hombres A., Carrie C., Montbarbon X. Phase III randomized trial of Calendula officinalis compared with trolamine for the prevention of acute dermatitis during irradiation for breast cancer. J Clin Oncol. 2004;22(8):1447–1453. doi: 10.1200/JCO.2004.07.063. [DOI] [PubMed] [Google Scholar]
- 116.Sharp L., Finnilä K., Johansson H., Abrahamsson M., Hatschek T., Bergenmar M. No differences between Calendula cream and aqueous cream in the prevention of acute radiation skin reactions – results from a randomised blinded trial. Eur J Oncol Nurs. 2013;17(4):429–435. doi: 10.1016/j.ejon.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 117.Palatty P.L., Azmidah A., Rao S., et al. Topical application of a sandal wood oil and turmeric based cream prevents radiodermatitis in head and neck cancer patients undergoing external beam radiotherapy: a pilot study. Br J Radiol. 2014;87(1038):20130490. doi: 10.1259/bjr.20130490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rao S., Hegde S.K., Baliga-Rao M.P., et al. Sandalwood oil and turmeric-based cream prevents ionizing radiation-induced dermatitis in breast cancer patients: clinical study. Medicines (Basel) 2017;4(3):E43. doi: 10.3390/medicines4030043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ryan Wolf J., Heckler C.E., Guido J.J., et al. Oral curcumin for radiation dermatitis: a URCC NCORP study of 686 breast cancer patients. Support Care Cancer. 2018;26(5):1543–1552. doi: 10.1007/s00520-017-3957-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ryan J.L., Heckler C.E., Ling M., et al. Curcumin for radiation dermatitis: a randomized, double-blind, placebo-controlled clinical trial of thirty breast cancer patients. Radiat Res. 2013;180(1):34–43. doi: 10.1667/RR3255.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ryan Wolf J., Gewandter J.S., Bautista J., et al. Utility of topical agents for radiation dermatitis and pain: a randomized clinical trial. Support Care Cancer. 2020;28(7):3303–3311. doi: 10.1007/s00520-019-05166-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hemati S., Saeedi A. Clinical evaluation of Oral curcumin in prevention of acute dermatitis in breast cancer radiotherapy. J Isfahan Med School. 2011;29(152):1129–1136. [Google Scholar]
- 123.Huang C.J., Huang M.Y., Fang P.T., et al. Randomized double-blind, placebo-controlled trial evaluating oral glutamine on radiation-induced oral mucositis and dermatitis in head and neck cancer patients. Am J Clin Nutr. 2019;109(3):606–614. doi: 10.1093/ajcn/nqy329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Imai T., Matsuura K., Asada Y., et al. Effect of HMB/Arg/Gln on the prevention of radiation dermatitis in head and neck cancer patients treated with concurrent chemoradiotherapy. Jpn J Clin Oncol. 2014;44(5):422–427. doi: 10.1093/jjco/hyu027. [DOI] [PubMed] [Google Scholar]
- 125.Kwok R., O'Brien S., Lunz L., et al. Prospective randomized pilot study of standard skin care versus medihoney in the prophylactic and acute management of radiation dermatitis in patients receiving adjuvant radiation therapy for breast cancer. Int J Radiat Oncol. 2015;93(3):E468–E469. [Google Scholar]
- 126.Naidoo N., Molan P., Littler R., Mok G., Jameson M., Round G. A phase II randomized controlled trial of manuka honey as prophylaxis against radiation-induced dermatitis in breast cancer patients. Eur J Cancer. 2011;47:S367. [Google Scholar]
- 127.Becker-Schiebe M., Mengs U., Schaefer M., Bulitta M., Hoffmann W. Topical use of a silymarin-based preparation to prevent radiodermatitis : results of a prospective study in breast cancer patients. Strahlenther Onkol. 2011;187(8):485–491. doi: 10.1007/s00066-011-2204-z. [DOI] [PubMed] [Google Scholar]
- 128.Elyasi S., Karbasforushan H., Hosseini S., Fani Pakdel A. Topical silymarin administration for prevention of radiodermatitis in breast cancer patients: a randomized, double-blind, placebo-controlled clinical trial. Ann Oncol. 2018;29:ix2. doi: 10.1002/ptr.6231. [DOI] [PubMed] [Google Scholar]
- 129.Halperin E.C., Gaspar L., George S., Darr D., Pinnell S. A double-blind, randomized, prospective trial to evaluate topical vitamin C solution for the prevention of radiation dermatitis. CNS Cancer Consortium. Int J Radiat Oncol Biol Phys. 1993;26(3):413–416. doi: 10.1016/0360-3016(93)90958-x. [DOI] [PubMed] [Google Scholar]
- 130.Nasser N.J., Fenig S., Ravid A., et al. Vitamin D ointment for prevention of radiation dermatitis in breast cancer patients. NPJ Breast Cancer. 2017;3:10. doi: 10.1038/s41523-017-0006-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chou H.L., Shueng P.W., Liao L.J., et al. Prophylactic NS-21 maintains the skin moisture but does not reduce the severity of radiation dermatitis in patients with head and neck cancer: a randomized control trial. Radiat Oncol. 2019;14(1):90. doi: 10.1186/s13014-019-1302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hsieh C.H., Chou H.L., Shueng P.W., et al. A double-blind, randomized pilot study of NS-21 in the prevention of radiation dermatitis for patients with head and neck cancer. J Clin Oncol. 2018;36(15_suppl):6065. [Google Scholar]
