Abstract
An inverse correlation between colonization of the human nasopharynx by Streptococcus pneumoniae and Haemophilus influenzae, both common upper respiratory pathogens, has been reported. Studies were undertaken to determine if either of these organisms produces substances which inhibit growth of the other. Culture supernatants from S. pneumoniae inhibited growth of H. influenzae, whereas culture supernatants from H. influenzae had no effect on the growth of S. pneumoniae. Moreover, coculture of S. pneumoniae and H. influenzae led to a rapid decrease in viable counts of H. influenzae. The addition of purified catalase prevented killing of H. influenzae in coculture experiments, suggesting that hydrogen peroxide may be responsible for this bactericidal activity. H. influenzae was killed by concentrations of hydrogen peroxide similar to that produced by S. pneumoniae. Hydrogen peroxide is produced by the pneumococcus through the action of pyruvate oxidase (SpxB) under conditions of aerobic growth. Both an spxB mutant and a naturally occurring variant of S. pneumoniae, which is downregulated in SpxB expression, were unable to kill H. influenzae. A catalase-reversible inhibitory effect of S. pneumoniae on the growth of the respiratory tract pathogens Moraxella catarrhalis and Neisseria meningitidis was also observed. Elevated hydrogen peroxide production, therefore, may be a means by which S. pneumoniae is able to inhibit a variety of competing organisms in the aerobic environment of the upper respiratory tract.
Bacterial pathogens are generally studied individually, although in their natural environment they often coexist or compete with multiple other microbial species. The focus of this report is bacterial pathogens that commonly colonize and infect the respiratory tract of humans. The results of clinical studies that surveyed the etiologic agents in cases of otitis media in children and chronic bronchitis in adults showed that Streptococcus pneumoniae and Haemophilus influenzae are the most prevalent bacterial pathogens (14, 23). The frequency with which these two species are isolated from the same specimen, however, is significantly less than would be predicted based on their relative prevalence (25, 30). This suggests that there may be inhibitory effects of one species on the other in vivo. This would not be an unexpected finding considering our current understanding of the pathogenesis of colonization and infection by these species. For instance, since both S. pneumoniae and H. influenzae express cell surface phosphorylcholine, which mediates adherence to the receptor for platelet-activating factor, there may be competition for the same host cell receptor (12, 35, 46). In addition, phosphorylcholine is immunogenic, and antibody generated against phosphorylcholine from one species may promote clearance of a heterologous species bearing the same epitope (9, 31, 45). However, the presence of phosphorylcholine is required for viability in the case of the pneumococcus, while H. influenzae is able to switch off expression of this antigen (44, 51). Another example is the neuraminidase secreted by the pneumococcus, which has the potential to remove sialic acid residues from bacterial competitors known to express this as a cell surface structure (6, 10). The lipopolysaccharide of the respiratory tract pathogen, Neisseria meningitidis, and at least some strains of H. influenzae are sialylated and, in the case of the former, this modification acts to increase resistance to clearance mediated by complement (17, 21, 28, 29).
In order to begin to examine the interactions of the coinhabitants of the heavily colonized mucosal surface of the human upper respiratory tract, we tested the effect of coculture in vitro on growth and viability. These studies revealed that the pneumococcus produces an inhibitory substance that was shown to be hydrogen peroxide. This suggests that the production of H2O2 by S. pneumoniae, previously shown to be cytotoxic for cultured alveolar epithelial cells, may also be an effective mechanism for limiting or eliminating competitive flora, including common pathogens such as H. influenzae and N. meningitidis, which share the same microenvironment (15). These species, furthermore, are sensitive to levels of peroxide generated by the pneumococcus despite their production of catalase, an enzyme that acts to eliminate hydrogen peroxide (7, 8, 37).
MATERIALS AND METHODS
Bacterial strains, media, and chemicals.
Strains used in this study are described in Table 1. All strains were cultured in brain heart infusion broth (BHI) with or without 1.5% agar (Difco Laboratories, Detroit, Mich.). H. influenzae was grown in BHI medium supplemented with hemin and l-histidine (dissolved in 1% triethanolamine, each at a final concentration of 2.5 μg/ml) (sBHI) plus NAD (2.0 μg/ml) (Sigma Chemical Co., St. Louis, Mo.). All organisms were grown at 37°C with aeration except streptococci, which were grown without shaking. Plates containing streptococci and neisseriae were incubated in the presence of supplemental carbon dioxide using candle extinction jars. Pneumococci were plated on BHI containing 200 U of bovine liver catalase per ml (Worthington Biochemical, Freehold, N.J.).
TABLE 1.
Hydrogen peroxide sensitivity and production by various bacterial pathogens
| Species | MIC (mM)a | MBC (mM)b | H2O2 generated (mM)c | Source or referenced |
|---|---|---|---|---|
| Gram-negative | ||||
| H. influenzae Rd | 0.4 | 0.5 | <0.1 | 26 |
| H. influenzae Eagan | 0.4 | 0.5 | <0.1 | 26 |
| N. meningitidis MC58C3 | 0.4 | 5 | <0.1 | 32 |
| M. catarrhalis Bc1 | 1.1 | 160 | <0.1 | Clinical isolate |
| E. coli RS218 | ND | 15 | <0.1 | 1 |
| S. enterica serovar Typhimurium LT2 | ND | 20 | <0.1 | Collection of K. Sanderson |
| K. pneumoniae Kp1 | ND | 20 | <0.1 | Clinical isolate |
| P. aeruginosa PA01 | ND | 60 | <0.1 | ATCC 15692 |
| Gram-positive | ||||
| S. pyogenes P87 | ND | 40 | <0.1 | Clinical isolate |
| S. agalactiae P60 | ND | 80 | <0.1 | Clinical isolate |
| S. equisimilis P107 | ND | 20 | <0.1 | Clinical isolate |
| E. faecium P119 | ND | 80 | <0.1 | Clinical isolate |
| S. aureus A1 | ND | 10 | <0.1 | Clinical isolate |
| S. pneumoniae strains | ||||
| P394 (type 4) | 1.6 | 80 | TIGR genome strain | |
| D39 (type 2) | 1.2 | 80 | 0.44 ± 0.08 | 4 |
| P383 (type 6B) | ND | ND | 0.53 ± 0.08 | 22 |
| P384 (type 6A) | ND | ND | 0.71 ± 0.13 | 22 |
| P878 D39 (spxB::TnphoA) | 1.6 | 80 | <0.1 | 38 |
| P62 (type 9V opaque variant) | ND | ND | <0.1 | 22 |
| P64 (type 9V transparent variant) | ND | ND | 0.43 ± 0.13 | 22 |
The MIC was determined as the minimum concentration of H2O2 necessary to prevent turbid growth of a 1-in-50 inoculum of a stationary-phase culture following overnight incubation at 37°C. ND, not determined.
