Abstract
Liver transplantation (LT) is a highly curative therapy for patients with hepatocellular carcinoma (HCC). However, due to the shortage of donor livers and rapid progression of HCC, a majority of patients are dropped out from the waitlist. Recently, immunotherapy has shown great promise in the treatment of advanced HCC. However, the use of immunotherapy is limited in LT mainly due to the potentially increasing risk of graft rejection. One of the main challenges for researchers is the protection of donor graft from an immunotherapy-boosted immune response mounted by the host. Besides, the safety, availability, and costs of immunotherapy are other challenges that need to be addressed. Here, we reviewed the literature involving patients who received immunotherapy prior to transplant to avoid waitlist dropouts and following transplantation to prevent the progression of tumor recurrence and metastasis. Statistically, the incidence of rejection was 25.0% pre-transplant and 18.5% post-transplant. Based on the review of these clinical studies, we can conclude that conducting clinical trials on the safety and efficacy of currently available immunotherapy drugs and identifying novel immunotherapy targets through extensive research may be promising for patients who do not meet the selection criteria for LT and who experience post-transplant recurrence. To date, the clinical experience on the use of immunotherapy before or after LT comes from individual case studies. Although some of the reported results are promising, they are not sufficient to support the standardized use of immunotherapy in clinical practice.
Keywords: Liver transplantation, hepatocellular carcinoma, immunotherapy, immune checkpoint inhibitors, adoptive cell therapy
Introduction
Primary liver cancer is emerging as one of the leading malignancies worldwide and is the fourth major cause of cancer-related mortalities in China (1,2). Among all primary liver cancers, hepatocellular carcinoma (HCC) accounts for 80%−90% of the cases. Surgical tumor resection remains one of the promising curative treatments for a minority of patients with HCC if diagnosed at an early stage. However, most HCC patients are diagnosed at an advanced stage owing to the lack of specific clinical symptoms, leading to poor prognosis. In recent years, there have been advances in surgical strategies in liver transplantation (LT). Reports have shown that healthy donor transplantation for selected patients with HCC due to liver cirrhosis or with multi-tumor HCC was considered a superior curative treatment option compared to surgical resection (3-5). Several retrospective analyses have highlighted that the overall 5-year survival rate of patients with HCC following LT was significantly higher (59.3%−89.0%) than that of patients who underwent liver resection (41%−76%) (6-8). In China, HCC is responsible for 44.99% of LT cases (9). Currently, the Milan criteria (MC), proposed in 1996, represents the benchmark for selecting patients eligible for LT in HCC. Patients who may benefit from LT dropped out of the transplant waitlist due to the strict application (10).
Recent advances in identifying novel tumor antigens and the increased understanding of tumor immune microenvironment have led to the rapid development of immunotherapy-based approaches for various cancers such as metastatic melanoma and non-small cell lung cancer (11-13). Immune checkpoint inhibitors (ICIs) and adoptive cell transfer (ACT) therapies are included in the current forms of immunotherapy. According to the National Comprehensive Cancer Network (NCCN) guidelines, atezolizumab plus bevacizumab, was recently recommended as the preferred therapy for unresectable HCC (14). Further, research linking immunotherapy with organ transplantation has gained increased focus in recent years. Similar to other solid organ transplantation, in recipients of LT, the immune system mounts a response to the allograft primarily mediated by the infiltration of T lymphocytes and other immune cells along with varied inflammatory reactions. Immunotherapy may additionally pose a potential risk to the allograft by amplifying the immune response further (15). Blocking immune checkpoints through inhibitors of programmed cell death protein-1 (PD-1)/programmed cell death ligand-1 (PD-L1) and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) can prevent immune escape mechanisms of the tumor and induce sustainable antitumor responses (Figure 1) (16-18). Immunotherapy can be used in recipients of LT in two proposed scenarios (19). In the first scenario, immunotherapy may be used as conversion or neoadjuvant therapy in a pretransplant setting (20). Immunotherapy will boost the immune system and increase the cytotoxic T-cell activity against the cancer cells. When the immune system cools off and gradually returns to its normal state, LT can be safely conducted. In addition, pre-transplant immunosuppressants can be used to protect the graft. In the second scenario, immunotherapy can be used following transplantation to possibly mitigate cancer recurrence or de novo malignancies. Immunotherapy may be applied 1) to prevent tumor progression or reduce tumor staging before transplantation; 2) as therapeutic targets for preventing the recurrence of HCC following LT; and 3) to other organ tumors in recipients of LT who were under prolonged immunosuppression.
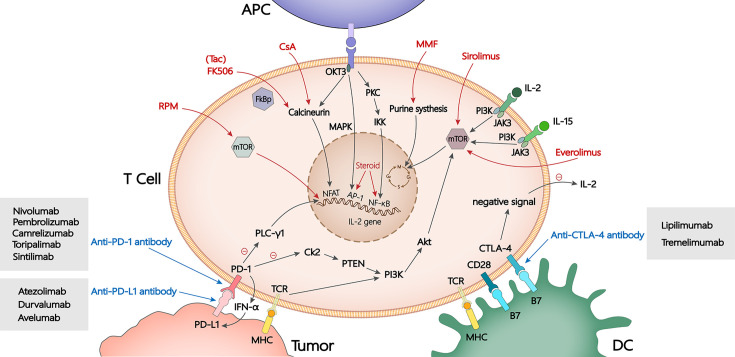
Figure 1.
Targets of immunosuppressants and immune checkpoint inhibitors. Steroids affect the activity of AP-1 and NF-κB which in turn influence T cell activation. Calcineurin inhibitors such as CsA, Tac and MMF inhibit T cell transcription, proliferation and synthesis. RPM including sirolimus and everolimus bind with mTOR, blocking IL-2 signal transmission. Immune checkpoint inhibitors such as anti-PD-1/PD-L1 antibody and anti-CTLA-4 antibody inhibit immune escape through the PD-1/PD-L1 and CTLA-4 pathway, so as to improve the anti-tumor effect. APC, antigen presenting cell; Tac (FK506), tacrolimus; CsA, cyclosporin A; MMF, mycophenolate mofetil; MHC, major histocompatibility complex; RPM, rapamycin; mTOR, mechanistic target of rapamycin; PD-1/PD-L1, programmed cell death protein-1/programmed cell death ligand-1; CTLA-4, cytotoxic T lymphocyte-associated antigen-4; IL, interleukin; DC, dendritic cell.
To achieve a better post-transplant outcome, downstaging before LT is preferred to reduce the tumor burden and meet the transplant criteria (21). Several multicenter clinical studies have shown that effective and sustained downstaging of HCC prior to LT could improve tumor-free and overall survival (OS) of patients compared to the other currently available non-transplantation therapies (22-24). Moreover, the NCCN guidelines recommend combining multiple adjuvant therapies to achieve a better downstaging effect (25). Immunotherapy, as a promising and feasible antitumor therapy, has gained increased success in preoperative downstaging treatment (26). Furthermore, locoregional therapies combined with immunotherapy were proposed to increase the therapeutic effect (27).
The 1- and 5-year survival rates following LT in HCC are 70% and 85%; however, up to 20% of the patients experienced recurrence following transplantation (28,29). A recurrence rate of 10%−20% in the liver and lung was reported (30-33). Recurrence is the rate-limiting factor for long-term survival in HCC (34). The median survival duration after recurrence was less than 1 year, and the highest tumor recurrence rate was reported about 2−3 years following transplant (35). There is emerging evidence that the choice of immunosuppressive therapy after LT in HCC can influence oncological survival and HCC recurrence (36). Conventional therapeutics have a limited effect on the prevention and treatment of tumor recurrence and metastasis. Therefore, an urgent need to develop effective therapies against tumor recurrence and metastasis exists (4). Until December 2022, thirty-one studies have reported using ICIs pre- and post-transplant, and the results exhibited remarkable efficiency (37,38).
Despite increasing evidence in favor of immunotherapy as a promising target in both pre- and post-transplant scenarios discussed above, there exists a concern with its use because of the unique immunological characteristics of patients eligible for LT. In this article, we have comprehensively reviewed the applications and deterrents of the use of immunotherapy in HCC for pre-transplant downstaging and post-transplant prevention and treatment of tumor recurrence and metastasis.
