Abstract
Purpose
This study aimed to report our 10-year experience with the management of iatrogenic (penetrating trauma) and traumatic (blunt or penetrating trauma) peripheral artery pseudoaneurysms, based on data from a tertiary referral center.
Methods
From January 2012 to December 2021, the medical records of consecutive patients with iatrogenic and traumatic peripheral artery pseudoaneurysms were retrospectively reviewed. Patient demographics, clinical features, imaging data, treatment details, and follow-up results were analyzed.
Results
Sixty-one consecutive patients were included in this study; 48 (79%) were men and 13 (21%) women, with a mean age of 49.4 ± 13.4 years (range 24–73 years). There were 42 patients (69%) who underwent open surgery, 18 (29%) undergoing endovascular embolization or stent implantation, and one (2%) undergoing ultrasound-guided thrombin injection. All patients successfully underwent open or interventional treatment. The median follow-up was 46.8 months (2.5–117.9 months), and the overall reintervention rate was 10%. Of these, one (5%) patient in the interventional treatment group and five (12%) patients in the open surgery group underwent reintervention. The overall complication rate was 8%, with complications occurring only in the open surgery group. No deaths occurred in the peri-operative period. No late complications, such as thrombosis or pseudoaneurysm recurrence, were observed.
Conclusion
Peripheral artery pseudoaneurysms arising from iatrogenic or traumatic causes can be effectively treated by both open surgery and interventional procedures in selected patients with acceptable mid- and long-term outcomes.
Keywords: Pseudoaneurysm, Trauma, Peripheral artery, Interventional therapy, Stent
1. Introduction
A pseudoaneurysm is a vascular abnormality resulting from a damaging force on the arterial wall, resulting in blood leakage to the extraluminal space that is wrapped by adjacent tissue.1,2 They can be caused by inflammation, infection, and iatrogenic and traumatic injuries.3, 4, 5 The exact incidence of overall peripheral artery pseudoaneurysms is unknown; however, it has been reported that the incidence of iatrogenic pseudoaneurysms is 0.44–1.8% following diagnostic catheterization and 3.2–7.7% after interventional treatment.6 In recent years, there has been an apparent increase in incidence due to increasing endovascular procedures.7 A pseudoaneurysm can lead to progressive sac enlargement, distal extremity ischemia, compression of surrounding tissue, and life-threatening events such as rupture and serious infection.8, 9, 10
Given the rarity of peripheral artery pseudoaneurysm, guidelines for management have not been well described.11 Open surgical resection is the mainstay treatment.12,13 However, with advances in interventional procedures and materials, minimally invasive endovascular treatment has been increasingly applied in recent years.14, 15, 16 In the current literature, most reports regarding peripheral artery pseudoaneurysms are case reports, and few medical centers have developed significant experience with optimal management. Thus, more studies with a relatively large cohort and long-term follow-up are needed.
The purpose of this study was to report our 10-year experience in the management of iatrogenic and traumatic peripheral artery pseudoaneurysms based on data from 61 patients at our medical center.
2. Methods
2.1. Study population
The study was an observational study which followed the STROBE guidelines17 and was approved by the institutional ethics review board. The need for written informed consent was waived due to the retrospective nature of the study. Medical records of consecutive patients with peripheral artery pseudoaneurysms, from January 2012 to December 2021, were retrospectively reviewed. The exclusion criteria were patients with nontraumatic/iatrogenic causes and insufficient medical data. Patient demographics, clinical features, imaging data, treatment details, and follow-up results were reviewed.
2.2. Treatment protocol
Pseudoaneurysms with a diameter <2 cm that were asymptomatic were treated conservatively and observed for spontaneous closure by regular duplex ultrasound examination for a duration of three weeks. For pseudoaneurysms with a diameter ≥2 cm or symptomatic, or pseudoaneurysms with a diameter <2 cm that did not close after three weeks of follow-up, an invasive intervention was indicated. Potential invasive interventions included open surgery or interventional treatments; the choice depended upon the doctor's experience and device availability. However, for pseudoaneurysms with infection, open wounds, or severe distal ischemia/neurological deficits due to compression by massive hematoma, open surgery should be performed.