The MBC was determined as the minimum concentration of H2O2 necessary for >99.9% killing of washed, log-phase cells in BHI medium after 30 min at 37°C.
H2O2 concentration present in culture supernatants after incubating approximately 5 × 107 washed, log-phase cells for 1 h in BHI medium at 37°C.
TIGR, The Institute for Genome Research.
Supernatant inhibition assays.
Cultures of S. pneumoniae P394 were grown in liquid BHI medium at 37°C under atmospheric conditions. After reaching mid-log phase (optical density at 620 nm [OD620] = 0.3 to 0.4), the cultures were harvested and spun at 10,000 × g for 2 min, and the supernatant was filtered through 0.2-μm (pore-size) filters. The target organism was grown in liquid BHI or sBHI medium to mid-log phase (OD620 = 0.3 to 0.4) and then diluted 10-fold in phosphate-buffered saline (PBS). Bacterial lawns were obtained by spreading 50 μl of diluted culture on BHI or sBHI agar with or without 200 U of catalase per ml. Then, 10-μl aliquots of supernatant were spotted onto these plates and allowed to dry prior to incubation at 37°C for 16 h. In some experiments, aliquots of supernatant were treated with proteinase K (final concentration, 50 μg/ml; Sigma) at 37°C for 1 h or heated to 65°C for 20 min prior to adding them to plates containing target organisms.
Coculture experiments.
Bacteria were grown in BHI medium at 37°C until mid-log phase (OD620 = 0.3 to 0.4), centrifuged for 2 min at 10,000 × g and 4°C, washed in ice-cold Hanks balanced saline solution (HBSS; Gibco BRL, Gaithersburg, Md.), and then resuspended in BHI at the original culture volume. Equal volumes of S. pneumoniae and the target strain were then mixed and incubated at 37°C in 96-well polystyrene microtiter plates (Dynex Technologies, Inc., Chantilly, Va.). As a negative control, each strain was mixed with an equal amount of BHI alone. Where indicated, individual wells were supplemented with catalase (final concentration, 1,000 U/ml). Serial dilutions were then prepared in HBSS, and an aliquot was plated on BHI agar plates containing catalase (final concentration, 200 U/ml) for viable counts. Dilutions of mixed cultures were spread on BHI plates supplemented with 2.0% Fildes enrichment (Difco) and grown under atmospheric conditions which selectively inhibited the growth of S. pneumoniae and allowed enumeration of the target species. Removal of the Fildes enrichment, which provides a source of hemin and NAD, provided selective conditions preventing the growth of H. influenzae.
Hydrogen peroxide sensitivity assays.
Bacteria were grown in BHI medium at 37°C until mid-log phase (OD620 = 0.3 to 0.4), centrifuged for 2 min at 10,000 × g and 4°C, washed in ice-cold HBSS, and resuspended in fresh BHI medium. Resuspended bacteria were added to microtiter plate wells in duplicate containing twofold dilutions of H2O2 (Sigma) in BHI medium and incubated at 37°C for 30 min. Aliquots from each well were applied to BHI agar plates containing 200 U of catalase per ml for viable counts. The concentration of H2O2 required to cause a 99.9% decrease in the number of colonies compared to the negative control without peroxide was recorded as the minimum bactericidal concentration (MBC). For MIC determination, 50-fold dilutions of stationary-phase cultures in BHI containing twofold dilutions of H2O2 were incubated at 37°C overnight. The minimum concentration necessary to prevent turbid growth was considered the MIC.
Hydrogen peroxide production assays.
Hydrogen peroxide production was measured in an assay developed by Pick and Keisari and modified by Duane and coworkers (15, 36). Bacteria were grown in BHI medium at 37°C until mid-log phase (OD620 = 0.3 to 0.4), centrifuged for 2 min at 10,000 × g and 4°C, washed in ice-cold HBSS, and resuspended in BHI medium to twice the original culture volume. Wells for negative controls contained 1,000 U of catalase per ml. After 1 h of incubation under atmospheric conditions at 37°C, the cultures were harvested, spun at 10,000 × g for 2 min, and filtered through a 0.2-μm (pore-size) membrane. Immediately prior to the assay, phenol red and horseradish peroxidase were added to peroxide assay buffer (5.0 mM K2HPO4, 1.0 mM KH2PO4, 140 mM NaCl, 0.5 mM glucose; pH 7.4) at final concentrations of 0.46 mM and 0.046 U/ml, respectively. Aliquots of filtered supernatant were added to the assay mixture at a ratio of 1 to 4 and incubated for 30 min at 37°C in duplicate. After the reactions were stopped by the addition of NaOH (final concentration, 0.004 N) the absorbance was recorded at a wavelength of 610 nm. Concentrations were calculated in comparison to a standard curve with known amounts of H2O2 added to control supernatant from wells containing catalase which had been heated to 100°C for 20 min to eliminate catalase activity.
Two-dimensional protein gel electrophoresis.
Two-dimensional protein gel electrophoresis followed by staining, computerized comparison, and mass spectrometric analysis of the proteins, was done as described elsewhere (K. Overweg, C. D. Pericone, L. G. C. Verhouf, J. N. Weiser, H. D. Meiring, A. D. P. J. M. De Jong, R. De Groot, and P. W. M. Hermans, submitted for publication).
Western transfer and immunoblotting.
P878 containing an in-frame fusion of TnphoA to the gene for pyruvate oxidase (spxB) was grown on tryptic soy agar plates containing catalase (200 U/ml) (38). Bacteria were grown for 16 h at 37°C under atmospheric conditions (20% O2, 0.03% CO2), in a candle extinction jar (17% O2, 3% CO2), or in the GasPak anaerobic system (<0.01% O2, 10% CO2) (Becton Dickinson, Cockeysville, Md.). Cells were harvested from plates, adjusted to equal density based on absorbance at 620 nm, washed in cold PBS, and treated at 100°C for 5 min in gel loading buffer (50 mM Tris-Cl, pH 6.8; 100 mM β-mercaptoethanol; 10% glycerol; 2% sodium dodecyl sulfate [SDS], 1% bromophenol blue) prior to separation in SDS–10% polyacrylamide gel electrophoresis (PAGE) gels. Equal loading was confirmed by measurement of total protein in whole-cell sonicates using the Micro BCA Protein Assay (Pierce Chemical Co., Rockford, Ill.). After transfer to Immobilon P membranes (Millipore Co., Bedford, Mass.), immunoblotting was carried out with an antibody raised against PhoA and detected with an antiserum to rabbit immunoglobulin G conjugated to alkaline phosphatase as described previously (43).
