Abstract
Questing ticks are usually collected by flagging or dragging. Mostly exophilic tick species are caught, such as Ixodes ricinus, the most common tick in Central Europe. In the present study, ticks collected from underground environments in the Grand Duchy of Luxembourg and in the Central German Uplands (Federal States of Hesse, Bavaria, Thuringia, Baden-Wuerttemberg, Rhineland-Palatinate, Saarland and Northrhine-Westphalia) were investigated. Six tick species were revealed among the 396 analyzed specimens: Ixodes ariadnae, Ixodes canisuga, Ixodes hexagonus, I. ricinus, Ixodes trianguliceps, and Dermacentor marginatus. Adults and immatures of I. hexagonus dominated the findings (57% of all specimens), especially in shelters acting as potential resting places of main hosts. Ixodes canisuga and I. trianguliceps were for the first time recorded in Luxembourg, and one nymph of the bat tick I. ariadnae represents only the second report for Germany. Collecting ticks in subterranean environments turned out to be a useful approach to increase knowledge about the occurrence of relatively rare tick species, including those that spend most of their lifetime on their hosts, but detach in such environmental settings.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10493-023-00795-2.
Keywords: Tick-borne diseases, Subterranean biology, Acari, Ixodidae, Underground environments
Introduction
Underground environments such as natural caves, abandoned mines and tunnels are often neglected sources of human and animal pathogens and the habitats of their respective arthropod vectors (Montagna et al. 2003; Jurado et al. 2010; Igreja 2011; Obame-Nkoghe et al. 2017). In Central Europe, underground environments harbor important arthropod vector groups, such as mosquitos (Diptera: Culicidae), fleas (Siphonaptera), and ticks (Acari: Ixodidae) (e.g., Zaenker et al. 2020), but taxonomically targeted and geographically more widespread studies in these environments are scarce (Kutzscher and Weber 2015; Dörge et al. 2020; Zittra et al. 2019, 2021; Zaenker et al. 2020).
Four ixodid tick species are currently listed for the Grand Duchy of Luxembourg, i.e., Ixodes ricinus, Ixodes frontalis, Ixodes hexagonus, and Dermacentor reticulatus (Reye et al. 2010; Reye 2011; Weigand et al. 2020). Additionally, Hyalomma marginatum was recently reported, but this species is considered a rare introduction without established populations in Luxembourg (Weigand et al. 2020). Population densities of D. reticulatus have significantly increased in the country in recent years, a phenomenon that can be attributed to an ongoing range expansion of this species in Central Europe (Rubel et al. 2016; Drehmann et al. 2020; Weigand et al. 2020). The only systematic national survey of ticks (2007–2009; >9,700 total specimens) revealed I. ricinus as the by far most widespread and common species in Luxembourg (Reye 2011). Ixodes hexagonus was occasionally collected from infested animals (predominantly hedgehogs, cats, and foxes), whereas I. frontalis was only rarely encountered on birds (Reye 2011).
Tick research in Germany is well established, and much more comprehensive data are available compared to Luxembourg. The German tick fauna currently includes 21 species, 19 ixodid species belonging to three genera: Dermacentor, Haemaphysalis, and Ixodes; and two soft tick species (Petney et al. 2012, 2015; Hornok et al. 2015a; Bröker et al. 2019; Hauck et al. 2020; Ott et al. 2020; Rubel et al. 2021). In addition, two ixodid species, H. marginatum and Hyalomma rufipes, are often imported into Germany by migratory birds (Chitimia-Dobler et al. 2019), whereas the cosmopolitan Rhipicephalus sanguineus enters the country with dogs (Petney et al. 2012). Ticks are also imported into Germany by humans returning from holidays abroad, e.g., Amblyomma mixtum from Cuba (Chitimia-Dobler et al. 2020), Dermacentor auratus from Cambodia (Chitimia-Dobler et al. 2021), and Rhipicephalus maculatus from South Africa (Chitimia-Dobler and Mans 2022).
Central European ticks are known from a variety of habitats (Mierzejewska et al. 2017), e.g., host nests in the case of Ixodes arboricola (Heylen et al. 2014a, b). However, most studies of German ticks have been based on flagging or dragging collection methods, especially investigations of I. ricinus abundance and/or activity, whether or not such studies focus on tick-borne pathogens (Bröker et al. 2019; Hauck et al. 2020; Ott et al. 2020). Similar techniques have been adopted for investigations involving Ixodes inopinatus, I. frontalis, I. hexagonus and D. reticulatus. In the special case of underground environments, four bat-infesting tick species have been reported: Carios vespertilionis, Ixodes simplex, Ixodes ariadnae, and Ixodes vespertilionis, in addition to several other non-ixodid species of parasitic or predatory mites (Zaenker et al. 2020). The tick Ixodes barbarossae, described by Schulze (1937) from the Kyffhäuserhöhle (Kyffhaeuser cave; Thuringia, Germany), is now considered a junior synonym of Ixodes canisuga. In the vast majority of cases, data on underground findings of tick species originate from focused ectoparasitic studies on bats (e.g., Schmidt 1987; Walter 1992; Rupp et al. 2004; Scheffler 2009). For Luxembourg, Weber (2013) conducted a comprehensive national survey of the cave-dwelling fauna, but no data so far exist on ticks collected from underground environments.
This contribution is a retrospective analysis of data on ticks collected in underground environments. Our results will contribute to the knowledge of the tick fauna of underground environments in the Central German Uplands of western Germany (mainly in the Federal States of Hesse, Rhineland-Palatinate, and Saarland) and Luxembourg. Tick findings from long-term biological assessments of subterranean environments are analyzed, and, for Germany, compared with the tick fauna of springs.
