Abstract
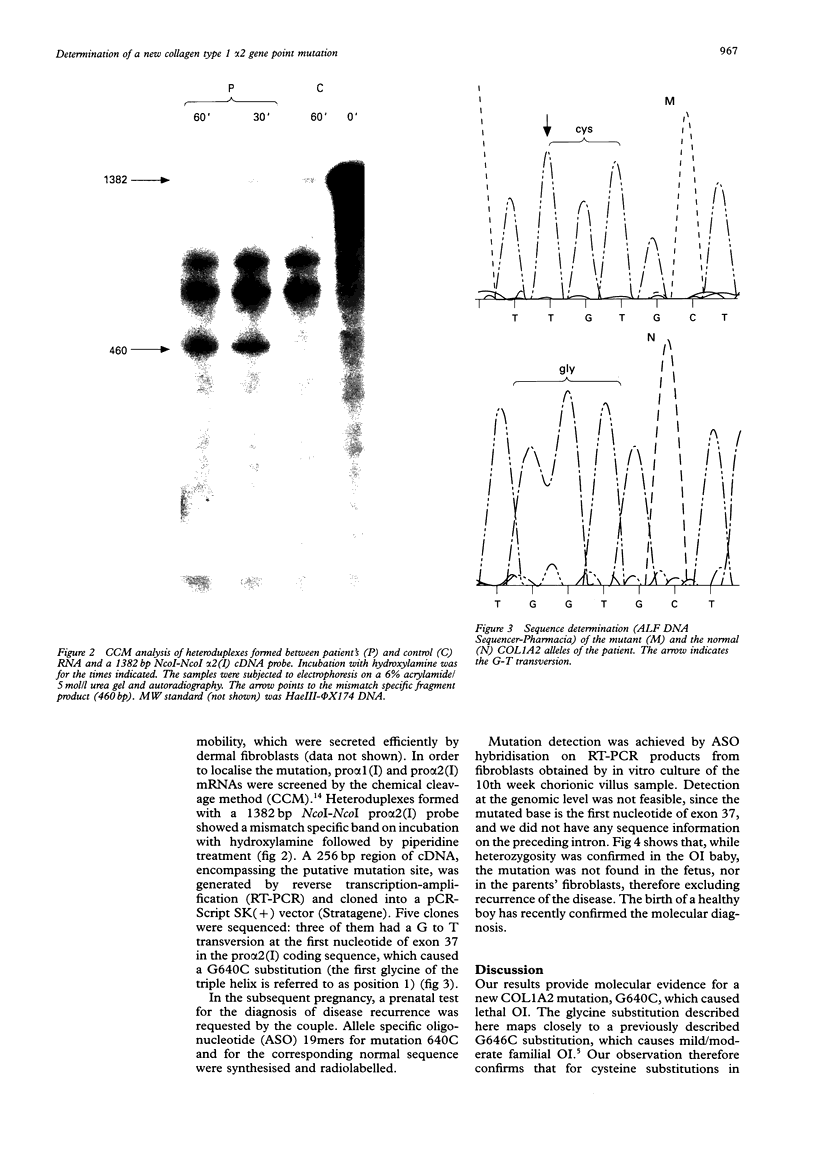
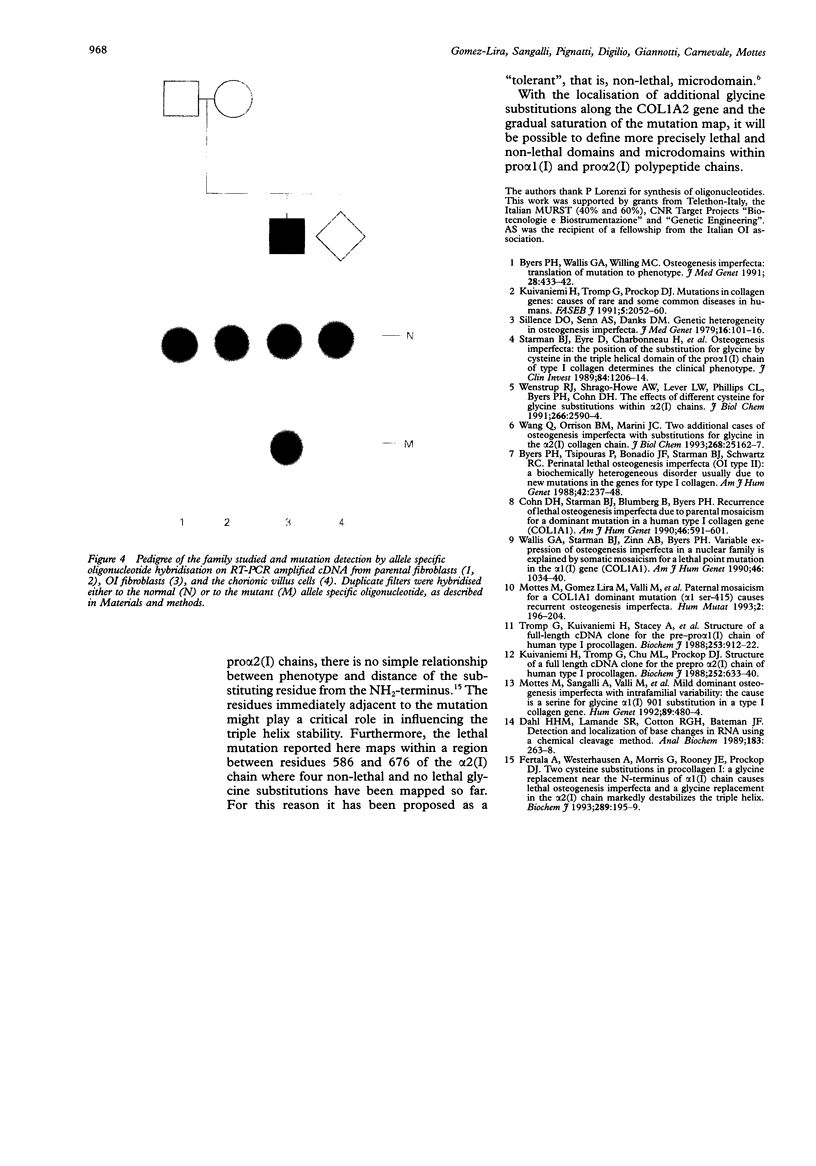
The molecular defect responsible for a sporadic case of extremely severe (type II/III) osteogenesis imperfecta was investigated. The mutation site was localised in the collagen type I pro alpha 2 mRNA molecules produced by the proband's skin fibroblasts by chemical cleavage of mismatch in heteroduplex nucleic acids. Reverse transcription-polymerase chain reaction DNA amplification, followed by cloning and sequencing, showed heterozygosity for a G to T transversion in the first nucleotide of exon 37 of the COL1A2 gene, which led to a cysteine for glycine substitution at position 640 of the triple helical domain. This newly characterised mutation is localised in a domain which contains several milder mutations, confirming that glycine substitutions within the alpha 2(I) chain do not follow a linear gradient pattern for genotype to phenotype correlations. In a subsequent pregnancy, absence of the G2327T mutation in the fetus was shown by allele specific oligonucleotide hybridisation to the trophoblast derived fibroblast mRNA after reverse transcription and in vitro amplification. (The nucleotide number assigned to the mutant base was inferred from the numbering system devised by the Osteogenesis Imperfecta Analysis Consortium (The OIAC Newsletter, 1 April 1994).)
