Abstract
INTRODUCTION
Alternatives such as remotely delivered therapy in the home environment or telehealth represent an opportunity to increase overall cardiac rehabilitation (CR) utilization. Implementing alternatives into regular practice is the next step in development; however, the cost aspect is essential for policymakers. Limited economic budgets lead to cost-effectiveness analyses before implementation. They are appropriate in cases where there is evidence that the compared intervention provides a similar health benefit to usual care. This systematic review aimed to compare the cost-effectiveness of exercise-based telehealth CR interventions compared to standard exercise-based CR.
EVIDENCE ACQUISITION
PubMed and Web of Science databases were systematically searched up to August 2022 to identify randomized controlled trials assessing patients undergoing telehealth CR. The intervention was compared to standard CR protocols. The primary intent was to identify the cost-effectiveness. Interventions that met the criteria were home-based telehealth CR interventions delivered by information and communications technology (telephone, computer, internet, or videoconferencing) and included the results of an economic evaluation, comparing interventions in terms of cost-effectiveness, utility, costs and benefits, or cost-minimization analysis. The systematic review protocol was registered in the PROSPERO Registry (CRD42022322531).
EVIDENCE SYNTHESIS
Out of 1525 identified studies, 67 articles were assessed for eligibility, and, at the end of the screening process, 12 studies were included in the present systematic review. Most studies (92%) included in this systematic review found strong evidence that exercise-based telehealth CR is cost-effective. Compared to CBCR, there were no major differences, except for three studies evaluating a significant difference in average cost per patient and intervention costs in favor of telehealth CR.
CONCLUSIONS
Telehealth CR based on exercise is as cost-effective as CBCR interventions. Funding telehealth CR by third-party payers may promote patient participation to increase overall CR utilization. High-quality research is needed to identify the most cost-effective design.
Key words: Telemedicine, Cardiac rehabilitation, Costs and cost analysis, Exercise
Introduction
The incidence of cardiovascular diseases is increasing on a European scale due to the aging of the population.1 After a cardiac event, cardiac rehabilitation (CR) is recommended to patients as part of secondary prevention, an intervention usually based on exercise and lifestyle changes.2, 3 It has been confirmed that exercise-based CR interventions reduce morbidity and mortality and positively affect overall well-being and quality of life.4, 5 In Europe, CR is available in 91% of countries, and approximately 655,000 patients start intervention annually.6 However, few eligible patients participate in these programs. The most common barriers are psychosocial, transactional, or financial.7 Alternatives such as remotely delivered therapy in the home environment or telehealth represent an opportunity to increase overall CR utilization. Especially during the covid-19 pandemic, the remote-guided approach was even more highlighted, as it was possible to provide CR in light of quarantine and public health restrictions.8, 9 Remotely delivered CR using telehealth represents the provision of CR in a home-based setting via modern Information and Communications Technology (ICT). For example, smartphone applications, web-based exercise platforms, heart rate (HR) sensors, or virtual reality. In addition, this approach enables telemonitoring and telesupervision to monitor safety and provide expert guidance.10, 11 A recent meta-analysis of telehealth CR studies by Antoniou et al. demonstrated a similar effect in increasing cardiorespiratory fitness and quality of life as standard CR in a hospital center (CBCR).12 Moreover, patients reported the high acceptability of the telehealth approach, more lasting effects, and high safety of home-based exercise.13, 14 Therefore, these alternatives supplement CBCR and could represent a strategy to increase participation.15, 16 Implementing alternatives into regular practice is the next step in development; however, the cost aspect is essential for policymakers. Limited economic budgets lead to cost-effectiveness analyses, i.e., the ratio of the health benefit of the intervention to the costs, before implementation, and are appropriate in cases where there is evidence that the compared intervention provides a similar health benefit to usual care. The resulting differences in economic benefits are usually expressed in monetary units. An earlier systematic review of economic evaluations of CR interventions demonstrated cost-effectivity, especially with exercise as a component.17 Telehealth CR analysis of 4 trials provided evidence ranging from dominant to $ 588,734 per QALY and was cost-effective in exercise in all relevant trials, whereas the results are subject to uncertainty. Inconclusive evidence that home-based alternatives to CR are not only less expensive but also similarly effective to hospital programs was provided by a review by Lee et al.18 This systematic review aimed to compare the cost-effectiveness of exercise-based telehealth CR interventions compared to CBCR. Our study provides a new perspective and recent evidence to the literature, which has increased significantly in the last five years,19 to enable a more accurate assessment of the cost-effectiveness of telehealth CR. Furthermore, the review critically analyzes the evidence,20-31 identifies research gaps, and provides a perspective for future research.
Evidence acquisition
A systematic literature search and review were conducted to identify economic evaluations of home-based telehealth CR interventions. This systematic review followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Guidelines. The systematic review protocol was registered in the PROSPERO registry (CRD42022322531).
Eligibility criteria
The Populations, Interventions, Comparisons, Outcomes, and Study designs (PICOS) framework was used to inform eligibility criteria. The inclusion criteria were: 1) P – people with a medical diagnosis of CVD; 2) I – intervention group received home-based telehealth CR intervention delivered by ICT (telephone, computer, internet, or videoconferencing) including the use of telemonitoring devices, and telephone calls; 3) C – active comparator, in our case, exercise-based CBCR; 4) O – results measured and reported cost-effectiveness; and 5) S – randomized controlled trial and English language. Only randomized controlled trial studies were included to generate the best evidence. The exclusion criteria were as follows: 1) quasi-experimental, qualitative, or case studies; 2) conference abstracts; and 3) unavailable full text even after contacting the authors.
Search methods
The search was structured to identify the cost-effectiveness of exercise-based telehealth CR interventions published since 2000 in English. An electronic literature search was conducted in August 2022 through the PubMed database and the Web of Science metasearch engine. Search terms included CR terminology and economic evaluation terms based on specific search filters for economic evaluation articles.32 The selection process involved a keyword search that is summarized in Supplementary Digital Material 1 (Supplementary Table I). After the initial literature search, relevant articles were selected according to keywords. Articles were then manually assessed by two independent reviewers (LB and KF) based on title and abstract. After the first round, the relevance of articles based on the inclusion criteria was assessed using full text; differences of opinion were discussed and resolved with a third reviewer (FD).
Data extraction and synthesis
Data extraction included intervention methods and outcomes. Interventions were critically analyzed using extracted data from reviewers (LB and KF), with final results subsequently discussed with a third reviewer (FD). Due to the heterogeneity between the included interventions, quantitative data synthesis was impossible, and a narrative approach was used to summarize the findings. The synthesis of systematic reviews of economic evaluations commonly takes a narrative approach, whereas a meta-analysis is a common step for reviews of clinical evidence.33 Cost values in included studies were converted to the single currency Euro (¤) according to the Current Exchange Index (September 21, 2022) for better comparison. To convert to USA ($) and Pounds (£), multiply by 0.98 and 1.16 (e.g., 1000 ¤ is equivalent to 980 USA $; 1000 USA $ is equivalent to 1020 ¤; 1000 ¤ is equivalent to 861 £; 1000 £ is equivalent to 1162 ¤).
