Abstract
Objective
To explore the change and feasibility of surgical techniques of laparoscopic transhiatal (TH)-lower mediastinal lymph node dissection (LMLND) for adenocarcinoma of the esophagogastric junction (AEG) according to Idea, Development, Exploration, Assessment, and Long-term follow-up (IDEAL) 2a standards.
Methods
Patients diagnosed with AEG who underwent laparoscopic TH-LMLND were prospectively included from April 14, 2020, to March 26, 2021. Clinical and pathological information as well as surgical outcomes were quantitatively analyzed. Semistructured interviews with the surgeon after each operation were qualitatively analyzed.
Results
Thirty-five patients were included. There were no cases of transition to open surgery, but three cases involved combination with transthoracic surgery. In qualitative analysis, 108 items under three main themes were detected: explosion, dissection, and reconstruction. Revised instruction was subsequently designed according to the change in surgical technique and the cognitive process behind it. Three patients had anastomotic leaks postoperatively, with one classified as Clavien-Dindo IIIa.
Conclusions
The surgical technique of laparoscopic TH-LMLND is stable and feasible; further IDEAL 2b research is warranted.
Keywords: Adenocarcinoma of esophagogastric junction, laparoscopic surgery, transhiatal approach, lower mediastinal lymph node dissection, IDEAL 2a research
Introduction
Previous studies have shown that the metastatic rate of lower mediastinal lymph nodes in adenocarcinoma of the esophagogastric junction (AEG) is high (1-6), with dissection required according to Japanese Gastric Cancer Treatment Guidelines (5th edition) (7).
Radical dissection and surgical safety should both be considered when designing the surgical route for AEG. According to JCOG9502 (8,9), transhiatal (TH) dissection is noninferior to left thoracoabdominal dissection with respect to overall survival for tumors with esophageal invasion less than 3 cm. Therefore, the TH approach is recommended for AEG with esophageal invasion less than 3 cm according to Japanese Gastric Cancer Treatment Guidelines (5th edition) (7).
Laparoscopic surgery for gastric cancer has been widely studied in recent years. High-quality evidence has proven the safety and noninferiority of laparoscopy to open surgery in the distal gastrectomy for both early and locally advanced gastric cancer (10-13). However, high-quality evidence is still lacking for laparoscopic total or proximal gastrectomy, which is required for AEG treatment. Although the JCOG1401, KLASS03, and CLASS02 trials have demonstrated its safety in early gastric cancer (14-16), AEG was excluded or included in only a small number of these studies. Therefore, the safety and feasibility of laparoscopic surgery for AEG treatment still need further exploration.
Laparoscopic TH lower mediastinal lymph node dissection (LMLND) is challenging in laparoscopic surgery for AEG. Indeed, due to the limited number of landmark structures, it is difficult to standardize the surgical procedures for LMLND (17). Most previous studies involved retrospective cohorts with small sample sizes, and the results showed significant heterogeneity (17-27). Similarly, the surgical techniques employed were only briefly described in the Methods section and were inconsistent among the studies, with the definition of the left and right borders being one of the most controversial steps. Costi et al. first described the procedure in 2004 and defined the left and right borders as the left and right mediastinal pleura (18). Prophylactic chest drainage has been used in cases of potential pleural injury. The incidence of pleural injury I previous studies was between 14.3% and 30.0% (19,22). Nevertheless, other studies have indicated that dissection of the left and right mediastinal pleura may allow for complete dissection of the lower mediastinal lymph nodes and adequate exposure of the surgical field, especially for proximal reconstruction (20,21,24,25,28).
The methodology for surgical innovation — the Idea, Development, Exploration, Assessment, and Long-term follow up (IDEAL) framework and recommendations — may be applied for exploration of surgical techniques for laparoscopic LMLND. The IDEAL framework and recommendation describe the development of a surgical innovation in five stages: pre-IDEAL, stage 1, the idea stage; stage 2a, the development stage; stage 2b, the exploration stage; stage 3, the assessment stage; and stage 4, evaluation in the long-term study stage (29-31). Accordingly, surgical techniques for LMLND should be at stage 2a, the development stage, and research should involve a prospective single-center study with a small sample size, aiming to present the safety and efficacy of the technique. Moreover, reporting results should focus on technique changes to determine stability and repeatability for further studies (31).
Materials and methods
Patients
From April 14, 2020, to March 26, 2021, patients diagnosed with AEG who underwent laparoscopic gastrectomy and LMLND with the TH approach in the First Ward, Gastrointestinal Cancer Center, Peking University Cancer Hospital, were included. The same surgeon (male) with 14 years of experience in gastrointestinal surgery, with a yearly average of 300 cases, performed all of the surgeries.
Inclusion criteria included the following: 1) diagnosis of adenocarcinoma preoperatively by endoscopy and biopsy; 2) tumors located at the esophagogastric junction (EGJ) according to the Siewert criteria with invasion of the EGJ, as confirmed by preoperative endoscopy or upper gastrointestinal radiography (32,33); 3) tumors at clinical stages of T2−T4a diagnosed by contrast-enhanced computed tomography (CT) scan; 4) no evidence of distant metastasis according to preoperative examinations, except for positive cytological results in peritoneal lavage; and 5) informed consent.
Exclusion criteria were as follows: 1) pregnancy or breastfeeding; 2) severe or uncontrolled diseases of other systems, including heart failure, renal failure, seizures, psychosis, and infectious diseases; 3) history of ischemic heart disease or cerebral vascular disease within 6 months; 4) organ transplants and needing immunosuppressive therapies; or 5) needing emergency surgery because of perforation, obstruction, or hemorrhage.
Elimination criteria included the following: 1) complete surgery not performed; 2) withdrawal from the study; or 3) inappropriateness of continuing the study because of severe adverse events.
The study was approved by the Institutional Review Board of Peking University Cancer Hospital, and written informed consent was obtained from every patient.
Surgical procedures
A 12 mm trocar was inserted subumbilically, and a 5 mm trocar was inserted at the anterior axillary line below the right costal margin. Laparoscopic exploration was regularly performed to rule out peritoneal metastasis; peritoneal lavage was also performed routinely. A 5 mm trocar and a 12 mm trocar were inserted at the left and right midclavicular lines, respectively, slightly above the umbilical level. A 12 mm trocar was inserted at the anterior axillary line below the left costal margin. Total or proximal gastrectomy was performed according to Japanese Gastric Cancer Treatment Guidelines (5th edition) (7). LMLND was carried out; details are presented in Figure 1 and video (http://www.cjcrcn.org/video/33.html). If laparoscopic-assisted surgery was chosen, an upper abdominal incision was made for digestive tract reconstruction. If total laparoscopic surgery was chosen, reconstruction was performed intracorporeally, and the specimen was removed using a specimen pocket through elongation of the subumbilical trocar incision.
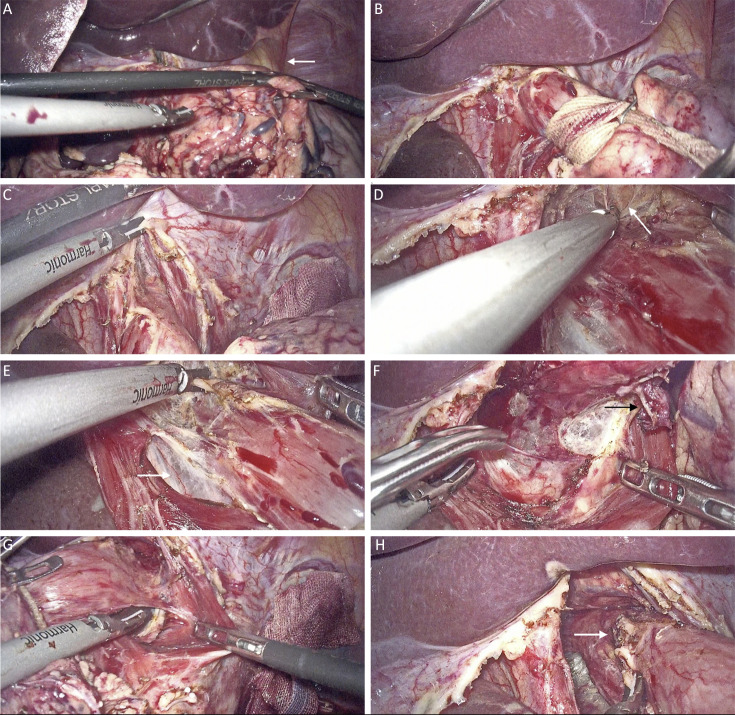
Figure 1.
