Abstract
Anthocyanins are a valuable source of antioxidants in the human diet and contribute to fruit coloration. In red-skinned pears, anthocyanin biosynthesis can be induced by light, in which the MYB–bHLH–WDR complex plays a critically important role in transcriptional regulation. However, knowledge of WRKY-mediated transcriptional regulation of light-induced anthocyanin biosynthesis is scarce in red pears. This work identified and functionally characterized a light-inducing WRKY transcription factor, PpWRKY44, in pear. Functional analysis based on overexpressed pear calli showed that PpWRKY44 promoted anthocyanin accumulation. Also, transiently overexpressed PpWRKY44 in pear leaves and fruit peels significantly enhanced the accumulation of anthocyanin, whereas silencing PpWRKY44 in pear fruit peels impaired induction of the accumulation of anthocyanin by light. By chromatin immunoprecipitation and electrophoretic mobility shift assay coupled to a quantitative polymerase chain reaction, we found that PpWRKY44 bound in vivo and in vitro to the PpMYB10 promoter, revealing it as a direct downstream target gene. Moreover, PpWRKY44 was activated by PpBBX18, a light signal transduction pathway component. Our results explained the mechanism mediating the impacts of PpWRKY44 on the transcriptional regulation of anthocyanin accumulation, with potential implications for fine-tuning the fruit peel coloration triggered by light in red pears.
Introduction
Pear (Pyrus L.), as one of the fruit crops produced in temperate regions, is economically significant thanks to the health benefits accompanying its edible fruit. In recent years, red-skinned pears have emerged as a fruit popular among consumers. The red pear fruit skin is attributable to anthocyanin accumulation [1]. Anthocyanins are an important secondary metabolite belonging to a class of phenylpropanoid compounds called flavonoids [2]. Their beneficial effects on humans are related to their health-promoting antioxidative properties, which can protect against cardiovascular disorders and degenerative diseases [3, 4]. In plants, anthocyanins perform various functions, such as fertility, defensive responses against plant pathogens, protection against UV light, and antioxidant activity [5, 6]. Thus, the regulatory systems controlling anthocyanin biosynthesis are the main focus of research.
Anthocyanin biosynthesis occurs within the phenylpropanoid pathway as part of the flavonoid branch. It is executed via a series of structural genes and is catalyzed by numerous well-documented enzymes. These enzymes include phenylalanine ammonia-lyase (PAL), chalcone synthase (CHS), chalcone isomerase (CHI), flavanone 3-hydroxylase (F3H), dihydroflavonol-reductase (DFR), anthocyanidin synthase (ANS), and UDP-glucose:flavonoid 3-glucosyltransferase (UFGT) [5, 7]. The transcriptional regulation of the genes encoding these enzymes is tuned by a conserved MYB–bHLH–WDR (MBW) complex, which comprises MYB transcription factors (TFs), basic helix–loop–helix (bHLH) TFs, and WD-repeat proteins (e.g. WD40) [7, 8]. R2R3-MYB TFs are among the best-characterized TFs as critical transcriptional regulators of anthocyanin structural genes [9]. The knockdown of FvMYB10 resulted in the production of white strawberry fruit [10]. In apple, three MYB genes, MdMYB10, MdMYB1, and MdMYBA, which are closely related homologs of Arabidopsis AtMYB75/PAP1 and AtMYB90/PAP2, encode TFs that directly trigger the transcriptional activation of anthocyanin structural genes [11–13]. In pear, both PpMYB10 [14] and PpMYB114 [15] are positively correlated with anthocyanin accumulation by directly acting upstream of anthocyanin structural genes. MYB-mediated regulation of anthocyanin accumulation at the transcriptional and post-translational levels depends on environmental stimuli, including low temperatures, water, salt, and light [16–18].
Light, an important environmental signal, strongly affects anthocyanin biosynthesis in several plant species [12, 19]. Light signals can be sensed by receptors and converted into physiological responses (e.g. anthocyanin biosynthesis) via various signal transduction pathways, in which MYB TFs play a critically essential role. In petunia (Petunia hybrida), anthocyanin accumulation in vegetative organs induced by light is tightly correlated to the expression levels of genes encoding anthocyanin-associated MYB TFs [16]. ELONGATED HYPOCOTYL 5 (HY5), the central regulator of the signaling transduction pathway responsive to light, increases anthocyanin accumulation due to the direct regulation of anthocyanin-associated genes (MYB and structural genes) in several plant species [20, 21]. Additionally, our previous reports showed that two B-box proteins, PpBBX16 and PpBBX18, are light-dependent and can function together with PpHY5 to mediate anthocyanin accumulation in pear. Both proteins require PpHY5 to induce the expression of the anthocyanin biosynthesis regulatory gene PpMYB10 in pear [22, 23]. Other TFs, such as NAC, ERF, and WRKY, also affect transcriptional regulation of light-dependent anthocyanin biosynthesis in various fruit species [9] by functioning alone or as part of multiprotein complexes.
The WRKY TF is one of the main TFs in plants. It has at least one conserved 60 amino acid domain, called the WRKY domain, that comprises a highly conserved polypeptide (WRKYGQK) and a zinc finger motif at its N- and C-terminus, respectively [24]. The WRKY TFs are grouped into three subfamilies (Groups I, II, and III) based on the WRKY and zinc finger motif types. The Group I members are characterized by two WRKY domains with a zinc finger motif (C2H2-type). In contrast, Group II and III members contain only one WRKY domain with zinc finger motifs (C2HC- and C2H2-type). Moreover, Group II members may be divided into subgroups IIa, IIb, IIc, IId, and IIe based on their conserved motifs [24] All WRKY proteins play regulatory functions by binding to the DNA sequence (C/T)TGAC(T/C), called W-box elements, in the promoter region of their target genes [25]. Earlier studies showed that WRKY proteins serve as important regulators in many developmental and physiological processes, including leaf development [26], root growth [27, 28], seed development [29, 30], and senescence [26, 31, 32], and in plant responses to biotic and abiotic stresses [33, 34]. There are indications that members of these protein groups are also involved in secondary metabolism in plants. For example, GaWRKY1 helps regulate sesquiterpene biosynthesis in cotton by modulating the expression of CAD1-A [35]. In grape, VvWRKY26 induces the accumulation of flavonoids by targeting the structural genes of the flavonoid biosynthesis pathway [36]. There are a few documents describing the role of these proteins related to light-mediated anthocyanin biosynthesis. A recent study determined that BnWRKY41-1 controls anthocyanin accumulation, similar to AtWRKY41 in Arabidopsis rosette leaves in the presence of light [37]. In the presence of light, apple MdWRKY41 modulates anthocyanin accumulation by negatively regulating the transcriptions of MdUFGT, MdANR, and MdMYB12 [38]. In contrast, apple MdWRKY11 activates MdMYB10-promoted anthocyanin accumulation [39]. Furthermore, in response to light, the MdWRKY1–MdLNC499–MdERF109 complex enhances anthocyanin accumulation by targeting the structural genes in the early stages of fruit coloration of apple. Briefly, light activates the expression of MdWRKY1, which leads to the upregulated transcription of MdLNC499 and the formation of the MdERF109 protein, which increases the induction of the transcription of anthocyanin structural genes in apple fruit [40]. However, whether WRKY TFs are implicated in light-induced anthocyanin accumulation is less documented and data from red pears are scarce. In this study, a light-responsive Group-I WRKY TF (PpWRKY44) in ‘Hongzaosu’ pear fruit was identified. Our analyses clarified that PpWRKY44 positively regulates anthocyanin biosynthesis via the transcriptional regulation of PpMYB10. Additionally, based on the observed high luciferase activity and β-galactosidase (GUS) staining, PpBBX18 likely activates the PpWRKY44 promoter. We determined that WRKY TFs are regulators of light-induced anthocyanin accumulation. Collectively, the findings of this study have further clarified the effects of WRKY-mediated transcriptional regulation of anthocyanin-related genes induced by light.