Open surgery methods included aneurysmectomy, disruption site repair, ligation, end-to-end anastomosis, and vein/synthetic graft interposition. Interventional treatment methods included endovascular stent implantation and embolization with coils or glue. Based on the patient's condition, anatomical characteristics, and collateral branches of the pseudoaneurysm, individualized treatment modalities should be performed. Aneurysmectomy with disruption-site repair or end-to-end anastomosis was performed in a pseudoaneurysm with a narrow neck, and vein/synthetic graft interposition was applied in a pseudoaneurysm with a wide neck, which could cause a large deficit in the arterial wall. Ligation or endovascular embolization was performed in a pseudoaneurysm originating from small or large arterial branches, where sufficient collaterals were formed. Sandwich embolization, which is embolization of the afferent artery, aneurysm cavity, and efferent artery of the associated artery, was preferred. Ultrasound-guided thrombin injection was performed in a narrow-neck, small, and suitable-depth pseudoaneurysm. Endovascular stent implantation was performed in wide-neck pseudoaneurysms or pseudoaneurysms arising from the arterial trunk. Surgical repair may be dangerous in older patients, hemodynamically unstable patients, or patients with a history of recent open surgery due to multiple traumas. In these conditions, endovascular treatment is recommended, and debridement to relieve the tension caused by the hematoma may be needed at certain times.
For patients undergoing stent implantation, clopidogrel (75 mg/day) and aspirin (100 mg/day) were used routinely for three months, followed by aspirin for six months or longer (for small arteries).
2.3. Study endpoints
The primary endpoint was successful exclusion of the pseudoaneurysm, defined as no blood leakage into the pseudoaneurysm on post-operative angiogram or ultrasound images. The secondary endpoints were reintervention and post-operative complications. Reintervention was defined as an aneurysm-related additional procedure after the primary treatment, including debridement and hematoma evacuation/wound drainage. Post-operative complications included wound infection, aneurysm rupture, thrombosis, stent migration, stent infolding, endoleak formation, amputation, and severe neurological deficits.
2.4. Follow-up protocol
All patients were prescribed a follow-up protocol, including assessment of clinical symptoms, survival, and ultrasound or computed tomographic angiography (CTA) or magnetic resonance imaging (MRA) images at two weeks (before discharge), three months, six months, and 12 months, and annually thereafter. We recommended ultrasound examination for most cases, whereas CTA was preferred within three months in patients who underwent stent implantation.
2.5. Statistical analysis
Continuous variables are expressed as numbers with percentages and categorical variables as mean with standard deviation (SD) or median with range. All statistical analyses were performed using IBM SPSS software, version 19.0 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Patient characteristics
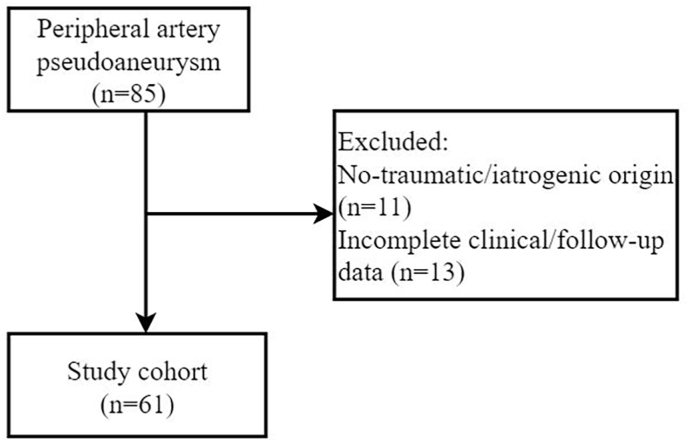
Sixty-one consecutive patients were included in this study (Fig. 1). Of these, 48 (79%) were men and 13 (21%) women, with a mean age of 49.4 ± 13.4 years (range 24–73 years). The pseudoaneurysm was caused by blunt or penetrating trauma in 40 patients (66%) and by arterial puncture for diagnostic and therapeutic procedures in 21 patients (34%). All patients (100%) were symptomatic, including pain, swelling, impaired extremity movement, dysphagia, hoarseness, pulsatile mass, or reduced hemoglobin level. The diagnosis of pseudoaneurysm was made using duplex ultrasound and/or CTA (detailed baseline and anatomical characteristics are provided in Table 1, Table 2).
Fig. 1.
Flowchart of the patient inclusion.
Table 1.