Immunotherapy for downstaging prior to LT
Recently, downstaging prior to LT has gained importance in the screening of patients to determine eligibility for transplantation. This is due to its benefits in minimizing the risk of waitlist dropout and decreasing tumor dimension to meet the acceptable criteria. Patients who do not meet the MC should undergo extensive attempts for downstaging without a prior exclusion to ensure a better long-term prognosis (39). A comprehensive cohort study indicated that successful downstaging of tumors that initially did not meet the MC resulted in post-transplant survival and recurrence-free survival rates comparable to the group that met the MC (40). Transarterial chemoembolization (TACE), transarterial radioembolization, radiofrequency ablation, tyrosine kinase inhibitors (TKIs), and combinations of the aforementioned treatments are some of the widely used downstaging methods in clinics. Considering the evidence of the excellent clinical outcomes for immunotherapy in primary liver cancer, several international clinics have implemented pre-transplant immunotherapy using ICIs as a neoadjuvant “bridging” therapy (41). The half-lives of the ICIs are listed in Table 1.
Table 1. Half-life of ICIs.
| ICIs | Target | Half-life (d) |
| ICI, immune checkpoint inhibitor; PD-1, programmed cell death-1; PD-L1, programmed cell death-ligand 1; CTLA-4, cytotoxic T lymphocyte-associated antigen-4. | ||
| Nivolumab | PD-1 | 25.0 |
| Pembrolizumab | PD-1 | 22.0 |
| Camrelizumab | PD-1 | 5.5 |
| Toripalimab | PD-1 | 12.6 |
| Sintilimab | PD-1 | 19.6 |
| Tislelizumab | PD-1 | 13.3 |
| Penpulimab | PD-1 | 23.3 |
| Cemiplimab | PD-1 | 19.0 |
| Atezolizumab | PD-L1 | 27.0 |
| Durvalumab | PD-L1 | 18.0 |
| Avelumab | PD-L1 | 6.1 |
| Ipilimumab | CTLA-4 | 14.7 |
ICIs for Downstaging
Reports stated that ICIs exhibit considerable benefits in improving life quality with a favorable safety profile in patients with advanced or metastatic cancer (42-44). To date, a total of 14 single-center clinical studies have reported using ICIs as a downstaging therapy prior to LT. PD-1 inhibitors were reported to be used most widely in clinics for downstaging. In early 2020, Schwacha-Eipper et al. were the first to successfully use an ICI in a patient with HCC not meeting the MC (26). Chen et al. described five patients treated with PD-1 inhibitors prior to transplant and found that none of them had experienced acute allograft rejections (45). Kang et al. administered pembrolizumab prior to LT in an adolescent. Recurrent disease or allograft rejection (46) was not observed, suggesting a significant curative effect of ICIs in HCC downstaging. Nevertheless, clinicians should not ignore the possible rise in the reports of allograft rejection rates following ICI administration. Dehghan et al. presented a retransplant case report describing the rescue from a severe acute rejection due to pre-transplant therapy with nivolumab, a PD-1 inhibitor (47). From the report by Abdelrahim et al., treatment with atezolizumab and bevacizumab has shown excellent responses in patient with poorly differentiated HCC (48).
It is worth noting that there are no definitive conclusions on whether the time interval between the administration of ICIs and LT is associated with allograft rejections or other immune responses. Some scholars have proposed that interval >28 or 90 d is relatively safe. Dave et al. (49), Sogbe et al. (50) and Schnickel et al. (51) reported that patients who received the last dose within 90 d developed acute cell rejection. No patient who underwent LT more than 90 d after the last dose of ICIs developed rejection. Tabrizian et al. reported nine patients at a single center receiving nivolumab as pre-transplant treatment. Among the nine patients, the shortest interval between nivolumab administration and LT was one day, and neither severe allograft rejection nor tumor recurrence was noted (52). Lizaola-Mayo demonstrated a patient received ipilimumab and nivolumab considering disease progression 9 weeks pre-transplant and no rejection was observed (53). Chen et al. reported one patient who received toripalimab 93 d before LT and experienced fatal acute hepatic necrosis (54). The large heterogeneity of hepatic cancer and the complex immunological environment in patients are probably the cause of the lack of guidelines or consensus on the method and time of use of ICIs in LT. In general, for downstaging before LT in HCC, reducing tumor volume to meet the MC and a drop in serum biomarkers, such as alpha-fetoprotein (AFP) and des-gamma-carboxy prothrombin are important clinical endpoints.
Additionally, there is an increased focus on the combination therapy of ICIs with TKIs or locoregional therapy, which has shown a synergistic effect against HCC. Qiao et al. investigated seven patients who were administered PD-1 inhibitors in combination with lenvatinib, as downstaging treatment, and underwent successful LT (55).
Safety of ICIs used for downstaging
According to the guidelines of the American Society of Clinical Oncology (ASCO), the adverse effects of ICIs can be divided into 10 categories. Cutaneous toxicities are the most common and reported in up to 71.5% of patients, followed by gastrointestinal, renal, and nervous system toxicities among others (56). These adverse effects are also likely to occur in HCC patients who were treated with ICIs as a downstaging treatment prior to LT. Allograft rejection caused by the administration of ICIs needs to be studied further in LT recipients. Graft-versus-host disease (GVHD), the most common type of transplant rejection complication, includes hyperacute, acute and chronic GVHD, host-versus-graft disease. Studies have reported that acute rejection following LT occurred in 11.2%−32.0% (57-59) of the patients. Fifteen out of 67 (22.4%) LT recipients who received immunotherapy experienced acute allograft rejection. The risk of rejection with immunotherapy is not sufficiently high compared to the overall risk of rejection following LT. This may be due to the small sample size.
Chen et al. (54), Nordness et al. (60), and Aby et al. (61) administered PD-1 inhibitors prior to transplantation which led to fatal, acute necrosis of the liver. Ten out of forty (25%) patients suffered allograft rejection (Table 2) (47,49,51,52,54,55,60,61). Furthermore, based on previous studies, postoperative PD-L1 expression in the allograft biopsy was shown to be closely associated with allograft rejection (54,55). PD-L1 was found to be expressed in postoperative biopsies in patients who experienced fatal acute rejection. Recent studies have highlighted the need to detect postoperative PD-L1 expression in LT recipients who received ICIs.
Table 2. Preoperative application of ICIs in LT.
| Authors | Year | No. of patients | ICIs | Mean immunotherapy course | Immunosuppression | Mean time between immunotherapy and LT | Rejection | Recurrence |
| ICI, immune checkpoint inhibitor; LT, liver transplantation; MMF, mycophenolate mofetil; Tac, tacrolimus; ATG, anti-thymocyte globulin; N, No; Y, Yes; N/A, not available. | ||||||||
| Schwacha-Eipper et al. (26) | 2020 | 1 | Nivolumab | 34 | N/A | 6 weeks | N | N |
| Chen et al. (45) | 2021 | 5 | Nivolumab | 3 | Tac, MMF | 9 weeks | N | 2/5 Y |
| Kang et al. (46) | 2021 | 1 | Pembrolizumab | 3 | Sirolimus, Tac | 138 d | N | N |
| Dehghan et al. (47) | 2021 | 1 | Nivolumab | 17 | Steroids, MMF, Tac | 5 weeks | Y | N/A |
| Abdelrahim et al. (48) | 2022 | 1 | Atezolizumab | 6 | Tac, MMF | 8 weeks | N | N |
| Dave et al. (49) | 2022 | 5 | Nivolumab | N/A | N/A | 105 d | 2/5 Y | N/A |
| Sogbe et al. (50) | 2021 | 1 | Durvalumab | N/A | Steroids, MMF, Tac | >3 months | N | N |
| Schnickel et al. (51) | 2022 | 5 | Nivolumab | N/A | Steroids, MMF, Tac | 14.6 months | 2/5 Y | N |
| Tabrizian et al. (52) | 2021 | 9 | Nivolumab | 13 | Steroids, MMF, Tac | 40.8 months | 1/9 Y | N |
| Lizaola-Mayo et al. (53) | 2021 | 1 | Ipilimumab Nivolumab | N/A | Tac, MMF, ATG, steriods | 9 weeks | N | N |
| Chen et al. (54) | 2021 | 1 | Toripalimab | 10 | Tac, steroids | 93 d | Y | N/A |
| Qiao et al. (55) | 2021 | 7 | Pembrolizumab Camrelizumab | 3 | Basiliximab, steroids, cyclosporine, Tac, sirolimus, MMF | 1.3 months | 1/7 Y | N/A |
| Nordness et al. (60) | 2020 | 1 | Nivolumab | 44 | Steroids, MMF, Tac | 8 d | Y | N/A |
| Aby et al. (61) | 2022 | 1 | Nivolumab | 23 | Steroids, MMF, Tac | 16 d | Y | N/A |
Immunotherapy for preventing and treating tumor recurrence and metastasis after LT
Since tumor recurrence and metastasis following LT severely affect the prognosis of LT recipients in HCC, there is an urgent need to identify high-risk LT recipients and develop newer effective treatment options for preventing and treating tumor recurrence and metastasis. Post-transplant immunotherapy is gaining increased importance due to its possible role in the elimination of occult tumor cells and treatment of tumor metastasis following transplantation (62,63). Both ICIs and ACT therapies are reported to be used as post-transplant immunotherapies. However, it should be noted that post-transplant immunotherapies can not only achieve a persistent antitumor immune response, but also expose patients to a high risk of graft rejection and loss of function (64,65).
