Abstract
Background
The HOST-EXAM (Harmonizing Optimal Strategy for Treatment of Coronary Artery Disease–Extended Antiplatelet Monotherapy) trial showed superior efficacy and safety of clopidogrel monotherapy compared with aspirin monotherapy during the chronic maintenance period after percutaneous coronary intervention (PCI).
Objectives
The goal of this study was to investigate the cost-effectiveness of clopidogrel monotherapy compared with that of aspirin monotherapy.
Methods
A Markov model was developed for patients in the stable phase after PCI. From the perspectives of the South Korean, UK, and U.S. health care systems, the lifetime health care costs and quality-adjusted life-years (QALYs) of each strategy were estimated. Transition probabilities were obtained from the HOST-EXAM trial, and health care costs and health-related utilities were obtained from data and literature for each country.
Results
From the perspective of the South Korean health care system, the base-case analysis showed that clopidogrel monotherapy was $3,192 higher in lifetime health care costs and 0.139 lower in QALYs compared with aspirin. This result was greatly influenced by the numerically but insignificantly higher cardiovascular mortality of clopidogrel compared with aspirin. In the analogous UK and U.S. models, clopidogrel monotherapy was projected to decrease health care costs by £1,122 and $8,920 per patient compared with aspirin monotherapy while reducing QALYs by 0.103 and 0.175, respectively.
Conclusions
Based on empirical data from the HOST-EXAM trial, clopidogrel monotherapy was projected to lead to reduced QALYs compared with aspirin during the chronic maintenance period after PCI. These results were affected by a numerically higher rate of cardiovascular mortality in clopidogrel monotherapy reported from the HOST-EXAM trial. (Harmonizing Optimal Strategy for Treatment of Coronary Artery Stenosis–Extended Antiplatelet Monotherapy [HOST-EXAM]; NCT02044250)
Key Words: aspirin, clopidogrel, coronary artery disease, cost-effectiveness, percutaneous coronary intervention
Central Illustration
The lifelong use of single antiplatelet therapy is currently recommended during the chronic maintenance period after percutaneous coronary intervention (PCI).1,2 However, data regarding the use of antiplatelet agents during this period are limited. For indefinite maintenance therapy with a single antiplatelet agent, aspirin is the most widely used and is recommended as standard therapy.3 Clopidogrel is often used as an alternative to aspirin in patients who cannot tolerate aspirin or who have allergies to aspirin.4
Recently, the HOST-EXAM (Harmonizing Optimal Strategy for Treatment of Coronary Artery Disease–Extended Antiplatelet Monotherapy) trial showed that clopidogrel monotherapy during the chronic maintenance period after PCI significantly reduced the risk of a composite of all-cause death, nonfatal myocardial infarction, stroke, readmission because of acute coronary syndrome (ACS), and Bleeding Academic Research Consortium bleeding type 3 or greater.5 However, because clopidogrel is more expensive than aspirin, data around the cost-effectiveness of clopidogrel and aspirin after PCI for their lifelong use are important, given the financial pressures being faced by health care systems in various countries.6, 7, 8, 9, 10, 11 We therefore performed a cost-effectiveness analysis of clopidogrel and aspirin monotherapy after PCI based on the results from the HOST-EXAM trial in 3 disparate health care systems.
Methods
The HOST-EXAM Trial
The efficacy and safety of clopidogrel monotherapy compared with aspirin monotherapy in the chronic maintenance period after PCI were reported in the HOST-EXAM trial.5,12 Briefly, the HOST-EXAM trial was an investigator-initiated, prospective, randomized, open-label, multicenter trial at 37 centers in South Korea. The trial randomly assigned patients who maintained dual antiplatelet therapy after PCI for 6 to 18 months without clinical events to receive clopidogrel 75 mg once daily or aspirin 100 mg once daily for 24 months between March 2014 and May 2018. The primary outcome was a composite of all-cause death, nonfatal myocardial infarction, stroke, readmission due to ACS, and Bleeding Academic Research Consortium bleeding type 3 or greater at 24 months. The risk of the primary outcome was significantly lower in the clopidogrel monotherapy group than in the aspirin monotherapy group (HR: 0.73; 95% CI: 0.59-0.90; P = 0.003).5
Analytic Overview of Cost-Effectiveness Analysis
To compare the long-term cost-effectiveness of clopidogrel monotherapy and aspirin monotherapy after PCI from a health care system perspective, a Markov model was developed by using TreeAge Pro 2020 (TreeAge Software). For each country, the model structure and clinical inputs were identical, but health care costs and utility weights differed based on local data. We applied the model to a hypothetical cohort of 60-year-old patients who maintained dual antiplatelet therapy without clinical events for 6 to 18 months after receiving PCI. The health outcome was quality-adjusted life-years (QALYs).
