Key Points
Question
What were the types of significant incidental findings (SIFs) detected on low-dose computed tomography screening examinations in the National Lung Screening Trial?
Findings
In this case series study of 26 455 participants who underwent screening with low-dose computed tomography in the National Lung Screening Trial, 8954 (33.8%) had a SIF reported. Most screening tests with a SIF (12 228 [89.1%]) had at least 1 abnormality considered reportable to the referring clinician; the most common SIFs included emphysema (8677 of 20 156 [43.0%]), coronary artery calcium (2432 [12.1%]), and masses (1493 [7.4%]).
Meaning
The results of this case series study suggest that SIFs should be reported in a consistent manner and that SIF management should follow established guidelines to potentially minimize costs to patients, clinicians, and the health care system.
Abstract
Importance
Low-dose computed tomography (LDCT) lung screening has been shown to reduce lung cancer mortality. Significant incidental findings (SIFs) have been widely reported in patients undergoing LDCT lung screening. However, the exact nature of these SIF findings has not been described.
Objective
To describe SIFs reported in the LDCT arm of the National Lung Screening Trial and classify SIFs as reportable or not reportable to the referring clinician (RC) using the American College of Radiology’s white papers on incidental findings.
Design, Setting, and Participants
This was a retrospective case series study of 26 455 participants in the National Lung Screening Trial who underwent at least 1 screening examination with LDCT. The trial was conducted from 2002 to 2009, and data were collected at 33 US academic medical centers.
Main Outcomes and Measures
Significant incident findings were defined as a final diagnosis of a negative screen result with significant abnormalities that were not suspicious for lung cancer or a positive screen result with emphysema, significant cardiovascular abnormality, or significant abnormality above or below the diaphragm.
Results
Of 26 455 participants, 10 833 (41.0%) were women, the mean (SD) age was 61.4 (5.0) years, and there were 1179 (4.5%) Black, 470 (1.8%) Hispanic/Latino, and 24 123 (91.2%) White individuals. Participants were scheduled to undergo 3 screenings during the course of the trial; the present study included 75 126 LDCT screening examinations performed for 26 455 participants. A SIF was reported for 8954 (33.8%) of 26 455 participants who were screened with LDCT. Of screening tests with a SIF detected, 12 228 (89.1%) had a SIF considered reportable to the RC, with a higher proportion of reportable SIFs among those with a positive screen result for lung cancer (7632 [94.1%]) compared with those with a negative screen result (4596 [81.8%]). The most common SIFs reported included emphysema (8677 [43.0%] of 20 156 SIFs reported), coronary artery calcium (2432 [12.1%]), and masses or suspicious lesions (1493 [7.4%]). Masses included kidney (647 [3.2%]), liver (420 [2.1%]), adrenal (265 [1.3%]), and breast (161 [0.8%]) abnormalities. Classification was based on free-text comments; 2205 of 13 299 comments (16.6%) could not be classified. The hierarchical reporting of final diagnosis in NLST may have been associated with an overestimate of severe emphysema in participants with a positive screen result for lung cancer.
Conclusions and Relevance
This case series study found that SIFs were commonly reported in the LDCT arm of the National Lung Screening Trial, and most of these SIFs were considered reportable to the RC and likely to require follow-up. Future screening trials should standardize SIF reporting.
This case series study examines significant incidental findings reported in the low-dose computed tomography arm of the National Lung Screening Trial.
Introduction
Low-dose computed tomography (LDCT) lung screening has been shown to reduce lung cancer mortality by up to 24%.1,2 Despite the evidence of clinical efficacy, the availability of screening covered by insurance,3,4,5,6 updated US Preventive Services Task Force guidelines expanding screening eligibility criteria, and expanded US Centers for Medicare & Medicaid Services coverage,7,8 widespread adoption of LDCT lung screening has lagged.9 One concern potentially limiting the widespread acceptance of screening is the high rate of significant incidental findings (SIFs) detected at screening and uncertainty as how to best address these findings.10
At LDCT screening, potentially significant abnormalities not associated with lung cancer, commonly referred to as SIFs, are often detected. These findings have potential clinical significance and are sufficiently concerning to the interpreting radiologist that they are reported to the referring clinician (RC). Significant incidental findings have been noted in as many as 14%11 of patients undergoing baseline LDCT lung screening in clinical trials and most individuals undergoing lung screening in the community.12,13 In the National Lung Screening Trial (NLST), SIFs were detected in 10% of LDCT participants at the first screen and 5% to 6% of participants at the second and third annual screens.1 Radiologists in the NLST were advised that SIFs that were reported at baseline and stable at repeated screening did not need to be reported again. In certain situations, SIF detection may be associated with the early diagnosis of life-altering conditions, such as extrapulmonary cancers, cardiovascular, or respiratory conditions8,9,14,15,16,17; however, SIFs may also be associated with unnecessary iatrogenic morbidity and mortality and low-value health services,18 particularly if the discovery of these findings stimulates a highly invasive diagnostic workup for what are ultimately determined to be benign conditions.19,20,21,22
Lung-RADS was introduced by the American College of Radiology (ACR) in 2015 to standardize LDCT lung cancer screening reporting and management recommendations.23 In addition, the ACR published a series of evidence-based white papers to provide a structured framework and clinical decision support guidance in managing SIFs.24,25,26,27,28,29 To our knowledge, information on specific SIFs detected in the NLST has not been reported. Publications to date have been limited, largely focusing on the organ in which the SIF was detected30; somewhat more detail has been provided for kidney abnormalities.31
In the NLST, detailed information on SIFs was reported as free text. In this article, we describe the SIFs detected in the LDCT arm of the NLST. We also delineate the approach that we used to categorize these SIFs, as well as the necessity of reporting these SIFs to the RC using a rubric based on the ACR white papers on incidental findings.24,25,26,27,28,29
Methods
We used data collected for the NLST. The NLST was a collaboration between the American College of Radiology Imaging Network (ACRIN), now part of the ECOG-ACRIN Cancer Research Group, and the Lung Screening Study (LSS). It has been described elsewhere.1,32,33 The NLST was a multi-institutional trial of 53 452 participants that was designed to determine whether screening with LDCT was associated with a reduction in lung cancer mortality compared with chest radiography (CXR).1 Eligible participants for the NLST were aged 55 to 74 years at study accrual with at least a 30–pack year cigarette smoking history who were either currently smoking or formerly smoked and quit within the previous 15 years. Participants were excluded if they had a history of lung cancer, hemoptysis, or unexplained weight loss during the preceding year. Participants were randomly assigned to undergo LDCT or CXR and were scheduled to receive 3 screening examinations: a baseline screen (T0) and 2 incidence screens (T1, T2) at 1-year intervals. Participants were followed for 5 to 7 years from recruitment, and detailed information on any cancer diagnosis and cause of death was collected.1,34,35 For this article, we focused on participants who were randomized to the LDCT arm of the trial.
