Abstract
The cytosolic superoxide dismutase (SOD) of Fasciola hepatica, a causative agent of fascioliasis, was purified and characterized. The enzyme consists of two identical subunits, each with an apparent molecular mass of 17.5 kDa. An analysis of the enzyme's primary structure and inhibition studies revealed that the enzyme is a copper/zinc-containing SOD (Cu/Zn-SOD). The enzyme activity was relatively stable in a broad pH range, from pH 7.0 to 10.0, and the enzyme showed maximum activity at pH 7.5. This enzyme also displayed strong antigenicity against sera of bovine and human subjects with fascioliasis. The SOD gene fragment was amplified by PCR with degenerate oligonucleotide primers derived from amino acid sequences conserved in the Cu/Zn-SODs of other organisms. An F. hepatica cDNA library was screened with the SOD gene fragment as a probe. As a result, a complete gene encoding the Cu/Zn-SOD was identified, and its nucleotide sequence was determined. The gene had an open reading frame of 438 bp and 146 deduced amino acids. Comparison of the deduced amino acid sequence of the enzyme with previously reported Cu/Zn-SOD amino acid sequences revealed considerably high homologies. The coding region of the F. hepatica Cu/Zn-SOD was cloned and expressed in Escherichia coli. Staining of native polyacrylamide gel for SOD activity of the expressed protein revealed SOD activity that was inactivated by potassium cyanide and hydrogen peroxide but not by sodium azide. This means that the presence of the recombinant fusion protein is indicative of Cu/Zn-SOD. The expressed protein also reacted with sera of bovine and human subjects with fascioliasis, but it did not react with sera of uninfected bovine and human subjects.
Worldwide, Fasciola hepatica, a liver fluke, is a causative agent of fascioliasis in mammals (11, 34, 49), including humans (2, 15, 38). Following ingestion of metacercaria by the hosts, the juvenile worms burrow through the host gut walls and migrate to the liver, where they cause extensive damage before moving into the bile ducts. Finally, the parasites pass the bile duct walls and develop into mature forms that live in the microenvironment of the bile ducts. Although the adult worms have a predominantly anaerobic metabolism and inhabit in the bile duct, where the oxygen tension is relatively low (58), oxygen is still required for other functions such as egg generation, which generate reactive oxygen species (59). In addition to this normal confrontation with oxidative stress, the parasite is exposed to reactive oxygen species generated by host effector cells such as macrophages, eosinophils, neutrophils, and platelets (3, 37).
Free oxygen radicals generated by these effector cells via the oxidative burst are thought to contribute to the killing of parasites by hosts (37, 42, 44). To defend themselves against oxygen-mediated killing mechanisms of hosts, parasites have developed antioxidant enzyme systems. It has been suggested that antioxidant suppression of host oxidative killing may play a protective role in the parasite life cycle (9). Therefore, antioxidant enzymes have been considered important virulence factors in a number of parasites (16, 33, 40, 43).
Prominent among antioxidants are superoxide dismutases (SODs), which catalyze the decomposition of superoxide, the first reactive species in the reduction of molecular oxygen into hydrogen peroxide and molecular oxygen (4, 7, 18, 19). SODs are postulated to play a role in the protection of parasites against the cellular, oxygen-mediated killing mechanisms of the hosts (10, 21, 23, 26, 27, 29).
Up to now, SODs have been characterized and cloned from various helminth parasites of different species such as Schistosoma mansoni, Onchocerca volvulus, Dirofilaria immitis, and Brugia pahangi (10, 21, 22, 25, 27, 29, 56). In these parasites, SODs have been found to be surface-located or secreted (23, 25, 26, 29, 56). This has led to the hypothesis that the enzymes play a special defensive role for the parasite at the interface of the host-parasite interaction. Like the above-mentioned parasites, F. hepatica has developed SODs to defend itself from oxidative killing mechanisms of the host (48). However, at present there is little information indicating that F. hepatica SOD is associated with the pathogenicity of the parasite. Therefore, in order to elucidate a possible role of the SOD of F. hepatica in the defense against the oxygen-dependent killing mechanisms of the host, we carried out the purification and characterization of cytosolic SOD from F. hepatica, and we cloned the gene and functionally expressed it in E. coli.
MATERIALS AND METHODS
Collection of parasite.
F. hepatica adult worms were obtained from the liver of an infected bovine. The collected worms were washed several times with phosphate-buffered saline (PBS [pH 7.4]) and then incubated in the same buffer at 37°C for 3 h to eliminate any residual host matter. After the parasites were washed with PBS several times, they were stored at −70°C until used.
Preparation of parasite extract.
F. hepatica adult worms were homogenized with a Teflon homogenizer in PBS supplemented with 1 mM phenylmethylsulfonyl fluoride, 10 mM iodoacetic acid, and 10 mM leupeptin. The homogenates were centrifuged at 28,000 × g for 30 min at 4°C, and the supernatants were collected and used for further studies. The protein concentration was determined by the method of Lowry et al. (35), with bovine serum albumin as a standard.
Purification of SOD.
F. hepatica extract was applied to a diethylaminoethyl (DEAE)-Sephacel column (1.6 by 12 cm; Pharmacia, Uppsala, Sweden) equilibrated with 50 mM sodium phosphate buffer (pH 7.5). The column was extensively washed with the same buffer, and the absorbed proteins were eluted with a linear gradient of 0.5 M NaCl. Fractions exhibiting SOD activity were pooled and purified further by successive carboxymethyl (CM) Sepharose Fast Flow chromatography (1.6- by 12-cm column; Pharmacia), equilibrated with 0.1 M sodium acetate buffer (pH 5.5), and eluted with a linear gradient of 0.5 M NaCl. Fractions exhibiting SOD activity were collected, concentrated, and applied to Superose 12 molecular-sieve chromatography columns (1.6 by 30 cm; Pharmacia) equilibrated with 50 mM sodium phosphate buffer (pH 7.5) containing 0.15 M NaCl. Active fractions were collected, concentrated, and applied to Mono-Q ion-exchange chromatography columns (0.5 by 5 cm; Pharmacia) equilibrated with 10 mM Tris-HCl buffer (pH 7.0). After extensive washing with the same buffer, absorbed proteins were eluted with a linear gradient of 0.5 M NaCl. All the purification procedures were performed at 4°C.
Enzyme assay.
SOD activity was determined by the neotetrazolium chloride (NTC) reduction assay based on the method of Noridaka et al. (45). The assay mixtures (0.5 ml) contained 50 μl of 0.5 M sodium phosphate (pH 7.5), 25 μl of 16% Triton X-100, 2.5 μl of 10 mM EDTA, 75 μl of 1.2 mM NTC, 2.5 μl of xanthine oxidase (1.0 U), 10 μl of sample, 25 μl of 4 mM hypoxanthine, and distilled water. The A540 was monitored with a spectrophotometer (model DU-600; Beckman, Palo Alto, Calif.) after the addition of 0.5 ml of a solution containing 1 M formate buffer (pH 3.5), 10% Triton X-100, and 40% formaldehyde. One unit of enzyme activity was defined as the amount of the enzyme required to cause 50% inhibition in the rate of reduction of NTC under the conditions of the assay.
