Characterization of the polymorphism of human insulin upon pH variation and structure determination of the cubic polymorph via Rietveld refinement are reported.
Keywords: human insulin, cubic polymorph, X-ray powder diffraction, synchrotron radiation
Abstract
The polymorphism of human insulin upon pH variation was characterized via X-ray powder diffraction, employing a crystallization protocol previously established for co-crystallization with phenolic derivatives. Two distinct rhombohedral (R3) polymorphs and one cubic (I213) polymorph were identified with increasing pH, corresponding to the T6, T3R3 f and T2 conformations of insulin, respectively. The structure of the cubic T2 polymorph was determined via multi-profile stereochemically restrained Rietveld refinement at 2.7 Å resolution. This constitutes the first cubic insulin structure to be determined from crystals grown in the presence of zinc ions, although no zinc binding was observed. The differences of the polycrystalline variant from other cubic insulin structures, as well as the nature of the pH-driven phase transitions, are discussed in detail.
1. Introduction
The story of the structural characterization of insulin has been tightly coupled with the development of macromolecular crystallography since the late 1920s (Vijayan, 2002 ▸). After a century of crystallographic research, insulin has proven to be one of the most polymorphic proteins, exhibiting a variety of molecular conformations, crystal forms and metal- and ligand-binding abilities.
At its core, insulin is a small protein comprising 51 amino acids in two polypeptide chains: A and B. Depending on the physicochemical environment, the N-terminal region of chain B can be found in a helical (R), intermediate (Rf) or extended (T) conformation, although only the extended conformation is observed for monomeric insulin in solution. The helical and intermediate conformations, which exhibit higher physicochemical stability (Rahuel-Clermont et al., 1997 ▸), are only observed in hexameric assemblies of insulin coordinated by divalent cations, physiologically zinc. The resulting hexamers can adopt one of the following combinations of conformations: T6,
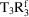 or R6 (Kaarsholm et al., 1989 ▸). Although the T6 conformation is predominantly observed in solution, the
or R6 (Kaarsholm et al., 1989 ▸). Although the T6 conformation is predominantly observed in solution, the
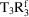 state can be stabilized in the presence of halides, pseudo-halides or organic carboxylates, while the further addition of phenol derivatives can stabilize the R6 conformation (Dunn, 2005 ▸; Whittingham et al., 1995 ▸).
state can be stabilized in the presence of halides, pseudo-halides or organic carboxylates, while the further addition of phenol derivatives can stabilize the R6 conformation (Dunn, 2005 ▸; Whittingham et al., 1995 ▸).
Two highly similar crystal forms or polymorphs of rhombohedral insulin have been observed to date for the T6 (Adams et al., 1969 ▸) and
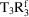 (Bentley et al., 1976 ▸) molecular conformations, respectively, while insulin hexamers complexed with phenolic ligands in the R6 conformation have been observed in a variety of crystal polymorphs, as briefly summarized by Spiliopoulou, Valmas et al. (2020 ▸). In the absence of zinc cations and in the millimolar concentration range insulin monomers cannot form hexamers and instead crystallize as dimers, predominately with cubic symmetry. This cubic polymorph was first identified by Abel et al. (1927 ▸), while the zinc-free nature of these crystals was only recognized after 31 years (Schlichtkrull, 1958 ▸) and the first porcine cubic insulin structure was solved a further 20 years later (Dodson et al., 1978 ▸).
(Bentley et al., 1976 ▸) molecular conformations, respectively, while insulin hexamers complexed with phenolic ligands in the R6 conformation have been observed in a variety of crystal polymorphs, as briefly summarized by Spiliopoulou, Valmas et al. (2020 ▸). In the absence of zinc cations and in the millimolar concentration range insulin monomers cannot form hexamers and instead crystallize as dimers, predominately with cubic symmetry. This cubic polymorph was first identified by Abel et al. (1927 ▸), while the zinc-free nature of these crystals was only recognized after 31 years (Schlichtkrull, 1958 ▸) and the first porcine cubic insulin structure was solved a further 20 years later (Dodson et al., 1978 ▸).
Owing to its high solvent content [∼65%(v/v)], the cubic polymorph has served as an ideal system to explore pH-dependent conformational changes (Gursky, Badger et al., 1992 ▸), monovalent metal-binding sites (Gursky, Li et al., 1992 ▸) and the protonation states of several structurally crucial amino acids (Diao, 2003 ▸; Ishikawa, Chatake, Morimoto et al., 2008 ▸; Ishikawa, Chatake, Ohnishi et al., 2008 ▸). More recently, the cubic polymorph of the human insulin (HI) variant was reported as a byproduct of co-crystallization efforts with polysialic acid (Timofeev et al., 2010 ▸), while multiple mutants and engineered constructs in a cubic setting are now available in the Protein Data Bank (PDB).
Our interest in the cubic insulin polymorph was a result of our crystallization studies of HI–ligand complexes in the search for stable and densely packed polymorphs that could serve as more potent microcrystalline antidiabetic formulations (Spiliopoulou, Valmas et al., 2020 ▸; Karavassili et al., 2017 ▸). Using X-ray powder diffraction (XRPD), we exploit the observation of novel crystalline polymorphs of insulin as a first strong indication of ligand binding. Thus, in our efforts to ascertain which polymorphs are suggestive of ligand binding, we pursued the crystallization of HI via our established protocol but in the absence of any ligands. During these experiments we observed the cubic HI polymorph and, motivated by the impressive quality of the collected XRPD data and the lack of a wild-type human cubic insulin structure in the PDB at the time, we set forth to determine its structure to explore the potential differences between cubic insulins from different species.
2. Experimental
2.1. Materials
Recombinant human insulin (batch No. SPIN-05-101B) was provided by Novo Nordisk A/S. Zinc acetate (CAS No. 5970-45-6) was purchased from Sigma–Aldrich (now part of Merck KGaA). Sodium thiocyanate (CAS No. 540-72-7) was purchased from Fluka Chemie (now part of Merck KGaA). Disodium hydrogen phosphate (CAS No. 10028-24-7) was purchased from PanReac AppliChem ITW Reagents. Potassium dihydrogen phosphate (CAS No. 7778-77-0) was purchased from Honeywell International Inc. Deionized water from an Aquatron A4D system equipped with a Zalion 200.4 column was used in all experiments.
2.2. Crystallization experiments
Human insulin was crystallized employing a previously established protocol for ligand co-crystallization experiments (Fili et al., 2015 ▸; Karavassili et al., 2012 ▸, 2017 ▸; Spiliopoulou, Valmas et al., 2015 ▸, 2021 ▸; Triandafillidis et al., 2020 ▸).
2.2.1. Buffer preparation
Two phosphate solutions were prepared: disodium hydrogen phosphate (Na2HPO4) and potassium dihydrogen phosphate (KH2PO4) at 2 M concentration. The phosphate-buffer stocks were mixed in appropriate ratios under a pH meter to create a systematic pH gradient of 2 M phosphate buffers. A total of 21 buffers were prepared with pH values between 4.5 and 8.5.
2.2.2. Crystallization
For 20 samples, 380 mg lyophilized zinc-free insulin was dissolved in 20 ml double-distilled water (ddH2O). 2.31 ml 0.01 M zinc acetate solution was added to form insulin hexamers and the solution was aspirated lightly until no clouding was observed. After 5 min, 0.299 ml 1 M sodium thiocyanate solution was added to the protein mixture.
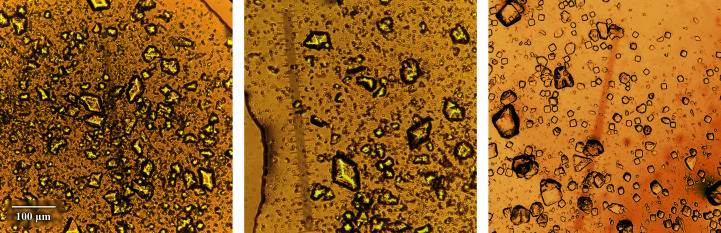
For each sample, 1 ml of the protein mixture along with 0.25 ml phosphate buffer solution were placed in an Eppendorf tube, resulting in a final protein concentration of 13.50 mg ml−1 or 2.32 mM (Table 1 ▸). Samples were stored at room temperature and crystals started appearing after 1–5 days (Fig. 1 ▸), depending on the pH. Powder diffraction patterns were collected at least one month after crystallization experiments.
Table 1. Crystallization parameters.
| Method | Batch |
| Container | 1.5 ml Eppendorf tubes |
| Temperature (K) | 298 |
| Buffer composition of protein solution | ddH2O |
| Final composition† | 13.50 mg ml−1 (2.32 mM) human insulin, 0.82 mM zinc acetate, 10.56 mM sodium thiocyanate, 0.4 M phosphate (Na2HPO4/KH2PO4) |
| Starting pH | 4.50–8.50 |
| Final volume (ml) | 1.25 |
Values calculated based on the crystallization protocol presented in Section 2.
Figure 1.
From left to right: polycrystalline HI samples corresponding to polymorphs R3T6 (nl18, pH 6.31), R3T3R3 (nl116, pH 7.06) and I213 (nl218, pH 7.70). The scale bar corresponds to all panels.
Each sample was labeled with a unique identifier starting with ‘nl’ (i.e. no ligand) followed by the experiment series number and an incremental sample number in order of increasing pH. For example, nl212 corresponds to sample 12 of the second crystallization experiment.