- 133.Huang C.J., Hou M.F., Kan J.Y., et al. Prophylactic treatment with adlay bran extract reduces the risk of severe acute radiation dermatitis: a prospective, randomized, double-blind study. Evid Based Complement Alternat Med. 2015;2015:1–8. doi: 10.1155/2015/312072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kong M., Hwang D.S., Lee J.Y., Yoon S.W. The efficacy and safety of jaungo, a traditional medicinal ointment, in preventing radiation dermatitis in patients with breast cancer: a prospective, single-blinded, randomized pilot study. Evid Based Complement Altern Med. 2016;2016:9481413. doi: 10.1155/2016/9481413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lin L.C., Que J., Lin L.K., Lin F.C. Zinc supplementation to improve mucositis and dermatitis in patients after radiotherapy for head-and-neck cancers: a double-blind, randomized study. Int J Radiat Oncol Biol Phys. 2006;65(3):745–750. doi: 10.1016/j.ijrobp.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 136.Salamzadeh J., Kamalinejad M., Mofid B., Mortazavi S.A., Sheikhlar A., Babaeian M. The effect of elaeagnus angustifolia L. Cream on radiation-induced skin reactions in women with breast cancer; A preliminary clinical trial. Iran J Pharm Sci. 2017;13(2) doi: 10.22034/ijps.2017.31130. Available from: [DOI] [Google Scholar]
- 137.Halm M.A., Baker C., Harshe V. Effect of an essential oil mixture on skin reactions in women undergoing radiotherapy for breast cancer: a pilot study. J Holist Nurs. 2014;32(4):290–303. doi: 10.1177/0898010114527184. [DOI] [PubMed] [Google Scholar]
- 138.Rollmann D.C., Novotny P.J., Petersen I.A., et al. Double-blind, placebo-controlled pilot study of processed ultra emu oil versus placebo in the prevention of radiation dermatitis. Int J Radiat Oncol Biol Phys. 2015;92(3):650–658. doi: 10.1016/j.ijrobp.2015.02.028. [DOI] [PubMed] [Google Scholar]
- 139.Thanthong S., Nanthong R., Kongwattanakul S., et al. Prophylaxis of radiation-induced dermatitis in patients with breast cancer using herbal creams: a prospective randomized controlled trial. Integr Cancer Ther. 2020;19 doi: 10.1177/1534735420920714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Togni S., Maramaldi G., Bonetta A., Giacomelli L., Di Pierro F. Clinical evaluation of safety and efficacy of Boswellia-based cream for prevention of adjuvant radiotherapy skin damage in mammary carcinoma: a randomized placebo controlled trial. Eur Rev Med Pharmacol Sci. 2015;19(8):1338–1344. [PubMed] [Google Scholar]
- 141.Maiche A.G., Gröhn P., Mäki-Hokkonen H. Effect of chamomile cream and almond ointment on acute radiation skin reaction. Acta Oncol. 1991;30(3):395–396. doi: 10.3109/02841869109092392. [DOI] [PubMed] [Google Scholar]
- 142.Chitapanarux I., Tovanabutra N., Chiewchanvit S., et al. Emulsion of olive oil and calcium hydroxide for the prevention of radiation dermatitis in hypofractionation post-mastectomy radiotherapy: a randomized controlled trial. Breast Care (Basel) 2019;14(6):394–400. doi: 10.1159/000496062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cui Z., Xin M., Yin H., Zhang J., Han F. Topical use of olive oil preparation to prevent radiodermatitis: results of a prospective study in nasopharyngeal carcinoma patients. Int J Clin Exp Med. 2015;8(7):11000–11006. [PMC free article] [PubMed] [Google Scholar]
- 144.Rafati M., Ghasemi A., Saeedi M., et al. Nigella sativa L. for prevention of acute radiation dermatitis in breast cancer: a randomized, double-blind, placebo-controlled, clinical trial. Complement Ther Med. 2019;47:102205. doi: 10.1016/j.ctim.2019.102205. [DOI] [PubMed] [Google Scholar]
- 145.Ma H., Zhang X., Bai M., Wang X. Clinical effects of lianbai liquid in prevention and treatment of dermal injury caused by radiotherapy. J Tradit Chin Med. 2007;27(3):193–196. [PubMed] [Google Scholar]
- 146.Chan R.J., Mann J., Tripcony L., et al. Natural oil-based emulsion containing allantoin versus aqueous cream for managing radiation-induced skin reactions in patients with cancer: a phase 3, double-blind, randomized, controlled trial. Int J Radiat Oncol Biol Phys. 2014;90(4):756–764. doi: 10.1016/j.ijrobp.2014.06.034. [DOI] [PubMed] [Google Scholar]
- 147.Koukourakis G., Pissakas G., Ganos C.G., Sivolapenko G., Kardamakis D. Effectiveness and tolerability of natural herbal formulations in the prevention of radiation-induced skin toxicity in patients undergoing radiotherapy. Int J Low Extrem Wounds. 2020 doi: 10.1177/1534734620923912. [DOI] [PubMed] [Google Scholar]
- 148.Balaji Subramanian S., Sathiya K., Balaji K., Thirunavukarasu M. Efficacy of cryptomphalus aspersa secretion cream in the treatment and prevention of radiodermatitis in cancer patients: better to move forward at a snail's pace. Support Care. 2018;26(2 Supplement 1):S243–S244. [Google Scholar]
- 149.Ferreira E.B., Bontempo P., dos Santos M.A., et al. Chamomilla recutita gel for patient skin reactions submitted to chemoradiation therapy: a case report. Estima. 2017;15(2):120–123. [Google Scholar]
- 150.Diniz Dos Reis P. Assessing the efficacy of chamomile gel as a prophylactic agent for radiation dermatitis. Support Care Cancer. 2017 [Google Scholar]
- 151.Näf G., Gasser U.E., Holzgang H.E., Schafroth S., Oehler C., Zwahlen D.R. Prevention of acute radiation-induced skin reaction with NPE® camellia sinensis nonfermentatum extract in female breast cancer patients undergoing postoperative radiotherapy: a single centre, prospective, open-label pilot study. Int J Breast Cancer. 2018;2018:2479274. doi: 10.1155/2018/2479274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu S., Li C., Shueng P.W., et al. Preventive effect of thixotropic gel containing tea tree oil from severe radiation dermatitis for head and neck cancer patients receiving chemoradiation. Int J Radiat Oncol. 2019 Sep;105(1):E377–E378. [Google Scholar]
- 153.Morganti A.G., Digesù C., Panunzi S., et al. Radioprotective effect of moderate wine consumption in patients with breast carcinoma. Int J Radiat Oncol Biol Phys. 2009;74(5):1501–1505. doi: 10.1016/j.ijrobp.2008.10.089. [DOI] [PubMed] [Google Scholar]