Polyacrylamide gel electrophoresis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed by the method of Laemmli (31). Native polyacrylamide gel electrophoresis was performed at 4°C in the absence of SDS. Gels were stained with Coomassie brilliant blue and destained.
Staining for SOD activity.
To identify the SOD activity on the native gel, the gel was stained by the riboflavin-nitroblue tetrazolium method (6). In brief, the gels were soaked simultaneously in a solution of 0.2% nitroblue tetrazolium, 0.028 M N,N,N′,N′-tetramethylethylenediamine (TEMED), and 2.8 × 10−5 M riboflavin in 50 mM potassium phosphate buffer (pH 7.8) for 30 min at room temperature. After that, the gels were illuminated until chromatic zones indicating SOD activity were visible in a uniformly blue background.
Determination of molecular weight of purified enzyme.
Apparent molecular weight of the purified enzyme was determined by SDS-PAGE as described above. The molecular weight standard proteins were phosphorylase b (94,000), bovine serum albumin (67,000), ovalbumin (43,000), carbonic anhydrase (30,000), soybean trypsin inhibitor (20,100), and α-lactalbumin (14,400) (Pharmacia). The native molecular weight of the enzyme was determined by molecular-sieve chromatography with a Superose 12 column.
Determination of the metallic cofactor of SOD.
To determine the types of SOD on the native gel, the purified enzyme was mixed with potassium cyanide (KCN; 3 and 6 mM), sodium azide (NaN3; 5 and 10 mM), or hydrogen peroxide (H2O2; 5 and 10 mM) and incubated at 37°C for 30 min. After incubation, the enzyme activity in each sample was assayed as described above.
Effect of pH on SOD activity.
To determine the optimal pH value for the SOD activity, the enzyme activity was measured by standard assay method, with 50 mM sodium phosphate buffers (pH 6.0 to 7.0), 50 mM Tris-HCl buffers (pH 8.0 to 9.0), or 50 mM glycine-NaOH buffers (pH 10.0 to 11.0) instead of standard buffer. Following 30 min of incubation at 37°C, the SOD activity was measured and compared.
N-terminal amino acid sequencing.
The purified enzymes (10 μg) were subjected to SDS–10% polyacrylamide gel electrophoresis and then electroblotted onto polyvinylidene difluoride membrane as described previously (39). Blots were briefly stained with Coomassie brilliant blue and destained, and the stained protein was excised and subjected to N-terminal amino acid sequencing with a MilliGen/Biosearch 6600 Prosequence system (Millipore, Bedford, Mass.).
Western blot analysis.
After SDS-PAGE, the proteins were electrotransferred from the gel to nitrocellulose membrane (0.45 μm; Bio-Rad) by the method of Towbin et al. (60). After transfer, the membrane was blocked in PBST (0.05% Tween 20 in PBS) containing 3% skim milk for 1 h at room temperature and then incubated with diluted (1:1,000) sera of bovine or human subjects with fascioliasis, paragonimiasis, and clonorchiasis or sera from healthy sera controls for 2 h at room temperature. After being washed three times with PBST, the membrane was incubated with peroxidase-conjugated anti-human immunoglobulin G (IgG) or peroxidase-conjugated anti-bovine IgG (Sigma) for 2 h at room temperature. After an additional three washes with PBST, the membrane was incubated in a freshly prepared mixture of substrate solution (4 mg of 3,3′-diaminobenzidine per ml, 0.01% of hydrogen peroxide in 0.1 M PBS [pH 7.2]) for 10 min at room temperature. The reaction was stopped by washing the membrane with distilled water several times. Bovine serum samples were collected from F. hepatica-positive and parasite-free bovines in a slaughterhouse in order to get the positive and negative controls. Human F. hepatica-positive sera were collected from three fascioliasis patients.
mRNA purification.
F. hepatica adult worms were homogenized in guanidium thiocyanate and layered on a CsCl step gradient, and total RNA was extracted by a method described previously (12). mRNA was selected by oligo(dT) chromatography (23).
Construction of cDNA library.
cDNA library of F. hepatica was constructed using the Librarian Express cDNA library kit (Invitrogen, San Diego, Calif.). The pcDNA3.1+ vector was linearized with BstXI and NotI enzymes. The first strand of cDNA was synthesized with avian myeloblastosis virus reverse transcriptase from mRNA using an oligo(dT) primer that contained a NotI restriction site. After the second strand of cDNA was synthesized, BstXI/EcoRI adapters were ligated to the double-stranded cDNA. This cDNA was then trimmed with NotI enzyme to produce cDNA with BstXI-NotI ends. All of this cDNA was then run out on a low-melt agarose gel with molecular weight markers. The cDNA with a size of >500 bp was cut out from the gel and recovered from the agarose. This size-enriched cDNA was then ligated into the linearized pcDNA3.1+ plasmid. The ligated plasmid-cDNA was then transformed into E. coli TOP10F′ (Invitrogen) and amplified by overnight growth on ampicillin plates. These plates were scraped and pooled into a glycerol stock that was aliquoted, and stored at −70°C. The number of primary recombinants in the library was determined to be 1.38 × 106 by serial dilution of the unamplified library. Restriction analysis of 10 clones showed that 10 out of 10 contained inserts. The inserts had the sizes listed above, and the average size of the 10 clones was 0.74 kb.
PCR.
Two degenerate oligonucleotide primers were designed based on conserved amino acids of copper/zinc-containing (Cu/Zn-SODs) from various eukaryotic organisms. The sequence of the forward primer (primer 1) was 5′-GC(T/G)GG(A/T)(G/C)C(T/G)CATTT(T/C)AATCC-3′, and the sequence of the reverse primer (primer 2) was 5′-CC(A/G)CA(A/T)GC(A/T)A(A/C)ACGA(G/C)(G/C)ACCAGCATT-3′. Using the two primers, PCR analysis was performed on an F. hepatica cDNA library to check the presence of SOD cDNA sequence. Two microliters of F. hepatica library was heated at 90°C for 5 min to denature the cell and was used as the DNA template in the PCR. A 50-μl volume of a reaction mixture containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, and 1.5 mM MgCl2, a 10 μM concentration of each deoxynucleoside triphosphate, 2.5 U of Taq DNA polymerase (Boehringer Mannheim GmbH, Mannheim, Germany), and 20 pmol of each primer was subjected to 45 cycles of amplification with a Thermal Cycler (model 480; Perkin Elmer, Foster City, Calif.). Each step was done at 94°C for 1 min in denaturation, 50°C for 2 min in annealing, and 72°C for 2 min in extension. The amplified product was purified from the gel, ligated into pCR2.1 vector, and transformed into competent E. coli INVα F′ cells, using an Original TA cloning kit (Invitrogen).