Materials and methods
Governmental permission and control
According to the German Federal Law on Nature Protection § 30, natural caves, semi-natural mining galleries and spring areas are protected biotopes in Germany. To conduct research in these biotopes and collect animals, the Regional Association for Research on Caves and Karst obtained permission from the Hesse Regional Authorities for Nature Protection, Environment and Geology. Data are reported yearly to the latter office and are archived in the Biospeleological Register (Reiss et al. 2009). Dieter Weber obtained permission from the Struktur- und Genehmigungsdirektion Süd for the Federal State of Rhineland-Palatinate and from the Landesamt für Umwelt- und Arbeitsschutz, Geschäftsbereich 3, Natur- und Umweltschutz for the Federal State of Saarland.
For Luxembourg, tick specimens originated from the study of Weber (2013) and subsequent visits to subterranean sites. Sampling was permitted by the respective owners of subterranean sites or due to the close collaboration of the National Museum of Natural History Luxembourg (MNHNL) with the Ministère de l’Environnement, du Climat et du Développement durable (MECDD) Luxembourg. Specimens are stored in the wet collection of the MNHNL (reference numbers MNHNL67125, MNHNL130068-MNHNL130307).
Datasets
Subterranean environments are frequently visited for bioassessment of cave-dwelling organisms (Weber 2013; Zaenker et al. 2020), e.g., in the context of monitoring habitat type 8310 (non-touristic caves) of the EU Habitats Directive (Council Directive 92/43/EEC; Weigand et al. 2022). Ticks are routinely observed, but have never been systematically evaluated. Here, tick findings from visits to subterranean sites (natural caves, mines, cellars, tunnels, etc.) in the Central German Uplands (mainly in the Federal States of Hesse, Rhineland-Palatinate and Saarland) and Luxembourg are analyzed. Ticks were not removed from their hosts (e.g., bats), but were directly collected from the environment or detected in Barber traps. Most collecting focused on microenvironments, such as the walls and ceilings of underground sites. Ticks were collected using fine forceps and placed in 70% ethanol in 1.5-mL Eppendorf tubes. In semi-aquatic biotopes, materials such as wood and stones were shaken over a white cup. At some localities, tick specimens were retrieved from Barber traps with 70% isopropanol used as a fixative and preservative.
Available tick findings from regular spring monitoring in the Central German Uplands were included for comparison. Focus was laid on the Rhön mountain region in eastern Hesse and the neighboring Federal States of Bavaria and Thuringia. Most specimens were collected from plants (net catch in spring vegetation), stones and rocks as well as organic and inorganic substrates such as mud, leaves and decaying wood in these aquatic biotopes.
Specimen identification
Ticks were examined with a Keyence VHX-900F microscope (Itasca, IL, USA) and identified to species level using morphological keys (Filippova 1977; Pérez-Eid 2007; Hornok et al. 2014). In cases of questionable specimens, those were objected to molecular analysis. DNA was extracted using the QIAamp mini DNA extraction kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. The 16S rRNA gene was amplified by PCR and Sanger sequenced according to Halos et al. (2004), using the tick-specific primer pair TQ16S + 1F (5’-CTGCTCAATGATTTTTTAAATTGCTGTGG-3’) and TQ16S-2R (5’-ACGCTGTTATCCCTAGAG-3’) of Black and Piesman (1994). Sequences were edited and primers trimmed using the Geneious Prime 2022.2.1 software (https://www.geneious.com). A BLASTN search (Zhang et al. 2000) in GenBank was performed to molecularly annotate the individual 16S rDNA sequences. 16S rDNA sequence data can be found in GenBank and the supplementary material.
Results
Germany
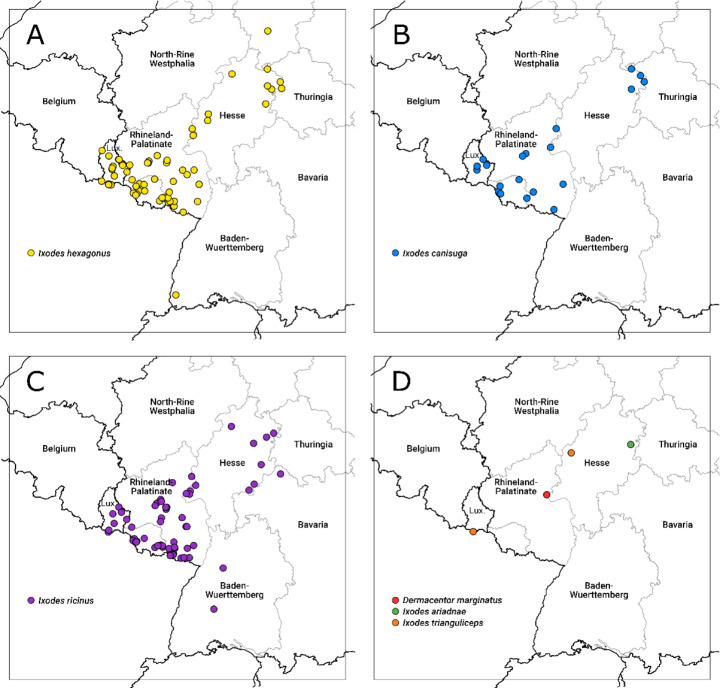
In total, 224 ixodid ticks were collected between August 1997 and January 2022 from 114 subterranean sites in the Central German Uplands, primarily the Federal States of Hesse, Rhineland-Palatinate and Saarland (Table 1). The most frequently observed species were Ixodes hexagonus (n = 83; 38 males, 30 females, 14 nymphs, 1 larva) and I. ricinus (n = 77; 10 males, 6 females, 39 nymphs, 22 larvae), followed by I. canisuga (n = 61; 22 males, 30 females, 4 nymphs, 5 larvae) (Tables 1 and 2, S1 and S2, Fig. 1). Single specimens of I. ariadnae (1 nymph), I. trianguliceps (1 female), and Dermacentor marginatus (1 male) were also recorded. The single I. ariadnae nymph was collected in 2021 on the wall of a water passage in Friedewald (Hesse) (Fig. 2). The analysis of the 16S rDNA marker of this specimen verified the initial morphological identification of I. ariadnae (GenBank acc. nr. OQ615884; Table S2). The I. trianguliceps female was found in 2003, on the wall of a mining tunnel in Dillenburg (Hesse). The D. marginatus male was found in 2012, also on the wall of a mining tunnel in Kaub (Rhineland-Palatinate). The tick findings of the spring monitoring data from the Rhön mountain region consisted only of I. ricinus specimens in all developmental stages (n = 290; Table S3).