Results
Identification of candidate gene PpWRKY44 and analysis of its expression in pear fruit and calli under light
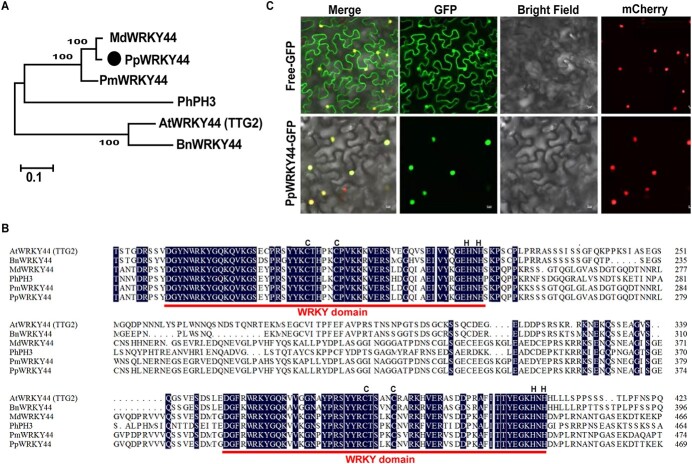
Previous studies in pear have shown that substantial induction of anthocyanin biosynthesis can be achieved by light [41]. To identify candidate regulators belonging to WRKY TFs that may be involved in this process, we analyzed our previous transcriptomic data on pear calli exposed to light to induce anthocyanin biosynthesis [23, 41]. The candidate light-inducible WRKY gene was identified, and its expression was upregulated in calli after 2 days of light treatment (Supplementary Data Fig. S1). Phylogenetic analysis revealed that the candidate WRKY gene is closely related to Arabidopsis AtWRKY44/TTG2, which is a Group-I WRKY TF that regulates proanthocyanidin synthesis in the seed coat [42] (Supplementary Data Fig. S2). Thus, we named this pear WRKY TF PpWRKY44. A multiple protein sequence alignment indicated that PpWRKY44 contains C2H2-type zinc finger motifs and two WRKY domains, which are conserved in the WRKY44 proteins of other species (Fig. 1A and B). To determine the subcellular localization of PpWRKY44, we fused the PpWRKY44 coding sequence with the green fluorescent protein (GFP)-encoding gene and transiently expressed the fusion construct in Nicotiana benthamiana leaves. A fluorescence examination based on GFP detection revealed that PpWRKY44–GFP is localized entirely to the nuclei. In contrast, GFP alone was found throughout the cell (Fig. 1C), demonstrating that PpWRKY44 is a nuclear protein.
Figure 1.
Sequence characteristics and subcellular localization of PpWRKY44. (A) Phylogenetic relationship of PpWRKY44 and other WRKYs from other species conducted on the basis of protein sequences. PpWRKY44 is marked by a black circle. (B) Sequence alignment of PpWRKY44 and other WRKY transcription factors. Pp, Pyrus pyrifolia; Md, Malus domestica (MdWRKY44: XP_008387690.2); Pm, Prunus mume (PmWRKY44: XP_008242029.1); Bn, Brassica napus (BnWRKY44: XP_022557932.1); At, Arabidopsis thaliana (AtTTG2: NP_181263.2); Ph, Petunia hybrida (PhPh3: AMR43368). Red lines represent the conserved WRKY amino acid domains, whereas black letters represent zinc finger motifs. (C) Nuclear localization of PpWRKY44 in tobacco leave cells. Scale bars = 10 μm.
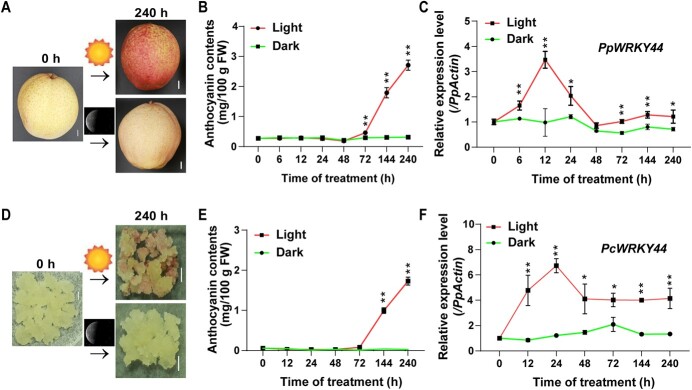
To analyze the PpWRKY44 expression pattern in response to light, ‘Hongzaosu’ pear fruits were subjected to a 10-day light treatment. As expected, upon visual inspection of the pear tissue types, the light treatment displayed a strong red coloring that was not observed in the dark treatment (Fig. 2). In brief, anthocyanins started to accumulate after 48 hours in light-treated pear fruit peels. The content of anthocyanin subsequently continued increasing for the duration of the treatment. In contrast, accumulated anthocyanins were scarce in the dark-treated fruit (Fig. 2A and B). The RT–qPCR analysis of the effects of the light treatment revealed that the PpWRKY44 expression level increased after 6 hours and peaked after 12 hours. Compared with its expression under light, PpWRKY44 was expressed at lower levels in darkness (Fig. 2C). Furthermore, most anthocyanin-related genes (PpBBX18, PpMYB10, PpMYB114, PpCHI, PpCHS, PpF3H, PpDFR, PpUFGT, and PpANS) were expressed similarly to PpWRKY44 following light and dark treatments (Supplementary Data Fig. S3). Considering that pear calli would be used as the plant material for subsequent experiments, we analyzed PcWRKY44 expression in light-treated pear calli over a 10-day period. The data indicated that PcWRKY44 expression increased after 12 hours and peaked after 24 hours, increasing ~6-fold, and the calli began to accumulate anthocyanins after 72 hours (Fig. 2D–F). Induction of the expression of PpWRKY44 by light treatment followed by increasing anthocyanin accumulation indicates that PpWRKY44 is likely a light-responsive regulator of anthocyanin biosynthesis in pear.
Figure 2.
Light-responsive PpWRKY44 expression pattern. (A) Light-induced phenotypes of ‘Hongzaosu’ pear fruits. Fruits at 0 and 240 hours are shown. Scale bars = 1 cm. (B) Content of anthocyanin for each sample time-point in fruit peel. (C) Expression patterns of PpWRKY44 at each sample time-point during treatment. (D) Light-induced phenotypes of pear calli. Pear calli at 0 and 240 hours are shown. Scale bars = 1 cm. (E) Content of anthocyanin in pear calli at each sample time-point. (F) PcWRKY44 expression patterns at each sample time-point. Error bars represent the standard deviation of three biological replicates. The expression level at 0 hours was used as the reference. *P < .05, **P < .01 (two-tailed Student’s t-test).
PpWRKY44 promotes anthocyanin biosynthesis
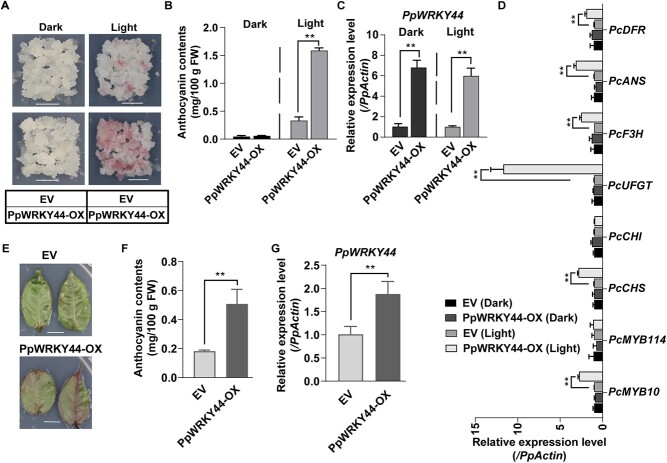
To explore the regulatory effects of PpWRKY44 on light-induced anthocyanin accumulation, we developed Pyrus communis ‘Clapp’s Favorite’ transgenic calli overexpressing PpWRKY44 (PpWRKY44-OX) via Agrobacterium tumefaciens-mediated transformation. The results of the RT–PCR and RT–qPCR analyses confirmed the presence of PpWRKY44 in the transgenic calli (Fig. 3C; Supplementary Data Fig. S4). We subsequently investigated whether PpWRKY44 mediates anthocyanin biosynthesis in response to light. Specifically, soft and fast-growing PpWRKY44-OX and control calli containing the empty vector (EV) were treated with continuous light for 6 days. Upon visual inspection of the overexpressing PpWRKY44, the red color appeared to be increased in various tissue types of pear (Fig. 3). Anthocyanins accumulated considerably more in the PpWRKY44-OX calli than in the EV calli. In contrast, anthocyanins did not accumulate in calli incubated in darkness (Fig. 3A and B). The overexpression of PpWRKY44 also dramatically led to an increase in PcMYB10 expression, but not in PcMYB114 expression (Fig. 3D). Besides, the anthocyanin biosynthetic pathway genes, including PcCHI, PcCHS, PcF3H PcUFGT, PcANS, and PcDFR, were also significantly upregulated in PpWRKY44-OX compared with EV in response to light. In contrast, when compared with EV, no significant differences were observed in expressions of anthocyanin biosynthetic pathway genes in PpWRKY44-OX under dark treatment (Fig. 3D). The PpWRKY44 function associated with anthocyanin biosynthesis regulation was validated by analyzing pear leaves transiently overexpressing PpWRKY44-OX or EV (Fig. 3E–G). Consistent with the examination results of pear calli, PpWRKY44-OX pear leaves accumulated substantially more anthocyanins than the pear leaves containing the EV (Fig. 3F).
Figure 3.
