Abstract
The S1P1 receptor is the target of four marketed drugs for the treatment of multiple sclerosis and ulcerative colitis. Targeting an S1P exporter, specifically Spns2, that is ‘upstream’ of S1P receptor engagement is an alternate strategy that might recapitulate the efficacy of S1P receptor modulators without the cardiac toxicity. We recently reported the first Spns2 inhibitor SLF1081851 (16d) that has modest potency with in vivo activity. To develop more potent compounds, we initiated a structure-activity relationship study that identified 2-aminobenzoxazole as a viable scaffold. Our studies revealed SLB1122168 (33p), which is a potent inhibitor (IC50 = 94 ± 6 nM) of Spns2-mediated S1P release. Administration of 33p to mice and rats resulted in a dose-dependent decrease in circulating lymphocytes, a pharmacodynamic indication of Spns2 inhibition. 33p provides a valuable tool compound to explore both the therapeutic potential of targeting Spns2 and the physiologic consequences of selective S1P export inhibition.
Keywords: Sphingosine-1-phosphate, S1P, Spns2, lymphopenia, transporter inhibitor, aminobenzoxazole
Introduction
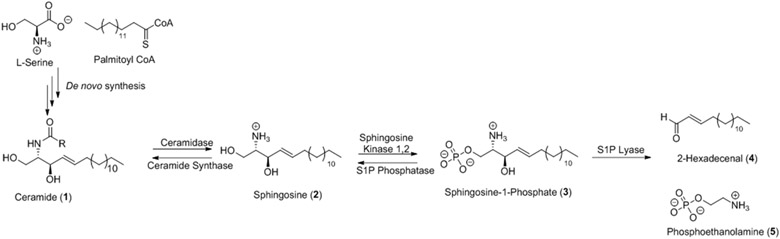
Sphingosine-1-phosphate (S1P) is a bioactive lipid that plays an important role in many biological processes.1-4 The S1P pathway starts with the de novo synthesis of ceramide (1) from the condensation of serine and palmitoyl-CoA enabled by serine palmitoyl-CoA transferase (Figure 1).5 The fatty acid group of ceramide is hydrolyzed by ceramidase to form sphingosine (Sph) (2) that is converted to S1P (3) by sphingosine kinases SphK1 and SphK2. S1P may be degraded by S1P lyase to form 2-hexadecenal (4) and phosphoethanolamine (5), de-phosphorylated by S1P phosphatase to regenerate Sph or, in some cells, extruded to chaperone proteins in the extracellular environment.5
Figure 1.
Abbreviated depiction of the S1P pathway.
In vertebrates, S1P is an extracellular lipid mediator that engages cellular signaling pathways via interaction with a set of cell surface G-protein coupled receptors (S1P1-5 in mammals). S1P is exported to the extracellular space by at least two transporters, which are members of the major facilitator superfamily (MFS). In red blood cells, S1P is exported by Mfsd2b6 whereas in other cells types including endothelial cells, the S1P exporter is Spinster homolog 2 (Spns2).7-9 Extracellular S1P binds to S1P1-5 resulting in a myriad of cellular responses, including cell migration such as lymphocyte egress (Figure 2).10-13
Figure 2.
Pathway of S1P transport
Lymphocyte trafficking from lymph nodes to efferent lymph is thought to be mediated by a S1P gradient, whereby the S1P concentration in lymph are high relative to secondary lymphoid tissue enabling egress of lymphocytes from secondary lymphoid tissues to efferent lymph.14-15 This biology was revealed by studies of the prodrug FTY720 fingolimod, an Sph analog that is converted in vivo to phospho-FTY720 (FTY720-P) by SphK2.16 FTY720-P is a potent agonist at four of five S1P receptors; desensitization of lymphocyte S1P1 ultimately results in immunosuppressive effects.17 Fingolimod was the first S1P receptor modulator to be granted marketing approval (for the indication relapsing remitting multiple sclerosis).18 Three follow-on S1P1 receptor-targeted drugs have been approved by the FDA including siponimod, ozanimod and ponesimod for treatment of multiple sclerosis 19-23 and, in the case of ozanimod, ulcerative colitis.24 In all cases, these drugs act as functional antagonists as the receptor is internalized upon receptor activation. All four drugs exhibit an on-target adverse effect – first dose bradycardia (slow heart rate).25 This effect is due to the engagement of S1P1 expressed by the cardiac conduction system, which was only observed in humans and not rats.2, 26
To circumvent the liability of S1P receptor modulators, other nodes of the S1P pathway have been assessed, including the inhibition of SphK127-28 or SphK2 28-29 as well as transporters Spns28 and Mfsd2b.6, 30 Ideally, decreasing levels of extracellular S1P by blocking S1P exporters would recapitulate the efficacy of S1PR modulators, which act by desensitizing lymphocytes to S1P. Mfsd2b is expressed solely in hematopoietic cells (erythrocytes and platelets) and gene ablation studies with mice suggest that plasma S1P is mostly exported via this transporter.6 Spns2, which is expressed by the endothelium, is expected to be both the source of lymph S1P and some fraction of plasma S1P. While studies using Spns2-knockout mice invariably report that Spns2-deficient mice have significantly diminished blood lymphocyte counts, the same literature is contradictory regarding both plasma and lymph [S1P] in these animals.9, 31-37 In the standard model of multiple sclerosis (EAE, Experimental Autoimmune Encephalomyelitis), germ line deletion of Spns2 in mice resulted in improvement of clinical scores.38 In addition, Spns2 ‘knockdown’ in human renal cell lines showed decreased expression of pro-fibrotic cytokines including fibronectin and connective tissue growth factor, suggesting Spns2 could have therapeutic benefits for renal fibrosis.39 Recently, we participated in a study that demonstrated Spns2 deficiency in kidney perivascular cells, but not tubular cells, ameliorates kidney fibrosis through modulation of inflammatory cells. This result was unexpected in that the an S1P modulator (fingolimod) was ineffective in these models.40 Taken together, these reports indicate that Spns2 might be a useful drug target.
Learning the therapeutic potential of targeting Spns2 requires validated tool compounds. Our laboratories recently reported SLF1081851 as a first generation Spns2 inhibitor with an IC50 value of 1.93 μM in an S1P release assay.41 Administration of SLF1081851 to mice evoked dose-dependent lymphopenia and modestly decreased plasma [S1P]. The former phenotype is reported in all mice strains rendered deficient in Spns2, while the literature is unsettled regarding the later phenotype (vide supra). In addition, SLF1081851 ameliorated kidney fibrosis in both a folic acid toxicity and a surgical (ischemia reperfusion injury) model at 10 mg/Kg.40 While SLF1081851 has proven to be an informative lead compound, its utility is limited by low potency and a narrow therapeutic window (maximum tolerable dose < 20 mg/kg). In this report, we performed a structure-activity relationship (SAR) study by modifying the hydrophobic tail, the heterocyclic linker (an oxadiazole in SLF1081851), and the primary amine head group to identify more potent Spns2 inhibitors (Figure 3). Our studies revealed that fused heterocycles (2-aminobenzoxazole) bearing a pyrrolidine head group and a decyl tail are potent in inhibiting Spns2. In particular, SLB1122168 has an IC50 of 94 nM with the demonstrated in vivo pharmacodynamic effect of lymphopenia in both mice and rats.
Figure 3.
Approach to the SAR of SLF1081851.
Results and Discussion
We initially focused our studies on the linker region of the pharmacophore.41 The fusion of phenyl and oxadiazole moieties produces attractive 5,6-bicyclic ring systems that enforce conformational rigidity and polarity. Benzoxazoles are an obvious choice because they are the amalgamation of the phenyl and oxadiazole rings. An alternative is benzimidazoles, which possess symmetrical NH in the fused ring system. Synthesis of the benzoxazole derivatives began with 1,1-carbonyldiimidazole (CDI) mediated coupling of the appropriate carboxylic acid with 2-amino-4-bromo-phenol (11) affording amide 12 (Scheme 1). An intramolecular Mitsunobu reaction was employed to form the benzoxazole intermediate 13 in 38-87% yield.42 Performing a one-pot hydroboration of 1-decene followed by Suzuki-Miyaura cross-coupling with 13 generates intermediate 14.43 Boc-deprotection using hydrochloric acid resulted in the desired product 15 as the hydrogen chloride salt.
Scheme 1.
Reagents and conditions: (a) N-Boc-amino acid (1.3 equiv), CDI (1.3 equiv), DIEA (1.6 equiv), DCM, rt, 16 h, 38-87%; (b) PPh3 (2.0 equiv), DIAD (2.0 equiv), THF, 70 °C, 4 h, 24-69%; (c) 9-BBN (2.2 equiv), 1-decene (2.0 equiv), Pd(dppf)Cl2·CH2Cl2 (0.075 equiv), 3 M KOH (3.0 equiv), THF, 70 °C, 16 h, 62-70%; (d) 4 M HCl, DCM, rt, 4 h. 59-92%
The synthetic approach towards 2-aminobenzoxazole and 2-aminobenzimidazole compounds are described in Scheme 2. Reaction of bromo-2-aminophenol (16) with carbon disulfide afforded mercaptobenzoxazole intermediate 17 in 84% yield. Derivatives of 17 where the bromine is in different positions allows for diversification of the substitution pattern in the final molecule. Intermediates 18-19 were successfully obtained by nucleophilic aromatic substitution of 17 with thionyl chloride in 82% yield. To generate benzimidazole 20, 21 was reacted with phosphoryl chloride. Nucleophilic aromatic substitution with various amines afforded the corresponding 2-amino-substituted derivatives (22-24). One-pot hydroboration of various alkenes followed by Suzuki-Miyaura cross-coupling with (22-24) yielded intermediates (25-31). Boc-deprotection using hydrochloric acid afforded final compounds 32-38 as the hydrogen chloride salt.
Scheme 2.
Reagents and conditions: (a) CS2 (1.2 equiv.), K2CO3 (1.2 equiv.), EtOH/H2O, 80 °C, 3 h, 84%; (b) SOCl2 (2.5 equiv.), DMF (0.04 equiv.), DCM, rt, 1 h, 82%; (c) POCl3 (neat), reflux, 16 h, 73%; (d) N-Boc-amine (1.2 equiv.), K2CO3 (2.0 equiv.), DMF, 120 °C, 2 h, 49-91%; (e) N-Boc-amine (1.2 equiv.), i-PrOAc, 130 °C, 16 h, 70%; (f) 9-BBN (2.2 equiv.), 1-decene (2.0 equiv.), Pd(dppf)Cl2·CH2Cl2 (0.15 equiv.), 3 M KOH (3.0 equiv.), THF, 70 °C, 16 h, 60-86%; (g) 4 M HCl, DCM, rt, 4 h, 60-95%.
We screened the putative inhibitors for Spns2 inhibition at either a 1 μM or 0.3 μM concentration (Table 1) using a mouse Spns2 HeLa cell-based S1P release assay.41 Our initial studies focused on the polar head group by comparing alkyl and cycloalkyl amines with various lengths and ring sizes. Decyl tails were investigated first since they were shown to have favorable potency as we have previously reported.41 Starting with a benzoxazole linker, a homologation study of linear alkyl amines from 2- to 5-carbon (15a-d) showed that the 3-carbon linker (15b) was preferred increasing the inhibitory activity from 0% to 40%. To further increase the polarity and number of hydrogen bond donors and acceptors in the positively charge head groups, a 2-amino group was introduced (32a-d). As shown in entries 5-8, increasing the carbon linker from 2 to 5 improved the activity to afford 32d with 35% inhibition. Tertiary amines bearing a methyl (32e) resulted in amelioration of activity while re-introducing polarity with aminoethyl (32f) restored potency. We next investigated cyclic amine moieties (32g-i) and found that exocyclic amines were not tolerated whereas the piperazine derivative (32i) showed 18% inhibition at 1 μM.
Table 1.
Spns2 inhibitory activity with different head groups.

| |||
|---|---|---|---|
| Entry | Cmpd | R3 | % Inh |
| 1 | SLF1081851 | 44 ± 5 30 ± 7c |
|
| 2 | 15a |
|
5 ± 0 |
| 3 | 15b |
|
40 ± 0 |
| 4 | 15c |
|
0 |
| 5 | 15d |
|
25 ± 0 |
| 6 | 32a |
|
16 ± 0 |
| 7 | 32b |
|
15 ± 0 |
| 8 | 32c |
|
32 ± 0 |
| 9 | 32d |
|
35 ± 0 |
| 10 | 32e |
|
0 |
| 11 | 32f |

|
25 ± 1 |
| 12 | 32g |

|
1 ± 0 |
| 13 | 32h |

|
4 ± 0 |
| 14 | 32i |

|
18 ± 2 |
| 15b | 32j |

|
20 ± 0 |
| 16 | 32k |

|
75 ± 4 |
| 17 | 32l |

|
47 ± 2 |
| 18 | 32m |

|
54 ± 2 |
| 19 | 32n |

|
50 ± 5 |
| 20 | 32o |

|
63 ± 6 |
| 21 | 32p |

|
87 ± 7 56 ± 4c |
| 22 | 32q |

|
50 ± 4 |
| 23 | 32r |
|
6 ± 0 |
| 24 | 32s |

|
0 |
| 25 | 32t |

|
14 ± 0 |
| 26 | 32u |

|
2 ± 0 |
| 27 | 32v |

|
32 ± 2 |
Percent inhibition relative to the control (no inhibitor). All compounds were tested at 1 μM concentration unless noted otherwise. Assays were performed in duplicate.
Tested as a TFA salt.
Tested at 0.3 μM.
To further explore the SAR of cyclic amines, the effect of ring size was probed. Our studies indicate that azetidine (32j), pyrrolidine (32k-l), and piperidine (32m-o) were efficient inhibitors with pyrrolidine 32k being the most potent at 75% inhibitory activity. Interestingly, the (S)-enantiomer was more potent than the (R)-enantiomer (32l). Further, we investigated the effect of inserting a methylene linker to generate homologated pyrrolidine and piperidine derivatives. For pyrrolidines, the methylene linker increased activity for the R isomer (32p). Importantly, putting the methylene tether at the beta position of the pyrrolidine ring (32p) had a marked increase in potency relative to the alpha position (32r). Further, it was determined that the inhibitory activity is changed markedly depending on the size of the ring; i.e., pyrrolidines (32p-q) are more potent than piperidines (32t-v). In contrast to the pyrrolidine series, the stereochemical preference changed to R rather than S in the piperidine series. 32p was identified to be the most potent compound in this series with 87% inhibition at 1 μM.
As the results for 32p were near the detection limit of the S1P release assay, retesting at 0.3 μM gave 56% inhibition suggesting an IC50 value of about 300 nM (Table 1, entry 20). To determine the optimal tail length, a homologation (octyl to dodecyl, 34-37p) study was performed (Table 2). We found that as the alkyl length increases from octyl to nonyl, a concomitant increase in potency was observed. In contrast, a further increase in chain length to dodecyl (37p) resulted in decreased potency. It was determined that nonyl-undecyl tails were equipotent and optimal for this scaffold. Because the benzoxazole scaffold is not symmetrical, we synthesized compound 33p where the decyl tail is ‘para to nitrogen’ (6 position) and found activity improved to 77% inhibition (at 0.3 μM) with this positional isomer. For completeness, the corresponding benzimidazole 38p was synthesized. No improvement in inhibitory activity was observed with this modification. These results indicate compound 33p is our most potent inhibitor.
Table 2.
Spns2 inhibitory activity of tail modifications.

| |||||
|---|---|---|---|---|---|
| Entry | Cmpd | X | R2 Position | R2 | % Inhibition |
| 1 | 34p | O | 5 |
|
42 ± 3 |
| 2 | 35p | O | 5 |
|
50 ± 4 |
| 3 | 33p | O | 6 |
|
77 ± 4 |
| 4 | 36p | O | 5 |
|
52 ± 4 |
| 5 | 37p | O | 5 |
|
36 ± 2 |
| 6 | 38p | N | 5/6 |
|
34 ± 1 |
Percent inhibition relative to the control (no inhibitor). All compounds were tested at 0.3 μM
A concentration-effect study was performed with the two most potent inhibitors, 32p and 33p. As shown in Figure 4, a sigmoidal curve is generated as the concentrations of 33p and 32p were increased (IC50 values of 94 ± 6 nM and 227 ± 13 nM, respectively) confirming that structural isomer 33p is significantly more potent than 32p. To establish the pharmacokinetic-pharmacodynamic relationship, pharmacokinetic profiling of 33p was undertaken in rats. At 10 mg/Kg, 33p achieved a maximum concentration of 4 μM at 2 hours post-dose with levels at ≥ 1 μM for 24 hours and a half-life of 8 ± 0.2 hours (Figure 5A). During the course of the experiment, lymphocyte levels were also determined. As shown in Figure 5B, concentration-dependent decrease in lymphocyte counts was observed, indicating pharmacokinetic-pharmacodynamic correlation. Our studies in rat revealed a 55% decrease of circulating lymphocytes, which is consistent with studies using knock-out mice.33
Figure 4.
Dose-response curve of 32p and 33p against Spns2.
Figure 5.
Pharmacodynamic and pharmacokinetic analysis of 33p. Male rats (Sprague Dawley strain, 4 weeks old, (n=6) were injected (IP) at a dose of 10 mg/Kg. (A) Blood 33p (B) Percent circulating lymphocytes after 33p administration. Shown is the percentage of white blood cells in peripheral blood that are lymphocytes. Error bars indicate standard deviations.
To determine in vivo activity, 32p and 33p were administered to cohorts of mice and circulating lymphocytes counts and plasma [S1P] were measured at 4 hours post dosing. As documented in Figure 6A, both compounds evoked a significant decrease in circulating lymphocytes relative to vehicle control. Further, 33p was found to exert this effect at 16 hours post administration in a dose-dependent manner (Figure 6B). These results are consistent with the phenotype that is observed with Spns2-knockout mice suggesting in vivo target engagement.31, 36 However, neither 32p nor 33p administration resulted in a significant decrease in plasma [S1P] (Figure 6C), which is unlike our experience with our first generation Spns2 inhibitor, SLF1081851.41 As mentioned previously, the literature regarding genetically modified, Spns2-deficient mice is contradictory on this point. Specifically, mice rendered deficient in Spns2 by genetic manipulation of its gene are variously reported to have no significant change,31,34,37 a ~23% decrease,36 a 25-30% decrease,35 a 40% decrease9 and a 45% decrease33 in plasma S1P concentrations. We do not understand what underlies these discrepancies and why our early Spns2 inhibitor, SLF1081851, consistently reduced plasma S1P concentrations by ~20%, while our subsequent inhibitors evoke no significant changes in mice, and we know of no other manipulation of the S1P pathway that changes plasma S1P concentrations without changing red blood cell S1P concentrations. Resolution regarding the issue of Spns2 inhibition on plasma [S1P] awaits discovery and testing of additional inhibitors on diverse chemical scaffolds.
Figure 6.