A variation of the sample pH was observed with time. Throughout this study, ‘starting pH’ will refer to the pH of the crystallization buffer prior to mixing with the protein mixture, while ‘final pH’ will refer to the pH of the sample prior to diffraction experiments, which was determined by directly measuring the pH of the supernatant crystallization solution.
2.3. X-ray powder diffraction (XRPD) measurements
2.3.1. Sample preparation
Prior to XRPD data collection, the polycrystalline protein slurries were transferred to borosilicate glass capillaries of 1 mm diameter and 0.1 mm thickness. Each capillary was first filled with mother liquor, i.e. the supernatant crystallization solution, and an amount of polycrystalline slurry was then transferred using a pipette or a syringe. The capillaries were then centrifuged to enhance crystal packing and topped up with more slurry if needed. A sample volume with a height of ∼1 cm is required per capillary, but preferably much more, depending on sample availability. Excess mother liquor was removed with a syringe and the capillaries were cut and sealed with silicone vacuum grease to prevent dehydration. Each sample was mounted onto the goniometer head of the diffractometer and rotated during data collection to ensure better powder averaging.
2.3.2. Data collection
High-resolution XRPD data were collected on the ID22 beamline (Fitch, 2004 ▸; Dejoie et al., 2018 ▸) at the European Synchrotron Radiation Facility (ESRF), Grenoble, France in Debye–Scherrer mode at room temperature employing a nine-channel LaBr3 scintillator detector equipped with nine Si(111) crystal analyzers, during two separate experiments [series 1, λ = 1.29974 (1) Å; series 2, λ = 1.299995 (3) Å]. Each capillary was measured at multiple adjacent positions along the capillary axis to minimize radiation-damage effects, collecting two successive scans per position. The detector was translated at 25° min−1 and each sample position was exposed to the beam for 2 min per scan. All first and second scans from all capillary positions were then merged separately. Only the merged first scans were used for further analysis since radiation damage was observed in subsequent scans. Each detector channel was also individually inspected for signs of radiation damage, such as peak shifts or loss of diffraction intensity, and was excluded from merging if necessary.
Additional XRPD data were collected on an in-house X’Pert Pro diffractometer (Malvern Panalytical) in Debye–Scherrer geometry with Cu Kα radiation [λ = 1.540585 (3) Å; Hölzer et al., 1997 ▸] at room temperature employing a PIXcel 1D detector. Each sample was repeatedly measured at a single capillary position for ∼24 scans per sample. The detector was translated at 1° min−1 and the sample was exposed for 30 min per scan. The individual scans were inspected for signs of radiation damage and were then averaged to enhance the data statistics. No noticeable radiation damage was observed even after 12 h of data collection, due to the significantly lower beam brilliance.
2.3.3. Data processing
For each crystallization series, the XRPD patterns were grouped manually via surface plots or semi-automatically via principal component analysis using HighScore Plus (Degen et al., 2014 ▸). Representative patterns from each cluster were then indexed using DICVOL04 (Boultif & Louër, 2004 ▸).
Pawley refinement (Pawley, 1981 ▸) was performed for each data set in HighScore Plus to extract peak intensities and accurately characterize the lattice dimensions and peak shape parameters. The peak profile was modeled using a pseudo-Voigt function (Thompson et al., 1987 ▸) with full-width at half-maximum (FWHM) and asymmetry type following the Caglioti–Paoletti–Ricci (Caglioti et al., 1958 ▸) and Finger–Cox–Jephcoat (Finger et al., 1994 ▸) models, respectively.
2.4. Structure refinement
Six data sets were selected for structure refinement of the, at the time, unknown human variant of the cubic insulin polymorph.
2.4.1. Data-set selection
Candidate data sets were initially subjected to multi-profile Pawley refinement in PRODD (Wright & Forsyth, 2000 ▸; Wright, 2004 ▸). In this process, intensities are extracted via a single refinement in which each diffraction profile is calculated as a sum of overlapping reflections, the intensities of which are variables in a least-squares procedure. All data sets are fitted using the same integrated intensities for each pattern but with different unit-cell parameters. This preliminary analysis verifies the isomorphism of the data sets employed for structure refinement by assessing the quality of the fit and the discrepancies in refinement statistics (for example R wp, χ2) across all data sets, while enabling the extraction of a more precise intensity set with higher effective completeness (Basso et al., 2005 ▸; Margiolaki et al., 2007 ▸; Margiolaki & Wright, 2008 ▸; Besnard et al., 2007 ▸; Wright et al., 2007 ▸; Margiolaki, 2019 ▸). A single set of intensities is extracted via this process.
Following preliminary analysis, a total of six data sets were selected for structure refinement: five synchrotron data sets and one laboratory data set (Supplementary Table S1). Reflections with a reasonable signal-to-noise ratio [I/σ(I) > 2] could be observed up to a d-spacing resolution of 2.7 Å.
2.4.2. Rietveld refinement
In order to initiate structure refinement, we employed PDB entry 9ins (Gursky, Li et al., 1992 ▸), corresponding to porcine cubic insulin, as a starting model. The model was initially stripped of solvent molecules and alternative conformations. In addition, ThrB30 was substituted with an alanine, which is physiologically found in HI. Owing to the absence of any clear indications of structural heterogeneity upon pH variation, a six-histogram, stereochemically restrained Rietveld refinement (Rietveld, 1969 ▸) was performed using GSAS (Larson & Von Dreele, 2004 ▸) to extract an average structural model of the cubic human insulin polymorph (Margiolaki, 2019 ▸; Margiolaki & Wright, 2008 ▸; Spiliopoulou, Triandafillidis et al., 2020 ▸). This procedure has previously been applied to sperm whale metmyoglobin (Von Dreele, 1999 ▸), hen and turkey egg-white lysozyme (Von Dreele, 2001 ▸, 2005 ▸, 2007 ▸; Basso et al., 2005 ▸; Margiolaki et al., 2005 ▸), human and bovine insulin (Von et al., 2000 ▸; Margiolaki et al., 2013 ▸), human ponsin domain SH3.2 (Margiolaki et al., 2007 ▸) and a pharmaceutical peptide, octreotide (Spiliopoulou, Karavassili et al., 2021 ▸; Fili et al., 2019 ▸).
A single set of unit-cell parameters was extracted from the first data set alone, while lattice differences with subsequent data sets were modeled via refinable hydroelastic strain terms of the reciprocal metric tensor elements (Larson & Von Dreele, 2004 ▸). The same peak profile used for Pawley refinement was also used for Rietveld refinement. Peak shape parameters, background coefficients, zero-shift, unit-cell and lattice-strain terms were first optimized in a multi-profile Le Bail refinement (Le Bail et al., 1988 ▸) in GSAS and then further refined during Rietveld refinement.
The solvent-content contribution to the diffracted intensities was accounted for using the exponential scaling model (Moews & Kretsinger, 1975 ▸; Weichenberger et al., 2015 ▸), which is a direct application of Babinet’s principle to the calculated structure factors. Although more sophisticated masking methods are typically employed in macromolecular crystallography, this approach has proven to be accurate enough for the medium resolution of macromolecular XRPD data (Von Dreele, 1999 ▸, 2005 ▸; Margiolaki et al., 2005 ▸, 2013 ▸).
In order to reduce the number of refinable parameters and to increase the robustness of the refinement process, protein atoms were described using the flexible rigid-body (FRB) approach (Margiolaki et al., 2013 ▸; Von Dreele, 2019 ▸; Spiliopoulou, Karavassili et al., 2021 ▸; Fili et al., 2019 ▸). Here, each residue is considered as a rigid body with its origin fixed at the location of the Cα atom and its orientation described via a quaternion, thus requiring a total of only six refinable parameters. The side chain and backbone carbonyl of the residue are considered to be flexible components; thus, additional refinable torsion angles are introduced for these. Bond lengths and angles are kept fixed at typical values. This approach requires ∼66% fewer refinable parameters than a free-atom refinement. In addition, the isotropic temperature factors used for refinement were constrained across all protein and solvent atoms, respectively.
After most non-atomic model parameters (for example histogram scale factors, solvent coefficients, profile parameters etc.) reached reasonable values, total OMIT maps (Bhat, 1988 ▸; Bhat & Cohen, 1984 ▸) were generated using SFCHECK (Vaguine et al., 1999 ▸) to evaluate the fit of the structure to the electron density. OMIT maps are typically the preferred type of map for macromolecular powder diffraction, due to the inherent model bias in the observed structure factors extracted via the Rietveld method (Toby, 2019 ▸; Spiliopoulou, Triandafillidis et al., 2020 ▸). However, due to the low effective completeness of the multi-data-set refinement (Supplementary Fig. S1) resulting from the exactly overlapping peaks of the cubic crystal system, standard 2F o − F c maps were also employed to aid the model-building process.
Using OMIT and 2F o − F c maps, water molecules were gradually added to the structure in Coot (Emsley & Cowtan, 2004 ▸; Emsley et al., 2010 ▸). The model was periodically subjected to stereochemical checks using PROCHECK (Laskowski et al., 1993 ▸) and MolProbity (Chen et al., 2010 ▸; Williams et al., 2018 ▸) to highlight incorrectly built regions of the structure. The final model reached a total R wp = 6.94% and χ2 = 2.06 and was deposited in the PDB under accession code 7qac.
2.5. Molecular-dynamics simulations
In order to assess the conformational plasticity of the polycrystalline cubic insulin structure, coarse-grained molecular-dynamics simulations were performed. For comparison, additional simulations were performed on HI structures solved via conventional single-crystal techniques: a cubic T2 structure (PDB entry 3i40; Timofeev et al., 2010 ▸), a rhombohedral T6 structure (PDB entry 1mso; Smith et al., 2003 ▸) and an orthorhombic T2 structure (PDB entry 1b9e; Yao et al., 1999 ▸).
