Bacteria were for a long time believed to exist as individual cells that sought primarily to find nutrients and multiply. The discovery of intercellular communication among bacteria has led to the realization that bacteria are capable of coordinated activity that was once believed to be restricted to multicellular organisms. The capacity to behave collectively as a group has obvious advantages, for example, the ability to migrate to a more suitable environment/better nutrient supply and to adopt new modes of growth, such as sporulation or biofilm formation, which may afford protection from deleterious environments. The “language” used for this intercellular communication is based on small, self-generated signal molecules called autoinducers. Through the use of autoinducers, bacteria can regulate their behavior according to population density. The phenomenon of quorum sensing, or cell-to-cell communication, relies on the principle that when a single bacterium releases autoinducers (AIs) into the environment, their concentration is too low to be detected. However, when sufficient bacteria are present, autoinducer concentrations reach a threshold level that allows the bacteria to sense a critical cell mass and, in response, to activate or repress target genes. Most of the bacteria thus far identified that utilize quorum-sensing systems are associated in some way with plants or animals. The nature of these relationships can be either amicable, as characterized by symbiotic bacteria, or adversarial, as seen with pathogenic bacteria. There are numerous bacteria that have components of a quorum-sensing system for which the phenotype regulated remains an enigma. Similarly, there are bacteria known to regulate a specific phenotype via quorum sensing for which one or more of the regulatory components have thus far eluded identification. In this review we give examples of pathogenic relationships, focusing on organisms for which many of the facets of their quorum-sensing systems have been elucidated.
QUORUM SENSING IN GRAM-NEGATIVE BACTERIA
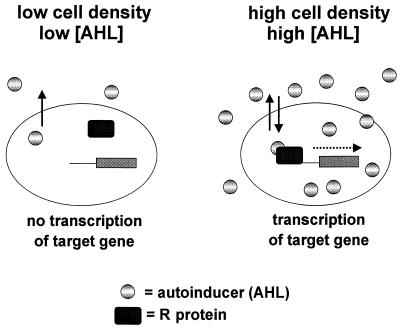
The vast majority of gram-negative quorum-sensing systems that have been studied thus far utilize N-acyl homoserine lactones (AHL) as signaling molecules. When in high enough concentration, these molecules can bind to and activate a transcriptional activator, or R protein, which in turn induces expression of target genes (Fig. 1). The use of biosensors to screen spent culture supernatants has led to the discovery that AHLs are produced by a plethora of unrelated bacteria (Table 1). Biosensors typically consist of a quorum-sensing-controlled promoter fused to a reporter such as lacZ or the lux operon. These biosensor strains contain a functional R protein but lack the AHL synthase enzyme; therefore, promoter activity depends on the presence of exogenous AHL. Despite the fact that R proteins are exquisitely sensitive to their cognate AHLs, some infidelity does exist and this infidelity enables R proteins to be responsive to a range of AHL molecules, albeit higher concentrations of noncognate AHL are usually required for activation. To date, AHL molecules have been identified containing 4- to 14-carbon acyl side chains and either an oxo, a hydroxy, or no substitution at the third carbon. Only two AHLs bearing double bonds have been identified: 7,8-cis-N-(3-hydroxytetradecenoyl)homoserine lactone from Rhizobium leguminosarum (47, 105) and 7,8-cis-N-(tetradecenoyl)homoserine lactone from Rhodobacter sphaerhoides (92).
FIG. 1.
Quorum sensing in gram-negative organisms involves two regulatory components: the transcriptional activator protein (R protein) and the AI molecule produced by the autoinducer synthase. Accumulation of AI occurs in a cell-density-dependent manner until a threshold level is reached. At this time the AI binds to and activates the R protein, which in turn induces gene expression. The R protein consists of two domains: the N terminus of the protein that interacts with AI and the C terminus that is involved in DNA binding. Typically, gram-negative AI molecules are N-acyl-HSLs; however, other types of signal molecules do exist.
TABLE 1.
Summary of quorum sensing in gram-negative bacteria
| Organism | Major signal molecule | Regulatory proteins | Phenotype | Reference |
|---|---|---|---|---|
| Vibrio fischeri | 3-Oxo-C6-HSL | LuxI/LuxR | Bioluminescence | 26, 31 |
| Vibrio harveyi | 3-Hydroxy-C4-HSL | LuxLM/LuxN | Bioluminescence | 7, 8, 13, 108 |
| ? | Lux?/LuxPQ | |||
| Vibrio anguillarum | ? | VanI/VanR | 3-Oxo-C10-HSL | 69 |
| Pseudomonas aeruginosa | 3-Oxo-C12-HSL | LasI/LasR | Multiple extracellular enzymes, RhlR, Xcp, biofilm formation | 14, 19, 42, 43, 76, 78, 121 |
| C4-HSL | RhlI/RhlR | Multiple extracellular enzymes, rhamnolipid, RpoS, secondary metabolites | 12, 61, 73, 74, 79, 126 | |
| Pseudomonas aureofaciens | C6-HSL | PhzI/PhzR | Phenazine antibiotics | 84, 85 |
| Agrobacterium tumefaciens | 3-Oxo-C8-HSL | TraI/TraR | Ti plasmid conjugation | 51, 87, 130 |
| Erwinia carotovora subsp. carotovora | 3-Oxo-C6-HSL | ExpI/ExpR | Exoenzymes | 4, 16, 55, 65, 88 |
| CarI/CarR | Carbapenem antibiotics | |||
| Erwinia chrysanthemi | 3-Oxo-C6-HSL | ExpI/ExpR | Pectate lyases | 72, 98 |
| C6-HSL | ||||
| Erwinia stewartii | 3-Oxo-C6-HSL | EsaI/EsaR | Exopolysaccharide, virulence factors | 9 |
| Rhizobium leguminosarum | C6-HSL | RhiI/RhiR | RhiABC rhizosphere-expressed genes, nodulation | 18, 47, 99 |
| C8-HSL | ||||
| 3-Hydroxy-7-cis-C14-HSL | ||||
| Rhizobium etli | ? | RaiI/RaiR | Restriction of number of nitrogen-fixing nodules | 100 |
| Chromobacterium violaceum | C6-HSL | CviI/CviR | Exoenzymes, antibiotics, cyanide, violacein | 67 |
| Burkholderia cepacia | C8-HSL | CepI/R | Protease, siderophores | 62 |
| Aeromonas hydrophila | C4-HSL | AhyI/AhyR | Exoprotease production | 114 |
| Aeromonas salmonicida | C4-HSL | AsaI/AsaR | Extracellular protease | 114 |
| Ralstonia solanacearum | C8-HSL | SolI/SolR | ? | 35 |
| Serratia liquifaciens | C4-HSL | SwrI/SwrR | Extracellular protease, swarming | 27, 44 |
| Rhodobacter sphaeroides | 7-cis-C14-HSL | CerI/CerR | Dispersal from bacterial aggregates | 92 |
| Enterobacter agglomerans | 3-Oxo-C6-HSL | EagI/EagR | ? | 115 |
| Escherichia coli | ? | ?/SdiA | Cell division, attachment and effacing lesion formation | 107, 110, 127 |
| Yersinia enterocholitica | C6-HSL | YenI/YenR | ? | 120 |
| Yersinia pseudotuberculosis | C8-HSL | YesI/YesR | ? | 3 |
It is becoming apparent that in addition to AHLs, alternative gram-negative signaling molecules do exist. For example, the plant pathogen Ralstonia solanacearum produces 3-hydroxypalmitic acid methyl ester as a novel signaling molecule which, together with AHLs, is used to regulate virulence (34). Xanthomonas campestris pv. campestris, a cabbage pathogen, produces a diffusible extracellular factor (DSF) which has yet to be chemically characterized but is not an AHL (5). In Pseudomonas aeruginosa, a third autoinducer, designated PQS (Pseudomonas quinolone signal), was identified that is distinct from the other two AHL autoinducers produced by this organism in that it is a 2-heptyl-3-hydroxy-4-quinolone (82). Butyrolactones have been isolated from Pseudomonas aureofaciens culture supernatants (41), and recently, a novel family of signaling compounds, identified as diketopiperazines (DKPs), were discovered in cell-free supernatants of P. aeruginosa, Pseudomonas fluorescens, Pseudomonas alcaligenes, Enterobacter agglomerans, and Citrobacter freundii (49). Although these molecules were capable of only weakly activating a number of LuxR-based biosensors, some of the DKPs were able to act antagonistically to reduce N-3-(oxohexanoyl)homoserine lactone (3-oxo-C6-HSL)-mediated bioluminescence, suggesting that they may be able to compete for LuxR binding. In nature, DKPs have been isolated from a wide range of sources and have been shown to have pharmacological effects in various mammals, including humans (91); however, the precise role played by DKPs in bacterial cell-to-cell signaling has yet to be established.
PSEUDOMONAS AERUGINOSA
With regard to bacteria that utilize quorum sensing as part of their pathogenic lifestyle, P. aeruginosa is perhaps the best understood in terms of the virulence factors regulated and the role quorum sensing plays in pathogenicity. Classified as an opportunistic pathogen, P. aeruginosa primarily infects individuals who are immunocompromised, such as patients with cancer or AIDS (33, 68) or those having breaches in normal barriers caused by burns, indwelling medical devices, or prolonged use of broad-spectrum antibiotics (11, 23). P. aeruginosa has an impressive armament of both cell-associated and extracellular virulence factors. Expression of many of the extracellular factors is not constitutive but rather cell-density dependent with maximum protease production occurring during the late logarithmic and early stationary phases of growth (123, 124). The genetic basis for this growth-phase regulation was uncovered with the discovery that P. aeruginosa contains genes, called lasR and lasI, with significant homology to the luxR and luxI genes of Vibrio fischeri (42, 76). In V. fischeri, luxR and luxI are involved in the cell-density-dependent regulation of light production (30, 109). The luxR gene encodes a transcriptional activator of the bioluminescence operon, and luxI codes for an autoinducer synthase that directs the synthesis of the autoinducer 3-oxo-C6-HSL (26). Upon binding 3-oxo-C6-HSL, the LuxR protein becomes activated, enabling it to induce transcription of the lux operon. Since the discovery of the lux quorum-sensing system, a number of gram-negative bacteria, including P. aeruginosa, have been found to produce LuxR- and LuxI-type proteins (for reviews, see references 39 and 40).
