Abstract
BACKGROUND & AIMS
Atherosclerosis and arteriosclerosis contribute to vascular aging and cardiovascular disease (CVD) risk. Both processes can be assessed simply in the lower-limbs and reflect systemic pathology. However, only atherosclerosis is routinely assessed, typically via ankle-brachial index (ABI). Arteriosclerosis can be assessed using femoral-ankle pulse wave velocity (faPWV), but no studies have identified whether ABI and faPWV similarly associate with overt CVD and risk factors, nor whether faPWV confers additional information. The aims of this study were to, (i) Compare associations of ABI and faPWV with traditional CVD risk factors, including age, sex, systolic blood pressure (SBP), high-density lipoprotein (HDL), total cholesterol (TC), smoking, and diabetes; and, ii) Determine the independent and additive associations of ABI and faPWV with a composite measure of prevalent CVD.
METHODS
We evaluated ABI and faPWV in 4,330 older-aged (75.3±5.0 years) adults using an oscillometric screening device. Associations between ABI and faPWV with CVD risk factors and CVD were determined using mixed-model linear- and logistic-regression.
RESULTS
ABI and faPWV were associated with age, HDL, and smoking. ABI was associated with sex, TC, diabetes. faPWV was associated with SBP. Both ABI and faPWV were inversely associated with CVD. Low ABI (≤0.9 vs. >0.9) and low faPWV (≤9.94 vs. >9.94) increased the odds of CVD by 2.41-fold (95% CI:1.85,3.17) and 1.46-fold (95% CI:1.23,1.74), respectively. The inverse association between faPWV and CVD was independent of ABI and CVD risk factors.
CONCLUSION
ABI and faPWV, measures of lower-limb atherosclerosis and arteriosclerosis, are independently associated with CVD risk factors and prevalent CVD. Assessment of faPWV may confer additional risk information beyond ABI.
Keywords: Ankle-brachial index, femoral-ankle pulse wave velocity, cardiovascular risk factors, arterial stiffness, peripheral artery disease, coronary heart disease, heart failure, stroke
INTRODUCTION
The determination of traditional cardiovascular disease (CVD) risk factors including age, blood pressure and diabetes, enables clinicians and epidemiological researchers to estimate an individual’s risk of developing CVD [1–3]. Novel biomarkers have been shown to improve patient risk stratification, including those indicating lower-limb arterial health [4]. The assessment of lower-limb arterial health provides clinicians with the opportunity to conveniently detect local disease pathology, but also determine general CVD risk, as poor lower-limb arterial health is a manifestation of systemic pathophysiology [5]. Importantly, such assessments also permit identification of high-risk patients, given that individuals with peripheral vascular diseases are at a higher risk of cardiovascular events and death, than those with isolated cardiac or cerebrovascular disease [6].
Ideally, lower-limb arterial health assessment would screen for both atherosclerosis and arteriosclerosis as both processes contribute to vascular aging, and therefore, CVD risk [7,8]. Atherosclerosis, the narrowing of the artery by the deposition of atheromatous plaque, typically occurs in the intima layer, and is principally characterized by the accumulation of lipids and fibrous elements, smooth muscle cell migration and foam cell development [9]. In contrast, arteriosclerosis, the stiffening and thickening of the arterial wall, reflects degenerative changes of the extra-cellular matrix in the media layer, and is principally characterized by elastin fatigue fracture, and collagen deposition and cross-linking [8]. Although these phenomena are typically viewed as distinct pathways, they do share common pathophysiological mechanisms. For example, increased luminal pressure induced by arterial stiffening impairs endothelial function and augments collagen production and deposition in the arterial wall, accelerating the formation of atheromatous plaque [8,10]. But whilst they typically co-exist, and may interact, their relative contribution to vascular aging, and therefore cardiovascular risk, may differ by arterial territory [10]. Whether atherosclerosis and arteriosclerosis of the femoral-tibial pathway equally associate with CVD risk factors and disease, or whether their combined assessment confers additional cardiovascular risk information is unknown.
The ankle-brachial index (ABI) is a simple biomarker of atherosclerosis in the legs [11]. Defined as the ratio of systolic blood pressure (SBP) measured at the ankle to SBP measured at the brachial artery, ABI was first employed as a non-invasive tool for the screening of occlusive peripheral artery disease (PAD) [8]. Whilst ABI indicates arterial stenosis in the lower-limbs, any occlusion typically indicates the presence of systemic atherosclerosis [11,12]. As such low resting ABI (<0.9) typically serves as an independent prognostic marker for all CVD [13–16]. Despite its popularity, evidence supporting the use of ABI as a screening or monitoring tool at a population level, particularly in asymptomatic adults, remains limited [4,12].
A simple and potentially complimentary measure of lower-limb arterial health, and a measure of arteriosclerosis, is arterial stiffness. Emerging evidence indicates that arterial stiffening is one of the earliest detectable markers of changes in vascular structure and function of the arterial wall [17]. Lower-limb arterial stiffness can be determined using femoral-ankle pulse wave velocity (faPWV). Importantly, faPWV is clinically viable as it can be assessed with accuracy and precision using simple oscillometry [18,19], and faPWV can be determined concurrently with ABI. Lower-limb(s) arterial stiffness is associated with CVD risk factors, including blood pressure, diabetes and dyslipidemia [20–22] and also reflects systemic CVD burden [22–26]. However, it is unknown whether ABI and faPWV are similarly associated with prevalent CVD, nor whether faPWV confers incremental information over and above ABI and established CVD risk factors [27].
As a proof of concept [27], the aims of the current study were: (i) to compare the independent associations of ABI and faPWV with traditional CVD risk factors, including age, sex, systolic blood pressure (SBP), high-density lipoprotein (HDL), total cholesterol (TC), smoking, and diabetes; and, ii) determine the independent and additive associations of ABI and faPWV with a composite measure of prevalent CVD status that includes coronary heart disease (CHD), heart failure (HF), and stroke. These aims were undertaken using a well characterized community-dwelling population of older men and women from the Atherosclerosis Risk in Communities (ARIC) Study cohort.
PATIENTS AND METHODS
This observational study is reported in accordance with STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) guidelines [28]. Participants provided written informed consent, and the study was approved by the Institutional Review Boards at all field centers, coordinating center, and central labs and reading centers.
STUDY POPULATION
The ARIC Study is a population-based, longitudinal study of 15,792 men and women aged 45–64 years enrolled between 1987 and 1989 from 4 US communities (Forsyth County, North Carolina; Jackson, Mississippi; Minneapolis, Minnesota; and Washington County, Maryland). Details of the baseline visit have been previously described [29]. Prior to exclusions, the current analysis includes 6,538 participants who attended visit 5 between 2011 and 2013 and had ABI and PWV measures completed (5,683 total participants at visit 5).
