Abstract
In daily clinical practice, physicians often encounter patients with angina or those with evidence of myocardial ischemia from noninvasive tests but not having obstructive coronary artery disease. This type of ischemic heart disease is referred to as ischemia with nonobstructive coronary arteries (INOCA). INOCA patients often suffer from recurrent chest pain without adequate management and are associated with poor clinical outcomes. There are several endotypes of INOCA, and each endotype should be treated based on its specific underlying mechanism. Therefore, identifying INOCA and discriminating its underlying mechanisms are important issues and of clinical interest. Invasive physiologic assessment is the first step in the diagnosis of INOCA and discriminating the underlying mechanism; additional provocation tests help physicians identify the vasospastic component in INOCA patients. Comprehensive information acquired from these invasive tests can provide a template for mechanism-specific management for patients with INOCA.
Key Words: coronary microvascular disease, coronary spasm, invasive physiologic assessment, ischemia with nonobstructive coronary artery, ischemic heart disease, provocation test
Central Illustration
Highlights
-
•
Patients with INOCA are underdiagnosed in daily practice.
-
•
Because the presence of INOCA is associated with poor clinical outcomes, identifying INOCA and discriminating the underlying mechanisms are clinically important.
-
•
Invasive coronary evaluation with physiologic assessment and provocation tests is needed to diagnose INOCA.
-
•
Mechanism-specific management can improve the prognosis of INOCA patients.
Patients suffering from exertional chest pain with or without signs of ischemic heart disease but without obstructive coronary artery disease are frequently encountered in daily practice (Figure 1).1 Because the usual tests do not readily identify an atherosclerotic lesion on angiography, these patients are often dismissed as having noncardiac chest pain and may not receive sufficient explanation for their symptoms or receive adequate treatment for symptom relief. A common scenario may include continued recurrent angina, poor quality of life, repeated admission for un-necessary invasive tests or revascularization, and adverse clinical events.2, 3, 4, 5, 6 Recently, clinical interest in these patients defined as having ischemia with nonobstructive coronary arteries (INOCA) has gained significant momentum, stimulating the quest to define, classify, and treat these patients according to the endotypes of INOCA. In addition, several studies have reported a higher prevalence of microvascular dysfunction and coronary artery spasm in Asian patients, emphasizing the clinical importance of INOCA in Asian patients.7,8 The current review focuses on the clinical importance, mechanism, assessment, and management of INOCA according to its endotypes driven by the contribution of clinically relevant physiologic data acquired from specific invasive tests.
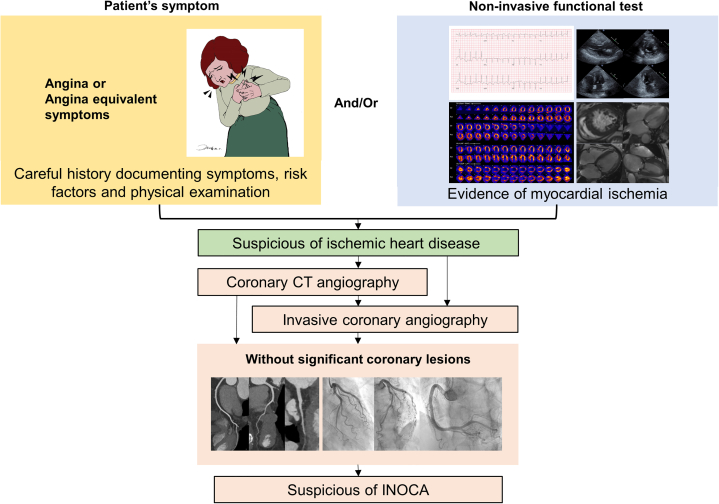
Figure 1.
Clinical Diagnostic Flow of INOCA
Ischemic heart disease is suspected when patients have symptoms and/or signs of ischemic heart disease. When there is no flow-limiting obstructive coronary artery disease, ischemia with nonobstructive coronary arteries (INOCA) should be suspected. CT = computed tomography.
Clinical importance of INOCA
Angina pectoris is the most common symptom of ischemic heart disease and is caused by the mismatch of demand and supply of coronary artery blood flow to the myocardium.9 Although the prevalence of INOCA has not been well-documented, the American College of Cardiology National Cardiovascular Data Registry reported that only 37.6% of patients had obstructive coronary artery diseases, and 39.2% of patients were without evidence of coronary artery disease among about 400,000 patients with suspected ischemic heart disease.1 More recently, a post hoc analysis of the ISCHEMIA (International Study of Comparative Health Effectiveness With Medical and Invasive Approaches) trial reported that 13% of patients were without obstructive coronary artery disease even though these patients had evidence of moderate to severe ischemia according to noninvasive tests.10 These patients often suffer from repeated chest pain that causes hospital readmission for invasive tests, leading to increased medical burden and cost. It is reported that about 50% of patients with nonobstructive coronary arteries experienced recurrent chest pain and impairment in functional capacity and quality of life.11, 12, 13 A previous study reported that South Asians with heart failure were more frequently associated with impaired macrovascular and microvascular endothelial dysfunctions than others,8 and other studies reported a higher prevalence of variant angina in Japanese cohorts than in White patients.14,15 A retrospective cohort from Denmark with 11,223 patients showed that symptomatic patients with normal coronary arteries had a 3-fold higher risk for rehospitalization and a 2.3-fold higher risk of repeat angiography.4 The WISE (Women’s Ischemia Syndrome Evaluation) study also documented the increased medical costs of caring for patients with INOCA.16 A recent meta-analysis reported that approximately half of the nonobstructive coronary artery disease patients were confirmed to have coronary microvascular disease and/or coronary artery spasm, indicating that a substantial number of INOCA patients are underdiagnosed in daily clinical practice.17
Even though previous studies have reported heterogeneous results on the prognosis of INOCA because of its various endotypes, it is evident that INOCA is not a benign disease. A recent meta-analysis of 54 studies with 35,039 INOCA patients reported a pooled incidence of all-cause death and nonfatal myocardial infarction of 0.98 per 100 person-years, which was higher than that of a similarly aged North American general population.18 In a retrospective cohort study from eastern Denmark, INOCA was associated with a 1.3- to 1.8-fold higher risk of major adverse cardiovascular events, including cardiovascular death, myocardial infarction, stroke or heart failure, and all-cause death.6 More specifically, microvascular angina with impaired coronary flow reserve (CFR) increased the risk of adverse cardiac events, including all-cause death, target vessel myocardial infarction, and clinically driven target vessel revascularization, about 4.0-fold.19 A prospective registry from Japan also reported a 6% rate of major adverse cardiac events in patients with vasospastic angina during a median follow-up period of 32 months.20
Coronary Circulation and Endotypes of INOCA
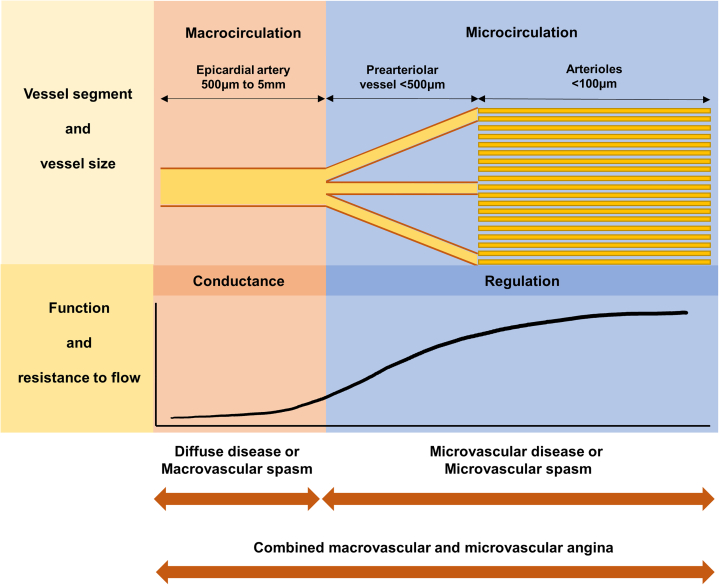
The coronary arterial system consists of epicardial coronary arteries, prearterioles, and arterioles with different sizes, distinct functions, and uninterrupted borders (Figure 2).21 Myocardial ischemia from the mismatch of demand and supply of coronary artery blood flow to the myocardium can originate from any part of this coronary arterial system (Table 1).9 The epicardial coronary artery is the most proximal compartment of the system with a diameter >500 μm and acts as a conduit for coronary blood flow with little resistance. The intermediate compartment is composed of prearteriolar vessels; they have a diameter of 500 to 100 μm and are characterized by measurable pressure drops along their path. This compartment maintains the coronary pressure at the proximal end of arterioles within a narrow range in the variations in flow and pressure. The distal compartment is represented by intramural arterioles with a diameter <100 μm and also generates a considerable pressure drop. This part plays a key role in matching the demand and supply of coronary artery blood flow to the myocardium. Prearteriolar vessels and arterioles compose the coronary microcirculation.
Figure 2.
Coronary Anatomy and Possible Causes of INOCA
The coronary arterial system is composed of 3 compartments: epicardial coronary arteries, prearterioles, and arterioles. Each compartment has different sizes and functions with uninterrupted borders. Myocardial ischemia can originate from any part of this coronary arterial system. INOCA = ischemia with non-obstructive coronary arteries.
Table 1.
