Abstract
The gram-negative respiratory pathogen Legionella pneumophila infects and grows within mammalian macrophages and protozoan host cells. Upon uptake into macrophages, L. pneumophila establishes a replicative organelle that avoids fusion with endocytic vesicles. There are 24 dot/icm genes on the L. pneumophila chromosome required for biogenesis of this vacuole. Many of the Dot/Icm proteins are predicted to be components of a membrane-bound secretion apparatus similar to type IV conjugal transfer systems. We have been investigating the function of L. pneumophila dot/icm gene products that do not have obvious orthologs in other type IV transfer systems, since these determinants could govern processes unique to phagosome biogenesis. The icmX gene product falls into this category. To understand the role of the IcmX protein in pathogenesis, we have detailed interactions between an L. pneumophila icmX deletion mutant and murine bone marrow-derived macrophages. These data demonstrate that icmX is required for biogenesis of the L. pneumophila replicative organelle. Immunoblot analysis indicates that the icmX gene product is a polypeptide with an estimated molecular mass of 50 kDa. The IcmX protein was localized to the bacterial periplasm, and periplasmic translocation was mediated by an N-terminal sec-dependent leader peptide. A truncated IcmX product was secreted into culture supernatants by wild-type L. pneumophila growing extracellularly in liquid media; however, transport of the IcmX protein into eukaryotic host cells was not detected. Proteins similar in molecular weight to IcmX were identified in other Legionella species by immunoblot analysis using a monoclonal antibody specific for L. pneumophila IcmX protein. From these data, we conclude that the IcmX protein is an essential component of the dot/icm secretion apparatus, and that a conserved mechanism of host cell parasitism exists for members of the Legionellaceae family.
The respiratory pathogen Legionella pneumophila is a facultative intracellular bacterium that can grow within human alveolar macrophages (29). When virulent L. pneumophila bacteria come in contact with host macrophages, they induce the formation of pores in the macrophage membrane (31) and enter a vacuole that evades fusion with late endosomes and lysosomes (28, 46). Phagosomes containing L. pneumophila mature into specialized organelles that support intracellular growth (14, 27). Events that permit L. pneumophila to alter endocytic trafficking of the phagosome in which it resides are mediated through the actions of products encoded by the dot/icm genes (5, 35, 49, 55).
Phagosomes containing L. pneumophila dot/icm mutants traffic to lysosomes as efficiently as and with kinetics similar to vacuoles harboring dead or avirulent microorganisms (46). The inability of L. pneumophila dot/icm mutants to modulate phagosome trafficking is manifested as a severe defect in growth and survival within macrophages (3, 5, 48, 50), as well as a pronounced reduction in virulence (18, 35). Interestingly, when these mutants reside in the same vacuole as wild-type L. pneumophila, they are capable of replicating within this compartment (14). Thus, the dot/icm genes are not required for intracellular growth per se, but play a fundamental role in creating a nutrient rich organelle that is isolated from endocytic traffic.
How the dot/icm gene products control trafficking of phagosomes containing L. pneumophila is unclear. It has been postulated that the dot/icm genes encode a transport apparatus that exports protein molecules into the host cell which directly affect phagosome maturation (45, 51, 56). Evidence in support of this hypothesis comes from DNA sequence analysis indicating that a number of the dot/icm products are similar to components of type IV secretion systems. Recently, it was shown that 14 of the dot/icm products have significant sequence similarities to proteins in the transfer region of the IncI plasmid colIb-P9 (53). Thus, these dot/icm products and their col1b-P9 counterparts are likely orthologous components of a type IV secretion apparatus with a common ancestral progenitor.
Endogenous substrates secreted by the dot/icm apparatus have eluded detection; however, the observation that conjugal transfer of plasmid RSF1010 from L. pneumophila to other gram-negative bacteria requires several of the dot/icm gene products demonstrates that this apparatus has transport activity (49, 55). It seems unlikely that the transfer of genetic material from L. pneumophila into the host cell plays a fundamental role in preventing fusion of the phagosome with lysosomes, since this process happens either during or immediately after bacterial uptake (46, 57). It is apparent that further analysis of the numerous dot/icm proteins is required to understand the function of these genes and determine how they regulate biogenesis of phagosomes in which L. pneumophila resides.
To understand L. pneumophila pathogenesis and elucidate the molecular mechanisms that bacteria employ to alter vesicle trafficking, we have been focusing on dot/icm genes that are not present in other type IV secretion systems. Our hypothesis is that these genes may encode factors that are either exported by the bacteria or tailor a specific function of the type IV apparatus that is directly related to phagosome trafficking. Gene products encoded within the icmWXYZ operon appear to be unique to the L. pneumophila dot/icm system. Recently, we demonstrated that the icmW gene encodes a protein that is not required for the formation of pores in the macrophage plasma membrane upon contact, even though it is necessary for phagosome trafficking and intracellular growth (58). It was postulated that IcmW could be a chaperone that pilots protein molecules to the Dot/Icm apparatus for directed secretion through the pore formed in the host cell plasma membrane.
In this study, we extend our molecular and genetic analysis of the icmWXYZ gene cluster. Our results demonstrate that icmX is required for L. pneumophila phagosome trafficking and intracellular growth. Translational gene fusions and immunoblot analysis indicate that the icmXYZ region encodes a single protein, IcmX, located in the bacterial periplasm. From these data, we conclude that the IcmX protein is an essential component of the Dot/Icm transporter.
MATERIALS AND METHODS
Bacterial strains and media.
The L. pneumophila strains used in this study (Table 1) were grown on charcoal-yeast extract (CYE) plates or in ACES [N-(2-acetamido-2-aminoethanesulfonic acid]-buffered yeast extract (AYE) broth as described previously (19, 47). As required, antibiotics were added to L. pneumophila media at the following concentrations: streptomycin, 100 μg ml−1; kanamycin, 20 μg ml−1; and chloramphenicol, 10 μg ml−1. Escherichia coli strains were grown on L-agar plates or in L broth, and antibiotics were added to growth media at the following concentrations as required: ampicillin, 100 μg ml−1; kanamycin, 40 μg ml−1; and chloramphenicol, 25 μg ml−1.
TABLE 1.
Legionella strains used in this study
| Strain | Parent strain | Important trait(s) | Reference |
|---|---|---|---|
| CR24 | LP02 | rpsL thyA | 5 |
| CR26 | JV374 | rpsL thyA ΔdotB | 55 |
| CR39 | LP01 | rpsL | 5 |
| CR51 | LP02 | rpsL thyA ΔdotA | 14 |
| CR58 | LP01 | rpsL ΔdotA | 47 |
| CR157 | LP01 | rpsL ΔicmW | 58 |
| CR174 | CR157 | rpsL ΔicmW (pIcmWM45) | 58 |
| MM101 | LP01 | rpsL ΔicmX | This study |
| L. pneumophila SG1 | ATCC 33152 | Serogroup 1 | 9 |
| L. pneumophila SG2 | ATCC 33154 | Serogroup 2 | 9 |
| L. pneumophila SG3 | ATCC 33155 | Serogroup 3 | 9 |
| L. bozemanii | ATCC 33217 | 36 | |
| L. gratiana | ATCC 49413 | 6 | |
| L. micdadei (Pittsburgh) | ATCC 33204 | 42 | |
| L. micdadei (Tatlock) | ATCC 33218 | 25 |
Cell culture.
U937 cells (American Type Culture Collection) were cultured in RPMI 1640 with 10% fetal bovine serum (Gibco). U937 cells were differentiated with phorbol 12-myristate 13-acetate (Sigma) for 48 h as described elsewhere (43). Bone marrow-derived macrophages were cultured from female A/J mice (Jackson Laboratory) as described elsewhere (10).
Plasmid constructions.
The in-frame icmX deletion was constructed by joining 5′ and 3′ icmX DNA fragments together in the gene replacement vector pSR47S (39). The 5′ DNA fragment was generated by PCR amplification using a forward primer (5′-GGGAGCTCCTCTTACGATCCTTGATCC-3′) and a reverse primer (5′-CCACGCGTGGCCAAGGCCAGTTTAGGTAA-3′). The 3′ DNA fragment was generated by PCR amplification using a forward primer (5′-CCACGCGTCCCACTGCTGATTTTTCAAGCT-3′) and a reverse primer (5′-CCTCTAGAGCCGGGGAATGATTTAAACCAT-3′). MluI restriction sites that were incorporated into primers are in boldface. The 5′ icmX DNA product was digested with MluI and XbaI. The 3′ icmX DNA product was digested with MluI and SacI. The DNA fragments were ligated into the vector pSR47S, which had been digested with XbaI and SacI. The resulting plasmid contains an icmX deletion allele that has the coding region for IcmX replaced by a MluI restriction site. All pSR47S plasmid constructs were propagated in E. coli DH5α (λpir). This strain encodes the RK6 π protein, which is required for plasmid replication (32).
The icmX deletion was introduced onto the chromosome of L. pneumophila CR39 by allelic exchange. The deletion plasmid was mated from E. coli into CR39 as previously described (47). Mating mixtures were plated on CYE containing kanamycin and streptomycin to select for L. pneumophila that had integrated the deletion plasmid onto the chromosome. Kanamycin-resistant colonies were then plated on CYE containing 5% sucrose to select for bacteria that had lost the plasmid. Sucrose-resistant colonies were screened by PCR using primers that flank the icmX gene to identify clones that had incorporated the deletion. Incorporation of the deletion was further confirmed by digesting the PCR products with MluI. Several ΔicmX clones were assayed for growth in U937 cells, and all were incapable of intracellular replication. Clone MM101 was selected for future studies.