Quality of reporting
Economic evaluation Quality of reporting for CTR and CBCR interventions was conducted using the Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022). These standards include an updated checklist of 28 items.34 The CHEERS 2022 checklist aims to ensure that health economic evaluations are identifiable, interpretable, and useful for decision-making. The 28 item-checklist is divided into seven main sections: 1) title (one item); 2) abstract (one item); 3) introduction (one item); 4) methods (eighteen items); 5) results (four items); 6) discussion (one item); and 7) other relevant information (two items). Several studies in the health economic evaluation in the rehabilitation field have used this checklist.35, 36 The economic Quality of reporting of included studies was conducted independently by two reviewers (KF and FD). Disagreements were discussed with a third reviewer (LB) until a consensus was reached. Consistency of item ratings was ensured by following the CHEERS 2022 Checklist Guidelines. Individual items were rated as “yes,” “no,” “partially,” “unclear,” or “not applicable.” Finally, the number of studies that received a yes rating for the overall Quality of Reporting evaluation was counted. A score was given for each applicable item, and a total score (out of 28) was obtained for each study. The higher the score, the better the quality of study reporting. The total score was obtained, ranging from 0 to 28 points. The studies were classified according to the average score: high-quality as 22 points or more (80%), good-quality as 12 to 21 points, and low-quality as 11 points or less (40%). High-quality studies were defined as highly relevant, reproducible, and excellent methodically described results reports. Good-quality studies were defined as moderately clinically relevant, with limitations in reporting results. The low-quality studies reported substantial relevance limitations and methods with low reproducibility. This individual approach was used since the CHEERS cut-off scales were not yet recommended.
Evidence synthesis
A total of 1525 records were identified in the database and metasearch engine search (Figure 1). Screening of titles and abstracts showed that 1458 publications did not meet the inclusion criteria. The remaining 67 publications were subjected to a detailed full-text examination, from which 55 were excluded. Thus, there were twelve eligible articles for inclusion in the qualitative synthesis of this systematic review.20-31 An overview of study characteristics is presented in Table I.20-31
Figure 1.
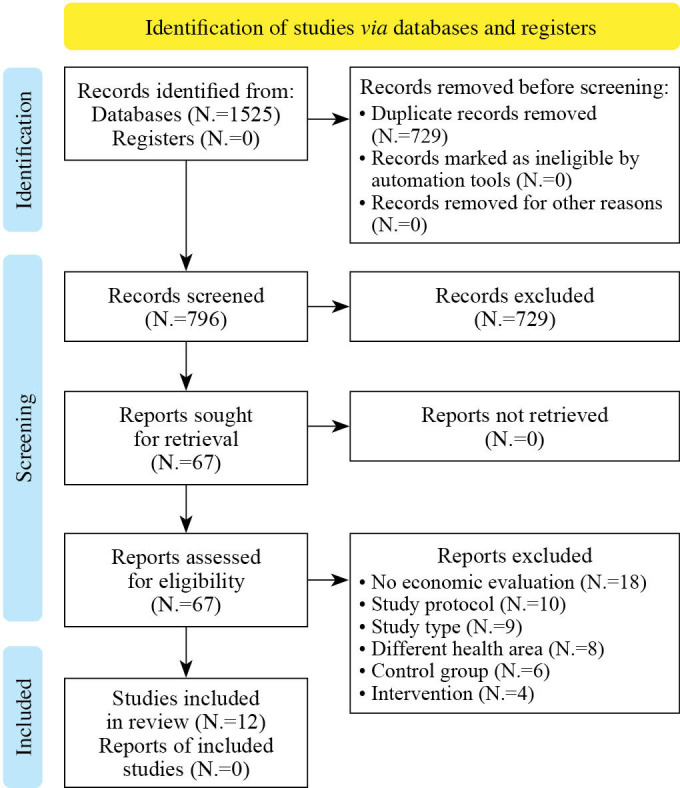
—Flow diagram detailing the search strategy.
Table I. —Study characteristics.
| Study | Population | Intervention | QALY | Costs (¤) | ICER per QALY (¤) | Time horizon (weeks) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| TCR | CBCR | Difference | TCR | CBCR | Difference | |||||
| Brouwers et al.20 | CAD (N.=300) | CTR (HR monitor, telesupervision, ICT platform, 2-5 exercise/week, 20–60 min) | 0.841 | 0.844 | -0.004 | 4787 | 5507 | -720 | 0 | 36 |
| Carlson et al.21 | MI; CABG (N.=80) | Hybrid CR; 0-4weeks CBCR + 5-25 weeks HBCR (3 exercise/week, 30-40 min) | NR | NR | NR | 1549 | 2396 | -847 | NR | 24 |
| Frederix et al.22 | CAD; CHF (N.=140) | Internet-based CTR (accelerometer, telemonitoring, coaching, >2 exercise/week, 45-60 min) | 0.07 | -0.15 | 0.22 | 3262 | 4140 | -878 | -3993 | 12 |
| Frederix et al.23 | CAD; CHF (N.=126) | Comprehensive CTR (accelerometer, telemonitoring, coaching, >2 exercise/week, 45-60 min) | 0.39 | 0.36 | 0.026 | 2155 | 2720 | -565 | –21707 | 24 |
| Hwang et al.24 | CHF (N.=53) | CTR (online videoconferencing platform, telesupervision, 2 exercise/week, 60min) | 0.36 | 0.36 | 0 | 2372 | 3994 | -1622 | -4240 | 12 |
| Kidholm et al.25 | CAD; MI; CHF (N.=151) | CTR (ICT platform, digital exercise diary, one personal supervision, 2 exercise/week) |
0.089 | 0.085 | 0.004 | 5724 | 4057 | 1667 | 483608 | 12 |
| Kraal et al.26 | I-II CVR (N.=90) | HBCR (HR monitor, web application, telemonitoring, telefeedback, >2 exercise/week, 45-60 min) | 0.77 | 0.78 | 0.01 | 2855 | 2419 | -436 | NR | 12 |
| Lima et al.27 | MI; CABG (N.=49) | HBCR (HR monitor, personal diary, telesupervision, 5 exercise/week, 50-60 min) | NR | NR | NR | 61 | 138 | -77 | NR | 12 |
| Maddison et al.28 | IHD (N.=171) | mobile phone CR (web-video and text messages, >5 exercise/week, 30 min) | NR | NR | NR | NR | NR | NR | 15247 | 12 |
| Maddison et al.29 | CAD (N.=162) | real-time CTR (web platform, smartphone, HR monitor, remote monitoring and coaching) | NR | NR | -0.03 | 2894 | 5610 | -2716 | NR | 12 |
| Southard et al.30 | CAD (N.=104) | Internet-based CR (web-education modules, online chat discussions, telefeedback) | NR | NR | NR | 462 | 1446 | -990 | NR | 24 |
| Whittaker et al.31 | MI (N.=120) | HBCR (mobile phone, Wellness Diary and web portal, daily text messaging, exercise most days/week, 30 min) | NR | NR | NR | 1747 | 2290 | -543 | NR | 24 |
CAD: coronary artery disease; MI: myocardial infarction; CHF: chronic heart failure; CR: cardiac rehabilitation; CTR: cardiac telerehabilitation; HBCR: home-based cardiac telerehabilitation; HR: heart rate monitor; CBCR: center-based cardiac rehabilitation; ICT: information and communications technology; QALY: quality-adjusted life year; ICER: Incremental Cost-Effectiveness Ratio; TCR: telehealth cardiac rehabilitation.
