Abstract
CONTEXT:
Hemostatic nanoparticles (hNPs) have shown efficacy in decreasing intracerebral hemorrhage (ICH) in animal models and are suggested to be of use to counter tissue plasminogen activator (tPA)-induced acute ICH.
AIMS:
The objective of this study was to test the ability of an hNP preparation to alter the clotting properties of blood exposed to tPA ex vivo.
MATERIALS AND METHODS:
Fresh blood samples were obtained from normal male Sprague-Dawley rats (~300 g; n = 6) and prepared for coagulation assays by thromboelastography (TEG) methods. Samples were untreated, exposed to tPA, or exposed to tPA and then to hNP. TEG parameters included reaction time (R, time in minutes elapsed from test initiation to initial fibrin formation), coagulation time (K, time in minutes from R until initial clot formation), angle (α, a measure in degrees of the rate of clot formation), maximum amplitude (MA, the point when the clot reaches its MA in mm), lysis at 30 min after MA (LY30, %), and clot strength (G, dynes/cm2), an index of clot strength.
STATISTICAL ANALYSIS USED:
Kruskal–Wallis test was employed to compare TEG parameters measured for untreated control samples versus those exposed to tPA and to compare tPA-exposed samples to samples treated with tPA + hNPs. Significances were inferred at P ≤ 0.05.
RESULTS:
Compared to untreated samples, tPA-treated samples showed a trend toward decreased angle and G suggesting potentially clot formation rate and clot strength. The addition of hNP did not affect any of these or other measured indices.
CONCLUSIONS:
The data demonstrated no hemostatic effects when the hNP was used in the presence of tPA. The lack of change in any of the TEG parameters measured in the present study may indicate limitations of the hNPs to reverse the thrombolytic cascade initiated by tPA.
Keywords: Cerebral ischemia, coagulation, intracerebral hemorrhage, thromboelastography, thrombolysis
Introduction
Reperfusion through intravenous tissue plasminogen activator (tPA) is the only thrombolytic therapy presently approved by the Food and Drug Administration to treat acute ischemic stroke (AIS).[1] It needs to be administered within 4.5 h after stroke onset, or within 6 h with mechanical thrombectomy.[2] Successful reperfusion performed within the therapeutic time window can rescue reversibly damaged ischemic penumbra and decrease the extent of chronic brain injury and consequent disability. However, even within these narrow treatment windows, earlier treatment with tPA is more beneficial for stroke patients because of delay-associated, increased risk of symptomatic intracerebral hemorrhage (ICH), or hemorrhagic transformation (HT).[3,4,5] Only 5%–10% of all AIS patients are presently considered eligible for recanalization therapy due to this serious limitation. However, a larger percentage of stroke patients that present outside the optimal treatment window can be a candidate, but require advanced imaging to determine their eligibility.[6] More patients could potentially be eligible for reperfusion with tPA if reliable adjunctive therapies to mitigate HT were available.[7] Another use for tPA is in dissolving blood clots in the ventricular system. However, it may also result in blood derivatives entering the parenchyma and adversely affect brain function.[8] Limiting options for its dose variations, a tPA dose escalation-dependent increase in blood–brain barrier damage and hemoglobin extravasation is also reported.[9]
In this regard, nanoparticle preparations have shown promise as hemostatic agents through their actions on the clotting cascade.[10,11,12] Compared to biologicals, these agents have the advantages of being relatively less expensive and functional even with ambient storage.[13] They also offer the additional advantages of precise control of size to attenuate or prevent mechanical filtration in lung capillary beds and trapping by the liver reticuloendothelial system.[13] Intravenous administration of one such hemostatic nanoparticle (hNP) preparation showed decreased clotting time, with no effect on clot firmness, significantly decreased blood loss after liver injury, and increased survival at 1 h.[11] hNPs used in another in vivo multiorgan hemorrhage model following blast injury also showed hemostatic efficacy and increased animal survival acutely with no sub-chronic side effects.[12] The effects of these hNPs on the clotting cascade were tested in this study using normal blood samples that were prior exposed to tPA.
