Abstract
Brucella abortus RB51 is a stable rough, attenuated mutant vaccine strain derived from the virulent strain 2308. Recently, we demonstrated that the wboA gene in RB51 is disrupted by an IS711 element (R. Vemulapalli, J. R. McQuiston, G. G. Schurig, N. Srirauganathan, S. M. Halling, and S. M. Boyle, Clin. Diagn. Lab. Immunol. 6:760–764, 1999). Disruption of the wboA gene in smooth, virulent B. abortus, Brucella melitensis, and Brucella suis results in rough, attenuated mutants which fail to produce the O polysaccharide (O antigen). In this study, we explored whether the wboA gene disruption is responsible for the rough phenotype of RB51. We complemented RB51 with a functional wboA gene, and the resulting strain was designated RB51WboA. Colony and Western blot analyses indicated that RB51WboA expressed the O antigen; immunoelectron microscopy revealed that the O antigen was present in the cytoplasm. Crystal violet staining, acryflavin agglutination, and polymyxin B sensitivity studies indicated that RB51WboA had rough phenotypic characteristics similar to those of RB51. Bacterial clearance studies of BALB/c mice indicated no increase in the survival ability of RB51WboA in vivo compared to that of RB51. Vaccination of mice with live RB51WboA induced antibodies to the O antigen which were predominantly of the immunoglobulin G2a (IgG2a) and IgG3 isotypes. After in vitro stimulation of splenocytes with killed bacterial cells, quantitation of gamma interferon in the culture supernatants indicated that RB51WboA immunization induced higher levels of gamma interferon than immunization with RB51. Mice vaccinated with RB51WboA were better protected against a challenge infection with the virulent strain 2308 than those vaccinated with RB51. These studies indicate that in addition to the disruption of the wboA gene there is at least one other mutation in RB51 responsible for its rough phenotype. These studies also suggest that the expressed O antigen in RB51WboA is responsible either directly or indirectly for the observed enhancement in the T-cell response.
Brucella abortus is a gram-negative, facultatively intracellular bacterial pathogen that can cause abortion in pregnant cattle and undulant fever in humans (11). In the infected host, B. abortus multiplies within the phagosomes of macrophage-monocyte lineage cells by inhibiting phagolysosome fusion (17, 28). In pregnant animals, B. abortus also replicates in placental and fetal tissues, causing abortions (30). B. abortus strains exhibiting a smooth phenotype contain a surface-exposed O polysaccharide chain (O antigen) as part of their lipopolysaccharide (LPS) structure (7). Truly rough strains do not contain O antigen in their LPSs. The O antigen of Brucella is a homopolymer of 4,6-dideoxy-4-formamido-α-d-mannopyranosyl residues joined by an α-1,2 linkage in A-epitope-dominant strains, but every fifth residue is joined by an α-1,3 linkage in M-epitope-dominant strains (5, 6). In B. abortus, the smooth LPS has been shown to be a virulence factor, since rough strains that were derived from smooth virulent strains are attenuated (1, 18, 25, 32, 37). This virulence property of the smooth LPS has been ascribed to its ability to protect B. abortus from complement-mediated killing and several bactericidal properties of professional phagocytes; hence, only smooth B. abortus strains can readily replicate within macrophages (1, 12). The O antigen is also an immunodominant component of B. abortus; infected animals develop antibodies to this antigen. At least in some animal species, such as mice, antibodies to the O antigen can confer passive as well as acquired partial protection against a challenge infection (4, 26). However, as in most intracellular bacterial infections, cell-mediated immunity appears to play a major role in the acquired resistance to brucellosis (3, 4, 8). This finding is substantiated by the effective protection afforded by vaccination with live rough strains, such as B. abortus RB51. It is well demonstrated that the protection conferred by RB51 vaccination can only be transferred by immune T cells and not by antibodies (19).
B. abortus RB51 is an attenuated stable rough mutant derived from the virulent strain 2308 (32). This strain is currently employed as the official vaccine for cattle brucellosis in the United States and other countries. The vaccine efficacy and stability of RB51 are well proven under experimental as well as field conditions (8, 19, 20, 22, 27). Recently, we discovered that the wboA gene of RB51 is interrupted by an IS711 element (35). The wboA gene is capable of encoding a glycosyltransferase that has been demonstrated to be essential for the biosynthesis of the Brucella O antigen (25). Disruption of the wboA gene in B. abortus 2308, Brucella melitensis 16M, and Brucella suis resulted in rough mutants that were unable to synthesize the O antigen (37). In this study, we asked whether the IS711 interruption of this gene is responsible for the rough phenotype of RB51. We demonstrate that the complementation of RB51 with a functional wboA gene results in O-antigen synthesis but does not change either its rough phenotype or its attenuation characteristics. We further show that vaccination of mice with the wboA-complemented RB51 results in the induction of significantly enhanced protection.
MATERIALS AND METHODS
Bacterial strains.
B. abortus strains 2308 and RB51 were from our culture collection. All bacteria were grown either in tryptic soy broth (TSB) or on tryptic soy agar (TSA) plates. Chloramphenicol at a concentration of 30 μg/ml was added to the medium when bacteria containing the plasmid pBBR1MCS (21) or pAB3 (25) were cultured.