RESULTS
Bactericidal effect of S. pneumoniae on H. influenzae.
The hypothesis that pathogens inhabiting the same host environment might generate growth-inhibitory substances was examined. Initial experiments tested the effect of culture supernatant of S. pneumoniae P394 and H. influenzae Rd on the growth of the other species. Aliquots of culture supernatant filtrates from one organism were added to a lawn of the other organism which had been spread on solidified medium which supports the growth of only that species. A zone of completely inhibited growth was observed when supernatants from S. pneumoniae were added to lawns of H. influenzae, while the inverse showed no observable effect on growth (data not shown). This demonstrated that S. pneumoniae produced a substance that inhibited the growth of H. influenzae. Similar results were obtained using three nontypeable clinical isolates of H. influenzae, as well as the type b isolate, Eagan. Likewise, unrelated S. pneumoniae clinical isolates of types 2, 6A, and 6B were all capable of inhibiting the above-mentioned strains of H. influenzae, demonstrating that the observed effect was not strain specific.
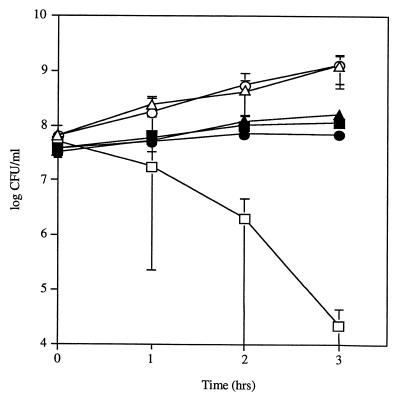
To test whether this growth-inhibitory effect was also bactericidal, both species were grown to mid-log phase and cocultured in liquid medium. When 108 CFU of H. influenzae Rd per ml were cocultured with 5 × 107 CFU of S. pneumoniae P394 per ml, the viable count of H. influenzae decreased to below detectable levels (104 CFU/ml) within 3 h, whereas the viable count of H. influenzae cultured in the absence of S. pneumoniae under the same conditions increased to 109 CFU/ml (Fig. 1). In contrast, the viable count of S. pneumoniae increased to 108 CFU/ml, whether cultured with H. influenzae or in the absence of H. influenzae. These observations showed that the substance produced by S. pneumoniae was not only inhibitory but also bactericidal against H. influenzae.
FIG. 1.
Effect of coculture of S. pneumoniae P394 and H. influenzae Rd. Following growth to mid-log phase, H. influenzae was washed and incubated in sBHI containing heat-inactivated catalase either with (□) or without (○) S. pneumoniae for the time indicated, and viable counts were determined in duplicate on selective media. Viable counts of S. pneumoniae incubated in coculture with (■) or without (●) H. influenzae were determined in duplicate by plating on selective media. The same amount of active catalase (1,000 U/ml) was included during coculture of S. pneumoniae (▴) and H. influenzae (▵). Values represent the average of three independent determinations in duplicate, and the error bars represent the standard deviations.
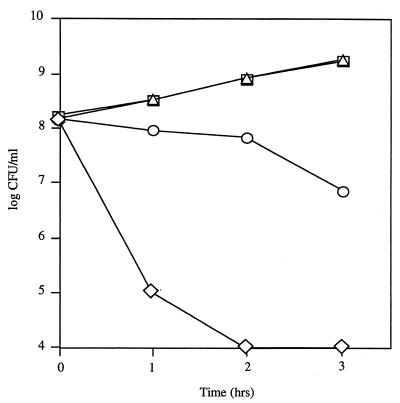
In similar dose-response experiments, 107 CFU of S. pneumoniae per ml reduced the number of H. influenzae from 108 to <104 CFU/ml within 3 h (Fig. 2). S. pneumoniae at 106 CFU/ml reduced the equivalent number of H. influenzae approximately 10-fold within 3 h. The growth of the equivalent number of H. influenzae with 105 CFU of S. pneumoniae per ml was comparable to that of H. influenzae grown in the absence of S. pneumoniae.
FIG. 2.
Dose-dependent killing of H. influenzae Rd by S. pneumoniae P394. Following growth to mid-log phase, H. influenzae was washed and cultured alone (triangles) or with 105 (squares), 106 (circles), or 107 (diamonds) CFU of S. pneumoniae per ml and incubated in sBHI for the times indicated; viable counts were determined on selective media. Values represent the average of two independent determinations in duplicate.
The bactericidal effect of S. pneumoniae is due to hydrogen peroxide production.
Supernatants from cultures of S. pneumoniae treated with proteinase K or heated to 65°C for 20 min retained inhibitory activity, suggesting that the inhibitory substance was not likely to be a protein (data not shown). In addition, the inhibitory effect was diminished when S. pneumoniae was grown under less-than-atmospheric levels of environmental oxygen (data not shown). It had previously been shown that S. pneumoniae makes substantial amounts of H2O2 when grown aerobically (2, 34). It was therefore suspected that the inhibitory effect of S. pneumoniae supernatant might be due to H2O2 production. Further support for this possibility came from the observation that the inhibitory effect was inversely proportional to the level of hemin in the growth medium (data not shown). Hemin had previously been shown to mitigate the effects of oxidative stress on H. influenzae, presumably because of its ability to decompose hydrogen peroxide (24, 27). Catalase, a heme-containing enzyme which specifically degrades H2O2, was then added to BHI plates at a concentration of 200 U/ml. This eliminated the inhibitory effect of S. pneumoniae culture supernatants on H. influenzae (data not shown).
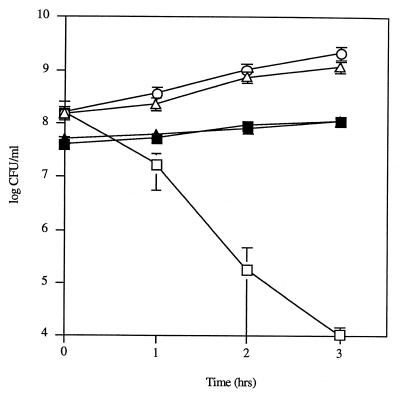
The effect of catalase on the bactericidal activity of S. pneumoniae was then explored using quantitative coculture experiments with bacteria grown in liquid medium. H. influenzae cultured with S. pneumoniae in the presence of 1,000 U of catalase per ml grew at the same rate as H. influenzae cultured alone, whereas heat-inactivated catalase (100°C for 20 min) was unable to eliminate the bactericidal activity of S. pneumoniae (Fig. 1). To confirm that hydrogen peroxide was responsible for the bactericidal activity of the pneumococcus, 108 CFU of H. influenzae per ml were cocultured with 5 × 107 CFU of an S. pneumoniae strain per ml in which the pyruvate oxidase gene (spxB) was insertionally inactivated. This mutant has previously been shown to produce <1% of the H2O2 of its parent strain, D39 (38). As expected, the spxB mutant was unable to kill H. influenzae in coculture experiments, in contrast to its parent strain D39 (Fig. 3). The growth of D39 and that of the spxB mutant were indistinguishable under these conditions.