All costs are expressed in 2020 U.S. dollars in South Korea and the United States and in the 2020 British pound in the United Kingdom. Future costs and QALYs were discounted at an annual rate of 4.5%, based on the economic evaluation guidelines of pharmaceuticals in South Korea.13 For analyses from the UK and U.S. health care systems, a discount rate of 3.5% for the United Kingdom and 3.0% for the United States was applied.14,15
The main outcome measure was the incremental cost-effectiveness ratio (ICER) calculated as incremental costs per QALY gained, compared with the less expensive strategy. Deterministic and probabilistic sensitivity analyses (PSAs) were performed to explore the effects of parameter uncertainty on the results. Subgroup analyses were performed to assess the influence of patient characteristics. In addition, considering the uncertainty of the relative impact of clopidogrel on mortality compared with aspirin, we performed a scenario analysis with the assumption of no difference in mortality. The current study complied with the Consolidated Health Economic Evaluation Reporting Standards statement (Supplemental Table 1).
This analysis was approved by the ethics committee at Seoul National University Hospital and was conducted according to the principles of the Declaration of Helsinki. This study is registered with ClinicalTrials.gov number (NCT02044250).
Model
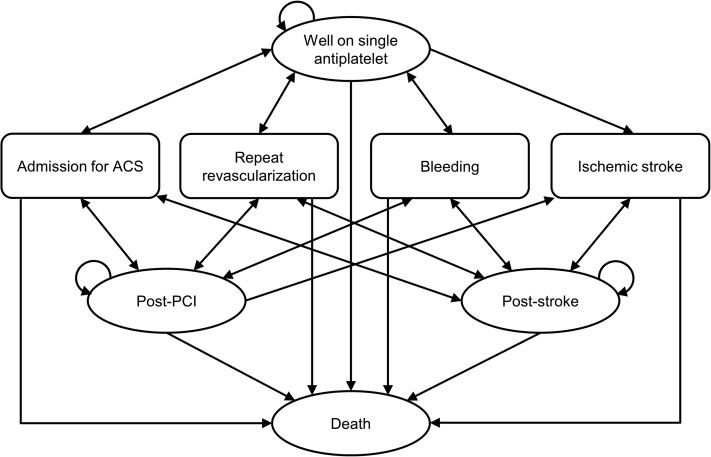
Figure 1 displays the schematic representation of the model used in the current study. The model structure was designed to consider the clinical pathways of the disease and key clinical outcomes in the HOST-EXAM trial. The cycle length of the model was set to 1 year based on the nature of the disease. A time horizon of 30 years was adopted to evaluate the long-term cost-effectiveness. The cohort entered the model in the “well on single antiplatelet” state. During the chronic maintenance period after PCI, patients were at risk of admission for ACS, repeat revascularization, bleeding, ischemic stroke, and cardiovascular and noncardiovascular deaths. “Post-PCI,” “post-stroke,” and “death” were modeled as health states that the patient could move to, according to the occurrence of a clinical event. It was assumed that dual antiplatelet therapy was maintained for 1 year after moving to the “post-PCI” state; the subsequent risk of clinical events, follow-up cost, and utility were to be the same as those of patients in the “well on single antiplatelet” state. The “post-stroke” state reflected reduced utility and increased follow-up cost; and the risk of clinical events was considered the same as that of patients in the “well on single antiplatelet” state unless the patient underwent additional PCI. Because the cycle length of the model was 1 year, transient events (eg, nonfatal bleeding, coronary artery bypass graft surgery, admission for ACS but not receiving repeat revascularization) were reflected as transition states in the model. When these events occurred, treatment costs and utility reductions were counted; however, there was no shift in the health state.
Figure 1.
Model Schematic
The structure of the Markov model is shown. The cohort entered the model in the “well on single antiplatelet” state. Yearly, patients were at risk of admission for acute coronary syndrome (ACS), repeat revascularization, bleeding, ischemic stroke, and death. “Post-PCI,” “post-stroke,” and “death” were modeled as health states that the patient could move into according to the occurrence of a clinical event. PCI = percutaneous coronary intervention.
Model Input
The base-case values and ranges of key model inputs are summarized in Supplemental Tables 2 to 6.
Transition Probabilities
Annual transition probabilities under single antiplatelet therapy were calculated from the HOST-EXAM trial (Supplemental Table 2). Survival analysis with Kaplan-Meier estimates was used to determine the risk of clinical pathways and derive transition probabilities for each event. For patients who moved to the “post-PCI” state, the transition probabilities for 1 year under dual antiplatelet therapy were retrieved from the Grand Drug-Eluting Stent Registry, which enrolled 17,286 patients from 55 participating centers in South Korea.16,17 For noncardiovascular mortality, data on the age-specific mortality obtained from the 2019 South Korean life table were applied.18 For the subgroup analyses, values for the transition probabilities under a single antiplatelet, which could be obtained from the HOST-EXAM trial, were estimated for each subgroup (Supplemental Table 3).
Costs and Health State Utilities
From the perspective of the South Korean health care system, costs include those paid by the insurer and out-of-pocket costs paid by patients. For drug costs, base-case values were based on the weighted average price of each component in 2020.19 The range of drug costs used in the sensitivity analysis was based on the prices of individual products covered by national health insurance.20 Annual follow-up costs, treatment costs when clinical events occurred, and medical costs for 1 year before death were estimated through analysis of health insurance claims data using the National Health Insurance Service–National Health Information Database (approval no. for data access: NHIS-2021-1-249). To include the cost of uncovered services paid by patients, the proportions of such costs were applied to the estimated medical costs (Supplemental Table 4).21 All costs were adjusted to 2020 using the South Korean health care consumer price index and expressed in U.S. dollars ($1 = 1,086 South Korean Won in 2020).