Screening of F. hepatica cDNA library.
The cDNA library was screened by a standard colony hybridization assay using the PCR product amplified with degenerate oligonucleotide primers as a probe. The probe was labeled to a specific activity of >109 cpm/μg with [α-32P]dCTP (Amersham, Arlington Heights, Ill.) by random priming method (16). A total of 106 CFU of F. hepatica cDNA library was plated, and the cells were transferred onto a nylon membrane (0.2-μm pore size; Amersham). The transferred cells were hybridized with probe in a standard buffer solution at 50°C for 16 h. Membranes were washed two times for 15 min each time with a solution containing 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% (wt/vol) SDS at 68°C and washed twice for 15 min each time with maleic acid buffer solution after being subject to reaction with 1× blocking solution for 30 min. After that, additional washings were carried out for 20 min each in 2×, 1×, and 0.5× SSC solution. Ten positive clones were identified, and two of them that yielded larger sizes were subjected to a second round of screening to isolate larger cDNAs. Finally, one F. hepatica Cu/Zn-SOD cDNA was identified and sequenced. The cDNA contained the complete coding region and 5′ and 3′ untranslated regions.
DNA sequencing and sequence analysis.
The nucleotide sequence of the cloned gene was determined by the dideoxynucleotide chain termination method (53) using the Sequenase version 2.0 DNA sequencing kit (Amersham) and also by using the ABI PRISM Dye Terminator Cycle Sequencing Ready Reaction kit (Perkin Elmer, Beaconsfield, United Kingdom), following the manufacturer's instruction.
Northern blot analysis.
Total RNA was purified from adult worms of F. hepatica by the method described earlier. Northern blot analysis was performed by standard procedure (51). The purified RNA (10 μg) was separated on a 1% agarose gel containing 0.67 M formaldehyde and transferred onto a Hybond-N nylon membrane (Amersham). The membrane was prehybridized and hybridized using an F. hepatica SOD cDNA labeled with 32P by random-priming method as a probe (17).
Expression and purification of the fusion protein.
F. hepatica SOD protein was expressed in E. coli with prokaryotic expression vector pGEX-4T-2 (Pharmacia), which contains an isopropyl-β-d-thiogalactoside (IPTG)-inducible tac promotor, the T5 promotor transcription-translation system and a glutathione S-transferase (GST) coding sequence. The whole coding region of F. hepatica Cu/Zn-SOD cDNA was amplified by PCR, using forward primer CP1 (5′-GGATCCATGTCGGGTTCCAGTGGC-3′), which contains a BamHI site upstream of the start codon, and reverse primer CP2, (5′-GAATTCTTATTCCGTCAGACCAATTAC-3′), which contains an EcoRI site downstream of the stop codon. The CP1 and CP2 primers were designed such that the ATG and TAA of the amplified product would be in frame with the GST. The PCR product was purified, ligated into pCR2.1 vector, and transformed into competent E. coli INVα F′ (Invitrogen) again. After purification of plasmid DNA, the nucleotide sequence of insert was confirmed by sequencing. The plasmid DNA was digested with BamHI and EcoRI and ligated to pGEX-4T-2 vector predigested with the same enzymes using standard techniques (51). The resulting plasmid (named pGEX/FhSOD) was transformed into competent E. coli BL-21 cells (Pharmacia) and spread on Luria-Bertani agar plates containing 100 μg of ampicillin per ml. The expression of fusion protein was performed by adding IPTG to a final concentration of 1 mM, and the fusion protein was purified with a glutathione-Sepharose 4B column (Pharmacia). To cleave the fusion protein from the GST carrier, the protein was incubated with thrombin (Sigma), 1:500 (wt/wt), in cleavage buffer (50 mM Tris-HCl [pH 8.0], 100 mM NaCl, 2.5 mM CaCl2, 0.1% β-mercaptoethanol) for 4 h at room temperature.
Nucleotide sequence accession number.
F. hepatica Cu/Zn-SOD cDNA has been assigned EMBL/GenBank accession number AF071229.
RESULTS
Purification of SOD.
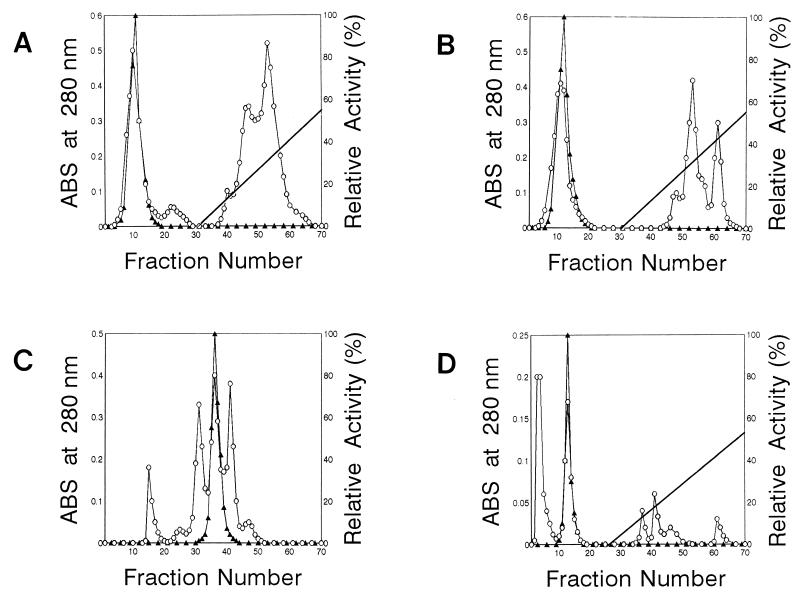
When extract of F. hepatica was subjected to DEAE-Sephacel ion-exchange chromatography, one SOD-active peak was detected (Fig. 1A). These fractions were collected, desalted, and concentrated. The enzyme was purified further by successive CM Sepharose Fast Flow ion-exchange chromatography, Superose 12 molecular-sieve chromatography, and Mono-Q ion-exchange chromatography (Fig. 1B, C, and D). The purified enzyme was found to have a molecular mass of approximately 17.5 kDa on SDS-PAGE (Fig. 2). The native molecular mass of the enzyme was 34 kDa when estimated by Superose 12 molecular-sieve chromatography (data not shown). This indicates that the enzyme has dimeric structure consisted of two identical subunits. Table 1 summarizes the purification of the SOD. SOD was purified to a specific activity of about 727.1 U/mg protein, with a yield of 14.6%.
FIG. 1.
Purification profiles of SOD from F. hepatica. (A) DEAE-Sephacel ion-exchange chromatography; (B) CM Sepharose Fast Flow ion-exchange chromatography; (C) Superose 12 molecular sieve chromatography, (D) Mono-Q ion-exchange chromatography. ▴, SOD activity; ○, protein concentration. The straight line represents a 0 to 0.5 M NaCl gradient. For detailed explanations, see Materials and Methods. ABS, absorbance.