- 154.Xenos K., Koliarakis N., Markantonis S., et al. Effect of pomegranate extract (PFE) in human radiodermatitis patients with squamous cell carcinoma (SCC) after radiotherapy. Melanoma Res. 2010;20:e71. [Google Scholar]
- 155.Ravo V., Calvanese M.G., Di Franco R., et al. Prevention of cutaneous damages induced by radiotherapy in breast cancer: an institutional experience. Tumori. 2011;97(6):732–736. doi: 10.1177/030089161109700609. [DOI] [PubMed] [Google Scholar]
- 156.Ferreira E.B., Ciol M.A., de Meneses A.G., Bontempo P., Hoffman J.M., Reis P.E.D.D. Chamomile gel versus urea cream to prevent acute radiation dermatitis in head and neck cancer patients: results from a preliminary clinical trial. Integr Cancer Ther. 2020;19 doi: 10.1177/1534735420962174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ghasemi A., Danesh B., Yazdani-Charati J., Hosseinimehr S.J. Randomized double-blind placebo-controlled trial of celecoxib for the prevention of skin toxicity in patients receiving radiation therapy for breast cancer. Anti-Inflammatory Anti-Allergy Agents Med Chem. 2018;17(1):57–67. doi: 10.2174/1871523017666180411162114. [DOI] [PubMed] [Google Scholar]
- 158.Lievens Y., Haustermans K., Van den Weyngaert D., et al. Does sucralfate reduce the acute side-effects in head and neck cancer treated with radiotherapy? A double-blind randomized trial. Radiother Oncol. 1998;47(2):149–153. doi: 10.1016/s0167-8140(97)00231-4. [DOI] [PubMed] [Google Scholar]
- 159.Aygenc E., Celikkanat S., Kaymakci M., Aksaray F., Ozdem C. Prophylactic effect of pentoxifylline on radiotherapy complications: a clinical study. Otolaryngol Head Neck Surg. 2004 Mar;130(3):351–356. doi: 10.1016/j.otohns.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 160.Furusawa M., Baba Y., Murakami R., et al. Azelastine: its clinical application for radiation dermatitis. Radiat Med. 1996;14(3):151–154. [PubMed] [Google Scholar]
- 161.Dale P.S., Tamhankar C.P., George D., Daftary G.V. Co-medication with hydrolytic enzymes in radiation therapy of uterine cervix: evidence of the reduction of acute side effects. Cancer Chemother Pharmacol. 2001;47(Suppl):S29–S34. doi: 10.1007/s002800170006. [DOI] [PubMed] [Google Scholar]
- 162.Gujral M.S., Patnaik P.M., Kaul R., et al. Efficacy of hydrolytic enzymes in preventing radiation therapy-induced side effects in patients with head and neck cancers. Cancer Chemother Pharmacol. 2001;47(Suppl):S23–S28. doi: 10.1007/s002800170005. [DOI] [PubMed] [Google Scholar]
- 163.Kaul R., Mishra B.K., Sutradar P., Choudhary V., Gujral M.S. The role of Wobe-Mugos in reducing acute sequele of radiation in head and neck cancers--a clinical phase-III randomized trial. Indian J Cancer. 1999;36(2–4):141–148. [PubMed] [Google Scholar]
- 164.Kong M., Hong S.E. Topical use of recombinant human epidermal growth factor (EGF)-based cream to prevent radiation dermatitis in breast cancer patients: a single-blind randomized preliminary study. Asian Pac J Cancer Prev. 2013;14(8):4859–4864. doi: 10.7314/apjcp.2013.14.8.4859. [DOI] [PubMed] [Google Scholar]
- 165.Kang H.C., Ahn S.D., Choi D.H., Kang M.K., Chung W.K., Wu H.G. The safety and efficacy of EGF-based cream for the prevention of radiotherapy-induced skin injury: results from a multicenter observational study. Radiat Oncol J. 2014;32(3):156–162. doi: 10.3857/roj.2014.32.3.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kong M., Shin S.H., Lee E., Yun E.K. The effect of laughter therapy on radiation dermatitis in patients with breast cancer: a single-blind prospective pilot study. OncoTargets Ther. 2014;7:2053–2059. doi: 10.2147/OTT.S72973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lewis L., Carson S., Bydder S., Athifa M., Williams A.M., Bremner A. Evaluating the effects of aluminum-containing and non-aluminum containing deodorants on axillary skin toxicity during radiation therapy for breast cancer: a 3-armed randomized controlled trial. Int J Radiat Oncol Biol Phys. 2014;90(4):765–771. doi: 10.1016/j.ijrobp.2014.06.054. [DOI] [PubMed] [Google Scholar]
- 168.Watson L.C., Gies D., Thompson E., Thomas B. Randomized control trial: evaluating aluminum-based antiperspirant use, axilla skin toxicity, and reported quality of life in women receiving external beam radiotherapy for treatment of Stage 0, I, and II breast cancer. Int J Radiat Oncol Biol Phys. 2012;83(1):e29–e34. doi: 10.1016/j.ijrobp.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 169.Bennett C. An investigation into the use of a non-metallic deodorant during radiotherapy treatment: a randomised controlled trial. J Radiother Pract. 2009;8(1):3–9. [Google Scholar]
- 170.Gee A., Moffitt D., Churn M., Errington R.D. A randomised controlled trial to test a non-metallic deodorant used during a course of radiotherapy. J Radiother Pract. 2000;1(4):205–212. [Google Scholar]
- 171.Théberge V., Harel F., Dagnault A. Use of axillary deodorant and effect on acute skin toxicity during radiotherapy for breast cancer: a prospective randomized noninferiority trial. Int J Radiat Oncol Biol Phys. 2009;75(4):1048–1052. doi: 10.1016/j.ijrobp.2008.12.046. [DOI] [PubMed] [Google Scholar]
- 172.Kunos C.A., Abdallah R.R., Lyons J.A. Hydration during breast radiotherapy may lower skin toxicity. Breast J. 2014;20(6):679–681. doi: 10.1111/tbj.12348. [DOI] [PubMed] [Google Scholar]
- 173.Kouvaris J., Kouloulias V., Kokakis J., Matsopoulos G., Myrsini B., Vlahos L. The cytoprotective effect of amifostine in acute radiation dermatitis: a retrospective analysis. Eur J Dermatol. 2002;12(5):458–462. [PubMed] [Google Scholar]