Included studies
Two studies from samples were conducted in the USA,21, 30 New Zealand,28, 29 Australia,24, 31 Belgium,22, 23 and the Netherlands;20, 26 one each was conducted in Brazil27 and Denmark.25 Most studies (10/12, 83%) were published in the last ten years, and six (50%) were published in the last five years. The number of participants in the included studies ranged from 53 to 300 participants. The average age was 60.6 years (range: 55.6-67.0), and the representation of men was, on average, 82.7% (range: 75.0-88.9). Most studies included a wide range of age groups. A total of 1,546 participants were included in the systematic review. Participants in the included studies survived different types of CVD. As expected, Table I20-31 shows significant differences in study populations, as CR is recommended for multiple groups of patients. Four studies only included participants with coronary artery disease (CAD).20, 28-30 Another two studies included a combination of participants after myocardial infarction (MI) or coronary artery bypass graft (CABG),21, 27 and two studies included a combination of CAD and chronic heart failure (CHF).22, 23 The remaining studies included participants with CHF,24 cardiovascular risk stage I-II,26 MI, or a combination of participants with cardiac disease,25 such as CAD or MI or CHF or CABG. Differences in the characteristics of CVD between studies did not allow a more precise distinction of subgroups of the population with CVD. Therefore, the study set is considered as a whole.
Intervention
All studies compared telehealth CR with an active control group of CBCR or community-based CR. The design of the telehealth CR intervention varied considerably across studies (Table I).20-31 The intervention period ranged from 3 to 9 months; most often (58%), the 3-month CR model was used. All studies included various forms of remote monitoring and telehealth CR counseling. However, comparing exercise prescriptions is complicated because of the diversity. Ten studies (83%) reported a specific exercise prescription.29, 30 The prescribed weekly exercises ranged from 2 to “exercise most days a week.” Most often, two or more exercises per week20-26 were recommended. Session durations were prescribed in nine of the twelve studies; the time range of the exercise session was 20-60 min. Two studies included real-time exercise monitoring.24, 29 Other studies used telemonitoring postexercise. An ICT platform was used for telemonitoring20, 22, 26, 28-31 or a personal exercise diary combined with telesupervision.27, 30 Patients used HR monitor,20, 21, 26, 27, 29 accelerometer,22, 23, 26 web-application28, 29, 31 to monitor exercise intensity. Telesupervision was provided through text messages,22, 23, 28, 30, 31 telephone calls,20, 26, 27 audiovisual calls,24, 29 or personal visits.21, 25 In studies, monitoring and teleconsultation are mostly performed by physiotherapists,20, 24, 26, 27 community care teams,21, 28, 29, 31 nurses,25, 30 or semi-automatic web systems.22, 23 Teleconsultations included monitoring adherence to the exercise intervention through training diaries, feedback, motivation for the next period, and identifying barriers to exercise or adverse events.
Control groups
Control group participants were given instructions in 8/12 (67%) trials. Other studies included an active control group under supervision in a community-based CR program28, 31 or CBCR supervision without a detailed description of the exercise intervention or recommendations.25, 30 Most studies provided a CR intervention according to guidelines for the control group.21-27, 29 Five studies (42%) designed a similar exercise prescription regarding the duration and frequency of sessions.22, 23, 26, 27, 29 One study described the prescription as individually tailored, and intensity sessions varied among patients20 or only as a comprehensive CR program.21
Characteristics of the methods
Twelve trials were included.20-31 The blinding method was used in 7/12 (58%) studies. The primary outcome investigators24, 26-29 or all investigators were blinded.22, 23 Four studies did not include the method of blinding.25, 28, 30, 31 Blinding is a crucial method to limit the distortion of study results. However, by the nature of training interventions (e.g., exercises, devices, manual therapy), blinding for therapists and patients in Physical Therapy Trials may be challenging.37
Characteristics of study outcomes
The primary outcome in most studies was the effect on functional capacity.22,23,26,28,29. Four studies had cost-effectiveness20, 24, 25, 31 or adherence27 as the primary outcome. Secondary outcomes in the studies were quality of life,20, 22-31 cost-effectiveness,20, 24, 25, 31 cardiovascular rehospitalizations,23-26 blood sampling,21-23, 27, 28, 30 blood pressure,21-23, 27-30 functional capacity,21, 27, 30, 31 physical activity22, 23, 26, 29 and anxiety and depression.26
Cost methods
The description of cost-effectiveness was made from a societal and patient perspective. This included the costs of the intervention and the costs of healthcare resources extracted from the studies. Direct health care costs were assessed, i.e., costs of using health care or costs directly related to the delivery of the intervention. In more detail, healthcare costs were categorized as intervention 12/12 (100%),20-31 hospitalization 8/12 (67%),20, 22, 23, 25, 26, 29-31 outpatient 8/12 (67%),20, 22-26, 29, 30 diagnostics 3/12 (25%),22, 23, 29 community care 2/12 (17%),25, 31 medication 2/12 (17%).20, 26, 29 Other costs included presenteeism 2/12 (17%)20.26, absenteeism at work 2/12 (17%)20.26, travel costs 2/12 (17%)25.31, and unpaid labor 1/12 (8%).26 Sensitivity analysis was performed in 7/12 (58%) studies.20, 22-26, 29 A cost-utility analysis with quality-adjusted life-years (QALYs) as an outcome measure was performed in 8/12 (67%) studies.20, 22-26, 29 Moreover, cost-utility data were compared by calculating of the incremental cost-effectiveness ratio (ICER) in 8/12 (67%) studies.20, 22-26, 29 The incremental effectiveness represents the change in total average QALY between the telehealth and CBCR groups. ICER’s plane was generated by bootstrapping the point estimates of the costs and utilities. Data for generating QALYs included health-related quality of life questionnaire surveys. Therefore, the most frequently used EuroQol Five-Dimensional (EQ-5D) Questionnaire 6/12 (50%),20, 22-24, 29, 31 Short Form 36 Health Survey (SF-36) 4/12 (33%),25-28 or one study used the Dartmouth COOP Charts Questionnaire 1/12 (8%).30 The EQ-5D and SF-36 questionnaires are considered valid and reliable instruments for surveying health-related quality of life in patients with CVD.38, 39
Study results
Costs in providing the intervention
Costs varied between the studies included in the review and are shown in Table I.20-31 The majority of studies (11/12, 92%) comparing telehealth CR and exercise-based CBCR reported net costs of the intervention and were associated in 10/12 (83%) with lower costs for providing telehealth CR (range -2716 to -77 ¤).20-24, 26, 27, 29-31 Three also stated that the average cost per patient was significantly lower (-565 ¤, P=0.01, -1622 ¤, P=0.001, respectively -847 ¤, P=0.05).21, 22, 24 Another study reported significantly lower costs for providing the intervention with CBCR (-1023 ¤).25 However, the authors judged that a higher number of patient scenarios might result in reduced costs of the devices used in the intervention and make the telehealth program more cost-effective over the long term. One study reported only intervention costs without comparison.28
Costs in unplanned healthcare services utilization
In addition to intervention costs, one study estimated hospital medication costs, which revealed a significant difference (-159 ¤, P=0.02) in favor of telehealth CR.29
The incremental Cost-Effectiveness Ratio
ICER estimation was performed in half of the cases,20, 22-25, 28 and in 5/6 (83%), the telehealth CR intervention was considered cost-effective (from -21.707 ¤ per QALY to 15.247 ¤ per QALY). The probability of cost-effectiveness of telehealth CR was explicitly presented in three studies and was similarly assessed as 69% and 86%, 72 and 90%, and 97% and 75%, respectively.20, 26, 29 Three other studies rated the probability of cost-effectivity telehealth CR as highly probable, whereas they did not provide a rate.22-24
Cost in social aspects of CR participation
A comparison of costs per patient from a societal perspective consisting of absenteeism from paid and unpaid work was demonstrated by two studies. A similar result in favor of patients in telehealth CR was recorded, in savings, from Absence from work (-2691 and -607¤).20, 26 Patient travel costs were analyzed in two studies for telehealth CR were substantially less than for CBCR attendance (82 vs. 408 ¤), respectively. One study revealed significantly lower extra costs (43 ¤ in telehealth CR vs. 68 ¤ in CBCR, P=0.03).25, 31