FIG. 2.
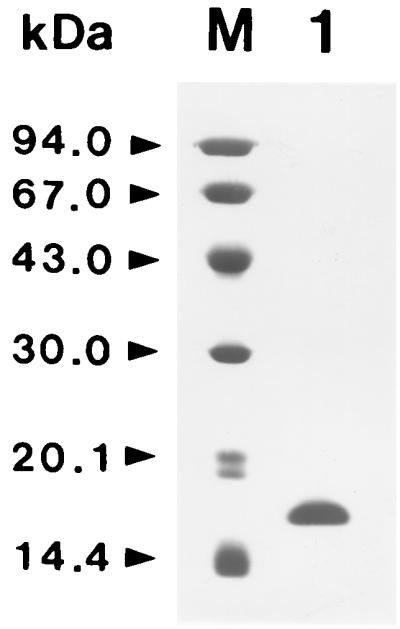
SDS-PAGE analysis of purified F. hepatica Cu/Zn-SOD. Lane M, molecular weight marker proteins; lane 1, purified F. hepatica Cu/Zn-SOD.
TABLE 1.
Purification scheme of F. hepatica SOD
| Step | Total protein (mg) | Total activity (U)a | Specific activity (U/mg)a | Purification (fold) | Yield (%) |
|---|---|---|---|---|---|
| Crude extract | 920.0 | 5,980.0 | 6.5 | 1.0 | 100.0 |
| DEAE-Sephacelb | 349.8 | 3,818.2 | 10.9 | 1.7 | 63.8 |
| CM Sepharosec | 100.8 | 2,666.4 | 26.5 | 4.1 | 44.6 |
| Superose 12d | 13.5 | 1,790.0 | 132.6 | 20.4 | 29.9 |
| Mono-Qc | 1.2 | 872.5 | 727.1 | 111.9 | 14.6 |
One unit is defined as the amount of SOD needed to cause a 50% inhibition in the rate of oxidation of neotetrazolium chloride at 37°C.
DEAE-Sephacel ion-exchange chromatography.
CM Sepharose Fast Flow ion-exchange chromatography.
Superose 12 molecular-sieve chromatography.
Mono-Q ion-exchange chromatography.
Characterization of the F. hepatica SOD.
To determine the metal cofactor of the F. hepatica SOD, studies on the inhibitors of SODs containing various cofactors (Cu/Zn, Mn, or Fe) were performed. It was inhibited by KCN and H2O2, which are both known to inhibit Cu/Zn-SOD, but not by NaN3 (Table 2). This suggests that F. hepatica SOD was Cu/Zn-SOD. The pH profile of the SOD was determined. It showed activity over a broad pH range of 7.0 to 11.0, and the maximum level of activity was at pH 7.5 (data not shown). The activities below pH 7.0 were not assayed, because the xanthine oxidase is inactive below a pH of 6.5. The N-terminal amino acid sequence of the first 12 residues of the enzyme was Met-Ser-Gly-Ser-Ser-Gly-Val-Gln-Gly-Thr-Val-Lys. The purified enzyme reacted with sera from bovine and human subjects with fascioliasis but not with sera from healthy controls (Fig. 3).
TABLE 2.
Sensitivity of the F. hepatica SOD to various inhibitors
| Inhibitor and concn | Inhibition rate (%) |
|---|---|
| KCN | |
| 3 mM | 53.3 |
| 6 mM | 98.2 |
| NaN3 | |
| 5 mM | 0 |
| 10 mM | 2.2 |
| H2O2 | |
| 3 mM | 34.7 |
| 6 mM | 51.4 |
FIG. 3.
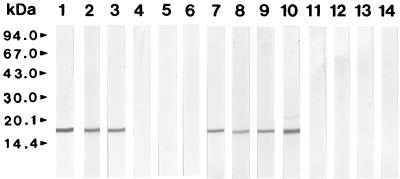
Western blot analysis of the purified enzyme with sera collected from bovine and human subjects with fascioliasis Lanes 1 to 3, sera from bovines with fascioliasis; lanes 4 to 6, sera from healthy bovine; lanes 7 to 10, sera from humans with fascioliasis; lanes 11 to 14, sera from healthy humans.
Cloning and characterization of F. hepatica Cu/Zn-SOD cDNA.
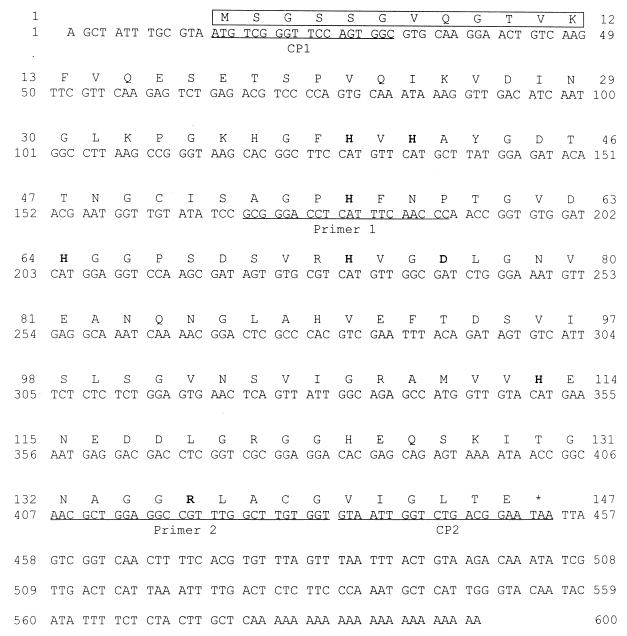
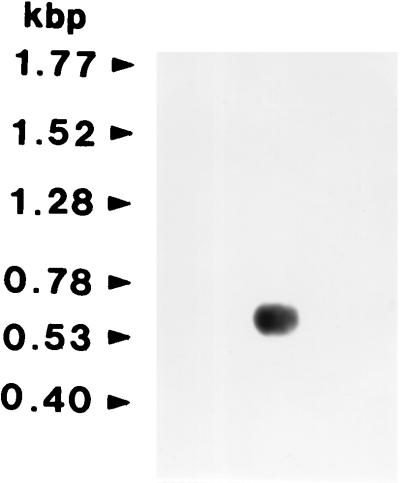
A comparison of the amino acid sequences of known Cu/Zn-SODs from various organisms revealed certain regions of the protein that are strongly conserved (4, 21, 24, 25, 29). By using these regions, we designed two degenerate oligonucleotide primers. PCR amplification of an aliquot of the F. hepatica cDNA library using these primers yielded a product with the expected size of 264 bp. Sequence analysis confirmed that the 264-bp fragment contained nucleotide and deduced amino acid sequence homologies to the Cu/Zn-SODs of the other organisms. To obtain full-length cDNA clones, we used the 264-bp product as a probe for screening of F. hepatica cDNA library. The primary screening of the cDNA library yielded 10 hybridizing colonies that consisted of mixed populations of full-size and truncated colonies. Two of the 10 colonies that yielded larger sizes were used as a probe in a second round of screening to isolate larger cDNAs. Finally, one F. hepatica cDNA was selected and sequenced. It was 600 bp and contained the complete open reading frame of 438 bp with 5′ and 3′ nontranslated regions (Fig. 4). The 3′ nontranslated region included a poly(A) tail and possessed the modified 5-TACTGAAA-3 conserved octamer-like sequence present in the 3′ nontranslated regions of the O. volvulus and S. mansoni Cu/Zn-SOD cDNAs at positions 490 to 497 (5′-TACTGTAA-3′). In Northern blot hybridization analysis, a strong hybridized signal of around 600 bp was identified (Fig. 5). This size is consistent with that expected based upon the size of the F. hepatica Cu/Zn-SOD gene.