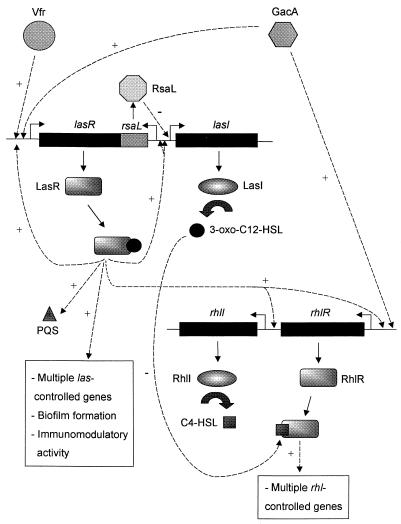
In P. aeruginosa, the transcriptional activator LasR functions in conjunction with its cognate AHL, N-(3-oxododecanoyl)-l-homoserine lactone (3-oxo-C12-HSL), synthesized by the LasI autoinducer synthase (76, 78). LasR–3-oxo-C12-HSL regulates expression of a number of P. aeruginosa virulence genes including lasB, lasA, aprA, and toxA (42, 43, 78, 121) as well as lasI itself, creating an autoinduction feedback loop (106) (Fig. 2). An additional gene, rsaL, is under the regulatory control of LasR–3-oxo-C12-HSL, the product of which negatively regulates P. aeruginosa quorum sensing by inhibiting lasI expression (20).
FIG. 2.
The quorum-sensing circuitry of P. aeruginosa is illustrated. Expression of the lasR gene is subject to at least two levels of control: the global regulators Vfr and GacA (1, 97) and the las quorum-sensing system, which regulates expression of both lasR and lasI. The latter creates an autoinduction feedback loop. Regulation of the rhl system is similar to las in that GacA affects expression of rhlR (97), and the rhlR and rhlI genes are controlled to some degree by the las system. Interestingly, the las quorum-sensing system was shown to elicit an additional level of control over the rhl system; the las signal molecule, 3-oxo-C12-HSL, can act posttranslationally to block RhlR activation by C4-HSL. The las and rhl quorum-sensing systems regulate expression of numerous genes that contribute to the virulence of P. aeruginosa. In addition, the las signal molecule, 3-oxo-C12-HSL, is required for biofilm differentiation and exhibits immunomodulatory activity.
The discovery of a second signaling system revealed that quorum sensing in P. aeruginosa is more complex than originally believed (12, 73, 74, 126). The rhl quorum-sensing system consists of the transcriptional activator RhlR and the autoinducer synthase RhlI which directs the synthesis of N-butyryl-l-homoserine lactone (C4-HSL) (79). The RhlR–C4-HSL complex regulates expression of rhlAB, required for rhamnolipid production, lasB, aprA, the stationary-phase sigma factor RpoS, and production of the secondary metabolites pyocyanin and cyanide (12, 60, 61, 73, 79, 126).
With the finding that P. aeruginosa has two separate quorum-sensing circuits came the question of whether the two were capable of interaction. In spite of the predicted structural similarities between LasR and RhlR and the similarities between the two AHLs, there is little interchangeability between the two systems. The R-proteins are not significantly activated by their noncognate AHLs; LasR is not activated by C4-HSL and 3-oxo-C12-HSL is capable of only low-level RhlR activation (80). Thus it appears that the R proteins show high specificity with regard to the AHL required for their activation. Similarly, genes that are primarily activated by one system are only minimally activated by the other (80), indicating that specific recognition sequences must be present in the operator regions of these target genes that dictate which quorum-sensing system is required for induction. Despite the high fidelity of these systems for their regulatory components and gene targets, a link between the two systems does exist. The las system positively regulates expression of both rhlR and rhlI (60, 83) (Fig. 2). Furthermore, 3-oxo-C12-HSL is able to compete with C4-HSL for RhlR binding, indicating that 3-oxo-C12-HSL is able to act as an antagonist of the rhl system (83). Thus, it appears that in P. aeruginosa, quorum sensing is arranged in a hierarchical fashion with the las system being the dominant regulator.
In addition to 3-oxo-C12-HSL and C4-HSL, which are the major AHLs produced by P. aeruginosa grown in the laboratory, minor AHL products can also be detected (78). A complete description of the AHL biosynthetic pathways is beyond the scope of this review (for a review, see reference 37); however, the autoinducer synthase molecules examined to date have been found to use S-adenosylmethionine and the appropriate fatty acid conjugated to acyl carrier protein (ACL) as substrates. In P. aeruginosa, in vitro studies of AHL synthesis have revealed that the majority, if not all, of the 3-oxo-HSLs found in culture supernatants are synthesized by LasI (H. Schweizer, personal communication). Furthermore, when one of the enzymatic steps of the fatty acid biosynthetic pathway becomes rate limiting, 3-oxo-C12-HSL is no longer produced at detectable levels; instead, the shorter-chain-length HSLs 3-oxo-C10-HSL, 3-oxo-C8-HSL and 3-oxo-C6-HSL are preferentially generated (H. Schweizer, personal communication). These findings indicate that the acyl chain lengths of the HSL products are at least in part regulated by the availability of the 3-oxo-acyl-ACP substrate precursors.
To date, the biological function of these noncognate AHLs remains an enigma. One possible role for these minor AHL molecules is to activate additional LuxR-type proteins. In P. aeruginosa, two genes encoding proteins with significant homology to LasR and RhlR have been identified; however, at this time it is unclear whether the minor signal molecules present in P. aeruginosa culture supernatants can activate either of these R proteins. A second possible role for noncognate AHLs arises from the fact that these molecules can frequently activate a given R protein, albeit at lower induction levels than for the cognate AHL. In this manner, minor AHLs may function as competitive inhibitors of autoinduction. An example of this is seen in P. aeruginosa where the las signal molecule 3-oxo-C12-HSL can efficiently compete with C4-HSL for RhlR binding (83). Similarly in V. fischeri, a second AHL synthase, AinS, directs the synthesis of N-octanoyl-l-HSL (C8-HSL) (59). Despite the fact that C8-HSL can activate LuxR to some degree, it appears that this molecule functions as a competitive inhibitor of V. fischeri bioluminescence. In ainS mutants, induction of bioluminescence occurs at a lower cell density than in the parental strain (59). Furthermore, addition of C8-HSL to cultures of either the wild-type strain or ainS mutants results in delayed onset of bioluminescence (59). Thus, in both P. aeruginosa and V. fischeri, the inhibitory effect of noncognate AHLs may represent a means of “fine tuning” these quorum-sensing systems to precisely control expression of target genes.
Recently, a third autoinducer molecule was identified in P. aeruginosa (82). This molecule is structurally very different from the other two P. aeruginosa autoinducers in that it is a 2-heptyl-3-hydroxy-4-quinolone, designated PQS. Preliminary studies have revealed that PQS is involved in lasB expression and that although expression of PQS is under control of the las system, RhlR is required for PQS activity. At present, many aspects of PQS have yet to be uncovered, including the role it plays in P. aeruginosa quorum sensing and virulence and the R protein with which it reacts. The structural similarity between PQS and antimicrobial quinolones is quite intriguing, although preliminary studies have not shown any antimicrobial activity associated with PQS (82). The discovery of PQS reveals yet another layer in the increasingly complex system used by this organism to maintain tight control of its virulence factors. This tight regulation is a common theme in P. aeruginosa quorum sensing, evidenced by the fact that the xcp genes involved in type II secretion are under control of both the las and rhl quorum-sensing systems (14). This pathway is utilized in secretion of quorum-sensing controlled enzymes, such as elastase and proteases, indicating that P. aeruginosa is extremely vigilant about regulating these factors at both the levels of production and export.
P. aeruginosa is intrinsically resistant to numerous antimicrobial agents, including antibiotics, organic solvents, and detergents. Low outer membrane permeability together with the presence of multidrug efflux pumps that export a wide range of antimicrobial agents is thought to contribute to this intrinsic resistance. Three well-studied P. aeruginosa pumps have been described: MexAB-OprM, MexCD-OprJ, and MexEF-OprN encoded by the mexAB-oprM, mexCD-oprJ, and mexEF-oprN operons, respectively (58, 89, 90). During a study to investigate whether AIs freely diffuse in and out of P. aeruginosa cells, it was discovered that in addition to its slow diffusion, 3-oxo-C12-HSL is actively pumped from cells by the MexAB-OprM pump (81). In contrast, C4-HSL diffuses rapidly across the cell membranes and is not actively transported (81). Presumably, the difference in the length of the acyl chains accounts for the differences in cellular accumulation of the two AIs, with the more hydrophobic 3-oxo-C12-HSL partitioning into the cytoplasmic membrane, thereby facilitating its export by the MexAB-OprM pump. These findings are intriguing because they suggest that antimicrobial therapy designed to interfere with MexAB-OprM drug efflux will also affect las-controlled gene expression. In cells lacking a functional MexAB-OprM pump, a higher accumulation of 3-oxo-C12-HSL would be expected to occur sooner, which should result in earlier expression of target genes. It has been theorized that bacteria employ quorum sensing for regulation of virulence to ensure that toxic immune response-activating factors are elicited only after a sufficient number of bacteria have been amassed to overwhelm host defenses. If the bacteria are forced to prematurely produce virulence factors, the host may recognize the invading bacteria sooner and eradicate the infection. Thus, antimicrobial strategies designed to disarm efflux pumps and increase the antibiotic susceptibility of P. aeruginosa may prove even more effective if they cause premature expression of virulence products.
Quorum sensing in P. aeruginosa is involved in regulating expression of a number of virulence factors, and as such, this regulation is believed to play an important role in the pathogenicity of this organism. Using a number of different animal models, this presumption has been confirmed. In the neonatal mouse model of pneumonia, a lasR-deficient strain of P. aeruginosa was found to have significantly decreased virulence compared to that in the parent (117). Analysis of a lasI mutant, a rhlI mutant, and a lasI rhlI double mutant in the same model revealed markedly decreased virulence, with the most notable reduction seen in the double I mutant (77). In a burned mouse model, strains deficient in lasR, lasI, rhlI, or both lasI and rhlI were found to be less virulent in vivo than in the parental strain (101, 102). In addition, the total number of bacteria recovered from the spleens, livers, and skin of mice infected with the different mutants were significantly lower than those for the parent strain (102). These findings indicate that quorum sensing plays an important role in the dissemination of P. aeruginosa throughout the body of burned mice. In the double I mutant, which was the least virulent strain, complementation with lasI, rhlI, or both lasI and rhlI on multicopy plasmid significantly increased both in vivo virulence and the ability to spread within the burned skin of the infected animals (102).