Endotypes of INOCA
| Endotypes | Features | Diagnosis |
|---|---|---|
| Coronary microvascular disease | Structural and/or functional abnormalities in the microvascular system A limitation in the vasodilatory ability and absolute conductance ability of the microvascular system Associated with risk factors of cardiovascular disease, ventricular hypertrophy, or cardiomyopathies |
Based on invasive physiologic assessment
|
| Epicardial vasospastic angina | Hyper-reactive response of the epicardial coronary artery segment to vasoconstrictive stimuli | Based on provocation test using ergonovine or acetylcholine
|
| Microvascular vasospastic angina | Spasm of vascular smooth muscle cells in prearteriolar vessels and arterioles | Based on the provocation test using acetylcholine
|
| Masked diffuse disease | Coronary angiography can underestimate diffuse coronary atherosclerosis. Invasive physiologic assessment and/or intravascular coronary imaging can reveal hidden coronary atherosclerosis. |
Based on invasive physiologic assessment
|
CFR = coronary flow reserve; ECG = electrocardiogram; FFR = fractional flow reserve; HMR = hyperemic microvascular resistance; IMR = index of microcirculatory resistance; INOCA = ischemia with nonobstructive coronary artery disease; NHPR = nonhyperemic pressure ratio.
Coronary microvascular disease
Although obstructive epicardial coronary artery disease is the most common cause of myocardial ischemia, not all visually obstructive lesions cause flow limitation to the myocardium. Therefore, in patients with non–flow-limiting coronary lesions, myocardial ischemia or angina can be caused by abnormalities in coronary microvasculature. The abnormalities in the microvascular system can be explained by 2 mechanisms: structural and functional abnormalities, which can occur independently or concomitantly. The possible causes of structural microvascular disease are luminal narrowing of microvessels by medial wall or intimal thickening, luminal obstruction caused by thromboembolism, capillary rarefaction, extrinsic vascular compression, and vascular wall infiltration.21,22 These microvasculature abnormalities, which are associated with the risk factors of cardiovascular disease, underlying ventricular hypertrophy, or underlying cardiomyopathies, can cause a limitation in the vasodilatory ability and absolute conductance ability of the microvascular system, thereby reducing blood and oxygen supply to the myocardium.21, 22, 23 Impaired vasodilatory ability is known to be associated with endothelium-dependent and/or endothelium-independent mechanisms.21,23,24
Vasospastic angina
Epicardial coronary artery spasm, also known as variant angina or vasospastic angina, is characterized by resting chest pain not associated with increased myocardial oxygen demand, chest pain with diurnal variation frequently at night or early morning, and a prompt response to nitroglycerin.7,25 Compared with Western countries, the incidence of epicardial coronary artery spasm is reported to be higher in Asian countries.25, 26, 27, 28 Epicardial coronary artery spasm is associated with a hyper-reactive response of the epicardial coronary artery segment to vasoconstrictive stimuli.29 Non–endothelial-dependent contraction of vascular smooth muscle cells has been consistently demonstrated in patients with epicardial coronary artery spasm, but endothelial dysfunction is also associated with epicardial coronary artery spasm.7
Vasospastic angina can also be caused by coronary microvascular spasm, which is associated with the spasm of vascular smooth muscle cells in prearteriolar vessels and arterioles.30 The potential mechanisms of microvascular spasm are an increased release of vasoconstrictive substances, increased susceptibility of vascular smooth muscle cells, or an abnormal increase of sympathetic tone.22 Both epicardial and microvascular spasms can occur simultaneously.31
Masked diffuse disease
Even in INOCA patients, the presence of coronary atherosclerosis is common in intravascular imaging studies.32,33 An intravascular ultrasound substudy of the WISE study reported that 79% of patients had coronary atherosclerosis with a percent atheroma volume of 27%, whereas coronary angiography demonstrated only 30% of patients had minimal coronary artery disease among 100 women with nonobstructive coronary artery disease.33 Because most of these lesions showed positive remodeling and a preserved lumen size, diffuse coronary atherosclerosis can be unrevealed or underestimated by coronary angiography. When clinically suspected, the use of intracoronary imaging or invasive physiologic studies can be helpful in assessing the presence and functional significance of these masked diffuse diseases.32
Diagnosis of INOCA
Identifying patients with INOCA is not simple because there are multiple causes of chest pain and myocardial ischemia. Therefore, careful and detailed history taking, the evaluation of risk factors, and physical examination are prerequisites for discriminating the noncardiac origin of chest pain. If a noncardiac origin of chest pain is suspected, proper further evaluation is needed to exclude the diagnosis of ischemic heart disease. When the noncardiac cause of chest pain is excluded, the evidence of myocardial ischemia should be evaluated. Typical symptoms, ischemic electrocardiograms, impaired perfusion identified by myocardial perfusion imaging, and stress-induced regional wall motion abnormality are clinical surrogate markers for myocardial ischemia. Because the diagnosis of INOCA requires both myocardial ischemia and no obstruction in epicardial coronary arteries, defining myocardial ischemia represents the first step in the diagnosis of INOCA (Figure 1).
Noninvasive tests for evaluating INOCA
Various modalities to evaluate the presence of myocardial ischemia include an exercise electrocardiogram, resting or stress echocardiography, stress nuclear myocardial perfusion imaging, and cardiac magnetic resonance.34 Because current noninvasive functional tests rely on detecting large regional differences in myocardial perfusion or regional wall motion abnormalities in the left ventricle, these tests are hampered in their ability to diagnose coronary microvascular disease that can affect the whole myocardium or vasospastic angina that occurs in a given specific situation.25,35,36 In addition, most of the noninvasive functional tests cannot provide anatomical information, and anatomical assessment to exclude obstructive coronary artery diseases is needed to diagnose INOCA.
Coronary computed tomography angiography (CCTA) is a representative noninvasive diagnostic tool that can detect significant coronary artery stenoses and coronary atherosclerotic plaque.37, 38, 39 Its negative predictive value for excluding significant plaque or coronary stenosis is very high.40 However, the presence of significant stenosis in CCTA does not always mean the presence of ischemia-causing stenosis.41,42 Therefore, it is important to recognize that the presence of epicardial stenosis or atherosclerotic plaque on CCTA does not exclude the possibility of INOCA.
Invasive coronary angiography and physiologic tests to diagnose coronary microvascular disease
Invasive coronary angiography is the current gold standard method for evaluation and treatment planning for obstructive coronary artery disease.34,43 Invasive coronary angiography can intuitively provide information on microvascular dysfunction by showing slow coronary flow.44 However, invasive coronary angiography alone cannot provide adequate information regarding whether coronary artery stenoses cause flow limitation or objective evidence of microvascular dysfunction. Furthermore, the diagnosis of coronary vasospasm generally requires provocation testing.
Comprehensive physiologic assessment using a pressure sensor guidewire is needed to discriminate myocardial ischemia caused by epicardial coronary artery lesions and coronary microvascular disease (Figure 3, Table 2). Fractional flow reserve (FFR) or nonhyperemic pressure ratio (NHPR) is a standard invasive method to define ischemia-causing epicardial coronary artery lesions.34,43,45,46 These indexes estimate flow reduction caused by the epicardial coronary artery lesions, and FFR ≤0.80 and NHPR ≤0.89 indicate flow-limiting or ischemia-causing epicardial coronary artery stenosis. If there is evidence of flow-limiting epicardial coronary artery stenosis, careful pull back tracings help physicians in their judgment of plaque distribution, along with the coronary artery, whether it is focal or diffuse. Therefore, the first step in evaluating INOCA during invasive coronary angiography is detecting the flow-limiting epicardial coronary artery stenosis and assessing its disease pattern using FFR or NHPR. Intracoronary imaging studies can also reveal hidden diffuse coronary atherosclerosis (Figure 4A). Recently, quantitative flow ratio, a 3-dimensional quantitative coronary angiography–based computation of FFR, has been proposed, which also can help to define the degree and disease pattern of flow-limiting epicardial coronary artery disease.47,48
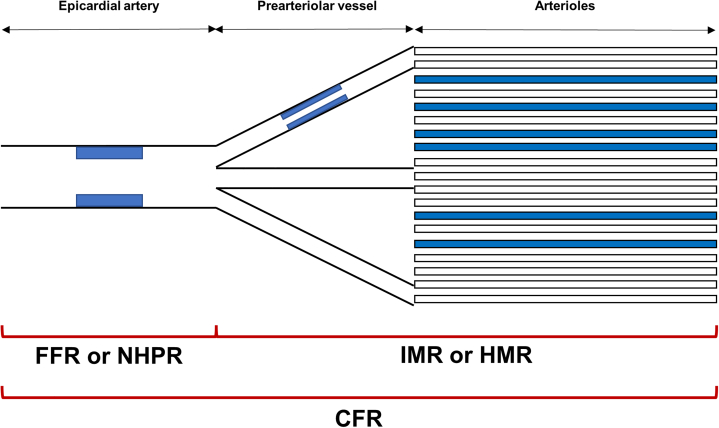
Figure 3.
Schematic Concept of Invasive Physiologic Indexes
Invasive physiologic indexes are helpful in identifying the presence of myocardial ischemia and discriminating the mechanisms of myocardial ischemia. Fractional flow reserve (FFR) or nonhyperemic pressure ratio (NHPR) reflects the degree of flow limitation caused by epicardial coronary artery lesions, and the index of microcirculatory resistance (IMR) or hyperemic microvascular resistance (HMR) represents the status of the microcirculatory system. Coronary flow reserve (CFR) is influenced by both macrovascular and microvascular disease status.
Table 2.