We excluded participants with the following conditions due to concerns of ABI and PWV data quality: BMI ≥40 kg/m2, major arrhythmias (atrial or ventricular premature beats, atrial fibrillation or flutter), major arrhythmias with evidence of biased PWV waveforms, aortic aneurysms, abdominal aorta ≥5 cm, history of aortic or peripheral revascularization or aortic graft, aortic stenosis, moderate or greater aortic regurgitation and an ABI ≥ 1.5, which indicates incompressible arteries. Additionally, we excluded participants whose race was other than white or African American (due to small sample size) as well as those with missing ABI, PWV or vascular risk factor data. Participants were asked not to consume food or drink, and refrain from tobacco and vigorous physical activity after midnight prior to the clinic visit or for 8 hours prior to the visit. Visit 5 study examination included interviewer-administered questionnaires to obtain demographic data, medical history and lifestyle information, blood and urine collection, and assessment of vascular risk factors and cardiovascular phenotypes, including ABI and PWV.
EXPERIMENTAL MEASURES
ANKLE-BRACHIAL INDEX AND PULSE-WAVE VELOCITY
Technicians measured ABI and faPWV following a standardized protocol using the automated cardiovascular screening device VP-1000 Plus (Omron, Kyoto, Japan)[30], after participants were supine for 5–10 minutes. Quality assurance for PWV included central training and recertification, quarterly equipment calibration, and ongoing quality control reviews by one of the authors (H.T.) on a stratified random sample of 40 records per month with feedback provided to technicians. Approximately 78% of records were considered optimal quality, 17% were good quality, 3% were acceptable, and none were poor or unacceptable.
Ankle-brachial index.
Assessments were completed with the participant in a supine position with both arms resting along his/her side while bent to 90 degrees at the elbows. Two electrocardiogram clips were attached on the inner side of both wrists, and blood pressure cuffs were placed on both arms and ankles. Blood pressure was measured simultaneously in the four limbs at least twice at an interval of 5 minutes. Using the higher value of the right or left brachial systolic blood pressure as the denominator, the ABI, the ratio of ankle systolic blood pressure to brachial systolic blood pressure [11], was calculated for right and left legs. The mean ABI of the two measurements was recorded for each leg. The minimum ABI, of left or right leg, was used for analyses.
Femoral-ankle pulse-wave velocity.
Bilateral femoral arterial waveforms were acquired by applanation tonometry sensors on the common femoral arterials (via elastic tape around the hip). Bilateral posterior-tibial arterial pressure waveforms were detected by extremities cuffs connected to plethysmographic and oscillometric pressure sensors wrapped on both ankles. Distance for faPWV was automatically calculated by the VP-1000 Plus using height-based formulas, as previously described [31]. A minimum of two PWV measurements were taken per participant and the last two measurements were averaged. The maximum of left and right faPWV measures was included for analysis.
MEASUREMENT OF CVD RISK FACTORS AND COVARIATES
Demographics.
Age was calculated from date of birth. Sex and race were self-reported. History of smoking was self-reported and analyzed as dichotomous (current versus noncurrent).
Anthropometrics.
Body weight was measured to the nearest 0.1 kg, and height was recorded to the nearest centimeter (cm). Body mass index (BMI) was calculated using weight (kg) divided by height squared (m2).
Blood Pressure.
Three seated BP measurements were obtained after a 5-minute rest using an oscillometric automated sphygmomanometer (Omron HEM-907 XL, Omron, Kyoto, Japan), and the average of the last two measurements was used. Hypertension was defined as SBP ≥140 mm Hg, diastolic BP (DBP) ≥90 mm Hg, or antihypertensive medication use.
Blood Markers.
Blood samples were obtained following a standardized venipuncture protocol and shipped weekly to ARIC central laboratories where assays for total cholesterol (TC) and high-density lipoprotein (HDL), and fasting glucose concentration were performed. Total plasma cholesterol concentrations were determined enzymatically using a Cobas-Bio analyzer with reagents purchased from Boehringer Mannheim Biochemicals, (Indianapolis, IN). Plasma HDL concentrations were measured using the method of Warnick et al. [32]. Diabetes was defined as fasting glucose ≥126 mg/dl, non-fasting glucose ≥200 mg/dl, antidiabetic medication use, or self-reported diagnosis of diabetes by a physician.
Medications.
Participants were asked to bring to the clinical visit all prescription and nonprescription medications taken within the two preceding weeks. That information was transcribed and categorized using MediSPAN prescription codes and classified into medication categories. Participants also self-reported medication use. Medications used included β-blockers, α-blockers, calcium channel blockers, diuretics, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers.
PREVALENT CARDIOVASCULAR DISEASE
Prevalent CVD was defined as composite measure of CHD, stroke and HF [3]. Prevalent CHD was defined by self‐reported prior physician diagnosis of myocardial infarction or coronary revascularization, or prevalent myocardial infarction. Prevalent HF was classified by having at least one of the following: an adjudicated diagnosis of a HF event, any physician report of HF, self-reported HF or self-report of HF medication with pro-BNP greater than 125 pg/mL, or subsequent self-report of HF or HF medication (defined as medications participants reported taking for the treatment of HF). Prevalent stroke was defined by self‐reported prior physician diagnosis of stroke or trans ischemic attack.
STATISTICAL ANALYSIS
Statistical analyses were performed using R Statistical Software. The α-level was set a-priori for all statistical procedures at α = 0.05. Cumulative frequency and Q-Q plots were used to compare the distributions of ABI and faPWV. Participant characteristics were stratified by faPWV quartiles and were estimated as mean and standard deviation (SD), or frequencies and percent. Descriptive data across faPWV quartiles were compared using one-way analysis of variance (ANOVA) for continuous outcomes and Kruskal-Wallis for categorical outcomes. We initially explored associations between ABI and faPWV with 5-year age groups using Spearman correlations (r). For linear regression we report unstandardized and standardized β coefficient estimates and 95% confidence intervals (95% CI), and the R2 values for model fit.
For aim 1, ABI and faPWV associations with traditional CVD risk factors were evaluated using mixed model linear regression, with race and field center specified as random intercepts. Independent variables included age, sex, SBP, HDL, TC, smoking, and diabetes. All independent variables were initially entered into the model (model 1), and variables significantly associated with ABI and faPWV (P<0.1) were retained using a backward step-wise method (final model). For Aim 2, we investigated the associations between ABI and faPWV with a composite measure of CVD status that included CHD, HF and stroke using univariable and multi-variable binomial logistic regression. Univariable models included ABI or faPWV only. Multivariable model 1 included all CVD risk factors only. Multivariable model 2 included CVD risk factors and ABI or faPWV independently. Multivariable model 3 included all CVD risk factors, ABI, and faPWV.