PpWRKY44 functional assay through its overexpression in pear calli and pear leaves. (A) Phenotypes of EV and PpWRKY44-OX calli after a 6-day light treatment. Scale bars = 1 cm. (B) Content of anthocyanin in EV and PpWRKY44-OX calli. (C) PpWRKY44 expression level in EV and PpWRKY44-OX calli. (D) Relative transcript levels of PcMYB10, PcMYB114, PcCHS, PcCHI, PcDFR, PpF3H, and PpANS in EV and PpWRKY44-OX calli. (E) Phenotypes of leaves transiently transformed with PpWRKY44-OX or EV and (F) their anthocyanin contents after 3-day light treatment. (G) PpWRKY44 expression level in transgenic pear leaves. Error bars represent the standard deviation of three biological replicates. The expression level of EV was used as the reference. **P < .01 (two-tailed Student’s t-test).
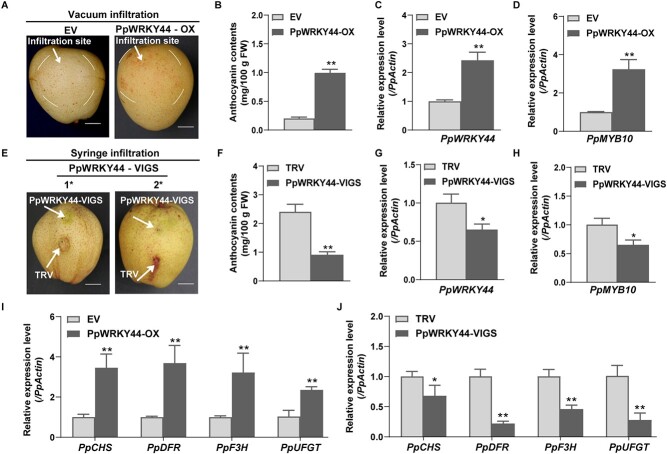
To further verify that PpWRKY44 modulates anthocyanin biosynthesis, the PpWRKY44-OX vector was vacuum-infiltratedinto ‘Meirensu’ pear fruit, whereas a virus-induced gene silencing (VIGS) vector (TRV2-PpWRKY44) was injected into ‘Hongzaosu’ pear fruit. Red coloration was detected around the infiltration site after 5 days of light treatment, but only for the PpWRKY44-OX fruit (Fig. 4A), which was in accordance with the anthocyanin content (Fig. 4B). Regarding the VIGS analysis, compared with the effectsof EV after a 7-day light treatment, the silencing of PpWRKY44 had a suppressive effect on coloration and decreased the anthocyanin content around the injection site (Fig. 4E, F). Relative to the control, PpWRKY44-OX fruit displayed a significantly higher level of PpMYB10 expression, while the PpMYB10 expression level was significantly lower in TRV2-PpWRKY44 fruit (Fig. 4D and H). Furthermore, a comparison with control fruit carrying the EV revealed that the PpCHS, PpF3H, PpDFR, and PpUFGT expression levels increased in the PpWRKY44-OX fruit but decreased in the TRV2-PpWRKY44 fruit (Fig 4I and J). These results demonstrated that PpWRKY44 acts upstream of the regulation of PpMYB10 transcription for light-induced anthocyanin biosynthesis in red pear fruit.
Figure 4.
Transient expressions and silencing of PpWRKY44 in pear fruit. (A) ‘Meirensu’ fruit anthocyanin accumulation in the transient overexpression of PpWRKY44 after a 5-day light treatment. Scale bars = 1 cm. (B) Anthocyanin contents around infiltrated sites of fruit peel transiently overexpressing PpWRKY44. (C) Relative PpWRKY44 transcript level in fruit transiently overexpressing PpWRKY44. (D) Relative PpMYB10 transcript level in fruit transiently overexpressing PpWRKY44. (E) Transient silencing of PpWRKY44 reduced the accumulation of anthocyanin in mature ‘Hongzaosu’ fruit after a 7-day light treatment. Scale bars = 1 cm. (F) Anthocyanin contents around the infiltrated sites of pear fruit in which PpWRKY44 was transiently silenced. (G) PpWRKY44 expression in pear fruit in which PpWRKY44 was transiently silenced. (H) Relative PpMYB10 transcript level in pear fruit in which PpWRKY44 was transiently silenced. (I) Relative transcript levels of PpCHS, PpDFR, PpF3H, and PpUFGT genes in pear fruit transiently overexpressing PpWRKY44. (J) Relative transcript levels of PpCHS, PpDFR, PpF3H, and PpUFGT genes in pear fruit in which PpWRKY44 was transiently silenced. Error bars represent the standard deviation of three biological replicates. The expression level of EV was used as the reference. *P < .05, **P < .01 (two-tailed Student’s t-test).
PpWRKY44 activates the PpMYB10 promoter
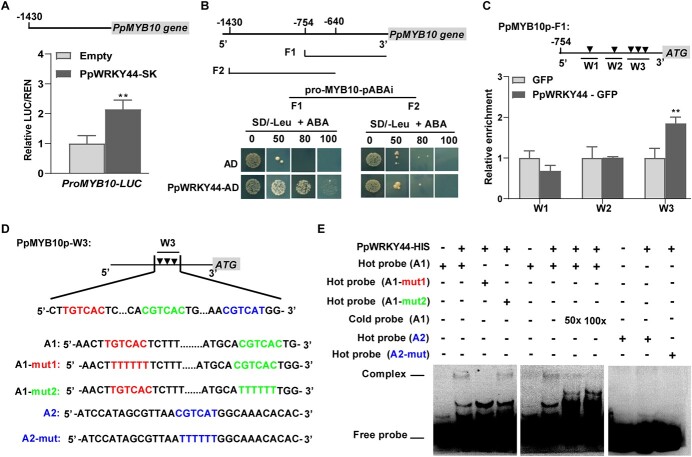
We recently confirmed that PpMYB10, a key regulator of light-induced anthocyanin biosynthesis, can bind to most anthocyanin structural gene promoters [41]. Because PpWRKY44 can induce the expression of PpMYB10, we imagined that PpWRKY44 protein can bind to the PpMYB10 promoter to regulate transcription (Figs 3D and D). Hence, we analyzed the PpMYB10 promoter region and identified many W-box elements, potential binding sites for WRKY TFs. A transient dual-luciferase assay revealed an increase in PpMYB10 promoter activity in the presence of PpWRKY44 (Fig. 5A). Similarly, PpWRKY44 activated the promoters of most of the anthocyanin structural genes (Supplementary Data Fig. S5). For the yeast one-hybrid (Y1H) assays, we first constructed PpMYB10 promoter fragments F1 (start codon to −754 bp) and F2 (−640 to −1430 bp). The two fragments were fused into the pAbAi vector (Fig. 5B). In the Y1H assays, PpWRKY44 was able to bind to F1 but not to F2, suggesting that PpWRKY44 can only bind to the W-box elements in F1 (Fig. 5B). The fragment F1 was further divided into three fragments (W1, W2, and W3) containing W-box elements for chromatin immunoprecipitation (ChIP)–qPCR assays. Specifically, we used the PpWRKY44–GFP transgenic calli and performed the ChIP–qPCR analysis using anti-GFP antibodies. The results confirmed that PpWRKY44 enriched fragment W3, irrespective of fragments W1 and W2 (Fig. 5C), indicating that PpWRKY44 recognizes fragment W3 in the PpMYB10 promoter. Next, we examined fragment W3 for the presence of W-box elements and their reverse-complemented sequences, which resulted in the detection of three W-box elements (TGTCAC, CGTCAC, and CGTCAT) (Fig. 5D). Electrophoretic mobility shift assay (EMSA)s, which were performed using the recombinant PpWRKY44-His fusion protein, indicated that PpWRKY44 was able to bind to the probe of sequence A1 and cause a mobility shift, but it failed to bind to the probe of sequence A2 (Fig. 5E). Moreover, PpWRKY44 was still able to bind to the probe of sequence A1 when CGTCAC was mutated to TTTTTT, but not when TGTCAC was mutated to TTTTTT (Fig. 5D), implying that PpWRKY44 could bind directly to the W-box (TGTCAC) within the sequence A1 of the PpMYB10 promoter. Additionally, an increase in the amount of the unlabeled probe of sequence A1 resulted in a decrease in the ability of PpWRKY44 to bind to the probe of sequence A1 (Fig. 5D). These results suggested that PpWRKY44 transcriptionally regulates the PpMYB10 gene by binding directly to the W-box in its promoter.
Figure 5.