Antisera.
The Brucella O-antigen-specific rat monoclonal antibody Bru38 was previously developed in our laboratory (31). Goat antiserum to B. abortus RB51 was available in our laboratory (34). Antiserum to B. abortus strain 19 was obtained from five mice at 2 and 4 weeks after inoculating them intraperitoneally (i.p.) with 3 × 106 CFU/mouse; the serum samples were used in the enzyme-linked immunosorbent assay (ELISA) to detect O-antigen-specific antibodies.
Complementation of strain RB51.
B. abortus RB51 was electroporated with a previously constructed plasmid, pAB3, a pBBR1MCS plasmid containing the wild-type wboA gene along with its promoter from B. abortus 2308 (25). Previously described methods were followed for the electroporation of strain RB51 (24). Bacteria containing the plasmids pBBR1MCS and pAB3 were selected by plating them on TSA plates containing chloramphenicol.
Serological tests.
The colony immunoblot assay and Western blotting were performed according to previously published procedures (29, 34). For the Western blot analysis, extracts were prepared with equal numbers of bacteria of each strain, and 150 μg of protein was loaded in each lane. An indirect ELISA was performed to measure the isotypes of O-antigen-specific antibodies in the sera of mice (13). Yersinia enterocolitica O:9 LPS, already available in our laboratory (32), was used as the antigen, since the O-polysaccharide chain of this LPS is identical in structure to that of B. abortus (7). Y. enterocolitica O:9 LPS was adsorbed to wells of polystyrene plates (Nunc Maxisorp) at a concentration of 0.5 μg/well in 50 μl of bicarbonate buffer (pH 9.6). After overnight incubation at 4°C, the plates were blocked with 2% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) (pH 7.4) for 2 h at room temperature. Mouse serum samples at 1:100 dilution were added to the wells in duplicate and incubated for 3 h at room temperature. The plates were washed three times with PBS containing 0.05% Tween 20. Isotype-specific goat anti-mouse horseradish peroxidase conjugates (ICN pharmaceuticals, Inc.) were added for 30 min at room temperature. After the plates were washed three times, 100 μl of TMB substrate solution (KPL, Gaithersburg, Md.) was added and incubated in the dark for 20 min. The reaction was stopped by adding 100 μl of 0.18 M sulfuric acid/well, and the absorbence of the developed color was measured at 450 nm.
Polymyxin B sensitivity assay.
The bactericidal effects of polymyxin B on B. abortus strains 2308, RB51, and RB51WboA (RB51 harboring plasmid pAB3) were determined according to published procedures (1, 23). Briefly, brucellae were grown to log phase in TSB and pelleted by centrifugation at 2,400 × g for 15 min. The pellets were resuspended in PBS to obtain approximately 7 × 104 CFU of bacteria/μl and incubated for 1.5 h with polymyxin B (Sigma) at different concentrations, and 0.5-ml aliquots of the bacterial suspensions were diluted to 1/10 and 1/100. From each dilution, 5 drops of 10 μl each were placed on TSA plates and incubated for 3 to 5 days at 37°C. Experiments were performed in triplicate, and the results were expressed as a percentage of brucellae surviving in suspensions containing no polymyxin B.
Immunoelectron microscopy.
B. abortus strains 19, RB51, and RB51WboA were grown in TSB and washed twice in PBS by centrifuging them at 2,400 × g for 15 min. The bacteria were fixed in 1 ml of 2.5% (vol/vol) glutaraldehyde in PBS overnight at 4°C, and samples were set in 2% (wt/vol) agar and washed in PBS. The agarose plugs were then dehydrated via stepwise alcohol series, embedded in the acrylic resin LR White in gelatin capsules, and baked at 55°C for 24 h under vacuum. Ultrathin sections were obtained by cutting the embedded material and deposited on 200-mesh nickel grids. Immunogold labeling of the grids was performed according to published procedures (10). Bru38, a rat monoclonal antibody to the O antigen, and gold-labeled rabbit anti-rat immunoglobulin G (IgG) (Sigma) were used as the primary and secondary antibody, respectively. Prior to reacting with the primary antibody, the grids were blocked with PBS containing 2% BSA and 10% normal rabbit serum. After immunogold labeling, the grids were stained with 4% uranyl acetate in water and 0.4% lead citrate in 0.1 M NaOH. Then the grids were observed with a transmission electron microscope (JEOL 100 CX II).
Survival of strain RB51WboA in mice.
Six-week-old female BALB/c mice (Charles River Laboratories, Wilmington, Mass.) were used. Groups of 15 mice were inoculated i.p. with 3 × 108 CFU of strains RB51 and RB51WboA. At 2, 4, and 6 weeks after inoculation, the Brucella CFU per spleen were determined as described previously (32). Briefly, at each time point, five mice from each group were sacrificed and their spleens were collected and homogenized in TSB. Serial dilutions of each spleen's homogenates were plated on TSA plates as well as on chloramphenicol-containing TSA plates in the case of homogenates of the spleens of strain RB51WboA-inoculated mice.
Protection experiment with mice.
