Abstract
A semisynthetic antibody phage display library was used to select recombinant antibodies directed against surface components of a pathogenic strain of Streptococcus suis serotype 2 and against extracellular factor (EF), a protein known to be exclusively associated with pathogenic S. suis serotype 2 strains. Three distinct monoclonal phage antibodies directed against conformational epitopes of surface protein components of S. suis were selected. In addition, three different monoclonal phage antibodies were isolated that recognized EF. To isolate antibody fragments that recognize epitopes specific for a pathogenic S. suis serotype 2 strain, compared to a nonpathogenic serotype 2 strain, we applied a subtractive selection procedure. With this procedure, only one distinct phage antibody was found, and it was shown to be directed against EF. This demonstrates the selectivity of the applied procedure and confirms that EF is indeed differentially expressed by pathogenic and nonpathogenic strains. It also shows that EF is a very dominant antigen in phage antibody selections.
Antibody phage display is a very powerful technique for selecting recombinant antibodies from a large library (15, 18, 30). An antibody phage library consists of the variable regions of heavy (VH) and light (VL) chains of human antibodies, which are randomly combined and linked together by a polypeptide linker to form a single-chain fragment (scFv). These scFvs are fused to a minor coat protein of bacteriophage M13, pIII, resulting in phages displaying antibody fragments. The display of scFvs on a filamentous phage offers the possibility to select phage antibodies without using hybridoma technology. Phage antibodies are selected by panning the library for several rounds on an immobilized antigen. At present, large synthetic libraries are available, which are created from unrearranged V gene segments from nonimmunized healthy human donors. These libraries can be used to select antibodies against any given antigen, including foreign antigens, self antigens, nonimmunogenic antigens, and toxic antigens (30). In addition, subtractive selection strategies to select for phage antibodies against differentially expressed structures on the surface of different cell types, like thymic cells (25) and human blood cells (16), as well as against proteins differentially expressed on two types of strains of the gram-negative bacterium Moraxella catarrhalis (2), have been described.
Streptococcus suis is a gram-positive bacterium that can cause severe infections in pigs. Young pigs can suffer from meningitis, septicemia, and arthritis and often do not survive an S. suis infection (3, 27). Occasionally, S. suis can also cause meningitis in humans (1). Until now, no effective vaccines have been available. Besides, very little is known of S. suis in general and its pathogenesis in particular. This makes it difficult to control the disease. So far, 35 capsular serotypes of S. suis have been described (6, 7, 12, 19). Worldwide, S. suis serotype 2 is the most frequently isolated serotype. Strains of serotype 2 can differ in their virulence: pathogenic, weak-pathogenic, and nonpathogenic strains are recognized (26, 28). Previously, we showed that the expression of two proteins, muramidase-released protein (MRP) and extracellular factor (EF) is strongly associated with pathogenic strains of serotype 2 (28, 29). Therefore, these proteins are considered virulence markers for S. suis serotype 2. However, besides MRP and EF, other proteins may be important in the pathogenesis of an S. suis infection (5, 8, 9, 13, 14, 28, 29). The use of a phage display library may be of great help in identifying these proteins and in determining the difference between pathogenic and nonpathogenic S. suis strains.
Since a considerable number of virulence factors of pathogenic bacteria are either secreted or located on the cell surface, we first tried to select phage antibodies against whole cells of a virulent strain of S. suis serotype 2. In addition, phage antibodies were selected against EF. Finally, phage antibodies were selected against cell-associated structures of a pathogenic strain of S. suis after subtraction with a nonpathogenic strain. Three distinct anti-EF phage antibodies, as well as three distinct anti-S. suis phage antibodies, were selected. After subtraction, one phage antibody remained, which recognized EF. These data clearly show the successful selection of a phage antibody directed against a protein exclusively expressed by a pathogenic strain of S. suis serotype 2 and that EF is a very dominant protein in phage antibody selections.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Two Escherichia coli strains were used in this study as hosts for bacteriophages: TG1 [K-12 Δ(lac-pro) supE thi hsdD5/F′ traD36 proA+ B+ lacIq lacZΔM15] and HB2151 [K-12 ara Δ(lac-pro) thi/F′ proA+ B+ lacIq ZΔM15]. Both strains were grown on tryptone yeast extract (TYE) plates (17) containing 1% glucose and antibiotics when required. Cultures were grown in 2× TYE broth (2× TY) (17). S. suis strain 10 expresses EF and MRP, while S. suis strain T15 does not (23, 29). Strain 10 was proven to be pathogenic, while strain T15 was proven to be nonpathogenic in an experimental pig model (23, 29). S. suis strain 10cpsΔEF is an isogenic mutant of S. suis strain 10 that is deficient in capsular polysaccharide production (21). S. suis strains were grown on Columbia agar blood base plates (code CM331; Oxoid, Ltd., London, United Kingdom), containing 6% (vol/vol) horse blood. Cultures were grown in Todd-Hewitt broth (code CM 189; Oxoid, Ltd.).
Preparation of antigens.
Stationary-phase S. suis cells (100 ml) were centrifuged for 20 min at 2,500 × g and washed twice with 100 ml of phosphate-buffered saline (PBS) (0.1 M NaCl, 33 mM Na2HPO4, 17 mM NaH2PO4 · 2H2O; pH 7.4). The cells were resuspended in 50 ml of PBS. This suspension was used for coatings, both for the selection procedure and for the enzyme-linked immunosorbent assay (ELISA). The supernatant was collected for use on Western blots. To prepare protoplast supernatant, exponential-phase S. suis cells (100 ml) were centrifuged for 30 min at 2,500 × g and washed in 100 ml of PBS. The pellet was resuspended in 10 ml of protoplast buffer (30 mM Tris, 3 mM MgCl2, 25% sucrose, 30 μg of lysozyme ml−1 [Boehringer GmbH, Mannheim, Germany]), incubated for 1 h at 37°C, and centrifuged for 10 min at 11,600 × g. The supernatant was collected to coat immunotubes and microtiter plates. It was also used as an antigen on Western blots.