Table 1.
Overview of tick species in underground environments in Germany (DE) and Luxembourg (LU). Also shown are the ixodid ticks encountered in the frame of the German spring monitoring in the Rhön mountains
| Species | No. specimens | Underground habitat type | Spring | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Natural | Artificial | ||||||||||
| Total | DE | LU | Total | DE | LU | DE | |||||
| Ixodes ariadnae | 1 | 1 | 1 | ||||||||
| Ixodes canisuga | 71 | 49 | 39 | 10 | 22 | 22 | |||||
| Ixodes hexagonus | 237 | 90 | 18 | 72 | 147 | 65 | 82 | ||||
| Ixodes ricinus | 374 | 20 | 16 | 4 | 64 | 61 | 3 | 290 | |||
| Ixodes trianguliceps | 2 | 2 | 1 | 1 | |||||||
| Dermacentor marginatus | 1 | 1 | 1 | ||||||||
Table 2.
Overview of gender proportions and developmental stages of ixodid ticks found in underground environments in Germany (DE) and Luxembourg (LU).
| Species | No. specimens | Males | Females | Nymphs | Larvae | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | DE | LU | Total | DE | LU | Total | DE | LU | Total | DE | LU | |||||||
| Ixodes ariadnae | 1 | 1 | 1 | |||||||||||||||
| Ixodes canisuga | 71 | 25 | 22 | 3 | 36 | 30 | 6 | 4 | 4 | 0 | 6 | 5 | 1 | |||||
| Ixodes hexagonus | 237 | 102 | 38 | 64 | 74 | 30 | 44 | 51 | 14 | 37 | 10 | 1 | 9 | |||||
| Ixodes ricinus | 84 | 12 | 10 | 2 | 7 | 6 | 1 | 40 | 39 | 1 | 25 | 22 | 3 | |||||
| Ixodes trianguliceps | 2 | 2 | 1 | 1 | ||||||||||||||
| Dermacentor marginatus | 1 | 1 | 1 | |||||||||||||||
Fig. 1.
Distribution of tick species detected in subterranean environments of the Central German Uplands and Luxembourg. A: Ixodes hexagonus, B: Ixodes canisuga, C: Ixodes ricinus, D: Dermacentor marginatus, Ixodes ariadnae, Ixodes trianguliceps
Fig. 2.
Ixodes ariadnae nymph, reference code Mi5737 of the Biospeleological Register of Hesse (Germany). A: dorsal view; B: ventral view
Luxembourg
In total, 172 ixodid ticks were obtained from 27 underground sites in Luxembourg visited between January 1996 and June 2014 (Table 1). The records were dominated by I. hexagonus (n = 154; 64 males, 44 females, 37 nymphs and 9 larvae), followed by I. canisuga (n = 10; 3 males, 6 females, 1 larva), I. ricinus (n = 7; 2 males, 1 female, 1 nymph, 3 larvae) and I. trianguliceps (1 female) (Fig. 1; Tables 1 and 2, S1 and S2). However, more than one-third of all I. hexagonus specimens were found at a single site (n = 57, Méischtrefer Hiel), predominantly resulting from three sampling events in winter 2010/2011 (90%). Findings of I. canisuga and I. trianguliceps represent the first records for this country. The single I. trianguliceps female was found in 2007 in the entrance area of Minière Hutbierg. Ixodes canisuga records originated from five caves: Fusselach, Bitzmaschinn (both part of the Mamerlayen), Grande Fleur, Déiwepetz and the Méischtrefer Hiel. Records usually were made in the entrance area, but all three findings in the Méischtrefer Hiel stem from the dark zone.
Discussion
New and rare faunistic findings
In total, six tick species were detected in the investigated underground environments (I. ariadnae, I. canisuga, I. hexagonus, I. ricinus, I. trianguliceps, D. marginatus) of the Central German Uplands and Luxembourg, with I. hexagonus adults and nymphs dominating the findings, followed by adult I. canisuga and I. ricinus nymphs. This was particularly true for sites known as shelters or overwintering places of their preferred hosts, e.g., carnivorous mammals (red foxes and badgers) or hedgehogs (Arthur 1953; Harris and Thompson 1978). However, at this point, it also must be noted that a few (or even single) sampling events or underground sites can bias the proportions of individual tick species in the overall dataset. For example, 46% of all I. canisuga specimens collected in Germany originated from the 7 March 1998 collection event in the JOMI-Höhle, or in the case of I. hexagonus specimens from Luxembourg, 37% were collected in winter 2010/11 in the Méischtrefer Hiel. Only I. ricinus was found in tick samples from springs in the Central German Uplands. This species was occasionally encountered in underground sites, both in Germany (n = 77) and Luxembourg (7). The findings of I. canisuga and I. trianguliceps in Luxembourg constitute the first records for that country, thereby adding to the known tick fauna of Luxembourg.