Biological evaluation of 32p and 33p in female mice. (A) Dosing with 32p and 33p resulted in a decrease in circulating lymphocytes. Blood was drawn 4 h postdose (10 mg/Kg). Age-matched female mice (C57BL/6j strain) were injected (IP) with 32p and 33p or the vehicle. (B) Blood drawn after 16 h postdose (10 mg/Kg and 30 mg/Kg of 33p. Shown is the percentage of white blood cells in peripheral blood that are lymphocytes. (C) plasma S1P levels 4 h post dose t-test: * ≤ 0.5; *** ≤ 0.001; **** ≤ 0.0001; ns = not statistically significant
Finally, we tested 33p as a potential inhibitor of S1P release from mouse red blood cells. We did not observe any decrease in S1P release from RBCs at concentrations up to 10 μM (data not shown). This result is consistent both with the lack of amino acid sequence conservation between Mfsd2b and Spns2 and with our failure to observe a decrease in plasma [S1P] in response to 33p.
Conclusion
The S1P pathway has been investigated intensively since the discovery that S1P receptors are the target of phospho-FTY720. The development of fingolimod and other S1P modulators such as siponimod, ozanimod, and ponesimod as medicines for treating autoimmune diseases fully validated the pathway as a drug target. However, on-target adverse effects such as first dose bradycardia and, rarely, macular edema presents an argument for targeting alternative nodes in the pathway. In this study, we investigated a node upstream of S1P receptors, that is, the S1P transporter Spns2. Because Spns2 plays a role in both lymphocyte trafficking and S1P-mediated inflammation, we hypothesize that blocking S1P transport could be another mechanism for manipulating the S1P pathway that circumvents the adverse effects of S1P modulators. Recent studies have shown that Spns2 is a potential therapeutic target for multiple sclerosis38, and kidney fibrosis.39-40 Unfortunately, small molecule inhibitors of Spns2 are lacking, which impedes the understanding of the physiological role of this transporter. In the current structure-activity relationship study, we interrogated three regions of a pharmacophore based on 16d (SLF1081851).41 Our studies reveal that (i) a benzoxazole linker is an excellent mimetic of ‘phenyl-oxadiazole’, (ii) a positively charged cyclic secondary amine such as a pyrrolidine is preferred, (iii) decyl tail is optimal for inhibition, and (iv) an alkyl tail in a position ‘para to nitrogen’ improves potency. In particular, we identified 33p as the most potent Spns2 inhibitor to date with an IC50 of 94 nM in vitro. In vivo administration of 33p in mice resulted in the expected decrease of circulating lymphocytes in rodents as observed with Spns2 knockout mice suggesting inhibition of Spns2 in vivo. Pharmacokinetic profiling in rats indicated a favorable half-life of 8 h where drug levels correlated with lymphopenia. Thus, 33p affords a chemical tool that will be useful to investigate the effects of inhibiting Spns2 both in vitro and in vivo. The benzoxazole scaffold might be useful also as a platform for the development of future inhibitors.
Experimental Section
General Materials and Synthetic Procedures.
Reactions were performed using the Schlenk technique under an argon or nitrogen atmosphere, unless otherwise specified. All glassware used was flame-dried or oven-dried overnight. Chemicals were obtained from commercial sources and used without further purification, unless otherwise noted. THF, toluene, and DCM were dried using the Innovative Technology Pure SolvMD solvent purification system prior to use. Column chromatography was performed using SiliaFlash P60 40–63 μm, 60 Å. Thin-layer chromatography (TLC) analyses were performed using Silicycle aluminum-backed silica gel F-254 plates. NMR spectroscopic experiments were performed using a Bruker AVANCE III 600 MHz, Bruker AVANCE II 500 MHz, Agilent 400-MR 400 MHz, or a Varian Inova 400 MHz spectrometer. Chemical shifts are reported in δ ppm and 1H and 13C NMR are referenced using the residual protonated solvent or an internal standard (TMS). Data are reported as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, quin = quintet, dd = doublet of doublets, dt = doublet of triplets, m = multiplet), coupling constants (Hz), rotamer splitting (*) and integration. ESI mass spectra were obtained using an Agilent 6220 TOF LC─MS or Waters Synapt-G2S Q-TOF LCMS. Purity assessments were performed by Waters UPLC analysis. UPLC conditions: Solvent A: Water (0.1% TFA); solvent B: acetonitrile (0.1% TFA); column: Acquity BEH C18 1.7 μm 2.1 x 50 mm; method: isocratic 60% A, 40% B from 0-3.50 min then linear gradient from 40-95% B by 5 minutes, return to 40% B by 6 minutes, then hold for 2 minutes at 60% A, 40% B; UV wavelength = 280 nm; flow rate: 0.613 mL/min. All compounds tested in biological assays were assessed to have ≥95% purity by HPLC, unless otherwise noted. Compounds evaluated in vivo were HPLC-purified prior to injection into animals and were of ≥95% purity by HPLC.
In Vitro Spns2 Assay.
HeLa cells were transfected with pcDNA3.1 plasmids encoding mouse Spns2 or mouse Spns2Arg200Ser (transport dead mutant). Transfected cell pools were selected by inclusion of geneticin (G418) in the cell media. To assess S1P release, HeLa cells were plated onto 12-well plates and grown to near confluence. Growth media were removed by aspiration and 1.5 mL of release assay medium (RPMI 1640 with 0.2% fatty acid-free BSA) supplemented with 4-deoxypyridoxine (1 mM), NaF (2 mM), and Na3VO4 (0.2 mM) to retard degradation of S1P by S1P lyase and S1P phosphatases was added to each well. Test articles were introduced into duplicate wells (0-2 μM), and plates were placed in a tissue culture incubator for 18–20 h. Following this incubation, the medium was collected, internal standard (5 μL of 0.5 μM d7-S1P in methanol) and 150 μL of 100% trichloroacetic acid were added and, after mixing, the tubes were placed on ice for 45–60 min. The precipitated material was collected by centrifugation, the pellets washed with water, recentrifuged, and the final pellet mixed vigorously after adding 0.3 mL methanol. After further centrifugation, 0.16 mL of the supernatant fluid was added to UPLC vials and S1P and d7-S1P were quantified by LC-MS/MS by injecting 3-9 μL on column. Spns2-expressing cells were found to release S1P into the culture media in ca. 20-fold excess of Spns2Arg200Ser-transfected or non-transfected cells.
In Vivo Studies.
Compounds 32p and 33p (10 or 30 mg/Kg) or an equal volume of vehicle (36.1% PEG400 / 9.1% ethanol / 4.6% solutol / 50% H2O) were administered by intraperitoneal injection into female mice (C57BL/6j strain) or male rats (Sprague-Dawley strain) from Jackson Labs. Blood samples were analyzed via LC─MS/MS as described for analysis using the in vitro Spns2 assay above. Lymphocyte counts were obtained from 20 μL of mouse or rat blood using a Heska HT5 Element blood analyzer. For PK analysis in rats, whole blood collections via tail nick (ca. 70 μL) were performed at 0, 1, 2, 4, 8, and 24 h post dose and plasma samples prepared for LC─MS/MS analysis for drug and S1P levels. All animal protocols were approved prior to experimentation by the University of Virginia School of Medicine’s Animal Care and Use Committee.
Synthetic Procedures
General Procedure 1: CDI Coupling
To a vial containing N-Boc-amino acid (1.3 equiv.) was added DCM (0.3 M) and carbonyldiimidazole (CDI) (1.3 equiv.). The resulting mixture was allowed to stir at rt for 2 hours, followed by addition of 2-amino-4-bromo-phenol 11 (1.0 equiv.) and DIEA (1.6 equiv.). The resulting mixture was allowed to stir at rt for 16 hours, when the starting material was determined to be completely consumed based on TLC. The resulting mixture was concentrated in vacuo to afford a brown oil which was then subjected to flash chromatography on silica gel with an appropriate ethyl acetate and hexanes solvent system to afford the product.
General Procedure 2: Mitsunobu Coupling
To a sealed microwave vial containing PPh3 (2.0 equiv.) was added THF (0.3 M), phenol 12a-d and N-Boc protected amine (1.0 equiv.) under inert atmosphere. The resulting mixture was allowed to stir at 0 °C for 20 minutes then diisopropyl azodicarboxylate (DIAD) (2.0 equiv.) was added. The mixture was then heated to 70 °C until the starting material was determined to be completely consumed based on TLC (4 hours). The mixture was diluted in ethyl acetate and extracted fromwater. The organic layer was dried over anhydrous sodium sulfate and concentrated in vacuo to afford an orange oil. The crude mixture was then subjected to flash chromatography on silica gel with ethyl acetate and hexanes solvent system to afford the product.
General Procedure 3a: Suzuki Cross-Coupling
To a sealed microwave vial containing alkene (2.0 equiv.) was added THF (0.1 M) and 9-BBN (2.2 equiv.) under inert atmosphere. The resulting mixture was allowed to stir at 70 °C for 1 hour, followed by the addition of requisite benzoxazole (1.0 equiv.), 3M KOH (3.0 equiv.), and Pd(dppf)Cl2·CH2Cl2 (0.0075 equiv.). The resulting mixture was allowed to stir at 70 °C until starting material was determined to be completely consumed based on TLC (16 hours). The resulting mixture was concentrated in vacuo to afford a brown oil which was then subjected to flash chromatography on silica gel with an appropriate ethyl acetate and hexanes solvent system to afford the pure product.
General Procedure 3b: Suzuki Cross-Coupling
To a sealed microwave vial containing alkene (2.0 equiv.) was added THF (0.1 M) and 9-BBN (2.2 equiv) under inert atmosphere. The resulting mixture was allowed to stir at 70 °C for 1 hour, followed by the addition of requisite aminobenzoxazole (1.0 equiv.), 3M KOH (3.0 equiv.), and Pd(dppf)Cl2·CH2Cl2 (0.015 equiv.). The resulting mixture was allowed to stir at 70 °C until starting material was determined to be completely consumed based on TLC (16 hours). The resulting mixture was concentrated in vacuo to afford a brown oil which was then subjected to flash chromatography on silica gel with an appropriate ethyl acetate and hexanes solvent system to afford the pure product.
General Procedure 4: HCl Boc Deprotection
To a vial containing N-Boc- protected amine (1.0 equiv.) was added DCM (0.1 M) and 4 M HCl in dioxane (10 equiv). The resulting mixture was stirred at rt until starting material was determined to be completely consumed based on TLC (4 hours). The resulting mixture was triturated with diethyl ether to afford the pure product as the hydrochloride salt.
General Procedure 5: 2-Mercaptobenzoxazole Ring Formation
To a sealed microwave vial containing 2-amino-4-bromo-phenol (1.0 equiv.) was added EtOH/H2O (5:1, 0.3 M), K2CO3 (1.2 equiv.), and CS2 (1.2 equiv.). The resulting mixture was allowed to stir at 80 °C until starting material was determined to be completely consumed based on TLC (3 hours). The resulting mixture was diluted in water, and acetic acid was added in dropwise until a white solid was precipitated. The precipitate was solubilized in ethyl acetate and concentrated in vacuo to afford a blue oil which was then subjected to flash chromatography on silica gel with an appropriate ethyl acetate and hexanes solvent system to afford the pure product.
General Procedure 6a: Chlorination
To a round bottom flask containing 5-bromobenzo[d]oxazole-2-thiol (1.0 equiv.) was sequentially added DCM (0.3 M), SOCl2 (2.5 equiv.), and DMF (0.04 equiv.) under inert atmosphere. The resulting mixture was allowed to stir at rt until starting material was determined to be completely consumed based on TLC (1 hour). The result mixture was diluted in ethyl acetate and washed with water. The organic layer was then dried over anhydrous sodium sulfate and concentrated in vacuo to afford a light pink oil which was then subjected to flash chromatography on silica gel with an appropriate ethyl acetate and hexanes solvent system to afford the pure product.
General Procedure 6b: Chlorination
To a microwave vial containing 5-bromo-1,3-dihydro-2H-benzo[d]imidazol-2-one (1.0 equiv.) was added POCl3 (25.0 equiv.) under inert atmosphere. The resulting mixture was allowed to stir at reflux until starting material was determined to be completed consumed based on TLC (16 hours). The reaction mixture was slowly poured into ice cold water and the pH was adjusted to 7 using 6M NaOH. The yellow solid was filtered and washed with water to afford the pure product.
General Procedure 7a: Nucleophilic Aromatic Substitution
To a sealed microwave vial containing the requisite benzoxazole (1.0 equiv.) was added DMF (0.3 M), N-Boc-amine (1.2 equiv.), and K2CO3 (2.0 equiv.). The resulting mixture was allowed to stir at 120 °C until starting material was determined to be completely consumed based on TLC (2 hours). The resulting reaction mixture was diluted in ethyl acetate and washed with a saturated lithium bromide solution. The organic layer was then dried over anhydrous sodium sulfate and concentrated in vacuo to afford a clear oil which was then subjected to flash chromatography on silica gel with an appropriate ethyl acetate and hexanes solvent system to afford the pure product.
General Procedure 7b: Nucleophilic Aromatic Substitution
To a sealed microwave vial containing the requisite benzimidazole (1.0 equiv.) was added isopropyl acetate (0.3 M) and N-Boc-amine (1.2 equiv.). The resulting mixture was allowed to stir at 130 °C until starting material was determined to be completely consumed based on TLC (16 hours). The resulting reaction was diluted in dichloromethane and washed with a saturated sodium bicarbonate solution. The organic layer was then dried over anhydrous sodium sulfate and concentrated in vacuo to afford a clear oil which was then subjected to flash chromatography on silica gel with an appropriate ethyl acetate and hexanes solvent system to afford the pure product.
General Procedure 8: TFA Boc Deprotection