Surgical process of LMLND. (A) Nathanson liver retractor was adjusted. LTL (white arrow) was not divided in this case; (B) Esophagus was denudated and retracted with tape; (C) Hiatus was split; (D) Dissection of the anterior esophagus and exposure of the pericardium (white arrow); (E) Dissection of the right side of the esophagus, with exposure of the infra-cardiac bursa (white arrow); (F) Dissection of the posterior esophagus. A lymph node is exposed (white arrow); (G) Dissection of the left side of the esophagus; (H) Dissection is complete, and a lymph node is exposed (white arrow). LMLND, lower mediastinal lymph node dissection.
Data collection
Baseline information, intraoperative and postoperative information, and pathological information were collected from the electronic medical records system. One researcher performed a face-to-face semistructured interview with the surgeon after each surgery, with audio recording, in the surgeon’s office. The researcher (male) is at the same department as the surgeon, has an MD degree, and had finished residential and fellowship training in general surgery and gastrointestinal tumor surgery. The surgeon was aware of the purpose and goal of this study. The interviews included the following: 1) an overall evaluation of the difficulty level; 2) changes in each procedure and the reason; 3) potential risks and mistakes in each procedure; and 4) potential improvements for ensuing surgeries and the reasons. The duration of the interviews was not predetermined. Data saturation was considered when no technique changes were undertaken for at least 5 consecutive cases. After transcribing the audio records, two researchers reviewed all the records for accuracy. Then, the surgeon reviewed the records to confirm the transcription. Videos were taken during the surgeries using the recording function of the laparoscope. All the videos were reviewed to extract the following information: 1) the dissection time of the LMLND (starting with opening the diaphragm and ending with freeing the esophagus) and 2) injury to the mediastinal pleura and subsequent management.
Data analysis and qualitative methodology
Regular quantitative analysis of the data was performed using SPSS software (Version 26; IBM Corp., NewYork, USA).
Qualitative methodology was applied for analysis of the postoperative interviews. Qualitative methodology aims to categorize information, including texts, pictures, and videos, which are difficult to analyze with calculation in quantitative methodology (34). The results are reported in line with the Consolidated Criteria for Reporting Qualitative Research (COREQ) criteria (35).
The framework approach was utilized in the current research (36) with the following steps, all of which were performed by two researchers: 1) read all of the transcriptions; 2) extracted the key themes and constructed the framework; 3) coded each transcription according to the framework using NVivo software (Version 12; QSR International, Burlington, USA); 4) reviewed and revised all contents under each theme and subtheme; 5) summarized all contents and prepared a chart of technical changes; and 6) revised technical changes (done by the surgeon) and generated optimized technical instruction.
The framework was constructed with task analysis methodology (37,38), which analyzes each task in the following two dimensions: 1) hierarchical task analysis, listing the main surgical steps; and 2) cognitive task analysis, describing the cognitive process using the naturalistic model. The latter includes the following: task (T), the specific manipulation taken; situation awareness (SA), understanding of specific surgical situations; decision making (DM), the change in plan according to different conditions; potential errors (E), avoidable or already existing risks and errors. We also analyzed the root cause (R) of E according to T, SA and DM.
Results
Clinical and pathological characteristics of included patients
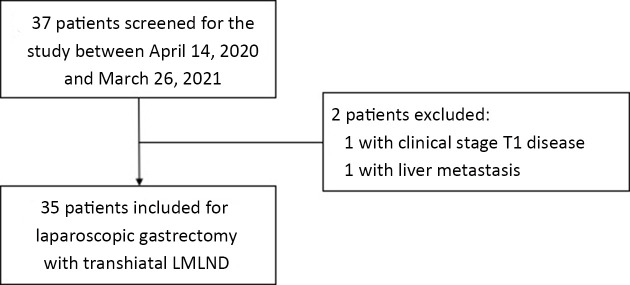
Thirty-five patients were prospectively included in the current study (Figure 2, Table 1). According to preoperative examinations, most cases (74.3%) were Siewert type II and cT3 (60.0%). Twenty-seven patients (77.1%) received total gastrectomy and eight (22.9%) proximal gastrectomy. Three patients underwent combined trans-thoracic surgery. Two of these cases were because of the positive intraoperative proximal margin; the other patient had a history of esophageal injury, and the esophageal resection was extended to avoid risks of anastomotic complications. One case was transferred to open surgery because of difficulty in proximal reconstruction. The dissection time for LMLND was (18.2±6.3) min. Thirteen (38.2%) patients sustained pleural injury under the TH approach.
Figure 2.
Flowchart of patient inclusion. LMLND, lower mediastinal lymph node dissection.
Table 1. Clinical and pathological characteristics of included patients.
| Variables | n (%) |
| BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; LMLND, lower mediastinal lymph node dissection; TH, transhiatal. *, Because of the technical errors of the surgical videos, three patients didnot have the data of the dissection time of the LMLND, and one patient didnot have the data of pleural injuries under TH approach. #, Patients combined with trans-thoracic surgeries were excluded. | |
| Sex | |
| Male | 33 (94.3) |
| Female | 2 (5.7) |
Age (year) (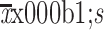 ) ) |
64.7±5.9 |
BMI (kg/m2) (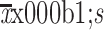 ) ) |
23.7±2.6 |
| ECOG score | |
| 0 | 29 (82.9) |
| 1 | 5 (14.3) |
| 3 | 1 (2.9) |
| Preoperative treatment | 19 (54.3) |
| (y)cT | |
| (y)cT2 | 4 (11.4) |
| (y)cT3 | 21 (60.0) |
| (y)cT4a | 10 (28.6) |
| (y)cN | |
| (y)cN0 | 4 (11.4) |
| (y)cN1 | 12 (34.3) |
| (y)cN2 | 19 (54.3) |
| Surgical approach | |
| Laparoscopic-assisted | 31 (88.6) |
| Total laparoscopic | 4 (11.4) |
| Extension of gastrectomy | |
| Total gastrectomy | 27 (77.1) |
| Proximal gastrectomy | 8 (22.9) |
| Extension of lymph node dissection | |
| D1+ | 2 (5.7) |
| D2 | 33 (94.3) |
| Extension of LMLND | |
| Stations 110 and 111 | 7 (20.0) |
| Stations 110, 111, and part of 112 | 28 (80.0) |
| Combined organ resection | 2 (5.7) |
| Combined trans-thoracic surgery | 3 (8.6) |
| Transfer to open surgery | 1 (2.9) |
| Pleural injury under TH approach* | |
| No injuries | 21 (61.8) |
| Injuries to both sides | 2 (5.9) |
| Injuries to left side | 9 (26.5) |
| Injuries to right side | 2 (5.9) |
Operation time (min) (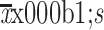 )# )#
|
309.8±69.5 |
Dissection time of LMLND (min) (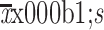 )* )* |
18.2±6.3 |
Incision length (cm) (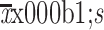 )# )#
|
9.5±4.0 |
Intra-operative blood loss (mL) (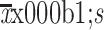 )# )#
|
100±70.4 |
| Intra-operative blood transfusion | 0 (0) |
| Siewert type | |
| Type I | 1 (2.9) |
| Type II | 26 (74.3) |
| Type III | 8 (22.9) |
| Esophageal resection (cm)# | 2.5±1.0 |
| Tumor length (cm) | 4.7±2.8 |
| Proximal margin (cm)# | 1.7±0.7 |
| Esophageal invasion (cm) | 1.1±1.0 |
| Bormann type | |
| Type 1 | 2 (5.7) |
| Type 2 | 7 (20.0) |
| Type 3 | 24 (68.6) |
| Type 4 | 2 (5.7) |
No. of LMLND (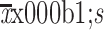 ) ) |
2.0±2.8 |
| Lower mediastinal metastasis | 4 (11.4) |
Framework of changes in surgical technique and optimized technical instruction
Twenty interviews were conducted after the surgery for 24 of the patients. The characteristics of each patient as well as the overall evaluation of the difficulty level are shown in Supplementary Table S1. There were three main themes in the framework according to hierarchical task analysis: exposure, dissection, and reconstruction. Five, three, and two subthemes were categorized under these three main themes. The contents under each subtheme were summarized into several points and categorized according to the naturalistic model, and points similar to those in the prior interview were deleted. Overall, there were 108 points, 44 of which were under the theme “exposure”, 50 under “dissection”, and 14 under “reconstruction” (Supplementary Table S2,S3). Similarly, when categorized according to cognitive task analysis, of the 108 points, 38 were under “subtask”, 36 under “situation-awareness”, 19 under “decision-making” and 15 under “potential error”.