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byers P. H., Tsipouras P., Bonadio J. F., Starman B. J., Schwartz R. C. Perinatal lethal osteogenesis imperfecta (OI type II): a biochemically heterogeneous disorder usually due to new mutations in the genes for type I collagen. Am J Hum Genet. 1988 Feb;42(2):237–248. [PMC free article] [PubMed] [Google Scholar]
- Byers P. H., Wallis G. A., Willing M. C. Osteogenesis imperfecta: translation of mutation to phenotype. J Med Genet. 1991 Jul;28(7):433–442. doi: 10.1136/jmg.28.7.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn D. H., Starman B. J., Blumberg B., Byers P. H. Recurrence of lethal osteogenesis imperfecta due to parental mosaicism for a dominant mutation in a human type I collagen gene (COL1A1). Am J Hum Genet. 1990 Mar;46(3):591–601. [PMC free article] [PubMed] [Google Scholar]
- Dahl H. H., Lamande S. R., Cotton R. G., Bateman J. F. Detection and localization of base changes in RNA using a chemical cleavage method. Anal Biochem. 1989 Dec;183(2):263–268. doi: 10.1016/0003-2697(89)90477-6. [DOI] [PubMed] [Google Scholar]
- Fertala A., Westerhausen A., Morris G., Rooney J. E., Prockop D. J. Two cysteine substitutions in procollagen I: a glycine replacement near the N-terminus of alpha 1(I) chain causes lethal osteogenesis imperfecta and a glycine replacement in the alpha 2(I) chain markedly destabilizes the triple helix. Biochem J. 1993 Jan 1;289(Pt 1):195–199. doi: 10.1042/bj2890195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuivaniemi H., Tromp G., Chu M. L., Prockop D. J. Structure of a full-length cDNA clone for the prepro alpha 2(I) chain of human type I procollagen. Comparison with the chicken gene confirms unusual patterns of gene conservation. Biochem J. 1988 Jun 15;252(3):633–640. doi: 10.1042/bj2520633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuivaniemi H., Tromp G., Prockop D. J. Mutations in collagen genes: causes of rare and some common diseases in humans. FASEB J. 1991 Apr;5(7):2052–2060. doi: 10.1096/fasebj.5.7.2010058. [DOI] [PubMed] [Google Scholar]
- Mottes M., Gomez Lira M. M., Valli M., Scarano G., Lonardo F., Forlino A., Cetta G., Pignatti P. F. Paternal mosaicism for a COL1A1 dominant mutation (alpha 1 Ser-415) causes recurrent osteogenesis imperfecta. Hum Mutat. 1993;2(3):196–204. doi: 10.1002/humu.1380020308. [DOI] [PubMed] [Google Scholar]
- Mottes M., Sangalli A., Valli M., Gomez Lira M., Tenni R., Buttitta P., Pignatti P. F., Cetta G. Mild dominant osteogenesis imperfecta with intrafamilial variability: the cause is a serine for glycine alpha 1(I) 901 substitution in a type-I collagen gene. Hum Genet. 1992 Jul;89(5):480–484. doi: 10.1007/BF00219169. [DOI] [PubMed] [Google Scholar]
- Sillence D. O., Senn A., Danks D. M. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979 Apr;16(2):101–116. doi: 10.1136/jmg.16.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starman B. J., Eyre D., Charbonneau H., Harrylock M., Weis M. A., Weiss L., Graham J. M., Jr, Byers P. H. Osteogenesis imperfecta. The position of substitution for glycine by cysteine in the triple helical domain of the pro alpha 1(I) chains of type I collagen determines the clinical phenotype. J Clin Invest. 1989 Oct;84(4):1206–1214. doi: 10.1172/JCI114286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis G. A., Starman B. J., Zinn A. B., Byers P. H. Variable expression of osteogenesis imperfecta in a nuclear family is explained by somatic mosaicism for a lethal point mutation in the alpha 1(I) gene (COL1A1) of type I collagen in a parent. Am J Hum Genet. 1990 Jun;46(6):1034–1040. [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Orrison B. M., Marini J. C. Two additional cases of osteogenesis imperfecta with substitutions for glycine in the alpha 2(I) collagen chain. A regional model relating mutation location with phenotype. J Biol Chem. 1993 Nov 25;268(33):25162–25167. [PubMed] [Google Scholar]
- Wenstrup R. J., Shrago-Howe A. W., Lever L. W., Phillips C. L., Byers P. H., Cohn D. H. The effects of different cysteine for glycine substitutions within alpha 2(I) chains. Evidence of distinct structural domains within the type I collagen triple helix. J Biol Chem. 1991 Feb 5;266(4):2590–2594. [PubMed] [Google Scholar]
- de Moor M. M., Human D. G., Reichart B. Management of pulmonary atresia or critical pulmonary stenosis and intact ventricular septum with a small or hypoplastic right ventricle. Int J Cardiol. 1988 May;19(2):245–253. doi: 10.1016/0167-5273(88)90085-x. [DOI] [PubMed] [Google Scholar]