Key Points
Question
Is place of birth associated with the efficacy of whole-body hypothermia in neonates with hypoxic-ischemic encephalopathy (HIE) in low- and middle-income countries?
Findings
In this nested cohort study within a randomized clinical trial recruiting 408 neonates with moderate or severe HIE from 7 tertiary neonatal intensive care units in South Asia, whole-body hypothermia was not associated with reductions in brain injury measured by magnetic resonance biomarkers at age 2 weeks among neonates born at either a tertiary care center or other facilities.
Meaning
These findings do not support the use of whole-body hypothermia for neonates with HIE in low- and middle-income countries.
Abstract
Importance
The association between place of birth and hypothermic neuroprotection after hypoxic-ischemic encephalopathy (HIE) in low- and middle-income countries (LMICs) is unknown.
Objective
To ascertain the association between place of birth and the efficacy of whole-body hypothermia for protection against brain injury measured by magnetic resonance (MR) biomarkers among neonates born at a tertiary care center (inborn) or other facilities (outborn).
Design, Setting, and Participants
This nested cohort study within a randomized clinical trial involved neonates at 7 tertiary neonatal intensive care units in India, Sri Lanka, and Bangladesh between August 15, 2015, and February 15, 2019. A total of 408 neonates born at or after 36 weeks’ gestation with moderate or severe HIE were randomized to receive whole-body hypothermia (reduction of rectal temperatures to between 33.0 °C and 34.0 °C; hypothermia group) for 72 hours or no whole-body hypothermia (rectal temperatures maintained between 36.0 °C and 37.0 °C; control group) within 6 hours of birth, with follow-up until September 27, 2020.
Exposure
3T MR imaging, MR spectroscopy, and diffusion tensor imaging.
Main Outcomes and Measures
Thalamic N-acetyl aspartate (NAA) mmol/kg wet weight, thalamic lactate to NAA peak area ratios, brain injury scores, and white matter fractional anisotropy at 1 to 2 weeks and death or moderate or severe disability at 18 to 22 months.
Results
Among 408 neonates, the mean (SD) gestational age was 38.7 (1.3) weeks; 267 (65.4%) were male. A total of 123 neonates were inborn and 285 were outborn. Inborn neonates were smaller (mean [SD], 2.8 [0.5] kg vs 2.9 [0.4] kg; P = .02), more likely to have instrumental or cesarean deliveries (43.1% vs 24.7%; P = .01), and more likely to be intubated at birth (78.9% vs 29.1%; P = .001) than outborn neonates, although the rate of severe HIE was not different (23.6% vs 17.9%; P = .22). Magnetic resonance data from 267 neonates (80 inborn and 187 outborn) were analyzed. In the hypothermia vs control groups, the mean (SD) thalamic NAA levels were 8.04 (1.98) vs 8.31 (1.13) among inborn neonates (odds ratio [OR], −0.28; 95% CI, −1.62 to 1.07; P = .68) and 8.03 (1.89) vs 7.99 (1.72) among outborn neonates (OR, 0.05; 95% CI, −0.62 to 0.71; P = .89); the median (IQR) thalamic lactate to NAA peak area ratios were 0.13 (0.10-0.20) vs 0.12 (0.09-0.18) among inborn neonates (OR, 1.02; 95% CI, 0.96-1.08; P = .59) and 0.14 (0.11-0.20) vs 0.14 (0.10-0.17) among outborn neonates (OR, 1.03; 95% CI, 0.98-1.09; P = .18). There was no difference in brain injury scores or white matter fractional anisotropy between the hypothermia and control groups among inborn or outborn neonates. Whole-body hypothermia was not associated with reductions in death or disability, either among 123 inborn neonates (hypothermia vs control group: 34 neonates [58.6%] vs 34 [56.7%]; risk ratio, 1.03; 95% CI, 0.76-1.41), or 285 outborn neonates (hypothermia vs control group: 64 neonates [46.7%] vs 60 [43.2%]; risk ratio, 1.08; 95% CI, 0.83-1.41).
Conclusions and Relevance
In this nested cohort study, whole-body hypothermia was not associated with reductions in brain injury after HIE among neonates in South Asia, irrespective of place of birth. These findings do not support the use of whole-body hypothermia for HIE among neonates in LMICs.
Trial Registration
ClinicalTrials.gov Identifier: NCT02387385
This nested cohort study within a clinical trial assesses the association of whole-body hypothermia with magnetic resonance biomarkers and outcomes among neonates born at a tertiary care center vs other facilities in South Asia.
Introduction
Brain injury associated with birth depression, otherwise known as hypoxic-ischemic encephalopathy (HIE), is the most common cause of death or lifelong neurodisability among neonates born at full term,1 accounting for approximately 1 million deaths every year. More than 90% of the disease burden is in low- and middle-income countries (LMICs). In high-income countries (HICs), several clinical trials2,3,4 have reported that selective head or whole-body hypothermia reduces brain injury, death, or neurodisability at ages 18 to 24 months. These trials have included both neonates born within (inborn) or outside (outborn) a tertiary neonatal intensive care unit (NICU) with facilities for induced hypothermia, and hypothermia was found to improve clinical outcomes irrespective of place of birth.5 Unlike tertiary care centers, nontertiary care centers in LMICs often have clinicians with limited neonatal expertise in addition to limited resources and a lack of dedicated neonatal transport, which may contribute to suboptimal postresuscitation care and aggravation of the brain injury before transfer to a cooling center.6
Magnetic resonance (MR) spectroscopic biomarkers provide an objective quantitative assessment of brain injury7,8 and can quantify neuroprotection using substantially smaller samples than clinical outcome measures. In this subgroup analysis of the Hypothermia for Encephalopathy in Low- and Middle-Income Countries (HELIX) trial, we examined the association of place of birth with the efficacy of whole-body hypothermia for protection against brain injury using quantitative 3T MR biomarkers.
Methods
Study Design and Participants
HELIX was an open-label randomized clinical trial (RCT) that recruited 408 neonates with moderate or severe HIE from 7 tertiary NICUs in India, Sri Lanka, and Bangladesh between August 15, 2015, and February 15, 2019,9 with follow-up data through September 27, 2020 (the trial protocol is provided in Supplement 1, and the study flowchart is available in eFigure 1 in Supplement 2). The trial was approved by the Imperial College Research Ethics Committee and the ethics committees at all participating sites (Supplement 1). Parental informed consent was obtained for all neonates. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline for RCTs.
Neonates born at or after 36 weeks’ gestation with a birth weight of 1.8 kg or more who required continued resuscitation at age 5 minutes, had a 5-minute Apgar score less than 6, or both (or had lack of cry by age 5 minutes for home birth) were recruited if there was evidence of moderate or severe HIE on structured neurological examination performed between 1 and 6 hours after birth by a certified examiner. We excluded neonates who had no heart rate at age 10 minutes despite adequate resuscitation, those with major life-threatening congenital malformations, or those with parents who were unable to attend follow-up assessments. Neonates allocated to the hypothermia group had a controlled reduction of rectal temperatures to between 33.0 °C and 34.0 °C within 6 hours of birth that was continued for 72 hours followed by rewarming at 0.5 °C per hour using an neonate cooling and rewarming system (Tecotherm Neo; Inspiration Health Care [now International Biomedical]). The control group had rectal temperatures maintained between 36.0 °C and 37.0 °C. Neonates born at the participating tertiary NICUs with facilities for whole-body hypothermia were defined as inborn, and neonates born elsewhere (including other tertiary care centers, secondary care centers, primary care facilities, or at home) were defined as outborn. The primary outcome of the HELIX trial was death or moderate or severe disability assessed between 18 and 22 months by neurodevelopmental pediatricians masked to the allocation and neuroimaging data.9
Procedures
Before recruitment, all MR scanners were harmonized using phantom studies and adult volunteers, and spectroscopic data were acquired using previously validated cross-platform sequences that can be used in 3T scanners from all 3 common manufacturers.8 This included Magnetom Skyra 3T (Siemens Healthineers) (4 sites), Magnetom Verio 3T (Siemens Healthineers) (1 site), Philips Achieva 3T (Philips Medical Systems) (1 site), and SIGNA 3T (GE Healthcare) (1 site) MR scanners.All neonates underwent MR scans at age 2 weeks unless they died before the scan or parents declined consent.