Invasive Physiologic Indexes for Evaluating INOCA
| Physiologic Index | Definition | Cutoff Value | Features |
|---|---|---|---|
| FFR | Ratio of distal coronary pressure to aortic pressure during hyperemia | ≤0.80 | Reflecting disease burden of epicardial coronary artery |
| NHPR | Ratio of distal coronary pressure to aortic pressure during the resting state | ≤0.89 | Reflecting disease burden of epicardial coronary artery Several NHPRs: instantaneous wave-free ratio, resting full cycle ratio, and diastolic pressure ratio. |
| CFR | Ratio of hyperemic coronary flow and resting coronary flow | <2.0-2.5 | Reflecting both epicardial coronary artery disease and microvascular dysfunction |
| IMR | Distal coronary artery pressure multiplied by hyperemic mean transit time | >25 U | Microvascular-specific index |
| HMR | Ratio of maximal coronary flow velocity to distal coronary artery pressure during hyperemia | >2.5 mm Hg/cm/s | Microvascular-specific index |
INOCA = ischemia with nonobstructive coronary arteries.
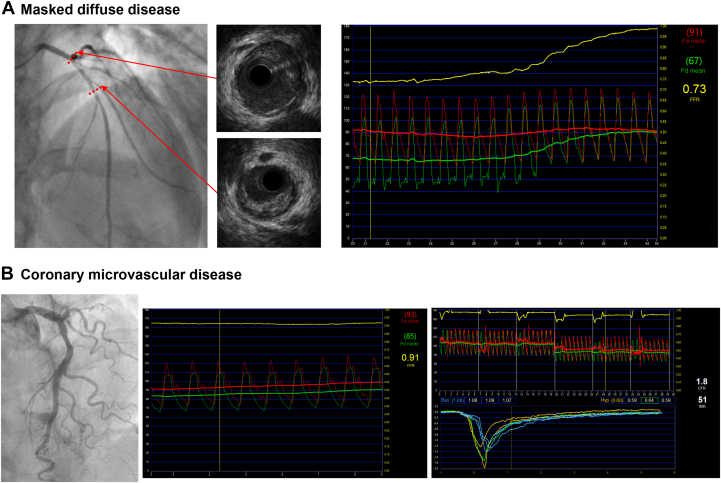
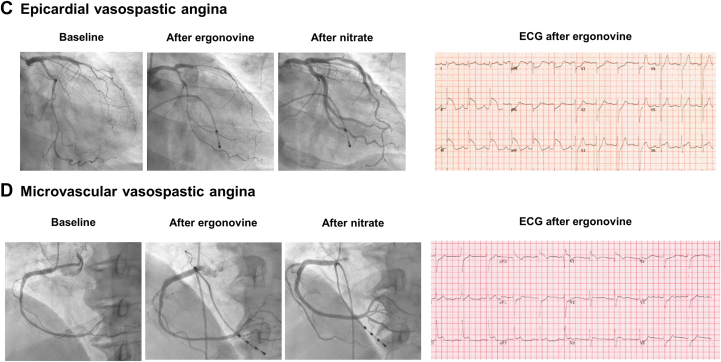
Figure 4.
Representative Cases for INOCA
(A) Masked diffuse disease. Invasive coronary angiography showed an insignificant coronary lesion at the proximal left anterior descending (LAD) artery. However, FFR was 0.73 with a gradual step-up at pressure pull back tracing. Intracoronary ultrasound showed diffuse atherosclerotic plaque at the proximal LAD. (B) Coronary microvascular disease. Invasive coronary angiography showed insignificant epicardial coronary stenosis. FFR was 0.91 at the distal LAD artery, but CFR was 1.8 and IMR was 51 U. (C) Epicardial vasospastic angina. Baseline angiography showed spastic coronary arteries. After intravenous ergonovine infusion, the patient complained of severe chest pain, and significant coronary spasms (>90% constriction) occurred in the LAD and right coronary arteries, whereas the electrocardiogram showed significant ST-segment elevation. (D) Microvascular vasospastic angina. The ergonovine provocation test showed diffuse insignificant epicardial artery constriction (<90%). However, the patient complained of severe chest pain, and the electrocardiogram showed significant ST-segment elevation at inferior leads. Abbreviations as in Figures 2 and 3.
After excluding the presence of flow-limiting obstructive coronary artery disease, the assessment of microvascular function is needed to define coronary microvascular disease (Figures 3 and 4B, Tables 1 and 2). CFR is an index of how coronary blood flow can be increased during a hyperemic state compared with a resting state, and it is affected by both epicardial coronary artery stenosis and microvascular function.46,49, 50, 51 CFR can be measured in a cardiac catheterization laboratory using a Doppler wire or a pressure-temperature sensor-equipped guidewire.46,52, 53, 54, 55 Using the Doppler wire, CFR can be obtained as the ratio of hyperemic average peak coronary flow velocity to resting average peak coronary flow velocity.51,55 The thermodilution technique is used to calculate CFR via the saline bolus transit time (Tmn), which is a surrogate marker of coronary blood flow. Thermodilution curves are obtained by 3 injections of 3 to 4 mL room temperature saline during the resting state and hyperemic state to derive the mean Tmn. CFR is a ratio of the resting Tmn and the hyperemic Tmn.49,50 CFR ≤2.0 or 2.5 indicates the presence of flow-limiting stenosis, microvascular dysfunction, or both.52, 53, 54, 55 Therefore, CFR represents the presence of microvascular dysfunction in the absence of flow-limiting epicardial disease.
The thermodilution technique can derive a more microvascular-specific index, the index of microcirculatory resistance (IMR) (Figure 3, Table 2). The uncorrected IMR is defined as distal coronary artery pressure multiplied by hyperemic Tmn. In the presence of significant epicardial stenosis, collateral flow is substantial, and this leads to a decrease in coronary flow and an increase in distal coronary pressure; therefore, uncorrected IMR can overestimate the microvascular resistance.56 To correct this phenomenon, Yong’s formula is often used; the corrected IMR with this formula is calculated as: proximal aortic pressure × Tmn × ([1.35 × distal coronary artery pressure/proximal aortic pressure] − 0.32).56 An IMR value >25 is considered to indicate the presence of microvascular dysfunction.57 In the international registry study, patients with low CFR and high IMR showed an increased risk of all-cause death, any myocardial infarction, and any revascularization (HR: 2.873; 95% CI: 1.476-5.594; P = 0.002) compared with those with high CFR and low IMR among high FFR patients.58 Another microvascular-specific index is hyperemic microvascular resistance (HMR).59, 60, 61 HMR is defined as the ratio between the distal coronary artery pressure and maximal coronary flow velocity during hyperemia (Figure 3, Table 2). Previous studies reported that HMR >2.5 mm Hg/cm/s has a good predictive value for microvascular disease.61,62
Provocation test to diagnose vasospastic angina
Even though invasive coronary angiography with comprehensive physiologic assessment may help physicians to diagnose coronary microvascular disease, it cannot provide information on vasospastic angina, another endotype of INOCA (Table 1). If physicians cannot find the specific mechanism of INOCA based on a comprehensive invasive physiologic assessment or if there are specific features of vasospastic angina, such as resting chest pain, diurnal variation, or a prompt response to nitroglycerin, a provocation test is needed to diagnose vasospastic angina.25 The acetylcholine provocation test and the ergonovine provocation test during coronary angiography are the most widely used methods to confirm the presence of coronary artery spasm (Figure 4C, Table 1).63,64 Acetylcholine acts on the muscarinic cholinergic receptors in vascular smooth muscle cells25,63,65 and ergonovine on the serotonin receptors in vascular smooth muscle cells.25,63 Both have high sensitivity and specificity to confirm the presence of epicardial vasospasm.25,28,63,65 Because these 2 drugs use different mediators, there is a possibility of different coronary responses to the drugs. Previous studies reported that spasms caused by acetylcholine are distal and diffuse, whereas those caused by ergonovine are proximal and focal.65 In addition to these different patterns of spasms according to the drugs, different test results between acetylcholine and ergonovine were also reported.65 Because of this, the supplementary use of both drugs can be useful when vasospastic angina is strongly suspected, but the provocation test is negative with 1 drug.
Microvascular vasospastic angina can be diagnosed with acetylcholine infusion during coronary angiography, and it occurs with a lower dose of acetylcholine compared with that of epicardial coronary spasm.30 When angina and ischemic electrocardiographic changes (ST-segment depression or elevation ≥0.1 mV) in at least 2 contiguous leads occur after acetylcholine infusion without significant epicardial coronary artery constriction (<90%), the presence of microvascular spasm can be diagnosed (Table 1).44,64 Epicardial vasospastic angina and microvascular vasospastic angina can occur concomitantly,31 and their coexistence can be confirmed in cases with microvascular vasospasm occurring with a lower dose of acetylcholine than epicardial artery spasm. Recently, the acetylcholine rechallenge test was introduced as a novel method to define coexisting epicardial and microvascular vasospasm.66 When there is transient total or subtotal coronary artery occlusion (>90% constriction) after intracoronary acetylcholine infusion, intracoronary nitroglycerin is injected, and intracoronary acetylcholine is reinfused after 3 minutes to confirm the microvascular vasospasm. Recently, the application of an intracoronary ergonovine test to diagnose microvascular spasms was also reported (Figure 4D).67 Sueda and Sakaue67 defined microvascular vasospasm as <75% stenosis, usual chest symptoms, and ischemic electrocardiographic changes during the ergonovine provocation test; they found 12 patients (2%) among 505 patients with suspected vasospastic angina. Even though there can be complications with provocation tests, such as refractory spasm by provocation test or fatal arrhythmia,65 a recent meta-analysis reported its safety during daily practice.68 Takahashi et al68 reported that major complications, including death, ventricular fibrillation or ventricular tachycardia, myocardial infarction, and shock requiring resuscitation, occurred in 0.5% of the pooled 12,585 patients without any reports of deaths.