To a vial containing N-Boc-protected amine (1.0 equiv.) was added DCM and TFA (10 equiv.). The resulting mixture was stirred at rt until starting material was determined to be completely consumed based on TLC (16 hours). The resulting mixture was triturated with diethyl ether to afford the pure product.
tert-butyl-(3-((5-bromo-2-hydroxyphenyl)amino)-3-oxopropyl)carbamate (12a).
Synthesized according to General Procedure 1. Purified via column chromatography (50% ethyl acetate/hexanes). Orange solid (56%, 322 mg). 1H NMR (500 MHz, DMSO-d6) δ 10.10 (s, 1H), 9.19 (s, 1H), 8.03 (s, 1H), 7.03 (dd, J = 8.6, 2.5 Hz, 1H), 6.77 (d, J = 8.5 Hz, 2H), 3.16 (q, J = 6.6 Hz, 2H), 2.49 (t, J = 7.0 Hz, 2H), 1.33 (s, 9H). 13C NMR (126 MHz, DMSO-d6) δ 170.2, 155.5, 146.9, 128.0, 126.5, 124.1, 116.9, 109.6, 77.7, 36.7, 36.6, 28.3. HRMS (ESI+) m/z calc’d. for C14H20BrN2O4+ (M+H)+ 359.0601, found 359.0596.
tert-butyl-(4-((5-bromo-2-hydroxyphenyl)amino)-4-oxobutyl)carbamate (12b).
Synthesized according to General Procedure 1. Purified via column chromatography (50% ethyl acetate/hexanes). Light yellow solid (38%, 228 mg). 1H NMR (600 MHz, DMSO-d6) δ 10.17 (s, 1H), 9.22 (s, 1H), 8.09 (d, J = 2.5 Hz, 1H), 7.07 (dd, J = 8.6, 2.5 Hz, 1H), 6.85 (t, J = 5.7 Hz, 1H), 6.81 (d, J = 8.6 Hz, 1H), 2.95 (q, J = 6.6 Hz, 2H), 2.39 (t, J = 7.4 Hz, 2H), 1.67 (quin, J = 7.2 Hz, 2H), 1.38 (s, 9H). 13C NMR (151 MHz, DMSO-d6) δ 171.7, 155.6, 146.8, 128.2, 126.4, 123.9, 116.9, 109.7, 77.5, 39.5, 33.6, 28.3, 25.8. HRMS (ESI+) m/z calc’d. for C15H22BrN2O4+ (M+H)+ 373.0757, found 373.0739.
tert-butyl-(5-((5-bromo-2-hydroxyphenyl)amino)-5-oxopentyl)carbamate (12c).
Synthesized according to General Procedure 1. Purified via column chromatography (50% ethyl acetate/hexanes). White solid (66%, 411 mg). 1H NMR (400 MHz, DMSO-d6) δ 10.12 (s, 1H), 9.14 (s, 1H), 8.04 (d, J = 2.5 Hz, 1H), 7.03 (dd, J = 8.6, 2.5 Hz, 1H), 6.79 – 6.74 (m, 2H), 2.89 (q, J = 6.6 Hz, 2H), 2.36 (t, J = 7.4 Hz, 2H), 1.51 (quin, J = 7.5 Hz, 2H), 1.40 – 1.35 (m, 2H), 1.34 (s, 9H). 13C NMR (101 MHz, DMSO-d6) δ 172.4, 156.0, 147.2, 128.6, 126.8, 124.2, 117.4, 110.1, 77.8, 39.9, 36.1, 29.5, 28.7, 23.0. HRMS (ESI+) m/z calc’d. for C16H24BrN2O4+ (M+H)+ 387.0914, found 387.0915.
tert-butyl-(6-((5-bromo-2-hydroxyphenyl)amino)-6-oxohexyl)carbamate (12d).
Synthesized according to General Procedure 1. Purified via column chromatography (50% ethyl acetate/hexanes). Yellow solid (87%, 26 mg). 1H NMR (400 MHz, DMSO-d6) δ 10.12 (s, 1H), 9.15 (s, 1H), 8.05 (d, J = 2.5 Hz, 1H), 7.04 (dd, J = 8.6, 2.5 Hz, 1H), 6.78 (d, J = 8.5 Hz, 1H), 6.75 – 6.71 (m, 1H), 2.87 (q, J = 6.8 Hz, 2H), 2.36 (t, J = 7.4 Hz, 2H), 1.52 (quin, J = 7.4 Hz, 2H), 1.39 – 1.32 (m, 11H), 1.27 – 1.19 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ 172.0, 155.6, 146.7, 128.2, 126.7, 123.8, 116.9, 109.7, 77.3, 36.0, 29.3, 28.3, 26.0, 26.0, 25.0. HRMS (ESI+) m/z calc’d. for C17H25BrN2NaO4+ (M+Na)+ 423.0890, found 423.0922.
tert-butyl-(2-(5-bromobenzo[d]oxazol-2-yl)ethyl)carbamate (13a).
Synthesized according to General Procedure 2. Purified via column chromatography (20% ethyl acetate/hexanes). Pink solid (69%, 230 mg). 1H NMR (500 MHz, CDCl3) δ 7.81 (d, J = 1.8 Hz, 1H), 7.43 (dd, J = 8.6, 1.9 Hz, 1H), 7.37 (d, J = 8.6 Hz, 1H), 5.21 (s, 1H), 3.68 (q, J = 6.2 Hz, 2H), 3.12 (t, J = 6.2 Hz, 2H), 1.42 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 166.4, 155.8, 149.8, 142.8, 127.8, 122.7, 117.0, 111.7, 79.6, 37.2, 29.4, 28.4. HRMS (ESI+) m/z calc’d. for C14H18BrN2O3+ (M+H)+ 341.0495, found 341.0493.
tert-butyl-(3-(5-bromobenzo[d]oxazol-2-yl)propyl)carbamate (13b).
Synthesized according to General Procedure 2. Purified via column chromatography (20% ethyl acetate/hexanes). Light yellow solid (45%, 117 mg). Synthesized according to General Procedure 2. 1H NMR (400 MHz, CDCl3) δ 7.79 – 7.75 (m, 1H), 7.41 – 7.37 (m, 1H), 7.33 (d, J = 8.6 Hz, 1H), 4.70 (s, 1H), 3.24 (q, J = 6.6 Hz, 2H), 2.96 (t, J = 7.4 Hz, 2H), 2.05 (quin, J = 7.1 Hz, 2H), 1.40 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 168.9, 156.1, 150.0, 143.1, 127.8, 122.8, 117.1, 111.7, 78.6, 28.5, 27.1, 26.1, 21.9. HRMS (ESI+) m/z calc’d. for C15H20BrN2O3+ (M+H)+ 355.0652, found 355.0653.
tert-butyl-(4-(5-bromobenzo[d]oxazol-2-yl)butyl)carbamate (13c).
Synthesized according to General Procedure 2. Purified via column chromatography (20% ethyl acetate/hexanes). Pink solid (53%, 211 mg). 1H NMR (500 MHz, CDCl3) δ 7.80 (d, J = 1.9 Hz, 1H), 7.41 (dd, J = 8.6, 2.0 Hz, 1H), 7.35 (d, J = 8.5 Hz, 1H), 4.65 (s, 1H), 3.19 (q, J = 6.7 Hz, 2H), 2.96 (t, J = 7.5 Hz, 2H), 1.92 (quin, J = 7.3 Hz, 2H), 1.62 (quin, J = 7.2 Hz, 2H), 1.44 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 168.2, 156.1, 149.9, 143.0, 127.7, 122.7, 117.0, 111.7, 79.4, 40.2, 29.6, 28.5, 28.3, 23.9. HRMS (ESI+) m/z calc’d. for C16H22BrN2O3+ (M+H)+ 369.0808, found 369.0837.
tert-butyl-(5-(5-bromobenzo[d]oxazol-2-yl)pentyl)carbamate (13d).
Synthesized according to General Procedure 2. Purified via column chromatography (30% ethyl acetate/hexanes). Pink solid (24%, 75 mg). 1H NMR (500 MHz, CDCl3) δ 7.80 (d, J = 1.9 Hz, 1H), 7.42 (dd, J = 8.5, 1.9 Hz, 1H), 7.36 (d, J = 8.6 Hz, 1H), 5.10 – 4.95 (m, 2H), 4.65 (s, 1H), 3.14 (q, J = 6.7 Hz, 2H), 2.94 (t, J = 7.5 Hz, 2H), 1.90 (quin, J = 7.5 Hz, 2H), 1.55 (quin, J = 7.1 Hz, 2H), 1.44 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 168.3, 156.0, 149.8, 142.9, 127.5, 122.6, 116.8, 111.5, 79.1, 40.2, 29.6, 28.5, 28.4, 26.2, 26.1. HRMS (ESI+) m/z calc’d. for C17H24BrN2O3+ (M+H)+ 383.0965, found 383.0973.
tert-butyl-(2-(5-decylbenzo[d]oxazol-2-yl)ethyl)carbamate (14a).
Synthesized according to General Procedure 3. Purified via column chromatography (20% ethyl acetate/hexanes). Clear oil (53%, 62 mg). 1H NMR (400 MHz, CDCl3) δ 7.46 (s, 1H), 7.36 (d, J = 8.2 Hz, 1H), 7.11 (d, J = 8.4 Hz, 1H), 5.33 (s, 1H), 3.66 (q, J = 6.3 Hz, 2H), 3.10 (t, J = 6.2 Hz, 2H), 2.70 (t, J = 7.7 Hz, 2H), 1.64 (quin, J = 7.0 Hz, 2H), 1.43 (s, 9H), 1.33 – 1.23 (m, 14H), 0.88 (t, J = 6.8 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 165.3, 155.9, 149.3, 141.4, 139.4, 125.3, 119.0, 109.9, 79.5, 36.0, 32.2, 32.0, 29.7, 29.7, 29.6, 29.4, 29.3, 28.5, 26.4, 22.8, 22.1, 14.2. HRMS (ESI+) m/z calc’d. for C24H39N2O3+ (M+H)+ 403.2955, found 403.2933.
tert-butyl-(3-(5-decylbenzo[d]oxazol-2-yl)propyl)carbamate (14b).
Synthesized according to General Procedure 3. Purified via column chromatography (20% ethyl acetate/hexanes). Off-white solid (99 mg, 70%). 1H NMR (500 MHz, CDCl3) δ 7.42 (s, 1H), 7.32 (d, J = 8.3 Hz, 1H), 7.07 (d, J = 8.4 Hz, 1H), 4.89 (s, 1H), 3.28 – 3.18 (m, 2H), 2.93 (t, J = 7.3 Hz, 2H), 2.66 (t, J = 7.6 Hz, 2H), 2.07 – 2.01 (m, 2H), 1.88 – 1.75 (m, 2H), 1.39 (s, 9H), 1.29 – 1.18 (m, 14H), 0.84 (t, J = 6.9 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 166.6, 156.1, 149.2, 141.4, 139.2, 125.1, 118.9, 109.7, 79.2, 39.9, 36.0, 32.2, 32.1, 32.0, 29.7, 29.6, 29.4, 29.2, 28.4, 27.1, 26.3, 22.7, 14.2. HRMS (ESI+) m/z calc’d. for C15H41N2O3+ (M+H)+ 417.3112, found 417.3106.
tert-butyl-(4-(5-decylbenzo[d]oxazol-2-yl)butyl)carbamate (14c).
Synthesized according to General Procedure 3. Purified via column chromatography (20% ethyl acetate/hexanes). Off-white solid (62%, 54 mg). 1H NMR (500 MHz, CDCl3) δ 7.45 (d, J = 1.7 Hz, 1H), 7.35 (d, J = 8.2 Hz, 1H), 7.10 (dd, J = 8.3, 1.7 Hz, 1H), 4.69 (s, 1H), 3.18 (q, J = 6.7 Hz, 2H), 2.93 (t, J = 7.5 Hz, 2H), 2.69 (t, J = 7.8 Hz, 2H), 1.91 (quin, J = 7.6 Hz, 2H), 1.67 – 1.60 (m, 4H), 1.44 (s, 9H), 1.33 – 1.23 (m, 14H), 0.88 (t, J = 7.0 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 166.9, 156.1, 149.2, 141.5, 139.3, 125.1, 119.0, 109.8, 79.2, 40.2, 36.0, 32.1, 32.0, 29.7, 29.7, 29.6, 29.4, 29.3, 28.5, 28.4, 26.4, 24.0, 22.8, 14.2. HRMS (ESI+) m/z calc’d. for C26H43N2O3+ (M+H)+ 431.3268, found 431.3257.
tert-butyl-(5-(5-decylbenzo[d]oxazol-2-yl)pentyl)carbamate (14d).
Synthesized according to General Procedure 3. Purified via column chromatography (30% ethyl acetate/hexanes). Off-white solid (68%, 59 mg). 1H NMR (500 MHz, CDCl3) δ 7.45 (s, 1H), 7.35 (d, J = 8.3 Hz, 1H), 7.10 (dd, J = 8.3, 1.7 Hz, 1H), 4.63 (s, 1H), 3.13 (q, J = 6.7 Hz, 2H), 2.91 (t, J = 7.5 Hz, 2H), 2.69 (t, J = 7.8 Hz, 2H), 1.89 (quin, J = 7.6 Hz, 2H), 1.69 – 1.60 (m, 2H), 1.54 (q, J = 7.2 Hz, 2H), 1.45 – 1.43 (m, 11H), 1.35 – 1.22 (m, 14H), 0.88 (t, J = 6.9 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 167.1, 156.1, 149.2, 141.6, 139.2, 125.1, 119.0, 109.8, 79.3, 40.4, 36.0, 32.2, 32.0, 29.8, 29.7, 29.7, 29.6, 29.4, 29.3, 28.6, 28.5, 26.4, 26.4, 22.8, 14.2. HRMS (ESI+) m/z calc’d. for C27H45N2O3+ (M+H)+ 445.3425, found 445.3417.
2-(5-decylbenzo[d]oxazol-2-yl)ethan-1-amine hydrochloride (15a).
Synthesized according to General Procedure 4a. Purified via trituration with diethyl ether. White solid (73%, 38 mg). 1H NMR (500 MHz, CD3OD) δ 7.55 – 7.42 (m, 2H), 7.22 (d, J = 8.5 Hz, 1H), 3.50 (t, J = 6.8 Hz, 2H), 3.34 (t, J = 6.9 Hz, 2H), 2.72 (t, J = 7.6 Hz, 2H), 1.64 (quin, J = 7.5 Hz, 2H), 1.35 – 1.23 (m, 14H), 0.88 (t, J = 6.9 Hz, 3H). 13C NMR (126 MHz, CD3OD) δ 164.8, 150.7, 142.1, 141.1, 127.1, 119.8, 111.2, 37.6, 36.8, 33.2, 33.1, 30.7, 30.7, 30.6, 30.5, 30.2, 27.1, 23.7, 14.4. HRMS (ESI+) m/z calc’d. for C19H31N2O+ (M+H)+ 303.2431, found 303.2422.
3-(5-decylbenzo[d]oxazol-2-yl)propan-1-amine hydrochloride (15b).
Synthesized according to General Procedure 4a. Purified via trituration with diethyl ether. White solid (92%, 70 mg). 1H NMR (500 MHz, CD3OD) δ 7.48 – 7.42 (m, 2H), 7.21 (dd, J = 8.4, 1.7 Hz, 1H), 3.12 – 3.07 (m, 4H), 2.72 (t, J = 8.0 Hz, 2H), 2.22 (quin, J = 7.4 Hz, 2H), 1.64 (quin, J = 7.2 Hz, 2H), 1.34 – 1.24 (m, 14H), 0.89 (t, J = 6.9 Hz, 3H). 13C NMR (126 MHz, CD3OD) δ 167.6, 150.5, 142.0, 141.0, 126.9, 119.5, 111.0, 40.0, 36.8, 33.2, 33.1, 30.7, 30.7, 30.6, 30.4, 30.2, 26.3, 25.3, 23.7, 14.4. HRMS (ESI+) m/z calc’d. for C20H33N2O+ (M+H)+ 317.2587, found 317.2585.
4-(5-decylbenzo[d]oxazol-2-yl)butan-1-amine hydrochloride (15c).
Synthesized according to General Procedure 4a. Purified via trituration with diethyl ether. White solid (87%, 26 mg). 1H NMR (500 MHz, CD3OD) δ 7.45 (d, J = 8.4 Hz, 1H), 7.42 (s, 1H), 7.20 (dd, J = 8.4, 1.7 Hz, 1H), 3.05 – 2.97 (m, 4H), 2.72 (t, J = 8.2 Hz, 2H), 2.02 – 1.92 (m, 2H), 1.77 (quin, J = 7.7 Hz, 2H), 1.64 (quin, J = 7.0 Hz, 2H), 1.37 – 1.21 (m, 14H), 0.89 (t, J = 6.9 Hz, 3H). 13C NMR (126 MHz, CD3OD) δ 168.5, 150.5, 142.0, 141.0, 126.8, 119.4, 111.0, 40.3, 36.8, 33.2, 33.1, 30.7, 30.7, 30.6, 30.5, 30.2, 28.6, 27.9, 24.4, 23.7, 14.4. HRMS (ESI+) m/z calc’d. for C21H35N2O+ (M+H)+ 331.2744, found 331.2745.
5-(5-decylbenzo[d]oxazol-2-yl)pentan-1-amine hyrdrochloride (15d).
Synthesized according to General Procedure 4a. Purified via trituration with diethyl ether. Off-white solid (59%, 30 mg). 1H NMR (500 MHz, CD3OD) δ 7.45 (d, J = 8.4 Hz, 1H), 7.41 (s, 1H), 7.19 (dd, J = 8.4, 1.7 Hz, 1H), 2.99 (t, J = 7.4 Hz, 2H), 2.94 (t, J = 7.6 Hz, 2H), 2.72 (t, J = 7.8 Hz, 2H), 1.93 (quin, J = 7.5 Hz, 2H), 1.72 (quin, J = 7.6 Hz, 2H), 1.65 (quin, J = 7.1 Hz, 2H), 1.55 – 1.47 (m, 2H), 1.35 – 1.24 (m, 14H), 0.89 (t, J = 6.9 Hz, 3H). 13C NMR (126 MHz, CD3OD) δ 169.0, 150.4, 141.9, 141.0, 126.7, 119.3, 111.0, 40.5, 36.8, 33.2, 33.1, 30.7, 30.7, 30.6, 30.4, 30.2, 28.9, 28.2, 27.1, 26.8, 23.7, 14.4. HRMS (ESI +) m/z calc’d. for C22H37N2O+ (M+H)+ 345.2900, found 345.2879.
5-bromobenzo[d]oxazole-2-thiol (17).
Synthesized according to General Procedure 5. Purified via column chromatography (35% ethyl acetate/hexanes). Pink solid (84%, 572 mg). 1H NMR (400 MHz, DMSO-d6) δ 13.99 (s, 1H), 7.52 – 7.31 (m, 3H). 13C NMR (101 MHz, DMSO-d6) δ 180.5, 147.4, 132.9, 126.3, 117.0, 113.1, 111.7. HRMS (ESI+) m/z calc’d. for C7H5NOS+ (M+H)+ 229.9270, found 229.9265.
6-bromobenzo[d]oxazole-2-thiol (17).
Synthesized according to General Procedure 5. Purified via column chromatography (35% ethyl acetate/hexanes). Pink solid (72%, 401 mg). 1H NMR (400 MHz, DMSO-d6) δ 7.81 (dd, J = 1.9, 0.5 Hz, 1H), 7.45 (dd, J = 8.4, 1.8 Hz, 1H), 7.17 (dd, J = 8.4, 0.5 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 180.7, 149.2, 131.3, 128.4, 115.9, 113.7, 112.2. HRMS (ESI+) m/z calc’d. for C7H5BrNOS+ (M+H)+ 229.9270, found 229.9277.
5-bromo-2-chlorobenzo[d]oxazole (18).
Synthesized according to General Procedure 6a. Purified via column chromatography (5% ethyl acetate/hexanes). White solid (86%, 638 mg). 1H NMR (500 MHz, CDCl3) δ 7.83 (d, J = 2.0 Hz, 1H), 7.50 (dd, J = 8.7, 1.9 Hz, 1H), 7.39 (d, J = 8.7 Hz, 1H). 13C NMR (126 MHz, CDCl3) δ 152.3, 150.7, 142.6, 128.8, 122.9, 118.1, 111.8.
6-bromo-2-chlorobenzo[d]oxazole (19).
Synthesized according to General Procedure 6a. Purified via column chromatography (5% ethyl acetate/hexanes). White solid (87%, 220 mg). 1H NMR (400 MHz, CDCl3) δ 7.69 (dt, J = 1.8, 0.6 Hz, 1H), 7.56 – 7.48 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 152.0, 151.6, 140.4, 128.8, 120.9, 118.8, 114.1.
5-bromo-2-chloro-1H-benzo[d]imidazole (20).
Synthesized according to General Procedure 6b. Purified via column chromatography (35% ethyl acetate/hexanes). Off-white solid (73%, 317 mg). 1H NMR (600 MHz, DMSO) δ 13.51 (s, 1H), 7.73 (s, 1H), 7.54 – 7.41 (m, 1H), 7.36 (dd, J = 8.5, 1.9 Hz, 1H). 13C NMR (126 MHz, DMSO) δ 152.3, 140.0, 136.1, 130.3, 125.5, 114.8, 112.3. HRMS (ESI+) m/z calc’d. for C7H5BrClN2+ (M+H)+ 230.9319, found 230.9337.
tert-butyl-(2-((5-bromobenzo[d]oxazol-2-yl)amino)ethyl)carbamate (22a).
Synthesized according to General Procedure 7a. Purified via column chromatography (60% ethyl acetate/hexanes). Off-white solid (67%, 92 mg). 1H NMR (500 MHz, CDCl3) δ 7.46 (s, 1H), 7.14 (dd, J = 8.4, 1.9 Hz, 1H), 7.09 (d, J = 8.4 Hz, 1H), 5.96 (s, 1H), 4.99 (s, 1H), 3.59 (q, J = 5.3 Hz, 2H), 3.44 (q, J = 5.8 Hz, 2H), 1.43 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 162.9, 157.2, 147.8, 144.9, 123.7, 119.5, 116.7, 110.0, 80.2, 44.4, 40.4, 28.5. HRMS (ESI+) m/z calc’d. for C14H19BrN3O3+ (M+H)+ 356.0604, found 356.0617.
tert-butyl-(3-((5-bromobenzo[d]oxazol-2-yl)amino)propyl)carbamate (22b).