This study was reviewed by the Brown University institutional review board and received an expedited review and approval under expedited category 5. Informed consent was waived due to the study being a secondary analysis.
Screening Test Interpretation in the NLST
The process used to interpret NLST LDCT screening examinations, describe abnormalities, and assign a final diagnosis is described in Figure 1. In the NLST, the focus was on detecting and diagnosing lung cancer. The final result at each screening visit was classified as either a positive (suspicious for lung cancer) or a negative (no findings suggestive of lung cancer) screen result. Participants with a negative screen result were further classified as negative screen, significant abnormalities not suspicious for lung cancer (NEG/SIF) or negative screen, no significant abnormalities (NEG). Participants with a positive screen result may also have had SIFs detected, and these are indicated as positive screen/significant abnormalities (POS/SIF). We referred to participants with a positive screen result for lung cancer, but with no SIFs, as POS (eFigure in Supplement 1).
Figure 1. Process Used to Interpret National Lung Screening Trial Screening Examinations During the Course of the Trial, Assess Abnormalities Observed, and Assign a Final Diagnosis.
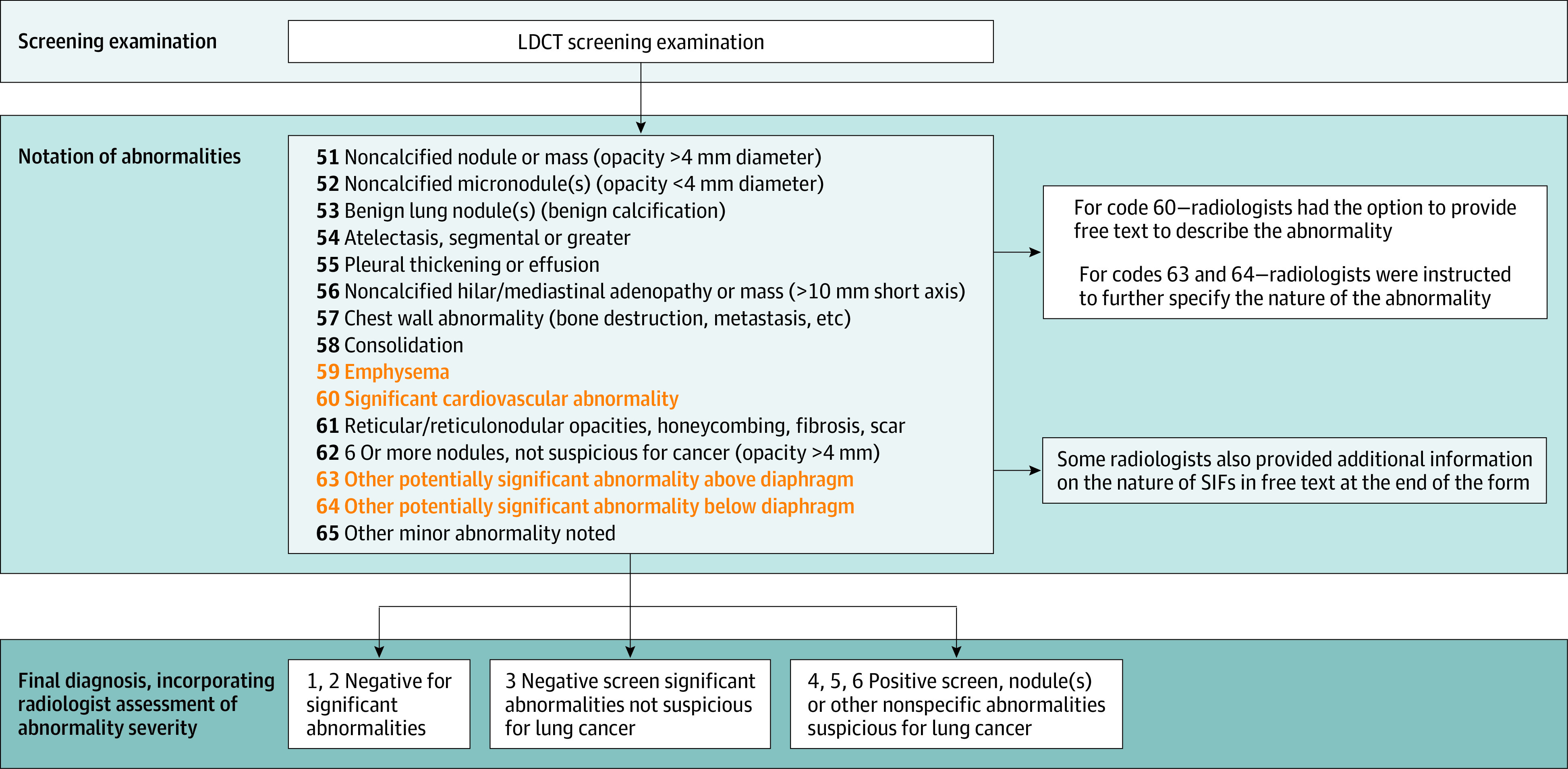
Abnormalities highlighted were reported for screening examinations with a final diagnosis of a negative screen result, significant abnormalities not suspicious for lung cancer or positive screen result, nodule(s) or other nonspecific abnormalities suspicious for lung cancer were considered significant incidental findings; and their descriptions were examined in this study. LDCT indicates low-dose computed tomography; SIF, significant incidental finding.