FIG. 3.
Effect of coculture of H. influenzae Rd with S. pneumoniae D39 and its spxB mutant, P878. Following growth to mid-log phase, H. influenzae was washed and incubated in sBHI alone (○), with D39 (□), or with P878 (▵) for the times indicated, and viable counts were determined in duplicate on selective media. Viable counts of D39 (■) or P878 (▴) incubated in coculture with H. influenzae were determined in duplicate by plating on selective media. Values represent the average of three independent determinations in duplicate, and the error bars represent the standard deviations.
Bactericidal effect of S. pneumoniae on other respiratory tract pathogens.
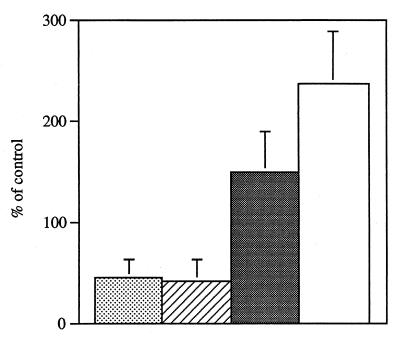
The inhibitory effect of S. pneumoniae was tested on two other common inhabitants of the human respiratory tract: a clinical isolate of Moraxella catarrhalis and an unencapsulated mutant of a type b N. meningitidis strain (MC58C3). Catalase-reversible inhibition of N. meningitidis by supernatants from S. pneumoniae culture was observed on BHI agar. While an inhibitory effect of pneumococcal supernatant was not seen on lawns of M. catarrhalis, cross-streaking of S. pneumoniae and M. catarrhalis on BHI agar revealed a catalase-reversible inhibitory effect on M. catarrhalis only in the immediate vicinity of S. pneumoniae. Coculture experiments to examine the bactericidal effect on these species showed that 108 CFU of N. meningitidis per ml incubated with 5 × 107 CFU/ml S. pneumoniae for 1.5 h resulted in a catalase-reversible 45 ± 19% decrease in viable count compared to N. meningitidis cultured in the absence of S. pneumoniae (Fig. 4). M. catarrhalis grown at 108 CFU/ml in the presence of 5 × 107 CFU of S. pneumoniae per ml for 3 h resulted in a catalase-reversible 43 ± 21% decrease in viable counts compared to M. catarrhalis grown alone (Fig. 4). In contrast, the viable count of S. pneumoniae increased substantially when grown with either N. meningitidis or M. catarrhalis compared to S. pneumoniae grown alone (Fig. 4).
FIG. 4.
Effect of coculture of S. pneumoniae P394 with either M. catarrhalis (Bc1) or N. meningitidis (MC58C3). Following growth to mid-log phase, S. pneumoniae (P394) was washed and incubated in BHI alone, with N. meningitidis (MC58C3) for 1.5 h, or with M. catarrhalis (Bc1) for 3 h. Viable counts of N. meningitidis (stippled bar) or M. catarrhalis (hatched bar) incubated in coculture with S. pneumoniae were determined in duplicate by plating on selective media. Viable counts of S. pneumoniae in coculture with N. meningitidis (black bar) or M. catarrhalis (white bar) were determined in duplicate on selective media. Values represent the change in viable count expressed as a percentage of a control culture containing that organism alone. Values are the average of three experiments, and error bars represent the standard deviations.
Hydrogen peroxide production and sensitivity to hydrogen peroxide.
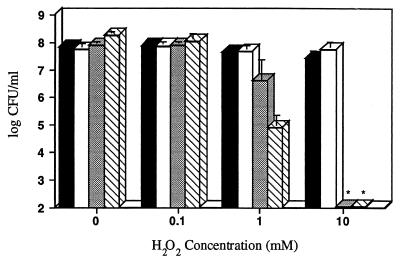
The relative sensitivities of S. pneumoniae P394 and the three other respiratory tract pathogens to hydrogen peroxide were examined using quantitative H2O2 challenge assays (Fig. 5). After a 30-min exposure to 0.1 mM H2O2, the survival of S. pneumoniae, M. catarrhalis, and N. meningitidis was unaffected, whereas the number of viable H. influenzae decreased by approximately twofold. At a concentration of 1.0 mM H2O2, the survival of S. pneumoniae and M. catarrhalis was unaffected, whereas the number of H. influenzae decreased approximately 2,000-fold, and the number of N. meningitidis decreased approximately 20-fold. At a concentration of 10 mM H2O2, H. influenzae and N. meningitidis decreased to undetectable levels (<100 CFU/ml), whereas the number of S. pneumoniae decreased only threefold, and M. catarrhalis was unaffected.
FIG. 5.
Effect of H2O2 on the survival of S. pneumoniae (P394), M. catarrhalis (Bc1), N. meningitidis (MC58C3), and H. influenzae (Rd). Following growth to mid-log phase, S. pneumoniae (black bars), M. catarrhalis (white bars), N. meningitidis (stippled bars), or H. influenzae (hatched bars) were washed and incubated at 37°C in BHI or sBHI containing the indicated concentration of H2O2. After 30 min, viable counts were determined on BHI or sBHI plates containing 200 U of catalase per ml. Values represent the average of three independent determinations in duplicate, and the error bars represent the standard deviations. ∗, Below the limit of detection.
A survey of bacterial species was made to determine if the levels of hydrogen peroxide production and resistance exhibited by S. pneumoniae are unusual among human pathogens. Of the species tested for peroxide generation, only S. pneumoniae isolates exhibited production of detectable levels (>0.1 mM) of hydrogen peroxide using a horseradish peroxidase-phenol red assay (Table 1). Survival in different concentrations of exogenously added H2O2 varied widely among the species of gram-negative and gram-positive bacteria tested. The species most susceptible to growth inhibition and killing by H2O2 was H. influenzae (MIC, 0.4 mM; MBC, 0.5 mM). N. meningitidis was also relatively sensitive (MIC, 0.4 mM; MBC, 5.0 mM). M. catarrhalis was relatively insensitive to the effects of hydrogen peroxide (MIC, 1.1 mM; MBC, 160 mM). The pneumococcus was also relatively insensitive (MIC, 1.6 mM; MBC, 80 mM), thus explaining its ability to survive endogenously produced hydrogen peroxide.
Factors affecting hydrogen peroxide production by S. pneumoniae.