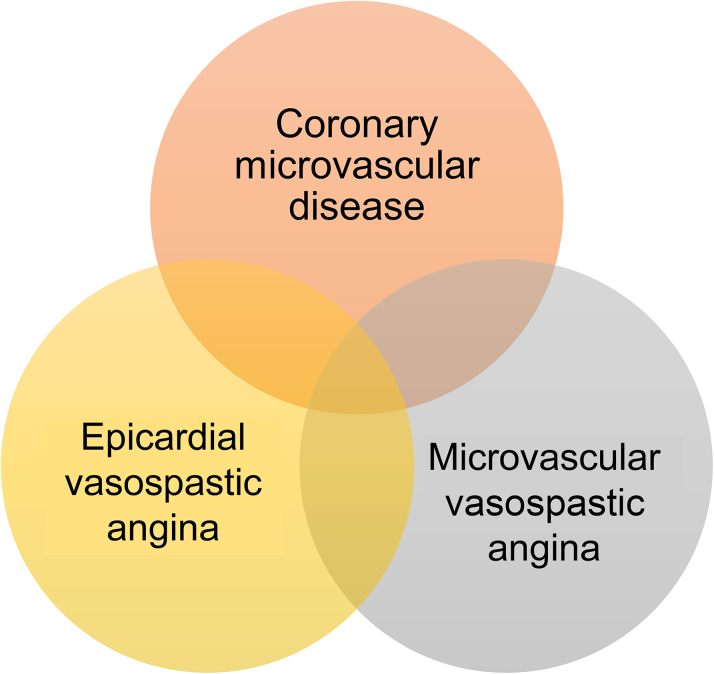
Diagnostic flow of INOCA
Recent studies reported that multiple mechanisms could coexist in patients with INOCA (Figure 5). Feenstra et al69 reported that endothelial dysfunction was prevalent in patients with vasospastic angina and dilatory microvascular dysfunction. Seitz et al66 reported that concomitant epicardial artery vasospasm and microvascular vasospasm are more common than previously reported. Furthermore, previous studies reported that comprehensive physiologic assessment is helpful not only in the diagnosis of INOCA but also in risk stratification.19,52 Therefore, when INOCA is suspected based on symptoms and/or noninvasive tests, a systematic approach to diagnose INOCA and define underlying mechanisms is needed. After the careful assessment of the presence of obstructive coronary artery disease based on coronary angiography with or without intracoronary imaging or physiologic studies, comprehensive physiologic tests are needed to define the coronary microvascular disease. After these tests, provocation tests are helpful in finding the concomitant vasospastic angina (Central Illustration).
Figure 5.
Occurrence and Overlap of INOCA Endotypes
There are several endotypes of ischemia with nonobstructive coronary arteries (INOCA), and multiple mechanisms can coexist in patients with INOCA.
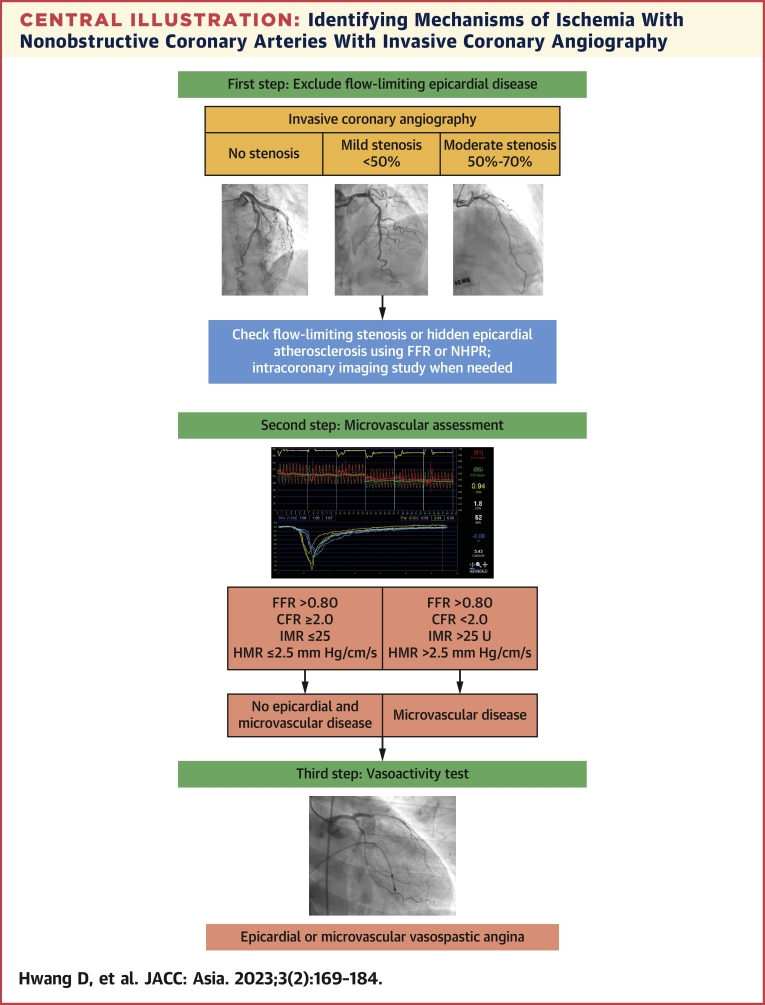
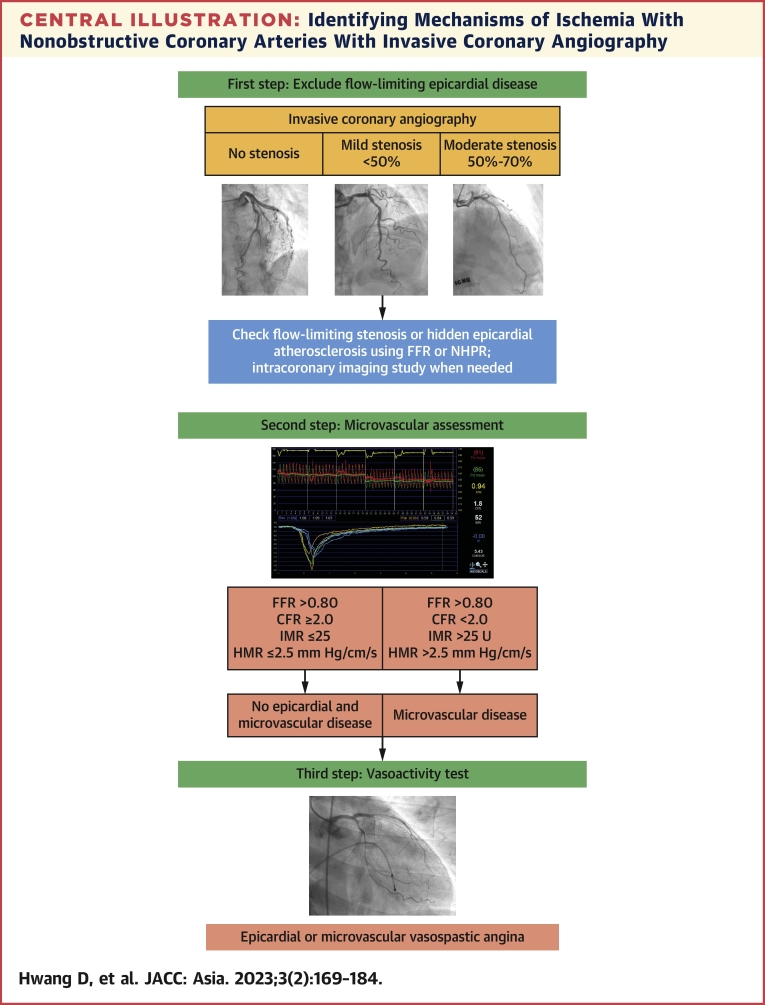
Central Illustration.
Identifying Mechanisms of Ischemia With Nonobstructive Coronary Arteries With Invasive Coronary Angiography
A systematic approach to diagnose ischemia with nonobstructive coronary arteries (INOCA) and define the underlying mechanisms is presented. The first step is excluding flow-limiting epicardial coronary artery disease using invasive coronary angiography. Invasive physiologic assessment using fractional flow reserve (FFR) or nonhyperemic pressure ratio (NHPR) and/or invasive coronary imaging is helpful. After excluding the presence of ischemia-causing epicardial lesions, comprehensive physiologic assessments should be performed to identify the microvascular dysfunction. Finally, provocation tests are helpful in the diagnosis of vasospastic angina. HMR = hyperemic microvascular resistance; IMR = index of microvascular resistance.
Management of INOCA
To date, there is no standard evidence-based treatment of INOCA because of its heterogeneous mechanisms and the lack of well-designed clinical trials. Because the underlying mechanisms in each endotype of INOCA are different, a patient-centered treatment strategy based on the mechanism of INOCA should be recommended. The CorMicA (Coronary Microvascular Angina) study evaluated the benefits of invasive coronary functional tests in INOCA patients.70 The investigator performed invasive diagnostic tests to discriminate the underlying mechanisms of angina in INOCA patients and applied specific treatment strategies according to the test results. Briefly, if there was evidence of coronary microvascular disease, aspirin, statin, and angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) were considered as a baseline therapy, and a beta-blocker was considered as first-line therapy and a calcium-channel blocker as second-line therapy for antianginal effects. In the case of vasospastic angina, a calcium-channel blocker was considered as first-line therapy and nitrates as second-line therapy. They reported that stratified management in INOCA patients leads to significant and sustained angina improvement and quality of life at 1 year compared with standard care of patients with guideline-directed medical therapy and antianginal treatment depending on the physician’s discretion.70
Coronary microvascular disease
Coronary microvascular disease is characterized by the impaired vasodilatory function of the microvascular system, and its causes are heterogeneous.21 Conventional cardiovascular risk factors, including hypertension, diabetes, hypercholesterolemia, obesity, and smoking, are associated with coronary anatomical and functional microvascular disease.23 Optimal management of these cardiovascular risk factors may prevent the progression of microvascular disease with or without improving microvascular vasodilatory function. The proper management of hypertension is known to improve CFR, and insulin sensitizer metformin has been shown to improve endothelial function.71,72 Statins may be beneficial because of their pleiotropic effects improving endothelial function.73,74 Weight reduction with exercise and smoking cessation also have consistent benefits in improving CFR and clinical outcomes.75, 76, 77, 78 Therefore, the management of INOCA should start with the proper assessment and management of cardiovascular risk factors.