Table S1. Characteristics of each case.
| Case | BMI (kg/m2) | Siewert type | Tumor length (cm) | Extension of gastrectomy | Pleural injury and management | Overall evaluation by the surgeon |
| NACT, neoadjuvant chemo-therapy; *, combined with trans-thoracic surgery. | ||||||
| 1 | 24 | II | 3.0 | Proximal | No injury | Increase of difficulty due to NACT |
| 2 | 18 | II | 8.0 | Proximal | No injury | Increase of proficiency in spite of insufficient exposure |
| 3 | 21 | II | 5.0 | Total | No injury | Stable |
| 4* | 23 | III | 3.0 | Total | Injury to the left, no management | Increase of proficiency, decrease of delicacy |
| 5 | 26 | II | 5.5 | Total | No injury | Increase of difficulty due to a small physique of the patient |
| 6 | 24 | III | 7.0 | Total | No injury | Stable |
| 7 | 21 | II | 2.0 | Proximal | No injury | Un-evaluated |
| 8 | 26 | III | 6.0 | Total | Injury to the left, clipping | Stable |
| 9 | 26 | II | 4.0 | Total | Injury to the left, suturing and placing drainage | Long interval with previous case but stable, a little influence due to inexperienced assistance |
| 10 | 22 | III | 10.0 | Total | Injury to the left, suturing | Stable |
| 11* | 24 | III | 13.0 | Total | Injury to the left, suturing | Long interval with previous case but stable |
| 12 | 27 | II | 5.0 | Total | Injury to both sides, suturing | Certain influence due to inexperienced assistance |
| 13* | 23 | II | 12.0 | Total | Loss of data | Un-evaluated |
| 14 | 25 | II | 5.0 | Total | No injury | Stable |
| 15 | 22 | II | 2.5 | Proximal | No injury | Stable |
| 16 | 24 | II | 1.0 | Proximal | No injury | Stable |
| 17 | 21 | II | 1.0 | Total | Injury to the left, suturing | Un-evaluated |
| 18 | 25 | I | 3.0 | Proximal | Injury to both sides, no management | Stable |
| 19 | 25 | II | 2.0 | Proximal | No injury | Stable |
| 20 | 21 | II | 2.0 | Total | No injury | Stable |
| 21 | 29 | II | 4.0 | Total | No injury | Un-evaluated |
| 22 | 22 | II | 3.0 | Total | No injury | Stable |
| 23 | 24 | II | 2.5 | Total | No injury | Un-evaluated |
| 24 | 20 | II | 6.5 | Total | No injury | Increase of difficulty due to a small physique of the patient |
| 25 | 22 | II | 2.0 | Proximal | No injury | Un-explained |
| 26 | 20 | III | 7.0 | Total | Injury to the right, suturing | Increase of difficulty due to adhesion of the tumor to the diaphragm |
| 27 | 29 | II | 5.0 | Total | Injury to the left, clipping | Increase of proficiency |
| 28 | 19 | II | 4.0 | Total | No injury | Stable |
| 29 | 25 | III | 2.0 | Total | Injury to the left, suturing | Stable |
| 30 | 24 | II | 3.0 | Total | No injury | Insufficient surgical field due to a large tumor |
| 31 | 27 | III | 5.0 | Total | No injury | Un-evaluated |
| 32 | 26 | II | 6.0 | Total | No injury | Un-evaluated |
| 33 | 23 | II | 6.0 | Total | Injury to the right, suturing | Un-evaluated |
| 34 | 25 | II | 5.0 | Total | Injury to the left, no management | Un-evaluated |
| 35 | 25 | II | 5.0 | Total | No injury | Un-evaluated |
Table S2. No. of points under each sub-theme.
| Sub-theme | No. of points | Distribution of points according to the naturalistic model | |||
| Sub-task | Situation awareness | Decision-making | Potential error | ||
| LTL, left triangular ligament. | |||||
| Total | 108 | 38 | 36 | 19 | 15 |
| 1 Exposure | 44 | 18 | 12 | 11 | 3 |
| 1-1 Adjusting the Nathanson liver retractor | 3 | 2 | 1 | 0 | 0 |
| 1-2 Dividing the LTL | 13 | 4 | 3 | 5 | 1 |
| 1-3 Splitting the hiatus | 8 | 4 | 2 | 1 | 1 |
| 1-4 Retracting the esophagus | 8 | 3 | 4 | 1 | 0 |
| 1-5 Hanging the diaphragmatic crura | 12 | 5 | 2 | 4 | 1 |
| 2 Dissection | 50 | 16 | 18 | 5 | 11 |
| 2-1 Route of dissection | 9 | 3 | 4 | 1 | 1 |
| 2-2 Margin of dissection | 31 | 7 | 13 | 4 | 7 |
| 2-3 Definition of certain lymph node stations | 10 | 6 | 1 | 0 | 3 |
| 3 Reconstruction | 14 | 4 | 6 | 3 | 1 |
| 3-1 Esophago-jejunal/gastric anastomosis | 12 | 4 | 6 | 2 | 0 |
| 3-2 Jejuno-jejunal anastomosis | 2 | 0 | 0 | 1 | 1 |
Table S3. Change of points in order of cases.
| Theme | Case No. | Sub-task | Situation awareness | Decision-making | Potential errors |
| LTL, left triangular ligament. | |||||
| 1 Exposure | |||||
| 1-1 Adjusting the Nathanson liver retractor | 1&2 | Optimize the retraction angle for better exposure | − | − | − |
| 6 | Switch to a smaller size retractor and only retract the part at the LTL | Adequate mobility of a retractor is required for a better exposure | − | − | |
| 1-2 Dividing the left triangular ligament | 1&2 | 1 Only divide the part above the hiatus2 Leave the LTL un-divided | Dividing the LTL enables better exposure but is of technical difficulty | If the division is difficult to perform, e.g., because of a plump left hepatic lobe or if the surgical field is already well-exposed, e.g., a wide sub-diaphragmic space, consider leaving it un-divided | Division risks injuring the surrounding structures including the liver and the diaphragm |
| 3 | − | Splitting the hiatus towards the direction of the pericardium could help avoid the influence of the LTL. Thus, there is no need to divide the LTL routinely | If there is over 1cm interval between the LTL and the hiatus, consider leaving the LTL un-divided; | − | |
| 5 | LTL divided | Dividing the LTL enables better exposure for reconstruction; | When the division is difficult to perform, e.g., when the left hepatic lobe is closely associated with the diaphragm, consider leaving the LTL un-divided | − | |
| 6 | Adjust the liver retractor after division | − | If the LTL is compacted, consider leaving it un-divided | − | |
| 1-3 Splitting the hiatus | 1&2 | Split the hiatus vertically for 1−1.5 cm;Split the hiatus after retracting the esophagus | The pericardium is easy to be recognized (white and fibrotic) and is surrounded by large amount of adipose tissue, protecting it from injuries | When the LTL is left un-divided,retracting the esophagus could help determine the position of the pericardium and further determine the direction of splitting the hiatus | − |
| 6 | Determine the plane of dissection after splitting, then divide the adipose tissue surrounding the pericardium before further splitting | The adipose tissue surrounding the pericardium belongs to LN station No. 111 | − | A sufficient splitting of the hiatus benefits exposure and subsequently the determination of the plane of dissection | |
| 8 | Splitting for approximately 1cm until reaching the adipose tissue below the pericardium | − | − | − | |
| 1-4 Retracting the esophagus | 1&2 | Retract the esophagus with a tape | − | If the LTL is left un-divided, retract the esophagus before splitting the hiatus | − |
| 5 | Optimize the retraction angle before passing the tape to the assistantRetract with moderate force | Optimizing the angle before passing makes it easier for the assistant to assist even with less experience | − | − | |
| 8 | − | The retraction makes the anatomy of the lower mediastinum change accordingly | − | − | |
| 12 | − | The distance between the esophagus and the mediastinal pleura changes with the retraction and the physique of the patient | − | − | |
| 14&15 | − | Retracting the esophagus helps expose the infra-cardiac bursa, which is an important anatomic landmark | − | − | |
| 1-5 Hanging the diaphragmatic crura | 1&2 | Leave them un-hanged | Hanging is for better exposure | If the surgical field is already well-exposed, e.g., a wide sub-diaphragmic space, consider leaving it un-hanged | − |
| 12 | Hang the right crus, send the suture through the right subcostal trocar before fixation | − | If the surgical field is poorly exposed, e.g., when the tumor invades the EGJ, hanging is necessary for better exposure | If the surgical field is poorly exposed, the anastomosis will be influenced or cause injury to the diaphragm | |
| 14&15 | 14 Hang both of the diaphragmatic crura;15 Hang the left crus just before approaching the left margin of dissection | − | If the surgical field is poorly exposed, e.g., in a patient with a sharp acute costal angle, hanging is necessary;If the surgical field is well exposed, re-consider the necessity and timing of hanging | − | |