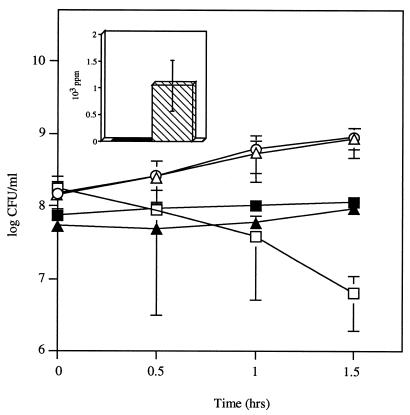
Strains P62 and P64, two naturally occurring phase variants of the same strain, were tested for H2O2 production after it was determined by comparison of two-dimensional gel electrophoresis of whole bacterial proteins followed by microsequencing that the major difference in whole-cell protein expression was in the higher SpxB expression in P64 compared to P62 (Fig. 6, insert) (Overweg et al., submitted). Phase variation in SpxB expression correlated with difference in H2O2 generation, with P64 producing significant amounts of H2O2, whereas production by P62 was undetectable (Table 1). The bactericidal effect of these variants on H. influenzae was then compared in coculture experiments (Fig. 6). After 1.5 h of coculture, the decrease in the viable counts of H. influenzae in the presence of P64 was approximately 100-fold, whereas P62 had no effect.
FIG. 6.
Effect of coculture of H. influenzae Rd with S. pneumoniae opaque (P62) or transparent (P64) variants of a type 9V isolate. Following growth to mid-log phase, H. influenzae was washed and incubated in sBHI either alone (○), with P62 (▵), or with P64 (□) for the times indicated, and viable counts were determined in duplicate on selective media. Viable counts of P62 (▴) or P64 (■) incubated in coculture with H. influenzae were determined in duplicate by plating on selective media. Values represent the average of three independent determinations in duplicate, and the error bars represent the standard deviations. (Inset) Relative expression of SpxB in S. pneumoniae variants P62 (black bar) and P64 (hatched bar) as determined by two-dimensional gel electrophoresis followed by mass spectrometric analysis. Results represent the average of four independent experiments, with error bars representing the standard deviations.
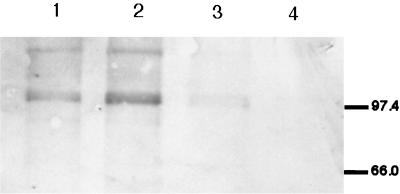
The production of H2O2 by the pneumococcus correlated with the concentration of O2 in the environment, being decreased in microaerobic conditions (data not shown). In order to determine the effect of environmental oxygen on SpxB expression, Western blots were performed on lysates from strain P878, which contains an in-frame fusion of PhoA to SpxB, using an antibody to bacterial alkaline phosphatase. Equal amounts of whole-cell lysates of P878 cultured under various concentrations of O2 and CO2 were separated by SDS-PAGE, transferred to a membrane, and immunoblotted. A band corresponding to the SpxB-PhoA fusion protein was detected in samples grown aerobically but was almost completely absent from samples grown anaerobically (Fig. 7). The highest level of expression of SpxB was noted in the conditions of high oxygen and increased carbon dioxide, which correspond to the conditions expected of the mucosal surface of the respiratory tract.
FIG. 7.
Western blot showing the effect of environmental oxygen and carbon dioxide tension on pyruvate oxidase (SpxB) expression in S. pneumoniae P878, which contains an in-frame fusion to PhoA. Cell lysates of spxB::phoA mutant (P878) grown under 20% O2–0.03% CO2 (lane 1), 17% O2–3% CO2 (lane 2), or <0.01% O2–10% CO2 (lane 3) were electrophoresed on an SDS–10% polyacrylamide gel, transferred to a polyvinylidene difluoride membrane, and immunoblotted with an antibody to PhoA. As a negative control, cell lysates from the parent strain (D39) grown under 17% O2–3% CO2 (lane 4) were included. Size markers are in kilodaltons.
DISCUSSION
This study documents the production of a soluble antimicrobial substance by S. pneumoniae. Several lines of evidence demonstrate that this substance is hydrogen peroxide. The effect of the pneumococcus in coculture experiments was completely eliminated by the addition of active but not inactivated catalase. A similar antimicrobial effect was reproduced by the addition of exogenous H2O2 at concentrations shown to be generated by the pneumococcus in liquid culture. This effect, furthermore, was absent in a pyruvate oxidase (spxB) mutant that synthesizes <1% of parental levels of H2O2 as well as a spontaneous variant that is downregulated in expression of SpxB (Overweg et al., submitted). Anaerobic growth conditions also lead to a diminished expression of SpxB which correlated with a loss of antimicrobial effect (data not shown). Finally, the degree of antimicrobial effect against three species was proportional to their sensitivity to both growth inhibition and killing mediated by exogenous hydrogen peroxide.
Among the gram-positive (n = 6) and gram-negative (n = 7) species tested, the pneumococcus was the only species that generated concentrations of H2O2 that were >0.1 mM in liquid culture when at mid-log phase growth in aerobic conditions. For one of the S. pneumoniae strains tested, the average H2O2 concentration after 1 h of culture was 1.1 mM. This is consistent with the observation that S. pneumoniae produce approximately the same amount of H2O2 per gram of total cellular protein as neutrophils during the oxidative burst (15). The calculated concentrations of H2O2 produced by S. pneumoniae in the present study agree with those previously reported for this species (2, 5, 38). Our results, furthermore, confirmed that the spxB mutant was deficient in H2O2 production (38). The mechanism that allows for the survival and growth of the pneumococcus, a catalase-negative organism, in substantial concentrations of hydrogen peroxide is unknown. S. pneumoniae contains NADH oxidase but lacks other systems involved in the oxidative stress response, such as OxyR (3). It was noted in this study that the mutant deficient in pyruvate oxidase activity often grew to a higher density in liquid culture. A similar effect on pneumococcal growth in liquid culture was observed in the presence of exogenous catalase and in coculture with M. catarrhalis or N. meningitidis, species that both produce high levels of catalase (37). Furthermore, the pneumococcus requires catalase for optimal growth on solid surfaces where the density of organisms is high (42). These observations support previous findings that endogenous production of hydrogen peroxide is permissive for growth but may have an adverse effect on its rate (2, 20, 34). This negative effect of hydrogen peroxide on growth raises the question as to why the pneumococcus, an organism that does not express catalase activity, synthesizes copious amounts of this highly toxic substance. It has been suggested that H2O2 generated by S. pneumoniae contributes to the pathogenesis of disease in the respiratory tract by its cytotoxic effects on the epithelial barrier of the host (15, 19). This effect, however, required ≥108 CFU/ml, a density of bacteria unlikely to occur in the commensal state for this organism. In contrast, the antimicrobial effect was evident in coculture experiments with as few as 106 CFU/ml. Data presented here support the hypothesis that the pneumococcus generates unusually high amounts of hydrogen peroxide as a means of inhibiting and/or killing other species that may compete for the same environmental niche in the heavily colonized human nasopharynx.