To construct plasmid pMM1.3, the icmX gene was amplified from L. pneumophila CR157 by using a forward primer (5′-GGGAGCTCCAATAACCCTTGCCTGTAC-3′) and a reverse primer (5′-CCTCTAGAGCCGGGGAATGATTTAAACCAT-3′). The PCR product was digested with SacI and XbaI and then ligated into the broad-host-range cloning vector pMMB207 (40), which had been digested similarly. The resulting plasmid contains the icmX gene and the promoter for the icmWX operon but lacks the icmW gene.
To construct plasmid pMM5, the icmX gene was amplified from strain CR157 by using a forward primer (5′-CCGAATTCTCTTTCTCACCCAATAACC-3′) and a reverse primer (5′-GGCCATGGCTTGCTCAGAAGGAGAGCCTTG-3′). The NcoI site in the reverse primer will fuse the last codon of icmX in frame with the M45 epitope tag present in the vector pSB616 (16). The PCR product was digested with EcoRI and NcoI and then ligated into pSB616, which had been digested with the same enzymes. The icmXM45 allele was removed from this plasmid using enzymes EcoRI and SalI. This fragment was ligated into pMMB207, which had been digested with the same enzymes. The resulting plasmid, pMM5, contains the icmXM45 allele and the icmWX promoter.
Plasmid pCR2-1, which produces the IcmX48-PhoA protein, was constructed by first generating a PCR product using a forward primer (5′-GGGAGCTCCTCTTACGATCCTTGATCCTG-3′) and a reverse primer (5′-CCGTCTAGATTTTCCCATCTGCGCCGG-3′). The PCR product was digested with EcoRV and SacI and then ligated into the vector pDot450::PhoA (47), which was first cut with XbaI, blunted with Klenow enzyme, and then cut with SacI. Plasmid pCR3140, which expresses the IcmX399-PhoA protein, was constructed by generating a PCR product using the same forward primer and the reverse primer 5′-CCGTCTAGACCATTGTTGGTTTGGTTGTC-3′. This PCR product was digested with SacI and XbaI and then ligated into the vector pDot450::PhoA, which had been digested with the same enzymes. Plasmid picmX1231::phoA was constructed using the same forward primer and the reverse primer 5′-CCGTCTAGACTGTAGCAGGGGATGCGT-3′. This PCR product was also digested with SacI and XbaI and cloned into the vector pDot450::PhoA, which had been digested with the same enzymes.
Intracellular growth assay.
Growth of L. pneumophila in U937 cells and bone marrow-derived macrophages was determined by using a previously described standard intracellular growth assay (47). Confluent monolayers of bone marrow-derived macrophages or phorbol ester-treated U937 cells in 24-well tissue culture dishes were infected with L. pneumophila at a multiplicity of infection (MOI) of 0.1. The bacteria were harvested in early stationary phase by either growth for 16 to 18 h in AYE broth or isolation from a heavy region of growth on a CYE-agar plate 48 h after inoculation. The infected macrophages were incubated at 37°C and 5% CO2 for 1 h and washed three times with phosphate-buffered saline (PBS) to remove extracellular bacteria. Fresh tissue culture medium was added prior to further incubation. At the appropriate time points, macrophages were lysed in sterile distilled H2O, and dilutions were plated on CYE-agarose plates.
Macrophage permeability assay.
Formation of pores in macrophage membranes upon contact with L. pneumophila was determined as described previously (31). L. pneumophila strains were grown for 48 h on CYE-agar plates and opsonized with polyclonal rabbit anti-L. pneumophila antibody (1:2,000) prior to infections (antibody provided by Ralph Isberg, Tufts University). Bacteria were added at the indicated MOI to 1.5 × 105 murine bone marrow-derived macrophages that were plated on coverslips in 24-well tissue culture dishes. The tissue culture plates were centrifuged at 150 × g for 5 min at room temperature and then incubated for 1 h at 37°C in 5% CO2. The coverslips were inverted onto 5 μl of PBS containing ethidium bromide (25 μg ml−1) and acridine orange (5 μg ml−1). Acridine orange enters all cells, but ethidium bromide is excluded from cells with an intact plasma membrane. Pore-forming activity was measured as the percentage of total cells that stained positive for ethidium bromide. Overlapping color images were recorded for each coverslip using a Zeiss LSM510 confocal microscope and 10× objective. Each image was saved as a TIFF file and exported into NIH Image 1.62 for analysis. The cell counting macro for NIH Image 1.62 was used to determine the number of macrophages that stained positive with ethidium bromide in each image.
Immunofluorescence microscopy.
L. pneumophila was grown overnight to saturation at 37°C in AYE broth. The bacteria were washed and resuspended in PBS (optical density at 600 nm [OD600] = 1.0). L. pneumophila at an MOI of 2 to 10 was added to 105 mouse bone marrow-derived macrophages plated on coverslips in 24-well tissue culture dishes. The tissue culture plates were centrifuged at 150 × g for 5 min at room temperature and incubated for 1 h at 37°C in 5% CO2. Extracellular bacteria were removed by washing multiple times with PBS. Fresh medium was added to each well, and the plates were returned to the incubator for 1 or 8 h. Cells were fixed for 20 min at room temperature in PBS containing 2% paraformaldehyde and 4.5% sucrose. The cells were washed with PBS containing 4.5% sucrose (PBS-S) and permeabilized in ice-cold methanol for 10 s. Coverslips were blocked in PBS containing 2% goat serum (Gibco) and 4.5% sucrose (PBS-GS) at room temperature for 1 h. Lysosomes and late endosomes were stained with the rat monoclonal antibody (MAb) 1D4B (1:100) specific for LAMP-1 (11), followed by fluorescein isothiocyanate-labeled goat anti-rat secondary antibody (1:500). All antibody washes were in PBS-S, and all antibody dilutions were in PBS-GS. Coverslips were treated with RNase A (100 μg ml−1 [Roche]) at 37°C for 30 min, and then bacterial and macrophage DNA was stained with 0.05 μg of propidium iodide (Sigma) ml−1 in PBS at 37°C for 15 min. Coverslips were washed in PBS and mounted onto 1 μl of mounting medium (90% glycerol, 10% PBS, 1 mg of phenylenediamine ml−1) on glass slides. Bacterial phagosomes were imaged using a Zeiss LSM510 confocal microscope. TIFF files were transferred, and images were labeled using Adobe Illustrator 7.0.
Immunoblot analysis.
N-terminal glutathione S-transferase (GST) fusion proteins were constructed with the IcmX and DotA proteins. A DNA fragment encoding the C-terminal 100 amino acid residues of the DotA protein was generated by PCR using the forward primer 5′-CGGAATTCCGGAATCTTTTGGTCAAG-3′ and the reverse primer 5′-GGGTCGACTGAATGTTATTCGGGAGGTGG-3′. The fragment was digested with EcoRI and SalI and then ligated into the similarly digested vector pGEX-KG (23). The GST-DotA fusion protein was expressed in E. coli and affinity purified on glutathione-Sepharose 4B as recommended by the manufacturer (Pharmacia Biotech). The GST-IcmX fusion protein was constructed by cloning an icmX restriction fragment into the vector pGEX-KG (23). The in-frame fusion junction was created by ligating the internal EcoRV site in icmX to the EcoRI site in pGEX-KG after a Klenow reaction was performed to fill in the 5′ EcoRI overhang. The resulting GST-IcmX fusion protein was insoluble when expressed in E. coli and was isolated from inclusion bodies as described elsewhere (24). These GST fusion proteins were used for all animal immunizations (24). Mouse MAbs were produced by the Cell Culture and Hybridoma Facility at State University of New York at Stony Brook. Antibodies from the supernatants of cloned hybridomas were screened by enzyme-linked immunosorbent assay using plates coated with the GST-DotA and GST-IcmX fusion proteins. Clones 5.1 and 11.29 were found to be specific for the IcmX protein, clones 2.29 and 37.29 were found to be specific for the DotA protein, and clones 99 and 107 were found to be specific for the GST protein. Rabbit polyclonal antibodies against IcmX were produced by the Yale University Animal Resources Center Immunization Service using the same antigen.
For immunoblot analysis, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels containing E. coli or Legionella protein extracts in Laemmli sample buffer (33) were transferred to Immobilon P membranes (Millipore) in transfer buffer (50 mM Tris, 380 mM glycine, 0.1% SDS, 20% methanol [pH 8.3]) at either 20 V for 12 h or 100 V for 1 h. The membranes were blocked in BLOTTO (PBS, 5% nonfat dry milk, 0.1% Tween 20) for 1 h at room temperature and incubated overnight with the designated primary antibodies at the following dilutions: rabbit polyclonal IcmX antibody (1:10,000), IcmX MAb 5.1 (1:10), M45 MAb 45 (1:1,000) (41), and DotA MAb 37.29 (1:1,000). Horseradish peroxidase conjugated anti-rabbit and anti-mouse secondary antibodies (Zymed) were used at a dilution of 1:2,000. Renaissance chemiluminescnece reagent (NEN) was used for antibody detection.
Cell fractionation.
Subcellular localization of the IcmX protein was conducted using L. pneumophila cells harvested from 50-ml cultures that were grown to saturation in AYE broth at 37°C. Bacterial cells were disrupted, and lysates were fractionated into soluble and membrane-associated proteins as detailed previously (47). Briefly, bacterial membranes were isolated by centrifugation onto a 60% sucrose cushion and then washed in a high-salt buffer (10 mM HEPES, 5 mM MgSO4, 0.5 M NaCl [pH 7.4]). Inner membrane proteins were solubilized by treating the washed membranes in a Triton X-100 buffer (10 mM HEPES, 5 mM MgSO4, 2% Triton X-100 [pH 7.4]). Outer membrane proteins and Triton X-100-insoluble debris were isolated by centrifugation at 100,000 × g for 30 min. Proteins from an estimated 108 L. pneumophila cells were separated by SDS-PAGE (12% gel), and IcmX protein in each fraction was identified by immunoblot analysis using MAb 5.1. The scanned immunoblots were analyzed using NIH Image 1.62 to determine the relative amounts of immunoreactive product in each lane.