| 16 | − | Due to the vertical splitting of the hiatus,using the trocar wound closure device to cephalically hanging the crura is better for exposure | − | − | |
| 2 Dissection | |||||
| 2-1 Route of dissection | 1&2 | Dissect the anterior and posterior margin before the lateral margins | There is little space between the esophagus and the pleura. Dissection of the anterior margin benefits the exposure of the side margins and helps prevent pleural injuries | − | The pleura is prone to be injured if the posterior margin or the lateral margins were dissected first |
| 14&15 | 14 Dissect in the sequence of anterior, right, posterior, and left | 14 Because of the in-advanced exposure of the right margin by the in-experienced assistant,its subsequent dissection avoided repeated manipulations. Another reason is the safety of the right pleura by the protection of the infra-cardiac bursa | If the exposure is different from expected, adjust the route accordingly to avoid repeated manipulations | − | |
| 16 | − | Safety and efficiency should both be taken into account for the arrangement of the dissection route | − | − | |
| 27 | Freely arrange the route ,ensuring that the left margin is dissected last | The left pleura is more prone to be injured than the right. Thus, the dissection of the left margin should be in the last place | − | − | |
| 2-2 The margin of the dissection | |||||
| 2-2-1 The superior margin | 1&2 | The superior margin should be defined by the lower pulmonary vein. But full exposure of the lower pulmonary vein is not required | Exposure of the lower pulmonary vein requires dissection of the pleura;It is difficult to estimate the distance between the pericardium and the lower pulmonary vein transhiatally;The pericardium is safe from injuries | − | It is hard to Manage the injuries of the lower pulmonary vein |
| 26 | − | An empiric standard could be made similar to the TME surgery, i.e., defining the superior margin as the para-esophageal tissue 3cm above the upper limit of the tumor, without crossing the pericardium | − | − | |
| 2-2-2 The anterior inferior margin | 1&2 | − | It is easy to expose the anterior space | − | − |
| 26 | The embracement of the diaphragm forms the inferior margin | − | − | − | |
| 2-2-3 The posterior margin | 1&2 | Lift the esophagus at the intersection with the pericardium in order to enter the posterior space;Dissect vertically;Perform the lateral expansion moderately; | The bilateral pleural line could be well exposed with the retraction of the esophagus | If the aorta is not exposed after dissection, the direction of the dissection could be tilted and should be promptly adjusted | There are several arterioles directly coming from the aorta with a high risk of hemorrhage;Caution should be raised not to injure the pleura when the lateral expansion is approaching 2 cm |
| 14&15 | − | − | − | The relative position of the esophagus is not constant. The pleura is prone to be injured with inadequate exposure | |
| 2-2-4 The lateral margin | 1&2 | − | − | − | The mediastinal pleura is prone to be injured |
| 3 | The left and right margin is defined by the left and right mediastinal pleura. The limit of the dissection is until the denudation of the pleura | − | − | − | |
| 4 | − | − | − | The pleura is prone to be injured by the ultrasonic scalpel | |
| 5 | − | The right pleura is protected by the infra-cardiac bursa, making it safe from injuries | − | The pleura is sheer and prone to be injured, especially when there is scant adipose tissue making the exposure of the pleural line difficult, or with improper retraction and manipulation | |
| 8 | − | − | If the pleura is injured, use the clip for repairment | − | |
| 9&10 | − | Simple pleural injury does not impact postoperative recovery | If the pleura is injured, place the chest tube instead of repairing it due to the difficulty of the maneuver as well as the possibility to release the air and the reactive pleural effusion | − | |
| 12 | − | − | If it is feasible to repair the injury, suture with PROLENE in order to control the spread of the possible anastomotic leak | − | |
| 14&15 | Keep close to the esophagus with the dissection of the left margin | The underlying purpose of pleural protection is to protect the lung, and the pleura is considered a landmark of entering the pleural cavity;The left mediastinal pleura adheres to the esophagus and is prone to be injured. Thus, keeping close to the esophagus while dissecting should be considered a thorough one | − | − | |
| 19&20 | Using the LIGASURE for dissection | The LIGASURE is quite blunt, with a smaller risk of pleural injuries.The assistant stands on the left, making the retraction of the esophagus to the left side easier and thus better exposure of the right margin and lower risk of injuries | − | − | |
| 22 | − | The LIGASURE is no better than the ultrasonic scalpel according to several practices | − | − | |
| 2-3 Definition of certain lymph node stations | − | − | − | − | |
| 2-3-1 Station No. 111 | 1&2 | 112pul could be approached by continual dissection after reaching 3cm lateral to the pericardium;Pushing the surrounding lymphatic and adipose tissue to the esophagus before dissection benefits en bloc resection;The inferior vena cava is the margin of station No. 111. The dissection only requires exposure of the caval opening. There is no need to expose the inferior vena cava;The inferior margin is the anterior esophageal wall | − | − | Complete exposure of the vena cava brings additional risk |
| 5 | Only requires dissecting the pericardium adipose tissue | − | − | The pleura is prone to be injured during dissection of Station No. 111 on the left | |
| 2-3-2 Station No. 112pul | 1&2 | Station No. 112pul could not be completedly dissected because the pulmonary ligament is preserved | The lymphatic and adipose tissue of Station No.112pul adheres to the pleura, which requires dissection of the pleura for a complete dissection; Caution should be raised not to injure the lungs | − | The pleura is prone to be injured during dissection |
| 3 Reconstruction | |||||
| 3-1 Esophago-jejunal/gastric anastomosis | 1&2 | Overlap reconstruction | Retraction of the esophagus impacts the estimation of its resection length.The esophageal invasion length of the tumor significantly impacts the reconstruction due to the limited operating space;The overlap reconstruction is relatively simple, but caution should be raised not to be disturbed by the heart beating when closing the common opening below the pericardium | − | − |
| 3 | OrVil reconstruction | The safety of the anastomosis is significant for postoperative recovery | OrVil should be used after a more extended esophageal transection. Linear stapler should be preferentially chosen otherwise | − | |
| 9&10 | − | The esophageal dissection length is limited in obese patients or patients with a plump liver | − | − | |
| 11 | Purse-string anastomosis trans-thoracically | − | Difficulty from the trans-abdominal anastomosis prompt combined trans-thoracic surgery | − | |
| 16 | Esophago-gastric delta anastomosis with seromuscular valva cardioplastics | An attempt in a relatively early case | − | − | |
| 3-2 Jejuno-jejunal anastomosis | 1&2 | − | − | Total laparoscopic surgery should be chosen for obese patients, dissecting the mesentery intracorporeally. If laparoscopic-assisted surgery is chosen, a longer assistive abdominal incision (12−15 cm) should be made | A smaller assistive incision brings additional risk |
We derived optimized technical instruction based on the changes in technical details according to the above framework as well as descriptions in previous studies (18-26) (Table 2). For each item under “potential error”, root causes were mapped to failures in “subtask”, “situation-awareness” and “decision-making”. Of the 11 potential errors raised, all of which were rooted in lacking “situation awareness”, two were rooted in the failure to complete “subtask” or correct “decision-making”.
