Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection caused worldwide health problems, and coronavirus disease 2019 (COVID-19) was declared a pandemic by the World Health Organization in March 2020. Cardiovascular complications of COVID-19 are not uncommon; among them, arrhythmia is considered a significant risk factor for poor health outcomes in adults. However, data are scarce on the arrhythmia of pediatric patients with SARS-CoV-2 infection, possibly due to their mild symptoms and low incidence of cardiovascular involvement. Multisystem inflammatory syndrome in children reportedly features increased cardiovascular involvement, but arrhythmic complications remain unidentified. Thus, here we review the epidemiology, manifestations, and outcomes of pediatric arrhythmia associated with COVID-19.
Keywords: COVID-19, MIS-C, SARS-CoV-2, Arrhythmia, Child
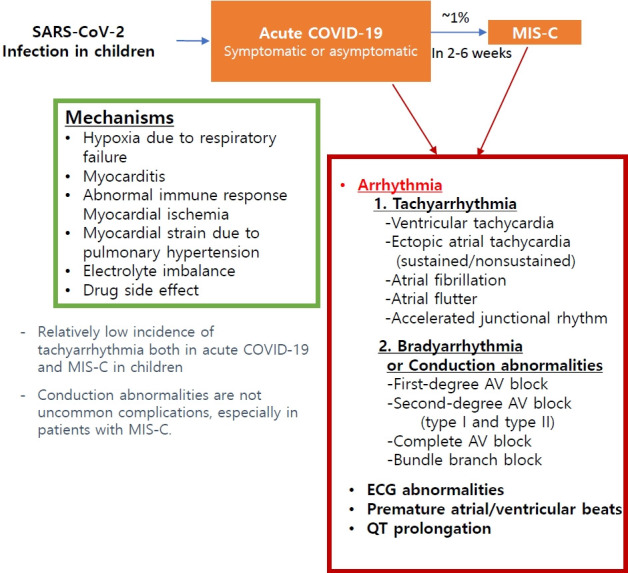
Graphical abstract. Arrhythmia associated with SARS-CoV-2 infection in pediatric patients. SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; COVID-19, coronavirus disease 2019; MIS-C, multisystem inflammatory syndrome in children; AV, atrioventricular; ECG, electrocardiogram.
Introduction
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). The rapid spread of the disease resulted in a global pandemic with 634 million confirmed cases and 6.6 million deaths reported as of late November 2022 by World Health Organization (WHO) [1]. Its clinical presentation ranges from asymptomatic to mild to severe respiratory illness, while its systemic complications include cardiovascular involvement. Its cardiovascular complications include acute myocarditis, pericarditis, vasculitis, heart failure, venous thromboembolism, and arrhythmia, all of which are associated with increased mortality in adults [2-5]. Children generally have milder symptoms than adults, but severe pediatric cases have been described [6]. Furthermore, multisystem inflammatory syndrome in children (MIS-C) with the feature of Kawasaki disease and toxic shock syndrome is a severe postinflammatory complication of SARS-CoV-2 infection reported mainly in children and adolescents [7-9]. Arrhythmia is among the critical cardiovascular complications reported in 18%–44% of adult patients [4,5,10,11]. However, there have been only a few reports of arrhythmia in pediatric patients, possibly related to the lower complication rates and milder disease course in this population. Therefore, the characterization, clinical course, and outcome of arrhythmia in the pediatric population have not been entirely clarified. This review aims to present the current state of knowledge and summarize the literature on arrhythmia associated with COVID-19 in pediatric patients.
Mechanism of cardiovascular complications of COVID-19
Coronaviruses are large and enveloped viruses with singlestranded RNA. While human coronaviruses cause common cold-like respiratory illnesses, SARS-CoV from China during 2002–2003 and Middle East respiratory syndrome coronavirus from the Middle East in 2012 and 2015 made outbreaks with increased fatality rates of 9.6% and 36%, respectively [12,13]. These viruses manifest not only mild respiratory symptoms but also severe illnesses such as acute respiratory distress syndrome and end organ damage.
SARS-CoV-2 utilizes human angiotensin-converting enzyme 2 (ACE2) as a host surface cellular receptor for virus entry similar to SARS-CoV.5,10) SARS-CoV-2 has a surface-located spike glycoprotein that includes a receptor-binding domain that is responsible for membrane fusion followed by endocytosis into target cells [14]. ACE2 expression is tissue-specific with distribution in the heart, intestines, kidneys, testes, and respiratory system, suggesting a correlation with extrapulmonary involvement [15,16]. Once the virus infects cells in the respiratory tract, viral replication and circulation can occur, leading to cardiac uptake and replication of the virus [17]. ACE2, together with ACE, plays a vital role in blood flow and volume via the renin-angiotensin system. ACE2 mRNA expression is upregulated in patients with heart failure, making those individuals more susceptible to SARS-CoV-2 infection and possibly explaining their worse outcomes [18].
The proposed mechanisms of myocardial injury include direct ACE2-mediated injury, hypoxia-induced injury from respiratory failure, microvascular thrombosis, and systemic inflammatory injury from an exaggerated immune response, including cytokine storm [19]. A reported 20%–30% of patients with COVID-19 showed elevated cardiac biomarkers such as troponin and B-type natriuretic peptide in the acute phase, suggesting that direct myocardial injury is not uncommon, especially in moderate to severely ill patients and non-survivors [3,20,21]. Elevated cardiac biomarkers are associated with COVID-19 severity and independent predictors of mortality. Cardiac magnetic resonance imaging has shown myocardial edema, impaired ventricular function, and myocardial fibrosis even after recovery from COVID-19 [19]. In cardiac autopsies, chamber dilatation, lymphocytic myocarditis, focal pericarditis, endocardial thrombosis, and small vessel thrombosis have been reported. Thus, SARS-CoV-2 may directly damage infected cardiac cells with inflammation, triggering severe cellular pathology and organ dysfunction [19].
Cardiovascular involvement of SARS-CoV-2 in children
Children infected with SARS-CoV-2 generally have mild symptoms, with reported mortality rates of <0.1% [6,22-25]. A significant number of children (15%–42%) are asymptomatic, whereas 18%–57% require hospitalization [25-29]. Typical symptoms and signs include fever (40%–64%), cough (33%–56%), fatigue, tachypnea, nasal congestion, feeding difficulty or intolerance, shortness of breath, sore throat, and headache [25-28]. However, severe cases requiring intensive care unit (ICU) admission have also been reported. In a European multicenter study of 582 individuals <18 years of age with polymerase chain reaction (PCR)-confirmed COVID-19, 8% of study participants required ICU admission, 4% required mechanical ventilation, 3% required inotropic support, and 1% required extracorporeal membrane oxygenation (ECMO) support [26]. A meta-analysis by Sumner et al. [6] demonstrated that, among 973 hospitalized school-aged patients, 10.1% required ICU admission, 4.2% had a severe outcome, and 1.1% (95% confidence interval, 0.2%–2.3%) died.
Cardiovascular involvement in children can occur in the setting of acute SARS-CoV-2 infection and post-infectious MIS-C [30]. During acute COVID-19 in children, cardiovascular complications are rare. Only a few case reports or case series have reported myocarditis, pericarditis, cardiogenic shock, and arrhythmia [31-33].