Phage display library.
The Griffin.1 library (a generous gift from Greg Winter, Centre for Protein Engineering, Cambridge, United Kingdom) was used to select phage antibodies. Griffin.1 is a semisynthetic phage library containing more than 109 clones. The library was constructed by recloning the VH and VL variable regions from the lox library vectors into the phagemid vector pHEN2 (11).
Rescue of the phage library.
Rescue of the library was essentially done as described previously (11). Briefly, over 1010 CFU of the Griffin.1 library in strain TG1 were inoculated in 500 ml of 2× TY broth containing 100 μg of ampicillin (AMP) ml−1 (Boehringer) and 1% glucose (GLU) (2× TY-AMP-GLU) and incubated at 37°C (with shaking at 200 rpm) until the optical density at 600 nm (OD600) was approximately 0.5. Twenty-five milliliters of this culture was infected with 1010 PFU of VCS-M13 helper phage (Stratagene, La Jolla, Calif.) and incubated for 30 min at 37°C. The infected cells were collected by centrifugation (10 min at 2,500 × g) and resuspended in 300 ml of 2× TY broth containing 100 μg of AMP ml−1 and 25 μg of kanamycin ml−1 (Boehringer) (2× TY-AMP-kanamycin). Subsequently, the cells were incubated overnight at 37°C and with shaking at 200 rpm. Bacterial cells were removed by centrifugation (30 min at 2,500 × g), and phages present in the supernatant were precipitated with polyethylene glycol-NaCl (20% polyethylene glycol 6000, 2.5 M NaCl) twice and resuspended in PBS to a final concentration of about 1013 PFU ml−1.
Selection procedure.
The selection procedure was performed with immunotubes and was carried out as described previously (18). Briefly, Maxisorp immunotubes (5.0-ml; Nunc, Roskilde, Denmark) were either incubated with 4 ml of purified EF protein (H. J. Wisselink et al., submitted for publication) at a concentration of 10 μg ml−1 or with 4 ml of intact S. suis cells. The tubes were incubated for 1 h at 37°C, followed by an incubation for 16 h at 4°C. Subsequently, the immunotubes were blocked with PBS containing 2% skimmed milk (MPBS) for 2 h at 37°C and washed three times with PBS. The phage library (about 1013 PFU in 2% MPBS) was added, and the tubes were incubated on a turntable for 30 min at room temperature, followed by a standing incubation for 90 min at room temperature. Unbound phages were removed by washing the tubes 20 times with PBS containing 0.1% Tween 20 and 20 times with PBS. Bound phages were eluted in 1 ml of 100 mM triethylamine (for 10 min, while being rotated on a turntable at room temperature). Eluted phages were neutralized with 0.5 ml of 1 M Tris-HCl (pH 7.4) and used to infect E. coli strain TG1. To determine the number of eluted phages, infected E. coli cells were plated in a serial dilution on TYE-AMP-GLU plates. For the subtractive selection, the immunotubes were incubated for 1 h at 37°C either with 4 ml of protoplast supernatant of S. suis strain 10, diluted 1:100 in PBS, or with 4 ml of intact S. suis cells, followed by an incubation for 16 h at 4°C. The phage library (about 1013 PFU of library cells) was preincubated in 2% MPBS for 15 min at room temperature either with 1 ml of undiluted protoplast supernatant or with 1 ml of intact cells of S. suis strain T15, and subsequently the mixture was added to the immunotube. The selection procedure was then performed as described above.
PCR and restriction enzyme analysis of phagemid DNA.
Individual colonies of phage-infected TG1 cells were transferred into a PCR mixture containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 2 mM MgCl2, 10 pmol of each deoxynucleoside triphosphate, 10 pmol of the forward primer fdSeq1 (5′-GAATTTTCTGTATGAGG-3′), 10 pmol of the backward primer LMB3 (5′-CAGGAAACAGCTATGAC-3′), and 2 U of Taq polymerase (Perkin-Elmer, Foster City, Calif.). DNA amplification was carried out with a Perkin-Elmer 9600 thermal cycler, and the program consisted of an incubation for 10 min at 94°C and 30 cycles of 1 min at 94°C, 1 min at 55°C, and 1.5 min at 72°C, followed and 5 min at 72°C. PCR products were analyzed on a 1.5% agarose gel containing 0.5 μg of ethidium bromide ml−1. PCR products were digested with BstNI (New England Biolabs, Beverly, Mass.) or AvaII (Promega, Madison, Wis.). The restriction mixture, containing 50 mM NaCl, 10 mM Tris-HCl (pH 7.9), 10 mM MgCl2, 1 mM dithiothreitol, and 4 U of BstNI or AvaII, was added to the PCR mixture in a 1:1 ratio. The mixture was incubated for 2 h at 60°C for BstNI and for 2 h at 37°C for AvaII. The restriction products were analyzed on a 4% agarose gel containing 50% multipurpose agarose and 50% Metaphor agarose (FMC Bioproducts, Rockland, Maine) and 0.5 μg of ethidium bromide ml−1.
Phage ELISA.
Microtiter plates (Greiner Labortechnik, Frickenhausen, Germany) were coated with purified EF protein or with intact S. suis cells as described above for the coating of the immunotubes. Before being used, the plates were washed three times with PBS and blocked with 2% MPBS for 2 h at 37°C. Polyethylene glycol-precipitated polyclonal phage antibodies (PoPhAbs) or a culture supernatant of superinfected TG1 cells containing monoclonal phage antibodies (MoPhAbs) was used in serial dilutions in 2% MPBS and incubated for 90 min at 37°C. Plates were washed three times with PBS containing 0.05% Tween 20 and three times with PBS. Phages were detected by using the Detection Module Recombinant Phage Antibody system (Pharmacia, Uppsala, Sweden) according to the manufacturer's recommendations.
Expression of soluble antibody fragments.