There are several medical treatment options for coronary microvascular disease (Table 3). An ACE inhibitor or ARB has demonstrated the improvement of microvascular vasodilatory function, exercise tolerance, and angina symptoms.79, 80, 81 In some studies, an ACE inhibitor or ARB also demonstrated reverse microvascular remodeling and regression of periarteriolar fibrosis on biopsy.81,82 Beta-blockers reduce myocardial oxygen consumption by reducing heart rate and myocardial contractility and increase the diastolic filling time. These effects reduce the number of ischemic episodes and increase the threshold of ischemic symptoms.83, 84, 85, 86 In a previous report, beta-blockers were also associated with an improvement of endothelial functions.87 Calcium-channel blockers can also be used because of their vasodilatory effects. Despite limited data regarding the effects of calcium-channel blockers on endothelial and microvascular function, their use is associated with improved exercise tolerance and symptoms.88 Nitrates can also be used to relieve angina, but their effects on microvascular disease are inconclusive and are often reported as ineffective and poorly tolerated because of their stealing effect.89 Ranolazine inhibits the late sodium current and reduces intracellular calcium levels, leading to the improvement of ventricular relaxation.90 A previous study reported symptom improvement in women with INOCA and CFR improvement with ranolazine.91 Nicorandil is a vasodilating agent via nitrate and potassium channel activation with minimal side effects.92 Other drugs, such as ivabradine, a specific Rho-kinase inhibitor, or an endothelin receptor antagonist, are also suggested as potential treatment options for treating microvascular disease.93, 94, 95 Even though there are various options for treating coronary microvascular disease, they have not been evaluated in well-designed clinical trials, and further study is warranted.
Table 3.
Currently Available Medical Treatment Options for INOCA
| Treatment Agents | Clinical Effects | Applicable Endotypes of INOCA |
||
|---|---|---|---|---|
| Coronary Microvascular Disease | Epicardial Vasospastic Angina | Microvascular Vasospastic Angina | ||
| ACE inhibitor/ARB |
|
+ | − | − |
| Beta-blocker |
|
+ | − | − |
| Nitrates |
|
− | + | ± |
| CCB |
|
± | + | + |
| Statin |
|
+ | − | − |
| Nicorandil |
|
± | + | + |
| Ranolazine |
|
+ | − | − |
| Trimetazidine |
|
+ | - | - |
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; CCB = calcium-channel blocker; INOCA = ischemia with nonobstructive coronary arteries.
Vasospastic angina
Vasospastic angina is characterized by the spasm of vascular smooth muscle cells in the epicardial coronary artery or microvasculature, including the prearteriolar vessels and arterioles. Therefore, vasodilating drugs, such as calcium-channel blockers and nitrates, are the most important treatment options for vasospastic angina (Table 3).25 Nondihydropyridine calcium-channel blockers are preferred in vasospastic angina, but dihydropyridine calcium-channel blockers also can be used in vasospastic angina.25,96,97 Long-acting nitrates are beneficial in patients with persistent symptoms with calcium-channel blockers, and short-acting nitrates are useful to relieve acute episodes of vasospasm.25,96,97 Considering the diurnal variation of vasospastic attack, prescription time can be an important issue. To prevent angina in the early morning or at midnight, medication before sleep, not after dinner, can be useful to prevent the symptom.25 In patients with refractory symptoms during treatment with calcium-channel blockers and nitrates, nicorandil, alpha-1 receptor blockers, or Rho-kinase inhibitors can be additional options for vasospastic angina.25,92,96,97 For patients with vasospastic angina, the use of beta-blockers, both selective or nonselective, should be avoided because of the possible effects on smooth muscle vasospasm with blocking beta-2 receptors.25,96,97
Most medical options for vasospastic angina have focused on epicardial coronary vasospasm, and the evidence for microvascular spasms is limited.98,99 Because the coronary vessel tone regulation by endothelium-derived relaxing factors varies according to vessel size, the effects of vasodilatory drugs on the coronary microvasculature can be diminished.99 Seitz et al99 reported that nitrates could prevent microvascular vasospasm only in 20% of patients and attenuate microvascular vasospasm in 49% of patients. Even though calcium-channel blockers and nitrates are the recommended treatment options for microvascular vasospastic angina, like epicardial vasospastic angina, different treatment strategies may be needed, considering the different underlying pathophysiologic mechanisms. However, a trial-and-error principle is still needed to find optimal, tailored medical treatment for microvascular vasospastic angina.
Future Perspectives
It is evident that the diagnosis of INOCA is often dismissed, leading to inadequate management of patients with possible INOCA. The prevalence of INOCA according to its endotypes, the proportion of concomitant mechanisms, and clinical relevance according to its mechanisms are limited. The ongoing INOCAIT (Ischemia in Patients With Non-obstructive Disease [INOCA] in Italy INOCA IT Multicenter Registry; NCT05164640), DISCOVER INOCA (Determining the Cause of Coronary Vasomotor Disorders in Patients With Ischemia and No Obstructive Coronary Artery Disease; NCT05288361), and CorCTCA (Coronary Microvascular Function and CT Coronary Angiography; NCT03477890) trials will reveal the prevalence and patterns of overlapping mechanisms and their prognosis (Table 4). However, a standard definition of INOCA and systematic diagnostic flow are still lacking, and these are needed to uniformly define the disease and classify its endotypes while considering their concomitant occurrence. Recently, there have been several efforts to establish the methods and criteria for diagnosing INOCA. The COVADIS (Coronary Vasomotor Disorders International Study) group and the Japanese Circulation Society proposed standardized diagnostic criteria for microvascular angina and vasospastic angina.44,64,100,101 However, because several different mechanisms can be concurrently present in INOCA patients, a systematic diagnostic flow to define INOCA is needed. Using future well-designed, adequately powered, population-based prospective registry data may help physicians understand INOCA regarding its prevalence according to its endotypes, distribution of overlapping mechanisms, and prognosis according to its endotypes. For adequate management of INOCA, targeting the underlying mechanisms of INOCA is needed, but a stratified management strategy and its efficacy for INOCA still remain controversial. Even though the recent CorMicA trial demonstrated the benefits of invasive diagnostic tests for discriminating the underlying mechanisms of INOCA patients in their treatments, further studies are needed to verify the underlying mechanism-guided treatment strategy. The ongoing iCorMicA (International Study of Coronary Microvascular Angina; NCT04674449) will provide an answer to this issue. Furthermore, to date, there is no disease-modifying management of INOCA (Table 4). The randomized WARRIOR (Women’s Ischemia Trial to Reduce Events in Non-Obstructive CAD; NCT03417388) trial is investigating the impact of statin and ACE inhibitor/ARB therapy on major adverse cardiovascular events in women INOCA patients (Table 4). Other potential drugs for treating INOCA, such as endothelin receptor antagonists (PRIZE [Precision Medicine With Zibotentan in Microvascular Angina; NCT04097314), are also under investigation (Table 4).
Table 4.
Ongoing Studies for Evaluating the Prevalence, Prognosis, and Management of INOCA
| Diagnosis | Diagnosis | Diagnosis |
|---|---|---|
| INOCAIT (NCT05164640) | Prospective registry | Prevalence, proportion of endotypes, and prognosis of INOCA |
| DISCOVER INOCA (NCT05288361) | Prospective registry | Prevalence, proportion of endotypes, and prognosis of INOCA |
| CorCTCA (NCT03477890) | Randomized controlled trial | Impact of invasive diagnostic tests for INOCA in classifying and managing INOCA patients |
| iCorMicA (NCT04674449) | Randomized controlled trial | Benefit of stratified management of INOCA based on invasive tests |
| WARRIOR (NCT03417388) | Randomized controlled trial | Prognostic impact of intensive statin/ACE inhibitor/ARB treatment in INOCA patients |
| PRIZE (NCT04097314) | Randomized controlled trial | Antianginal effect of zibotentan in patients with coronary slow flow phenomenon |
CorCTCA = Coronary Microvascular Function and CT Coronary Angiography; DISCOVER INOCA = Determining the Cause of Coronary Vasomotor Disorders in Patients With Ischemia and No Obstructive Coronary Artery Disease; iCorMicA = International Study of Coronary Microvascular Angina; INOCAIT = Ischemia in Patients With Non-obstructive Disease [INOCA] in Italy INOCA IT Multicenter Registry; PRIZE = Precision Medicine With Zibotentan in Microvascular Angina; WARRIOR = Women’s Ischemia Trial to Reduce Events in Non-Obstructive CAD; other abbreviations as in Table 3.
Conclusions
Patients whose symptoms are suspicious of INOCA are often dismissed and underdiagnosed in clinical practice. Considering the clinical importance of INOCA and its prognosis, this should be diagnosed adequately, and invasive physiologic assessment is mandatory to define the presence of INOCA and discriminate its underlying mechanisms. Mechanism-specific management of INOCA might be more beneficial, and ongoing studies will shed light on this issue. Further treatment options with disease-modifying drugs are under investigation; these might further enhance INOCA patients’ outcomes.