Quality of reporting according to CHEERS 2022 Guidelines
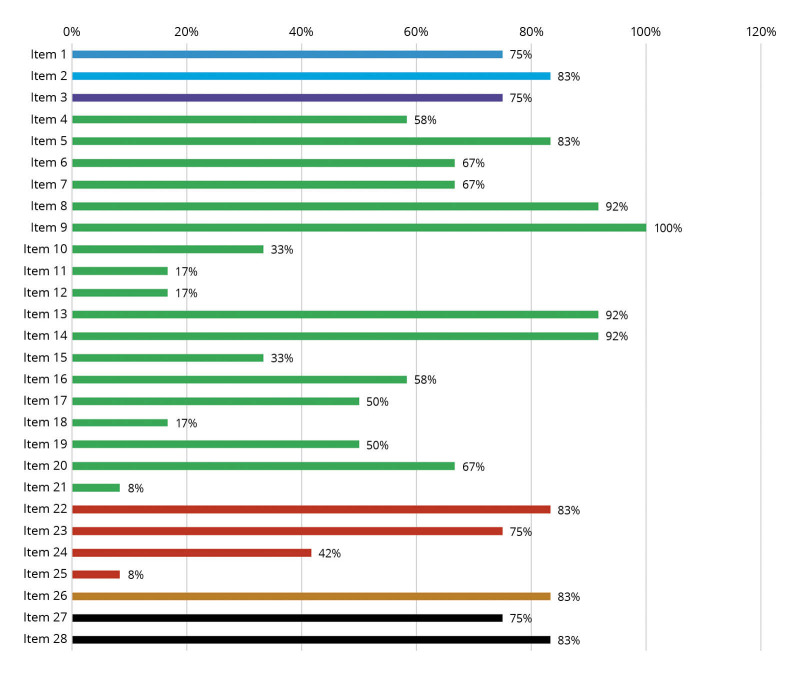
The quality of reporting economic evaluations (Supplementary Digital Material 2: Supplementary Table II) showed inconsistent results. Overall quality was “good,” with an average score of 16.8 points (59.8%) ranging from 5 to 24 points. Two studies met the requirements of the high-quality category.20, 24 Six were, on average,22-26, 28, 29 and four studies were in the low-quality category.21, 27, 30, 31 There were several items, particularly in the methodology section, that showed low reporting in the selected studies, especially the item discount rate (17%), selection of outcomes (17%), measurement of outcomes (33%), characterizing heterogeneity (17%), approach to engagement with patients and others affected by the study (8%). Other items from the results section that were not frequently reported were the effect of uncertainty (42%) and the effect of engagement with patients and others affected by the study (8%). On the other hand, some of the items that were excellently reported in the included studies were the inclusion of abstract (83%), followed by the item study population (83%), perspective (92%), time horizon (100%), valuation of outcomes (92%), measurement and valuation of resources and costs (92%), study parameters (83%), study findings, limitations, generalizability, and current knowledge (83%), and item conflicts of interest (83%). The percentage of studies recorded for each item on the CHEERS 2022 Checklist is shown in Figure 2.
Figure 2.
—Quality assessment of included studies. The figure shows the quality of reporting of studies through the CHEERS 2022 Checklist. The vertical axis shows each item on the checklist from 1 to 28. The horizontal axis shows the percentage of studies that met each item. Shades of gray (colors in the online version) indicate different sections of the checklist.
Discussion
This systematic review assessed the evidence evaluating the cost-effectiveness of telehealth CR compared with CBCR. It is the first systematic review of currently available literature that evaluates health-related costs in telehealth CR interventions. Half of the included studies are from the last five years, showing the early nature of this approach. However, the results support actions to implement into regular practice and provide policymakers with an up-to-date evidence base of the potential cost savings that can be achieved. This work suggests similar clinical outcomes of telehealth CR and CBCR interventions for physiological and psychosocial parameters. In this regard, some studies have reported further improvements in quality-of-life parameters and promoting lifestyle-related CR interventions based on eHealth.40-44 Most studies included in this systematic review found strong evidence that exercise-based telehealth CR is cost-effective. Compared to CBCR, there were no major differences, except for three studies evaluating a significant difference in average cost per patient and intervention costs in favor of telehealth CR. The evidence provides unclear certainty, whereas it shows a more robust view of alternatives compared to recent reviews.17, 18, 45 Further studies will be needed, especially in real-world settings, to confirm more robust significance. The other result of this systematic review is that the examination of the quality of economic reporting was variable. The results of this study correspond with the current state of the art of economic evaluation in the rehabilitation field. A systematic review by Flemming et al. noted inconsistent reporting, especially a mean score of 17.5 (72.9%), indicating good-quality reporting across several rehabilitation domains.35 Indeed, these results highlight the need to optimize the economic reporting of telehealth CR to increase transparency and accuracy for decision-making. Poor quality reporting can create concerns when determining whether reported results are valid. In order to expand the CR field with alternatives, there is a need to produce high-quality research that proves cost-effectiveness. Telehealth CRs are considered effective and practical interventions with the potential for greater self-responsibility and self-efficacy than CBCRs.46-51 On the other hand, the disadvantage of telehealth CR is the lack of social contact and fewer face-to-face interactions associated with the group approach of CBCR.8, 16, 52 The expanding CVD base represented by different patient subgroups requires the inclusion of diverse CR models to optimize health outcomes. Elderly patients have been found to prefer home-based interventions over centrally-based ones.53 Logistical barriers are a common reason for non-participation; telehealth interventions may increase uptake.54 The chronic nature of CVD usually requires readmission or additional hospital treatment.55, 56 Indeed, reducing the number of readmissions is a necessary result of cost-saving measures in providing curative-preventive care. It is also a critical outcome for patients and their families because of the potential additional stress burden associated with CVD.57, 58 Moreover, remotely guided delivery strategies may be a valuable option for patients from rural areas to prevent recurrent diseases associated with further rehospitalization. The telehealth CR interventions described in this systematic review suggest potential benefits, including a lower economic burden for preventive healthcare provision and increased access to CR. The expanded access and increase in the overall use of CR can lead to economic gains and cost savings for CVD treatment. Implementation of telehealth CR may also address patient logistical barriers or issues with lower availability of preventive healthcare services in this community. Furthermore, exercise-based telehealth CR interventions using telemonitoring and telesupervision can be effective on a broader scale. Since most patients have internet access and ICT at home,59 a clinical or Physical and Rehabilitation Medicine specialist can relatively easily use it in their clinical practice at a relatively low cost.60, 61 Especially in the current technological boom, the price of ICT is expected to decrease, which should facilitate development on a larger scale in the future.62-64
Limitations of the study
One thing to note is the heterogeneity among included telehealth CR interventions. The studies differed in design, specifically in the duration and frequency of the exercise intervention, the telehealth ICT used, or the individual price costs and their analyses. This fact limits the overall generalization of the results. Furthermore, the difference in preventive components in telehealth CR models was found. Some studies were based on exercise alone compared with other studies providing a comprehensive prevention approach.3 In addition, the cost-effectiveness of telehealth interventions may differ significantly across patient groups.65 Our results may be influenced by the diversity of characteristics between the patient groups. Specifically, diverse groups of patients after CVD were included. Therefore, interventions should be targeted at a selected subgroup in future studies. This review points to a lack of evidence for low-middle-income countries, despite the significant rates of global CVD mortality in these countries.66 The research and practice in telehealth CR have recently experienced significant expansion.19 This exponential growth is also evident in the recent published study protocols.67-73 Further evidence will be essential to determine which telehealth forms of nutritional and/or psychological therapy might be cost-effective. ICT can provide a more individualized approach focused on several preventive components. Therefore, providing telehealth CR should be more than remote telemonitoring exercises. This would make the telehealth approach suitable for integration among other subgroups of patients with CVD or other chronic diseases in which the health benefit of providing physical exercise was found.74-76 Given the recent calls for comprehensive integration of the essential preventive components of CR, this should support the expected preventive health outcomes.77, 78
Conclusions
Telehealth CR based on exercise is as cost-effective as CBCR interventions. Funding telehealth CR by third-party payers may promote patient participation to increase overall CR utilization. High-quality research is needed to identify the most cost-effective design.