The E. coli nonsuppressor strain, HB2151, was infected with phages as described for TG1. Individual HB2151 colonies were transferred into 150 μl of 2× TY-AMP-GLU. Plates were incubated at 37°C (with shaking at 200 rpm) until the OD600 of the cells was about 0.9. Isopropyl-β-d-thiogalactopyranoside (IPTG) (Eurogentec, Seraing, Belgium) was added to a final concentration of 1 mM. Plates were incubated for 16 to 24 h at 30°C (with shaking at 200 rpm). Bacteria were centrifuged at 1,800 × g for 10 min. Supernatants containing scFvs were collected for further use.
Western blot analysis.
Proteins were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane by standard procedures (20). The membrane was blocked in Tris-buffered saline (TBS) (50 mM Tris-HCl [pH 7.5], 150 mM NaCl) containing 4% skimmed milk and 0.05% Tween 20 (Blotto) for 1 h. To detect specific antigens, the membranes were incubated with a 1:1 dilution of scFvs in Blotto for at least 90 min. Bound scFvs were visualized with a 1:1,000 dilution of anti-c-myc monoclonal antibody (MAb) (Boehringer) in Blotto-TBS (1:1), followed by an incubation with a 1:1,000 dilution of alkaline phosphatase-conjugated anti-mouse antibody. As a substrate, we used Nitro Blue Tetrazolium (Merck, Darmstadt, Germany)-bromochloroindolyl phosphate (Sigma, St. Louis, Mo.). All washing steps were performed in Blotto-TBS (1:1). A hybridoma-derived MAb directed against EF and convalescent serum raised against S. suis strain 10 in swine were used as positive controls (28).
Dotblot analysis.
Proteins were spotted onto nitrocellulose by using a Bio Dot Apparatus (Bio-Rad Laboratories, Richmond, Calif.) according to the manufacturer's recommendations. Three different protein samples were used: native protoplast supernatant diluted 1:1 in TBS, protoplast supernatant diluted 1:1 in SDS loading buffer (100 mM Tris-HCl [pH 6.8], 4% SDS, 0.2% bromophenol blue, 20% glycerol), and protoplast supernatant diluted 1:1 in native loading buffer (100 mM Tris-HCl [pH 6.8], 10% glycerol, and 0.25‰ bromophenol blue). The blots were blocked in Blotto for 1 h. To detect specific proteins, the membrane was incubated for at least 90 min with a 1:1 dilution of MoPhAbs in Blotto. Phages were detected with the Detection Module Recombinant Phage Antibody System (Pharmacia), according to the manufacturer's recommendations. The signal was visualized by using ECL+ (Amersham Pharmacia Biotech, N.J.) according to the manufacturer's recommendations. Signals were detected on the Storm (Molecular Dynamics, Sunnyvale, Calif.). All washing steps were performed in Blotto-TBS (1:1).
Nucleotide sequence analysis.
Nucleotide sequences were determined with a 373A DNA Sequencing System (Applied Biosystems, Warrington, United Kingdom). Samples were prepared with an ABI/PRISM Dye Terminator Cycle Sequencing Ready Reaction kit (Applied Biosystems). Custom-made sequencing primers were purchased from Life Technologies. Primers used were as follows: Boli 189 (5′-GCCTACGGCAGCCGCTCGAT-3′), FOR_LinkSeq (5′-GCCACCTCCGCCTGAACC-3′), Boli 101 (5′-GGTGGAGGCGGTTCACGCGCAGGTGGCTCT-3′), and fdSeq1 (5′-GAATTTTCTGTATGAGG-3′). Sequencing data were assembled and analyzed with the Lasergene program (DNASTAR). The V-BASE sequence directory described by Tomlinson et al. (24) was used to assign germ line VH and VL segments.
RESULTS
Selection of phage antibodies.
The Griffin.1 phage library was used to select phage antibodies against purified EF protein. Six rounds of selection were performed on EF. Input phage titers were very uniform, between 5.5 × 1012 and 4.7 × 1013. After each round of selection, the number of eluted phages was determined. The results (Table 1) show that phage titers decreased in the first two rounds of selection and rose again in the third round. This indicates antigen-specific phage antibodies were selected and enriched.
TABLE 1.
Phage titers obtained after biopanning on purified EF protein and intact S. suis cells and after a subtractive selection procedure
| Selection round | Phage titer (CFU ml−1)
|
|||
|---|---|---|---|---|
| Standard selection procedure
|
Subtractive selection procedureb
|
|||
| EFa | Intact cells of S. suis strain 10 | Intact cells of S. suis strain 10 | Protoplast supernatant of S. suis strain 10 | |
| 0 | 4.7 × 1013 | 4.7 × 1013 | 5.0 × 1012 | 5.0 × 1012 |
| 1 | 3.0 × 105 | 3.5 × 104 | 1.0 × 105 | 1.5 × 105 |
| 2 | 3.5 × 103 | 6.5 × 103 | 4.5 × 105 | 5.0 × 104 |
| 3 | 2.0 × 106 | 5.3 × 104 | 4.0 × 104 | 7.0 × 105 |
| 4 | 2.0 × 106 | 1.5 × 105 | 5.0 × 103 | 1.0 × 107 |
| 5 | 4.1 × 105 | 3.0 × 105 | 8.3 × 107 | |
| 6 | 1.0 × 108 | |||
Purified protein.
Subtraction with S. suis strain T15.
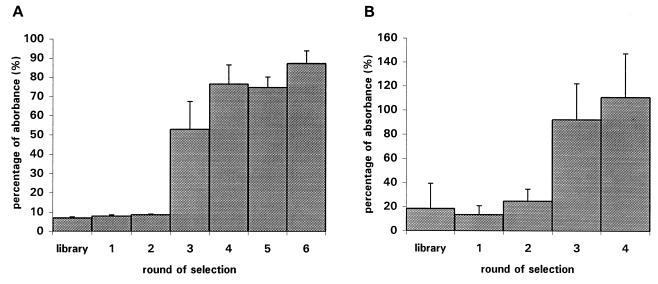
PoPhAbs derived from the library and the successive selection rounds were subsequently tested for their capacity to bind to purified EF protein in an ELISA (Fig. 1). An increase in the absorption signal was observed for the phage antibodies obtained after the second selection round. This indicates that antigen-specific PoPhAbs had been selected and enriched after round 2. To confirm this data further, 96 individual, randomly chosen colonies from each selection round were used to prepare MoPhAbs. These MoPhAbs were subsequently tested with an ELISA for their capacity to bind to EF. As shown in Table 2, the number of MoPhAbs which showed specific binding to EF increased after the successive rounds of selection. The MoPhAbs did not bind to 3% bovine serum albumin, 2% MPBS, Todd-Hewitt medium, or uncoated plates (OD600 < 0.150).