Materials and Methods
All the reagents used were of either analytical or pharmaceutical grade. Male Sprague-Dawley rats of about 8 months of age were used (~300 g; n = 6) and their handling was done under an institutionally approved protocol (IACUC #1440). Blood samples were obtained from the tail vein for coagulation assays through thromboelastography (TEG) methods using a Thromboelastograph Coagulation Analyzer (Model 5000; Haemoscope Corporation, Niles, IL, USA).
TEG is a viscoelastic technique that allows for the quantitative measurement of the efficiency of blood coagulation. It measures five primary parameters. The reaction time measures the time of latency from the start of the test to initial fibrin formation. The kinetics (K) measures the time taken to achieve a certain level of clot strength. The alpha angle (A) measures the speed of fibrin build-up and cross-linking, i.e. the rate of clot formation. The maximum amplitude (MA) measures the ultimate strength of the contracted platelet-fibrin clot. Finally, the thrombolysis (Ly30) measures the clot stability versus breakdown over 30 min from the establishment of maximal clot strength. We also report clot strength, G, which is derived from the MA. Clinicians have used TEG to guide blood product therapy in traumatic and acquired coagulopathy for over two decades.[14] TEG is frequently used to guide such therapy in the emergency trauma and perioperative areas of medicine.[15]
Samples were mixed with either tPA or tPA + hNP immediately before TEG. tPA was used at a concentration of 636 ng/ml blood and hNP at 8 mg/ml blood. Recorded TEG parameters included the following [Figure 1]: reaction time (R, time in minutes elapsed from test initiation to initial fibrin formation); coagulation time (K, time in minutes from R until initial clot formation); angle (α, a measure in degrees of the rate of clot formation); (MA, the point when the clot reaches its MA in millimeters); lysis at 30 min (LY30, the percentage of fibrinolysis 30 min after MA); and clot strength (G, dynes/cm2), an index of clot strength derived from the amplitude.
Figure 1.
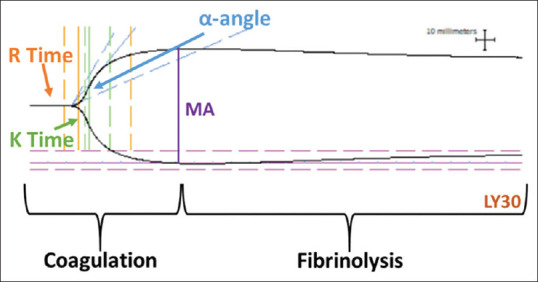
A typical blood coagulation and fibrinolysis curve obtained from thromboelastography analysis of blood samples. Various quantitative parameters used to measure the clotting cascade are indicated on the relevant parts of the curve
We performed analyses using the Kruskal–Wallis test to compare TEG parameters measured for untreated control samples versus those exposed to tPA and to compare tPA-exposed samples to samples treated with tPA + hNPs. Significances were inferred at P ≤ 0.05.
Results and Discussion
There was no statistically significant difference between control samples and tPA-exposed samples in all measurements: R (P = 0.263), angle (P = 0.631), K (P = 0.873), MA (P = 0.230), LY30 (P = 0.200), and G (P = 0.109). There was also no statistically significant difference between tPA-exposed samples and tPA + hNP samples all measurements: R (P = 0.810, angle (P = 1.0), K (P = 0.936), MA (P = 0.749), LY30 (P = 0.689), and G (P = 0.810). No statistically significant differences were observed among the measured coagulation cascade indices in these samples [Figure 2]. Two of the measured TEG parameters, alpha angle (A) and clot strength (G), showed a trend toward a decrease, but did not attain statistical significance [Figure 2]. None of the coagulation parameters studied showed any significant differences between the tPA and tPA + hNP groups [Figure 2]. Thus, the results did not demonstrate that these hNPs were effective in attenuating or reversing the thrombolysis set off by prior tPA treatment. These data are not in agreement with previous reports of increased survival of rats exposed to blast injury and then treated with hNPs.[11] The main mechanism of hNP-induced increased animal survival observed in this study was suggested to be due to more rapid clot formation and an increase in clot strength.[11] An examination of data presented in Figure 2 shows that angle (rate of clot formation) and G (clot strength) were among the main parameters likely to be affected by tPA in its thrombolytic effects. Therefore, it stands to reason that the main mechanisms of hNP action were affected by tPA, and thus, the succeeding addition of hNP did not show enhanced blood clotting effects. One caveat is that we used normal rats in this preliminary study. The effectiveness of tPA is reported to be altered/decreased in cases with existing metabolic syndrome due to the propensity of forming denser clots.[16] Although higher tPA dosing is suggested to overcome this deficiency, it is also accompanied by increased hemorrhage risk. Whether hNPS could be more effective under such circumstances through the preexisting physiological bias toward thrombosis needs to be investigated. Furthermore, it is possible that the efficacy of hNP would be greater in higher doses.