To determine whether IcmX is secreted into culture supernatants, 50-ml cultures of L. pneumophila were grown in AYE broth at 37°C. Exponentially growing bacteria taken from a CYE-agar plate were used to inoculate the cultures (starting OD600 = 0.2). Bacterial growth was monitored by measuring the OD600 of the cultures at regular intervals. After each OD600 reading, an aliquot of liquid culture containing 109 bacterial cells was removed. Bacterial cells were isolated by centrifugation at 8,000 × g for 15 min at 4°C. The clarified culture supernatants were centrifuged at 15,000 × g for 20 min at 4°C to remove any remaining bacterial cells or insoluble debris. Proteins in the culture supernatants were precipitated by adding ice-cold 100% trichloroacetic acid (TCA) to a final concentration of 12%. After incubation on ice for 1 h, the TCA precipitate was pelleted at 15,000 × g for 30 min at 4°C. Whole cell bacterial pellets and TCA-precipitated supernatant proteins were resuspended in 200 μl of Laemmli buffer and boiled for 5 min, and 20 μl of each sample was separated by SDS-PAGE (12% gel). IcmX protein was detected by immunoblot analysis using a rabbit polyclonal antibody specific for IcmX.
To determine whether the IcmX protein is secreted upon contact with eukaryotic host cells, murine bone marrow-derived macrophages were infected and fractionated as described previously (15, 58). Briefly, a confluent monolayer of murine bone marrow-derived macrophages in 100-mm-diameter tissue culture dishes were infected with L. pneumophila at an MOI of 50 in 2 ml of RPMI medium without fetal bovine serum. The infected macrophages were incubated at 37°C in 5% CO2 for 2 h to allow bacterial uptake. After infection, the tissue culture medium was removed. To separate extracellular bacteria from proteins in the tissue culture supernatant, the collected medium was centrifuged at 15,000 × g for 15 min. The pellet, which contains extracellular bacteria, was resuspended in 50 μl of PBS. Proteins in the tissue culture supernatant were precipitated with TCA (12% final), pelleted, and resuspended in 50 μl of PBS. The macrophage monolayer was lysed on ice in 2 ml of Hanks' balanced salt solution (Gibco) containing 0.1% Triton X-100 and a 1:1,000 dilution of a protease inhibitor cocktail (Sigma P-8340). The macrophage lysate was transferred to a microcentrifuge tube and treated with RNase A (10 μg ml−1) and DNase I (10 μg ml−1) for 15 min at room temperature. Intracellular bacteria and Triton X-100-insoluble material were pelleted by centrifugation at 15,000 × g. The pellet was resuspended in 50 μl of PBS. Triton X-100-soluble proteins, which would presumably include factors secreted or translocated by the bacteria upon contact with host cells, were precipitated in TCA (12% final), and pelleted at 15,000 × g. The TCA pellet was resuspended in 50 μl of PBS; 50 μl of 2× Laemmli buffer was added to each fraction. Samples to be probed for IcmX protein were boiled for 5 min before loading, and samples to be probed for DotA protein were incubated at 37°C for 5 min prior to being loaded. A 20-μl aliquot of each sample was separated by SDS-PAGE (12% gel) for immunoblot analysis. IcmX protein was identified in each fraction by immunoblot analysis using a polyclonal antibody specific for IcmX, and the DotA protein was identified using the MAb 37.29.
Alkaline phosphatase assay.
Plasmids pCR2-1, pCR3140, and picmX1231::phoA were transformed into the E. coli phoA mutant strain CC118 (30) and L. pneumophila strains CR24 (thyA), CR26 (thyA ΔdotB), and CR51 (thyA ΔdotA). The resulting bacterial strains were grown in liquid broth. Alkaline phosphatase activity was determined by using the substrate Sigma 104 and measuring production of the colorimetric reaction product as described previously (47).
Biotin labeling.
L. pneumophila strain CR174 was grown to early stationary phase in AYE broth at 37°C. CR174 expresses the IcmWM45 epitope-tagged protein, which has been shown previously to be located in the bacterial cytoplasm (58). Bacterial cells were pelleted at 8,000 × g for 15 min and washed in ice-cold PBS. To label bacterial proteins, 2 × 109 L. pneumophila cells were resuspended in 1 ml of PBS. Sulfonsuccinimidyl-6-(biotinamido)hexanoate (sulfo-NHS-LC-biotin; Pierce) was added at a final concentration of 10 μg ml−1. The cells were incubated on ice for 30 min. Excess biotin was removed by three successive washes where the cells were pelleted at 15,000 × g for 2 min and resuspended in 1 ml of ice-cold PBS. After the final wash, the cells were lysed in 100 μl of cracking buffer (1% SDS, 10 mM Tris, 1 mM EDTA [pH 8.0]) and boiled for 2 min. The lysates were cooled at room temperature, and 900 μl of ice-cold immunoprecipitation buffer (2% Triton X-100, 50 mM Tris, 1 mM EDTA, 150 mM NaCl [pH 8.0]) was added. The lysates were cleared by pelleting insoluble debris at 15,000 × g for 10 min. TCA (12% final) was added to 400 μl of the cleared lysate, and the precipitated proteins were resuspended in 100 μl of protein loading buffer. To isolate biotin-labeled proteins, 50 μl of streptavidin-agarose (Sigma) was added to 400 μl of the remaining cleared lysate, and the mixture was incubated overnight at 4°C on a rotating shaker. The streptavidin beads were pelleted for 30 s in a microcentrifuge, and the beads were washed three times in immunoprecipitation buffer. After the final wash, the beads were resuspended in 100 μl of protein loading buffer and boiled for 5 min. Then 20 μl of the TCA-precipitated lysate and 20 μl of the streptavidin agarose precipitate were separated by SDS-PAGE (12% gel). The IcmX protein was identified in each fraction by immunoblot analysis using a rabbit polyclonal antibody specific for IcmX, and the IcmWM45 protein was identified with MAb 45.
Protease protection.
L. pneumophila strain CR174 was grown to mid-exponential phase (OD600 = 2.0) in AYE broth at 37°C. Bacterial cells were pelleted at 15,000 × g for 5 min and washed with either a sucrose buffer (30 mM Tris, 20% sucrose [pH 8.0]) or a Tris-buffered saline solution (30 mM Tris, 100 mM NaCl, 1 mM MgCl2 [pH 8.0]). For each time point, approximately 109 washed bacterial cells were pelleted and resuspended in 100 μl of similar wash buffer. To permeabilize the outer membrane and peptidoglycan layer, EDTA (1 mM final) and lysozyme (10 μg ml−1 final) were added to the bacterial cells in sucrose buffer. After a 10-min incubation period on ice, pronase (Sigma) was added to all tubes (0.05 mg ml−1 final), and the reaction mixtures were incubated at room temperature. Reactions were stopped just before and 10, 30, and 60 min after the addition of pronase. The pronase digestions were terminated upon addition of 1.4 ml of 12% TCA to the reaction tube. TCA-precipitated proteins were collected for each time point and resuspended 400 μl of Laemmli buffer, and 20 μl of each fraction was separated by SDS-PAGE (12% gel) for immunoblot analysis. To demonstrate that IcmX protein in cellular extracts is protease sensitive, 109 bacteria in sucrose buffer and Tris-buffered saline were lysed on ice by sonication for five bursts of 30 s each (Branson Sonifier 250 with microtip), and the resulting extracts were digested with pronase (0.05 mg ml−1 final) for 60 min at room temperature. IcmX protein was identified by immunoblot analysis using a rabbit polyclonal antibody specific for IcmX.
Identification of IcmX protein in other Legionella serogroups and species.
Legionella species and serogroups were obtained from the American Type Culture Collection. The bacteria were first passaged on CYE-agar plates and then grown overnight in AYE broth at 37°C to saturation. Approximately 109 bacterial cells were pelleted, washed once in PBS, and lysed in 200 μl of Laemmli buffer. The bacterial extracts were separated by SDS-PAGE (12% gel), and IcmX-immunoreactive products were identified by immunoblot analysis using MAb 5.1. For nucleic hybridization studies, digested chromosomal DNA from each of the Legionella species and serogroups was probed using 32P-labeled DNA fragments encoding dotA (nucleotides 40 to 454), icmX (nucleotides 1 to 519), and mip (nucleotides 267 to 696) as described previously (12). To detect DNA fragments with at least 70% nucleotide identity, the filters were hybridized with the probes at 37°C in the presence of 5× SSC (1× SSC is 0.15 M NaCl plus 0.15 M sodium citrate)–18% deionized formamide and were washed at 50°C in 5.3× SSC containing 0.1% SDS.
RESULTS
Identification of the IcmX protein.