The MR protocol (acquisition time) included 3-dimensional T1- and T2-weighted MR imaging (15 minutes) in the axial and sagittal planes, diffusion tensor imaging (DTI) (7 minutes), proton MR spectroscopic metabolite peak area ratios (PARs) (7 minutes), and metabolite absolute concentrations (13 minutes). We acquired MR spectroscopy in a single 15 × 15 × 15 mm3 voxel centered on the left thalamus, and we centrally postprocessed the pseudonymized raw MR data while masked to the treatment allocation and clinical outcomes.10 We excluded poor-quality data based on predefined criteria before data analysis to avoid any selection bias. We analyzed the MR spectroscopic data using LCModel software, version 6.3 (Stephen Provencher),11 and we analyzed the DTI data using functional MR imaging in the FMRIB Software Library, version 6.0 (FMRIB Analysis Group).12 Voxelwise statistical analysis of the fractional anisotropic (FA) data was conducted using tract-based spatial statistics. All MR images were centrally reported using a previously validated scoring system.13
Statistical Analysis
The baseline demographic and clinical characteristics of inborn and outborn neonates in the hypothermia and control groups were compared using the unpaired t test or Mann-Whitney test for continuous variables and the χ2 test or Fisher exact test for categorical variables. Race and ethnicity data were not collected because the study population was all from India, Sri Lanka, and Bangladesh (all of Asian race). Binary outcomes were compared between groups using the χ2 test, with group differences expressed as risk ratios (RRs) with 95% CIs. Ordinal logistic regression was used for the analysis of ordinal outcomes (including MR biomarkers), with the group differences expressed as odds ratios (ORs) with corresponding 95% CIs. Continuous outcomes were compared between groups using the unpaired t test. Outcomes with positively skewed distribution were log transformed for the analysis. Cox regression was used for the analysis of time of death, with survival as the outcome. The significance threshold was 2-tailed P = .05. Data were analyzed using Stata software, version 17.0 (StataCorp LLC).
Results
Study Population
Among 408 neonates, the mean (SD) gestational age was 38.7 (1.3) weeks; 267 (65.4%) were male and 141 (34.6%) were female. A total of 123 neonates were inborn and 285 were outborn. Although the baseline clinical characteristics of the hypothermia and control groups were not significantly different (Table 1), there were some differences between inborn and outborn neonates. Compared with the outborn neonates, the inborn neonates were born to older mothers (mean [SD] age, 25.7 [5.0] years vs 23.7 [4.2] years; P = .001); more of them had meconium-stained amniotic fluid (47 of 123 neonates [38.2%] vs 63 of 269 [23.4%]; P < .001), reduced fetal movements (13 of 114 neonates [11.4%] vs 11 of 221 [5.0%]; P < .001), and fetal heart rate decelerations (16 of 116 neonates [13.8%] vs 7 of 208 [3.4%]; P < .001); and more of them had instrumental or cesarean deliveries (53 of 123 neonates [43.1%] vs 70 of 283 [24.7%]; P = .01). The inborn neonates also had lower birth weight (mean [SD], 2.8 [0.5] kg vs 2.9 [0.4] kg; P = .02) and more often required intubation at birth (97 of 123 neonates [78.9%] vs 81 of 278 [29.1%]; P = .001) than the outborn neonates. The inborn neonates were also admitted to the NICU earlier (median [IQR], 30 [15-50] minutes vs 180 [123-240] minutes; P = .001) and had lower rates of seizures at randomization (75 of 122 neonates [61.5%] vs 224 of 285 [78.6%]; P = .001) than outborn neonates (eTable 1 in Supplement 2). However, the rate of severe HIE was not different between inborn and outborn groups (29 neonates [23.6%] vs 51 [17.9%]; P = .22).
Table 1. Baseline Participant Characteristics.
| Characteristic | Participants, No./total No. (%) | ||
|---|---|---|---|
| Hypothermia group | Control group | P value | |
| Inborn neonates (n = 123) | |||
| Total participants, No. | 62 | 61 | NA |
| Maternal characteristics and prenatal conditions | |||
| Age, mean (SD), ya | 26.1 (5.0) | 25.4 (5.2) | .45 |
| Booked pregnanciesb | 62/62 (100) | 60/61 (98.4) | .50 |
| Primigravida | 37/62 (59.7) | 30/61 (49.2) | .24 |
| Diabetes | 0 | 1/61 (1.6) | .50 |
| Pregnancy-induced hypertension | 5/62 (8.1) | 3/61 (4.9) | .47 |
| Thyroid disorders | 0 | 0 | >.99 |
| Complications of deliveryc | 20/62 (32.3) | 23/60 (38.3) | .48 |
| Maternal pyrexia | 2/61 (3.3) | 3/60 (5.0) | .68 |
| Rupture of membranes >24 h | 0 | 0 | >.99 |
| Meconium-stained amniotic fluid | 28/62 (45.2) | 19/61 (31.1) | .11 |
| Reduced fetal movements | 10/60 (16.7) | 3/54 (5.6) | .06 |
| Fetal heart rate decelerations | 8/58 (13.8) | 8/58 (13.8) | >.99 |
| Funisitis | 12/61 (19.7) | 9/60 (15.0) | .50 |
| Perinatal sentinel eventsc | |||
| Cord prolapse | 1/62 (1.6) | 3/61 (4.9) | .37 |
| Cord around neck | 1/62 (1.6) | 1/61 (1.6) | >.99 |
| Prolonged second stage | 1/62 (1.6) | 6/61 (9.8) | .06 |
| Obstructed labor | 1/62 (1.6) | 1/61 (1.6) | >.99 |
| Shoulder dystocia | 0 | 0 | >.99 |
| Antepartum hemorrhage | 3/62 (4.8) | 2/61 (3.3) | .66 |
| Mode of delivery | |||
| Instrumental | 8/62 (12.9) | 10/61 (16.4) | .62 |
| Prelabor cesarean | 1/62 (1.6) | 2/61 (3.3) | |
| In-labor cesarean | 19/62 (30.6) | 13/61 (21.3) | |
| Spontaneous vaginal | 34/62 (54.8) | 36/61 (59.0) | |
| Condition at birth | |||
| Cord blood pH at delivery, mean (SD)d | 6.91 (0.16) | 6.93 (0.21) | .73 |
| Apgar score at 5 min, median (IQR)e | 5 (3-5) | 4 (4-5) | .68 |
| Apgar score at 10 min, median (IQR)f | 6 (4-7) | 6 (5-7) | .84 |
| Endotracheal ventilation at birth | 48/62 (77.4) | 49/61 (80.3) | .69 |
| Neonate characteristics and conditions | |||
| Birth weight, mean (SD), gg | 2735 (447) | 2896 (464) | .05 |
| Birth weight <2 SDs | 16/62 (25.8) | 11/61 (18.0) | .30 |
| Head circumference, mean (SD), cmh | 33.9 (1.6) | 34.3 (1.9) | .31 |
| Head circumference <2 SDs | 4/62 (6.5) | 2/60 (3.3) | .68 |
| Gestational age of neonate, mean (SD), wkg | 39.0 (1.4) | 38.9 (1.6) | .91 |
| Age at admission to the NICU, median (IQR), ming | 31 (15-46) | 33 (18-50) | .85 |
| Sex | |||
| Male | 39/62 (62.9) | 41/61 (67.2) | .62 |
| Female | 23/62 (37.1) | 20/61 (32.8) | |
| Moderate encephalopathy | 44/62 (71.0) | 50/61 (82.0) | .15 |
| Severe encephalopathy | 18/62 (29.0) | 11/61 (18.0) | |
| Clinical seizures at admission | 39/62 (62.9) | 36/61 (59.0) | .66 |
| Outborn neonates (n = 285) i | |||
| Total participants, No. | 140 | 145 | NA |
| Maternal characteristics and prenatal conditions | |||
| Age, mean (SD), yj | 23.8 (4.2) | 23.8 (4.3) | .94 |
| Booked pregnanciesb | 134/138 (97.1) | 130/144 (90.3) | .03 |
| Primigravida | 81/139 (58.3) | 87/145 (60.0) | .77 |
| Diabetes | 1/140 (0.7) | 0 | .49 |
| Pregnancy-induced hypertension | 4/140 (2.9) | 1/145 (0.7) | .16 |
| Thyroid disorders | 0 | 1/145 (0.7) | >.99 |
| Complications of deliveryc | 18/130 (13.8) | 17/137 (12.4) | .73 |
| Maternal pyrexia | 1/128 (0.8) | 2/139 (1.4) | >.99 |
| Rupture of membranes >24 h | 1/126 (0.8) | 3/136 (2.2) | .35 |
| Meconium-stained amniotic fluid | 32/130 (24.6) | 31/139 (22.3) | .65 |
| Reduced fetal movements | 6/109 (5.5) | 5/112 (4.5) | .72 |
| Fetal heart rate decelerations | 6/106 (5.7) | 1/102 (1.0) | .12 |
| Funisitis | 25/120 (20.8) | 20/125 (16.0) | .33 |
| Perinatal sentinel eventsc | |||
| Cord prolapse | 3/140 (2.1) | 3/145 (2.1) | .96 |
| Cord around neck | 1/140 (0.7) | 5/145 (3.4) | .21 |
| Prolonged second stage | 1/140 (0.7) | 3/145 (2.1) | .62 |
| Obstructed labor | 1/140 (0.7) | 2/145 (1.4) | >.99 |
| Shoulder dystocia | 1/140 (0.7) | 0 | .49 |
| Antepartum hemorrhagek | 3/140 (2.1) | 0 | .07 |
| Mode of delivery | |||
| Instrumental | 13/139 (9.4) | 9/144 (6.3) | .29 |
| Prelabor cesarean | 0 | 0 | |
| In-labor cesarean | 27/139 (19.4) | 21/144 (14.6) | |
| Spontaneous vaginal | 99/139 (71.2) | 114/144 (79.2) | |
| Condition at birth | |||
| Cord blood pH at delivery, mean (SD)l | 7.02 (0.41) | 7.06 (0.21) | .79 |
| Apgar score at 5 min, median (IQR)m | 5 (4-6) | 5 (4-6) | .09 |
| Apgar score at 10 min, median (IQR)n | 5 (4-7) | 6 (4-7) | .39 |
| Endotracheal ventilation at birth | 41/138 (29.7) | 40/140 (28.6) | .84 |
| Neonate characteristics and conditions | |||
| Birth weight, mean (SD), g | 2892 (444) | 2956 (452) | .23 |
| Birth weight <2 SDs | 20/140 (14.3) | 16/145 (11.0) | .41 |
| Head circumference, mean (SD), cmo | 34.3 (1.4) | 34.3 (1.4) | .59 |
| Head circumference <2 SDs | 4/139 (2.9) | 4/145 (2.8) | >.99 |
| Gestational age of neonate, mean (SD), wkp | 38.8 (1.2) | 39.0 (1.1) | .18 |
| Age at admission to the NICU, median (IQR), min | 180 (120-240) | 189 (139-255) | .16 |
| Sex | |||
| Male | 93/140 (66.4) | 94/145 (64.8) | .78 |
| Female | 47/140 (33.6) | 51/145 (35.2) | |
| Moderate encephalopathy | 117/140 (83.6) | 117/145 (80.7) | .53 |
| Severe encephalopathy | 23/140 (16.4) | 28/145 (19.3) | |
| Clinical seizures at admission | 110/140 (78.6) | 114/145 (78.6) | .99 |
Abbreviations: NA, not applicable; NICU, neonatal intensive care unit.