FIG. 4.
Nucleotide and deduced amino acid sequences of F. hepatica Cu/Zn-SOD cDNA. Conserved amino acid residues for Cu and Zn binding and active site formation are shown in boldface. The positions of the primers used in this study are highlighted by underlining. Primers 1 and 2 were used to amplify the 264-bp PCR product employed for screening the cDNA library. Primers CP1 and CP2 were used for construction of the expression plasmid. The boxed amino acid residues indicate the N-terminal amino acid sequence of the purified enzyme, as determined by protein sequencing.
FIG. 5.
Northern blot analysis of F. hepatica Cu/Zn-SOD mRNA transcripts. A 10-μg quantity of poly(A)+ mRNA was electrophoresed on a 1.2% formaldehyde-agarose gel, transferred to nylon membrane and hybridized with a 32P-labeled probe. The size of the hybridizing mRNA was about 600 kb. The positions of RNA size markers are indicated on the left.
Analysis of the deduced amino acid sequence of F. hepatica Cu/Zn-SOD cDNA.
The complete F. hepatica Cu/Zn-SOD cDNA encodes a 146-amino-acid protein with a predicted molecular mass of 17,592 Da. As shown in Fig. 4, the amino acid residues known to be responsible for binding to copper/zinc (His-39, His-41, His-56, His-64, His-73, His-113, and Asp-76) were present in the sequence (4, 19, 55). The arginine residue (Arg-136) which is believed to be necessary to guide the superoxide anion to the active site (4, 19) was also present. Therefore, all of the Cu/Zn binding sites and active sites were conserved. The two cysteine residues (Cys-50 and Cys-139) which are believed to form a disulfide bond were present. Residues involved in Cu/Zn-SOD dimer formation (Gly-30, Leu-31, Gly-34, His-36, Arg-72, Gly-78, Ile-106, Leu-137, and Val-141) were also conserved (14). It had a putative N-glycosylation site at Phe-59. When the deduced amino acid sequence of the F. hepatica Cu/Zn-SOD was aligned with Cu/Zn-SODs of the other parasites for sequence homology comparison, a significant amount of sequence identity was found (Fig. 6). When gaps were used in all of the sequences to improve the alignments, the homologies were 64.8% for the F. hepatica and S. mansoni Cu/Zn-SODs, 51.6% for the F. hepatica and B. pahangi Cu/Zn-SODs, and 48.4% for the F. hepatica and O. volvulus Cu/Zn-SODs.
FIG. 6.
Alignment of the deduced amino acid sequence of Cu/Zn-SOD of F. hepatica with those of other known CU/Zn-SODs. Gaps are introduced to maximize alignment. FHSOD, F. hepatica Cu/Zn-SOD (in this study); SMSOD, S. mansoni Cu/Zn-SOD (25); BPSOD, B. pahangi Cu/Zn-SOD (56); OVSOD, O. volvulus Cu/Zn-SOD (20); HUMANSOD, human Cu/Zn-SOD (54).
Expression and purification of F. hepatica Cu/Zn-SOD.
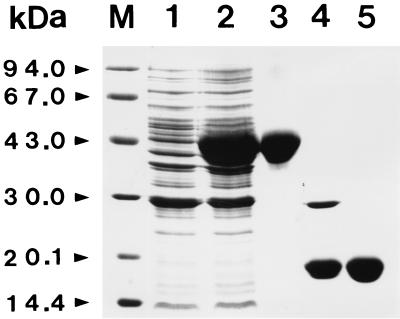
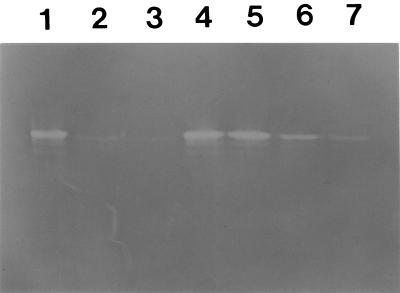
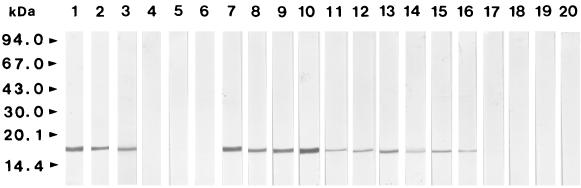
The expression of the cloned F. hepatica Cu/Zn-SOD gene was induced by adding IPTG and analyzed on SDS-PAGE followed by Coomassie blue staining. The size of the expressed protein was approximately 43 kDa, which corresponded with the predicted molecular weight of the protein, with 26 kDa being from the GST and 17.6 kDa being from F. hepatica Cu/Zn-SOD, as predicted from the cDNA (Fig. 7). F. hepatica Cu/Zn-SOD obtained from cleavage of the fusion protein with thrombin did run as 17.5 kDa. A nondenaturing PAGE analysis of the protein showed a SOD activity. The SOD activity was sensitive to KCN and H2O2 but resistant to NaN3, which is indicative of Cu/Zn-SOD (Fig. 8). The expressed protein reacted strongly to sera from bovine and human subjects with fascioliasis, but not to sera from uninfected subjects (Fig. 9). These suggested that the expressed protein might be a useful antigen for the diagnosis of fascioliasis. However, the expressed F. hepatica Cu/Zn-SOD showed cross reactivities with sera from human paragonimiasis and clonorchiasis patients. These may be due to high homologies observed in that Cu/Zn-SODs. Therefore, before F. hepatica Cu/Zn-SOD is used for diagnostic purposes in connection with fascioliasis, more specific monoclonal antibodies against the F. hepatica Cu/Zn-SOD should be made.
FIG. 7.
Expression and purification of the GST-F. hepatica SOD (FhSOD) fusion protein. Lane M, molecular weight marker proteins; lane 1, uninduced E. coli lysate; lane 2, IPTG-induced E. coli lysate; lane 3, purified GST-FhSOD fusion protein; lane 4, GST-FhSOD fusion protein after treatment of thrombin; lane 5, purified FhSOD.
FIG. 8.