Synthesized according to General Procedure 7a. Purified via column chromatography (60% ethyl acetate/hexanes). White solid (91%, 90 mg). 1H NMR (500 MHz, CDCl3) δ 7.44 (s, 1H), 7.11 (dd, J = 8.4, 1.9 Hz, 1H), 7.07 (d, J = 8.4 Hz, 1H), 6.24 (s, 1H), 4.96 (s, 1H), 3.52 (q, J = 6.1 Hz, 2H), 3.26 (q, J = 6.3 Hz, 2H), 1.79 (quin, J = 6.2 Hz, 2H), 1.45 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 162.9, 157.0, 147.6, 144.9, 123.3, 119.1, 116.4, 109.7, 79.7, 39.6, 37.0, 30.1, 28.4. HRMS (ESI+) m/z calc’d. for C15H21BrN3O3+ (M+H)+ 370.0761, found 370.0754.
tert-butyl-(4-((5-bromobenzo[d]oxazol-2-yl)amino)butyl)carbamate diamine (22c).
Synthesized according to General Procedure 7a. Purified via column chromatography (60% ethyl acetate/hexanes). White solid (51%, 174 mg). 1H NMR (400 MHz, CDCl3) δ 7.47 (s, 1H), 7.14 (dd, J = 8.4, 1.9 Hz, 1H), 7.09 (d, J = 8.4 Hz, 1H), 5.23 (s, 1H), 4.68 (s, 1H), 3.50 (q, J = 6.6 Hz, 2H), 3.19 (q, J = 6.6 Hz, 2H), 1.76 – 1.67 (m, 2H), 1.66 – 1.57 (m, 2H), 1.45 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 163.8, 156.2, 147.6, 144.8, 123.4, 119.2, 116.5, 109.8, 79.3, 42.8, 39.9, 28.4, 27.5, 26.6. HRMS (ESI+) m/z calc’d. for C16H23BrN3O3+ (M+H)+ 384.0917, found 384.0906.
tert-butyl-(5-((5-bromobenzo[d]oxazol-2-yl)amino)pentyl)carbamate (22d).
Synthesized according to General Procedure 7a. Purified via column chromatography (60% ethyl acetate/hexanes). Off-white solid (64%, 66 mg). 1H NMR (500 MHz, CDCl3) δ 7.46 (s, 1H), 7.13 (dd, J = 8.4, 1.9 Hz, 1H), 7.08 (d, J = 8.4 Hz, 1H), 5.40 (s, 1H), 4.58 (s, 1H), 3.47 (q, J = 6.6 Hz, 2H), 3.14 (q, J = 6.6 Hz, 2H), 1.71 (quin, J = 7.1 Hz, 2H), 1.54 (quin, J = 7.0 Hz, 2H), 1.44 (s, 9H), 1.44 – 1.40 (m, 2H). 13C NMR (126 MHz, CDCl3) δ 162.9, 156.3, 147.7, 145.0, 123.5, 119.4, 116.7, 109.9, 79.4, 43.2, 40.2, 30.0, 29.1, 28.6, 23.8. HRMS (ESI+) m/z calc’d. for C17H25BrN3O3+ (M+H)+ 398.1074, found 398.1053.
tert-butyl-(2-((5-bromobenzo[d]oxazol-2-yl)(methyl)amino)ethyl)carbamate (22e).
Synthesized according to General Procedure 7a. Purified via column chromatography (50% ethyl acetate/hexanes). White solid (68%, 100 mg). 1H NMR (400 MHz, CDCl3) δ 7.45 (s, 1H), 7.14 – 7.08 (m, 2H), 4.91 (s, 1H), 3.68 (t, J = 6.2 Hz, 2H), 3.43 (q, J = 6.2 Hz, 2H), 3.24 (s, 3H), 1.37 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 180.2, 163.1, 156.0, 147.9, 123.1, 119.0, 116.8, 109.8, 79.6, 50.2, 38.5, 36.2, 28.3. HRMS (ESI+) m/z calc’d. for C15H21BrN3O3+ (M+H)+ 370.0761, found 370.0736.
di-tert-butyl-(((5-bromobenzo[d]oxazol-2-yl)azanediyl)bis(ethane-2,1-diyl))dicarbamate (22f).
Synthesized according to General Procedure 7a. Purified via column chromatography (50% ethyl acetate/hexanes). White solid (72%, 142 mg). 1H NMR (400 MHz, CDCl3) δ 7.44 (d, J = 1.7 Hz, 1H), 7.14 – 7.06 (m, 2H), 5.14 (s, 2H), 3.67 (t, J = 5.9 Hz, 4H), 3.43 (q, J = 6.0 Hz, 4H), 1.36 (s, 18H). 13C NMR (101 MHz, CDCl3) δ 156.2, 156.0, 147.8, 145.0, 123.2, 119.1, 116.7, 109.8, 79.5, 49.6, 39.3, 28.3. HRMS (ESI+) m/z calc’d. for C21H32BrN4O5+ (M+H)+ 499.1551, found 499.1591.
tert-butyl-(S)-(1-(5-bromobenzo[d]oxazol-2-yl)pyrrolidin-3-yl)carbamate (22g).
Synthesized according to General Procedure 7a. Purified via column chromatography (60% ethyl acetate/hexanes). White solid (66%, 82 mg). 1H NMR (400 MHz, CDCl3) δ 7.46 (s, 1H), 7.12 – 7.11 (m, 2H), 4.71 (s, 1H), 4.37 (s, 1H), 3.89 (dd, J = 11.0, 6.0 Hz, 1H), 3.78 – 3.70 (m, 2H), 3.53 (dd, J = 11.2, 4.2 Hz, 1H), 2.38 – 2.23 (m, 1H), 2.07 – 1.95 (m, 1H), 1.46 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 161.2, 155.2, 148.1, 145.1, 123.1, 119.2, 116.7, 109.8, 80.1, 53.3, 50.5, 45.6, 31.7, 28.4. HRMS (ESI+) m/z calc’d. for C16H21BrN3O3+ (M+H)+ 382.0766, found 382.0737.
tert-butyl-(R)-(1-(5-bromobenzo[d]oxazol-2-yl)pyrrolidin-3-yl)carbamate (22h).
Synthesized according to General Procedure 7a. Purified via column chromatography (60% ethyl acetate/hexanes). Off-white solid (50%, 70 mg). 1H NMR (400 MHz, CDCl3) δ 7.47 (s, 1H), 7.16 – 7.05 (m, 2H), 4.71 (s, 1H), 4.37 (s, 1H), 3.89 (dd, J = 11.0, 6.0 Hz, 1H), 3.78 – 3.67 (m, 2H), 3.53 (dd, J = 11.1, 4.2 Hz, 1H), 2.37 – 2.24 (m, 1H), 2.07 – 1.96 (m, 1H), 1.46 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 161.2, 155.2, 148.1, 145.2, 123.1, 119.2, 116.7, 109.8, 80.1, 53.3, 50.5, 45.6, 31.7, 28.4. HRMS (ESI+) m/z calc’d. for C16H21BrN3O3+ (M+H)+ 382.0766, found 382.0737.
tert-butyl-4-(5-bromobenzo[d]oxazol-2-yl)piperazine-1-carboxylate (22i).
Synthesized according to General Procedure 7a. Purified via column chromatography (45% ethyl acetate/hexanes). White solid (71%, 105 mg). 1H NMR (500 MHz, CDCl3) δ 7.47 (s, 1H), 7.16 – 7.09 (m, 2H), 3.67 (t, J = 6.6 Hz, 4H), 3.57 (t, J = 6.7 Hz, 4H), 1.49 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 162.5, 154.5, 147.8, 144.8, 123.6, 119.4, 116.8, 109.9, 80.5, 45.4, 43.3, 28.4. HRMS (ESI+) m/z calc’d. for C16H21BrN3O3+ (M+H)+ 382.0766, found 382.0737.
tert-butyl-3-((5-bromobenzo[d]oxazol-2-yl)amino)azetidine-1-carboxylate (22j).
Synthesized according to General Procedure 7a. Purified via column chromatography (45% ethyl acetate/hexanes). Off-white solid (66%, 90 mg). 1H NMR (500 MHz, CDCl3) δ 7.48 (d, J = 2.0 Hz, 1H), 7.18 (dd, J = 8.4, 1.9 Hz, 1H), 7.13 (d, J = 8.5 Hz, 1H), 6.44 (s, 1H), 4.64 (quin, J = 6.3 Hz, 1H), 4.38 (dd, J = 9.4, 7.5 Hz, 2H), 3.94 (dd, J = 9.5, 5.0 Hz, 2H), 1.46 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 161.4, 156.2, 147.6, 144.2, 124.1, 119.6, 116.9, 110.1, 80.2, 56.8, 42.7, 28.4. HRMS (ESI+) m/z calc’d. for C15H18BrN3O3+ (M+H)+ 368.0610, found 368.0587.
tert-butyl-(S)-3-((5-bromobenzo[d]oxazol-2-yl)amino)pyrrolidine-1-carboxylate (22k).
Synthesized according to General Procedure 7a. Purified via column chromatography (45% ethyl acetate/hexanes). White solid (73%, 201 mg). 1H NMR (500 MHz, CDCl3) δ 7.49 (s, 1H), 7.16 (d, J = 8.4 Hz, 1H), 7.11 (d, J = 8.4 Hz, 1H), 5.60 (s, 1H), 4.46 (s, 1H), 3.74 (dd, J = 11.6, 5.9 Hz, 1H), 3.58 – 3.31 (m, 3H), 2.35 – 2.20 (m, 1H), 2.18 – 1.96 (m, 1H), 1.47 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 161.8, 154.5, 147.6, 144.5, 123.9, 119.6, 116.7, 110.0, 79.9, 52.7*, 51.6*, 43.8*, 31.5*, 28.5. HRMS (ESI+) m/z calc’d. for C16H21BrN3O3+ (M+H)+ 382.0761, found 382.0763.
tert-butyl-(R)-3-((5-bromobenzo[d]oxazol-2-yl)amino)pyrrolidine-1-carboxylate (22l).
Synthesized according to General Procedure 7a. Purified via column chromatography (45% ethyl acetate/hexanes). White solid (83%, 175 mg). 1H NMR (400 MHz, CDCl3) δ 7.50 (d, J = 1.8 Hz, 1H), 7.17 (dd, J = 8.5, 1.9 Hz, 1H), 7.11 (d, J = 8.4 Hz, 1H), 5.06 (s, 1H), 4.46 (s, 1H), 3.74 (dd, J = 11.6, 5.9 Hz, 1H), 3.57 – 3.32 (m, 4H), 2.33 – 2.23 (m, 1H), 2.12 – 1.96 (m, 1H), 1.47 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 161.7, 154.5, 147.6, 144.5, 123.8, 119.5, 116.7, 109.9, 79.8, 52.7*, 51.5*, 43.8*, 31.4*, 28.5. HRMS (ESI+) m/z calc’d. for C16H21BrN3O3+ (M+H)+ 382.0749, found 382.0763.
tert-butyl-4-((5-bromobenzo[d]oxazol-2-yl)amino)piperidine-1-carboxylate (22m).
Synthesized according to General Procedure 7a. Purified via column chromatography (45% ethyl acetate/hexanes). White solid (65%, 95 mg). 1H NMR (500 MHz, CDCl3) δ 7.47 (s, 1H), 7.15 (dd, J = 8.4, 1.8 Hz, 1H), 7.10 (d, J = 8.5 Hz, 1H), 5.14 (d, J = 7.8 Hz, 1H), 4.10 (s, 2H), 3.94 – 3.85 (m, 1H), 2.95 (t, J = 12.9 Hz, 2H), 2.17 – 2.07 (m, 2H), 1.53 – 1.40 (m, 11H). 13C NMR (126 MHz, CDCl3) δ 161.8, 154.8, 147.6, 144.8, 123.8, 119.6, 116.8, 110.0, 80.0, 50.8, 42.6, 32.4, 28.6. HRMS (ESI+) m/z calc’d. for C17H23BrN3O3+ (M+H)+ 396.0917, found 396.0896.
tert-butyl-(S)-3-((5-bromobenzo[d]oxazol-2-yl)amino)piperidine-1-carboxylate (22n).
Synthesized according to General Procedure 7a. Purified via column chromatography (45% ethyl acetate/hexanes). Off-white solid (59%, 88 mg). 1H NMR (400 MHz, CDCl3) δ 7.47 (s, 1H), 7.15 (dd, J = 8.4, 2.9 Hz, 1H), 7.10 (d, J = 8.4 Hz, 1H), 5.44 (s, 1H), 4.04 – 3.87 (m, 1H), 3.73 – 3.64 (m, 1H), 3.56 – 3.44 (m, 2H), 3.42 – 3.34 (m, 1H), 2.05 – 1.94 (m, 1H), 1.87 – 1.68 (m, 3H), 1.43 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 161.9, 155.4, 147.6, 144.9, 123.8, 119.6, 116.8, 110.0, 80.3, 49.0, 48.6, 29.8, 28.8, 28.5, 22.2. HRMS (ESI+) m/z calc’d. for C17H23BrN3O3+ (M+H)+ 396.0917, found 396.0925.
tert-butyl-(R)-3-((5-bromobenzo[d]oxazol-2-yl)amino)piperidine-1-carboxylate (22o).
Synthesized according to General Procedure 7a. Purified via column chromatography (45% ethyl acetate/hexanes). Off-white solid (74%, 143 mg). 1H NMR (400 MHz, CDCl3) δ 7.44 (s, 1H), 7.16 – 7.09 (m, 1H), 7.07 (d, J = 10.4 Hz, 1H), 5.50 (s, 1H), 3.95 – 3.85 (m, 1H), 3.67 (d, J = 13.3 Hz, 1H), 3.54 – 3.30 (m, 4H), 2.03 – 1.90 (m, 2H), 1.85 – 1.67 (m, 2H), 1.63 – 1.52 (m, 2H), 1.40 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 161.8, 155.1, 147.5, 144.7, 123.6, 119.4, 116.6, 109.8, 80.1, 48.8, 48.5, 29.7, 28.3, 28.3, 22.1. HRMS (ESI+) m/z calc’d. for C17H23BrN3O3+ (M+H)+ 396.0917, found 396.0916.
tert-butyl-(R)-3-(((5-bromobenzo[d]oxazol-2-yl)amino)methyl)pyrrolidine-1-carboxylate (22p).
Synthesized according to General Procedure 7a. Purified via column chromatography (45% ethyl acetate/hexanes). Off-white solid (73%, 1.142 g). 1H NMR (400 MHz, CDCl3) δ 7.42 (s, 1H), 7.16 – 7.01 (m, 2H), 6.04 – 5.75 (m, 1H), 3.60 – 3.27 (m, 5H), 3.20 – 3.02 (m, 1H), 2.59 (quin, J = 7.3 Hz, 1H), 2.09 – 1.97 (m, 1H), 1.74 – 1.62 (m, 1H), 1.43 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 162.7, 154.5, 147.5, 144.6, 123.6, 119.3, 116.6, 109.8, 79.4, 49.2*, 45.5*, 45.1*, 38.4*, 29.2, 28.5. HRMS (ESI+) m/z calc’d. for C17H23BrN3O3+ (M+H)+ 396.0917, found 396.0919
tert-butyl-(S)-3-(((5-bromobenzo[d]oxazol-2-yl)amino)methyl)pyrrolidine-1-carboxylate (22q).
Synthesized according to General Procedure 7a. Purified via column chromatography (45% ethyl acetate/hexanes). Off-white solid (75%, 113 mg). 1H NMR (500 MHz, CDCl3) δ 7.45 (s, 1H), 7.17 – 7.07 (m, 2H), 5.88 – 5.61 (m, 1H), 3.63 – 3.27 (m, 5H), 3.21 – 3.06 (m, 1H), 2.62 (q, J = 7.1 Hz, 1H), 2.14 – 1.99 (m, 1H), 1.78 – 1.64 (m, 1H), 1.46 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 162.8, 154.7, 147.7, 144.8, 123.8, 119.5, 116.8, 110.0, 79.6, 49.3*, 45.6*, 45.2*, 38.6*, 29.3, 28.6. HRMS (ESI+) m/z calc’d. for C17H23BrN3O3+ (M+H)+ 396.0917, found 396.0913.
tert-butyl-(S)-2-(((5-bromobenzo[d]oxazol-2-yl)amino)methyl)pyrrolidine-1-carboxylate (22r).
Synthesized according to General Procedure 7a. Purified via column chromatography (45% ethyl acetate/hexanes). Off-white solid (88%, 134 mg). 1H NMR (600 MHz, CDCl3) δ 7.45 (d, J = 12.0 Hz, 1H), 7.31 (s, 1H), 7.20 – 7.05 (m, 1H), 4.27 – 4.07 (m, 1H), 3.67 – 3.27 (m, 4H), 2.18 – 2.03 (m, 2H), 1.98 – 1.85 (m, 2H), 1.82 – 1.68 (m, 1H), 1.50 (s, 9H). 13C NMR (151 MHz, CDCl3) δ 163.0, 157.2, 147.8, 145.2, 123.3, 119.1, 116.4, 109.9, 80.6, 56.6, 49.8, 47.5, 29.8, 28.5, 24.1. HRMS (ESI+) m/z calc’d. for C17H23BrN3O3+ (M+H)+ 396.0917, found 396.0923.
tert-butyl-(R)-2-(((5-bromobenzo[d]oxazol-2-yl)amino)methyl)pyrrolidine-1-carboxylate (22s).
Synthesized according to General Procedure 7a. Purified via column chromatography (45% ethyl acetate/hexanes). Off-white solid (68%, 98 mg). 1H NMR (600 MHz, CDCl3) δ 7.43 (s, 1H), 7.31 (s, 1H), 7.16 – 7.05 (m, 2H), 4.18 (t, J = 9.3 Hz, 1H), 3.63 – 3.33 (m, 4H), 2.13 – 2.03 (m, 1H), 1.98 – 1.85 (m, 2H), 1.79 – 1.70 (m, 1H), 1.48 (s, 9H). 13C NMR (151 MHz, CDCl3) δ 162.8, 157.1, 147.7, 145.0, 123.2, 119.0, 116.3, 109.7, 80.5, 56.4, 49.6, 47.3, 29.6, 28.4, 24.0. HRMS (ESI+) m/z calc’d. for C17H23BrN3O3+ (M+H)+ 396.0917, found 396.0918.
tert-butyl-4-(((5-bromobenzo[d]oxazol-2-yl)amino)methyl)piperidine-1-carboxylate (22t).
Synthesized according to General Procedure 7a. Purified via column chromatography (45% ethyl acetate/hexanes). White solid (72%, 116 mg). 1H NMR (400 MHz, CDCl3) δ 7.45 (t, J = 1.8 Hz, 1H), 7.14 (dt, J = 8.4, 1.9 Hz, 1H), 7.09 (dd, J = 8.4, 1.6 Hz, 1H), 5.67 (s, 1H), 4.24 – 4.06 (m, 3H), 3.45 – 3.32 (m, 2H), 2.71 (t, J = 12.9 Hz, 2H), 1.88 – 1.71 (m, 4H), 1.46 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 163.0, 154.9, 147.6, 144.9, 123.6, 119.4, 116.8, 109.9, 79.7, 48.6, 43.6, 36.5, 29.7, 28.6. HRMS (ESI+) m/z calc’d. for C18H25BrN3O3+ (M+H)+ 410.1074, found 410.1076.
tert-butyl-(R)-3-(((5-bromobenzo[d]oxazol-2-yl)amino)methyl)piperidine-1-carboxylate (22u)
Synthesized according to General Procedure 7. Purified via column chromatography (45% ethyl acetate/hexanes). Off-white solid (80%, 116 mg). 1H NMR (500 MHz, CDCl3) δ 7.45 (s, 1H), 7.13 (d, J = 10.3 Hz, 1H), 7.08 (d, J = 8.4 Hz, 1H), 5.82 (s, 1H), 3.73 (dd, J = 14.4, 3.7 Hz, 1H), 3.64 – 3.38 (m, 2H), 3.37 – 2.98 (m, 3H), 2.00 – 1.91 (m, 1H), 1.89 – 1.83 (m, 1H), 1.70 – 1.61 (m, 2H), 1.45 (s, 9H), 1.37 (q, J = 14.5 Hz, 1H). 13C NMR (126 MHz, CDCl3) δ 162.9, 155.6, 147.7, 145.0, 123.6, 119.4, 116.7, 109.9, 79.9, 46.5, 45.4, 45.1, 35.2, 28.6, 28.0, 23.6. HRMS (ESI+) m/z calc’d. for C18H25BrN3O3+ (M+H)+ 410.1074, found 410.1070.
tert-butyl-(S)-3-(((5-bromobenzo[d]oxazol-2-yl)amino)methyl)piperidine-1-carboxylate (22v)
Synthesized according to General Procedure 7a. Purified via column chromatography (45% ethyl acetate/hexanes). Off-white solid (51%, 80 mg). 1H NMR (600 MHz, CDCl3) δ 7.46 (s, 1H), 7.14 (d, J = 8.3 Hz, 1H), 7.10 (d, J = 8.8 Hz, 1H), 5.84 (s, 1H), 3.74 (d, J = 13.5 Hz, 1H), 3.62 – 3.37 (m, 2H), 3.33 – 3.18 (m, 1H), 3.15 – 2.94 (m, 2H), 2.02 – 1.92 (m, 1H), 1.92 – 1.85 (m, 1H), 1.74 – 1.63 (m, 1H), 1.47 (s, 9H), 1.42 – 1.34 (m, 2H). 13C NMR (151 MHz, CDCl3) δ 162.9, 155.5, 147.7, 144.9, 123.6, 119.4, 116.6, 109.9, 79.9, 46.4, 45.4, 45.0, 35.1, 28.5, 28.0, 23.6. HRMS (ESI+) m/z calc’d. for C18H25BrN3O3+ (M+H)+ 410.1074, found 410.1074
tert-butyl-(R)-3-((6-bromobenzo[d]oxazol-2-yl)amino)pyrrolidine-1-carboxylate (23p).
Synthesized according to General Procedure 7a. Purified via column chromatography (45% ethyl acetate/hexanes). White solid (76%, 132 mg). 1H NMR (500 MHz, CDCl3) δ 7.41 (s, 1H), 7.30 (dd, J = 8.4, 1.9 Hz, 1H), 7.23 (d, J = 8.3 Hz, 1H), 5.46 (d, J = 19.9 Hz, 1H), 4.46 (s, 1H), 3.74 (dd, J = 11.6, 5.9 Hz, 1H), 3.59 – 3.32 (m, 3H), 2.34 – 2.22 (m, 1H), 2.15 – 1.98 (m, 1H), 1.47 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 161.2, 154.5, 149.0, 142.1, 127.2, 117.5, 113.2, 112.4, 79.9, 53.1*, 51.6*, 43.6*, 31.4*, 28.5. HRMS (ESI+) m/z calc’d. for C16H21BrN3O3+ (M+H)+ 382.0761, found 382.0764.