From the perspective of the UK health care system, drug prices were based on the tariff prices of nonproprietary drugs from the British National Formulary.22 Most of the other costs in the United Kingdom were derived from the National Schedule of NHS costs,23 and some costs that were difficult to obtain from these data were obtained from previously published literature. The costs derived from previous literature were adjusted to the 2020 British pound by using the UK Medical Service Consumer Price Index (Supplemental Table 5). From the perspective of the U.S. health care system, drug prices were based on the current generic prices in the United States,24 and other costs were derived from previously published literature. All costs were adjusted to 2020 U.S. dollars by using the U.S. Medical Care Consumer Price Index (Supplemental Table 6).
To calculate QALYs, utility weights for each health state and disutility due to event occurrence were required. All utilities were derived from the published literature with a similar study population in each country. Values that could not be obtained from studies in that country were derived from expert opinion. The duration of disutility for each event was determined based on previous studies and expert opinions (Supplemental Tables 4 to 6).
Sensitivity Analyses
To explore parameter uncertainty, 2 types of sensitivity analyses were conducted. First, we conducted one-way sensitivity analysis for the plausible range of each parameter (Supplemental Tables 2 and 4). For some parameters, the value of the ICER seemed infinite owing to the change in the direction of incremental effects. Therefore, the tornado diagram was presented as an incremental net monetary benefit (INMB). The willingness-to-pay (WTP) threshold in South Korea, U.S. $30,581 per QALY gained, was obtained from a previous study and was adjusted to 2020.25 Second, to explore the joint uncertainty of all parameters, we assigned distributions to parameters and conducted PSA based on 10,000 second-order Monte Carlo simulations. The distributions of model inputs are presented in Supplemental Tables 2 and 4.
Scenario Analysis
With the uncertainties in the relative impact of clopidogrel and aspirin on cardiovascular mortality rates, we performed a scenario analysis in which we assumed no differences in short- and long-term cardiovascular mortality between the clopidogrel monotherapy and aspirin monotherapy groups. In this scenario, a threshold analysis for the price of generic clopidogrel was performed to identify the price at which clopidogrel monotherapy would be cost-effective under the WTP per QALY gained in South Korea.
Results
Trial Population and Clinical Outcomes
The HOST-EXAM trial included 5,438 patients, with 2,710 patients in the clopidogrel group and 2,728 patients in the aspirin group. The baseline characteristics were well balanced between the two groups and are presented in Supplemental Table 7. After 24 months of follow-up, clopidogrel monotherapy reduced the risk of the primary outcome (HR: 0.73; 95% CI: 0.59-0.90; P = 0.003) (Supplemental Table 8). For individual outcomes, clopidogrel monotherapy reduced the risk of readmission due to ACS (HR: 0.61; 95% CI: 0.45-0.82; P = 0.001), stroke (HR: 0.42; 95% CI: 0.24-0.73; P = 0.002), and any bleeding events (HR: 0.70; 95% CI: 0.52-0.95; P = 0.021), including major bleeding events (HR: 0.63; 95% CI: 0.41-0.97; P = 0.035) (Supplemental Table 8). However, the rates of all-cause deaths and cardiovascular deaths were numerically higher in the clopidogrel group than in the aspirin group.
Cost-Effectiveness Analyses From the South Korean Health Care System Perspective
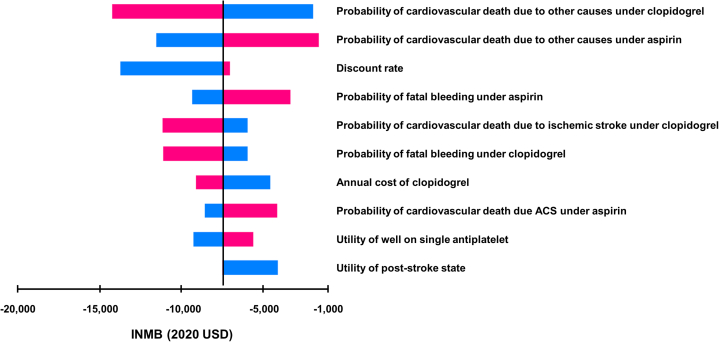
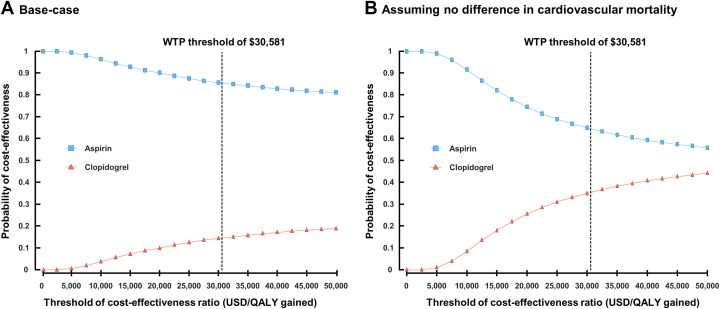
In our model, clopidogrel monotherapy was associated with an increase in health care costs of $3,192 but did not lead to QALY gained compared with aspirin monotherapy from the perspective of the South Korean health care system (Table 1). Clopidogrel monotherapy had 0.139 lower QALY than aspirin monotherapy. In the one-way sensitivity analysis, the cardiovascular death rates had the greatest impact on the ICER (Figure 2). However, clopidogrel monotherapy did not become cost-effective. In the PSA, the probability that clopidogrel monotherapy would be cost-effective under a WTP of $30,581 per QALY gained was 14.5% (Figure 3A, Supplemental Figure 1A). This opposite direction of QALYs compared with the primary outcome in the trial was mainly driven by the higher number of cardiovascular deaths reported in patients receiving clopidogrel monotherapy, which was not statistically significant (Supplemental Table 8). In subgroup analyses, health care costs were higher in the clopidogrel group than in the aspirin group in all subgroups (Supplemental Table 9). However, clopidogrel monotherapy was associated with an increase in QALYs in patients aged <65 years, male patients, or patients with single-vessel disease due to the absence of difference in cardiovascular mortality between the clopidogrel and aspirin groups (Supplemental Table 10).