Demographics and clinical features of the patients with peripheral artery pseudoaneurysm.
| Variables | Patients (N = 61) |
|---|---|
| Age(year), mean ± SD | 49.4 ± 13.4 |
| Gender, n (%) | |
| Male | 48 (79) |
| Female | 13 (21) |
| Smoking, n (%) | 16 (26) |
| Comorbidities, n (%) | |
| Hypertension | 29 (48) |
| Diabetes mellitus | 6 (10) |
| Coronary heart disease | 6 (10) |
| Renal failure | 2 (3) |
| Type of injury, n (%) | |
| Traumatic | 40 (66) |
| Iatrogenic | 21 (34) |
| Symptoms/signs, n (%) | |
| Pulsatile mass | 31 (51) |
| Pain | 32 (52) |
| Swelling | 28 (46) |
| Impaired extremity movement | 16 (26) |
| Dysphagia | 1 (2) |
| Hoarseness | 1 (2) |
| Duration between injury and diagnosis (d), median (range) | 4 (1–730) |
SD = standard deviation.
Table 2.
Anatomic characteristics of the peripheral artery pseudoaneurysm.
| Variables | Patients (N = 61) |
|---|---|
| Location of the PA, n (%) | |
| Superficial femoral artery | 15 (25) |
| Deep femoral artery | 6 (10) |
| Common femoral artery | 5 (8) |
| Brachial artery | 9 (15) |
| Radial artery | 3 (5) |
| Ulnar artery | 2 (3) |
| Subclavian artery | 7 (11) |
| Popliteal artery | 4 (7) |
| Peroneal artery | 1 (2) |
| Anterior tibial artery | 1 (2) |
| Posterior tibial artery | 5 (8) |
| Superior gluteal artery | 1 (2) |
| Inferior gluteal artery | 2 (3) |
| PA diameter (cm), mean ± SD | 6.0 ± 3.1 |
| Neck diameter (mm), mean ± SD | 5.5 ± 3.4 |
| Concomitant AVF, n (%) | 9 (15) |
PA = pseudoaneurysm; SD = standard deviation.
3.2. Treatment details
There were 42 patients (69%) who underwent open surgical procedures including aneurysmectomy + ligation (n = 5), aneurysmectomy + saphenous vein/synthetic graft interposition (n = 8), aneurysmectomy + end-to-end anastomosis (n = 7), and lateral repair (n = 22); 18 patients (29%) underwent endovascular embolization (n = 6) or stent implantation (n = 12); and one patient (2%) underwent ultrasound-guided thrombin injection. Open surgical procedures were performed under general anesthesia in 40 (95%) patients and under local anesthesia in two (5%) patients. All endovascular procedures were performed under local anesthesia (Fig. 2, Fig. 3).
Fig. 2.
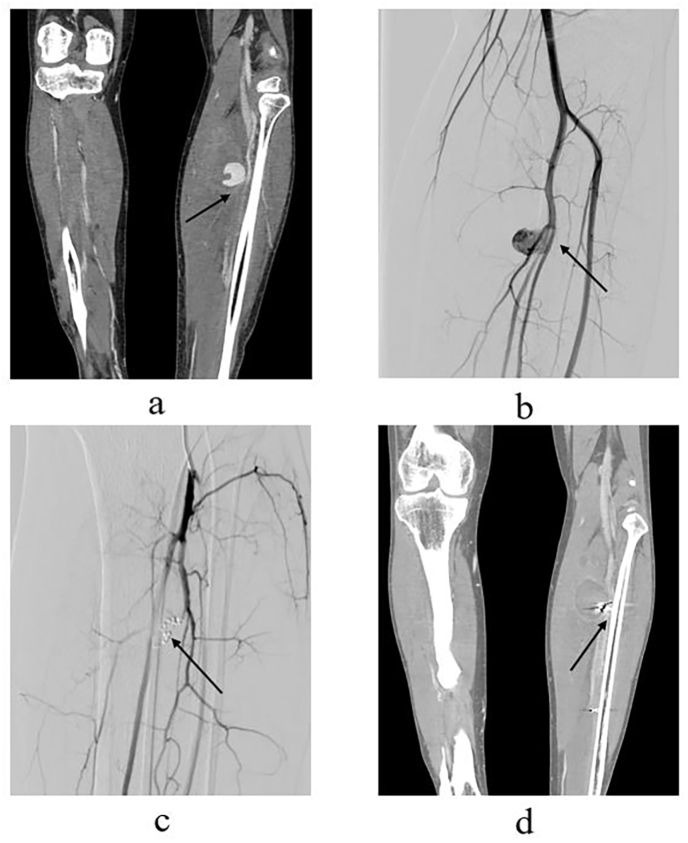
(a, b) Computed tomography angiography and digital subtraction angiography images of a 39-year-old patient demonstrating a traumatic pseudoaneurysm (4.3 × 3.8 cm) arising from small branches of the left posterior tibial artery (arrow). (c) Post-operative angiography image, showing that the proximal and distal of the parent artery of the pseudoaneurysm were embolized by coils (Cook Medical, Bloomington, IN, USA), with no blood flow into the pseudoaneurysm (arrow). (d) Computed tomography angiography image two months after embolization, demonstrating there is no active bleeding into the pseudoaneurysm, and the pseudoaneurysm is completely thrombosed (arrow).