Among 60 participants in the hypothermia group and 57 in the control group.
Booked pregnancies had antenatal follow-up visits at the recruiting hospital or any other health-care facility.
Not mutually exclusive.
Among 16 participants in the hypothermia group and 17 in the control group.
Among 61 participants in the hypothermia group and 61 in the control group.
Among 52 participants in the hypothermia group and 46 in the control group.
Among 62 participants in the hypothermia group and 61 in the control group.
Among 62 participants in the hypothermia group and 60 in the control group.
Born at other tertiary medical college hospitals (65 neonates), secondary district hospitals (124 neonates), primary care centers (44 neonates), private hospitals (40 neonates), unnamed hospitals (2 neonates), or home (10 neonates) and transferred to the cooling center within 6 hours of birth.
Among 139 participants in the hypothermia group and 139 in the control group.
Three participants had abruption.
Among 6 participants in the hypothermia group and 7 in the control group.
Among 76 participants in the hypothermia group and 74 in the control group.
Among 13 participants in the hypothermia group and 21 in the control group.
Among 139 participants in the hypothermia group and 145 in the control group.
Among 140 participants in the hypothermia group and 144 in the control group.
Temperature profiles are provided in Figure 1 and eFigure 2 in Supplement 2. Among 202 neonates, the target rectal temperature of 34 °C or lower was achieved by 6 hours in 144 neonates (71.3%), between 6 and 7 hours in 39 (19.3%), between 7 and 8 hours in 12 (5.9%), between 8 and 9 hours in 4 (2.0%), and between 9 and 10 hours in 2 (1.0%). Among the 62 inborn neonates in the hypothermia group, the target rectal temperature of 34 °C or lower was achieved by 6 hours in 46 neonates (74.2%), between 6 and 7 hours in 12 (19.4%), and between 7 and 8 hours in 4 (6.5%). Among the 140 outborn neonates in the hypothermia group, the target rectal temperature of 34 °C or lower was achieved by 6 hours in 98 neonates (70.0%), between 6 and 7 hours in 27 (19.3%), between 7 and 8 hours in 8 (5.7%), between 8 and 9 hours in 4 (2.9%), and between 9 and 10 hours in 2 (1.4%). One outborn neonate in the hypothermia group did not receive cooling.
Figure 1. Association of Whole-Body Hypothermia With Magnetic Resonance Spectroscopic Biomarkers and Time to Target Temperature Among Inborn vs Outborn Neonates.
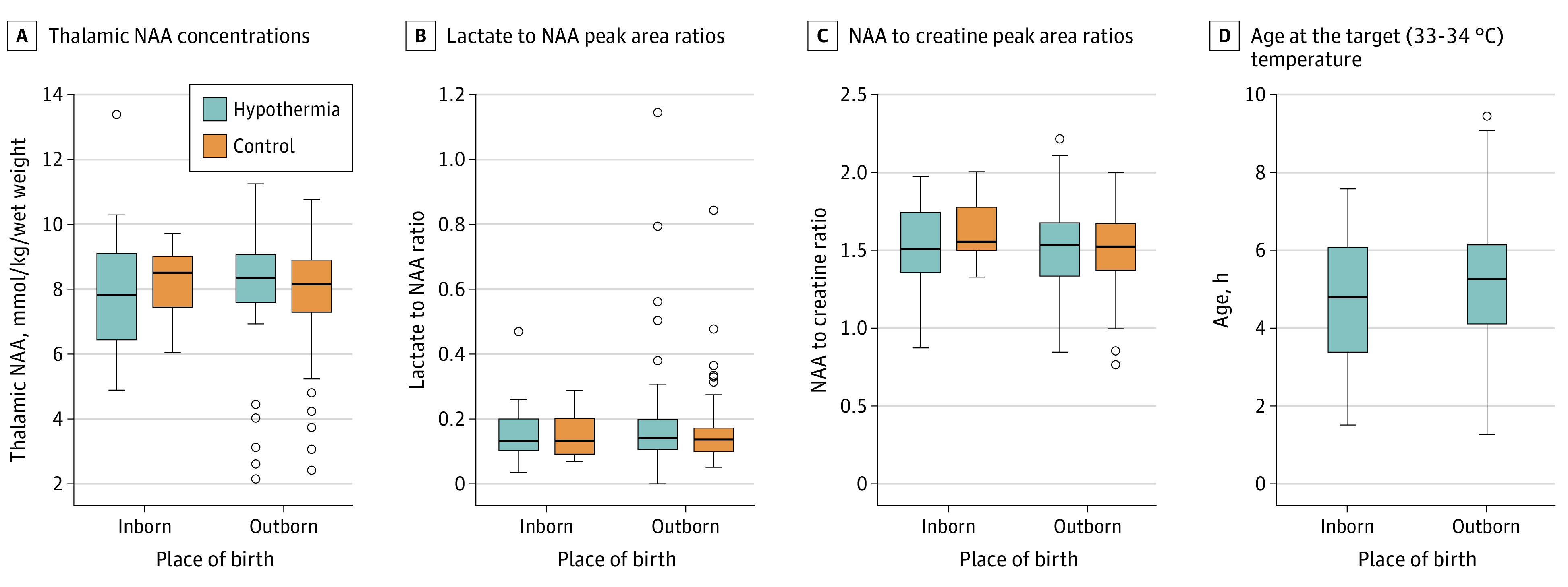
Horizontal lines across bars represent medians, circles represent outliers, and whiskers represent ranges. NAA indicates N-acetyl aspartate.
MR Biomarker Analysis Based on Place of Birth
Of 408 neonates recruited, 114 (27.9%) died before MR imaging, and 10 parents declined consent. The remaining 284 neonates received MR imaging; of those, data from 267 (94.0%) were analyzed. Neonates who did not receive MR imaging (n = 124) had more severe encephalopathy (50 neonates [40.3%] vs 30 [10.6%]), a higher mortality rate (122 neonates [98.4%] vs 25 [8.8%]), and a higher rate of death or disability (122 neonates [98.4%] vs 70 [24.6%]) compared with those who received MR imaging (P < .001 for all comparisons) (eTable 2 in Supplement 2).
Of the 123 inborn neonates, 40 died and 1 was discharged against medical advice before the scan. The remaining 82 neonates (66.7%) received MR imaging; of those, data from 80 neonates (37 in the hypothermia group and 43 in the control group) were available for analysis. The scans were performed at a median (IQR) age of 12.0 (9.7-16.2) days in the hypothermia group and 12.0 (10.0-13.0) days in the control group.
Among inborn neonates, the mean (SD) absolute levels of thalamic N-acetyl aspartate (NAA) were 8.04 (1.98) mmol/kg wet weight in the hypothermia group and 8.31 (1.13) mmol/kg wet weight in the control group (OR, −0.28; 95% CI, −1.62 to 1.07; P = .68), and the median (IQR) thalamic lactate to NAA PARs were 0.13 (0.10-0.20) in the hypothermia group and 0.12 (0.09-0.18) in the control group (OR, 1.02; 95% CI, 0.96-1.08; P = .59). There was no difference in white matter FA at any brain region between inborn neonates in the hypothermia and control groups (eFigure 3 in Supplement 2). The brain injury scores based on conventional T1- and T2-weighted images in the basal ganglia, white matter, or cerebral cortex did not reveal any differences between inborn neonates in the hypothermia and control groups (Table 2).