Cardiovascular involvement in children with MIS-C
MIS-C is characterized by hyperinflammatory shock syndrome with multiorgan involvement in previously asymptomatic children with SARS-CoV-2 infection and was first reported in case series in the United Kingdom in April 2020. It showed similar features of atypical Kawasaki disease, Kawasaki disease shock syndrome, or toxic shock syndrome [7]. Subsequently, similar cases began to be reported in series [34-36]. The United States Centers for Disease Control and the WHO termed this syndrome MIS-C [37,38]. It is also called pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 [39]. Among the pediatric patients with PCR-confirmed SARS-CoV-2 infection in an Australian multicenter study, 1.3% had MIS-C.
MIS-C reportedly occurs 2–6 weeks after COVID-19 symptom onset or contact with infected individuals [35,40-42]. Cardiac involvement in MIS-C occurs in up to 80% of patients [42]. Additionally, cardiogenic or vasodilatory shock is present in 37%–60% of MIS-C patients [9,43-46]. Half of one population of patients had reduced left ventricular (LV) ejection fraction on echocardiography, while 9%–17% developed coronary artery dilatation or aneurysm [9,35,44,46]. Myocarditis, valvular regurgitation, and pericardial effusion have also been identified. Arrhythmias or electrocardiographic abnormalities are common, reported in up to 60% of patients [35,47-49]. Outcomes are generally positive, with a resolution of inflammatory and cardiac abnormalities within 1–4 weeks. However, 0.3%–5% of patients received ECMO support, while 0%–2% of patients, most of whom were previously healthy individuals, died [9,35,43,46,48,50].
Arrhythmia in children with COVID-19 or MIS-C
Arrhythmia, reported in 18%–44% of adult patients with COVID-19, is associated with worse clinical health outcomes [4,5,10,11,51]. Among them, the majority (82%) developed atrial arrhythmias, 21% developed ventricular arrhythmias, and 23% had bradyarrhythmia [11]. Potential mechanisms of arrhythmia are hypoxia due to direct viral involvement in the lung, myocarditis, an abnormal host immune response, myocardial ischemia, myocardial strain due to pulmonary hypertension, electrolyte derangements, intravascular volume imbalances, and drugrelated side effects [52]. Arrhythmias are likely not only a direct effect of viral infection but also a complication of systemic illness [51].
The incidence of arrhythmia associated with SARS-CoV-2 in children is much lower, with only a few case series or case reports to date. Tachyarrhythmia in pediatric patients was reported at a rate of 0%–17%, albeit some studies did not specify the type of arrhythmia and included isolated premature ventricular/atrial complexes [35,44,47,48,53,54]. On the other hand, multicenter studies reported the incidence of tachyarrhythmia as 1.7%–1.8%4 [3,55]. Variations in the incidence of arrhythmia may exist among studies because measures depend on the included patient population, such as hospitalized patients, public surveillance, or PCR-confirmed SARS-CoV-2 patients. Moreover, monitoring methods, such as 12-lead electrocardiography and continuous telemetry, may influence the variations. Published articles on tachyarrhythmia in children are summarized in Table 1; these include only those that demonstrated specified types of arrhythmia as well as their management, clinical details, and outcomes. Data for the patients with isolated premature atrial or ventricular complex and studies lacking descriptions of specific types of arrhythmia were omitted.
Table 1.
Summary of studies of patients with tachyarrhythmia
| Study | Age (yr) | No. | Arrhythmia | Condition related to SARS-CoV-2 | Underlying disease | Clinical detail | Management (for antiarrhythmiaa)+COVID-19 treatment) | Outcome | Study type | Country |
|---|---|---|---|---|---|---|---|---|---|---|
| Samuel et al. [54] 2020 November | Median age, 14.5 yr (range, 12-20 yr) | 6/36 (17%) | Nonsustained monomorphic VT (n=5), sus- tained AT (n= 1) | Acute COVID-19 (6), acute myocarditis (2) | No previous heart disease | LV dysfunction (2), large pericardial effusion (1), normal LV function (4) | All hemodynamically tolerated and sefl-lmit- ed arrhythmia.prophy- lactic antiarrhythmic drug; amiodarone (1), beta-blocker (2). Hy- droxychloroquine±azi- thromycin | Self-resolving, no mortality | Observation study | New York, USA |
| Cantarutti et al. [65] 2021 August | Total cohort mean, 9±5.9 | 3/294 (1%) | Nonsustained VT (2), AF (1) | Acute COVID-19 (248), MIS-C (46) | NA | NA | Not requiring emer- gency treatment for arrhythmia. IVIG, cor- ticosteriod, anakinra in most severe pa- teints. | All patients recovered. | Multicenter observation study | Rome, Italy |
| Dionne et al. [55] 2022 | Median, 15.4 yr (range, 10.4–17.4 yr) | 63/3,600 (1.8%) | SVT (28, 44%): reentrant SVT (2), ectopic AT (10), AFL (8), AF (9), accelerated junctional rhy- thm (9, 14%), VT (38, 60%) | Acute COVID-19 (22/1257,1,8%), MIS-C (41/ 2343, 1.7%) | More patients with underlying heart disease in acute COVID-19 | Severe LV dysfunction (31%), more respiratory support (81%), more vaso- pressor requirement and ECMO | No intervention (41%), Antiarrhythmic medi- cation (49%), electri- cal cardioversion (17 %), CPR (13%), ECMO (14%) | 9/63 (14%) died. 22% were discarged with medication. | Multicenter | USA, multicenter |
| Tseng et al. [79] 2021 March | 5 | 1 | Monomorphic VT | Acute COVID-19, fulminant myocarditis | Previously healthy | Cardiogenic shock, biventricular dysfunction | Cardioversion, lidoca- ine, amiodarone → no effect, VA ECMO on HD 4 | Complete reco- very and discharged | Case report | Michgan, USA |
| Kohli et al. [80] 2022 | 15 | 1 | AF | Acute COVID-19, fulminant myocarditis | Previously healthy | Severe LV dysfunction, cardiogenic shock → milrinone, epinephrine, AF on HD2 | Initropics, IVIG, steriod, anakinra, cardiover- sion followed by amio- darone for AF → no recur | NSR, normaliz- ed LV function, no recur, discharged | Case report | Chicago, USA |
| Hopkins and Webster [81] 2021 April | 9 Days, newborn | 1 | SVT | Acute COVID-19 | Normal heart. mother had acute COVID-19 | Orthodromic SVT with aberrancy. Normal LV function | Transesophageal over- drive pacing, oral pro- pranolol 2 mg/kg/day | No recur of SVT, discharged | Case report | Chicago, USA |
| Whittaker et al. [9] 2020 June | NA | 4/58 (6.9%) | Broad complex tachycardia (n=1), AF (n=1), second-degree AVB (n= 1), and first-degree AVB (n=1) | MIS-C | Most were previously healthy (88%) | (1) A patient with wide complex ta- chycardia → low cardiac output- → ECMO, (2) a patient with AF → amiodarone, (3) a patient with 2nd degree AVB NSR | For the total cohort: inotropics in 47%, IVIG in 71%, steroid in 64 %, Anakinra in 5%, and infliximab in 14%, supportive care alone in 22% | NA | Multicenter observation study | England |
| Riollano‐Cruz et al. [82] 2020 June | 14 | 1/15 (6.7%) | VT, QT prolongation | MIS-C | NA | Mild LV dysfunction 48% | Inotropics, amiodarone (not specified for the management of arrhythmia), lidocaine, anakinra, tocilizumab, remdesivir | Recovered LV function, discharged on HD 13 | Obsrvasion study | New York, USA |