Table 1.
Results of Cost-Effectiveness Analyses Between Clopidogrel and Aspirin Monotherapy
| Costs | Incremental Costs | Effectiveness | Incremental Effectiveness | ICER | |
|---|---|---|---|---|---|
| Korean health care system perspective | |||||
| Aspirin | 14,451 | – | 12.447 | – | Dominant |
| Clopidogrel | 17,643 | 3,192 | 12.309 | –0.139 | Dominated |
| UK health care system perspective | |||||
| Aspirin | 7,371 | 1,122 | 13.492 | 0.103 | 10,931 |
| Clopidogrel | 6,249 | – | 13.390 | – | – |
| U.S. health care system perspective | |||||
| Aspirin | 112,915 | 8,920 | 15.280 | 0.175 | 50,933 |
| Clopidogrel | 103,996 | – | 15.105 | – | – |
For the analyses from the South Korean and U.S. health care system perspectives, the unit of the costs was the U.S. dollar. For the analysis from the UK health care system perspective, the unit of the cost was the British pound. For all analyses, the unit of effectiveness was quality-adjusted life-year. Incremental cost-effectiveness ratio (ICER) was defined as incremental costs/quality-adjusted life-year gained.
Figure 2.
Selected Results of 1-Way Sensitivity Analysis
A tornado diagram for clopidogrel vs aspirin monotherapy is presented to visualize the one-way sensitivity analysis. For some parameters, the value of the incremental cost-effectiveness ratio seemed infinite due to the change in the direction of incremental effects. The tornado diagram was therefore presented as an incremental net monetary benefit (INMB), assuming a willingness-to-pay threshold of $30,581. The top 10 variables with considerable INMB variation are shown. The vertical line represents the base-case INMB. The x-axis represents the ranges of INMB when the parameter values were varied over plausible ranges. Blue color indicates when each parameter has values lower than the base-case value within the range, and red color indicates when each parameter has higher values. ACS = acute coronary syndrome; USD = U.S. dollar.
Figure 3.
Cost-Effectiveness Acceptability Curves
Probabilistic sensitivity analyses were performed on base-case and scenario, assuming no difference in cardiovascular mortality. The dashed lines represent the willingness-to-pay (WTP) threshold of $30,581. The curves show the probabilities that each monotherapy is cost-effective at varying cost-effectiveness threshold ratios. QALY = quality-adjusted life-year; USD = U.S. dollar.
To address the uncertainties in the relative impact of clopidogrel and aspirin on mortality, we performed a scenario analysis with the assumption of no difference in short- and long-term cardiovascular deaths between the clopidogrel and aspirin monotherapy groups (Table 2). In this scenario analysis, clopidogrel monotherapy was projected to increase QALYs by 0.042 compared with aspirin monotherapy at an incremental cost of $3,194, resulting in an ICER of $75,428 in South Korea. PSA revealed that the probability of clopidogrel monotherapy being cost-effective under the WTP threshold was 35.4% (Figure 3B, Supplemental Figure 1B). The cost of clopidogrel would need to be decreased by 36% (from $1.03 to $0.66 per tablet) to be less than the WTP threshold used for South Korea in this study.
Table 2.
Scenario Analyses With the Assumption of No Difference in Mortality
| Costs | Incremental Costs | Effectiveness | Incremental Effectiveness | ICER | |
|---|---|---|---|---|---|
| Korean health care system perspective | |||||
| Aspirin | 14,462 | – | 12.363 | – | – |
| Clopidogrel | 17,656 | 3,194 | 12.405 | 0.042 | 75,428 |
| UK health care system perspective | |||||
| Aspirin | 7,333 | 1,050 | 13.396 | –0.105 | Dominated |
| Clopidogrel | 6,283 | – | 13.500 | – | Dominant |
| U.S. health care system perspective | |||||
| Aspirin | 112,479 | 8,071 | 15.167 | –0.066 | Dominated |
| Clopidogrel | 104,408 | – | 15.233 | – | Dominant |
For the analyses from the South Korean and U.S. health care system perspectives, the unit of costs was U.S. dollars. For the analysis from the UK health care system perspective, the unit of the cost was the British pound. For all analyses, the unit of effectiveness was quality-adjusted life-years.