Table 2. Association of Whole-Body Hypothermia With Conventional MR Biomarkers and MR Spectroscopic Biomarkers.
| Outcome | Category | Participants, No. (%) | OR or MD (95% CI)a | P value | |
|---|---|---|---|---|---|
| Hypothermia group | Control group | ||||
| Inborn neonates (n = 76) | |||||
| Total participants, No. | 37 | 39 | |||
| Conventional MR imaging | |||||
| Basal ganglia and thalamic injury | 0 | 27 (73.0) | 30 (76.9) | 1.02 (0.37 to 2.86) | .97 |
| 1 | 4 (10.8) | 0 | |||
| 2 | 3 (8.1) | 2 (5.1) | |||
| 3 | 3 (8.1) | 7 (17.9) | |||
| Posterior limb of internal capsule | Normal | 30 (81.1) | 30 (76.9) | 0.74 (0.25 to 2.25) | .60 |
| Equivocal | 2 (5.4) | 1 (2.6) | |||
| Abnormal | 5 (13.5) | 8 (20.5) | |||
| White matter injury | 0 | 7 (18.9) | 3 (7.7) | 0.49 (0.20 to 1.21) | .12 |
| 1 | 4 (10.8) | 5 (12.8) | |||
| 2 | 22 (59.5) | 22 (56.4) | |||
| 3 | 4 (10.8) | 9 (23.1) | |||
| Cortical injury | 0 | 24 (64.9) | 27 (69.2) | 1.02 (0.40 to 2.61) | .97 |
| 1 | 8 (21.6) | 3 (7.7) | |||
| 2 | 1 (2.7) | 2 (5.1) | |||
| 3 | 4 (10.8) | 7 (17.9) | |||
| MR spectroscopy | |||||
| Thalamic NAA, mean (SD), mmol/kg wet weight | 8.04 (1.98) | 8.31 (1.13) | −0.28 (−1.62 to 1.07) | .68 | |
| NAA to choline PAR, mean (SD) | 0.86 (0.11) | 0.92 (0.12) | −0.06 (−0.15 to 0.03) | .16 | |
| NAA to creatinine PAR, mean (SD) | 1.54 (0.28) | 1.65 (0.21) | −0.11 (−0.30 to 0.09) | .26 | |
| Thalamic lactate to NAA PAR, median (IQR) | 0.13 (0.10 to 0.20) | 0.12 (0.09 to 0.18) | 1.02 (0.96 to 1.08) | .59 | |
| Outborn neonates (n = 173) | |||||
| Total participants, No. | 79 | 94 | |||
| Conventional MR imaging | |||||
| Basal ganglia and thalamic injury | 0 | 63 (79.7) | 72 (76.6) | 0.91 (0.44 to 1.87) | .80 |
| 1 | 3 (3.8) | 10 (10.6) | |||
| 2 | 7 (8.9) | 8 (8.5) | |||
| 3 | 6 (7.6) | 4 (4.3) | |||
| Posterior limb of internal capsule | Normal | 65 (82.3) | 76 (80.9) | 0.92 (0.43 to 1.99) | .84 |
| Equivocal | 3 (3.8) | 5 (5.3) | |||
| Abnormal | 11 (13.9) | 13 (13.8) | |||
| White matter injury | 0 | 15 (19.0) | 27 (28.7) | 1.26 (0.73 to 2.17) | .41 |
| 1 | 23 (29.1) | 20 (21.3) | |||
| 2 | 33 (41.8) | 39 (41.5) | |||
| 3 | 8 (10.1) | 8 (8.5) | |||
| Cortical injury | 0 | 62 (78.5) | 64 (68.1) | 0.62 (0.31 to 1.23) | .17 |
| 1 | 10 (12.7) | 20 (21.3) | |||
| 2 | 2 (2.5) | 7 (7.4) | |||
| 3 | 5 (6.3) | 3 (3.2) | |||
| MR spectroscopy | |||||
| Thalamic NAA, mean (SD), mmol/kg wet weight | 8.03 (1.89) | 7.99 (1.72) | 0.05 (−0.62 to 0.71) | .89 | |
| NAA to choline PAR, mean (SD) | 0.83 (0.21) | 0.84 (0.16) | −0.01 (−0.07 to 0.06) | .85 | |
| NAA to creatinine PAR, mean (SD) | 1.51 (0.30) | 1.50 (0.27) | 0.02 (−0.08 to 0.11) | .74 | |
| Thalamic lactate to NAA PAR, median (IQR) | 0.14 (0.11 to 0.20) | 0.14 (0.10 to 0.17) | 1.03 (0.98 to 1.09) | .18 | |
Abbreviations: MD, mean difference; MR, magnetic resonance; NAA, N-acetyl aspartate; OR, odds ratio; PAR, peak area ratio.
Odds ratios (calculated as the odds of being in next highest outcome category for the hypothermia group relative to the odds of being in next highest outcome category for the control group) were reported for conventional MR imaging. MDs (calculated as cooling group values minus control group values) were reported for MR spectroscopy.
Of the 285 outborn neonates, 74 died, 7 were discharged against medical advice before MR imaging, and 2 parents declined consent. The remaining 202 neonates (70.9%) received MR imaging; of those, data from 187 neonates (85 in the hypothermia group and 102 in the control group) were available for analysis. The MR imaging was performed at a median (IQR) age of 15.5 (12.2-24.0) days in the hypothermia group and 14.0 (11.0-19.0) days in the control group.
Among outborn neonates, the mean (SD) absolute levels of thalamic NAA were 8.03 (1.89) mmol/kg wet weight in the hypothermia group and 7.99 (1.72) in the control group (OR, 0.05; 95% CI, −0.62 to 0.71; P = .89), and the median (IQR) thalamic lactate to NAA PARs were 0.14 (0.11-0.20) in the hypothermia group and 0.14 (0.10-0.17) in the control group (OR, 1.03; 95% CI, 0.98-1.09; P = .18). There was no difference in white matter FA at any brain region between outborn neonates in the hypothermia and control groups (eFigure 3 in Supplement 2). The brain injury scores based on conventional T1- and T2-weighted images in the basal ganglia, white matter, or cerebral cortex did not reveal any differences between the hypothermia and control groups.
Clinical Outcome Analysis Based on Place of Birth
Clinical outcome analysis included all recruited neonates, irrespective of the MR data. Among the 123 inborn neonates in the hypothermia (n = 62) vs control (n = 61) groups, the rates of gastric bleeding (17 neonates [27.4%] vs 7 [11.5%]), pulmonary hemorrhage (13 neonates [21.0%] vs 4 [6.6%]), coagulopathy (24 neonates [38.7%] vs 13 [21.3.%]), severe thrombocytopenia (10 neonates [16.1%] vs 3 [4.9%]), persistent metabolic acidosis (9 neonates [14.5%] vs 4 [6.6%]), and persistent pulmonary hypertension (11 neonates [17.7%] vs 7 [11.5%]) during neonatal hospitalization and mortality at discharge (24 neonates [38.7%] vs 19 [31.1%]) revealed higher occurrence in the hypothermia group, although these differences were not statistically significant. At 22 months, no statistically significant differences were noted in mortality (25 of 59 neonates [42.4%] vs 20 of 61 [32.8%]; P = .28), survival without neurodisability (16 of 33 neonates [48.5%] vs 21 of 40 [52.5%]; P = .73), or the composite of death or moderate or severe disability (34 of 58 neonates [58.6%] vs 34 of 60 [56.7%]; RR, 1.03; 95% CI, 0.76-1.41; P = .83) among inborn neonates in the hypothermia vs control groups (Table 3).
Table 3. Short- and Long-term Outcomes After Whole-Body Hypothermia Among Inborn vs Outborn Neonates.