- 174.Samuels M.A., Chico I.M., Hirsch R.L., Fullmer K., Ethyol S.Q., Safety Study Group Ongoing prospective multicenter safety study of the cytoprotectant amifostine given subcutaneously: overview of trial design. Semin Oncol. 2003;30(6 Suppl 18):94–95. doi: 10.1053/j.seminoncol.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 175.Thongkhao P., Peerawong T., Bridhikitti J., Jiratrachu R., Suwanraksa C., Geater A. Effects of regular bra-wearing on acute skin toxicity in breast-conserving radiotherapy women. Asian Pac J Cancer Care. 2019;4(4):157–164. [Google Scholar]
- 176.Campbell I.R., Illingworth M.H. Can patients wash during radiotherapy to the breast or chest wall? A randomized controlled trial. Clin Oncol (R Coll Radiol) 1992;4(2):78–82. doi: 10.1016/s0936-6555(05)80971-9. [DOI] [PubMed] [Google Scholar]
- 177.Roy I., Fortin A., Larochelle M. The impact of skin washing with water and soap during breast irradiation: a randomized study. Radiother Oncol. 2001;58(3):333–339. doi: 10.1016/s0167-8140(00)00322-4. [DOI] [PubMed] [Google Scholar]
- 178.Westbury C., Hines F., Hawkes E., Ashley S., Brada M. Advice on hair and scalp care during cranial radiotherapy: a prospective randomized trial. Radiother Oncol. 2000;54(2):109–116. doi: 10.1016/s0167-8140(99)00146-2. [DOI] [PubMed] [Google Scholar]
- 179.Pardo J., Murcia M., Soto R., et al. An emulsion containing hyaluronic acid and chondroitin sulfate for prevention and treatment of radiation dermatitis in breast cancer patients - a randomized study. Int J Radiat Oncol. 2015;93(3):E12. [Google Scholar]
- 180.Rese A., D'Ippolito E., Piccolo F., et al. T-lysyal based cream (Repalysyal) in the prevention of acute skin toxicity in breast cancer patients. Radiother Oncol. 2016;119:S564–S565. [Google Scholar]
- 181.Pardo Masferrer J., Murcia Mejía M., Vidal Fernández M., et al. Prophylaxis with a cream containing urea reduces the incidence and severity of radio-induced dermatitis. Clin Transl Oncol. 2010;12(1):43–48. doi: 10.1007/s12094-010-0465-0. [DOI] [PubMed] [Google Scholar]
- 182.Perez M., Cabeza M.A., Murillo M.T., et al. A specific 3% urea hydrating lotion reduces radiation-induced dermatitis compared with hydration alone. Eur J Cancer Suppl. 2009;7(2):251. [Google Scholar]
- 183.Bayard L.G., Buzon M.C.S., Pain E.A., Calvo E.G., Alvarez E.M. Incidence of radio-dermatitis in head and neck cancer with 3D conformal radiotherapy and prophylaxis with topic cream. Eur J Cancer. 2011;47:S562. [Google Scholar]
- 184.Sezen D., Bolukbasi Y., Corak Cebi C., Selek U. Poster Session Abstracts [Internet] American Association for Cancer Research; 2019. Comparison of three different topical agents on prevention of acute radiodermatitis during breast cancer radiotherapy.http://cancerres.aacrjournals.org/lookup/doi/10.1158/1538-7445.SABCS18-P3-12-18 [cited 2021 Dec 23]. p. P3-12-18-P3-12–8. Available from: [Google Scholar]
- 185.Matceyevsky D., Hahoshen N.Y., Vexler A., Noam A., Khafif A., Ben-Yosef R. Assessing the effectiveness of Dead Sea products as prophylactic agents for acute radiochemotherapy-induced skin and mucosal toxicity in patients with head and neck cancers: a phase 2 study. Isr Med Assoc J. 2007;9(6):439–442. [PubMed] [Google Scholar]
- 186.Rossi E., Noberasco C., Picchi M., et al. Complementary and integrative medicine to reduce adverse effects of anticancer therapy. J Altern Complement Med. 2018;24(9–10):933–941. doi: 10.1089/acm.2018.0143. [DOI] [PubMed] [Google Scholar]
- 187.Ferro A.C., Haffty B.G., Omabegho M., Schwartz S., Goyal S. Prevention of radiation dermatitis in breast cancer patients. Int J Radiat Oncol. 2013;87(2):S219. [Google Scholar]
- 188.Häfner M.F., Fetzner L., Hassel J.C., Debus J., Potthoff K. Prophylaxis of acute radiation dermatitis with an innovative FDA-approved two-step skin care system in a patient with head and neck cancer undergoing a platin-based radiochemotherapy: a case report and review of the literature. Dermatology. 2013;227(2):171–174. doi: 10.1159/000353974. [DOI] [PubMed] [Google Scholar]
- 189.Potthoff K., Fetzner L., Nejad-Asgari S., et al. First clinical experiences with R1 and R2, a lactokine-based medical device, for prophylaxis of radiation dermatitis in cancer patients. Support Care Cancer. 2011;19(Suppl 2):S281. [Google Scholar]
- 190.Di Franco R., Calvanese M., Murino P., et al. Skin toxicity from external beam radiation therapy in breast cancer patients: protective effects of Resveratrol, Lycopene, Vitamin C and anthocianin (Ixor®) Radiat Oncol. 2012;7:12. doi: 10.1186/1748-717X-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Miko Enomoto T., Johnson T., Peterson N., Homer L., Walts D., Johnson N. Combination glutathione and anthocyanins as an alternative for skin care during external-beam radiation. Am J Surg. 2005;189(5):627–630. doi: 10.1016/j.amjsurg.2005.02.001. discussion 630-631. [DOI] [PubMed] [Google Scholar]
- 192.Röper B., Kaisig D., Auer F., Mergen E., Molls M. Thêta-Cream versus Bepanthol lotion in breast cancer patients under radiotherapy. A new prophylactic agent in skin care? Strahlenther Onkol. 2004;180(5):315–322. doi: 10.1007/s00066-004-1174-9. [DOI] [PubMed] [Google Scholar]
- 193.Lee J., Lee S.W., Hong J.P., Shon M.W., Ryu S.H., Ahn S.D. Foam dressing with epidermal growth factor for severe radiation dermatitis in head and neck cancer patients. Int Wound J. 2016;13(3):390–393. doi: 10.1111/iwj.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Balzarini A., Felisi E., Martini A., De Conno F. Efficacy of homeopathic treatment of skin reactions during radiotherapy for breast cancer: a randomised, double-blind clinical trial. Br Homeopath J. 2000;89(1):8–12. doi: 10.1054/homp.1999.0328. [DOI] [PubMed] [Google Scholar]