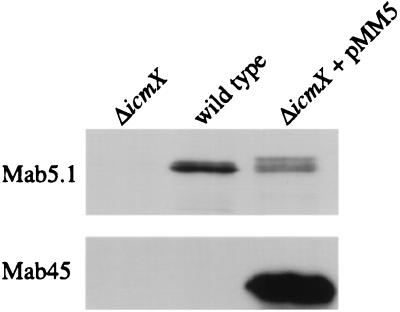
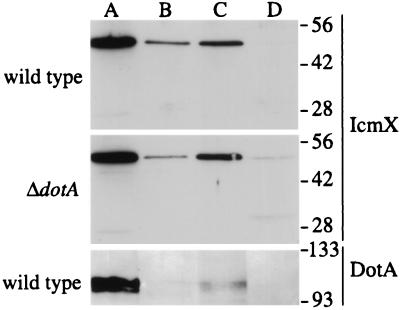
The icmX gene was initially reported to be a 420-bp open reading frame located within the icmWXYZ operon, and it was predicted to encode a protein of 140 amino acids (8). Recent sequence corrections suggest that the icmXYZ region contains a single gene, icmX, predicted to encode a 1,398-bp open reading frame that would produce a protein with a molecular mass of 50.6 kDa (GenBank accession no. U07354). To determine whether a 50.6-kDa IcmX protein is produced by this open reading frame, the M45 epitope tag was fused to the 3′ end of the putative 1,398-bp icmX gene. An epitope-tagged product with an estimated molecular mass of 50 kDa was identified by immunoblot analysis in L. pneumophila extracts expressing the icmXM45 gene fusion encoded on plasmid pMM5 (Fig. 1, bottom). To identify an endogenously produced icmX product, MAbs were generated against a GST-IcmX fusion protein and used to detect a product with an estimated molecular mass of 50 kDa in L. pneumophila extracts (Fig. 1, top). To determine whether this product was encoded by the icmX gene, an in-frame chromosomal deletion of icmX was created in L. pneumophila, resulting in strain MM101. The 50-kDa protein identified by MAb 5.1 was not found in extracts prepared from the L. pneumophila icmX deletion mutant; however, the MAb did recognized the epitope-tagged IcmX-M45 protein (Fig. 1). As expected, the apparent molecular weight of the IcmXM45 protein was slightly larger due to the addition of 18 C-terminal amino acid residues that comprise the M45 peptide. These data demonstrate that the icmX gene encodes a 50-kDa polypeptide, indicating that the most recent icmX sequence is correct (GenBank accession no. U07354).
FIG. 1.
The L. pneumophila icmX gene encodes a polypeptide with an estimated molecular mass of 50 kDa. Immunoblots of cellular extracts from L. pneumophila strains MM101 (ΔicmX), CR39 (wild type), and MM101 expressing the epitope-tagged IcmX-M45 protein (ΔicmX + pMM5) were probed with either MAb 5.1 specific for IcmX or MAb 45 specific for the M45 epitope tag. The gel region illustrated was located between protein standards with molecular masses of 56 and 42 kDa.
IcmX protein is required for phagosome trafficking and intracellular growth.
Previous studies suggest that icmX is essential for L. pneumophila pathogenesis (18, 35). To delineate the role of icmX during uptake and trafficking of phagosomes containing L. pneumophila, we first measured the capacity of MM101 to grow intracellularly. To complement the ΔicmX mutation in MM101, a DNA fragment encompassing the icmWX operon was amplified from the chromosome of L. pneumophila strain CR157, which has an in-frame deletion in the icmW gene (58). The plasmid containing this amplified DNA fragment, pMM1.3, contains the entire icmX gene transcribed by the endogenous L. pneumophila promoter for the icmWX operon but does not produce the IcmW protein.
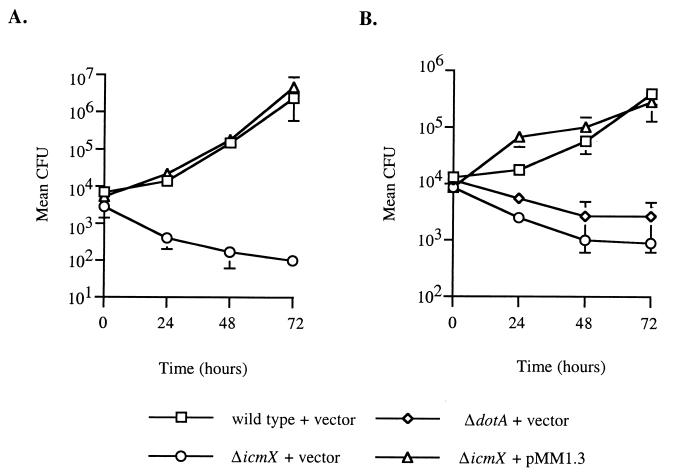
We investigated whether the ΔicmX mutant, MM101, could replicate in mammalian phagocytic cells by measuring the CFUs recovered from infected host cells over 72 h. MM101 (ΔicmX) was not capable of replicating in either the human U937 macrophage-like cell line (Fig. 2A) or bone marrow-derived macrophages cultured from A/J mice (Fig. 2B). Intracellular survival of MM101 in murine bone marrow-derived macrophages paralleled that of an isogenic ΔdotA mutant. When plasmid pMM1.3 was introduced in trans to the chromosomal icmX deletion, intracellular replication of MM101 was restored to near wild-type levels in both U937 cells and murine macrophages. These data demonstrate that icmX is essential for intracellular growth of L. pneumophila within mammalian cells.
FIG. 2.
The IcmX protein is required for replication of L. pneumophila within macrophages. Intracellular growth of L. pneumophila in differentiated U937 cells (A) or in mouse bone marrow-derived macrophages (B) was measured over 72 h. Host cells were infected with CR39 (wild type), CR58 (ΔdotA), and MM101 (ΔicmX) containing the cloning vector pMMB207 (vector) and with MM101 containing the icmX gene in trans (pMM1.3). Each data point represents mean CFU recovered from eukaryotic host cells in triplicate wells ± standard deviation.
Virulent L. pneumophila will form pores in the plasma membrane of host cells upon contact (31). Mutations in several of the dot/icm genes have been shown to eliminate pore-forming activity (31, 58). To determine whether icmX plays an essential role in the formation of pores in the macrophage membrane, we examined whether macrophages infected with MM101 (ΔicmX) would exclude the membrane-impermeable dye ethidium bromide. Nuclear fluorescence after ethidium bromide staining was not observed for macrophages infected with MM101 at an MOI as high as 1,000 (Fig. 3). Thus, ethidium bromide is excluded from these cells, indicating that the ΔicmX mutant does not create pores in the macrophage plasma membrane. Pore-forming activity was fully restored to the ΔicmX strain when the icmX gene was returned in trans on the plasmid pMM1.3. A similar number of macrophages infected with MM101 (pMM1.3) were stained with ethidium bromide as macrophages infected with wild-type L. pneumophila. These data demonstrate that icmX is required for the formation of pores in the membrane of eukaryotic host cells.
FIG. 3.
L. pneumophila IcmX protein is required for the formation of pores in the plasma membrane of eukaryotic host cells. Bone marrow-derived macrophages were infected with L. pneumophila at MOIs of 0, 20, 200, and 1,000 for 1 h and stained with ethidium bromide and acridine orange. Fluorescence micrographs were used to calculate the percentage of macrophages that stained positive for ethidium bromide in random fields. Data shown are the average of three independent readings ± standard deviation. The L. pneumophila strains used were CR39 (wild type), CR58 (ΔdotA), MM101 (ΔicmX), and MM101 containing plasmid pMM1.3.
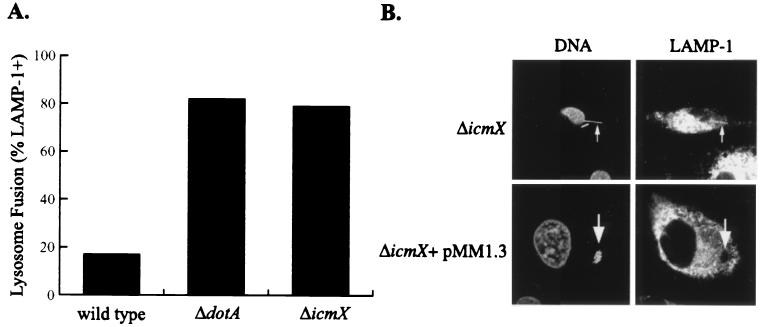
We examined bone marrow-derived macrophages infected with MM101 to determine whether icmX is required for inhibition of phagosome lysosome fusion and establishment of a replicative vacuole. Mouse bone marrow-derived macrophages were infected with L. pneumophila for 1 h. Fixed macrophages were stained with MAb 1D4B, specific for the late endosomal/lysosomal protein LAMP-1 (11). Bacterial and macrophage nuclei were labeled with propidium iodide. The stained macrophages were examined by confocal microscopy, and the percentage of bacterial phagosomes that had acquired LAMP-1 was determined. The majority of phagosomes containing MM101 (ΔicmX) were found to have intense circumferential LAMP-1 staining, whereas LAMP-1 was absent from most phagosomes containing wild-type L. pneumophila (Fig. 4A). To investigate whether an icmX mutant can form a replicative niche, murine bone marrow-derived macrophages were pulsed with MM101 and the complemented strain MM101 (pMM1.3) for 1 h. Infected macrophages were fixed after an 8-h chase and then stained with 1D4B and propidium iodide. MM101 (ΔicmX) was unable to establish replicative organelles. The ΔicmX mutants were found as single bacterial rods inside phagosomes that stained positive for LAMP-1 (Fig. 4B). In contrast, when the icmX gene was reintroduced on plasmid pMM1.3, the bacteria were found replicating inside large vacuoles that were devoid of LAMP-1 staining (Fig. 4B). These results were consistent in three independent experiments in which more than 500 infected macrophages were examined for each strain. From these data, we conclude that the IcmX protein plays an important role in the early signaling events that regulate trafficking of the L. pneumophila phagosome, processes that are essential for the establishment of a replicative niche in eukaryotic host cells.
FIG. 4.