Table 2. Optimized technical instruction.
| Theme | Sub-task | Situation awareness | Decision-making | Potential errors |
| LTL, left triangular ligament; T, tast; SA, situation awareness; DM, decision making; E, potential error; R, root cause; LN, lymph node. | ||||
| 1 Exposure | T1-1: Routinely use the Nathanson liver retractor, switch to a smaller size retractor after diving the LTL, optimize the retraction angle and only retract the part at the LTL | SA1-1: Adequate mobility and suitable size of a retractor are required for better exposure | − | − |
| T1-2: Divide the LTL according to certain conditions | SA1-2-1: There is a high risk of dividing the LTLSA1-2-2: Dividing the LTL provides better exposure and enough space for subsequent anastomosis | DM1-2-1: If the division is difficult to perform, e.g., due to a plump left hepatic lobe, or a compacted LTL, consider leaving it un-divided. If there is a need for better exposure, only divide the part above the hiatusDM1-2-2: If the surgical field is already well-exposed, e.g., a wide sub-diaphragmic space, enough interval between the LTL and the hiatus, consider leaving it un-divided | E1-2: Division risks injuring the surrounding structures including the liver and the diaphragm — R: SA1-2-1, SA1-2-2 | |
| T1-3-1: Split the hiatus vertically for 1−1.5 cm | SA1-3-1: The pericardium looks white and fibrotic, and is protected by surrounding adipose tissue.SA1-3-2: The adipose tissue surrounding the pericardium belongs to LN station No. 111.SA1-3-3: The purpose of splitting the hiatus is to determine the dissection plane. | DM1-3-1: If there is not enough exposure, retracting the esophagus to form an angle between the diaphragm could help determine the position of the pericardium and further determine the direction of splitting the hiatus.DM1-3-2: Extend the splitting according to the exposure | E1-3: Insufficient splitting impact the determination of the dissection plane, and subsequently, incomplete dissection — R: SA1-3-2, SA1-3-3, DM1-3-2 | |
| T1-4: Retract the esophagus with a tape, optimize the retraction angle before passing the tape to the assistant, who should retract with moderate force | SA1-4-1: Retracting the esophagus helps expose the infra-cardiac bursa, which is an important anatomic landmark.SA1-4-2: The retraction makes the anatomy of the lower mediastinum change accordinglySA1-4-3: Optimizing the angle before passing makes it easier for the assistant to assist even with less experience | − | − | |
| T1-5: Hang both of the diaphragmatic crura with sutures, send the suture through the subcostal trocars or trocar wound closure device before fixation | SA1-5-1: The purpose and timing of hanging the crura are for better exposure.SA1-5-2: Hanging the crura eliminates the need for retraction by the assistant.SA1-5-3: Adjust the suture to make better exposure before fixation | DM1-5: If there is a wide sub-diaphragmic space, or the tumor has not invaded the EGJ, making a well-exposed surgical field, consider leaving the crura un-hanged or hanging one of the crura | E1-5: The un-hanged or improperly hanged crura leads to a poorly-exposed surgical field and subsequent risk of diaphragmatic injuries during anastomosis —R: T1-5, SA1-5-1, SA1-5-3 | |
| 2 Dissection | T2-1: Unsophisticated surgeons should dissect in the sequence of anterior, right, posterior, and left. Skillful surgeons can adjust the route according to certain surgical conditions and personal habits | SA2-1-1: Safety and efficiency of maneuvers should both be taken into account for the arrangement of the dissection route.SA2-1-2: Dissection of the anterior margin benefits the exposure of the side margins | − | E2-1: An improper route impact the exposure and increase the risk of pleural injuries — R: T2-1, SA2-1-1,SA2-1-2 |
| T2-2-1: Dissect the upper anterior of the esophagus and expand laterally for approximately 3 cm before reaching the pulmonary ligament. The superior margin is the lower pulmonary vein, but full exposure is not required.T2-2-2: Dissect the lower anterior of the esophagus. The inferior margin is the diaphragm | SA2-2-1: There is no need to dissect LN station No. 112 completely. Exposure of the lower pulmonary vein requires dissection of pulmonary ligament, leading to higher risk than benefit.SA2-2-2: There is no need to dissect LN station No. 111 completely. Its upper limit is the adipose tissue surrounding the pericardium, not crossing the defined superior margin. The inferior caval opening is the right inferior limit of the LN station No. 111. The dissection only requires exposure of the caval opening. There is no need to expose the inferior vena cava | DM2-2: If the tumor is located lower, the superior margin can be defined as the para-esophageal tissue 3 cm above the upper limit of the tumor, without crossing the pericardium. | E2-2-1: Pleural injuries — R: SA2-2-1E2-2-2: Injuries to the inferior vena cava — R:SA2-2-2 | |
| T2-3: Dissect the right side of the esophagus. The right margin is the right mediastinal pleura | SA2-3-1: Consider denudation of the pleura as a marker of complete dissection. There is no need to dissect the pleura.SA2-3-2: The pleura is sheer and prone to be injured, especially with inadequate exposure, improper manipulation of energy devices, or over-retraction.SA2-3-3: Simple pleural injury does not impact postoperative recovery. The underlying purpose of pleural protection is to prevent lung injuries.SA2-3-4: The right pleura is protected by the infra-cardiac bursa, making it safe from injuries | DM2-3-1: If the pleura is injured, choose from the following according to the severity and feasibility of repairment. (1) clipping (2) suturing with PROLENE (3) placing a chest tube without repairing | E2-3-1: Pleural injuries — R: SA2-3-2E2-3-2: Lung injuries — R: SA2-3-3 | |
| T2-4: Lift the esophagus at the intersection with the pericardium to enter the posterior space;Dissect vertically;Perform the lateral expansion moderately until reaching the pleura | SA2-4: The bilateral pleural line could be well exposed with the retraction of the esophagus. Take care not to injure | DM2-4: If the aorta is not exposed after moderate dissection, the direction of the dissection could be tilted and should be promptly adjusted | E2-4: Pleural injuries — R: SA2-4, DM2-4 | |
| T2-5: Dissect the left side of the esophagus. Keep close to the esophagus while dissecting. The left margin is the left mediastinal pleura | SA2-5-1: Same as SA2-3-1SA2-5-2: Same as SA2-3-2SA2-5-3: Same as SA2-3-3SA2-5-4: The left mediastinal pleura adheres to the esophagus and is prone to be injured | DM2-5-1: Same as SA2-3-1 | E2-5-1: Pleural injuries — R: SA2-5-2, SA2-5-4E2-5-2: Lung injuries — R: SA2-5-3 | |
| 3 Reconstruction | T3-1: Perform esophago-jejunal/gastric anastomosis. The choice of anastomotic method is based on the location of the esophageal transection. Overlap reconstruction should be the first-line option | SA3-1-1: Retraction of the esophagus impacts the estimation of the location of the transaction.SA3-1-2: The safety of the anastomosis is significant for postoperative recovery | DM3-1-1: OrVil should be used after a higher esophageal transection.DM3-1-2: Difficulty from the trans-abdominal anastomosis prompt combined trans-thoracic surgery | − |
| T3-2: Perform jejuno-jejunal anastomosis through the assistive incision or intra-corporeally | − | DM3-2: Total laparoscopic surgery should be chosen for obese patients, dissecting the mesentery intracorporeally. If laparoscopic-assisted surgery is chosen, a longer assistive abdominal incision (12−15 cm) should be made | − | |
Postoperative outcomes
Postoperative recovery and postoperative complications are shown in Supplementary Table S4, Table 3, respectively. The postoperative hospital stay was 19.0±12.0 d. The incidence of postoperative complications was 60.0%. The incidence of anastomotic leakage was 8.6%, and one case (2.9%) was classified as Clavien-Dindo (CD) IIIa. There was one case of anastomotic stenosis (2.9%), which was classified as CD I. There was no perioperative death or reoperation. Postoperative complications are reported in detail for each case in Supplementary Table S5.