Fig. 3.
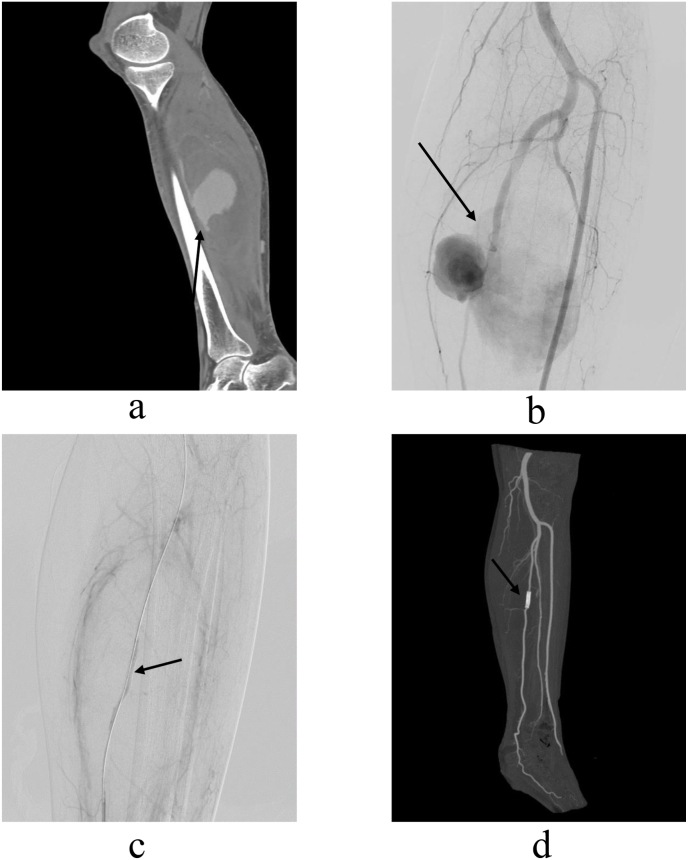
(a, b) Computed tomography angiography and digital subtraction angiography images of a 45-year-old patient demonstrating a traumatic pseudoaneurysm (8.6 × 4.5 cm) arising from the trunk of the left posterior tibial artery (arrow). (c) Post-operative angiography image, showing that a covered stent (5 mm × 25 mm, Viabahn, W. L. Gore & Associates Inc, Newark, Del) was implanted in a good position with no blood flow into the pseudoaneurysm (arrow). (d) Computed tomography angiography image three months after the stent was implanted, demonstrating the following: the stent is in good shape; the left posterior tibial artery is well reconstructed; there is no active blood into the pseudoaneurysm; and the pseudoaneurysm is completely thrombosed (arrow).
Systemic heparinization at a dose of 3000–5000 units was routinely used during endovascular stent implantation. However, patients with concomitant injuries who had a high risk of bleeding received a low dose of heparin. During the open procedures, three (7%) patients required a blood transfusion.
Endovascular embolization materials in this cohort included coils (Tornado coils: Cook Medical, Bloomington, IN, USA; Nester Coils: Cook Medical, Bloomington, IN, USA) and gelfoam sponge (Hengrui Medical, Nanjing, Jiangsu, China), or both (n = 5). Stents used in this cohort included Viabahn (W. L. Gore & Associates Inc, Newark, Del) and Fluency (C.R. Bard, Murray Hill, NJ) covered stents (other operative information is shown in Table 3).
Table 3.
Treatment and outcomes of the patients with peripheral artery pseudoaneurysm.
| Variables | Patients (N = 61) |
|---|---|
| Treatment modalities, n (%) | |
| Ultrasound-guided thrombin injection | 1 (2) |
| Open surgery | 42 (69) |
| Endovascular embolization/stent insertion | 18 (29) |
| Operation time (min), mean ± SD | |
| Open surgery | 142.9 ± 63.4 |
| Endovascular embolization/stent insertion | 58.6 ± 32.0 |
| Hospital stay (d), mean ± SD | |
| Open surgery | 19.8 ± 11.8 |
| Endovascular embolization/stent insertion | 17.4 ± 12.1 |
| Follow-up time (month), median (range) | 46.8 (2.5–117.9) |
| Reintervention, n (%) | |
| Open surgery | 5 (12) |
| Endovascular embolization/stent insertion | 1 (6) |
| Wound infection | |
| Open surgery | 5 (12) |
| Endovascular embolization/stent insertion | 0 (0) |
SD = standard deviation.