Figure 2.
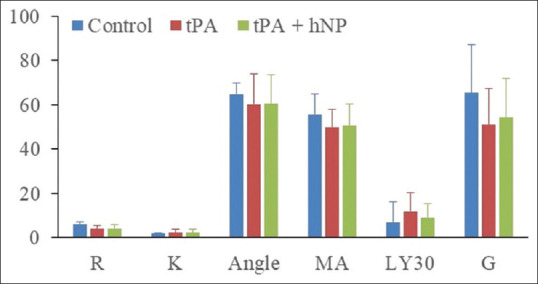
A bar graph of the measured parameters from control and tPA-added blood samples without and with hemostatic nanoparticle treatment. Compared to control samples, rate of clot formation (Angle) and clot strength (g) showed a trend toward decrease, but not statistical significance in tPA and in tPA + HNP samples. Units of measurements for the parameters are explained in the methods. tPA: Tissue plasminogen activator, hNP: hemostatic nanoparticle
In summary, unlike previous in vivo and in vitro studies using hNPs that have reported effects of promoting blood clotting and of improving survival in trauma models, the present ex vivo study did not demonstrate hemostatic effects when hNPs were used in the presence of tPA. The lack of change in the measured TEG parameters in the present study is suggestive of the limitations of such hNPs to reverse the thrombolytic cascade initiated by tPA. For effective clinical translation, further studies are needed to evaluate the effects of hNPs and other similar preparations in presence of nonprescription drugs such as aspirin and prescription blood thinners. Data from these interactions may help in preparing hNPs that are specifically designed to exert their effects even in the presence of routinely used blood thinners and prescription thrombolytics. In addition, combating deleterious dose–response effects of tPA[9] and side effects of localized applications such as in ventricular hemorrhage[8] may need specially formulated agents. Of note here is that microvascular functionality toward thrombosis and hemostasis in the brain is different from most peripheral organ systems.[17] It is reported that the inactivation of tPA and urokinase-type plasminogen activator genes in mouse models led to thrombosis and fibrin deposition in most organs except in brain and kidneys.[18] Thus, comparatively low reliance of cerebral vasculature on antithrombotic and fibrinolytic pathways and dependence on tissue factor suggests a basic property of protection against brain hemorrhage.[17] Thus, agents that may counteract tPA in peripheral organs may need to be customized for use in brain hemorrhage with these properties of cerebral vasculature as the target mechanisms.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors thank Jun Xu (HFH) for expert technical assistance in performing the studies and Dr. I. Lopez-Plaza (HFH) for allowing the use of TEG apparatus.