In a study employing three different models of infection, namely Caenorhabditis elegans (nematode), Arabidopsis thaliana (plant), and a burned mouse model, a lasR-deficient mutant generated through random mutagenesis exhibited greatly reduced virulence in all three models (116). Intriguingly, a gacA mutant and a toxA mutant also exhibited decreased virulence in the three models (93, 94, 116). GacA is a global activator in P. aeruginosa that has previously been shown to regulate expression of lasR and rhlR and production of the rhl AHL, C4-HSL (97); toxA encodes exotoxin A, which is regulated by the las quorum-sensing system (43). These studies are extremely exciting because they suggest that the three aforementioned genes, which are all linked to quorum sensing, contribute to the trans-kindom virulence of P. aeruginosa. Moreover, using the less costly and simpler plant or nematode model of infection enables identification of genes required for infection of other species. In the future, it will be intriguing to see if other bacteria that infect multiple species. In the future, it will be intriguing to see if other bacteria that infect multiple species and employ quorum sensing as part of their pathogenic lifestyles have genes that contribute to virulence in such diverse hosts.
In a study designed to assess the role of P. aeruginosa quorum sensing in human infections, sputum samples from the lungs of cystic fibrosis (CF) patients infected with P. aeruginosa were assayed for lasR, lasA, lasB, and toxA expression (111). A correlation was observed between lasA, lasB, and toxA transcript accumulation, suggesting coordinated regulation of these genes. Moreover, accumulation of the lasR transcript correlated with that of the other genes; thus, it appears that LasR–3-oxo-C12-HSL actively regulates gene expression during chronic lung infection.
BURKHOLDERIA CEPACIA: EVIDENCE OF A ROLE FOR INTERSPECIES COMMUNICATION IN PATHOGENICITY?
B. cepacia (formerly Pseudomonas cepacia) has emerged as a formidable pathogen in individuals with CF (46). In most instances, patients colonized with B. cepacia are coinfected with P. aeruginosa (118), and this has led to speculation whether interspecies communication using P. aeruginosa AHLs can enhance the pathogenicity of B. cepacia. Addition of P. aeruginosa-spent media to cultures of B. cepacia resulted in a substantial increase in both protease synthesis (twofold) and siderophore production (sevenfold), suggesting the presence of a quorum-sensing system (66). Subsequently, luxRI homologs have been identified in B. cepacia, called cepR and cepI (62). The CepRI quorum-sensing system was found to have both a positive and negative regulatory role in B. cepacia, increasing protease production while simultaneously decreasing siderophore synthesis (62). In culture supernatants, the concentration of B. cepacia AHL, identified as C8-HSL, was found to be 1,000-fold less than the concentration of 3-oxo-C12-HSL and C4-HSL in P. aeruginosa culture supernatants (62). Whether B. cepacia actually produces C8-HSL in minute amounts or the conditions that were used for AHL production were not optimal has yet to be determined. However, it is possible that B. cepacia colonization of the CF lung succeeds infection with other microorganisms, like P. aeruginosa, because B. cepacia can utilize the exogenous AHLs produced by other bacteria to initiate infection. As such, B. cepacia represents an example of an organism that profits from the energy investment made by others to regulate its own pathogenicity and may provide evidence for communication between different bacterial species.
ERWINIA CAROTOVORA
E. carotovora is a phytopathogen that causes soft rot in a variety of plants (6). The pathogenicity of E. carotovora depends on the production of various plant tissue-degrading enzymes, including pectate lyases, polygalacturonase, cellulase, and proteases. These enzymes are involved in maceration of plant tissue necessary for bacterial colonization of the host. Production of enzymes by only a few cells of E. carotovora would not have an effect on the plant tissue, and more likely, it would activate the plant phytodefense mechanisms. Therefore, E. carotovora uses quorum sensing, which ensures that exoenzyme production does not occur until sufficient bacterial numbers have been achieved for successful tissue destruction and evasion of plant defenses (55, 88). This regulation relies on the LuxRI homologs ExpR and ExpI (55, 88) that control expression of the tissue-macerating enzymes in a cell-density-dependent manner. The exact roles of ExpR and its cognate AHL, 3-oxo-C6-HSL, in exoenzyme regulation are not yet clearly defined. Studies have shown that an expI mutant is deficient in exoenzyme production and unable to macerate plant tissues. In contrast, a mutation in expR does not affect enzyme production, and surprisingly, overexpression of expR results in decreased enzyme production (65). These findings have led to the proposal that ExpR may act as a repressor of exoenzyme synthesis by sequestering the levels of 3-oxo-C6-HSL. E. carotovora quorum sensing is made even more complex by the finding that synthesis of the broad-spectrum antibiotic carbapenem is regulated using a second quorum-sensing system. Carbapenem production is regulated by CarR and CarI; the latter catalyzes the synthesis of 3-oxo-C6-HSL (4, 16, 65). When sufficient 3-oxo-C6-HSL is present, it binds to and activates CarR, enabling it to induce expression of the carbapenem biosynthetic genes. Since the release of nutrient-rich constituents from the plant likely promotes the growth of competing microflora, it appears that E. carotovora has developed a sophisticated strategy to counteract this competition by coordinating production of carbapenem with the tissue-macerating enzymes. Two additional regulatory systems, known as RsmA and Aep, have recently been linked to the ExpR/I and CarR/I quorum-sensing circuits. For more details on RsmA and Aep, see reference 86.
AGROBACTERIUM TUMEFACIENS
A. tumefaciens is a pathogen that is capable of causing crown gall tumors in plants through the transfer of oncogenic DNA from its tumor-inducing Ti plasmid to the nuclei of the plant. In addition to the vir genes required for plant transformation, the Ti plasmids also contain a complete set of tra genes that facilitate interbacterial transfer of the Ti plasmid (2, 32). Conjugation in A. tumefaciens is actually regulated by two different signaling mechanisms; one is plant based and the other is bacterium associated. The plant-produced signal regulating expression of the tra genes is a conjugal opine that is produced by crown gall tumors. Opines act as a nutrient source for the infecting bacteria, and production of these compounds is under direction of the Ti-plasmid, as are the enzymes necessary for the import and catabolism of these compounds by the bacteria. The two types of Ti plasmids present in A. tumefaciens differ with respect to the opine that acts as the conjugal signal. Nopaline-type Ti plasmids are induced by agrocinopines A and B (29), whereas conjugation of octopine-type Ti plasmids is induced by octopine (56). The discovery that A. tumefaciens produces a diffusible compound that dramatically stimulates plasmid conjugation (129) together with the identification of a regulator, called TraR, capable of activating expression of the tra genes (87) suggested that conjugal transfer in A. tumefaciens is regulated by a quorum-sensing system. The bacterial compound that stimulated conjugation was identified to be 3-oxo-C8-HSL (130) which is synthesized by the autoinducer synthase TraI (51). TraR–3-oxo-C8-HSL regulates expression of the tra regulon as well as the traI gene itself, thereby creating a positive feedback loop (2, 32, 51, 87). An additional gene, traM, positively regulated by TraR–3-oxo-HSL was found to play a role in A. tumefaciens quorum sensing (50). Overexpression of traM on a multicopy plasmid in the presence of wild-type levels of TraR abolished tra gene expression. However, upon overexpression of TraR, tra gene expression was restored, suggesting that TraM may interact stoichiometrically with TraR to act as an antagonist of the tra regulon.
The A. tumefaciens opine and quorum-sensing signal pathways are linked to one another in a hierarchical fashion, with opines being the dominant regulator. For TraR–3-oxo-C8-HSL signaling to occur, the appropriate opine must be present. In the octopine-type Ti plasmids, this control is mediated by placing the traR gene under regulatory control of an octopine-responsive activator, called OccR (38). It is only when sufficient octopine is present that OccR induces transcription of traR. After the cell density has increased to the point where sufficient 3-oxo-C8-HSL has accumulated to activate TraR, the tra operon is expressed. In the case of the nopaline-type Ti plasmids, the traR gene is also regulated by opines but through a different mechanism. Agrocinopines A and B induce expression of the traR gene; while a mutation in accR, which is believed to encode a repressor, causes constitutive tra gene expression (10). These findings have led to the proposal that AccR directly represses traR expression and that the agrocinopines can act as antagonists of this repression (125).
QUORUM SENSING IN GRAM-POSITIVE ORGANISMS
A number of gram-positive bacteria are known to employ quorum-sensing systems. The nature of the signal molecules used in these systems differs from those of gram-negative organisms, and thus far, no gram-positive bacteria have been shown to produce AHLs. Gram-positive quorum-sensing systems typically make use of small posttranslationally processed peptide signal molecules. These peptide signals interact with the sensor element of a histidine kinase two-component signal transduction system. Quorum sensing is used to regulate the development of bacterial competence in Bacillus subtilis and Streptococcus pneumoniae, conjugation in Enterococcus faecalis, and virulence in Staphylococcus aureus (24, 57). S. aureus causes a wide range of disease states that range from mild to life-threatening. The virulence of this organism is dependent on the temporal expression of a diverse array of virulence factors, including both cell-associated products, such as protein A, collagen- and fibronectin-binding protein, and secreted products including lipases, proteases, alpha-toxin, toxin-1, beta-hemolysin, and enterotoxin (25, and references therein). During the early stages of S. aureus infection, surface proteins involved in attachment (collagen- and fibronectin-binding protein) and defense (protein A) predominate. However, once a high cell density is achieved at the infection site, expression of S. aureus surface proteins is decreased and secreted proteins are preferentially expressed. The genetic basis for this temporal gene expression depends on two pleiotropic regulatory loci called agr (accessory gene regulator) (71, 96) and sar (staphylococcal accessory gene regulator) (15).
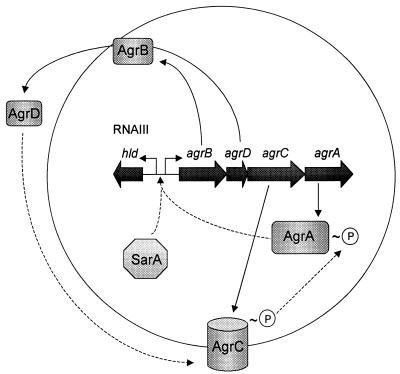
The agr locus consists of two divergently transcribed operons, RNAII and RNAIII (52, 53) (Fig. 3). The RNAII operon contains the agrBDCA genes that encode the signal transducer (AgrC) and response regulator (AgrA), and AgrB and AgrD which are involved in generating the quorum-sensing signal molecule. The RNAIII operon encodes a δ-hemolysin and is itself a regulatory RNA that plays a key role in the agr response. During S. aureus quorum sensing, the AgrC signal transducer is autophosphorylated in response to the octopeptide signal molecule, which in turn leads to the phosphorylation of the AgrA response regulator (63). Phosphorylated AgrA stimulates transcription of RNAIII and RNAIII, in turn, upregulates expression of numerous S. aureus exoproteins as well as the agrBDCA locus (52, 53). The latter leads to a rapid increase in the synthesis and export of the octopeptide signal molecule. At the second regulatory locus, the sar gene product (SarA) functions as a regulatory DNA-binding protein to induce expression of both the RNAII and RNAIII operons of the agr locus (95).