ICIs as postoperative treatments
Similar to strategies to minimize treatment doses for immunosuppression, a minimum therapeutic dose of ICIs was suggested for preventing and treating tumor recurrence and metastasis following LT in clinical situations where rejection is one of the major concerns (66). Until December 2022, sixteen clinical studies reported the use of ICIs following transplantation (Table 3). The rejection rate was 18.5% (5/27), and the progressive disease (PD) rate was 37.0% (10/27).
Table 3. Postoperative application of ICIs in LT.
| Authors | Year | No. of patients | ICIs | Mean immunotherapy course | Immunosuppression | Mean time between immunotherapy and LT (year) | Rejection | Recurrence/outcomes |
| ICI, immune checkpoint inhibitor; LT, liver transplantation; Tac, tacrolimus; MMF, mycophenolate mofetil; N, No; Y, Yes; N/A, not available; PR, partial response; PD, progressive disease; CR, complete response; SD, stable disease. | ||||||||
| Morales et al. (67) | 2015 | 1 | Ipilimumab | 4 | Tac, MMF | 8.0 | N | PR |
| Ranganath et al. (68) | 2015 | 1 | Ipilimumab | 4 | Tac | 8.0 | N | N/A |
| De Toni et al. (69) | 2017 | 1 | Nivolumab | 7 | Tac | 0.9 | N | PD |
| Chen et al. (70) | 2019 | 1 | Pembrolizumab | 15 | Everolimus, MMF, steroids | 3.6 | N | PR |
| Varkaris et al. (71) | 2017 | 1 | Pembrolizumab | N/A | Tac | 8.0 | N | PD |
| Zhuang et al. (72) | 2019 | 1 | Nivolumab | 31 | Tac | 2.7 | N | PD |
| Qiu et al. (73) | 2020 | 1 | Camrelizumab | 5 | Steroids, MMF, Tac | 4.0 | N | PD |
| Rammohan et al. (74) | 2018 | 1 | Pembrolizumab | N/A | Steroids, MMF, Tac | 4.0 | N | N |
| Amjad et al. (75) | 2020 | 1 | Nivolumab | N/A | Tac, MMF | 1.3 | N | N |
| Jarroudi et al. (76) | 2020 | 3 | Nivolumab | 3 | Tac | 1.9 | N | PD |
| Wang et al. (77) | 2016 | 1 | Pembrolizumab | 1 | Tac, sirolimus | 1.0 | Y | N |
| Gassmann et al. (78) | 2018 | 1 | Nivolumab | 1 | Steroids, MMF, everolimus | 2.0 | Y | N/A |
| DeLeon et al. (79) | 2018 | 5 | Nivolumab | 3.2 | Tac, sirolimus, MMF | 3.3 | 1/5 Y | 3/5 PD1/5 CR |
| Lee et al. (80) | 2020 | 1 | Nivolumab | 2 | Cyclosporine, everolimus, MMF, steroids, sirolimus | 12.0 | Y | N/A |
| Shi et al. (81) | 2021 | 5 | Toripalimab | 4.2 | N/A | N/A | N | 2/5 PD1/5 SD |
| Friend et al. (82) | 2017 | 2 | Nivolumab | 1.5 | Sirolimus, Tac | 1.0 | Y | N/A |
Several clinical studies with small sample sizes have investigated the therapeutic efficacy of ICIs in controlling tumor progression and prolonging the survival of LT recipients after tumor recurrence or metastasis. To date, there is no clinical evidence of graft rejection in patients who received post-transplant treatment with the CTLA-4 inhibitor ipilimumab (Figure 1) (83,84). Early in 2015, Morales et al. reported one patient who received ipilimumab (a CTLA-4 inhibitor) following transplant showed a partial response and no evidence of graft rejection (67). Ranganath et al. also reported an LT recipient who received ipilimumab and did not experience organ rejection, immune-related adverse events (AEs), or tumor regression (68). According to reports, treatment with ICIs and an immunosuppressive regimen of tacrolimus and prednisone along with close monitoring of liver function in patients with recurrent or metastatic HCC after transplant may be beneficial (69-71). Benefits in survival with recurrent HCC without AEs could be achieved with ICIs in an LT recipient under immunosuppression (72). However, considering the risk of graft rejection, it should be used with caution in allograft recipients (73). Rammohan et al. reported a patient with metastatic HCC who received pembrolizumab after LT and had a complete radiological response. No liver graft rejection or dysfunction was noted (74). Based on the available data, although immunotherapy has some risks, it may be used as a salvage therapy showing favorable benefits if no existing treatment options can prolong survival (75,76). In brief, several case reports and clinical studies showed that the administration of PD-1 inhibitors had acceptable clinical benefits. Further studies are warranted to understand the use, duration, and dose of ICIs for LT recipients. In conclusion, although several clinical studies found that ICIs were efficient in treating tumor recurrence and metastasis following LT, the risk:benefit ratio between the antitumor efficiency and AEs should be considered.
Adoptive cell transfer therapy for postoperative use
In ACT, T cells are genetically engineered ex vivo and injected into the patient’s body for antitumor, antiviral, or anti-inflammatory effects (85). This approach has significantly helped in enhancing treatment options for treating multiple types of tumors (86). Chimeric antigen receptor-T (CAR-T)cells, cytokine-induced killer (CIK) cells, T-cell receptor-gene-engineered T (TCR-T) cells, and tumor-infiltrating lymphocytes (TILs) are the most commonly used cell types for treating cancer. Gene-editing technology enables CAR-T cells and TCR-T cells to identify antigens on the cell surface of tumors, thus improving the specificity and reactivity of immune cells (87). Adoptive cell therapies, using CIK cells (15) and natural killer (NK) cell therapy (88) have been used as a prophylactic treatment in patients or mice models to prevent recurrence following LT, depending on the augmentation of NK cell response through interleukin-2 and other stimulated factors (89,90). The key inclusion criteria for patient selection were: 1) primary LT recipients with HCC who were ≥18 years old; 2) pathologist-confirmed possible tumors in the liver before cell administration; 3) the absence of serious cardiovascular disease and a satisfactory renal function, as indicated by receipt of hemodialysis more than twice a week and bone marrow competence; and 4) examinations with satisfactory liver functions and no acute AE. The key exclusion criteria were: 1) multiple organ transplant, prior solid organ or bone marrow transplant, fulminant hepatic failure, ABO-incompatible transplant, and intercurrent chemotherapy at enrollment; or 2) positive for infectious diseases [hepatitis C virus (HCV), hepatitis B core antibody (HBcAb), hepatitis B core antigen (HBcAg) and human immunodeficiency virus].
CIK cell therapy
Treatment with CIK cell therapy has been shown to prolong OS and progression-free survival (PFS) among patients with HCC (91,92). Studies have shown that the comprehensive application of CIK therapy in individuals, with a clinical assessment of complete remission and significant reduction of tumor load, was found to be an effective strategy to prevent tumor recurrence and enhance survival (93). Li et al. administered CIK cells to one patient following LT to eliminate any remaining tumor cells while also stimulating the immune system. This was shown to decrease the tumor volume, and clinical symptoms, such as fever and gastrointestinal symptoms, and CIK-associated AEs, were not observed (15). CIK cells could be a promising novel therapeutic option in the fight against cancer (94). Clinical trials have shown that CIK cell infusion has lower toxicity in LT recipients, suggesting a favorable safety profile.