Initially, the structures were energy-minimized to avoid steric clashes or invalid stereochemistry using the mdrun engine in GROMACS (Pronk et al., 2013 ▸). Molecular-dynamics simulations were performed using the UNRES web server (Czaplewski et al., 2018 ▸) for coarse-grained protein dynamics at a temperature of 300 K for a total of 100 ns. UNRES relies on the highly reduced united-residue model with only two interaction sites: the united peptide backbone, which consists of pseudobond-linked Cα atoms, and united side chains, which are pseudobond-linked to the respective Cα atoms (Khalili et al., 2005 ▸). The coarse-grained UNRES force field has been developed on a solid statistical-mechanical basis by expanding the potential of mean force of polypeptide-containing systems in water into a cluster-cumulant series and by parameterization of the series terms for simple model systems (Sieradzan et al., 2015 ▸). During the simulations, the root-mean-square deviations of the structures with respect to the initial crystal structures began to stabilize after 20 ns.
3. Results
3.1. Insulin polymorphism as a function of pH
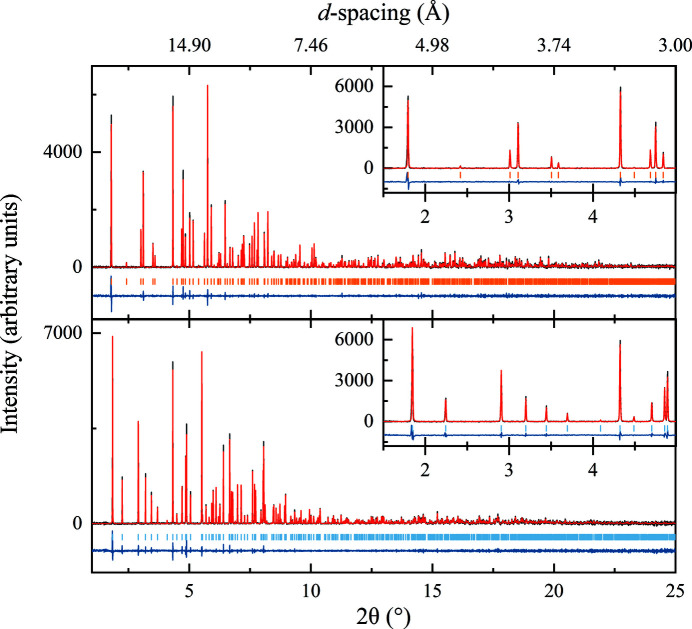
Three polymorphs of HI were observed at different pH values. At acidic pH (final pH 5.33–6.77) HI adopts a rhombohedral symmetry (space group R3; Fig. 2 ▸, upper panel) with unit-cell parameters a = 82.9870 (5), c = 34.0604 (3) Å for sample nl15 (R wp = 7.61%, χ2 = 1.4844). The unit-cell parameters suggest that HI adopts the extended T6 hexameric molecular conformation, based on the literature (Adams et al., 1969 ▸; Blundell et al., 1971 ▸; Smith et al., 2003 ▸). From now on, this polymorph will be referred to as R3T6.
Figure 2.
Pawley refinement of polycrystalline human insulin for polymorphs R3T6 (upper panel; final pH 5.87, R wp = 7.61%, χ2 = 1.48) and R3T3R3 (lower panel; final pH 6.84, R wp = 6.10%, χ2 = 1.40). Black, red and blue lines represent the experimental data, the calculated profile and the difference between them, respectively. The vertical lines correspond to indexed peak positions. The insets correspond to magnifications of the low-resolution region, highlighting the differences in peak positions despite the closely related unit-cell parameters. Data were collected on beamline ID22 at ESRF [λ = 1.29974 (1) Å] at room temperature.
Around neutral pH (final pH 6.76–7.36) HI again adopts a rhombohedral symmetry (space group R3; Fig. 2 ▸, lower panel), but with unit-cell parameters a = 80.5817 (6), c = 37.6980 (3) Å for sample nl114 (R
wp = 6.10%, χ2 = 1.4038). The unit-cell parameters suggest that insulin adopts the intermediate
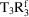 hexameric conformation (Bentley et al., 1976 ▸; Ciszak & Smith, 1994 ▸; Von Dreele et al., 2000 ▸; Frankær et al., 2012 ▸). From now on, this polymorph will be referred to as R3T3R3.
hexameric conformation (Bentley et al., 1976 ▸; Ciszak & Smith, 1994 ▸; Von Dreele et al., 2000 ▸; Frankær et al., 2012 ▸). From now on, this polymorph will be referred to as R3T3R3.
At basic pH (final pH 7.35–8.43) HI adopts a cubic symmetry (space group I213) with unit-cell parameter a = 78.8635 (3) Å for sample nl218 (R wp = 15.26%, χ2 = 1.6777). This polymorph has previously been observed for porcine (Badger et al., 1991 ▸; Dodson et al., 1978 ▸) and bovine insulin (Gursky, Li et al., 1992 ▸; Gursky, Badger et al., 1992 ▸) and more recently for the human variant (Timofeev et al., 2010 ▸).
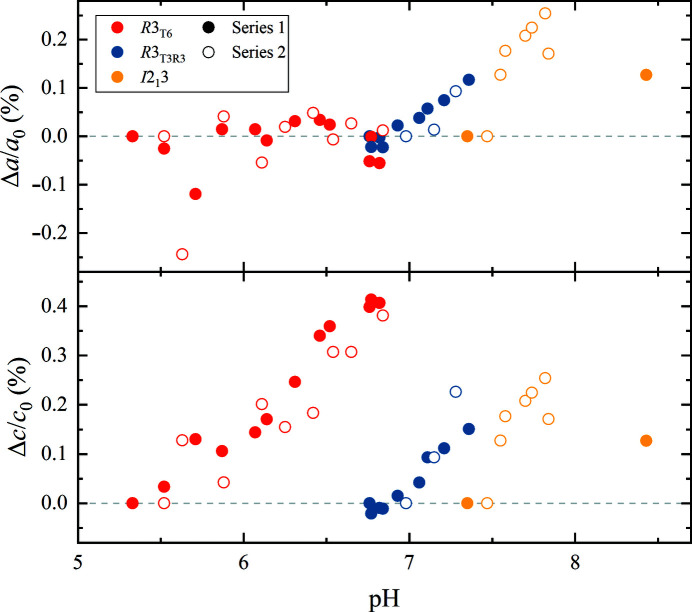
For each of the three polymorphs, a systematic variation in unit-cell parameters was observed as a function of pH (Fig. 3 ▸). Polymorph R3T6 exhibited a 0.41% (∼0.14 Å) increase along the c axis over 1.44 pH units (0.29% increase per pH unit), while no significant variation was observed for the a axis. Polymorph R3T3R3 exhibited a 0.12% (∼0.1 Å) increase along the a axis and a 0.15% (∼0.06 Å) increase along the c axis over 0.60 pH units (0.20% and 0.25% increase per pH unit, respectively). Lastly, polymorph I213 exhibited a 0.25% (∼0.2 Å) increase along the a axis over 0.80 pH units (0.31% increase per pH unit).
Figure 3.
Evolution of unit-cell parameters for the three insulin polymorphs as a function of pH. The percentage change was calculated with respect to the first (i.e. lowest pH) sample of each polymorph for each crystallization series, independently. pH values refer to final pH prior to data collection.
The extracted unit-cell parameters for each sample are reported in Supplementary Tables S2 and S3.
3.2. Cubic human insulin structure
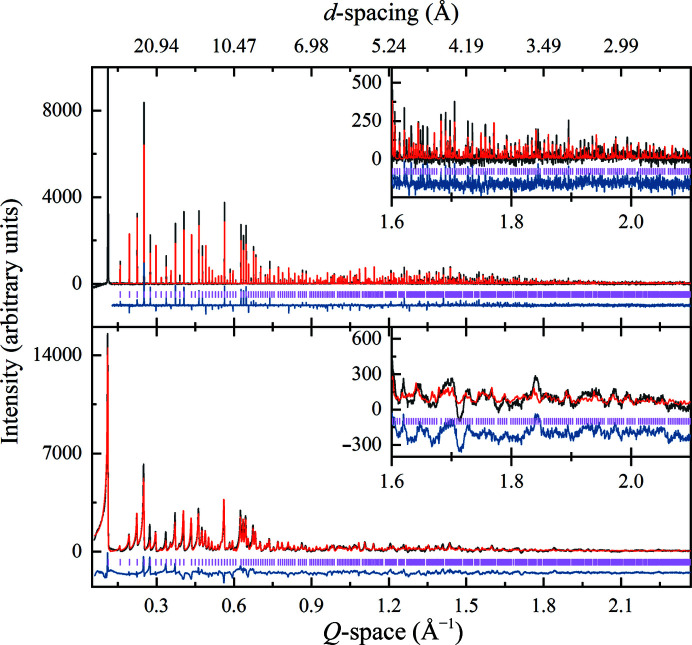
The crystal structure of the cubic human insulin polymorph was obtained via a multi-profile and stereochemically restrained Rietveld refinement (Fig. 4 ▸) at 2.7 Å resolution (total R wp = 6.94% and total χ2 = 2.06). The unit-cell parameter a was determined to be 78.86015 (7) Å. Overall, 18 158 reflections were extracted from the six XRPD patterns and 1951 restraints were imposed on the structural model. A total of 63 water molecules were identified in 2F o − F c and OMIT maps and an overall reasonable model fit to the electron density was observed (Fig. 5 ▸ c). The final model yielded a MolProbity score of 3.78 (Chen et al., 2010 ▸; Williams et al., 2018 ▸). Detailed statistics can be found in Table 2 ▸.