FIG. 1.
Enrichment for phages recognizing purified EF protein (A) or S. suis cells (B) as determined by ELISA. The absorbance of the positive control present in the phage detection kit was set at 100%. The data were expressed as percentages of absorbance relative to that of the control. Every column represents three individual experiments; error bars indicate the standard error of the mean.
TABLE 2.
Specificity of MoPhAbs selected against indicated antigens as determined by ELISA
| Selection round | No. (%)a of MoPhAbs which bind to:
|
|||
|---|---|---|---|---|
| EFb | Intact cells of S. suis strain 10 | Intact cells of S. suis strain 10c | Protoplast supernatant of S. suis strain 10c | |
| 0 | 0 (<0.2) | 0 (<0.2) | 0 (<0.2) | 0 (<0.2) |
| 1 | 0 (<0.2) | Not done | Not done | Not done |
| 2 | 0 (<0.2) | Not done | Not done | 7 (7) |
| 3 | 3 (3) | 50 (52) | Not done | 31 (32) |
| 4 | 22 (23) | 59 (61) | 0 (<0.2) | 41 (43) |
| 5 | 36 (38) | 3 (3) | 22 (23) | |
| 6 | 45 (47) | |||
n = 96.
Purified protein.
After subtraction with S. suis strain T15.
Subsequently, the Griffin.1 library was used to select phage antibodies against intact S. suis strain 10 cells. Four rounds of selection were performed on intact bacterial cells. Input phage titers were between 5.5 × 1012 and 4.7 × 1013. After each selection round, the eluted phage titers were determined. The titers decreased in the first two rounds but increased again in the third round (Table 1), indicating that enrichment of antigen-specific phages had taken place.
PoPhAbs derived from the library and the four rounds of selection were tested for their capacity to bind to intact S. suis cells. Figure 1 shows an increase in the absorption signal for PoPhAbs derived from the third selection round, confirming the selection of antigen-specific phage antibodies. Ninety-six individual, randomly chosen colonies from each selection round were induced to produce MoPhAbs. These MoPhAbs were tested with an ELISA on intact S. suis cells. The number of MoPhAbs that specifically recognized S. suis increased after the successive rounds of selection. The selected MoPhAbs did not bind to 3% bovine serum albumin, 2% MPBS, Todd-Hewitt medium, or uncoated plates (OD600 < 0.150). In conclusion, these data indicate that MoPhAbs specific for purified EF protein and intact S. suis strain 10 cells have been selected and enriched.
Diversity of isolated phage antibodies.
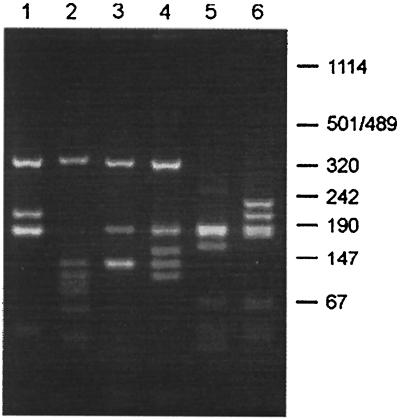
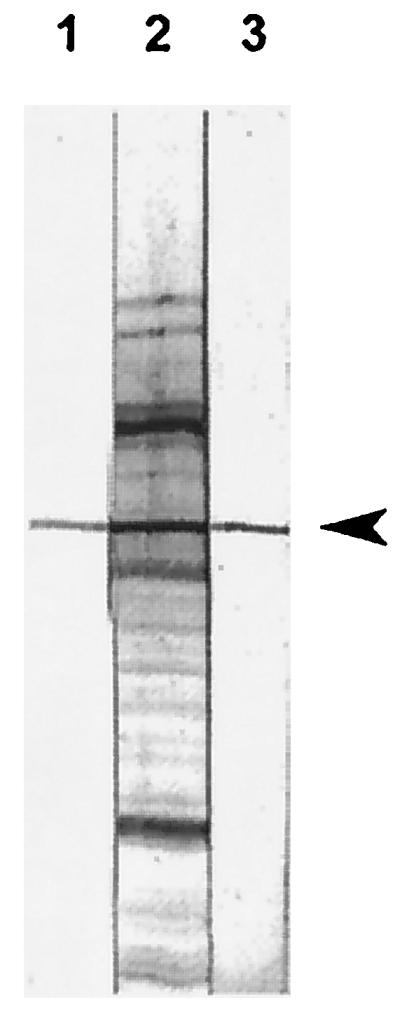
To determine the diversity among the selected phage antibodies, 96 individual clones obtained after the fifth round of selection against purified EF protein and after the third round of selection against S. suis cells were subjected to PCR. The PCR products were analyzed by restriction enzyme analysis. As a control, 10 randomly chosen clones of the original library were analyzed in the same way. Amplification of a complete scFv fragment will result in a PCR product of about 1 kb. Of 96 clones obtained after selection against EF, 36 showed a PCR product of 1 kb and 11 showed a PCR product of 0.7 to 0.8 kb; for 51 clones, no PCR product was obtained. Of the 96 clones obtained after selection against S. suis cells, 52 showed a PCR product of 1 kb and 24 showed a product of 0.7 to 0.8 kb; for 20 clones, no PCR product was obtained. Moreover, nearly all clones which yielded a PCR product of 1 kb were positive by ELISA. In contrast, clones which did not result in a PCR product were never positive by ELISA. From clones which yielded a PCR product of 0.7 to 0.8 kb, the ELISA signal varied between negative and weakly positive (data not shown). From all 10 clones randomly selected from the library, a 1-kb PCR product was obtained. As expected, these 10 clones showed 10 different BstNI restriction patterns. Among the 36 anti-EF clones, three distinct BstNI restriction patterns were found (E-H1, E-D9, and E-H3) (Fig. 2). Of the anti-EF clones, 92% were of the E-H1 type, 6% of the E-H3 type, and 3% of the E-D9 type. Surprisingly, after PCR and restriction analysis of the sixth selection round, only one clone was found, E-H1 (data not shown). This indicates that the increase of phage titers observed after round 6 was the result of enrichment of a subpopulation of the EF-binding clones. Among the 50 anti-S. suis clones, three other unique BstNI restriction patterns were found (S-A7, S-B1, and S-F7) (Fig. 2). Of the anti-S. suis clones, 63% were of the S-B1 type, 12% of the S-F9 type, and 4% of the S-A7 type.