| Clark et al. [44] 2020 September | Total cohort mean 7±5.2 | 6/55 (11%) | cAVB (n=3), transient 2nd AVB, sinus pause, 1 st degree AVB, and VT (1) | MIS-C | Previously healthy | All had decreased LV EF (27%–55%) | IVIG, steroid. Not specified for the antiarrhythmic therapy | cAVB normalized within 2 weeks. Other arrhtyhmia out come is not described | Multicenter observation study | International (USA, UK, spain, pakistan) |
| Santi et al. [83] 2020 October | 17 | 1 | AF, nonsustained VT | MIS-C | Previously healthy | Hypotensive → normal saline and epinephrine. Normal heart function, no pulmonary hypertension | AF on HD 3 → DC cardioversion, recurrence of AF → cardioversion and amiodarone, anakinra, IVIG, methylPd | Recovery, discharged home on HD 16 | Case report | Califonia, USA |
| Regan et al. [58] 2021 | 6 | 2/63 (3.2%) | Nonsustained ectopic AT | MIS-C | NA | Asymptomatic | No treatment | Live | Observation study | London, UK |
| 14 | Ectopic AT with RBBB | MIS-C | NA | Cardiogenic shock → ECMO | ECMO support and rate control with amiodarone → died following complications from the ECMO support | Died following complications from the ECMO support | ||||
| Tomlinson et al. [84] 2021 March | 13 | 1 | Accelerated idioventricular rhythm, sinus node dysfunction | MIS-C | Previously healthy | Normal LV EF, hypotension → epinephrine. Sinus node dysfunction, idioventricular rhythym → HD2, sinus ta- chycardia with left axis deviation | No antiarrhythmic drug. IVIG | Normal sinus rhythm on discharge, HD 9 | Case report | Virginia, USA |
| Schneider et al. [85] 2022 | 6 | 1 | VT | MIS-C | Previously healthy | LV dysfunction, cardiogenic shock, brief cardiac arrest VT → VA ECMO | VA ECMO, IVIG, steroid, tosilizumab, and remdesivir | Complete recovery and discharged | Case series | Michgan, USA |
| 15 | 1 | VT | MIS-C | TIDM | Severe both ventricular dysfunction → cardiogenic shock and development of VT | VA ECMO, IVIG, steroid, infliximab, and remdesivir | Decanulated after 4 days of ECMO. Discharged | |||
| Simpson et al. [57] 2020 July | 18 Years | 1 | VT | Acute COVID-19 | HCM, obesity, TIIDM, HTN | Preserved biventricular function→ VVECMO d/t respiratory failure→ escalated to VA ECMO d/t acute decompensated→ HF→VT→ stabilizaed after management | Defibrillation, infusion of amiodarone and lidocaine. Hydroxychloroquine, azithromycin, tocilizumab, convalescent plasma, IVIG, methylPd | Death d/t recurrence of VT on HD 31 | Case series | Multicenter, USA |
| 6 Months | 1 | VT | Acute COVID-19 | Repaired ALCAPA with severe ven- tricular dysfunction | New severe PHT with RV dysfunction, LV EF 20%. During intubation, bradycardia and VT → epinephrine, CPR | Not specified. epinephrine, milrinone, iNO for PHT, tocilizumab, remdesivir | Dischargedhome on HD 35 | Case series | Multicenter, USA |
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; COVID-19, coronavirus disease 2019; LV, left ventricular; VT, ventricular tachycardia; AF, atrial fibrillation; MIS-C, multisystem inflammatory syndrome in children; NA, not available; IVIG, intravenous immunoglobulin; SVT, supraventricular tachycardia; AT, atrial tachycardia; AFL, atrial flutter; CPR, cardiopulmonary resuscitation; ECMO, extracorporeal membrane oxygenation; VA, venoarterial; HD, hospital day; NSR, normal sinus rhythm; AVB, atrioventricular block; cAVB, complete atrioventricular block; RBBB, right bundle branch block; EF, ejection fraction; VV, venovenous; HCM, hypertrophic cardiomyopathy; TIDM, type I diabetes mellitus; TIIDM, type II diabetes mellitus; HF, heart failure; d/t, due to; HTN, hypertension; ALCAPA, anomalous left coronary artery from the pulmonary artery; PHT, pulmonary hypertension; RV, right ventricular; iNO, inhaled nitric oxide.
Arrhythmia management is underlined.
Four patients had underlying extracardiac disease: sickle cell disease (n=1), hematologic malignancy (n=2), Bloom syndrome (n=1)
Samuel et al. [54] reported that 6 patients (17%) among a sample of 36 hospitalized pediatric patients with acute COVID-19 had tachyarrhythmia on continuous telemetry; 5 had nonsustained monomorphic ventricular tachycardia (VT), and one had sustained atrial tachycardia. None had bradyarrhythmia. Among the 6 patients, 4 had underlying disease such as sickle cell anemia, hematologic malignancy, and Bloom syndrome. Two had mild to moderate LV dysfunction and 4 had normal LV systolic function. All arrhythmias were self-limiting, but amiodarone in one and beta-blockers in 2 patients were used for prophylactic purposes.
Most of the reported cases showed a benign disease course or complete recovery with timely and aggressive treatment. However, mortality in pediatric patients with tachyarrhythmia has been reported, and large multicenter studies demonstrated that tachyarrhythmia was associated with poor outcomes [56-58]. Using a public health surveillance registry of 63 hospitals in the United States, Dionne et al. [55] showed the characteristics and outcomes of tachyarrhythmia in patients aged <21 years and hospitalized with acute COVID-19 or MIS-C. Twenty-two of 1,257 patients (1.8%) with acute COVID-19 and 41 of 2,343 patients (1.7%) with MIS-C had tachyarrhythmia, including supra VT (44%), accelerated junctional rhythm (14%), and VT (60%). Among them, 8 (13%) required cardiopulmonary resuscitation, while 9 (14%) required ECMO support because of refractory tachyarrhythmia. Patients with tachyarrhythmia were older and more frequently required mechanical ventilation and ECMO support. They also had higher illness severity on hospital admission, a longer hospital length of stay, and higher mortality rates (14% vs. 2%, P<0.001). Among the patients with tachyarrhythmia, those with acute COVID-19 showed higher mortality rates than those with MIS-C (7 of 22 [32%] vs. 2 of 41 [5%], P=0.006). Although tachyarrhythmia rarely develops in pediatric patients based on the large population data, it may have a substantial clinical impact and require close monitoring and aggressive treatment. Arrhythmia can increase an individual’s risk of death by deteriorating their clinical condition, while tachyarrhythmia can reflect a severe underlying cardiac and respiratory condition.
Conduction abnormalities associated with SARS-CoV-2 (e.g., first-degree atrioventricular [AV] block, second-degree AV block, complete AV block, sinus bradycardia, and bundle branch block) are more common complications than tachyarrhythmia in children. Table 2 shows the clinical characteristics, management, and outcomes of the patients reported in the literature. Bradyarrhythmia was reported in 16%–20% of patients with MIS-C [56,59,60]. MIS-C usually presents several weeks after a viral infection, and a dysregulated inflammatory response is the presumed pathophysiology [41]. Therefore, inflammation of conduction tissue and edema adjacent to the AV nodal or His-Purkinje system can be assumed to contribute to the conduction abnormalities. Coronary insufficiency to the AV node and other conduction systems is also a possible mechanism. Conduction abnormalities such as sinus bradycardia and bundle branch block reportedly developed after the administration of anti-inflammatory drugs during admission in some patients, suggesting drug-related bradyarrhythmia as one etiology [59].