3.3. Outcomes
All patients successfully underwent open or endovascular treatment, and all symptoms were completely relieved within two weeks. Median follow-up was 46.8 months (2.5–117.9 months). During this period, debridement and hematoma evacuation were performed in patients who underwent endovascular stent implantation, and five additional debridement procedures were performed in patients who underwent open surgical repair due to post-operative wound infection. Thus, the overall reintervention rate was 10% (n = 6), occurring in 2% of patients (n = 1) in the endovascular embolization/stent implantation group and 8% (n = 5) in the open surgery group. The overall complication rate was 8% (n = 5), which all occurred in the open surgery group. No deaths occurred during the peri-operative period, although six patients who underwent open surgery died from non-pseudoaneurysm-related causes, including heart disease (n = 1), aortic rupture (n = 1), liver cancer (n = 2), and uremia (n = 2). No late complications were observed, such as thrombosis, pseudoaneurysm recurrence, thrombosis, stent migration, stent infolding, or endoleak formation (Table 3, Table 4).
Table 4.
Characteristics of patients undergoing endovascular therapy.
| No. | Sex | Age | Cause | T-PA(d) | Location | Size (cm) | Nd(mm) | Treatment | Reintervention | Follow-up time (m) | AE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 69 | T | 7 | SGA | 2.4 × 1.3 | 2 | E | No | 7.9 | None |
| 2 | M | 52 | T | 30 | SA | 7.1 × 3.5 | 4 | S | No | 11.2 | None |
| 3 | M | 48 | T | 35 | PA | 2.6 × 1.5 | 3.8 | E | No | 43.5 | None |
| 4 | M | 71 | I | 2 | SFA | 2.6 × 1.7 | 2 | S | No | 6.4 | None |
| 5 | M | 45 | T | 10 | PTA | 8.6 × 4.5 | 5 | S | No | 47.6 | None |
| 6 | M | 60 | T | 60 | SFA | 12.1 × 11.6 | 4.5 | S | No | 46.2 | None |
| 7 | M | 39 | T | 7 | PTA | 7.2 × 3.8 | 4 | E | No | 50.3 | None |
| 8 | M | 43 | T | 4 | DFA | 5.2 × 3.0 | 3 | E | No | 36 | None |
| 9 | M | 51 | T | 180 | SFA | 2.6 × 1.6 | 3 | E | No | 73.4 | None |
| 10 | M | 55 | I | 4 | CFA | 4.0 × 3.1 | 8 | S | No | 63.6 | None |
| 11 | M | 59 | T | 3 | SA | 7.2 × 5.3 | 5.7 | S | No | 63.8 | None |
| 12 | M | 53 | T | 3 | CFA | 4.6 × 3.6 | 5 | S | Yes | 65.3 | None |
| 13 | M | 65 | T | 7 | SA | 4.4 × 2.9 | 3.8 | S | No | 71.7 | None |
| 14 | F | 55 | T | 30 | IGA | 5.3 × 3.1 | 3 | E | No | 72.8 | None |
| 15 | M | 51 | T | 5 | SA | 4.4 × 1.8 | 3.5 | S | No | 49.8 | None |
| 16 | M | 49 | T | 4 | SA | 5.3 × 4.6 | 4 | S | No | 81.1 | None |
| 17 | M | 42 | T | 180 | SA | 5.1 × 4.6 | 4 | S | No | 4.5 | None |
| 18 | F | 45 | T | 730 | SA | 2.3 × 1.3 | 3 | S | No | 117.9 | None |
F= Female; M = Male; T = Traumatic; I= Iatrogenic; SGA= Superior gluteal artery; SA= Subclavian artery; T-PA= Time from injury to diagnosis of pseudoaneurysm; PA= Peroneal artery; SFA= Superficial femoral artery; PTA= Posterior tibial artery; DFA = Deep femoral artery; CFA= Common femoral artery; IGA= Inferior gluteal artery; E = Embolization; S= Stent; Nd= Neck diameter; AE = Adverse events.