FIG. 2.
BstNI fingerprints of the inserts of phages selected against purified EF protein and S. suis cells. Individual clones were subjected to PCR and restricted with BstNI. Lane 1, EF-specific clone E-H1; lane 2, EF-specific clone E-D9; lane 3, EF-specific clone E-H3; lane 4, S. suis-specific clone S-A7; lane 5, S. suis-specific clone S-B1; lane 6, S. suis-specific clone S-F9. The size of products is indicated in base pairs.
Immunoblot analysis.
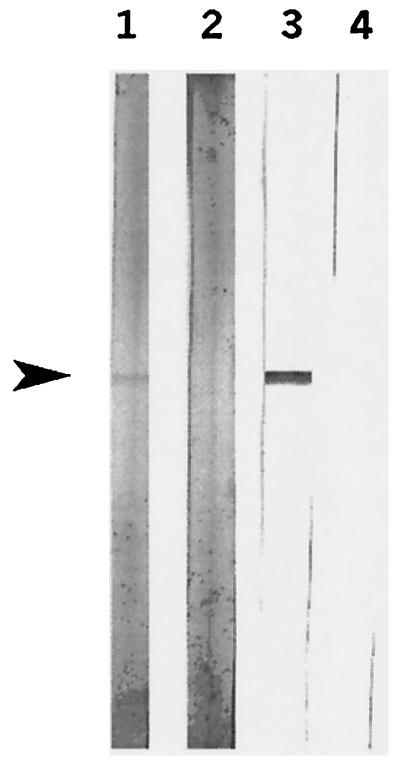
The binding specificity of MoPhAb E-H1 was analyzed as representative for anti-EF selected MoPhAbs with a Western blot of culture supernatant of S. suis strain 10. A hybridoma-derived MAb raised against EF and convalescent serum raised against S. suis strain 10 were included as controls. Figure 3 shows that EF, a 110-kDa protein (28), was clearly detected with the MAb as well as with the convalescent serum. A protein band of the same size was detected by using the scFv preparation of the selected MoPhAb, E-H1. These results show that E-H1 was indeed specifically directed against EF. Therefore, the antibody phage display is a fast and efficient method to select MoPhAbs against purified EF.
FIG. 3.
Western blot analysis of the culture supernatant of S. suis strain 10, probed with a classical MAb raised against EF (lane 1); convalescent serum raised against S. suis (lane 2); and scFvs of clone E-H1 (lane 3). Arrowhead, 110-kDa EF protein.
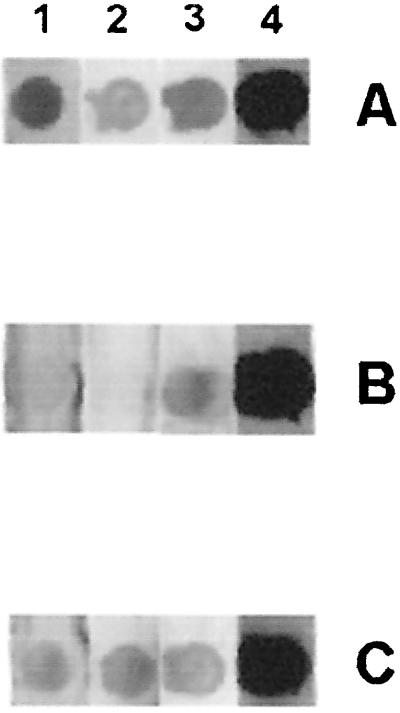
To characterize the MoPhAbs selected against intact S. suis cells, Western blots containing either culture supernatant or protoplast supernatant of S. suis strain 10 were incubated with MoPhAbs or scFvs of the three S. suis-specific clones. No specific band was detected. One possible explanation for this is that the selected MoPhAbs are directed against cytoplasmic components and that these components were not present on the blots used. However, because intact S. suis cells were used in the selection procedure, it is not very likely that the antibodies react with cytoplasmic proteins. Since strain 10 of S. suis serotype 2 is highly encapsulated, phage antibodies selected against intact S. suis cells may be directed against capsular polysaccharides. To test this possibility, we determined the capacity of the three anti-S. suis MoPhAbs to bind to strain 10cpsΔEF, an isogenic mutant of strain 10 deficient in capsular polysaccharide production (20). All three phage antibodies bound to the capsular mutant strain as efficiently as to the wild-type strain (OD600 > 0.350), thereby excluding the possibility that the phage antibodies are directed against capsular polysaccharide components. A further possibility is that the selected MoPhAbs recognize conformational epitopes and therefore do not recognize the denatured and reduced antigens on the Western blot. Protoplast supernatant was spotted on a blot under denaturing and nondenaturing conditions, and the dot blot was subsequently incubated with MoPhAbs prepared from clones S-A7, S-B1, and S-F9. Figure 4 shows that nondenatured proteins present in protoplast supernatant were recognized by the three selected MoPhAbs, whereas the denatured proteins were not recognized, except for clone S-F9, which showed a weak reaction with denatured proteins. This result shows that the MoPhAbs were directed against cell surface proteins of S. suis and that they recognize conformational epitopes.