Figure 4.
Multi-profile and stereochemically restrained Rietveld refinement of polycrystalline human insulin (polymorph I213). A synchrotron [upper panel; ID22 at ESRF, λ = 1.29974 (1) Å] and a laboratory [lower panel; Malvern Panalytical X’Pert Pro, λ = 1.540585 (3) Å] profile are presented. Upper panel: final pH 8.43, R p = 7.70%, R wp = 10.98%. The first peak was excluded from the refinement due to artificial distortions caused to the structure. Lower panel: final pH 7.84, R p = 2.12%, R wp = 3.34%. Black, red and blue lines represent the experimental data, the calculated profile and the difference between them, respectively. The vertical lines correspond to indexed peak positions. Data collection was performed at room temperature. A magnification of the high d-spacing resolution region of each data set is shown as an inset.
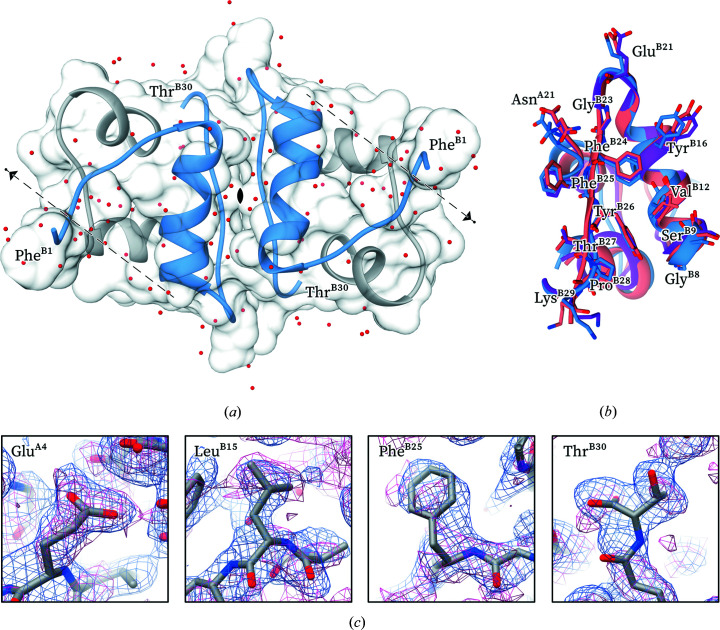
Figure 5.
(a) The polycrystalline T2 insulin dimer structure (PDB entry 7qac). The relevant symmetry elements are shown. Type A and type B chains are shown in white and blue, respectively. (b) Overview of the residues involved in the dimerization interface. Blue, polycrystalline cubic structure (PDB entry 7qac); purple, single-crystal cubic structure (PDB entry 3i40); pink, rhombohedral T6 structure (PDB entry 1mso). (c) 2F o − F c (blue) and OMIT (magenta) maps contoured at 1σ around specific residues of the polycrystalline structure.
Table 2. Crystallization, data collection, refinement and model-quality statistics of polycrystalline cubic HI (PDB entry 7qac) at 2.7 Å resolution.
| Sample | nl121 | nl218 | nl219 | nl220 | nl221 | nl220 |
|---|---|---|---|---|---|---|
| Crystallization | ||||||
| Final batch composition | 13.50 mg ml−1 insulin, 0.82 mM zinc acetate, 10.56 mM sodium thiocyanate, 0.4 M sodium potassium phosphate (Na2HPO4/KH2PO4) | |||||
| pH | ||||||
| Average | 8.56 | 7.88 | 8.02 | 8.17 | 8.26 | 8.17 |
| Starting/final | 8.69/8.43 | 8.06/7.70 | 8.30/7.74 | 8.50/7.84 | 8.70/7.82 | 8.50/7.84 |
| Data collection | ||||||
| Diffraction source | ID22, ESRF | X’Pert Pro | ||||
| Detector | Nine Si(111) analyzer crystals with LaBr3 scintillator detectors | PIXcel 1D | ||||
| Wavelength (Å) | 1.29974 (1) | 1.29986 (3) | 1.299995 (3) | 1.299995 (3) | 1.299995 (3) | 1.540585 (3)† |
| Temperature (K) | 298 | 298 | 298 | 298 | 298 | 298 |
| Total exposure time (min) | 2 | 2 | 2 | 2 | 2 | 720 |
| Resolution range (Å) | 39.43–2.29‡ | 39.43–2.70‡ | 39.43–2.70‡ | 39.43–2.70‡ | 39.43–2.72‡ | 55.76–2.64 |
| Refinement | ||||||
| Space group | I213 (No. 199) | |||||
| Unit-cell parameter a (Å) | 78.86015 (7) | |||||
| Lattice strain parameter δ11 (Å) | 0 | 0.00031788 | 0.00087738 | −0.00016805 | 0.00019147 | 0.0021313 |
| Scale factor | 0.18854 | 0.07749 | 0.05598 | 0.12654 | 0.17352 | 0.89767 |
| Solvent scattering coefficients | ||||||
| A S | 6.124 | 6.121 | 6.019 | 6.036 | 6.052 | 6.351 |
| U S | 2.739 | 2.503 | 2.920 | 2.859 | 2.775 | 6.437 |
| No. of reflections | 3816 | 2345 | 2349 | 2345 | 2305 | 4998 |
| No. of data points | 8256 | 7003 | 7002 | 7002 | 7003 | 4740 |
| No. of restraints | 1951 | |||||
| No. of parameters | 786 | |||||
| R p (%) | 7.70 | 11.54 | 12.16 | 8.80 | 7.61 | 2.12 |
| R wp (%) | 10.98 | 14.75 | 15.17 | 12.12 | 10.95 | 3.34 |
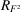 (%)
(%) |
42.02 | 38.68 | 44.09 | 38.89 | 35.52 | 24.00 |
| No. of non-H atoms | ||||||
| Protein | 406 | |||||
| Solvent | 63 | |||||
| Heterogen | 0 | |||||
| Model quality | ||||||
| Ramachandran analysis | ||||||
| Favored (%) | 91.49 | |||||
| Allowed (%) | 8.51 | |||||
| Outliers (%) | 0.00 | |||||
| Rotamer outliers (%) | 13.04 | |||||
| Root-mean-square deviations | ||||||
| Bond lengths (Å) | 0.015 | |||||
| Bond angles (°) | 1.43 | |||||
| Clashscore | 92.88 | |||||
| MolProbity score | 3.78 | |||||
Cu Kα radiation.
Peak (110) was excluded from refinement due to artificial distortions caused to the structure.
The model obtained revealed the T2 dimeric conformation of insulin (Fig. 5 ▸ a) in line with earlier studies [PDB entries 9ins (Gursky, Li et al., 1992 ▸), 3i40 and 3i3z (Timofeev et al., 2010 ▸)]. The asymmetric unit contains a single insulin molecule, which forms a dimer with a symmetry-related molecule. The I213 polymorph exhibits the largest observed solvent content [∼65%(v/v)] of all insulin polymorphs, followed by the hexameric C2221 polymorph (PDB entry 2om1; Norrman & Schluckebier, 2007 ▸; Karavassili et al., 2012 ▸). Contacts between insulin dimers extend in all three directions along the twofold screw axes, forming solvent channels parallel to each crystallographic axis, but also parallel to the threefold diagonal axis.
The insulin dimers in the I213 unit cell are mostly stabilized via an antiparallel β-sheet consisting of the two C-terminal regions of chain B of each monomer. This dimer interface involves residues AsnA21, GlyB8, SerB9, ValB12, TyrB16, GluB21 and GlyB23–LysB29. The hydrogen-bonding network formed between these residues is highly conserved in all cubic insulin structures, as well as in the hexameric assemblies. Despite an overall all-atom r.m.s.d. of 1.84 Å, no significant deviations in backbone or side-chain atoms are observed between the single-crystal (PDB entry 3i40) and the polycrystalline (PDB entry 7qac) cubic HI models for the residues participating in the dimer interface (Fig. 5 ▸ b), given the 2.7 Å resolution of the polycrystalline structure.
3.3. Rietveld refinement at high symmetries
Typically, in macromolecular powder diffraction multiple data sets are employed for structure refinement to exploit the variation in unit-cell parameters which results in anisotropic peak shifts and allows partial deconvolution of overlapping peaks. Such variation in unit-cell parameters can be a result of thermal variation (Brunelli et al., 2003 ▸; Shankland et al., 1997 ▸), controlled radiation damage (Besnard et al., 2007 ▸; Margiolaki et al., 2013 ▸) or variation in crystallization conditions (for example pH; Basso et al., 2005 ▸, 2010 ▸; Margiolaki et al., 2007 ▸), as was the case in the present study. However, the cubic crystal system exhibits exact peak overlap, and therefore any lattice variation results in an isotropic peak shift without any deconvolution of overlapping peaks. This makes it a remarkably challenging case for structure refinement, especially for such a complex molecule.