Particularly noteworthy discoveries include a single nymph of the bat tick I. ariadnae, found on 01.02.2021 on the wall of a water passage in Friedewald (Hesse, Germany). The initial morphological identification was confirmed by sequencing its 16S rDNA marker. The specimen of our study showed 100% genetic sequence identity to I. ariadnae specimens collected in Germany, Hungary and Turkey (Hornok et al. 2015a, b; Hekimoglu et al. 2022). We recorded the bat species Plecotus auritus, Myotis myotis and Myotis nattereri at the same sampling site where I. ariadnae was found. Ixodes ariadnae is known to be associated with various bat species (Hornok et al. 2014; Sándor et al. 2019), including P. auritus and Myotis spp. This finding represents only the second report of this bat tick species in Germany. Given the relatively recent discovery and formal taxonomic description of I. ariadnae (Hornok et al. 2014), it can be expected that the species is probably more widely distributed in Germany than these two records indicate.
A single male of D. marginatus was found in a slate tunnel in Rhineland-Palatinate (Germany), on the right bank of the Rhine. Recent studies have shown that the distribution of D. marginatus in Central Europe has not changed much over the years and is relatively limited compared with D. reticulatus (Drehmann et al. 2020; Weigand et al. 2020; Rubel et al. 2021; Walter et al. 2016) determined that most of the Rhineland-Palatinate region is suitable habitat for D. marginatus, as this species prefers relatively dry and sparsely vegetated areas, often alongside rivers. A further westward range expansion also reaching Luxembourg can be expected in coming years.
Two rare findings of I. trianguliceps (2 females) were made in underground environments of Germany and Luxembourg. The mouse tick feeds exclusively on small animals (Brown et al. 2008) and is a very rare parasite of humans (Guglielmone and Robbins 2018). Most commonly, its life stages infest soricomorph mammals (Soricidae, Talpidae) and rodents (Cricetidae, Muridae), but this tick occasionally parasitizes other hosts, such as birds, sciurid rodents, chiropterans, and squamatans (Guglielmone et al. 2014; Kovalevskii et al. 2013). The species has a Palearctic distribution, occurring in many European countries and parts of northwestern Asia (Petney et al. 2012). However, georeferenced data for Central Europe are generally scarce (Estrada-Peña et al. 2018; Rubel et al. 2021).
Ecological notes on the predominance of adult Ixodes hexagonus and I. canisuga in underground environments
Adults of I. canisuga and I. hexagonus, the two tick species most frequently represented by this stage in underground environments during our study, both mate off their hosts. Furthermore, I. hexagonus is known to detach from its hosts with the approach of darkness (Matuschka et al. 1990), and is quiescent for several days after molting. This behavior might explain why we encountered a much higher proportion of males than normally observed when directly collecting these two ticks from their preferred hosts (Arthur 1953; Harris and Thompson 1978; Walker 2018). Also, I. hexagonus was chiefly collected in non-natural subterranean environments in suburban areas of Luxembourg, which could be attributed to the ecology of its preferred host, hedgehogs, whose populations are often highest in suburban and urban areas because of reduced predation and higher food availability (Hubert et al. 2011; Walker 2018). This is also in accordance with the results of Neumann (1916), who recorded I. vespertilionis and I. hexagonus from 43 natural caves mostly located in France. However, only four specimens of I. hexagonus were recorded, suggesting that this species may indeed be more likely to occur in non-natural subterranean environments, which tend to be more common in (sub)urban areas and thus overlap with the environmental preferences of its preferred host. Red foxes (Vulpes vulpes) are the preferred final host for I. canisuga, but badgers (Meles meles), stone martens (Martes foina) or polecats (Mustela putorius) are also infested regularly. These hosts all have in common their use of self-constructed burrows or natural underground sites for shelter (e.g., crevices, holes, caves), which explains the high frequency of adult I. canisuga in the underground environments that we investigated. However, these data differ from those of Meyer-Kayser et al. (2012), who studied tick species and their developmental stages collected directly from red foxes in Thuringia, finding that adults of I. ricinus were most common (82% of all adult ticks), followed by nymphs of I. canisuga and I. hexagonus (85% of all nymphs). Notably, only a single male, but 485 female I. canisuga (ratio 0.2/99.8), and four males and 299 females of I. hexagonus (ratio 1.3/98.7) were detected among the 13,227 ticks collected from red foxes. Such results sharply contrast with our study of these two tick species when collected from underground environments, with gender ratios of 0.41/0.59 and 0.58/0.42 for I. canisuga and I. hexagonus, respectively. This indicates that collecting ticks from underground environments represents a promising strategy for obtaining males of these two species. Our results also suggest that I. ricinus adults apparently do not survive well in subterranean habitats, as they only comprised 7% of all adult ticks, but 47% of all nymphs and larvae.