Many lactic acid bacteria produce significant amounts of hydrogen peroxide during aerobic growth (50). In fact, several species of lactobacilli and oral streptococci have been shown to produce levels of H2O2 in liquid culture similar to that of S. pneumoniae (1 to 10 mM) (5, 16, 18, 47). Organisms shown to be killed or inhibited in vitro due to peroxide production by lactic acid bacteria include Neisseria gonorrhoeae, Staphylococcus aureus, Corynebacterium diphtheriae, and various other members of the oral flora (13, 16, 41, 50, 52). In the case of the pneumococcus, Colebrook was the first to describe its inhibitory activity by cross-streaking it with N. meningitidis and M. catarrhalis on solid medium (11). Similarly, McLeod and Gordon reported in 1922 the inhibition of growth of S. aureus due to S. pneumoniae culture supernatants, an effect they attributed to the presence of hydrogen peroxide (34). Our own study was able to take advantage of a genetically defined mutant that is essentially deficient in H2O2 production to confirm this hypothesis about the nature of the inhibitory substance generated by S. pneumoniae (38). Moreover, we demonstrate here that this antimicrobial effect may be a factor in the ability of the pneumococcus to compete against the other major pathogens residing in the upper respiratory tract of humans. The antimicrobial effect of the pneumococcus against three gram-negative, catalase-positive species that also colonize the mucosal surface of the human nasopharynx was assessed. The most dramatic effect was seen in coculture experiments with H. influenzae, where there was a 4-log decline in viable counts over 3 h due to the presence of 5 × 107 CFU of S. pneumoniae per ml. This was the most sensitive bacterial species among those tested to both the inhibitory and the bactericidal effects of the pneumococcus. If a similar effect occurs in vivo, this could at least in part account for the previously noted lower-than-expected rates of coinfection with S. pneumoniae and H. influenzae in otitis media and chronic bronchitis (25, 30). The inhibitory and bactericidal effects of H2O2 on H. influenzae occur despite the measurable expression of catalase by this species (8). In other words, a catalase-negative species, S. pneumoniae, is able to efficiently kill a catalase-positive species, H. influenzae, using H2O2. The level of catalase activity as measured by the ability to catalyze the decomposition of hydrogen peroxide, however, varies widely from species to species, and H. influenzae seems to be an example of a catalase-positive organism with relatively low catalase activity as measured in vitro (7, 33). H. influenzae possesses only one gene for catalase, unlike the other gram-negative species E. coli, Salmonella enterica serovar Typhimurium, and Shigella flexneri, which produce two catalases (8). A previously reported catalase-deficient mutant of H. influenzae, strain AB2593 (Rd::hktE−) was not significantly more sensitive to the antimicrobial effect of the pneumococcus compared to its parent strain, implying that catalase does little to protect H. influenzae under these conditions (data not shown) (8). H. influenzae may possess an impaired ability to upregulate catalase production in response to elevated levels of H2O2, possibly as a result of H. influenzae's inability to synthesize protoporphyrin IX, the biosynthetic precursor of heme, a required component of catalase (48). This finding is consistent with the observation that 108 CFU of exponentially growing H. influenzae produce only 5.7 U of catalase, and this expression level is induced only threefold by oxidative stress (8). Furthermore, the addition of H. influenzae to cultures of S. pneumoniae had only a small effect on the hydrogen peroxide concentration, suggesting that the endogenous production of catalase by H. influenzae was insufficient for these levels of H2O2 (data not shown). The effect of the pneumococcus was less dramatic against the meningococcus, where growth inhibition and minimal killing were observed after 1.5 h of coculture. When M. catarrhalis, a target species with markedly greater catalase activity, was tested, only a slight inhibitory bactericidal effect was evident after 3 h of coculture, although a catalase-reversible effect was noted with a higher density of pneumococci when the two organisms were cross-streaked on BHI agar.
In considering the contribution of hydrogen peroxide production to pneumococcal carriage, it should be noted that the studies presented here are based exclusively on in vitro effects. The synthesis of H2O2 by the pneumococcus in vivo has not been determined, although maximal expression of SpxB was noted in an oxygen and carbon dioxide rich environment, as would be expected on the surface of the upper respiratory tract. In addition, the antimicrobial effect correlated with variability in the expression of SpxB and was present in a variant with a transparent colony phenotype but not the opaque variant of the same isolate (Overweg et al., submitted). Only the transparent form has been shown to persistently colonize the nasopharynx in an animal model of carriage (42). This suggests that the increased production of H2O2 associated with this phenotype may contribute to its ability to efficiently colonize a host, whereas the opaque phenotype may be outcompeted by the other flora. Another consideration in extrapolating these results to the situation in vivo is that host factors on the mucosal surface may act to inactivate bacterial hydrogen peroxide. In this regard, viridans streptococci, which may generate concentrations of hydrogen peroxide similar to that of S. pneumoniae, have been suggested to prevent colonization of gram-negative bacilli, including H. influenzae, in the human oropharynx by a mechanism that may be mediated in part by H2O2 production (39, 40). In addition, the spxB mutant of S. pneumoniae does not persist within the airway in an animal model of colonization in rabbits (38). Although the mechanism for this defect in carriage is unknown and there are several plausible explanations, it is possible that it results from an inability of the mutant to suppress local competitors. Future studies will address the significance of these observations to pneumococcal carriage and the maintenance of the normal microflora of the upper respiratory tract.
ACKNOWLEDGMENTS
We thank H. R. Masure for providing strain P878 (D39 spxB mutant) and W. R. Bishai for providing strain AB2593 (Rd::hktE−). Expert technical assistance was provided by Miki Kapoor and Gregory Moy.
This work was supported by grants from the Sophia Foundation for Medical Research (grant 183) and the U.S. Public Health Service (AI38436 and AI44231).