Initially, ABI and faPWV were entered as continuous variables. Comparisons were also made whereby ABI and faPWV were entered as categorical variables. For ABI, data was categorized according to the recognized criteria for classifying PAD (ABI: ≤0.9 vs. >0.9). Given that no clinical threshold for faPWV has been identified, faPWV was categorized by quartiles, with the reference level and direction of association being determined following continuous analysis findings. For logistical regression, we report odds ratios as well as indicators of overall model fit including: Akaike’s Information Criteria (AIC), McFadden’s Pseudo R2 (R2McF) and Chi-square statistic (X2). Likelihood ratio tests were used to compare the goodness-of-fit between nested logistic regression models. The Vuong likelihood ratio test was used to compared non-nested logistic regression models, within the nonnest2 package in R. All multivariable logistical regression models were adjusted for antihypertensive medication and race-field center. Assumptions of linearity, collinearity, homoscedasticity, and outliers were assessed for every model.
RESULTS
CHARACTERISTICS OF THE STUDY POPULATION
Descriptive characteristics are reported in Table 1. Following exclusions, the sample included 4,330 cohort participants between the ages of 66 and 90 years, of which 59.5% were women and 22.3% were African American. Of the 5,683 participants who attended visit 5 and underwent ABI and PWV measurements: 1353 were excluded using the following criteria: pre-existing condition (n=579), race other than white or African American due to low numbers (n=15), missing ABI data (n=13), missing PWV data (n=433), missing risk factor data (n=83), and missing covariates (n=230).
TABLE 1.
Descriptive characteristics of ARIC visit 5 participants, overall and stratified by femoral-ankle pulse wave velocity (faPWV) quartiles.
| Overall | Q1 | Q2 | Q3 | Q4 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 4330 | n = 1081 | n = 1045 | n = 1138 | n = 1066 | P Value | ||||||
| Continuous Variables (Mean, SD) | |||||||||||
| Age (years) | 75.3 | (5.0) | 75.2 | (5.0) | 74.9 | (4.88)d | 75.2 | (5.02)d | 75.7 | (5.2) | 0.002 |
| Body Mass Index (kg/m2) | 29.2 | (4.7) | 28.1 | (4.5) | 27.4 | (4.3) | 26.7 | (4.1) | 28.2 | (4.6) | <0.001a |
| Diastolic blood pressure (mm Hg) | 66 | (10) | 62 | (10) | 65 | (10) | 67 | (10) | 70 | (10.7) | <0.001a |
| Systolic blood pressure (mm Hg) | 130 | (18) | 126 | (17) | 128 | (17) | 130 | (16) | 135 | (18) | <0.001a |
| Heart rate (bpm) | 65 | (11) | 63 | (10.1)c,d | 64 | (10)c,d | 65 | (11.2)d | 67 | (11) | <0.001a |
| Fasting glucose (mg/dL) | 112 | (26) | 110 | (25) | 112 | (26) | 111 | (26) | 112 | (26) | <0.001 |
| Total Cholesterol (mg/dL) | 183 | (41) | 181 | (40)d | 182 | (41)d | 183 | (41) | 187 | (43) | 0.001 |
| High-density lipoproteins (mg/dL) | 53 | (14) | 51 | (13)b,c,d | 53 | (14) | 54 | (15) | 54 | (15) | <0.001 |
| Ankle Brachial Index | 1.11 | (0.13) | 1.06 | (0.17)b,cd | 1.11 | (0.12)c,d | 1.12 | (0.11) | 1.13 | (0.11) | <0.001 |
| Femoral-ankle PWV (m/s) | 11.2 | (1.9) | 9.0 | (0.8) | 10.5 | (0.3) | 11.6 | (0.4) | 13.8 | (1.5) | - |
| Categorical Variables (No., %) | |||||||||||
| Sex | |||||||||||
| Men | 1750 | (41) | 443 | (41) | 417 | (40) | 468 | (41) | 422 | (40) | 0.821 |
| Women | 2580 | (59) | 638 | (59) | 628 | (60) | 670 | (59) | 644 | (60) | |
| Race | b,c,d | d | |||||||||
| African American | 967 | (22) | 333 | (31) | 241 | (23) | 219 | (19) | 174 | (16) | <0.001 |
| White | 3363 | (78) | 748 | (69) | 804 | (77) | 919 | (81) | 892 | (84) | |
| Current smoker | 248 | (6) | 82 | (8)d | 57 | (5) | 57 | (5) | 52 | (5) | 0.021 |
| Prevalent Cardiovascular Disease | |||||||||||
| Coronary heart disease | 459 | (11) | 184 | (17)c,d | 147 | (14) | 147 | (13) | 131 | (12) | <0.011 |
| Heart failure | 125 | (3) | 170 | (16)b,c,d | 100 | (10) | 102 | (9) | 87 | (8) | <0.001 |
| Stroke | 2835 | (66) | 32 | (3) | 38 | (4) | 26 | (2) | 29 | (3) | 0.296 |
| Cardiovascular Disease Risk Factors | |||||||||||
| Hypertension | 610 | (14) | 751 | (70)c | 685 | (66) | 741 | (65) | 654 | (61) | <0.001 |
| Diabetes | 1276 | (29) | 358 | (33)c,d | 327 | (31) | 306 | (27) | 283 | (27) | <0.001 |
| Ankle Brachial Index ≤ 0.9 | 298 | (6.9) | 158 | (14.8)b,cd | 64 | (6.1) d | 44 | (3.9) | 32 | (3.0) | <0.001 |
| Medication use | |||||||||||
| β-Blocker | 1311 | (30) | 410 | (38)c,d | 328 | 31d | 333 | (29)d | 240 | (23) | <0.001 |
| α-Blocker | 140 | (3) | 60 | (6)b,c,d | 27 | (3) | 30 | (3) | 23 | (2) | <0.001 |
| Diuretic | 1666 | (38) | 499 | (46)b,c,d | 418 | 40d | 406 | (36) | 343 | (32) | <0.001 |
| ACE Inhibitor | 986 | (23) | 245 | (23) | 231 | (22) | 281 | (25) | 227 | (21) | 0.278 |
| ANG II receptor blocker | 443 | (10) | 125 | (12) | 105 | (10) | 108 | (9) | 103 | (10) | 0.327 |
| Calcium channel blocker | 1068 | (25) | 345 | (32)b,c,d | 267 | (26)d | 256 | (23) | 199 | (19) | 0.327 |
Abbreviations: PWV, pulse-wave velocity. faPWV quartiles Q1, <9.94 m/s; Q2, 9.94 m/s to 11.0 m/s; Q3, >11.0 m/s to 12.3 m/s Q4 >12.3 m/s.
Comparisons: All groups significantly different;
vs. Q2;
vs. Q3;
vs. Q4.
