Abstract
Background
Lymphovascular invasion (LVI) and perineural invasion (PNI) are associated with poorer prognosis in several human malignancies, but their significance in gastric cancer (GC) remains to be clearly defined. Our study aimed to investigate the prognostic value of LVI/PNI in patients with curative resected GC.
Methods
Records of 1488 patients with stage I‐–III GC and 3327 patients with stage I–III colorectal cancer (CRC) were reviewed retrospectively, and difference in the incidence of LVI/PNI between GC and CRC was compared. Univariate and multivariate analyses were used to evaluate whether LVI/PNI was an independent risk factor for lymph node metastasis (LNM) and overall survival (OS) in GC.
Results
Patients with stage I–III GC had a significantly higher incidence of LVI/PNI than patients with stage I–III CRC (50.54% vs. 21.91%, p < 0.001). LVI/PNI was significantly associated with higher CEA, higher CA199, deeper tumor invasion, more lymph node metastasis, and advanced TNM stage in GC ( p < 0.05). Multivariate logistic regression analysis identified LVI/PNI (OR = 2.64, 95%CI: 2.05–3.40, p < 0.001) as an independent risk factor for LNM in GC. The OS rate was significantly lower in the LVI/PNI‐positive GC group than that in the LVI/PNI‐negative GC group ( p < 0.001). On multivariate Cox regression analysis, LVI/PNI (HR = 1.34, 95%CI: 1.04–1.71, p = 0.023) was an independent prognostic factor for OS in GC.
Conclusion
GC has a high incidence of LVI/PNI, which was closely associated with disease progression. LVI/PNI could serve as an independent risk factor for LNM and the prognosis of patients with curative resected GC. These findings will be helpful in predicting survival outcomes more accurately and establishing individualized treatment plans.
Keywords: gastric cancer, lymph node metastasis, lymphovascular invasion, perineural invasion, prognosis
Gastric cancer has a high incidence of LVI/PNI, which was closely associated with disease progression. LVI/PNI could serve as an independent risk factor for lymph node metastasis and the prognosis of patients with curative resected gastric cancer. These findings will be helpful in predicting survival outcomes more accurately and establishing individualized treatment plans.
1. INTRODUCTION
Gastric cancer (GC) is the fifth most common malignancy and the fourth leading cause of cancer‐related deaths globally, which has the highest incidence and mortality rates in East Asian countries. 1 GC is often diagnosed at an advanced stage with a poor prognosis, and lymph node metastasis (LNM) is an essential factor that negatively impacts the prognosis and determines clinical management for GC. 2 , 3 , 4
Lymphovascular invasion (LVI) was histologically defined as the presence of tumor emboli within either the lymphatic or vascular channels or the destruction of the lymphatic or vascular wall by cancer cells. 5 Perineural invasion (PNI) was histologically defined as tumor invasion of nerve structures and spread along nerve sheaths. 6 LVI and PNI have been identified as a harbinger of poor prognosis for many malignancies; More interestingly, GC seems to have a relatively high incidence of LVI/PNI among various kinds of cancers, including colorectal cancer (CRC), esophageal squamous cell carcinoma, etc. 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 However, the prognostic value of LVI and PNI in GC is still under debate. Some researchers have found that LVI and PNI were independent risk factors for survival outcomes of patients with GC. 10 , 15 , 16 , 17 , 18 Others found that LVI and PNI were not independent prognostic factors despite their strong association with disease progression in GC. 9 , 19 , 20 , 21 , 22 , 23 , 24
We hypothesized that LVI/PNI is associated with LNM and survival outcomes in GC patients. In this study, we first compared the difference in the incidence of LVI/PNI between GC and CRC. Then, we analyzed associations between LVI/PNI and other clinicopathologic features in GC. Next, we identified clinicopathologic prognostic factors for lymph node metastasis. Finally, we evaluated the prognostic significance of LVI/PNI in GC patients who underwent curative gastrectomy.
2. METHODS
2.1. Patients
This is a retrospective study. Ethical Committee of The Sixth Affiliated Hospital, Sun Yat‐sen University, had approved this retrospective research, and the requirement for written informed consent was waived. Records of 2474 consecutive patients with GC who had surgical resection from January 2009 to May 2021 at The Sixth Affiliated Hospital, Sun Yat‐sen University were reviewed. The inclusion criteria were as follows: (1) primary adenocarcinoma of the stomach confirmed by histopathology; (2) pathologic stage I–III according to the criteria of the American Joint Committee on Cancer TNM staging system (seventh edition) 25 ; (3) patients who underwent curative gastrectomy. Patients with incomplete clinicopathologic data, stage IV disease, noncurative operation, remnant GC, and patients who were lost to follow‐up were excluded. As a comparison, patients with pathologic stage I–III primary colorectal adenocarcinoma and complete LVI and PNI data between January 2017 and December 2018 were included.