Supplementary Digital Material 1
Supplementary Table I
Search strategy.
Supplementary Digital Material 2
Supplementary Table II
Quality assessment of included economic evaluations.
References
- 1.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group . Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J Am Coll Cardiol 2020;76:2982–3021. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33309175&dopt=Abstract 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winnige P, Vysoky R, Dosbaba F, Batalik L. Cardiac rehabilitation and its essential role in the secondary prevention of cardiovascular diseases. World J Clin Cases 2021;9:1761–84. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33748226&dopt=Abstract 10.12998/wjcc.v9.i8.1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandesara PB, Lambert CT, Gordon NF, Fletcher GF, Franklin BA, Wenger NK, et al. Cardiac rehabilitation and risk reduction: time to “rebrand and reinvigorate”. J Am Coll Cardiol 2015;65:389–95. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25634839&dopt=Abstract 10.1016/j.jacc.2014.10.059 [DOI] [PubMed] [Google Scholar]
- 4.Graham HL, Lac A, Lee H, Benton MJ. Predicting Long-Term Mortality, Morbidity, and Survival Outcomes Following a Cardiac Event: A Cardiac Rehabilitation Study. Rehabil Process Outcome 2019;8:1179572719827610. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34497458&dopt=Abstract 10.1177/1179572719827610 [DOI] [PMC free article] [PubMed]
- 5.Shepherd CW, While AE. Cardiac rehabilitation and quality of life: a systematic review. Int J Nurs Stud 2012;49:755–71. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22197653&dopt=Abstract 10.1016/j.ijnurstu.2011.11.019 [DOI] [PubMed] [Google Scholar]
- 6.Abreu A, Pesah E, Supervia M, Turk-Adawi K, Bjarnason-Wehrens B, Lopez-Jimenez F, et al. Cardiac rehabilitation availability and delivery in Europe: How does it differ by region and compare with other high-income countries?: Endorsed by the European Association of Preventive Cardiology. Eur J Prev Cardiol 2019;26:1131–46. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30782007&dopt=Abstract 10.1177/2047487319827453 [DOI] [PubMed] [Google Scholar]
- 7.Dunlay SM, Witt BJ, Allison TG, Hayes SN, Weston SA, Koepsell E, et al. Barriers to participation in cardiac rehabilitation. Am Heart J 2009;158:852–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19853708&dopt=Abstract https://doi.org/ 10.1016/j.ahj.2009.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stefanakis M, Batalik L, Papathanasiou J, Dipla L, Antoniou V, Pepera G. Exercise-based cardiac rehabilitation programs in the era of COVID-19: a critical review. Rev Cardiovasc Med 2021;22:1143–55. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34957758&dopt=Abstract 10.31083/j.rcm2204123 [DOI] [PubMed] [Google Scholar]
- 9.Besnier F, Gayda M, Nigam A, Juneau M, Bherer L. Cardiac Rehabilitation During Quarantine in COVID-19 Pandemic: Challenges for Center-Based Programs. Arch Phys Med Rehabil 2020;101:1835–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32599060&dopt=Abstract 10.1016/j.apmr.2020.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batalik L, Filakova K, Batalikova K, Dosbaba F. Remotely monitored telerehabilitation for cardiac patients: A review of the current situation. World J Clin Cases 2020;8:1818–31. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32518772&dopt=Abstract 10.12998/wjcc.v8.i10.1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su JJ, Yu DS, Paguio JT. Effect of eHealth cardiac rehabilitation on health outcomes of coronary heart disease patients: A systematic review and meta-analysis. J Adv Nurs 2020;76:754–72. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31769527&dopt=Abstract 10.1111/jan.14272 [DOI] [PubMed] [Google Scholar]
- 12.Antoniou V, Davos CH, Kapreli E, Batalik L, Panagiotakos DB, Pepera G. Effectiveness of Home-Based Cardiac Rehabilitation, Using Wearable Sensors, as a Multicomponent, Cutting-Edge Intervention: A Systematic Review and Meta-Analysis. J Clin Med 2022;11:3772. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35807055&dopt=Abstract 10.3390/jcm11133772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stefanakis M, Batalik L, Antoniou V, Pepera G. Safety of home-based cardiac rehabilitation: A systematic review. Heart Lung 2022;55:117–26. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35533492&dopt=Abstract 10.1016/j.hrtlng.2022.04.016 [DOI] [PubMed] [Google Scholar]
- 14.Batalik L, Dosbaba F, Hartman M, Konecny V, Batalikova K, Spinar J. Long-term exercise effects after cardiac telerehabilitation in patients with coronary artery disease: 1-year follow-up results of the randomized study. Eur J Phys Rehabil Med 2021;57:807–14. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33619944&dopt=Abstract 10.23736/S1973-9087.21.06653-3 [DOI] [PubMed] [Google Scholar]
- 15.Ades PA, Keteyian SJ, Wright JS, Hamm LF, Lui K, Newlin K, et al. Increasing Cardiac Rehabilitation Participation From 20% to 70%: A Road Map From the Million Hearts Cardiac Rehabilitation Collaborative. Mayo Clin Proc 2017;92:234–42. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27855953&dopt=Abstract 10.1016/j.mayocp.2016.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scherrenberg M, Wilhelm M, Hansen D, Völler H, Cornelissen V, Frederix I, et al. The future is now: a call for action for cardiac telerehabilitation in the COVID-19 pandemic from the secondary prevention and rehabilitation section of the European Association of Preventive Cardiology. Eur J Prev Cardiol 2020;2047487320939671. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32615796&dopt=Abstract [DOI] [PMC free article] [PubMed]
- 17.Shields GE, Wells A, Doherty P, Heagerty A, Buck D, Davies LM. Cost-effectiveness of cardiac rehabilitation: a systematic review. Heart 2018;104:1403–10. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29654096&dopt=Abstract 10.1136/heartjnl-2017-312809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee AJ, Strickler GK, Shepard DS. The economics of cardiac rehabilitation and lifestyle modification: a review of literature. J Cardiopulm Rehabil Prev 2007;27:135–42. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17558193&dopt=Abstract 10.1097/01.HCR.0000270694.94010.8b [DOI] [PubMed] [Google Scholar]
- 19.Batalik L, Pepera G, Su JJ. Cardiac telerehabilitation improves lipid profile in the long term: insights and implications. Int J Cardiol 2022;367:117–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=36055472&dopt=Abstract 10.1016/j.ijcard.2022.08.055 [DOI] [PubMed] [Google Scholar]