FIG. 3.
Quorum sensing in S. aureus is depicted. The agr locus consists of two divergent transcriptional units. One operon (agrBDCA) encodes the proteins responsible for generating and sensing the peptide signal molecule (AgrD), and the other encodes δ-hemolysin and RNAIII. The signal molecule is an octapeptide cleaved from the middle of the agrD gene product and is exported from the cell via the membrane-associated AgrB protein. Once the extracellular concentration of AgrD reaches a threshold level, it stimulates autophosphorylation of the transmembrane AgrC protein, which in turn phosphorylates the response regulator (AgrA). In its phosphorylated state, AgrA activates expression of RNAIII. The rise in the level of regulatory RNAIII leads to increased secretion of numerous factors, reduced expression of specific surface proteins, and induced expression of the agrBDCA operon. SarA is a DNA-binding protein, encoded by the sar locus, that upregulates expression of both agr operons.
EXPLOITING BACTERIAL QUORUM SENSING
Quorum sensing, a novel target for antimicrobial therapy.
The continuing emergence of multiple-drug-resistant strains of bacteria has necessitated finding novel strategies for treating bacterial infections. The discovery that a wide spectrum of organisms use quorum sensing to control virulence factor production makes it an attractive target for antimicrobial therapy. Through blocking this cell-to-cell signaling mechanism, pathogenic organisms that use quorum sensing to control virulence could potentially be rendered avirulent. Several possible ways of interrupting the quorum-sensing circuitry exist; for example, as described earlier, autoinducers and R proteins have a unique specificity for one another. Noncognate autoinducers typically only weakly activate or may inhibit R protein activation all together. Therefore, analogs that bind to but do not activate R proteins could act as antagonists to prevent autoinducer binding, which in turn would shut down the quorum-sensing cascade. The ability of autoinducer analogs to inhibit activation of R proteins has already been demonstrated in a number of bacteria, including V. fischeri, A. tumefaciens, Chromobacterium violaceum, and Aeromonas salmonicida (69, 103, 114, 131). Examples of this inhibition have been found to exist in nature. In P. aeruginosa, 3-oxo-C12-HSL is able to antagonize RhlR activation by C4-HSL, and production of C8-HSL by V. fischeri delays the onset of bioluminescence, presumably by competing with 3-oxo-C6-HSL for LuxR binding. Moreover the seaweed Delisea pulchra produces furanone compounds, structurally similar to AHLs, that are capable of interfering with the quorum-sensing systems of Serratia liquefaciens, V. fischeri, and Vibrio harveyi (45).
In an exciting recent report, an enzyme from an isolate of Bacillus that is capable of degrading AHLs was discovered (22). This enzyme is encoded by the aiiA gene (autoinducer inactivation) and contains two domains that are homologous to the active sites of the following metalloenzymes: glyoxalase II, metalloβ-lactamase, and arylsulfatase (22). Expression of aiiA in E. carotovora decreased generation of pectolytic enzymes and significantly reduced AI production. Even more noteworthy was the finding that expression of the AiiA enzyme in E. carotovora attenuated soft rot disease on all of the plants tested (22). In the future it may be possible to confer resistance to soft-rot and other diseases brought on by bacteria that regulate virulence via autoinduction by providing plants with the aiiA gene.
As a third means of interfering with quorum sensing, the biosynthetic pathways of some AHL molecules have been elucidated (48, 54, 70, 104). Interrupting the AHL biosynthetic pathway and shutting down AHL synthesis, perhaps through the use of analogs of AHL precursors, would be a highly effective means of blocking the quorum-sensing cascade.
In organisms that employ more than one quorum-sensing system for virulence regulation, it may be necessary to disarm all of the systems present to attenuate virulence. This was found to be the case with P. aeruginosa. In this organism, the las quorum-sensing system controls expression of the rhl system, suggesting that shutting down the las circuit should be sufficient to abolish quorum-sensing regulated virulence factor production. However, after growth under high stress conditions, spontaneous mutants of a lasR-deficient strain capable of elastase and rhamnolipid production were isolated (122). Analysis of one of these mutants, PR1-E4, revealed increased rhlI expression relative to the parent and likely accounts for the increased production of rhamnolipid and elastase (122). Conversely, when both the las and rhl systems were nonfunctional, mutants with restored production of virulence factors could not be recovered. These findings suggest that for P. aeruginosa, therapeutic strategies will have to target both the las and the rhl quorum-sensing systems to be most effective.
Recently, quorum sensing was found to regulate expression of the type III secretion systems of both enterohemorrhagic Escherichia coli (EHEC) and enteropathogenic E. coli (EPEC) (110). Detection of AI molecules produced by E. coli was first reported because of the ability of these signals to activate one of the two V. harveyi quorum-sensing systems, called the autoinducer 2 (AI-2) system (112). Subsequently, synthesis of AI-2 was shown to be dependent on the luxS gene (113) and homologues of luxS have been identified in a number of gram-negative and gram-positive bacteria (113). Outside of V. harveyi and now EHEC and EPEC, little is known about the functions that are controlled by this class of signaling molecules. Both EHEC and EPEC interact with intestinal epithelia to cause attaching and effacing lesions. These lesions result from gene products encoded by a pathogenicity island called the locus of enterocyte effacement (LEE) (28, 64), which encodes a type III secretion system and other products involved in lesion formation. Using lacZ fusions, it was discovered that the majority of the LEE-encoded genes were quorum-sensing regulated (110). These findings suggest that strategies designed to interfere with quorum sensing may be useful for treating and preventing the devastating effects of EPEC and EHEC infections. Furthermore, many gram-negative bacteria employ both quorum-sensing and type III secretion systems, and one might speculate that these two systems are intimately associated, both with each other and with the regulation of virulence in many pathogenic bacteria.
Quorum sensing and biofilm formation.
In nature, bacteria are frequently found encased in a polysaccharide matrix attached to a solid surface. This mode of growth, referred to as a biofilm, offers protection from environmental agents that would otherwise threaten their planktonic counterparts. P. aeruginosa is an example of an organism frequently found growing in biofilms. Microscopic analysis of P. aeruginosa biofilm communities reveals that they are not just sugar-encased masses of cells. Rather distinct mushroom and stalk-like structures that contain intervening water channels to allow nutrients to flow in and waste products to flow out are present. Because they pose problems of both medical and industrial importance, the ability of bacteria, such as P. aeruginosa, to form biofilms is of profound interest. In the clinical setting, biofilms formed on medical devices and in bacterial infections can wreak havoc, largely because bacteria growing as a biofilm are refractile to host defenses including phagocytes, antibodies, and complement (17). Moreover, these organisms are highly resistant to antibiotics, making eradication by using conventional chemotherapy virtually ineffectual. These findings underscore the need to find novel ways of preventing biofilm formation and eradicating those already established. Recently, a link between biofilm formation and quorum sensing was discovered in P. aeruginosa. Analysis of biofilms formed by a P. aeruginosa mutant deficient in the production of the las signal molecule, 3-oxo-C12-HSL, revealed a biofilm that was much thinner and lacked the three-dimensional architecture observed in that of the parent (19). Even more noteworthy was the fact that, while the parental biofilm was resistant to the detergent sodium dodecyl sulfate (SDS), the mutant biofilm rapidly dispersed from the underlying surface after SDS exposure. When grown in the presence of exogenous 3-oxo-C12-HSL, the mutant biofilm resembled that of the parent and was resistant to SDS. Thus, it appears that quorum sensing plays a critical role in the formation of mature, differentiated biofilm structures. It is not known at this time if other bacteria use quorum sensing during biofilm formation; however these findings suggest that, at least in the case of P. aeruginosa, strategies designed to block quorum sensing may be an effective means of preventing biofilm formation.
Quorum sensing as a means of biological control in agriculture.
Many plant-associated bacteria employ quorum sensing for regulation of specific phenotypes as part of their pathogenic or symbiotic lifestyles. As such, the ability to block or promote these quorum-sensing systems may offer new strategies for managing plant diseases and increasing crop productivity. In a recent study in which plants were genetically modified to produce AHLs, the feasibility of using plant-produced AHLs to manipulate bacterium-plant associations was realized (36). In these studies, plasmids containing the Yersinia enterocolitica yenI gene were expressed in the chloroplasts of tobacco plants. YenI directs the synthesis of C6-HSL and 3-oxo-C6-HSL in a 1:1 ratio, and these compounds are the cognate AHLs for the plant symbiont P. aureofaciens and the plant pathogen E. carotovora, respectively. E. carotovora, as discussed earlier, is classed as a plant pathogen due to its quorum-sensing-regulated production of plant-degrading enzymes. In contrast, P. aureofaciens 30-84 is a symbiotic bacterium that can protect wheat from take-all, a disease caused by the fungus Gaeumannomyces graminis var. tritici (85). P. aureofaciens 30-84 produces three phenazine antibiotics that contribute to this disease suppression, production of which is regulated by the PhzR/I quorum-sensing system (84, 128).
Intriguingly, Fray and coworkers (36) discovered that AHLs diffused from the chloroplastic organs of the tobacco plant and from the roots as well. The plant-produced AHLs induced bioluminescence in an E. coli strain containing an AHL-activated lux reporter. Furthermore, the AHLs restored the antifungal activity of a “disarmed” P. aureofaciens 30-84 phzI strain and enabled an avirulent E. carotovora carI mutant to infect the transgenic plants (36). Thus, the plant-produced AHLs appear to behave in a manner similar to that of their bacterial counterparts.
The benefits of using plant-produced AHLs for modifying the behavior of symbiotic bacteria are clear. For example, these AHLs could be used to promote an antifungal environment by P. aureofaciens, or alternatively, they might enhance the ability of nitrogen-fixing bacteria such as rhizobial species. In the case of pathogenic E. carotovora, the ability to regulate expression of plant-degrading enzymes in a cell-density-dependent manner is believed to contribute to the virulence of this organism. It is only after high cell densities have been achieved that the bacteria are able to successfully compete with the plant host defenses. If the production of plant-degrading enzymes were induced prematurely, when bacterial numbers were low, then the plant might be able to mount an effective defense. Indeed, resistance to E. carotovora infection has been observed in plants treated with salicylic acid, which induces the plant phytodefense system (75). Therefore, production of AHLs in plants that are hosts for E. carotovora, such as potatoes and carrots, may afford protection from the consequences of bacterial infection.