FIG. 4.
Dot blot analysis of the protoplast supernatant of strain 10, probed with three anti-S. suis MoPhAbs selected against S. suis cells. (A) protoplast supernatant in TBS; (B) protoplast supernatant in SDS loading buffer; (C) native loading buffer. Proteins were either incubated with S-A7 (lane 1), S-B1 (lane 2), or S-F9 (lane 3). Convalescent serum raised against S. suis strain 10 was used as a positive control (lane 4).
Subtractive selection on S. suis strain 10.
To identify proteins exclusively expressed by a pathogenic S. suis serotype 2 strain, phage antibodies were selected against intact cells and against protoplast supernatant of the pathogenic S. suis strain 10, after subtraction with the nonpathogenic S. suis strain T15, lacking protein EF. Five rounds of selection were performed on both antigens. Input phage titers for both selections were fairly constant, between 5 × 1012 and 3.8 × 1013.
For selection on intact cells of S. suis strain 10, phage titers decreased in the first four rounds of selection and increased slightly after the fifth round (Table 1). To test the specificity of the selected phage antibodies, 96 randomly chosen colonies from the library and from selection rounds four and five were induced to produce MoPhAbs. The binding of these MoPhAbs to proteins present on intact cells of S. suis strain 10 was determined by an ELISA. After five rounds of selection, three positive clones were found (Table 2).
For selection on protoplast supernatant of S. suis strain 10, phage titers decreased in the first two rounds of selection and increased again after the fourth round of selection (Table 1), indicating enrichment of antigen-binding phages. To test the specificity of the selected phage antibodies, 96 randomly chosen colonies from each selection round were induced to produce MoPhAbs. The binding of the MoPhAbs to proteins present in the protoplast supernatant of S. suis strain 10 was determined. The number of MoPhAbs which showed specific binding to the protoplast supernatant of S. suis strain 10 in ELISA increased in the successive selection rounds (Table 2), indicating enrichment of S. suis-specific clones.
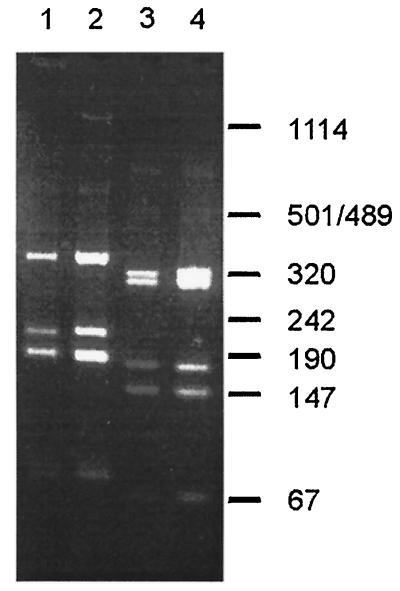
Clones that were positive in the monoclonal phage ELISAs on intact cells or on protoplast supernatant of S. suis strain 10 were subsequently tested by PCR and fingerprint analysis. Ninety-seven of these clones, including the three clones selected on intact S. suis cells, showed a PCR product of 1 kb. Moreover, all 97 clones showed an identical BstNI restriction pattern. Surprisingly, this pattern was identical to the pattern of clone E-H1, indicating that clone E-H1 and the subtractive clone (Sub-B3) are identical. Clones E-H1 and Sub-B3 were further characterized by AvaII fingerprints. Figure 5 shows that the AvaII restriction patterns of E-H1 and Sub-B3 were identical. These data strongly indicate that the phage antibodies selected by the subtractive procedure are similar to those of E-H1 and therefore directed against EF. Since strains 10 and T15 are known to differ in the expression of EF (28, 29), these data suggest that the subtractive selection procedure has succeeded. This was further confirmed by Western blots of culture supernatants of S. suis strains 10 and T15 that were incubated with scFvs derived from Sub-B3. As control, a MAb raised against EF was used. The 110-kDa EF protein was recognized by Sub-B3 and the MAb against EF in culture supernatant of strain 10 but not in culture supernatant of strain T15 (Fig. 6).
FIG. 5.
BstNI and AvaII fingerprints of the inserts of EF-specific clone E-H1 and S. suis-specific clone Sub-B3. Individual clones were subjected to PCR and restricted with BstNI and AvaII. Lane 1, EF specific-clone E-H1 restricted with BstNI; lane 2, S. suis-specific clone Sub-B3 restricted with BstNI; lane 3, EF-specific clone E-H1 restricted with AvaII; lane 4, S. suis-specific clone Sub-B3 restricted with AvaII. The size of products is indicated in base pairs.
FIG. 6.
Western blot analysis of the culture supernatant of S. suis strain 10 (lanes 1 and 3) and strain T15 (lanes 2 and 4) with scFvs of clone Sub-B3 (lanes 1 and 2) and with a classical MAb raised against EF (lanes 3 and 4). Arrowhead, 110-kDa EF protein.
Nucleotide sequence analysis of V regions of selected MoPhAbs.
The nucleotide sequence of all seven selected MoPhAbs was determined and analyzed by use of the V-BASE sequence directory described by Tomlinson et al. (24) (Table 3). As expected, based on their identical restriction patterns, clones E-H1 and Sub-B3, both recognizing EF but selected on different antigens, used the same V genes. Remarkably, however, the CDR3 region of both clones was different, both in amino acid composition and in charge. Both the V genes and the CDR3 sequences of the other five clones were very variable, as was expected based on the large differences between the restriction patterns of those clones.
TABLE 3.