Table 2.
Summary of studies of patients with bradyarrhythmia
| Study | Age (yr) | No. of patients | Arrhythmia | Condition related to SARS-CoV-2 | Past medical history | Clinical detail | Laboratory findings | Management | Outcome | Study type | Country |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lara et al. [62] 2020 | 12 | 1 | Complete AVB | Acute COVID-19, fulminant myocarditis | Previously healthy | Cardiac arrest following severe bradycardia with complete heart block, and hypotension. De- creased LV EF 27% | Elevated BNP, and troponin I | Epinephrine, IVIG | NSR on HD 4, and improved LV function to normal | Case report | Louisiana, USA |
| El-Assaad et al. [86] 2020 May | 10 | 1 | First-degree AVB → complete AVB | Acute COVID-19, myocarditis | Pityriasis lichenoides chronica | Sinus tachy, severe LV dysfunction EF 32% → 1st degree AVB on HD 2. complete degree AVB and 2nd degree AVB on HD 3. | Elevated CRP, D- dimer, BNP, and high-sensitive troponin. | IVIG, anakinra, methylPd, remdesivir. no intervention for bradyarrhythmia | Spontaneously resolved | Case report | Boston, USA |
| Dionne et al. [56] 2020 August | Median 12.1 | 5/25 (20%) | First-degree AV block (n=5) → progressed to second- (n=3) or third- AVB (n=1) | MIS-C | NA | All had ventricular dys- function (LV EF 40%– 55% in 4, <40% in 1). Inotropics support d/t hypotension or shock in 4 patients | Elevated BNP (4/5), normal troponin (5) | No treatment required for the AVB. Inotropics for shock | NSR in all patients | Single center observational study | Boston, USA |
| Carmona et al. [59] 2021 October | 19 | 3 | 1st AVB with RBBB on admission → complete AVB → type 2nd-degree AVB type I 2nddegree AVB → first-degree AVB | MIS-C | Previously healthy | LV EF 40% improved to 50%. Cardiac MRI: sub- epicardial enhancement along the basal inferior wall | Hypotensive, elevated CRP, ESR, and BNP. Mildly elevated troponin | Isoproterenol for cAVB, IVIG, methylPd, azithromycin, Anakinra, tocilizumab | Discharge, normal LV function, persistent firstdegree AVB | Case report | Florida, USA |
| 9 | Sinus bradycardia on HD 8 with prolonged QTc 545 msec | MIS-C | Previously healthy | LV EF 35%–40%, hypotension | Mildly elevated troponin, elevated BNP, CRP | IVIG, methylPd, anakinra, inotropics | Discharged with normal QTc and normal LV EF | ||||
| 9 | RBBB on HD 4 (initially NSR → sinus bradycardia) | MIS-C | Obese | Inicial ormal LV function On HD 3, mild LV dysfunction with RBBB → junctional rhythm, sinus brady 38–48 bpm | Elevated BNP, IL- 6, lactic acid, and CRP. Normal troponin | IVIG, methylPd, anakinra | Discharged with NSR and normal LV function | ||||
| Domico et al. [63] 2020 | 11 | 1 | Sinus bradycardia with 1st and 2nd degree on HD 4 type II 2nd-degree AVB, nonspecific intraventricular conduction delay, nonsustained VT | MIS-C, giant aneurysm in coronary arterie | Previously healthy | Vasogenic shock, normal LV EF → intubation, inotropes → On HD 4, sinus brady with varying degree AV block (1st and 2nd degree) type II 2nd degree AVB | Elevated CRP, ESR, IL-6, and lactate. Serial troponin during admission: within normal range | Temporary transvenous pacing and methylPd, IVIG, infliximab | NSR. Complete recovery before discharge | Case report | Califonia, USA |
| Choi et al. [61] 2020 December | Median 11.5 (range 9–17) | 6/32 (19%) | First-degree AVB (n=6), RBBB (n=1) | MIS-C | NA | Onset of AVB: median 8 days after the initial symptom. No advanced AVB | Elevated CRP, IL- 6, NT pro-BNP, high-sensitive troponin T, LDH, D-dimer | No management for first-degree AVB, IVIG, methylPd, anakinra (13%) | NSR 3 days there after | obs. Study | New York, USA |
| Mehta et al. [64] 2021 | 6 | 1 | Complete AVB with a HR of 32 bpm on admission | MIS-C | Previously healthy | 4 Days after fever onset, Shock with HR 32 bpm with poor perfusion on admission. Mild LV dysfunction | Elevated CRP, LDH, NT pro-BNP, and tropoin I | Isoproterenol/adrenaline followed by temporary PM implantation. IVIG, methylPd, | NSR after 5 days. NSR during fol- lo w-up at 2 months after the illness | Case report | West Bengal, India |
| 7 | 1 | Complete AVB with HR of 26/min on admission | MIS-C | NA | 6 Days after fever onset, shock with HR 26 bpm on admission. mild LV dilatation and dysfunction | Elevated CRP, LDH, NT pro-BNP, and tropoin I | Isoproterenol/adrenaline followed by temporary PM implantation → no recovery for 12 days → permanent PM implantation | Permanent PM. Remained pace- maker dependent at 1 month of follow-up | |||
| Giordano et al. [87] 2021 | 14 | 1 | First-degree AVB | MIS-C | Previously healthy | Hypotensive shock | Elevated CRP, ESR, LDH, D-dimer, and troponin | IVIG, methylPd | Recovered to NSR | Case report | Italy |
| Di Filippo et al. [88] 2021 August | 12 | 1 | First-degree AVB | MIS-C | Previously healthy | Improved clincial condition after IVIG and methylPd, but on day 8 after fever worsening EF 53% with increased BNP, moderate MR and first-degree AVB appeared | Elevated CRP, Ddimer, IL-6, BNP, and troponin | IVIG, methypPd | 12 Days after fever, normal ECG and normal LV EF | Case report | Italy |
| Sisko et al. [89] 2021 October | 8 | 1 | Complete AVB with ventricular escaped beat 30 bpm | Chronic phase of COVID-19 (COVID-19 infection 4 months ago) | Previously healthy | Severe RV dysfunction, severe TR, abdominal pain, marked bradycardia, hepatomegaly | Normal CRP, ESR, and torponin I. Elevated BNP. Diffuse late gadolinium enhancement in RV free wall in MRI | Dopamine, milrinone, IVIG, methylPd, favipiravir, temporary PM implantation on HD#7 | Permanent PM implantation and TV repair on the 19th day on admission | Case report | Izmir, Turkey |
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; AVB, atrioventricular block; COVID-19, coronavirus disease 2019; LV, left ventricular; EF, ejection fraction; BNP, brain natriuretic peptide; bpm, beat for minute; IVIG, intravenous immunoglobulin; NSR, normal sinus rhythm; HD, hospital day; CRP, C-reactive protein; methylPd, methylprednisolone; NA, not available; MIS-C, multisystem inflammatory syndrome in children; MRI, magnetic resonance imaging; ESR, erythrocyte sedimentation rate; cAVB, complete atrioventricular block; RBBB, right bundle branch block; IL-6, interleukin-6; ECG, electrocardiogram; MIS-C, giant aneurysm in coronary arteries; NT pro-BNP, N-terminal-pro-hormone brain natriuretic peptide; HR, heart rate; PM, pacemaker; LDH, lactate dehydrogenase; MR, mitral regurgitation: TR, tricuspid regurgitation; RV, right ventricular.