- 20.Brouwers RW, van der Poort EK, Kemps HM, van den Akker-van Marle ME, Kraal JJ. Cost-effectiveness of Cardiac Telerehabilitation With Relapse Prevention for the Treatment of Patients With Coronary Artery Disease in the Netherlands. JAMA Netw Open 2021;4:e2136652. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34854907&dopt=Abstract 10.1001/jamanetworkopen.2021.36652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlson JJ, Johnson JA, Franklin BA, VanderLaan RL. Program participation, exercise adherence, cardiovascular outcomes, and program cost of traditional versus modified cardiac rehabilitation. Am J Cardiol 2000;86:17–23. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10867086&dopt=Abstract 10.1016/S0002-9149(00)00822-5 [DOI] [PubMed] [Google Scholar]
- 22.Frederix I, Hansen D, Coninx K, Vandervoort P, Vandijck D, Hens N, et al. Effect of comprehensive cardiac telerehabilitation on one-year cardiovascular rehospitalization rate, medical costs and quality of life: A cost-effectiveness analysis. Eur J Prev Cardiol 2016;23:674–82. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26289723&dopt=Abstract 10.1177/2047487315602257 [DOI] [PubMed] [Google Scholar]
- 23.Frederix I, Solmi F, Piepoli MF, Dendale P. Cardiac telerehabilitation: A novel cost-efficient care delivery strategy that can induce long-term health benefits. Eur J Prev Cardiol 2017;24:1708–17. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28925749&dopt=Abstract 10.1177/2047487317732274 [DOI] [PubMed] [Google Scholar]
- 24.Hwang R, Morris NR, Mandrusiak A, Bruning J, Peters R, Korczyk D, et al. Cost-Utility Analysis of Home-Based Telerehabilitation Compared With Centre-Based Rehabilitation in Patients With Heart Failure. Heart Lung Circ 2019;28:1795–803. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30528811&dopt=Abstract 10.1016/j.hlc.2018.11.010 [DOI] [PubMed] [Google Scholar]
- 25.Kidholm K, Rasmussen MK, Andreasen JJ, Hansen J, Nielsen G, Spindler H, et al. Cost-Utility Analysis of a Cardiac Telerehabilitation Program: The Teledialog Project. Telemed J E Health 2016;22:553–63. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26713491&dopt=Abstract 10.1089/tmj.2015.0194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kraal JJ, Van den Akker-Van Marle ME, Abu-Hanna A, Stut W, Peek N, Kemps HM. Clinical and cost-effectiveness of home-based cardiac rehabilitation compared to conventional, centre-based cardiac rehabilitation: results of the FIT@Home study. Eur J Prev Cardiol 2017;24:1260–73. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28534417&dopt=Abstract 10.1177/2047487317710803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DE Lima AP, Pereira DG, Nascimento IO, Martins TH, Oliveira AC, Nogueira TS, et al. Cardiac telerehabilitation in a middle-income country: analysis of adherence, effectiveness and cost through a randomized clinical trial. Eur J Phys Rehabil Med 2022;58:598–605. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35634888&dopt=Abstract 10.23736/S1973-9087.22.07340-3 [DOI] [PMC free article] [PubMed]
- 28.Maddison R, Pfaeffli L, Whittaker R, Stewart R, Kerr A, Jiang Y, et al. A mobile phone intervention increases physical activity in people with cardiovascular disease: results from the HEART randomized controlled trial. Eur J Prev Cardiol 2015;22:701–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24817694&dopt=Abstract 10.1177/2047487314535076 [DOI] [PubMed] [Google Scholar]
- 29.Maddison R, Rawstorn JC, Stewart RA, Benatar J, Whittaker R, Rolleston A, et al. Effects and costs of real-time cardiac telerehabilitation: randomised controlled non-inferiority trial. Heart 2019;105:122–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30150328&dopt=Abstract 10.1136/heartjnl-2018-313189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Southard BH, Southard DR, Nuckolls J. Clinical trial of an Internet-based case management system for secondary prevention of heart disease. J Cardiopulm Rehabil 2003;23:341–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=14512778&dopt=Abstract 10.1097/00008483-200309000-00003 [DOI] [PubMed] [Google Scholar]
- 31.Whittaker F, Wade V. The costs and benefits of technology-enabled, home-based cardiac rehabilitation measured in a randomised controlled trial. J Telemed Telecare 2014;20:419–22. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25400004&dopt=Abstract 10.1177/1357633X14552376 [DOI] [PubMed] [Google Scholar]
- 32.Walker DG, Wilson RF, Sharma R, Bridges J, Niessen L, Bass EB, et al. Best Practices for Conducting Economic Evaluations in Health Care: A Systematic Review of Quality Assessment Tools. Rockville, MD: Agency for Healthcare Research and Quality; 2012. [PubMed] [Google Scholar]
- 33.Shields GE, Elvidge J. Challenges in synthesising cost-effectiveness estimates. Syst Rev 2020;9:289. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33298168&dopt=Abstract 10.1186/s13643-020-01536-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. ; CHEERS 2022 ISPOR Good Research Practices Task Force. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) Statement: Updated Reporting Guidance for Health Economic Evaluations. Value Health 2022;25:3–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35031096&dopt=Abstract 10.1016/j.jval.2021.11.1351 [DOI] [PubMed] [Google Scholar]
- 35.Flemming J, Chojecki D, Tjosvold L, Paulden M, Armijo-Olivo S. Quality of reporting of economic evaluations in rehabilitation research: a systematic review. Disabil Rehabil 2022;44:2233–40. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33126829&dopt=Abstract https://doi.org/ 10.1080/09638288.2020.1830441 [DOI] [PubMed] [Google Scholar]
- 36.Edwards K, Jones N, Newton J, Foster C, Judge A, Jackson K, et al. The cost-effectiveness of exercise-based cardiac rehabilitation: a systematic review of the characteristics and methodological quality of published literature. Health Econ Rev 2017;7:37. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29052044&dopt=Abstract 10.1186/s13561-017-0173-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Armijo-Olivo S, Fuentes J, da Costa BR, Saltaji H, Ha C, Cummings GG. Blinding in Physical Therapy Trials and Its Association with Treatment Effects: A Meta-epidemiological Study. Am J Phys Med Rehabil 2017;96:34–44. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27149591&dopt=Abstract 10.1097/PHM.0000000000000521 [DOI] [PubMed] [Google Scholar]
- 38.Nowels D, McGloin J, Westfall JM, Holcomb S. Validation of the EQ-5D quality of life instrument in patients after myocardial infarction. Qual Life Res 2005;14:95–105. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15789944&dopt=Abstract 10.1007/s11136-004-0614-4 [DOI] [PubMed] [Google Scholar]