Demographic and clinicopathologic characteristics were obtained from the Cancer Database of The Sixth Affiliated Hospital, Sun Yat‐sen University, including age (<60 years old, ≥60 years old), sex (male, female), body mass index (BMI) (<18.5 kg/m2, ≥18.5 kg/m2), preoperative hemoglobin (HGB) level (<90 g/L, ≥90 g/L), preoperative serum albumin level (<35 g/L, ≥35 g/L), preoperative carcinoembryonic antigen (CEA) level (<5 μg/L, ≥5 μg/L), preoperative carbohydrate antigen 125 (CA125) level (<35 U/mL, ≥35 U/mL), preoperative carbohydrate antigen 19–9 (CA199) level (<37 U/mL, ≥37 U/mL), tumor location (upper, middle, lower, or total), pathologic TNM stage, LVI, PNI, and most recent follow‐up data. Postoperative telephone follow‐up by the staff of the Cancer Database took place every 6 months up to 3 years, and annually thereafter. Preoperative laboratory tests were routinely performed for cancer patients, including HGB, serum albumin, and tumor markers. Gastrectomy resection specimens are routinely stained with hematoxylin and eosin (H&E) by Department of Pathology, and all specimens were analyzed by two experienced pathologists. LVI was considered positive when there were tumor emboli in lumina of endothelial‐lined spaces on H&E‐stained slides, and PNI was considered positive when tumor cells were found in perineural or intraneural spaces on H&E‐stained slides. In this study, LVI/PNI (+) means whether LVI or PNI was positive, and LVI/PNI (−) means both LVI and PNI were negative. Overall survival (OS) was calculated from the date of surgical resection to the date of last follow‐up or death for any cause.
2.2. Statistical analysis
Statistical analyses were performed using GraphPad Prism (version 8.0) and R software (version 4.0.2). Comparisons of categorical variables between groups were performed using the chi‐square test. Univariate and multivariate logistic regression analyses were used to identify prognostic factors for lymph node metastasis. Univariate and multivariate analyses of prognostic factors for OS in GC were performed using Cox proportional hazards models. Kaplan–Meier curves were generated for OS. Then differences in survival rates between groups were compared using the log‐rank test. All p values <0.05 were considered statistically significant.
3. RESULTS
3.1. Difference in the incidence of LVI/PNI between GC and CRC
A total of 1488 patients with GC and 3327 patients with CRC were finally enrolled in this study, as shown in Figure 1. The incidences of LVI/PNI in both GC and CRC showed a growing tendency as the diseases progressed (Figure 2A). In detail, incidences of LVI/PNI in patients with stage I, II, and III GC were 10.32%, 53.47%, and 76.91%, respectively, while incidences of LVI/PNI in patients with stage I, II, and III CRC were 5.17%, 14.80%, and 38.41%, respectively. The GC patients had a significantly higher proportion of positive LVI/PNI than the CRC patients with the same pathologic TNM stage (p < 0.005). Besides, the overall incidence of LVI/PNI in stage I–III GC patients was also significantly higher than that in stage I–III CRC patients (50.54% vs. 21.91%, p < 0.001) (Table 1).
FIGURE 1.
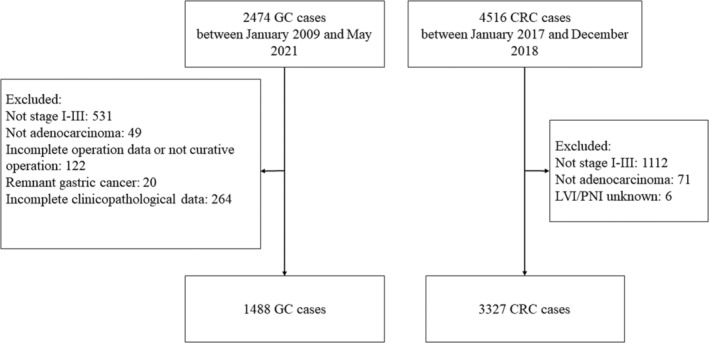
Flowchart of the study.
FIGURE 2.
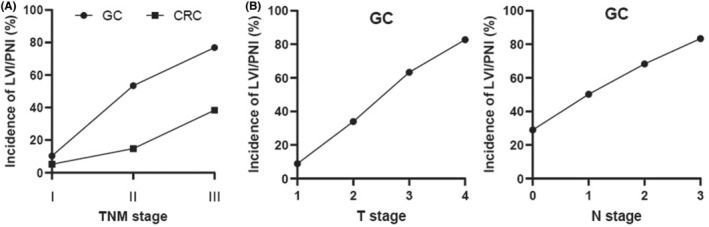
The incidence of LVI/PNI in gastric cancer (GC) and colorectal cancer. (A) Difference in the incidence of LVI/PNI between GC and colorectal cancer. (B) The incidence of LVI/PNI was increasing with advanced T stage or N stage in GC.