CONCLUDING REMARKS
The ability to coordinate behavior in a cell-density-dependent fashion has several obvious advantages. In the case of pathogenic microorganisms, the regulation of virulence determinants throughout the infection process is believed to play an important role in pathogenicity. Evading host defenses is a major goal of pathogens, and as such, quorum sensing is an important asset because it enables bacteria to appropriately time expression of immune response-activating products. Using quorum sensing, bacteria can amass a high cell density before virulence determinants are expressed, and in so doing, the bacteria are able to make a concerted attack and produce ample virulence factors to overwhelm the host defenses.
In this minireview we have discussed the diverse role of AHL signaling molecules in bacterial cell-to-cell communication, as well as their potential role in the interaction of bacteria with eucaryotic hosts. To think that these AHL molecules, which readily diffuse across cell membranes, have no direct effect on the eucaryotic cells is somewhat naïve. Indeed evidence exists to suggest that these AHL signal molecules interact directly with eucaryotic cells to modulate host immune responses. As an example, the P. aeruginosa AHL 3-oxo-C12-HSL was shown to elicit interleukin-8 production in a respiratory epithelial cell line (21). Because interleukin-8 is a neutrophil chemoattractant, it seems improbable that this response affords any benefit to P. aeruginosa. It is more likely the AHL is having the unintentional effect of acting as a signal to warn the host of the presence of this bacterium. In another study, Telford and coworkers (119) discovered that 3-oxo-C12-HSL suppressed release of interleukin-12 and tumor necrosis factor alpha from lipopolysaccharide-stimulated macrophages. In this instance, 3-oxo-C12-HSL may be behaving as a virulence factor directly, by modulating the inflammatory responses of the host. The ability of these signal molecules to act as virulence determinants themselves suggests a possible role for AHLs produced by organisms for which a quorum-sensing-regulated phenotype has not been ascribed, such as in Yersinia.
As the list of bacteria that employ quorum-sensing systems continues to grow, so does the number of possibilities for exploiting these regulatory mechanisms. Because many important animal and plant pathogens use quorum sensing to regulate virulence, strategies designed to interfere with these signaling systems will likely have broad applicability for biological control of disease-causing organisms. In the future, it will be intriguing to see whether additional human pathogens utilize quorum sensing as part of their pathogenic lifestyle and, if so, whether production of the signal molecules, AHL or otherwise, can be exploited to control infections. The discovery that P. aeruginosa uses quorum sensing to regulate biofilm production suggests that agents capable of blocking quorum sensing may also be useful for preventing biofilm formation. The recent production of AHLs in plants represents an exciting new approach to controlling crop diseases as well as to manipulating plant-microbe interactions for improved crop production in the future.
REFERENCES
- 1.Albus A M, Runyen-Janecky L J, West S E H, Iglewski B H. Vfr controls quorum sensing in Pseudomonas aeruginosa. J Bacteriol. 1997;179:3928–3935. doi: 10.1128/jb.179.12.3928-3935.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alt-Mörbe J, Stryker J L, Fuqua C, Farrand S K, Winans S C. The conjugal transfer system of A. tumefaciens octopine-type Ti-plasmids is closely related to the transfer system of an IncP plasmid and distantly related to Ti plasmid vir genes. J Bacteriol. 1996;178:4248–4257. doi: 10.1128/jb.178.14.4248-4257.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkinson S, Throup J P, Stewart G S, Williams P. A hierarchical quorum-sensing system in Yersinia pseudotuberculosis is involved in the regulation of motility and clumping. Mol Microbiol. 1999;33:1267–1277. doi: 10.1046/j.1365-2958.1999.01578.x. [DOI] [PubMed] [Google Scholar]
- 4.Bainton N J, Stead P, Chhabra S R, Bycroft B W, Salmond G P C, Steward G S A B, Williams P. N-(3-Oxohexanoyl)-l-homoserine lactone regulates carbapenem antibiotic production in Erwinia carotovora. Biochem J. 1992;288:997–1004. doi: 10.1042/bj2880997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barber C E, Tang J L, Fend J X, Pan M Q, Wilson T J G, Slater H, Dow J M, Williams P, Daniels M J. A novel regulatory system required for pathogenicity of Xanthomonas campestris is mediated by a small diffusible signal molecule. Mol Microbiol. 1997;24:555–566. doi: 10.1046/j.1365-2958.1997.3721736.x. [DOI] [PubMed] [Google Scholar]
- 6.Barras F, van Gijsegem F, Chatterjee A K. Extracellular enzymes and pathogenesis of soft-rot Erwinia. Annu Rev Phytopathol. 1994;32:201–234. [Google Scholar]
- 7.Bassler B L, Wright M, Showalter R E, Silverman M R. Intercellular signalling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol Microbiol. 1993;9:773–786. doi: 10.1111/j.1365-2958.1993.tb01737.x. [DOI] [PubMed] [Google Scholar]
- 8.Bassler B L, Wright M, Silverman M R. Multiple signalling systems controlling expression of luminescence in Vibrio harveyi: sequence and function of genes encoding a second sensory pathway. Mol Microbiol. 1994;13:273–286. doi: 10.1111/j.1365-2958.1994.tb00422.x. [DOI] [PubMed] [Google Scholar]
- 9.Beck von Bodman S, Farrand S K. Capsular polysaccharide biosynthesis and pathogenicity in Erwinia stewartii require induction by a N-acyl-homoserine lactone autoinducer. J Bacteriol. 1995;177:5000–5008. doi: 10.1128/jb.177.17.5000-5008.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beck von Bodman S, Hayman G T, Farrand S K. Opine catabolism and conjugal transfer of the nopaline Ti plasmid pTiC58 are coordinately regulated by a single repressor. Proc Natl Acad Sci USA. 1992;89:643–647. doi: 10.1073/pnas.89.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brewer S C, Wunderink R G, Jones C B, Leeper K V J. Ventilator-associated pneumonia due to Pseudomonas aeruginosa. Chest. 1996;109:1019–1029. doi: 10.1378/chest.109.4.1019. [DOI] [PubMed] [Google Scholar]
- 12.Brint J M, Ohman D E. Synthesis of multiple exoproducts in Pseudomonas aeruginosa is under the control of RhlR-RhlI, another set of regulators in strain PAO1 with homology to the autoinducer-responsive LuxR-LuxI family. J Bacteriol. 1995;177:7155–7163. doi: 10.1128/jb.177.24.7155-7163.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao J, Meighen E A. Purification and structural identification of an autoinducer for the luminescence system of Vibrio harveyi. J Biol Chem. 1989;264:21670–21676. [PubMed] [Google Scholar]
- 14.Chapon-Herve V, Akrim M, Latifi A, Williams P, Lazdunski A, Bally M. Regulation of the xcp secretion pathway by multiple quorum-sensing modulons in Pseudomonas aeruginosa. Mol Microbiol. 1997;24:1169–1178. doi: 10.1046/j.1365-2958.1997.4271794.x. [DOI] [PubMed] [Google Scholar]
- 15.Cheung A L, Koomey J M, Butler C A, Projan S J, Fischetti V A. Regulation of exoprotein expression in Staphylococcus aureus by a locus (sar) distinct from agr. Proc Natl Acad Sci USA. 1992;89:6462–6466. doi: 10.1073/pnas.89.14.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chhabra S R, Stead P, Bainton N J, Salmond G P C, Stewart G S A B, Williams P, Bycroft B W. Autoregulation of carbapenem biosynthesis in Erwinia carotovora ATCC 39048 by analogues of N-3-(oxohexanoyl)-l-homoserine lactone. J Antibiot. 1993;46:441–454. doi: 10.7164/antibiotics.46.441. [DOI] [PubMed] [Google Scholar]
- 17.Costerton J W, Stewart P S, Greenberg E P. Bacterial biofilms: a common cause of persistant infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 18.Cubo M T, Economou A, Murphy G, Johnston A W B, Downie J A. Molecular characterization and regulation of the rhizosphere-expressed genes rhiABCR that can influence nodulation by Rhizobium leguminosarum biovar viciae. J Bacteriol. 1992;174:4026–4035. doi: 10.1128/jb.174.12.4026-4035.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies D G, Parsek M R, Pearson J P, Iglewski B H, Costerton J W, Greenberg E P. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1997;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 20.de Kievit T R, Seed P C, Passador L, Nezezon J, Iglewski B H. RsaL, a novel repressor of virulence gene expression in Pseudomonas aeruginosa. J Bacteriol. 1999;181:2175–2184. doi: 10.1128/jb.181.7.2175-2184.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DiMango E, Zar H J, Bryan R, Prince A. Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin-8. J Clin Investig. 1995;96:2204–2210. doi: 10.1172/JCI118275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong Y-H, Xu J-L, Li X-Z, Zhang L-H. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc Natl Acad Sci USA. 2000;97:3526–3531. doi: 10.1073/pnas.060023897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunn M, Wunderink R G. Ventilator-associated pneumonia caused by Pseudomonas infection. Clinics Chest Med. 1995;16:95–109. [PubMed] [Google Scholar]
- 24.Dunny G M, Hirt H, Erlandsen S. Multiple roles for enterococcal sex pheromone peptides in conjugation, plasmid maintenance and pathogenesis. In: England R, Hobbs G, Bainton N, Roberts D M, editors. Microbial signalling and communication. Cambridge, United Kingdom: University Press; 1999. pp. 117–138. [Google Scholar]
- 25.Dunny G M, Leonard B A B. Cell-cell communication in Gram-positive bacteria. Annu Rev Microbiol. 1997;51:527–564. doi: 10.1146/annurev.micro.51.1.527. [DOI] [PubMed] [Google Scholar]