PpWRKY44 protein binds to the promoter of PpMYB10. (A) PpWRKY44 induced PpMYB10 transcription in dual-luciferase assays. (B) Schematic diagram of PpMYB10 promoter fragments F1 (start codon to −754 bp) and F2 (−640 to −1430 bp) and the interactions between PpWRKY44 and these fragments in yeast cells. (C) ChIP–qPCR assays showed that PpWRKY44 protein bound to the PpMYB10 promoter. Chromatins from GFP and PpWRKY44–GFP pear calli were immunoprecipitated with or without a GFP antibody. Three regions (W1, W2, and W3) were analyzed by RT–qPCR. Enrichment of GFP was set to 1. (D) Schematic diagram of PpMYB10 promoter fragment W3 used for the EMSAs. (E) EMSA results revealed the binding of PpWRKY44 protein to the W-box (TGTCAC) within the sequence A1 of the PpMYB10 promoter. Error bars represent the standard deviation of three biological replicates. **P < .01 (two-tailed Student’s t-test).
PpBBX18 activates the transcription of PpWRKY44
We previously demonstrated that light-induced PpBBX18 regulates PpMYB10 transcription, thereby promoting anthocyanin biosynthesis [23]. Interestingly, we note that the PpWRKY44 expression level significantly increased in the pear calli overexpressing PpBBX18 (Supplementary Data Fig. S6). To test whether silencing of PpBBX18 also affects the expression of PpWRKY44, we performed a transient transformation assay on immature ‘Hongzaosu’ pear fruit with the VIGS vector (TRV2-PpBBX18). As shown in Supplementary Data Fig. S7A and B, silencing of PpBBX18 suppressed coloration. RT–qPCR analysis revealed that the expression level of PpWRKY44 was significantly lower in PpBBX18-silenced fruit compared with control fruit (Supplementary Data Fig. S7D). Furthermore, the expression levels of PpMYB10 and anthocyanin-related genes were significantly lower in the PpBBX18-silenced fruit compared with control fruit (Supplementary Data Fig. S8). Furthermore, we fused the PpWRKY44 promoter to a LUC reporter gene for a dual-luciferase assay. The observed high luciferase activity implied that PpBBX18 can activate the PpWRKY44 promoter (Fig. 6A). Moreover, the construct containing the PpBBX18 coding sequence was co-transformed into the leaves of N. benthamiana together with the construct carrying the promoter of PpWRKY44 fused to the GUS reporter gene (Fig. 6B). We found that coexpressing PpBBX18 with the PpWRKY44 promoter increased GUS staining (Fig. 6C) and the relative expression level of GUS (Fig. 6D). Taken together, these results demonstrate that PpBBX18 stimulates the transcription of PpWRKY44 to enhance its expression.
Figure 6.
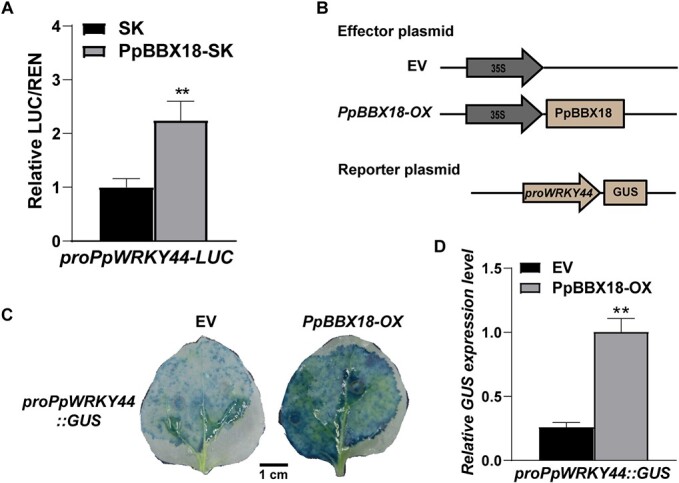
PpBBX18 activates the transcription of PpWRKY44. (A) PpBBX18 induced PpWRKY44 transcription in the dual-luciferase assay. (B) Schematic diagram showing the constructs of effector and reporter used in GUS analysis. (C) Image of GUS staining results for tobacco leaves co-transformed with PpBBX18 and the PpWRKY44 promoter. Scale bars = 1 cm. (D) Detection of relative GUS expression level in tobacco leaves presented in (C) by RT–qPCR. Error bars represent the standard deviation of three biological replicates. **P < .01 (two-tailed Student’s t-test).
Discussion
Light-induced PpWRKY44 is a positive regulator of anthocyanin biosynthesis in pear
The WRKY TF superfamily is exclusive to the plant kingdom; its members modulate numerous physiological processes, including flowering, seed and trichome development, and senescence, via indispensable transcriptional regulatory networks [29, 32, 33]. Emerging evidence indicated that WRKY TFs are vital for regulating biotic and abiotic stress responses [24, 43, 44]. There is also convincing evidence that WRKY TFs are implicated in the context of light signaling pathways. In Arabidopsis, AtWRKY22 expression is repressed by light and induced by exposure to darkness [45]. Additionally, AtWRKY63 and AtWRKY40 activate and repress the transcription of genes implicated in the signaling pathway responsive to high-intensity light [46]. In apple, light induces MdWRKY1 expression, whereas it has the opposite effect on MdWRKY41 expression [38, 40]. Here we showed that PpWRKY44 is a component of light signaling, with at least three factors that may explain that. First, the expression of PpWRKY44 was induced when previously bagged pear fruit and pear calli (wild-type) were exposed to light (Fig. 2). Second, the promoter analysis of PpWRKY44 displayed several light-responsive elements (Supplementary Data Table S1). Third, PpWRKY44 was activated by PpBBX18, which is one of the light signal transduction pathway components (Fig. 6).
Prior and current studies demonstrated that WRKY TFs negatively or positively affect light-depended anthocyanin biosynthesis in plants [39, 47]. In apple, MdWRKY41 suppressed the expression of MdMYB12, MdANR, and MdUFGT, and negatively regulated the accumulation of anthocyanin in response to light [38]. A recent investigation confirmed that MdWRKY11 enhances fruit coloration by upregulating the transcription levels of their downstream genes MdMYB10 and structural genes, which are required for anthocyanin biosynthesis in apple [39]. Furthermore, in response to light, apple MdWRKY1 activates MdLNC499 expression, which leads to upregulated MdERF109 expression. The generated MdERF109 led to significantly elevated anthocyanin accumulation via binding directly to the MdbHLH3, MdUFGT, and MdCHS promoters [40]. Our previous transcriptomic analysis discovered that the expression levels of more than 25 WRKY-encoding genes in pear are upregulated in response to light. However, their functions in light-responsive processes remain unclear [41]. Similarly, the regulatory functions of WRKY TFs during anthocyanin biosynthesis in red pears have not been thoroughly characterized. In this work, phylogenetic analysis revealed the close relationship between PpWRKY44 and AtWRKY44, which is a Group-I WRKY TF in Arabidopsis (Supplementary Data Fig. S1). Moreover, AtWRKY44 influences trichome formation and regulates seed coat tannin production by modulating the vacuolar transport steps in the proanthocyanidin pathway [29, 42]. Furthermore, phenotypic and molecular analyses of the overexpression of PpWRKY44 in various pear tissues (e.g. leaves and fruit) and in pear calli as well as the effects of silencing PpWRKY44 in pear fruit indicated that PpWRKY44 positively regulated light-dependent anthocyanin accumulation by increasing the transcription of the regulatory gene PpMYB10. These findings prove that PpWRKY44 is indeed a light-responsive gene, and its expression correlates with light-dependent anthocyanin accumulation in red pears.
PpWRKY44 promotes anthocyanin biosynthesis by activating PpMYB10 expression
Light-dependent anthocyanin biosynthesis is indeed a complex process involving the coordinated regulation of several key structural genes, such as CHS, DFR, and UFGT [5, 48, 49], which are mainly transcriptionally regulated by MYB TFs [51]. MYB TFs also act as bridges between specific TFs of environmental signaling components and the anthocyanin structural genes and thus link different signaling pathways before channeling the transcriptional instructions to the structural genes. In this manner, MYB TFs help plants decode environmental cues (e.g. light) into physiological responses (e.g. anthocyanin accumulation) [5, 7, 8, 51]. In apple, MdMYB1 responds to light and affects apple fruit coloration by regulating the transcription of their downstream genes MdDFR and MdUFGT [12]. Recent studies showed that WRKY TFs regulate the transcription of MYB TF-encoding genes to modulate anthocyanin biosynthesis. For example, anthocyanin production was inhibited by AtWRKY41 in Arabidopsis rosette leaves by transcriptionally regulating the three MYB TF genes (AtMYB75, AtMYB111, and AtMYBD) [37]. In apple, proanthocyanidin biosynthesis is inhibited by MdWRKY41, which functions directly upstream of MdMYB12, which encodes a positive modulator of proanthocyanidin biosynthesis, to repress its expression [38]. Earlier research indicated that the MdMYB1 promoter is transcriptionally regulated by MdWRKY72 to promote anthocyanin synthesis in apple [52]. A similar transcriptional regulation was observed in pear. More specifically, in red-skinned pear fruit, PpWRKY26 transcriptionally activates the PpMYB114 promoter and promotes anthocyanin biosynthesis [53]. The PpMYB10 TF is a critical regulator of anthocyanin biosynthesis in pear because it can directly act upstream of most anthocyanin structural genes [21]. In the current work, PpWRKY44 activated the transcription of PpMYB10 (Fig. 3). A series of in vitro and in vivo analyses demonstrated that PpMYB10 directly acts downstream of PpWRKY44, thereby positively regulating anthocyanin accumulation (Fig. 5).