Protection experiments were carried out as previously described (32). Groups of eight mice were vaccinated by i.p. inoculation of the viable strains RB51 (3 × 108 CFU/mouse), RB51pBB (5 × 108 CFU/mouse), and RB51WboA (2.5 × 108 CFU/mouse). The exact doses were determined retrospectively by plating the serial dilutions of the bacterial suspensions used for inoculating the mice. As a control, one group of mice was inoculated with saline alone. At 2, 4, and 6 weeks after inoculation, three mice from each group were bled retroorbitally, and the sera were collected and stored at −40°C until they were used in ELISAs. Seven weeks after inoculation, five mice from each group were challenged i.p. with 3 × 104 CFU of virulent B. abortus 2308/mouse. The remaining three mice from each group were sacrificed 7 to 8 weeks postvaccination to collect spleens for in vitro culture work as described below. Two weeks after challenge infection, the mice were sacrificed and the CFU in their spleens were determined.
Splenocyte cultures and quantitation of IFN-γ.
Between 7 and 8 weeks postinoculation, mice vaccinated with strains RB51, RB51WboA, and RB51pBB or with saline were sacrificed by CO2 asphyxiation, and their spleens were removed under aseptic conditions. Single-cell suspensions were prepared from the spleens according to previously described procedures (34). Red blood cells were lysed with ACK solution (150 mM NH4Cl, 1 mM KHCO3, 0.1 mM Na2EDTA [pH 7.3]), and the splenocytes were cultured in 96-well flat-bottom plates at a concentration of 5 × 105/well in the presence of heat-killed RB51 or RB51WboA cells equivalent to 106 CFU, 0.5 μg of concanavalin A, or no additives (unstimulated control). RPMI 1640 medium (GIBCO BRL) supplemented with 2 mM l-glutamine, 10% heat-inactivated fetal calf serum, and 50 μM 2-mercaptoethanol was used for culturing the splenocytes. The cells were cultured for 5 days, and the plates were centrifuged at 1,500 × g for 10 min. The clear culture supernatants were transferred to a new 96-well plate and stored at −70°C until ELISA was performed to determine the gamma interferon (IFN-γ) concentrations. Mouse IFN-γ-specific antigen capture ELISA was performed as previously described (34). Purified recombinant mouse IFN-γ was used as a standard each time an assay was performed. All assays were performed in duplicate, and the concentration of IFN-γ in the culture supernatants was calculated by using a linear-regression equation obtained from the absorbance values of the standards.
Statistical analysis.
Student's t test was used to analyze the data for bacterial clearance and protection experiments. The concentrations of IFN-γ were log transformed, and the differences among the groups were analyzed by performing analysis of variance and Dunnett's tests.
RESULTS
O-antigen synthesis and rough phenotype of RB51 complemented with wboA gene.
B. abortus RB51 transformed with pAB3, a broad-host-range plasmid containing the wild-type wboA gene from strain 2308, resulted in several thousand chloramphenicol-resistant colonies. One hundred of these RB51 recombinants were randomly selected and analyzed for their rough or smooth phenotype by crystal violet staining and agglutination in acryflavin solution (2, 36). All the recombinants retained crystal violet stain and agglutinated in acryflavin solution, indicating their rough phenotypic characteristic (data not shown). However, in a colony immunoblot assay, all the recombinants reacted with Bru38 (a Brucella O-antigen-specific monoclonal antibody) while RB51 and RB51 containing the pBBR1MCS plasmid alone did not react (not shown). One of the recombinants, designated RB51WboA, was selected for further analysis.
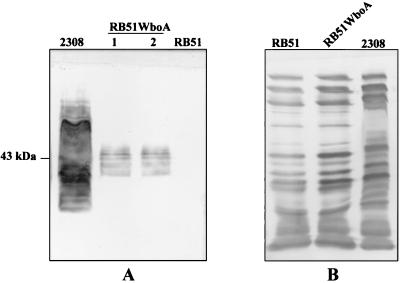
Western blotting of RB51WboA with Bru38 revealed several reactive bands 35 to 45 kDa in size (Fig. 1A). As expected, Bru38 did not show any reactivity with RB51, whereas its reactive pattern with 2308 was similar to previous descriptions (32). No major qualitative difference in the protein profiles of RB51 and RB51WboA was detected on the Western blot using goat serum hyperimmunized to RB51 (Fig. 1B).
FIG. 1.
Western blot reactivity of antigens from whole organisms of B. abortus strains RB51WboA, RB51, and 2308 with Bru38 (A) and goat serum hyperimmunized to RB51 antigens (B). Antigens of strains 2308, RB51WboA, and RB51 were separated by sodium dodecyl sulfate–12.5% polyacrylamide gel electrophoresis, transferred onto a nitrocellulose membrane, and reacted with the indicated antibodies. Lanes labeled 1 and 2 contain antigens from two different colonies of strain RB51WboA.
The B. abortus strains RB51 and RB51WboA showed similar sensitivities to the bactericidal effect of polymyxin B (data not shown). As anticipated, the tested concentrations of polymyxin B had no effect on the survival of B. abortus 2308 (1).