AIM 1: ASSOCIATIONS of ABI and faPWV WITH CVD RISK FACTORS
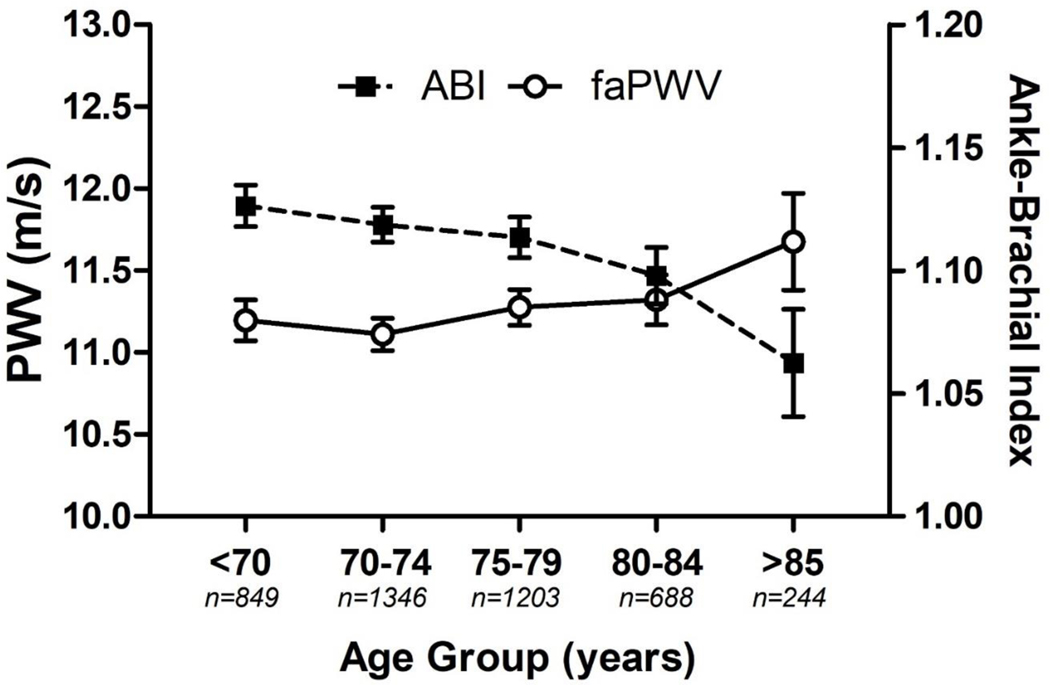
Linear regression analysis revealed a positive association between faPWV and ABI (R2=0.039). Non-linearity between faPWV and ABI was explored by specifying the faPWV quadratic term. The quadratic term was significant (P<0.001), and the change in R2 due to the quadratic term was significant (ΔR2=0.04, P<0.001). Figure 1 presents ABI and faPWV values stratified by age categories. ABI had a negative correlation with age (r = −0.08, 95% CI: −0.11, −0.05, P < 0.001) and faPWV had a positive correlation with age (r = 0.04, 95% CI: 0.01, 0.07, P = 0.004).
FIGURE 1.
Mean ankle-brachial index (ABI) and femoral-ankle pulse wave velocity (faPWV) in 5-year age groups, with 95% confidence intervals. n = 4,330.
Backwards stepwise regression analysis was used to identify CVD risk factors associated with ABI and faPWV (Table 2). For ABI we observed positive associations with male sex and HDL, and negative associations with age, TC, smoking and diabetes. Overall, faPWV was associated with fewer CVD risk factors, demonstrating positive associations with age, SBP, and HDL, and a negative association with smoking. Accordingly, only age, HDL, and smoking status were mutual covariates. The highest standardized beta coefficients were also different between measures, with ABI demonstrating the strongest association with smoking, male sex and age and faPWV demonstrating the strongest association with SBP, HDL and smoking.
Table 2.
Linear regression models for association of ankle-brachial index (ABI) and femoral-ankle pulse-wave velocity (faPWV) with traditional cardiovascular disease risk factors at visit 5.
| ABI |
faPWV |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | Std. β | 95% CI | P Value | β | Std. β | 95% CI | P Value | |||
| Model 1 | ||||||||||
| Age (years) | −0.004 | −0.139 | −0.167 | −0.110 | <0.001 | 0.011 | 0.030 | 0.001 | 0.059 | 0.045 |
| Male Sex | 0.042 | 0.156 | 0.125 | 0.186 | <0.001 | 0.109 | 0.028 | −0.004 | 0.059 | 0.083 |
| Systolic blood pressure (mm Hg) | 0.000 | 0.005 | −0.023 | 0.033 | 0.724 | 0.023 | 0.208 | 0.179 | 0.237 | <0.001 |
| High-density lipoproteins (mg/dL) | 0.001 | 0.067 | 0.034 | 0.100 | <0.001 | 0.009 | 0.066 | 0.031 | 0.100 | <0.001 |
| Total Cholesterol (mg/dL) | 0.000 | −0.072 | −0.105 | −0.040 | <0.001 | 0.000 | 0.003 | −0.031 | 0.036 | 0.869 |
| Smoker | −0.099 | −0.173 | −0.200 | −0.145 | <0.001 | −0.308 | −0.037 | −0.066 | −0.008 | 0.011 |
| Diabetes | −0.009 | −0.030 | −0.060 | −0.002 | 0.039 | −0.099 | −0.023 | −0.053 | 0.007 | 0.128 |
| Overall Model Fit | mR2 = .073 | cR2 = .189 | mR2 = .058 | cR2 = .103 | ||||||
| Final Model * | ||||||||||
| Age (years) | −0.004 | −0.138 | −0.166 | −0.110 | <0.001 | 0.012 | 0.030 | 0.001 | 0.059 | 0.042 |
| Male sex | 0.042 | 0.155 | 0.125 | 0.186 | <0.001 | 0.106 | 0.027 | −0.004 | 0.058 | 0.089 |
| Systolic blood pressure (mm Hg) | 0.023 | 0.208 | 0.179 | 0.237 | <0.001 | |||||
| High-density lipoproteins (mg/dL) | 0.001 | 0.067 | 0.034 | 0.100 | <0.001 | 0.010 | 0.071 | 0.039 | 0.102 | <0.001 |
| Total Cholesterol (mg/dL) | 0.000 | −0.072 | −0.104 | −0.040 | <0.001 | |||||
| Smoker | −0.099 | −0.173 | −0.201 | −0.145 | <0.001 | −0.305 | −0.037 | −0.066 | −0.008 | 0.012 |
| Diabetes | −0.009 | −0.030 | −0.060 | −0.002 | 0.039 | |||||
| Overall Model Fit | mR2 = −0.073 | cR2 = .189 | mR2 = .058 | cR2 = .103 | ||||||
Abbreviations: β, beta coefficient; CI, confidence interval; std. β, standardized beta coefficient; mR2, marginal R squared coefficient; cR2, conditional R squared coefficient.