In plants, anthocyanin biosynthesis is transcriptionally regulated by the MBW complex, which has been widely studied [7, 8]. Recent reports described how WRKY TFs might influence the regulatory effects of the MBW complex [42, 54]. In apple, MdWRKY40 interacts with the vital component of the MBW complex, MdMYB1, to enhance its expression and binding to target genes in response to wounding [47]. Another study revealed that MdWRKY75 stimulates the accumulation of anthocyanins in apples by binding to the promoter of the MYB transcription factor MdMYB1 and enhancing its activity [55]. PpWRKY26 directly activates PpMYB114 transcription and interacts with PpbHLH3 to target the PpMYB114 promoter, ultimately leading to anthocyanin accumulation in red-skinned pear [53]. The novel WRKY–MBW module may be essential for regulating anthocyanin biosynthesis. The results presented herein suggest that PpWRKY44 can positively regulate anthocyanin accumulation via transcriptional regulation of PpMYB10, which encodes a key factor of the MYB10–bHLH3–WD40 (i.e. MBW) complex, which regulates anthocyanin biosynthesis in pear.
PpWRKY44 is part of the light-induced anthocyanin biosynthesis cascade
Recently, BBX proteins have been identified as inducers of anthocyanin biosynthesis in several plants [22, 56]. In a previous study, we revealed that PpBBX18 contributes to the light-induced coloration of pear fruit by regulating the expression of PpMYB10, although it cannot bind directly to the PpMYB10 promoter [23]. Interestingly, we detected a highly significant expression of PpWRKY44 in calli overexpressing PpBBX18 (Supplementary Data Fig. S5), suggesting that PpBBX18 might regulate PpWRKY44 expression. Transient silencing of PpBBX18 expression in pear fruit confirmed this finding (Supplementary Data Fig. S7). We next performed a dual-luciferase assay and GUS staining. The analysis revealed that PpBBX18 could activate the expression of PpWRKY44 (Fig. 6). Therefore, in response to light, PpBBX18 may increase the transcription of PpWRKY44, which encodes a direct regulator of PpMYB10 expression. Although further in vivo experiments are needed, these results generate the interesting hypothesis that PpWRKY44 might involve the light-induced anthocyanin biosynthesis cascade (PpBBX18–PpWRKY44–PpMYB10) in red pears.
In conclusion, a light-responsive Group-I WRKY TF (PpWRKY44) in ‘Hongzaosu’ pear fruit was identified. In response to light, PpWRKY44 is highly expressed downstream of PpBBX18. The encoded TF targets PpMYB10 promoter fragment W3, containing three W-box elements. The EMSA data indicated that TGTCAC is the specific W-box element in fragment W3 that binds to PpWRKY44, leading to transcriptional regulation (Fig. 7). Hence, we have demonstrated that PpWRKY44 positively regulates light-induced anthocyanin biosynthesis through direct activation of the PpMYB10 promoter in red pear fruit. Our findings have further elucidated the molecular mechanism underlying WRKY-mediated transcriptional regulation of light-induced anthocyanin biosynthesis regulatory genes in red pear fruit.
Figure 7.
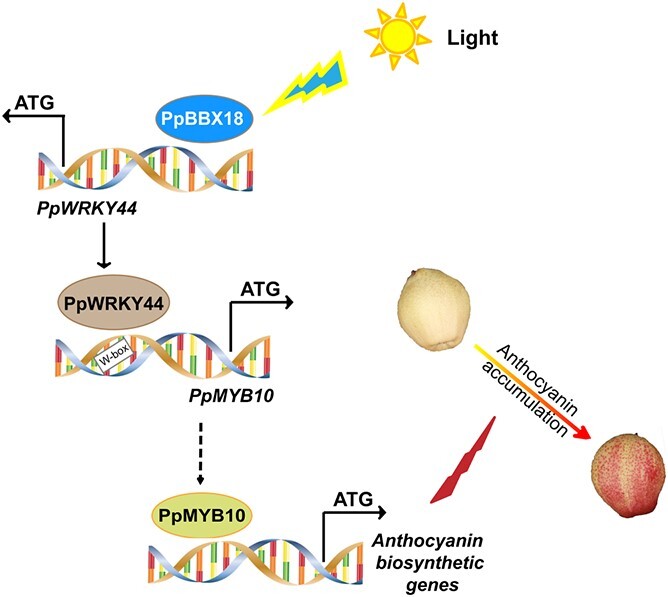
A simplified model for the regulation by PpWRKY44 of light-induced anthocyanin accumulation. Under light, PpBBX18 targets the promoter of PpWRKY44 to activate its gene expression. PpWRKY44 proteins activate the expression of anthocyanins regulatory gene, upstream of anthocyanins structural genes, PpMYB10.
Materials and methods
Identification and phylogenetic analysis of PpWRKY44 TF
Transcriptome sequencing data from our previous studies investigating light-induced anthocyanin accumulation in pear fruit and calli [23, 43] were used to identify the light-induced WRKY TF. The database of The Arabidopsis Information Resource (TAIR, https://www.arabidopsis.org/) was used for Arabidopsis WRKY protein sequences. In contrast, the pear WRKY protein sequences from pear genome data were identified using local BLAST analysis. The sequence alignment of pear and Arabidopsis WRKY proteins was constructed using ClustalW in MEGA 7.0. IQ-TREE was used for the inferred phylogenetic tree via the maximum likelihood method, with 1000 ultrafast bootstrap replicates [58]. The phylogenetic tree was visualized by using the iTOL program (https://itol.embl.de/). Multiple sequence alignment and characterization of the conserved WRKY domains in the proteins of pear and other species were performed using DNAMAN software.
Subcellular localization
To determine the subcellular localization of PpWRKY44, the coding sequences of PpWRKY44 were amplified without a stop codon from skin cDNA prepared from the pear cultivar ‘Hongzaosu’ using primer sequences shown in Supplementary Data Table S2 and fused into the pCAMBIA1300 vector, including the GFP tag sequence. The empty vector of pCAMBIA1300 was employed as control. The constructs were introduced into strain GV3101 of A. tumefaciens cells. Nicotiana benthamiana (mCherry nuclear expression) leaf infiltration was conducted as described previously [58]. GFP fluorescence in the transiently transformed leaves was analyzed and imaged using the A1 confocal laser scanning microscope (Nikon, Japan).
Plant materials and light treatments
Bagged pear (Pyrus pyrifolia × P. communis cultivar ’Hongzaosu’) fruits used in the current study were collected from an orchard 150 days after full bloom. Then, the bagged fruit was quickly taken to the laboratory and maintained in the dark at 22°C overnight. Dedifferentiated pear calli were easy to prepare from the flesh cells of young P. communis ‘Clapp’s Favorite’ fruit compared with P. pyrifolia and were used in this study. They were cultured on Murashige and Skoog (MS) solid medium containing 30 g l−1 sucrose, 1.0 mg l−1 2,4-dichlorophenoxyacetic acid, and 0.5 mg l−1 6-benzylaminopurine at 22°C in the dark. The calli were subcultured before being used for light treatment and genetic transformation three times at 20-day intervals.
For the light response assay, harvested fruits were treated with light as described previously [59]. Briefly, the bagged fruits were separated into two groups and placed in a phytotron at 17°C. One group was exposed to light (60 μmol m−2 s−1), whereas the fruits in the other group were not exposed to light (i.e. control fruits). After starting the light treatment, the exposed side of the peel of each fruit was scraped at 0, 6, 12, 24, 48, 72, 144, and 240 hours. Three biological replicates were prepared for each sample time-point, with three fruits used for one biological replicate.
For the light response assay, pear calli were treated with light in a phytotron at 17°C. Samples were collected after starting the light treatment at 0, 12, 24, 48, 72, 144, and 240 hours. The pear calli used as controls were covered with aluminum foil. For further analysis, the pear fruit and calli samples were maintained at −80°C after being quickly frozen in liquid nitrogen.