TABLE 1.
Difference in the incidence of LVI/PNI between gastric cancer and colorectal cancer.
| pTNM stage | Cancer | Overall | LVI/PNI | p‐value | |
|---|---|---|---|---|---|
| (+) (%) | (−) (%) | ||||
| I | Colorectal cancer | 619 | 32 (5.17) | 587 (94.83) | 0.003 |
| Gastric cancer | 407 | 42 (10.32) | 365 (89.68) | ||
| II | Colorectal cancer | 1453 | 215 (14.80) | 1238 (85.20) | <0.001 |
| Gastric cancer | 518 | 277 (53.47) | 241 (46.53) | ||
| III | Colorectal cancer | 1255 | 482 (38.41) | 773 (61.59) | <0.001 |
| Gastric cancer | 563 | 433 (76.91) | 130 (23.09) | ||
| I–III | Colorectal cancer | 3327 | 729 (21.91) | 2598 (78.09) | <0.001 |
| Gastric cancer | 1488 | 752 (50.54) | 736 (49.46) | ||
Abbreviations: LVI, lymphovascular invasion; PNI, perineural invasion.
3.2. Clinicopathologic features of stage I–III GC patients according to LVI/PNI status
Among the 1488 GC patients included in our study, 752 (50.54%) cases had LVI/PNI‐positive disease and 736 (49.46%) cases had LVI/PNI‐negative disease. As shown in Table 2, for GC patients with LVI/PNI‐positive disease, higher CEA level (p = 0.019), higher CA199 level (p < 0.001), deeper tumor invasion (p < 0.001), more LNM (p < 0.001), and advanced TNM stage (p < 0.001) were noted compared to those with LVI/PNI‐negative disease. Besides, the incidence of LVI/PNI was increasing in GC patients with advanced T stage or N stage (Figure 2B). There were no significant differences in terms of BMI, sex, age, HGB level, ALB level, CA125 level, and tumor location between the patients with and without LVI/PNI.
TABLE 2.
Clinicopathologic characteristics of patients undergoing curative gastrectomy for stage I–III gastric cancer based on LVI/PNI status.
| Characteristics | Overall n = 1488 | LVI/PNI | p‐value | |
|---|---|---|---|---|
| (−) n = 736 | (+) n = 752 | |||
| Sex (%) | ||||
| Male | 1001 (67.27) | 493 (66.98) | 508 (67.55) | 0.858 |
| Female | 487 (32.73) | 243 (33.02) | 244 (32.45) | |
| Age (years) (%) | ||||
| <60 | 688 (46.24) | 335 (45.52) | 353 (46.94) | 0.618 |
| ≥60 | 800 (53.76) | 401 (54.48) | 399 (53.06) | |
| BMI (kg/m2) (%) | ||||
| <18.5 | 184 (12.37) | 85 (11.55) | 99 (13.16) | 0.385 |
| ≥18.5 | 1304 (87.63) | 651 (88.45) | 653 (86.84) | |
| HGB (g/L) (%) | ||||
| <90 | 226 (15.19) | 106 (14.40) | 120 (15.96) | 0.445 |
| ≥90 | 1262 (84.81) | 630 (85.60) | 632 (84.04) | |
| ALB (g/L) (%) | ||||
| <35 | 228 (15.32) | 106 (14.40) | 122 (16.22) | 0.366 |
| ≥35 | 1260 (84.68) | 630 (85.60) | 630 (83.78) | |
| CEA (μg/L) (%) | ||||
| ≤5 | 1247 (83.80) | 634 (86.14) | 613 (81.52) | 0.019 |
| >5 | 241 (16.20) | 102 (13.86) | 139 (18.48) | |
| CA199 (U/mL) (%) | ||||
| ≤37 | 1294 (86.96) | 665 (90.35) | 629 (83.64) | <0.001 |
| >37 | 194 (13.04) | 71 (9.65) | 123 (16.36) | |
| CA125 (U/mL) (%) | ||||
| ≤35 | 1418 (95.30) | 706 (95.92) | 712 (94.68) | 0.313 |
| >35 | 70 (4.70) | 30 (4.08) | 40 (5.32) | |
| Tumor location (%) | ||||
| Total | 12 (0.81) | 5 (0.68) | 7 (0.93) | 0.838 |
| Upper | 429 (28.83) | 211 (28.67) | 218 (28.99) | |
| Middle | 323 (21.71) | 155 (21.06) | 168 (22.34) | |
| Lower | 724 (48.66) | 365 (49.59) | 359 (47.74) | |
| pT stage (%) | ||||
| T1 | 339 (22.78) | 309 (41.98) | 30 (3.99) | <0.001 |
| T2 | 171 (11.49) | 113 (15.35) | 58 (7.71) | |
| T3 | 747 (50.20) | 274 (37.23) | 473 (62.90) | |
| T4 | 231 (15.52) | 40 (5.43) | 191 (25.40) | |
| pN stage (%) | ||||
| N0 | 642 (43.15) | 456 (61.96) | 186 (24.73) | <0.001 |
| N1 | 295 (19.83) | 147 (19.97) | 148 (19.68) | |
| N2 | 274 (18.41) | 87 (11.82) | 187 (24.87) | |
| N3 | 277 (18.62) | 46 (6.25) | 231 (30.72) | |
| pTNM_stage (%) | ||||
| I | 407 (27.35) | 365 (49.59) | 42 (5.59) | <0.001 |
| II | 518 (34.81) | 241 (32.74) | 277 (36.84) | |
| III | 563 (37.84) | 130 (17.66) | 433 (57.58) | |
Abbreviations: ALB, albumin; BMI, body mass index; CA, carbohydrate antigen; CEA, carcinoembryonic antigen; HGB, hemoglobin; LVI, lymphovascular invasion; PNI, perineural invasion.