Adjustments: antihypertensive medication, race/field center
AIM 2: ASSOCIATIONS of ABI and faPWV WITH PREVALENT CVD
Table 3 presents associations for ABI and faPWV with prevalent CVD (CHD, HF and stroke) following univariable and multivariable logistic regression analyses. When specified as continuous variables, both ABI and faPWV were negatively associated with prevalent CVD (univariable) and were negligibly impacted with adjustment for traditional CVD risk factors (multivariable 2). Similarly, when specified as categorical variables, both a low ABI (<0.9) and a low faPWV (<9.94 m/s) were associated with prevalent CVD (univariable), and these associations remained significant after adjustment for CVD risk factors (multivariable 2). After adjustment for CVD risk factors, a low ABI increased the odds of CVD by 2.41-fold, whilst a low faPWV increased the odds of CVD by 1.46-fold. Comparison of non-nested models, whereby ABI or faPWV were entered independently (univariable and multivariable), showed that ABI explained a significantly greater portion of the variance in prevalent CVD than faPWV.
Table 3.
Logistic regression models for association of ankle-brachial index (ABI) and femoral-ankle pulse-wave velocity (faPWV) with cardiovascular disease at visit 5.
| Model Coefficients |
Overall Model Fit |
|||||||
|---|---|---|---|---|---|---|---|---|
| Continuous | OR | 95% CI | P Value | AIC | R2McF | X2 (df) | P Value | |
| Univariable | ||||||||
| ABI | 0.12 | 0.07 | 0.20 | <0.001 | 4407 | .015 | 64.30 | <0.001 |
| faPWV | 0.91 | 0.87 | 0.94 | <0.001 | 4447 | .005 | 23.81a | <0.001 |
| Model 1 | ||||||||
| CVD risk factors | 3971 | .117 | 522.00 | <0.001 | ||||
| Model 2 | ||||||||
| ABI | 0.12 | 0.07 | 0.21 | <0.001 | 3921 | .129 | 574.00b | <0.001 |
| faPWV | 0.92 | 0.88 | 0.96 | <0.001 | 3960 | .120 | 534.52a,b | <0.001 |
| Model 3 | ||||||||
| ABI | 0.13 | 0.07 | 0.24 | <0.001 | 3917 | .130 | 579.41c | <0.001 |
| faPWV | 0.95 | 0.91 | 0.99 | 0.021 | ||||
| Categorical | ||||||||
| Univariable | ||||||||
| ABI | 3.12 | 2.45 | 3.98 | <0.001 | 4393 | .018 | 78.20 | <0.001 |
| faPWV | 1.57 | 1.34 | 1.84 | 0.008 | 4441 | .006 | 30.02a | <0.001 |
| Model 1 | ||||||||
| CVD risk factors | 3971 | .117 | 522.00 | <0.001 | ||||
| Model 2 | ||||||||
| ABI | 2.41 | 1.85 | 3.17 | <0.001 | 3932 | .126 | 562.46b | <0.001 |
| faPWV | 1.46 | 1.23 | 1.74 | <0.001 | 3955 | .121 | 539.89a,b | <0.001 |
| Model 3 | ||||||||
| ABI | 2.25 | 1.71 | 2.96 | <0.001 | 3924 | .128 | 572.86c | <0.001 |
| faPWV | 1.35 | 1.12 | 1.61 | 0.001 | ||||
Categorical comparisons: categorical analyses, comparisons represent ≤0.9 vs. >0.9 for ABI, and Q1 (≤9.94 m/s) vs. Q2-Q4 (>9.94 m/s) for faPWV, whereby higher values were set as the reference level following continuous analysis.
Model descriptions: Model 1 - CVD risk factors only including: age, sex, systolic blood pressure, HDL cholesterol, total cholesterol, smoking status and diabetes. Model 2 - CVD risk factors plus ABI or faPWV separately. Model 3 - CVD risk factors plus ABI and faPWV concurrently. Adjustments: All multivariable models were adjusted for antihypertensive medication use, and race/field center.
Comparisons: vs univariable and multivariable 2 ABI models (non-nested);
vs. multivariable 1(P<0.001).
vs. multivariable 2~ABI (P<0.001).
Abbreviations: OR, odds ratio; CI, confidence interval; R2McF, McFadden’s Pseudo R2; X2, Chi square statistic, df, degrees of freedom.
For continuous and categorical analyses, the inverse relationships with CVD remained significant when ABI and faPWV were included in the model together (multivariable 3). Goodness of fit comparisons of nested models showed that when ABI and faPWV were entered together, the model explained significantly more variation in prevalent CVD than when ABI was entered independently with CVD risk factors. This indicates that faPWV does explain variation in prevalent CVD beyond ABI and CVD risk factors.
SENSITIVITY AND ANCILLARY ANALYSIS
Associations of ABI and faPWV with CVD risk factors and prevalent CVD were repeated following exclusion of participants with low ABI ≤ 0.9 (n=298) and high ABI≥1.4 (n=12), as the presence of PAD or arterial calcification, respectively, do have the potential to impact faPWV measures. This included logistical regression analysis where ABI was entered as a categorical variable comparing quartiles (Q1 vs. Q2, Q3 and Q4) rather than using the clinically used cut-off of ≤0.9. This data analysis demonstrated no differences when compared to the primary analysis. Analysis of mean faPWV, and left and right faPWV measures independently had no impact on findings when compared to those determined in primary analysis. Finally, given that high rather than low PWV is typically associated with CVD, the association between high faPWV (Q1-Q3: ≤ 12.3 m/s vs. Q4: > 12.3 m/s) and the composite measure of CVD was explored, but no significant associations were observed.
DISCUSSION
The aims of the current study were: (i) to compare the independent associations of ABI and faPWV with traditional CVD risk factors, and ii) determine the independent and additive associations of ABI and faPWV with a composite measure of prevalent CVD. Our findings indicate that in older adults, ABI is associated with age, sex, HDL, TC, diabetes and smoking, whilst faPWV was associated with age, HDL, smoking and uniquely SBP, but any mutual associations were more strongly associated with ABI. However, both ABI and faPWV were independently associated with CVD beyond traditional risk factors. Further, the association between faPWV and CVD persisted beyond ABI and CVD risk factors, indicating that faPWV may confer unique and additive clinical CVD risk information.