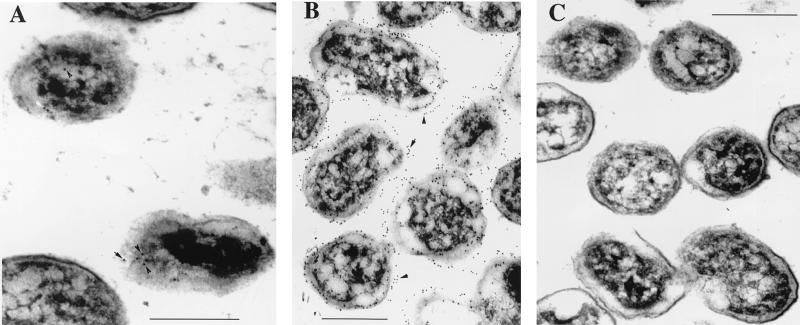
Immunoelectron microscopy of thin sections of B. abortus RB51WboA revealed the absence of O antigen on the bacterial surface but demonstrated small amounts of the expressed O antigen in the cytoplasm (Fig. 2A). In contrast, thin sections of B. abortus 2308 demonstrated the presence of O antigen mostly on the surfaces of the organisms, while no O antigen was detected in RB51 (Fig. 2B and C).
FIG. 2.
Immunoelectron microscopy detection of Brucella O antigen in the cytoplasm of strain RB51WboA. Thin sections of RB51WboA (A), 2308 (B), and RB51 (C) were reacted with Bru38 followed by gold-labeled rabbit anti-rat IgG. The arrowheads and arrows in panels A and B indicate the labeled gold particles. Bars = 0.45 μm.
Bacterial persistence.
To verify whether there were any differences in the attenuation characteristics of RB51WboA and RB51, we compared the bacterial clearance from the spleens of mice inoculated i.p. with these strains. The spleens of mice inoculated with either RB51 or RB51WboA were free of detectable brucellae by 40 days postinoculation, and no differences were observed at 14 and 30 days postinoculation. (The mice were inoculated i.p. with 6 × 108 CFU of B. abortus RB51 or 5.8 × 108 CFU of B. abortus RB51WboA. After 14 days, the spleens of mice inoculated with RB51 and RB51WboA contained 2.5 × 104 ± 1.2 × 104 and 1.5 × 104 ± 0.2 × 104 CFU, respectively; after 30 days, they contained 0.4 × 102 ± 0.3 × 102 and 0.6 × 102 ± 0.3 × 102 CFU. After 40 days, no bacteria were isolated.)
O-antigen-specific antibody subisotypes.
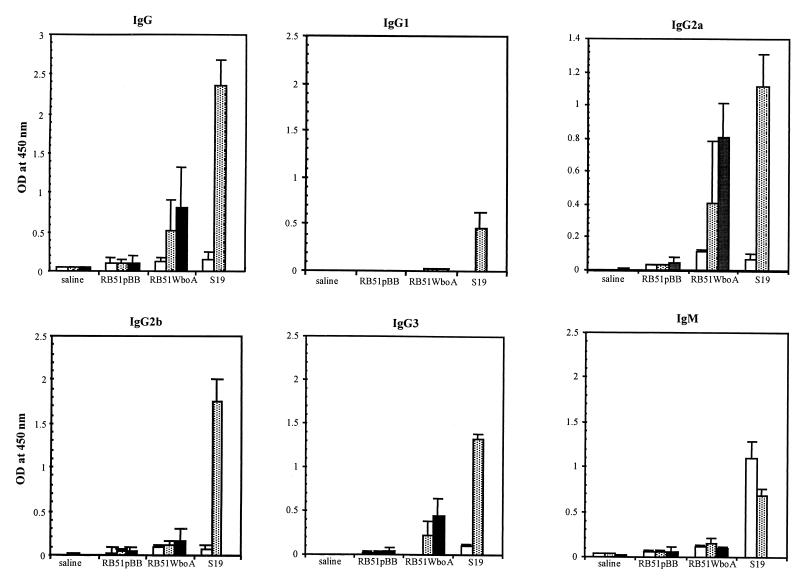
Mice vaccinated with RB51WboA developed antibodies to the O antigen which were detectable 4 weeks postvaccination, and the antibody levels increased slightly at 6 weeks (Fig. 3). The induced antibodies were mainly of the IgG2a and IgG3 subisotypes. Only low levels of IgM antibodies and no IgG1 antibodies specific to the O antigen were detected. Mice vaccinated with S19 developed higher levels of O-antigen-specific antibodies than those vaccinated with RB51WboA. In contrast to RB51WboA-vaccinated mice, S19-vaccinated mice developed substantial levels of O-antigen-specific IgG1, IgG2b, and IgM (Fig. 3). Vaccination with strains RB51 and RB51pBB did not induce antibodies specific to the O antigen; the levels of IgG, IgG2a, and IgM antibodies detected in these groups were not significantly different from those of the saline group (the high background obtained with these sera could be due to the nature of the antigen used in the ELISA).
FIG. 3.
ELISA detection of selected isotypes of O-antigen-specific antibodies in sera of mice vaccinated with RB51pBB, RB51WboA, and S19 or inoculated with saline alone. Sera collected from three mice of each group at 2 weeks (open bars), 4 weeks (shaded bars), and 6 weeks (solid bars) postvaccination were diluted 1 in 100 and assayed for the presence of antibodies to the O antigen. The results were shown as mean ± standard deviation of the optical density (OD) at 450 nm of the color developed. The results with strain RB51 were similar to those with strain RB51pBB (not shown).