tert-butyl-(R)-3-(((6-bromo-1H-benzo[d]imidazol-2-yl)amino)methyl)pyrrolidine-1-carboxylate (24p).
Synthesized according to General Procedure 7b. Purified via column chromatography (100% ethyl acetate). Brown solid (69%, 122 mg). 1H NMR (500 MHz, CDCl3) δ 10.64 (s, 1H), 7.40 – 7.31 (m, 1H), 7.15 – 7.02 (m, 2H), 5.80 – 5.51 (m, 1H), 3.63 – 3.54 (m, 1H), 3.53 – 3.45 (m, 1H), 3.44 – 3.38 (m, 1H), 3.32 – 3.21 (m, 2H), 3.14 – 3.00 (m, 1H), 2.63 – 2.48 (m, 1H), 2.05 – 1.86 (m, 1H), 1.64 – 1.54 (m, 1H), 1.43 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 156.7, 155.9, 155.4, 155.0, 123.4, 115.5, 113.2, 80.3, 66.0, 49.5, 45.6, 38.2, 29.2, 28.7, 15.4.
tert-butyl-(2-((5-decylbenzo[d]oxazol-2-yl)amino)ethyl)carbamate (25a).
Synthesized according to General Procedure 3b. Purified via column chromatography (60% ethyl acetate/hexanes). White solid (60%, 56 mg). 1H NMR (500 MHz, CDCl3) δ 7.15 (s, 1H), 7.10 (d, J = 8.1 Hz, 1H), 6.81 (dd, J = 8.1, 1.7 Hz, 1H), 6.22 (s, 1H), 5.21 (s, 1H), 3.58 (t, J = 5.6 Hz, 2H), 3.42 (q, J = 5.9 Hz, 2H), 2.61 (t, J = 7.7 Hz, 2H), 1.59 (quin, J = 7.3 Hz, 2H), 1.40 (s, 9H), 1.31 – 1.21 (m, 14H), 0.87 (t, J = 6.9 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 162.6, 156.8, 146.9, 142.9, 139.0, 121.1, 116.0, 108.2, 79.8, 43.8, 40.5, 36.1, 32.1, 32.0, 29.7, 29.7, 29.6, 29.4, 29.3, 28.4, 22.8, 14.2. HRMS (ESI+) m/z calc’d. for C24H40N3O3+ (M+H)+ 418.3064, found 418.3078.
tert-butyl-(3-((5-decylbenzo[d]oxazol-2-yl)amino)propyl)carbamate (25b).
Synthesized according to General Procedure 3b. Purified via column chromatography (60% ethyl acetate/hexanes). White solid (91%, 90 mg). 1H NMR (500 MHz, CDCl3) δ 7.42 (s, 1H), 7.32 (d, J = 8.3 Hz, 1H), 7.07 (d, J = 8.4 Hz, 1H), 4.89 (s, 1H), 3.36 – 3.15 (m, 1H), 2.93 (t, J = 7.3 Hz, 2H), 2.66 (t, J = 7.6 Hz, 2H), 2.11 – 1.99 (m, 2H), 1.91 – 1.55 (m, 4H), 1.39 (s, 9H), 1.29 – 1.18 (m, 14H), 0.84 (t, J = 6.9 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 166.6, 156.1, 149.2, 141.4, 139.2, 125.1, 118.9, 109.7, 79.2, 39.9, 36.0, 32.2, 32.1, 32.0, 29.7, 29.6, 29.4, 29.2, 28.4, 27.1, 26.3, 22.7, 14.2. HRMS (ESI+) m/z calc’d. for C25H42N3O3+ (M+H)+ 432.3221, found 432.3227.
tert-butyl-(4-((5-decylbenzo[d]oxazol-2-yl)amino)butyl)carbamate (25c).
Synthesized according to General Procedure 3b. Purified via column chromatography (60% ethyl acetate/hexanes). Off-white solid (77%, 89 mg). 1H NMR (500 MHz, CDCl3) δ 7.15 (s, 1H), 7.11 (d, J = 8.1 Hz, 1H), 6.82 (dd, J = 8.2, 1.7 Hz, 1H), 5.94 (s, 1H), 4.81 (s, 1H), 3.47 (t, J = 7.1 Hz, 2H), 3.16 (q, J = 6.8 Hz, 2H), 2.62 (t, J = 7.7 Hz, 2H), 1.73 – 1.66 (m, 2H), 1.63 – 1.55 (m, 4H), 1.43 (s, 9H), 1.32 – 1.23 (m, 14H), 0.87 (t, J = 6.9 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 162.5, 156.2, 146.8, 143.0, 138.9, 120.9, 115.9, 108.1, 79.3, 42.7, 40.1, 36.1, 32.1, 32.0, 29.7, 29.7, 29.6, 29.4, 29.3, 28.5, 27.4, 26.9, 22.8, 14.2. HRMS (ESI+) m/z calc’d. for C26H44N3O3+ (M+H)+ 446.3377, found 446.3351.
tert-butyl-(5-((5-decylbenzo[d]oxazol-2-yl)amino)pentyl)carbamate (25d).
Synthesized according to General Procedure 3b. Purified via column chromatography (60% ethyl acetate/hexanes). Off-white solid (65%, 51 mg). 1H NMR (500 MHz, CDCl3) δ 7.17 (s, 1H), 7.11 (d, J = 8.1 Hz, 1H), 6.83 (dd, J = 8.1, 1.7 Hz, 1H), 5.00 (s, 1H), 4.55 (s, 1H), 3.47 (q, J = 6.6 Hz, 2H), 3.14 (q, J = 6.9 Hz, 2H), 2.67 – 2.58 (m, 2H), 1.70 (quin, J = 7.2 Hz, 2H), 1.64 – 1.57 (m, 2H), 1.52 (q, J = 7.1 Hz, 2H), 1.44 (s, 9H), 1.44 – 1.38 (m, 2H), 1.32 – 1.22 (m, 14H), 0.87 (t, J = 6.9 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 162.4, 156.3, 146.9, 143.2, 139.0, 121.1, 116.3, 108.2, 79.4, 43.2, 40.3, 36.1, 32.2, 32.0, 30.0, 29.8, 29.8, 29.7, 29.5, 29.4, 28.6, 23.9, 22.8, 14.3. HRMS (ESI+) m/z calc’d. for C27H46N3O3+ (M+H)+ 460.3534, found 460.3529.
tert-butyl-(2-((5-decylbenzo[d]oxazol-2-yl)(methyl)amino)ethyl)carbamate (25e).
Synthesized according to General Procedure 3b. Purified via column chromatography (50% ethyl acetate/hexanes). White solid (69%, 80 mg). 1H NMR (400 MHz, CDCl3) δ 7.15 (s, 1H), 7.12 (d, J = 8.1 Hz, 1H), 6.81 (d, J = 8.2 Hz, 1H), 4.92 (s, 1H), 3.66 (t, J = 6.2 Hz, 2H), 3.42 (q, J = 6.1 Hz, 2H), 3.21 (s, 3H), 2.63 (t, J = 7.6 Hz, 2H), 1.60 (quin, J = 7.3 Hz, 2H), 1.38 (s, 9H), 1.32 – 1.24 (m, 14H), 0.88 (t, J = 6.9, 3H). 13C NMR (101 MHz, CDCl3) δ 163.1, 156.2, 147.3, 143.5, 139.1, 120.7, 116.0, 108.2, 79.6, 50.2, 38.8, 36.3, 36.1, 32.1, 32.0, 29.8, 29.7, 29.7, 29.5, 29.3, 28.4, 22.8, 14.3. HRMS (ESI+) m/z calc’d. for C25H42N3O3+ (M+H)+ 432.3221, found 432.3226.
di-tert-butyl-(((5-decylbenzo[d]oxazol-2-yl)azanediyl)bis(ethane-2,1-diyl))dicarbamate (25f).
Synthesized according to General Procedure 3b. Purified via column chromatography (50% ethyl acetate/hexanes). Off-white solid (70%, 112 mg). 1H NMR (400 MHz, CDCl3) δ 7.12 (d, J = 1.6 Hz, 1H), 7.09 (d, J = 8.1 Hz, 1H), 6.79 (dd, J = 8.2, 1.7 Hz, 1H), 5.15 (s, 2H), 3.65 (t, J = 6.0 Hz, 4H), 3.41 (q, J = 6.0 Hz, 4H), 2.61 (t, J = 7.6 Hz, 2H), 1.58 (quin, J = 6.9 Hz, 2H), 1.33 (s, 18H), 1.29 – 1.20 (m, 14H), 0.84 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 159.4, 156.4, 147.2, 143.2, 139.1, 120.9, 116.0, 108.3, 79.8, 49.5, 39.3, 36.1, 32.1, 32.0, 29.8, 29.8, 29.7, 29.5, 29.3, 28.4, 22.8, 14.3. HRMS (ESI+) m/z calc’d. for C31H53N4O5+ (M+H)+ 561.4010, found 561.3996.
tert-butyl-(S)-(1-(5-decylbenzo[d]oxazol-2-yl)pyrrolidin-3-yl)carbamate (25g).
Synthesized according to General Procedure 3b. Purified via column chromatography (60% ethyl acetate/hexanes). White solid (66%, 82 mg). 1H NMR (500 MHz, CDCl3) δ 7.17 (s, 1H), 7.14 (d, J = 8.1 Hz, 1H), 6.82 (dd, J = 8.1, 1.7 Hz, 1H), 4.75 (s, 1H), 4.37 (s, 1H), 3.87 (dd, J = 10.9, 6.0 Hz, 1H), 3.78 – 3.67 (m, 2H), 3.52 (d, J = 10.9 Hz, 1H), 2.63 (t, J = 8.0 Hz, 2H), 2.35 – 2.22 (m, 1H), 2.04 – 1.93 (m, 1H), 1.60 (quin, J = 7.1 Hz, 2H), 1.45 (s, 9H), 1.33 – 1.21 (m, 14H), 0.87 (t, J = 6.9 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 161.0, 155.3, 147.4, 143.4, 139.1, 120.8, 116.1, 108.2, 80.1, 53.4, 50.7, 45.6, 36.1, 32.1, 32.0, 31.9, 29.8, 29.7, 29.7, 29.5, 29.3, 28.5, 22.8, 14.3. HRMS (ESI+) m/z calc’d. for C26H42N3O3+ (M+H)+ 444.3226, found 444.3195.
tert-butyl-(R)-(1-(5-decylbenzo[d]oxazol-2-yl)pyrrolidin-3-yl)carbamate (25h).
Synthesized according to General Procedure 3b. Purified via column chromatography (60% ethyl acetate/hexanes). Off-white solid (70%, 53 mg). 1H NMR (400 MHz, CDCl3) δ 7.18 (s, 1H), 7.14 (d, J = 8.1 Hz, 1H), 6.82 (dd, J = 8.1, 1.7 Hz, 1H), 4.72 (s, 1H), 4.37 (s, 1H), 3.88 (dd, J = 10.9, 6.0 Hz, 1H), 3.80 – 3.68 (m, 2H), 3.58 – 3.47 (m, 1H), 2.63 (t, J = 7.6 Hz, 2H), 2.37 – 2.24 (m, 1H), 2.07 – 1.95 (m, 1H), 1.61 (quin, J = 7.4 Hz, 2H), 1.46 (s, 9H), 1.32 – 1.22 (m, 14H), 0.88 (t, J = 6.7 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 160.8, 155.2, 147.2, 143.1, 139.0, 120.7, 116.0, 108.1, 80.0, 53.3, 50.5, 45.5, 36.0, 32.0, 31.9, 31.8, 29.6, 29.6, 29.5, 29.3, 29.2, 28.4, 22.7, 14.1. HRMS (ESI+) m/z calc’d. for C26H42N3O3+ (M+H)+ 444.3226, found 444.3195.
tert-butyl-4-(5-decylbenzo[d]oxazol-2-yl)piperazine-1-carboxylate (25i).
Synthesized according to General Procedure 3b. Purified via column chromatography (45% ethyl acetate/hexanes). White solid (79%, 84 mg). 1H NMR (500 MHz, CDCl3) δ 7.18 (s, 1H), 7.15 (d, J = 8.1 Hz, 1H), 6.85 (dd, J = 8.1, 1.7 Hz, 1H), 3.66 (t, J = 6.6 Hz, 3H), 3.56 (t, J = 6.7 Hz, 3H), 2.64 (t, J = 7.6 Hz, 2H), 1.65 – 1.56 (m, 2H), 1.49 (s, 9H), 1.34 – 1.19 (m, 14H), 0.87 (t, J = 7.0 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 162.2, 154.6, 147.0, 142.9, 139.1, 121.2, 116.2, 108.2, 80.4, 45.5, 42.7, 36.0, 32.0, 31.9, 29.6, 29.6, 29.5, 29.3, 29.2, 28.4, 22.7, 14.1. HRMS (ESI+) m/z calc’d. for C26H42N3O3+ (M+H)+ 444.3221, found 444.3195.
tert-butyl-3-((5-decylbenzo[d]oxazol-2-yl)amino)azetidine-1-carboxylate (25j).
Synthesized according to General Procedure 3b. Purified via column chromatography (45% ethyl acetate/hexanes). Yellow solid (66%, 90 mg). 1H NMR (400 MHz, CDCl3) δ 7.19 (s, 1H), 7.15 (d, J = 8.2 Hz, 1H), 6.88 (dd, J = 8.3, 2.2 Hz, 1H), 5.35 (s, 1H), 4.63 (s, 1H), 4.37 (dd, J = 9.4, 7.5 Hz, 2H), 3.89 (dd, J = 9.4, 7.5 Hz, 1H), 2.64 (t, J = 7.6 Hz, 2H), 1.45 (s, 9H), 1.34 – 1.21 (m, 14H), 0.87 (t, J = 6.8 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 160.7, 156.2, 146.7, 141.9, 139.3, 121.7, 116.4, 108.4, 80.0, 42.7, 36.0, 32.0, 31.9, 29.7, 29.6, 29.6, 29.5, 29.3, 29.2, 28.4, 22.7, 14.1. HRMS (ESI+) m/z calc’d. for C25H39N3O3+ (M+H)+ 430.3070, found 430.3100.
tert-butyl-(S)-3-((5-decylbenzo[d]oxazol-2-yl)amino)pyrrolidine-1-carboxylate (25k).
Synthesized according to General Procedure 3b. Purified via column chromatography (45% ethyl acetate/hexanes). Yellow oil (78%, 178 mg). 1H NMR (500 MHz, CDCl3) δ 7.20 (s, 1H), 7.13 (d, J = 8.0 Hz, 1H), 6.85 (d, J = 8.1 Hz, 1H), 6.17 (s, 1H), 4.45 (s, 1H), 3.74 (dd, J = 11.5, 6.0 Hz, 1H), 3.58 – 3.34 (m, 3H), 2.63 (t, J = 7.7 Hz, 2H), 2.29 – 2.23 (m, 1H), 2.14 – 1.98 (m, 1H), 1.61 (quin, J = 7.2 Hz, 2H), 1.46 (s, 9H), 1.34 – 1.20 (m, 14H), 0.88 (t, J = 7.0 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 161.5, 154.7, 146.7, 142.4, 139.2, 121.4, 116.2, 108.3, 79.9, 52.6 (d), 51.8 (d), 44.1, 43.8, 36.1, 32.1, 32.0, 31.3, 29.7, 29.6, 29.4, 29.3, 28.6, 22.8, 14.2. HRMS (ESI+) m/z calc’d. for C26H42N3O3+ (M+H)+ 444.3321, found 444.3320.
tert-butyl-(R)-3-((5-decylbenzo[d]oxazol-2-yl)amino)pyrrolidine-1-carboxylate (22l).
Synthesized according to General Procedure 6a. Purified via column chromatography (45% ethyl acetate/hexanes). Yellow oil (60%, 122 mg). 1H NMR (400 MHz, CDCl3) δ 7.19 (s, 1H), 7.14 (d, J = 8.2 Hz, 1H), 6.86 (dd, J = 8.2, 1.7 Hz, 1H), 5.29 (s, 1H), 4.47 (s, 1H), 3.74 (dd, J = 11.5, 6.0 Hz, 1H), 3.57 – 3.31 (m, 3H), 2.68 – 2.60 (m, 2H), 2.34 – 2.21 (m, 1H), 2.14 – 1.95 (m, 1H), 1.61 (quin, J = 7.2 Hz, 2H), 1.47 (s, 9H), 1.33 – 1.23 (m, 14H), 0.88 (t, J = 6.9 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 161.2, 154.5, 146.8, 142.7, 139.0, 121.4, 116.3, 108.2, 79.7, 43.6, 36.0, 32.1, 32.0, 31.9, 29.6, 29.6, 29.5, 29.3, 29.2, 28.5, 26.2, 22.7, 22.0, 14.0. HRMS (ESI+) m/z calc’d. for C26H42N3O3+ (M+H)+ 444.3221, found 444.3229.
tert-butyl-4-((5-decylbenzo[d]oxazol-2-yl)amino)piperidine-1-carboxylate (25m).
Synthesized according to General Procedure 3b. Purified via column chromatography (45% ethyl acetate/hexanes). Yellow oil (85%, 93 mg). 1H NMR (500 MHz, CDCl3) δ 7.21 (s, 1H), 7.15 (d, J = 8.2 Hz, 1H), 6.87 (dd, J = 8.1, 1.7 Hz, 1H), 4.92 (s, 1H), 4.10 (bs, 2H), 3.96 – 3.88 (m, 1H), 2.97 (t, J = 10.2 Hz, 2H), 2.65 (t, J = 7.3 Hz, 2H), 2.22 – 2.12 (m, 2H), 1.66 – 1.58 (m, 2H), 1.51 – 1.42 (m, 11H), 1.34 – 1.23 (m, 14H), 0.90 (t, J = 6.9 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 161.1, 154.7, 146.6, 142.8, 139.0, 121.2, 116.2, 108.1, 79.8, 50.5, 36.0, 32.3, 32.0, 31.9, 29.6, 29.6, 29.5, 29.3, 29.2, 28.4, 22.7, 14.1. HRMS (ESI+) m/z calc’d. for C27H44N3O3+ (M+H)+ 458.3377, found 458.3371.
tert-butyl-(S)-3-((5-decylbenzo[d]oxazol-2-yl)amino)piperidine-1-carboxylate (25n).
Synthesized according to General Procedure 3b. Purified via column chromatography (45% ethyl acetate/hexanes). Yellow oil (62%, 70 mg). 1H NMR (400 MHz, CDCl3) δ 7.18 (s, 1H), 7.13 (d, J = 8.1 Hz, 1H), 6.84 (dd, J = 8.2, 1.7 Hz, 1H), 5.65 (s, 1H), 3.99 – 3.87 (m, 1H), 3.80 – 3.72 (m, 1H), 3.50 – 3.36 (m, 3H), 2.63 (t, J = 8.0 Hz, 2H), 2.06 – 1.93 (m, 1H), 1.84 – 1.69 (m, 2H), 1.60 (quin, J = 7.4 Hz, 3H), 1.43 (s, 9H), 1.32 – 1.20 (m, 14H), 0.88 (t, J = 6.8 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 161.4, 155.2, 146.7, 142.7, 139.1, 121.2, 116.2, 108.3, 80.1, 48.8, 44.0, 36.1, 32.1, 32.0, 30.1, 29.7, 29.7, 29.6, 29.5, 29.4, 29.3, 28.4, 22.8, 22.4, 14.2. HRMS (ESI+) m/z calc’d. for C27H44N3O3+ (M+H)+ 458.3377, found 458.3381.
tert-butyl-(R)-3-((5-decylbenzo[d]oxazol-2-yl)amino)piperidine-1-carboxylate (25o).
Synthesized according to General Procedure 3b. Purified via column chromatography (45% ethyl acetate/hexanes). Yellow oil (83%, 48 mg). 1H NMR (400 MHz, CDCl3) δ 7.15 (s, 1H), 7.10 (d, J = 8.1 Hz, 1H), 6.81 (dd, J = 8.2, 1.7 Hz, 1H), 5.76 (s, 1H), 3.94 – 3.83 (m, 1H), 3.80 – 3.65 (m, 1H), 3.48 – 3.30 (m, 3H), 2.60 (t, J = 7.6 Hz, 2H), 2.03 – 1.92 (m, 1H), 1.82 – 1.65 (m, 2H), 1.63 – 1.49 (m, 3H), 1.40 (s, 9H), 1.29 – 1.19 (m, 14H), 0.85 (t, J = 6.8 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 161.4, 155.2, 146.7, 142.7, 139.1, 121.2, 116.1, 108.2, 80.1, 48.9, 43.9, 36.1, 32.1, 32.0, 30.1, 29.7, 29.7, 29.6, 29.4, 29.3, 28.5, 28.4, 22.8, 22.4, 14.2. HRMS (ESI+) m/z calc’d. for C27H44N3O3+ (M+H)+ 458.3377, found 458.3391
tert-butyl-(R)-3-(((5-decylbenzo[d]oxazol-2-yl)amino)methyl)pyrrolidine-1-carboxylate (25p).
Synthesized according to General Procedure 3b. Purified via column chromatography (45% ethyl acetate/hexanes). Yellow oil (67%, 79 mg). 1H NMR (400 MHz, CDCl3) δ 7.19 – 7.05 (m, 2H), 6.82 (d, J = 8.1 Hz, 1H), 6.60 (s, 1H), 3.62 – 3.39 (m, 4H), 3.39 – 3.23 (m, 1H), 3.24 – 3.04 (m, 1H), 2.62 (t, J = 7.7 Hz, 3H), 2.10 – 1.97 (m, 1H), 1.80 – 1.65 (m, 1H), 1.61 (quin, J = 7.9 Hz, 2H), 1.45 (s, 9H), 1.33 – 1.20 (m, 14H), 0.87 (t, J = 6.9 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 162.5, 154.6, 146.7, 142.9, 138.9, 120.9, 115.8, 108.1, 79.3, 49.3*, 45.4*, 45.2*, 38.6*, 36.0, 32.0, 31.9, 29.6, 29.6, 29.5, 29.3, 29.2, 28.5, 28.4, 22.7, 14.1. HRMS (ESI+) m/z calc’d. for C27H44N3O3+ (M+H)+ 458.3377, found 458.3381.
tert-butyl-(S)-3-(((5-decylbenzo[d]oxazol-2-yl)amino)methyl)pyrrolidine-1-carboxylate (25r).
Synthesized according to General Procedure 3b. Purified via column chromatography (45% ethyl acetate/hexanes). Yellow oil (71%, 948 mg). 1H NMR (400 MHz, CDCl3) δ 7.16 – 7.06 (m, 2H), 6.82 (d, J = 8.1 Hz, 1H), 6.44 (s, 1H), 3.65 – 3.38 (m, 3H), 3.33 (q, J = 9.4 Hz, 1H), 3.24 – 3.04 (m, 1H), 2.70 – 2.56 (m, 3H), 2.09 – 1.96 (m, 1H), 1.81 – 1.55 (m, 4H), 1.45 (s, 9H), 1.35 – 1.20 (m, 14H), 0.88 (t, J = 6.7 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 162.5, 154.6, 146.7, 142.9, 139.0, 121.0, 115.9, 108.1, 79.4, 49.3*, 45.5*, 45.3*, 45.0*, 38.2*, 36.3, 36.0, 29.7, 29.7, 29.6, 29.4, 29.3, 28.6, 28.4, 22.7, 14.2. HRMS (ESI+) m/z calc’d. for C27H44N3O3+ (M+H)+ 458.3377, found 458.3371.