NK cells therapy
NK cells act as guardians to the surveillant microenvironment of the immune system. They can rapidly destroy or “kill” multiple adjacent cells (95). NK cell-mediated immunotherapy has emerged as a safe and effective treatment option in advanced-stage leukemia (96). In 2009, Ohira et al. initially hypothesized that adoptive immunotherapy employing activated NK cells in living donor recipients with HCC can be a unique method to restore the suppressed immune system (97). And it was demonstrated in a mouse model. In 2012, they reported the use of NK and natural killer T (NKT) cells in patients and mouse models. The results showed that this adoptive immunotherapy could mount remarkable anti-HCV responses in HCV-infected LT recipients (89). Furthermore, they later used NK cells from a deceased donor liver in clinical cell transplantation, hoping to benefit patients with HCC who underwent LT for preventing tumor recurrence with this strategy (90). Most recently, an intravenous infusion of NK cells derived from deceased donor liver was used 3−5 d after LT to prevent HCC recurrence in patients. No severe cell infusion-related AEs, acute rejection episodes, or any evidence of HCC recurrence were noted (98). The infusion of liver NK cells was well tolerated and effective in avoiding HCC recurrence following LT. A possible explanation is that under immunosuppressed conditions, activated allogenic liver NK cells can prevent HCC recurrence (99). Thus, adoptive immunotherapy employed after transplantation appears to be a feasible option with no recurrence. This strategy was first reported in a male who developed recurrence following LT. He was treated with iodine-125 seed implantation and allogenic NK cell immunotherapy and showed improved immune function and reduced tumor size (100). Preliminary clinical studies have indicated that NK cell infusions for preventing or treating HCC recurrence following LT have a good safety profile and promising prospects.
Dendritic cell (DC) therapy
DCs are highly specialized antigen-presenting cells and play a major role in the initiation and regulation of responses in innate and adaptive immunity, the generation of robust antigen-specific T-cell immune responses, and the induction of immunological memory responses in cancer (101). The results of a phase I/II randomized, controlled trial (UMIN000010691) confirmed that DC adjuvant therapy was safe after curative liver resection in HCC to prevent recurrence (102). Kayashima et al. infused interleukin-12 combined with DCs in mice. Results showed no tumor recurrence or metastasis, but prolonged survival under immunosuppression (103). Although the DC therapy strategies have not been applied in LT, the beneficial results in the HCC mouse model suggest that intratumor neoadjuvant immunotherapy using DCs is potent and effective to control HCC recurrence in patients following LT (103). This novel immunotherapy has a promising potential in improving survival and preventing HCC recurrence following transplantation.
TCR-T cells
TCR-T cells are genetically modified cells that can be directed against specific tumor antigens and have shown promising therapeutic results in preventing tumor growth (104). In a novel case report, autologous-armored hepatitis B virus (HBV) TCR-T cells were infused following LT. The results confirmed that TCR-T cells can target HBsAg-expressing HCC cells and prevent recurrence (105). In another case report, metastatic lesions were reported to be decreased in the first year following TCR-T cell administration (106). Hafezi et al. revealed that engineered TCR-T cells that can attack relapse-induced tumor cells in LT recipients, while briefly escaping the immunosuppressive medications, can be a novel immunotherapeutic strategy (107). TCR-T-cell therapy shows specificity in evading immunosuppression and provides better antitumor therapy.
CAR-T cells
Chimeric antigen receptors combine antigen specificity with T-cell activation in a single fusion molecule. Four generations of CARs therapies have been developed to date (108). CAR-T-cell therapy has phenomenal potential for the treatment of relapsed or refractory hematologic malignancies (18,109-112). T and NK cells transduced with a CAR can detect the CD147 surface marker and kill different malignant HCC cells (113). CAR-T cells in mouse models showed a visible effect (114). Only one report has published the initial safety profile and antitumor activities of CAR-T cell therapy in 13 patients with HCC. The 1-year survival rate was 42.0%, with a median OS of 278 d (115). CAR-T cells significantly enhanced the antitumor activity against solid tumors (116). Although CAR-T cell therapy has not yet been used in LT, its antitumor ability in HCC appears promising in transplant scenarios.
Postoperative safety of immunotherapy
The use of immunotherapy for LT recipients in a postoperative setting is attractive and promising. Although, studies have reported considerable benefits of postoperative immunotherapy with ICIs so far, AEs and the risk of fatal acute rejection restrict its use (77,117,118). This calls for caution with the use of ICIs in a postoperative setting in LT.
According to a systematic review, among four patients with recurrent HCC treated with ICIs, two deaths were reported due to graft rejection and two due to PD (119). The rates of allograft rejection and mortality continue to remain high in solid organ transplant recipients. Gassmann reviewed 29 published cases and reported a graft loss rate of 36%−54% (78). Decisions for its uses must be made considering the clinical benefit of PD-1 inhibitor therapy and the risk of graft rejection (79). Lee et al. reported an eventual outcome of severe acute T cell-mediated rejection 12 years after transplantation in a male who received nivolumab (80). Shi et al. conducted a prospective study on patients with recurrent HCC after transplant. Five patients with negative PD-L1 expression in the graft received anti-PD-1 therapy and showed no graft rejection, whereas one patient with PD-L1 expression in the graft showed rejection (81). Acute rejection was observed in two patients with PD-L1 positive (82). As with the use of PD-1 inhibitors before transplantation, positive PD-L1 expression in grafts post-transplant may be mainly responsible for rejection. Data on the use of PD-1/PD-L1 inhibitors in LT recipients are inadequate. Additional research with larger, prospective trials are needed to determine the best approach for selection and care among LT recipients to be administered PD-1 inhibitors. Furthermore, despite immunosuppression, the therapeutic benefit of PD-1 inhibitor therapy may be achieved. However, the risk of graft rejection outweighs the benefit; therefore, patients must be cautiously screened before exploring this option in LT.
The ACT therapy in a post-transplant setting not only has the feasibility in treating recurrent and metastatic tumors but also can contribute uniquely to prevent recurrence and metastasis. According to data from clinical studies with a small sample size, ACT-associated AEs were not observed. ACTs may be considered safer than ICIs. Further research is warranted owing to the lack of larger studies using ACTs.
Conclusions and prospects
Due to the shortage of donor livers and rapid progression of HCC, a large number of patients with HCC are dropped out from the LT waitlist. In addition, challenges in treating and preventing tumor recurrence and metastasis after LT exist due to a lack of effective therapeutics (120). With a better understanding of tumor immunology and advances in biotechnology through the development of monoclonal antibodies, immunotherapy has become a popular option in cancer treatment. Atezolizumab in combination with bevacizumab is approved by the Food and Drug Administration (FDA) as a first-line treatment for patients with HCC. Future research in the use of innovative adoptive cell treatments for HCC is being explored. Moreover, with remarkable clinical outcomes, different immunotherapy strategies have been applied in pre-transplant downstaging and treatments and in the prevention of recurrent or metastatic HCC following transplantation.
The criteria for selecting patients eligible for LT are summarized here. Patients are included if: 1) received TACE prior to transplant, radiofrequency ablation, transarterial Y90 radioembolization, and multikinase inhibitors or underwent resection for advanced stage HCC, with insufficient efficacy (elevated AFP and PD), minimal response rates, and associated intolerability; 2) advanced, recurrent, de novo malignancy or metastasis of lymph node was confirmed through surveillance imaging or histology; 3) tumor progressed after using first-line treatment (sorafenib) and second-line treatment (gemcitabine and oxaliplatin/regorafenib chemotherapy); 4) intolerable to sorafenib; 5) immunotherapy is used as salvage treatment for HCC recurrence following LT; and 6) a high tumor mutational burden was confirmed using a total exon gene test, indicating that immunotherapy might be effective. Patients are excluded if: 1) negative PD-L1 expression was noted in grafts; or 2) bowel obstruction and gastrointestinal bleeding were present. Patients with advanced, recurrent, de novo malignancy or metastasis and PD after conventional locoregional or systemic therapy might achieve maximum benefits.
In this article, we reviewed fourteen studies including forty patients prior to LT until December 2022. The rejection rate was reported to be 25% and recurrence was noted in 2 patients. Clinical trials (No. NCT05027425) exploring the safety of the interval between last dose of ICIs and LT are ongoing.
Sixteen studies reported the use of ICIs after transplantation. The rejection rate was 18.5%, and the PD rate was 37% in 27 patients. Two trials (No. NCT03966209 and NCT04564313) assessed the safety and efficacy of PD-1 inhibitors (toripalimab and camrelizumab) in patients with recurrent or metastatic cancer following LT. The primary outcome measures the included rate of occurrence of serious AEs, acute graft rejection rate, and objective response rate. Other trials (No. NCT01147380 and NCT02849015) aim to assess the efficacy and safety of NK cells following LT. Immunotherapy plays a unique role in preventing tumor recurrence or metastasis. Considering the challenges, although the path to successfully applying immunotherapy treatment strategies in LT for HCC is bumpy, it is promising.