3.3. Independent risk factors for lymph node metastasis in GC
As shown in Table 3, results of univariate logistic regression analysis showed that BMI, CEA level, CA199 level, T stage, and LVI/PNI status were associated with LNM in patients with GC (p < 0.05). In multivariate analysis, positive LVI/PNI (OR = 2.64, 95%CI: 2.05–3.40, p < 0.001) was identified as an independent risk factor for LNM in GC. Besides, lower BMI, higher CA199 level, and advanced T stage were also independent risk factors for LNM in GC.
TABLE 3.
Results of univariate and multivariable logistic regression analyses for lymph node metastasis in gastric cancer.
| Characteristics | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p‐value | |
| Sex | ||||
| Female | Reference | |||
| Male | 1.06 (0.85–1.32) | 0.586 | ||
| Age (years) | ||||
| ≥60 | Reference | |||
| <60 | 1.09 (0.89–1.34) | 0.411 | ||
| BMI (kg/m2) | ||||
| ≥18.5 | Reference | |||
| <18.5 | 1.62 (1.17–2.24) | 0.004 | 1.50 (1.04–2.17) | 0.030 |
| HGB (g/L) | ||||
| ≥90 | Reference | |||
| <90 | 1.31 (0.98–1.75) | 0.069 | ||
| ALB (g/L) | ||||
| ≥35 | Reference | |||
| <35 | 1.22 (0.92–1.63) | 0.174 | ||
| CEA (μg/L) | ||||
| ≤5 | Reference | |||
| >5 | 1.48 (1.11–1.97) | 0.007 | 1.06 (0.77–1.47) | 0.713 |
| CA199 (U/mL) | ||||
| ≤37 | Reference | |||
| >37 | 2.43 (1.73–3.41) | <0.001 | 1.50 (1.04–2.18) | 0.032 |
| CA125 (U/mL) | ||||
| ≤35 | Reference | |||
| >35 | 1.58 (0.95–2.64) | 0.077 | ||
| Tumor location | ||||
| Total | Reference | |||
| Upper | 1.55 (0.49–4.90) | 0.452 | ||
| Middle | 1.14 (0.36–3.61) | 0.825 | ||
| Lower | 1.28 (0.41–4.02) | 0.668 | ||
| pT stage | ||||
| T1 | Reference | |||
| T2 | 2.90 (1.96–4.31) | <0.001 | 2.26 (1.51–3.40) | <0.001 |
| T3 | 6.92 (5.14–9.32) | <0.001 | 4.04 (2.91–5.60) | <0.001 |
| T4 | 18.14 (11.75–28.02) | <0.001 | 8.68 (5.42–13.91) | <0.001 |
| LVI/PNI | ||||
| (−) | Reference | |||
| (+) | 4.96 (3.97–6.19) | <0.001 | 2.64 (2.05–3.40) | <0.001 |
Abbreviations: ALB, albumin; BMI, body mass index; CA, carbohydrate antigen; CEA, carcinoembryonic antigen; HGB, hemoglobin; LVI, lymphovascular invasion; PNI, perineural invasion.