Deduced amino acid sequences of heavy-chain CDR3 and usage of VH and VL genes by seven MoPhAbs selected against EF or S. suis
| Clone | CDR3 sequence | Germ line segmenta
|
|
|---|---|---|---|
| VH | VL | ||
| E-H1 | NKEMP | DP49 | Vλ3 |
| E-D9 | LRETS | DP23 | VλII |
| E-H3 | PALPAFWNT | DP73 | VκI |
| S-A7 | NYVNAPSR | DP15 | VκI |
| S-B1 | LGLPG | DP44 | VλI |
| S-F9 | GTNRP | DP47 | VλI |
| Sub-B3 | ANSNRKF | DP49 | Vλ3 |
Assignment of germ line VH and VL segments is according to the V-BASE sequence directory described by Tomlinson et al. (24).
DISCUSSION
A semisynthetic antibody phage library was used to select recombinant antibodies directed against surface components of a pathogenic strain of S. suis serotype 2, including EF, a protein known to be exclusively associated with pathogenic S. suis serotype 2, including EF, a protein known to be exclusively associated with pathogenic S. suis serotype 2 strains (28, 29). Using purified EF protein as an antigen, three unique anti-EF phage antibodies were selected, probably directed against different linear epitopes of EF. On a Western blot, anti-EF clone E-H1 recognized EF as efficiently as a hybridoma-derived MAb raised against EF. This clearly shows that selection on purified EF protein yielded MoPhAbs specific for EF.
Using intact S. suis serotype 2 cells as an antigen, three distinct phage antibodies were selected. These MoPhAbs recognized proteins present in the protoplast supernatant fraction of S. suis strain 10, indicating that the MoPhAbs are directed against proteins present on the cell surface of S. suis. The phages reacted with nondenatured proteins of encapsulated S. suis and a nonencapsulated mutant, indicating that the MoPhAbs were directed against conformational protein epitopes.
As determined by PCR, only 50% of the selected clones contained a full-sized insert of 1 kb. Twenty-five percent of the clones showed a small-sized product of about 0.7 to 0.8 kb, and 25% did not contain an insert at all. Similar results were described previously by de Bruin et al. (4). These authors also described the selection of phages containing small-sized inserts after using the Griffin.1 library. In addition, they showed that these phages were already present in the original library and represented a few phages that did not obtain a VH region during the construction of the library. In mixed cultures, phages containing small-sized inserts tended to overgrow the phages containing full-sized inserts (4).
With a pathogenic and a nonpathogenic strain in a subtractive selection procedure, one distinct phage antibody (Sub-B3) was selected that seemed to be identical to the phage antibody selected on EF-coated immunotubes (E-H1), as determined by PCR and fingerprint analysis. Nucleotide sequence analysis confirmed that both clones used the same V genes. Remarkably, clones E-H1 and Sub-B3 used different CDR3 regions. Therefore, Sub-B3 may bind to EF with a different affinity or to another epitope than E-H1. Whether this is true remains to be determined. Sub-B3 was shown to recognize EF on a Western blot. This clone was found both after subtractive selection on intact S. suis cells and on protoplast supernatant of S. suis. Since no EF-recognizing scFvs were selected when the library was panned on intact S. suis cells without subtraction, it can be concluded that the subtractive selection procedure was successful.
Clones E-H1 and Sub-B3 seemed to be very dominant in the selection procedure: after selection on purified EF protein, 92% of the anti-EF clones were of the E-H1 type; after subtractive selection both on intact cells and on protoplast supernatant, 100% of the clones were of the Sub-B3 type. Taken together, this indicates that EF is very capable of catching specific phage antibodies. This is the first example of the selection of phage antibodies directed against differentially expressed proteins on gram-positive bacteria after a subtractive selection procedure. So far, no clones against MRP or other differentially expressed proteins were selected, although strain T15 used for subtraction lacks MRP and EF.
To select phages specific for proteins other than EF exclusively expressed by the pathogenic S. suis serotype 2 strains, an isogenic mutant of pathogenic strain 10 deficient in the expression of EF may be helpful.
REFERENCES
- 1.Arends J P, Zanen H C. Meningitis caused by Streptococcus suis in humans. Rev Infect Dis. 1988;10:131–137. doi: 10.1093/clinids/10.1.131. [DOI] [PubMed] [Google Scholar]
- 2.Boel E, Bootsma H, de Kruif J, Jansze M, Klingman K L, van Dijk H, Logtenberg T. Phage antibodies obtained by competitive selection on complement-resistant Moraxella (Branhamella) catarrhalis recognize the high-molecular-weight outer membrane protein. Infect Immun. 1998;66:83–88. doi: 10.1128/iai.66.1.83-88.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clifton-Hadley F A. Streptococcus suis type 2 infections. Br Vet J. 1983;139:1–5. doi: 10.1016/s0007-1935(17)30581-x. [DOI] [PubMed] [Google Scholar]
- 4.de Bruin R, Spelt K, Mol J, Quattrocchio F. Selection of high-affinity antibodies from phage display libraries. Nat Biotechnol. 1999;17:397–399. doi: 10.1038/7959. [DOI] [PubMed] [Google Scholar]
- 5.Feder I, Chengappa M M, Fenwick B, Rider M, Staats J. Partial characterization of Streptococcus suis type 2 hemolysin. J Clin Microbiol. 1994;32:1256–1260. doi: 10.1128/jcm.32.5.1256-1260.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gottschalk M, Higgins R, Jacques M, Beaudoin M, Henrichsen J. Characterization of six new capsular types (23 through 28) of Streptococcus suis. J Clin Microbiol. 1991;29:2590–2594. doi: 10.1128/jcm.29.11.2590-2594.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gottschalk M, Higgins R, Jacques M, Mittal K R, Henrichsen J. Description of 14 new capsular types of Streptococcus suis. J Clin Microbiol. 1989;27:2633–2636. doi: 10.1128/jcm.27.12.2633-2636.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gottschalk M, Lacouture S, Dubreuil J D. Characterization of Streptococcus suis type 2 hemolysin. Microbiology. 1995;141:189–195. doi: 10.1099/00221287-141-1-189. [DOI] [PubMed] [Google Scholar]