Considering the clinical benefits and possible AEs of immunotherapy, clinicians should focus on the following four aspects. First, it is crucial to detect the expression of biomarkers, such as PD-L1, after immunotherapy to prevent post-transplant acute allograft rejection. Patients with positive allograft PD-L1 expression should not be considered for immunotherapy. Second, experts recommend that the interval between immunotherapy and LT should be more than its half-life. According to clinical experience, it may be safe to withdraw immunotherapy 28 or 90 d before LT. There is no consensus on the courses and withdrawal time of immunotherapy. Third, the clinical benefits of using immunosuppressants and immunotherapy agents should be balanced. Immunosuppressants should be tapered to the lowest effective dose to protect against rejection (121). Finally, a multidisciplinary team (MDT) including a hepatobiliary surgeon, an oncologist, a pathologist, radiologists, and other specialists contributes to comprehensive treatment and prognosis of cancers. The MDT is encouraged to develop customized treatment protocols for LT recipients who received immunotherapy.
Immunotherapy has the potential to improve the prognosis of LT in HCC. However, large-scale trial data to guide its clinical application are lacking. Several clinical trials are ongoing (Table 4). We believe in the bright future of immunotherapy with extensive ongoing research.
Table 4. Clinical trials of immunotherapy for LT.
| No. of NCT | PI | Intervention model description | Intervention/treatment | Primary outcomes measures | Secondary outcome measures | Status |
| LT, liver transplantation; PI, principal investigator; RFS, recurrence-free survival; OS, overall survival; I.V., intravenous; AE, adverse event; CTCAE, Common Terminology Criteria for Adverse Events; ORR, objective response rate; PFS, progression-free survival; TTP, time to progression; SAE, serious adverse event; GR, graft rejection; NK, nature killer. | ||||||
| NCT05027425 | Davendra Sohal et al. | Single-arm, open-label, multicenter clinical trial, phase II | Four months durvalumab plus tremelimumab, after 72 d undergo LT | Cellular rejection rates | 1. AEs during treatment, and graft loss and mortality rates2. Radiologic responses via RECIST 1.1 and/or mRECIST3. Pathologic responses via explanted liver assessment4. RFS and OS outcomes based on survival follow-up reporting | Not yet recruiting |
| NCT03966209 | Huang et al. | Single group assignment, open label, phase I | JS001(PD-1 inhibitor) 240 mg I.V. Q3W | 1. Serious AEs rate2. Acute graft rejection rate | ORR, PFS, OS | Recruiting |
| NCT04564313 | Wang et al. | Single group assignment, open label, phase I | Camrelizumab (SHR-1210), 200 mg, I.V., Q3W | ORR | OS, PFS, TTP, SAE, GR | Recruiting |
| NCT01147380 | Seigo Nishida et al. | Non-randomized, parallel assignment, open label, phase I | Small dose: inoculation NK cells between 10 and 100 million cellsLarge dose: inoculation cells between 100 and 1,000 million cells | Side effects of cadaveric donor liver NK cell infusion | 1. NK cell infusion-related toxicity2. Anti-HCC effect of this treatment3. Anti-HCV effect of this treatment (if applicable) | Completed |
| NCT02849015 | Chen et al. | Randomized, parallel assignment, open label phase I, phase II | Device: CryosurgeryBiological: NK immunotherapy | No. of participants with treatment-related AEs as assessed by CTCAE v4.0 (Time frame: 3 months) | PFS, OS | Completed |
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Acknowledgements
This study was supported by grants from National Key Research and Development Program of China (No. 2021YFA1100500); the Major Research Plan of the National Natural Science Foundation of China (No. 92159202); Key Program, National Natural Science Foundation of China (No. 81930016); Key Research & Development Plan of Zhejiang Province (No. 2019C0350); Key Research & Development Program of Zhejiang Province (No. 2022C03108) and Young Program of National Natural Science Funds (No. 81802889).
Contributor Information
Zhikun Liu, Email: zjxu@zju.edu.cn.
Xiao Xu, Email: zjxu@zju.edu.cn.
References
- 1.Sung H, Ferlay J, Siegel RL, et al Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Wei W, Zeng H, Zheng R, et al Cancer registration in China and its role in cancer prevention and control. Lancet Oncol. 2020;21:e342–9. doi: 10.1016/s1470-2045(20)30073-5. [DOI] [PubMed] [Google Scholar]
- 3.de Lope CR, Tremosini S, Forner A, et al. Management of HCC. J Hepatol 2012;56 Suppl 1: S75-87.
- 4.Forner A, Reig M, Bruix J Hepatocellular carcinoma. Lancet. 2018;391:1301–14. doi: 10.1016/s0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 5.Mazzaferro V, Llovet JM, Miceli R, et al Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43. doi: 10.1016/s1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- 6.Zaydfudim VM, Vachharajani N, Klintmalm GB, et al Liver resection and transplantation for patients with hepatocellular carcinoma beyond Milan criteria. Ann Surg. 2016;264:650–8. doi: 10.1097/sla.0000000000001866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menahem B, Lubrano J, Duvoux C, et al Liver transplantation versus liver resection for hepatocellular carcinoma in intention to treat: An attempt to perform an ideal meta-analysis. Liver Transpl. 2017;23:836–44. doi: 10.1002/lt.24758. [DOI] [PubMed] [Google Scholar]
- 8.Zhang K, Chen R, Gong X, et al Survival outcomes of liver transplantation versus liver resection among patients with hepatocellular carcinoma: A SEER-based longitudinal study. J Formos Med Assoc. 2019;118:790–6. doi: 10.1016/j.jfma.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Ling S, Jiang G, Que Q, et al Liver transplantation in patients with liver failure: Twenty years of experience from China. Liver Int. 2022;42:2110–6. doi: 10.1111/liv.15288. [DOI] [PubMed] [Google Scholar]
- 10.Silva MF, Sherman M Criteria for liver transplantation for HCC: what should the limits be. J Hepatol. 2011;55:1137–47. doi: 10.1016/j.jhep.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Larkin J, Chiarion-Sileni V, Gonzalez R, et al Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381:1535–46. doi: 10.1056/NEJMoa1910836. [DOI] [PubMed] [Google Scholar]
- 12.Reck M, Rodríguez-Abreu D, Robinson AG, et al Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥50. J Clin Oncol. 2021;39:2339–49. doi: 10.1200/jco.21.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo H, Li W, Qian L, et al Clinical challenges in neoadjuvant immunotherapy for non-small cell lung cancer. Chin J Cancer Res. 2021;33:203–15. doi: 10.21147/j.issn.1000-9604.2021.02.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finn RS, Qin S, Ikeda M, et al Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 15.Li R, Yan F, Liu L, et al Cytokine-induced killer cell therapy for the treatment of primary hepatocellular carcinoma subsequent to liver transplantation: A case report. Oncol Lett. 2016;11:1885–8. doi: 10.3892/ol.2016.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun C, Mezzadra R, Schumacher TN Regulation and function of the PD-L1 checkpoint. Immunity. 2018;48:434–52. doi: 10.1016/j.immuni.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodman A, Patel SP, Kurzrock R PD-1-PD-L1 immune-checkpoint blockade in B-cell lymphomas. Nat Rev Clin Oncol. 2017;14:203–20. doi: 10.1038/nrclinonc.2016.168. [DOI] [PubMed] [Google Scholar]
- 18.Qian Y, Yang T, Liang H, et al Myeloid checkpoints for cancer immunotherapy. Chin J Cancer Res. 2022;34:460–82. doi: 10.21147/j.issn.1000-9604.2022.05.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdelrahim M, Esmail A, Saharia A, et al Utilization of immunotherapy for the treatment of hepatocellular carcinoma in the peri-transplant setting: Transplant oncology view. Cancers (Basel) 2022;14:1760. doi: 10.3390/cancers14071760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang C, Sun XD, Qiu W, et al Conversion therapy in liver transplantation for hepatocellular carcinoma: What’s new in the era of molecular and immune therapy. Hepatobiliary Pancreat Dis Int. 2023;22:7–13. doi: 10.1016/j.hbpd.2022.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Jiang G, Ling S, Zhan Q, et al Downstaging treatment for patients with hepatocelluar carcinoma before transplantation. Transplant Rev (Orlando) 2021;35:100606. doi: 10.1016/j.trre.2021.100606. [DOI] [PubMed] [Google Scholar]