Table S4. Postoperative recovery.
| Items |
|
| *, visual analogue scale. | |
| Time to first ambulation (d) | 1.2±0.7 |
| Time to first flatus (d) | 4.1±1.1 |
| Time to first liquid resumption (d) | 4.3±6.0 |
| Time to first liquid diet (d) | 7.5±5.8 |
| Postoperative hospital stay (d) | 19.0±12.0 |
| Highest pain level* | 5.1±1.9 |
Table 3. Postoperative complications and CD classification.
| Complications | n (%) | ||||
| CD I | CD II | CD IIIa | CD IIIb | CD IV | |
| CD, Clavien-Dindo. | |||||
| Anastomotic leak | 0 (0) | 2 (5.7) | 1 (2.9) | 0 (0) | 0 (0) |
| Anastomotic stenosis | 1 (2.9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Intra-abdominal hemorrhage | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Intra-abdominal infection | 0 (0) | 1 (2.9) | 1 (2.9) | 0 (0) | 0 (0) |
| Pleural effusion | 0 (0) | 0 (0) | 3 (8.6) | 0 (0) | 0 (0) |
| Pulmonary infection | 0 (0) | 6 (17.1) | 0 (0) | 0 (0) | 0 (0) |
| Pancreatic leak | 1 (2.9) | 1 (2.9) | 0 (0) | 0 (0) | 0 (0) |
Table S5. Postoperative complications by case.
| Case No. | Time of diagnosis (postoperative day) | Diagnosis and CD classification | Management | Outcome |
| CD, Clavien-Dindo; TPN, total parenteral nutrition. | ||||
| 1 | 1 | Liver injury II | Liver protecting agents | Recovered |
| 3 | Anastomotic leak, intra-abdominal infection II | TPN, antibiotics | Recovered | |
| 4 | Pleural effusion IIIa | Thoracentesis with placement of indwelling catheter | Recovered | |
| 24 | Intestinal infection II | Antibiotics, enemas | Recovered | |
| 2 | 1 | Pancreatic leak II | Octreotide | Recovered |
| 1 | Arrhythmia II | Antiarrhythmics | Recovered | |
| 3 | Anastomotic leak, intra-abdominal infection IIIa | Adjustment of the abdominal drainage and placement of the nasal-jejunal feeding tube via endoscopic | Recovered | |
| 3 | Pleural effusion IIIa | Thoracentesis with placement of indwelling catheter | Recovered | |
| 3 | 5 | Intestinal infection II | Antibiotics, enemas | Recovered |
| 15 | Acute cholecystitis II | Antibiotics | Recovered | |
| 6 | 3 | Pulmonary infection II | Antibiotics | Recovered |
| 7 | 10 | Intestinal infection II | Antibiotics, enemas | Recovered |
| 8 | 1 | Infection of an unknown origin II | Antibiotics | Recovered |
| 9 | 1 | Liver injury II | Liver protecting agents | Recovered |
| 11 | 3 | Lymphatic leak II | TPN, preventative antibiotics | Recovered |
| 12 | 1 | Gastrointestinal hemorrhage IIIa | Endoscopic hemostasis | Recovered |
| 11 | Bloodstream infection II | Antibiotics | Recovered | |
| 13 | Gastroparesis IIIb | Placement of the nasal-jejunal feeding tube via endoscopic under general anesthesia | Recovered | |
| 13 | 7 | Anastomotic leak II | TPN, preventative antibiotics | Recovered |
| 14 | 25 | Impaired wound healing I | Wound debridement and drainage | Recovered |
| 16 | 10 | Anastomotic stenosis I | No specific management | Recovered |
| 17 | 1 | Postoperative pain | Endoscopic examination under general anesthesia, analgesics | Recovered |
| 8 | Pleural effusion IIIa | Thoracentesis with placement of indwelling catheter | Recovered | |
| 19 | 1 | Fever with an unknown cause II | Antibiotics | Recovered |
| 21 | 1 | Pulmonary infection II | Antibiotics | Recovered |
| 1 | Atrial fibrillation II | Antiarrhythmics | Recovered | |
| 7 | Lymphatic leak II | TPN | Recovered | |
| 10 | Impaired wound healing I | Wound debridement and drainage | Recovered | |
| 24 | 2 | Pulmonary infection II | Antibiotics | Recovered |
| 30 | 1 | Pulmonary infection II | Antibiotics | Recovered |
| 32 | 2 | Fever with an unknown cause II | Antibiotics | Recovered |
| 33 | 1 | Fever with an unknown cause II | Antibiotics | Recovered |
| 1 | Pancreatic leak I | No specific management | Recovered | |
| 34 | 2 | Pulmonary infection II | Antibiotics | Recovered |
| 35 | 12 | Partial intestinal obstruction II | TPN | Recovered |
| 13 | Pulmonary infection II | Antibiotics | Recovered | |
Discussion
There is a consensus regarding the necessity of LMLND for AEG (39,40), though a technical standard of surgical procedures is lacking. According to the IDEAL framework and recommendations, research regarding laparoscopic LMLND under the TH approach is at stage 2a, the development stage. However, previous studies on LMLND have only reported surgical outcomes of small cohorts and briefly described the techniques applied in the Methods section, without presenting technique changes (17,19-21,24,25). The current study reports technique changes of LMLND following the standard of IDEAL 2a (41) and is the first IDEAL 2a study on laparoscopic LMLND under the TH approach.
Qualitative methodology was applied for analysis of postoperative interviews. The framework constructed by the task analysis approach presents changes in technical details. Moreover, the cognitive process is shown in the framework, including the interpretation and decision-making of certain surgical conditions and potential errors and risks. Because there is yet no technical standard for laparoscopic LMLND, the optimized technical instruction derived from the framework may help inexperienced surgeons to better understand the what and why of the surgical process. Training and evaluation programs based on this instruction may also be the foundation of a further multicenter IDEAL 2b study. The current study, to our knowledge, is the first IDEAL 2a study using/qualitative methodology to report technique changes in a particular surgical process (42).
The significant changes are described and discussed below.
Sufficient exposure to the surgical field is prerequisite for a successful surgery. There were five subtasks regarding exposure in the framework, including 1) adjusting the Nathanson liver retractor; 2) dividing the left triangular ligament (LTL); 3) splitting the hiatus; 4) retracting the esophagus; and 5) hanging the diaphragmatic crura. The surgeon adjusted these subtasks according to different anatomy and tissue conditions. Additionally, techniques to decrease the difficulty for an in-experienced assistant to help with the exposure were noted. Nevertheless, they all have the same purpose: to ensure better exposure and a sufficient operative space.
In general, dissection of the lower mediastinal lymph nodes is the most important and most disputed aspect of this surgery. The number of points under the theme “Dissection” was greater than that the other two and focused on the subtheme “The margin of the dissection”. According to Japanese Classification of Esophageal Cancer (43), the lower mediastinal lymph nodes include station 110, 111, and 112, and station 112 includes 112aoA and 112pul. However, complete dissection of all lymph node stations under the TH approach may lead to potential injuries to the pleura, lung, lower pulmonary vein, and inferior vena cava. Because most AEG patients in China receive treatment from gastrointestinal surgeons, with the TH approach being widely adopted, it is reasonable to consider limited dissection to lower surgical risk. According to previous high-quality evidence, station 110 is the only lymph node station among the lower mediastinal lymph nodes that warrant dissection for AEG with esophageal invasion less than 4 cm (3,6), and its dissection does not require opening the pleura. Thus, the final instruction defined the following margin of dissection: 1) the superior margin is the lower pulmonary vein, but full exposure is not needed; 2) the inferior margin is the diaphragm with exposure of the caval opening; 3) the posterior margin is the aorta; and 4) the lateral margins are the left and right mediastinal pleura with a recommendation to keep close to the esophagus while dissecting the left margin.
The most vulnerable structure during LMLND was the mediastinal pleura. There were also changes in the cognitive process regarding the significance and management of its injury. Because the pleura is relatively sheer and exposure and recognition of the pleura during surgery are difficult, the surgeon quickly noted the potential risk of injury during the first few cohort cases. Additionally, due to the protection of the infra-cardiac bursa, the right pleura is less risky, as supported by the current study’s quantitative analysis (Table 1). Nevertheless, its impact on postoperative recovery and management was not determined until the last cases. The first pleural injury occurred in case No. 4; with a history of esophageal injury, this patient eventually underwent trans-thoracic surgery, and the pleural injury was left without management. The second injury was in case No. 8, involving clipping during surgery. Neither of these cases involved postoperative pulmonary complications. After discussion with thoracic surgeons and reviewing the literature, we believe that simple pleural injuries are not a significant factor for poor postoperative recovery. More cases of pleural injuries emerged as the surgeon became more efficient with the maneuver and less careful. Thus, the surgeon further noticed that the pleural mediastinum may serve as a marker for entering the pleural cavity, preventing further injury to the lung. Moreover, the integrity of the pleura may be a barrier to the spread of possible anastomotic leaks. Therefore, the optimized instruction recommends various choices according to the severity of injuries, including clipping, suturing, and chest tube placement without repairment if repair is not feasible.