L. pneumophila requires the IcmX protein to establish a replicative organelle that evades fusion with late endosomes and lysosomes. (A) Murine bone marrow-derived macrophages were infected with L. pneumophila for 1 h, and phagosomes were scored for the presence of LAMP-1. Results show that phagosomes containing wild-type L. pneumophila strain CR39 (wild type) were able to avoid fusion with LAMP-1-containing organelles, whereas phagosomes containing the mutant CR58 (ΔdotA) or MM101 (ΔicmX) accumulated LAMP-1. Data are the average of two independent experiments that did not differ by more than 10% in which 100 phagosomes were scored for each strain. (B) Macrophages were infected with L. pneumophila for 8 h and then stained with propidium iodide to label bacteria and macrophage nuclei and MAb (1D4B) specific for LAMP-1. L. pneumophila ΔicmX mutants were found as single rods inside phagosomes that stained positive for LAMP-1 (small arrows). The complemented L. pneumophila mutants (ΔicmX + pMM1.3) were localized to organelles containing multiple bacteria that did not exhibit appreciable levels of LAMP-1 staining on the surrounding membrane (large arrows).
Bacterium-associated IcmX protein is located in the periplasm.
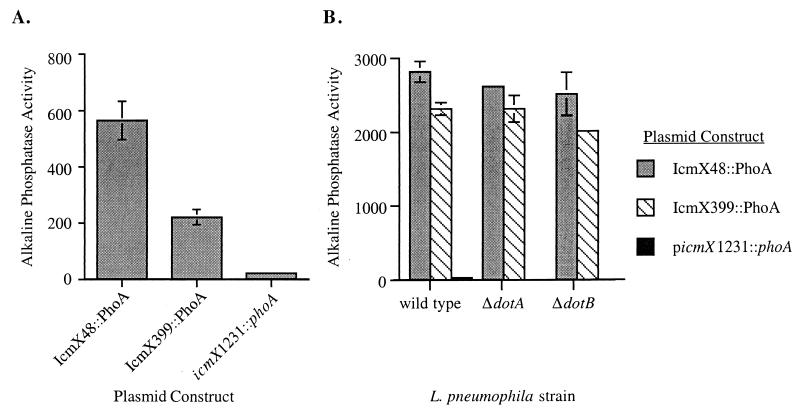
Predictions based on sequence analysis suggested that icmX encodes a sec-dependent N-terminal signal peptide that could mediate translocation of the protein across the bacterial inner membrane into the periplasmic space. To examine whether the IcmX protein is translocated across the inner membrane by a sec-mediated process, E. coli alkaline phosphatase gene fusions were constructed (26, 34). The phoA gene was ligated in frame to the icmX gene to create translational C-terminal alkaline phosphatase fusions at amino acid positions 48 and 399 of the IcmX protein. High levels of alkaline phosphatase activity were detected in E. coli expressing the IcmX48-PhoA protein and the IcmX399-PhoA protein (Fig. 5A). Plasmid picmX1231::phoA has the phoA gene ligated to the icmX +1 reading frame, resulting in an out-of-frame fusion. Alkaline phosphatase activity above background levels was not detected in E. coli containing picmX1231::phoA (Fig. 5A). To determine whether the Dot/Icm apparatus plays a role in translocation of the IcmX-PhoA hybrid proteins into the bacterial periplasm, the fusion plasmids were introduced into L. pneumophila strain CR24 and into isogenic ΔdotA (CR51) and ΔdotB (CR26) mutant strains. Alkaline phosphatase activities were over 100-fold above background in CR24, CR51, and CR26 expressing the IcmX48-PhoA protein and the IcmX399-PhoA protein (Fig. 5B). These data demonstrate that the IcmX protein has an N-terminal sequence that can mediate translocation of polypeptides across the bacterial cytoplasmic membrane by a process that does not require the Dot/Icm transporter, indicating that this is a sec-mediated transport event.
FIG. 5.
The N-terminal domain of IcmX can direct translocation of alkaline phosphatase fusion proteins into the bacterial periplasm. (A) Alkaline phosphatase activity was measured for E. coli strain CC118 harboring plasmids pCR2-1 (IcmX48::PhoA), pCR3140 (IcmX399::PhoA), and picmX1231::phoA. (B) Plasmids containing the icmX::phoA fusions were transferred into L. pneumophila strains CR24 (wild type), CR26 (ΔdotB), and CR51 (ΔdotA). Alkaline phosphatase activity for each fusion protein expressed in these isogenic L. pneumophila strains was determined. Data are the average of three independent assays ± standard deviation.
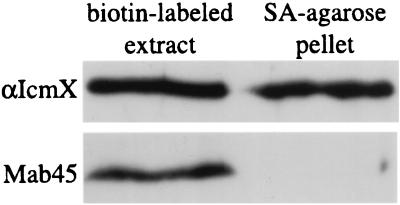
To determine whether IcmX protein expressed endogenously is translocated into the bacterial periplasm, intact L. pneumophila cells were labeled with sulfo-NHS-LC-biotin. This relatively small molecule can cross the bacterial envelope to gain access to proteins in the periplasm, but it cannot diffuse across the inner membrane to label cytoplasmic proteins (1, 7). L. pneumophila extracts were prepared after treatment of bacterial cells with sulfo-NHS-LC-biotin, and labeled proteins were precipitated on streptavidin-conjugated agarose beads. Immunoblot analysis of proteins precipitated on streptavidin-agarose indicate that the IcmX protein is accessible to sulfo-NHS-LC-biotin labeling, but the cytoplasmic IcmW protein is not biotin labeled (Fig. 6). These data demonstrate that the IcmX protein is translocated across the inner membrane into the bacterial periplasm in L. pneumophila.
FIG. 6.
The IcmX protein is translocated across the L. pneumophila plasma membrane. L. pneumophila strain CR174 was biotin labeled with the membrane-impermeable analog sulfo-NHS-LC-biotin. Biotin-labeled proteins were precipitated from the CR174 extract by using streptavidin-agarose. Immunoblots containing samples of total cell extract (biotin-labeled extract) and proteins precipitated on streptavidin-agarose (SA-agarose pellet) were probed using either a polyclonal antibody specific for IcmX or MAb 45, which will detect the cytoplasmic IcmWM45 protein.
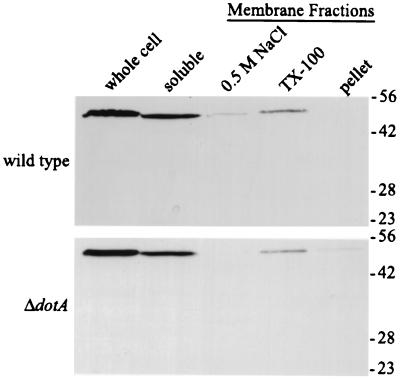
To investigate the subcellular localization of the IcmX protein, we divided L. pneumophila extracts into soluble and membrane-associated fractions and then determined the amount of IcmX protein present in each fraction by immunoblot analysis (Fig. 7). An estimated 78% of the IcmX protein was soluble after bacterial lysis. The remaining IcmX protein was recovered in the bacterial membrane fraction and could be dissolved in 2% Triton X-100, which suggests that some of the IcmX protein is associated with the inner membrane. In agreement with previously published data (47), the DotA protein was found in the Triton X-100-soluble fraction when these fractions were probed with DotA-specific MAb 37.29 (data not shown).
FIG. 7.
The IcmX protein in L. pneumophila is primarily soluble and not membrane associated. Proteins from L. pneumophila strain CR39 (wild type) and CR58 (ΔdotA) were separated into soluble and membrane-associated fractions. The bacterial lysates (whole cell), proteins in the cytoplasm and periplasm (soluble), proteins obtained after salt (0.5 M NaCl) and detergent (Triton X-100 [TX-100]) extraction of isolated membranes, and the Triton X-100-insoluble membrane proteins (pellet) were analyzed. The amount of IcmX protein in each fraction was determined by immunoblot analysis using MAb 5.1. Indicated on the right are the positions of molecular mass standards in kilodaltons.
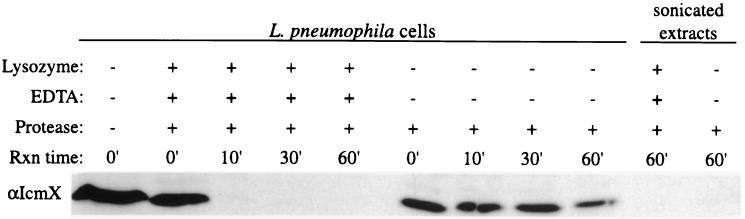
One interpretation that can be drawn from the subcellular localization studies is that bacterial cell-associated IcmX protein resides primarily in the periplasmic space. Alternatively, IcmX protein may be located at the cell surface and associate weakly with the bacterial envelope. To distinguish between these two possibilities, we subjected L. pneumophila cells to protease digestion and then measured the degradation of IcmX protein by immunoblot analysis. Cell-associated IcmX protein was degraded rapidly when the cells were pretreated with EDTA and lysozyme, agents that allow the protease to enter the periplasmic space by selectively disrupting the outer membrane and peptidoglycan layer (Fig. 8). There was no detectable degradation of IcmX protein over the first 30 min in bacterial cells that were not treated with EDTA-lysozyme, which suggests that IcmX protein does not accumulate on the bacterial surface. Treatment of the bacterial cells with EDTA-lysozyme resulted in rapid proteolytic digestion of the polytopic inner membrane protein DotA but did not facilitate degradation of the cytoplasmic IcmW protein (data not shown). Based on these data, we conclude that cell-associated IcmX protein resides primarily in the bacterial periplasm.
FIG. 8.