3.4. Independent prognostic factors for survival outcomes in GC
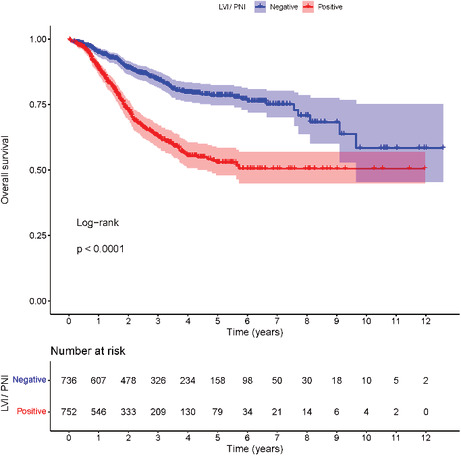
The median follow‐up time was 34.8 months. For GC patients with stage I–III disease, the 1‐year, 3‐year, and 5‐year OS rates in the LVI/PNI‐positive group were 88.9%, 62.8%, and 53.0%, respectively; and the 1‐year, 3‐year, and 5‐year OS rates in the LVI/PNI‐negative group were 94.9%, 84.3%, and 78.4%, respectively. The OS rate was significantly worse in the LVI/PNI‐positive group than that in the LVI/PNI‐negative group (p < 0.001) (Figure 3).
FIGURE 3.
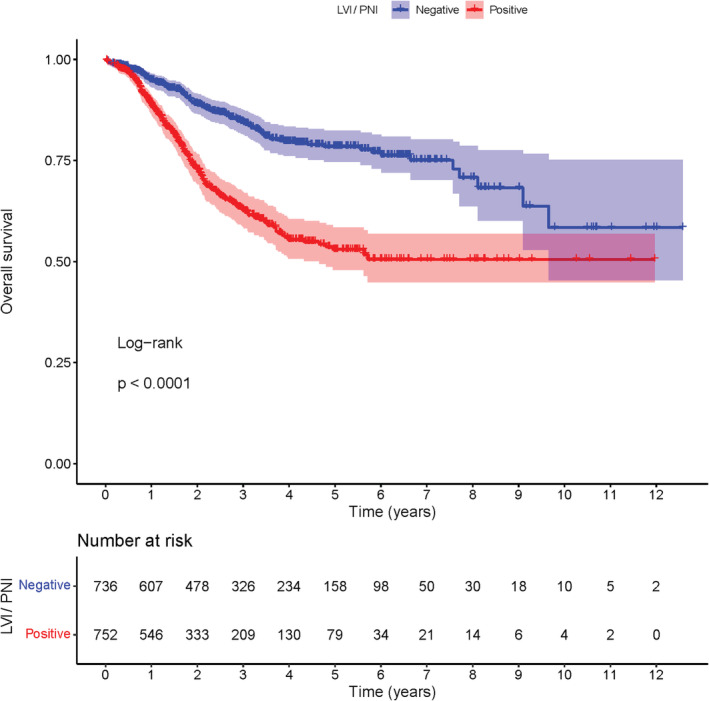
Kaplan–Meier analysis of overall survival based on LVI/PNI status among 1488 patients with stage I–III gastric cancer who underwent curative gastrectomy.
As shown in Table 4, univariate Cox regression analysis found that age, BMI, HGB, serum ALB level, CEA level, CA199 level, CA125 level, T stage, N stage, and LVI/PNI status were associated with OS of patients with stage I–III GC after curative resection (p < 0.05). Then multivariate analysis further identified positive LVI/PNI (HR = 1.34, 95%CI: 1.04–1.71, p = 0.023) as an independent prognostic factor for OS in GC patients. Besides, age, BMI, CA199 level, CA125 level, T stage, and N stage were also independent prognostic factors for OS of GC patients (p < 0.05).
TABLE 4.
Results of univariate and multivariable Cox regression analyses for overall survival in gastric cancer.