Enhanced vaccine efficacy.
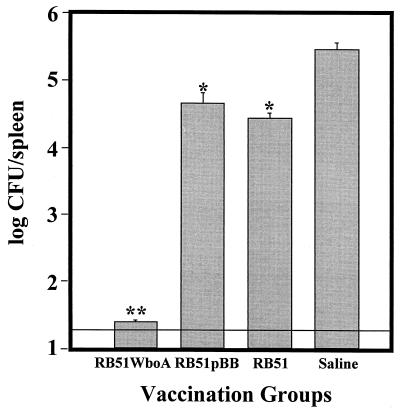
Compared to the saline-inoculated control, vaccination of mice with RB51, RB51pBB, or RB51WboA prior to challenge with the virulent strain 2308 significantly reduced the number of virulent brucellae in the spleens 2 weeks after challenge (Fig. 4). Spleens from mice vaccinated with RB51WboA were the least infected and were significantly different from those of all other groups. In fact, only two mice of that group contained 40 CFU, and no bacteria were isolated from the other three mice (Fig. 4). Although the mice vaccinated with RB51pBB appeared to have higher CFU than the RB51-vaccinated mice, this difference was not statistically significant. Based on the splenic bacterial counts, RB51 and RB51pBB vaccinations conferred 1.5 and 1 log units of protection, respectively, whereas RB51WboA induced at least 4 log units of protection.
FIG. 4.
Protection against B. abortus 2308 challenge of mice vaccinated with RB51WboA, RB51, and RB51pBB. Vaccinated and saline-inoculated mice were challenged by i.p. inoculation of 3 × 104 CFU of the virulent strain 2308, and after 2 weeks, the mice were sacrificed and the Brucella CFU in their spleens were determined. The parallel line above the y axis indicates the lower limit of detection. Groups with one asterisk are significantly different from the saline control group (P < 0.001) but not from each other. The group with two asterisks is significantly different from the saline control group and also from the groups with one asterisk (P < 0.0001). The error bars indicate standard deviations.
The protection experiment was repeated two more times, and the results obtained were similar to those described above.
Increased IFN-γ secretion.
In response to stimulation with killed RB51 equivalent to 107 CFU, splenocytes of RB51WboA-vaccinated mice secreted significantly more IFN-γ than the splenocytes of RB51-vaccinated mice (Table 1). Also, upon in vitro stimulation with killed RB51WboA, splenocytes from mice vaccinated with RB51WboA produced significantly more IFN-γ than splenocytes from RB51-vaccinated mice (Table 1). Since it is not possible to quantify and verify the numbers of bacterial cells after they are killed by heat, we did not compare the levels of IFN-γ secreted by splenocytes of one group in response to stimulation with RB51 and RB51WboA.
TABLE 1.
Production of IFN-γ by splenocytes of vaccinated mice upon in vitro stimulation with killed RB51 or RB51WboA
| Splenocytes from mice vaccinated with: | IFN-γ (ng/ml) in culture supernatants after stimulation with killed Brucellaa
|
|||
|---|---|---|---|---|
| 106 CFU
|
107 CFU
|
|||
| RB51 | RB51WboA | RB51 | RB51WboA | |
| RB51 | 18.7 ± 3.1 | 57.2 ± 8.9 | 98.4 ± 9.4 | 129.1 ± 23.9 |
| RB51WboA | 27.2 ± 3.6 | 104.4 ± 11.6 | 155.8 ± 17.7 | 227.4 ± 32.9 |
| P value | 0.0825 | 0.0071* | 0.0369* | 0.0112* |
Equivalent to the indicated number of live bacteria; mean ± standard deviation (n = 3). *, significant difference between the two groups in the column.
Splenocytes from nonvaccinated mice produced <1 ng of IFN-γ/μl when stimulated with killed cells of either Brucella strain (data not shown). No IFN-γ was detected in any of the negative-control cultures containing no stimulant, whereas all cultures stimulated with concanavalin A produced similar levels of IFN-γ (80 to 90 ng/ml) (data not shown).