Cellular IcmX protein is not exposed on the bacterial surface. L. pneumophila cells were suspended in either a sucrose buffer containing EDTA and lysozyme to make the cell envelope permeable or a Tris-saline buffer that will maintain outer membrane integrity. The bacterial cells were digested with pronase (Protease) for 0 to 60 min (Rxn time). Sonicated L. pneumophila extracts were prepared in these same buffers as a control for protease activity. Immunoblots containing samples from each reaction were probed with a polyclonal antibody specific for IcmX. The region of the blot containing the 50-kDa IcmX-immunoreactive product is shown (αIcmX).
An IcmX fragment is secreted into culture supernatants during L. pneumophila growth in liquid broth.
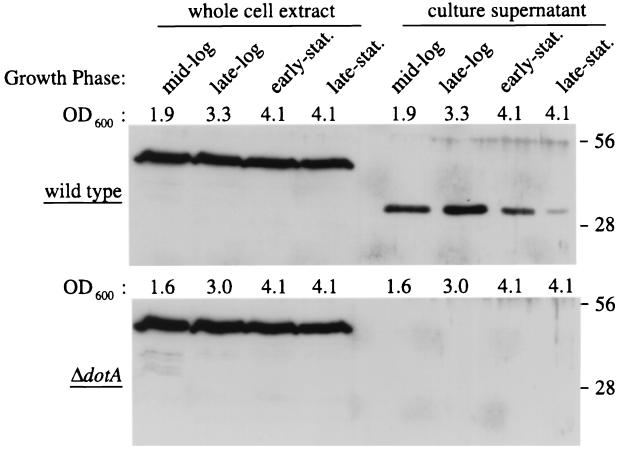
It is possible that proteins secreted by the Dot/Icm apparatus will be present in supernatants isolated from broth-grown L. pneumophila cultures. To determine whether periplasmic IcmX protein is secreted by a dot/icm-dependent mechanism, culture supernatants were harvested during growth of wild-type and dotA mutant L. pneumophila in liquid broth. Immunoblot analysis of whole cell bacteria and culture supernatants indicate that full-length IcmX protein, with an estimated molecular mass of 50 kDa, is found associated with intact bacterial cells but is not present in culture supernatants (Fig. 9). A truncated IcmX-immunoreactive product was observed in wild-type L. pneumophila culture supernatants. This product was notably absent in supernatants isolated from dotA mutant cultures. The truncated immunoreactive product was never detected in culture supernatants from MM101 (ΔicmX) (data not shown). The IcmX-immunoreactive product became less abundant as the bacteria enter stationary phase. This decrease in protein level was likely due to degradation of the IcmX product by the potent zinc metalloprotease that accumulates in L. pneumophila culture supernatants (17). These data indicate that after translocation into the periplasm, the IcmX protein is processed and secreted by a process that requires the Dot/Icm apparatus.
FIG. 9.
Wild-type L. pneumophila secretes a truncated IcmX fragment. L. pneumophila strains CR39 (wild type) and CR58 (ΔdotA) were harvested during extracellular growth in liquid culture. The growth phase of bacteria in each sample was determined by plotting the OD600 of the culture over time. Protein extracts were prepared from bacteria in mid-exponential, late exponential, early stationary (stat.), and late stationary phases of growth. Proteins were isolated from intact bacteria (whole cell extract) and the growth media (culture supernatant). Immunoblots containing these protein samples were probed with a polyclonal antibody specific for IcmX. The positions of molecular mass standards with masses of 56 and 28 kDa are indicated on the right.
DotA-dependent secretion and translocation of IcmX is not detected upon contact with macrophages.
If secretion of the IcmX protein by the Dot/Icm apparatus is important for host cell parasitism, detection of this product may be enhanced upon L. pneumophila contact with macrophages. To determine whether dot/icm-dependent secretion and/or translocation of IcmX occurs during contact with host cells, monolayers of bone marrow-derived macrophages were infected with wild-type L. pneumophila and an isogenic dotA mutant for 2 h. Fractions enriched for proteins located in tissue culture supernatant, macrophage cytoplasm, extracellular bacteria, and intracellular bacteria were examined by immunoblot analysis using a polyclonal antibody specific for the IcmX protein (Fig. 10). The IcmX protein was found predominantly in fractions containing intact bacteria (Fig. 10, lanes A and C). There were no apparent differences in IcmX localization when fractions isolated from macrophages infected with wild-type L. pneumophila were compared with fractions isolated after infection with a ΔdotA mutant (Fig. 10). In contrast to extracellular growth in liquid broth, wild-type L. pneumophila did not secrete appreciable levels of the truncated IcmX product during host cell infection. When the fractions were probed for the inner membrane protein DotA, there was no detectable signal observed in the samples enriched for proteins secreted into the tissue culture supernatant or macrophage cytoplasm, even though there was detectable full-length IcmX protein in these same fractions (Fig. 10, wild type, lanes B and D). These data indicate that IcmX protein detected in lanes B and D was not derived from lysed L. pneumophila cells or intact bacteria. Thus, this pool of IcmX protein was released from the bacterial periplasm by a dot/icm-independent mechanism. We were unable to detect staining of the phagosome lumen, phagosome membrane, or macrophage cytoplasm when macrophages containing replicating L. pneumophila were stained with polyclonal or monoclonal IcmX antibodies and then examined by immunofluorescence microscopy (data not shown). These data suggest that targeted secretion of either full-length or the truncated IcmX product by the Dot/Icm apparatus may not be specifically induced upon bacterial contact with eukaryotic host cells.
FIG. 10.
Secretion of the IcmX protein is not enhanced upon L. pneumophila contact with eukaryotic host cells. Bone marrow-derived macrophages were infected separately with L. pneumophila strains CR39 (wild type) and CR58 (ΔdotA) for 2 h. After infection, fractions enriched for extracellular bacteria (lane A), proteins secreted into the tissue culture media (lane B), intracellular bacteria (lane C), and proteins secreted intracellularly (lane D) were collected (see Materials and Methods). IcmX and DotA protein levels were determined by immunoblot analysis using a rabbit polyclonal antibody specific for IcmX and the DotA-specific MAb 37.29.
The IcmX protein is conserved among different Legionellaceae family members.
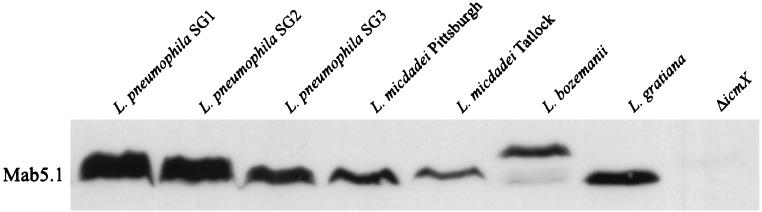
Homology searches indicate that the icmX product has no known counterparts in other secretion systems. To determine whether the IcmX protein is expressed by other Legionella isolates, several different L. pneumophila serogroups and other Legionella species were analyzed for the presence of IcmX protein by probing immunoblots with MAb 5.1. Immunoreactive products with a molecular mass similar to that of the L. pneumophila serogroup 1 IcmX protein were identified in all other L. pneumophila serogroups and Legionella species examined (Fig. 11). Nucleic acid hybridization studies using both the L. pneumophila serogroup 1 icmX and dotA genes as probes confirm that the dotA/icmWX region is present in these other Legionella serogroups and species (data not shown). These data indicate that an IcmX homolog is expressed by distantly related Legionella species, suggesting that the dot/icm secretion system is likely to play a role in host cell parasitism by all Legionellaceae family members.
FIG. 11.
A protein homologous to IcmX is expressed ubiquitously by Legionellaceae family members. Immunoblots containing whole cell protein extracts from various L. pneumophila serogroups and Legionella species were probed with MAb 5.1 specific for IcmX.
DISCUSSION
Modulation of phagosome biogenesis is an intracellular survival strategy that numerous microbial pathogens have adopted (38, 54). This process allows microorganisms to modify endocytic vacuoles into specialized organelles that will protect them from degradation and provide nutrients for intracellular growth. L. pneumophila is an interesting example of a facultative intracellular pathogen that can infect and multiply within evolutionarily diverse eukaryotic host cells (21, 52). In nature, freshwater protozoa are the principal reservoir for the bacteria (20). Aerosols containing L. pneumophila can result in human infections which are manifested by replication of the bacteria in alveolar macrophages (29, 37). The isolation of L. pneumophila mutants that are unable to replicate in eukaryotic host cells has identified a number of factors that play a role in pathogenesis (3, 5, 13, 22, 48). The dot/icm genes encode factors that enable L. pneumophila to alter trafficking of phagosomes formed upon uptake of the bacteria into macrophages (46, 57). Based on the capacity of L. pneumophila to replicate within different hosts, we would predict that the function of the dot/icm genes is to interfere with a fundamental cellular process that has been conserved across evolutionary boundaries. In an effort to understand how the dot/icm gene products can modulate biogenesis of the L. pneumophila phagosome, we have focused our attention on those products that are unique to L. pneumophila and are not found as components of other transport systems.
In this study, we identified the IcmX protein. Protein fusions and immunoblot analysis indicate that the icmX gene encodes a protein with an estimated mass of 50 kDa. These data confirm the most recent GenBank DNA sequence entry reporting that the icmX gene is 1,398 bp in length. We found that L. pneumophila mutants containing an in-frame deletion in the icmX gene were unable to replicate intracellularly and that bacterial growth in macrophages could be restored by introducing the icmX gene in trans to this mutation. These data demonstrate that icmX is essential for host cell pathogenesis. These findings agree with earlier reports demonstrating that icmX transposon insertion mutants were defective for replication in human monocytic cell lines (48) and were unable to cause pneumonia in a guinea pig model of disease (18).