REFERENCES
- 1.Achtman M, Mercer A, Kusecek B, Pohl A, Heuzenroede R M, Aaronson W, Sutton A, Silver R P. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect Immun. 1983;39:315–335. doi: 10.1128/iai.39.1.315-335.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Annear D I, Dorman D C. Hydrogen peroxide accumulation during growth of the pneumococcus. Aust J Exp Bio Med Sci. 1952;30:191–195. doi: 10.1038/icb.1952.18. [DOI] [PubMed] [Google Scholar]
- 3.Auzat I, Chapuy-Regaud S, Le Bras G, Dos Santos D, Ogunniyi A, Le Thomas I, Garel J R, Paton J C, Trombe M C. The NADH oxidase of Streptococcus pneumoniae: its involvement in competence and virulence. Mol Microbiol. 1999;34:1018–1028. doi: 10.1046/j.1365-2958.1999.01663.x. [DOI] [PubMed] [Google Scholar]
- 4.Avery O T, MacLeod C M, McCarty M. Studies on the nature of the chemical nature of the substance inducing transformation of pneumococcal types. J Exp Med. 1944;79:137–157. doi: 10.1084/jem.79.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnard J P, Stinson M W. The alpha-hemolysin of Streptococcus gordonii is hydrogen peroxide. Infect Immun. 1996;64:3853–3857. doi: 10.1128/iai.64.9.3853-3857.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berry A, Lock R, Paton J. Cloning and characterization of nanB, a second Streptococcus pneumoniae neuraminidase gene, and purification of the NanB enzyme from recombinant Escherichia coli. J Bacteriol. 1996;178:4854–4860. doi: 10.1128/jb.178.16.4854-4860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bisaillon J, Dubois G, Beaudet R, Sylvestre M, Charbonneau R, Gagnon M. Quantitative determination of catalase activity produced by Neisseria gonorrhoeae, Staphylococcus epidermidis, Neisseria meningitidis, and other bacterial strains using the Catalasemeter. Exp Biol. 1985;43:225–230. [PubMed] [Google Scholar]
- 8.Bishai W, Howard N, Winkelstein J, Smith H. Characterization and virulence analysis of catalase mutants of Haemophilus influenzae. Infect Immun. 1994;62:4855–60. doi: 10.1128/iai.62.11.4855-4860.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Briles D E, Nahm M, Schroer K, Davie J, Baker P, Kearney J, Barletta R. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 Streptococcus pneumoniae. J Exp Med. 1981;153:694–705. doi: 10.1084/jem.153.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camara M, Boulnois G J, Andrew P W, Mitchell T J. A neuraminidase from Streptococcus pneumoniae has the features of a surface protein. Infect Immun. 1994;62:3688–3695. doi: 10.1128/iai.62.9.3688-3695.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colebrook L. Bacterial antagonism, with particular reference to meningococcus. Lancet. 1915;ii:1136–1138. [Google Scholar]
- 12.Cundell D R, Gerard N P, Gerard C, Idanpaan-Heikkila I, Tuomanen E I. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature. 1995;377:435–438. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- 13.Dahiya R S, Speck M L. Hydrogen peroxide formation by lactobacilli and its effect on Staphylococcus aureus. J Dairy Sci. 1968;51:1568–1572. doi: 10.3168/jds.S0022-0302(68)87232-7. [DOI] [PubMed] [Google Scholar]
- 14.Del Beccaro M A, Mendelman P M, Inglis A F, Richardson M A, Duncan N O, Clausen C R, Stull T L. Bacteriology of acute otitis media: a new perspective. J Pediatr. 1992;120:81–84. doi: 10.1016/s0022-3476(05)80605-5. [DOI] [PubMed] [Google Scholar]
- 15.Duane P G, Rubins J B, Weisel H R, Janoff E N. Identification of hydrogen peroxide as a Streptococcus pneumoniae toxin for rat alveolar epithelial cells. Infect Immun. 1993;61:4392–4397. doi: 10.1128/iai.61.10.4392-4397.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubreuil D, Bisaillon J G, Beaudet R. Inhibition of Neisseria gonorrhoeae growth due to hydrogen peroxide production by urogenital streptococci. Microbios. 1984;39:159–167. [PubMed] [Google Scholar]
- 17.Estabrook M M, Griffiss J M, Jarvis G A. Sialylation of Neisseria meningitidis lipooligosaccharide inhibits serum bactericidal activity masking lacto-N-neotraose. Infect Immun. 1997;65:4436–4444. doi: 10.1128/iai.65.11.4436-4444.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Mendoa A, Liebana J, Castillo A M, De La Higuera A, Piedrola G. Evaluation of the capacity of oral streptococci to produce hydrogen peroxide. J Med Microbiol. 1993;39:434–439. doi: 10.1099/00222615-39-6-434. [DOI] [PubMed] [Google Scholar]
- 19.Hirst R A, Sikand K S, Rutman A, Mitchell T J, Andrew P W, O'Callaghan C. Relative roles of pneumolysin and hydrogen peroxide from Streptococcus pneumoniae in inhibition of ependymal ciliary beat frequency. Infect Immun. 2000;68:1557–1562. doi: 10.1128/iai.68.3.1557-1562.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holt L B. The culture of Streptococcus pneumoniae. J Gen Microbiol. 1962;27:327–330. doi: 10.1099/00221287-27-2-327. [DOI] [PubMed] [Google Scholar]
- 21.Hood D W, Makepeace K, Deadman M E, Rest R F, Thibault P, Martin A, Richards J C, Moxon E R. Sialic acid in the lipopolysaccharide of Haemophilus influenzae: strain distribution, influence on serum resistance and structural characterization. Mol Microbiol. 1999;33:679–692. doi: 10.1046/j.1365-2958.1999.01509.x. [DOI] [PubMed] [Google Scholar]
- 22.Kim J O, Romero-Steiner S, Sørensen U, Blom J, Carvalho M, Barnardi S, Carlone G, Weiser J N. Relationship between cell-surface carbohydrates and intrastrain variation on opsonophagocytosis of Streptococcus pneumoniae. Infect Immun. 1999;67:2327–2333. doi: 10.1128/iai.67.5.2327-2333.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein J O. Role of nontypeable Haemophilus influenzae in pediatric respiratory tract infections. Pediatr Infect Dis. 1997;16:S5–S8. doi: 10.1097/00006454-199702001-00002. [DOI] [PubMed] [Google Scholar]
- 24.Kremer M L. The reaction of hemin with H2O2. Eur J Biochem. 1989;185:651–658. doi: 10.1111/j.1432-1033.1989.tb15162.x. [DOI] [PubMed] [Google Scholar]
- 25.Luotonen J. Streptococcus pneumoniae and Haemophilus influenzae in nasal cultures during acute otitis media. Acta Otolaryngol. 1982;93:295–299. doi: 10.3109/00016488209130886. [DOI] [PubMed] [Google Scholar]