- 26.Eberhard A, Burlingame A L, Eberhard C, Kenyon G L, Nealson K H, Oppenheimer J. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry. 1981;20:2444–2449. doi: 10.1021/bi00512a013. [DOI] [PubMed] [Google Scholar]
- 27.Eberl L, Winson M K, Sternberg C, Stewart G S A B, Christiansen G, Chhabra S R, Bycroft B W, Williams P, Molin S, Givskov M. Involvement of N-acyl-l-homoserine lactone autoinducers in controlling the multicellular behavior of Serratia liquifaciens. Mol Microbiol. 1996;20:127–136. doi: 10.1111/j.1365-2958.1996.tb02495.x. [DOI] [PubMed] [Google Scholar]
- 28.Elliott S J, Wainwright L A, McDaniel T K, Jarvis K G, Deng Y K, Lai L C, McNamara B P, Donnenberg M S, Kaper J B. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coli E2348/69. Mol Microbiol. 1998;28:1–4. doi: 10.1046/j.1365-2958.1998.00783.x. [DOI] [PubMed] [Google Scholar]
- 29.Ellis J G, Kerr A, Petit A, Tempé J. Conjugal transfer of nopaline and agropine Ti-plasmids—the role of agrocinopines. Mol Gen Genet. 1982;186:269–273. [Google Scholar]
- 30.Engebrecht J, Nealson K H, Silverman M. Bacterial bioluminescence: isolation and genetic analysis of the functions from Vibrio fischeri. Cell. 1983;32:773–781. doi: 10.1016/0092-8674(83)90063-6. [DOI] [PubMed] [Google Scholar]
- 31.Engebrecht J, Silverman M. Nucleotide sequence of the regulatory locus controlling expression of bacterial genes for bioluminescence. Nucleic Acids Res. 1987;15:10455–10467. doi: 10.1093/nar/15.24.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farrand S K, Hwang I, Cook D M. The tra region of the nopaline-type Ti plasmid is a chimera with elements related to the transfer systems of RSF1010, RP4, and F. J Bacteriol. 1996;178:4233–4247. doi: 10.1128/jb.178.14.4233-4247.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fergie J E, Shema S J, Lott L, Crawford R, Patrick C C. Pseudomonas aeruginosa bacteremia in immunocompromised children: analysis of factors associated with a poor outcome. Clin Infect Dis. 1994;18:390–394. doi: 10.1093/clinids/18.3.390. [DOI] [PubMed] [Google Scholar]
- 34.Flavier A B, Clough S J, Schell M A, Denny T P. Identification of 3-hydroxypalmitic acid methyl ester as a novel autoregulator controlling virulence in Ralstonia solanacearum. Mol Microbiol. 1997;26:251–259. doi: 10.1046/j.1365-2958.1997.5661945.x. [DOI] [PubMed] [Google Scholar]
- 35.Flavier A B, Ganova-Raeva L M, Schell M A, Denny T P. Hierarchical autoinduction in Ralstonia solanacearum: control of acyl-homoserine lactone production by a novel autoregulatory system responsive to 3-hydroxypalmitic acid ester. J Bacteriol. 1997;179:7089–7097. doi: 10.1128/jb.179.22.7089-7097.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fray R G, Throup J P, Daykin M, Wallace A, Williams P, Stewart G S A B, Grierson D. Plants genetically modified to produce N-acylhomoserine lactones communicate with bacteria. Nat Biotechnol. 1999;17:1017–1020. doi: 10.1038/13717. [DOI] [PubMed] [Google Scholar]
- 37.Fuqua C, Eberhard A. Signal generation in autoinduction systems: synthesis of acylated homoserine lactones by LuxI-type proteins. In: Dunny G M, Winans S C, editors. Cell-cell signaling in bacteria. Washington, D.C.: ASM Press; 1999. pp. 211–230. [Google Scholar]
- 38.Fuqua C, Winans S C. Localization of OccR-activated and TraR-activated promoters that express two ABC-type permeases and the traR gene of Ti plasmid pTiR10. Mol Microbiol. 1996;20:1199–1210. doi: 10.1111/j.1365-2958.1996.tb02640.x. [DOI] [PubMed] [Google Scholar]
- 39.Fuqua W C, Winans S C, Greenberg E P. Quorum sensing in bacteria: the LuxR/LuxI family of cell density-responsive transcriptional regulators. J Bacteriol. 1994;176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fuqua W C, Winans S C, Greenberg E P. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu Rev Microbiol. 1996;50:727–751. doi: 10.1146/annurev.micro.50.1.727. [DOI] [PubMed] [Google Scholar]
- 41.Gamard P, Sauriol F, Benhamou N, Belanger R R, Paulitz T C. Novel butyrolactones with antifungal activity produced by Pseudomonas aureofaciens strain 63-28. J Antibiot. 1997;50:742–749. doi: 10.7164/antibiotics.50.742. [DOI] [PubMed] [Google Scholar]
- 42.Gambello M J, Iglewski B H. Cloning and characterization of the Pseudomonas aeruginosa lasR gene: a transcriptional activator of elastase expression. J Bacteriol. 1991;173:3000–3009. doi: 10.1128/jb.173.9.3000-3009.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gambello M J, Kaye S, Iglewski B H. LasR of Pseudomonas aeruginosa is a transcriptional activator of the alkaline protease gene (apr) and an enhancer of exotoxin A expression. Infect Immun. 1993;61:1180–1184. doi: 10.1128/iai.61.4.1180-1184.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Givskov M, Eberl L, Molin S. Control of exoenzyme production, motility and cell differentiation in Serratia liquifaciens. FEMS Microbiol Lett. 1997;148:115–122. [Google Scholar]
- 45.Givskov M, de Nys R, Manefield M, Gram L, Maximilien R, Eberl L, Molin S, Steinberg P D, Kjelleberg S. Eukaryotic interference with homoserine lactone-mediated prokaryotic signalling. J Bacteriol. 1996;178:6618–6622. doi: 10.1128/jb.178.22.6618-6622.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Govan J R W, Hughes J E, Vandamme P. Burkholderia cepacia: medical, taxonomic and ecological issues. J Med Microbiol. 1996;45:395–407. doi: 10.1099/00222615-45-6-395. [DOI] [PubMed] [Google Scholar]
- 47.Gray K M, Pearson J P, Downie J A, Boboye B E A, Greenberg E P. Cell-to-cell signaling in the symbiotic nitrogen-fixing bacterium Rhizobium leguminosarum: autoinduction of stationary phase and rhizosphere-expressed genes. J Bacteriol. 1996;178:372–376. doi: 10.1128/jb.178.2.372-376.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanzelka B L, Greenberg E P. Quorum sensing in Vibrio fischeri: evidence that S-adenosyl methionine is the amino acid substrate for autoinducer synthesis. J Bacteriol. 1996;178:5291–5294. doi: 10.1128/jb.178.17.5291-5294.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holden M T G, Chhabra S R, de Nys R, Stead P, Bainton N J, Hill P J, Manefield M, Kumar N, Labatte M, England D, Rice S, Givskov M, Salmond G P C, Stewart G S A B, Bycroft B W, Kjelleberg S, Williams P. Quorum-sensing cross talk: isolation and chemical characterization of cyclic dipeptides from Pseudomonas aeruginosa and other Gram-negative bacteria. Mol Microbiol. 1999;33:1254–1266. doi: 10.1046/j.1365-2958.1999.01577.x. [DOI] [PubMed] [Google Scholar]
- 50.Hwang I, Cook D M, Farrand S K. A new regulatory element modulates homoserine lactone-mediated autoinduction of Ti plasmid conjugal transfer. J Bacteriol. 1995;177:449–458. doi: 10.1128/jb.177.2.449-458.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hwang I, Pei-Li L, Zhang L, Piper K R, Cook D M, Tate M E, Farrand S K. TraI, a LuxI homolog, is responsible for production of conjugation factor, the Ti plasmid N-acylhomoserine lactone autoinducer. Proc Natl Acad Sci USA. 1994;91:4639–4643. doi: 10.1073/pnas.91.11.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ji G, Beavis R C, Novick R P. Cell density control of staphylococcal virulence mediated by an octapeptide pheromone. Proc Natl Acad Sci USA. 1995;92:12055–12059. doi: 10.1073/pnas.92.26.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ji G, Beavis R, Novick R P. Bacterial interference caused by autoinducing peptide variants. Science. 1997;276:2027–2030. doi: 10.1126/science.276.5321.2027. [DOI] [PubMed] [Google Scholar]
- 54.Jiang Y, Camara M, Chhabra S R, Hardie K R, Bycroft B W, Lazdunski A, Salmond G P C, Stewart G S A B, Williams P. In vitro biosynthesis of the Pseudomonas aeruginosa quorum-sensing signal molecule, N-butanoyl-l-homoserine lactone. Mol Microbiol. 1998;28:193–204. doi: 10.1046/j.1365-2958.1998.00789.x. [DOI] [PubMed] [Google Scholar]
- 55.Jones S, Yu B, Bainton N J, Birdsall M, Bycroft B W, Chhabra S R, Cox A J R, Golby P, Reeves P J, Stephens S, Winson M K, Salmond G P C, Stewart G S A B, Williams P. The lux autoinducer regulates the production of exoenzyme virulence determinants in Erwinia carotovora and Pseudomonas aeruginosa. EMBO J. 1993;12:2477–2482. doi: 10.1002/j.1460-2075.1993.tb05902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klapwijk P M, Scheulderman T, Schilperoort R. Coordinated regulation of octopine degradation and conjugative transfer of Ti plasmids in Agrobacterium tumefaciens: evidence for a common regulatory gene and separate operons. J Bacteriol. 1978;136:775–785. doi: 10.1128/jb.136.2.775-785.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kleerebezem M, Quadri L E N, Kuipers O P, de Vos W M. Quorum sensing by peptide pheromones and two component signal transduction systems in Gram-positive bacteria. Mol Microbiol. 1997;24:895–904. doi: 10.1046/j.1365-2958.1997.4251782.x. [DOI] [PubMed] [Google Scholar]
- 58.Kohler T, Michea-Hamzehpour M, Henze U, Gotoh N, Curty L K, Pechere J C. Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol Microbiol. 1997;23:345–354. doi: 10.1046/j.1365-2958.1997.2281594.x. [DOI] [PubMed] [Google Scholar]
- 59.Kuo A, Callahan N S M, Dunlap P V. Modulation of luminescence operon expression by N-octanoyl-l-homoserine lactone in ainS mutants of Vibrio fischeri. J Bacteriol. 1996;178:971–976. doi: 10.1128/jb.178.4.971-976.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Latifi A, Foglino M, Tanaka K, Williams P, Lazdunski A. A hierarchical quorum-sensing cascade in Pseudomonas aeruginosa links the transcriptional activators LasR and RhlR to expression of the stationary-phase sigma factor RpoS. Mol Microbiol. 1996;21:1137–1146. doi: 10.1046/j.1365-2958.1996.00063.x. [DOI] [PubMed] [Google Scholar]