Abbreviations as in Table 1.
Cost-Effectiveness Analyses From the UK and U.S. Health Care System Perspectives
The analyses were conducted in the UK and U.S. health care systems to explore cost-effectiveness from the perspectives of other health care systems. In contrast to the results from South Korea, clopidogrel monotherapy was projected to decrease lifetime health care costs by £1,122 in the United Kingdom and by $8,920 in the United States (Table 1). These results were driven by differences in drug and direct medical costs, such as annual follow-up, admission due to clinical events, procedures, or surgery (Supplemental Table 11). Although clopidogrel monotherapy decreased health care costs by reducing nonfatal clinical events in the United Kingdom and the United States, it was not associated with QALY gains in either country, as in South Korea. Clopidogrel monotherapy was associated with 0.103 and 0.175 lower QALYs in the United Kingdom and the United States, respectively, compared with aspirin monotherapy.
When the scenario assuming no difference in cardiovascular deaths between clopidogrel and aspirin monotherapy was applied to the United Kingdom and the United States, clopidogrel monotherapy was projected to increase QALYs by 0.105 and 0.066, respectively, compared with aspirin monotherapy without an increase in health care costs (Table 2). Clopidogrel monotherapy decreased health care costs by £1,050 in the United Kingdom and $8,071 in the United States. These results indicate that clopidogrel monotherapy was the dominant treatment strategy in the United Kingdom and the United States in this scenario.
Discussion
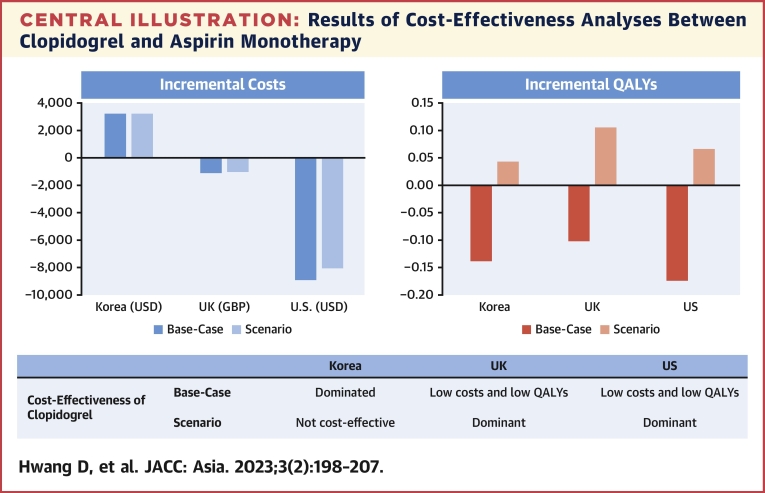
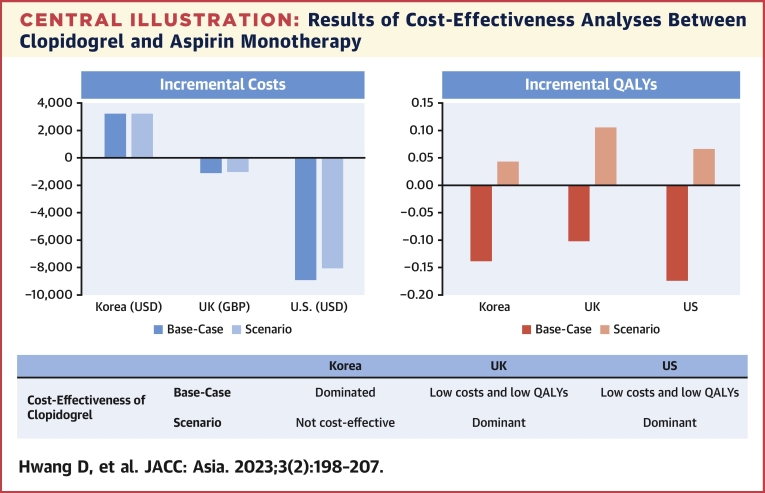
The current cost-effectiveness analysis based on the HOST-EXAM trial showed that clopidogrel monotherapy was associated with increased health care costs compared with aspirin monotherapy in South Korea. However, the increased costs of clopidogrel monotherapy did not lead to an increase in QALY gain. These results were mainly driven by the numerically higher number of cardiovascular deaths reported in the clopidogrel group. In the United Kingdom and the United States, clopidogrel monotherapy decreased health care costs but did not increase QALYs. In the scenario analysis assuming no difference in cardiovascular mortality between the 2 groups, clopidogrel monotherapy increased QALYs but was not cost-effective in South Korea. However, it was a dominant treatment strategy in the United Kingdom and the United States (Central Illustration).
Central Illustration.
Results of Cost-Effectiveness Analyses Between Clopidogrel and Aspirin Monotherapy
During the chronic maintenance period after percutaneous coronary intervention, clopidogrel monotherapy did not increase quality-adjusted life-years (QALYs) compared with aspirin monotherapy, despite increased costs in South Korea. In the United Kingdom and the United States, clopidogrel monotherapy decreased health care costs but was not associated with QALY gains. Scenario analysis with the assumption of no difference in mortality between the 2 groups show that clopidogrel was the dominant treatment strategy in the United Kingdom and the United States, but it was not cost-effective in South Korea. GBP = British pound; USD = U.S. dollar.