- 26.Lysenko E S, Richards J C, Cox A D, Stewart A, Martin A, Kapoor M, Weiser J N. The position of phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae affects binding and sensitivity to C-reactive protein mediated killing. Mol Microbiol. 2000;35:234–245. doi: 10.1046/j.1365-2958.2000.01707.x. [DOI] [PubMed] [Google Scholar]
- 27.MacIver I, Hansen E J. Lack of expression of the global regulator OxyR in Haemophilus influenzae has a profound effect on growth phenotype. Infect Immun. 1996;64:4618–4629. doi: 10.1128/iai.64.11.4618-4629.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mandrell R E, Kim J J, John C M, Gibson B W, Sugai J V, Apicella M A, Griffiss J M, Yamasaki R. Endogenous sialylation of the lipooligosaccharides of Neisseria meningitidis. J Bacteriol. 1991;173:2823–2832. doi: 10.1128/jb.173.9.2823-2832.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandrell R E, McLaughlin R, Kwaik Y A, Lesse A, Yamasaki R, Gibson B, Spinola S M, Apicella M A. Lipooligosaccharides (LOS) of some Haemophilus species mimic human glycosphingolipids, and some LOS are sialylated. Infect Immun. 1992;60:1322–1328. doi: 10.1128/iai.60.4.1322-1328.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.May R J. Pathogenic bacteria in chronic bronchitis. Lancet. 1954;ii:839–842. doi: 10.1016/s0140-6736(54)91931-5. [DOI] [PubMed] [Google Scholar]
- 31.McDaniel L S, Benjamin W H J, Forman C, Briles D E. Blood clearance by anti-phosphocholine antibodies as a mechanism of protection in experimental pneumococcal bacteremia. J Immunol. 1984;133:3308–12. [PubMed] [Google Scholar]
- 32.McGuinness B, Clarke I, Lambden P, Barlow A, Poolman J, Jones D, Heckels J. Point mutation in meningococcal porA gene associated with increased endemic disease. Lancet. 1991;337:514–517. doi: 10.1016/0140-6736(91)91297-8. [DOI] [PubMed] [Google Scholar]
- 33.McLeod J W, Gordon J. Catalase production and sensitiveness to hydrogen peroxide amongst bacteria: with a scheme of classification based on these properties. J Pathol Bacteriol. 1923;26:326–331. [Google Scholar]
- 34.McLeod J W, Gordon J. Production of hydrogen peroxide by bacteria. Biochem J. 1922;16:499–506. doi: 10.1042/bj0160499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mosser J L, Tomasz A. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an enzyme. J Biol Chem. 1970;245:287–298. [PubMed] [Google Scholar]
- 36.Pick E, Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods. 1980;38:161–170. doi: 10.1016/0022-1759(80)90340-3. [DOI] [PubMed] [Google Scholar]
- 37.Singh S, Cisera K M, Turnidge J D, Russell E G. Selection of optimum laboratory tests for the identification of Moraxella catarrhalis. Pathology. 1997;29:206–208. doi: 10.1080/00313029700169874. [DOI] [PubMed] [Google Scholar]
- 38.Spellerberg B, Cundell D R, Sandros J, Pearce B J, Idanpaan-Heikkila I, Rosenow C, Masure H R. Pyruvate oxidase, as a determinant of virulence in Streptococcus pneumoniae. Mol Microbiol. 1996;19:803–813. doi: 10.1046/j.1365-2958.1996.425954.x. [DOI] [PubMed] [Google Scholar]
- 39.Sprunt K, Leidy G A, Redman W. Prevention of bacterial overgrowth. J Infect Dis. 1971;123:1–10. doi: 10.1093/infdis/123.1.1. [DOI] [PubMed] [Google Scholar]
- 40.Sprunt K, Redman W. Evidence suggesting importance of role of interbacterial inhibition in maintaining balance of normal flora. Ann Intern Med. 1968;68:579–590. doi: 10.7326/0003-4819-68-3-579. [DOI] [PubMed] [Google Scholar]
- 41.Thompson R, Johnson A. The inhibitory action of saliva on the diphtheria bacillus: hydrogen peroxide, the inhibitory agent produced by salivary streptococci. J Infect Dis. 1951;88:81–85. doi: 10.1093/infdis/88.1.81. [DOI] [PubMed] [Google Scholar]
- 42.Weiser J N, Austrian R, Sreenivasan P K, Masure H R. Phase variation in pneumococcal opacity: relationship between colonial morphology and nasopharyngeal colonization. Infect Immun. 1994;62:2582–2589. doi: 10.1128/iai.62.6.2582-2589.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiser J N, Chong S T H, Greenberg D, Fong W. Identification and characterization of a cell envelope protein of Haemophilus influenzae contributing to phase variation in colony opacity and nasopharyngeal colonization. Mol Microbiol. 1995;17:555–564. doi: 10.1111/j.1365-2958.1995.mmi_17030555.x. [DOI] [PubMed] [Google Scholar]
- 44.Weiser J N, Love J M, Moxon E R. The molecular mechanism of phase variation of H. influenzae lipopolysaccharide. Cell. 1989;59:657–665. doi: 10.1016/0092-8674(89)90011-1. [DOI] [PubMed] [Google Scholar]
- 45.Weiser J N, Pan N, McGowan K L, Musher D, Martin A, Richards J C. Phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae contributes to persistence in the respiratory tract and sensitivity to serum killing mediated by C-reactive protein. J Exp Med. 1998;187:631–640. doi: 10.1084/jem.187.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiser J N, Shchepetov M, Chong S T H. Decoration of lipopolysaccaride with phosphorylcholine: a phase-variable characteristic of Haemophilus influenzae. Infect Immun. 1997;65:943–950. doi: 10.1128/iai.65.3.943-950.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wheater D M, Hirsch A, Mattick A T R. Possible identity of “lactobacillin” with hydrogen peroxide produced by lactobacilli. Nature. 1952;170:623–624. doi: 10.1038/170623a0. [DOI] [PubMed] [Google Scholar]
- 48.White D C, White G S. Hemin biosynthesis in Haemophilus. J Bacteriol. 1963;85:842–850. doi: 10.1128/jb.85.4.842-850.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whittenbury R. Hydrogen peroxide formation and catalase activity in the lactic acid bacteria. J Gen Microbiol. 1964;35:13–26. doi: 10.1099/00221287-35-1-13. [DOI] [PubMed] [Google Scholar]
- 50.Wilcox M D P, Drucker D B. Partial characterisation of the inhibitory substances produced by Streptococcus oralis and related species. Microbios. 1988;55:135–145. [PubMed] [Google Scholar]
- 51.Yother J, Leopold K, White J, Fischer W. Generation and properties of a Streptococcus pneumoniae mutant which does not require choline or analogs for growth. J Bacteriol. 1998;180:2093–2101. doi: 10.1128/jb.180.8.2093-2101.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng H Y, Alcorn T M, Cohen M S. Effects of H2O2-producing lactobacilli on Neisseria gonorrhoeae growth and catalase activity. J Infect Dis. 1994;170:1209–1215. doi: 10.1093/infdis/170.5.1209. [DOI] [PubMed] [Google Scholar]