The nature of each SIF reported for participants with positive or negative screen results for lung cancer was indicated on the case report forms as (1) emphysema, (2) a significant cardiovascular abnormality, (3) an other potentially significant abnormality above diaphragm, or (4) an other potentially significant abnormality below the diaphragm. Radiologists had the option of specifying the specific nature of the abnormality for emphysema and cardiovascular abnormalities (1 and 2) and were asked to specify the nature of the abnormality for abnormalities above or below the diaphragm (3 and 4). These abnormality descriptions were entered as free text.
Creation of Classifications
To develop classifications, we focused on abnormalities detected in NEG/SIF screens, because these were clearly reported by the trial radiologists as significant abnormalities that were not suspicious for lung cancer. Three fellowship-trained cardiothoracic radiologists (C.C., T.D.T., and E.F.) reviewed the comments to assess their organ locations and types. One radiologist (C.C.) then created a classification grid based on the ACR white papers on incidental findings.24,25,26,27,28,29 The multistep iterative process to categorize the free-text comments was as follows:
Include free-text comments accompanying abnormalities that were previously described (1, 2, 3, and 4), as well as additional comment fields on each form in which radiologists were instructed to describe abnormalities not associated with lung cancer.
Separate abnormalities divided by punctuation (eg, / and+) or an “and.”
Classify each unique comment into specific categories. Incorporate abbreviations typically used by radiologists.
Authors (I.G., T.D.T., E.F., and C.C.) visually reviewed comment classifications to ensure accuracy.
The expert panel reviewed unclassified comments and placed them in categories as appropriate. New categories were created if needed. This was then reviewed again (on a second iteration) by 2 authors (C.C. and T.D.T.).
Apply categories derived from participants designated as NEG/SIF to POS/SIF participants.
Repeat step 4. Two radiologists (C.C. and T.D.T.) made a final determination on these abnormalities.
Assessment of Classifications as Requiring Reporting to the Primary Care Clinician
We convened the expert panel (C.C., T.D.T., E.F., A.N.T., and R.H.) to determine whether each SIF classification category should be classified as reportable or not reportable to the RC. The former indicated the expert panel’s opinion that the SIF probably necessitated further diagnostic evaluation.
Participants in the NLST may have had more than 1 SIF detected at each screening examination (T0, T1, T2) and/or the same or different SIFs detected at more than 1 screening examination. To capture this information, we reported SIFs in several ways: per participant, in which we focused on whether each participant had a SIF detected ever in the NLST; per screen, in which we reported whether a participant had a SIF detected at each screening visit; and per SIF (abnormality reported), in which we reported all SIFs detected.
Statistical Analyses
We compared demographic characteristics using the t test for continuous variables and χ2 tests for categorical variables. Bonferroni-correction with a familywise error rate of .05 was used to address multiple comparisons. Thus, statistical significance was established at P < .01. All analyses were conducted using SAS (SAS Institute).
Results
There were 26 722 participants in the LDCT arm of the NLST, and 267 (1.0%) of these did not receive a screening examination. The final sample included the 26 455 participants with at least 1 LDCT screening examination. During the course of the trial, 8108 screens (10.8%) in 5401 participants (20.4%) reported POS/SIF and 5620 screens (7.5%) in 4303 participants (16.3%) reported NEG/SIF findings. These represented 18.3% of all screens and 33.8% of all NLST LDCT participants. Some participants received POS/SIF and NEG/SIF screen results during the course of the trial.
Table 1 shows demographic characteristics of LDCT participants in the NLST by SIF status. Participants were classified as either ever SIF (diagnosed as an NEG/SIF or POS/SIF at any screening examination) or never SIF. Participants with SIFs were more likely to be older (mean [SD] age, 62.2 [5.2] years for ever SIF vs 61.0 [4.9] years for never SIF), White (8297 [92.7%] for ever SIF vs 15 826 [90.4%] for never SIF), non-Hispanic (8796 [98.2%] for ever SIF vs 17 082 [97.6%] for never SIF), male (5443 [60.8%] for ever SIF vs 10 179 [58.2%] for never SIF), and less educated (P < .001 for all comparisons).
Table 1. Demographic Characteristics of National Lung Screening Trial LDCT Arm Study Participantsa Among Those Who Ever Had a SIF Reported at Least Once During the Screening Trial vs Those Who Did Not.
| Characteristic | No. (%) | |
|---|---|---|
| Ever SIF | Never SIF | |
| Age, mean (SD), yb | 62.2 (5.2) | 61.0 (4.9) |
| Raceb | ||
| Black | 368 (4.1) | 811 (4.6) |
| White | 8297 (92.7) | 15 826 (90.4) |
| Otherc | 289 (3.2) | 864 (4.9) |
| Ethnicityb | ||
| Hispanic/Latino | 113 (1.3) | 357 (2.0) |
| Not Hispanic/Latino | 8796 (98.2) | 17 082 (97.6) |
| Other | 45 (0.5) | 62 (0.4) |
| Sexb | ||
| Female | 3511 (39.2) | 7322 (41.8) |
| Male | 5443 (60.8) | 10 179 (58.2) |
| Educationb | ||
| Eighth grade or less | 141 (1.6) | 219 (1.3) |
| Ninth through eleventh grade | 494 (5.5) | 772 (4.4) |
| High school graduate/GED | 2273 (25.4) | 3943 (22.5) |
| Post–high school training, excluding college | 1280 (14.3) | 2426 (13.9) |
| Associate degree/some college | 2041 (22.8) | 4104 (23.5) |
| Bachelor degree | 1361 (15.2) | 3104 (17.7) |
| Graduate school | 1178 (13.2) | 2584 (14.8) |
| Other | 186 (2.1) | 349 (2.0) |
| Total | 8954 (100) | 17 501 (100) |
Abbreviations: GED, General Educational Development test; LDCT, low-dose computed tomography; SIF, significant incidental finding.