| Outcome | Participants, No./total No. (%) | ||
|---|---|---|---|
| Hypothermia group | Control group | RR (95% CI) | |
| Inborn neonates (n = 123) | |||
| Total participants, No. | 62 | 61 | |
| Outcomes at hospital discharge | |||
| Gastric bleeding | 17/62 (27.4) | 7/61 (11.5) | 2.39 (1.07-5.35) |
| Pulmonary hemorrhage | 13/62 (21.0) | 4/61 (6.6) | 3.20 (1.10-9.26) |
| Major hemorrhage | 1/62 (1.6) | 1/61 (1.6) | 0.98 (0.06-15.30) |
| Extracranial bleeding | 3/62 (4.8) | 7/61 (11.5) | 0.42 (0.11-1.56) |
| Prolonged coagulation | 24/62 (38.7) | 13/61 (21.3) | 1.82 (1.02-3.22) |
| Severe thrombocytopenia | 10/62 (16.1) | 3/61 (4.9) | 3.28 (0.95-11.30) |
| Persistent metabolic acidosis | 9/62 (14.5) | 4/61 (6.6) | 2.21 (0.72-6.81) |
| Persistent hypotension | 16/62 (25.8) | 8/61 (13.1) | 1.97 (0.91-4.26) |
| Persistent pulmonary hypertension | 11/62 (17.7) | 7/61 (11.5) | 1.55 (0.64-3.75) |
| Culture-positive early-onset sepsis | 8/62 (12.9) | 4/61 (6.6) | 1.97 (0.62-6.20) |
| Culture-positive late-onset sepsis | 2/62 (3.2) | 7/61 (11.5) | 0.28 (0.06-1.30) |
| Necrotizing enterocolitis | 0 | 0 | NA |
| Cardiac arrythmia | 1/62 (1.6) | 0 | NAa |
| Kidney failure | 7/62 (11.3) | 6/61 (9.8) | 1.15 (0.41-3.22) |
| Pneumonia | 7/62 (11.3) | 9/61 (14.8) | 0.76 (0.30-1.92) |
| Inotropic support in first 4 d | 48/62 (77.4) | 38/61 (62.3) | 1.24 (0.98-1.58) |
| >1 Inotrope | 26/62 (41.9) | 18/61 (29.5) | 1.42 (0.87-2.31) |
| Anticonvulsant treatment in first 4 d | 47/62 (75.8) | 51/61 (83.6) | 0.91 (0.76-1.08) |
| >1 Anticonvulsant | 11/62 (17.7) | 15/61 (24.6) | 0.72 (0.36-1.44) |
| Death by time of discharge | 24/62 (38.7) | 19/61 (31.1) | 1.24 (0.76-2.02) |
| Duration of hospitalization, median (IQR), db | 14.6 (11.8-21.0) | 14.8 (11.4-18.4) | 1.04 (0.86-1.27) |
| Outcomes at age 18-22 mo | |||
| Unavailable for follow-up | 3/62 (4.8) | 0 | NAa |
| Microcephaly | 17/33 (51.5) | 15/40 (37.5) | 1.37 (0.82-2.31) |
| Survival without disability | 16/33 (48.5) | 21/40 (52.5) | 0.92 (0.58-1.46) |
| Bayley cognitive composite score <85 | 10/32 (31.3) | 14/39 (35.9) | 0.87 (0.45-1.70) |
| Bayley motor composite score <85 | 9/32 (28.1) | 13/39 (33.3) | 0.84 (0.41-1.72) |
| Bayley language composite score <85 | 13/32 (40.6) | 16/39 (41.0) | 0.99 (0.56-1.74) |
| Cerebral palsy | 8/33 (24.2) | 13/40 (32.5) | 0.75 (0.35-1.58) |
| Death | 25/59 (42.4) | 20/61 (32.8) | 1.29 (0.81-2.06) |
| Death or moderate or severe disability (ITT)c,d | 34/58 (58.6) | 34/60 (56.7) | 1.03 (0.76-1.41) |
| Death or moderate or severe disability (per protocol)c,d | 34/58 (58.6) | 34/60 (56.7) | 1.03 (0.76-1.41) |
| Outborn neonates (n = 285) | |||
| Total participants, No. | 140 | 145 | |
| Outcomes at hospital discharge | |||
| Gastric bleeding | 45/140 (32.1) | 27/145 (18.6) | 1.73 (1.14-2.62) |
| Pulmonary hemorrhage | 29/140 (20.7) | 24/145 (16.6) | 1.25 (0.77-2.04) |
| Major hemorrhage | 1/140 (0.7) | 3/145 (2.1) | 0.35 (0.04-3.28) |
| Extracranial bleeding | 4/140 (2.9) | 4/145 (2.8) | 1.04 (0.26-4.06) |
| Prolonged coagulation | 55/140 (39.3) | 39/145 (26.9) | 1.46 (1.04-2.05) |
| Severe thrombocytopenia | 23/140 (16.4) | 12/145 (8.3) | 1.99 (1.03-3.83) |
| Persistent metabolic acidosis | 37/140 (26.4) | 20/145 (13.8) | 1.92 (1.17-3.13) |
| Persistent hypotension | 29/140 (20.7) | 17/145 (11.7) | 1.77 (1.02-3.07) |
| Persistent pulmonary hypertension | 13/140 (9.3) | 9/145 (6.2) | 1.50 (0.66-3.39) |
| Culture-positive early-onset sepsis | 4/140 (2.9) | 6/145 (4.1) | 0.69 (0.20-2.39) |
| Culture-positive late-onset sepsis | 12/140 (8.6) | 4/145 (2.8) | 3.11 (1.03-9.40) |
| Necrotizing enterocolitis | 5/140 (3.6) | 1/145 (0.7) | 5.18 (0.61-43.80) |
| Cardiac arrythmia | 4/140 (2.9) | 0 | NAa |
| Kidney failure | 15/140 (10.7) | 10/145 (6.9) | 1.55 (0.72-3.34) |
| Pneumonia | 19/140 (13.6) | 16/145 (11.0) | 1.23 (0.65-2.29) |
| Inotropic support in first 4 d | 113/140 (80.7) | 88/145 (60.7) | 1.33 (1.14-1.55) |
| >1 Inotrope | 78/140 (55.7) | 52/145 (35.9) | 1.55 (1.19-2.02) |
| Anticonvulsant treatment in first 4 d | 127/140 (90.7) | 132/145 (91.0) | 1.00 (0.93-1.07) |
| >1 Anticonvulsant | 21/140 (15.0) | 31/145 (21.4) | 0.70 (0.42-1.16) |
| Death by time of discharge | 48/140 (34.3) | 30/145 (20.7) | 1.66 (1.12-2.45) |
| Duration of hospitalization, median (IQR), de | 17.0 (13.1-23.3) | 13.7 (10.3-18.8) | 1.24 (1.07-1.44) |
| Outcomes at age 18-22 mo | |||
| Unavailable for follow-up | 1/140 (0.7) | 5/145 (3.4) | 0.21 (0.02-1.75) |
| Microcephaly | 16/77 (20.8) | 22/95 (23.2) | 0.90 (0.51-1.59) |
| Survival without disability | 31/78 (39.7) | 26/96 (27.1) | 1.48 (0.96-2.25) |
| Bayley cognitive composite score <85 | 26/76 (34.2) | 32/94 (34.0) | 1.00 (0.66-1.53) |
| Bayley motor composite score <85 | 8/76 (10.5) | 20/94 (21.3) | 0.49 (0.23-1.06) |
| Bayley language composite score <85 | 34/76 (44.7) | 58/94 (61.7) | 0.73 (0.54-0.98) |
| Cerebral palsy | 4/78 (5.1) | 15/96 (15.6) | 0.33 (0.11-0.95) |
| Death | 59/139 (42.4) | 43/140 (30.7) | 1.38 (1.01-1.89) |
| Death or moderate or severe disability (ITT)c,f | 64/137 (46.7) | 60/139 (43.2) | 1.08 (0.83-1.41) |
| Death or moderate or severe disability (per protocol)c,g | 64/136 (47.1) | 60/139 (43.2) | 1.09 (0.84-1.42) |
| Death or moderate or severe disability (ITT)h,i | 61/133 (45.9) | 55/133 (41.4) | 1.11 (0.84-1.46) |
| Death or moderate or severe disability (per protocol)h,j | 61/132 (46.2) | 55/133 (41.4) | 1.12 (0.85-1.47) |
Abbreviations: ITT, intention to treat; NA, not applicable; RR, risk ratio.
Unable to calculate RRs due to no occurrence of outcome in 1 group.
Among 38 participants in the hypothermia group and 41 in the control group.
Severe disability was defined as any 1 of the following: a cognitive composite score of less than 70 on the Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III)14; a gross motor function classification system level of 3 to 5; a profound hearing impairment requiring hearing aids or a cochlear implant; or blindness. Moderate disability was defined as a cognitive composite score of 70 to 84 on the Bayley-III and 1 or more of the following: a gross motor function classification system level of 2, a hearing impairment with no amplification, or a persistent seizure disorder.
P = .83.
Among 92 participants in the hypothermia group and 115 in the control group.
P = .55.
P = .52.
Excluding neonates who were born at home.
P = .46.
P = .43.
Among the 285 outborn neonates in the hypothermia (n = 140) vs control (n = 145) groups, the rates of gastric bleeding (45 neonates [32.1%] vs 27 [18.6%]; P = .01), coagulopathy (55 neonates [39.3%] vs 39 [26.9%]; P = .03), severe thrombocytopenia (23 neonates [16.4%] vs 12 [8.3%]; P = .04), persistent metabolic acidosis (37 neonates [26.4%] vs 20 [13.8%]; P = .01), and inotropic use (113 neonates [80.7%] vs 88 [60.7%]; P = .001) during neonatal hospitalization mortality at hospital discharge (48 neonates [34.3%] vs 30 [20.7%]; P = .01) and at 22 months (59 of 139 neonates [42.4%] vs 43 of 140 [30.7%]; P = .04) were significantly higher in the hypothermia group (Table 3 and Figure 2). Conversely, neonates in the hypothermia group had better rates of survival without neurodisability than those in the control group (31 of 78 neonates [39.7%] vs 26 of 96 [27.1%]; P = .08), although this difference was not statistically significant. The composite outcome of death or moderate or severe disability was not significantly different in the hypothermia vs control groups (64 of 137 neonates [46.7%] vs 60 of 139 [43.2%]; RR, 1.08; 95% CI, 0.83-1.41; P = .55). The results were similar when 10 neonates who were born at home were excluded from the analysis (Table 3).