Genetic transformation
To generate the transgenic pear calli, the constructs 35S:PpWRKY44–GFP and 35S:GFP (i.e. GFP alone) were used. The constructs were separately introduced into strain EHA105 of A. tumefaciens cells, followed by transformation into pear calli by means of the A. tumefaciens-mediated method as described previously [23]. The transgenic calli were cultured under continuous dark conditions on MS-based solid medium at 22°C. The medium was supplemented with 10 mg/l hygromycin and 200 mg/l timentin. After confirming that they were transformed correctly, the transgenic calli were subcultured onto fresh regeneration medium every 15–20 days. Regarding the light treatment, transgenic pear calli were exposed for 6 days to continuous light.
Anthocyanin measurements
The contents of anthocyanin in pear peel and calli were measured with slight modifications as described previously [60]. In brief, pear peel and calli were powdered in liquid nitrogen. Then, 0.1 g was weighed and maintained in the dark at 4°C overnight in 1 ml of extraction solution (acetic acid:methanol = 1:99, v/v). The absorbance of each 100-μl sample was measured (at 530, 620, and 650 nm) with a DU800 spectrophotometer (Beckman Coulter, USA). The formula [[(A530 − A650) − 0.2 × (A650 − A620)]/sample quantity] was employed to determine the anthocyanin content.
RNA extraction, cDNA synthesis, and gene expression analysis
RNAs from the pear peel and pear calli of WT and transgenic lines were isolated based on a modified CTAB method as described previously [61]. First-strand cDNA was synthesized from 1 μg of isolated RNA with the HiScript® II Q RT SuperMix for qPCR (+gDNA wiper; Vazyme Biotech). The generated cDNA was a template for RT–qPCR assays with gene-specific primers (Supplementary Data Table S2) using iTaq™ Universal SYBR® Green Supermix (Bio-Rad, https://www.bio-rad.com/). The 2−ΔΔCT method was utilized to estimate the relative transcription values for RT–qPCR normalization using pear PpActin (JN684184) as the reference gene.
Transient transformation of pear leaves and fruits
A transient gene expression assay was employed to overexpress PpWRKY44 in mature ‘Meirensu’ pear fruit. The coding sequences of PpWRKY44 were amplified from skin cDNA of ‘Hongzaosu’ using primer sequences shown in Supplementary Data Table S2, and fused into the pGreenII0029 62-SK vector to construct PpWRKY44–SK. After the empty SK and PpWRKY44–SK constructs were delivered into strain GV3101 of A. tumefaciens cells, transient overexpression experiments were performed as described previously [23] by means of the GM-0.33A vacuum pump (Jinteng, China). For the pear fruit infiltration, 15 bagged fruits were infiltrated with the EV, while 15 bagged fruits were infiltrated with PpWRKY44-SK (PpWRKY44-OX). VIGS assays were used to silence PpWRKY44 in the ‘Hongzaosu’ pear fruit. A specific 318 bp-long DNA fragment of the coding sequences region of PpWRKY44 was amplified from skin cDNA of ‘Hongzaosu’ using primer sequences shown in Supplementary Data Table S2, and fused into the pTRV2 vector to construct pTRV2–PpWRKY44. VIGS experiments were performed after the pTRV2–PpWRKY44, pTRV1, and pTRV2 vectors were introduced into strain EHA105 of A. tumefaciens, as described previously [23]. For pear fruit injection, 15 bagged fruits were injected with the EV (pTRV1:pTRV2 = 1:1, v/v), while 15 bagged fruits were injected with pTRV2–PpWRKY44 (pTRV1:pTRV2–PpWRKY44 = 1:1, v/v). The ‘Meirensu’-infiltrated fruits and ‘Hongzaosu’-injected fruits were then kept in darkness for 24 hours and then placed in a continuous light incubator for 5 days for gene overexpression assays and 7 days for VIGS assays. After photographing them, fruit peels near the infiltration site were scraped and kept at −80°C after being quickly frozen in liquid nitrogen. For the transient transformation of pear leaves (Pyrus ussuriensis), transient pear fruit vectors were also used. The detached leaves were mixed with A. tumefaciens cells (GV3101) containing the recombinant vectors and then infiltrated for 20 minutes using a GM-0.33A vacuum pump (Jinteng, China), and placed in darkness for 1 day. After 2 days of light treatment, pear leaves were photographed and sampled to extract RNA and measure anthocyanin content.
Dual-luciferase assay
The transient expression assay followed the protocol described previously [62]. In brief, the coding sequences of PpWRKY44 were amplified from skin cDNA of ‘Hongzaosu’ and ligated with the pGreenII0029 62–SK vector, creating the effector construct. The promoter of PpMYB10 was cloned from genomic DNA of ‘Hongzaosu’ into the pGreenII0800–LUC vector, creating the reporter construct. Both constructs were separately delivered into strain GV3101 of A. tumefaciens cells. Agrobacterium strains containing recombinant constructs were combined at a volume ratio of 10:1 (10 PpWRKY44–SK, 1 ProPpMYB10–LUC) before co-transformation into N. benthamiana leaves. For the negative control, the leaves were injected with a combination of cells containing pGreenII0029 62–SK and ProPpMYB10–LUC. Renilla and firefly luciferase activities were tested 2.5 days after injection by means of a Dual-Luciferase Reporter Assay Kit (Promega, https://www.promega.com) based on the operating instructions. Primers provided in Supplementary Data Table S2 were utilized to amplify the promoter of PpMYB10 and the coding sequence of PpWRKY44.
Yeast one-hybrid assays
According to the Yeast Protocols Handbook (Clontech), a Y1H assay was performed. The PpMYB10 promoter fragments were amplified from genomic DNA of ‘Hongzaosu’ by means of the primers provided in Supplementary Data Table S2, and incorporated into the pAbAi vector. The vector was then sequenced and inserted into Y1HGold yeast cells. Y1HGold cells harboring the PpMYB10–pAbAi vector were added to SD/−Ura plates to test promoter auto-activation and select positive colonies. The coding sequences of PpWRKY44 were ligated with the pGADT7 prey vector (AD). The Y1HGold strain harboring the PpMYB10–pAbAi vector was re-transformed with PpWRKY44–AD or the empty AD plasmid. Positive interactions were selected at 30°C for 5 days on SD/−Leu plates containing aureobasidin A (AbA).
Chromatin immunoprecipitation–qPCR assays
The ChIP–qPCR assays were conducted as described previously [63]. Light-treated transgenic pear calli containing PpWRKY44–GFP or GFP alone were collected for subsequent cross-linking with formaldehyde (1%) under vacuum conditions for 15 minutes. Cross-linking was stopped by adding glycine (125 mM final concentration) and maintaining vacuum conditions for 10 minutes. The chromatin DNA was then extracted via sucrose gradient centrifugation, and sonicated at 4°C for 30 minutes (30 seconds with 30-second intervals) using the Bioruptor Plus device (Diagenode) to produce 200- to 300-bp random fragments. The sonicated chromatin was immunoprecipitated overnight using anti-GFP antibodies (Abcam, China), after which qPCR analysis was used to determine the amount of immunoprecipitated chromatin.
Electrophoretic mobility shift assay
The coding sequences of PpWRKY44 were amplified from skin cDNA of ‘Hongzaosu’ using the primers provided in Supplementary Data Table S2. It was then ligated with the pET-32a vector containing a His tag using BamHI and HindIII restriction enzymes. For protein induction, recombinant vector was introduced into strain BL21 Escherichia coli cells and the cells were incubated overnight at 16°C with 0.2 mM isopropyl-β-d-thiogalactopyranoside. The fusion protein was purified utilizing Ni-NTA Sefinose™ Resin (Sangon Biotech, China). For preparing the probes, probes labeled with biotin at the 3′ end were synthesized (Genebio, China), followed by the preparation of double-stranded DNA probes as described previously [64]. An EMSA was performed using a LightShift™ Chemiluminescent EMSA Kit (Thermo Fisher Scientific, USA). Briefly, purified recombinant His-PpWRKY44 was incubated with biotin-labeled probes for 30 minutes at room temperature. Then, the reaction mixture was separated by PAGE at 200 V, transferred to a nylon membrane (Millipore, http://www.merckmillipore.com/), and subjected to UV cross-linking. Finally, anti-biotin antibody was used to detect the biotin-labeled probes.
GUS staining assays
The promoter of PpWRKY44 (~1500 bp) was cloned from genomic DNA of ‘Hongzaosu’ into the pCAMBIA1301 vector upstream of the GUS gene, creating the reporter vector. The coding sequences of PpBBX18 were amplified from skin cDNA of ‘Hongzaosu’ and inserted into pCAMBIA1300–GFP, creating the effector vector. Agrobacterium (A. tumefaciens GV3101-pSoup) cells containing the reporter and effector vectors, after mixing equally (v/v), were transiently expressed in N. benthamiana using 4-week-old tobacco plants with three leaves as described previously [63]. For GUS staining, the leaves were dipped in GUS staining solution, infiltrated for 15 minutes using a GM-0.33A vacuum pump (Jinteng, China), placed in darkness overnight at 37°C, and then quickly frozen in liquid nitrogen to analyze the GUS expression level by RT–qPCR according to a previously described method [65]. The other leaves were washed in ethanol (80%) to remove chlorophyll before photographing.