tert-butyl-(S)-2-(((5-decylbenzo[d]oxazol-2-yl)amino)methyl)pyrrolidine-1-carboxylate (25r).
Synthesized according to General Procedure 3b. Purified via column chromatography (45% ethyl acetate/hexanes). Yellow oil (69%, 100 mg). 1H NMR (400 MHz, CDCl3) δ 7.19 (s, 1H), 7.11 (d, J = 8.2 Hz, 1H), 7.07 (s, 1H), 6.81 (d, J = 8.2 Hz, 1H), 4.23 – 4.00 (m, 1H), 3.67 – 3.31 (m, 4H), 2.63 (t, J = 7.7 Hz, 2H), 2.12 – 2.00 (m, 1H), 1.99 – 1.80 (m, 2H), 1.79 – 1.71 (m, 1H), 1.61 (quin, J = 7.2 Hz, 2H), 1.49 (s, 9H), 1.33 – 1.21 (m, 14H), 0.88 (t, J = 6.7 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 162.4, 156.8, 146.8, 142.8, 138.8, 120.9, 116.0, 108.2, 80.4, 56.8, 49.1, 47.4, 36.1, 32.1, 32.0, 29.9, 29.7, 29.7, 29.6, 29.6, 29.4, 29.3, 28.5, 22.8, 14.2. HRMS (ESI+) m/z calc’d. for C27H44N3O3+ (M+H)+ 458.3377, found 458.3383.
tert-butyl-(R)-2-(((5-decylbenzo[d]oxazol-2-yl)amino)methyl)pyrrolidine-1-carboxylate (25s).
Synthesized according to General Procedure 3b. Purified via column chromatography (45% ethyl acetate/hexanes). Yellow oil (90%, 102 mg). 1H NMR (400 MHz, CDCl3) δ 7.17 (s, 1H), 7.10 (d, J = 8.1 Hz, 1H), 7.00 (s, 1H), 6.81 (d, J = 8.7 Hz, 1H), 4.26 – 4.04 (m, 1H), 3.67 – 3.31 (m, 4H), 2.62 (t, J = 7.7 Hz, 2H), 2.13 – 2.00 (m, 1H), 1.99 – 1.70 (m, 3H), 1.61 (quin, J = 7.5 Hz, 2H), 1.48 (s, 9H), 1.34 – 1.23 (m, 14H), 0.88 (t, J = 6.8 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 162.3, 156.7, 146.7, 142.9, 138.9, 138.6, 120.7, 115.8, 108.0, 80.2, 56.6, 49.2, 47.2, 36.0, 32.0, 31.9, 29.6, 29.6, 29.5, 29.5, 29.3, 29.2, 24.0, 22.6, 14.1. HRMS (ESI+) m/z calc’d. for C27H44N3O3+ (M+H)+ 458.3377, found 458.3388.
tert-butyl-4-(((5-decylbenzo[d]oxazol-2-yl)amino)methyl)piperidine-1-carboxylate (25t).
Synthesized according to General Procedure 3b. Purified via column chromatography (45% ethyl acetate/hexanes). Yellow oil (77%, 88 mg). 1H NMR (400 MHz, CDCl3) δ 7.17 (s, 1H), 7.11 (d, J = 8.1 Hz, 1H), 6.83 (dd, J = 8.2, 1.6 Hz, 1H), 5.78 (s, 1H), 4.12 (s, 2H), 3.35 (d, J = 6.7 Hz, 2H), 2.75 – 2.58 (m, 4H), 1.89 – 1.71 (m, 4H), 1.60 (quin, J = 7.5 Hz, 2H), 1.45 (s, 9H), 1.33 – 1.22 (m, 14H), 1.21 – 1.11 (m, 1H), 0.87 (t, J = 6.9 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 162.4, 154.9, 146.7, 142.7, 139.1, 121.1, 116.1, 108.2, 79.6, 48.6, 43.7, 36.5, 36.1, 32.1, 32.0, 29.8, 29.7, 29.7, 29.6, 29.4, 29.3, 28.6, 22.8, 14.2. HRMS (ESI+) m/z calc’d. for C28H46N3O3+ (M+H)+ 472.3534, found 472.3526.
tert-butyl-(R)-3-(((5-decylbenzo[d]oxazol-2-yl)amino)methyl)piperidine-1-carboxylate (25u).
Synthesized according to General Procedure 3b. Purified via column chromatography (45% ethyl acetate/hexanes). Yellow oil (70%, 57 mg). 1H NMR (400 MHz, CDCl3) δ 7.18 (s, 1H), 7.12 (d, J = 8.1 Hz, 1H), 6.83 (dd, J = 8.2, 1.7 Hz, 1H), 5.75 (s, 1H), 3.77 (dd, J = 13.3, 3.6 Hz, 1H), 3.69 – 3.57 (m, 2H), 3.50 – 3.38 (m, 1H), 3.36 – 3.23 (m, 1H), 3.12 (s, 1H), 2.98 (s, 1H), 2.63 (t, J = 2H), 1.99 – 1.91 (m, 1H), 1.90 – 1.82 (m, 1H), 1.70 – 1.56 (m, 3H), 1.45 (s, 9H), 1.40 – 1.32 (m, 1H), 1.32 – 1.22 (m, 14H), 0.87 (t, J = 6.9 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 162.3, 155.8, 146.8, 142.6, 139.1, 121.2, 116.2, 108.3, 79.8, 45.3, 36.1, 35.6, 32.1, 30.0, 29.9, 29.8, 29.8, 29.7, 29.7, 29.5, 29.3, 28.6, 28.1, 23.8, 22.8, 14.2. HRMS (ESI+) m/z calc’d. for C28H46N3O3+ (M+H)+ 472.3534, found 472.3524.
tert-butyl-(S)-3-(((5-decylbenzo[d]oxazol-2-yl)amino)methyl)piperidine-1-carboxylate (25v).
Synthesized according to General Procedure 3b. Purified via column chromatography (45% ethyl acetate/hexanes). Yellow oil (79%, 84 mg). 1H NMR (500 MHz, CDCl3) δ 7.18 (s, 1H), 7.11 (d, J = 8.2 Hz, 1H), 6.82 (d, J = 8.1 Hz, 1H), 6.04 (s, 1H), 3.79 (dd, J = 13.3, 3.6 Hz, 1H), 3.70 – 3.57 (m, 1H), 3.47 – 3.39 (m, 1H), 3.36 – 3.24 (m, 1H), 3.16 – 3.04 (m, 1H), 2.96 (t, J = 11.0 Hz, 1H), 2.62 (t, J = 7.7 Hz, 2H), 1.97 – 1.90 (m, 1H), 1.90 – 1.83 (m, 1H), 1.70 – 1.56 (m, 3H), 1.45 (s, 9H), 1.39 – 1.32 (m, 2H), 1.33 – 1.21 (m, 14H), 0.87 (t, J = 6.9 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 162.2, 155.2, 146.6, 142.6, 138.9, 121.0, 116.0, 108.1, 79.7, 45.2, 36.0, 35.5, 32.0, 31.9, 29.9, 29.8, 29.6, 29.6, 29.5, 29.3, 29.2, 28.4, 28.1, 23.7, 22.7, 14.1. HRMS (ESI+) m/z calc’d. for C28H46N3O3+ (M+H)+ 472.3534, found 472.3524.
tert-butyl-(R)-3-(((6-decylbenzo[d]oxazol-2-yl)amino)methyl)pyrrolidine-1-carboxylate (26p).
Synthesized according to General Procedure 3b. Purified via column chromatography (45% ethyl acetate/hexanes). Yellow oil (69%, 48 mg) 1H NMR (600 MHz, CDCl3) δ 7.23 (d, J = 7.9 Hz, 1H), 7.07 (d, J = 7.1 Hz, 1H), 6.98 (s, 1H), 6.04 (s, 1H), 3.62 – 3.40 (m, 4H), 3.34 (dq, J = 30.2, 8.5 Hz, 1H), 3.18 – 3.07 (m, 1H), 2.63 (t, J = 7.8 Hz, 3H), 2.10 – 2.00 (m, 1H), 1.76 – 1.64 (m, 1H), 1.60 (quin, J = 7.3 Hz, 2H), 1.45 (s, 9H), 1.36 – 1.21 (m, 14H), 0.88 (t, J = 7.1 Hz, 3H). 13C NMR (151 MHz, CDCl3) δ 162.0, 154.7, 148.7, 140.6, 136.4, 124.2, 115.7, 108.7, 79.4, 49.4*, 45.5*, 45.2*, 38.6*, 36.1, 32.1, 32.0, 29.7, 29.7, 29.6, 29.4, 29.3, 28.6, 28.6, 22.8, 14.2. HRMS (ESI+) m/z calc’d. for C27H44N3O3+ (M+H)+ 458.3377, found 458.3372.
tert-butyl-(R)-3-(((5-octylbenzo[d]oxazol-2-yl)amino)methyl)pyrrolidine-1-carboxylate (27p).
Synthesized according to General Procedure 3b. Purified via column chromatography (45% ethyl acetate/hexanes). Yellow oil (30%, 51 mg). 1H NMR (600 MHz, CDCl3) δ 7.20 – 7.11 (m, 2H), 6.94 – 6.85 (m, 1H), 3.62 – 3.49 (m, 4H), 3.48 – 3.40 (m, 1H), 3.40 – 3.28 (m, 1H), 3.23 – 3.06 (m, 1H), 2.71 – 2.59 (m, 3H), 2.16 – 2.01 (m, 1H), 1.79 – 1.65 (m, 1H), 1.59 (quin, J = 7.4 Hz, 2H), 1.45 (s, 9H), 1.33 – 1.20 (m, 10H), 0.87 (t, J = 7.0 Hz, 3H). 13C NMR (151 MHz, CDCl3) δ 161.29, 154.64, 145.75, 139.95, 138.93, 122.05, 115.13, 108.78, 79.45, 49.28*, 45.53*, 45.14*, 38.90, 38.05, 36.03, 32.01, 31.94, 29.54, 29.34, 29.26, 28.58, 22.74, 14.19. HRMS (ESI+) m/z calc’d. for C25H40N3O3+ (M+H)+ 430.3064, found 430.3063.
tert-butyl-(R)-3-(((5-nonylbenzo[d]oxazol-2-yl)amino)methyl)pyrrolidine-1-carboxylate (28p).
Synthesized according to General Procedure 3b. Purified via column chromatography (45% ethyl acetate/hexanes). Yellow oil (30%, 52 mg). 1H NMR (500 MHz, CDCl3) δ 7.18 – 7.09 (m, 2H), 6.83 (d, J = 7.8 Hz, 1H), 5.90 (s, 1H), 3.62 – 3.40 (m, 2H), 3.40 – 3.27 (m, 1H), 3.21 – 3.04 (m, 1H), 2.63 (t, J = 7.6 Hz, 5H), 2.05 (tt, J = 12.4, 5.6 Hz, 1H), 1.76 – 1.65 (m, 1H), 1.61 (quin, J = 7.1 Hz, 2H), 1.45 (s, 9H), 1.32 – 1.23 (m, 12H), 0.87 (t, J = 6.9 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 162.3, 154.7, 146.8, 142.9, 139.1, 121.2, 116.1, 108.2, 79.4, 49.4*, 45.6*, 45.2*, 38.6*, 36.1, 32.1, 32.0, 29.8, 29.7, 29.6, 29.4, 29.3, 28.6, 22.8, 14.2. HRMS (ESI+) m/z calc’d. for C26H42N3O3+ (M+H)+ 444.3221, found 444.3219.
tert-butyl-(R)-3-(((5-undecylbenzo[d]oxazol-2-yl)amino)methyl)pyrrolidine-1-carboxylate (29p).
Synthesized according to General Procedure 3b. Purified via column chromatography (45% ethyl acetate/hexanes). Yellow oil (65%, 54 mg). 1H NMR (600 MHz, CDCl3) δ 7.17 – 7.09 (m, 2H), 6.83 (s, 1H), 6.11 (s, 1H), 3.61 – 3.40 (m, 4H), 3.41 – 3.26 (m, 1H), 3.21 – 3.02 (m, 1H), 2.63 (t, J = 7.6 Hz, 3H), 2.09 – 2.01 (m, 1H), 1.78 – 1.64 (m, 1H), 1.60 (quin, J = 7.3 Hz, 2H), 1.45 (s, 9H), 1.37 – 1.18 (m, 16H), 0.88 (t, J = 7.0 Hz, 3H). 13C NMR (151 MHz, CDCl3) δ 162.4, 154.7, 146.8, 142.9, 139.0, 121.1, 116.0, 108.2, 79.4, 49.3*, 45.5*, 45.1*, 38.6*, 36.1, 32.1, 32.0, 29.8, 29.7, 29.7, 29.6, 29.4, 29.3, 28.6, 22.8, 14.2. HRMS (ESI+) m/z calc’d. for C28H46N3O3+ (M+H)+ 472.3534, found 472.3530.
tert-butyl-(R)-3-(((5-dodecylbenzo[d]oxazol-2-yl)amino)methyl)pyrrolidine-1-carboxylate (30p).
Synthesized according to General Procedure 3b. Purified via column chromatography (45% ethyl acetate/hexanes). Yellow oil (65%, 40 mg). 1H NMR (600 MHz, CDCl3) δ 7.16 (s, 1H), 7.13 (d, J = 7.4 Hz, 1H), 6.87 – 6.81 (m, 1H), 5.65 (s, 1H), 3.63 – 3.41 (m, 4H), 3.40 – 3.27 (m, 1H), 3.20 – 3.05 (m, 1H), 2.63 (t, J = 7.7 Hz, 3H), 2.05 (quin, J = 6.3 Hz, 1H), 1.74 – 1.64 (m, 1H), 1.60 (quin, J = 7.4 Hz, 2H), 1.45 (s, 9H), 1.34 – 1.22 (m, 18H), 0.88 (t, J = 7.0 Hz, 3H). 13C NMR (151 MHz, CDCl3) δ 162.3, 154.7, 146.8, 142.9, 139.1, 121.3, 116.2, 108.3, 79.5, 49.3*, 45.6*, 45.2*, 38.6*, 36.1, 32.2, 32.0, 29.8, 29.8, 29.7, 29.7, 29.5, 29.3, 28.6, 22.8, 14.3. HRMS (ESI+) m/z calc’d. for C29H48N3O3+ (M+H)+ 486.3690, found 486.3696.
tert-butyl-(R)-3-(((5-decyl-1H-benzo[d]imidazol-2-yl)amino)methyl)pyrrolidine-1-carboxylate (31p).
Synthesized according to General Procedure 3b. Purified via column chromatography (100% ethyl acetate). 1H NMR (600 MHz, CDCl3) δ 7.65 – 7.53 (m, 1H), 7.49 – 7.43 (m, 1H), 7.21 – 7.00 (m, 2H), 6.86 (s, 1H), 3.57 – 3.18 (m, 5H), 3.01 (d, J = 30.2 Hz, 1H), 2.68 – 2.43 (m, 3H), 1.91 (d, J = 19.7 Hz, 1H), 1.61 – 1.54 (m, 3H), 1.44 (s, 9H), 1.34 – 1.18 (m, 14H), 0.88 (t, J = 6.9 Hz, 3H). 13C NMR (151 MHz, CDCl3) δ 155.0, 136.2, 133.2*, 132.3, 131.3*, 128.7*, 121.5, 111.8, 79.6, 49.4*, 45.6*, 45.1, 39.1, 38.2, 36.3, 32.4, 32.0, 29.7, 29.7, 29.5, 29.4, 29.3, 28.6, 22.8, 14.2. HRMS (ESI+) m/z calc’d. for C27H45N4O2+ (M+H)+ 457.3537, found 457.3540.
N1-(5-decylbenzo[d]oxazol-2-yl)ethane-1,2-diamine hydrochloride (32a).
Synthesized according to General Procedure 4. Purified via trituration with diethyl ether. White solid (51%, 35 mg). 1H NMR (500 MHz, CD3OD) δ 7.45 (d, J = 8.4 Hz, 1H), 7.27 (s, 1H), 7.18 (dd, J = 8.5, 1.7 Hz, 1H), 3.86 (t, J = 6.1 Hz, 2H), 3.33 – 3.30 (m, 2H), 2.72 (t, J = 7.8 Hz, 2H), 1.64 (quin, J = 7.5 Hz, 2H), 1.36 – 1.23 (m, 14H), 0.88 (t, J = 6.9 Hz, 3H). 13C NMR (101 MHz, CD3OD) δ 160.6, 145.4, 143.4, 131.6, 126.0, 113.5, 111.6, 41.7, 39.5, 36.7, 33.0, 32.9, 30.7, 30.7, 30.6, 30.4, 30.2, 23.7, 14.4. HRMS (ESI+) m/z calc’d. for C19H32N3O+ (M+H)+ 318.2540, found 318.2550.
N1-(5-decylbenzo[d]oxazol-2-yl)propane-1,3-diamine hydrochloride (32b).
Synthesized according to General Procedure 4. Purified via trituration with diethyl ether. White solid (94%, 40 mg, 94% purity by UPLC). 1H NMR (500 MHz, CD3OD) δ 7.44 (d, J = 8.5 Hz, 1H), 7.27 (s, 1H), 7.18 (dd, J = 8.5, 1.6 Hz, 1H), 3.65 (t, J = 7.0 Hz, 2H), 3.09 (t, J = 7.0 Hz, 2H), 2.71 (t, J = 7.6 Hz, 2H), 2.11 (quin, J = 7.1 Hz, 2H), 1.63 (quin, J = 6.9 Hz, 2H), 1.37 – 1.20 (m, 14H), 0.88 (t, J = 6.8 Hz, 3H). 13C NMR (126 MHz, CD3OD) δ 160.3, 145.4, 143.3, 131.4, 125.9, 113.2, 111.5, 41.4, 38.1, 36.7, 33.1, 32.9, 30.7, 30.7, 30.6, 30.5, 30.2, 27.8, 23.7, 14.4. HRMS (ESI+) m/z calc’d. for C20H34N3O+ (M+H)+ 332.2696, found 332.2694.
N1-(5-decylbenzo[d]oxazol-2-yl)butane-1,4-diamine hydrochloride (32c).
Synthesized according to General Procedure 4. Purified via trituration with diethyl ether. White solid (87%, 66 mg). 1H NMR (500 MHz, CD3OD) δ 7.44 (d, J = 8.4 Hz, 1H), 7.26 (s, 1H), 7.19 (d, J = 8.4 Hz, 1H), 3.58 (t, J = 6.7 Hz, 2H), 3.01 (t, J = 7.0 Hz, 2H), 2.72 (t, J = 7.7 Hz, 2H), 1.87 – 1.77 (m, 4H), 1.63 (quin, J = 7.8 Hz, 2H), 1.36 – 1.24 (m, 14H), 0.89 (t, J = 6.9 Hz, 3H). 13C NMR (126 MHz, CD3OD) δ 160.1, 145.2, 143.3, 130.9, 126.0, 113.0, 111.6, 43.8, 40.2, 36.7, 33.1, 32.9, 30.7, 30.7, 30.6, 30.4, 30.2, 26.7, 25.7, 23.7, 14.4. HRMS (ESI+) m/z calc’d. for C21H36N3O+ (M+H)+ 346.2853, found 346.2862.
N1-(5-decylbenzo[d]oxazol-2-yl)pentane-1,5-diamine hydrochloride (32d).
Synthesized according to General Procedure 4. Purified via trituration with diethyl ether. Yellow solid (83%, 40 mg). 1H NMR (500 MHz, CD3OD) δ 7.44 (d, J = 8.4 Hz, 1H), 7.26 (s, 1H), 7.18 (dd, J = 8.5, 1.7 Hz, 1H), 3.56 (t, J = 7.2 Hz, 2H), 2.96 (t, J = 7.7 Hz, 2H), 2.71 (t, J = 7.6 Hz, 2H), 1.84 – 1.70 (m, 4H), 1.63 (quin, J = 7.4 Hz, 2H), 1.58 – 1.49 (m, 2H), 1.29 (d, J = 28.2 Hz, 14H), 0.88 (t, J = 6.9 Hz, 3H). 13C NMR (101 MHz, CD3OD) δ 160.0, 145.2, 143.2, 131.0, 125.9, 113.0, 111.5, 44.2, 40.5, 36.7, 33.0, 32.9, 30.7, 30.7, 30.5, 30.4, 30.2, 29.1, 28.0, 24.5, 23.7, 14.4. HRMS (ESI+) m/z calc’d. for C22H38N3O+ (M+H)+ 360.3009, found 360.3009.
N1-(5-decylbenzo[d]oxazol-2-yl)-N1-methylethane-1,2-diamine hydrochloride (32e).
Synthesized according to General Procedure 4. Purified via trituration with diethyl ether. White solid (32%, 56 mg). 1H NMR (400 MHz, CD3OD) δ 7.49 – 7.41 (m, 1H), 7.29 – 7.26 (m, 1H), 7.20 – 7.13 (m, 1H), 4.01 (q, J = 6.3 Hz, 2H), 3.41 – 3.36 (m, 5H), 2.73 (quin, J = 7.6 Hz, 2H), 1.65 (quin, J = 7.8 Hz, 2H), 1.42 – 1.22 (m, 14H), 0.91 (t, J = 6.6 Hz, 3H). 13C NMR (101 MHz, CD3OD) δ 160.6, 145.9, 143.3, 132.8, 125.8, 113.6, 111.6, 49.8, 38.0, 37.3, 36.7, 33.0, 32.9, 30.7, 30.7, 30.6, 30.4, 30.2, 23.7, 14.4. HRMS (ESI+) m/z calc’d. for C20H34N3O+ (M+H)+ 332.2696, found 332.2694.
N1-(2-aminoethyl)-N1-(5-decylbenzo[d]oxazol-2-yl)ethane-1,2-diamine hydrochloride (32f).
Synthesized according to General Procedure 4. Purified via trituration with diethyl ether. White solid (78%, 54 mg). 1H NMR (400 MHz, CD3OD) δ 7.39 – 7.29 (m, 1H), 7.26 – 7.18 (m, 1H), 7.08 – 6.95 (m, 1H), 4.03 – 3.92 (m, 4H), 3.38 – 3.31 (m, 4H), 2.70 (quin, J = 7.6 Hz, 2H), 1.63 (quin, J = 7.8 Hz, 2H), 1.33 – 1.22 (m, 14H), 0.88 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CD3OD) δ 161.3, 146.5, 142.9, 135.1, 125.5, 114.7, 111.3, 66.9, 48.1, 38.1, 36.8, 33.0, 33.0, 30.7, 30.7, 30.6, 30.4, 30.2, 14.4. HRMS (ESI+) m/z calc’d. for C21H37N4O+ (M+H)+ 361.2962, found 361.2933.
(S)-1-(5-decylbenzo[d]oxazol-2-yl)pyrrolidin-3-amine hydrochloride (32g).
Synthesized according to General Procedure 4. Purified via trituration with diethyl ether. Off-white solid (76%, 35 mg). 1H NMR (400 MHz, CD3OD) δ 7.50 (d, J = 8.4 Hz, 1H), 7.29 (s, 1H), 7.20 (dd, J = 8.5, 1.7 Hz, 1H), 4.25 – 4.16 (m, 2H), 4.09 – 3.92 (m, 4H), 2.74 (t, J = 7.9 Hz, 2H), 2.69 – 2.59 (m, 1H), 2.43 – 2.33 (m, 1H), 1.65 (quin, J = 6.6 Hz, 2H), 1.37 – 1.24 (m, 14H), 0.89 (t, J = 6.9 Hz, 3H). 13C NMR (126 MHz, CD3OD) δ 158.2, 146.2, 143.3, 132.9, 125.8, 113.6, 111.5, 52.7, 51.4, 47.7, 36.7, 33.1, 33.0, 30.7, 30.7, 30.6, 30.5, 30.3, 30.2, 23.7, 14.4. HRMS (ESI+) m/z calc’d. for C21H34N3O+ (M+H)+ 344.2702, found 344.2679.