- 61.Latifi A, Winson M K, Foglino M, Bycroft B W, Stewart G S A B, Lazdunski L, Williams P. Multiple homologues of LuxR and LuxI control expression of virulence determinants and secondary metabolites through quorum sensing in Pseudomonas aeruginosa PAO1. Mol Microbiol. 1995;17:333–343. doi: 10.1111/j.1365-2958.1995.mmi_17020333.x. [DOI] [PubMed] [Google Scholar]
- 62.Lewenza S, Conway B, Greenberg E P, Sokol P A. Quorum sensing in Burkholderia cepacia: identification of the LuxRI homologs CepRI. J Bacteriol. 1999;181:748–756. doi: 10.1128/jb.181.3.748-756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lina G, Jarraud S, Ji G, Greenland T, Pedraza A, Etienne J, Novick R P, Vandenesch F. Transmembrane topology and histidine protein kinase activity of AgrC, the agr signal receptor in Staphylococcus aureus. Mol Microbiol. 1998;28:655–662. doi: 10.1046/j.1365-2958.1998.00830.x. [DOI] [PubMed] [Google Scholar]
- 64.McDaniel T K, Jarvis K G, Donnenberg M S, Kaper J B. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci USA. 1995;92:1664–1668. doi: 10.1073/pnas.92.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McGowan S, Sebaihia M, Jones S, Yu S, Bainton N, Chan P F, Bycroft B W, Stewart G S A B, Salmond G P C, Williams P. Carbapenem antibiotic production in Erwinia carotovora is regulated by CarR, a homologue of the LuxR transcriptional activator. Microbiology. 1995;141:541–550. doi: 10.1099/13500872-141-3-541. [DOI] [PubMed] [Google Scholar]
- 66.McKenney D, Brown K E, Allison D G. Influence of Pseudomonas aeruginosa exoproducts on virulence factor production in Burkholderia cepacia: evidence of interspecies communication. J Bacteriol. 1995;177:6989–6992. doi: 10.1128/jb.177.23.6989-6992.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McLean K H, Winson M K, Fish L, Taylor A, Chhabra S R, Camara M, Daykin M, Lamb J H, Swift S, Bycroft B W, Stewart G S A B, Williams P. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology. 1997;143:3703–3711. doi: 10.1099/00221287-143-12-3703. [DOI] [PubMed] [Google Scholar]
- 68.Mendelson M H, Gurtman A, Szabo S, Neibart E, Meyers B R, Policar M, Cheung T W, Lillienfeld D, Hammer G, Reddy S, Choi K, Hirschman S Z. Pseudomonas aeruginosa bacteremia in patients with AIDS. Clin Infect Dis. 1994;18:886–895. doi: 10.1093/clinids/18.6.886. [DOI] [PubMed] [Google Scholar]
- 69.Milton D L, Hardman A, Camara M, Chhabra S R, Bycroft B W, Stewart G S A B, Williams P. Quorum sensing in Vibrio anguillarum: characterization of the vanI/vanR locus and identification of the autoinducer N-(3-oxododecanoyl)-l-homoserine lactone. J Bacteriol. 1997;179:3004–3012. doi: 10.1128/jb.179.9.3004-3012.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moré M I, Finger L D, Stryker J L, Fuqua C, Eberhard A, Winans S C. Enzymatic synthesis of a quorum-sensing autoinducer through use of defined substrates. Science. 1996;272:1655–1658. doi: 10.1126/science.272.5268.1655. [DOI] [PubMed] [Google Scholar]
- 71.Morfeldt E, Janzon L, Arvidson S, Lofdahl S. Cloning of a chromosomal locus (exp) which regulates the expression of several exoprotein genes in Staphylococcus aureus. Mol Gen Genet. 1988;211:435–440. doi: 10.1007/BF00425697. [DOI] [PubMed] [Google Scholar]
- 72.Nasser W, Bouillant M L, Salmond G, Reverchon S. Characterization of the Erwinia chrysanthemi expI-expR locus directing the synthesis of two N-acyl-homoserine lactone signal molecules. Mol Microbiol. 1998;29:1391–1405. doi: 10.1046/j.1365-2958.1998.01022.x. [DOI] [PubMed] [Google Scholar]
- 73.Ochsner U A, Koch A K, Fiechter A, Reiser J. Isolation and characterization of a regulatory gene affecting rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. J Bacteriol. 1994;176:2044–2054. doi: 10.1128/jb.176.7.2044-2054.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ochsner U A, Reiser J. Autoinducer-mediated regulation of rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1995;92:6424–6428. doi: 10.1073/pnas.92.14.6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Palva T K, Hurtig M, Saindrenan P, Palva E T. Salicylic acid induced resistance to Erwinia carotovora subsp. carotovora in tobacco. Mol Plant-Microbe Interact. 1994;7:356–363. [Google Scholar]
- 76.Passador L, Cook J M, Gambello M J, Rust L, Iglewski B H. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science. 1993;260:1127–1130. doi: 10.1126/science.8493556. [DOI] [PubMed] [Google Scholar]
- 77.Pearson J P, Feldman M, Iglewski B H, Prince A. Pseudomonas aeruginosa cell-to-cell signaling is required for virulence in a model of acute pulmonary infection. Infect Immun. 2000;68:4331–4334. doi: 10.1128/iai.68.7.4331-4334.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pearson J P, Gray K M, Passador L, Tucker K D, Eberhard A, Iglewski B H, Greenberg E P. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc Natl Acad Sci USA. 1994;91:197–201. doi: 10.1073/pnas.91.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pearson J P, Passador L, Iglewski B H, Greenberg E P. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1995;92:1490–1494. doi: 10.1073/pnas.92.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pearson J P, Pesci E C, Iglewski B H. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J Bacteriol. 1997;179:5756–5767. doi: 10.1128/jb.179.18.5756-5767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pearson J P, Van Delden C, Iglewski B H. Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J Bacteriol. 1999;181:1203–1210. doi: 10.1128/jb.181.4.1203-1210.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pesci E C, Milbank J B, Pearson J P, McKnight S, Kende A S, Greenberg E P, Iglewski B H. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1999;96:11229–11234. doi: 10.1073/pnas.96.20.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pesci E C, Pearson J P, Seed P C, Iglewski B H. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J Bacteriol. 1997;179:3127–3132. doi: 10.1128/jb.179.10.3127-3132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pierson L S, III, Keppenne V D, Wood D W. Phenazine antibiotic biosynthesis in Pseudomonas aureofaciens 30-84 is regulated by PhzR in response to cell density. J Bacteriol. 1994;176:3966–3974. doi: 10.1128/jb.176.13.3966-3974.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pierson L S, III, Thomashow L S. Cloning and heterologous expression of the phenazine biosynthetic locus of Pseudomonas aureofaciens 30-84. Mol Plant-Microbe Interact. 1992;5:330–339. doi: 10.1094/mpmi-5-330. [DOI] [PubMed] [Google Scholar]
- 86.Pierson L S, III, Wood D W, Beck von Bodman S. Quorum sensing in plant-associated bacteria. In: Dunny G M, Winans S C, editors. Cell-cell signaling in bacteria. Washington, D.C.: ASM Press; 1999. pp. 101–116. [Google Scholar]
- 87.Piper K R, Beck von Bodman S, Farrand S K. Conjugation factor of Agrobacterium tumefaciens regulates Ti plasmid transfer by autoinduction. Nature. 1993;362:448–450. doi: 10.1038/362448a0. [DOI] [PubMed] [Google Scholar]
- 88.Pirhonen M, Flego D, Heikinheimo R, Palva E T. A small diffusible signal molecule is responsible for the global control of virulence and exoenzyme production in the plant pathogen Erwinia carotovora. EMBO J. 1993;12:2467–2476. doi: 10.1002/j.1460-2075.1993.tb05901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Poole K, Gotoh N, Tsujimoto H, Zhao Q, Wada A, Yamasaki T, Neshat S, Yamagishi J, Li X Z, Nishino T. Overexpression of the mexC-mexC-oprJ efflux operon in nfxB-type multidrug-resistant strains of Pseudomonas aeruginosa. Mol Microbiol. 1996;21:713–724. doi: 10.1046/j.1365-2958.1996.281397.x. [DOI] [PubMed] [Google Scholar]
- 90.Poole K, Krebes K, McNally C, Neshat S. Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J Bacteriol. 1993;175:7363–7372. doi: 10.1128/jb.175.22.7363-7372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Prasad C. Bioactive cyclic dipeptides. Peptides. 1995;16:151–164. doi: 10.1016/0196-9781(94)00017-z. [DOI] [PubMed] [Google Scholar]
- 92.Puskas A, Greenberg E P, Kaplan S, Schaefer A L. A quorum-sensing system in the free-living photosynthetic bacterium Rhodobacter sphaerhoides. J Bacteriol. 1997;179:7530–7537. doi: 10.1128/jb.179.23.7530-7537.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rahme L G, Stevens E J, Wolfort S F, Shao J, Tompkins R G, Ausubel F M. Common virulence factors for bacterial pathogenicity in plants and animals. Science. 1995;268:1899–1902. doi: 10.1126/science.7604262. [DOI] [PubMed] [Google Scholar]
- 94.Rahme L G, Tan M-W, Le L, Wong S M, Tompkins R G, Calderwood S B, Ausubel F M. Use of model plant hosts to identify Pseudomonas aeruginosa virulence factors. Proc Natl Acad Sci USA. 1997;94:13245–13250. doi: 10.1073/pnas.94.24.13245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rechtin T M, Gillaspy A F, Schumacher M A, Brennan R G, Smeltzer M S, Hurlburt B K. Characterization of the SarA virulence gene regulator of Staphylococcus aureus. Mol Microbiol. 1999;33:307–316. doi: 10.1046/j.1365-2958.1999.01474.x. [DOI] [PubMed] [Google Scholar]
- 96.Recsei P, Kreiswirth B, O'Reilly M, Schlievert P, Gruss A, Novick R P. Regulation of exoprotein gene expression in Staphylococcus aureus by agr. Mol Gen Genet. 1986;202:58–61. doi: 10.1007/BF00330517. [DOI] [PubMed] [Google Scholar]
- 97.Reimmann C, Beyeler M, Latifi A, Winteler H, Foglino M, Lazdunski A, Haas D. The global activator GacA of Pseudomonas aeruginosa PAO positively controls the production of the autoinducer N-butyryl-homoserine lactone and the formation of the virulence factors pyocyanin, cyanide, and lipase. Mol Microbiol. 1997;24:309–319. doi: 10.1046/j.1365-2958.1997.3291701.x. [DOI] [PubMed] [Google Scholar]
- 98.Reverchon S, Bouillant M L, Salmond G, Nasser W. Integration of the quorum-sensing system in the regulatory networks controlling virulence factor synthesis in Erwinia chrysanthemi. Mol Microbiol. 1998;29:1407–1418. doi: 10.1046/j.1365-2958.1998.01023.x. [DOI] [PubMed] [Google Scholar]