Statistical analysis
Samples were statistically analyzed with two-tailed Student’s t-test using GraphPad Prism version 8.0.
Acknowledgements
This work was supported by the National Key Research and Development Program (2018YFD1000200), the China Agriculture Research System of MOF and MARA and the Fundamental Research Funds for the Central Universities (2021QNA6022).
Author contributions
Y.T. and S.B. planned and designed the research; A.A. conducted most of the experiments with help from X.Z., L.P., and L.Z.; Y.G. contributed to the bioinformatics analysis; M.A. and J.N. provided help and advice; A.A., M.A., J.N., S.B., and Y.T. wrote the manuscript. All authors read and approved the manuscript for submission.
Data availability
All relevant data in this study are provided in the article and its supplementary files.
Conflicts of interest
The authors declare no competing interests.
Supplementary Data
Supplementary data is available at Horticulture Research online.
Supplementary Material
Contributor Information
Ahmed Alabd, College of Agriculture and Biotechnology, Zhejiang University, Hangzhou, Zhejiang 310058, China; Department of Pomology, Faculty of Agriculture, Alexandria University, Alexandria 21545, Egypt.
Mudassar Ahmad, College of Agriculture and Biotechnology, Zhejiang University, Hangzhou, Zhejiang 310058, China.
Xiao Zhang, College of Agriculture and Biotechnology, Zhejiang University, Hangzhou, Zhejiang 310058, China.
Yuhao Gao, College of Agriculture and Biotechnology, Zhejiang University, Hangzhou, Zhejiang 310058, China.
Lin Peng, College of Agriculture and Biotechnology, Zhejiang University, Hangzhou, Zhejiang 310058, China.
Lu Zhang, College of Agriculture and Biotechnology, Zhejiang University, Hangzhou, Zhejiang 310058, China.
Junbei Ni, College of Agriculture and Biotechnology, Zhejiang University, Hangzhou, Zhejiang 310058, China.
Songling Bai, College of Agriculture and Biotechnology, Zhejiang University, Hangzhou, Zhejiang 310058, China.
Yuanwen Teng, College of Agriculture and Biotechnology, Zhejiang University, Hangzhou, Zhejiang 310058, China; Hainan Institute of Zhejiang University, Sanya, Hainan 572025, China.
References
- 1. Steyn WJ, Wand SJE, Holcroft DMet al. Red colour development and loss in pears. Acta Hortic. 2005;671:79–85. [Google Scholar]
- 2. Winkel-Shirley B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001;126:485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. He Y, Li D, Li Set al. SmBICs inhibit anthocyanin biosynthesis in eggplant (Solanum melongena L.). Plant Cell Physiol. 2021;62:1001–11. [DOI] [PubMed] [Google Scholar]
- 4. Wu T, Guo X, Zhang Met al. Anthocyanins in black rice, soybean and purple corn increase fecal butyric acid and prevent liver inflammation in high fat diet-induced obese mice. Food Funct. 2017;8:3178–86. [DOI] [PubMed] [Google Scholar]
- 5. Koes R, Verweij WQuattrocchio F. Flavonoids: a colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci. 2005;10:236–42. [DOI] [PubMed] [Google Scholar]
- 6. Kitamura S. Transport of flavonoids: from cytosolic synthesis to vacuolar accumulation. In: Grotewold E, ed. The Science of Flavonoids. Springer: New York, 2006,123–46. [Google Scholar]
- 7. Xu W, Dubos CLepiniec L. Transcriptional control of flavonoid biosynthesis by MYB-bHLH-WDR complexes. Trends Plant Sci. 2015;20:176–85. [DOI] [PubMed] [Google Scholar]
- 8. Jaakola L. New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci. 2013;18:477–83. [DOI] [PubMed] [Google Scholar]
- 9. Allan AC, Espley RV. MYBs drive novel consumer traits in fruits and vegetables. Trends Plant Sci. 2018;23:693–705. [DOI] [PubMed] [Google Scholar]
- 10. Lin-Wang K, McGhie TK, Wang Met al. Engineering the anthocyanin regulatory complex of strawberry (Fragaria vesca). Front Plant Sci. 2014;5:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Espley RV, Hellens RP, Putterill Jet al. Red colouration in apple fruit is due to the activity of the MYB transcription factor, MdMYB10. Plant J. 2007;49:414–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takos AM, Jaffé FW, Jacob SRet al. Light-induced expression of a MYB gene regulates anthocyanin biosynthesis in red apples. Plant Physiol. 2006;142:1216–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ban Y, Honda C, Hatsuyama Yet al. Isolation and functional analysis of a MYB transcription factor gene that is a key regulator for the development of red coloration in apple skin. Plant Cell Physiol. 2007;48:958–70. [DOI] [PubMed] [Google Scholar]
- 14. Feng S, Wang Y, Yang Set al. Anthocyanin biosynthesis in pears is regulated by a R2R3-MYB transcription factor PyMYB10. Planta. 2010;232:245–55. [DOI] [PubMed] [Google Scholar]
- 15. Yao G, Ming M, Allan ACet al. Map-based cloning of the pear gene MYB114 identifies an interaction with other transcription factors to coordinately regulate fruit anthocyanin biosynthesis. Plant J. 2017;92:437–51. [DOI] [PubMed] [Google Scholar]
- 16. Albert NW, Lewis DH, Zhang Het al. Light-induced vegetative anthocyanin pigmentation in petunia. J Exp Bot. 2009;60:2191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Albert NW, Lewis DH, Zhang Het al. Members of an R2R3-MYB transcription factor family in petunia are developmentally and environmentally regulated to control complex floral and vegetative pigmentation patterning. Plant J. 2011;65:771–84. [DOI] [PubMed] [Google Scholar]
- 18. Gonzalez A. Pigment loss in response to the environment: a new role for the WD/bHLH/MYB anthocyanin regulatory complex. New Phytol. 2009;182:1–3. [DOI] [PubMed] [Google Scholar]
- 19. Azuma A, Kobayashi S, Mitani Net al. Genomic and genetic analysis of Myb-related genes that regulate anthocyanin biosynthesis in grape berry skin. Theor Appl Genet. 2008;117:1009–19. [DOI] [PubMed] [Google Scholar]
- 20. Shin D, Choi M, Kim Ket al. HY5 regulates anthocyanin biosynthesis by inducing the transcriptional activation of the MYB75/PAP1 transcription factor in Arabidopsis. FEBS Lett. 2013;587:1543–7. [DOI] [PubMed] [Google Scholar]
- 21. Tao R, Bai S, Ni Jet al. The blue light signal transduction pathway is involved in anthocyanin accumulation in ‘Red Zaosu’ pear. Planta. 2018;248:37–48. [DOI] [PubMed] [Google Scholar]
- 22. Bai S, Tao R, Tang Yet al. BBX16, a B-box protein, positively regulates light-induced anthocyanin accumulation by activating MYB10 in red pear. Plant Biotechnol J. 2019;17:1985–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bai S, Tao R, Yin Let al. Two B-box proteins, PpBBX18 and PpBBX21, antagonistically regulate anthocyanin biosynthesis via competitive association with Pyrus pyrifolia ELONGATED HYPOCOTYL 5 in the peel of pear fruit. Plant J. 2019;100:1208–23. [DOI] [PubMed] [Google Scholar]
- 24. Eulgem T, Somssich IE. Networks of WRKY transcription factors in defense signaling. Curr Opin Plant Biol. 2007;10:366–71. [DOI] [PubMed] [Google Scholar]
- 25. Ülker B, Somssich IE. WRKY transcription factors: from DNA binding towards biological function. Curr Opin Plant Biol. 2004;7:491–8. [DOI] [PubMed] [Google Scholar]
- 26. Besseau S, Li JPalva ET. WRKY54 and WRKY70 co-operate as negative regulators of leaf senescence in Arabidopsis thaliana. J Exp Bot. 2012;63:2667–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Devaiah BN, Karthikeyan ASRaghothama KG. WRKY75 transcription factor is a modulator of phosphate acquisition and root development in Arabidopsis. Plant Physiol. 2007;143:1789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou QY, Tian AG, Zou HFet al. Soybean WRKY-type transcription factor genes, GmWRKY13, GmWRKY21, and GmWRKY54, confer differential tolerance to abiotic stresses in transgenic Arabidopsis plants. Plant Biotechnol J. 2008;6:486–503. [DOI] [PubMed] [Google Scholar]