Our results show that the IcmX protein is necessary for biogenesis of an organelle that supports L. pneumophila intracellular growth. L. pneumophila icmX deletion mutants were defective in the ability to form pores in the macrophage plasma membrane, and they reside in phagosomes that fuse with vesicles containing the late endosomal/lysosomal protein LAMP-1. These data indicate that the IcmX protein is required to modulate trafficking of the phagosome in which L. pneumophila resides. In a recent study we found that icmX mutants can replicate intracellularly if they reside in a vacuole formed by wild-type L. pneumophila, indicating that icmX is not required to assimilate nutrients from a niche that is permissive for growth (14). Thus, the icmX product is a factor that plays an essential role in the transmission of a signal to the host cell that modulates phagosome biogenesis.
C-terminal alkaline phosphatase protein fusions to the IcmX protein had phosphatase activity in both E. coli and L. pneumophila, and their activity was not dependent on a functional Dot/Icm transport apparatus. Sulfo-NHS-LC-biotin labeling experiments confirm that the IcmX protein is translocated across the bacterial inner membrane. Fractionation of L. pneumophila lysates determined that most of the bacterium-associated IcmX protein remains soluble and does not sediment with the inner and outer membranes. In addition, bacterium-associated IcmX protein was not accessible to extracellular proteases. From these data, we conclude that an N-terminal signal peptide mediates translocation of the IcmX protein into the bacterial periplasm by a sec-dependent process and that the bacterial-associated IcmX protein resides primarily in the periplasmic space.
Supernatants from wild-type L. pneumophila grown in liquid culture contain a truncated IcmX fragment. We have recently purified a protein from L. pneumophila with an estimated mass of approximately 30 kDa that is present in wild-type but not dotA mutant culture supernatants (H. Nagai and C. R. Roy, unpublished data). N-terminal amino acid sequence data obtained for this protein was 100% identical to amino acids 165 to 177 of the IcmX protein, indicating that this protein is the secreted IcmX-immunoreactive product identified in Fig. 9. Thus, the secreted IcmX product is a C-terminal fragment that results from processing events that remove the first 164 amino acid residues of the full-length IcmX protein. The IcmX 165-466 protein would have a calculated mass of 33.2 kDa, in close agreement with the mobility of the truncated IcmX product identified by immunoblot analysis. Interestingly, the VirB1 protein from Agrobacterium tumefaciens is secreted by an analogous mechanism (4). VirB1 has an N-terminal transmembrane domain that mediates translocation of the protein into the bacterial periplasm, where the protein becomes a substrate for the VirB secretion apparatus. Like IcmX, the secreted VirB1* product is a truncated C-terminal polypeptide. The first 172 amino-terminal residues are removed from VirB1 to generate the VirB1* product.
Secretion of the truncated IcmX protein could not be detected during infection of eukaryotic host cells. We cannot rule out the possibility that during eukaryotic host cell infection, the half-life of secreted IcmX protein is very short. This could limit our ability to detect secretion of IcmX protein into host cellular compartments by the Dot/Icm apparatus. Alternatively, the secreted IcmX protein could represent a spent form of the protein that is no longer needed by the bacteria. Future studies will focus on whether the secreted IcmX protein is required for host cell pathogenesis.
Immunoblot analysis indicates that other Legionella species express a protein homologous to IcmX from L. pneumophila serogroup 1. These data suggest that the Dot/Icm transport system has been conserved in other virulent Legionella species. There is evidence to suggest that Legionella micdadei creates a replicative organelle that is morphologically distinct from the L. pneumophila growth niche (2). It has also been reported that a number of L. pneumophila virulence traits that require the Dot/Icm apparatus, such as pore formation and evasion of phagosome lysosome fusion, are attenuated in strains of L. micdadei (30). In contrast, our data demonstrate that L. micdadei, one of the most distant Legionella species evolutionarily (44), expresses an IcmX homolog. In addition, we have identified genes with homology to L. pneumophila dotA and icmX in L. micdadei by using low-stringency nucleic acid hybridization techniques. These data suggest that functionally similar Dot/Icm secretion systems are expressed by most Legionellaceae family members. It is possible that putative Dot/Icm effector proteins secreted by these type IV-related transporters have divergent functions. This could explain the attenuation in virulence and differences in host cell interactions that have been observed for evolutionarily distant Legionella species. A more detailed understanding of how the Dot/Icm apparatus functions is certain to help elucidate the molecular mechanisms underlying these phenotypic variations.
ACKNOWLEDGMENTS
This work was supported by NIH grant R29 AI41699.
We thank Jonathan Kagan and Jörn Coers for helpful suggestions during manuscript preparation, and we thank Hiroki Nagai for communicating unpublished data.
REFERENCES
- 1.Abath F G, Almeida A M, Ferreira L C. Identification of surface-exposed Yersinia pestis proteins by radio-iodination and biotinylation. J Med Microbiol. 1992;37:420–424. doi: 10.1099/00222615-37-6-420. [DOI] [PubMed] [Google Scholar]
- 2.Abu Kwaik Y, Venkataraman C, Harb O S, Gao L Y. Signal transduction in the protozoan host Hartmannella vermiformis upon attachment and invasion by Legionella micdadei. Appl Environ Microbiol. 1998;64:3134–3139. doi: 10.1128/aem.64.9.3134-3139.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews H L, Vogel J P, Isberg R R. Identification of linked Legionella pneumophila genes essential for intracellular growth and evasion of the endocytic pathway. Infect Immun. 1998;66:950–958. doi: 10.1128/iai.66.3.950-958.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baron C, Llosa M, Zhou S, Zambryski P C. VirB1, a component of the T-complex transfer machinery of Agrobacterium tumefaciens, is processed to a C-terminal secreted product, VirB1. J Bacteriol. 1997;179:1203–1210. doi: 10.1128/jb.179.4.1203-1210.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger K H, Isberg R R. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol Microbiol. 1993;7:7–19. doi: 10.1111/j.1365-2958.1993.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 6.Bornstein N, Marmet D, Surgot M, Nowicki M, Meugnier H, Fleurette J, Ageron E, Grimont F, Grimont P A, Thacker W L, et al. Legionella gratiana sp. nov. isolated from French spa water. Res Microbiol. 1989;140:541–552. doi: 10.1016/0923-2508(89)90086-7. [DOI] [PubMed] [Google Scholar]
- 7.Bradburne J A, Godfrey P, Choi J H, Mathis J N. In vivo labeling of Escherichia coli cell envelope proteins with N-hydroxysuccinimide esters of biotin. Appl Environ Microbiol. 1993;59:663–668. doi: 10.1128/aem.59.3.663-668.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brand B C, Sadosky A B, Shuman H A. The Legionella pneumophila icm locus: a set of genes required for intracellular multiplication in human macrophages. Mol Microbiol. 1994;14:797–808. doi: 10.1111/j.1365-2958.1994.tb01316.x. [DOI] [PubMed] [Google Scholar]
- 9.Brenner D J, Steigerwalt A G, McDade J E. Classification of the Legionnaires' disease bacterium: Legionella pneumophila, genus novum, species nova, of the family Legionellaceae, family nova. Ann Intern Med. 1979;90:656–658. doi: 10.7326/0003-4819-90-4-656. [DOI] [PubMed] [Google Scholar]
- 10.Celada A, Gray P W, Rinderknecht E, Schreiber R D. Evidence for a gamma-interferon receptor that regulates macrophage tumoricidal activity. J Exp Med. 1984;160:55–74. doi: 10.1084/jem.160.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J W, Pan W, D'Souza M P, August J T. Lysosome-associated membrane proteins: characterization of LAMP-1 of macrophage P388 and mouse embryo 3T3 cultured cells. Arch Biochem Biophys. 1985;239:574–586. doi: 10.1016/0003-9861(85)90727-1. [DOI] [PubMed] [Google Scholar]
- 12.Cianciotto N P, Bangsborg J M, Eisenstein B I, Engleberg N C. Identification of mip-like genes in the genus Legionella. Infect Immun. 1990;58:2912–2918. doi: 10.1128/iai.58.9.2912-2918.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cianciotto N P, Fields B S. Legionella pneumophila mip gene potentiates intracellular infection of protozoa and human macrophages. Proc Natl Acad Sci USA. 1992;89:5188–5191. doi: 10.1073/pnas.89.11.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coers J, Monahan C, Roy C R. Modulation of phagosome biogenesis by Legionella pneumophila creates an organelle permissive for intracellular growth. Nat Cell Biol. 1999;1:451–453. doi: 10.1038/15687. [DOI] [PubMed] [Google Scholar]
- 15.Collazo C M, Galan J E. The invasion-associated type III system of Salmonella typhimurium directs the translocation of Sip proteins into the host cell. Mol Microbiol. 1997;24:747–756. doi: 10.1046/j.1365-2958.1997.3781740.x. [DOI] [PubMed] [Google Scholar]