Includes 26 455 participants with at least 1 screening examination; 267 of 26 722 participants (1.0%) randomized to the LDCT arm of the trial had no screening examination.
P < .001 comparing each covariate between ever SIF and never SIF participants. P values were univariate t test (age) and χ2 (categorical variables) Bonferroni-adjusted.
Includes individuals of American Indian or Alaska Native, Asian, Native Hawaiian or other Pacific Islander, multiracial, or unknown race.
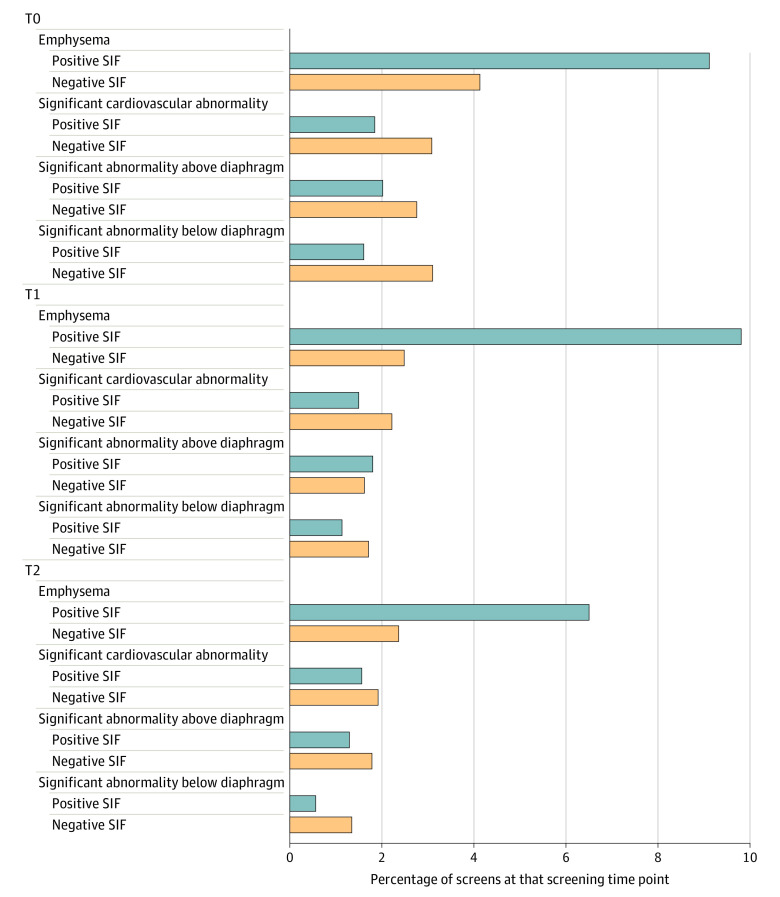
Figure 2 shows the types of SIFs reported for participants with POS/SIF vs NEG/SIF at each screening visit. The same abnormality for a single participant may have been reported at more than 1 screening examination. Emphysema was the most common SIF detected; depending on the screening visit, emphysema represented 62.5% to 69.0% of the SIFs detected for participants with positive screen results, but only 30.9% to 31.9% of the SIFs for participants with negative screen results. The rate of most abnormalities was lower at the incidence (T1 and T2) screens, as opposed to the baseline screen (T0).
Figure 2. Abnormalities Associated With a Significant Incidental Finding (SIF) Classification.
Includes participants with a positive screen result for lung cancer with significant abnormalities and negative screen result for lung cancer with significant abnormalities. Participants may have received a diagnosis of more than 1 SIF at each screening visit and may appear in the figure more than once for each screening visit. The same SIF for a participant may appear at more than 1 screening visit if the radiologist felt that it remained a concern.
Table 2 shows the number of SIFs reported for participants with a positive vs negative screen result by screening visit. A larger proportion of participants with a positive screen result for lung cancer had SIFs detected than those with a negative screen result for lung cancer (3172 [44.1%] vs 2694 [14.1%] at T0; 2950 [42.7%] vs 1517 [8.5%] at T1; and 1986 [49.1%] vs 1409 [7.0%] at T2). Across all 75 126 LDCT screens, 13 728 (18.3%) had SIFs detected. Most of these screening tests had at least 1 SIF (89.1%) that was considered reportable to the RC (eTable 1 in Supplement 1).
Table 2. Number of Screening Tests With SIFs as Stratified by Screening Visit and Resulta.
| Screening visit | No. (%) | Total | |||
|---|---|---|---|---|---|
| POS | NEG | ||||
| SIF | No SIF | SIF | No SIF | ||
| T0 | 3172 (44.1) | 4019 (55.9) | 2694 (14.1) | 16 424 (85.9) | 26 309 |
| T1 | 2950 (42.7) | 3954 (57.3) | 1517 (8.5) | 16 294 (91.5) | 24 715 |
| T2 | 1986 (49.1) | 2060 (50.9) | 1409 (7.0) | 18 647 (93.0) | 24 102 |
| Total | 8108 (44.7) | 10 033 (55.3) | 5620 (9.9) | 51 365 (90.1) | 75 126 |
Abbreviations: NEG, negative screen result for lung cancer; POS, positive screen result for lung cancer; SIF, significant incidental finding; T0, baseline screen; T1, first incidence screen; T2, second incidence screen.
Participants enter the table once per screening visit.
Table 3 describes the most common abnormalities that were considered reportable to the RC. eTable 2 in Supplement 1 contains a list of these abnormalities. The denominator for the full table (n = 20 156) included all abnormalities noted for screens with abnormalities of emphysema or a significant cardiovascular abnormality regardless of whether they contained a comment describing the specific nature of the abnormality (Figure 1), as well as all other potentially significant abnormalities above the diaphragm or an other potentially significant abnormality below the diaphragm, for which a comment was provided. Emphysema was reported for 8677 screens and represented 43% of all abnormalities reported.