| Characteristics | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p‐value | |
| Sex | ||||
| Female | Reference | |||
| Male | 1.19 (0.94–1.50) | 0.157 | ||
| Age (years) | ||||
| ≥60 | Reference | |||
| <60 | 0.60 (0.48–0.75) | <0.001 | 0.64 (0.51–0.80) | <0.001 |
| BMI (kg/m2) | ||||
| ≥18.5 | Reference | |||
| <18.5 | 2.34 (1.81–3.02) | <0.001 | 1.89 (1.46–2.45) | <0.001 |
| HGB (g/L) | ||||
| ≥90 | Reference | |||
| <90 | 1.59 (1.22–2.07) | 0.001 | 1.25 (0.94–1.65) | 0.128 |
| ALB (g/L) | ||||
| ≥35 | Reference | |||
| <35 | 1.79 (1.37–2.34) | <0.001 | 1.26 (0.94–1.69) | 0.117 |
| CEA (μg/L) | ||||
| ≤5 | Reference | |||
| >5 | 1.73 (1.35–2.22) | <0.001 | 1.24 (0.97–1.60) | 0.091 |
| CA199 (U/mL) | ||||
| ≤37 | Reference | |||
| >37 | 2.29 (1.77–2.95) | <0.001 | 1.45 (1.11–1.88) | 0.006 |
| CA125 (U/mL | ||||
| ≤35 | Reference | |||
| >35 | 2.52 (1.71–3.71) | <0.001 | 1.92 (1.29–2.86) | 0.001 |
| Tumor location | ||||
| Total | Reference | |||
| Upper | 1.22 (0.39–3.83) | 0.736 | ||
| Middle | 1.13 (0.35–3.57) | 0.840 | ||
| Lower | 0.90 (0.29–2.83) | 0.860 | ||
| pT stage | ||||
| T1 | Reference | |||
| T2 | 2.55 (1.35–4.83) | 0.004 | 1.80 (0.94–3.44) | 0.076 |
| T3 | 6.32 (3.85–10.35) | <0.001 | 3.37 (2.00–5.69) | <0.001 |
| T4 | 10.92 (6.5–18.32) | <0.001 | 4.58 (2.59–8.07) | <0.001 |
| pN stage | ||||
| N0 | Reference | |||
| N1 | 2.15 (1.56–2.96) | <0.001 | 1.63 (1.17–2.26) | 0.004 |
| N2 | 3.16 (2.32–4.29) | <0.001 | 1.94 (1.41–2.68) | <0.001 |
| N3 | 4.73 (3.51–6.39) | <0.001 | 2.38 (1.71–3.31) | <0.001 |
| LVI/PNI | ||||
| (−) | Reference | |||
| (+) | 2.50 (2.00–3.13) | <0.001 | 1.34 (1.04–1.71) | 0.023 |
Abbreviations: ALB, albumin; BMI, body mass index; CA, carbohydrate antigen; CEA, carcinoembryonic antigen; HGB, hemoglobin; LVI, lymphovascular invasion; PNI, perineural invasion.
4. DISCUSSION
Gastric cancer is the fifth most common malignancy and the fourth leading cause of cancer‐related deaths worldwide. 1 Previous studies have discussed the role of LVI/PNI in GC; however, the results are controversial and its prognostic value is still under debate. In the current research, we found that the incidence of LVI/PNI was significantly higher in GC compared with CRC. Our results revealed that LVI/PNI was associated with deeper tumor invasion and more lymph node metastasis. Moreover, we identified that LVI/PNI was an independent prognostic factor for survival outcomes in GC.
For many malignancies, LVI and PNI have emerged as important pathologic features and they have been identified as a harbinger of poor prognosis. 7 , 8 , 11 , 12 , 13 , 14 Interestingly, when we reviewed the literature, we found that in terms of LVI/PNI incidence, GC 9 , 10 seems to rank high among various kinds of cancers, including CRC, 7 , 8 esophageal squamous cell carcinoma, 11 bladder cancer, 12 prostate cancer. 13 , 14 The current study confirmed what we surmised. Our finding demonstrated that the GC patients did have a significantly higher proportion of positive LVI/PNI than the CRC patients with the same TNM stage. Moreover, incidences of LVI/PNI in GC patients with T4, N3, and stage III diseases were up to 82.68%, 83.39%, and 76.91%, respectively. This unusual phenomenon indicated that LVI/PNI might play a critical role in the development and progression of GC.
GC is often diagnosed at an advanced stage, and LNM is the major diffusion route that predicts a worse prognosis. 2 , 3 , 4 However, the mechanism of LNM in GC is still not fully understood. In our study, 56.85% (846/1488) GC patients were diagnosed with lymph node metastasis, and incidences of LVI/PNI in patients with stage N0, N1, N2, and N3 GC were 28.97%, 50.17%, 68.25%, and 83.39%, respectively. Our study also found that 75.27% of the 752 patients with LVI/PNI had lymph node metastasis, and LVI/PNI was an independent risk factor for LNM in GC. One possible explanation is that the migration of cancer cells into vessels is an early step for nodal or distant metastasis. 26 It is also reasonable to assume that the presence of LVI/PNI may indicate a more malignant phenotype with stronger invasiveness. Although the role of LVI/PNI in the occurrence of LNM is still unknown, our results suggest that occult metastasis should be suspected and close follow‐up should be considered when LVI/PNI is detected.
Prognostic value of LVI/PNI in GC is still under debate. Some researchers argued that LVI/PNI was not an independent prognostic factor despite its strong association with disease progression, including larger size of the neoplasm, deeper tumor invasion, more lymph node metastasis, and more distant metastasis. 9 , 19 , 20 , 21 , 22 , 23 , 24 Conversely, other studies have identified LVI/PNI as an independent risk factor for survival outcome of patients with GC. 10 , 15 , 16 , 17 , 18 Our results from the Kaplan–Meier analysis indicated that the OS rate was significantly worse in the LVI/PNI‐positive group than in the LVI/PNI‐negative group. Multivariate Cox regression model further identified LVI/PNI as an independent prognostic factor, which disproves the belief that the poor prognosis of the LVI/PNI‐positive GC patients is due to its close association with other established prognostic factors.