First-degree AV block was the most common bradyarrhythmia [56,60,61]. It presented at a median 6–8 days after symptom onset, and most patients returned to a normal sinus rhythm spontaneously. A transient complete AV block or second-degree AV block (type I or type II) has been also reported, and some patients required temporary pacemaker insertion [44,56,62]. The incidence of a complete AV block was as low as 2.1%–5%, and most cases recovered a normal sinus rhythm [44,56]. However, some patients who developed a first-degree AV block progressed to a second- or third-degree AV block [56,59,63]. In rare instances, some children required permanent pacemaker implantation, and the first-degree AV block persisted at the time of discharge in some, suggesting the possibility of irreversible damage to the conduction tissue [59,64]. Therefore, patients with conduction abnormalities require intensive electrocardiogram monitoring during hospitalization and long-term follow-up after discharge [56,60,61]. The therapeutic effect of anti-inflammatory drugs on AV block or the preventive effect on the progression to a complete AV block remains unknown because all related studies were retrospective.
Electrocardiographic changes such as nonspecific T wave changes, low QRS amplitudes, an abnormal QRS axis, ventricular hypertrophy, and significant ST-segment changes were findings in 26%–35% of patients with acute COVID-19 and 35%–60% of those with MIS-C [43,44,49,54,58,65].
Repolarization abnormality
There have been reports of QT prolongation or repolarization abnormalities in patients with COVID-19 and MIS-C irrespective of QT-prolonging drug use [44,59,61,65-68]. Repolarization abnormalities, with myocardial injury from acute myocarditis in COVID-19 and a hyperinflammatory state in MIS-C, can increase the risk of malignant arrhythmia in patients with inherited arrhythmia despite no reports to date. Pawar et al. [69] reported neonates with multiple inflammatory syndromes presenting with QTc prolongation with a 2:1 AV block who were born to mothers with a history of COVID-19. An AV block with 2:1 conduction was a functional block due to QT prolongation and disappeared with intravenous immunoglobulin and methylprednisolone, followed by normalization of the QTc interval in all patients.
Furthermore, some drugs used in patients with COVID-19, such as remdesivir, azithromycin, and hydroxychloroquine, can prolong the QTc interval even in patients without underlying disease (www.crediblemeds.org) [38]. An electrolyte imbalance (hypokalemia, hypocalcemia, and hypomagnesemia) and dehydration from a poor oral intake, diarrhea, or vomiting may further increase the QTc interval [52]. So, patients with COVID-19 and an increased risk of QT prolongation require electrocardiography and QTc change monitoring during the illness. As patients with congenital long QT syndrome have an extended QT interval with the increased risk of torsades de pointes, they require electrocardiography monitoring during acute COVID-19 or MIS-C and must avoid QT-prolonging drugs and hypokalemia with COVID-19-associated diarrhea.
Autonomic dysfunction
Palpitations, dizziness, and orthostatic intolerance have been noted in some patients for weeks to months after the initial SARS-CoV-2 infection [70-72]. Postural orthostatic tachycardia syndrome and autonomic dysfunction are among the causes of such symptoms. The proposed mechanisms are dehydration, increased cardiac sympathetic outflow from damaged or altered autonomic nervous system function, and autoimmunity [71]. Although the mechanism is not yet clarified, the symptoms usually significantly improved with lifestyle modifications and medications such as fludrocortisone, midodrine, or beta-blockers [70].
Cardiovascular complications including arrhythmia in patients with congenital heart disease
Patients with underlying cardiac diseases, such as congenital heart disease (CHD) and cardiomyopathy, were initially considered at high risk for mortality and poor outcomes [57,73,74]. Strah et al. [75] demonstrated that pediatric patients with COVID-19 and moderate to severe CHD were younger at admission (1 vs. 11 years), had a longer length of stay, and had higher morbidity and mortality rates than those without CHD. However, other studies exhibited that worse New York Heart Association Functional class ≥III, genetic syndrome, and adults with CHD were significantly associated with the need for hospitalization/respiratory support as well as higher morbidity and mortality rates rather than CHD presence or severity [76,77]. A multicenter study from 58 adult CHD centers that included 1,044 infected patients also demonstrated that the COVID-19 case/fatality rate of 2.3% was similar to that (2.2%) of the general population. Cyanosis, previous heart failure admission, a worse physiological stage, pulmonary arterial hypertension, male sex, renal insufficiency, and diabetes were associated with death, while anatomic complexity was not predictive [78].
Patients with CHD are at increased risk for arrhythmia, but only one case series of arrhythmic events was published during the COVID-19 pandemic [57]. Simpson et al. presented 6 patients with CHD and one patient with hypertrophic cardiomyopathy for acute COVID-19, of whom 2 had newly developed VT requiring cardiopulmonary resuscitation or defibrillation. Both had respiratory failure and decompensated heart failure before the development of VT, and one eventually died of VT recurrence. Although the association between arrhythmia development in CHD patients and COVID-19 has not been identified, a proarrhythmic substrate in repaired or unrepaired CHD in a patient with diastolic and/or systolic dysfunction combined with right ventricular failure from respiratory failure may result in poor outcomes.
Conclusion
Cardiovascular complications are uncommon in children with SARS-CoV-2 infection, whereas those with MIS-C had increased cardiovascular involvement. Tachyarrhythmia was associated with poor clinical outcomes in a large multicenter study. Many patients improved with/without medical treatment, but some required ECMO support. Bradyarrhythmia was relatively common, reported in up to 20% of patients, and more frequently occurred in patients with MIS-C than acute COVID-19. Most patients with conduction abnormalities recovered a normal sinus rhythm, and permanent pacemaker implantation was rarely required.
Pediatric patients have a relatively low incidence of arrhythmic complications, but those with arrhythmia require a timely diagnosis with immediate aggressive treatment. Electrocardiography abnormalities and QT prolongation were not uncommon. Thus, among pediatric patients with acute COVID-19 or MIS-C, especially those who are critically ill, close monitoring for arrhythmia and QT prolongation is required as appropriate management. There are limited data for cardiovascular involvement in pediatric patients, and the effects of SARS-CoV-2 infection are still emerging. Therefore, continuous research with long-term follow-up is required.
Key message
· Pediatric patients have a relatively low incidence of tachyarrhythmia both in acute coronavirus disease 2019 and multisystem inflammatory syndrome in children (MIS-C), but it was associated with an increased risk of poor outcomes.
· Conduction abnormalities were not uncommon, especially in those with MIS-C. Most patients recovered to normal sinus rhythm; however, some progressed to advanced atrioventricular block and rarely required permanent pacemaker implantation.