4. Discussion
The reports of experience in the management of peripheral artery pseudoaneurysm remain limited to small case reports.12, 13, 14,18 Choosing the optimal management strategies for peripheral artery pseudoaneurysms is an urgent clinical problem. In the present study, we presented a retrospective study of 61 patients undergoing open surgical repair or interventional procedures for the management of iatrogenic and traumatic peripheral artery pseudoaneurysm at a tertiary referral center. We found that peripheral artery pseudoaneurysm arising from iatrogenic or traumatic causes could be effectively treated by both open surgery and interventional procedures in selected patients with acceptable mid- and long-term outcomes (overall reintervention rate was 10% and overall complication rate was 8%). Compared with open surgery, endovascular treatment methods might entail a shorter hospital stay (19.8 ± 11.8 d vs 17.4 ± 12.1 d), shorter operative time (142.9 ± 63.4 min vs 58.6 ± 32.0 min), lower reintervention rate (12% vs. 6%), and lower complication rate (12% vs. 0%), respectively. However, the exact comparison between them should be explored in future studies, given the small sample size and patient heterogeneity in the present study.
Ultrasonography has become the first-line imaging modality in the diagnosis of peripheral artery pseudoaneurysms due to its convenience and low cost; however, it is highly operator-dependent and susceptible to intestinal gas, subcutaneous fat, and limb swelling, and images of the distal branch of the artery and deeply located lesions are inferior.19,20 The CTA is another widely used imaging modality for the diagnosis of pseudoaneurysms, as it has a three-dimensional ability that allows visualization of pseudoaneurysms from different angles. It can detect the exact location, origin, surrounding hematoma, collateral circulation, and associated injuries, which is helpful in planning treatment modalities. Moreover, CTA can be used in cases where the ultrasound could not give full details of the pseudoaneurysm, such as large and irregular-shaped pseudoaneurysms in the subclavian artery, pseudoaneurysms within small artery crevasses, or those that are deep-seated.21 Regardless of availability, MRA is seldom used due to its high monetary and time cost, particularly for iatrogenic and traumatic pseudoaneurysms in urgent conditions.22 Pseudoaneurysms with a diameter <2 cm could be treated conservatively and followed up by regular duplex ultrasound examination, as most pseudoaneurysms this size have the potential for spontaneous thrombosis.23 Follow-up can be three to four weeks according to the current literature.11,24 For pseudoaneurysms with a diameter ≥2 cm that are symptomatic or a diameter <2 cm that did not close after follow-up, invasive intervention is indicated.
The mainstay treatment of peripheral artery pseudoaneurysm remains open surgery.12,13 The technical and clinical success rates are reported to be 50–100% and 86.36–100%, respectively.16 Operative complications include wound infections, massive hemorrhage, lymphorrhea, nerve injury, skin compromise, and thrombosis.6 The complication rate has been reported to be up to 50%.16,25 In the present study, the success rate was 100% in patients undergoing open surgery and the complication rate was 12%, which was an acceptable outcome and consistent with previously reported results.
With advances in interventional procedures and materials, minimally invasive endovascular treatment methods including embolization and stent implantation have been increasingly applied in recent years, and have shown comparable prognosis to open surgery.14,16 At our center, the decision to perform open surgery or interventional treatment depends on the doctor's experience and device availability. However, for pseudoaneurysms with infection, open wounds, or severe distal ischemia/neurological deficits due to compression by massive hematoma, open surgery should be performed. In addition, open surgery may be dangerous in older patients, hemodynamically unstable patients, or patients with a history of recent open surgery due to multiple traumas. In these conditions, endovascular treatment is recommended, and debridement to relieve the tension caused by the hematoma may be needed at certain times. Pseudoaneurysms arising from the subclavian artery are also recommended for endovascular treatment due to the complex anatomical conditions of the subclavian artery area, which makes it difficult to expose the subclavian artery and increases the risk of nerve injury and massive hemorrhage.26 In the present study, all (n = 7) pseudoaneurysms arising from the subclavian artery underwent endovascular stent implantation and were clinically successful without any complications. Moreover, compared with open surgery, endovascular treatment methods might entail a shorter hospital stay (19.8 ± 11.8 d vs 17.4 ± 12.1 d), shorter operative time (142.9 ± 63.4 min vs 58.6 ± 32.0 min), lower reintervention rate (12% vs. 6%), and lower complication rate (12% vs. 0%). We could not compare the two groups statistically due to the diverse types of pseudoaneurysms in each group. More comparison studies to evaluate outcomes between the two therapeutic modalities are needed in the future.