Figure 2. Kaplan-Meier Survival Curves for Inborn and Outborn Neonates.
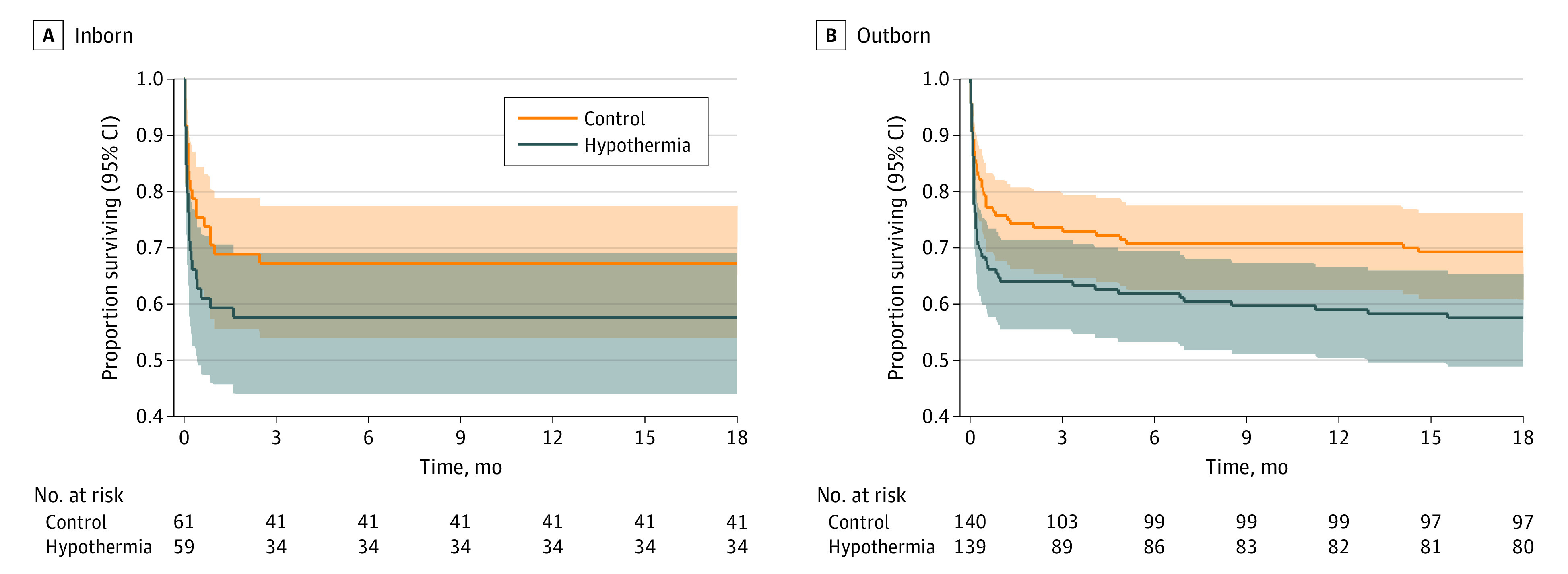
Whole-body hypothermia was associated with increased mortality (RR, 1.35; 95% CI, 1.04-1.76; number needed to harm, 9) and reduced cerebral palsy (RR, 0.53; 95% CI, 0.28-0.98; number needed to treat, 10) at 18 to 22 months among all 408 neonates recruited to the HELIX trial. Among inborn neonates, the relative risk of mortality was 29% higher (RR, 1.29; 95% CI, 0.81-2.06; P = .28) and cerebral palsy was 25% lower (RR, 0.75; 95% CI, 0.35-1.58; P = .44) in the hypothermia group than the control group, although these differences were not statistically significant. Among outborn neonates, the relative risk of mortality was 38% higher (RR, 1.38; 95% CI, 1.01-1.89; P = .01) and cerebral palsy was 67% lower (RR, 0.33; 95% CI, 0.11-0.95; P = .05) in the hypothermia group than the control group.
Discussion
This nested cohort study involved a subgroup analysis of data from the HELIX trial, which was, to our knowledge, the largest clinical trial of hypothermia reported to date and the first to use quantitative MR biomarkers in a multicountry setting using harmonized cross-platform sequences. Findings revealed that whole-body hypothermia was not associated with reductions in brain injury measured by thalamic NAA levels, thalamic lactate to NAA or NAA to creatinine PARs, tract-based spatial statistics of whole-brain white matter FA, or conventional MR imaging injury scores, irrespective of place of birth. The direction of treatment effect (or lack of treatment effect) measured using thalamic NAA levels, thalamic lactate to NAA PARs, and NAA to creatinine PARs was associated with the clinical outcomes. Whole-body hypothermia was not associated with reductions in death or disability at 18 to 22 months among both inborn and outborn neonates. Because the HELIX trial was adequately powered to assess the clinical outcomes, these data provide the first validation, to our knowledge, of MR spectroscopic biomarkers as a surrogate end point in HIE neuroprotection trials.
Although earlier clinical trials of whole-body hypothermia have reported MR imaging data obtained as part of standard clinical care,13,15,16 none used MR spectroscopy or DTI. While MR imaging scoring systems do estimate later outcomes,17 they are subjective and user dependent. The Total Body Hypothermia Plus Xenon (TOBY-Xe) trial18 acquired MR spectroscopic biomarkers from 78 neonates with HIE at 3 UK sites using Philips Achieva 3T (Philips Medical Systems) MR scanners. The trial found no change in thalamic lactate to NAA PARs with inhaled xenon. The TOBY-Xe trial was not powered to examine clinical outcomes nor did it perform NAA analysis.18
The first Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network (NRN) hypothermia trial3 included 45% outborn neonates, the Infant Cooling Evaluation (ICE) trial19 included 61% outborn neonates, and the more recent High-Dose Erythropoietin for Asphyxia and Encephalopathy (HEAL) trial20 included 83% outborn neonates. In the NICHD NRN trial,5 neonates born outside an NRN cooling center were more unwell, required longer resuscitation at birth, and more often had severe encephalopathy than inborn neonates. In contrast, the outborn neonates recruited to the HELIX trial were less unwell than the inborn neonates, and many did not require endotracheal intubation at birth. One possible explanation is that outborn neonates requiring extensive resuscitation died soon after birth or were too unwell to be transferred to a tertiary cooling center within 6 hours and hence were not captured in the trial.
An inverse association was seen between mortality and cerebral palsy at 18 to 22 months. For every 10 neonates treated with whole-body hypothermia, at least 1 additional neonate died and 1 case of cerebral palsy was averted. Therefore, it is important that the primary outcome of any neuroprotective intervention for HIE, particularly in NICUs in LMICs, remains the composite of death or moderate or severe disability, and reduction in cerebral palsy should always be considered in the context of shifts in mortality within the same trial and not in isolation.
Although many observational studies from LMICs primarily recruiting neonates with mild brain injury21,22 or normal amplitude–integrated electroencephalography23 have reported short-term benefits, no inferences about the safety or efficacy of hypothermia can be made because these studies did not include randomized control groups.21,23,24 While more than 15 small single-center pilot RCTs have reported short-term benefits of whole-body hypothermia among neonates in LMICs,25 the HELIX trial is, to our knowledge, the only well-designed and rigorously conducted multicenter trial to report neurodevelopmental outcomes. Small single-center studies overestimate treatment effects; hence, the inclusion of pilot RCTs in meta-analyses may result in inaccurate estimates.26 Moreover, many pilot RCTs are difficult to interpret. For example, 6 trials27,28,29,30,31,32 with different event rates but overlapping recruitment periods were reported from the same hospital, 1 trial33 reported outcomes at 8 years within a year of completing neonatal recruitment, and another trial34 reported a 68% reduction in adverse outcomes using ice cubes on the neonate’s head.
It is unlikely that the lack of hypothermic neuroprotection seen in the HELIX trial35 can be explained by (1) delay in achieving target temperature, (2) use of simplified inclusion criteria for neonates born at home (ie, absence of cry by 5 minutes), (3) coexistent infection, or (4) lack of 1:1 nursing care. First, of all the major induced hypothermia trials, age at randomization (4.3 hours) was shortest in the HELIX trial (compared with age 4.8 hours in the Selective Head Cooling With Mild Systemic Hypothermia After Neonatal Encephalopathy [CoolCap] multicenter RCT,2 age 4.3 hours in the NICHD NRN trial,3 and age 4.7 hours in the Total Body Hypothermia [TOBY] trial4), and the cooling device was the most efficient. Thus, 72% of the neonates reached the target temperature (≤34 °C) by 6 hours in the HELIX trial compared with 53% in the NICHD NRN trial.3 In the TOBY trial,4 target temperatures were achieved much later because 68% of neonates were randomized after 4 hours, although randomization age (<4 hours vs 4-6 hours) had no effect on neuroprotection.