The route of dissection is rarely mentioned in previous studies. However, it still needs consideration in actual practice. Safety and efficiency should both be taken into account when designing a procedure. In the early stages, when the maneuvers were not highly sophisticated and the significance of pleural injuries was not fully understood, safety should be placed first. Because dissection of the anterior and posterior margins favors exposure of the lateral margins and the left pleura is more prone to injury than the right, we recommended dissecting in the order of anterior, posterior, right and left. With the number of successful cases increasing, the surgeon may adjust the order according to specific surgical conditions. The only requirement is to leave the left margin in the last place.
As there were no technique changes after case 30, we considered that the procedure had reached stability and is feasible for further research according to IDEAL stages. Because the current study was in an early IDEAL stage, there would inevitably be limitations. Although implemented in as standardized a manner as possible, the qualitative method is inevitably objective, especially when the review was conducted with a single surgeon, due to the innovativeness of the technique. On the other hand, with a small number of cases, there was limited potential for quantitative research. We calculated the overall complication rate to be 60%, but it was difficult to perform comparisons due to the one-arm design. According to our previous data of laparoscopic total gastrectomy without LMLND, the complication rate is 34.7% in patients undergoing TLTG with either π-shaped or the modified overlap method using knotless barbed sutures (44). However, 48.2% of the patients had tumors located in the middle-third of the stomach, clearly lower than the patients included in the current study, who had tumors in the EGJ. According to another study, patients undergoing laparoscopic gastrectomy with OrVilTM had a complication rate of 46.7%, and 78.8% of them had AEG (45). Thus, in the current study focusing on AEG patients with an even higher tumor location on average, the higher complication rate is acceptable. Overall, the current result showed the safety of this procedure. A further IDEAL 2b study to verify this procedure in a larger number of cases with randomized clinical trials is in progress (No. NCT04443478) (46).
Conclusions
In the current study, there were no technique changes after case 30, showing the feasibility and stability of the procedure. An optimized technical instruction was eventually produced. Moreover, quantitative analysis showed an acceptable postoperative outcome and thus the safety of this procedure. In conclusion, the IDEAL 2a study for laparoscopic LMLND under the TH approach has reached its prospective outcome. A further IDEAL 2b study for this surgery may be performed in the future.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Acknowledgements
This study was supported by Beijing Municipal Administration of Hospitals (No. DFL20181103) and Beijing Hospitals Authority Innovation Studio of Young Staff Funding Support (No. 202123).
Contributor Information
Ziyu Li, Email: ziyu_li@hsc.pku.edu.cn.
Jiafu Ji, Email: jijiafu@hsc.pku.edu.cn.
References
- 1.Feith M, Stein HJ, Siewert JR Adenocarcinoma of the esophagogastric junction: surgical therapy based on 1602 consecutive resected patients. Surg Oncol Clin N Am. 2006;15:751–64. doi: 10.1016/j.soc.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 2.Hosoda K, Yamashita K, Katada N, et al Impact of lower mediastinal lymphadenectomy for the treatment of esophagogastric junction carcinoma. Anticancer Res. 2015;35:445–56. [PubMed] [Google Scholar]
- 3.Yamashita H, Seto Y, Sano T, et al Results of a nation-wide retrospective study of lymphadenectomy for esophagogastric junction carcinoma. Gastric Cancer. 2017;20:69–83. doi: 10.1007/s10120-016-0663-8. [DOI] [PubMed] [Google Scholar]
- 4.Yoshikawa T, Takeuchi H, Hasegawa S, et al Theoretical therapeutic impact of lymph node dissection on adenocarcinoma and squamous cell carcinoma of the esophagogastric junction. Gastric Cancer. 2016;19:143–9. doi: 10.1007/s10120-014-0439-y. [DOI] [PubMed] [Google Scholar]
- 5.Koyanagi K, Kato F, Kanamori J, et al Clinical significance of esophageal invasion length for the prediction of mediastinal lymph node metastasis in Siewert type II adenocarcinoma: A retrospective single-institution study. Ann Gastroenterol Surg. 2018;2:187–96. doi: 10.1002/ags3.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurokawa Y, Takeuchi H, Doki Y, et al Mapping of lymph node metastasis from esophagogastric junction tumors: A prospective nationwide multicenter study. Ann Surg. 2021;274:120–7. doi: 10.1097/SLA.0000000000003499. [DOI] [PubMed] [Google Scholar]
- 7.Japanese Gastric Cancer Association Japanese Gastric Cancer Treatment Guidelines 2018 (5th edition) Gastric Cancer. 2021;24:1–21. doi: 10.1007/s10120-020-01042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sasako M, Sano T, Yamamoto S, et al Left thoracoabdominal approach versus abdominal-transhiatal approach for gastric cancer of the cardia or subcardia: a randomised controlled trial. Lancet Oncol. 2006;7:644–51. doi: 10.1016/s1470-2045(06)70766-5. [DOI] [PubMed] [Google Scholar]
- 9.Kurokawa Y, Sasako M, Sano T, et al Ten-year follow-up results of a randomized clinical trial comparing left thoracoabdominal and abdominal transhiatal approaches to total gastrectomy for adenocarcinoma of the oesophagogastric junction or gastric cardia. Br J Surg. 2015;102:341–8. doi: 10.1002/bjs.9764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim HH, Han SU, Kim MC, et al Effect of laparoscopic distal gastrectomy vs. open distal gastrectomy on long-term survival among patients with stage I gastric cancer: The KLASS-01 randomized clinical trial. JAMA Oncol. 2019;5:506–13. doi: 10.1001/jamaoncol.2018.6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu J, Huang C, Sun Y, et al Effect of laparoscopic vs. open distal gastrectomy on 3-year disease-free survival in patients with locally advanced gastric cancer: The CLASS-01 randomized clinical trial. JAMA. 2019;321:1983–92. doi: 10.1001/jama.2019.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katai H, Mizusawa J, Katayama H, et al Survival outcomes after laparoscopy-assisted distal gastrectomy versus open distal gastrectomy with nodal dissection for clinical stage IA or IB gastric cancer (JCOG0912): a multicentre, non-inferiority, phase 3 randomised controlled trial. Lancet Gastroenterol Hepatol. 2020;5:142–51. doi: 10.1016/s2468-1253(19)30332-2. [DOI] [PubMed] [Google Scholar]
- 13.Xiong W, Xu Y, Chen T, et al Laparoscopic vs. open surgery for gastrointestinal stromal tumors of esophagogastric junction: A multicenter, retrospective cohort analysis with propensity score weighting. Chin J Cancer Res. 2021;33:42–52. doi: 10.21147/j.issn.1000-9604.2021.01.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hyung WJ, Yang HK, Han SU, et al A feasibility study of laparoscopic total gastrectomy for clinical stage I gastric cancer: a prospective multi-center phase II clinical trial, KLASS 03. Gastric Cancer. 2019;22:214–22. doi: 10.1007/s10120-018-0864-4. [DOI] [PubMed] [Google Scholar]
- 15.Katai H, Mizusawa J, Katayama H, et al Single-arm confirmatory trial of laparoscopy-assisted total or proximal gastrectomy with nodal dissection for clinical stage I gastric cancer: Japan Clinical Oncology Group study JCOG1401. Gastric Cancer. 2019;22:999–1008. doi: 10.1007/s10120-019-00929-9. [DOI] [PubMed] [Google Scholar]