Footnotes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.World Health Organization . Geneva (Switzerland): World Health Organization; Weekly epidemiological update on COVID-19 [Internet] c2020 [cited 2022 Nov 30]. Available from: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---23-november-2022. [Google Scholar]
- 2.Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:819–24. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–8. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, et al. Clinical characteristics of COVID-19 in New York City. N Engl J Med. 2020;382:2372–4. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sumner MW, Kanngiesser A, Lotfali-Khani K, Lodha N, Lorenzetti D, Funk AL, et al. Severe outcomes associated with SARS-CoV-2 infection in children: a systematic review and meta-analysis. Front Pediatr. 2022;10:916655. doi: 10.3389/fped.2022.916655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–8. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiotos K, Bassiri H, Behrens EM, Blatz AM, Chang J, Diorio C, et al. Multisystem inflammatory syndrome in children during the coronavirus 2019 pandemic: a case series. J Pediatric Infect Dis Soc. 2020;9:393–8. doi: 10.1093/jpids/piaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–69. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colon CM, Barrios JG, Chiles JW, McElwee SK, Russell DW, Maddox WR, et al. Atrial arrhythmias in COVID-19 patients. JACC Clin Electrophysiol. 2020;6:1189–90. doi: 10.1016/j.jacep.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coromilas EJ, Kochav S, Goldenthal I, Biviano A, Garan H, Goldbarg S, et al. Worldwide survey of COVID-19-associated arrhythmias. Circ Arrhythm Electrophysiol. 2021;14:e009458. doi: 10.1161/CIRCEP.120.009458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention . Atlanta (GA): Centers for Disease Control and Prevention; 2011. Severe acute respiratory syndrome [Internet] [cited 2022 Nov 30]. Available from: https://www.cdc.gov/sars/who/clinicians.html. [Google Scholar]
- 13.World Health Organization . Geneva (Switzerland): World Health Organization; c2022. Middle East respiratory syndrome [Internet] [cited 2022 Nov 30]. Available from: http://www.emro.who.int/health-topics/merscov/mers-outbreaks.html. [Google Scholar]
- 14.Derespina KR, Kaushik S, Plichta A, Conway EE, Jr, Bercow A, Choi J, et al. Clinical manifestations and outcomes of critically ill children and adolescents with coronavirus disease 2019 in New York City. J Pediatr. 2020;226:55–63. doi: 10.1016/j.jpeds.2020.07.039. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:E1–9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 16.Sungnak W, Huang N, Becavin C, Berg M, Queen R, Litvinukova M, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681–7. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tucker NR, Chaffin M, Bedi KC, Jr, Papangeli I, Akkad AD, Arduini A, et al. Myocyte-specific upregulation of ACE2 in cardiovascular disease: implications for SARS-CoV-2-mediated myocarditis. Circulation. 2020;142:708–10. doi: 10.1161/CIRCULATIONAHA.120.047911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bristow MR, Zisman LS, Altman NL, Gilbert EM, Lowes BD, Minobe WA, et al. Dynamic regulation of SARS-Cov-2 binding and cell entry mechanisms in remodeled human ventricular myocardium. JACC Basic Transl Sci. 2020;5:871–83. doi: 10.1016/j.jacbts.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung MK, Zidar DA, Bristow MR, Cameron SJ, Chan T, Harding CV, 3rd, et al. COVID-19 and cardiovascular disease: from bench to bedside. Circ Res. 2021;128:1214–36. doi: 10.1161/CIRCRESAHA.121.317997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia de Guadiana-Romualdo L, Morell-Garcia D, Rodriguez-Fraga O, Morales-Indiano C, Maria Lourdes Padilla Jimenez A, Gutierrez Revilla JI, et al. Cardiac troponin and COVID-19 severity: results from BIOCOVID study. Eur J Clin Invest. 2021;51:e13532. doi: 10.1111/eci.13532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheth A, Modi M, Dawson D, Dominic P. Prognostic value of cardiac biomarkers in COVID-19 infection. Sci Rep. 2021;11:4930. doi: 10.1038/s41598-021-84643-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta NS, Mytton OT, Mullins EWS, Fowler TA, Falconer CL, Murphy OB, et al. SARS-CoV-2 (COVID-19): what do we know about children? A systematic review. Clin Infect Dis. 2020;71:2469–79. doi: 10.1093/cid/ciaa556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parcha V, Booker KS, Kalra R, Kuranz S, Berra L, Arora G, et al. A retrospective cohort study of 12,306 pediatric COVID-19 patients in the United States. Sci Rep. 2021;11:10231. doi: 10.1038/s41598-021-89553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith C, Odd D, Harwood R, Ward J, Linney M, Clark M, et al. Deaths in children and young people in England after SARS-CoV-2 infection during the first pandemic year. Nat Med. 2022;28:185–92. doi: 10.1038/s41591-021-01578-1. [DOI] [PubMed] [Google Scholar]
- 25.Irfan O, Muttalib F, Tang K, Jiang L, Lassi ZS, Bhutta Z. Clinical characteristics, treatment and outcomes of paediatric COVID-19: a systematic review and meta-analysis. Arch Dis Child. 2021;106:440–8. doi: 10.1136/archdischild-2020-321385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gotzinger F, Santiago-Garcia B, Noguera-Julian A, Lanaspa M, Lancella L, Calo Carducci FI, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4:653–61. doi: 10.1016/S2352-4642(20)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yasuhara J, Kuno T, Takagi H, Sumitomo N. Clinical characteristics of COVID-19 in children: a systematic review. Pediatr Pulmonol. 2020;55:2565–75. doi: 10.1002/ppul.24991. [DOI] [PubMed] [Google Scholar]
- 28.Li B, Zhang S, Zhang R, Chen X, Wang Y, Zhu C. Epidemiological and clinical characteristics of COVID-19 in children: a systematic review and meta-analysis. Front Pediatr. 2020;8:591132. doi: 10.3389/fped.2020.591132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wurzel D, McMinn A, Hoq M, Blyth CC, Burgner D, Tosif S, et al. Prospective characterisation of SARS-CoV-2 infections among children presenting to tertiary paediatric hospitals across Australia in 2020: a national cohort study. BMJ Open. 2021;11:e054510. doi: 10.1136/bmjopen-2021-054510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jone PN, John A, Oster ME, Allen K, Tremoulet AH, Saarel EV, et al. SARS-CoV-2 infection and associated cardiovascular manifestations and complications in children and young adults: a scientific statement from the American Heart Association. Circulation. 2022;145:e1037–52. doi: 10.1161/CIR.0000000000001064. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez-Gonzalez M, Castellano-Martinez A, Cascales-Poyatos HM, Perez-Reviriego AA. Cardiovascular impact of COVID-19 with a focus on children: a systematic review. World J Clin Cases. 2020;8:5250–83. doi: 10.12998/wjcc.v8.i21.5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dimopoulou D, Spyridis N, Dasoula F, Krepis P, Eleftheriou E, Liaska M, et al. Pericarditis as the main clinical manifestation of COVID-19 in adolescents. Pediatr Infect Dis J. 2021;40:e197–9. doi: 10.1097/INF.0000000000003096. [DOI] [PubMed] [Google Scholar]