In this study, we performed combined synchrotron and laboratory refinement of a protein structure. Due to the lower photon flux of laboratory instruments samples can be irradiated for more than 24 h, resulting in excellent counting statistics even in the highest resolution shells. On the other hand, the unparalleled angular resolution of synchrotron diffractometers is crucial for deconvolution of the heavily overlapping peaks in protein samples, especially in the early stages of indexing and structure solution. In the absence of a single instrument with excellent angular resolution, which mitigates radiation-related sample degradation, this combinatorial approach of synchrotron and laboratory diffraction data for protein structure refinement has proven to be of great value for macromolecular powder diffraction.
Even though a multi-profile Rietveld refinement does not alleviate peak overlap in the case of cubic symmetry, it does however increase the information content available for refinement in terms of d-spacing resolution and counting statistics. In contrast to our initial concerns, the refinement process proved to be robust and relatively rapid, overcoming the expected difficulties of powder crystallography in such high symmetries.
4. Discussion
4.1. pH-driven conformational transitions
A variety of solution studies have demonstrated that insulin hexamers exist in an equilibrium between the T6,
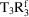 and R6 states (Renscheidt et al., 1984 ▸; Wollmer et al., 1987 ▸; Thomas & Wollmer, 1989 ▸; Roy et al., 1989 ▸; Brader et al., 1991 ▸; Gross & Dunn, 1992 ▸; Choi et al., 1993 ▸). Although T6 is the predominant state, the equilibrium can be shifted towards the
and R6 states (Renscheidt et al., 1984 ▸; Wollmer et al., 1987 ▸; Thomas & Wollmer, 1989 ▸; Roy et al., 1989 ▸; Brader et al., 1991 ▸; Gross & Dunn, 1992 ▸; Choi et al., 1993 ▸). Although T6 is the predominant state, the equilibrium can be shifted towards the
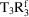 state by the addition of anions and towards the R6 state by the addition of phenol derivatives. In this study, we have observed two phase transitions that are entirely pH-driven.
state by the addition of anions and towards the R6 state by the addition of phenol derivatives. In this study, we have observed two phase transitions that are entirely pH-driven.
Although the crystallization solution contains thiocyanate ion, a strongly chaotropic anion that is capable of stabilizing the B1–B8 helix in the R state (Whittingham et al., 1995 ▸), the T6 conformation of insulin is observed at pH < 6.77, while a further increase in the pH seems to favor the
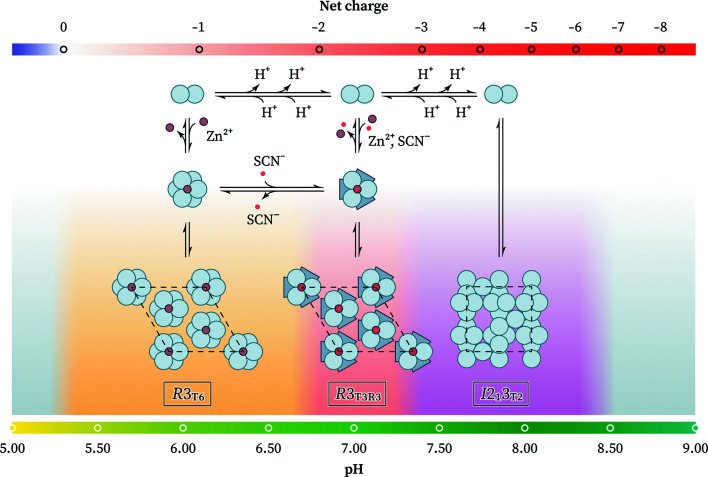
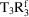 state. More interestingly, at pH > 7.35 zinc-free cubic crystals of dimers are observed. Importantly, in contrast to all other cubic insulin structures available in the literature, in this study the cubic crystals were grown in the presence of zinc.
state. More interestingly, at pH > 7.35 zinc-free cubic crystals of dimers are observed. Importantly, in contrast to all other cubic insulin structures available in the literature, in this study the cubic crystals were grown in the presence of zinc.
As the pH increases from 5.5 to 8.5, insulin acquires a progressively more negative overall charge (pI 5.3) and thus increasingly stronger electrostatic repulsions develop between the insulin molecules. The effective charge of insulin is considerably affected by the ionic strength of the solution, as well as the oligomerization state, which alters the microenvironment of certain residues. We hypothesize that at the 2.1 Zn2+:insulin hexamer ratio used in this study the overall repulsions between insulin dimers at higher pH overcome the attractive forces of the zinc ions that coordinate with three HisB10 to form hexamers, thus favoring the formation of dimeric cubic crystals. We speculate that an increase in the zinc content would shift the equilibrium towards the hexameric state, and thus that the R3T3R3 polymorph would be the predominant form even at pH > 7.35, but further experiments are required to test this hypothesis. By a similar argument, an increase in thiocyanate content would be expected to induce the R3T6 → R3T3R3 transition at lower pH (Fig. 6 ▸).
Figure 6.
Mechanistic hypothesis behind the pH-driven conformational transitions of insulin.
A potential pH-sensitive candidate residue driving these phase transitions is GluB13. In the hexameric assembly, six GluB13 residues are closely located to each other at the center of the hexamer. Given a pK a of 4.3, the six glutamate residues are likely to be mostly deprotonated and negatively charged at physiological pH; thus, the strongly attractive interaction between zinc ions and three HisB10 residues is necessary for the formation of hexamers. It has previously been shown that the binding of calcium ions in the central hexamer cavity counteracts most of the repulsive GluB13 interactions and further stabilizes the hexameric Zn-insulin assembly (Storm & Dunn, 1985 ▸).
At pH 6.0 the six GluB13 residues form a well defined hydrogen-bond network in the T6 state (PDB entry 1mso; Smith et al., 2003 ▸), while in the
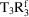 and R6 states the GluB13 side chains are less well ordered and are typically found in multiple conformations, suggesting a role contributing to the T → R transition (Fig. 7 ▸
b). In fact, mutation of GluB13 to Gln yields
and R6 states the GluB13 side chains are less well ordered and are typically found in multiple conformations, suggesting a role contributing to the T → R transition (Fig. 7 ▸
b). In fact, mutation of GluB13 to Gln yields
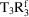 hexamers at pH 7.0 even in the absence of zinc or other helix-stabilizing anions, further supporting this hypothesis (Bentley et al., 1992 ▸).
hexamers at pH 7.0 even in the absence of zinc or other helix-stabilizing anions, further supporting this hypothesis (Bentley et al., 1992 ▸).
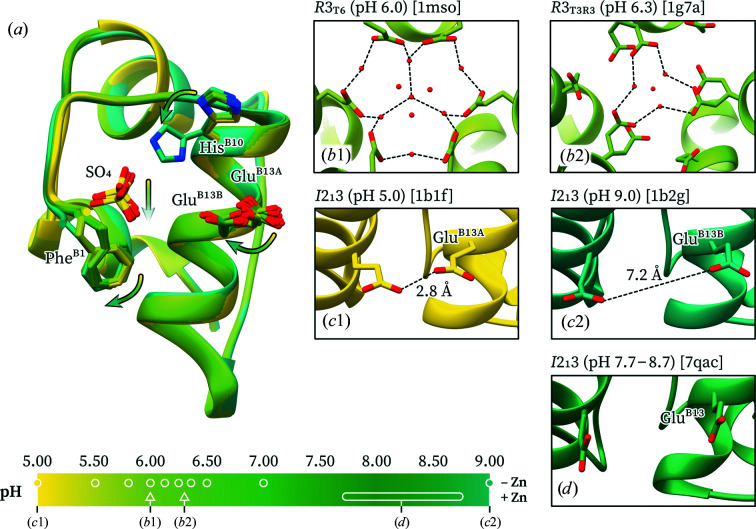
Figure 7.
Variation of the GluB13 conformation with pH. (a) Overlay of ten cubic insulin structures from pH 5.0 to 9.0. The residues that undergo major structural changes are highlighted. The arrows indicate the trend upon pH increase. (b1) Six GluB13 residues in the T6 hexamer form a highly ordered hydrogen-bond network with neighboring water molecules. (b2) In the
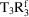 hexamer three of the GluB13 residues exhibit double conformations, while the other three are retracted away from the hexamer core. (c1) At pH 5.0, the two GluB13 residues of the cubic polymorph are hydrogen-bonded (proximal conformation). (c2) At pH 9.0, the GluB13 residues in the cubic polymorph have completely switched to a new conformation that considerably separates them (distant conformation). (d) In the polycrystalline cubic structure the two GluB13 residues are found in the distant conformation. Structures are colored based on pH. PDB codes are shown in parentheses.
hexamer three of the GluB13 residues exhibit double conformations, while the other three are retracted away from the hexamer core. (c1) At pH 5.0, the two GluB13 residues of the cubic polymorph are hydrogen-bonded (proximal conformation). (c2) At pH 9.0, the GluB13 residues in the cubic polymorph have completely switched to a new conformation that considerably separates them (distant conformation). (d) In the polycrystalline cubic structure the two GluB13 residues are found in the distant conformation. Structures are colored based on pH. PDB codes are shown in parentheses.
Progressively increasing electrostatic repulsions between GluB13 with increasing pH have also been observed crystallographically in titration studies of cubic insulin crystals (Diao, 2003 ▸). At pH < 5.8 the two symmetry-related GluB13 residues in the insulin dimer are hydrogen-bonded (proximal conformation, 2.8 Å distance; PDB entry 1b17). At pH 6.0–6.98 a second, distant conformation is observed with a progressively increasing occupancy, while at pH 9.0 only the second conformation is present, in which the two GluB13 side-chain carboxylates are located 7.2 Å apart (distant conformation; PDB entry 1b2g; Fig. 7 ▸ a). Neutron diffraction studies on the cubic polymorph have confirmed that GluB13 is deprotonated at pD 9.0 (pH ≃ 8.6; PDB entry 2zpp; Ishikawa, Chatake, Morimoto et al., 2008 ▸). In this study (final pH 7.70–8.70) the two GluB13 residues in the cubic polymorph are found in the distant conformation (Fig. 7 ▸ d), which is consistent with the repulsion hypothesis.