- 9.Gottschalk M, Lebrun A, Jacques M, Higgins R. Hemagglutination properties of Streptococcus suis. J Clin Microbiol. 1990;28:2156–2158. doi: 10.1128/jcm.28.9.2156-2158.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffiths A D, Malmqvist M, Marks J D, Bye J M, Embleton M J, McCafferty J, Baier M, Holliger K P, Gorick B D, Hughes-Jones N C, Hoogenboom H R, Winter G. Human anti-self antibodies with high specificity from phage display libraries. EMBO J. 1993;12:725–734. doi: 10.1002/j.1460-2075.1993.tb05706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffiths A D, Williams S C, Hartley O, Tomlinson I M, Waterhouse P, Crosby W L, Kontermann R E, Jones P T, Low N M, Allison T J, et al. Isolation of high affinity human antibodies directly from large synthetic repertoires. EMBO J. 1994;13:3245–3260. doi: 10.1002/j.1460-2075.1994.tb06626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins R, Gottschalk M, Boudreau M, Lebrun A, Henrichsen J. Description of six new capsular types (20 through 34) of Streptococcus suis. J Vet Diagn Investig. 1995;7:405–406. doi: 10.1177/104063879500700322. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs A, Loeffen A C P L, van den Berg A J G, Storm P K. Identification, purification, and characterization of a thiol-activated hemolysin (suilysin) of Streptococcus suis. Infect Immun. 1994;62:1742–1748. doi: 10.1093/benz/9780199773787.article.b00034458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacques M, Gottschalk M, Foiry B, Higgins R. Ultrastructural study of surface components of Streptococcus suis. J Bacteriol. 1990;172:2833–2838. doi: 10.1128/jb.172.6.2833-2838.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marks J D, Hoogenboom H R, Bonnert T P, McCafferty J, Griffiths A D, Winter G. By-passing immunization. Human antibodies from V-gene libraries displayed on phage. J Mol Biol. 1991;222:581–597. doi: 10.1016/0022-2836(91)90498-u. [DOI] [PubMed] [Google Scholar]
- 16.Marks J D, Ouwehand W H, Bye J M, Finnern R, Gorick B D, Boak D, Thorpe S, Hugh-Jones N C, Winter G. Human antibody fragments specific for human blood group antigens from a phage display library. Bio/Technology. 1993;11:1145–1149. doi: 10.1038/nbt1093-1145. [DOI] [PubMed] [Google Scholar]
- 17.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 18.Nissim A, Hoogenboom H R, Tomlinson I M, Midgley C, Lane D, Winter G. Antibody fragments from a ‘single pot’ phage display library as immunochemical reagents. EMBO J. 1994;13:692–698. doi: 10.1002/j.1460-2075.1994.tb06308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perch B, Pedersen K B, Henrichsen J. Serology of capsulated streptococci pathogenic for pigs: six new serotypes of Streptococcus suis. J Clin Microbiol. 1983;19:993–996. doi: 10.1128/jcm.17.6.993-996.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sambrook J, Fritsch E, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 21.Smith H E, Damman M, van der Velde J, Wagenaars F, Wisselink H J, Stockhofe-Zurwieden N, Smits M A. Identification and characterization of the cps locus of Streptococcus suis serotype 2: the capsule protects against phagocytosis and is an important virulence factor. Infect Immun. 1999;67:1750–1756. doi: 10.1128/iai.67.4.1750-1756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith H E, Rijnsburger M, Stockhofe-Zurwieden N, Wisselink H J, Vecht U, Smits M A. Virulent strains of Streptococcus suis serotype 2 and highly virulent strains of Streptococcus suis serotype 1 can be recognized by a unique ribotype profile. J Clin Microbiol. 1997;35:1049–1053. doi: 10.1128/jcm.35.5.1049-1053.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith H E, Vecht U, Wisselink H J, Stockhofe-Zurwieden N, Biermann Y, Smits M A. Mutants of Streptococcus suis types 1 and 2 impaired in expression of muramidase-related protein and extracellular protein induce disease in newborn germfree pigs. Infect Immun. 1996;64:4409–4412. doi: 10.1128/iai.64.10.4409-4412.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomlinson I M, Walter G, Marks J D, Llewleyn M B, Winter G. The repertoire of human germline VH sequences reveals about fifty groups of VH segments with different hypervariable loops. J Mol Biol. 1992;227:776–798. doi: 10.1016/0022-2836(92)90223-7. [DOI] [PubMed] [Google Scholar]
- 25.van Ewijk W, de Kruif J, Germeraad W T, Berendes P, Ropke C, Platenburg P P, Logtenberg T. Subtractive isolation of phage-displayed single-chain antibodies to thymic stromal cells by using intact thymic fragments. Proc Natl Acad Sci USA. 1997;94:3903–3908. doi: 10.1073/pnas.94.8.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vecht U, Arends J P, van der Molen E J, van Leengoed L A M G. Differences in virulence between two strains of Streptococcus suis type 2 after experimentally induced infection of newborn germfree pigs. Am J Vet Res. 1989;50:1037–1043. [PubMed] [Google Scholar]
- 27.Vecht U, van Leengoed L A M G, Verheyen E R M. Streptococcus suis infections in pigs in The Netherlands (Part I) Vet Q. 1985;7:315–321. doi: 10.1080/01652176.1985.9694005. [DOI] [PubMed] [Google Scholar]
- 28.Vecht U, Wisselink H J, van Dijk J E, Smith H E. Virulence of Streptococcus suis type 2 strains in newborn germfree pigs depends on phenotype. Infect Immun. 1992;60:550–556. doi: 10.1128/iai.60.2.550-556.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vecht U, Wisselink H J, Jellema M L, Smith H E. Identification of two proteins associated with virulence of Streptococcus suis type 2. Infect Immun. 1991;59:3156–3162. doi: 10.1128/iai.59.9.3156-3162.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winter G, Griffiths A D, Hawkins R E, Hoogenboom H R. Making antibodies by phage display technology. Annu Rev Immunol. 1994;12:433–455. doi: 10.1146/annurev.iy.12.040194.002245. [DOI] [PubMed] [Google Scholar]