- 33.Garau G, Joachim S, Duliere GL, Melissopoulou M, Boccar S, Fraipont V, et al. Sudden cardiogenic shock mimicking fulminant myocarditis in a surviving teenager affected by severe acute respiratory syndrome coronavirus 2 infection. ESC Heart Fail. 2021;8:766–73. doi: 10.1002/ehf2.13049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–8. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belhadjer Z, Meot M, Bajolle F, Khraiche D, Legendre A, Abakka S, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142:429–36. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 36.Toubiana J, Poirault C, Corsia A, Bajolle F, Fourgeaud J, Angoulvant F, et al. Kawasaki-like multisystem inflammatory syndrome in children during the COVID-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention . Atlanta (GA): Centers for Disease Control and Prevention; 2020. Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19), 2020 [Internet] [cited 2022 Nov 30]. Available from: https://emergency.cdc.gov/han/2020/han00432.asp. [Google Scholar]
- 38.World Health Organization . Geneva (Switzerland): World Health Organization; 2020. Multisystem inflammatory syndrome in children and adolescents with COVID-19: scientific brief, 15 May 2020 [Internet] [cited 2022 Nov 30]. Available from: https://apps.who.int/iris/handle/10665/332095. [Google Scholar]
- 39.Harwood R, Allin B, Jones CE, Whittaker E, Ramnarayan P, Ramanan AV, et al. A national consensus management pathway for paediatric inflammatory multisystem syndrome temporally associated with COVID-19 (PIMS-TS): results of a national Delphi process. Lancet Child Adolesc Health. 2021;5:133–41. doi: 10.1016/S2352-4642(20)30304-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belot A, Antona D, Renolleau S, Javouhey E, Hentgen V, Angoulvant F, et al. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May 2020. Euro Surveill. 2020;25:2001010. doi: 10.2807/1560-7917.ES.2020.25.22.2001010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alsaied T, Tremoulet AH, Burns JC, Saidi A, Dionne A, Lang SM, et al. Review of cardiac involvement in multisystem inflammatory syndrome in children. Circulation. 2021;143:78–88. doi: 10.1161/CIRCULATIONAHA.120.049836. [DOI] [PubMed] [Google Scholar]
- 42.Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–46. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valverde I, Singh Y, Sanchez-de-Toledo J, Theocharis P, Chikermane A, Di Filippo S, et al. Acute cardiovascular manifestations in 286 Children with multisystem inflammatory syndrome associated with COVID-19 infection in Europe. Circulation. 2021;143:21–32. doi: 10.1161/CIRCULATIONAHA.120.050065. [DOI] [PubMed] [Google Scholar]
- 44.Clark BC, Sanchez-de-Toledo J, Bautista-Rodriguez C, Choueiter N, Lara D, Kang H, et al. Cardiac abnormalities seen in pediatric patients during the SARS-CoV2 pandemic: an international experience. J Am Heart Assoc. 2020;9:e018007. doi: 10.1161/JAHA.120.018007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aggarwal A, Cohen E, Figueira M, Sabharwal V, Herlihy JM, Bronwen C, et al. Multisystem inflammatory syndrome in an adult with COVID-19-a trial of anakinra: a case report. Infect Dis Clin Pract (Baltim Md) 2021;29:e420–3. doi: 10.1097/IPC.0000000000001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Belay ED, Abrams J, Oster ME, Giovanni J, Pierce T, Meng L, et al. Trends in geographic and temporal distribution of US children with multisystem inflammatory syndrome during the COVID-19 pandemic. JAMA Pediatr. 2021;175:837–45. doi: 10.1001/jamapediatrics.2021.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheung EW, Zachariah P, Gorelik M, Boneparth A, Kernie SG, Orange JS, et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA. 2020;324:294–6. doi: 10.1001/jama.2020.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feldstein LR, Tenforde MW, Friedman KG, Newhams M, Rose EB, Dapul H, et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. 2021;325:1074–87. doi: 10.1001/jama.2021.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramcharan T, Nolan O, Lai CY, Prabhu N, Krishnamurthy R, Richter AG, et al. Paediatric inflammatory multisystem syndrome: temporally associated with SARS-CoV-2 (PIMS-TS): cardiac features, management and short-term outcomes at a UK tertiary paediatric hospital. Pediatr Cardiol. 2020;41:1391–401. doi: 10.1007/s00246-020-02391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Farooqi KM, Chan A, Weller RJ, Mi J, Jiang P, Abrahams E, et al. Longitudinal outcomes for multisystem inflammatory syndrome in children. Pediatrics. 2021;148:e2021051155. doi: 10.1542/peds.2021-051155. [DOI] [PubMed] [Google Scholar]
- 51.Bhatla A, Mayer MM, Adusumalli S, Hyman MC, Oh E, Tierney A, et al. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020;17:1439–44. doi: 10.1016/j.hrthm.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dherange P, Lang J, Qian P, Oberfeld B, Sauer WH, Koplan B, et al. Arrhythmias and COVID-19: a review. JACC Clin Electrophysiol. 2020;6:1193–204. doi: 10.1016/j.jacep.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sperotto F, Friedman KG, Son MBF, VanderPluym CJ, Newburger JW, Dionne A. Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: a comprehensive review and proposed clinical approach. Eur J Pediatr. 2021;180:307–22. doi: 10.1007/s00431-020-03766-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Samuel S, Friedman RA, Sharma C, Ganigara M, Mitchell E, Schleien C, et al. Incidence of arrhythmias and electrocardiographic abnormalities in symptomatic pediatric patients with PCR-positive SARS-CoV-2 infection, including drug-induced changes in the corrected QT interval. Heart Rhythm. 2020;17:1960–6. doi: 10.1016/j.hrthm.2020.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dionne A, Friedman KG, Young CC, Newhams MM, Kucukak S, Jackson AM, et al. Tachyarrhythmias during hospitalization for COVID-19 or multisystem inflammatory syndrome in children and adolescents. J Am Heart Assoc. 2022;11:e025915. doi: 10.1161/JAHA.122.025915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dionne A, Mah DY, Son MBF, Lee PY, Henderson L, Baker AL, et al. Atrioventricular block in children with multisystem inflammatory syndrome. Pediatrics. 2020;146:e2020009704. doi: 10.1542/peds.2020-009704. [DOI] [PubMed] [Google Scholar]
- 57.Simpson M, Collins C, Nash DB, Panesar LE, Oster ME. Coronavirus disease 2019 infection in children with pre-existing heart disease. J Pediatr. 2020;227:302–7.e2. doi: 10.1016/j.jpeds.2020.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Regan W, O'Byrne L, Stewart K, Miller O, Pushparajah K, Theocharis P, et al. Electrocardiographic changes in children with multisystem inflammation associated with COVID-19: associated with coronavirus disease 2019. J Pediatr. 2021;234:27–32.e2. doi: 10.1016/j.jpeds.2020.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carmona CA, Levent F, Lee K, Trivedi B. Atrioventricular conduction abnormalities in multisystem inflammatory syndrome in children. Case Rep Pediatr. 2021;2021:6124898. doi: 10.1155/2021/6124898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sokunbi O, Akinbolagbe Y, Akintan P, Oyeleke G, Kusimo O, Owowo U, et al. Clinical presentation and short-term outcomes of multisystemic inflammatory syndrome in children in Lagos, Nigeria during the COVID-19 pandemic: a case series. EClinicalMedicine. 2022;49:101475. doi: 10.1016/j.eclinm.2022.101475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Choi NH, Fremed M, Starc T, Weller R, Cheung E, Ferris A, et al. MIS-C and cardiac conduction abnormalities. Pediatrics. 2020;146:e2020009738. doi: 10.1542/peds.2020-009738. [DOI] [PubMed] [Google Scholar]