Current guidelines recommend the indefinite use of a single antiplatelet agent for secondary prevention of cardiovascular events after a specific period of dual antiplatelet therapy in patients undergoing PCI.1,3 Aspirin is the most widely used antiplatelet agent in patients after PCI, and clopidogrel is recommended as an alternative to aspirin.3,4 We recently reported the superior efficacy and safety of clopidogrel monotherapy compared with aspirin monotherapy during the chronic maintenance period after PCI in the HOST-EXAM trial.5 However, considering the marked difference in drug costs between clopidogrel and aspirin and health care costs in various countries, it is unclear whether clopidogrel monotherapy is a cost-effective treatment strategy for secondary prevention in these patients.
In this cost-effectiveness analysis based on the HOST-EXAM trial, clopidogrel monotherapy increased lifetime health care costs, but the benefit of clopidogrel monotherapy in the composite clinical outcomes did not translate into an increase in QALYs in South Korea. Aspirin monotherapy is therefore projected to be the dominant strategy from the viewpoint of economic evaluation. These results were mainly due to the higher cost of clopidogrel in South Korea, as well as the numerically higher numbers of cardiovascular deaths in the clopidogrel group than in the aspirin group from the trial. Although direct health care costs were higher in the aspirin group (excluding drug costs), the higher cost of clopidogrel exceeded this difference. Although the difference in the cardiovascular death rate between the 2 groups was only 0.2% at 24 months and was not statistically significant, this difference outweighed the benefit of clopidogrel on nonfatal events in QALYs. This finding was supported by the fact that cardiovascular deaths had the greatest impact on ICER in the sensitivity analysis, and several subgroups with minimal differences in cardiovascular death rates reported higher QALYs in the clopidogrel group than in the aspirin group.
The results of the cost-effectiveness analysis may vary according to a given country as the economy, health care system, and medical costs differ across countries. With this in mind, cost-effectiveness analyses from the perspectives of the UK and U.S. health care systems were performed in the current study. Although necessary assumptions and data from external sources were inevitably applied to estimate cost-effectiveness, the results from both countries were similar. Clopidogrel monotherapy decreased health care costs compared with aspirin monotherapy, driven by differences in both drug prices and costs associated with adverse clinical events across the different health care systems. In the United Kingdom, the drug costs for clopidogrel and aspirin are similar. Therefore, direct medical costs, including annual follow-up, admission due to clinical events, procedures, or surgery, mainly affect the results. In the United States, although generic clopidogrel is 8 times more expensive than aspirin, the high direct medical costs for clinical events exceed the difference in drug costs.
The relative effects of aspirin and clopidogrel on mortality remain unclear, however. In the CAPRIE (Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events) trial, all-cause mortality and vascular mortality were numerically higher in the aspirin group than in the clopidogrel group.26 Another study from a pooled analysis of STOPDAPT-1 and STOPDAPT-2 (Short and Optimal Duration of Dual Antiplatelet Therapy-1 and -2, respectively) trials also reported a numerically higher but comparable risk of cardiovascular and noncardiovascular mortalities in aspirin monotherapy compared with clopidogrel monotherapy after short-term dual antiplatelet therapy in PCI patients.27 A recent meta-analysis comparing the effects of aspirin and P2Y12 inhibitors on secondary prevention also found comparable risks of cardiovascular and noncardiovascular mortalities between them.28
Based on the unclear relative effects of clopidogrel and aspirin on mortality, the cost-effectiveness of clopidogrel vs aspirin monotherapy must be compared based on the assumption that there is no difference in short- or long-term mortality. In our scenario analysis, assuming no difference in cardiovascular deaths between the 2 treatment strategies, clopidogrel monotherapy was not a cost-effective treatment strategy, although it increased QALYs compared with aspirin monotherapy in South Korea. However, clopidogrel was associated with QALY gain and cost savings in the United Kingdom and the United States, indicating that clopidogrel monotherapy was the dominant treatment strategy in both countries, with the assumption of no difference in mortality. These results suggest that clopidogrel monotherapy can be the dominant treatment strategy from a cost-effectiveness perspective in the countries where the difference in costs of clopidogrel and aspirin is minimal or requires higher medical costs for treating adverse cardiovascular events, without evidence of the relative effects of clopidogrel and aspirin on mortality.
Study Limitations
First, utilities and disutilities for quality-of-life measures were not collected in the HOST-EXAM trial. Second, we used transition probabilities calculated from the HOST-EXAM trial and previous registry data and assumed that the risk of clinical events did not change over 30 years in the model. Third, the possibility that the values of disutilities may increase if the event is repeated was not taken into consideration. Fourth, the long-term effects of clopidogrel and aspirin monotherapy on mortality remain uncertain. If the long-term follow-up results of the HOST-EXAM trial are available, the uncertainties related to cardiovascular death observed in the HOST-EXAM may be clarified.