- 29. Johnson CS, Kolevski BSmyth DR. TRANSPARENT TESTA GLABRA2, a trichome and seed coat development gene of Arabidopsis, encodes a WRKY transcription factor. Plant Cell. 2002;14:1359–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Luo M, Dennis ES, Berger Fet al. MINISEED3 (MINI3), a WRKY family gene, and HAIKU2 (IKU2), a leucine-rich repeat (LRR) KINASE gene, are regulators of seed size in Arabidopsis. Proc Natl Acad Sci USA. 2005;102:17531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Robatzek S, Somssich IE. A new member of the Arabidopsis WRKY transcription factor family, AtWRKY6, is associated with both senescence- and defence-related processes. Plant J. 2001;28:123–33. [DOI] [PubMed] [Google Scholar]
- 32. Ülker B, Shahid Mukhtar MSomssich IE. The WRKY70 transcription factor of Arabidopsis influences both the plant senescence and defense signaling pathways. Planta. 2007;226:125–37. [DOI] [PubMed] [Google Scholar]
- 33. Rushton PJ, Somssich IE, Ringler Pet al. WRKY transcription factors. Trends Plant Sci. 2010;15:247–58. [DOI] [PubMed] [Google Scholar]
- 34. Chen L, Song Y, Li Set al. The role of WRKY transcription factors in plant abiotic stresses. Biochim Biophys Acta. 2012;1819:120–8. [DOI] [PubMed] [Google Scholar]
- 35. Xu Y-H, Wang J-W, Wang Set al. Characterization of GaWRKY1, a cotton transcription factor that regulates the sesquiterpene synthase gene (+)-δ-cadinene synthase-A. Plant Physiol. 2004;135:507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Amato A, Cavallini E, Zenoni Set al. A grapevine TTG2-like WRKY transcription factor is involved in regulating vacuolar transport and flavonoid biosynthesis. Front Plant Sci. 2017;7:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Duan S, Wang J, Gao Cet al. Functional characterization of a heterologously expressed Brassica napus WRKY41-1 transcription factor in regulating anthocyanin biosynthesis in Arabidopsis thaliana. Plant Sci. 2018;268:47–53. [DOI] [PubMed] [Google Scholar]
- 38. Mao Z, Jiang H, Wang Set al. The MdHY5-MdWRKY41-MdMYB transcription factor cascade regulates the anthocyanin and proanthocyanidin biosynthesis in red-fleshed apple. Plant Sci. 2021;306:110848. [DOI] [PubMed] [Google Scholar]
- 39. Liu W, Wang Y, Yu Let al. MdWRKY11 participates in anthocyanin accumulation in red-fleshed apples by affecting MYB transcription factors and the photoresponse factor MdHY5. J Agric Food Chem. 2019;67:8783–93. [DOI] [PubMed] [Google Scholar]
- 40. Ma H, Yang T, Li Yet al. The long noncoding RNA MdLNC499 bridges MdWRKY1 and MdERF109 function to regulate early-stage light-induced anthocyanin accumulation in apple fruit. Plant Cell. 2021;33:3309–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bai S, Sun Y, Qian Met al. Transcriptome analysis of bagging-treated red Chinese sand pear peels reveals light-responsive pathway functions in anthocyanin accumulation. Sci Rep. 2017;7:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gonzalez A, Brown M, Hatlestad Get al. TTG2 controls the developmental regulation of seed coat tannins in Arabidopsis by regulating vacuolar transport steps in the proanthocyanidin pathway. Dev Biol. 2016;419:54–63. [DOI] [PubMed] [Google Scholar]
- 43. Liu Y, Yang T, Lin Zet al. A WRKY transcription factor PbrWRKY53 from Pyrus betulaefolia is involved in drought tolerance and AsA accumulation. Plant Biotechnol J. 2019;17:1770–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jang JY, Choi CHHwang DJ. The WRKY superfamily of rice transcription factors. Plant Pathol J. 2010;26:110–4. [Google Scholar]
- 45. Zhou X, Jiang YYu D. WRKY22 transcription factor mediates dark-induced leaf senescence in Arabidopsis. Mol Cells. 2011;31:303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Aken Van O, Zhang B, Law Set al. AtWRKY40 and AtWRKY63 modulate the expression of stress-responsive nuclear genes encoding mitochondrial and chloroplast proteins. Plant Physiol. 2013;162:254–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. An JP, Zhang XW, You CXet al. MdWRKY40 promotes wounding-induced anthocyanin biosynthesis in association with MdMYB1 and undergoes MdBT2-mediated degradation. New Phytol. 2019;224:380–95. [DOI] [PubMed] [Google Scholar]
- 48. Kim S-H, Lee J-R, Hong S-Tet al. Molecular cloning and analysis of anthocyanin biosynthesis genes preferentially expressed in apple skin. Plant Sci. 2003;165:403–13. [Google Scholar]
- 49. Qian M, Yu B, Li Xet al. Isolation and expression analysis of anthocyanin biosynthesis genes from the red Chinese sand pear, Pyrus pyrifolia Nakai cv. Mantianhong, in response to methyl jasmonate treatment and UV-B/VIS conditions. Plant Mol Biol Report. 2014;32:428–37. [Google Scholar]
- 50. Ramsay NA, Glover BJ. MYB–bHLH–WD40 protein complex and the evolution of cellular diversity. Trends Plant Sci. 2005;10:63–70. [DOI] [PubMed] [Google Scholar]
- 51. Maier A, Schrader A, Kokkelink Let al. Light and the E3 ubiquitin ligase COP1/SPA control the protein stability of the MYB transcription factors PAP1 and PAP2 involved in anthocyanin accumulation in Arabidopsis. Plant J. 2013;74:638–51. [DOI] [PubMed] [Google Scholar]
- 52. Hu J, Fang H, Wang Jet al. Ultraviolet B-induced MdWRKY72 expression promotes anthocyanin synthesis in apple. Plant Sci. 2020;292:110377. [DOI] [PubMed] [Google Scholar]
- 53. Li C, Wu J, Hu K-Det al. PyWRKY26 and PybHLH3 cotargeted the PyMYB114 promoter to regulate anthocyanin biosynthesis and transport in red-skinned pears. Hortic Res. 2020;7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pesch M, Dartan B, Birkenbihl Ret al. Arabidopsis TTG2 regulates TRY expression through enhancement of activator complex-triggered activation. Plant Cell. 2014;26:4067–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Su M, Zuo W, Wang Yet al. The WKRY transcription factor MdWRKY75 regulates anthocyanins accumulation in apples (Malus domestica). Funct Plant Biol. 2022;49:799–809. [DOI] [PubMed] [Google Scholar]
- 56. Plunkett BJ, Henry-Kirk R, Friend Aet al. Apple B-box factors regulate light-responsive anthocyanin biosynthesis genes. Sci Rep. 2019;9:17762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nguyen L-T, Schmidt HA, Haeseler Aet al. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2015;32:268–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yang Q, Niu Q, Li Jet al. PpHB22, a member of HD-zip proteins, activates PpDAM1 to regulate bud dormancy transition in ‘Suli’ pear (Pyrus pyrifolia White Pear Group). Plant Physiol Biochem. 2018;127:355–65. [DOI] [PubMed] [Google Scholar]
- 59. Sun Y, Qian M, Wu Ret al. Postharvest pigmentation in red Chinese sand pears (Pyrus pyrifolia Nakai) in response to optimum light and temperature. Postharvest Biol Technol. 2014;91:64–71. [Google Scholar]
- 60. Huang C, Yu B, Teng Yet al. Effects of fruit bagging on coloring and related physiology, and qualities of red Chinese sand pears during fruit maturation. Sci Hortic. 2009;121:149–58. [Google Scholar]
- 61. Premathilake AT, Ni J, Shen Jet al. Transcriptome analysis provides new insights into the transcriptional regulation of methyl jasmonate-induced flavonoid biosynthesis in pear calli. BMC Plant Biol. 2020;20:388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ahmad M, Li J, Yang Qet al. Phylogenetic, molecular, and functional characterization of PpyCBF proteins in Asian pears (Pyrus pyrifolia). Int J Mol Sci. 2019;20:2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tao R, Yu W, Gao Yet al. Light-induced basic/helix-loop-Helix64 enhances anthocyanin biosynthesis and undergoes CONSTITUTIVELY PHOTOMORPHOGENIC1-mediated degradation in pear. Plant Physiol. 2020;184:1684–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yang Q, Yang B, Li Jet al. ABA-responsive ABRE-BINDING FACTOR3 activates DAM3 expression to promote bud dormancy in Asian pear. Plant Cell Environ. 2020;43:1360–75. [DOI] [PubMed] [Google Scholar]
- 65. Liu D, Yang Q. Expression patterns of NbrgsCaM family genes in Nicotiana benthamiana and their potential roles in development and stress responses. Sci Rep. 2020;10:9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data in this study are provided in the article and its supplementary files.