Activity staining of expressed F. hepatica SOD. Lane 1, purified SOD only; lane 2, with 3 mM KCN; lane 3, with 6 mM KCN; lane 4, with 5 mM NaN3; lane 5, with 10 mM NaN3; lane 6, with 5 mM H2O2; lane 7, with 10 mM H2O2.
FIG. 9.
Western blot analysis of the expressed protein with sera of bovine and human subjects with fascioliasis. Lanes 1 to 3, sera from bovines with fascioliasis; lanes 4 to 6, sera of healthy bovines; lanes 7 to 10, sera from humans with fascioliasis; lanes 11 to 13, sera from humans with paragonimiasis; lanes 14 to 16, sera from humans with clonorchiasis; lanes 17 to 20, sera of healthy humans.
DISCUSSION
Parasites have been known to develop several possible mechanisms to circumvent the immune responses of the hosts during invasion and maintenance. One possible mechanism of the immune evasion could be the expression of antioxidant enzymes that would suppress oxidative killing by the host effector cells (9). Prominent among the antioxidant enzymes are SODs that are believed to play a major role in the antioxidant system of the parasites. Studies which demonstrate the presence of SOD activity in many parasites support the hypothesis that parasites use SOD not only in its usual function but also as a way of protecting themselves against the cellular, oxygen-mediated killing mechanisms of the hosts (10, 21, 28, 41, 52, 57).
In this study, we have characterized biochemical properties of a purified SOD from F. hepatica adult worms and cloned and expressed its gene as a prerequisite for further investigations of its possible pathophysiological role in F. hepatica pathogenesis. The enzymatic and sequence analysis of the purified F. hepatica SOD revealed characteristics and a structure similar to that of cytosolic Cu/Zn-SODs from the other species (10, 21, 25, 26, 27, 32, 41, 50, 56). It reacted with sera from bovine and human subjects with fascioliasis but not with sera from healthy controls. Recently, it has been reported that two forms of Cu/Zn-SODs were detected in F. hepatica (48). One is a 60-kDa extracellular SOD found in in vitro culture medium of F. hepatica adult worms and the other is a 32-kDa cytosolic SOD detected in a detergent-extractable fraction of the parasite. Although the enzyme that we describe here showed enzymatic characteristics similar to those of the 32-kDa cytosolic SOD, it seems unlikely that the enzyme is homologous to the 32-kDa SOD, since their molecular weights and N-terminal amino acid sequences were not matched. Therefore, it is possible to postulate that the two enzymes are isoenzymes of F. hepatica.
The N-terminal amino acid sequence and molecular weight of the purified native F. hepatica Cu/Zn-SOD correspond exactly to those of the deduced amino acid sequence, demonstrating the homogeneity of the purified enzyme and indicating that no maturation or modification of the N-terminal region is functionally necessary.
It is known that there are two forms of Cu/Zn-SODs, extracellular and cytosolic SOD, in helminth parasites which are analogous to the human SODs (21, 25, 26, 29, 48). Since superoxide anions are not able to penetrate biological membranes (36), it seems likely that extracellular SOD is more effective as a defense mechanism of parasites against oxidative killing of hosts than cytosolic SOD. Therefore, the hypothesis that extracellular SOD plays a more important protection role against oxidative killing of hosts than cytosolic SOD is acceptable. However, if the cytosolic SOD is located with surface-associated form, it also provides sufficient protection against extracellular superoxide toxicity. The purified and cloned F. hepatica Cu/Zn-SOD in this study was thought to be a cytosolic enzyme when it was determined that its N-terminal amino acid sequence did not include a signal peptide sequence. However, the fact that the cloned gene contained a potential site for N-linked glycosylation enhances the possibility that the enzyme may locate in surface-associated form. However, to more precisely define the physiological role of the enzyme, more studies on localization of the enzyme should be performed.
The whole open reading frame region of F. hepatica Cu/Zn-SOD was cloned and expressed in E. coli. As a result, we were able to produce a large quantity of a highly purified, enzymatically active protein that behaves essentially the same as the native enzyme purified from F. hepatica. It also showed strong antigenicity against the sera from bovine and human subjects with fascioliasis but not against sera from healthy controls. On the basis of these results, the enzyme will be useful for various further studies. First, it will facilitate functional and structural studies of the protein, including its crystallization. Second, it will be used for the design of the drugs that can specifically inhibit the parasite enzyme. SODs of parasites have been identified as attractive targets for chemotherapy (1, 61). Since the effective dismutation of the superoxide anion is important to the survival of the parasites, inhibition of parasite SODs would very likely lead to the parasites being more susceptible to oxidative killing by hosts. Indeed, many chemotherapeutic drugs for various parasites are based on their effects through free-radical-mediated mechanisms (13). Therefore, comparison of the inhibition characteristics of F. hepatica Cu/Zn-SOD and mammalian SODs may ultimately lead to the design of drugs for treatment of fascioliasis. Finally, that parasite SODs are recognized as antigens by infected hosts and, more notably, SODs in administered vaccines that have an immunoprotective role are ideas that have been described earlier (8, 30, 47). Furthermore, vaccination of mice with Brucella abortus SOD induced a significant level of protection against virulent B. abortus infection and Norcardia asteroides with an antibody to the extracellular SOD increased its susceptibility to killing by leukocytes (5, 46). Such observations indicate that F. hepatica Cu/Zn-SOD may also be an attractive vaccine candidate and target for immunodiagnosis of fascioliasis.
ACKNOWLEDGMENTS
This work was supported by the 2-year Basic Medical Research grant 1997-021-F0020 (1997 to 1999) from the Ministry of Education of the Republic of Korea.
We are deeply grateful to Myung-Kee Hwang for his technical assistance and to Young-Hwan Chung for collecting experimental materials in the public butcheries of Kangwon province.