- 195.Schratter-Sehn A.U., Brinda K., Kahrer M., Novak M. Improvement of skin care during radiotherapy. Onkologie. 2001;24(1):44–46. doi: 10.1159/000050281. [DOI] [PubMed] [Google Scholar]
- 196.Younus J., Lock M., Vujovic O., et al. A case-control, mono-center, open-label, pilot study to evaluate the feasibility of therapeutic touch in preventing radiation dermatitis in women with breast cancer receiving adjuvant radiation therapy. Complement Ther Med. 2015;23(4):612–616. doi: 10.1016/j.ctim.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 197.Ogita M., Sekiguchi K., Akahane K., et al. Association of moisturization and topical corticosteroid usage during and after whole-breast radiation therapy: a post hoc analysis of a randomized controlled trial. Int J Radiat Oncol. 2016;96(2):E42. [Google Scholar]
- 198.Shariati L., Amouheidari A., Naji Esfahani H., et al. Protective effects of doxepin cream on radiation dermatitis in breast cancer: a single arm double-blind randomized clinical trial. Br J Clin Pharmacol. 2020;86(9):1875–1881. doi: 10.1111/bcp.14238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Delaney G., Fisher R., Hook C., Barton M. Sucralfate cream in the management of moist desquamation during radiotherapy. Australas Radiol. 1997;41(3):270–275. doi: 10.1111/j.1440-1673.1997.tb00672.x. [DOI] [PubMed] [Google Scholar]
- 200.Rothenberger J., Constantinescu M.A., Held M., et al. Use of a polylactide-based copolymer as a temporary skin substitute for a patient with moist desquamation due to radiation. Wounds Compend Clin Res Pract. 2016;28(7):E26–E30. [PubMed] [Google Scholar]
- 201.Zhu W., Jia L., Chen G., et al. Epigallocatechin-3-gallate ameliorates radiation-induced acute skin damage in breast cancer patients undergoing adjuvant radiotherapy. Oncotarget. 2016;7(30):48607–48613. doi: 10.18632/oncotarget.9495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kirova Y.M., Fromantin I., De Rycke Y., et al. Can we decrease the skin reaction in breast cancer patients using hyaluronic acid during radiation therapy? Results of phase III randomised trial. Radiother Oncol. 2011;100(2):205–209. doi: 10.1016/j.radonc.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 203.Presta G., Puliatti A., Bonetti L., Tolotti A., Sari D., Valcarenghi D. Effectiveness of hyaluronic acid gel (Jalosome soothing gel) for the treatment of radiodermatitis in a patient receiving head and neck radiotherapy associated with cetuximab: a case report and review. Int Wound J. 2019;16(6):1433–1439. doi: 10.1111/iwj.13210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wunnasaweg A. Effectiveness of moisturizing barrier cream during external beam radiation therapy. J Thai Assoc Radiat Oncol. 2016;22(1):55–61. [Google Scholar]
- 205.Sekiguchi K., Ogita M., Akahane K., et al. Randomized, prospective assessment of moisturizer efficacy for the treatment of radiation dermatitis following radiotherapy after breast-conserving surgery. Jpn J Clin Oncol. 2015;45(12):1146–1153. doi: 10.1093/jjco/hyv155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Censabella S., Claes S., Orlandini M., Braekers R., Thijs H., Bulens P. Retrospective study of radiotherapy-induced skin reactions in breast cancer patients: reduced incidence of moist desquamation with a hydroactive colloid gel versus dexpanthenol. Eur J Oncol Nurs. 2014;18(5):499–504. doi: 10.1016/j.ejon.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 207.Claes S., Thijs H., Braekers R., Orlandini M., Sente F., Bulens P. A clinical study comparing a hydroactive colloid gel to a dexpanthenol cream for the treatment of skin reactions in breast irradiation. Int J Radiat Oncol. 2012;84(3):S229. [Google Scholar]
- 208.Manzanas García A., López Carrizosa M.C., Vallejo Ocaña C., et al. Superoxidase dismutase (SOD) topical use in oncologic patients: treatment of acute cutaneous toxicity secondary to radiotherapy. Clin Transl Oncol. 2008;10(3):163–167. doi: 10.1007/s12094-008-0174-0. [DOI] [PubMed] [Google Scholar]
- 209.Uzaraga I., Gerbis B., Holwerda E., Gillis D., Wai E. Topical amitriptyline, ketamine, and lidocaine in neuropathic pain caused by radiation skin reaction: a pilot study. Support Care Cancer. 2012;20(7):1515–1524. doi: 10.1007/s00520-011-1240-7. [DOI] [PubMed] [Google Scholar]
- 210.Gollins S., Gaffney C., Slade S., Swindell R. RCT on gentian violet versus a hydrogel dressing for radiotherapy-induced moist skin desquamation. J Wound Care. 2008;17(6):268–270. doi: 10.12968/jowc.2008.17.6.29589. 272, 274–270. [DOI] [PubMed] [Google Scholar]
- 211.Potera M.E., Lookingbill D.P., Stryker J.A. Prophylaxis of radiation dermatitis with a topical cortisone cream. Radiology. 1982;143(3):775–777. doi: 10.1148/radiology.143.3.7079509. [DOI] [PubMed] [Google Scholar]
- 212.Yokota T., Zenda S., Ota I., et al. Phase 3 randomized trial of topical steroid versus placebo for prevention of radiation dermatitis in patients with head and neck cancer receiving chemoradiation. Int J Radiat Oncol Biol Phys. 2021;111(3):794–803. doi: 10.1016/j.ijrobp.2021.05.133. [DOI] [PubMed] [Google Scholar]