- 16.Liu F, Huang C, Xu Z, et al Morbidity and mortality of laparoscopic vs open total gastrectomy for clinical stage I gastric cancer: The CLASS02 multicenter randomized clinical trial. JAMA Oncol. 2020;6:1590–7. doi: 10.1001/jamaoncol.2020.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurahashi Y, Nakamura T, Ishida Y, et al Transhiatal lower mediastinal lymph node dissection for esophagogastric junction carcinoma by interconnecting four body cavities. Surg Oncol. 2022;43:101793. doi: 10.1016/j.suronc.2022.101793. [DOI] [PubMed] [Google Scholar]
- 18.Costi R, Himpens J, Bruyns J, et al Totally laparoscopic transhiatal esophago-gastrectomy without thoracic or cervical access. The least invasive surgery for adenocarcinoma of the cardia? Surg Endosc. 2004;18:629–32. doi: 10.1007/s00464-003-9053-5. [DOI] [PubMed] [Google Scholar]
- 19.Ren MY, Huang B, Zhang J, et al Laparoscopic transhiatal proximal gastrectomy for adenocarcinoma of the esophagogastric junction: report of 98 cases. Zhonghua Wei Chang Wai Ke Za Zhi. 2012;15:906–9. [PubMed] [Google Scholar]
- 20.Takiguchi S, Miyazaki Y, Shinno N, et al Laparoscopic mediastinal dissection via an open left diaphragm approach for advanced Siewert type II adenocarcinoma. Surg Today. 2016;46:129–34. doi: 10.1007/s00595-015-1247-7. [DOI] [PubMed] [Google Scholar]
- 21.Kinjo Y, Satoh S, Ochi S, et al Laparoscopic transhiatal lymphadenectomy in the lower mediastinum for adenocarcinoma of the esophagogastric junction. Int Cancer Conf J. 2018;7:37–9. doi: 10.1007/s13691-018-0318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugita S, Kinoshita T, Kaito A, et al Short-term outcomes after laparoscopic versus open transhiatal resection of Siewert type II adenocarcinoma of the esophagogastric junction. Surg Endosc. 2018;32:383–90. doi: 10.1007/s00464-017-5687-6. [DOI] [PubMed] [Google Scholar]
- 23.Wang D, Cao S, Tan X, et al Effects of robotic and laparoscopic-assisted surgery on lymph node dissection and short-term outcomes in patients with Siewert II adenocarcinoma of esophagogastric junction. Zhonghua Wei Chang Wai Ke Za Zhi (in Chinese) 2019;22:156–63. [PubMed] [Google Scholar]
- 24.Pang W, Liu G, Zhang Y, et al Total laparoscopic transabdominal-transdiaphragmatic approach for treating Siewert II tumors: a prospective analysis of a case series. World J Surg Oncol. 2021;19:26. doi: 10.1186/s12957-021-02136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakaguchi M, Hosogi H, Kanaya S Laparoscopic en bloc lower mediastinal lymph node dissection via transhiatal approach for adenocarcinoma of esophagogastric junction. Surg Oncol. 2021;36:34–5. doi: 10.1016/j.suronc.2020.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Sugita S, Kinoshita T, Kuwata T, et al Long-term oncological outcomes of laparoscopic versus open transhiatal resection for patients with Siewert type II adenocarcinoma of the esophagogastric junction. Surg Endosc. 2021;35:340–8. doi: 10.1007/s00464-020-07406-w. [DOI] [PubMed] [Google Scholar]
- 27.Hoshino A, Tokunaga M, Kinugasa Y Totally laparoscopic transhiatal middle and lower mediastinal lymphadenectomy for esophageal cancer. Surg Laparosc Endosc & Percutan Tech. 2021;31:808–11. doi: 10.1097/sle.0000000000000964. [DOI] [PubMed] [Google Scholar]
- 28.Xu Z, Hu C, Zhang Y, et al Efficacy analysis of Cheng’s GIRAFFE reconstruction after proximal gastrectomy for adenocarcinoma of esophagogastric junction. Chin J Cancer Res. 2022;34:289–97. doi: 10.21147/j.issn.1000-9604.2022.03.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cook JA, McCulloch P, Blazeby JM, et al IDEAL framework for surgical innovation 3: randomised controlled trials in the assessment stage and evaluations in the long term study stage. BMJ. 2013;346:f2820. doi: 10.1136/bmj.f2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ergina PL, Barkun JS, McCulloch P, et al IDEAL framework for surgical innovation 2: observational studies in the exploration and assessment stages. BMJ. 2013;346:f3011. doi: 10.1136/bmj.f3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCulloch P, Cook JA, Altman DG, et al IDEAL framework for surgical innovation 1: the idea and development stages. BMJ. 2013;346:f3012. doi: 10.1136/bmj.f3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siewert JR, Stein HJ Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg. 1998;85:1457–9. doi: 10.1046/j.1365-2168.1998.00940.x. [DOI] [PubMed] [Google Scholar]
- 33.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Gastric Cancer Version 4. 2020. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1434
- 34.Mays N, Pope C. Introduction. In. Qualitative Research in Health Care. NewYork: John Wiley & Sons Ltd., 2020. p1-13.
- 35.Tong A, Sainsbury P, Craig J Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19:349–57. doi: 10.1093/intqhc/mzm042. [DOI] [PubMed] [Google Scholar]
- 36.Pope C, Ziebland S, Mays N. Analysis. In. Qualitative Research in Health Care NewYork: John Wiley & Sons Ltd., 2020. p111-33.
- 37.Madani A, Watanabe Y, Feldman LS, et al Expert intraoperative judgment and decision-making: Defining the cognitive competencies for safe laparoscopic cholecystectomy. J Am Coll Surg. 2015;221:931–40.e8. doi: 10.1016/j.jamcollsurg.2015.07.450. [DOI] [PubMed] [Google Scholar]
- 38.Madani A, Grover K, Kuo JH, et al Defining the competencies for laparoscopic transabdominal adrenalectomy: An investigation of intraoperative behaviors and decisions of experts. Surgery. 2020;167:241–9. doi: 10.1016/j.surg.2019.03.035. [DOI] [PubMed] [Google Scholar]
- 39.Multidisciplinary Union for Esophagogastric Junction Disease of Chinese Societyfor Deseases of the Esophagus (CSDE), Laparoscopic Surgery Committee of the Endoscopist Branch in the Chinese Medical Doctor Association (CMDA), Upper Digestive Tract Surgeons Committee of the Surgeon Branch in the Chinese Mdical Dotor Association (CMDA), et al. Chinese expert consensus on the surgical treatment for adenocarcinoma of esophagogastric junction (2018 edition). Zhonghua Wei Chang Wai Ke Za Zhi (in Chinese) 2018;21:961-75.
- 40.Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma (15th edition). Tokyo: Japanese Gastric Cancer Association, 2017.
- 41.Yu J, Shan F, McCulloch P, et al The methodological framework of surgical innovation: The interpretation of IDEAL reporting guideline. Zhongguo Xiong Xin Xue Guan Wai Ke Lin Chuang Za Zhi. 2021;28:131–6. doi: 10.7507/1007-4848.202011088. [DOI] [Google Scholar]
- 42.Khachane A, Philippou Y, Hirst A, et al Appraising the uptake and use of the IDEAL Framework and Recommendations: A review of the literature. Int J Surg. 2018;57:84–90. doi: 10.1016/j.ijsu.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 43.Japan Esophageal Society Japanese Classification of Esophageal Cancer, 11th Edition: part I. Esophagus. 2017;14:1–36. doi: 10.1007/s10388-016-0551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Liu Z, Shan F, et al Short-term outcomes after totally laparoscopic total gastrectomy with esophagojejunostomy constructed by π-shaped method versus overlap method. J Surg Oncol. 2021;124:1329–37. doi: 10.1002/jso.26642. [DOI] [PubMed] [Google Scholar]
- 45.Hong F, Wang Y, Zhang Y, et al Comparison of the short-term outcomes of laparoscopic and open total or proximal gastrectomy using the transorally inserted anvil (OrVil(TM)) for the proximal reconstruction: a propensity score matching analysis. Langenbecks Arch Surg. 2021;406:651–8. doi: 10.1007/s00423-021-02126-8. [DOI] [PubMed] [Google Scholar]
- 46.Li S, Ying X, Shan F, et al Laparoscopic vs. open lower mediastinal lymphadenectomy for Siewert type II/III adenocarcinoma of esophagogastric junction: An exploratory, observational, prospective, IDEAL stage 2b cohort study (CLASS-10 study) Chin J Cancer Res. 2022;34:406–14. doi: 10.21147/j.issn.1000-9604.2022.04.08. [DOI] [PMC free article] [PubMed] [Google Scholar]