Table 3. Specific SIFs Considered Reportable to the Referring Physician for the LDCT Arm of the NLST Across All 3 Screens by Frequency of Reporta.
| Organ system | Classification | No. (% of all SIFs reported) |
|---|---|---|
| Abdomen | Kidney mass | 647 (3.2) |
| Liver lesion, no size | 420 (2.1) | |
| Adrenal nodule or mass and not further characterized as benign | 265 (1.3) | |
| Cardiovascular | Coronary artery calcification, no evidence of a prior CABG or stent | 2432 (12.1) |
| Significant cardiovascular abnormality, not specified | 904 (4.5) | |
| Aortic aneurysm | 198 (1.0) | |
| Pulmonary | Emphysema, COPD, hyperinflation, code 59 with no comments | 8677 (43.0) |
| Diffuse or patchy ground glass opacification | 253 (1.3) | |
| Thoracic and chest wall | Breast: nodule, mass | 161 (0.8) |
| Total | NA | 20 156 (100) |
Abbreviations: CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; LDCT, low-dose computed tomography; NA, not applicable; NLST, National Lung Screening Trial; SIFs, significant incidental findings.
Total represents the total number of SIFs reported by radiologists, as reported in eTable 2 in Supplement 1. This table includes SIFs occurring with a frequency of 0.8% or more of all reported SIFs observed in the LDCT arm of the NLST. The same SIF can appear more than once if detected at more than 1 screening visit.
There were 13 299 SIFs with free-text comments; of these, 11 094 (83.4%) were classified according to the process outlined previously, and 7860 classifiable comments (70.8%) were considered reportable. Abnormalities are organized by organ system. Coronary artery calcium (CAC) was detected 2432 times (12.1% of 20 156 SIFs reported), and there were 198 occurrences of aortic aneurysms (1.0% of SIFs). Indications of a potentially malignant finding were relatively common, including kidney (647 [3.2%]), liver (420 [2.1%]), adrenal (265 [1.3%]), and breast (161 [0.8%]) abnormalities. Other SIFs that potentially indicated cancer were also reported for these and other organs (eTable 2 in Supplement 1 reports all abnormalities, and eTable 3 in Supplement 1 reports abnormalities once per participant).
Discussion
In this case series study, SIFs were frequently reported for NLST LDCT participants; 33.8% of participants had a SIF reported on at least 1 screening examination and 18.3% of LDCT screening examinations had at least 1 SIF reported. Most screening tests with a SIF (89.1%) had at least 1 abnormality considered reportable to the RC. This represented a substantial number of findings for which further evaluation was likely needed. At the same time, it may also represent an opportunity for early intervention for clinically important extrapulmonary cancers, cardiovascular, and respiratory conditions. Optimal classification and reporting of SIFs, together with a tailored response to these abnormalities, can potentially minimize the unnecessary burden and costs borne by patients and RCs and avoid iatrogenic morbidity and medically inappropriate care. Building on the classification of SIFs presented in this article may improve SIF management.
Significant incidental findings were more common in participants with a screening result that was positive for lung cancer (44.7%) than for participants with a negative screen result for lung cancer (9.9%). However, given the many participants with a negative LDCT screen result for lung cancer (56 985 at 3 screening examinations), this is a substantial number of potentially clinically significant findings (5620).
Despite the high rate of SIFs detected in the LDCT (as opposed to the CXR) arm of the NLST, LDCT lung screening has been shown to be cost-effective compared with no screening,36 and, in a Medicare population, the overall and diagnostic per-patient costs did not differ significantly between the 2 arms of the study.37 Yet this cost-effectiveness relies on ensuring that SIFs are judiciously reported and appropriately managed as lung screening diffuses into the community, where higher rates of SIFs have been reported than in the NLST.12,38,39 Optimizing management of SIFs may even improve lung screening cost-effectiveness.
Detecting SIFs has the potential to facilitate an early diagnosis of extrapulmonary cancer. Death due to extrapulmonary cancers, including kidney, liver, pancreatic, and mediastinal cancers, comprised 22.3% of the certified deaths in the LDCT arm of the NLST.40 Earlier publications provided limited detail on the nature of the SIF abnormalities, largely focusing on the organ in which the SIF was located.30,31 In contrast to these earlier articles, we classified the nature of the SIFs detected using criteria based on the ACR white papers on incidental findings,24,25,26,27,28,29 as well as classifying the findings by necessity of reporting to the RC. We reported higher rates of findings that were suspicious for potential cancer than did Nguyen et al30 or Pinsky et al.31 The higher numbers of SIFs may be because we included data from the LSS and ACRIN arms of the NLST, whereas Nguyen et al30 examined only LSS data. We examined additional comment fields describing SIFs located in several locations on the ACRIN and LSS forms, whereas Pinsky et al31 did not appear to include these fields. Even if these findings that were suspicious for extrapulmonary cancers are associated with cancer diagnoses, it remains to be seen whether early detection of these extrapulmonary cancers will be associated with a reduction in cancer-specific mortality.
Additionally, CAC has been associated with cardiovascular death in a reanalysis of a subset of NLST participants.41 We reported that 2432 of 75 126 LDCT screens (3.2%; 1776 of 26 455 LDCT participants [6.7%]) indicated CAC, in addition to other cardiac abnormalities. Coronary artery calcium has been reported with higher frequency in clinical screening populations, with up to 80% of screened patients exhibiting CAC in clinical screening settings.12,13 The higher rate of CAC in clinical screening settings compared with the NLST may partially reflect differences in patient populations. Namely, individuals in the US who are eligible for screening have a higher incidence of medical comorbidities than NLST participants.42 Notwithstanding, the implications of LDCT-detected CAC are uncertain. Although prior work demonstrates that the presence and severity of CAC are associated with adverse cardiac events in the NLST population, uncertainty remains about the true value of LDCT-detected CAC in the lung screening population.41,43 This uncertainty arises from the clinical characteristics of the screen-eligible population, namely their advanced age and heavy smoking history, which, irrespective of CAC, places most of the LCS-eligible population at high cardiovascular risk.44,45 Whether CAC adds to the discriminative power of existing clinical cardiovascular risk tools to predict cardiovascular risk in this population is also uncertain.46 Thus, early detection of CAC and other cardiac abnormalities may provide an opportunity for intervention, but it also has the potential to stimulate unnecessary medical testing and intervention.