The major limitation of the current research is its design. It was a retrospective study from a single institution. Only patients with stage I–III GC who had curative gastrectomy were included in the study, and among them, patients with incomplete data were excluded. All of these can cause bias in the results. In this study, we aimed to demonstrate that GC had an unusually high incidence of LVI/PNI. Due to our limited research conditions, we only compared incidence of LVI/PNI of GC with that of CRC based on the Cancer Database of our hospital. As a supplement, we compared incidence of LVI/PNI of GC with that of other cancers reported by previous works of literature. And the collection time of GC patients and CRC patients was different. These factors might weaken the conclusion. In addition, chemotherapy regimen of patients, including data of neoadjuvant chemotherapy, could not be accurately tracked in the current cancer database and thus was not analyzed, which may have an impact on the survival outcomes in patients with advanced disease.
5. CONCLUSION
In conclusion, LVI/PNI is an underreported phenomenon in GC. Our study demonstrated that GC had a high incidence of LVI/PNI, which was closely associated with disease progression. LVI/PNI was identified as an independent risk factor for LNM and the prognosis of patients with stage I–III GC. These findings will be helpful to predict survival outcomes more accurately and establish individualized treatment plans.
AUTHOR CONTRIBUTIONS
Fengxiang Zhang: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); project administration (equal); resources (equal); software (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Huaxian Chen: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); project administration (equal); resources (equal); software (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Dandong Luo: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); project administration (equal); resources (equal); software (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Zhizhong Xiong: Data curation (supporting); methodology (supporting); software (supporting); writing – review and editing (supporting). Xianzhe Li: Data curation (supporting); methodology (supporting); software (supporting); writing – review and editing (supporting). Shi Yin: Data curation (supporting); methodology (supporting); software (supporting); writing – review and editing (supporting). Longyang Jin: Data curation (supporting); methodology (supporting); software (supporting); writing – review and editing (supporting). Shi Chen: Conceptualization (equal); funding acquisition (equal); resources (equal); supervision (equal). Junsheng Peng: Conceptualization (equal); funding acquisition (equal); resources (equal); supervision (equal). Lei Lian: Conceptualization (equal); funding acquisition (equal); resources (equal); supervision (equal).
FUNDING INFORMATION
This study was supported by the Guangdong Natural Science Fund for Outstanding Youth Scholars (Grant No. 2020B151502067) and National Key Clinical Discipline.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest regarding the content of the article.
ETHICS STATEMENT
The current research complies with the standards of the Declaration of Helsinki. Ethical Committee of The Sixth Affiliated Hospital, Sun Yat‐sen University had approved this retrospective research (No. 2022ZSLYEC‐232), and the requirement for written informed consent was waived.
ACKNOWLEDGMENTS
We thank all the staff of the Cancer Database of the Sixth Affiliated Hospital, Sun Yat‐sen University.
Zhang F, Chen H, Luo D, et al. Lymphovascular or perineural invasion is associated with lymph node metastasis and survival outcomes in patients with gastric cancer. Cancer Med. 2023;12:9401‐9408. doi: 10.1002/cam4.5701
Fengxiang Zhang, Huaxian Chen, and Dandong Luo contributed equally to this work.