Conclusions
The current study could not show the cost-effectiveness of clopidogrel monotherapy compared with aspirin monotherapy during the chronic maintenance period after PCI. However, clopidogrel monotherapy can be the dominant treatment strategy from a cost-effectiveness perspective in countries where the difference in costs of both drugs is small or medical costs for treating adverse cardiovascular events are high, based on the unclear relative effects of clopidogrel and aspirin on mortality.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: The indefinite use of a single antiplatelet agent after a specific period of dual antiplatelet therapy in patients with coronary intervention is recommended in the current guidelines. However, data on which antiplatelet agent to use during this period are limited. Recently, the HOST-EXAM trial reported superior efficacy and safety of clopidogrel monotherapy compared with aspirin monotherapy. In the current cost-effectiveness analysis of the HOST-EXAM trial, the benefit of clopidogrel monotherapy in the composite clinical outcomes was not translated into an increase in QALYs. This result was mainly due to numerically higher cardiovascular deaths in the clopidogrel group than in the aspirin group in the trial. Health care costs with clopidogrel monotherapy increased in South Korea but decreased in the United Kingdom and the United States. These results were mainly due to differences in the health care systems, expenses for adverse clinical events, and drug prices. The scenario analysis assuming no difference in cardiovascular mortality showed that clopidogrel monotherapy was not cost-effective in South Korea but could be a dominant treatment strategy in the United Kingdom and the United States.
TRANSLATIONAL OUTLOOK: Current cost-effectiveness analyses based on the HOST-EXAM trial could not show the cost-effectiveness of clopidogrel monotherapy. Considering the influence of cardiovascular mortality on these results, clopidogrel monotherapy can be the dominant treatment strategy from a cost-effectiveness perspective in countries where the difference in costs of both drugs is small or the medical costs for treating adverse cardiovascular events are high.
Funding Support and Author Disclosures
The funding sources were a consortium of 4 pharmaceutical Companies (ChongKunDang, SamJin, HanMi, and DaeWoong), grants from the Patient-Centered Clinical Research Coordinating Center (grant numbers HI19C0481 and HC19C0305), grants from Seoul National University Hospital (06-2011-3280, 06-2011-3680, and 06-2010-1560), and Korea Health Technology R&D Project (grant number HI17C2085) funded by the Ministry of Health & Welfare, South Korea. The funders had no role in study design, data collection, analysis, interpretation, or writing of the manuscript. Dr H.-S. Kim has received research grants or speaker fees from Daiichi Sankyo, Boston Scientific, Terumo, Biotronik, and Dio, as well as Medtronic, Abbott Vascular, Edwards Lifesciences, AmGen, and Boehringer Ingelheim. Dr Koo has received institutional research grants from Abbott Vascular and Philips. Dr Park has received fees from Daiichi Sankyo, AstraZeneca, Sanofi, Bristol Myers Squibb, Bayer AG, and Pfizer outside of the submitted work. Dr Mamas has received unrestricted education grants from Abbott, Terumo, and Medtronic; and speaker fees from Daiichi Sankyo, Bristol Myers Squibb, and Terumo. Dr Cohen has received research grant support from AstraZeneca and Daiichi Sankyo; and consulting income from MyoKardia, a subsidiary of Bristol Myers Squibb. There was no financial relationships with any organizations that might have an interest in this work in the previous 3 years. No other relationships or activities that could appear to have influenced the submitted work. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental figure and tables, please see the online version of this paper.
Contributor Information
Tae-Jin Lee, Email: tjlee@snu.ac.kr.
Hyo-Soo Kim, Email: hyosoo@snu.ac.kr.
Appendix
References
- 1.Levine G., Bates E., Bittl J., et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. J Am Coll Cardiol. 2016;68:1082–1115. doi: 10.1016/j.jacc.2016.03.513. [DOI] [PubMed] [Google Scholar]
- 2.Valgimigli M., Bueno H., Byrne R.A., et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2018;39:213–260. doi: 10.1093/eurheartj/ehx419. [DOI] [PubMed] [Google Scholar]
- 3.Neumann F.J., Sousa-Uva M., Ahlsson A., et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. doi: 10.1093/eurheartj/ehy855. [DOI] [PubMed] [Google Scholar]
- 4.Smith S., Benjamin E., Bonow R., et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update. J Am Coll Cardiol. 2011;58:2432–2446. doi: 10.1016/j.jacc.2011.10.824. [DOI] [PubMed] [Google Scholar]
- 5.Koo B.K., Kang J., Park K.W., et al. Aspirin versus clopidogrel for chronic maintenance monotherapy after percutaneous coronary intervention (HOST-EXAM): an investigator-initiated, prospective, randomised, open-label, multicentre trial. Lancet. 2021;397:2487–2496. doi: 10.1016/S0140-6736(21)01063-1. [DOI] [PubMed] [Google Scholar]