Funding Support and Author Disclosures
Dr Koo has received an institutional research grant from Abbott Vascular and Philips Volcanumber. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Patel M.R., Peterson E.D., Dai D., et al. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362(10):886–895. doi: 10.1056/NEJMoa0907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bairey Merz C.N., Shaw L.J., Reis S.E., et al. Insights from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study: part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol. 2006;47(suppl):S21–S29. doi: 10.1016/j.jacc.2004.12.084. [DOI] [PubMed] [Google Scholar]
- 3.Gulati M., Cooper-DeHoff R.M., McClure C., et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women’s Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch Intern Med. 2009;169(9):843–850. doi: 10.1001/archinternmed.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jespersen L., Abildstrom S.Z., Hvelplund A., et al. Burden of hospital admission and repeat angiography in angina pectoris patients with and without coronary artery disease: a registry-based cohort study. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0093170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jespersen L., Abildstrøm S.Z., Hvelplund A., Prescott E. Persistent angina: highly prevalent and associated with long-term anxiety, depression, low physical functioning, and quality of life in stable angina pectoris. Clin Res Cardiol. 2013;102(8):571–581. doi: 10.1007/s00392-013-0568-z. [DOI] [PubMed] [Google Scholar]
- 6.Jespersen L., Hvelplund A., Abildstrøm S.Z., et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33(6):734–744. doi: 10.1093/eurheartj/ehr331. [DOI] [PubMed] [Google Scholar]
- 7.Lanza G.A., Careri G., Crea F. Mechanisms of coronary artery spasm. Circulation. 2011;124(16):1774–1782. doi: 10.1161/CIRCULATIONAHA.111.037283. [DOI] [PubMed] [Google Scholar]
- 8.Shantsila E., Wrigley B., Shantsila A., et al. Ethnic differences in macrovascular and microvascular function in systolic heart failure. Circ Heart Fail. 2011;4(6):754–762. doi: 10.1161/CIRCHEARTFAILURE.111.962365. [DOI] [PubMed] [Google Scholar]
- 9.Ford T.J., Corcoran D., Berry C. Stable coronary syndromes: pathophysiology, diagnostic advances and therapeutic need. Heart. 2018;104(4):284–292. doi: 10.1136/heartjnl-2017-311446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reynolds H.R., Diaz A., Cyr D.D., et al. Ischemia with nonobstructive coronary arteries: insights from the ISCHEMIA trial. J Am Coll Cardiol Img. 2023;16(1):63–74. doi: 10.1016/j.jcmg.2022.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rumsfeld J.S. Health status and clinical practice: when will they meet? Circulation. 2002;106(1):5–7. doi: 10.1161/01.cir.0000020805.31531.48. [DOI] [PubMed] [Google Scholar]
- 12.Spertus J.A. Evolving applications for patient-centered health status measures. Circulation. 2008;118(20):2103–2110. doi: 10.1161/CIRCULATIONAHA.107.747568. [DOI] [PubMed] [Google Scholar]
- 13.Di Fiore D.P., Beltrame J.F. Chest pain in patients with 'normal angiography': could it be cardiac? Int J Evid Based Healthc. 2013;11(1):56–68. doi: 10.1111/1744-1609.12002. [DOI] [PubMed] [Google Scholar]
- 14.Shimokawa H., Nagasawa K., Irie T., et al. Clinical characteristics and long-term prognosis of patients with variant angina. A comparative study between western and Japanese populations. Int J Cardiol. 1988;18(3):331–349. doi: 10.1016/0167-5273(88)90052-6. [DOI] [PubMed] [Google Scholar]
- 15.Beltrame J.F., Sasayama S., Maseri A. Racial heterogeneity in coronary artery vasomotor reactivity: differences between Japanese and Caucasian patients. J Am Coll Cardiol. 1999;33(6):1442–1452. doi: 10.1016/s0735-1097(99)00073-x. [DOI] [PubMed] [Google Scholar]
- 16.Shaw L.J., Merz C.N., Pepine C.J., et al. The economic burden of angina in women with suspected ischemic heart disease: results from the National Institutes of Health--National Heart, Lung, and Blood Institute--sponsored Women's Ischemia Syndrome Evaluation. Circulation. 2006;114(9):894–904. doi: 10.1161/CIRCULATIONAHA.105.609990. [DOI] [PubMed] [Google Scholar]
- 17.Mileva N., Nagumo S., Mizukami T., et al. Prevalence of coronary microvascular disease and coronary vasospasm in patients with nonobstructive coronary artery disease: systematic review and meta-analysis. J Am Heart Assoc. 2022;11(7) doi: 10.1161/JAHA.121.023207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radico F., Zimarino M., Fulgenzi F., et al. Determinants of long-term clinical outcomes in patients with angina but without obstructive coronary artery disease: a systematic review and meta-analysis. Eur Heart J. 2018;39(23):2135–2146. doi: 10.1093/eurheartj/ehy185. [DOI] [PubMed] [Google Scholar]
- 19.Lee S.H., Shin D., Lee J.M., et al. Clinical relevance of ischemia with nonobstructive coronary arteries according to coronary microvascular dysfunction. J Am Heart Assoc. 2022;11(9) doi: 10.1161/JAHA.121.025171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takagi Y., Takahashi J., Yasuda S., et al. Prognostic stratification of patients with vasospastic angina: a comprehensive clinical risk score developed by the Japanese Coronary Spasm Association. J Am Coll Cardiol. 2013;62(13):1144–1153. doi: 10.1016/j.jacc.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 21.Camici P.G., Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356(8):830–840. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 22.Taqueti V.R., Di Carli M.F. Coronary microvascular disease pathogenic mechanisms and therapeutic options: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72(21):2625–2641. doi: 10.1016/j.jacc.2018.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crea F., Camici P.G., Bairey Merz C.N. Coronary microvascular dysfunction: an update. Eur Heart J. 2014;35(17):1101–1111. doi: 10.1093/eurheartj/eht513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuo L., Davis M.J., Chilian W.M. Longitudinal gradients for endothelium-dependent and -independent vascular responses in the coronary microcirculation. Circulation. 1995;92(3):518–525. doi: 10.1161/01.cir.92.3.518. [DOI] [PubMed] [Google Scholar]
- 25.Song J.K. Coronary artery vasospasm. Korean Circ J. 2018;48(9):767–777. doi: 10.4070/kcj.2018.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sueda S., Kohno H., Fukuda H., et al. Frequency of provoked coronary spasms in patients undergoing coronary arteriography using a spasm provocation test via intracoronary administration of ergonovine. Angiology. 2004;55(4):403–411. doi: 10.1177/000331970405500407. [DOI] [PubMed] [Google Scholar]
- 27.Hung M.Y., Hsu K.H., Hung M.J., Cheng C.W., Cherng W.J. Interactions among gender, age, hypertension and C-reactive protein in coronary vasospasm. Eur J Clin Invest. 2010;40(12):1094–1103. doi: 10.1111/j.1365-2362.2010.02360.x. [DOI] [PubMed] [Google Scholar]
- 28.Bertrand M.E., LaBlanche J.M., Tilmant P.Y., et al. Frequency of provoked coronary arterial spasm in 1089 consecutive patients undergoing coronary arteriography. Circulation. 1982;65(7):1299–1306. doi: 10.1161/01.cir.65.7.1299. [DOI] [PubMed] [Google Scholar]
- 29.Ong P., Athanasiadis A., Perne A., et al. Coronary vasomotor abnormalities in patients with stable angina after successful stent implantation but without in-stent restenosis. Clin Res Cardiol. 2014;103(1):11–19. doi: 10.1007/s00392-013-0615-9. [DOI] [PubMed] [Google Scholar]
- 30.Mohri M., Koyanagi M., Egashira K., et al. Angina pectoris caused by coronary microvascular spasm. Lancet. 1998;351(9110):1165–1169. doi: 10.1016/S0140-6736(97)07329-7. [DOI] [PubMed] [Google Scholar]
- 31.Martínez Pereyra V., Hubert A., Seitz A., Bekeredjian R., Sechtem U., Ong P. Epicardial and microvascular coronary spasm in the same patient?—acetylcholine testing pointing towards a common pathophysiological background. Coron Artery Dis. 2020;31(4):398–399. doi: 10.1097/MCA.0000000000000829. [DOI] [PubMed] [Google Scholar]
- 32.Lee B.K., Lim H.S., Fearon W.F., et al. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation. 2015;131(12):1054–1060. doi: 10.1161/CIRCULATIONAHA.114.012636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khuddus M.A., Pepine C.J., Handberg E.M., et al. An intravascular ultrasound analysis in women experiencing chest pain in the absence of obstructive coronary artery disease: a substudy from the National Heart, Lung and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) J Interv Cardiol. 2010;23(6):511–519. doi: 10.1111/j.1540-8183.2010.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gulati M., Levy P.D., Mukherjee D., et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;78(22):2218–2261. doi: 10.1016/j.jacc.2021.07.052. [DOI] [PubMed] [Google Scholar]
- 35.Galassi A.R., Crea F., Araujo L.I., et al. Comparison of regional myocardial blood flow in syndrome X and one-vessel coronary artery disease. Am J Cardiol. 1993;72(2):134–139. doi: 10.1016/0002-9149(93)90148-6. [DOI] [PubMed] [Google Scholar]