Co-occurrences of ticks in underground environments
The importance of spatio-temporal co-occurrences of tick species must be highlighted, such as in the case of the JOMI-Höhle in Rhineland-Palatinate (Germany), where I. canisuga, I. hexagonus, and I. ricinus were collected on a single day. In Luxembourg, all three species were also detected at single sites, but not on the same day, e.g., in the Fusselach (Mamerlayen) during summer 2007 and in the Méischtrefer Hiel during summer 2010. Co-occurrences of tick species can favor occasional transfers between hosts (Jahfari et al. 2017) and point to the importance of conducting further tick surveys in underground environments. This is relevant to public health, as I. hexagonus and I. canisuga may act as vectors of Borrelia burgdorferi and can transmit Babesia missiroli and tick-borne encephalitis (TBE) virus, respectively (Gern et al. 1991; Labuda and Randolph 1999; Skuballa et al. 2007; Jahfari et al. 2017; Arthur 1953) noted the risk of tick infestation at underground sites frequently visited by humans, such as air-raid shelters and similar constructions, especially if such sites are inhabited by I. hexagonus, which is a more frequent parasite of humans and pet animals than I. canisuga (Liebisch and Walter 1986; Guglielmone and Robbins 2018). The underground sites of the Mamerlayen in Luxembourg are known tourist attractions, so that the risk of tick bite there should be emphasized. Finally, as already stated by Jahfari et al. (2017), hedgehogs must be seen as a central element in the spread of ticks and tick-borne diseases, especially in urban and suburban settings.
Sampling in underground environments
Tick populations are mostly monitored using dragging or flagging, methods that involve moving white fabric over vegetation to collect questing ticks. Alternatively, ticks can be collected from captured animals or from carcasses. Specimens may also be collected using dry ice traps (Yans et al. 2022). Flagging/dragging is highly effective when working with exophilic ticks, such as I. ricinus, but it is ineffective when seeking endophilic species, some of which are frequent in subterranean environments, as demonstrated by the results of our study. Host animals that visit these biotopes, either temporarily or for longer periods (e.g., for hibernation or dormancy), may leave detached endophilic species where they can be relatively easily collected, thereby adding to our knowledge of their distribution. A limitation to this approach is determining the hosts of the encountered specimens – without blood meal analysis. Exceptions to this are bat-infesting ticks, which are highly host specific (Sándor et al. 2019). Additionally, collecting in subterranean environments lacks a temporal dimension, as it is not possible to determine when tick specimens have dropped from their hosts after feeding. A tick found in a cave in early spring may have dropped from an active host just days before or months ago, while the host was hibernating (e.g., hedgehog or bats), in winter dormancy (e.g., badger) or resting (e.g., fox). Adding to this, larvae can be very long-lived, and prolonged periods of molting are observed under low temperatures, e.g., a mean of 60 days at 15 °C in a saturated atmosphere for I. hexagonus (Arthur 1951). Given that mean temperatures in Central European caves are between 8 and 10 °C (Zaenker et al. 2020), an even longer time period can be expected.
Conclusions
Caves, mines, tunnels and other smaller underground cavities are often used as shelters or resting places (incl. hibernation and winter dormancy) by small to large mammals and their associated parasites and, as such, can contribute to the spread of ticks and tick-borne diseases. Despite limitations when collecting ticks in underground environments, our study results highlight the importance of obtaining taxonomic and distributional data from these often neglected habitats. Adult ticks of both sexes of I. hexagonus and I. canisuga, as well as nymphs of I. ricinus, were the most abundant specimens, but likewise rare findings were made (e.g., I. ariadnae, I. trianguliceps). The data at hand suggest that underground environments could act as reservoirs and sites of multiple tick-borne diseases by co-locating different tick species and their suitable hosts. Collecting ticks in such environments may help to increase our knowledge about the distribution of individual tick species, alongside classical flagging/dragging, thus gaining further insights into their ecologies and spatial dynamics – especially in suburban areas. Hedgehogs and their associated tick community might play a key role here.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the members of the Hesse Federation for Cave and Karst Research (Germany) for their assistance in collecting invertebrates while conducting biospeleological research. We are also greatful to Hannes Köble and Stefan Meyer for collecting ticks in the caves of Baden-Wuerttemberg and Lower Saxony. Special appreciation is extended to the staffs of Kellerwald-Edersee National Park and the Rhön Biosphere Reserve, who have provided ongoing support of our spring biotope investigations. We appreciate the comments of Richard G. Robbins made on an earlier and actual version of our manuscript.
Author contribution statement
A.W. and L.C.-D. wrote the main manuscript text. C.Z. and A.W. prepared the figures. A.W. generated the tables. L.C.-D., S.Z., D.W., S.S., M.B. and A.W. generated, analyzed and interpreted data. All authors reviewed the manuscript.