LIMITATIONS AND STRENGTHS
The strengths and limitations of this study need to be addressed to contextualize the findings and better facilitate comparisons to the existing literature. Firstly, the cross-sectional design precludes the assessment of causality in the observed associations. Additionally, as with any observational study, we cannot rule out the possibility of residual confounding - though we did include several important confounders in our models. Secondly, the generalizability of our findings is limited to older populations and cannot be extended to younger, healthier cohorts. Further, the predominate inclusion of participants who had survived from baseline (1987–1989) and attended the Visit 5 examination (2011–2013), and were thus likely healthier compared to those who did not participate in the visit, may have generated a bias within the study population. Finally, the use of height-based formulas to calculate faPWV were validated in a Japanese population and may not be applicable to other racial or ethnic groups. A major strength is that this is the first study to directly compare the associations ABI and faPWV, two bio-markers of lower-extremity arterial health, with traditional CVD risk factors and prevalent CVD, and does so using a large community-dwelling population.
COMPARISON TO THE LITERATURE: CVD RISK FACTORS
Consistent with previous findings, both ABI [33–35] and faPWV [23,26,36] were associated with age. Aged vessels demonstrate elevated expression of pro-inflammatory molecules, encouraging the accumulation of atherogenic lipoproteins, and thus, plaque development [37]. It is also expected that distensible elastin fibres in the arterial wall become fragmented and discontinuous with age, this, coupled with an attenuation of the elastin-collagen ratio, promotes arterial stiffening [20,39]. However, the stronger association between age and ABI compared to faPWV may indicate a greater propensity for atherosclerotic, rather than arteriosclerotic, processes with age in the lower-limb vasculature. Lower susceptibility to arteriosclerotic pathology in the lower limbs may, in part, be due to peripheral muscular arteries inherently exhibiting higher stiffness, compromising chiefly of vascular smooth muscle cells and low elastin [38].
Vascular aging may also be differentially accelerated by lifestyle behaviours or the presence of disease. For example, there is strong evidence linking smoking with atherosclerosis, particularly of the lower-limb [39–41]. But the weaker association of smoking with faPWV compared to ABI in the present study is indicative of the weaker association between smoking and arterial stiffness more broadly [42]. Interestingly, consistent with previous observations in the ARIC study [43], we report a negative association between faPWV and smoking. Smoking-induced dysregulation of the metalloproteinase system, leading to the breakdown of collagen, and thus arterial wall hemodynamic properties, has been presented as a cause of this phenomenon [44]. Both faPWV and ABI were equally associated with HDL, with the analogous positive association highlighting the key role of this antiatherogenic lipoprotein. But faPWV was not associated with TC, which is perhaps not surprising given that broadly cross-sectional studies have typically found weak [20] or no relationship [26,36,45] between single lipid parameters and arterial stiffness. In contrast, the link between dyslipidemia and atherosclerosis is well established, with elevated LDLs stimulating endothelial cells to secrete atherogenic cytokines, encouraging foam cell formation in the intima, a first step in the formation of an atherosclerotic lesion [46]. Dyslipidemia is also exasperated by hyperglycemia, further promoting atherogenesis in diabetic individuals [46], particularly of the lower-limb [47], supporting the positive association between ABI and diabetes in this study. Although prevalent diabetes can also accelerate arterial remodeling [48], we did not observe an association between faPWV and diabetes. This may be because diabetes preferentially leads to stiffening of central over peripheral arteries [26].
Unlike ABI, faPWV was associated with SBP. The strong positive association between faPWV and SBP is consisted with previous literature [27,32,49,52] and reflects the inter-dependency of blood pressure and arterial stiffness. Our failure to observe an association between ABI and brachial SBP has also been reported by others [49] and likely indicates that the variation in ABI is principally driven by ankle SBP. However, like faPWV [36], ABI has reliably been associated with hypertension [33,50], including in ancillary analysis of the present study, reflecting the systemic impact of both arteriosclerosis and atherosclerosis pathology in the lower-limb. Collectively, our findings suggest that ABI and faPWV may be complimentary indexes of vascular aging in older adults, with some unique risk factors associations, however, ABI may be more sensitive to both fixed (age, sex) and modifiable (smoking, diabetes) CVD risk factors that accelerate vascular aging.
COMPARISON TO THE LITERATURE: PREVALENT CVD
Both ABI and faPWV were negatively associated with prevalent CVD, a composite measure that included HF, CHD, and stroke, beyond traditional CVD risk factors. Low ABI (≤0.9) and low faPWV (≤9.94 m/s) increased the odds of CVD by 141% and 46%, respectively. Further, low faPWV remained associated with CVD in fully adjusted models, indicating that faPWV may provide independent incremental prognostic value beyond both traditional CVD risk factors and ABI. Low ABI has reliably been associated with CVD [13–15], but the link between lower-limb arterial stiffness and CVD is less clear. Arteriosclerosis, the stiffening and thickening of the arterial wall, is typically indicated by high PWV and has a positive association with disease. For example, Kawai [24] reported that high faPWV was associated with higher stroke incidence, whilst faPWV is also found to be higher in type 2 diabetes patients than healthy controls [26], both intuitive, positive, associations. But, aligned with our, perhaps unexpected, findings, low faPWV has been associated with myocardial stress [22] and coronary artery disease [25] in older adults, as well as ischemic heart disease in diabetes patients [23]. The association between faPWV and CVD may therefore be differentially linked to CVD type and severity.
Although a few studies have reported inverse relationships between faPWV and CVD [22,23,25], the underlying pathophysiological mechanism(s) are unclear. Residual confounding may have contributed to the inverse relationship, given that some CVD risk factors (e.g. age vs. smoking) were differentially associated with faPWV. However, our multivariable models were adjusted for known confounders and widely recognized CVD risk factors. Alternatively, reduced lower-limb arterial stiffness may chiefly be due to the presence of significant arterial atherosclerosis leading to arterial stenosis [21], or PAD, which is prevalent in older adults [11]. Significant stenosis reduces BP downstream, on which PWV is dependent, suppressing pulse wave propagation [51]. To account for this possibility, we excluded participants with ABI ≤ 0.9, however, an inverse association with CVD persisted. Finally, it has been suggested that loss of elasticity and recoil may not necessarily translate to arterial stiffening, and may occur in the absence of stenosis [52]. But whether through atherosclerotic-dependent or -independent means, low faPWV may contribute to a reduction in the central to peripheral arterial stiffness gradient, augmenting the transmission of excessive forward pressure into the microcirculation, a pathophysiological basis for cardiovascular events and target organ damage [53,54].