Ultrasound-guided thrombin injection is a common treatment for peripheral artery pseudoaneurysms, with a success rate of 93–100%.6 It is often performed in narrow-neck, small, and suitable-depth pseudoaneurysms.9,25 The complications include distal embolization, massive bleeding, infection, and death.7,25,27 Thus, some doctors have attempted to perform ultrasound-guided thrombin injection with the assistance of balloon occlusion and have achieved satisfactory results.11 In the present study, only one patient received this treatment, and the follow-up outcome was satisfactory without any complications.
The present study has some limitations. First, the results of the present study have an inherent bias due to its retrospective nature and small sample size. Second, the study did not compare interventional treatment and open surgery due to the heterogeneity of types of pseudoaneurysms in each group. Therefore, future multi-center studies are needed to evaluate the outcomes of each therapeutic modality.
5. Conclusion
Peripheral artery pseudoaneurysms arising from iatrogenic or traumatic causes can be effectively treated by both open surgery and interventional procedures in selected patients with acceptable mid- and long-term outcomes.
Authors' contributions
Study conception and design: Yingliang Wang, Hai Zheng, Bin Xiong. Acquisition of data: Wei Yao, Hai Zheng, Shuguang Ju, Yaowei Bai. Analysis and interpretation of data: Yingliang Wang, Chongtu Yang, Songjiang Huang, Jiacheng Liu. Drafting of the manuscript: Yingliang Wang, Hai Zheng, Tongqiang Li, Chaoyang Wang. Critical revision: Bin Xiong, Yang Chen, Jiacheng Liu, Tongqiang Li, Chaoyang Wang. All authors read and approved the final manuscript.
Declaration of competing interest
The authors have no relevant financial or non-financial interests to disclose.
References
- 1.Kronzon I. Diagnosis and treatment of iatrogenic femoral artery pseudoaneurysm: a review. J Am Soc Echocardiogr. 1997;10:236–245. doi: 10.1016/s0894-7317(97)70061-0. [DOI] [PubMed] [Google Scholar]
- 2.Sueyoshi E., Sakamoto I., Nakashima K., et al. Visceral and peripheral arterial pseudoaneurysms. AJR Am J Roentgenol. 2005;185:741–749. doi: 10.2214/ajr.185.3.01850741. [DOI] [PubMed] [Google Scholar]
- 3.Saad N.E., Saad W.E., Davies M.G., et al. Pseudoaneurysms and the role of minimally invasive techniques in their management. Radiographics. 2005;25:S173–S189. doi: 10.1148/rg.25si055503. [DOI] [PubMed] [Google Scholar]
- 4.Güneyli S., Gök M., Bozkaya H., et al. Endovascular management of iatrogenic renal arterial lesions and clinical outcomes. Diagn Interv Radiol. 2015;21:229–234. doi: 10.5152/dir.2014.14286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeMartino R.R., Walsh T.R., Powell R.J. Large traumatic thigh pseudoaneurysm with associated arteriovenous fistula. J Vasc Surg. 2016;63:1375. doi: 10.1016/j.jvs.2014.07.096. [DOI] [PubMed] [Google Scholar]
- 6.Henry J.C., Franz R.W. Pseudoaneurysms of the peripheral arteries. Int J Angiol. 2019;28:20–24. doi: 10.1055/s-0039-1677676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bansal A., Gorsi U., Farook S., et al. Interventional radiology management of extremity pseudoaneurysms: a pictorial essay. Emerg Radiol. 2021;28:1029–1039. doi: 10.1007/s10140-021-01939-w. [DOI] [PubMed] [Google Scholar]
- 8.Wygant C.M., Cohle S.D. Fatal rupture of pseudoaneurysm following angioplasty. Cardiovasc Pathol. 2021;50 doi: 10.1016/j.carpath.2020.107268. [DOI] [PubMed] [Google Scholar]
- 9.Kurzawski J., Janion-Sadowska A., Zandecki L., et al. Comparison of the efficacy and safety of two dosing protocols for ultrasound guided thrombin injection in patients with iatrogenic femoral pseudoaneurysms. Eur J Vasc Endovasc Surg. 2020;59:1019–1025. doi: 10.1016/j.ejvs.2020.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Chun E.J. Ultrasonographic evaluation of complications related to transfemoral arterial procedures. Ultrasonography. 2018;37:164–173. doi: 10.14366/usg.17047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vowels T.J., Zubair M.M., Bismuth J., et al. Balloon-assisted ultrasound-guided percutaneous thrombin injection of iatrogenic femoral artery pseudoaneurysms: a case report and description of the technique. Vasc Endovasc Surg. 2020;54:532–535. doi: 10.1177/1538574420927861. [DOI] [PubMed] [Google Scholar]