(R)-1-(5-decylbenzo[d]oxazol-2-yl)pyrrolidin-3-amine hydrochloride (32h).
Synthesized according to General Procedure 4. Purified via trituration with diethyl ether. Off-white solid (99%, 38 mg). 1H NMR (400 MHz, CD3OD) δ 7.51 (d, J = 8.5 Hz, 1H), 7.31 (s, 1H), 7.22 (dd, J = 8.5, 1.7 Hz, 1H), 4.28 – 4.15 (m, 2H), 4.12 – 3.93 (m, 3H), 2.75 (t, J = 7.9 Hz, 2H), 2.71 – 2.60 (m, 1H), 2.44 – 2.35 (m, 1H), 1.66 (quin, J = 6.6 Hz, 2H), 1.36 – 1.27 (m, 14H), 0.90 (t, J = 6.8 Hz, 3H). 13C NMR (101 MHz, CD3OD) δ 158.0, 146.1, 143.5, 132.4, 125.9, 113.5, 111.6, 52.7, 51.4, 47.8, 36.7, 33.0, 32.9, 30.7, 30.7, 30.6, 30.4, 30.4, 30.2, 23.7, 14.4. HRMS (ESI+) m/z calc’d. for C21H34N3O+ (M+H)+ 344.2702, found 344.2679.
5-decyl-2-(piperazin-1-yl)benzo[d]oxazole hydrochloride (32i).
Synthesized according to General Procedure 4. Purified via trituration with diethyl ether. White solid (81%, 55 mg). 1H NMR (500 MHz, CD3OD) δ 7.30 (d, J = 8.2 Hz, 1H), 7.18 (d, J = 1.7 Hz, 1H), 6.99 (dd, J = 8.2, 1.7 Hz, 1H), 4.01 – 3.94 (m, 4H), 3.43 – 3.39 (m, 4H), 2.66 (t, J = 7.6 Hz, 2H), 1.62 (quin, J = 7.4 Hz, 2H), 1.34 – 1.22 (m, 14H), 0.88 (t, J = 6.9 Hz, 3H). 13C NMR (126 MHz, CD3OD) δ 162.3, 148.0 141.4, 141.2, 123.7, 116.5, 110.1, 43.7, 36.8, 33.1, 33.0, 30.7, 30.7, 30.6, 30.4, 30.2, 23.7, 14.4. HRMS (ESI+) m/z calc’d. for C21H34N3O+ (M+H)+ 344.2696, found 344.2679.
N-(azetidin-3-yl)-5-decylbenzo[d]oxazol-2-amine 2,2,2-trifluoroacetate (32j).
Synthesized according to General Procedure 8. Purified via trituration with diethyl ether. White solid (33%, 31 mg). 1H NMR (400 MHz, CD3OD) δ 7.23 (d, J = 8.2 Hz, 1H), 7.16 (d, J = 1.6 Hz, 1H), 6.94 (dd, J = 8.2, 1.7 Hz, 1H), 4.77 (quin, J = 7.4 Hz, 1H), 4.47 – 4.28 (m, 4H), 2.67 (t, J = 7.2 Hz, 2H), 1.64 (quin, J = 7.3 Hz, 2H), 1.36 – 1.27 (m, 14H), 0.90 (t, J = 6.9 Hz, 3H). 13C NMR (101 MHz, CD3OD) δ 162.3, 148.1, 143.3, 140.5, 123.1, 117.0, 109.5, 53.9, 46.0, 36.9, 33.2, 33.1, 30.7, 30.7, 30.6, 30.4, 30.2, 23.7, 14.4. HRMS (ESI+) m/z calc’d. for C20H32N3O+ (M+H)+ 330.2545, found 330.2558.
(S)-5-decyl-N-(pyrrolidin-3-yl)benzo[d]oxazol-2-amine hydrochloride (32k).
Synthesized according to General Procedure 4. Purified via trituration with diethyl ether. White solid (66%, 100 mg). 1H NMR (500 MHz, CD3OD) δ 7.40 (d, J = 8.3 Hz, 1H), 7.25 (s, 1H), 7.12 (dd, J = 8.4, 1.7 Hz, 1H), 4.66 – 4.59 (m, 1H), 3.70 (dd, J = 12.7, 6.6 Hz, 1H), 3.59 – 3.45 (m, 3H), 2.70 (t, J = 7.6 Hz, 2H), 2.55 – 2.45 (m, 1H), 2.32 – 2.22 (m, 1H), 1.63 (quin, J = 8.2 Hz, 2H), 1.37 – 1.22 (m, 14H), 0.88 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CD3OD) δ 161.8, 147.0, 141.6, 138.4, 124.2, 115.4, 110.3, 53.5, 51.0, 45.6, 36.8, 33.0, 33.0, 31.3, 30.7, 30.7, 30.6, 30.4, 30.2, 23.7, 14.4. HRMS (ESI+) m/z calc’d. for C21H34N3O+ (M+H)+ 344.2696, found 344.2669.
(R)-5-decyl-N-(pyrrolidin-3-yl)benzo[d]oxazol-2-amine hydrochloride (32l).
Synthesized according to General Procedure 4. Purified via trituration with diethyl ether. White solid (76%, 35 mg). 1H NMR (500 MHz, CD3OD) δ 7.47 (d, J = 8.5 Hz, 1H), 7.29 (s, 1H), 7.20 (dd, J = 8.5, 1.7 Hz, 1H), 4.73 – 4.66 (m, 1H), 3.74 (dd, J = 12.8, 6.8 Hz, 1H), 3.58 – 3.46 (m, 3H), 2.72 (t, J = 7.2 Hz, 2H), 2.59 – 2.45 (m, 1H), 2.37 – 2.19 (m, 1H), 1.63 (quin, J = 7.6 Hz, 2H), 1.34 – 1.23 (m, 14H), 0.87 (t, J = 7.0 Hz, 3H). 13C NMR (126 MHz, CD3OD) δ 160.0, 145.5, 143.4, 131.7, 126.1, 113.5, 111.6, 53.9, 50.7, 45.6, 36.7, 33.1, 33.0, 31.3, 30.7, 30.7, 30.6, 30.5, 30.2, 23.7, 14.4. HRMS (ESI+) m/z calc’d. for C21H34N3O+ (M+H)+ 344.2696, found 344.2697.
5-decyl-N-(piperidin-4-yl)benzo[d]oxazol-2-amine hydrochloride (32m).
Synthesized according to General Procedure 4. Purified via trituration with diethyl ether. White solid (80%, 64 mg). 1H NMR (400 MHz, CD3OD) δ 7.38 (d, J = 8.4 Hz, 1H), 7.24 (s, 1H), 7.11 (dd, J = 8.4, 1.7 Hz, 1H), 4.13 – 4.02 (m, 1H), 3.56 – 3.48 (m, 2H), 3.27 – 3.15 (m, 2H), 2.71 (t, J = 7.1 Hz, 2H), 2.39 – 2.30 (m, 2H), 2.02 – 1.88 (m, 2H), 1.64 (quin, J = 7.1 Hz, 3H), 1.38 – 1.24 (m, 14H), 0.88 (t, J = 6.9 Hz, 3H). 13C NMR (126 MHz, CD3OD) δ 160.7, 146.1, 142.4, 135.0, 125.0, 114.2, 110.9, 49.6, 43.8, 36.8, 33.1, 33.0, 30.7, 30.7, 30.6, 30.4, 30.2, 29.4, 23.7, 14.4. HRMS (ESI+) m/z calc’d. for C22H36N3O+ (M+H)+ 358.2853, found 358.2898.
(S)-5-decyl-N-(piperidin-3-yl)benzo[d]oxazol-2-amine hydrochloride (32n).
Synthesized according to General Procedure 4. Purified via trituration with diethyl ether. Off-white solid (81%, 43 mg). 1H NMR (400 MHz, CD3OD) δ 7.39 (d, J = 8.2 Hz, 1H), 7.25 (s, 1H), 7.14 – 7.07 (m, 1H), 4.22 – 4.07 (m, 1H), 3.65 (d, J = 12.8 Hz, 1H), 3.44 – 3.35 (m, 1H), 3.17 – 3.02 (m, 2H), 2.77 – 2.67 (m, 2H), 2.27 (d, J = 12.8 Hz, 1H), 2.13 (d, J = 14.7 Hz, 1H), 1.99 – 1.76 (m, 2H), 1.71 – 1.59 (m, 2H), 1.37 – 1.26 (m, 14H), 0.91 (t, J = 6.1 Hz, 3H). 13C NMR (101 MHz, CD3OD) δ 161.3, 146.6, 141.9, 137.1, 124.5, 114.9, 110.6, 49.4, 48.7, 47.5, 44.7, 36.8, 33.0, 30.7, 30.7, 30.6, 30.4, 30.2, 29.1, 23.7, 21.7, 14.4. HRMS (ESI+) m/z calc’d. for C22H36N3O+ (M+H)+ 358.2853, found 358.2855.
(R)-5-decyl-N-(piperidin-3-yl)benzo[d]oxazol-2-amine hydrochloride (32o).
Synthesized according to General Procedure 4. Purified via trituration with diethyl ether. Off-white solid (60%, 25 mg, 94% purity by UPLC). 1H NMR (400 MHz, CD3OD) δ 7.41 (d, J = 8.4 Hz, 1H), 7.26 (s, 1H), 7.15 (dd, J = 8.5, 3.8 Hz, 1H), 4.24 – 4.13 (m, 1H), 3.66 (dd, J = 12.4, 3.9 Hz, 1H), 3.42 – 3.34 (m, 1H), 3.16 – 3.02 (m, 2H), 2.73 (t, J = 7.6 Hz, 2H), 2.33 – 2.24 (m, 1H), 2.18 – 2.08 (m, 1H), 2.01 – 1.87 (m, 1H), 1.87 – 1.76 (m, 1H), 1.65 (quin, J = 8.4 Hz, 2H), 1.39 – 1.25 (m, 14H), 0.91 (t, J = 7.0 Hz, 3H). 13C NMR (101 MHz, CD3OD) δ 160.6, 145.5, 143.1, 132.0, 125.8, 113.4, 111.4, 49.2, 46.4, 45.9, 38.7, 36.7, 33.0, 32.9, 30.7, 30.7, 30.5, 30.4, 30.2, 29.1, 23.7, 14.4. HRMS (ESI+) m/z calc’d. for C22H36N3O+ (M+H)+ 358.2853, found 358.2852.
(S)-5-decyl-N-(pyrrolidin-3-ylmethyl)benzo[d]oxazol-2-amine hydrochloride (32p).
Synthesized according to General Procedure 4. Purified via trituration with diethyl ether. Off-white solid (83%, 678 mg). 1H NMR (400 MHz, CD3OD) δ 7.44 – 7.38 (m, 1H), 7.26 – 7.21 (m, 1H), 7.19 – 7.12 (m, 1H), 3.66 – 3.59 (m, 2H), 3.54 (dd, J = 11.9, 7.9 Hz, 1H), 3.48 – 3.39 (m, 1H), 3.36 – 3.30 (m, 1H), 3.14 – 3.03 (m, 1H), 2.79 (quin, J = 7.9 Hz, 1H), 2.70 (t, J = 7.6 Hz, 2H), 2.36 – 2.25 (m, 1H), 1.91 – 1.77 (m, 1H), 1.63 (t, J = 7.5 Hz, 2H), 1.34 – 1.23 (m, 14H), 0.87 (t, J = 6.9 Hz, 3H). 13C NMR (101 MHz, CD3OD) δ 160.6, 145.5, 143.1, 132.0, 125.8, 113.4, 111.4, 49.2, 46.4, 45.9, 38.7, 36.7, 33.0, 32.9, 30.7, 30.7, 30.5, 30.4, 30.2, 29.1, 23.7, 14.4. HRMS (ESI+) m/z calc’d. for C22H36N3O+ (M+H)+ 358.2853, found 358.2849.
(R)-5-decyl-N-(pyrrolidin-3-ylmethyl)benzo[d]oxazol-2-amine hydrochloride (32q).
Synthesized according to General Procedure 4. Purified via trituration with diethyl ether. Off-white solid (68%, 46 mg). 1H NMR (400 MHz, CD3OD) δ 7.47 (dd, J = 8.4, 3.3 Hz, 1H), 7.33 – 7.27 (m, 1H), 7.23 – 7.16 (m, 1H), 3.70 – 3.66 (m, 2H), 3.62 – 3.53 (m, 1H), 3.52 – 3.44 (m, 1H), 3.41 – 3.31 (m, 1H), 3.18 – 3.08 (m, 1H), 2.90 – 2.78 (m, 1H), 2.74 (t, J = 7.7 Hz, 2H), 2.43 – 2.27 (m, 1H), 1.93 – 1.80 (m, 1H), 1.66 (quin, J = 6.6 Hz, 2H), 1.39 – 1.25 (m, 14H), 0.91 (t, J = 6.8 Hz, 3H). 13C NMR (101 MHz, CD3OD) δ 160.6, 145.4, 143.2, 131.9, 125.8, 113.4, 111.4, 49.1, 46.4, 45.9, 38.7, 36.7, 33.0, 32.9, 30.7, 30.7, 30.5, 30.4, 30.2, 29.1, 23.7, 14.4. HRMS (ESI+) m/z calc’d. for C22H36N3O+ (M+H)+ 358.2853, found 358.2854.
(S)-5-decyl-N-(pyrrolidin-2-ylmethyl)benzo[d]oxazol-2-amine hydrochloride (32r).
Synthesized according to General Procedure 4. Purified via trituration with diethyl ether. Off-white solid (50%, 43 mg). 1H NMR (500 MHz, CD3OD) δ 7.37 (d, J = 8.3 Hz, 1H), 7.22 (s, 1H), 7.08 (dd, J = 8.4, 1.7 Hz, 1H), 3.93 (quin, J = 6.6 Hz, 1H), 3.86 (d, J = 6.4 Hz, 2H), 3.42 – 3.35 (m, 1H), 3.34 – 3.27 (m, 1H), 2.65 (t, J = 7.6 Hz, 2H), 2.31 – 2.23 (m, 1H), 2.16 – 1.98 (m, 2H), 1.83 (dq, J = 13.2, 8.6 Hz, 1H), 1.58 (quin, J = 7.5 Hz, 2H), 1.30 – 1.17 (m, 14H), 0.83 (t, J = 6.9 Hz, 3H). 13C NMR (126 MHz, CD3OD) δ 161.3, 146.0, 142.7, 134.0, 125.3, 114.2, 111.2, 60.5, 46.7, 44.6, 36.7, 33.0, 33.0, 30.7, 30.7, 30.6, 30.4, 30.2, 28.7, 24.1, 23.7, 14.5. HRMS (ESI+) m/z calc’d. for C22H36N3O+ (M+H)+ 358.2853, found 358.2878.
(R)-5-decyl-N-(pyrrolidin-2-ylmethyl)benzo[d]oxazol-2-amine hydrochloride (32s).
Synthesized according to General Procedure 4. Purified via trituration with diethyl ether. Off-white solid (68%, 46 mg). 1H NMR (500 MHz, CD3OD) δ 7.34 (d, J = 8.4 Hz, 1H), 7.21 (s, 1H), 7.06 (dd, J = 8.4, 1.7 Hz, 1H), 3.98 – 3.88 (m, 1H), 3.84 (s, 1H), 3.83 (d, J = 2.4 Hz, 1H), 3.43 – 3.29 (m, 2H), 2.66 (t, J = 7.6 Hz, 2H), 2.33 – 2.23 (m, 1H), 2.18 – 1.97 (m, 2H), 1.84 (dq, J = 13.2, 8.6 Hz, 1H), 1.60 (quin, J = 7.8 Hz, 2H), 1.34 – 1.18 (m, 14H), 0.86 (t, J = 6.9 Hz, 3H). 13C NMR (126 MHz, CD3OD) δ 162.0, 146.5, 142.2, 135.9, 124.8, 114.7, 110.8, 60.7, 46.7, 44.5, 36.8, 33.0, 33.0, 30.7, 30.7, 30.6, 30.4, 30.2, 28.6, 24.2, 23.7, 14.4. HRMS (ESI+) m/z calc’d. for C22H36N3O+ (M+H)+ 358.2853, found 358.2849.
5-decyl-N-(piperidin-4-ylmethyl)benzo[d]oxazol-2-amine hydrochloride (32t).
Synthesized according to General Procedure 4. Purified via trituration with diethyl ether. White solid (70%, 53 mg). 1H NMR (400 MHz, CD3OD) δ 7.46 (d, J = 8.4 Hz, 1H), 7.29 (s, 1H), 7.20 (d, J = 8.5 Hz, 1H), 3.55 – 3.40 (m, 4H), 3.04 (td, J = 13.0, 2.8 Hz, 2H), 2.72 (t, J = 7.6 Hz, 2H), 2.18 – 2.04 (m, 3H), 1.73 – 1.50 (m, 4H), 1.37 – 1.22 (m, 14H), 0.88 (t, J = 6.6 Hz, 3H). 13C NMR (101 MHz, CD3OD) δ 160.3, 145.2, 143.3, 131.0, 126.0, 113.1, 111.5, 44.7, 36.7, 34.6, 33.0, 32.9, 30.7, 30.7, 30.6, 30.4, 30.2, 27.3, 23.7, 14.4. HRMS (ESI+) m/z calc’d. for C23H38N3O3+ (M+H)+ 372.3009, found 372.3012.
(S)-5-decyl-N-(piperidin-3-ylmethyl)benzo[d]oxazol-2-amine hydrochloride (32u).
Synthesized according to General Procedure 4. Purified via trituration with diethyl ether. Off-white solid (82%, 67 mg). 1H NMR (500 MHz, CD3OD) δ 7.41 (d, J = 8.4 Hz, 1H), 7.24 (s, 1H), 7.15 (dd, J = 8.4, 3.0 Hz, 1H), 3.52 – 3.46 (m, 3H), 3.40 – 3.35 (m, 1H), 2.94 (td, J = 12.9, 3.1 Hz, 1H), 2.83 (t, J = 12.2 Hz, 1H), 2.71 (t, J = 7.8 Hz, 2H), 2.29 – 2.18 (m, 1H), 2.07 – 1.95 (m, 2H), 1.84 – 1.71 (m, 1H), 1.63 (quin, J = 7.5 Hz, 2H), 1.46 – 1.36 (m, 1H), 1.34 – 1.24 (m, 14H), 0.89 (t, J = 6.9 Hz, 3H). 13C NMR (101 MHz, CD3OD) δ 160.7, 145.5, 143.1, 132.3, 125.7, 113.5, 111.3, 47.5, 46.8, 45.3, 36.7, 34.9, 33.0, 32.9, 30.7, 30.7, 30.6, 30.4, 30.2, 27.0, 23.7, 22.8, 14.4. HRMS (ESI+) m/z calc’d. for C23H38N3O3+ (M+H)+ 372.3009, found 372.3029.
(R)-5-decyl-N-(piperidin-3-ylmethyl)benzo[d]oxazol-2-amine hydrochloride (32v).
Synthesized according to General Procedure 4. Purified via trituration with diethyl ether. Off-white solid (65%, 32 mg). 1H NMR (500 MHz, CD3OD) δ 7.31 – 7.23 (m, 1H), 7.18 – 7.12 (m, 1H), 7.03 – 6.94 (m, 1H), 3.55 – 3.21 (m, 5H), 2.99 – 2.87 (m, 1H), 2.85 – 2.72 (m, 1H), 2.70 – 2.56 (m, 2H), 2.30 – 2.14 (m, 1H), 2.04 – 1.93 (m, 2H), 1.83 – 1.68 (m, 1H), 1.66 – 1.53 (m, 2H), 1.32 – 1.15 (m, 14H), 0.85 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CD3OD) δ 162.4, 146.6, 141.7, 137.7, 124.1, 114.8, 110.3, 47.8, 46.6, 45.3, 36.8 (d, J = 3.2 Hz), 35.1, 33.1, 33.1, 30.7, 30.6, 30.4, 30.2, 27.1, 23.7, 22.9, 14.4. HRMS (ESI+) m/z calc’d. for C23H38N3O3+ (M+H)+ 372.3009, found 372.3010.
(S)-6-decyl-N-(pyrrolidin-3-ylmethyl)benzo[d]oxazol-2-amine hydrochloride (33p).
Synthesized according to General Procedure 4. Purified via trituration with diethyl ether. Off-white solid (56%, 22 mg). 1H NMR (600 MHz, CD3OD) δ 7.24 (s, 1H), 7.21 (d, J = 8.1 Hz, 1H), 7.10 (d, J = 8.1 Hz, 1H), 3.50 (d, J = 7.1 Hz, 2H), 3.45 (dd, J = 11.9, 7.9 Hz, 1H), 3.40 – 3.34 (m, 1H), 3.25 – 3.25 (m, 1H), 3.00 (dd, J = 11.9, 8.2 Hz, 1H), 2.72 (hept, J = 7.7 Hz, 1H), 2.63 (t, J = 7.6 Hz, 2H), 2.25 – 2.18 (m, 1H), 1.82 – 1.72 (m, 1H), 1.57 (quin, J = 7.6 Hz, 2H), 1.29 – 1.17 (m, 14H), 0.82 (t, J = 7.0 Hz, 3H). 13C NMR (151 MHz, CD3OD) δ 161.9, 148.4, 140.1, 134.6, 126.6, 114.4, 110.8, 49.2, 46.4, 45.7, 39.0, 36.8, 33.1, 33.0, 30.7, 30.7, 30.6, 30.5, 30.2, 29.1, 23.7, 14.5. HRMS (ESI+) m/z calc’d. for C22H36N3O+ (M+H)+ 358.2853, found 358.2847.
(S)-5-octyl-N-(pyrrolidin-3-ylmethyl)benzo[d]oxazol-2-amine hydrochloride (34p).
Synthesized according to General Procedure 4. Purified via trituration with diethyl ether. Off-white solid (67%, 29 mg). 1H NMR (400 MHz, CD3OD) δ 7.37 (d, J = 8.4 Hz, 1H), 7.22 (s, 1H), 7.09 (d, J = 8.4 Hz, 1H), 3.62 (d, J = 7.2 Hz, 2H), 3.53 (dd, J = 11.9, 7.8 Hz, 1H), 3.49 – 3.42 (m, 1H), 3.36 – 3.30 (m, 1H), 3.10 (dd, J = 11.9, 7.9 Hz, 1H), 2.80 (quin, J = 7.6 Hz, 1H), 2.66 (t, J = 7.6 Hz, 2H), 2.36 – 2.24 (m, 1H), 1.94 – 1.79 (m, 1H), 1.60 (quin, J = 7.2 Hz, 2H), 1.35 – 1.22 (m, 10H), 0.85 (t, J = 6.8 Hz, 3H). 13C NMR (126 MHz, Methanol-d4) δ 161.0, 145.8, 142.7, 133.5, 125.3, 113.8, 111.1, 49.1, 46.3, 45.9, 38.8, 36.7, 33.0, 33.0, 30.5, 30.4, 30.2, 29.1, 23.7, 14.4. HRMS (ESI+) m/z calc’d. for C20H32N3O+ (M+H)+ 330.2540, found 330.2539.
(S)-5-nonyl-N-(pyrrolidin-3-ylmethyl)benzo[d]oxazol-2-amine hydrochloride (35p).
Synthesized according to General Procedure 4. Purified via trituration with diethyl ether. Off-white solid (29%, 32 mg, 93% purity by UPLC). 1H NMR (500 MHz, CD3OD) δ 7.16 (d, J = 8.1 Hz, 1H), 7.08 (s, 1H), 6.88 (dd, J = 8.2, 1.7 Hz, 1H), 3.48 (d, J = 7.1 Hz, 3H), 3.46 – 3.37 (m, 1H), 3.34 (s, 1H), 3.06 (dd, J = 11.9, 8.0 Hz, 1H), 2.76 (quin, J = 7.6 Hz, 1H), 2.64 (t, J = 7.6 Hz, 2H), 2.27 – 2.19 (m, 1H), 1.87 – 1.78 (m, 1H), 1.62 (quin, J = 7.4 Hz, 2H), 1.34 – 1.26 (m, 12H), 0.89 (t, J = 6.9 Hz, 3H). 13C NMR (151 MHz, CD3OD) δ 160.9, 145.7, 142.9, 132.9, 125.5, 113.6, 111.2, 49.6, 46.4, 45.9, 38.8, 36.7, 33.0, 33.0, 30.7, 30.6, 30.4, 30.2, 29.1, 23.7, 14.4. HRMS (ESI+) m/z calc’d. for C21H34N3O+ (M+H)+ 344.2696, found 334.2698.
(S)-N-(pyrrolidin-3-ylmethyl)-5-undecylbenzo[d]oxazol-2-amine hydrochloride (36p).