IMPLICATIONS AND CONCLUSIONS
The assessment of lower-limb arterial health permits clinicians to accurately assess CVD risk, and identify individuals at higher risk of cardiovascular events [4]. However, CVD screening has typically focused on the determination of atherosclerosis in the lower-limbs using ABI [11,27], and likely ignores the contribution that lower-limb arteriosclerosis may contribute to CVD risk. The current study extends the literature by reporting that whilst lower-limb atherosclerosis may be a more sensitive marker of CVD, faPWV, an assessment of lower-limb arteriosclerosis, is independently associated CVD beyond ABI, and therefore may confer unique and additive CVD risk information. But of note, it is low faPWV, perhaps representing a loss of arterial elasticity and breakdown in wall structure with disease, rather than arteriosclerosis per se, that is more strongly associated with CVD. A regression of peripheral arterial stiffness may contribute to a reduction in the physiologically advantageous central to peripheral arterial stiffness gradient, a pathophysiological basis for cardiovascular events and target organ damage [56,57]. Importantly, faPWV can be measured accurately and reliably using simple oscillometry, and undertaken concurrently with ABI, meaning it is viable for time-sensitive clinical and epidemiological research settings. However, future work is warranted to; i) confirm if combined faPWV and ABI measures improve prediction of CVD outcome beyond ABI alone, and ii) identify the mechanisms leading to low faPWV and its role in heightening CVD risk.
ACKNOWLEDGEMENTS
The authors thank the staff and participants of the ARIC study for their important contributions. The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). The study was also supported by R01AG053938.
FINANCIAL SUPPORT
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). The study was also supported by R01AG053938.
Footnotes
CONFLICTS OF INTEREST
NONE
REFERENCES
- 1.British Cardiac Society, British Hypertension Society, Diabetes UK, Heart UK, Primary Care Cardiovascular Society, Stroke Association. JBS 2: Joint British Societies’ guidelines on prevention of cardiovascular disease in clinical practice. Heart 2005; 91 Suppl 5:v1–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016; 37 (29):2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Agostino RB Sr., Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008; 117 (6):743–753. [DOI] [PubMed] [Google Scholar]
- 4.Lindholt JS, Sogaard R. Population screening and intervention for vascular disease in Danish men (VIVA): a randomised controlled trial. Lancet 2017; 390 (10109):2256–2265. [DOI] [PubMed] [Google Scholar]
- 5.Perlstein TS, Creager MA. The ankle-brachial index as a biomarker of cardiovascular risk: it’s not just about the legs. Circulation 2009; 120 (21):2033–2035. [DOI] [PubMed] [Google Scholar]
- 6.Alberts MJ, Bhatt DL, Mas JL, Ohman EM, Hirsch AT, Rother J, et al. Three-year follow-up and event rates in the international REduction of Atherothrombosis for Continued Health Registry. Eur Heart J 2009; 30 (19):2318–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nichols WW, Nichols WW, McDonald DA. McDonald’s blood flow in arteries : theoretic, experimental, and clinical principles. London: Hodder Arnold; 2011. [Google Scholar]
- 8.Palombo C, Kozakova M. Arterial stiffness, atherosclerosis and cardiovascular risk: Pathophysiologic mechanisms and emerging clinical indications. Vascul Pharmacol 2016; 77:1–7. [DOI] [PubMed] [Google Scholar]
- 9.Lusis AJ. Atherosclerosis. Nature 2000; 407 (6801):233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim HL, Kim SH. Pulse Wave Velocity in Atherosclerosis. Front Cardiovasc Med 2019; 6:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation 2012; 126 (24):2890–2909. [DOI] [PubMed] [Google Scholar]
- 12.U. S. Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, et al. Screening for Peripheral Artery Disease and Cardiovascular Disease Risk Assessment With the Ankle-Brachial Index: US Preventive Services Task Force Recommendation Statement. JAMA 2018; 320 (2):177–183. [DOI] [PubMed] [Google Scholar]
- 13.Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA 2008; 300 (2):197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newman AB, Shemanski L, Manolio TA, Cushman M, Mittelmark M, Polak JF, et al. Ankle-arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. The Cardiovascular Health Study Group. Arterioscler Thromb Vasc Biol 1999; 19 (3):538–545. [DOI] [PubMed] [Google Scholar]
- 15.Criqui MH, McClelland RL, McDermott MM, Allison MA, Blumenthal RS, Aboyans V, et al. The ankle-brachial index and incident cardiovascular events in the MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2010; 56 (18):1506–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.U. S. Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, et al. Risk Assessment for Cardiovascular Disease With Nontraditional Risk Factors: US Preventive Services Task Force Recommendation Statement. JAMA 2018; 320 (3):272–280. [DOI] [PubMed] [Google Scholar]
- 17.Chirinos JA, Segers P, Hughes T, Townsend R. Large-Artery Stiffness in Health and Disease: JACC State-of-the-Art Review. J Am Coll Cardiol 2019; 74 (9):1237–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stone K, Fryer S, Kelsch E, Burnet K, Zieff G, Faulkner J, et al. Validity and reliability of lower-limb pulse-wave velocity assessments using an oscillometric technique. Exp Physiol 2019; 104 (5):765–774. [DOI] [PubMed] [Google Scholar]
- 19.Stone K, Fryer S, Faulkner J, Meyer ML, Heffernan K, Zieff G, et al. The aortic-femoral arterial stiffness gradient demonstrates good between-day reliability. Hypertens Res 2021. [DOI] [PubMed]
- 20.Choo J, Shin C, Barinas-Mitchell E, Masaki K, Willcox BJ, Seto TB, et al. Regional pulse wave velocities and their cardiovascular risk factors among healthy middle-aged men: a cross-sectional population-based study. BMC Cardiovasc Disord 2014; 14:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wohlfahrt P, Krajcoviechova A, Seidlerova J, Galovcova M, Bruthans J, Filipovsky J, et al. Lower-extremity arterial stiffness vs. aortic stiffness in the general population. Hypertens Res 2013; 36 (8):718–724. [DOI] [PubMed] [Google Scholar]
- 22.Liu S, Kim ED, Wu A, Meyer ML, Cheng S, Hoogeveen RC, et al. Central and peripheral pulse wave velocity and subclinical myocardial stress and damage in older adults. PLoS One 2019; 14 (2):e0212892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hatsuda S, Shoji T, Shinohara K, Kimoto E, Mori K, Fukumoto S, et al. Regional arterial stiffness associated with ischemic heart disease in type 2 diabetes mellitus. J Atheroscler Thromb 2006; 13 (2):114–121. [DOI] [PubMed] [Google Scholar]