- 62.Lara D, Young T, Del Toro K, Chan V, Ianiro C, Hunt K, et al. Acute fulminant myocarditis in a pediatric patient with COVID-19 infection. Pediatrics. 2020;146:e20201509. doi: 10.1542/peds.2020-1509. [DOI] [PubMed] [Google Scholar]
- 63.Domico M, McCanta AC, Hunt JL, Ashouri N, Nugent D, Kelly RB. High-grade heart block requiring transvenous pacing associated with multisystem inflammatory syndrome in children during the COVID-19 pandemic. HeartRhythm Case Rep. 2020;6:811–4. doi: 10.1016/j.hrcr.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mehta R, Ghosh S, Nandy JD, Das S, Chattopadhyay A. Atypical presentation of complete heart block in children with pediatric inflammatory multisystem syndrome: a case series of two patients. Ann Pediatr Cardiol. 2021;14:408–11. doi: 10.4103/apc.apc_96_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cantarutti N, Battista V, Adorisio R, Cicenia M, Campanello C, Listo E, et al. Cardiac manifestations in children with SARS-COV-2 infection: 1-year pediatric multicenter experience. Children (Basel) 2021;8:717. doi: 10.3390/children8080717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mahmoudi E, Mollazadeh R, Mansouri P, Keykhaei M, Mirshafiee S, Hedayat B, et al. Ventricular repolarization heterogeneity in patients with COVID-19: original data, systematic review, and meta-analysis. Clin Cardiol. 2022;45:110–8. doi: 10.1002/clc.23767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cozzolino D, Romano C, Nevola R, Marrone A, Umano GR, Cuomo G, et al. COVID-19 and arrhythmia: The factors associated and the role of myocardial electrical impulse propagation. An observational study based on cardiac telemetric monitoring. Front Cardiovasc Med. 2022;9:912474. doi: 10.3389/fcvm.2022.912474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ece I, Kocoglu M, Kavurt AV, Bagrul D, Gul AEK, Koca S, et al. Assessment of cardiac arrhythmic risk in children with COVID-19 infection. Pediatr Cardiol. 2021;42:264–8. doi: 10.1007/s00246-020-02474-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pawar R, Gavade V, Patil N, Mali V, Girwalkar A, Tarkasband V, et al. Neonatal multisystem inflammatory syndrome (MIS-N) associated with prenatal maternal SARS-CoV-2: a case series. Children (Basel) 2021;8:572. doi: 10.3390/children8070572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Drogalis-Kim D, Kramer C, Duran S. Ongoing dizziness following acute COVID-19 infection: a single center pediatric case series. Pediatrics. 2022;150:e2022056860. doi: 10.1542/peds.2022-056860. [DOI] [PubMed] [Google Scholar]
- 71.Goldstein DS. The possible association between COVID-19 and postural tachycardia syndrome. Heart Rhythm. 2021;18:508–9. doi: 10.1016/j.hrthm.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blitshteyn S, Whitelaw S. Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID-19 infection: a case series of 20 patients. Immunol Res. 2021;69:205–11. doi: 10.1007/s12026-021-09185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rakha S, Sobh A, Hager AH, Hafez M, Alsawah GA, Abuelkheir MM, et al. Cardiac implications of multisystem inflammatory syndrome associated with COVID-19 in children under the age of 5 years. Cardiol Young. 2022;32:800–5. doi: 10.1017/S1047951121003140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID-19 infection: different points from adults. Pediatr Pulmonol. 2020;55:1169–74. doi: 10.1002/ppul.24718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Strah DD, Kowalek KA, Weinberger K, Mendelson J, Hoyer AW, Klewer SE, et al. Worse hospital outcomes for children and adults with COVID-19 and congenital heart disease. Pediatr Cardiol. 2022;43:541–6. doi: 10.1007/s00246-021-02751-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lewis MJ, Anderson BR, Fremed M, Argenio M, Krishnan U, Weller R, et al. Impact of coronavirus disease 2019 (COVID-19) on patients with congenital heart disease across the Lifespan: the experience of an academic congenital heart disease center in New York City. J Am Heart Assoc. 2020;9:e017580. doi: 10.1161/JAHA.120.017580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sabatino J, Ferrero P, Chessa M, Bianco F, Ciliberti P, Secinaro A, et al. COVID-19 and congenital heart disease: results from a nationwide survey. J Clin Med. 2020;9:1774. doi: 10.3390/jcm9061774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Broberg CS, Kovacs AH, Sadeghi S, Rosenbaum MS, Lewis MJ, Carazo MR, et al. COVID-19 in adults with congenital heart disease. J Am Coll Cardiol. 2021;77:1644–55. doi: 10.1016/j.jacc.2021.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tseng YS, Herron C, Garcia R, Cashen K. Sustained ventricular tachycardia in a paediatric patient with acute COVID-19 myocarditis. Cardiol Young. 2021;31:1510–2. doi: 10.1017/S1047951121000792. [DOI] [PubMed] [Google Scholar]
- 80.Kohli U, Meinert E, Chong G, Tesher M, Jani P. Fulminant myocarditis and atrial fibrillation in child with acute COVID-19. J Electrocardiol. 2022;73:150–2. doi: 10.1016/j.jelectrocard.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hopkins KA, Webster G. Aberrated supraventricular tachycardia associated with neonatal fever and COVID-19 infection. BMJ Case Rep. 2021;14:e241846. doi: 10.1136/bcr-2021-241846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Riollano-Cruz M, Akkoyun E, Briceno-Brito E, Kowalsky S, Reed J, Posada R, et al. Multisystem inflammatory syndrome in children related to COVID-19: a New York City experience. J Med Virol. 2021;93:424–33. doi: 10.1002/jmv.26224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Santi AD, Aquino P, Dorfman M. Atrial fibrillation in a child with COVID-19 infection. Cardiol Young. 2021;31:318–21. doi: 10.1017/S1047951120003893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tomlinson LG, Cohen MI, Levorson RE, Tzeng MB. COVID-19-associated multisystem inflammatory syndrome in children presenting uniquely with sinus node dysfunction in the setting of shock. Cardiol Young. 2021;31:1202–4. doi: 10.1017/S1047951121000354. [DOI] [PubMed] [Google Scholar]
- 85.Schneider J, Tilford B, Safa R, Dentel J, Veenstra M, Ang J, et al. Extracorporeal membrane oxygenation for multisystem inflammatory syndrome in children. Perfusion. 2022;37:639–42. doi: 10.1177/02676591211020904. [DOI] [PubMed] [Google Scholar]
- 86.El-Assaad I, Hood-Pishchany MI, Kheir J, Mistry K, Dixit A, Halyabar O, et al. Complete heart block, severe ventricular dysfunction, and myocardial inflammation in a child with COVID-19 infection. JACC Case Rep. 2020;2:1351–5. doi: 10.1016/j.jaccas.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Giordano S, Failla MC, Li Cavoli MG, Romano D, Vanella V, Caruso C, et al. A 7-year-old goy and a 14-year-old girl initially diagnosed with toxic shock syndrome and tested positive for SARS-CoV-2 infection, supporting a diagnosis of multisystem inflammatory syndrome in children (MIS-C) Am J Case Rep. 2021;22:e931570. doi: 10.12659/AJCR.931570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Di Filippo P, Raso M, Cacciatore M, Patacchiola R, Renda G, Rossi N, et al. Case report: mitral valve involvement and first-degree atrial-ventricular block in two patients with multisystem inflammatory syndrome in children. Front Pediatr. 2021;9:676934. doi: 10.3389/fped.2021.676934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sisko SG, Atik SU, Karadeniz C, Guzeltas A, Ergul Y. Complete heart block, severe right ventricular dysfunction in a child with COVID-19 infection. Cardiol Young. 2022;32:1001–3. doi: 10.1017/S1047951121004248. [DOI] [PMC free article] [PubMed] [Google Scholar]