- 22.Mazzaferro V, Citterio D, Bhoori S, et al Liver transplantation in hepatocellular carcinoma after tumour downstaging (XXL): a randomised, controlled, phase 2b/3 trial. Lancet Oncol. 2020;21:947–56. doi: 10.1016/s1470-2045(20)30224-2. [DOI] [PubMed] [Google Scholar]
- 23.Chapman WC, Majella Doyle MB, Stuart JE, et al Outcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantation. Ann Surg. 2008;248:617–25. doi: 10.1097/SLA.0b013e31818a07d4. [DOI] [PubMed] [Google Scholar]
- 24.Mehta N, Guy J, Frenette CT, et al Excellent outcomes of liver transplantation following down-staging of hepatocellular carcinoma to within Milan criteria: A multicenter study. Clin Gastroenterol Hepatol. 2018;16:955–64. doi: 10.1016/j.cgh.2017.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu X, Chen J, Wei Q, et al Clinical practice guidelines on liver transplantation for hepatocellular carcinoma in China (2018 edition) Hepatobiliary & Pancreatic Diseases International. 2019;18:307–12. doi: 10.1016/j.hbpd.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Schwacha-Eipper B, Minciuna I, Banz V, et al Immunotherapy as a downstaging therapy for liver transplantation. Hepatology. 2020;72:1488–90. doi: 10.1002/hep.31234. [DOI] [PubMed] [Google Scholar]
- 27.Greten TF, Mauda-Havakuk M, Heinrich B, et al Combined locoregional-immunotherapy for liver cancer. J Hepatol. 2019;70:999–1007. doi: 10.1016/j.jhep.2019.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugawara Y, Hibi T Surgical treatment of hepatocellular carcinoma. Biosci Trends. 2021;15:138–41. doi: 10.5582/bst.2021.01094. [DOI] [PubMed] [Google Scholar]
- 29.Citores MJ, Lucena JL, de la Fuente S, et al Serum biomarkers and risk of hepatocellular carcinoma recurrence after liver transplantation. World J Hepatol. 2019;11:50–64. doi: 10.4254/wjh.v11.i1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehta N, Heimbach J, Harnois DM, et al Validation of a risk estimation of tumor recurrence after transplant (RETREAT) score for hepatocellular carcinoma recurrence after liver transplant. JAMA Oncol. 2017;3:493–500. doi: 10.1001/jamaoncol.2016.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffman D, Mehta N Recurrence of hepatocellular carcinoma following liver transplantation. Expert Rev Gastroenterol Hepatol. 2021;15:91–102. doi: 10.1080/17474124.2021.1823213. [DOI] [PubMed] [Google Scholar]
- 32.Tan DJH, Wong C, Ng CH, et al A meta-analysis on the rate of hepatocellular carcinoma recurrence after liver transplant and associations to etiology, alpha-fetoprotein, income and ethnicity. J Clin Med. 2021;10:238. doi: 10.3390/jcm10020238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maccali C, Chagas AL, Boin I, et al Recurrence of hepatocellular carcinoma after liver transplantation: Prognostic and predictive factors of survival in a Latin American cohort. Liver Int. 2021;41:851–62. doi: 10.1111/liv.14736. [DOI] [PubMed] [Google Scholar]
- 34.Zimmerman MA, Ghobrial RM, Tong MJ, et al. Recurrence of hepatocellular carcinoma following liver transplantation: a review of preoperative and postoperative prognostic indicators. Arch Surg 2008;143:182-8;discussion 188.
- 35.Sapisochin G, Bruix J Liver transplantation for hepatocellular carcinoma: outcomes and novel surgical approaches. Nat Rev Gastroenterol Hepatol. 2017;14:203–17. doi: 10.1038/nrgastro.2016.193. [DOI] [PubMed] [Google Scholar]
- 36.Khorsandi SE, Heaton N Optimization of immunosuppressive medication upon liver transplantation against HCC recurrence. Transl Gastroenterol Hepatol. 2016;1:25. doi: 10.21037/tgh.2016.03.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang T, Zhang L, Xu Y, et al Neoadjuvant therapy and immunotherapy strategies for hepatocellular carcinoma. Am J Cancer Res. 2020;10:1658–67. [PMC free article] [PubMed] [Google Scholar]
- 38.Damiris K, Abbad H, Pyrsopoulos N Cellular based treatment modalities for unresectable hepatocellular carcinoma. World J Clin Oncol. 2021;12:290–308. doi: 10.5306/wjco.v12.i5.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chapman WC, Garcia-Aroz S, Vachharajani N, et al Liver transplantation for advanced hepatocellular carcinoma after downstaging without up-front stage restrictions. J Am Coll Surg. 2017;224:610–21. doi: 10.1016/j.jamcollsurg.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 40.Yao FY, Mehta N, Flemming J, et al Downstaging of hepatocellular cancer before liver transplant: long-term outcome compared to tumors within Milan criteria. Hepatology. 2015;61:1968–77. doi: 10.1002/hep.27752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abdelrahim M, Esmail A, Abudayyeh A, et al Transplant oncology: an evolving field in cancer care. Cancers (Basel) 2021;13:4911. doi: 10.3390/cancers13194911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ryoo BY, Merle P, Kulkarni AS, et al Health-related quality-of-life impact of pembrolizumab versus best supportive care in previously systemically treated patients with advanced hepatocellular carcinoma: KEYNOTE-240. Cancer. 2021;127:865–74. doi: 10.1002/cncr.33317. [DOI] [PubMed] [Google Scholar]
- 43.Bang YJ, Golan T, Dahan L, et al Ramucirumab and durvalumab for previously treated, advanced non-small-cell lung cancer, gastric/gastro-oesophageal junction adenocarcinoma, or hepatocellular carcinoma: An open-label, phase Ia/b study (JVDJ) Eur J Cancer. 2020;137:272–84. doi: 10.1016/j.ejca.2020.06.007. [DOI] [PubMed] [Google Scholar]
- 44.Segal NH, Ou SI, Balmanoukian A, et al Safety and efficacy of durvalumab in patients with head and neck squamous cell carcinoma: results from a phase I/II expansion cohort. Eur J Cancer. 2019;109:154–61. doi: 10.1016/j.ejca.2018.12.029. [DOI] [PubMed] [Google Scholar]
- 45.Chen Z, Hong X, Wang T, et al Prognosis after liver transplantation in patients treated with anti- PD-1 immunotherapy for advanced hepatocellular carcinoma: case series. Ann Palliat Med. 2021;10:9354–61. doi: 10.21037/apm-21-999. [DOI] [PubMed] [Google Scholar]
- 46.Kang E, Martinez M, Moisander-Joyce H, et al Stable liver graft post anti-PD1 therapy as a bridge to transplantation in an adolescent with hepatocellular carcinoma. Pediatr Transplant. 2022;26:e14209. doi: 10.1111/petr.14209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dehghan Y, Schnickel GT, Hosseini M, et al Rescue liver re-transplantation after graft loss due to severe rejection in the setting of pre-transplant nivolumab therapy. Clin J Gastroenterol. 2021;14:1718–24. doi: 10.1007/s12328-021-01521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abdelrahim M, Esmail A, Umoru G, et al Immunotherapy as a neoadjuvant therapy for a patient with hepatocellular carcinoma in the pretransplant setting: A case report. Curr Oncol. 2022;29:4267–73. doi: 10.3390/curroncol29060341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dave S, Yang K, Schnickel GT, et al The impact of treatment of hepatocellular carcinoma with immune checkpoint inhibitors on pre- and post-liver transplant outcomes. Transplantation. 2022;106:e308–9. doi: 10.1097/tp.0000000000004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sogbe M, López-Guerra D, Blanco-Fernández G, et al. Durvalumab as a successful downstaging therapy for liver transplantation in hepatocellular carcinoma: The importance of a washout period. Transplantation. 2021;105:e398–400. doi: 10.1097/tp.0000000000003855. [DOI] [PubMed] [Google Scholar]