- 24.Kawai T, Ohishi M, Onishi M, Ito N, Takeya Y, Oguro R, et al. Prognostic impact of regional arterial stiffness in hypertensive patients. Heart Vessels 2015; 30 (3):338–346. [DOI] [PubMed] [Google Scholar]
- 25.Tsuchikura S, Shoji T, Kimoto E, Shinohara K, Hatsuda S, Koyama H, et al. Central versus peripheral arterial stiffness in association with coronary, cerebral and peripheral arterial disease. Atherosclerosis 2010; 211 (2):480–485. [DOI] [PubMed] [Google Scholar]
- 26.Kimoto E, Shoji T, Shinohara K, Inaba M, Okuno Y, Miki T, et al. Preferential stiffening of central over peripheral arteries in type 2 diabetes. Diabetes 2003; 52 (2):448–452. [DOI] [PubMed] [Google Scholar]
- 27.Vlachopoulos C, Xaplanteris P, Aboyans V, Brodmann M, Cifkova R, Cosentino F, et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis 2015; 241 (2):507–532. [DOI] [PubMed] [Google Scholar]
- 28.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007; 335 (7624):806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The Atherosclerosis Risk in Communities (ARIC) study: Design and Objectives. The ARIC Investigators. American Journal of Epidemiology 1989; 129 (4):687–702. [PubMed] [Google Scholar]
- 30.Cortez-Cooper MY, Supak JA, Tanaka H. A new device for automatic measurements of arterial stiffness and ankle-brachial index. Am J Cardiol 2003; 91 (12):1519–1522, A1519. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka H, Munakata M, Kawano Y, Ohishi M, Shoji T, Sugawara J, et al. Comparison between carotid-femoral and brachial-ankle pulse wave velocity as measures of arterial stiffness. J Hypertens 2009; 27 (10):2022–2027. [DOI] [PubMed] [Google Scholar]
- 32.Warnick GR, Mayfield C, Benderson J, Chen JS, Albers JJ. HDL cholesterol quantitation by phosphotungstate-Mg2+ and by dextran sulfate-Mn2+-polyethylene glycol precipitation, both with enzymic cholesterol assay compared with the lipid research method. Am J Clin Pathol 1982; 78 (5):718–723. [DOI] [PubMed] [Google Scholar]
- 33.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation 2004; 110 (6):738–743. [DOI] [PubMed] [Google Scholar]
- 34.Kennedy M, Solomon C, Manolio TA, Criqui MH, Newman AB, Polak JF, et al. Risk factors for declining ankle-brachial index in men and women 65 years or older: the Cardiovascular Health Study. Arch Intern Med 2005; 165 (16):1896–1902. [DOI] [PubMed] [Google Scholar]
- 35.Newman AB, Siscovick DS, TA M, Polak J, Fried LP, Borhani N, et al. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group. Circulation 1993; 88 (3):37–45. [DOI] [PubMed] [Google Scholar]
- 36.Tsuchikura S, Shoji T, Kimoto E, Shinohara K, Hatsuda S, Koyama H, et al. Brachial-ankle pulse wave velocity as an index of central arterial stiffness. J Atheroscler Thromb 2010; 17 (6):658–665. [DOI] [PubMed] [Google Scholar]
- 37.Wang JC, Bennett M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res 2012; 111 (2):245–259. [DOI] [PubMed] [Google Scholar]
- 38.Greenwald SE. Ageing of the conduit arteries. J Pathol 2007; 211 (2):157–172. [DOI] [PubMed] [Google Scholar]
- 39.Lu JT, Creager MA. The relationship of cigarette smoking to peripheral arterial disease. Rev Cardiovasc Med 2004; 5 (4):189–193. [PubMed] [Google Scholar]
- 40.Adams MR, Jessup W, Celermajer DS. Cigarette smoking is associated with increased human monocyte adhesion to endothelial cells: reversibility with oral L-arginine but not vitamin C. J Am Coll Cardiol 1997; 29 (3):491–497. [DOI] [PubMed] [Google Scholar]
- 41.Lu L, Mackay DF, Pell JP. Meta-analysis of the association between cigarette smoking and peripheral arterial disease. Heart 2014; 100 (5):414–423. [DOI] [PubMed] [Google Scholar]
- 42.Doonan RJ, Hausvater A, Scallan C, Mikhailidis DP, Pilote L, Daskalopoulou SS. The effect of smoking on arterial stiffness. Hypertens Res 2010; 33 (5):398–410. [DOI] [PubMed] [Google Scholar]
- 43.Camplain R, Meyer ML, Tanaka H, Palta P, Agarwal SK, Aguilar D, et al. Smoking Behaviors and Arterial Stiffness Measured by Pulse Wave Velocity in Older Adults: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Hypertens 2016; 29 (11):1268–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Furmanski P. Revealing the mechanism of tissue damage due to tobacco use: finally, a smoking gun? Am J Pathol 2013; 182 (5):1489–1493. [DOI] [PubMed] [Google Scholar]
- 45.Meyer ML, Tanaka H, Palta P, Cheng S, Gouskova N, Aguilar D, et al. Correlates of Segmental Pulse Wave Velocity in Older Adults: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Hypertens 2016; 29 (1):114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choy PC, Siow YL, Mymin D, O K. Lipids and atherosclerosis. Biochem Cell Biol 2004; 82 (1):212–224. [DOI] [PubMed] [Google Scholar]
- 47.Marso SP, Hiatt WR. Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol 2006; 47 (5):921–929. [DOI] [PubMed] [Google Scholar]
- 48.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation 2006; 114 (6):597–605. [DOI] [PubMed] [Google Scholar]
- 49.Hendriks EJ, Westerink J, de Jong PA, de Borst GJ, Nathoe HM, Mali WP, et al. Association of High Ankle Brachial Index With Incident Cardiovascular Disease and Mortality in a High-Risk Population. Arterioscler Thromb Vasc Biol 2016; 36 (2):412–417. [DOI] [PubMed] [Google Scholar]
- 50.Zheng ZJ, Rosamond WD, Chambless LE, Nieto FJ, Barnes RW, Hutchinson RG, et al. Lower extremity arterial disease assessed by ankle-brachial index in a middle-aged population of African Americans and whites: the Atherosclerosis Risk in Communities (ARIC) Study. American journal of preventive medicine 2005; 29 (5 Suppl 1):42–49. [DOI] [PubMed] [Google Scholar]
- 51.Yokoyama H, Shoji T, Kimoto E, Shinohara K, Tanaka S, Koyama H, et al. Pulse wave velocity in lower-limb arteries among diabetic patients with peripheral arterial disease. J Atheroscler Thromb 2003; 10 (4):253–258. [DOI] [PubMed] [Google Scholar]
- 52.Loehr LR, Meyer ML, Poon AK, Selvin E, Palta P, Tanaka H, et al. Prediabetes and Diabetes Are Associated With Arterial Stiffness in Older Adults: The ARIC Study. Am J Hypertens 2016; 29 (9):1038–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stone K, Fryer S, Meyer M, Kucharska-newton A, Faulkner J, Zieff G, et al. The Aortic-Femoral Arterial Stiffness Gradient: An Atherosclerosis Risk in Communities (ARIC) Study. Journal of Hypertension in press. [DOI] [PMC free article] [PubMed]
- 54.Fortier C, Mac-Way F, Desmeules S, Marquis K, De Serres SA, Lebel M, et al. Aortic-brachial stiffness mismatch and mortality in dialysis population. Hypertension 2015; 65 (2):378–384. [DOI] [PubMed] [Google Scholar]