- 6.Karnon J., Brennan A., Pandor A., et al. Modelling the long term cost effectiveness of clopidogrel for the secondary prevention of occlusive vascular events in the UK. Curr Med Res Opin. 2005;21:101–112. doi: 10.1185/030079904x18036. [DOI] [PubMed] [Google Scholar]
- 7.Berger K., Hessel F., Kreuzer J., Smala A., Diener H.C. Clopidogrel versus aspirin in patients with atherothrombosis: CAPRIE-based calculation of cost-effectiveness for Germany. Curr Med Res Opin. 2008;24:267–274. doi: 10.1185/030079908x253762. [DOI] [PubMed] [Google Scholar]
- 8.Logman J.F., Heeg B.M., Herlitz J., van Hout B.A. Costs and consequences of clopidogrel versus aspirin for secondary prevention of ischaemic events in (high-risk) atherosclerotic patients in Sweden: a lifetime model based on the CAPRIE trial and high-risk CAPRIE subpopulations. Appl Health Econ Health Policy. 2010;8:251–265. doi: 10.2165/11535520-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Kourlaba G., Fragoulakis V., Maniadakis N. Clopidogrel versus aspirin in patients with atherothrombosis: a CAPRIE-based cost-effectiveness model for Greece. Appl Health Econ Health Policy. 2012;10:331–342. doi: 10.1007/BF03261867. [DOI] [PubMed] [Google Scholar]
- 10.Lin Z., Zhang L., Yang X., Liu L., Xuan J. Cost-effective analysis of clopidogrel versus aspirin for high risk patients with established peripheral arterial disease in China. J Med Econ. 2020;23:659–666. doi: 10.1080/13696998.2020.1724119. [DOI] [PubMed] [Google Scholar]
- 11.Gaspoz J.M., Coxson P.G., Goldman P.A., et al. Cost effectiveness of aspirin, clopidogrel, or both for secondary prevention of coronary heart disease. N Engl J Med. 2002;346:1800–1806. doi: 10.1056/NEJM200206063462309. [DOI] [PubMed] [Google Scholar]
- 12.Lee H., Koo B.K., Park K.W., et al. A randomized clinical trial comparing long-term clopidogrel vs aspirin monotherapy beyond dual antiplatelet therapy after drug-eluting coronary stent implantation: design and rationale of the Harmonizing Optimal Strategy for Treatment of Coronary Artery Stenosis–Extended Antiplatelet Monotherapy (HOST-EXAM) trial. Am Heart J. 2017;185:17–25. doi: 10.1016/j.ahj.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Guidelines for Economic Evaluation of Pharmaceuticals in Korea. Health Insurance Review & Assessment Service; 2021. [Google Scholar]
- 14.Basu A., Ganiats T.G. Cost-Effectiveness in Health and Medicine. Oxford University Press; 2017. Discounting in cost-effectiveness analysis; pp. 277–288. [Google Scholar]
- 15.National Institute for Health and Care Excellence . National Institute for Health and Care Excellence; 2013. Guide to the Methods of Technology Appraisal 2013. [PubMed] [Google Scholar]
- 16.Hwang D., Kang J., Yang H.M., et al. Better prognosis after complete revascularization using contemporary coronary stents in patients with chronic kidney disease. Circ Cardiovasc Interv. 2019;12 doi: 10.1161/CIRCINTERVENTIONS.119.007907. [DOI] [PubMed] [Google Scholar]
- 17.Kang J., Park K.W., Lee H.S., et al. Relative impact of clinical risk versus procedural risk on clinical outcomes after percutaneous coronary intervention. Circ Cardiovasc Interv. 2021;14 doi: 10.1161/CIRCINTERVENTIONS.120.009642. [DOI] [PubMed] [Google Scholar]
- 18.Statistics Korea Life Tables. 2019. https://kosis.kr/publication/publicationThema.do?pubcode=LL
- 19.Health Insurance Review and Assessment Service The weighted price of drug by component in the 2020. https://www.hira.or.kr
- 20.Health Insurance Review and Assessment Service Currently applied drug price file (2017.12.01-2020.12.01) https://www.hira.or.kr
- 21.Lee O.H., Park G.H., Kim J.H., et al. Health Insurance Research Institute, National Health Insurance Service; 2020. Health Insurance Patient Medical Expenditure Survey in 2019. [Google Scholar]
- 22.Joint Formulary Committee, Royal Pharmaceutical Society of Great Britain . BMJ Group and Pharmaceutical Press; 2020. British National Formulary 80 (September 2020-March 2021) [Google Scholar]
- 23.NHS England and NHS Improvement . NHS England and NHS Improvement; 2020. National Schedule of NHS Costs—Year 2019-20—NHS Trust and NHS Foundation Trusts. [Google Scholar]
- 24.Costco. http://www.costco.com
- 25.Ahn J.H., Kim Y.H., Shin S.J., Park J.Y. National Evidence-based Healthcare Collaborating Agency; 2012. Asian Collaboration on Cost-Effectiveness in Health Care Decision Making. [Google Scholar]
- 26.CAPRIE Steering Committee A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE) Lancet. 1996;348:1329–1339. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 27.Natsuaki M., Morimoto T., Watanabe H., et al. Clopidogrel monotherapy vs. aspirin monotherapy following short-term dual antiplatelet therapy in patients receiving everolimus-eluting coronary stent implantation. Circ J. 2020;84:1483–1492. doi: 10.1253/circj.CJ-20-0298. [DOI] [PubMed] [Google Scholar]
- 28.Chiarito M., Sanz-Sánchez J., Cannata F., et al. Monotherapy with a P2Y(12) inhibitor or aspirin for secondary prevention in patients with established atherosclerosis: a systematic review and meta-analysis. Lancet. 2020;395:1487–1495. doi: 10.1016/S0140-6736(20)30315-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.