Synthesized according to General Procedure 4. Purified via trituration with diethyl ether. Off-white solid (83%, 39 mg). 1H NMR (500 MHz, CD3OD) δ 7.29 (d, J = 8.3 Hz, 1H), 7.17 (s, 1H), 7.02 (dd, J = 8.3, 1.6 Hz, 1H), 3.55 (d, J = 7.2 Hz, 2H), 3.51 (dd, J = 11.9, 7.9 Hz, 1H), 3.46 – 3.40 (m, 1H), 3.35 – 3.32 (m, 1H), 3.07 (dd, J = 11.9, 8.2 Hz, 1H), 2.77 (quin, J = 7.6 Hz, 1H), 2.68 (t, J = 7.6 Hz, 2H), 2.33 – 2.23 (m, 1H), 1.89 – 1.78 (m, 1H), 1.62 (quin, J = 7.6 Hz, 2H), 1.35 – 1.24 (m, 16H), 0.89 (t, J = 6.9 Hz, 3H). 13C NMR (151 MHz, CD3OD) δ 162.5, 146.7, 141.6, 137.9, 124.1, 114.9, 110.3, 49.2, 46.4, 45.6, 39.1, 36.8, 33.1, 33.1, 30.7, 30.7, 30.7, 30.6, 30.5, 30.2, 29.0, 23.7, 14.4. HRMS (ESI+) m/z calc’d. for C23H38N3O+ (M+H)+ 372.3009, found 372.3007.
(S)-5-dodecyl-N-(pyrrolidin-3-ylmethyl)benzo[d]oxazol-2-amine hydrochloride (37p).
Synthesized according to General Procedure 4. Purified via trituration with diethyl ether. Off-white solid (60%, 21 mg). 1H NMR (500 MHz, CD3OD) δ 7.36 (d, J = 8.2 Hz, 1H), 7.21 (s, 1H), 7.08 (d, J = 8.4 Hz, 1H), 3.61 (d, J = 6.8 Hz, 2H), 3.52 (dd, J = 11.8, 7.7 Hz, 1H), 3.46 – 3.38 (m, 1H), 3.33 – 3.25 (m, 1H), 3.08 (dd, J = 11.9, 7.8 Hz, 1H), 2.80 (h, J = 7.4 Hz, 1H), 2.66 (t, J = 7.6 Hz, 1H), 2.34 – 2.23 (m, 1H), 1.89 – 1.78 (m, 1H), 1.59 (quin, J = 7.3 Hz, 2H), 1.31 – 1.20 (m, 18H), 0.85 (t, J = 6.6 Hz, 3H). 13C NMR (126 MHz, CD3OD) δ 161.1, 145.8, 142.6, 133.7, 125.2, 113.8, 111.1, 46.4, 45.9, 38.8, 36.7, 33.1, 33.0, 30.8, 30.7, 30.7, 30.6, 30.5, 30.2, 29.1, 23.7, 14.5. HRMS (ESI+) m/z calc’d. for C24H40N3O+ (M+H)+ 386.3166, found 386.3170
(S)-5-decyl-N-(pyrrolidin-3-ylmethyl)-1H-benzo[d]imidazol-2-amine hydrochloride (38p).
Synthesized according to General Procedure 4. Purified via trituration with diethyl ether. Off-white solid (100%, 15 mg). 1H NMR (500 MHz, CD3OD) δ 7.30 (d, J = 8.2 Hz, 1H), 7.22 (s, 1H), 7.12 (d, J = 7.3 Hz, 1H), 3.59 – 3.53 (m, 3H), 3.51 – 3.41 (m, 1H), 3.36 – 3.31 (m, 1H), 3.15 – 3.07 (m, 1H), 2.80 (quin, J = 7.7 Hz, 1H), 2.70 (t, J = 7.6 Hz, 2H), 2.37 – 2.26 (m, 1H), 1.92 – 1.80 (m, 1H), 1.63 (quin, J = 7.1 Hz, 2H), 1.35 – 1.22 (m, 14H), 0.88 (t, J = 6.9 Hz, 3H). 13C NMR (126 MHz, CD3OD) δ 151.5, 140.6, 131.2, 129.1, 125.4, 112.1, 112.0, 49.2, 46.4, 46.1, 38.9, 36.8, 33.1, 30.7, 30.7, 30.6, 30.4, 30.2, 29.1, 23.7, 14.4. HRMS (ESI+) m/z calc’d. for C22H37N4+ (M+H)+ 357.3013, found 357.3016.
Supplementary Material
ACKNOWLEDGMENTS
We thank the National Institutes of Health (R01 AI144026) for support of these studies.
ABBREVIATIONS
- dppf
1,1′-bis(diphenylphosphino)ferrocene
- EAE
experimental autoimmune encephalomyelitis
- MFS
major facilitator superfamily
- MS
multiple sclerosis
- mSphK1
mouse sphingosine kinase 1
- mSphK2
mouse sphingosine kinase 2
- S1P
sphingosine-1-phosphate
- S1P1-5
sphingosine-1-phosphate receptors 1-5
- SphK1
sphingosine kinase 1
- SphK2
sphingosine kinase 2
- Spns2
spinster homolog 2
Footnotes
Supporting Information.
The supporting information is available free of charge.
Characterization data (1H, 13C, and UPLC traces) for 15a-d, 32a-v, 33-38p.
Molecular formula strings and the associated biological data (CSV).
The authors declare the following competing financial interest(s): W.L.S. and K.R.L. are the co-founders of Flux Therapeutics Inc, which was created to commercialize S1P-related discoveries, including Spns2 inhibitors, discovered and characterized in their laboratories.
References:
- 1.Stepanovska B; Huwiler A, Targeting the S1P receptor signaling pathways as a promising approach for treatment of autoimmune and inflammatory diseases. Pharmacol. Res 2020, 154, 104170. [DOI] [PubMed] [Google Scholar]
- 2.Cartier A; Hla T, Sphingosine 1-phosphate: Lipid signaling in pathology and therapy. Science 2019, 366 (6463), eaar5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kunkel GT; Maceyka M; Milstien S; Spiegel S, Targeting the sphingosine-1-phosphate axis in cancer, inflammation and beyond. Nat. Rev. Drug Disc 2013, 12 (9), 688–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maceyka M; Spiegel S, Sphingolipid metabolites in inflammatory disease. Nature 2014, 510 (7503), 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chalfant CE; Spiegel S, Sphingosine 1-phosphate and ceramide 1-phosphate: expanding roles in cell signaling. J. Cell. Sci 2005, 118 (20), 4605–4612. [DOI] [PubMed] [Google Scholar]
- 6.Vu TM; Ishizu A-N; Foo JC; Toh XR; Zhang F; Whee DM; Torta F; Cazenave-Gassiot A; Matsumura T; Kim S; Toh S-AES; Suda T; Silver DL; Wenk MR; Nguyen LN, Mfsd2b is essential for the sphingosine-1-phosphate export in erythrocytes and platelets. Nature 2017, 550 (7677), 524–528. [DOI] [PubMed] [Google Scholar]
- 7.Osborne N; Brand-Arzamendi K; Ober EA; Jin S-W; Verkade H; Holtzman NG; Yelon D; Stainier DYR, The Spinster Homolog, Two of Hearts, Is Required for Sphingosine 1-Phosphate Signaling in Zebrafish. Curr. Biol 2008, 18 (23), 1882–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawahara A; Nishi T; Hisano Y; Fukui H; Yamaguchi A; Mochizuki N, The Sphingolipid Transporter Spns2 Functions in Migration of Zebrafish Myocardial Precursors. Science 2009, 323 (5913), 524–527. [DOI] [PubMed] [Google Scholar]
- 9.Hisano Y; Kobayashi N; Yamaguchi A; Nishi T, Mouse SPNS2 functions as a sphingosine-1-phosphate transporter in vascular endothelial cells. PLoS One 2012, 7 (6), e38941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brinkmann V; Cyster JG; Hla T, FTY720: sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am. J. Transplant 2004, 4 (7), 1019–1025. [DOI] [PubMed] [Google Scholar]
- 11.Cuvillier O; Pirianov G; Kleuser B; Vanek PG; Coso OA; Gutkind S; Spiegel S, Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 1996, 381 (6585), 800–3. [DOI] [PubMed] [Google Scholar]
- 12.Spiegel S; Milstien S, The outs and the ins of sphingosine-1-phosphate in immunity. Nat. Rev. Immunol 2011, 11 (6), 403–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cyster JG; Schwab SR, Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu. Rev. Immunol 2012, 30, 69–94. [DOI] [PubMed] [Google Scholar]
- 14.Hla T; Venkataraman K; Michaud J, The vascular S1P gradient—Cellular sources and biological significance. Biochim. Biophys. Acta - Mol. Cell. Biol. Lipids 2008, 1781 (9), 477–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwab SR; Pereira JP; Matloubian M; Xu Y; Huang Y; Cyster JG, Lymphocyte Sequestration Through S1P Lyase Inhibition and Disruption of S1P Gradients. Science 2005, 309 (5741), 1735–1739. [DOI] [PubMed] [Google Scholar]
- 16.Billich A; Bornancin F; Dévay P; Mechtcheriakova D; Urtz N; Baumruker T, Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. J. Biol. Chem 2003, 278 (48), 47408–47415. [DOI] [PubMed] [Google Scholar]
- 17.Brinkmann V; Davis MD; Heise CE; Albert R; Cottens S; Hof R; Bruns C; Prieschl E; Baumruker T; Hiestand P; Foster CA; Zollinger M; Lynch KR, The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J. Biol. Chem 2002, 277 (24), 21453–21457. [DOI] [PubMed] [Google Scholar]
- 18.Chun J; Brinkmann V, A mechanistically novel, first oral therapy for multiple sclerosis: the development of fingolimod (FTY720, Gilenya). Discov. Med 2011, 12 (64), 213–228. [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen JA; Khatri B; Barkhof F; Comi G; Hartung H-P; Montalban X; Pelletier J; Stites T; Ritter S; von Rosenstiel P; Tomic D; Kappos L, Long-term (up to 4.5 years) treatment with fingolimod in multiple sclerosis: results from the extension of the randomised TRANSFORMS study. J. Neurol. Neurosurg. Psychiatry 2016, 87 (5), 468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaudhry BZ; Cohen JA; Conway DS, Sphingosine 1-Phosphate Receptor Modulators for the Treatment of Multiple Sclerosis. Neurotherapeutics 2017, 14 (4), 859–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott LJ, Siponimod: A Review in Secondary Progressive Multiple Sclerosis. CNS Drugs 2020, 34 (11), 1191–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olsson T; Boster A; Fernández Ó; Freedman MS; Pozzilli C; Bach D; Berkani O; Mueller MS; Sidorenko T; Radue E-W; Melanson M, Oral ponesimod in relapsing-remitting multiple sclerosis: a randomised phase II trial. J. Neurol. Neurosurg. Psychiatry 2014, 85 (11), 1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lassiter G; Melancon C; Rooney T; Murat A-M; Kaye JS; Kaye AM; Kaye RJ; Cornett EM; Kaye AD; Shah RJ; Viswanath O; Urits I, Ozanimod to Treat Relapsing Forms of Multiple Sclerosis: A Comprehensive Review of Disease, Drug Efficacy and Side Effects. Neurol. Int 2020, 12 (3), 89–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandborn WJ; Feagan BG; D’Haens G; Wolf DC; Jovanovic I; Hanauer SB; Ghosh S; Petersen A; Hua SY; Lee JH; Charles L; Chitkara D; Usiskin K; Colombel J-F; Laine L; Danese S, Ozanimod as Induction and Maintenance Therapy for Ulcerative Colitis. New Engl. J. Med 2021, 385 (14), 1280–1291. [DOI] [PubMed] [Google Scholar]
- 25.Camm J; Hla T; Bakshi R; Brinkmann V, Cardiac and vascular effects of fingolimod: mechanistic basis and clinical implications. Am. Heart J 2014, 168 (5), 632–644. [DOI] [PubMed] [Google Scholar]
- 26.Cohen JA; Chun J, Mechanisms of fingolimod's efficacy and adverse effects in multiple sclerosis. Ann. Neurol 2011, 69 (5), 759–77. [DOI] [PubMed] [Google Scholar]
- 27.Bu Y; Wu H; Deng R; Wang Y, Therapeutic Potential of SphK1 Inhibitors Based on Abnormal Expression of SphK1 in Inflammatory Immune Related-Diseases. Front. Pharmacol 2021, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lynch KR; Thorpe SB; Santos WL, Sphingosine kinase inhibitors: a review of patent literature (2006-2015). Expert Opin Ther Pat. 2016, 26 (12), 1409–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neubauer HA; Pitson SM, Roles, regulation and inhibitors of sphingosine kinase 2. FEBS J. 2013, 280 (21), 5317–5336. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi N; Kawasaki-Nishi S; Otsuka M; Hisano Y; Yamaguchi A; Nishi T, MFSD2B is a sphingosine 1-phosphate transporter in erythroid cells. Sci. Rep 2018, 8 (1), 4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hasan Z; Nguyen TQ; Lam BWS; Wong JHX; Wong CCY; Tan CKH; Yu J; Thiam CH; Zhang Y; Angeli V; Nguyen LN, Postnatal deletion of Spns2 prevents neuroinflammation without compromising blood vascular functions. Cell. Mol. Life Sci 2022, 79 (11), 541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simmons S; Sasaki N; Umemoto E; Uchida Y; Fukuhara S; Kitazawa Y; Okudaira M; Inoue A; Tohya K; Aoi K; Aoki J; Mochizuki N; Matsuno K; Takeda K; Miyasaka M; Ishii M, High-endothelial cell-derived S1P regulates dendritic cell localization and vascular integrity in the lymph node. eLife 2019, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukuhara S; Simmons S; Kawamura S; Inoue A; Orba Y; Tokudome T; Sunden Y; Arai Y; Moriwaki K; Ishida J; Uemura A; Kiyonari H; Abe T; Fukamizu A; Hirashima M; Sawa H; Aoki J; Ishii M; Mochizuki N, The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J. Clin. Invest 2012, 122 (4), 1416–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nijnik A; Clare S; Hale C; Chen J; Raisen C; Mottram L; Lucas M; Estabel J; Ryder E; Adissu H; Project SMG; Adams NC; Ramirez-Solis R; White JK; Steel KP; Dougan G; Hancock REW, The Role of Sphingosine-1-Phosphate Transporter Spns2 in Immune System Function. J. Immunol. Res 2012, 189 (1), 102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagahashi M; Kim EY; Yamada A; Ramachandran S; Allegood JC; Hait NC; Maceyka M; Milstien S; Takabe K; Spiegel S, Spns2, a transporter of phosphorylated sphingoid bases, regulates their blood and lymph levels, and the lymphatic network. FASEB J. 2013, 27 (3), 1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mendoza A; Bréart B; Ramos-Perez Willy D.; Pitt Lauren A.; Gobert M; Sunkara M; Lafaille Juan J.; Morris Andrew J.; Schwab Susan R., The Transporter Spns2 Is Required for Secretion of Lymph but Not Plasma Sphingosine-1-Phosphate. Cell Rep. 2012, 2 (5), 1104–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le TNU; Nguyen TQ; Kalailingam P; Nguyen YTK; Sukumar VK; Tan CKH; Tukijan F; Couty L; Hasan Z; Del Gaudio I; Wenk MR; Cazenave-Gassiot A; Camerer E; Nguyen LN, Mfsd2b and Spns2 are essential for maintenance of blood vessels during development and in anaphylactic shock. Cell Rep. 2022, 40 (7), 111208. [DOI] [PubMed] [Google Scholar]
- 38.Donoviel MS; Hait NC; Ramachandran S; Maceyka M; Takabe K; Milstien S; Oravecz T; Spiegel S, Spinster 2, a sphingosine-1-phosphate transporter, plays a critical role in inflammatory and autoimmune diseases. FASEB J. 2015, (12), 5018–5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blanchard O; Stepanovska B; Starck M; Erhardt M; Römer I; Meyer Zu Heringdorf D; Pfeilschifter J; Zangemeister-Wittke U; Huwiler A, Downregulation of the S1P Transporter Spinster Homology Protein 2 (Spns2) Exerts an Anti-Fibrotic and Anti-Inflammatory Effect in Human Renal Proximal Tubular Epithelial Cells. Int. J. Mol. Sci 2018, 19 (5), 1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanaka S; Zheng S; Kharel Y; Fritzemeier RG; Huang T; Foster D; Poudel N; Goggins E; Yamaoka Y; Rudnicka KP; Lipsey JE; Radel HV; Ryuh SM; Inoue T; Yao J; Rosin DL; Schwab SR; Santos WL; Lynch KR; Okusa MD, Sphingosine 1-phosphate signaling in perivascular cells enhances inflammation and fibrosis in the kidney. Sci. Transl. Med 2022, 14 (658), eabj2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fritzemeier R; Foster D; Peralta A; Payette M; Kharel Y; Huang T; Lynch KR; Santos WL, Discovery of In Vivo Active Sphingosine-1-phosphate Transporter (Spns2) Inhibitors. J. Med. Chem 2022, 65 (11), 7656–7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swamy KCK; Kumar NNB; Balaraman E; Kumar KVPP, Mitsunobu and Related Reactions: Advances and Applications. Chem. Rev 2009, 109 (6), 2551–2651. [DOI] [PubMed] [Google Scholar]
- 43.Bellina F; Carpita A; Rossi R, Palladium Catalysts for the Suzuki Cross-Coupling Reaction: An Overview of Recent Advances. Synthesis 2004, 2004 (15), 2419–2440. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.