- 51.Schnickel GT, Fabbri K, Hosseini M, et al Liver transplantation for hepatocellular carcinoma following checkpoint inhibitor therapy with nivolumab. Am J Transplant. 2022;22:1699–704. doi: 10.1111/ajt.16965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tabrizian P, Florman S, Schwartz M PD-1 inhibitor as bridge therapy to liver transplantation. Am J Transplant. 2021;21:1979–80. doi: 10.1111/ajt.16448. [DOI] [PubMed] [Google Scholar]
- 53.Lizaola-Mayo BC, Mathur AK, Borad MJ, et al Immunotherapy as a downstaging tool for liver transplantation in hepatocellular carcinoma. Am J Gastroenterol. 2021;116:2478–80. doi: 10.14309/ajg.0000000000001391. [DOI] [PubMed] [Google Scholar]
- 54.Chen GH, Wang GB, Huang F, et al Pretransplant use of toripalimab for hepatocellular carcinoma resulting in fatal acute hepatic necrosis in the immediate postoperative period. Transpl Immunol. 2021;66:101386. doi: 10.1016/j.trim.2021.101386. [DOI] [PubMed] [Google Scholar]
- 55.Qiao ZY, Zhang ZJ, Lv ZC, et al Neoadjuvant programmed cell death 1 (PD-1) inhibitor treatment in patients with hepatocellular carcinoma before liver transplant: A cohort study and literature review. Front Immunol. 2021;12:653437. doi: 10.3389/fimmu.2021.653437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schneider BJ, Lacchetti C, Bollin K Management of the Top 10 most common immune-related adverse events in patients treated with immune checkpoint inhibitor therapy. JCO Oncol Pract. 2022;18:431–44. doi: 10.1200/op.21.00776. [DOI] [PubMed] [Google Scholar]
- 57.Yamashiki N, Sugawara Y, Tamura S, et al Living-donor liver transplantation for autoimmune hepatitis and autoimmune hepatitis-primary biliary cirrhosis overlap syndrome. Hepatol Res. 2012;42:1016–23. doi: 10.1111/j.1872-034X.2012.01018.x. [DOI] [PubMed] [Google Scholar]
- 58.Heneghan MA, Zolfino T, Muiesan P, et al An evaluation of long-term outcomes after liver transplantation for cryptogenic cirrhosis. Liver Transpl. 2003;9:921–8. doi: 10.1053/jlts.2003.50165. [DOI] [PubMed] [Google Scholar]
- 59.Navez J, Iesari S, Kourta D, et al The real incidence of biliary tract complications after adult liver transplantation: the role of the prospective routine use of cholangiography during post-transplant follow-up. Transpl Int. 2021;34:245–58. doi: 10.1111/tri.13786. [DOI] [PubMed] [Google Scholar]
- 60.Nordness MF, Hamel S, Godfrey CM, et al Fatal hepatic necrosis after nivolumab as a bridge to liver transplant for HCC: Are checkpoint inhibitors safe for the pretransplant patient. Am J Transplant. 2020;20:879–83. doi: 10.1111/ajt.15617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aby ES, Lake JR Immune checkpoint inhibitor therapy before liver transplantation-case and literature review. Transplant Direct. 2022;8:e1304. doi: 10.1097/txd.0000000000001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Verna EC, Patel YA, Aggarwal A, et al Liver transplantation for hepatocellular carcinoma: Management after the transplant. Am J Transplant. 2020;20:333–47. doi: 10.1111/ajt.15697. [DOI] [PubMed] [Google Scholar]
- 63.Wang ZY, Geng L, Zheng SS Current strategies for preventing the recurrence of hepatocellular carcinoma after liver transplantation. Hepatobiliary Pancreat Dis Int. 2015;14:145–9. doi: 10.1016/s1499-3872(15)60345-9. [DOI] [PubMed] [Google Scholar]
- 64.Hu B, Yang XB, Sang XT Liver graft rejection following immune checkpoint inhibitors treatment: a review. Med Oncol. 2019;36:94. doi: 10.1007/s12032-019-1316-7. [DOI] [PubMed] [Google Scholar]
- 65.Lin Z, Lu D, Wei X, et al Heterogeneous responses in hepatocellular carcinoma: the achilles heel of immune checkpoint inhibitors. Am J Cancer Res. 2020;10:1085–102. [PMC free article] [PubMed] [Google Scholar]
- 66.Ho CM, Chen HL, Hu RH, et al. Harnessing immunotherapy for liver recipients with hepatocellular carcinoma: a review from a transplant oncology perspective. Ther Adv Med Oncol 2019;11:1758835919843463.
- 67.Morales RE, Shoushtari AN, Walsh MM, et al Safety and efficacy of ipilimumab to treat advanced melanoma in the setting of liver transplantation. J Immunother Cancer. 2015;3:22. doi: 10.1186/s40425-015-0066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ranganath HA, Panella TJ Administration of ipilimumab to a liver transplant recipient with unresectable metastatic melanoma. J Immunother. 2015;38:211. doi: 10.1097/cji.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 69.De Toni EN, Gerbes AL Tapering of immunosuppression and sustained treatment with nivolumab in a liver transplant recipient. Gastroenterology. 2017;152:1631–3. doi: 10.1053/j.gastro.2017.01.063. [DOI] [PubMed] [Google Scholar]
- 70.Chen JA, Esteghamat N, Kim EJ, et al PD-1 blockade in a liver transplant recipient with microsatellite unstable metastatic colorectal cancer and hepatic impairment. J Natl Compr Canc Netw. 2019;17:1026–30. doi: 10.6004/jnccn.2019.7328. [DOI] [PubMed] [Google Scholar]
- 71.Varkaris A, Lewis DW, Nugent FW Preserved liver transplant after PD-1 pathway inhibitor for hepatocellular carcinoma. Am J Gastroenterol. 2017;112:1895–6. doi: 10.1038/ajg.2017.387. [DOI] [PubMed] [Google Scholar]
- 72.Zhuang L, Mou HB, Yu LF, et al Immune checkpoint inhibitor for hepatocellular carcinoma recurrence after liver transplantation. Hepatobiliary Pancreat Dis Int. 2020;19:91–3. doi: 10.1016/j.hbpd.2019.09.011. [DOI] [PubMed] [Google Scholar]
- 73.Qiu J, Tang W, Du C Immune checkpoint inhibitors in patients with recurrent hepatocellular carcinoma after liver transplantation: A case report and literature review. Curr Cancer Drug Targets. 2020;20:720–7. doi: 10.2174/1568009620666200520084415. [DOI] [PubMed] [Google Scholar]
- 74.Rammohan A, Reddy MS, Farouk M, et al Pembrolizumab for metastatic hepatocellular carcinoma following live donor liver transplantation: The silver bullet. Hepatology. 2018;67:1166–8. doi: 10.1002/hep.29575. [DOI] [PubMed] [Google Scholar]
- 75.Amjad W, Kotiah S, Gupta A, et al Successful treatment of disseminated hepatocellular carcinoma after liver transplantation with nivolumab. J Clin Exp Hepatol. 2020;10:185–7. doi: 10.1016/j.jceh.2019.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Al Jarroudi O, Ulusakarya A, Almohamad W, et al Anti-programmed cell death protein 1 (PD-1) immunotherapy for metastatic hepatocellular carcinoma after liver transplantation: A report of three cases. Cureus. 2020;12:e11150. doi: 10.7759/cureus.11150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang G, Tang H, Zhang Y, et al Programmed death receptor(PD)-1 monoclonal antibody induced acute immune hepatitis in the treatment of recurrent hepatocellular carcinoma after liver transplantation a case report. Qi Guan Yi Zhi. 2016;7:44–7. doi: 10.3969/j.issn.1674-7445.2016.01.008. [DOI] [Google Scholar]
- 78.Gassmann D, Weiler S, Mertens JC, et al Liver allograft failure after nivolumab treatment — A case report with systematic literature research. Transplant Direct. 2018;4:e376. doi: 10.1097/txd.0000000000000814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.DeLeon TT, Salomao MA, Aqel BA, et al Pilot evaluation of PD-1 inhibition in metastatic cancer patients with a history of liver transplantation: the Mayo Clinic experience. J Gastrointest Oncol. 2018;9:1054–62. doi: 10.21037/jgo.2018.07.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee BT, Horwich BH, Chopra S, et al Checkpoint inhibitor-induced rejection of a liver allograft: A combination of acute T cell-mediated and antibody-mediated rejection. Liver Transpl. 2019;25:1845–8. doi: 10.1002/lt.25622. [DOI] [PubMed] [Google Scholar]
- 81.Shi GM, Wang J, Huang XW, et al Graft programmed death ligand 1 expression as a marker for transplant rejection following anti-programmed death 1 immunotherapy for recurrent liver tumors. Liver Transpl. 2021;27:444–9. doi: 10.1002/lt.25887. [DOI] [PubMed] [Google Scholar]
- 82.Friend BD, Venick RS, McDiarmid SV, et al. Fatal orthotopic liver transplant organ rejection induced by a checkpoint inhibitor in two patients with refractory, metastatic hepatocellular carcinoma. Pediatr Blood Cancer 2017;64.