Respiratory disease, and emphysema in particular, has been associated with death due to respiratory causes in NLST participants. This study’s findings are consistent with earlier work that found evidence of emphysema and/or chronic obstructive pulmonary disease in participants who did not report these conditions at baseline.47 We reported that more than 10% of all LDCT screens showed signs of emphysema and/or chronic obstructive pulmonary disease. Early detection of these conditions may offer an opportunity for effective early intervention, including smoking cessation among those currently smoking, potentially reducing respiratory morbidity in these participants.
Although it is possible that SIFs detected in the NLST represented known conditions, NLST participants were healthier than most individuals undergoing lung screening in actual clinical settings.48 Individuals were excluded from NLST enrollment if they had been treated for, or told by a physician that they had, evidence of any cancer within the 5 years preceding study entry; thus, SIFs indicative of cancer were likely to be a novel finding.32,49
In 2015, Lung-RADS was introduced to standardize LDCT lung cancer screening reporting and management recommendations.23 Using Lung-RADS, radiologists are directed to use the “S” modifier to report “clinically significant or potentially clinically significant findings unrelated to lung cancer.” Our description of SIFs detected in NLST potentially provides insight into the volume of community-administered lung cancer screening examinations that may require an “S” modifier.50
Limitations
This study had limitations. We were able to categorize most of the free-text comments; however, 2205 of 13 299 comments (16.6%) remained uncategorized. Many of these appeared to be data entry errors, referring to lung abnormalities associated with lung cancer, rather than a SIF. Some comments lacked the specificity needed to allow classification (eg, “recommend kidney ultrasonography” without an indication for that ultrasonography). In current community lung cancer screening practice, standardized reporting with Lung-RADS and increasing use of clinical decision support tools may ameliorate this issue. The classifications presented in this article may serve as a template for more standardized reporting of SIFs detected at lung screening.
In addition, NEG/SIFs were clearly designated by the reading radiologists as significant abnormalities not suspicious for lung cancer, but POS/SIFs were not. Cardiac SIFs and SIFS above and below the diaphragm (eFigure in Supplement 1) were labeled as significant, but emphysema was not. Thus, we may have overestimated the number of POS/SIFs with emphysema that were considered a significant abnormality.
Conclusions
In this case series study, slightly more than one-third of all NLST LDCT screening participants had a SIF detected. Most of these SIFs were classified as reportable to the RC for additional evaluation. This large number of potentially clinically important SIFs may be associated with a heavy burden of follow-up care and testing, with implications for patients, clinicians, and the health care system. At the same time, the discovery of these SIFs can potentially present an opportunity for early detection of non–lung cancer conditions in a high-risk population. Judicious classification and reporting of SIFs may be used to assess the benefits or harms associated with their detection and guide improved management of participants with SIFs detected at lung screening. Future screening trials should consider classification of SIFs in the design phase of the trial. Future work needs to assess the association of these SIFs with patient morbidity and mortality, as well as focus on the most appropriate approach to assessing these SIFs once discovered.
eTable 1. Number of significant incidental findings (SIFs) assessed by screening result and whether SIF should be reported to the primary care provider
eTable 2. Specific SIFs by whether they were considered reportable to the referring physician for the LDCT arm of the NLST, across all three screens (T0, T1, T2) by frequency of report
eTable 3. Specific SIFs considered reportable to the referring physician for the LDCT arm of the NLST, across all three screens (T0, T1, T2) by frequency of report
eFigure. Procedure used to categorize each screening examination
Data sharing statement
References
- 1.Aberle DR, Adams AM, Berg CD, et al. ; National Lung Screening Trial Research Team . Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. doi: 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized Trial. N Engl J Med. 2020;382(6):503-513. doi: 10.1056/NEJMoa1911793 [DOI] [PubMed] [Google Scholar]
- 3.Moyer VA; US Preventive Services Task Force . Screening for lung cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330-338. doi: 10.7326/M13-2771 [DOI] [PubMed] [Google Scholar]
- 4.Fleming C. Health policy brief: preventive services without cost sharing. Accessed April 5, 2023. https://www.healthaffairs.org/do/10.1377/forefront.20101229.008423
- 5.US Government . Patient Protection and Affordable Care Act. Accessed June 1, 2016. https://www.gpo.gov/fdsys/pkg/PLAW-111publ148/content-detail.html
- 6.US Centers for Medicare & Medicaid Services . Decision memo for screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439N). Accessed April 5, 2023. https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&NCAId=274
- 7.Landy R, Young CD, Skarzynski M, et al. Using prediction models to reduce persistent racial and ethnic disparities in the draft 2020 USPSTF lung cancer screening guidelines. J Natl Cancer Inst. 2021;113(11):1590-1594. doi: 10.1093/jnci/djaa211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krist AH, Davidson KW, Mangione CM, et al. ; US Preventive Services Task Force . Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(10):962-970. doi: 10.1001/jama.2021.1117 [DOI] [PubMed] [Google Scholar]