DATA AVAILABILITY STATEMENT
All analyzed data are included in this article. The original data are available upon reasonable request to the corresponding author.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. [DOI] [PubMed] [Google Scholar]
- 2. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396(10251):635‐648. [DOI] [PubMed] [Google Scholar]
- 3. Wang FH, Zhang XT, Li YF, et al. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond). 2021;41(8):747‐795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ajani JA, D'Amico TA, Bentrem DJ, et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(2):167‐192. [DOI] [PubMed] [Google Scholar]
- 5. Dicken BJ, Graham K, Hamilton SM, et al. Lymphovascular invasion is associated with poor survival in gastric cancer: an application of gene‐expression and tissue array techniques. Ann Surg. 2006;243(1):64‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: a review of the literature. Cancer. 2009;115(15):3379‐3391. [DOI] [PubMed] [Google Scholar]
- 7. Skancke M, Arnott SM, Amdur RL, Siegel RS, Obias VJ, Umapathi BA. Lymphovascular invasion and perineural invasion negatively impact overall survival for stage II adenocarcinoma of the colon. Dis Colon Rectum. 2019;62(2):181‐188. [DOI] [PubMed] [Google Scholar]
- 8. Poeschl EM, Pollheimer MJ, Kornprat P, et al. Perineural invasion: correlation with aggressive phenotype and independent prognostic variable in both colon and rectum cancer. J Clin Oncol. 2010;28(21):e358‐e360. author reply e361‐352. [DOI] [PubMed] [Google Scholar]
- 9. Duraker N, Sişman S, Can G. The significance of perineural invasion as a prognostic factor in patients with gastric carcinoma. Surg Today. 2003;33(2):95‐100. [DOI] [PubMed] [Google Scholar]
- 10. Hwang JE, Hong JY, Kim JE, et al. Prognostic significance of the concomitant existence of lymphovascular and perineural invasion in locally advanced gastric cancer patients who underwent curative gastrectomy and adjuvant chemotherapy. Jpn J Clin Oncol. 2015;45(6):541‐546. [DOI] [PubMed] [Google Scholar]
- 11. Wu J, Chen QX. Prognostic and predictive significance of tumor length in patients with esophageal squamous cell carcinoma undergoing radical resection. BMC Cancer. 2016;16:394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Muppa P, Gupta S, Frank I, et al. Prognostic significance of lymphatic, vascular and perineural invasion for bladder cancer patients treated by radical cystectomy. Pathology. 2017;49(3):259‐266. [DOI] [PubMed] [Google Scholar]
- 13. Zareba P, Flavin R, Isikbay M, et al. Perineural invasion and risk of lethal prostate cancer. Cancer Epidemiol Biomarkers Prev. 2017;26(5):719‐726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saeter T, Vlatkovic L, Waaler G, et al. Combining lymphovascular invasion with reactive stromal grade predicts prostate cancer mortality. Prostate. 2016;76(12):1088‐1094. [DOI] [PubMed] [Google Scholar]
- 15. Lu J, Dai Y, Xie JW, et al. Combination of lymphovascular invasion and the AJCC TNM staging system improves prediction of prognosis in N0 stage gastric cancer: results from a high‐volume institution. BMC Cancer. 2019;19(1):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao B, Huang X, Zhang J, et al. Clinicopathologic factors associated with recurrence and long‐term survival in node‐negative advanced gastric cancer patients. Rev Esp Enferm Dig. 2019;111(2):111‐120. [DOI] [PubMed] [Google Scholar]
- 17. Kunisaki C, Makino H, Kimura J, et al. Impact of lymphovascular invasion in patients with stage I gastric cancer. Surgery. 2010;147(2):204‐211. [DOI] [PubMed] [Google Scholar]
- 18. Bilici A, Seker M, Ustaalioglu BB, et al. Prognostic significance of perineural invasion in patients with gastric cancer who underwent curative resection. Ann Surg Oncol. 2010;17(8):2037‐2044. [DOI] [PubMed] [Google Scholar]
- 19. Chiaravalli AM, Cornaggia M, Furlan D, et al. The role of histological investigation in prognostic evaluation of advanced gastric cancer. Analysis of histological structure and molecular changes compared with invasive pattern and stage. Virchows Arch. 2001;439(2):158‐169. [DOI] [PubMed] [Google Scholar]
- 20. Ryu WS, Kim JH, Jang YJ, et al. Expression of estrogen receptors in gastric cancer and their clinical significance. J Surg Oncol. 2012;106(4):456‐461. [DOI] [PubMed] [Google Scholar]
- 21. De Franco L, Marrelli D, Voglino C, et al. Prognostic value of perineural invasion in resected gastric cancer patients according to Lauren histotype. Pathol Oncol Res. 2018;24(2):393‐400. [DOI] [PubMed] [Google Scholar]
- 22. Kwon KJ, Shim KN, Song EM, et al. Clinicopathological characteristics and prognosis of signet ring cell carcinoma of the stomach. Gastric Cancer. 2014;17(1):43‐53. [DOI] [PubMed] [Google Scholar]
- 23. Liu E, Zhong M, Xu F, et al. Impact of lymphatic vessel invasion on survival in curative resected gastric cancer. J Gastrointest Surg. 2011;15(9):1526‐1531. [DOI] [PubMed] [Google Scholar]
- 24. Kim JH, Park SS, Park SH, et al. Clinical significance of immunohistochemically‐identified lymphatic and/or blood vessel tumor invasion in gastric cancer. J Surg Res. 2010;162(2):177‐183. [DOI] [PubMed] [Google Scholar]
- 25. Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17(12):3077‐3079. [DOI] [PubMed] [Google Scholar]
- 26. Kang YJ, Kim HS, Jang WS, et al. Impact of lymphovascular invasion on lymph node metastasis for patients undergoing radical prostatectomy with negative resection margin. BMC Cancer. 2017;17(1):321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All analyzed data are included in this article. The original data are available upon reasonable request to the corresponding author.