Second, the HELIX trial results did not change when the 10 neonates born at home were excluded. While the inclusion criteria of the HELIX trial (which required resuscitation at birth) were less stringent than those of the NICHD NRN trial3 (which required acidosis and resuscitation at birth), they were more stringent than other trials in HICs that required only acidosis or resuscitation at birth and not necessarily both.2,4 The NICHD NRN trial3 and the HELIX trial were the only clinical trials to perform standardized neurological assessments. Third, the occurrence of coexistent infection and funisitis in the HELIX trial was similar to previous trials conducted in HICs.2,3,4 Furthermore, observational studies36,37 in LMICs using both polymerase chain reaction testing and blood cultures have reported coexistent infection in fewer than 10% of the neonates with encephalopathy37; these findings are again similar to studies in HICs.2,3,4,36 Hence, preclinical models of combined infection and ischemia may not represent HIE scenarios in LMICs. Fourth, the HELIX trial was conducted in accredited tertiary NICUs managed by dedicated neonatologists, mostly with 1:2 to 1:3 nursing care ratios. All previous pilot trials in LMICs had neonate to nurse ratios of 1:438 or worse.24 Any intervention that requires 1:1 nursing care and is unsafe outside a tertiary NICU setting has no generalizability in LMICs. For example, the highest burden of HIE in LMICs occurs in secondary (district-level) care neonatal units with neonate to nurse ratios of 1:15 to 1:30.39
One possible explanation for the lack of hypothermic neuroprotection is difference in the nature and timing of intrapartum hypoxia. In preclinical models, hypothermia was associated with the prevention of secondary energy failure only when the hypoxic injury was single, acute, and occurred in a previously healthy animal40; this scenario closely mimicked acute intrapartum sentinel events among individuals in HICs. Because we did not have detailed intrapartum monitoring data, no definite conclusions about the timing of fetal brain injury can be made. Nevertheless, a constellation of factors comprising infrequent major intrapartum sentinel events, low birth weight, coagulopathy, early-onset clinical seizures, and cerebral white matter injury suggests that the unborn neonates may have already been compromised (eg, due to suboptimal nutrition and other socioeconomic factors). Hence, brain injury might occur from intermittent cerebral hypoxia during active labor, especially if labor is medically augmented41—“vulnerable baby in the womb.” Preclinical models of growth-restricted animals and intermittent umbilical cord occlusion42 may represent this scenario. If confirmed by subsequent mechanistic studies, this hypothesis may lead to a paradigm shift in our understanding of HIE in LMICs and aid in the development of appropriate neuroprotective therapies.
Limitations
This study has several limitations. As with all previous studies,13,15,16 we were unable to obtain MR imaging in neonates who died soon after birth. The mortality rate among neonates who had MR imaging was 8.8%, while those who did not have MR imaging was 98.4%; therefore, deep brain nuclei and brainstem injury might have been underreported. Furthermore, the HELIX trial recruited only populations in LMICs with low income and high HIE burden and did not recruit those receiving care from private hospitals. Private-sector hospitals primarily cater to the middle and upper socioeconomic classes, have small maternity units with fewer than 2000 births per year, have substantially more neonates who are born by elective cesarean than vaginal delivery, and thus have very few inborn neonates with HIE.43 Nevertheless, interventions in HICs may not directly translate to those settings; for example, the survival of premature neonates is substantially lower and the occurrence of neonatal sepsis is 19 times higher (1.7% vs 32.0%) in even well-resourced private-sector tertiary NICUs in India than public-sector NICUs in the UK.44
Conclusions
In this nested cohort study within the HELIX trial, whole-body hypothermia was not associated with reductions in brain injury as measured by quantitative MR biomarkers, irrespective of place of birth, among neonates in South Asian NICUs. Quantitative MR spectroscopic biomarkers can be used as surrogate outcome measures for future clinical trials and for further comparison between populations in HICs and LMICs. Policy makers should be aware that continued use of whole-body hypothermia may harm the population with the highest disease burden and worsen inequalities in LMICs.
HELIX Trial Protocol
eTable 1. Baseline Characteristics of Inborn and Outborn Neonates
eTable 2. Clinical Characteristics of Neonates With and Without MR Imaging (MRI)
eFigure 1. Flowchart
eFigure 2. Mean (SD) Rectal Temperature of Inborn (Top Panel) and Outborn (Bottom Panel) Neonates
eFigure 3. Treatment Effects of Whole-Body Hypothermia on Whole-Brain White Matter Fractional Anisotropy Quantified by Tract-Based Spatial Statistics Among Inborn (A) and Outborn (B) Neonates
Data Sharing Statement
References
- 1.Lawn J, Shibuya K, Stein C. No cry at birth: global estimates of intrapartum stillbirths and intrapartum-related neonatal deaths. Bull World Health Organ. 2005;83(6):409-417. [PMC free article] [PubMed] [Google Scholar]
- 2.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365(9460):663-670. doi: 10.1016/S0140-6736(05)17946-X [DOI] [PubMed] [Google Scholar]
- 3.Shankaran S, Laptook AR, Ehrenkranz RA, et al. ; National Institute of Child Health and Human Development Neonatal Research Network . Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574-1584. doi: 10.1056/NEJMcps050929 [DOI] [PubMed] [Google Scholar]
- 4.Azzopardi DV, Strohm B, Edwards AD, et al. ; TOBY Study Group . Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361(14):1349-1358. doi: 10.1056/NEJMoa0900854 [DOI] [PubMed] [Google Scholar]
- 5.Natarajan G, Pappas A, Shankaran S, et al. Effect of inborn vs. outborn delivery on neurodevelopmental outcomes in infants with hypoxic-ischemic encephalopathy: secondary analyses of the NICHD whole-body cooling trial. Pediatr Res. 2012;72(4):414-419. doi: 10.1038/pr.2012.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robertson NJ, Kendall GS, Thayyil S. Techniques for therapeutic hypothermia during transport and in hospital for perinatal asphyxial encephalopathy. Semin Fetal Neonatal Med. 2010;15(5):276-286. doi: 10.1016/j.siny.2010.03.006 [DOI] [PubMed] [Google Scholar]
- 7.Thayyil S, Chandrasekaran M, Taylor A, et al. Cerebral magnetic resonance biomarkers in neonatal encephalopathy: a meta-analysis. Pediatrics. 2010;125(2):e382-e395. doi: 10.1542/peds.2009-1046 [DOI] [PubMed] [Google Scholar]
- 8.Lally PJ, Montaldo P, Oliveira V, et al. ; MARBLE Consortium . Magnetic resonance spectroscopy assessment of brain injury after moderate hypothermia in neonatal encephalopathy: a prospective multicentre cohort study. Lancet Neurol. 2019;18(1):35-45. doi: 10.1016/S1474-4422(18)30325-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thayyil S, Pant S, Montaldo P, et al. ; HELIX Consortium . Hypothermia for moderate or severe neonatal encephalopathy in low-income and middle-income countries (HELIX): a randomised controlled trial in India, Sri Lanka, and Bangladesh. Lancet Glob Health. 2021;9(9):e1273-e1285. doi: 10.1016/S2214-109X(21)00264-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thayyil S, Oliveira V, Lally PJ, et al. ; HELIX Trial Group . Hypothermia for encephalopathy in low and middle-income countries (HELIX): study protocol for a randomised controlled trial. Trials. 2017;18(1):432. doi: 10.1186/s13063-017-2165-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LCModel. Version 6.3. Stephen Provencher. 1992-2021. Accessed April 6, 2023. https://github.com/eborisch/LCModel/blob/main/LCModel.f
- 12.FMRIB Software Library. Version 6.0. FMRIB Analysis Group. 2018. Accessed March 6, 2023. https://fsl.fmrib.ox.ac.uk/fsl/fslwiki
- 13.Rutherford M, Ramenghi LA, Edwards AD, et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol. 2010;9(1):39-45. doi: 10.1016/S1474-4422(09)70295-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albers CA, Grieve AJ. Test review: Bayley, N. (2006). Bayley Scales of Infant and Toddler Development–Third Edition. San Antonio, TX: Harcourt Assessment. J Psychoeduc Assess. 2007;25(2):180-190. doi: 10.1177/0734282906297199 [DOI] [Google Scholar]