- 39.McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care 1994;32:40–66. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8277801&dopt=Abstract 10.1097/00005650-199401000-00004 [DOI] [PubMed] [Google Scholar]
- 40.Su JJ, Paguio J, Baratedi WM, Abu-Odah H, Batalik L. Experience of coronary heart disease patients with a nurse-led eHealth cardiac rehabilitation: qualitative process evaluation of a randomized controlled trial. Heart Lung 2023;57:214–21. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=36265371&dopt=Abstract 10.1016/j.hrtlng.2022.10.005 [DOI] [PubMed] [Google Scholar]
- 41.Subedi N, Rawstorn JC, Gao L, Koorts H, Maddison R. Implementation of Telerehabilitation Interventions for the Self-Management of Cardiovascular Disease: systematic Review. JMIR Mhealth Uhealth 2020;8:e17957. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33245286&dopt=Abstract 10.2196/17957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su JJ, Yu DS. Effects of a nurse-led eHealth cardiac rehabilitation programme on health outcomes of patients with coronary heart disease: A randomised controlled trial. Int J Nurs Stud 2021;122:104040. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34333211&dopt=Abstract 10.1016/j.ijnurstu.2021.104040 [DOI] [PubMed] [Google Scholar]
- 43.Rawstorn JC, Gant N, Direito A, Beckmann C, Maddison R. Telehealth exercise-based cardiac rehabilitation: a systematic review and meta-analysis. Heart 2016;102:1183–92. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26936337&dopt=Abstract 10.1136/heartjnl-2015-308966 [DOI] [PubMed] [Google Scholar]
- 44.Wongvibulsin S, Habeos EE, Huynh PP, Xun H, Shan R, Porosnicu Rodriguez KA, et al. Digital Health Interventions for Cardiac Rehabilitation: Systematic Literature Review. J Med Internet Res 2021;23:e18773. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33555259&dopt=Abstract 10.2196/18773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Collins L, Scuffham P, Gargett S. Cost-analysis of gym-based versus home-based cardiac rehabilitation programs. Aust Health Rev 2001;24:51–61. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11357742&dopt=Abstract 10.1071/AH010051 [DOI] [PubMed] [Google Scholar]
- 46.Su JJ, Yu DS. Effectiveness of eHealth cardiac rehabilitation on health outcomes of coronary heart disease patients: a randomized controlled trial protocol. BMC Cardiovasc Disord 2019;19:274. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31783800&dopt=Abstract 10.1186/s12872-019-1262-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsumoto ME, Wilske GC, Tapia R. Innovative Approaches to Delivering Telehealth. Phys Med Rehabil Clin N Am 2021;32:451–65. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33814069&dopt=Abstract 10.1016/j.pmr.2020.12.008 [DOI] [PubMed] [Google Scholar]
- 48.Chong MS, Sit JW, Karthikesu K, Chair SY. Effectiveness of technology-assisted cardiac rehabilitation: A systematic review and meta-analysis. Int J Nurs Stud 2021;124:104087. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34562846&dopt=Abstract 10.1016/j.ijnurstu.2021.104087 [DOI] [PubMed] [Google Scholar]
- 49.Kikuchi A, Taniguchi T, Nakamoto K, Sera F, Ohtani T, Yamada T, et al. Feasibility of home-based cardiac rehabilitation using an integrated telerehabilitation platform in elderly patients with heart failure: A pilot study. J Cardiol 2021;78:66–71. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33579602&dopt=Abstract 10.1016/j.jjcc.2021.01.010 [DOI] [PubMed] [Google Scholar]
- 50.Saito T, Izawa KP. Effectiveness and feasibility of home-based telerehabilitation for community-dwelling elderly people in Southeast Asian countries and regions: a systematic review. Aging Clin Exp Res 2021;33:2657–69. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33765258&dopt=Abstract 10.1007/s40520-021-01820-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Owen O, O’Carroll V. The effectiveness of cardiac telerehabilitation in comparison to centre-based cardiac rehabilitation programmes: A literature review. J Telemed Telecare 2022;X221085865. [Epub ahead of print] https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35369770&dopt=Abstract 10.1177/1357633X221085865 [DOI] [PMC free article] [PubMed]
- 52.Thamman R, Janardhanan R. Cardiac rehabilitation using telemedicine: the need for tele cardiac rehabilitation. Rev Cardiovasc Med 2020;21:497–500. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33387993&dopt=Abstract 10.31083/j.rcm.2020.04.201 [DOI] [PubMed] [Google Scholar]
- 53.Daly RM, Gianoudis J, Hall T, Mundell NL, Maddison R. Feasibility, Usability, and Enjoyment of a Home-Based Exercise Program Delivered via an Exercise App for Musculoskeletal Health in Community-Dwelling Older Adults: Short-term Prospective Pilot Study. JMIR Mhealth Uhealth 2021;9:e21094. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33439147&dopt=Abstract 10.2196/21094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winnige P, Filakova K, Hnatiak J, Dosbaba F, Bocek O, Pepera G, et al. Validity and Reliability of the Cardiac Rehabilitation Barriers Scale in the Czech Republic (CRBS-CZE): Determination of Key Barriers in East-Central Europe. Int J Environ Res Public Health 2021;18:13113. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34948722&dopt=Abstract 10.3390/ijerph182413113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lawson C, Crothers H, Remsing S, Squire I, Zaccardi F, Davies M, et al. Trends in 30-day readmissions following hospitalisation for heart failure by sex, socioeconomic status and ethnicity. EClinicalMedicine 2021;38:101008. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34308315&dopt=Abstract 10.1016/j.eclinm.2021.101008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heydari A, Ziaee ES, Ebrahimzade S. The frequency of rehospitalization and its contributing factors in patient with cardiovascular diseases hospitalized in selected hospitals in Mashhad in 2010. Hor Med Sci 2011;17:65–71. [Google Scholar]
- 57.Rosa GM, Scagliola R, Ghione P, Valbusa A, Brunelli C, Carbone F, et al. Predictors of cardiovascular outcome and rehospitalization in elderly patients with heart failure. Eur J Clin Invest 2019;49:e13044. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30368802&dopt=Abstract 10.1111/eci.13044 [DOI] [PubMed] [Google Scholar]
- 58.Gheorghiade M, Vaduganathan M, Fonarow GC, Bonow RO. Rehospitalization for heart failure: problems and perspectives. J Am Coll Cardiol 2013;61:391–403. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23219302&dopt=Abstract 10.1016/j.jacc.2012.09.038 [DOI] [PubMed] [Google Scholar]
- 59.Internet/Broadband Fact Sheet. Pew Research Center; [Internet]. Available from: https://www.pewresearch.org/internet/fact-sheet/internet-broadband/ [cited 2023, Jan 10].