REFERENCES
- 1.Asada K, Kanematsu S, Okaka S, Hayakawa T. Phylogenetic distribution of three types of superoxide dismutase in organisms and in cell organelles. In: Bannister J V, Hill H, editors. Chemical and biochemical aspects of superoxide and superoxide dismutases. New York, N.Y: Elsevier/North-Holland; 1980. pp. 136–153. [Google Scholar]
- 2.Ashton W L G, Boardman P L, D'Sa C J, Everall P H, Houghton A W J. Human fascioliasis in Shropshire. Br Med J. 1970;3:500–502. doi: 10.1136/bmj.3.5721.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badwey J A, Karnovsky M L. Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem. 1980;49:695–726. doi: 10.1146/annurev.bi.49.070180.003403. [DOI] [PubMed] [Google Scholar]
- 4.Bannister J V, Bannister W H, Rottilio G. Aspects of the structure, function and application of superoxide dismutases. Crit Rev Biochem. 1987;22:111–180. doi: 10.3109/10409238709083738. [DOI] [PubMed] [Google Scholar]
- 5.Beaman L V, Beaman B I. Monoclonal antibodies demonstrate that superoxide dismutase contributes to protection of Norcardia asteroides within the intact host. Infect Immun. 1990;58:3122–3128. doi: 10.1128/iai.58.9.3122-3128.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beauchamp C O, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 7.Beyer W, Imlay J, Fridovich I. Superoxide dismutases. Prog Nucleic Acids Res Mol Biol. 1991;40:221–253. doi: 10.1016/s0079-6603(08)60843-0. [DOI] [PubMed] [Google Scholar]
- 8.Bruchhaus I, Brattig N W, Tannich E. Recombinant expression, purification and biochemical characterization of a superoxide dismutase from Entamoeba histolytica. Arch Med Res. 1992;23:27–29. [PubMed] [Google Scholar]
- 9.Callahan H L, Crouch R K, James E R. Helminth anti-oxidant enzymes: a protective mechanism against host oxidants? Parasitol Today. 1988;4:218–225. doi: 10.1016/0169-4758(88)90162-7. [DOI] [PubMed] [Google Scholar]
- 10.Callahan H L, Crouch R K, James E R. Dirofilaria immitis superoxide dismutase: purification and characterization. Mol Biochem Parasitol. 1991;49:245–252. doi: 10.1016/0166-6851(91)90068-h. [DOI] [PubMed] [Google Scholar]
- 11.Chauvin A, Moreau E, Boulard C. Diagnosis of bovine fascioliasis using serology of pools of sera. Vet Res. 1997;28:37–43. [PubMed] [Google Scholar]
- 12.Chirgwin J M, Przybyla A E, MacDonald R J, Rutter W J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 13.Decampo R, Moreno S N J. Free radical metabolism of antiparasitic agents. Fed Proc. 1986;45:2471–2476. [PubMed] [Google Scholar]
- 14.Deng H-X, Hentati A, Tainer J A, Iqgal Z, Cayabyab A, Hung W-Y, Getzoff E D, Hu P, Herzfeldt B, Ross R P, Warner C, Deng G, Soriano E, Smyth C, Parge H E, Ahmed A, Roses A D, Hallewell R A, Pericak-Vance M A, Siddique T. Amyotrophic lateral sclerosis and structural defects in Cu, Zn superoxide dismutase. Science. 1993;261:1047–1051. doi: 10.1126/science.8351519. [DOI] [PubMed] [Google Scholar]
- 15.el-Shabrawi M, el-Karaksy H, Okasha S, el-Hennacoy A. Human fascioliasis: clinical features and diagnostic difficulties in Egyptian children. J Trop Pediatr. 1997;43:162–166. doi: 10.1093/tropej/43.3.162. [DOI] [PubMed] [Google Scholar]
- 16.Fairfield A S, Abosch A, Ranz A, Eaton J W, Meshnick S R. Oxidant defense enzymes of Plasmodium falciparum. Mol Biochem Parasitol. 1988;30:77–82. doi: 10.1016/0166-6851(88)90134-x. [DOI] [PubMed] [Google Scholar]
- 17.Feinberg A P, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- 18.Fridovich I. Superoxide radical: an endogeneous toxicant. Annu Rev Toxicol. 1983;23:239–257. doi: 10.1146/annurev.pa.23.040183.001323. [DOI] [PubMed] [Google Scholar]
- 19.Fridovich I. Superoxide dismutases. Adv Enzymol. 1986;58:61–97. doi: 10.1002/9780470123041.ch2. [DOI] [PubMed] [Google Scholar]
- 20.Getzoff E D, Tainer J A, Weiner P K, Kollman P A, Richardson J S, Richardson D C. Electrostatic recognition between superoxide and copper, zinc superoxide dismutase. Nature (London) 1983;306:287–290. doi: 10.1038/306287a0. [DOI] [PubMed] [Google Scholar]
- 21.Henkle K J, Liebau E, Müller S, Bergmann B, Walter R D. Characterization and molecular cloning of a Cu/Zn superoxide dismutase from the human parasite Onchocerca volvulus. Infect Immun. 1991;59:2063–2069. doi: 10.1128/iai.59.6.2063-2069.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henkle-Duhrsen K, Tawe W, Warnecko C, Walter R D. Characterization of the manganese superoxide dismutase cDNA and gene from the human parasite Onchocerca volvulvus. Biochem J. 1995;308:441–446. doi: 10.1042/bj3080441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henkle-Duhrsen K, Tuan R S, Wildenberg G, Eschbach M L, Tawe W, Zipfel P, Walter R D. Localization and functional analysis of the cytosolic and extracellular CuZn superoxide dismutases in the human parasitic nematode Onchocerca volvulus. Mol Biochem Parasitol. 1997;88:187–202. doi: 10.1016/s0166-6851(97)00092-3. [DOI] [PubMed] [Google Scholar]
- 24.Heussler V T, Eichhorn M, Dobbelaere D A. Cloning of a full-length cDNA encoding bovine interleukin 4 by the polymerase chain reaction. Gene. 1992;114:273–278. doi: 10.1016/0378-1119(92)90587-f. [DOI] [PubMed] [Google Scholar]
- 25.Hong Z, Kosman D J, Thakur A, Rekosh D, Loverde P T. Identification and purification of a second form of Cu/Zn superoxide dismutase from Schistosoma mansoni. Infect Immun. 1992;60:3641–3651. doi: 10.1128/iai.60.9.3641-3651.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong Z, Loverde P T, Hammarskjold M L, Rekosh D. Schistosoma mansoni: cloning of a complementary DNA encoding a cytosolic Cu/Zn superoxide dismutase and high-yield expression of the enzymatically active gene product in Escherichia coli. Exp Parasitol. 1992;75:308–322. doi: 10.1016/0014-4894(92)90216-w. [DOI] [PubMed] [Google Scholar]
- 27.Hong Z, Loverde P T, Thakur A, Hammarskjold M L, Rekosh D. Schistosoma mansoni: a Cu/Zn superoxide dismutase is glycosylated when expressed mammalian cells and localized to a subtegumental region in adult schistosomes. Exp Parasitol. 1993;76:101–114. doi: 10.1006/expr.1993.1012. [DOI] [PubMed] [Google Scholar]