Funding Declaration
This study has not received any specific grant funding.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Arthur DR. The bionomics of Ixodes hexagonus Leach in Britain. Parasit. 1951;41:82–90. doi: 10.1017/S0031182000016607. [DOI] [PubMed] [Google Scholar]
- Arthur DR. The host relationships of Ixodes hexagonus Leach in Britain. Parasit. 1953;43(3–4):227–238. doi: 10.1017/S003118200001859X. [DOI] [PubMed] [Google Scholar]
- Black WC, Piesman J. Phylogeny of hard-and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rDNA sequences. Proc Natl Acad Sci USA. 1994;91(21):10034. doi: 10.1073/pnas.91.21.10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bröker M, Chitimia-Dobler L, Dobler G. Zecken und Frühsommer-Meningoenzephalitis im Landkreis Marburg-Biedenkopf. Philippia. 2019;17:279–288. [Google Scholar]
- Brown KJ, Lambin X, Telford GR, Ogden NH, Telfer S, Woldehiwet Z, Birtles RJ (2008) Relative importance of Ixodes ricinus and Ixodes trianguliceps as vectors for Anaplasma phagocytophilum and Babesia microti in field vole (Microtus agrestis) populations. Appl Environ Microbiol 74:7118–7125 10.1128/AEM.00625-08 [DOI] [PMC free article] [PubMed]
- Chitimia-Dobler L, Mans B. An exotic souvenir on a german traveler returning from South Africa. Parasitol Res. 2022;121:1527–1531. doi: 10.1007/s00436-022-07480-0. [DOI] [PubMed] [Google Scholar]
- Chitimia-Dobler L, Schaper S, Rieß R, Bitterwolf K, Frangoulidis D, Bestehorn M, Springer A, Oehme R, Drehmann M, Lindau A, Mackenstedt U, Strube C, Dobler G. Imported Hyalomma ticks in Germany in 2018. Parasit Vectors. 2019;12:134. doi: 10.1186/s13071-019-3380-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitimia-Dobler L, Schaper S, Mansfeld P, Gonschorrek J, Bröker M, Nava S. Detection of Amblyomma mixtum (Acari: Ixodidae) in Germany on a human traveller returning from Cuba. J Med Entomol. 2020;57:962–964. doi: 10.1093/jme/tjz225. [DOI] [PubMed] [Google Scholar]
- Chitimia-Dobler L, Schaper S, Bröker M, Nava S. Long-term itching in a tourist following bite by a nymph of Dermacentor auratus (Acari: Ixodidae) in Cambodia. J Med Entomol. 2021;58(6):2495–2498. doi: 10.1093/jme/tjab088. [DOI] [PubMed] [Google Scholar]
- Dörge DD, Cunze S, Schleifenbaum H, Zaenker S, Klimpel S. An investigation of hibernating members from the Culex pipiens complex (Diptera, Culicidae) in subterranean habitats of central Germany. Sci Rep. 2020;10:10276. doi: 10.1038/s41598-020-67422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drehmann M, Springer A, Lindau A, Fachet K, Mai S, Thoma D, Schneider CR, Chitimia-Dobler L, Bröker M, Dobler G, Mackenstedt U, Strube C. The spatial distribution of Dermacentor ticks (Ixodidae) in Germany - evidence of a continuing spread of Dermacentor reticulatus. Front Vet Sci. 2020;7:578220. doi: 10.3389/fvets.2020.578220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippova NA (1977) Ixodid ticks (Ixodinae). Fauna USSR New Series 4(4), Nauka, Moscow, Leningrad
- Gern L, Toutoungi LN, Hu CM, Aeschlimann A. Ixodes (Pholeoixodes) hexagonus, an efficient vector of Borrelia burgdorferi in the laboratory. Med Vet Entomol. 1991;5(4):431–443. doi: 10.1111/j.1365-2915.1991.tb00571.x. [DOI] [PubMed] [Google Scholar]
- Guglielmone AA, Robbins RG (2018) Hard ticks (Acari: Ixodida: Ixodoidea) parasitizing humans. A global overview. Springer, p 313
- Guglielmone AA, Robbins RG, Apanaskevich DA, Petney TN, Estrada-Peña A, Horak IG (2014) The hard ticks of the world (Acari: Ixodida: Ixodoidea). Springer, p 738
- Halos L, Jamal T, Vial L, Maillard R, Suau A, Le Menach A, Boulouis H-J, Vayssier-Taussat M. Determination of an efficient and reliable method for DNA extraction from ticks. Vet Res. 2004;35:709–713. doi: 10.1051/vetres:2004038. [DOI] [PubMed] [Google Scholar]
- Harris S, Thompson GB (1978) Populations of the ticks Ixodes (Pholeoixodes) hexagonus and Ixodes (Pholeoixodes) canisuga infesting suburban foxes, Vulpes vulpes. J Zool 186(1):83–93
- Hauck D, Springer A, Chitimia-Dobler L, Strube C. Two-year monitoring of tick abundance and influencing factors in an urban area (city of Hanover, Germany) Ticks Tick Borne Dis. 2020;11(5):101464. doi: 10.1016/j.ttbdis.2020.101464. [DOI] [PubMed] [Google Scholar]
- Hekimoglu O, Elverici M, Yorulmaz T. A survey of hard ticks associated with cave dwelling mammals in Turkey. Ticks Tick Borne Dis. 2022;13(6):102008. doi: 10.1016/j.ttbdis.2022.102008. [DOI] [PubMed] [Google Scholar]
- Heylen D, Sprong H, van Oers K, Fonville M, Leirs H, Matthysen E (2014a) Are the specialized bird ticks, Ixodes arboricola and I. frontalis, competent vectors for Borrelia burgdorferi sensu lato? Environ Microbiol 16:1081–1089. 10.1111/1462-2920.12332 [DOI] [PubMed]
- Heylen D, van Oosten R, Devriendt N, Elst J, De Bruyn L, Matthysen E. Seasonal feeding activity of the tree-hole tick, Ixodes arboricola. Parasit. 2014;141:1044–1051. doi: 10.1017/s0031182014000225. [DOI] [PubMed] [Google Scholar]