- 60.Negrini S, Kiekens C, Bernetti A, Capecci M, Ceravolo MG, Lavezzi S, et al. Telemedicine from research to practice during the pandemic. “Instant paper from the field” on rehabilitation answers to the COVID-19 emergency. Eur J Phys Rehabil Med 2020;56:327–30. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32329593&dopt=Abstract 10.23736/S1973-9087.20.06331-5 [DOI] [PubMed] [Google Scholar]
- 61.The affordability of ICT services 2021 Policy brief. ITU; [Internet]. Available from: https://www.itu.int/en/ITU-D/Statistics/Documents/publications/prices2021/ITU_A4AI_Price_Brief_2021.pdf [cited 2023, Jan 10].
- 62.Marques-Sule E, Sempere-Rubio N, Esparcia-Sánchez S, Deka P, Sentandreu-Mañó T, Sánchez-González JL, et al. Physical Therapy Programs in Older Adults with Coronary Artery Disease: Preferences to Technology-Based Cardiac Physical Therapy Programs. Int J Environ Res Public Health 2022;19:13130. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=36293707&dopt=Abstract 10.3390/ijerph192013130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Su J, Zhang Y, Ke QQ, Su JK, Yang QH. Mobilizing artificial intelligence to cardiac telerehabilitation. Rev Cardiovasc Med 2022;23:45. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35229536&dopt=Abstract 10.31083/j.rcm2302045 [DOI] [PubMed] [Google Scholar]
- 64.Antoniou V, Xanthopoulos A, Giamouzis G, Davos C, Batalik L, Stavrou V, et al. Efficacy, efficiency and safety of a cardiac telerehabilitation programme using wearable sensors in patients with coronary heart disease: the TELEWEAR-CR study protocol. BMJ Open 2022;12:e059945. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35738643&dopt=Abstract 10.1136/bmjopen-2021-059945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oksman E, Linna M, Hörhammer I, Lammintakanen J, Talja M. Cost-effectiveness analysis for a tele-based health coaching program for chronic disease in primary care. BMC Health Serv Res 2017;17:138. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28202032&dopt=Abstract 10.1186/s12913-017-2088-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bowry AD, Lewey J, Dugani SB, Choudhry NK. The Burden of Cardiovascular Disease in Low- and Middle-Income Countries: epidemiology and Management. Can J Cardiol 2015;31:1151–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26321437&dopt=Abstract 10.1016/j.cjca.2015.06.028 [DOI] [PubMed] [Google Scholar]
- 67.Serón P, Oliveros MJ, Marzuca-Nassr GN, Lanas F, Morales G, Román C, et al. Hybrid cardiac rehabilitation trial (HYCARET): protocol of a randomised, multicentre, non-inferiority trial in South America. BMJ Open 2019;9:e031213. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31662385&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pastora-Bernal JM, Hernández-Fernández JJ, Estebanez-Pérez MJ, Molina-Torres G, García-López FJ, Martín-Valero R. Efficacy, Feasibility, Adherence, and Cost Effectiveness of a mHealth Telerehabilitation Program in Low Risk Cardiac Patients: A Study Protocol. Int J Environ Res Public Health 2021;18:4038. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33921310&dopt=Abstract 10.3390/ijerph18084038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao L, Maddison R, Rawstorn J, Ball K, Oldenburg B, Chow C, et al. Economic evaluation protocol for a multicentre randomised controlled trial to compare Smartphone Cardiac Rehabilitation, Assisted self-Management (SCRAM) versus usual care cardiac rehabilitation among people with coronary heart disease. BMJ Open 2020;10:e038178. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32847918&dopt=Abstract 10.1136/bmjopen-2020-038178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang C, Soliman-Hamad M, Robijns R, Verberkmoes N, Verstappen F, IJsselsteijn WA. Promoting Physical Activity With Self-Tracking and Mobile-Based Coaching for Cardiac Surgery Patients During the Discharge-Rehabilitation Gap: Protocol for a Randomized Controlled Trial. JMIR Res Protoc 2020;9:e16737. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32812886&dopt=Abstract 10.2196/16737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rawstorn JC, Ball K, Oldenburg B, Chow CK, McNaughton SA, Lamb KE, et al. Smartphone Cardiac Rehabilitation, Assisted Self-Management Versus Usual Care: Protocol for a Multicenter Randomized Controlled Trial to Compare Effects and Costs Among People With Coronary Heart Disease. JMIR Res Protoc 2020;9:e15022. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32012103&dopt=Abstract 10.2196/15022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gonzalez-Garcia MC, Fatehi F, Scherrenberg M, Henriksson R, Maciejewski A, Salamanca Viloria J, et al. International feasibility trial on the use of an interactive mobile health platform for cardiac rehabilitation: protocol of the Diversity 1 study. BMJ Health Care Inform 2019;26:e100042. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31488496&dopt=Abstract 10.1136/bmjhci-2019-100042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pedersen SS, Andersen CM, Ahm R, Skovbakke SJ, Kok R, Helmark C, et al. Efficacy and cost-effectiveness of a therapist-assisted web-based intervention for depression and anxiety in patients with ischemic heart disease attending cardiac rehabilitation [eMindYourHeart trial]: a randomised controlled trial protocol. BMC Cardiovasc Disord 2021;21:20. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33413109&dopt=Abstract 10.1186/s12872-020-01801-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bryant MS, Fedson SE, Sharafkhaneh A. Using Telehealth Cardiopulmonary Rehabilitation during the COVID-19 Pandemic. J Med Syst 2020;44:125. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32462352&dopt=Abstract 10.1007/s10916-020-01593-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seron P, Oliveros MJ, Gutierrez-Arias R, Fuentes-Aspe R, Torres-Castro RC, Merino-Osorio C, et al. Effectiveness of Telerehabilitation in Physical Therapy: A Rapid Overview. Phys Ther 2021;101:pzab053. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33561280&dopt=Abstract 10.1093/ptj/pzab053 [DOI] [PMC free article] [PubMed]
- 76.Batalik L, Filakova K, Radkovcova I, Dosbaba F, Winnige P, Vlazna D, et al. Cardio-Oncology Rehabilitation and Telehealth: Rationale for Future Integration in Supportive Care of Cancer Survivors. Front Cardiovasc Med 2022;9:858334. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35497988&dopt=Abstract 10.3389/fcvm.2022.858334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ambrosetti M, Abreu A, Corrà U, Davos CH, Hansen D, Frederix I, et al. Secondary prevention through comprehensive cardiovascular rehabilitation: From knowledge to implementation. 2020 update. A position paper from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur J Prev Cardiol 2020;2047487320913379. [Epub ahead of print] https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33611446&dopt=Abstract [DOI] [PubMed]
- 78.Pepera G, Tribali MS, Batalik L, Petrov I, Papathanasiou J. Epidemiology, risk factors and prognosis of cardiovascular disease in the Coronavirus Disease 2019 (COVID-19) pandemic era: a systematic review. Rev Cardiovasc Med 2022;23:28. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35092220&dopt=Abstract 10.31083/j.rcm2301028 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table I
Search strategy.
Supplementary Table II
Quality assessment of included economic evaluations.