Taken together, the previous work discussed above and the data from this study suggest that pH-dependent deprotonations are the driving force of the hexamer-to-dimer oligomerization transition and the related shift in crystal form (from rhombohedral to cubic) at pH 7.35.
4.2. Conformational plasticity of insulin
Although found in an extended conformation, the N-terminal region of chain B of the cubic polymorph does not adopt the same conformation as in the hexameric T state. While the T conformation is mostly preserved up to HisB5, a sharp double bend is observed at GlnB4 and AsnB3 which tethers PheB1 closer to the insulin core, residing in a hydrophobic cavity comprised of LeuA16, ValB2, AlaB14, LeuB17 and ValB18. In particular, the distance between PheB1 Cɛ1 and AlaB14 Cβ contracts from 11.2 Å in the hexagonal T6 state (PDB entry 1mso) to only 4.5 Å in the cubic polymorph (PDB entry 3i40).
In the case of the polycrystalline cubic insulin structure reported here (PDB entry 7qac) only a single bend is observed at AsnB3, resulting in a semi-extended conformation of chain B (Table 3 ▸). Rather than retracting towards the α-helix of chain B, PheB1 is instead anchored to a hydrophobic interface formed by two symmetry-related PheB1 residues along the crystallographic threefold symmetry axis and a symmetry-related GlnB4. This results in a 7 Å translocation of PheB1 Cα with respect to the single-crystal cubic structure (PDB entry 3i40). Although the N-terminal fold of chain B is quite conserved among various cubic insulin structures, human or otherwise, this alternate conformation of the polycrystalline structure could be a result of the translocation of the αII helix of chain A, which transposes LeuA16 about 2 Å further back, leading to an increase in the size of the hydrophobic cavity and potentially allowing ValB2 and PheB1 to untether from it (Supplementary Fig. S2).
Table 3. Secondary-structure annotation of several insulin structures with a T-like fold on chain B.
| PDB code | State | Space group | Secondary structure† | Reference |
|---|---|---|---|---|
| Chain B sequence | FVNQHLCGSH LVEALYLVCG ERGFFYTPKT | |||
| 1mso | T | R3 | ...B...THH HHHHHHHHHG GG.EEE.... | Smith et al. (2003 ▸) |
| 3i40 | Ic | I213 | ..SS...THH HHHHHHHHHG GG.B...S.. | Timofeev et al. (2010 ▸) |
| 7qac | Oc | I213 | ..S....TTH HHHHHHHHHT TS.....S.. | This study |
| 2ws0 | I | I4122 | .......HHH HHHHHHHHHG GG..TT. | Jiráček et al. (2010 ▸) |
| 4cy7 | I | P212121 | .......HHH HHHHHHHHHG GG.B.... | Kosinová et al. (2014 ▸) |
| 1b9e | O | P212121 | .......HHH HHHHHHHHHG GG.EEE.... | Yao et al. (1999 ▸) |
H, α-helix; G, 310-helix; E, β-sheet; B, isolated β-bridge; T, hydrogen-bonded turn; S, bend.
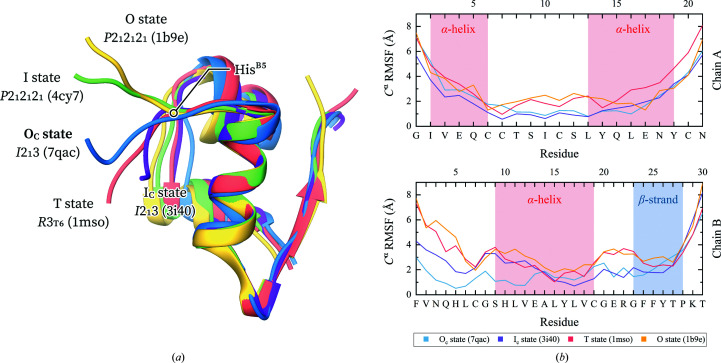
Apart from the distinct T, Rf and R states, the N-terminal region of chain B is capable of folding into a variety of extended, T-like states. Following the previously established nomenclature, the N-terminus can adopt an open, i.e. O, state, in which residues B1–B4 are detached from the rest of the molecule (PDB entry 1b9e; Yao et al., 1999 ▸), or an intermediate, i.e. I, state, in which residues B1 and B2 are positioned further away from the molecule than in the O state [PDB entries 2ws0 (Jiráček et al., 2010 ▸) and 4cy7 (Kosinová et al., 2014 ▸)]. The fold observed in the cubic structures has previously been referred to as a compact intermediate state, i.e. the Ic state (Kosinová et al., 2014 ▸). The polycrystalline cubic structure determined here retains the AsnB3 bend of the Ic fold; however, since residues B1–B2 extend away rather than towards the hydrophobic AlaB14 core, we describe it as a compact open state (i.e. the Oc state). In all these T-like states HisB5 acts as a hinge between the highly flexible B1–B4 residues and the rigid B6–B8 residues (Fig. 8 ▸ a).
Figure 8.
(a) Overlay of insulin structures in a variety of T-like states. (b) Root-mean-square fluctuations of the Cα position during coarse-grained molecular-dynamics simulations. Blue, polycrystalline cubic structure (PDB entry 7qac); purple, single-crystal cubic structure (PDB entry 3i40); pink, rhombohedral T6 structure (PDB entry 1mso); green, orthorhombic AlaB8NMeAla T2 structure (PDB entry 4cy7); yellow, orthorhombic SerB9Glu T2 structure (PDB entry 1b9e).
It is therefore evident that although only the T state is observed in solution for the insulin monomer, a wide range of T-like states can be stabilized in a crystal setting (as dimers or hexamers). Our molecular-dynamics simulations on these crystalline structures revealed significant variation in their flexibility. Specifically, the N-terminal region of chain B is highly rigid in the polycrystalline cubic structure compared with the more mobile Ic state of other cubic insulin structures. The detached B1–B4 conformation of the T and O states results in higher flexibility of the N-terminus. While the Ic, O and T states exhibit a mostly invariant mobility after CysB7, the polycrystalline Oc state is again more rigid. No significant variation was observed in the flexibility of chain A. Monitoring the LeuB15–PheB24, ValB12–TyrB26 and GlyB8–ProB28 Cα-atom distances throughout the simulation (Papaioannou et al., 2015 ▸) provided further evidence that the C-terminus of chain B retains the inactive closed conformation (Supplementary Table S4), which is stabilized by hydrophobic interactions with the α-helix of chain B. An open conformation of the C-terminus is required for insulin to bind to its receptor (Lawrence, 2021 ▸).
5. Conclusions
In the present study, we explored the polymorphism of human insulin in the presence of zinc and thiocyanate ions in the pH range 4.5–8.5. With increasing pH HI forms T6 and
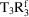 hexamers, while at pH > 7.35 only T2 dimers were observed in a cubic crystal symmetry.
hexamers, while at pH > 7.35 only T2 dimers were observed in a cubic crystal symmetry.
The angular and d-spacing resolution of the collected XRPD data motivated us to refine the structure of the cubic polymorph by employing a multi-profile and stereochemically restrained Rietveld refinement. The flexible rigid-body description of amino acids in GSAS proved to be essential for a robust refinement at 2.7 Å resolution, while we employed synchrotron and laboratory diffraction data in a combined refinement to benefit from the strengths of each instrumentation setup. Despite our concerns due to the high symmetry of the crystals, refinement proved to be feasible.
The polycrystalline cubic insulin structure exhibits some notable differences from other single-crystal structures of the same polymorph, especially in the conformation of the N-terminal region of chain B. We hypothesize that a translocation in LeuA16 increases the size of the hydrophobic cavity which typically houses PheB1, allowing it to adopt a new conformation, termed the Oc state. Our molecular-dynamics simulations suggested that this Oc state is less flexible than the typical Ic state of the cubic polymorph and other T-like states, implying that this is a more rigid conformation of human insulin.
The observed phase transitions are entirely pH-driven. Strikingly, we observed a transition to a zinc-free cubic insulin state despite the presence of zinc in solution. We argue that these transitions are a result of the increase in electrostatic repulsion between neighboring GluB13 residues as a result of pH variation. Further investigations of these pH-driven phase transitions, in terms of varying zinc-to-insulin and thiocyanate-to-insulin ratios, are required to test our hypotheses, as well as single-crystal X-ray diffraction experiments to yield higher resolution structural models.
6. Data availability
Atomic coordinates for the polycrystalline cubic human insulin have been deposited in the Protein Data Bank under accession code 7qac. Experimental and calculated diffraction profiles have been deposited in the structure-factors file.
Supplementary Material
PDB reference: polycrystalline cubic human insulin, 7qac
Supplementary Figures and Tables. DOI: 10.1107/S2059798323001328/tz5109sup1.pdf
Acknowledgments
We would like to thank Novo Nordisk A/S, Dr Matthias Norrman and Dr Gerd Schluckebier for the provision of human insulin and their invaluable support in crystallization procedures and Malvern Panalytical, Dr Thomas Degen and Dr Detlef Beckers for instrumentation and software support. We are grateful to the ESRF for the provision of beamtime on the ID22 beamline. We also thank Dr Arwen R. Pearson for her valuable input into the manuscript.