DISCUSSION
Previous studies indicated that disruption of the wboA gene in virulent, smooth B. abortus, B. melitensis, and B. suis resulted in attenuated, rough mutants which were unable to synthesize the O antigen (25, 37). We recently discovered that the wboA gene in B. abortus RB51 is disrupted by an IS711 element (35). The studies presented in this paper establish that complementation of RB51 with a functional wboA gene results in O-antigen synthesis but does not change the rough phenotype. This could be explained through a failure in exporting the O antigen to the surface of the organism, so that the surface-exposed LPS of RB51WboA would still be rough. Our immunoelectron microscopy study suggests that the synthesized O antigen in RB51WboA is present intracellularly but not on the bacterial surface (Fig. 2). However, in comparison with strain 2308, RB51WboA expressed only low levels of the O antigen (Fig. 1); hence, it is possible that the low levels of expression might have affected the sensitivity of our localization study. Nevertheless, based on these findings, it is reasonable to propose that B. abortus RB51 contains another genetic mutation(s), in addition to the disrupted wboA gene, that either affects the export of O antigen and smooth LPS to the bacterial surface or impedes the appropriate coupling of O antigen to the core LPS or both. Though several genes that are potentially involved in the synthesis of O antigen in Brucella were recently identified (1, 18, 25), the actual process and the steps leading to the synthesis of the Brucella smooth LPS are not known. Hence, it is difficult to predict which other gene(s) is mutated to generate the rough phenotype of RB51, and it is also not possible to identify any specific reason for the observed low levels of O-antigen production in RB51WboA. Our present studies did not establish whether the O antigen in RB51WboA is present in free form or bound to any lipid or protein carrier. However, based on the Bru38 reactive bands on the Western blot (Fig. 1), it appears that at least some of the O antigen is present in association with some lipid or protein component(s). B. melitensis B115 is a rough strain that also expresses the O antigen in its cytoplasm (9, 10). In this strain, at least some of the expressed O antigen was shown to be lipid bound and associated with the cell wall (10). A comparative study of strains B115 and RB51WboA with regard to their cytoplasmic O antigen expression may provide us with clues for further unraveling the process of smooth-LPS synthesis in Brucella.
The identical pattern of bacterial clearance of strains RB51 and RB51WboA by the inoculated mice clearly indicates that the mere synthesis and intracellular localization of the O antigen does not change the attenuation characteristics of RB51. This finding is in agreement with the previously proposed role for the surface-exposed O antigen of Brucella smooth LPS in counteracting the bactericidal effects of the host phagocytes and increasing intracellular survival (1, 17, 18, 25, 28).
The presence of IgG2a and absence of IgG1 subisotype antibodies specific to the O antigen in the sera of RB51WboA-vaccinated mice indicate the induction of a Th1 type of immune response (33). When stimulated in vitro with killed RB51 or RB51WboA strains, splenocytes obtained from the mice vaccinated with RB51WboA secreted larger amounts of IFN-γ than the splenocytes from RB51-vaccinated mice. A precise explanation for this observation is not available at this time, but some speculations are possible. The increased IFN-γ production could be attributed to a higher number of NK cells present in the splenocyte mixture or to an enhanced T-cell activation by the O antigen. It has been well demonstrated that the smooth LPS of B. abortus and its O antigen can form stable complexes with mouse major histocompatibility complex (MHC) II molecules independent of the MHC haplotype (14, 15, 16). It is therefore possible that the O antigen from RB51WboA is binding to the MHC II molecule of antigen-presenting cells and specifically activating T cells, as previously proposed by Forestier et al. (15). Such activation of T cells may account for the increased IFN-γ production observed in our study; the enhanced IFN-γ production potentially plays a role in the increased protective effect of RB51WboA. Also, Forestier et al. (15) reported that only B. abortus smooth LPS fragments of a specific size (∼45 kDa) can be detected in association with MHC II molecules. Interestingly, the size of the molecules containing the O antigen detected in RB51WboA was within this range (35 to 45 kDa), suggesting that it could bind efficiently to the MHC II molecules and be presented to T cells. Vaccination with RB51WboA could result in T cells specific for a variety of Brucella antigens, including the O antigen. The O-antigen-specific T cells, not inducible by RB51, and the T cells specific for other antigens, inducible by RB51, could become activated during the challenge with a smooth, virulent B. abortus, resulting in enhanced cell-mediated-immunity responses which may have contributed to the increased protection observed with RB51WboA. It is also possible that, even if the O antigen associates with the MHC II molecules, it may not be able to induce O-antigen-specific T cells. However, if small Brucella peptides are bound to the O antigen, even in minute quantities, the O antigen may serve to focus or concentrate these peptides onto the MHC II molecules and allow efficient, specific sensitization of T cells to the peptides. Induction of an enhanced response to the peptides may also partially explain the enhanced protective ability of RB51WboA.
The enhanced vaccine efficacy of RB51WboA could be due solely to the induction of antibodies to the O antigen and/or increased activation of specific T cells. Only protection experiments involving adoptive transfer of antibodies and/or immune T cells can delineate their protective role.
In conclusion, an important finding of this study is that RB51 carries mutations in more than one of the genes necessary for the expression of a smooth phenotype. Also, the availability of RB51WboA, which can produce O-antigen-containing molecules of the appropriate size to fit into MHC II molecules (15) while maintaining the attenuation characteristics of strain RB51, should further facilitate studies aimed at understanding the role of B. abortus O antigen in antigen presentation and immunomodulation.