References
- 1.Jilani TN, Siddiqui AH. StatPearls. Treasure Island (FL): StatPearls Publishing LLC; 2021. Tissue plasminogen activator. [PubMed] [Google Scholar]
- 2.Vivien D. Can the benefits of rtPA treatment for stroke be improved? Rev Neurol (Paris) 2017;173:566–71. doi: 10.1016/j.neurol.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Flick MJ. Mechanism of ICH with tPA thrombolysis. Blood. 2021;138:8–9. doi: 10.1182/blood.2021011268. [DOI] [PubMed] [Google Scholar]
- 4.Murray V, Norrving B, Sandercock PA, Terént A, Wardlaw JM, Wester P. The molecular basis of thrombolysis and its clinical application in stroke. J Intern Med. 2010;267:191–208. doi: 10.1111/j.1365-2796.2009.02205.x. [DOI] [PubMed] [Google Scholar]
- 5.Yepes M, Roussel BD, Ali C, Vivien D. Tissue-type plasminogen activator in the ischemic brain: More than a thrombolytic. Trends Neurosci. 2009;32:48–55. doi: 10.1016/j.tins.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Heidari P, Blayney S, Butler J, Hitomi E, Luby M, Leigh R. Frequency of thrombolytic targets in stroke patients presenting in an extended time window. Brain Circ. 2020;6:163–8. doi: 10.4103/bc.bc_12_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otsu Y, Namekawa M, Toriyabe M, Ninomiya I, Hatakeyama M, Uemura M, et al. Strategies to prevent hemorrhagic transformation after reperfusion therapies for acute ischemic stroke: A literature review. J Neurol Sci. 2020;419:117217. doi: 10.1016/j.jns.2020.117217. [DOI] [PubMed] [Google Scholar]
- 8.Bosche B, Mergenthaler P, Doeppner TR, Hescheler J, Molcanyi M. Complex clearance mechanisms after intraventricular hemorrhage and rt-PA treatment – A review on clinical trials. Transl Stroke Res. 2020;11:337–44. doi: 10.1007/s12975-019-00735-6. [DOI] [PubMed] [Google Scholar]
- 9.Burggraf D, Martens HK, Dichgans M, Hamann GF. rt-PA causes a dose-dependent increase in the extravasation of cellular and non-cellular blood elements after focal cerebral ischemia. Brain Res. 2007;1164:55–62. doi: 10.1016/j.brainres.2007.05.066. [DOI] [PubMed] [Google Scholar]
- 10.Bertram JP, Williams CA, Robinson R, Segal SS, Flynn NT, Lavik EB. Intravenous hemostat: Nanotechnology to halt bleeding. Sci Transl Med. 2009;1:11ra22. doi: 10.1126/scitranslmed.3000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shoffstall AJ, Atkins KT, Groynom RE, Varley ME, Everhart LM, Lashof-Sullivan MM, et al. Intravenous hemostatic nanoparticles increase survival following blunt trauma injury. Biomacromolecules. 2012;13:3850–7. doi: 10.1021/bm3013023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lashof-Sullivan MM, Shoffstall E, Atkins KT, Keane N, Bir C, VandeVord P, et al. Intravenously administered nanoparticles increase survival following blast trauma. Proc Natl Acad Sci U S A. 2014;111:10293–8. doi: 10.1073/pnas.1406979111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lashof-Sullivan M, Shoffstall A, Lavik E. Intravenous hemostats: Challenges in translation to patients. Nanoscale. 2013;5:10719–28. doi: 10.1039/c3nr03595f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pretorius E, Swanepoel AC, DeVilliers S, Bester J. Blood clot parameters: Thromboelastography and scanning electron microscopy in research and clinical practice. Thromb Res. 2017;154:59–63. doi: 10.1016/j.thromres.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Walsh M, Fritz S, Hake D, Son M, Greve S, Jbara M, et al. Targeted thromboelastographic (TEG) blood component and pharmacologic hemostatic therapy in traumatic and acquired coagulopathy. Curr Drug Targets. 2016;17:954–70. doi: 10.2174/1389450117666160310153211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mosimah CI, Murray PJ, Simpkins JW. Not all clots are created equal: A review of deficient thrombolysis with tissue plasminogen activator (tPA) in patients with metabolic syndrome. Int J Neurosci. 2019;129:612–8. doi: 10.1080/00207454.2018.1550400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher MJ. Brain regulation of thrombosis and hemostasis: From theory to practice. Stroke. 2013;44:3275–85. doi: 10.1161/STROKEAHA.113.000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carmeliet P, Schoonjans L, Kieckens L, Ream B, Degen J, Bronson R, et al. Physiological consequences of loss of plasminogen activator gene function in mice. Nature. 1994;368:419–24. doi: 10.1038/368419a0. [DOI] [PubMed] [Google Scholar]