- 36.Panting J.R., Gatehouse P.D., Yang G.Z., et al. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med. 2002;346(25):1948–1953. doi: 10.1056/NEJMoa012369. [DOI] [PubMed] [Google Scholar]
- 37.Hamon M., Biondi-Zoccai G.G., Malagutti P., et al. Diagnostic performance of multislice spiral computed tomography of coronary arteries as compared with conventional invasive coronary angiography: a meta-analysis. J Am Coll Cardiol. 2006;48(9):1896–1910. doi: 10.1016/j.jacc.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 38.Achenbach S., Moselewski F., Ropers D., et al. Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography: a segment-based comparison with intravascular ultrasound. Circulation. 2004;109(1):14–17. doi: 10.1161/01.CIR.0000111517.69230.0F. [DOI] [PubMed] [Google Scholar]
- 39.Leber A.W., Knez A., von Ziegler F., et al. Quantification of obstructive and nonobstructive coronary lesions by 64-slice computed tomography: a comparative study with quantitative coronary angiography and intravascular ultrasound. J Am Coll Cardiol. 2005;46(1):147–154. doi: 10.1016/j.jacc.2005.03.071. [DOI] [PubMed] [Google Scholar]
- 40.Hoffmann U., Bamberg F., Chae C.U., et al. Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) trial. J Am Coll Cardiol. 2009;53(18):1642–1650. doi: 10.1016/j.jacc.2009.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kern M.J., Samady H. Current concepts of integrated coronary physiology in the catheterization laboratory. J Am Coll Cardiol. 2010;55(3):173–185. doi: 10.1016/j.jacc.2009.06.062. [DOI] [PubMed] [Google Scholar]
- 42.Park S.J., Kang S.J., Ahn J.M., et al. Visual-functional mismatch between coronary angiography and fractional flow reserve. J Am Coll Cardiol Intv. 2012;5(10):1029–1036. doi: 10.1016/j.jcin.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 43.Knuuti J., Wijns W., Saraste A., et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 44.Ong P., Camici P.G., Beltrame J.F., et al. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. 2018;250:16–20. doi: 10.1016/j.ijcard.2017.08.068. [DOI] [PubMed] [Google Scholar]
- 45.Lee J.M., Choi K.H., Park J., et al. Physiological and clinical assessment of resting physiological indexes. Circulation. 2019;139(7):889–900. doi: 10.1161/CIRCULATIONAHA.118.037021. [DOI] [PubMed] [Google Scholar]
- 46.Lee J.M., Doh J.H., Nam C.W., Shin E.S., Koo B.K. Functional approach for coronary artery disease: filling the gap between evidence and practice. Korean Circ J. 2018;48(3):179–190. doi: 10.4070/kcj.2017.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Collet C., Sonck J., Vandeloo B., et al. Measurement of hyperemic pullback pressure gradients to characterize patterns of coronary atherosclerosis. J Am Coll Cardiol. 2019;74(14):1772–1784. doi: 10.1016/j.jacc.2019.07.072. [DOI] [PubMed] [Google Scholar]
- 48.Dai N., Hwang D., Lee J.M., et al. Feasibility of quantitative flow ratio-derived pullback pressure gradient index and its impact on diagnostic performance. J Am Coll Cardiol Intv. 2021;14(3):353–355. doi: 10.1016/j.jcin.2020.10.036. [DOI] [PubMed] [Google Scholar]
- 49.Barbato E., Aarnoudse W., Aengevaeren W.R., et al. Validation of coronary flow reserve measurements by thermodilution in clinical practice. Eur Heart J. 2004;25(3):219–223. doi: 10.1016/j.ehj.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 50.Pijls N.H., De Bruyne B., Smith L., et al. Coronary thermodilution to assess flow reserve: validation in humans. Circulation. 2002;105(21):2482–2486. doi: 10.1161/01.cir.0000017199.09457.3d. [DOI] [PubMed] [Google Scholar]
- 51.Everaars H., de Waard G.A., Driessen R.S., et al. Doppler flow velocity and thermodilution to assess coronary flow reserve: a head-to-head comparison with [(15)O]H(2)O PET. J Am Coll Cardiol Intv. 2018;11(20):2044–2054. doi: 10.1016/j.jcin.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 52.Lee J.M., Jung J.H., Hwang D., et al. Coronary flow reserve and microcirculatory resistance in patients with intermediate coronary stenosis. J Am Coll Cardiol. 2016;67(10):1158–1169. doi: 10.1016/j.jacc.2015.12.053. [DOI] [PubMed] [Google Scholar]
- 53.Usui E., Murai T., Kanaji Y., et al. Clinical significance of concordance or discordance between fractional flow reserve and coronary flow reserve for coronary physiological indices, microvascular resistance, and prognosis after elective percutaneous coronary intervention. EuroIntervention. 2018;14(7):798–805. doi: 10.4244/EIJ-D-17-00449. [DOI] [PubMed] [Google Scholar]
- 54.Sara J.D., Widmer R.J., Matsuzawa Y., Lennon R.J., Lerman L.O., Lerman A. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. J Am Coll Cardiol Intv. 2015;8(11):1445–1453. doi: 10.1016/j.jcin.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 55.Reis S.E., Holubkov R., Lee J.S., et al. Coronary flow velocity response to adenosine characterizes coronary microvascular function in women with chest pain and no obstructive coronary disease. Results from the pilot phase of the Women’s Ischemia Syndrome Evaluation (WISE) study. J Am Coll Cardiol. 1999;33(6):1469–1475. doi: 10.1016/s0735-1097(99)00072-8. [DOI] [PubMed] [Google Scholar]
- 56.Yong A.S., Layland J., Fearon W.F., et al. Calculation of the index of microcirculatory resistance without coronary wedge pressure measurement in the presence of epicardial stenosis. J Am Coll Cardiol Intv. 2013;6(1):53–58. doi: 10.1016/j.jcin.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 57.Fearon W.F., Kobayashi Y. Invasive assessment of the coronary microvasculature: the index of microcirculatory resistance. Circ Cardiovasc Interv. 2017;10(12) doi: 10.1161/CIRCINTERVENTIONS.117.005361. [DOI] [PubMed] [Google Scholar]
- 58.Lee J.M., Choi K.H., Doh J.H., et al. Long-term patient prognostication by coronary flow reserve and index of microcirculatory resistance: International Registry of Comprehensive Physiologic Assessment. Korean Circ J. 2020;50(10):890–903. doi: 10.4070/kcj.2020.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahn S.G., Suh J., Hung O.Y., et al. Discordance between fractional flow reserve and coronary flow reserve: insights from intracoronary imaging and physiological assessment. J Am Coll Cardiol Intv. 2017;10(10):999–1007. doi: 10.1016/j.jcin.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 60.Eftekhari A., Westra J., Stegehuis V., et al. Prognostic value of microvascular resistance and its association to fractional flow reserve: a DEFINE-FLOW substudy. Open Heart. 2022;9(1) doi: 10.1136/openhrt-2022-001981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williams R.P., de Waard G.A., De Silva K., et al. Doppler versus thermodilution-derived coronary microvascular resistance to predict coronary microvascular dysfunction in patients with acute myocardial infarction or stable angina pectoris. Am J Cardiol. 2018;121(1):1–8. doi: 10.1016/j.amjcard.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Teunissen P.F., de Waard G.A., Hollander M.R., et al. Doppler-derived intracoronary physiology indices predict the occurrence of microvascular injury and microvascular perfusion deficits after angiographically successful primary percutaneous coronary intervention. Circ Cardiovasc Interv. 2015;8(3) doi: 10.1161/CIRCINTERVENTIONS.114.001786. [DOI] [PubMed] [Google Scholar]
- 63.Zaya M., Mehta P.K., Merz C.N. Provocative testing for coronary reactivity and spasm. J Am Coll Cardiol. 2014;63(2):103–109. doi: 10.1016/j.jacc.2013.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beltrame J.F., Crea F., Kaski J.C., et al. International standardization of diagnostic criteria for vasospastic angina. Eur Heart J. 2017;38(33):2565–2568. doi: 10.1093/eurheartj/ehv351. [DOI] [PubMed] [Google Scholar]
- 65.Sueda S., Kohno H., Ochi T., Uraoka T., Tsunemitsu K. Overview of the pharmacological spasm provocation test: comparisons between acetylcholine and ergonovine. J Cardiol. 2017;69(1):57–65. doi: 10.1016/j.jjcc.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 66.Seitz A., Feenstra R., Konst R.E., et al. Acetylcholine rechallenge: a first step toward tailored treatment in patients with coronary artery spasm. J Am Coll Cardiol Intv. 2022;15(1):65–75. doi: 10.1016/j.jcin.2021.10.003. [DOI] [PubMed] [Google Scholar]
- 67.Sueda S., Sakaue T. Intracoronary ergonovine testing among 505 consecutive Japanese patients with angina-like chest pain and unobstructed coronary artery disease. Heart Vessels. 2022;37(6):931–941. doi: 10.1007/s00380-021-02002-x. [DOI] [PubMed] [Google Scholar]
- 68.Takahashi T., Samuels B.A., Li W., et al. Safety of provocative testing with intracoronary acetylcholine and implications for standard protocols. J Am Coll Cardiol. 2022;79(24):2367–2378. doi: 10.1016/S0735-1097(22)03358-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Feenstra R.G.T., Boerhout C.K.M., Woudstra J., et al. Presence of coronary endothelial dysfunction, coronary vasospasm, and adenosine-mediated vasodilatory disorders in patients with ischemia and nonobstructive coronary arteries. Circ Cardiovasc Interv. 2022;15(8) doi: 10.1161/CIRCINTERVENTIONS.122.012017. [DOI] [PubMed] [Google Scholar]