REFERENCES
- 1.Allen C A, Garry Adams L, Ficht T A. Transposon-derived Brucella abortus rough mutants are attenuated and exhibit reduced intracellular survival. Infect Immun. 1998;66:1008–1016. doi: 10.1128/iai.66.3.1008-1016.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alton G G, Jones L M, Pietz D E. Laboratory techniques in Brucellosis. World Health Organization monograph series no. 55. Geneva, Switzerland: World Health Organization; 1975. [PubMed] [Google Scholar]
- 3.Araya L N, Elzer P H, Rowe G E, Enright F M, Winter A J. Temporal development of protective cell-mediated and humoral immunity in BALB/c mice infected with Brucella abortus. J Immunol. 1989;143:3330–3337. [PubMed] [Google Scholar]
- 4.Araya L N, Winter A J. Comparative protection of mice against virulent and attenuated strains of Brucella abortus by passive transfer of immune T cells or serum. Infect Immun. 1990;58:254–256. doi: 10.1128/iai.58.1.254-256.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bundle D R, Cherwonogrodzky J W, Perry M B. The structure of the lipopolysaccharide O-chain (M-antigen) and polysaccharide B produced by Brucella melitensis 16M. FEMS Microbiol Lett. 1987;216:261–264. doi: 10.1016/0014-5793(87)80702-0. [DOI] [PubMed] [Google Scholar]
- 6.Caroff M, Bundle D R, Perry M B, Cherwonogrodzky J W, Duncan J R. Antigenic S-type lipopolysaccharide of Brucella abortus 1119-3. Infect Immun. 1984;46:384–389. doi: 10.1128/iai.46.2.384-388.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cherwonogrodzky J W, Dubray G, Moreno E, Mayer H. Antigens of Brucella. In: Nielsen K, Duncan J R, editors. Animal brucellosis. Boca Raton, Fla: CRC Press, Inc.; 1990. pp. 19–64. [Google Scholar]
- 8.Cheville N F, Stevens M G, Jensen A E, Tatum F M, Halling S M. Immune responses and protection against infection and abortion in cattle experimentally vaccinated with mutant strains of Brucella abortus. Am J Vet Res. 1993;54:1591–1597. [PubMed] [Google Scholar]
- 9.Cloeckaert A, Zygmunt M S, Dubray G, Limet J N. Characterization of O-polysaccharide specific monoclonal antibodies derived from mice infected with the rough Brucella melitensis strain B115. J Gen Microbiol. 1993;139:1551–1556. doi: 10.1099/00221287-139-7-1551. [DOI] [PubMed] [Google Scholar]
- 10.Cloeckaert A, Zygmunt M S, Nicolle J C, Dubray G, Limet J N. O-chain expression in the rough Brucella melitensis strain B115: induction of O-polysaccharide-specific monoclonal antibodies and intracellular localization demonstrated by immunoelectron microscopy. J Gen Microbiol. 1992;138:1211–1219. doi: 10.1099/00221287-138-6-1211. [DOI] [PubMed] [Google Scholar]
- 11.Corbel M J. Brucellosis: an overview. Emerg Infect Dis. 1997;3:213–221. doi: 10.3201/eid0302.970219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenschenk F C, Houle J J, Hoffmann E M. Serum sensitivity of field isolates and laboratory strains of Brucella abortus. Am J Vet Res. 1995;56:1592–1598. [PubMed] [Google Scholar]
- 13.Elzer P H, Jacobson R H, Nielsen K H, Douglas J T, Winter A J. BALB/c mice infected with Brucella abortus express protracted polyclonal responses of both IgG2a and IgG3 isotypes. Immunol Lett. 1994;42:145–150. doi: 10.1016/0165-2478(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 14.Escola J M, Moreno E, Chavrier P, Gorvel J P. The O-chain of Brucella abortus lipopolysaccharide induces SDS-resistant MHC II molecules in mouse B cells. Biochem Biophys Res Commun. 1994;203:1230–1236. doi: 10.1006/bbrc.1994.2314. [DOI] [PubMed] [Google Scholar]
- 15.Forestier C, Moreno E, Meresse S, Phalipon A, Olive D, Sansonetti P, Gorvel J P. Interaction of Brucella abortus lipopolysaccharide with major histocompatibility complex class II molecules in B lymphocytes. Infect Immun. 1999;67:4048–4054. doi: 10.1128/iai.67.8.4048-4054.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forestier C, Moreno E, Pizarro-Cerola J, Gorvel J P. Lysosomal accumulation and recycling of lipopolysaccharide to the cell surface of murine macrophages, an in vitro and in vivo study. J Immunol. 1999;162:6789–6791. [PubMed] [Google Scholar]
- 17.Frenchick P J, Markham R J F, Cochrane A H. Inhibition of phagosome-lysosome fusion in macrophages by soluble extracts of virulent Brucella abortus. Am J Vet Res. 1985;46:332–335. [PubMed] [Google Scholar]
- 18.Godfroid F, Taminiau B, Danese I, Denoel P, Tibor A, Weynants V, Cloeckaert A, Godfroid J, Letesson J-J. Identification of the perosamine synthetase gene of Brucella melitensis 16M and involvement of lipopolysaccharide O side chain in Brucella survival in mice and in macrophages. Infect Immun. 1998;66:5485–5493. doi: 10.1128/iai.66.11.5485-5493.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jimenez de Bagues M P, Elzer P H, Jones S M, Blasco J M, Enright F M, Schurig G G, Winter A J. Vaccination with Brucella abortus rough mutant RB51 protects BALB/c mice against virulent strains of Brucella abortus, Brucella melitensis, and Brucella ovis. Infect Immun. 1994;62:4990–4996. doi: 10.1128/iai.62.11.4990-4996.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen A E, Ewalt D R, Cheville N F, Thoen C O, Payeur J B. Determination of stability of Brucella abortus RB51 by use of genomic fingerprint, oxidative metabolism, and colonial morphology and differentiation of strain RB51 from B. abortus isolates from bison and elk. J Clin Microbiol. 1996;34:628–633. doi: 10.1128/jcm.34.3.628-633.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kovach M E, Phillips R W, Elzer P H, Roop R M, Peterson K M. pBBR1MCS: a broad-host-range cloning vector. BioTechniques. 1994;16:800–801. [PubMed] [Google Scholar]