Funding Statement
This research work was supported by the Hellenic Foundation for Research and Innovation (HFRI) under the ‘First call for HFRI research projects to support faculty members and researchers and the procurement of high-cost research equipment grant’ (Project No. 3051).
References
- Abel, J. J., Geiling, E. M. K., Rouiller, C. A., Bell, F. K. & Wintersteiner, O. (1927). J. Pharmacol. Exp. Ther. 31, 65–85.
- Adams, M. J., Blundell, T. L., Dodson, E. J., Dodson, G. G., Vijayan, M., Baker, E. N., Harding, M. M., Hodgkin, D. C., Rimmer, B. & Sheat, S. (1969). Nature, 224, 491–495.
- Badger, J., Haris, M. R., Reynolds, C. D., Evans, A. C., Dodson, E. J., Dodson, G. G. & North, A. C. T. (1991). Acta Cryst. B47, 127–136. [DOI] [PubMed]
- Basso, S., Besnard, C., Wright, J. P., Margiolaki, I., Fitch, A., Pattison, P. & Schiltz, M. (2010). Acta Cryst. D66, 756–761. [DOI] [PubMed]
- Basso, S., Fitch, A. N., Fox, G. C., Margiolaki, I. & Wright, J. P. (2005). Acta Cryst. D61, 1612–1625. [DOI] [PubMed]
- Bentley, G. A., Brange, J., Derewenda, Z., Dodson, E. J., Dodson, G. G., Markussen, J., Wilkinson, A. J., Wollmer, A. & Xiao, B. (1992). J. Mol. Biol. 228, 1163–1176. [DOI] [PubMed]
- Bentley, G., Dodson, E., Dodson, G., Hodgkin, D. & Mercola, D. (1976). Nature, 261, 166–168. [DOI] [PubMed]
- Besnard, C., Camus, F., Fleurant, M., Dahlström, A., Wright, J. P., Margiolaki, I., Pattison, P. & Schiltz, M. (2007). Z. Kristallogr. Suppl. 2007, 39–44.
- Bhat, T. N. (1988). J. Appl. Cryst. 21, 279–281.
- Bhat, T. N. & Cohen, G. H. (1984). J. Appl. Cryst. 17, 244–248.
- Blundell, T. L., Cutfield, J. F., Cutfield, S. M., Dodson, E. J., Dodson, G. G., Hodgkin, D. C., Mercola, D. A. & Vijayan, M. (1971). Nature, 231, 506–511. [DOI] [PubMed]
- Boultif, A. & Louër, D. (2004). J. Appl. Cryst. 37, 724–731.
- Brader, M. L., Kaarsholm, N. C., Lee, R. W. K. & Dunn, M. F. (1991). Biochemistry, 30, 6636–6645. [DOI] [PubMed]
- Brunelli, M., Wright, J. P., Vaughan, G. B. M., Mora, A. J. & Fitch, A. N. (2003). Angew. Chem. Int. Ed. 42, 2029–2032. [DOI] [PubMed]
- Caglioti, G., Paoletti, A. & Ricci, F. P. (1958). Nucl. Instrum. 3, 223–228.
- Chen, V. B., Arendall, W. B., Headd, J. J., Keedy, D. A., Immormino, R. M., Kapral, G. J., Murray, L. W., Richardson, J. S. & Richardson, D. C. (2010). Acta Cryst. D66, 12–21. [DOI] [PMC free article] [PubMed]
- Choi, W. E., Brader, M. L., Aguilar, V., Kaarsholm, N. C. & Dunn, M. F. (1993). Biochemistry, 32, 11638–11645. [DOI] [PubMed]
- Ciszak, E. & Smith, G. D. (1994). Biochemistry, 33, 1512–1517. [DOI] [PubMed]
- Czaplewski, C., Karczyńska, A., Sieradzan, A. K. & Liwo, A. (2018). Nucleic Acids Res. 46, W304–W309. [DOI] [PMC free article] [PubMed]
- Degen, T., Sadki, M., Bron, E., König, U. & Nénert, G. (2014). Powder Diffr. 29, S13–S18.
- Dejoie, C., Coduri, M., Petitdemange, S., Giacobbe, C., Covacci, E., Grimaldi, O., Autran, P.-O., Mogodi, M. W., Šišak Jung, D. & Fitch, A. N. (2018). J. Appl. Cryst. 51, 1721–1733.
- Diao, J. (2003). Acta Cryst. D59, 670–676. [DOI] [PubMed]
- Dodson, E. J., Dodson, G. G., Lewitova, A. & Sabesan, M. (1978). J. Mol. Biol. 125, 387–396. [DOI] [PubMed]
- Dunn, M. F. (2005). Biometals, 18, 295–303. [DOI] [PubMed]
- Emsley, P. & Cowtan, K. (2004). Acta Cryst. D60, 2126–2132. [DOI] [PubMed]
- Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. (2010). Acta Cryst. D66, 486–501. [DOI] [PMC free article] [PubMed]
- Fili, S., Valmas, A., Norrman, M., Schluckebier, G., Beckers, D., Degen, T., Wright, J., Fitch, A., Gozzo, F., Giannopoulou, A. E., Karavassili, F. & Margiolaki, I. (2015). IUCrJ, 2, 534–544. [DOI] [PMC free article] [PubMed]
- Fili, S., Valmas, A., Spiliopoulou, M., Kontou, P., Fitch, A., Beckers, D., Degen, T., Barlos, K., Barlos, K. K., Karavassili, F. & Margiolaki, I. (2019). Acta Cryst. B75, 611–620. [DOI] [PubMed]
- Finger, L. W., Cox, D. E. & Jephcoat, A. P. (1994). J. Appl. Cryst. 27, 892–900.
- Fitch, A. N. (2004). J. Res. Natl Inst. Stand. Technol. 109, 133–142. [DOI] [PMC free article] [PubMed]
- Frankær, C. G., Knudsen, M. V., Norén, K., Nazarenko, E., Ståhl, K. & Harris, P. (2012). Acta Cryst. D68, 1259–1271. [DOI] [PubMed]
- Gross, L. & Dunn, M. F. (1992). Biochemistry, 31, 1295–1301. [DOI] [PubMed]
- Gursky, O., Badger, J., Li, Y. & Caspar, D. L. (1992). Biophys. J. 63, 1210–1220. [DOI] [PMC free article] [PubMed]
- Gursky, O., Li, Y., Badger, J. & Caspar, D. L. (1992). Biophys. J. 61, 604–611. [DOI] [PMC free article] [PubMed]
- Hölzer, G., Fritsch, M., Deutsch, M., Härtwig, J. & Förster, E. (1997). Phys. Rev. A, 56, 4554–4568.
- Ishikawa, T., Chatake, T., Morimoto, Y., Maeda, M., Kurihara, K., Tanaka, I. & Niimura, N. (2008). Biochem. Biophys. Res. Commun. 376, 32–35. [DOI] [PubMed]
- Ishikawa, T., Chatake, T., Ohnishi, Y., Tanaka, I., Kurihara, K., Kuroki, R. & Niimura, N. (2008). Chem. Phys. 345, 152–158.
- Jiráček, J., Žáková, L., Antolíková, E., Watson, C. J., Turkenburg, J. P., Dodson, G. G. & Brzozowski, A. M. (2010). Proc. Natl Acad. Sci. USA, 107, 1966–1970. [DOI] [PMC free article] [PubMed]
- Kaarsholm, N. C., Ko, H. C. & Dunn, M. F. (1989). Biochemistry, 28, 4427–4435. [DOI] [PubMed]
- Kabsch, W. & Sander, C. (1983). Biopolymers, 22, 2577–2637. [DOI] [PubMed]
- Karavassili, F., Giannopoulou, A. E., Kotsiliti, E., Knight, L., Norrman, M., Schluckebier, G., Drube, L., Fitch, A. N., Wright, J. P. & Margiolaki, I. (2012). Acta Cryst. D68, 1632–1641. [DOI] [PubMed]
- Karavassili, F., Valmas, A., Fili, S., Georgiou, C. & Margiolaki, I. (2017). Biomolecules, 7, 63. [DOI] [PMC free article] [PubMed]
- Khalili, M., Liwo, A., Rakowski, F., Grochowski, P. & Scheraga, H. A. (2005). J. Phys. Chem. B, 109, 13785–13797. [DOI] [PMC free article] [PubMed]
- Kosinová, L., Veverka, V., Novotná, P., Collinsová, M., Urbanová, M., Moody, N. R., Turkenburg, J. P., Jiráček, J., Brzozowski, A. M. & Žáková, L. (2014). Biochemistry, 53, 3392–3402. [DOI] [PMC free article] [PubMed]
- Larson, A. C. & Von Dreele, R. B. (2004). GSAS: General Structure Analysis System. Los Alamos National Laboratory Report LAUR 86-748. Los Alamos National Laboratory, California, USA.
- Laskowski, R. A., MacArthur, M. W., Moss, D. S. & Thornton, J. M. (1993). J. Appl. Cryst. 26, 283–291.
- Lawrence, M. C. (2021). Mol. Metab. 52, 101255. [DOI] [PMC free article] [PubMed]
- Le Bail, A., Duroy, H. & Fourquet, J. L. (1988). Mater. Res. Bull. 23, 447–452.