- 213.Paterson D. Randomized intra-patient controlled trial of Mepilex lite dressings versus aqueous cream in managing radiation-induced skin reactions postmastectomy. J Cancer Sci Ther. 2012;4(11) https://www.omicsonline.org/randomized-intra-patient-controlled-trial-of-mepilex-lite-dressings-versus-aqueous-cream-in-managing-radiation-induced-skin-reactions-postmastectomy-1948-5956.1000166.php?aid=8898 Available from: [Google Scholar]
- 214.Zhong W.H., Tang Q.F., Hu L.Y., Feng H.X. Mepilex Lite dressings for managing acute radiation dermatitis in nasopharyngeal carcinoma patients: a systematic controlled clinical trial. Med Oncol. 2013;30(4):761. doi: 10.1007/s12032-013-0761-y. [DOI] [PubMed] [Google Scholar]
- 215.Diggelmann K.V., Zytkovicz A.E., Tuaine J.M., Bennett N.C., Kelly L.E., Herst P.M. Mepilex Lite dressings for the management of radiation-induced erythema: a systematic inpatient controlled clinical trial. Br J Radiol. 2010;83(995):971–978. doi: 10.1259/bjr/62011713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Bazire L., Fromantin I., Diallo A., et al. Hydrosorb® versus control (water based spray) in the management of radio-induced skin toxicity: results of multicentre controlled randomized trial. Radiother Oncol. 2015;117(2):229–233. doi: 10.1016/j.radonc.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 217.Mak S.S., Molassiotis A., Wan W.M., Lee I.Y., Chan E.S. The effects of hydrocolloid dressing and gentian violet on radiation-induced moist desquamation wound healing. Cancer Nurs. 2000;23(3):220–229. doi: 10.1097/00002820-200006000-00010. [DOI] [PubMed] [Google Scholar]
- 218.Macmillan M.S., Wells M., MacBride S., Raab G.M., Munro A., MacDougall H. Randomized comparison of dry dressings versus hydrogel in management of radiation-induced moist desquamation. Int J Radiat Oncol Biol Phys. 2007;68(3):864–872. doi: 10.1016/j.ijrobp.2006.12.049. [DOI] [PubMed] [Google Scholar]
- 219.Quilis A., Martín J., Rodríguez C., Sánchez P., Ribes J.L. Reducing radiation dermatitis during ongoing radiation therapy: an innovative film-forming wound dressing. J Radiat Oncol. 2018;7(3):255–264. [Google Scholar]
- 220.Vavassis P., Gelinas M., Chabot Tr J., Nguyen-Tân P.F. Phase 2 study of silver leaf dressing for treatment of radiation-induced dermatitis in patients receiving radiotherapy to the head and neck. J Otolaryngol Head Neck Surg. 2008;37(1):124–129. [PubMed] [Google Scholar]
- 221.Perea López P., Díaz Soriano P., Santiyán González A., Lozano Crespo B., Arregui López E., Morera López R. Silver-containing Hydrofiber® dressings to prevent progression of the radiation dermatitis in patients undergoing external beam radiotherapy and orthovoltage to the skin cancer. Rep Pract Oncol Radiother. 2013;18:S209. [Google Scholar]
- 222.Bonomo P., Desideri I., Loi M., et al. Management of severe bio-radiation dermatitis induced by radiotherapy and cetuximab in patients with head and neck cancer: emphasizing the role of calcium alginate dressings. Support Care Cancer. 2019;27(8):2957–2967. doi: 10.1007/s00520-018-4606-2. [DOI] [PubMed] [Google Scholar]
- 223.Hegarty F., Wong M. Polymeric membrane dressing for radiotherapy-induced skin reactions. Br J Nurs. 2014;23(Suppl 20):S38–S46. doi: 10.12968/bjon.2014.23.Sup20.S38. [DOI] [PubMed] [Google Scholar]
- 224.Lobo Gajiwala A., Sharma V. Use of irradiated amnion as a biological dressing in the treatment of radiation induced ulcers. Cell Tissue Bank. 2003;4(2–4):147–150. doi: 10.1023/B:CATB.0000007024.81019.03. [DOI] [PubMed] [Google Scholar]
- 225.Censabella S., Claes S., Robijns J., Bulens P., Mebis J. Photobiomodulation for the management of radiation dermatitis: the DERMIS trial, a pilot study of MLS(®) laser therapy in breast cancer patients. Support Care Cancer. 2016;24(9):3925–3933. doi: 10.1007/s00520-016-3232-0. [DOI] [PubMed] [Google Scholar]
- 226.Nugent M., Watson G., Welbury R. Early experience of low level laser therapy (LLLT) in the management of radiation dermatitis. Br J Oral Maxillofac Surg. 2015;53(10):e51. [Google Scholar]
- 227.Scharll M. Spectacular efficacy of low level laser therapy (LLLT) for the management of severe radiation-induced dermatitis: a case report. MASCC. 2015;23(1) [Google Scholar]
- 228.Gobbo M., Ottaviani G., Rupel K., Biasotto M., Guglielmi A. Can laser therapy be the answer for radiodermatitis in anal cancer patients? Two case reports. Photon Laser Med. 2016;5(3) https://www.degruyter.com/document/doi/10.1515/plm-2016-0009/html Available from: [Google Scholar]
- 229.Zacchigna S., Ciriello F., Gobbo M., et al. Off label use of laser therapy for the treatment of radiodermatitis in breast cancer patients. Support Care Cancer. 2013;21(1):S148. [Google Scholar]
- 230.Yogi V., Singh O., Mandloi V., Ahirwar M. Role of topical aloe vera gel in the recovery of high-grade, radiation-induced dermatitis. Clin Cancer Invest J. 2018;7(5):167. [Google Scholar]
- 231.Moolenaar M., Poorter R.L., van der Toorn P.P.G., Lenderink A.W., Poortmans P., Egberts A.C.G. The effect of honey compared to conventional treatment on healing of radiotherapy-induced skin toxicity in breast cancer patients. Acta Oncol. 2006;45(5):623–624. doi: 10.1080/02841860600781799. [DOI] [PubMed] [Google Scholar]
- 232.Ansari M., Farzin D., Mosalaei A., Omidvari S., Ahmadloo N., Mohammadianpanah M. Efficacy of topical alpha ointment (containing natural henna) compared to topical hydrocortisone (1%) in the healing of radiation-induced dermatitis in patients with breast cancer: a randomized controlled clinical trial. Iran J Med Sci. 2013;38(4):293–300. [PMC free article] [PubMed] [Google Scholar]