- 16.Collazo C M, Galan J E. Requirement for exported proteins in secretion through the invasion-associated type III system of Salmonella typhimurium. Infect Immun. 1996;64:3524–3531. doi: 10.1128/iai.64.9.3524-3531.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dreyfus L A, Iglewski B H. Purification and characterization of an extracellular protease of Legionella pneumophila. Infect Immun. 1986;51:736–743. doi: 10.1128/iai.51.3.736-743.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edelstein P H, Edelstein M A, Higa F, Falkow S. Discovery of virulence genes of Legionella pneumophila by using signature tagged mutagenesis in a guinea pig pneumonia model. Proc Natl Acad Sci USA. 1999;96:8190–8195. doi: 10.1073/pnas.96.14.8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feeley J C, Gibson R J, Gorman G W, Langford N C, Rasheed J K, Mackel D C, Blaine W B. Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila. J Clin Microbiol. 1979;10:437–441. doi: 10.1128/jcm.10.4.437-441.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fields B S. The molecular ecology of legionellae. Trends Microbiol. 1996;4:286–490. doi: 10.1016/0966-842x(96)10041-x. [DOI] [PubMed] [Google Scholar]
- 21.Gao L Y, Harb O S, Abu Kwaik Y. Utilization of similar mechanisms by Legionella pneumophila to parasitize two evolutionarily distant host cells, mammalian macrophages and protozoa. Infect Immun. 1997;65:4738–4746. doi: 10.1128/iai.65.11.4738-4746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao L Y, Harb O S, Kwaik Y A. Identification of macrophage-specific infectivity loci (mil) of Legionella pneumophila that are not required for infectivity of protozoa. Infect Immun. 1998;66:883–892. doi: 10.1128/iai.66.3.883-892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guan K L, Dixon J E. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- 24.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1988. [Google Scholar]
- 25.Hebert G A, Moss C W, McDougal L K, Bozeman F M, McKinney R M, Brenner D J. The rickettsia-like organisms TATLOCK (1943) and HEBA (1959): bacteria phenotypically similar to but genetically distinct from Legionella pneumophila and the WIGA bacterium. Ann Intern Med. 1980;92:45–52. doi: 10.7326/0003-4819-92-1-45. [DOI] [PubMed] [Google Scholar]
- 26.Hoffman C S, Wright A. Fusions of secreted proteins to alkaline phosphatase: an approach for studying protein secretion. Proc Natl Acad Sci USA. 1985;82:5107–5111. doi: 10.1073/pnas.82.15.5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horwitz M A. Formation of a novel phagosome by the Legionnaires' disease bacterium (Legionella pneumophila) in human monocytes. J Exp Med. 1983;158:1319–1331. doi: 10.1084/jem.158.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horwitz M A. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome lysosome fusion in human monocytes. J Exp Med. 1983;158:2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horwitz M A, Silverstein S C. Legionnaires' disease bacterium (Legionella pneumophila) multiplies intracellularly in human monocytes. J Clin Investig. 1980;66:441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joshi A D, Swanson M S. Comparative analysis of Legionella pneumophila and Legionella micdadei virulence traits. Infect Immun. 1999;67:4134–4142. doi: 10.1128/iai.67.8.4134-4142.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirby J E, Vogel J P, Andrews H L, Isberg R R. Evidence for pore-forming ability by Legionella pneumophila. Mol Microbiol. 1998;27:323–336. doi: 10.1046/j.1365-2958.1998.00680.x. [DOI] [PubMed] [Google Scholar]
- 32.Kolter R, Inuzuka M, Helinski D R. Trans-complementation-dependent replication of a low molecular weight origin fragment from plasmid R6K. Cell. 1978;15:1199–1208. doi: 10.1016/0092-8674(78)90046-6. [DOI] [PubMed] [Google Scholar]
- 33.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 34.Manoil C, Beckwith J. A genetic approach to analyzing membrane protein topology. Science. 1986;233:1403–1408. doi: 10.1126/science.3529391. [DOI] [PubMed] [Google Scholar]
- 35.Marra A, Blander S J, Horwitz M A, Shuman H A. Identification of a Legionella pneumophila locus required for intracellular multiplication in human macrophages. Proc Natl Acad Sci USA. 1992;89:9607–9611. doi: 10.1073/pnas.89.20.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDade J E, Brenner D J, Bozeman F M. Legionnaires' disease bacterium isolated in 1947. Ann Intern Med. 1979;90:659–661. doi: 10.7326/0003-4819-90-4-659. [DOI] [PubMed] [Google Scholar]
- 37.McDade J E, Shepard C C, Fraser D W, Tsai T R, Redus M A, Dowdle W R. Legionnaires' disease: isolation of a bacterium and demonstration of its role in other respiratory diseases. N Engl J Med. 1977;297:1197–1203. doi: 10.1056/NEJM197712012972202. [DOI] [PubMed] [Google Scholar]
- 38.Meresse S, Steele-Mortimer O, Moreno E, Desjardins M, Finlay B, Gorvel J P. Controlling the maturation of pathogen-containing vacuoles: a matter of life and death. Nat Cell Biol. 1999;1:E183–E188. doi: 10.1038/15620. [DOI] [PubMed] [Google Scholar]
- 39.Merriam J J, Mathur R, Maxfield-Boumil R, Isberg R R. Analysis of the Legionella pneumophila fliI gene: intracellular growth of a defined mutant defective for flagellum biosynthesis. Infect Immun. 1997;65:2497–2501. doi: 10.1128/iai.65.6.2497-2501.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morales V M, Backman A, Bagdasarian M. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene. 1991;97:39–47. doi: 10.1016/0378-1119(91)90007-x. [DOI] [PubMed] [Google Scholar]
- 41.Obert S O, Connor R J, Schmid S, Hearing P. The adenovirus E4-6/7 protein transactivates the E2 promoter by inducing dimerization of a heteromeric E2F complex. Mol Cell Biol. 1994;14:1333–1346. doi: 10.1128/mcb.14.2.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pasculle A W, Feeley J C, Gibson R J, Cordes L G, Myerowitz R L, Patton C M, Gorman G W, Carmack C L, Ezzell J W, Dowling J N. Pittsburgh pneumonia agent: direct isolation from human lung tissue. J Infect Dis. 1980;141:727–732. doi: 10.1093/infdis/141.6.727. [DOI] [PubMed] [Google Scholar]
- 43.Pearlman E, Engleberg N C, Eisenstein B I. Growth of Legionella pneumophila in a human macrophage-like (U937) cell line. Microb Pathog. 1988;5:87–95. doi: 10.1016/0882-4010(88)90011-3. [DOI] [PubMed] [Google Scholar]
- 44.Ratcliff R M, Donnellan S C, Lanser J A, Manning P A, Heuzenroeder M W. Interspecies sequence differences in the Mip protein from the genus Legionella: implications for function and evolutionary relatedness. Mol Microbiol. 1997;25:1149–1158. doi: 10.1046/j.1365-2958.1997.5471908.x. [DOI] [PubMed] [Google Scholar]
- 45.Roy C R. Trafficking of the Legionella pneumophila phagosome. ASM News. 1999;65:416–421. [Google Scholar]
- 46.Roy C R, Berger K, Isberg R R. Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol Microbiol. 1998;28:663–674. doi: 10.1046/j.1365-2958.1998.00841.x. [DOI] [PubMed] [Google Scholar]
- 47.Roy C R, Isberg R R. Topology of Legionella pneumophila DotA: an inner membrane protein required for replication in macrophages. Infect Immun. 1997;65:571–578. doi: 10.1128/iai.65.2.571-578.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sadosky A B, Wiater L A, Shuman H A. Identification of Legionella pneumophila genes required for growth within and killing of human macrophages. Infect Immun. 1993;61:5361–5373. doi: 10.1128/iai.61.12.5361-5373.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Segal G, Purcell M, Shuman H A. Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila genome. Proc Natl Acad Sci USA. 1998;95:1669–1674. doi: 10.1073/pnas.95.4.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Segal G, Shuman H A. Characterization of a new region required for macrophage killing by Legionella pneumophila. Infect Immun. 1997;65:5057–5066. doi: 10.1128/iai.65.12.5057-5066.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Segal G, Shuman H A. How is the intracellular fate of the Legionella pneumophila phagosome determined? Trends Microbiol. 1998;6:253–255. doi: 10.1016/s0966-842x(98)01308-0. [DOI] [PubMed] [Google Scholar]
- 52.Segal G, Shuman H A. Legionella pneumophila utilizes the same genes to multiply within Acanthamoeba castellanii and human macrophages. Infect Immun. 1999;67:2117–2124. doi: 10.1128/iai.67.5.2117-2124.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Segal G, Shuman H A. Possible origin of the Legionella pneumophila virulence genes and their relation to Coxiella burnetii. Mol Microbiol. 1999;33:669–670. doi: 10.1046/j.1365-2958.1999.01511.x. [DOI] [PubMed] [Google Scholar]
- 54.Sinai A P, Joiner K A. Safe haven: the cell biology of nonfusogenic pathogen vacuoles. Annu Rev Microbiol. 1997;51:415–462. doi: 10.1146/annurev.micro.51.1.415. [DOI] [PubMed] [Google Scholar]
- 55.Vogel J P, Andrews H L, Wong S K, Isberg R R. Conjugative transfer by the virulence system of Legionella pneumophila. Science. 1998;279:873–876. doi: 10.1126/science.279.5352.873. [DOI] [PubMed] [Google Scholar]
- 56.Vogel J P, Isberg R R. Cell biology of Legionella pneumophila. Curr Opin Microbiol. 1999;2:30–34. doi: 10.1016/s1369-5274(99)80005-8. [DOI] [PubMed] [Google Scholar]
- 57.Wiater L A, Dunn K, Maxfield F R, Shuman H A. Early events in phagosome establishment are required for intracellular survival of Legionella pneumophila. Infect Immun. 1998;66:4450–4460. doi: 10.1128/iai.66.9.4450-4460.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zuckman D M, Hung J B, Roy C R. Pore-forming activity is not sufficient for Legionella pneumophila phagosome trafficking and intracellular growth. Mol Microbiol. 1999;32:990–1001. doi: 10.1046/j.1365-2958.1999.01410.x. [DOI] [PubMed] [Google Scholar]