- 15.Shankaran S, Barnes PD, Hintz SR, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Brain injury following trial of hypothermia for neonatal hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2012;97(6):F398-F404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheong JLY, Coleman L, Hunt RW, et al. ; Infant Cooling Evaluation Collaboration . Prognostic utility of magnetic resonance imaging in neonatal hypoxic-ischemic encephalopathy: substudy of a randomized trial. Arch Pediatr Adolesc Med. 2012;166(7):634-640. doi: 10.1001/archpediatrics.2012.284 [DOI] [PubMed] [Google Scholar]
- 17.Shankaran S, McDonald SA, Laptook AR, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Neonatal magnetic resonance imaging pattern of brain injury as a biomarker of childhood outcomes following a trial of hypothermia for neonatal hypoxic-ischemic encephalopathy. J Pediatr. 2015;167(5):987-993. doi: 10.1016/j.jpeds.2015.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azzopardi D, Robertson NJ, Bainbridge A, et al. Moderate hypothermia within 6 h of birth plus inhaled xenon versus moderate hypothermia alone after birth asphyxia (TOBY-Xe): a proof-of-concept, open-label, randomised controlled trial. Lancet Neurol. 2016;15(2):145-153. doi: 10.1016/S1474-4422(15)00347-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobs SE, Morley CJ, Inder TE, et al. ; Infant Cooling Evaluation Collaboration . Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Arch Pediatr Adolesc Med. 2011;165(8):692-700. doi: 10.1001/archpediatrics.2011.43 [DOI] [PubMed] [Google Scholar]
- 20.Wu YW, Comstock BA, Gonzalez FF, et al. ; HEAL Consortium . Trial of erythropoietin for hypoxic-ischemic encephalopathy in newborns. N Engl J Med. 2022;387(2):148-159. doi: 10.1056/NEJMoa2119660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar C, Peruri G, Plakkal N, et al. Short-term outcome and predictors of survival among neonates with moderate or severe hypoxic ischemic encephalopathy: data from the Indian Neonatal Collaborative. Indian Pediatr. 2022;59(1):21-24. doi: 10.1007/s13312-022-2413-9 [DOI] [PubMed] [Google Scholar]
- 22.Thayyil S, Shankaran S. From therapeutic hypothermia to targeted temperature management in low-resource settings. Indian Pediatr. 2022;59(1):9-10. doi: 10.1007/s13312-022-2410-z [DOI] [PubMed] [Google Scholar]
- 23.Mascarenhas D, Goyal M, Nanavati R, Kirthana SB, Subhadarsini S. Short-term outcome and complications of therapeutic hypothermia in neonates with moderate-to-severe hypoxic ischaemic encephalopathy: a single-centre retrospective observational study in a hospital in Mumbai, India. Paediatr Int Child Health. Published online February 8, 2023. doi: 10.1080/20469047.2023.2171762 [DOI] [PubMed] [Google Scholar]
- 24.Nakwa FL, Sepeng L, van Kwawegen A, et al. Characteristics and outcomes of neonates with intrapartum asphyxia managed with therapeutic hypothermia in a public tertiary hospital in South Africa. BMC Pediatr. 2023;23(1):51. doi: 10.1186/s12887-023-03852-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abate BB, Bimerew M, Gebremichael B, et al. Effects of therapeutic hypothermia on death among asphyxiated neonates with hypoxic-ischemic encephalopathy: a systematic review and meta-analysis of randomized control trials. PLoS One. 2021;16(2):e0247229. doi: 10.1371/journal.pone.0247229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts I, Ker K. How systematic reviews cause research waste. Lancet. 2015;386(10003):1536. doi: 10.1016/S0140-6736(15)00489-4 [DOI] [PubMed] [Google Scholar]
- 27.Bharadwaj SK, Bhat BV. Therapeutic hypothermia using gel packs for term neonates with hypoxic ischaemic encephalopathy in resource-limited settings: a randomized controlled trial. J Trop Pediatr. 2012;58(5):382-388. doi: 10.1093/tropej/fms005 [DOI] [PubMed] [Google Scholar]
- 28.Joy R, Pournami F, Bethou A, Bhat VB, Bobby Z. Effect of therapeutic hypothermia on oxidative stress and outcome in term neonates with perinatal asphyxia: a randomized controlled trial. J Trop Pediatr. 2013;59(1):17-22. doi: 10.1093/tropej/fms036 [DOI] [PubMed] [Google Scholar]
- 29.Gane BD, Bhat V, Rao R, Nandhakumar S, Harichandrakumar KT, Adhisivam B. Effect of therapeutic hypothermia on DNA damage and neurodevelopmental outcome among term neonates with perinatal asphyxia: a randomized controlled trial. J Trop Pediatr. 2014;60(2):134-140. doi: 10.1093/tropej/fmt098 [DOI] [PubMed] [Google Scholar]
- 30.Tanigasalam V, Bhat V, Adhisivam B, Sridhar MG. Does therapeutic hypothermia reduce acute kidney injury among term neonates with perinatal asphyxia?—a randomized controlled trial. J Matern Fetal Neonatal Med. 2016;29(15):2545-2548. [DOI] [PubMed] [Google Scholar]
- 31.Rakesh K, Vishnu Bhat B, Adhisivam B, Ajith P. Effect of therapeutic hypothermia on myocardial dysfunction in term neonates with perinatal asphyxia—a randomized controlled trial. J Matern Fetal Neonatal Med. 2018;31(18):2418-2423. doi: 10.1080/14767058.2017.1344633 [DOI] [PubMed] [Google Scholar]
- 32.Catherine RC, Ballambattu VB, Adhisivam B, Bharadwaj SK, Palanivel C. Effect of therapeutic hypothermia on the outcome in term neonates with hypoxic ischemic encephalopathy—a randomized controlled trial. J Trop Pediatr. 2021;67(1):fmaa073. doi: 10.1093/tropej/fmaa073 [DOI] [PubMed] [Google Scholar]
- 33.Santhosh J, Mohamad MK. Effect of hypothermia for perinatal asphyxia on childhood outcomes. Int J Contemp Pediatrics. 2018;5(1):e5489. doi: 10.18203/2349-3291.ijcp20175489 [DOI] [Google Scholar]
- 34.Das S, Sarkar N, Bhattacharya M, et al. Neurological outcome at 30 months of age after mild hypothermia via selective head cooling in term neonates with perinatal asphyxia using low-cost CoolCap: a single-center randomized control pilot trial in India. J Pediatr Neurol. 2017;15(4):157-165. doi: 10.1055/s-0037-1603681 [DOI] [Google Scholar]
- 35.Thayyil S, Bassett P, Shankaran S. Questions about the HELIX trial—authors’ reply. Lancet Glob Health. 2021;9(12):e1654-e1655. doi: 10.1016/S2214-109X(21)00499-X [DOI] [PubMed] [Google Scholar]
- 36.Car KP, Nakwa F, Solomon F, et al. The association between early-onset sepsis and neonatal encephalopathy. J Perinatol. 2022;42(3):354-358. doi: 10.1038/s41372-021-01290-5 [DOI] [PubMed] [Google Scholar]
- 37.Tann CJ, Nkurunziza P, Nakakeeto M, et al. Prevalence of bloodstream pathogens is higher in neonatal encephalopathy cases vs. controls using a novel panel of real-time PCR assays. PLoS One. 2014;9(5):e97259. doi: 10.1371/journal.pone.0097259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aker K, Støen R, Eikenes L, et al. Therapeutic hypothermia for neonatal hypoxic-ischaemic encephalopathy in India (THIN study): a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2020;105(4):405-411. doi: 10.1136/archdischild-2019-317311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Narayanan I, Nsungwa-Sabiti J, Lusyati S, et al. Facility readiness in low and middle-income countries to address care of high risk/small and sick newborns. Matern Health Neonatol Perinatol. 2019;5:10. doi: 10.1186/s40748-019-0105-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thoresen M, Penrice J, Lorek A, et al. Mild hypothermia after severe transient hypoxia-ischemia ameliorates delayed cerebral energy failure in the newborn piglet. Pediatr Res. 1995;37(5):667-670. doi: 10.1203/00006450-199505000-00019 [DOI] [PubMed] [Google Scholar]
- 41.Burgod C, Pant S, Morales MM, et al. Effect of intra-partum oxytocin on neonatal encephalopathy: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2021;21(1):736. doi: 10.1186/s12884-021-04216-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wassink G, Bennet L, Davidson JO, Westgate JA, Gunn AJ. Pre-existing hypoxia is associated with greater EEG suppression and early onset of evolving seizure activity during brief repeated asphyxia in near-term fetal sheep. PLoS One. 2013;8(8):e73895. doi: 10.1371/journal.pone.0073895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chandrasekaran M, Swamy R, Ramji S, Shankaran S, Thayyil S. Therapeutic hypothermia for neonatal encephalopathy in Indian neonatal units: a survey of national practices. Indian Pediatr. 2017;54(11):969-970. doi: 10.1007/s13312-017-1194-z [DOI] [PubMed] [Google Scholar]
- 44.Diggikar S, Nagesh NK, Kumar NA, Aladangady N. A study comparing short-term outcome in preterm infants of ≤30 weeks gestation between a tertiary neonatal care unit in Bangalore, India and one in London, UK. Paediatr Int Child Health. 2022;42(1):5-11. doi: 10.1080/20469047.2022.2054916 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HELIX Trial Protocol
eTable 1. Baseline Characteristics of Inborn and Outborn Neonates
eTable 2. Clinical Characteristics of Neonates With and Without MR Imaging (MRI)
eFigure 1. Flowchart
eFigure 2. Mean (SD) Rectal Temperature of Inborn (Top Panel) and Outborn (Bottom Panel) Neonates
eFigure 3. Treatment Effects of Whole-Body Hypothermia on Whole-Brain White Matter Fractional Anisotropy Quantified by Tract-Based Spatial Statistics Among Inborn (A) and Outborn (B) Neonates
Data Sharing Statement