- Margiolaki, I. (2019). International Tables for Crystallography, Vol. H, 1st online ed., edited by C. J. Gilmore, J. A. Kaduk & H. Schenk, pp. 718–736. Chester: International Union of Crystallography.
- Margiolaki, I., Giannopoulou, A. E., Wright, J. P., Knight, L., Norrman, M., Schluckebier, G., Fitch, A. N. & Von Dreele, R. B. (2013). Acta Cryst. D69, 978–990. [DOI] [PubMed]
- Margiolaki, I. & Wright, J. P. (2008). Acta Cryst. A64, 169–180. [DOI] [PubMed]
- Margiolaki, I., Wright, J. P., Fitch, A. N., Fox, G. C. & Von Dreele, R. B. (2005). Acta Cryst. D61, 423–432. [DOI] [PubMed]
- Margiolaki, I., Wright, J. P., Wilmanns, M., Fitch, A. N. & Pinotsis, N. (2007). J. Am. Chem. Soc. 129, 11865–11871. [DOI] [PubMed]
- Moews, P. C. & Kretsinger, R. H. (1975). J. Mol. Biol. 91, 201–225. [DOI] [PubMed]
- Norrman, M. & Schluckebier, G. (2007). BMC Struct. Biol. 7, 83. [DOI] [PMC free article] [PubMed]
- Papaioannou, A., Kuyucak, S. & Kuncic, Z. (2015). PLoS One, 10, e0144058. [DOI] [PMC free article] [PubMed]
- Pawley, G. S. (1981). J. Appl. Cryst. 14, 357–361.
- Pronk, S., Páll, S., Schulz, R., Larsson, P., Bjelkmar, P., Apostolov, R., Shirts, M. R., Smith, J. C., Kasson, P. M., van der Spoel, D., Hess, B. & Lindahl, E. (2013). Bioinformatics, 29, 845–854. [DOI] [PMC free article] [PubMed]
- Rahuel-Clermont, S., French, C. A., Kaarsholm, N. C. & Dunn, M. F. (1997). Biochemistry, 36, 5837–5845. [DOI] [PubMed]
- Renscheidt, H., Strassburger, W., Glatter, U., Wollmer, A., Dodson, G. G. & Mercola, D. A. (1984). Eur. J. Biochem. 142, 7–14. [DOI] [PubMed]
- Rietveld, H. M. (1969). J. Appl. Cryst. 2, 65–71.
- Roy, M., Brader, M. L., Lee, R. W., Kaarsholm, N. C., Hansen, J. F. & Dunn, M. F. (1989). J. Biol. Chem. 264, 19081–19085. [PubMed]
- Schlichtkrull, J. (1958). Insulin Crystals: Chemical and Biological Studies on Insulin Crystals and Insulin Zinc Suspensions. Copenhagen: Munksgaard.
- Shankland, K., David, W. I. F. & Sivia, D. S. (1997). J. Mater. Chem. 7, 569–572.
- Sieradzan, A. K., Krupa, P., Scheraga, H. A., Liwo, A. & Czaplewski, C. (2015). J. Chem. Theory Comput. 11, 817–831. [DOI] [PMC free article] [PubMed]
- Smith, G. D., Pangborn, W. A. & Blessing, R. H. (2003). Acta Cryst. D59, 474–482. [DOI] [PubMed]
- Spiliopoulou, M., Karavassili, F., Triandafillidis, D.-P., Valmas, A., Fili, S., Kosinas, C., Barlos, K., Barlos, K. K., Morin, M., Reinle-Schmitt, M. L., Gozzo, F. & Margiolaki, I. (2021). Acta Cryst. A77, 186–195. [DOI] [PubMed]
- Spiliopoulou, M., Triandafillidis, D.-P., Valmas, A., Kosinas, C., Fitch, A. N., Von Dreele, R. B. & Margiolaki, I. (2020). Cryst. Growth Des. 20, 8101–8123.
- Spiliopoulou, M., Valmas, A., Triandafillidis, D.-P., Fili, S., Christopoulou, M., Filopoulou, A. J., Piskopou, A., Papadea, P., Fitch, A. N., Beckers, D., Degen, T., Gozzo, F., Morin, M., Reinle-Schmitt, M. L., Karavassili, F., Rosmaraki, E., Chasapis, C. T. & Margiolaki, I. (2021). J. Appl. Cryst. 54, 963–975.
- Spiliopoulou, M., Valmas, A., Triandafillidis, D.-P., Kosinas, C., Fitch, A., Karavassili, F. & Margiolaki, I. (2020). Crystals, 10, 54.
- Storm, M. C. & Dunn, M. F. (1985). Biochemistry, 24, 1749–1756. [DOI] [PubMed]
- Thomas, B. & Wollmer, A. (1989). Biol. Chem. Hoppe Seyler, 370, 1235–1244. [DOI] [PubMed]
- Thompson, P., Cox, D. E. & Hastings, J. B. (1987). J. Appl. Cryst. 20, 79–83.
- Timofeev, V. I., Chuprov-Netochin, R. N., Samigina, V. R., Bezuglov, V. V., Miroshnikov, K. A. & Kuranova, I. P. (2010). Acta Cryst. F66, 259–263. [DOI] [PMC free article] [PubMed]
- Toby, B. H. (2019). International Tables for Crystallography, Vol. H, 1st online ed., edited by C. J. Gilmore, J. A. Kaduk & H. Schenk, pp. 465–472. Chester: International Union of Crystallography.
- Touw, W. G., Baakman, C., Black, J., te Beek, T. A. H., Krieger, E., Joosten, R. P. & Vriend, G. (2015). Nucleic Acids Res. 43, D364–D368. [DOI] [PMC free article] [PubMed]
- Triandafillidis, D.-P., Parthenios, N., Spiliopoulou, M., Valmas, A., Kosinas, C., Gozzo, F., Reinle-Schmitt, M., Beckers, D., Degen, T., Pop, M., Fitch, A. N., Wollenhaupt, J., Weiss, M. S., Karavassili, F. & Margiolaki, I. (2020). Acta Cryst. D76, 1065–1079. [DOI] [PubMed]
- Vaguine, A. A., Richelle, J. & Wodak, S. J. (1999). Acta Cryst. D55, 191–205. [DOI] [PubMed]
- Valmas, A., Magiouf, K., Fili, S., Norrman, M., Schluckebier, G., Beckers, D., Degen, T., Wright, J., Fitch, A., Gozzo, F., Giannopoulou, A. E., Karavassili, F. & Margiolaki, I. (2015). Acta Cryst. D71, 819–828. [DOI] [PubMed]
- Vijayan, M. (2002). Curr. Sci. 83, 1598–1606.
- Von Dreele, R. B. (1999). J. Appl. Cryst. 32, 1084–1089.
- Von Dreele, R. B. (2001). Acta Cryst. D57, 1836–1842. [DOI] [PubMed]
- Von Dreele, R. B. (2005). Acta Cryst. D61, 22–32. [DOI] [PubMed]
- Von Dreele, R. B. (2007). J. Appl. Cryst. 40, 133–143.
- Von Dreele, R. B. (2019). Powder Diffr. 34, S32–S35.
- Von Dreele, R. B., Stephens, P. W., Smith, G. D. & Blessing, R. H. (2000). Acta Cryst. D56, 1549–1553. [DOI] [PubMed]
- Weichenberger, C. X., Afonine, P. V., Kantardjieff, K. & Rupp, B. (2015). Acta Cryst. D71, 1023–1038. [DOI] [PMC free article] [PubMed]
- Whittingham, J. L., Chaudhuri, S., Dodson, E. J., Moody, P. C. E. & Dodson, G. G. (1995). Biochemistry, 34, 15553–15563. [DOI] [PubMed]
- Williams, C. J., Headd, J. J., Moriarty, N. W., Prisant, M. G., Videau, L. L., Deis, L. N., Verma, V., Keedy, D. A., Hintze, B. J., Chen, V. B., Jain, S., Lewis, S. M., Arendall, W. B., Snoeyink, J., Adams, P. D., Lovell, S. C., Richardson, J. S. & Richardson, D. C. (2018). Protein Sci. 27, 293–315. [DOI] [PMC free article] [PubMed]
- Wollmer, A., Rannefeld, B., Johansen, R. R., Hejnaes, K. R., Balschmidt, P. & Hansen, F. B. (1987). Biol. Chem. Hoppe Seyler, 368, 903–912. [DOI] [PubMed]
- Wright, J. P. (2004). Z. Kristrallogr. 219, 791–802.
- Wright, J. P. & Forsyth, J. B. (2000). PRODD: Profile Refinement of Diffraction Data using the Cambridge Crystallographic Subroutine Library (CCSL). Rutherford Appleton Laboratory Report RAL-TR-2000-012. Rutherford Appleton Laboratory, Didcot, United Kingdom.
- Wright, J. P., Markvardsen, A. J. & Margiolaki, I. (2007). Z. Kristallogr. Suppl. 2007, 27–32.
- Yao, Z.-P., Zeng, Z.-H., Li, H.-M., Zhang, Y., Feng, Y.-M. & Wang, D.-C. (1999). Acta Cryst. D55, 1524–1532. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDB reference: polycrystalline cubic human insulin, 7qac
Supplementary Figures and Tables. DOI: 10.1107/S2059798323001328/tz5109sup1.pdf
Data Availability Statement
Atomic coordinates for the polycrystalline cubic human insulin have been deposited in the Protein Data Bank under accession code 7qac. Experimental and calculated diffraction profiles have been deposited in the structure-factors file.