- 233.Jensen J.M., Gau T., Schultze J., et al. Treatment of acute radiodermatitis with an oil-in-water emulsion following radiation therapy for breast cancer: a controlled, randomized trial. Strahlenther Onkol. 2011;187(6):378–384. doi: 10.1007/s00066-011-2224-8. [DOI] [PubMed] [Google Scholar]
- 234.Franco P., Potenza I., Moretto F., et al. Hypericum perforatum and neem oil for the management of acute skin toxicity in head and neck cancer patients undergoing radiation or chemo-radiation: a single-arm prospective observational study. Radiat Oncol. 2014;9:297. doi: 10.1186/s13014-014-0297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Pajonk F., Riedisser A., Henke M., McBride W.H., Fiebich B. The effects of tea extracts on proinflammatory signaling. BMC Med. 2006;4(1):28. doi: 10.1186/1741-7015-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zhou J., Fang L., Xie H., Yao W.X., Zhou X., Xiong Z.J. A pilot study using the Chinese herbal paste Liu-He-Dan to manage radiodermatitis associated with breast cancer radiotherapy. Curr Oncol. 2015;22(6):e453–e456. doi: 10.3747/co.22.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Franco P., Rampino M., Ostellino O., et al. Management of acute skin toxicity with Hypericum perforatum and neem oil during platinum-based concurrent chemo-radiation in head and neck cancer patients. Med Oncol. 2017;34(2):30. doi: 10.1007/s12032-017-0886-5. [DOI] [PubMed] [Google Scholar]
- 238.Garibaldi E., Gatti M., Gardes M.P., et al. Randomized trial on the efficacy of two non steroidal drugs in the prevention of skin damage induced by radiotherapy. J Plast Dermatol. 2009;5(3):233–239. [Google Scholar]
- 239.Martella S., Rietjens M., Lohsiriwat V., et al. Acute radiation dermatitis in breast cancer: topical therapy with vitamin E acetate in lipophilic gel base. Ecancermedicalscience. 2010;4:190. doi: 10.3332/ecancer.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Viswanath L., Bindhu J., Krishnamurthy B., Suresh K.P. Granulocyte-Colony Stimulating Factor (G-CSF) accelerates healing of radiation induced moist desquamation of the skin. Klin Onkol Cas Ceske Slov Onkol Spolecnosti. 2012;25(3):199–205. [PubMed] [Google Scholar]
- 241.Schlappack O. Homeopathic treatment of radiation-induced itching in breast cancer patients. A prospective observational study. Homeopathy. 2004;93(4):210–215. doi: 10.1016/j.homp.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 242.Dini D., Macchia R., Gozza A., et al. Management of acute radiodermatitis. Pharmacological or nonpharmacological remedies? Cancer Nurs. 1993;16(5):366–370. [PubMed] [Google Scholar]
- 243.Lupiáñez Pérez Y., Medina Carmona J., Lupiáñez Pérez I., Garcia Mata. Prevention and treatment of radiodermatitis using a nonadhesive foam dressing. J Wound Care. 2011;20(3):130–135. doi: 10.12968/jowc.2011.20.3.130. [DOI] [PubMed] [Google Scholar]
- 244.Dymackova R., Kazda T., Slavik M., Selingerova I., Slampa P., Slama O. Acupuncture in the treatment of acute toxicity during and after head and neck cancer radiotherapy: interim analysis of randomized prospective open-label trial. Biomed Pap Med Fac Univ Palacky Olomouc Czechoslov. 2020;164(4):454–460. doi: 10.5507/bp.2020.021. [DOI] [PubMed] [Google Scholar]
- 245.Yokota T., Zenda S., Ota I., et al. Topical steroid versus placebo for the prevention of radiation dermatitis in head and neck cancer patients receiving chemoradiotherapy: a phase III, randomized, double-blinded trial: J-SUPPORT 1602(TOPICS) Ann Oncol. 2020;31:S669. doi: 10.1186/s12885-018-4763-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Dalenc F., Ribet V., Rossi A.B., et al. Efficacy of a global supportive skin care programme with hydrotherapy after non-metastatic breast cancer treatment: a randomised, controlled study. Eur J Cancer Care. 2018;27(1) doi: 10.1111/ecc.12735. [DOI] [PubMed] [Google Scholar]
- 247.Chan R.J., Webster J., Chung B., Marquart L., Ahmed M., Garantziotis S. Prevention and treatment of acute radiation-induced skin reactions: a systematic review and meta-analysis of randomized controlled trials. BMC Cancer. 2014;14(1):53. doi: 10.1186/1471-2407-14-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kole A.J., Kole L., Moran M.S. Acute radiation dermatitis in breast cancer patients: challenges and solutions. Breast Cancer. 2017;9:313–323. doi: 10.2147/BCTT.S109763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Iacovelli N.A., Torrente Y., Ciuffreda A., et al. Topical treatment of radiation-induced dermatitis: current issues and potential solutions. Drugs Context. 2020;9:2020–2024. doi: 10.7573/dic.2020-4-7. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Yee C., Wang K., Asthana R., et al. Radiation-induced skin toxicity in breast cancer patients: a systematic review of randomized trials. Clin Breast Cancer. 2018;18(5):e825–e840. doi: 10.1016/j.clbc.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 251.Bolderston A., Lloyd N.S., Wong R.K.S., Holden L., Robb-Blenderman L. Supportive Care Guidelines Group of Cancer Care Ontario Program in Evidence-Based Care. The prevention and management of acute skin reactions related to radiation therapy: a systematic review and practice guideline. Support Care Cancer. 2006;14(8):802–817. doi: 10.1007/s00520-006-0063-4. [DOI] [PubMed] [Google Scholar]
- 252.Haruna F., Lipsett A., Marignol L. Topical management of acute radiation dermatitis in breast cancer patients: a systematic review and meta-analysis. Anticancer Res. 2017;37(10):5343–5353. doi: 10.21873/anticanres.11960. [DOI] [PubMed] [Google Scholar]