- 9.Richards TB, Soman A, Thomas CC, et al. Screening for lung cancer—10 states, 2017. MMWR Morb Mortal Wkly Rep. 2020;69(8):201-206. doi: 10.15585/mmwr.mm6908a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajupet S, Doshi D, Wisnivesky JP, Lin JJ. Attitudes about lung cancer screening: primary care providers versus specialists. Clin Lung Cancer. 2017;18(6):e417-e423. doi: 10.1016/j.cllc.2017.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swensen SJ, Jett JR, Sloan JA, et al. Screening for lung cancer with low-dose spiral computed tomography. Am J Respir Crit Care Med. 2002;165(4):508-513. doi: 10.1164/ajrccm.165.4.2107006 [DOI] [PubMed] [Google Scholar]
- 12.Morgan L, Choi H, Reid M, Khawaja A, Mazzone PJ. Frequency of incidental findings and subsequent evaluation in low-dose computed tomographic scans for lung cancer screening. Ann Am Thorac Soc. 2017;14(9):1450-1456. doi: 10.1513/AnnalsATS.201612-1023OC [DOI] [PubMed] [Google Scholar]
- 13.Tsai EB, Chiles C, Carter BW, et al. Incidental findings on lung cancer screening: significance and management. Semin Ultrasound CT MR. 2018;39(3):273-281. doi: 10.1053/j.sult.2018.02.005 [DOI] [PubMed] [Google Scholar]
- 14.MacRedmond R, McVey G, Lee M, et al. Screening for lung cancer using low dose CT scanning: results of 2 year follow up. Thorax. 2006;61(1):54-56. doi: 10.1136/thx.2004.037580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van de Wiel JC, Wang Y, Xu DM, et al. ; NELSON Study Group . Neglectable benefit of searching for incidental findings in the Dutch-Belgian lung cancer screening trial (NELSON) using low-dose multidetector CT. Eur Radiol. 2007;17(6):1474-1482. doi: 10.1007/s00330-006-0532-7 [DOI] [PubMed] [Google Scholar]
- 16.Rampinelli C, Preda L, Maniglio M, et al. Extrapulmonary malignancies detected at lung cancer screening. Radiology. 2011;261(1):293-299. doi: 10.1148/radiol.11102231 [DOI] [PubMed] [Google Scholar]
- 17.Chiles C, Paul NS. Beyond lung cancer: a strategic approach to interpreting screening computed tomography scans on the basis of mortality data from the National Lung Screening Trial. J Thorac Imaging. 2013;28(6):347-354. doi: 10.1097/RTI.0000000000000052 [DOI] [PubMed] [Google Scholar]
- 18.Mafi JN, Reid RO, Baseman LH, et al. Trends in low-value health service use and spending in the US Medicare fee-for-service program, 2014-2018. JAMA Netw Open. 2021;4(2):e2037328. doi: 10.1001/jamanetworkopen.2020.37328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Humphrey L, Deffebach M, Pappas M, et al. Screening for Lung Cancer: Systematic Review to Update the U.S. Preventive Services Task Force Recommendation. Agency for Healthcare Research and Quality; 2013. [PubMed] [Google Scholar]
- 20.Owens DK, Qaseem A, Chou R, Shekelle P; Clinical Guidelines Committee of the American College of Physicians . High-value, cost-conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med. 2011;154(3):174-180. doi: 10.7326/0003-4819-154-3-201102010-00007 [DOI] [PubMed] [Google Scholar]
- 21.Qaseem A, Alguire P, Dallas P, et al. Appropriate use of screening and diagnostic tests to foster high-value, cost-conscious care. Ann Intern Med. 2012;156(2):147-149. doi: 10.7326/0003-4819-156-2-201201170-00011 [DOI] [PubMed] [Google Scholar]
- 22.Harris RP, Wilt TJ, Qaseem A; High Value Care Task Force of the American College of Physicians . A value framework for cancer screening: advice for high-value care from the American College of Physicians. Ann Intern Med. 2015;162(10):712-717. doi: 10.7326/M14-2327 [DOI] [PubMed] [Google Scholar]
- 23.American College of Radiology . Lung-RADS assessment categories, version 1.1. Accessed January 1, 2020. https://www.acr.org/-/media/ACR/Files/RADS/Lung-RADS/LungRADSAssessmentCategoriesv1-1.pdf
- 24.Munden RF, Carter BW, Chiles C, et al. Managing incidental findings on thoracic CT: mediastinal and cardiovascular findings: a white paper of the ACR incidental findings committee. J Am Coll Radiol. 2018;15(8):1087-1096. doi: 10.1016/j.jacr.2018.04.029 [DOI] [PubMed] [Google Scholar]
- 25.Gore RM, Pickhardt PJ, Mortele KJ, et al. Management of incidental liver lesions on CT: a white paper of the ACR incidental findings committee. J Am Coll Radiol. 2017;14(11):1429-1437. doi: 10.1016/j.jacr.2017.07.018 [DOI] [PubMed] [Google Scholar]
- 26.Herts BR, Silverman SG, Hindman NM, et al. Management of the incidental renal mass on CT: a white paper of the ACR incidental findings committee. J Am Coll Radiol. 2018;15(2):264-273. doi: 10.1016/j.jacr.2017.04.028 [DOI] [PubMed] [Google Scholar]
- 27.Hoang JK, Langer JE, Middleton WD, et al. Managing incidental thyroid nodules detected on imaging: white paper of the ACR Incidental Thyroid Findings Committee. J Am Coll Radiol. 2015;12(2):143-150. doi: 10.1016/j.jacr.2014.09.038 [DOI] [PubMed] [Google Scholar]
- 28.Megibow AJ, Baker ME, Morgan DE, et al. Management of incidental pancreatic cysts: a white paper of the ACR incidental findings committee. J Am Coll Radiol. 2017;14(7):911-923. doi: 10.1016/j.jacr.2017.03.010 [DOI] [PubMed] [Google Scholar]
- 29.Mayo-Smith WW, Song JH, Boland GL, et al. Management of incidental adrenal masses: a white paper of the ACR incidental findings committee. J Am Coll Radiol. 2017;14(8):1038-1044. doi: 10.1016/j.jacr.2017.05.001 [DOI] [PubMed] [Google Scholar]
- 30.Nguyen XV, Davies L, Eastwood JD, Hoang JK. Extrapulmonary findings and malignancies in participants screened with chest CT in the National Lung Screening Trial. J Am Coll Radiol. 2017;14(3):324-330. doi: 10.1016/j.jacr.2016.09.044 [DOI] [PubMed] [Google Scholar]
- 31.Pinsky PF, Dunn B, Gierada D, et al. Incidental renal tumours on low-dose CT lung cancer screening exams. J Med Screen. 2017;24(2):104-109. doi: 10.1177/0969141316657115 [DOI] [PubMed] [Google Scholar]