- Hornok S, Kontschán J, Kováts D, Kovács R, Angyal D, Görföl T, Polacsek Z, Kalmár Z, Mihalca AD. Bat ticks revisited: Ixodes ariadnae sp. nov. and allopatric genotypes of I. vespertilionis in caves of Hungary. Parasit Vectors. 2014;7:202. doi: 10.1186/1756-3305-7-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornok S, Takács N, Szöke K, Kunz B. First record of Ixodes ariadnae in Germany. Acta Vet Hung. 2015;63:347–351. doi: 10.1556/004.2015.032. [DOI] [PubMed] [Google Scholar]
- Hornok S, Kontschán J, Estrada-Peña A, de Mera IGF, Tomanović S, de la Fuente J. Contributions to the morphology and phylogeny of the newly discovered bat tick species, Ixodes ariadnae in comparison with I. vespertilionis and I. simplex. Parasit Vectors. 2015;8(1):1–7. doi: 10.1186/s13071-015-0665-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert P, Julliard R, Biagianti S, Poulle ML. Ecological factors driving the higher hedgehog (Erinaceus europeaus) density in an urban area compared to the adjacent rural area. Landsc Urban Plan. 2011;103(1):34–43. doi: 10.1016/j.landurbplan.2011.05.010. [DOI] [Google Scholar]
- Igreja RP. Infectious diseases associated with caves. Wilderness Environ Med. 2011;22(2):115–121. doi: 10.1016/j.wem.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Jahfari S, Ruyts SC, Frazer-Mendelewska E, Jaarsma R, Verheyen K, Sprong H. Melting pot of tick-borne zoonoses: the european hedgehog contributes to the maintenance of various tick-borne diseases in natural cycles urban and suburban areas. Parasit Vectors. 2017;10(1):1–9. doi: 10.1186/s13071-017-2065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado V, Laiz L, Rodriguez-Nava V, Boiron P, Hermosin B, Sanchez-Moral S, Saiz-Jimenez C. Pathogenic and opportunistic microorganisms in caves. Int J Speleol. 2010;39(1):2. doi: 10.5038/1827-806X.39.1.2. [DOI] [Google Scholar]
- Kovalevskii YV, Korenberg EI, Gorelova NB, Nefedova VV. The ecology of Ixodes trianguliceps ticks and their role in the natural foci of ixodid tick-borne Borrelioses in the middle urals. Entomol Rev. 2013;93(8):1073–1083. doi: 10.1134/S0013873813080125. [DOI] [Google Scholar]
- Kutzscher C, Weber D. Flöhe (Siphonaptera) aus Höhlen Deutschlands, Frankreichs und Luxemburgs. Contrib Entomol. 2015;65(2):361–371. doi: 10.21248/contrib.entomol.65.2.361-371. [DOI] [Google Scholar]
- Labuda M, Randolph SE. Survival strategy of tick-borne encephalitis virus: cellular basis and environmental determinants. Zentralbl Bakteriol. 1999;289(5–7):513–524. doi: 10.1016/S0934-8840(99)80005-X. [DOI] [PubMed] [Google Scholar]
- Liebisch A, Walter G. Studies on the ticks of pets and wild animals in Germany: the occurrence and biology of the hedgehog tick (Ixodes hexagonus) and fox tick (Ixodes canisuga) Dtsch Tierarztl Wochenschr. 1986;93:447–450. [PubMed] [Google Scholar]
- Matuschka FR, Richter D, Fischer P, Spielman A. Nocturnal detachment of the tick Ixodes hexagonus from nocturnally active hosts. Med Vet Entomol. 1990;4:415–420. doi: 10.1111/j.1365-2915.1990.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Meyer-Kayser E, Hoffmann L, Silaghi C, Pfister K, Mahling M, Passos LM. Dynamics of tick infestations in foxes in Thuringia, Germany. Ticks Tick Borne Dis. 2012;3(4):232–239. doi: 10.1016/j.ttbdis.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Mierzejewska E, Estrada-Peña A, Bajer A. Spread of Dermacentor reticulatus is associated with the loss of forest area. Exp Appl Acarol. 2017;72:399–413. doi: 10.1007/s10493-017-0160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagna MT, Formicola W, Ragone G, Rizzello R, Chiriaco L, Beccarisi L, Tato D, Caggiano G, Santacroce MP. Caves as a new reservoir for potentially pathogenic yeasts. Ig Mod. 2003;119(1):1–9. [Google Scholar]
- Neumann LG. Ixodides (Acariens) Premiere Serie Arch Zool Exper et Gen Paris. 1916;55:517–527. [Google Scholar]
- Obame-Nkoghe J, Leroy EM, Paupy C. Diversity and role of cave-dwelling hematophagous insects in pathogen transmission in the afrotropical region. Emerg Microbes Infect. 2017;6(1):1–6. doi: 10.1038/emi.2017.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott D, Ulrich K, Ginsbach P, Öhme R, Lenhard T. Tick-borne encephalitis virus (TBEV) prevalence in field-collected ticks (Ixodes ricinus) and phylogenetic, structural and virulence analysis in a TBE high-risk endemic area in southwestern Germany. Parasit Vectors. 2020;13:303. doi: 10.1186/s13071-020-04146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Eid C. Les tiques: identification, biologie, importance médical vétérinaire. Paris: Lavoisier; 2007. [Google Scholar]
- Petney TN, Pfäffle MP, Skuballa JD. An annotated checklist of the ticks (Acari: Ixodida) of Germany. Syst Appl Acarol. 2012;17:15–170. doi: 10.11158/saa.17.2.2. [DOI] [Google Scholar]
- Petney TN, Moser E, Littwin N, Pfäffle MP, Muders SV, Taraschewski H. Additions to “Annotated checklist of the ticks (Acari: Ixodida) of Germany”: Ixodes acuminatus and Ixodes inopinatus. Syst Appl Acarol. 2015;20:221–224. doi: 10.11158/saa.20.2.9. [DOI] [Google Scholar]