- 83.Kuo JC, Lilly LB, Hogg D Immune checkpoint inhibitor therapy in a liver transplant recipient with a rare subtype of melanoma: a case report and literature review. Melanoma Res. 2018;28:61–4. doi: 10.1097/cmr.0000000000000410. [DOI] [PubMed] [Google Scholar]
- 84.Maggiore U, Pascual J The bad and the good news on cancer immunotherapy: Implications for organ transplant recipients. Adv Chronic Kidney Dis. 2016;23:312–6. doi: 10.1053/j.ackd.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 85.Lawal G, Xiao Y, Rahnemai-Azar AA, et al The immunology of hepatocellular carcinoma. Vaccines (Basel) 2021;9:1184. doi: 10.3390/vaccines9101184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang Z, Cao YJ Adoptive cell therapy targeting neoantigens: A frontier for cancer research. Front Immunol. 2020;11:176. doi: 10.3389/fimmu.2020.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lu F, Ma XJ, Jin WL, et al Neoantigen specific T cells derived from T cell-derived induced pluripotent stem cells for the treatment of hepatocellular carcinoma: potential and challenges. Front Immunol. 2021;12:690565. doi: 10.3389/fimmu.2021.690565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Harmon C, Sanchez-Fueyo A, O’Farrelly C, et al Natural killer cells and liver transplantation: Orchestrators of rejection or tolerance? Am J Transplant. 2016;16:751–7. doi: 10.1111/ajt.13565. [DOI] [PubMed] [Google Scholar]
- 89.Ohira M, Ishiyama K, Tanaka Y, et al Adoptive immunotherapy with liver allograft-derived lymphocytes induces anti-HCV activity after liver transplantation in humans and humanized mice. J Clin Invest. 2009;119:3226–35. doi: 10.1172/jci38374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ohira M, Nishida S, Tryphonopoulos P, et al Clinical-scale isolation of interleukin-2-stimulated liver natural killer cells for treatment of liver transplantation with hepatocellular carcinoma. Cell Transplant. 2012;21:1397–406. doi: 10.3727/096368911x627589. [DOI] [PubMed] [Google Scholar]
- 91.Yu X, Zhao H, Liu L, et al A randomized phase II study of autologous cytokine-induced killer cells in treatment of hepatocellular carcinoma. J Clin Immunol. 2014;34:194–203. doi: 10.1007/s10875-013-9976-0. [DOI] [PubMed] [Google Scholar]
- 92.Lee JH, Lee JH, Lim YS, et al Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148:1383–91.e6. doi: 10.1053/j.gastro.2015.02.055. [DOI] [PubMed] [Google Scholar]
- 93.Chen QF, Li W, Yu SC, et al Consensus of minimally invasive and multidisciplinary comprehensive treatment for hepatocellular carcinoma — 2020 Guangzhou recommendations. Front Oncol. 2021;11:621834. doi: 10.3389/fonc.2021.621834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Introna M CIK as therapeutic agents against tumors. J Autoimmun. 2017;85:32–44. doi: 10.1016/j.jaut.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 95.Shimasaki N, Jain A, Campana D NK cells for cancer immunotherapy. Nat Rev Drug Discov. 2020;19:200–18. doi: 10.1038/s41573-019-0052-1. [DOI] [PubMed] [Google Scholar]
- 96.Myers JA, Miller JS Exploring the NK cell platform for cancer immunotherapy. Nat Rev Clin Oncol. 2021;18:85–100. doi: 10.1038/s41571-020-0426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ohira M, Ohdan H, Mitsuta H, et al Adoptive transfer of TRAIL-expressing natural killer cells prevents recurrence of hepatocellular carcinoma after partial hepatectomy. Transplantation. 2006;82:1712–9. doi: 10.1097/01.tp.0000250935.41034.2d. [DOI] [PubMed] [Google Scholar]
- 98.Ohira M, Hotta R, Tanaka Y, et al Pilot study to determine the safety and feasibility of deceased donor liver natural killer cell infusion to liver transplant recipients with hepatocellular carcinoma. Cancer Immunol Immunother. 2022;71:589–99. doi: 10.1007/s00262-021-03005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nishida S, Levi DM, Tzakis AG Liver natural killer cell inoculum for liver transplantation with hepatocellular carcinoma. Curr Opin Organ Transplant. 2013;18:690–4. doi: 10.1097/mot.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 100.Xie S, Wu Z, Zhou L, et al Iodine-125 seed implantation and allogenic natural killer cell immunotherapy for hepatocellular carcinoma after liver transplantation: a case report. Onco Targets Ther. 2018;11:7345–52. doi: 10.2147/ott.S166962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wculek SK, Cueto FJ, Mujal AM, et al Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol. 2020;20:7–24. doi: 10.1038/s41577-019-0210-z. [DOI] [PubMed] [Google Scholar]
- 102.Matsui HM, Hazama S, Nakajima M, et al Novel adjuvant dendritic cell therapy with transfection of heat-shock protein 70 messenger RNA for patients with hepatocellular carcinoma: a phase I/II prospective randomized controlled clinical trial. Cancer Immunol Immunother. 2021;70:945–57. doi: 10.1007/s00262-020-02737-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kayashima H, Toshima T, Okano S, et al Intratumoral neoadjuvant immunotherapy using IL-12 and dendritic cells is an effective strategy to control recurrence of murine hepatocellular carcinoma in immunosuppressed mice. J Immunol. 2010;185:698–708. doi: 10.4049/jimmunol.0900187. [DOI] [PubMed] [Google Scholar]
- 104.Zhao L, Cao YJ Engineered T cell therapy for cancer in the clinic. Front Immunol. 2019;10:2250. doi: 10.3389/fimmu.2019.02250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Qasim W, Brunetto M, Gehring AJ, et al Immunotherapy of HCC metastases with autologous T cell receptor redirected T cells, targeting HBsAg in a liver transplant patient. J Hepatol. 2015;62:486–91. doi: 10.1016/j.jhep.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 106.Tan AT, Yang N, Lee Krishnamoorthy T, et al Use of expression profiles of HBV-DNA integrated into genomes of hepatocellular carcinoma cells to select T cells for immunotherapy. Gastroenterology. 2019;156:1862–76.e9. doi: 10.1053/j.gastro.2019.01.251. [DOI] [PubMed] [Google Scholar]
- 107.Hafezi M, Tan A, Bertoletti A Personalized armored TCR-redirected T cell therapy for liver/organ transplant with recurrent cancer. Cells. 2021;10:1861. doi: 10.3390/cells10081861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sadelain M, Brentjens R, Rivière I The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol. 2009;21:215–23. doi: 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang M, Munoz J, Goy A, et al KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382:1331–42. doi: 10.1056/NEJMoa1914347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Neelapu SS, Locke FL, Bartlett NL, et al Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–44. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Raje N, Berdeja J, Lin Y, et al Anti-BCMA CAR T-Cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. 2019;380:1726–37. doi: 10.1056/NEJMoa1817226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Elsallab M, Levine BL, Wayne AS, et al CAR T-cell product performance in haematological malignancies before and after marketing authorisation. Lancet Oncol. 2020;21:e104–16. doi: 10.1016/s1470-2045(19)30729-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tseng HC, Xiong W, Badeti S, et al Efficacy of anti-CD147 chimeric antigen receptors targeting hepatocellular carcinoma. Nat Commun. 2020;11:4810. doi: 10.1038/s41467-020-18444-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sun B, Yang D, Dai H, et al Eradication of hepatocellular carcinoma by NKG2D-based CAR-T cells. Cancer Immunol Res. 2019;7:1813–23. doi: 10.1158/2326-6066.Cir-19-0026. [DOI] [PubMed] [Google Scholar]
- 115.Shi D, Shi Y, Kaseb AO, et al Chimeric antigen receptor-glypican-3 T-cell therapy for advanced hepatocellular carcinoma: Results of phase I trials. Clin Cancer Res. 2020;26:3979–89. doi: 10.1158/1078-0432.Ccr-19-3259. [DOI] [PubMed] [Google Scholar]
- 116.Pang N, Shi J, Qin L, et al IL-7 and CCL19-secreting CAR-T cell therapy for tumors with positive glypican-3 or mesothelin. J Hematol Oncol. 2021;14:118. doi: 10.1186/s13045-021-01128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Munker S, De Toni EN Use of checkpoint inhibitors in liver transplant recipients. United European Gastroenterol J. 2018;6:970–3. doi: 10.1177/2050640618774631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang J, Li J, Tang G, et al Clinical outcomes and influencing factors of PD-1/PD-L1 in hepatocellular carcinoma. Oncol Lett. 2021;21:279. doi: 10.3892/ol.2021.12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Abdel-Wahab N, Safa H, Abudayyeh A, et al Checkpoint inhibitor therapy for cancer in solid organ transplantation recipients: an institutional experience and a systematic review of the literature. J Immunother Cancer. 2019;7:106. doi: 10.1186/s40425-019-0585-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Samuel D, Sanchez-Fueyo A Immunotherapy in liver transplantation. J Hepatol. 2017;67:874–5. doi: 10.1016/j.jhep.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 121.Au KP, Chok KSH Multidisciplinary approach for post-liver transplant recurrence of hepatocellular carcinoma: A proposed management algorithm. World J Gastroenterol. 2018;24:5081–94. doi: 10.3748/wjg.v24.i45.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]