- 32.Aberle DR, Berg CD, Black WC, et al. ; National Lung Screening Trial Research Team . The National Lung Screening Trial: overview and study design. Radiology. 2011;258(1):243-253. doi: 10.1148/radiol.10091808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aberle DR, Adams AM, Berg CD, et al. ; National Lung Screening Trial Research Team . Baseline characteristics of participants in the randomized National Lung Screening Trial. J Natl Cancer Inst. 2010;102(23):1771-1779. doi: 10.1093/jnci/djq434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gareen IF, Sicks JD, Jain AA, Moline D, Coffman-Kadish N, Aberle DR. Identifying and collecting pertinent medical records for centralized abstraction in a multi-center randomized clinical trial: the model used by the American College of Radiology arm of the National Lung Screening Trial. Contemp Clin Trials. 2013;34(1):36-44. doi: 10.1016/j.cct.2012.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marcus PM, Doria-Rose VP, Gareen IF, et al. Did death certificates and a death review process agree on lung cancer cause of death in the National Lung Screening Trial? Clin Trials. 2016;13(4):434-438. doi: 10.1177/1740774516638345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Black WC, Gareen IF, Soneji SS, et al. ; National Lung Screening Trial Research Team . Cost-effectiveness of CT screening in the National Lung Screening Trial. N Engl J Med. 2014;371(19):1793-1802. doi: 10.1056/NEJMoa1312547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gareen IF, Black WC, Tosteson T, Wang Q, Tosteson A. Medical care costs were similar across the low-dose computed tomography and chest x-ray arms of the National Lung Screening Trial despite different rates of significant incidental findings. Medical Care. 2018;56(5):403-409. doi: 10.1097/MLR.0000000000000900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janssen K, Schertz K, Rubin N, Begnaud A. Incidental findings in a decentralized lung cancer screening program. Ann Am Thorac Soc. 2019;16(9):1198-1201. doi: 10.1513/AnnalsATS.201812-908RL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kinsinger LS, Anderson C, Kim J, et al. Implementation of lung cancer screening in the Veterans Health Administration. JAMA Intern Med. 2017;177(3):399-406. doi: 10.1001/jamainternmed.2016.9022 [DOI] [PubMed] [Google Scholar]
- 40.Godoy MCB, White CS, Erasmus JJ, et al. Extrapulmonary neoplasms in lung cancer screening. Transl Lung Cancer Res. 2018;7(3):368-375. doi: 10.21037/tlcr.2018.06.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiles C, Duan F, Gladish GW, et al. ; NLST Study Team . Association of coronary artery calcification and mortality in the National Lung Screening Trial: a comparison of three scoring methods. Radiology. 2015;276(1):82-90. doi: 10.1148/radiol.15142062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Howard DH, Richards TB, Bach PB, Kegler MC, Berg CJ. Comorbidities, smoking status, and life expectancy among individuals eligible for lung cancer screening. Cancer. 2015;121(24):4341-4347. doi: 10.1002/cncr.29677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernheim A, Auffermann WF, Stillman AE. The dubious value of coronary calcium scoring on lung cancer screening CT. J Am Coll Radiol. 2017;14(3):343-344. doi: 10.1016/j.jacr.2016.08.011 [DOI] [PubMed] [Google Scholar]
- 44.Tailor TD, Chiles C, Yeboah J, et al. Cardiovascular risk in the lung cancer screening population: a multicenter study evaluating the association between coronary artery calcification and preventive statin prescription. J Am Coll Radiol. 2021;18(9):1258-1266. doi: 10.1016/j.jacr.2021.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yeboah J. Lung cancer screening eligible? Circulation. 2018;138(25):2867-2868. doi: 10.1161/CIRCULATIONAHA.118.037841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leigh A, McEvoy JW, Garg P, et al. Coronary artery calcium scores and atherosclerotic cardiovascular disease risk stratification in smokers. JACC Cardiovasc Imaging. 2019;12(5):852-861. doi: 10.1016/j.jcmg.2017.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pinsky PF, Lynch DA, Gierada DS. Incidental findings on low-dose CT scan lung cancer screenings and deaths from respiratory diseases. Chest. 2022;161(4):1092-1100. doi: 10.1016/j.chest.2021.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huo J, Xu Y, Sheu T, Volk RJ, Shih YT. Complication rates and downstream medical costs associated with invasive diagnostic procedures for lung abnormalities in the community setting. JAMA Intern Med. 2019;179(3):324-332. doi: 10.1001/jamainternmed.2018.6277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanoue LT, Sather P, Cortopassi I, et al. Standardizing the reporting of incidental, non–lung cancer (category S) findings identified on lung cancer screening low-dose CT imaging. Chest. 2022;161(6):1697-1706. doi: 10.1016/j.chest.2021.12.662 [DOI] [PubMed] [Google Scholar]
- 50.Dyer DS, White C, Conley Thomson C, et al. A quick reference guide for incidental findings on lung cancer screening CT examinations. J Am Coll Radiol. 2023;20(2):162-172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Number of significant incidental findings (SIFs) assessed by screening result and whether SIF should be reported to the primary care provider
eTable 2. Specific SIFs by whether they were considered reportable to the referring physician for the LDCT arm of the NLST, across all three screens (T0, T1, T2) by frequency of report
eTable 3. Specific SIFs considered reportable to the referring physician for the LDCT arm of the NLST, across all three screens (T0, T1, T2) by frequency of report
eFigure. Procedure used to categorize each screening examination
Data sharing statement