- 253.Kodiyan J., Amber K.T. A review of the use of topical calendula in the prevention and treatment of radiotherapy-induced skin reactions. Antioxidants (Basel) 2015;4(2):293–303. doi: 10.3390/antiox4020293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Farrugia C.J.E., Burke E.S., Haley M.E., Bedi K.T., Gandhi M.A. The use of aloe vera in cancer radiation: an updated comprehensive review. Complement Ther Clin Pract. 2019;35:126–130. doi: 10.1016/j.ctcp.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 255.Elad S., Cheng K.K.F., Lalla R.V., et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. 2020;126(19):4423–4431. doi: 10.1002/cncr.33100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Siddiquee S., McGee M.A., Vincent A.D., et al. Efficacy of topical Calendula officinalis on prevalence of radiation-induced dermatitis: a randomised controlled trial. Australas J Dermatol. 2021;62(1):e35–e40. doi: 10.1111/ajd.13434. [DOI] [PubMed] [Google Scholar]
- 257.Robijns J., Lodewijckx J., Claes S., et al. Photobiomodulation therapy for the prevention of acute radiation dermatitis in head and neck cancer patients (DERMISHEAD trial) Radiother Oncol. 2021;158:268–275. doi: 10.1016/j.radonc.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 258.Behroozian T., Milton L., Karam I., et al. Mepitel film for the prevention of acute radiation dermatitis in breast cancer: a randomized multicenter open-label phase III trial. J Clin Oncol. 2022 doi: 10.1200/JCO.22.01873. JCO.22.01873. [DOI] [PubMed] [Google Scholar]
- 259.Kianinia M., Roayaei M., Mahdavi H., Hemati S. A double-blind randomized trial on the effectiveness of mometasone 0.1% cream and hydrocortisone 1% cream on the prevention of acute radiation dermatitis in breast cancer patients following breast conserving surgery. Middle East J Cancer. 2021;12(3):406–414. [Google Scholar]
- 260.Sunku R., Kalita A.K., Bhattacharyya M., et al. Effect of corticosteroid ointment on radiation induced dermatitis in head and neck cancer patients: a prospective study. Indian J Cancer. 2021;58(1):69–75. doi: 10.4103/ijc.IJC_790_18. [DOI] [PubMed] [Google Scholar]
- 261.Kawamori J., Itazawa T., Fukushima S., Ito R., Yamauchi H., Sekiguchi K. Effect of heparinoid moisturizer on quality of life in patients with acute radiation skin damage following hypofractionated radiation therapy after breast-conserving surgery: a randomized controlled study. Breast Cancer. 2021;13:743–753. doi: 10.2147/BCTT.S347136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Fischer-Sgrott F., Sapienza L.G., Rech P., et al. Photobiomodulation for radiodermatitis prevention in breast cancer: results from a double blind randomized controlled trial (PHOTODERMIS trial) Int J Radiat Oncol Biol Phys. 2022;114(3):e35. [Google Scholar]
- 263.Robijns J., Lodewijckx J., Puts S., et al. Photobiomodulation therapy for the prevention of acute radiation dermatitis in breast cancer patients undergoing hypofractioned whole-breast irradiation (LABRA trial) Laser Surg Med. 2022;54(3):374–383. doi: 10.1002/lsm.23475. [DOI] [PubMed] [Google Scholar]
- 264.Tungkasamit T., Chakrabandhu S., Samakgarn V., et al. Reduction in severity of radiation-induced dermatitis in head and neck cancer patients treated with topical aloe vera gel: a randomized multicenter double-blind placebo-controlled trial. Eur J Oncol Nurs. 2022;59:102164. doi: 10.1016/j.ejon.2022.102164. [DOI] [PubMed] [Google Scholar]
- 265.Zheng J., Li G., Wang J., et al. Compound kushen injection protects skin from radiation injury via regulating bim. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.753068. https://www.frontiersin.org/articles/10.3389/fphar.2021.753068 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Zhu W., Zhao H. A randomized, double-blind, placebo-controlled phase 2 trial of epigallocatechin-gallate in the prevention of radiation-induced dermatitis. Int J Radiat Oncol Biol Phys. 2020;108(3):S12. [Google Scholar]
- 267.Kost Y., Mieczkowska K., Deutsch A., et al. Bacterial decolonization to prevent acute radiation dermatitis: a randomized controlled trial. J Clin Oncol. 2022;40(17_suppl):LBA12003. [Google Scholar]
- 268.Higgins J.P.T., Thomas J., Chandler J., et al., editors. Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition. John Wiley & Sons; Chichester, UK: 2019. [Google Scholar]
- 269.Pignol J.P., Olivotto I., Rakovitch E., et al. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol. 2008;26(13):2085–2092. doi: 10.1200/JCO.2007.15.2488. [DOI] [PubMed] [Google Scholar]
- 270.Joseph K., Vos L.J., Gabos Z., et al. Skin toxicity in early breast cancer patients treated with field-in-field breast intensity-modulated radiotherapy versus helical inverse breast intensity-modulated radiotherapy: results of a phase III randomised controlled trial. Clin Oncol. 2021;33(1):30–39. doi: 10.1016/j.clon.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 271.Mulliez T., Veldeman L., van Greveling A., et al. Hypofractionated whole breast irradiation for patients with large breasts: a randomized trial comparing prone and supine positions. Radiother Oncol. 2013;108(2):203–208. doi: 10.1016/j.radonc.2013.08.040. [DOI] [PubMed] [Google Scholar]
- 272.Li Y., Sakai M., Tsunoda A., et al. Normal tissue complication probability model for acute radiation dermatitis in patients with head and neck cancer treated with carbon ion radiation therapy. Int J Radiat Oncol. 2022 doi: 10.1016/j.ijrobp.2022.03.002. [DOI] [PubMed] [Google Scholar]
- 273.Murray Brunt A., Haviland J.S., Wheatley D.A., et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet. 2020;395(10237):1613–1626. doi: 10.1016/S0140-6736(20)30932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.