- Reiss M, Steiner H, Zaenker S. The biospeleological register of the Hesse Federation for Cave and Karst Research (Germany) Cave Karst Sci. 2009;35:25–34. [Google Scholar]
- Reye AL (2011) Prevalence and Diversity of Tick-Borne Pathogens from Central and Eastern Europe as well as West Africa. PhD thesis, Medizinische Fakultät der Universität des Saarlandes, Homburg/Saar. 149 pp
- Reye AL, Hübschen JM, Sausy A, Muller CP. Prevalence and seasonality of tick-borne pathogens in questing Ixodes ricinus ticks from Luxembourg. Appl Environm Microbiol. 2010;76:2923–2931. doi: 10.1128/AEM.03061-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubel F, Brugger K, Pfeffer M, Chitimia-Dobler L, Didyk YM, Leverenz S, Dautel H, Kahl O. Geographical distribution of Dermacentor marginatus and Dermacentor reticulatus in Europe. Ticks Tick Borne Dis. 2016;7:224–233. doi: 10.1016/j.ttbdis.2015.10.015. [DOI] [PubMed] [Google Scholar]
- Rubel F, Brugger K, Chitimia-Dobler L, Dautel H, Meyer-Kayser E, Kahl O. Atlas of ticks (Acari: Ixodidae) in Germany. Exp Appl Acarol. 2021;84:183–214. doi: 10.1007/s10493-021-00619-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp D, Zahn A, Ludwig P. Actual records of bat ectoparasites in Bavaria (Germany) Spixiana. 2004;27(2):185–190. [Google Scholar]
- Sándor AD, Corduneanu A, Péter Á, Mihalca AD, Barti L, Csősz I, Szőke K, Hornok S. Bats and ticks: host selection and seasonality of bat-specialist ticks in eastern Europe. Parasit Vectors. 2019;12:1–10. doi: 10.1186/s13071-019-3861-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffler I. Ektoparasiten der Fledermäuse in Deutschland-neue Erkenntnisse zur Verbreitung, Ökologie und Bedeutung. Beitr Jagd- Wildforsch. 2009;34:193–207. [Google Scholar]
- Schmidt E. Nachweise von Acari bei Chiropteren im Bezirk Neubrandenburg (DDR) Angew Parasitol. 1987;28(2):103–107. [PubMed] [Google Scholar]
- Schulze P. Die kleinhöhlenbewohnenden Zecken der Artengruppe um Ixodes autumnalis Leach 1815. Z Parasitenkd. 1937;9(3):351–372. doi: 10.1007/BF02119889. [DOI] [Google Scholar]
- Skuballa J, Öhme R, Hartelt K, Petney T, Bücher T, Kimmig P, Taraschewski H. European hedgehogs as hosts for Borrelia spp., Germany. Emerg Infect Dis. 2007;13(6):952–953. doi: 10.3201/eid1306.070224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MD. The hedgehog tick, Ixodes hexagonus (Leach, 1815) (Acari: Ixodidae); the natural history and ecology of a nest ectoparasite. Syst Appl Acarol. 2018;23(4):680–714. [Google Scholar]
- Walter G (1992) Verbreitung und Biologie von Argas vespertilionis, Ixodes simplex und Ixodes ricinus (Ixodoidea: Ixodidae; Argasidae) bei Fledermäusen (Chiroptera) in der Bundesrepublik Deutschland. Myotis 30:123–132
- Walter M, Brugger K, Rubel F. The ecological niche of Dermacentor marginatus in Germany. Parasitol Res. 2016;115(6):2165–2174. doi: 10.1007/s00436-016-4958-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber D. Die Höhlenfauna Luxemburgs. Ferrantia 69, Musée national d’histoire naturelle. Luxembourg: Luxembourg; 2013. p. 408. [Google Scholar]
- Weigand A, Teixeira J, Christian S. First record of Hyalomma marginatum sensu stricto C.L. Koch, 1844 and distribution of Dermacentor reticulatus (Fabricius, 1794) (Acari, Ixodidae) in Luxembourg. Bul Soc nat lux. 2020;122:253–263. [Google Scholar]
- Weigand A, Bücs SL, Deleva S, Bilela LL, Nyssen P, Paragamian K et al (2022) Current cave monitoring practices, their variation and recommendations for future improvement in Europe: A synopsis from the 6th EuroSpeleo Protection Symposium. Res Ideas Outcomes 8:e85859
- Yans MW, Branca AS, Hahn NG, Crawley SE, Figurskey AC, Hobson KR, Banfield MG, Borden JH. Development of a simple trap that captures ticks (Acari) on their dorsal surface. J Med Entomol. 2022;59(3):969–975. doi: 10.1093/jme/tjab23. [DOI] [PubMed] [Google Scholar]
- Zaenker S, Bogon K, Weigand A. Die Höhlentiere Deutschlands: Finden-Erkennen–Bestimmen. Wiebelsheim, Germany: Quelle & Meyer Verlag; 2020. p. 448. [Google Scholar]
- Zhang Z, Schwartz S, Wagner L, Miller W. A greedy algorithm for aligning DNA sequences. J Comput Biol. 2000;7(1–2):203–214. doi: 10.1089/10665270050081478. [DOI] [PubMed] [Google Scholar]
- Zittra C, Moog O, Christian E, Fuehrer HP. DNA-aided identification of Culex mosquitoes (Diptera: Culicidae) reveals unexpected diversity in underground cavities in Austria. Parasitol Res. 2019;118:1385–1391. doi: 10.1007/s00436-019-06277-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zittra C, Vitecek S, Teixeira J, Weber D, Schindelegger B, Schaffner F, Weigand AM. Mosquitoes (Diptera: Culicidae) in the Dark—Highlighting the importance of genetically identifying mosquito populations in subterranean environments of Central Europe. Pathogens. 2021;10(9):1090. doi: 10.3390/pathogens10091090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Peña A, Mihalca AD, Petney TN (eds) (eds) (2018) Ticks of Europe and North Africa: A guide to species identification. Springer. 368 pp
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.