- 99.Rodelas B, Lithgow J K, Wisniewski-Dye F, Hardman A, Wilkinson A, Economou A, Williams P, Downie J A. Analysis of quorum-sensing-dependent control of rhizosphere-expressed (rhi) genes in Rhizobium leguminosarum bv. viciae. J Bacteriol. 1999;181:3816–3823. doi: 10.1128/jb.181.12.3816-3823.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rosemeyeer V, Michiels J, Verreth C, Vanderleyden J. luxI- and luxR-homologous genes of Rhizobium etli CNPAF512 contribute to synthesis of autoinducer molecules and nodulation of Phaseolus vulgaris. J Bacteriol. 1998;180:815–821. doi: 10.1128/jb.180.4.815-821.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rumbaugh K P, Griswold J A, Hamood A N. Contribution of the regulatory gene lasR to the pathogenesis of Pseudomonas aeruginosa infection of burned mice. J Burn Care Rehabil. 1999;20:42–49. doi: 10.1097/00004630-199901001-00008. [DOI] [PubMed] [Google Scholar]
- 102.Rumbaugh K P, Griswold J A, Iglewski B H, Hamood A N. Contribution of quorum sensing to the virulence of Pseudomonas aeruginosa in burn wound infections. Infect Immun. 1999;67:5854–5862. doi: 10.1128/iai.67.11.5854-5862.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schaefer A L, Hanzelka B L, Eberhard A, Greenberg E P. Quorum sensing in Vibrio fischeri: probing autoinducer-LuxR interactions with autoinducer analogs. J Bacteriol. 1996;178:2897–2901. doi: 10.1128/jb.178.10.2897-2901.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schaefer A L, Val D L, Hanzelka B L, Cronan J E, Greenberg E P. Generation of cell-to-cell signals in quorum sensing: acylhomoserine synthase activity of a purified Vibrio fischeri LuxI protein. Proc Natl Acad Sci USA. 1996;93:9505–9509. doi: 10.1073/pnas.93.18.9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schripsema J, de Rudder K E E, van Vliet T B, Lankhorst P P, de Vroom E, Kijne J W, van Brussel A A N. Bacteriocin small of Rhizobium leguminosarum belongs to the class of N-acyl-l-homoserine lactone molecules, known as autoinducers and as quorum sensing co-transcriptional factors. J Bacteriol. 1996;178:366–371. doi: 10.1128/jb.178.2.366-371.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Seed P C, Passador L, Iglewski B H. Activation of the Pseudomonas aeruginosa lasI gene by LasR and the Pseudomonas autoinducer PAI: an autoinduction regulatory hierarchy. J Bacteriol. 1995;177:654–659. doi: 10.1128/jb.177.3.654-659.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sharma S, Stark T F, Beattie W G, Moses R E. Multiple control elements for the uvrC gene unit of Escherichia coli. Nucleic Acids Res. 1986;14:2301–2318. doi: 10.1093/nar/14.5.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Showalter R E, Martin M O, Silverman M R. Cloning and nucleotide sequence of luxR, a regulatory gene controlling luminescence in Vibrio harveyi. J Bacteriol. 1990;172:2946–2954. doi: 10.1128/jb.172.6.2946-2954.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sitnikov D M, Schineller J B, Baldwin T O. Transcriptional regulation of bioluminescence genes from Vibrio fischeri. Mol Microbiol. 1995;17:801–812. doi: 10.1111/j.1365-2958.1995.mmi_17050801.x. [DOI] [PubMed] [Google Scholar]
- 110.Sperandio V, Mellies J L, Nguyen W, Shin S, Kaper J B. Quorum sensing controls expression of the type III secretion gene transcription and protein secretion in enterohemorrhagic and enteropathogenic Escherichia coli. Proc Natl Acad Sci USA. 1999;96:15196–15201. doi: 10.1073/pnas.96.26.15196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Storey D G, Ujack E E, Rabin H R, Mitchell I. Pseudomonas aeruginosa lasR transcription correlates with the transcription of lasA, lasB, and toxA in chronic lung infections associated with cystic fibrosis. Infect Immun. 1998;66:2521–2528. doi: 10.1128/iai.66.6.2521-2528.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Surette M G, Bassler B L. Quorum sensing in Escherichia coli and Salmonella typhimurium. Proc Natl Acad Sci USA. 1998;95:7046–7050. doi: 10.1073/pnas.95.12.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Surette M G, Miller M B, Bassler B L. Quorum sensing in Escherichia coli, Salmonella typhimurium and Vibrio harveyi: a new family of genes responsible for autoinducer production. Proc Natl Acad Sci USA. 1999;96:1639–1644. doi: 10.1073/pnas.96.4.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Swift S, Karlyshev A V, Durant E L, Winson M K, Chhabra S R, Williams P, Macintyre S, Stewart G S A B. Quorum sensing in Aeromonas hydrophila and Aeromonas salmonicida: identification of the LuxRI homologues AhyRI and AsaRI and their cognate signal molecules. J Bacteriol. 1997;179:5271–5281. doi: 10.1128/jb.179.17.5271-5281.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Swift S, Winson M K, Chan P F, Bainton N J, Birdsall M, Reeves P J, Rees C E D, Chhabra S R, Hill P J, Throup J P, Bycroft B W, Salmond G P C, Williams P, Stewart G S A B. A novel strategy for the isolation of luxI homologues: evidence for the widespread distribution of a LuxR:LuxI superfamily in enteric bacteria. Mol Microbiol. 1993;10:511–520. doi: 10.1111/j.1365-2958.1993.tb00923.x. [DOI] [PubMed] [Google Scholar]
- 116.Tan M-W, Rahme L G, Sternberg J A, Tompkins R G, Ausubel F M. Pseudomonas aeruginosa killing of Caenorhabditis elegans used to identify P. aeruginosa virulence factors. Proc Natl Acad Sci USA. 1999;96:2408–2413. doi: 10.1073/pnas.96.5.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tang H B, DiMango E, Bryan R, Gambello M, Iglewski B H, Goldberg J B, Prince A. Contribution of specific Pseudomonas aeruginosa virulence factors to pathogenesis of pneumonia in a neonatal mouse model of infection. Infect Immun. 1996;64:37–43. doi: 10.1128/iai.64.1.37-43.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Taylor R F H, Gaya H, Hodson M E. Pseudomonas cepacia: pulmonary infection in patients with cystic fibrosis. Respir Med. 1993;87:187–192. doi: 10.1016/0954-6111(93)90090-m. [DOI] [PubMed] [Google Scholar]
- 119.Telford G, Wheeler D, Williams P, Tomkins P T, Appleby P, Sewell H, Stewart G S A B, Bycroft B W, Pritchard D I. The Pseudomonas aeruginosa quorum-sensing signal molecule N-(3-oxododecanoyl)-l-homoserine lactone has immunomodulatory activity. Infect Immun. 1998;66:36–42. doi: 10.1128/iai.66.1.36-42.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Throup J P, Camara M, Briggs G S, Winson M K, Chhabra S R, Bycroft B W, Williams P, Stewart G S A B. Characterisation of the yenI/R locus from Yersinia enterocolitica mediating the synthesis of two N-acyl-homoserine lactone signal molecules. Mol Microbiol. 1995;17:345–356. doi: 10.1111/j.1365-2958.1995.mmi_17020345.x. [DOI] [PubMed] [Google Scholar]
- 121.Toder D S, Gambello M J, Iglewski B H. Pseudomonas aeruginosa LasA: a second elastase gene under transcriptional control of lasR. Mol Microbiol. 1991;5:2003–2010. doi: 10.1111/j.1365-2958.1991.tb00822.x. [DOI] [PubMed] [Google Scholar]
- 122.Van Delden C, Pesci E C, Pearson J P, Iglewski B H. Starvation selection restores elastase and rhamnolipid production in a Pseudomonas aeruginosa quorum-sensing mutant. Infect Immun. 1998;66:4499–4502. doi: 10.1128/iai.66.9.4499-4502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Whooley M A, McLoughlin A. The proton motive force in Pseudomonas aeruginosa and its relationship to exoprotease production. J Gen Microbiol. 1983;129:989–996. doi: 10.1099/00221287-129-4-989. [DOI] [PubMed] [Google Scholar]
- 124.Whooley M A, O'Callaghan J A, McLoughlin A J. Effect of substrate on the regulation of exoprotease production by Pseudomonas aeruginosa ATCC 10145. J Gen Microbiol. 1983;129:981–988. doi: 10.1099/00221287-129-4-981. [DOI] [PubMed] [Google Scholar]
- 125.Winans S C, Zhu J, Moré M I. Cell density-dependent gene expression by Agrobacterium tumefaciens during colonization of crown gall tumors. In: Dunny G M, Winans S C, editors. Cell-cell signaling in bacteria. Washington, D.C.: ASM Press; 1999. pp. 117–128. [Google Scholar]
- 126.Winson M K, Camara M, Latifi A, Foglino M, Chhabra S R, Daykin M, Bally M, Chapon V, Salmond G P C, Bycroft B W, Lazdunski A, Stewart G S A B, Williams P. Multiple N-acyl-L-homoserine lactone signal molecules regulate production of virulence determinants and secondary metabolites in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1995;92:9427–9431. doi: 10.1073/pnas.92.20.9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Withers H L, Nordstrom K. Quorum-sensing acts at initiation of chromosomal replication in Escherichia coli. Proc Natl Acad Sci USA. 1998;95:15694–15699. doi: 10.1073/pnas.95.26.15694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wood D W, Pierson L S., III The phzI gene of Pseudomonas aureofaciens 30-84 is responsible for the production of a diffusible signal required for phenazine antibiotic production. Gene. 1996;168:49–53. doi: 10.1016/0378-1119(95)00754-7. [DOI] [PubMed] [Google Scholar]
- 129.Zhang L, Kerr A. A diffusible compound can enhance conjugal transfer of the Ti plasmid in Agrobacterium tumefaciens. J Bacteriol. 1991;173:1867–1872. doi: 10.1128/jb.173.6.1867-1872.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang L, Murphy P J, Kerr A, Tate M E. Agrobacterium conjugation and gene regulation by N-acyl-l-homoserine lactones. Nature. 1993;362:446–448. doi: 10.1038/362446a0. [DOI] [PubMed] [Google Scholar]
- 131.Zhu J, Beaber J W, Moré M I, Fuqua C, Eberhard A, Winans S C. Analogs of the autoinducer 3-oxooctanoyl-homoserine lactone strongly inhibit activity of the TraR protein of Agrobacterium tumefaciens. J Bacteriol. 1998;180:5398–5405. doi: 10.1128/jb.180.20.5398-5405.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]