- 70.Ford T.J., Stanley B., Sidik N., et al. 1-Year outcomes of angina management guided by invasive coronary function testing (CorMicA) J Am Coll Cardiol Intv. 2020;13(1):33–45. doi: 10.1016/j.jcin.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mizuno R., Fujimoto S., Saito Y., Okamoto Y. Optimal antihypertensive level for improvement of coronary microvascular dysfunction: the lower, the better? Hypertension. 2012;60(2):326–332. doi: 10.1161/HYPERTENSIONAHA.111.189209. [DOI] [PubMed] [Google Scholar]
- 72.Jadhav S., Ferrell W., Greer I.A., Petrie J.R., Cobbe S.M., Sattar N. Effects of metformin on microvascular function and exercise tolerance in women with angina and normal coronary arteries: a randomized, double-blind, placebo-controlled study. J Am Coll Cardiol. 2006;48(5):956–963. doi: 10.1016/j.jacc.2006.04.088. [DOI] [PubMed] [Google Scholar]
- 73.Ballantyne C.M., Raichlen J.S., Nicholls S.J., et al. Effect of rosuvastatin therapy on coronary artery stenoses assessed by quantitative coronary angiography: a study to evaluate the effect of rosuvastatin on intravascular ultrasound-derived coronary atheroma burden. Circulation. 2008;117(19):2458–2466. doi: 10.1161/CIRCULATIONAHA.108.773747. [DOI] [PubMed] [Google Scholar]
- 74.Ridker P.M., MacFadyen J., Libby P., Glynn R.J. Relation of baseline high-sensitivity C-reactive protein level to cardiovascular outcomes with rosuvastatin in the Justification for Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) Am J Cardiol. 2010;106(2):204–209. doi: 10.1016/j.amjcard.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 75.Carbone S., Del Buono M.G., Ozemek C., Lavie C.J. Obesity, risk of diabetes and role of physical activity, exercise training and cardiorespiratory fitness. Prog Cardiovasc Dis. 2019;62(4):327–333. doi: 10.1016/j.pcad.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 76.Olsen R.H., Pedersen L.R., Jürs A., Snoer M., Haugaard S.B., Prescott E. A randomised trial comparing the effect of exercise training and weight loss on microvascular function in coronary artery disease. Int J Cardiol. 2015;185:229–235. doi: 10.1016/j.ijcard.2015.03.118. [DOI] [PubMed] [Google Scholar]
- 77.Rooks C., Faber T., Votaw J., et al. Effects of smoking on coronary microcirculatory function: a twin study. Atherosclerosis. 2011;215(2):500–506. doi: 10.1016/j.atherosclerosis.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hambrecht R., Gielen S., Linke A., et al. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: a randomized trial. JAMA. 2000;283(23):3095–3101. doi: 10.1001/jama.283.23.3095. [DOI] [PubMed] [Google Scholar]
- 79.Pizzi C., Manfrini O., Fontana F., Bugiardini R. Angiotensin-converting enzyme inhibitors and 3-hydroxy-3-methylglutaryl coenzyme A reductase in cardiac Syndrome X: role of superoxide dismutase activity. Circulation. 2004;109(1):53–58. doi: 10.1161/01.CIR.0000100722.34034.E4. [DOI] [PubMed] [Google Scholar]
- 80.Pauly D.F., Johnson B.D., Anderson R.D., et al. In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, and microvascular dysfunction, angiotensin-converting enzyme inhibition is associated with improved microvascular function: a double-blind randomized study from the National Heart, Lung and Blood Institute Women's Ischemia Syndrome Evaluation (WISE) Am Heart J. 2011;162(4):678–684. doi: 10.1016/j.ahj.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schwartzkopff B., Brehm M., Mundhenke M., Strauer B.E. Repair of coronary arterioles after treatment with perindopril in hypertensive heart disease. Hypertension. 2000;36(2):220–225. doi: 10.1161/01.hyp.36.2.220. [DOI] [PubMed] [Google Scholar]
- 82.Neglia D., Fommei E., Varela-Carver A., et al. Perindopril and indapamide reverse coronary microvascular remodelling and improve flow in arterial hypertension. J Hypertens. 2011;29(2):364–372. doi: 10.1097/HJH.0b013e328340a08e. [DOI] [PubMed] [Google Scholar]
- 83.Lanza G.A., Colonna G., Pasceri V., Maseri A. Atenolol versus amlodipine versus isosorbide-5-mononitrate on anginal symptoms in syndrome X. Am J Cardiol. 1999;84(7):854–856. doi: 10.1016/s0002-9149(99)00450-6. A8. [DOI] [PubMed] [Google Scholar]
- 84.Leonardo F., Fragasso G., Rossetti E., et al. Comparison of trimetazidine with atenolol in patients with syndrome X: effects on diastolic function and exercise tolerance. Cardiologia. 1999;44(12):1065–1069. [PubMed] [Google Scholar]
- 85.Lanza G.A., Crea F. Primary coronary microvascular dysfunction: clinical presentation, pathophysiology, and management. Circulation. 2010;121(21):2317–2325. doi: 10.1161/CIRCULATIONAHA.109.900191. [DOI] [PubMed] [Google Scholar]
- 86.Padro T., Manfrini O., Bugiardini R., et al. ESC Working Group on Coronary Pathophysiology and Microcirculation position paper on ‘coronary microvascular dysfunction in cardiovascular disease’. Cardiovasc Res. 2020;116(4):741–755. doi: 10.1093/cvr/cvaa003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Matsuda Y., Akita H., Terashima M., Shiga N., Kanazawa K., Yokoyama M. Carvedilol improves endothelium-dependent dilatation in patients with coronary artery disease. Am Heart J. 2000;140(5):753–759. doi: 10.1067/mhj.2000.110093. [DOI] [PubMed] [Google Scholar]
- 88.Cannon R.O., 3rd, Watson R.M., Rosing D.R., Epstein S.E. Efficacy of calcium channel blocker therapy for angina pectoris resulting from small-vessel coronary artery disease and abnormal vasodilator reserve. Am J Cardiol. 1985;56(4):242–246. doi: 10.1016/0002-9149(85)90842-2. [DOI] [PubMed] [Google Scholar]
- 89.Kaski J.C., Crea F., Gersh B.J., Camici P.G. Reappraisal of ischemic heart disease. Circulation. 2018;138(14):1463–1480. doi: 10.1161/CIRCULATIONAHA.118.031373. [DOI] [PubMed] [Google Scholar]
- 90.Hasenfuss G., Maier L.S. Mechanism of action of the new anti-ischemia drug ranolazine. Clin Res Cardiol. 2008;97(4):222–226. doi: 10.1007/s00392-007-0612-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mehta P.K., Goykhman P., Thomson L.E., et al. Ranolazine improves angina in women with evidence of myocardial ischemia but no obstructive coronary artery disease. J Am Coll Cardiol Img. 2011;4(5):514–522. doi: 10.1016/j.jcmg.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen J.W., Lee W.L., Hsu N.W., et al. Effects of short-term treatment of nicorandil on exercise-induced myocardial ischemia and abnormal cardiac autonomic activity in microvascular angina. Am J Cardiol. 1997;80(1):32–38. doi: 10.1016/s0002-9149(97)00279-8. [DOI] [PubMed] [Google Scholar]
- 93.Denardo S.J., Wen X., Handberg E.M., et al. Effect of phosphodiesterase type 5 inhibition on microvascular coronary dysfunction in women: a Women’s Ischemia Syndrome Evaluation (WISE) ancillary study. Clin Cardiol. 2011;34(8):483–487. doi: 10.1002/clc.20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shi J., Wei L. Rho kinases in cardiovascular physiology and pathophysiology: the effect of fasudil. J Cardiovasc Pharmacol. 2013;62(4):341–354. doi: 10.1097/FJC.0b013e3182a3718f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Morrow A.J., Ford T.J., Mangion K., et al. Rationale and design of the Medical Research Council's Precision Medicine with Zibotentan in Microvascular Angina (PRIZE) trial. Am Heart J. 2020;229:70–80. doi: 10.1016/j.ahj.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Picard F., Sayah N., Spagnoli V., Adjedj J., Varenne O. Vasospastic angina: a literature review of current evidence. Arch Cardiovasc Dis. 2019;112(1):44–55. doi: 10.1016/j.acvd.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 97.Matta A., Bouisset F., Lhermusier T., et al. Coronary artery spasm: new insights. J Interv Cardiol. 2020;2020 doi: 10.1155/2020/5894586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bairey Merz C.N., Pepine C.J., Shimokawa H., Berry C. Treatment of coronary microvascular dysfunction. Cardiovasc Res. 2020;116(4):856–870. doi: 10.1093/cvr/cvaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Seitz A., Gardezy J., Pirozzolo G., et al. Long-term follow-up in patients with stable angina and unobstructed coronary arteries undergoing intracoronary acetylcholine testing. J Am Coll Cardiol Intv. 2020;13(16):1865–1876. doi: 10.1016/j.jcin.2020.05.009. [DOI] [PubMed] [Google Scholar]
- 100.Guidelines for diagnosis and treatment of patients with vasospastic angina (coronary spastic angina) (JCS 2008): digest version. Circ J. 2010;74(8):1745–1762. doi: 10.1253/circj.cj-10-74-0802. [DOI] [PubMed] [Google Scholar]
- 101.Guidelines for diagnosis and treatment of patients with vasospastic angina (coronary spastic angina) (JCS 2013) Circ J. 2014;78(11):2779–2801. doi: 10.1253/circj.cj-66-0098. [DOI] [PubMed] [Google Scholar]