- 28.Ismail S O, Skeiky Y A W, Bhatia A, Omara-Opyene L A, Gedamu L. Molecular cloning, characterization, and expression in Escherichia coli of iron superoxide dismutase cDNA from Leishmania donovani chagasi. Infect Immun. 1994;62:657–664. doi: 10.1128/iai.62.2.657-664.1994. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.James E R, McLean D C, Jr, Perler F. Molecular cloning of an Onchocerca volvulus extracellular Cu-Zn superoxide dismutase. Infect Immun. 1994;62:713–716. doi: 10.1128/iai.62.2.713-716.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lattemann C T, Yan Z X, Matzen A, Meyer T F, Apfel H. Immunogenicity of the extracellular copper/zinc superoxide dismutase of the filarial parasite Acanthochilonema viteae delivered by a two-phase vaccine strain of Salmonella typhimurium. Parasite Immunol. 1999;21:219–224. doi: 10.1046/j.1365-3024.1999.00207.x. [DOI] [PubMed] [Google Scholar]
- 31.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 32.Leid R W, Suquet C M. A superoxide dismutase of metacestodes of Taenia taeniaeformis. Mol Biochem Parasitol. 1986;18:301–311. doi: 10.1016/0166-6851(86)90087-3. [DOI] [PubMed] [Google Scholar]
- 33.Leid R W, Suquet C M, Tanigoshi L. Oxygen detoxifying enzymes in parasites: a review. Acta Leiden. 1989;57:107–114. [PubMed] [Google Scholar]
- 34.Lenton L M, Bygrave F L, Behm C A, Boray J C. Fasciola hepatica infection in sheep: changes in liver metabolism. Res Vet Sci. 1996;61:152–156. doi: 10.1016/s0034-5288(96)90091-0. [DOI] [PubMed] [Google Scholar]
- 35.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Foline phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 36.Lynch R E, Fridovich I. Permeation of the erythrocyte stroma by superoxide radical. J Biol Chem. 1978;253:4696–4699. [PubMed] [Google Scholar]
- 37.Maizels R A, Bundy D A P, Selkirk M E, Smith D F, Anderson R M. Immunological modulation and evasion by helminth parasites in human populations. Nature (London) 1993;365:797–805. doi: 10.1038/365797a0. [DOI] [PubMed] [Google Scholar]
- 38.Mas-Coma M S, Esteban J G, Bargues M D. Epidemiology of human fascioliasis: a review and proposed new classification. Bull W H O. 1999;77:340–346. [PMC free article] [PubMed] [Google Scholar]
- 39.Mastudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987;193:265–275. [PubMed] [Google Scholar]
- 40.Mei H, LoVerde P T. Schistosoma mansoni: the developmental regulation and immunolocalization of antioxidant enzymes. Exp Parasitol. 1997;86:69–78. doi: 10.1006/expr.1997.4150. [DOI] [PubMed] [Google Scholar]
- 41.Michalski W P, Prowse S J. Superoxide dismutases in Eimeria tenella. Mol Biochem Parasitol. 1991;47:189–195. doi: 10.1016/0166-6851(91)90178-9. [DOI] [PubMed] [Google Scholar]
- 42.Murray H W. Susceptibility of Leishmania to oxygen intermediates and killing by normal macrophages. J Exp Med. 1981;153:1302–1315. doi: 10.1084/jem.153.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nare B, Smith J M, Prichard R K. Schistosoma mansoni: levels of antioxidants and resistance to oxidants increase during development. Exp Parasitol. 1990;70:389–397. doi: 10.1016/0014-4894(90)90122-s. [DOI] [PubMed] [Google Scholar]
- 44.Nathan C, Nogueira N, Juangbhanich C, Ellis J, Cohn Z A. Activation of macrophages in vivo and in vitro. Correlation between hydrogen peroxide release and killing of Trypanosoma cruzi. J Exp Med. 1979;149:1056–1068. doi: 10.1084/jem.149.5.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Noridaka O, Sinbu M, Masto T, Akila O. Hyohininukem superoxide dismutase. Nippikaisi. 1982;92:583–590. . (In Japanese.) [Google Scholar]
- 46.Onate A A, Vemulapalli R, Andrews E, Schurig G G, Boyle S, Folch H. Vaccination with live Escherichia coli expressing Brucella abortus Cu/Zn superoxide dismutase protects mice against virulent B. abortus. Infect Immun. 1999;67:986–988. doi: 10.1128/iai.67.2.986-988.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Owhashi M, Tomiyoshi F, Hayashi H. Isolation and characterization of a neutrophil chemotactic factor from Tritrichomonas foetus organisms. Immunol Cell Biol. 1994;72:249–255. doi: 10.1038/icb.1994.37. [DOI] [PubMed] [Google Scholar]
- 48.Piacenza L, Raid R, Goni F, Carmona C. CuZn superoxide dismutase activities from Fasciola hepatica. Parasitology. 1998;117:555–562. doi: 10.1017/s0031182098003394. [DOI] [PubMed] [Google Scholar]
- 49.Rangel-Ruiz L J, Marquez-Izquierdo R, Bravo-Nogueira G. Bovine fascioliasis in Tabasco, Mexico. Vet Parasitol. 1999;81:119–127. doi: 10.1016/s0304-4017(98)00152-6. [DOI] [PubMed] [Google Scholar]
- 50.Rhoads M L. Trichinella spiralis: identification and purification of superoxide dismutase. Exp Parasitol. 1983;56:41–54. doi: 10.1016/0014-4894(83)90095-4. [DOI] [PubMed] [Google Scholar]
- 51.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 52.Sanchez M M, Monteoliva M, Fatou A, Garcia R M A. Superoxide dismutase from Ascaris suum. Parasitology. 1988;97:345–353. doi: 10.1017/s0031182000058546. [DOI] [PubMed] [Google Scholar]
- 53.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sherman L, Dafni N, Lieman-Hurwitz J, Groner Y. Nucleotide sequence and expression of human chromosome 21-encoded superoxide dismutase mRNA. Proc Natl Acad Sci USA. 1983;80:5465–5469. doi: 10.1073/pnas.80.18.5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tainer J A, Getzoff E D, Richardson J S, Richardson D C. Structure and mechanism of copper, zinc superoxide dismutase. Nature (London) 1983;306:284–287. doi: 10.1038/306284a0. [DOI] [PubMed] [Google Scholar]
- 56.Tang L, Ou X, Henkle-Duhrsen K, Selkirk M E. Extracellular and cytoplasmic CuZn superoxide dismutases from Brugia lymphatic filarial nematode parasites. Infect Immun. 1994;62:961–967. doi: 10.1128/iai.62.3.961-967.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tannich E, Bruchhaus I, Walter R D, Horstman R D. Pathogenic and nonpathogenic Entamoeba histolytica: identification and molecular cloning of an iron-containing superoxide dismutase. Mol Biochem Parasitol. 1991;49:61–72. doi: 10.1016/0166-6851(91)90130-x. [DOI] [PubMed] [Google Scholar]
- 58.Tielens A G M, van Den Heuvel J M, van Den Bergh S G. Changes in energy metabolism of the juvenile Fasciola hepatica during its development in the liver parenchyma. Mol Biochem Parasitol. 1982;6:277–286. doi: 10.1016/0166-6851(82)90060-3. [DOI] [PubMed] [Google Scholar]
- 59.Tielens A G M. Energy generation in parasitic helminthes. Parasitol Today. 1994;10:346–352. doi: 10.1016/0169-4758(94)90245-3. [DOI] [PubMed] [Google Scholar]
- 60.Towbin H, Staebelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilson C B, Tsai V, Remington J S. Failure to trigger the oxidative metabolic burst by normal macrophages: possible mechanism for survival of intracellular pathogens. J Exp Med. 1980;151:328–346. doi: 10.1084/jem.151.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]