- 12.Devendra A., Nishith P.G., Velmurugesan P., et al. Surgical management of peripheral artery pseudoaneurysm following orthopedic trauma: a report of 14 cases. Eur J Trauma Emerg Surg. 2022;48:637–645. doi: 10.1007/s00068-020-01546-3. [DOI] [PubMed] [Google Scholar]
- 13.Soto E., Ananthasekar S., Passman M.A., et al. Microsurgical management of a brachial artery pseudoaneurysm in a 41-day-old infant. J Vasc Surg Cases Innov Tech. 2021;7:133–136. doi: 10.1016/j.jvscit.2020.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan W., Dou S., Yang J., et al. Endovascular double-layer bare stent placement in the treatment of posttraumatic pseudoaneurysm. BioMed Res Int. 2021;2021 doi: 10.1155/2021/5575173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang C., Chen Z., Zhao Y., et al. Four-year outcomes following endovascular repair in patients with traumatic isolated popliteal artery injuries. J Vasc Surg. 2021;73:2064–2070. doi: 10.1016/j.jvs.2020.12.050. [DOI] [PubMed] [Google Scholar]
- 16.Shreve L., Jarmakani M., Javan H., et al. Endovascular management of traumatic pseudoaneurysms. CVIR Endovasc. 2020;3 doi: 10.1186/s42155-020-00182-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Elm E., Altman D.G., Egger M., et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4:e296. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohan B., Singal S., Bawa A.S., et al. Endovascular management of traumatic pseudoaneurysm: short & long term outcomes. J Clin Orthop Trauma. 2017;8:276–280. doi: 10.1016/j.jcot.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corvino A., Catalano O., de Magistris G., et al. Usefulness of Doppler techniques in the diagnosis of peripheral iatrogenic pseudoaneurysms secondary to minimally invasive interventional and surgical procedures: imaging findings and diagnostic performance study. J Ultrasound. 2020;23:563–573. doi: 10.1007/s40477-020-00475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coughlin B.F., Paushter D.M. Peripheral pseudoaneurysms: evaluation with duplex US. Radiology. 1988;168:339–342. doi: 10.1148/radiology.168.2.3293107. [DOI] [PubMed] [Google Scholar]
- 21.Busquéts A.R., Acosta J.A., Colón E., et al. Helical computed tomographic angiography for the diagnosis of traumatic arterial injuries of the extremities. J Trauma. 2004;56:625–628. doi: 10.1097/01.ta.0000053546.28739.cf. [DOI] [PubMed] [Google Scholar]
- 22.Toombs B.D., Jing J.M. Current concepts in the evaluation of vascular disease: magnetic resonance and computed tomographic angiography. Tex Heart Inst J. 2000;27:170–192. [PMC free article] [PubMed] [Google Scholar]
- 23.Toursarkissian B., Allen B.T., Petrinec D., et al. Spontaneous closure of selected iatrogenic pseudoaneurysms and arteriovenous fistulae. J Vasc Surg. 1997;25:803–808. doi: 10.1016/s0741-5214(97)70209-x. ; discussion 808-809. [DOI] [PubMed] [Google Scholar]
- 24.Kim D., Arbra C.A., Simon Ivey J., et al. Iatrogenic radial artery injuries: variable injury patterns, treatment times, and outcomes. Hand. 2021;16:93–98. doi: 10.1177/1558944719844348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saydam O., Serefli D., Engin A.Y., et al. Ultrasound-guided thrombin injection for treatment of iatrogenic femoral artery pseudoaneurysms compared with open surgery: first experiences from a single institution. Ann Surg Treat Res. 2020;98:270–276. doi: 10.4174/astr.2020.98.5.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y., Dong X., Liang H., et al. Endovascular treatment of subclavian artery pseudoaneurysm. Ann Vasc Surg. 2020;65:284.e1–284.e6. doi: 10.1016/j.avsg.2019.10.096. [DOI] [PubMed] [Google Scholar]
- 27.Ehieli W.L., Bozdogan E., Janas G., et al. Imaging-guided percutaneous thrombin injection for the treatment of iatrogenic femoral artery pseudoaneurysms. Abdom Radiol (NY) 2019;44:1120–1126. doi: 10.1007/s00261-019-01923-6. [DOI] [PubMed] [Google Scholar]