- 22.Lord V R, Schurig G G, Cherwonogrodzky J W, Marcano M J, Melendez G E. Field study of vaccination of cattle with Brucella abortus strains RB51 and 19 under high and low disease prevalence. Am J Vet Res. 1998;59:1016–1020. [PubMed] [Google Scholar]
- 23.Martinez de Tejada G, Pizarro-Cerda J, Moreno E, Moriyon I. The outer membranes of Brucella spp. are resistant to bactericidal cationic peptides. Infect Immun. 1995;63:3054–3061. doi: 10.1128/iai.63.8.3054-3061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McQuiston J R, Schurig G G, Sriranganathan N, Boyle S M. Transformation of Brucella species with suicide and broad host-range plasmids. Methods Mol Biol. 1995;47:143–148. doi: 10.1385/0-89603-310-4:143. [DOI] [PubMed] [Google Scholar]
- 25.Mcquiston J R, Vemulapalli R, Inzana T J, Schurig G G, Sriranganathan N, Fritzinger D, Hadfield T L, Warren R A, Snellings N, Hoover D, Halling S M, Boyle S M. Genetic characterization of a Tn5-disrupted glycosyltransferase gene homolog in Brucella abortus and its effect on lipopolysaccharide composition and virulence. Infect Immun. 1999;67:3830–3835. doi: 10.1128/iai.67.8.3830-3835.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montaraz J A, Winter A J, Hunter D M, Sowa B A, Wu A M, Adams L G. Protection against Brucella abortus in mice with O-polysaccharide-specific monoclonal antibodies. Infect Immun. 1986;51:961–963. doi: 10.1128/iai.51.3.961-963.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmer M V, Olsen S C, Cheville N F. Safety and immunogenicity of Brucella abortus strain RB51 vaccine in pregnant cattle. Am J Vet Res. 1997;58:472–477. [PubMed] [Google Scholar]
- 28.Pizarro-Cerda J, Moreno E, Sanguedolce V, Mege J-L, Gorvel J-P. Virulent Brucella abortus avoids lysosome fusion and distributes within autophagosome-like compartments. Infect Immun. 1998;66:2387–2392. doi: 10.1128/iai.66.5.2387-2392.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roop R M, Preston-Moore D, Bagchi T, Schurig G G. Rapid identification of smooth Brucella species with a monoclonal antibody. J Clin Microbiol. 1987;25:2090–2093. doi: 10.1128/jcm.25.11.2090-2093.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samartino L E, Enright F M. Pathogenesis of abortion of bovine brucellosis. Comp Immunol Microbiol Infect Dis. 1993;16:95–101. doi: 10.1016/0147-9571(93)90001-l. [DOI] [PubMed] [Google Scholar]
- 31.Schurig G G, Hammerberg C, Finkler B. Monoclonal antibodies to Brucella surface antigens associated with smooth lipopolysaccharide complex. Am J Vet Res. 1984;45:967–971. [PubMed] [Google Scholar]
- 32.Schurig G G, Roop II R M, Bagchi T, Boyle S, Buhrman D, Sriranganathan N. Biological properties of RB51; a stable rough strain of Brucella abortus. Vet Microbiol. 1991;28:171–188. doi: 10.1016/0378-1135(91)90091-s. [DOI] [PubMed] [Google Scholar]
- 33.Stevens T L, Bossie A, Sanders V M, Fernandez-Botran R, Coffman R L, Mosmann T R, Vitetta E S. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988;334:255–258. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- 34.Vemulapalli R, Duncan A J, Boyle S M, Sriranganathan N, Toth T E, Schurig G G. Cloning and sequencing of yajC and secD homologs of Brucella abortus and demonstration of immune responses to YajC in mice vaccinated with B. abortus RB51. Infect Immun. 1998;66:5684–5691. doi: 10.1128/iai.66.12.5684-5691.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vemulapalli R, McQuiston J R, Schurig G G, Sriranganathan N, Halling S M, Boyle S M. Identification of an IS711 element interrupting the wboA gene of Brucella abortus vaccine strain RB51 and a PCR assay to distinguish strain RB51 from other Brucella species and strains. Clin Diagn Lab Immunol. 1999;6:760–764. doi: 10.1128/cdli.6.5.760-764.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White P G, Wilson J B. Differentiation of smooth and nonsmooth colonies of brucellae. J Bacteriol. 1951;61:239–240. doi: 10.1128/jb.61.2.239-240.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winter A J, Schurig G G, Boyle S M, Sriranganathan N, Bevins J S, Enright F M, Elzer P H, Kopec J D. Protection of BALB/c mice against homologous and heterologous species of Brucella by rough strain vaccines derived from Brucella melitensis and Brucella suis biovar 4. Am J Vet Res. 1996;57:677–683. [PubMed] [Google Scholar]